NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, Dana T, Bougatsos C, et al. Pressure Ulcer Risk Assessment and Prevention: Comparative Effectiveness [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 May. (Comparative Effectiveness Reviews, No. 87.)
This publication is provided for historical reference only and the information may be out of date.

Pressure Ulcer Risk Assessment and Prevention: Comparative Effectiveness [Internet].
Show detailsOverview
The search and selection of articles are summarized in the study flow diagram (Figure 2). Database searches resulted in 4,773 potentially relevant articles. After dual review of abstracts and titles, 747 articles were selected for full-text review, and 120 studies (in 122 publications) were determined by dual review at the full-text level to meet inclusion criteria and were included in this review. Data extraction and quality assessment tables for all included studies per key question are available in Appendix H.
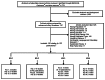
Figure 2
Literature flow diagram. a Cochrane databases include the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews. b Other sources include reference lists, peer reviewer suggestions, etc.
Key Question 1. For adults in various settings, is the use of any risk-assessment tool effective in reducing the incidence or severity of pressure ulcers, compared with other risk-assessment tools, clinical judgment alone, and/or usual care?
Key Points
- One good-quality, randomized trial (n=1,231) found no difference in pressure ulcer incidence between patients assessed with either the Waterlow scale or Ramstadius tool compared with clinical judgment alone (RR 1.4, 95% CI 0.82 to 2.4 and RR 0.77, 95% CI, 0.44 to 1.4, respectively) (strength of evidence: insufficient).
- One poor-quality, nonrandomized study (n=240) found use of a modified version of the Norton scale in conjunction with standardized use of preventive interventions based on risk score associated with lower risk of pressure ulcers compared with nurses’ clinical judgment alone (RR 0.11, 95% CI, 0.03 to 0.46) (strength of evidence: insufficient).
- One poor-quality, cluster randomized trial (n=521) found no difference between training in and use of the Braden score compared with nurses’ clinical judgment in risk of incident pressure ulcers, but included patients with prevalent ulcers (strength of evidence: insufficient).
Detailed Synthesis
One good-quality study and two poor-quality studies evaluated effects of using a formal risk assessment instrument compared with nurses’ judgment alone on subsequent risk of pressure ulcers (Appendix Tables H1, H2, and H3).13,45,46 The good-quality study was a randomized, controlled trial comparing the Waterlow scale and Ramstadius tool to clinical judgment.13 Of the two poor-quality studies, one was a nonrandomized study45 that evaluated a modified version of the Norton scale, and the other was a cluster randomized trial46 that evaluated the Braden scale. All three studies compared use of standardized instruments against nurses’ clinical judgment, which could introduce variability across studies due to differences in experience, training, skills, or other factors.
The good-quality trial (n=1,231) randomized newly admitted internal medicine or oncology patients to either the Waterlow scale, Ramstadius tool (an unvalidated risk assessment and intervention protocol) or nurses’ judgment.13 Baseline pressure ulcer risk scores were not reported, though 6 percent of patients had a pressure ulcer at baseline (primarily stage 1 or 2). There was no difference between interventions in risk of pressure ulcers after a mean of 9 days (8 vs. 5 vs. 7 percent for Waterlow vs. Ramstadius vs. clinical judgment; RR 1.4, 95% CI, 0.82 to 2.4 for Waterlow vs. clinical judgment and RR 0.77, 95% CI, 0.44 to 1.4 for Ramstadius vs. clinical judgment), or in length of stay (8.8 vs. 9.4 vs. 8.5 days, respectively). The proportion of patients that received more intensive preventive interventions (more advanced support surfaces, documented pressure ulcer care plan, skin integrity referral, or dietician referral) was similar across groups.
The nonrandomized study (n=240) evaluated hospice patients during an intervention period in which a modified Norton scale was applied and used to inform pressure ulcer prevention interventions (based on a standardized protocol), compared with a nonconcurrent control period in which the modified Norton scale was applied but not used to inform interventions.45 The modified Norton scale replaced the items “activity” and “mental conditions” with “nutritional status” and “pain,” and included additional items (diabetes, vascular disease, intravenous infusions or epidurals, altered mental status, lymphedema or ascites, fungating wound, and paraplegia), resulting in a possible range of scores of 5 to 39 (higher score indicating greater risk), compared with 5 to 20 on the original Norton scale. In the intervention period, patients with a score ≤10 received a hollow core fiber overlay; with a score between 11 and 15, a basic alternating air mattress overlay; and with a score ≥16, a more sophisticated alternating pressure mattress replacement. Patients in the comparison group received a hollow core fiber overlay unless they requested a special overlay or mattress used prior to admission. In addition, patients at high risk based on nurses’ judgment received the same alternating pressure mattress replacement as the highest risk patients (score ≥ 16) in the intervention group. The intervention was associated with a lower risk of incident pressure ulcers (2.5 vs. 22 percent, RR 0.11; 95% CI, 0.03 to 0.46), with more patients in the intervention compared with the comparison group receiving the sophisticated alternating pressure mattress (29 vs. 7.5 percent). Two-thirds of the ulcers were stage 1 and about one-third were stage 2. Methodological shortcomings included use of a nonrandomized design and an unvalidated modification of the Norton scale, higher baseline pressure ulcer risk scores in the intervention group (29 vs. 20 percent had scores ≥16), no statistical adjustment for confounders, and unclear blinding of nurses to modified Norton scores during the comparison period.
A cluster randomized trial (n=521) of patients with a Braden score ≤18 evaluated three interventions: a) pressure ulcer prevention training of nurses with education in use of the Braden scale, and mandatory use of the Braden scale; b) pressure ulcer prevention training of nurses with education in use of the Braden scale, but no mandatory use; and c) no additional pressure ulcer prevention training or training in use of the Braden scale, although pressure ulcer risk was assessed using an ad hoc five-level scale.46 Ward nurses in all three groups also participated in a one-day wound care management training. There was no difference in risk of incident pressure ulcers (22 vs. 22 vs. 15 percent, respectively, p=0.38). Differences between groups in use of preventive interventions were not reported. Methodological shortcomings in this study included unclear methods of randomization and allocation concealment, baseline differences in Braden scores, failure to evaluate cluster effects, and failure to blind outcome assessors to risk assessment scores. In addition, although incident pressure ulcers were reported, patients with pressure ulcers at baseline were included. Both the proportion of patients with ulcers at baseline and the proportion of incident ulcers that occurred in patients with ulcers at baseline were unclear.
A fourth study compared use of the Norton Scale with nurses’ clinical judgment in reducing pressure ulcers, but was excluded because it did not report incident pressure ulcers.47
Key Question 1a. Do the effectiveness and comparative effectiveness of risk-assessment tools differ according to setting?
- No study evaluated how effectiveness of risk assessment tools varies according to care setting (strength of evidence: insufficient).
Three trials on the effects of the use of a formal risk assessment instrument compared with nurses’ judgment on risk of pressure ulcers were conducted in different settings (acute care hospital vs. hospice care) but evaluated different risk assessment instruments and preventive interventions, and two of the studies had important methodological shortcomings, precluding judgments about whether effectiveness varied according to setting.13,45,46
Key Question 1b. Do the effectiveness and comparative effectiveness of risk-assessment tools differ according to patient characteristics, and other known risk factors for pressure ulcers, such as nutritional status or incontinence?
- No study evaluated how effectiveness of risk assessment tools varies in subgroups defined by patient characteristics (strength of evidence: insufficient).
Three trials on the effects of the use of a formal risk assessment instrument compared with nurses’ judgment on risk of pressure ulcers did not evaluate effectiveness in subgroups defined by patient characteristics.13,45,46
Key Question 2. How do various risk-assessment tools compare with one another in their ability to predict the incidence of pressure ulcers?
Key Points
- In two good- and five fair-quality studies (n=92 to 1,772), the median AUROC for the Braden scale was 0.77 (range 0.55 to 0.88). In 16 studies, based on a cutoff of ≤18, the median sensitivity was 0.74 (range 0.33 to 1.0) and median specificity 0.68 (range 0.34 to 0.86), for a positive likelihood ratio of 2.31 and negative likelihood ratio of 0.38 (strength of evidence: moderate).
- In three studies (one good- and two fair-quality; n=1,190 to 1,772), the median AUROC for the Norton scale was 0.74 (range 0.56 to 0.75). In five studies, using a cutoff of ≤14, median sensitivity was 0.75 (range 0.0 to 0.89) and median specificity 0.68 (range 0.59 to 0.95), for a positive likelihood ratio of 1.83 and negative likelihood ratio of 0.42 (strength of evidence: moderate).
- In four studies (one good- and three-fair quality; n=98 to 1,229), the median AUROC for the Waterlow scale was 0.61 (range 0.54 to 0.66). In two studies, based on a cutoff of ≥10, sensitivities were 0.88 and 1.0 and specificities 0.13 and 0.29, for positive likelihood ratios of 1.15 and 1.24 and negative likelihood ratios of 0.0 and 0.41 (strength of evidence: moderate).
- In three studies (one good- and two fair-quality; n=112 to 534), the median AUROC for the Cubbin and Jackson scale was 0.83 (range 0.72 to 0.90). In three studies, based on a cutoff of ≤24 to 29, median sensitivity was 0.89 (range 0.83 to 0.95) and median specificity was 0.61 (0.42 to 0.82), for positive likelihood ratios that ranged from 1.43 to 5.28 and negative likelihood ratios that ranged from 0.06 to 0.40 (strength of evidence: moderate).
- In six studies (two good- and four fair-quality) that directly compared risk assessment tools (n=112 to 1,772), there were no clear differences between scales based on the AUROC (strength of evidence: moderate).
