This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.66.1
Introduction
Despite progress in modelling human drug toxicity, many compounds fail during clinical trials due to unpredicted side effects. The cost of clinical studies are substantial, therefore it is essential that more predictive toxicology screens are developed and deployed early on in drug development (Greenhough et al 2010). Human hepatocytes represent the current gold standard model for evaluating drug toxicity, but are a limited resource that exhibit variable function. Therefore, the use of immortalised cell lines and animal tissue models are routinely employed due to their abundance. While both sources are informative, they are limited by poor function, species variability and/or instability in culture (Dalgetty et al 2009). Pluripotent stem cells (PSCs) are an attractive alternative source of human hepatocyte like cells (HLCs) (Medine et al 2010). PSCs are capable of self renewal and differentiation to all somatic cell types found in the adult and thereby represent a potentially inexhaustible source of differentiated cells. We have developed a procedure that is simple, highly efficient, amenable to automation and yields functional human HLCs (Hay et al 2008; Fletcher et al 2008; Hannoun et al 2010; Payne et al 2011 and Hay et al 2011). We believe our technology will lead to the scalable production of HLCs for drug discovery, disease modeling, the construction of extra-corporeal devices and possibly cell based transplantation therapies.
Flow Chart
Initial preparation of all chemical stocks and coating of culture plasticware
All steps to be carried out in a tissue culture hood under aseptic conditions.
Preparation of human basic Fibroblast Growth Factor (hbFGF)
- Prepare 10% BSA solution in PBS and filter through a 0.22 μm filter.
- From the 10% BSA solution prepare a 0.2% BSA solution.
- Add 10 mL 0.2% BSA solution/100 μg hbFGF.
- Pre-wet a 0.22 μm filter by filtering 5 mL 10% BSA solution through the filter. Discard the 10 mL BSA wash.
- Filter the hbFGF through the pre-washed filter.
- Aliquot the hbFGF in sterile eppendorfs and store at −20°C.Preparation of Human Activin A Stock Solution
- Add 1 mL of 0.2% BSA into a syringe and pre wet the filter.
- Dilute the Activin A in 0.2% BSA to a stock concentration of 100 μg/mL.
- Filter the Activin A solution and aliquot in sterile eppendorfs, store at −20°C.Preparation of Mouse Wnt3a Stock Solution
- Add 200 μl of PBS to a 2 μg vial of Wnt3a to a stock concentration of 10 μg/mL.
- Aliquot in sterile eppendorfs and store at −20°C.Preparation of Human HGF Stock Solution (1000X)
- Dilute the HGF in PBS to a stock concentration of 10 μg/mL.
- Filter the HGF solution and aliquot in sterile eppendorfs, store at −20°C.Preparation of Oncostatin M Stock Solution (1000X)
- Dilute the OSM in PBS to a stock concentration of 20 μg/mL.
- Filter the OSM solution and aliquot in sterile eppendorfs, store at −20°C.Coating of culture plasticware with Matrigel
- Thaw the 10 mL stock bottle of Matrigel overnight at 4°C on ice and then add 10 mL of KO-DMEM. Mix well using chilled pipettes and store 1 mL aliquots at −20°C.
- Thaw an aliquot of Matrigel at 4°C for at least 2 hours or overnight to avoid the formation of a gel.
- Add 5 mL of cold KO-DMEM to the matrigel, mix well with a pipette.
- Make up to 15 mL with cold KO-DMEM and mix using a pipette.
- Add matrigel to the plate or flask to be coated (Table 1)
- Incubate the coated plate or flask overnight at 4°C or room tempature for 1 hour before use.
- Plates or flasks which have been coated with matrigel can be stored at 4°C for up to 1 week. They should be clearly labelled with the date they were coated. Discard any plates or flasks not used within one week.
- Before use allow the coated culture container to come up to room temperature inside a tissue culture hood.
- Immediately prior to use aspirate the matrigel and add the cell suspension to the well or flask.
Routine maintenance of hESC cultures and characterization
Resuscitation of hESC lines
- 23. Remove hESCs from liquid nitrogen storage and quickly thaw in a 37°C water bath.
- 24. Transfer the cell suspension carefully to a sterile tube containing several mL of warm medium.
- 25. Pellet the cells by centrifugation @ low speed for 5 min (1000 RPM).
- 26. Aspirate off the supernatant and very gently resuspend the cells into warm ES medium and plate out on a MEF feeder layer.
