NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gutman SI, Oliansky DM, Belinson S, et al. PCA3 Testing in the Diagnosis and Management of Prostate Cancer: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 98 [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Apr. (Future Research Needs Papers, No. 24.)

PCA3 Testing in the Diagnosis and Management of Prostate Cancer: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 98 [Internet].
Show detailsBackground
Cancer of the prostate is the second most common cancer and the second leading cause of cancer deaths in men in the United States.1 Most patients have indolent tumors and may live for years with no or minimal effects, ultimately dying of other causes.2 However, some patients have aggressive tumors that spread beyond the prostate, resulting in significant morbidity and death.
The rationale for prostate cancer screening using serum total prostate-specific antigen (tPSA) levels was that early detection of prostate tumors would lead to timely intervention and reduced prevalence of disease.3,4 However, screening programs have generated considerable controversy, with concerns expressed that they lead to overdiagnosis and overtreatment of prostate cancer and associated harms. The United States Preventive Services Task Force has recently issued a recommendation against screening for prostate cancer based on PSA (prostate-specific antigen).5 However, the balance of benefits and harms of tPSA screening remains controversial.6
In 1999, researchers reported that the prostate cancer antigen 3 gene (PCA3; also known as DD3) was highly overexpressed in prostate cancer relative to normal prostate or benign prostatic hyperplasia tissue.7 Subsequently, noninvasive PCA3 tests on messenger RNA (ribonucleic acid) from urine were developed.
In April 2012, a draft Comparative Effectiveness Review (CER), PCA3 Testing for the Diagnosis and Management of Prostate Cancer, was completed. The review had two aims. The first was to evaluate the comparative effectiveness of replacing or supplementing existing testing approaches for decisionmaking on when to biopsy (Key Question 1) or rebiopsy (Key Question 2) men at risk for prostate cancer. Key Questions 1 and 2 were as follows.
Key Question 1: In patients with elevated tPSA and/or an abnormal digital rectal examination (DRE) who are candidates for initial prostate biopsy, what is the comparative effectiveness of PCA3 testing as a replacement for, or supplement to, standard tests, including diagnostic accuracy (clinical validity) for prostate cancer, intermediate outcomes (e.g., improved decisionmaking about biopsy), and long-term health outcomes (clinical utility), including mortality/morbidity, quality of life, and potential harms?
Key Question 2: In patients with elevated PSA and/or an abnormal DRE who are candidates for repeat prostate biopsy (when all previous biopsies were negative), what is the comparative effectiveness of PCA3 testing as a replacement for, or supplement to, standard tests, including diagnostic accuracy (clinical validity) for prostate cancer, intermediate outcomes (e.g., improved decisionmaking about biopsy), and long-term health outcomes (clinical utility), including mortality/morbidity, quality of life, and potential harms?
The second aim of the review (Key Question 3) was to evaluate the comparative effectiveness of replacing or supplementing existing approaches for categorizing men with a positive prostate cancer biopsy as having high- or low-risk cancer and making decisions about treatment (e.g., active surveillance or aggressive therapy). Key Question 3 was as follows.
Key Question 3: In patients with a positive biopsy for prostate cancer who are being evaluated to distinguish between indolent and aggressive disease, what is the effectiveness of using PCA3 testing alone, or in combination with the standard prognostic workup (e.g., tumor volume, Gleason score, clinical staging) or monitoring tests (e.g., tPSA, PSA velocity), with regard to diagnostic accuracy (clinical validity) for aggressive (high-risk) prostate cancer, intermediate outcomes (e.g., improved decisionmaking about prognosis and triage for active surveillance and/or aggressive treatment), and long-term health outcomes (clinical utility), including mortality/morbidity, quality of life, and potential harms?
The CER analyses revealed that PCA3 had improved diagnostic accuracy compared with tPSA in identifying the presence or absence of prostate cancer, with no differences resulting from biopsy status (initial vs. repeat biopsy). However, the strength of evidence was low. The CER data from matched studies were insufficient to answer all other questions posed. In addition, issues were raised about methodological flaws in current research approaches, including risk of biases related to selection of study subjects, the generally poor quality of individual studies, and the lack of longitudinal studies to investigate the impact of early decisionmaking on long-term health outcomes.