Detailed Synthesis
Forty-seven prospective cohort studies (assessing 53 separate populations in 48 publications) evaluated the diagnostic accuracy of pressure ulcer risk assessment tools (Appendix Table H4).17,18,20–25,39–43,45,48–81 Sample sizes ranged from 31 to over 3,000 patients; the mean age for participants in most studies was between 55 and 65 years. Seven studies assessed patients in community-based care facilities41,45,51,57,71,78,80 and four studies included populations from mixed settings;22,40,52,63 the remainder evaluated hospitalized patients. Twelve studies were rated good-quality,17,18,21,39,42,51,53,63,64,66,67,73,79 four studies poor-quality24,48,71,77 and the remainder fair-quality (Appendix Table H5). Common methodological shortcomings in the fair- or poor-quality studies included unclear methods of patient selection, failure to predefine cutoff scores, poorly described reference standards, and failure to blind outcomes assessment to risk assessment scores. Seventeen studies reported how use of interventions differed according to baseline risk score, but none adjusted for such differences in analyses.18,21,25,39,41–43,45,49,51,57,59–61,64,68,70 Duration of followup following risk assessment was generally not reported.
Braden Scale
The Braden scale was evaluated in 32 studies (in 33 publications) (Appendix Tables H4 and H5). 17,18,20–23,39–42,49–55,58–61,63,64,66–68,70–73,75,77,79 Two studies evaluated modified versions of the Braden in addition to the standard Braden: one added a blood circulation subscale,61 while the other added subscales for skin tone and body type.42
In seven studies of the standard Braden, the median AUROC was 0.77 (range 0.55 to 0.88) (Table 3).20,21,41,55,70,73,75 The other studies did not report the AUROC. Estimates for sensitivity and specificity varied depending on the cutoff (Appendix Table H6). At a cutoff of ≤15 on the standard Braden, median sensitivity was 0.33 (range 0.09 to 0.82) and median specificity was 0.91 (range 0.67 to 0.95) in 12 studies (Table 4).17,22,39,40,49,59,61,63,64,68,71,72 Based on the median sensitivity and specificity at this cutoff, the positive likelihood ratio was 3.67 and negative likelihood ratio 0.74. At a cutoff of ≤16, median sensitivity was 0.77 (range 0.35 to 1.0) and median specificity was 0.64 (range 0.14 to 1.0) in eight studies, for a positive likelihood ratio of 2.14 and negative likelihood ratio of 0.36.17,21,50,54,58,60,66,67,77 At a cutoff ≤18, median sensitivity was 0.74 (range 0.33 to 1.0) and median specificity was 0.68 (range 0.34 to 0.86) in 16 studies, for a positive likelihood ratio of 2.31 and negative likelihood ratio of 0.38.17,18,22,39–41,53,59,61,63,64,67,68,71–73 Excluding two poor-quality studies71,77 or including two studies that evaluated modified versions of the Braden42,61 resulted in similar estimates. One poor-quality study (n=291) that focused on heel ulcers found a Braden score of ≤12 associated with sensitivity of 0.14 and specificity of 0.94 and a Braden of ≤16 associated with sensitivity of 0.49 and specificity of 0.76.77
Table 3
Pressure ulcer risk assessment scales: area under the receiver operator characteristic.
Table 4
Sensitivity and specificity of pressure ulcer risk assessment scales.
Four fair-quality studies reported odds ratios for subsequent pressure ulcers based on Braden scale scores at baseline,41,52,54,61 but none adjusted for potential confounders. In addition, cutoffs varied between studies and studies that used the same cutoff reported inconsistent estimates (Appendix Table H4). For example, one study of 1,772 long-term care patients reported an odds ratio of 6.9 (CI not reported) at a Braden cutoff of ≤18,41 but a study of 813 hospitalized inpatients reported an odds ratio of 2.1 (p=0.03, CI not reported) at the same cutoff.52
Norton Scale
The Norton scale was evaluated in 12 studies (Appendix Tables H4 and H5).18,23,41,42,45,58,65,70,73,76,80,84 Three studies evaluated a modified Norton scale. In one of these studies, small clarifications were incorporated within existing items,76 one study added skin condition, motivation and age to the five existing items,58 and the third study added additional items (e.g. presence of diabetes) and reversed the scoring method, so that higher scores were associated with higher pressure ulcer risk.45 In three studies of the standard Norton, the median AUROC was 0.74 (range 0.56 to 0.75) (Table 3).41,70,73 At a cutoff of ≤14, median sensitivity was 0.75 (range 0.0 to 0.89) and median specificity was 0.68 (range 0.59 to 0.95) in five studies, for a positive likelihood ratio of 2.34 and negative likelihood ratio of 0.37 (Table 4).41,42,65,76,80 Two studies65,76 reported very low sensitivities (0.0 and 0.16) compared with the other three studies (range 0.75 to 0.89). One of these studies (sensitivity 0.16) evaluated a slightly modified version of the Norton scale in patients undergoing elective cardiovascular surgery or neurosurgery.76 The other study (sensitivity 0.0), which used the standard Norton scale, only reported five incident ulcers in 36 older patients in an acute care setting. Excluding these studies had little effect on median sensitivity or specificity (Appendix Table H6). At a cutoff of ≤16, median sensitivity and specificity was 0.75 (range 0.46 to 0.81) and 0.59 (range 0.55 to 0.60), respectively, in three studies, for a positive likelihood ratio of 1.83 and negative likelihood ratio of 0.42.18,73,84 None of the studies were rated poor-quality. One study reported an unadjusted odds ratios for incident pressure ulcers of 4.2 for a cutoff of 12 and 6.6 for a cutoff of 14 (CIs not reported).41
Waterlow Scale
The Waterlow scale was evaluated in ten studies (Appendix Tables H4 and H5).18,23,25,43,56,57,73,74,80,81 In four studies, the median AUROC was 0.61 (range 0.54 to 0.66) (Table 3).25,56,73,74 At a cutoff of ≥10, sensitivities were 0.88 and 1.0 and specificities were 0.13 and 0.29 in two studies, for positive likelihood ratios of 1.15 and 1.24 and negative likelihood ratios of 0 and 0.41.25,80 Sensitivity (0.81) and specificity (0.29) were similar in one study that evaluated a cutoff ≥15.43 However, another study that evaluated the same cutoff (≥15) reported a lower sensitivity (0.67) but higher specificity (0.79).81 In this study, 5 percent (15/274) of patients had pressure ulcers at baseline and 27 percent (74/274) of enrolled patients did not have a baseline Waterlow score; both factors may have affected these results. In another study, a cutoff score of ≥9 was associated with a sensitivity of 0.46 and a specificity of 0.60 (Table 4).73
Other Scales
Few other risk assessment scales were assessed in more than one study. The Cubbin and Jackson scale, consisting of 10 items with total scores ranging from 10 to 40, was associated with a median AUROC of 0.83 (range 0.72 to 0.9) in three studies (Table 3).20,21,25 Based on cutoffs of ≤24 to 29, median sensitivity was 0.89 (range 0.83 to 0.95) and specificity was 0.61 (0.42 to 0.82) in three studies (Table 4).20,21,25 Associated positive likelihood ratios ranged from 1.43 to 5.28 and negative likelihood ratios from 0.06 to 0.40. Two of the studies were rated fair-quality and the other good-quality; the good-quality study reported a sensitivity of 0.89 and specificity of 0.61 at a cutoff of ≤24, for a positive likelihood ratio of 2.28 and negative likelihood ratio of 0.18.21 Other risk assessment tools were evaluated in one study each, including the Gosnell,23 Song and Choi,20 Fragmment,70 Douglas,21 Knoll,78 Risk Assessment Pressure Score Scale (RAPS),24 Northern Hospital Pressure Ulcer Prevention Plan (TNH-PUPP),69 the Dutch CBO Score,84 and others,48,62 precluding reliable conclusions regarding diagnostic accuracy (Appendix Table H4).
Direct Comparisons
Five good-quality18,21,42,73,79 and nine fair-quality20,23,25,41,58,70,72,80,84 studies directly compared one pressure ulcer risk assessment scale to another (Appendix Tables H4 and H5).
Six studies directly compared the AUROC for two or more risk assessment scales (Table 5).20,21,25,41,70,73 In three studies, the AUROC was very similar for the Braden and Norton scales.41,70,73 Two studies that compared the Braden and the Cubbin and Jackson scales also reported similar AUROCs.20,21 One study reported similar AUROCs for the Waterlow compared with the Braden or Norton scales (range 0.55 to 0.61).73 One poor-quality study (n=291) that focused on heel ulcers found no difference in the AUROC for the Braden scale compared with several alternative, derived scales.77
Table 5
Direct comparisons of pressure ulcer risk assessment scales.
Eight studies directly compared sensitivity and specificity for different risk assessment scales based on the standard cutoffs discussed above (Braden ≤16 to 18, Norton ≤12 to 16, Waterlow ≥10 to 15 and/or Cubbin and Jackson ≤24 to 29) (Table 5).18,21,25,41,42,73,80,84 They reported comparable sensitivities and specificities for different risk assessment instruments,18,42,73,84 or the expected tradeoff of higher sensitivity for one scale compared with another, but lower specificity.21,25,41,80
Key Question 2a. Does the predictive validity of various risk-assessment tools differ according to setting?
Key Points
- One fair-quality study (n=843) found a Braden scale score of ≤18 associated with similar sensitivities and specificities in acute care and skilled nursing settings. Twenty-eight studies (10 good-, 16 fair- and two poor-quality) that evaluated the Braden scale in different settings found no clear differences in the AUROC or in sensitivities and specificities at standard (≤15 to 18) cutoffs (strength of evidence: low).
- Two studies (one good- and one fair-quality) found the Cubbin and Jackson scale associated with similar diagnostic accuracy compared with the Braden or Waterlow scales in intensive care patients (strength of evidence: low).