- 27. Refeed cells daily with fresh ES medium and upon subconfluence, the cells require passaging.Routine hESC maintenanceES cells are grown in plates or flasks coated with matrigel. The cells need to be examined and fed daily:
- 28. Examine under the microscope for contamination, cell morphology and confluence.
- 29. Aspirate the spent medium.
- 30. Add an appropriate volume of fresh Mouse Embryonic Fibroblast-Conditioned Medium (MEF-CM) + human bFGF (final concentration 4 ng/mL) or other serum free media6.Passaging cells with collagenasehESC lines (H1, H9 and RCM-1) will reach confluence every 5–7 days following passaging at a 1:3 split ratio. Early passage hESCs in the presence of stroma grow slower and the time on the matrigel can become an important factor. Human ESCs should not be left longer than 14 days on the same matrigel due to matrix degradation and in this instance it may be necessary to passage the cells at a 1:1 or 1:2 split ratio if the cells are subconfluent.Enzyme Incubation
- 31. All steps to be carried out in a tissue culture hood under aseptic conditions.
- 32. Ensure there is a new matrigel coated dish or flask prepared as per section 1.6.
- 33. Decide on the desired split ratio for the cells. A number of factors are involved in deciding the split ratio:
- High level of stroma with low numbers of colonies: can be passaged back to a smaller well size or can be passaged 1:1 which will get rid of some stroma and thus increase the colony to stroma ratio, promoting hESC growth.
- High level of stroma with large colonies, depending on the number of colonies, can be passaged 1:1 or 1:2 if there are enough large hESC colonies.
- Typical growing hESCs with a little stroma or no stroma and/or some differentiation can be passaged 1:2 or 3.
- Aspirate the media from the well or flask.
- Wash once with 2 mLs PBS (-MgCl2, -CaCl2).
- Add an appropriate volume of collagenase (200 U/mL diluted in KO-DMEM) and incubate at 37°C for 2–5 min. From 2 minutes onwards examine regularly under the microscope (1 minute intervals). At the point the differentiated cells begin to lift off and the colonies begin to lift at the edge the cells are ready to be passaged.
Scraping and Pooling the hESCs - 34. Aspirate the collagenase.
- 35. Wash once with 2 mLs PBS (-MgCl2, -CaCl2).
- 36. Add an appropriate volume of MEF-CM depending on the split ratio, and using a cell scraper physically remove the cells from the surface of the well or flask, then triturate gently by pipetting up and down 2–3 times using a 10 mL pipette. It is important that the hESCs are kept in clumps of cells and are not broken up into single cells.Replating the hESCs
- 37. Replate the resulting cell suspension onto the new matrigel coated flasks or wells.
- 38. Make the volume of MEF-CM up to 4 mL for a well of a 6-well plate.
- 39. When placing the cells in the incubator agitate the tissue culture container to ensure as even as possible a distribution of colonies as the colonies tend to settle in the centre of the tissue culture plate/flask affecting cell replating and differentiation.Routine characterisation of hESC populations by flow cytometry
- 40. hESC populations are examined via flow cytometry once a month for stem cell markers such as Oct 3/4, Tra 1-60 and SSEA-4.
- 41. hESC populations are removed from their substrate as single cell suspensions following a 5 minute treatment with trypsin/EDTA (Invitrogen).
- 42. Resuspend single cells at 1×106 cells/mL in PBS supplemented with 0.1% BSA and 0.1% sodium azide.
- 43. Incubate cell preparations at 4°C with the appropriate antibodies for 40 minutes.
- 44. Wash cells twice with PBS supplemented with 0.1% BSA and 0.1% sodium azide to remove unbound antibody and resuspend to a final volume of 100 μL and analyse by flow cytometry.
- 45. Data for 30000–40000 “live” events are acquired for each sample using a FACS Caliber cytometer equipped with a 488-nm laser and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). Unstained cells are included as controls. Dead and apoptotic cells along with debris were excluded from analysis using an electronic live gate on forward scatter and side scatter parameters.
Differentiation of hESCs to hepatic endoderm
Preparation of Media for differentiation of hESCs to hepatic endoderm.
All media preparation should be carried out in a tissue culture hood under aseptic conditions.
Preparation of RPMI:B27 priming medium for endoderm differentiation
- 46. For RPMI-B27 medium, mix RPMI 1640 (500 mL) and B27 (50×, 10 mL)
- 47. Swirl to mix components.