Several important evidence gaps were identified in the draft PCA3 CER:
- Lack of information on how much improvement in diagnostic accuracy is needed for any new test to impact biopsy decisionmaking
- Lack of information on the potential of adding PCA3 alone or with other biomarkers to change decisionmaking in practice
- Lack of information on how PCA3 compares in terms of diagnostic accuracy and clinical utility with the two more frequently used add-on tests (free PSA, PSA velocity) that have appeared in guidance documents
- Need for matched studies (studies in which results of testing between PCA3 and the comparators of interest are performed and reported on the same individuals rather than only on groups of individuals) not derived from “convenience” populations (e.g., biopsy referral centers) and more data on how key demographic factors (family history, race) impact the performance of PCA3 and comparators
- Need for outcome studies to determine how well PCA3 and other comparators used to categorize risk as insignificant/indolent or aggressive predict the behavior of tumors over time
- Lack of information on a range of methodological and statistical questions related to modeling, assessing the impact of verification bias, identifying most effective cutoffs for tests based on ROC (reviewer operating characteristic) analysis, and designs for future studies
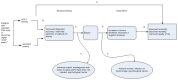
The analytical frameworks that guided the draft PCA3 CER are provided in Figures A and B.

Figure B
Future research needs for PCA3 testing: analytic framework for PCA3 used to distinguish indolent versus aggressive prostate cancer (Key Question 3). Abbreviation: PCA3 = prostate cancer antigen 3 gene a Diagnostic accuracy
Methods
Evidence gaps identified from the draft PCA3 CER, an update of the literature search, and a Stakeholder Panel were used to develop future research needs and preliminary questions. The Stakeholder Panel consisted of a group of eight individuals representing diverse perspectives, including methodological/research expertise, clinical experience (urology, oncology, epidemiology), clinical laboratory experience, and patient and payer representation. The Evidence-based Practice Center (EPC) staff compiled a list of research needs and questions, taking the Stakeholders’ comments into consideration. Through an iterative process including the use of teleconferences and SurveyMonkey®, an online survey tool, the EPC staff refined the research needs, and then the Stakeholder Panel prioritized them. In selecting criteria for prioritizing research needs and research questions, the Effective Health Care (EHC) Program Selection Criteria8 were modified to be applicable to primary research rather than to systematic reviews. The modified EHC Program Selection Criteria were distributed to the Stakeholders each time they were asked to prioritize research needs or research questions.
Research questions for each of the three research needs that were ranked the highest were generated from the CER and also through teleconferences and online input from Stakeholders. Research questions were characterized using the PICOTS (population, interventions, comparators, outcomes, timing, setting) framework.9 The Stakeholders again used SurveyMonkey® to prioritize the research questions for each research need. The EPC, with input from the Stakeholder Panel, evaluated a variety of study designs for their potential to address the research needs and questions in accordance with the recent Future Research Needs methods report authored by the EPCs for the Agency for Healthcare Research and Quality.10
Results
A total of nine research needs were identified through a combination of the CER findings, updated literature search, and input from the Stakeholders. Through the online prioritization process, the EPC generated a list of the three highest priority research needs, taking all Stakeholder comments into account. The Stakeholders prioritized the list of seven research questions (four for Research Need 1, two for Research Need 2, and one for Research Need 3) within each research need, resulting in six priority research questions (three for Research Need 1, two for Research Need 2, and one for Research Need 3). EPC staff evaluated the appropriateness of various study designs to address the research needs and further prioritized the research needs. The final prioritized list of three research needs, with associated research questions and PICOTS, is presented in Table A.
Table A
Priority PCA3 research needs with research questions and PICOTS.
Discussion
Based on the draft 2012 CER PCA3 Testing for the Diagnosis and Management of Prostate Cancer and with input from a diverse group of Stakeholders, a 10-step process was used for identifying and prioritizing clinically important research needs and research questions. The Stakeholders agreed with the findings of the CER and recognized that work needed to be done to better understand the clinical performance, impact on decisionmaking, and long-term health outcomes of PCA3 testing.
Given the complexity of topics discussed in the CER, the decision to limit the future research needs project to items within the clinical scope of testing, and to not address more general methodological and statistical issues, assured focus to the project.
There were several strengths to our process. First, Stakeholder Panel members came from a wide variety of relevant disciplines, which was important to provide a balanced and broad perspective on the research needs being discussed. Second, the use of a variety of interactive communication approaches, including a one-on-one orientation to the project, two teleconferences, and emails and Internet surveys, allowed work to proceed in an efficient and timely manner. Third, the Stakeholders actively and vigorously participated in all phases of the project.