- One good-quality study reported a lower optimal cutoff on the Braden scale in an acute care setting (sensitivity 0.55 and specificity 0.94 at a cutoff of ≤15) compared with a long-term care setting (sensitivity 0.57 and specificity 0.61 at a cutoff of ≤18), but the statistical significance of differences in diagnostic accuracy was not reported. Two studies (one good- and one fair-quality) found that optimal cutoff scores on the Braden scale were lower in surgical patients compared with optimal cutoff scores observed from other studies of patients in different settings, but no study directly compared optimal cutoffs in surgical compared with other care settings (strength of evidence: low).
Detailed Synthesis
Pressure ulcer risk assessment tools have been evaluated in various care settings, including five studies of nonsurgical intensive care patients,21,25,39,56,75 five studies of post-surgery patients,20,43,58,64,76 six studies of long-term care settings (including nursing homes and skilled care),22,40,41,51,63,84 two studies of home care settings,57,71 and one study of hospice patients (Appendix Table H4).45
Only one study evaluated the same risk assessment tool in patient subgroups defined by care setting in which the tool was applied. It found a Braden scale score of ≤18 associated with similar sensitivities and specificities in two acute care (sensitivities 0.88 and 0.60; specificities 0.68 and 0.81) and one skilled nursing setting (sensitivity 0.72; specificity 0.68) (Appendix Table H7).40
The usefulness of indirect comparisons across studies to assess how diagnostic accuracy might differ according to care setting was very limited. The AUROC was infrequently reported, differences in estimates across studies performed in different settings were small, and confidence intervals were not reported by most studies, making it difficult to determine the significance of any differences. For example, for the Braden scale, which was evaluated in the most studies, the AUROC was 0.71 and 0.80 in two studies of intensive care unit patients,21,75 0.88 in one study of surgical patients,20 and 0.77 in one study of long-term care patients41 (Appendix Table H8). Based on a cutoff of ≤15 on the Braden Scale, one study performed in an intensive care unit39 reported a higher sensitivity (0.75) and similar specificity (0.67) compared with studies in surgical (one study),64 long-term care (two studies),22,40 or home care (one study)71 settings, where sensitivities ranged from 0.14 to 0.33, and specificity from 0.83 to 0.95 (Appendix Table H7). Based on a cutoff of ≤18 on the Braden scale, the median sensitivity was 0.72 and median specificity 0.70 in acute care settings (eight studies18,39,40,53,59,61,68,72), compared with 0.76 and 0.65, respectively, in long-term care settings (four studies22,40,41,63). Other cutoffs and risk assessment instruments were evaluated in too few studies to assess differences in diagnostic accuracy across settings. The only study to evaluate hospice patients evaluated a modified version of the Norton scale in which scoring was reversed so that higher scores indicate higher risk and did not report the AUROC.45
Although the Cubbin and Jackson scale was specifically designed for use in intensive care patients, two studies reported a similar AUROC compared with the Braden or Waterlow scales.21,25
Some studies attempted to determine optimal cutoff scores for the Braden scale in specific settings, based on the best combination of sensitivity and specificity (Appendix Table H9). One study reported a lower optimal cutoff on the Braden scale in an acute care setting (sensitivity 0.55 and specificity 0.94 at a cutoff of ≤15) compared with a long-term care setting (sensitivity 0.57 and specificity 0.61 at a cutoff of ≤18), but the statistical significance of differences in diagnostic accuracy was not reported, and estimates were not reported at the same cutoff across settings.63 Two studies of surgical patients found that optimal Braden cutoff scores were lower (≤13 or 14)20,64 than the optimal cutoffs (≤15 to 18) observed in other studies of acute and long-term care settings.22,41,53,55,63,68 However, no study directly compared optimal Braden scale cutoffs in surgical compared with other care settings. Estimates of the optimal cutoff for the Norton, Waterlow and Cubbin and Jackson scales were not frequently reported.
Key Question 2b. Does the predictive validity of various risk-assessment tools differ according to patient characteristics?
Key Points
- One fair-quality study (n=834) reported similar AUROCs for the Braden scale in black and white patients in acute care or skilled nursing settings (strength of evidence: low).
- Three studies (one good- and two fair-quality; n=534 to 1,772) found no clear difference in AUROC estimates based on the presence of higher or lower mean baseline pressure ulcer risk scores (strength of evidence: moderate).
Detailed Synthesis
Few studies assessed the predictive validity of pressure ulcer risk assessment instruments in different patient subgroups defined by patient demographics or clinical characteristics. (Appendix Table H4). Two studies evaluated the predictive validity of a pressure ulcer risk assessment tool in subgroups defined by patient demographics or clinical characteristics.52,67 One study (n=834) reported similar AUROCs for the Braden scale in black (0.82) compared with white (0.75) patients in acute care or skilled nursing settings, as well as similar sensitivity and specificity using a cutoff of ≤18.52 The second study (n=74) found that in an acute care hospital setting, a Braden scale cutoff of ≤16 resulted in sensitivities of 0.77 and 0.9 in older (age 60–74) blacks and Hispanics, with low specificities (0.5 and 0.14).66
Although patient characteristics varied across studies of diagnostic accuracy, such differences are often associated with differences in care setting. In addition, few studies reported the AUROC, and studies applied different thresholds when estimating sensitivity and specificity. In three studies that reported the AUROC and mean baseline pressure ulcer risk scores, there was no clear difference in estimates based on the presence of higher or lower baseline pressure ulcer risk scores (Appendix Table H10).21,25,41,73 One small (n=36) study of younger trauma patients (mean age 32 years) found a Braden cutoff of ≤10 (lower than the usual cutoff range of 15–18) associated with high sensitivity (0.91) and specificity (0.96).49 No other studies exist in this specific population.
Key Question 3. In patients at increased risk of developing pressure ulcers, what are the effectiveness and comparative effectiveness of preventive interventions in reducing the incidence or severity of pressure ulcers?
Key Points
Support Surfaces
Mattresses, Overlays, and Bed Systems
- One good-quality trial (n=1166) and four fair-quality trials (n=83 to 543) found a more advanced static mattress or overlay associated with lower risk of incident pressure ulcers than a standard mattress (RR range 0.16 to 0.82), though the difference was not statistically significant in two trials. Six poor-quality trials reported results that were generally consistent with these findings, though one trial found no benefit. Three trials found no difference in length of stay. The static support surfaces evaluated in the trials varied, though a subgroup of three trials each found an Australian medical sheepskin overlay associated with lower risk of ulcers than a standard mattress (RR 0.30, 0.58, and 0.58) (strength of evidence: moderate).
- Three fair-quality trials (n=52 to 100) found no differences between different advanced static support mattresses or overlays in risk of pressure ulcers. One fair-quality trial (n=40) of nursing home patients found a foam replaceable parts mattress associated with lower risk of ulcers compared with a 4 inch thick, dimpled foam overlay (25 vs. 60 percent, RR 0.42, 95% CI, 0.18 to 0.96). Six poor-quality trials (n=37 to 407) also found no differences between different advanced static mattresses or overlays (strength of evidence: moderate).
- One fair-quality trial (n=98) found a low-air-loss bed associated with lower likelihood of one or more pressure ulcers in intensive care unit patients (12 vs. 51 percent, RR 0.23, 95% CI, 0.10 to 0.51), but a small (n=36), poor-quality trial found no difference between a low-air-loss mattress compared with a standard hospital bed following cardiovascular surgery (strength of evidence: low).
- One fair-quality trial (n=62) found no clear difference between a low-air-loss mattress compared with the Hill-Rom Duo mattress (options for constant low pressure or alternating-air) in risk of ulcers (strength of evidence: low).
- Three poor-quality trials (n=108 to 487) found lower incidence of pressure ulcers with use of an alternating air pressure mattress or overlay compared with a standard hospital mattress (strength of evidence: low).
- Six trials (n=32 to 487, one good-quality, one fair-quality, and four poor-quality) found no difference between an alternating air pressure overlay or mattress compared with various advanced static mattresses or overlays in pressure ulcer incidence or severity (strength of evidence: moderate).
- Four trials (n=44 to 1972; one good-quality, two fair-quality, and one poor-quality) found no clear differences between different alternating air mattresses or overlays. The good-quality (n=1972) trial found no difference in risk of stage 2 ulcers between an alternating air pressure overlay and an alternating air pressure mattress (RR 1.0, 95% CI, 0.81 to 1.3; adjusted OR 0.94, 95% CI, 0.68 to 1.3) (strength of evidence: moderate).
Heel Supports/Boots
- One fair-quality trial (n=239) of fracture patients found the Heelift Suspension Boot associated with decreased risk of heel, foot, or ankle ulcers compared with usual care without leg elevation (7 vs. 26 percent for any ulcer, RR 0.26, 95% CI, 0.12 to 0.53; 3.3 vs. 13.4 percent for stage 2 ulcers, RR 0.25, 95% CI, 0.09 to 0.72). One poor-quality trial (n=52) of hospitalized patients found no difference in risk of ulcers between a boot (Foot Waffle) and usual care (hospital pillow to prop up legs) (strength of evidence: low).
- One poor-quality trial (n=240) of hospitalized patients found no differences between three different types of boots (Bunny Boot, egg-crate heel lift positioner, and Foot Waffle) in risk of ulcers, though the overall incidence of ulcers was low (5 percent over 3 years) and results could have been confounded by differential use of cointerventions (strength of evidence: insufficient).
Wheelchair Cushions
- Four fair-quality trials (n=32 to 248) of older nursing home patients found inconsistent evidence on effects of more sophisticated wheelchair cushions compared with standard wheelchair cushions on risk of pressure ulcers, with the largest trial finding no difference between a contoured, individually customized foam cushion compared with a slab cushion. Results are difficult to interpret because the trials evaluated different cushions (strength of evidence: low).