- 48. Add all components to a filter unit and filter under vacuum, store at 4°C.Preparation of SR-DMSO medium for hepatocyte differentiation
- 49. For SR-DMSO medium, mix 80% KO-DMEM, 20% KO-SR, 0.5% L-glutamine, 1% non-essential amino acids, 0.1 mM β-Mercaptoethanol and 1% DMSO.
- 50. Filter the solution under vacum, store at 4°C and aliquot and store at −20°C if required.
- 51. Use 4 mL per well of a 6-well plate, and 6 mL per T25 flask.Preparation of L15 maturation medium for hepatocyte maturation
- 52. For L-15 medium, mix 500 mL Leibovitz L-15 medium, tryptose phosphate broth (final concentration 8.3%), heat inactivated foetal bovine serum (final concentration 8.3%), 10 μM hydrocortisone 21-hemisuccinate, 1 μM Insulin (bovine pancreas), 1% L-Glutamine, 0.2% ascorbic acid.
- 53. Filter the solution under vacum, store at 4°C and aliquot and store at −20°C if required.Preparation of final RPMI:B27 priming medium
- 54. Dispense the required volume of priming medium for the experiment (1 mL per well of a 6-well plate, and 2 mL per T25 flask).
- 55. Add Activin A to a final concentration of 100 ng/mL.
- 56. Add recombinant Wnt3a to a final concentration of 50 ng/mL.
- 57. Mix well and the media is now ready for use.
- 58. This final media should be made up fresh each day.Preparation of final L-15 maturation medium
- 59. Dispense the required volume of L-15 medium for the experiment (4 mL per well of a 6-well plate, and 6 mL per T25 flask).
- 60. Add HGF to a final concentration of 10 ng/mL.
- 61. Add OSM to a final concentration of 20 ng/mL.
- 62. Mix well and the media is now ready for use.
- 63. This final media should be made up fresh each day.Priming hESCs to definitive endoderm
- 64. Culture hESCs (H1, H9 and RCM-1) and propagate on matrigel coated plates with mouse embryonic fibroblast MEF-CM supplemented with bFGF.
- 65. Initiate hepatic differentiation when hESCs reach a confluency level of approximately 30%–60% (depending on the hESC line) by replacing the MEF-CM with priming medium (RPMI 1640-B27 supplemented with 100 ng/mL Activin A and 50 ng/mL Wnt3a.
- 66. The cells are cultured in priming medium for 3 days (changing the medium every 24 hours), and final priming medium with Activin A and Wnt3a is made up fresh each day.
- 67. After 72 hours in priming medium, change the medium to the second differentiation medium (SR-DMSO) for 5 days (changing the medium every 48 hours).
- 68. At day 8 culture the cells in maturation and maintenance medium (L-15) supplemented with 10 ng/mL hHGF and 20 ng/mL OSM for 9 days (changing medium every 48 hours). Maturation and maintenance medium with hHGF and OSM is made up fresh each day.
- 69. The cells gradually exhibit morphological changes from a spiky/triangular shape to a characteristic liver morphology displaying a polygonal appearance (Figure 2A).
Characterisation of hESC derived hepatic endoderm
Immunostaining
- 70. Wash hESC derived HLCs with PBS twice, 1 minute each wash.
- 71. Fix the HLCs with 4% PFA for 20 minutes at room temperature, (the cells can be stored in PBS at 4°C and stained at a later date). The cells can also be fixed with ice cold methanol for 10 mins at −20°C.
- 72. Wash the cells twice with PBS, 5 minutes each wash.
- 73. Incubate the cells for 2 minutes at room temperature with 100% ethanol for nuclear staining (this step is not required if methanol fixation is used).
- 74. Wash the cells twice with PBS, 5 minutes each wash.
- 75. Block the cells with PBS/T (0.1% tween)/10% BSA for 1 hour at room temperature.
- 76. Remove the blocking buffer and add the respective primary antibody diluted in PBS/T (0.1% tween)/1% BSA and incubate for 2 hours at room temperature, or overnight at 4°C with agitation.
- 77. Wash the cells 3 times with PBS/T (0.1% tween)/1% BSA at room temperature, 5 minutes each wash.
- 78. Add the appropriate secondary Alexa Fluor antibody (1:400) diluted in PBS to the cells and incubate at room temperature for 1 hour in the dark with agitation.
- 79. Wash the cells 3 times with PBS, 5 minutes each wash.