In evaluating the Stakeholders’ prioritization of the research needs, a logical pattern evolved that seemed to fit well with the development and credentialing of a new diagnostic test. Highest priority went to establishing the diagnostic accuracy of the test. This is a highly pragmatic starting point, since without a clinically validated signal, risk of failure in further exploration of the use of a new test is high. Second, priority went to defining what information the test signal conveyed about the aggressiveness of missed or identified disease and how this information might be used in decisionmaking. It is likely that a test that had a weak signal or that was poor at discriminating between indolent and aggressive disease might not convince physicians or patients in either real or simulated studies to make changes in management choices. Hence, the value of such a test would obviously be limited. Finally, in order to understand how a test impacts health outcomes, there is a need for either clinical studies or a strong chain of evidence based on carefully selected and documented surrogates for predicting outcomes.
It would be difficult to perform the randomized clinical trial that would be required to establish the ultimate benefits and risks of PCA3 testing. Therefore, the Stakeholders provided the pragmatic direction of considering mechanisms for looking at chains of evidence that could provide information about long-term health outcomes without waiting for completion of a long-term clinical trial. Panel suggestions included the correlation of PCA3 testing with prognostic features on biopsy or with changes in grading between biopsy and prostatectomy, short-term clinical studies fashioned after the REDUCE (Reduction by Dutasteride of Prostate Cancer Events) trial but with PCA3 testing as an intervention, and add-on studies to ongoing investigations of active surveillance in carefully chosen patients.
Conclusions
The following three prioritized research needs and six research questions were identified.
Research Need 1: Information on the comparative performance of PCA3 and currently used biomarkers to detect prostate cancer; “matched studies” on comparators.
- Research Question 1.1: What is the comparative effectiveness of PCA3 compared with the two commonly used add-on tests of fPSA (free prostate-specific antigen) and tPSA velocity/doubling time in predicting prostate biopsy results?
- Research Question 1.2: What are PCA3’s diagnostic performance characteristics in patients with elevated tPSA levels?
- Research Question 1.3: What is the comparative effectiveness of PCA3 compared with externally validated nomograms in predicting prostate biopsy results?
Research Need 2: Studies on how PCA3 actually helps in biopsy or treatment decisionmaking.
- Research Question 2.1: What information does PCA3 provide about the aggressiveness of prostate cancer? Do positive results correlate with tumors with aggressive features on biopsy or upgrading of tumors on prostatectomy? Do negative results correlate with tumors that may not require identification or aggressive treatment?
- Research Question 2.2: Does the addition of PCA3, either alone or in combination with other markers, change prostate cancer biopsy or treatment decisionmaking for the patient or physician?
Research Need 3: Information on impact of PCA3 in biopsy decisionmaking on long-term health outcomes.
- Research Question 3.1: Does the addition of PCA3 testing change long-term health outcomes in prostate screening?
References
- 1.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep–Oct;60(5):277–300. [PubMed: 20610543]
- 2.
- Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2011 Mar 15;117(6):1123–35. [PubMed: 20960523]
- 3.
- Sutcliffe P, Hummel S, Simpson E. Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review. Health Technol Assess. 2009;13:1–260. [PubMed: 19128541]
- 4.
- Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. Scientific World J. 2010;10:1919–31. [PMC free article: PMC5763794] [PubMed: 20890581]
- 5.
- Moyer VA. Screening for Prostate Cancer: U.S Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012 May 21 [Epub ahead of print] [PubMed: 22801674]
- 6.
- Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–78. [PubMed: 12813170]
- 7.
- Bussmakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999 Dec 1;59(23):5975–9. [PubMed: 10606244]
- 8.
- Whitlock EP, Lopez SA, Chang S, et al. AHRQ Series Paper 3: Identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the Effective Health-Care program. J Clin Epidemiol. 2010;63:491–501. [PubMed: 19540721]
- 9.
- Robinson KA, Saldanha IJ, Mckoy NA. Frameworks for Determining Research Gaps During Systematic Reviews. Methods Future Research Needs Report No. 2. Rockville, MD: Agency for Healthcare Research and Quality; Jun, 2011. (Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. HHSA 290-2007-10061-I.) AHRQ Publication No. 11-EHC043-EF. www
.effectivehealthcare .ahrq.gov/reports/final.cfm. [PubMed: 21977524] - 10.
- Carey T, Sanders G, Viswanathan M, et al. Framework for Considering Study Designs for Future Research Needs. Methods Future Research Needs Paper No. 8. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2012. (Prepared by the RTI–UNC Evidence-based Practice Center under Contract No. 290-2007-10056-I.) AHRQ Publication No. 12-EHC048-EF. www
.effectivehealthcare .ahrq.gov/reports/final.cfm. [PubMed: 22624168]
- Executive Summary - PCA3 Testing in the Diagnosis and Management of Prostate Can...Executive Summary - PCA3 Testing in the Diagnosis and Management of Prostate Cancer: Future Research Needs
Your browsing activity is empty.
Activity recording is turned off.
See more...