Nutritional Supplementation
- Five of six trials (one fair-quality and five poor-quality; n=59 to 672) found no difference between nutritional supplementation compared with standard hospital diet in risk of pressure ulcers. Four trials evaluated supplementation by mouth and two evaluated enteral supplementation (strength of evidence: low).
Repositioning
- One fair-quality cluster trial (n=213) found repositioning at a 30-degree tilt every 3 hours associated with lower risk of pressure ulcer compared with usual care (90-degree lateral repositioning every 6 hours during the night) after 28 days (3.0 vs. 11 percent, RR 0.27, 95% CI, 0.08 to 0.93) and one fair-quality trial (n=235) found no difference in risk of pressure ulcers between different repositioning intervals. Two other trials (n=46 and 838) evaluated repositioning interventions but only followed patients for one night or were susceptible to confounding due to differential use of support surfaces (strength of evidence: low).
- Two small (n=15 and 19), poor-quality trials found the addition of small, unscheduled shifts in body position (using a small rolled towel to designated areas during nurse-patient interactions) to standard repositioning every 2 hours had no effect on risk of pressure ulcers, but only reported one or two ulcers each. (strength of evidence: low)
Dressings
- One fair-quality (n=85) trial of patients undergoing cardiac surgery found a silicone border foam sacral dressing applied at intensive care unit (ICU) admission (the Mepilex Border sacrum) associated with lower likelihood of pressure ulcers compared with standard care (including preoperative placement of a silicone border foam dressing for surgery and use of a low air loss bed), but the difference was not statistically significant (2.0 vs. 12 percent, RR 0.18, 95% CI, 0.02 to 1.5) (strength of evidence: low)
- A poor-quality trial of 37 patients in a long-term care facility found use of the REMOIS Pad (consisting of a hydrocolloid skin adhesive layer, a support layer of urethane film, and an outer layer of multifilament nylon) on the greater trochanter associated with decreased risk of stage 1 ulcers compared with no pad on the contralateral trochanter after 4 weeks (5.4 vs. 30 percent, RR 0.18, 95% CI, 0.05 to 0.73) (strength of evidence: insufficient).
- One fair-quality cross-over trial (n=81) found no statistically significant difference in risk of pressure ulcers between changing incontinence pads three times compared with twice a night after 4 weeks (strength of evidence: low).
Intraoperative Warming
- One fair-quality trial (n=324) of patients undergoing major surgery found no statistically significant difference in risk of pressure ulcers between patients who received an intraoperative warming intervention (forced-air warming and warming of all intravenous fluids) compared with usual care (strength of evidence: low).
Drugs
- One poor-quality trial (n=85) of patients undergoing femur or hip surgery found no difference in risk of pressure ulcers between those who received 80 IU of corticotropin intramuscularly compared with a sham injection (strength of evidence: insufficient).
Polarized Light
- One small, poor-quality randomized trial (n=23) found no statistically significant difference between polarized light compared with standard care in risk of pressure ulcers (strength of evidence: insufficient).
Creams, Lotions and Cleansers
- One fair-quality (n=331) and one poor-quality (n=86) trial found creams with fatty acids associated with decreased risk of new pressure ulcers compared with placebo (RR 0.42, 95% C I 0.22 to 0.80 and RR 0.17, 95% CI, 0.04 to 0.70) (strength of evidence: low)
- Evidence from three poor-quality trials (n=79 to 258) was insufficient to determine effectiveness of other creams or lotions for preventing pressure ulcers (strength of evidence: insufficient).
- One fair-quality trial (n=93) found the Clinisan cleanser associated with lower risk of ulcer compared with standard soap and water in patients with incontinence at baseline (18 vs. 42 percent; RR 0.43, 95% CI, 0.19 to 0.98) (strength of evidence: low).
Detailed Synthesis
Support Surfaces
Forty-one randomized trials (in forty-two publications) evaluated various types of support surfaces for prevention of pressure ulcers in patients at increased risk85–126 (Appendix Table H11). Criteria for classifying support surfaces have historically included the material used (e.g., foam, air, gel, beads, water), whether the support surface is static or dynamic, including alternating-air, low-air-loss, or air-fluidized, and whether the support surface requires power.27 In this report, we classified support surfaces broadly as static, alternating air, or low-air-loss. Sample sizes ranged from 32 to 1,972 subjects, and followup ranged from 6 days to 6 months or until time to pressure ulcer development, hospital discharge, or death. Increased risk was based on risk assessment scale scores at baseline, including Braden ≤15–18, Norton ≤12–16, Waterlow ≥10–15, Cubbin and Jackson score ≤29, and others. When reported, mean Braden scores ranged from 9.4 to 15.9,86,87,94,95,97,106–108,112,117,123,125,126 Norton scores from 11.5 to 13.4,89–91,93,99,111,119 and Waterlow scores from 12.8 to 19.92,100,101,103,116,121 Trials of patients at lower baseline risk were typically conducted in surgical settings and are discussed below (see Key Question 3a).127–133
Three trials were rated good-quality,115,116,125 Twenty trials were rated fair-quality86,89–91,94–97,100,101,105,107–109,111,113,121,122,124,126 and 18 poor-quality;85–88,92,93,98,99,102–104,106,110,112,117–120,123 (Appendix Table H12). Many of the poor-quality trials were older and methods were inadequately reported, including unclear methods of randomization and allocation concealment and failure to report blinding of outcomes assessors. A challenge in interpreting the trials is that in some studies, patients who developed pressure ulcers received additional interventions to prevent further skin damage. Studies varied in how they accounted for these differences in treatments, but none reported adjusted risk estimates.
The support surfaces evaluated in the trials for both high- and low-risk patients varied (Table 6). They included static support surfaces such as mattresses or overlays filled with air, foam, gels, beads, silicone, or water; medical sheepskin overlays; and various static heel supports, boots, or wheelchair cushions. Trials also evaluated air-alternating mattresses or bed systems and some low-air-loss mattresses or bed systems. In addition, the “standard hospital mattress” comparator was not well described in a number of trials and probably differed. Previously, typical hospital mattresses were spring mattresses but more recently, foam mattresses.
Table 6
Types of support surfaces.
Mattresses, Overlays, and Bed Systems
Static Mattresses, Overlays, and Bed Systems
Twenty-two trials85,88,92,99–104,107–113,116,118–120,124,126 (sample sizes 36 to 543) compared static mattresses and/or mattress overlays with each other to prevent pressure ulcers. One was rated good quality,116 nine fair quality,100,101,107–109,111,113,124,126 and the other twelve poor quality.85,88,92,99,102–104,110,112,118–120 Duration of followup ranged from 7 days to 6 months. Trial settings included acute care hospitals (including the intensive care unit and post-operative settings)85,88,92,99–104,107–109,112,116,118–121 and long-term care nursing facilities.108,110,111,113,124,126
Twelve trials compared a more advanced static support surface to a standard hospital mattress control.85,88,99,100,102,104,107,112,113,116,120,124 One good-quality trial (n=1166) found a more advanced static mattress or overlay associated with lower risk of ulcers than a standard hospital mattress (8.5 vs. 10.9 percent, RR 0.78, 95% CI, 0.55 to 1.1), but the difference was not statistically significant.116 Four fair-quality trials (n=83 to 543) also found the more advanced static mattress or overlay associated with decreased risk of any (primarily stage 1) incident pressure ulcers (RR range 0.16 to 0.82),100,107,113,124 though the difference was not statistically significant in one trial (RR 0.28, 95% CI, 0.06 to 1.3) (Table 7).124 The static support surfaces evaluated in the trials were a viscoelastic and polyurethane form mattress,116 the Softform mattress,100 a sheepskin overlay,107,113 and an air overlay.124 There was no clear difference in results between trials published earlier compared with those published more recently, even though standard mattress comparators have changed over time.
Table 7
Effectiveness of pressure ulcer prevention support surfaces in at-risk patients—static mattresses, overlays, and bed systems.
Five poor-quality trials also found a more advanced static mattress or overlay (water mattress, bead overlay, cubed foam mattress, medical sheepskin, or low air pressure mattress) associated with decreased incidence of pressure ulcers compared with a standard hospital mattress (RR 0.08 to 0.32).85,99,104,112,120 One poor-quality trial found no difference between a visco-elastic foam mattress compared with a standard hospital mattress102 and one trial reported no ulcers in patients randomized to various static support surfaces, including a standard hospital mattress.88
Three of the trials found no difference between a more advanced static mattress or overlay and a standard mattress in length of stay.104,107,116 Three of the trials (two fair quality107,113 and one poor quality112) each found an Australian medical sheepskin overlay associated with lower risk of pressure ulcers compared with a standard mattress (RR 0.30, 0.58, and 0.58).
Eleven trials compared different advanced support surfaces.88,92,101,103,108–111,118,119,126 Three fair-quality trials (samples sizes 52 to 100) found no difference between the Transfoamwave and Transfoam mattresses,101 a convoluted compared with solid foam overlay,108 or a contoured compared with slab foam cushion111 in risk of pressure ulcers. One other fair-quality trial of newly admitted nursing home residents (n=40) found a foam replaceable parts mattress (Maxifloat; BG Industries, Northridge, CA) associated with lower risk of ulcers (all ulcers stage 1 or 2) compared with a 4-inch-thick, dimpled foam overlay (Iris 3999; Bio Clinic of Sunrise Medical Group, Ontario, CA) after 10 to 21 days (25 vs. 60 percent, RR 0.42, 95% CI, 0.18 to 0.96)126 (Table 7). Six poor-quality trials (n=37 to 407) found no differences between different various static support surfaces.88,92,103,110,118,119 However, in a subgroup analysis of patients ≥80 years of age, one of these trials found a polyether foam pad associated with greater risk of ulcers compared with the Spenco pad (63 vs. 32 percent; RR 1.99, 95% CI, 0.98 to 4.00; p=0.055).119
One fair-quality trial (n=70) found no pressure ulcers after a week in patients randomized to a profiling bed with a foam pressure relieving mattress compared with a nonprofiling bed with either a foam (n=25) or alternating air (n=10) mattress.109
Low-Air-Loss Mattresses, Overlays, and Bed Systems
One fair-quality105 and one poor-quality106 trial compared a low-air-loss mattress or bed compared with a standard hospital bed (Table 8). The fair-quality trial (n=98) found a low-air-loss bed associated with lower likelihood of one or more pressure ulcers in intensive care unit patients (12 vs. 51 percent, RR 0.23, 95% CI, 0.10 to 0.51).105 However, a small (n=36), poor-quality trial found no difference between a low-air-loss mattress compared with a standard hospital bed following cardiovascular surgery.106
Table 8
Effectiveness of pressure ulcer prevention support surfaces in at-risk patients—low-air-loss mattresses, overlays, and bed systems.