- 80. Mount each well with MOWIOL 4-88 and DAPI (1:1000). Cover the well with a cover slip, pressing the coverslip gently to ensure that all air bubbles are removed and store at 4°C in the dark.RNA isolation and extraction
- 81. Wash the hESC derived HLCs with PBS and aspirate.
- 82. Add 1 mL of TRIZOL reagent and incubate at room temperature for 5 minutes.
- 83. Scrape the cells and place in a 1.5 mL eppendorf (store at −80°C for later use if required).
- 84. Add 0.5 mL of Chloroform to the eppendorf and mix by inverting; make sure this is done in a fume hood.
- 85. Centrifuge the solution at 13,000 RPM for 15 minutes at 4°C.
- 86. Collect the aqueous layer and place into a clean eppendorf, make sure there is no contamination from the interface.
- 87. Add 1 mL of isopropanol and mix by inverting, leave at room temperature for 10 minutes to precipitate the RNA.
- 88. Centrifuge at 13,000 RPM for 10 minutes at 4°C.
- 89. Aspirate the supernatant and make sure not to disturb the RNA pellet. Wash with 0.5 mL of 70% ethanol and leave at room temperature for 5 minutes.
- 90. Centrifuge at 8,000 RPM for 5 minutes at 4°C.
- 91. Aspirate the ethanol and leave to dry at room temperature for 5–10 minutes.
- 92. Once all the ethanol has evaporated, resuspend the pellet in 30 μl of deionised water. Store the RNA at −80°C for later use.
- 93. Quantify the RNA concentration using a nanodrop.Reverse Transcription PCR
- 94. Set up a reaction using previously isolated RNA (200 ng), random hexamers, nucleotides (10 mM) reverse transcriptase and the respective buffer in a thin walled 0.5 mL eppendorf.
- 95. Set up a negative RT, the above reaction without the reverse transcriptase.
- 96. Place the tubes into a thermo cycler, PCR machine, and set up the following program:
- 37°C - 5 minutes (1 cycle)
- 42°C - 1 hour (1 cycle)
- 95°C - 5 minutes (1 cycle)
- 97. Store the cDNA at −20°C for later use if required.TAQMAN quantitative reverse transcriptase polymerase chain reaction
- 98. Harvest the cells at different time points throughout the differentiation protocol. Extract the RNA and carry out reverse transcription qPCR using the following primers and Assay on demand, (Applied Biosystems) protocol:
- Oct 4 Hs03005111_g1
- Nanog Hs02387400_g1
- Albumin Hs00910225_m1
- Alpha-fetoprotein Hs00173490_m1
Reverse transcription and TAQMAN qPCR - 99. Take 1 μg of RNA and reverse transcribe to cDNA using Invitrogen's Superscript III reverse transcription kit, as per manufacturer's instructions.
- 100. 1 μl of the cDNA used in a 25 μl TAQMAN reaction consisting of the appropriate primers from Applied Biosystems, 18S ribosomal control primers and Invitrogen's 2× platinum qPCR supermix UDG with rox and an appropriate volume of water.
- 101. Mix well and place 10 μl of each sample into two wells of either a 96 or 384 well qPCR plate.
- 102. Once all samples are loaded, plus the appropriate controls), seal the plate and analyse on the Applied Biosystems 7900HT TAQMAN machine.
- 103. Results are expressed as relative expression over a control sample.Functional Analysis of hESC Derived Hepatic Endoderm Cytochrome P450 Assays - http://www.promega.com/tbs/tb325/tb325.pdf
- 104. Incubate day 17 hESC derived HE with the specific substrate for 5 hours at 37°C (n = 3). Use tissue culture media as a negative control and incubate at 37°C for 5 hours.
- 105. Collect the supernatants and carry out the assay as per the manufacturer's instructions.
- 106. Measure the relative levels of basal activity and normalise to per mg protein as determined by the BCA Assay (http://www.piercenet.com/products/browse.cfm?fldID = 02020101).
Notes
- All volumes are based on a 6-well plate format. Adjust the volumes accordingly for the required plate or flask.
- All priming, differentiation and maturation medium is filtered under vacuum before use.
- Priming, differentiation and maturation medium is stored at 4°C for no longer than 2 weeks. Assess how much medium is required for the experiment and aliquot the remaining media and store at −20°C for future use.
- Matrigel is made up as per the manufacturer's instructions; 1 mL aliquots can be stored at −20°C until use.
- Growth factors once made up and aliquoted can be stored at −20°C and when thawed can be stored at 4°C for no longer than 2 weeks.
- The Primary antibody usually used to characterise hESC derived HLCs is albumin (1:250, Sigma-Aldrich, Saint Louis, MO).
Representative results
Characterisation of hESCs maintained prior to hepatic differentiation
In order to characterise the stem cell status of the H9 hESCs used in the study we studied a number of parameters. The cells exhibited hESC morphology, small, tightly packed cells growing in defined colonies (Figure 1A) and expressed the pluripotent stem cell gene markers, Oct-3/4 and Nanog (Figure 1B). We did not find significant differences in the expression of these genes compared to a H7 hESC line positive control. Additionally, 90.1% of the hESCs population was positive for the stem cell marker SSEA-4 (Figure 1C).
hESC differentiation to hepatic endoderm
Human embryonic stem cells can be efficiently differentiated to hepatic endoderm in vitro (Hay et al 2008). At day 9 of differentiation, cells were harvested and differentiation of hESC to HLCs was assessed. As previously reported the hESCs exhibited a series of profound morphological changes, and by day 9, exhibited early hepatocyte morphology developing a polygonal appearance (Figure 2A). Moreover, downregulation of Oct-3/4 over the 9 day time course was observed (Figure 2B). In contrast, liver transcripts AFP and albumin were up-regulated (Figure 2C) from day 7 onwards.
In vitro maturation of hepatic endoderm
Hepatic endoderm was matured in vitro using the established procedure (Hay et al 2008). On the final day of differentiation cultures were immunostained for human liver markers albumin, AFP and E-cadherin. The yield of HLCs using our procedure is typically 90% (Hay et al 2008; Hanoun et al 2010; Payne et al 2011). HLCs stained positive for Albumin, Alpha-fetoprotein and E-Cadherin (Figure 3).
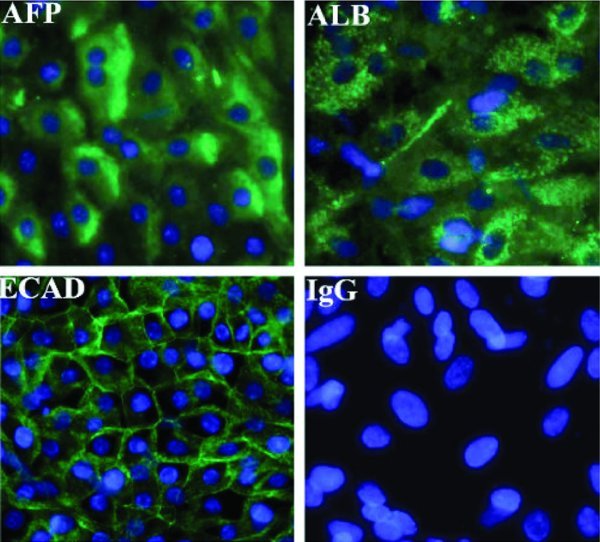
Figure 4
hESC (H9) derived HLCs exhibit metabolic activity. At Day 17, H9 differentiated to HLCs exhibited CYP3A metabolic activity (n = 6).
Discussion
We have developed a simple, homogeneous and highly reproducible in vitro model to generate scalable levels of human HLCs. Our model has been validated by a number of external collaborating laboratories. We routinely characterise stem cell derived HLCs using our in house tool box of developmental markers and liver specific functional assays (most of which are commercially available). The critical stages in our process are: the maintenance of stem cell pluripotency; the ability to direct stem cell differentiation to definitive endoderm; the specification of homogeneous cultures of hepatic endoderm and the ability to derive mature hepatic endoderm exhibiting broad range function in vitro.
One limitation to the large scale deployment of the stem cell derived HLC technology has been the short term differentiated function of mature hepatic endoderm in culture (∼4 days on matrigel). As such we have screened a polymer library for bio-active and novel cellular supports. This has led to the identification of a novel support which maintains hepatic function for at least 15 days. The future directions for this technology are initially industrial application in the drug discovery process (Hay et al 2011). Mid term gains form this technology are likely to be humanised extra-corporeal liver support devices for bridging or treating patients with liver disease. Long term gains from this technology could in cell based transplantation therapy for liver disease, however the current data demonstrates that this strategy requires significant effort before it can be used safely in the clinic (Payne et al 2011).
In conclusion, the significance of our in vitro technology is the ability to generate homogeneous and limitless cultures of high fidelity human HLCs for applied biology.