One fair-quality trial (n=62) found a low-air-loss mattress associated with lower risk of pressure ulcer compared with the Hill-Rom Duo mattress (options for constant low pressure or alternating-air), but the difference was not statistically significant (10 vs. 19 percent, RR 0.53, 95% CI, 0.15 to 1.9).122
Alternating Air Pressure Mattresses, Overlays, and Bed Systems
Eight trials (n=32 to 487, one good-quality,125 two fair-quality,89,96 and five poor-quality85,87,93,117,118) compared an alternating-air pressure mattress or overlay with static support surfaces (Table 9). Methodological shortcomings in the poor-quality trials included unclear methods of randomization and allocation concealment, failure to blind outcome assessors, high loss to followup, and failure to perform intention-to-treat analysis.
Table 9
Effectiveness of pressure ulcer prevention support surfaces in at-risk patients—alternating air pressure mattresses, overlays, and bed systems.
Three poor-quality trials found alternating air mattresses or overlays associated with lower risk of pressure ulcers compared with standard hospital mattresses.85,87,117 One trial (n=108) of stroke, post-operative, or terminally ill patients found an alternating double-layer air cell alternating air pressure overlay associated with decreased risk of pressure ulcers compared with a standard hospital mattress (3.4 vs. 37 percent for any ulcer, RR 0.10, 95% CI, 0.01 to 0.76; 3.4 vs. 22 percent for stage 2 ulcers, RR 0.17, 95% CI, 0.02 to 1.3).117 One trial (n=487) found an alternating air-pressure mattress associated with decreased risk of ulcers compared with a standard hospital mattress in risk of any pressure ulcer after 10 days (4.2 vs. 13 percent; RR 0.32, 95% CI, 0.14 to 0.74).85 Pressure ulcer severity was not reported in this trial. The third trial found a mattress with options for either alternating low pressure or continuous low pressure (Hill Rom Duo2) associated with lower risk of any new ulcer than a standard mattress (2.1 vs. 36 percent, RR 0.06, 95% CI, 0.02 to 0.20), though only 2 ulcers were higher than stage 1 (stage 2), and both occurred in the Duo2 arm (1.4 vs. 0 percent, RR 1.2, 95% CI, 0.06 to 25).87 Among patients in the Duo2 group, there was no difference in risk of pressure ulcers between patients randomized to the alternating compared with continuous low pressure settings (2.9 vs. 1.4 percent, RR 2.1, 95% CI, 0.19 to 22).
Six trials found no difference between an alternating air pressure overlay or mattress compared with various advanced static mattresses or overlays in pressure ulcer incidence or severity.85,87,89,93,118,125 The static support surfaces evaluated were a silicone overlay or mattress,89,93 water mattress,85 air mattress,118 constant low pressure air mattress,87, and viscoelastic foam mattress.125 In the good-quality trial (n=447), there was no difference in risk of stage 2 or higher ulcers between an alternating pressure air mattress and a visco-elastic foam mattress in hospitalized patients, though the foam mattress group also underwent scheduled turning every four hours (15 vs. 16 percent, RR 0.98, 95% CI, 0.64 to 1.5).125 There was also no difference in duration of hospitalization (22 vs. 18 days, p=0.11).
One fair-quality trial (n=43) of intensive care unit patients found stepped care (initial use of less advanced and expensive interventions followed by more advanced and expensive interventions if ulcers began to develop, based on a predefined algorithm) initially with alternating air pressure mattresses associated with decreased risk of pressure ulcers after 11 to 12 days compared with stepped care initially with primarily static support surfaces (4.3 vs. 55 percent for any ulcer; RR 0.08, 95% CI, 0.01 to 0.56; 0 vs. 35 percent excluding stage 1 ulcers, RR 0.06, 95% CI, 0.00 to 0.96).96 An earlier abstract for the same study that reported results for a larger sample that included intensive care unit as well as nonintensive care unit patients (n=230) also found the alternating pressure air mattress intervention associated with decreased risk of pressure ulcers (13 vs. 34 percent, RR 0.38, 95% CI, 0.22 to 0.66).134
Four trials (in five publications) compared different alternating air mattresses or overlays (Table 8).94,114,115,117,121 One good-quality (n=1972) trial of hospitalized patients found no difference in risk of incident stage 2 pressure ulcers between an alternating pressure overlay and an alternating pressure mattress (11 vs. 10 percent, RR 1.0, 95% CI, 0.81 to 1.3; adjusted OR 0.94, 95% CI, 0.68 to 1.3).115 Two fair-quality (n=44 and 610) trials of hospitalized patients found no differences in risk of pressure ulcers between different alternating pressure air mattresses121 or between a pulsating air suspension mattress compared with an air mattress with options for alternating pressure or constant low pressure.94 In both trials, the risk of stage 3 or higher ulcers was <2 percent. One of these trials also found no differences in length of stay.121 A poor-quality trial (n=108) found an alternating double-layer air cell overlay associated with decreased risk of pressure ulcers compared with an alternating single-layer air cell overlay, but the difference was not statistically significant (3.4 vs. 19 percent for any ulcer; RR 0.22, 95% CI, 0.03 to 1.8; 3.4 vs. 14 percent for stage 2 ulcers; RR 0.28, 95% CI, 0.03 to 2.3).117
Heel Supports/Boots
Three trials (n=52 to 240) evaluated static heel supports in hospital settings (Table 10).95,98,123 One fair-quality trial (n=239) of fracture patients found the Heelift Suspension Boot associated with decreased risk of heel, foot, or ankle ulcers compared with usual care without leg elevation (7 vs. 26 percent for any ulcer; RR 0.26, 95% CI, 0.12 to 0.53; 3.3 vs. 13 percent for stage 2 ulcers, RR 0.25, 95% CI, 0.09 to 0.72).95 One poor-quality trial (n=52) of hospitalized patients found no difference in risk of ulcers between a boot (Foot Waffle) and usual care (hospital pillow to prop up legs) in risk of incident ulcers (6 vs. 2 events, group sizes not reported).123 One other poor-quality (n=240) trial of hospitalized patients found no differences between three different types of boots (Bunny Boot, egg-crate heel lift positioner, and Foot Waffle) in risk of ulcers, though the overall incidence of ulcers was low (5 percent over 3 years) and nurses added pillows to the Bunny Boot, which could have confounded results.98 None of the trials evaluated length of stay or measures of resource utilization. Shortcomings in the poor-quality trials included unclear allocation concealment,123 significant differences between groups at baseline,98 failure to report attrition,98,123 lack of blinding of outcome assessors,98,123 and failure to perform intention-to-treat analysis.98,123
Table 10
Effectiveness of static heel supports for pressure ulcer prevention.
Wheelchair Cushions
Four trials evaluated static wheelchair cushions with more sophisticated cuts, materials, or shapes compared with standard wheelchair cushions (Table 11).86,90,91,97 All trials were rated fair-quality.86,90,91,97 All of the trials were conducted in older patients in extended care facilities or nursing homes and followed patients for three to six months. No trial focused on patients with spinal cord injury.
Table 11
Effectiveness of wheelchair cushions for pressure ulcer prevention.
Results of the trials were somewhat inconsistent and difficult to interpret because the trials evaluated different wheelchair cushion interventions. One (n=248) trial found no difference between a contoured, individually customized foam cushion compared with a slab cushion in risk of ulcers (68 vs. 68 percent; RR 1.0, 95% CI, 0.84 to 1.2).90 A small (n=32) pilot trial also found no difference between a pressure-reducing wheelchair cushion with incontinence cover compared with a generic foam cushion in risk of ulcers (40 vs. 59 percent; RR 0.68, 95% CI, 0.33 to 1.4).97 However, a third trial (n=141) found the Jay cushion (contoured urethane foam with a gel pad topper) associated with decreased risk of ulcers compared with a standard foam cushion (25 vs. 41 percent, RR 0.61, 95% CI, 0.37 to 1.0).91 The Jay cushion was also associated with decreased risk when the analysis was restricted to stage 2 or 3 ulcers (8.8 vs. 26 percent, RR 0.36, 95% CI, 0.15 to 0.85). Another trial (n=232) found various skin protection wheelchair cushions associated with lower risk of ischial tuberosity ulcers (primarily stage 2) compared with a standard segmented foam cushion when used with a fitted wheelchair (9.9 vs. 6.7 percent, RR 0.13, 95% CI, 0.02 to 1.0).86 None of the trials evaluated length of stay or measures of resource utilization.
Nutritional Supplementation
One fair-quality135 and five poor-quality randomized trials (n=59 to 672) examined nutritional interventions for preventing pressure ulcers (Table 12, Appendix Table H13).136–140 Four trials compared liquid nutritional supplements by mouth plus standard hospital diet compared with the standard hospital diet alone.136–139 One trial140 evaluated nutritional supplementation via tube feeding compared with a standard hospital diet by mouth and one trial135 a high fat, low-carbohydrate enteral formula enriched with lipids and vitamins compared with the same formulation without the lipid and vitamin supplementation. Methodological limitations in the trials included inadequate description of randomization and allocation concealment (Appendix Table H14). One trial also reported baseline differences between intervention groups in risk factors for pressure ulcers,136 and two had high attrition.137,138 Only one trial described measures to blind patients and caregivers to the nutritional intervention;139 no trial described blinding of outcomes assessors.