Materials and preparation
Matrigel coating plates and flasks
- Matrigel (10 mL, BD Biosciences, UK); store at −20°C.
- KO-DMEM (500 mL, Gibco, Invitrogen, UK); store at 4°C.
- Tissue culture plates (6 well, 12 well, Corning, UK)
- Tissue culture flask (25 cm2 vented, Corning, UK)
hESC Maintenance
- Mouse embryonic fibroblast conditioned medium (MEF-CM) (100 mL, R & D Systems, USA); store at −20°C.
- BSA solution (50 mL, Sigma Aldrich, UK); store at 4°C.
- Human basic fibroblast growth factor (100 μg, Peprotech, USA); store at −20°C.
Passaging hESCs with collagenase
- Confluent well or flask of hESCs.
- Matrigel coated wells or flasks as appropriate.
- Phospate buffered saline (-MgCl2, -CaCl2) (500 mL, Gibco, Invitrogen, UK); store at room temperature.
- Collagenase IV (1 g, Gibco, Invitrogen, UK); store at 4°C.
- Mouse embryonic fibroblast conditioned medium (MEF-CM) (100 mL, R & D Systems, USA).
- Human basic fibroblast growth factor (100 μg, Peprotech, USA).
Differentiation of hESCs to hepatic endoderm
- RPMI 1640 (500 mL, Gibco, Invitrogen, UK); store at 4°C.
- B27 Supplement (10 mL, Gibco, Invitrogen, UK); store at −20°C.
- Activin A (2 μg, Peprotech, USA); store at −20°C.
- Recombinant mouse Wnt3a (2 μg, R & D Systems, USA); store at −20°C.
- KO-DMEM (500 mL, Gibco, Invitrogen, UK); store at 4°C.
- KO-SR (500 mL, Gibco, Invitrogen, UK); store at −20°C.
- Non-essential amino acids (100 mL, Gibco, Invitrogen, UK); store at 4°C.
- β-Mercaptoethanol (10 mL, Gibco, Invitrogen, UK); store at 4°C.
- DMSO (Sigma Aldrich, UK); store at room temperature
- Leibovitz L-15 culture medium (500 mL, Sigma Aldrich, UK); store at 4°C.
- Tryptose phosphate broth (100 mL, Sigma Aldrich, UK); store at 4°C.
- Foetal bovine serum, heat inactivated (500 mL, Gibco, Invitrogen, UK); store at −20°C.
- Hydrocortisone 21-hemisuccinate (100 mg, Sigma Aldrich, UK); store at −20°C.
- Insulin (bovine pancreas) (100 mg, Sigma Aldrich, UK); store at −20°C.
- L-Glutamine (100 mL, Gibco, Invitrogen, UK); store at −20°C.
- Ascorbic acid (25 g, Sigma Aldrich, UK); store at −20°C.
- Human HGF (10 μg, Peprotech, USA); store at −20°C.
- Recombinant Human Oncostatin M (OSM) (50 μg, R & D Systems, USA); store at −20°C.
- Syringe driven filter unit 0.22 μm (Millipore, UK)
Characterisation of hESC derived hepatic endoderm
Immunostaining
- Phosphate buffer saline (-MgCl2, -CaCl2) (500 mL, Gibco, Invitrogen, UK); store at room temperature.
- PBST, PBS made up with 0.1% TWEEN 20 (Sigma-Aldrich, UK).
- Paraformaldehyde (PFA) (Sigma-Aldrich, UK) is made up in PBS, store −20°C.
- Glycerol (Sigma-Aldrich, UK), store at room temperature.
- Tris Base (Sigma-Aldrich, UK), store at room temperature.
- Ethanol
- Serum (AbD Serotech, UK), store at −20°C.
- Secondary Antibody, Alexa Fluorophores (Molecular Probes, Invitrogen, UK).
- MOWIOL 4-88 (Polysciences Inc, USA) is made up in Tris HCL and glycerol as per manufacturers instructions. DAPI (Pierce, Thermo Fisher Scientific, UK) is added to the MOWIOL solution at a 1:1000 dilution.
Primary antibodies
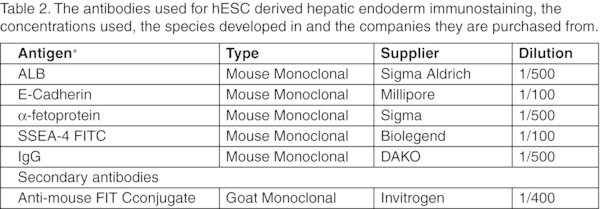
Table 2
The antibodies used for hESC derived hepatic endoderm immunostaining, the concentrations used, the species developed in and the companies they are purchased from.
Functional analysis of aepatic endoderm and normalisation (per mg protein)
Cytochrome P450 Assays
- p4-GLO CYP3A4, CYP1A2, Kits and luminometer (Promega, USA).
- White flat bottom 96 well assay plate (BD Biosciences, UK).
- BCA Assay Kit (Pierce, Thermo Fisher Scientific, UK).
- Transparent 96 well assay plate (IWAKI, UK)
References
- 1.
- Asgari S, Pournasr B, Salekdeh G.H, Ghodsizadeh A, Ott M, Baharvand H. Induced pluripotent stem cells: a new era for hepatology. J. Hepatol. 2010;53(4):738–51. [PubMed: 20621379] [CrossRef]
- 2.
- Hay D.C, Pernagallo S, Diaz-Mochon J.J, Medine C.N, Greenhough S, Hannoun Z, Schrader J, Black J.R, Fletcher J, Dalgetty D, Thompson A.I, Newsome P.N, Forbes S.J, Ross J.A, Bradley M, Iredale J.P. Unbiased Screening of Polymer Libraries to Define Novel Substrates for Functional Hepatocytes with Inducible Drug Metabolism. Stem Cell Research. 2011;6:92–101. [PubMed: 21277274] [CrossRef]
- 3.
- Payne C.M, Samuel K, Pryde A, King J, Brownstein D, Schrader J, Medine C.N, Forbes S.J, Iredale J.P, Newsome P.N, Hay D.C. Persistence of Functional Hepatocyte Like Cells in Immune Compromised Mice. Liver International. 2011;31(2):254–62. [PubMed: 21143581] [CrossRef]
- 4.
- Greenhough S, Medine C, Hay D.C. Pluripotent Stem Cell Derived Hepatocyte Like Cells and their Potential in Toxicity Screening. Toxicology. 2010;278:250–255. [PubMed: 20674645] [CrossRef]
- 5.
- Medine C.N, Greenhough S, Hay D.C. The Role of Stem Cell Derived Hepatic Endoderm in Human Drug Discovery. Biochemical Society Transactions. 2010;38(4):1033–6. [PubMed: 20658999] [CrossRef]
- 6.
- Hannoun Z, Fletcher J, Greenhough S, Medine C.N, Samuel K, Sharma R, Pryde A, Black J.R, Ross J.A, Wilmut I, Iredale J.P, Hay D.C. The Comparison between Conditioned Media and Serum Free Media in Human Embryonic Stem Cell Culture and Differentiation. Cellular Reprogramming. 2010;12(2):133–140. [PubMed: 20677928] [CrossRef]
- 7.
- Dalgetty D.M, Medine C, Iredale J.P, Hay D.C. Progress and Future Challenges in Stem Cell-Derived Liver Technologies. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2009;297(2):G241–8. [PubMed: 19520740] [CrossRef]
- 8.
- Hay D.C, Fletcher J, Payne C, Terrace J.D, Gallagher R.C.J, Snoeys J, Black J, Wojtacha D, Samuel K, Hannoun Z, Pryde A, Filippi C, Currie I.S, Forbes S.J, Ross J.A, Newsome P, Iredale J.P. Highly Efficient Differentiation of hESCs to Functional Hepatic Endoderm Requires Activin and Wnt3a Signalling. Proceedings of the National Academy of Sciences U. S. A. 2008;105(34):12301–12306. [PMC free article: PMC2518825] [PubMed: 18719101] [CrossRef]
- 9.
- Fletcher J, Cui W, Samuels K, Black J.R, Currie I.S, Terrace J.D, Payne C, Filippi C, Newsome P, Forbes S.J, Ross J.A, Iredale J.P, Hay D.C. The Inhibitory Role of Stromal Cell Mesenchyme on Human Embryonic Stem Cell Hepatocyte Differentiation is Overcome by Wnt3a Treatment. Cloning and Stem Cells. 2008;10(3):331–340. [PubMed: 18479212] [CrossRef]
- *
Last revised March 28, 2012. Published June 10, 2012. This chapter should be cited as: Medine, C. N., Lucendo-Villarin, B., Zhou, W., West, C. C., and Hay, D. C., Robust generation of hepatocyte-like cells from human embryonic stem cell populations (June 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.66.1, http://www
.stembook.org.