Table 12
Effectiveness of nutritional supplementation for pressure ulcer prevention.
The two largest trials of supplementation by mouth reported somewhat inconsistent results. One trial (n=672) found high-calorie oral liquid nutritional supplements plus standard hospital diet associated with slightly lower risk of pressure ulcers (AHCPR grading system) at 15 days compared with standard hospital diet alone in elderly patients (32 percent with Norton score of ≤10 at baseline) in the acute phase of a critical illness (40 vs. 48 percent, RR 0.83, 95% CI, 0.7 to 0.99).136 Although there were differences across intervention groups in markers of pressure ulcer risk, the nutritional intervention remained associated with lower risk after adjustment for these risk factors (RR 0.64, 95% CI, 0.42 to 0.97). Another trial (n=495, 28 percent classified as malnourished at baseline) found no difference between oral liquid nutritional supplements (200 ml twice daily) plus standard hospital diet compared with standard hospital diet alone in risk of pressure ulcers in newly admitted patients to long-term care after up to 6 months of followup (9.9 vs. 12 percent incidence of pressure ulcers in patients without ulcers at baseline, p>0.05).138 Two smaller trials also found no effects of a nutritional intervention on risk of pressures ulcers following hip fractures. One trial (n=103, mean CBO score 11 on a 0 to 39 scale) found no difference in risk of EPUAP stage 1 or 2 pressure ulcers (there were no stage 3 or 4 ulcers) between a standard hospital diet plus one daily oral liquid nutritional supplement (with protein, arginine, zinc, and antioxidants) compared with a standard hospital diet plus identical-appearing noncaloric water based placebo after 2 weeks (55 vs. 58 percent, RR 0.92, 95% CI, 0.65 to 1.3).139 There was also no difference in risk of stage 2 ulcers when they were evaluated separately (18 vs. 27 percent, RR 0.66, 95% CI, 0.31 to 1.4). Another trial (n=59, baseline pressure ulcer risk not assessed) found no statistically significant difference between a high-calorie oral nutritional supplement (mean 32 days of supplementation) plus hospital diet compared with hospital diet alone in risk of pressure ulcers at discharge (0 vs. 20 percent, RR 0.79, 95% CI, 0.14 to 4.4) or at 6 month followup (0 vs. 7 percent, RR 0.23, 95% CI, 0.01 to 4.3), although estimates were very imprecise due to small numbers of ulcers.137 In this trial, which was the only one to report length of stay, nutritional supplementation was associated with shorter median duration of hospitalization (24 vs. 40 days, p<0.04). Two trials found no clear effects of enteral supplementation on risk of pressure ulcers. One trial of patients with hip fracture (n=129, mean CBO score 9) found no difference between nutritional supplementation via tube feeding compared with standard hospital diet in risk of stage 2 or higher pressure ulcers after two weeks (52 vs. 57 percent, RR 0.92, 95% CI, 0.64 to 1.3) in risk of pressure ulcers.140 There was also no difference when the analysis was restricted to patients that received tube feeding for at least one week. One other trial of critically ill patients with acute lung injury (n=95) found no difference between an enteral formula enriched in lipids (eicosapentanoic acid and gamma-linolenic acid) and vitamins (vitamins A, C, and E) compared with without the enrichment in risk of new pressure ulcers after 4 days (11 vs. 18 percent, RR 0.59, 95% CI, 0.21 to 1.6) or 7 days (6.5 vs. 2.0 percent, RR 3.2, 95% CI, 0.34 to 30).135
Repositioning
Six randomized trials (n=15 to 838) examined the effectiveness of repositioning interventions for prevention of pressure ulcers (Table 13 and Appendices H15 and H16).141–146 All trials evaluated patients classified as higher-risk for ulcers based on the Braden, Norton or Waterlow scales. One good-quality,142 two fair-quality,143,146 and two poor-quality trials141,144 were conducted in long-term-care facilities of patients in their 80s. One fair-quality trial (attrition 15 percent and adherence 57 percent) was conducted in an acute care ward in a somewhat younger (mean age 70 years) population.145 The two poor-quality trials evaluated small, unscheduled shifts in body position plus repositioning every two hours compared with repositioning every two hours without the unscheduled shifts in body position.141,144 In the other trials, the repositioning interventions and standard care comparators varied (Appendix Table H15). Standard care always included less structured or frequent repositioning.
Table 13
Effectiveness of repositioning for pressure ulcer prevention.
One fair-quality cluster randomized trial (n=213) of higher-risk patients (baseline risk determined by the activity and mobility components of the Braden scale) in long-term-care facilities found repositioning at a 30-degree tilt every 3 hours associated with lower risk of pressure ulcer compared with usual care (90-degree lateral repositioning every 6 hours during the night) after 28 days (3.0 vs. 11 percent, RR 0.27, 95% CI, 0.08 to 0.93).143 Clustering effects were negligible. All of the ulcers were graded as stage 1 or 2 (EPUAP). A fair-quality randomized trial (n=46) of higher-risk (Waterlow score >10) patients in an acute-care ward found 30-degree tilt repositioning associated with no statistically significant difference in incidence of stage 1 ulcers (13 vs. 8.7 percent, RR 1.5, 95% CI, 0.28 to 8.2), but only followed patients for one night.145
A third, good-quality trial compared repositioning interventions that alternated the semi-Fowler position (30-degree elevation of the head and feet) and a lateral position (patient turned 30 degrees and supported by a pillow between the shoulders and pelvis) at four different intervals (2, 3, 4, or 6 hours) compared with usual preventive care (repositioning method not specified, based on nurse clinical judgment) in 838 at-risk (Braden score <17) patients in nursing homes.142 It found no difference between groups in risk of stage 1 ulcers (AHCPR) after 4 weeks, which ranged in incidence from 44 to 48 percent across groups. The 4 hour repositioning intervention was associated with the lowest risk of stage 2 or higher ulcers compared with the other interventions (3.0 percent vs. 14 to 24 percent; OR 0.12, 95% CI, 0.03 to 0.48). However, whether the difference was due to the repositioning interval is difficult to determine because the 4 and 6 hour repositioning interventions also included use of a pressure-reducing foam mattress (standard institutional mattresses were used in the other arms).
One fair-quality trial (n=235) found no difference between different repositioning intervals between the semi-Fowler 30 degree and lateral positions.146
Two small (n=15 and 19), poor-quality trials found the addition of small, unscheduled shifts in body position (using a small rolled towel to designated areas during nurse-patient interactions) to standard repositioning every 2 hours had no effect on risk of pressure ulcers, but only reported one or two ulcers each.141,144 Methodological shortcomings in the trials included inadequate description of randomization or allocation concealment methods, and lack of blinding of outcome assessors.
None of the trials reported length of stay or measures of resource utilization.
Dressings and Pads
Two fair-quality147,148 and one poor-quality149 trials evaluated dressings or pads for prevention of pressure ulcers (Appendix Tables H17 and H18). One trial compared a silicone border foam dressing with standard ICU care,147 one trial compared more with less frequent incontinence pad changes in women with incontinence,148 and the third trial compared use of a dressing (the REMOIS Pad) with no dressing.149 Methodological shortcomings in the trials included inadequate randomization147,149 or allocation concealment148,149 or failure to report intention-to-treat analysis.147 None of the trials reported length of stay or measures of resource utilization. A fair-quality randomized trial of cardiac surgery ICU patients (n=85, mean Braden 11 at baseline) found a silicone border foam sacral dressing applied at ICU admission (the Mepilex Border sacrum) associated with lower likelihood of pressure ulcers compared with standard ICU care (mean followup about 25 days), but the difference was not statistically significant (2.0 vs. 12 percent, RR 0.18, 95% CI, 0.02 to 1.5).147 Other components of standard care in both groups included preoperative placement of a silicone border foam dressing for surgery, and use of a low air loss bed. A poor-quality trial of 37 patients (mean Braden 10 at baseline) in a long-term care facility found use of the REMOIS Pad (consisting of a hydrocolloid skin adhesive layer, a support layer of urethane film, and an outer layer of multifilament nylon) on the greater trochanter associated with decreased risk of persistent erythema (stage 1 ulcer) compared with use of no pad on the contralateral trochanter after 4 weeks (5.4 vs. 30 percent, RR 0.18, 95% CI, 0.05 to 0.73).149
A fair-quality cross-over trial of incontinent female nursing home patients (n=81, mean Braden 13 at baseline) found no statistically significant difference in risk of stage 2 pressure ulcers (method used to classify pressure ulcers not reported) after 4 weeks between changing incontinence pads three times compared with twice a night, though no ulcers occurred in patients during the more frequent change period compared with five during the less frequent change period (odds ratio not reported, 95% CI, 0 to 1.1; p=0.1).148
Intraoperative Warming
One fair-quality (unclear randomization method) randomized trial (n=324) of patients undergoing major surgery found no statistically significant difference in risk of pressure ulcers (method for grading ulcers not specified and duration of postoperative followup not reported) between intraoperative warming (forced-air warming and warming of all intravenous fluids) compared with usual care, although results favored the warming intervention (5.6 vs. 10 percent, RR 0.54, 95% CI, 0.25 to 1.2) (Appendix Tables H19 and H20).150 Length of stay and measures of resource utilization were not reported.