Figures

Figure 1
Schematic for hepato-cellular differentiation.
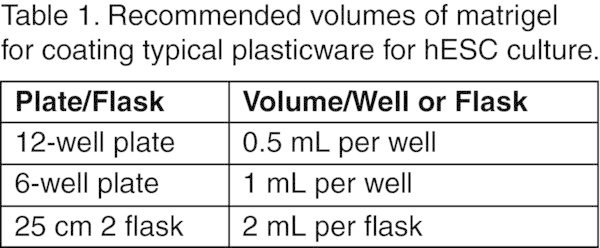
Table 1
Recommended volumes of matrigel for coating typical plasticware for hESC culture.
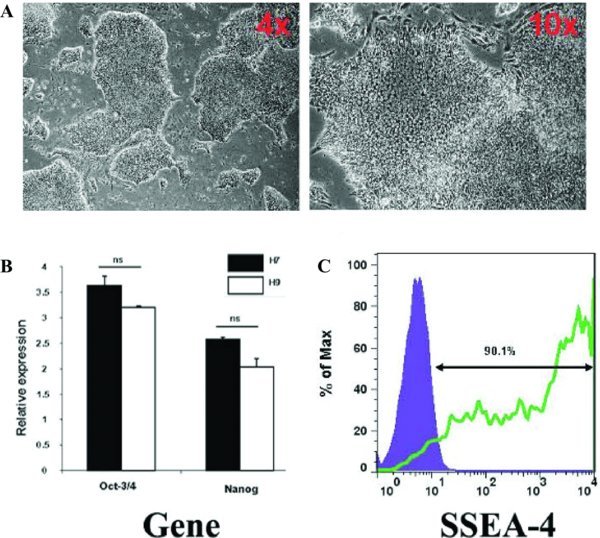
Figure 1
Characterisation of hESCs used in this study. (A) Phase contrast microscopy images representative of hESC morphology observed in culture at 4× and 10× magnification. The images were captured using a Nikon TE3000/U inverted microscope (B) hESCs cultured are Octamer (Oct-3/4) positive and Nanog positive. H7 hESCs were use as a control for pluripotency gene expression levels. Relative expression refers to folds of induction compared with the endogenous gene control, β-2-microglobulin. (C) FACS plots show hESC surface marker expression levels, including stage specific embryonic antigens SSEA 4.
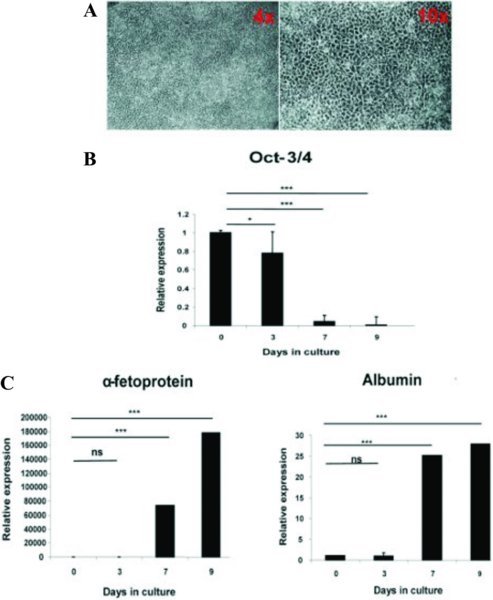
Figure 2
(A) Phase contrast microscopy representative images of hESC-derived hepatic endoderm morphology at day 9 of the differentiation observed in culture, at ×4 and ×10 magnification. (B) Characterization of changes in the gene expression. RNA was extracted and the cDNA was analysed by quantitative polymerase chain reaction, showing progressive downregulation of undifferentiated cell gene expression (Oct-3/4) and (C) Upregulation of hepatocyte gene expression (albumin and α-fetoprotein). Relative expression refers to folds of induction compared with the endogenous gene control, β-2-microglobulin at day 0 of differentiation. P<0.05 is denoted * and P<0.001 is denoted *** measured by students t-test in comparison to hESCs at day 0. Error bars represent 1 standard deviation.

Figure 3
Characterisation of hESC-derived hepatic endoderm. Immunocytochemistry showing expression of hepatocyte markers, albumin, AFP and E-Cadherin in hESC (H9) derived hepatic endoderm. Negative controls were performed with corresponding immunoglobulin G (IgG) and representatives images are shown.