Drugs
One poor-quality randomized trial (n=85) of patients undergoing femur or hip surgery found no difference in risk of pressure ulcers between those who received 80 IU of corticotropin intramuscularly compared with a sham injection (12 vs. 28 percent, RR 0.43, 95% CI, 0.16 to 1.1) (Appendix Tables H19 and H20).151 Length of stay and measures of resource utilization were not reported. Methodological shortcomings included unclear randomization technique, inadequate allocation concealment, unclear blinding methods, lack of intention-to-treat analysis, and failure to report demographic characteristics, ulcer risk, eligibility criteria, and attrition.
Polarized Light
One small, poor-quality randomized trial (n=23) of ICU patients found no statistically significant difference between polarized light compared with standard care (including use of a viscoelastic or low-air-loss mattress, repositioning, and viscoelastic pillow) in risk of any pressure ulcer (RR 0.43, 95% CI, 0.16 to 1.2) or stage 2 or greater ulcers (RR 0.08, 95% CI, 0.01 to 1.3).152 Methodological limitations included unclear randomization, high loss to followup, and lack of intention-to-treat analysis.
Creams, Lotions, and Cleansers
Two fair-quality153,154 and four poor-quality randomized trials (reported in five publications)155–159 evaluated lotions, creams, or cleansers in various settings, including nursing homes, long-term care facilities, and acute care hospitals (Table 14; Appendix Tables H21 and H22). None of the poor-quality trials155–159 reported adequate methods for randomization and/or allocation concealment, only two trials reported blinding of care providers or patients,155,157 and only one trial reported low loss to followup.155 In addition, one cluster randomized trial158,159 failed to assess cluster effects. Five trials evaluated older (mean age ≥80 years), predominantly female (range 67 to 81 percent) patients in long-term care settings or a geriatric care unit.153–156,158,159 The sixth trial evaluated younger (mean age 60 years) patients (proportion of female not reported) in an intensive care unit.157 Four trials compared a lotion or cream with placebo154,155,157–159 and a fifth156 compared two lotions. The creams and lotions evaluated in the trials varied (Table 13). The sixth trial compared a foam cleanser (Clinisan) to standard hospital soap.153
Table 14
Effectiveness of lotions and cleansers for pressure ulcer prevention.
One fair-quality trial (n=331) found a hyperoxygenated fatty acid cream (Mepentol) associated with lower risk of new pressure ulcers (severity not reported) compared with placebo after 30 days (7.3 vs. 17 percent, RR 0.42, 95% CI, 0.22 to 0.80).154 A poor-quality trial (n= 86) of patients in an intensive care unit (mean Norton score 9) found a lotion consisting of 1.6 grams of essential fatty acids associated with decreased risk of pressure ulcers after 3 weeks compared with a mineral oil placebo lotion (stage 1 or stage 2, 4.7 vs. 28 percent, RR 0.17, 95% CI, 0.04 to 0.70; stage 2 only 0 vs. 28 percent, RR 0.04, 95% CI, 0.002 to 0.66).157
A poor-quality trial (n=258) of patients in long-term care facilities found Conotrane cream (benzalkonium chloride [an antiseptic] plus dimeticone [a silicone fluid which is water repellant]) associated with lower risk of any pressure ulcer (Barbarel score) after 24 weeks compared with placebo cream, though the difference was not statistically significant (27 vs. 36 percent, RR 0.74, 95% CI, 0.52 to 1.1).155
A poor-quality crossover trial (n=79) of nursing home patients at higher risk for ulcers (Braden score at baseline ≤20) found no differences between 5 percent dimethyl sulfoxide cream (DMSO, a commercial solvent with various purported medicinal properties that is not approved by the Food and Drug Administration for treatment of ulcers) or a placebo cream (Vaseline-cetomacrogol) compared with neither cream in severity or incidence of pressure ulcers (any location) after 4 weeks (incidence 62, 31, and 39 percent), though the DMSO cream was associated with greater risk of ulcers than the placebo cream (RR 2.0, 95% CI, 1.1 to 3.6).158,159 Patients allocated to either cream also received a 2 to 3 minute massage during application of the cream, and all groups underwent 30° repositioning every 6 hours. The DMSO cream was also associated with greater risk of heel or ankle ulcers than either the placebo cream (RR 3.5, 95% CI, 1.5 to 8.4) or no cream (RR 3.3, 95% CI, 1.1 to 9.8).159
A poor-quality trial (n= 104) of higher-risk patients (mean Norton score 11 at baseline) in a hospital geriatric unit found no differences between the Prevasore (hexyl nicotinate, zinc stearate, isopropyl myristate, Dimethicone 350, cetrimide, and glycerol) compared with the Dermalex (hexachlorophene, squalene, and allantoin) creams in risk of skin deterioration after 3 weeks (13 vs. 22 percent, RR 0.59, 95% CI, 0.25 to 1.4).156
One fair-quality trial (n=93) found use of Clinisan cleanser associated with lower risk of ulcer compared with standard soap and water in patients with incontinence (18 vs. 42 percent; RR 0.43, 95% CI, 0.19 to 0.98).153 Three-quarters of the ulcers were stage 1.
None of the trials reported length of stay or measures of resource utilization.
Key Question 3a. Do the effectiveness and comparative effectiveness of preventive interventions differ according to risk level as determined by different risk-assessment methods and/or by particular risk factors?
Key Points
Lower Risk Populations
Static Support Surfaces
- Two trials (one good- and one fair-quality; n=175 and 413) found use of a static foam overlay associated with increased risk of pressure ulcers compared with standard care in lower-risk surgical patients, though the difference was not statistically significant in one trial (OR 1.9, 95% CI, 1.0 to 3.7 and RR 1.6, 95% CI, 0.76 to 3.3) (strength of evidence: moderate).
- Two trials (one good- and one poor-quality; n=416 and 505) found a static dry polymer overlay associated with decreased risk of pressure ulcers compared with standard care in lower-risk surgical patients (strength of evidence: low).
- One poor-quality trial (n=1,729) found no significant difference between a static foam block mattress and standard hospital mattress support surfaces in pressure ulcer incidence (strength of evidence: insufficient).
Alternating Support Surfaces
- Two trials (one good- and one poor-quality; n=198 and 217) found no differences between alternating compared with static support surfaces in risk of pressure ulcer incidence or severity (strength of evidence: low).
Detailed Synthesis
No studies directly evaluated the effectiveness or comparative effectiveness of preventive interventions in patients stratified by risk level. Most trials evaluated higher-risk patients and are summarized above (see Key Question 3).
Seven trials (n=175 to 505) evaluated pressure ulcer preventive interventions in lower-risk patients undergoing surgery (Table 15; Appendix Tables H11 and H12).127–133 Patients were lower-risk based on pressure ulcer risk assessment scores, using the Braden (score ≥20),131,133 Norton (score ≥20),129 modified Knoll (score ≤4)127,132 or modified Ek (score 3–4) scales.128 Interventions were given in the operating room in all studies except one,128 in which it was unclear if interventions were given in the operating room and post-operatively, or just post-operatively. Two studies continued interventions into the post-operative period.127,132 Post-operative followup ranged from 5 to 8 days, apart from one study that only evaluated patients in the immediate post-operative period130 and one study that did not report mean study duration.128 Four trials129–131,133 compared various static mattresses or overlays compared with standard operating room care and two compared an alternating air mattress to a static mattress.127,132 Two trials were rated good-quality,132,133 two fair-quality,129,131 and three poor-quality.127,128,130 Methodological shortcomings in the poor-quality trials included inadequate randomization, unclear methods of allocation concealment, and failure to blind outcome assessors. No trials reported length of stay or other resource utilization outcomes by treatment group.
Table 15
Effectiveness of pressure ulcer prevention support surfaces in lower-risk patients.
Static Mattresses, Overlays, or Bed Systems
Static mattresses or overlays were compared with standard operating room mattresses in one good-quality,133 two fair-quality129,131 and two poor-quality trials (Table 15).128,130 Two trials (n=175 and 413) found addition of a foam overlay to a standard operating mattress associated with increased risk of pressure ulcers (27 vs. 16 percent, OR 1.9, 95% CI, 1.0 to 3.7133 and 18 vs. 11 percent, RR 1.6, 95% CI, 0.76 to 3.3129) after five to six days, compared with a standard operating mattress alone, though the difference was not statistically significant in one of the trials. In both trials, about 90 percent of the ulcers were stage 1 and the remainder stage 2, based on the AHCPR or EPUAP grading systems.
One fair-quality trial (n=416) found addition of a dry polymer overlay to a standard operating room mattress associated with decreased risk of incident pressure ulcers compared with standard care (11 vs. 20 percent, OR 0.46; 95% CI, 0.26 to 0.82), based on assessments one day after surgery.131 Most (86 percent) of ulcers were blanching erythema, with no cases of frank ulceration. A poor-quality trial also found a dry polymer overlay in the operating room associated with decreased risk of subsequent ulcers.130
A poor-quality trial found no difference in development of post-operative pressure ulcers in groups receiving a foam block mattress or a standard hospital mattress (3.2 vs. 1.9 percent; RR 1.6; 95% CI, 0.90 to 3.0).128
Alternating Air Mattresses, Overlays, and Bed Systems
One good-quality trial132 and one poor-quality trial127 compared alternating support surfaces in the operating room with static, usual care surfaces and followed patients for 7 days post-operatively (Table 15). The good-quality trial found no statistically significant difference in pressure ulcer incidence or severity between the MicroPulse alternating air mattress system (in the operating room and continued post-operatively) compared with standard care, though results favored the alternating system (2 vs. 7 percent for any ulcer, RR 0.29, 95% CI, 0.06 to 1.4; 2 vs. 5 percent for stage 2 ulcer, RR 0.41, 95% CI, 0.08 to 2.0).132 A poor-quality trial similarly found an alternating pressure system associated with decreased risk of pressure ulcers compared with standard operating room care, though again results did not reach statistical significance (1 vs. 7 percent, RR 0.14, 95% CI, 0.02 to 1.1).127
Key Question 3b. Do the effectiveness and comparative effectiveness of preventive interventions differ according to setting?
- No study evaluated how effectiveness of preventive interventions varies according to care setting (strength of evidence: insufficient).
No study directly evaluated how effectiveness of preventive interventions varies according to care setting. Due to small numbers of studies, differences in interventions and comparisons, and methodological limitations in the studies, it was not possible to assess how effectiveness or comparative effectiveness of preventive interventions varies according to care setting based on indirect comparisons across studies. Studies of low-risk surgical patients are reviewed elsewhere (see Key Question 3a). Intraoperative warming therapy was also specifically evaluated in surgical patients.150
Key Question 3c. Do the effectiveness and comparative effectiveness of preventive interventions differ according to patient characteristics?
- No study evaluated how effectiveness of preventive interventions varies in subgroups defined by patient characteristics (strength of evidence: insufficient).
No study directly evaluated how effectiveness of preventive interventions varies in subgroups defined by patient characteristics. Due to small numbers of studies, differences in interventions and comparisons, and methodological limitations in the studies, it was not possible to assess how effectiveness or comparative effectiveness of preventive interventions varies according to patient characteristics based on indirect comparisons across studies.
Key Question 4. What are the harms of interventions for the prevention of pressure ulcers?
Key Points
- Nine of 48 trials of support surfaces reported harms (strength of evidence: low).
- Three trials (n=297 to 588) reported cases of heat-related discomfort with sheepskin overlays, with one trial reporting increased risk of withdrawal due to heat discomfort compared with a standard mattress (5 vs. 0 percent, RR 0.95, 95% CI, 0.93 to 0.98).
- One trial (n=39) that compared different dynamic mattresses reported some differences in pain and sleep disturbance and two trials (n=610 and 1972) found no differences in risk of withdrawal due to discomfort.
- One trial (n=198) reported no differences in risk of adverse events between a multi-cell pulsating dynamic mattress compared with a static gel pad overlay.
- One trial (n=239) of heel ulcer preventive interventions reported no difference in risk of adverse events between the Heelift Suspension Boot and standard care in hip fracture patients.
- One trial (n=141) reported that a urethane and gel wheel chair pad was associated with an increased risk of withdrawal due to discomfort compared with a standard foam wheel chair pad. (8 vs. 1 percent, RR 6.2, 95% CI 0.77 to 51).
- One trial of nutritional supplementation found that tube feeds were tolerated poorly, with 54 percent having the tube removed within 1 week, and 67 percent prior to completing the planned 2 week intervention. Four trials of nutritional supplementation by mouth did not report harms (strength of evidence: low).
- Two (n=46 and 838) of six trials of repositioning interventions reported harms. Both trials reported more nonadherence due to intolerability of a 30 degree tilt position compared with standard positioning (strength of evidence: low).
- Three (n=93 to 203) of six trials of lotions or creams reported harms. One trial found no differences in rash between different creams and two trials each reported one case of a wet sore or rash (strength of evidence: low).
- One (n=37) of three trials of dressings reported harms. One trial reported that application of the REMOIS pad resulted in pruritus in one patient (strength of evidence: low).
Detailed Synthesis
Harms were reported in only 1691,94,95,107,112,113,115,132,140,142,145,149,153,155,156,160 of 72 trials of preventive interventions. Of the trials reporting harms, few provided detailed information on specific harms, several only described single cases of harms, and none reported serious treatment-related harms.
Support Surfaces
Nine91,94,95,107,112–115,132,160 trials (in 10 publications) of 48 trials of support surfaces reported harms (Table 16; Appendix Tables H11 and H12). Three trials reported cases of heat-related discomfort with a sheepskin overlay, leading to some withdrawals in two trials.107,112,113 The only trial to report quantitative data found the sheepskin overlay associated with increased risk of withdrawal due to discomfort compared with a standard mattress (5 vs. 0 percent; RR 21, 95% CI, 1.3 to 364).107
Table 16
Harms of pressure ulcer prevention interventions.
One trial that compared dynamic mattresses reported less pain on the Nimbus II (p<0.05) and Quattro DC2000 (p<0.01) mattresses compared with the Pegasus Airwave Mattress.160 The same trial reported less sleep disturbance with the Quattro DC2000 compared with the Nimbus II (p<0.05) and Pegasus Airwave (p<0.01). Another trial reported no differences in risk of adverse events between a multi-cell, pulsating dynamic mattress compared with a static gel pad overlay, but data were not reported.132
Two trials that compared different alternating pressure mattresses or overlays found no difference in rate of withdrawal due to discomfort (5.1 vs. 3.7 percent in one study94 and 23 vs. 19 percent in the other115).
One trial of heel ulcer preventive interventions reported no difference in risk of adverse events between the Heelift Suspension Boot and standard care in hip fracture patients (20 vs. 23 adverse events, p=0.69; proportion of patients with adverse events not reported).95
One trial reported that a urethane and gel wheelchair pad (Jay cushion) was associated with an increased risk of withdrawal due to discomfort compared with a standard foam wheelchair pad (8 vs. 1 percent, RR 6.2, 95% CI 0.77 to 51).91
Nutritional Supplementation
One trial of nutritional supplementation found that patients tolerated tube feeds poorly. Six and a half percent (4/62) of patients removed the tube immediately, 54 percent (29/54) had the tube removed within 1 week, and 67 percent (32/48) had the tube removed prior to completing the planned two week intervention.140 Four trials that evaluated nutritional supplementation by mouth did not report harms.136–139
Repositioning
Two142,145 of three trials of repositioning reported harms (Table 16; Appendix Tables H15 and H16). One trial found a 30 degree tilt repositioning position more difficult to tolerate than a standard 90 degree position (87 vs. 24 percent; RR 0.17, 95% CI, 0.06 to 0.51).145 One other trial noted that not all patients could tolerate a 30 degree tilt position for the intended amount of time, but details regarding protocol violations were not reported.142
Creams, Lotions, and Cleansers
Three153,155,156 of six trials of lotions reported harms (Table 16; Appendix Tables H21 and H22). One trial found no differences between a silicone and antiseptic cream (Conotrane) and a placebo cream (Unguentine) in risk of redness (4 vs. 6 percent; RR 1.02, 95% CI, 0.96 to 1.09), rash (0 vs. 1 percent; RR 1.01, 95% CI, 0.98 to 1.04), or withdrawals due to redness or rash (3 vs. 2 percent; RR 0.99, 95% CI, 0.95 to 1.04).155 Two other trials of lotions or creams reported blisters or a wet sore in one patient each.153,156
Dressings
One of three trials of dressings reported harms. It reported pruritus in one patient following application of the REMOIS pad (Table 16; Appendix Tables H19 and H20).149
Key Question 4a. Do the harms of preventive interventions differ according to the type of intervention?
- No study evaluated how harms of preventive interventions vary according to the type of intervention (strength of evidence: insufficient).
No study directly compared harms in different categories of interventions (e.g., dressings vs. repositioning or support surfaces vs. lotions) or presumed mechanism of action (e.g., nutritional support vs. relief of pressure vs. skin protection). Across studies, reporting of harms was too limited (see Key Question 4) to draw conclusions about how harms may differ according to the type of intervention.
Key Question 4b. Do the harms of preventive interventions differ according to setting?
- No study evaluated how harms of preventive interventions vary according to care setting (strength of evidence: insufficient).
No study directly evaluated how estimates of harms varied according to care setting. Across studies, reporting of harms was too limited (see Key Question 4) to draw conclusions about how harms may differ according to care setting.
Key Question 4c. Do the harms of preventive interventions differ according to patient characteristics?
- No study evaluated how harms of preventive interventions vary in subgroups defined by patient characteristics (no evidence).
No study directly evaluated harms of preventive interventions in subgroups defined by specific patient characteristics such as underlying risk level, specific risk factors, or other factors. Across studies, reporting of harms was too limited (see Key Question 4a) to draw conclusions about how harms may differ according to care setting.
- Overview
- For adults in various settings, is the use of any risk-assessment tool effective in reducing the incidence or severity of pressure ulcers, compared with other risk-assessment tools, clinical judgment alone, and/or usual care?
- Do the effectiveness and comparative effectiveness of risk-assessment tools differ according to setting?
- Do the effectiveness and comparative effectiveness of risk-assessment tools differ according to patient characteristics, and other known risk factors for pressure ulcers, such as nutritional status or incontinence?
- How do various risk-assessment tools compare with one another in their ability to predict the incidence of pressure ulcers?
- Does the predictive validity of various risk-assessment tools differ according to setting?
- Does the predictive validity of various risk-assessment tools differ according to patient characteristics?
- In patients at increased risk of developing pressure ulcers, what are the effectiveness and comparative effectiveness of preventive interventions in reducing the incidence or severity of pressure ulcers?
- Do the effectiveness and comparative effectiveness of preventive interventions differ according to risk level as determined by different risk-assessment methods and/or by particular risk factors?
- Do the effectiveness and comparative effectiveness of preventive interventions differ according to setting?
- Do the effectiveness and comparative effectiveness of preventive interventions differ according to patient characteristics?
- What are the harms of interventions for the prevention of pressure ulcers?
- Do the harms of preventive interventions differ according to the type of intervention?
- Do the harms of preventive interventions differ according to setting?
- Do the harms of preventive interventions differ according to patient characteristics?
- Results - Pressure Ulcer Risk Assessment and Prevention: Comparative Effectivene...Results - Pressure Ulcer Risk Assessment and Prevention: Comparative Effectiveness
- Peer Reviewers - Radiation Therapy for Metastatic Bone Disease: Effectiveness an...Peer Reviewers - Radiation Therapy for Metastatic Bone Disease: Effectiveness and Harms
- Peer Reviewers - Diagnosis of GoutPeer Reviewers - Diagnosis of Gout
Your browsing activity is empty.
Activity recording is turned off.
See more...