NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glick SB, Samson DJ, Huang E, et al. Screening for Methicillin-Resistant Staphylococcus Aureus (MRSA) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jun. (Comparative Effectiveness Reviews, No. 102.)
This publication is provided for historical reference only and the information may be out of date.
Background
Methicillin-resistant Staphylococcus aureus (MRSA) emerged as a clinically relevant human pathogen more than five decades ago.1 The virulent bacterium was first detected in hospitals and other health care facilities where vulnerable hosts, frequent exposure to the selective pressure of intensive antimicrobial therapy, and the necessity for invasive procedures created a favorable environment for dissemination. MRSA emerged as an important cause of healthcare-associated infections, particularly central line–associated bloodstream infection, ventilator-associated pneumonia, and surgical site infection (SSI). Despite the adoption of infection-control measures, the incidence of MRSA infection at most U.S. hospitals steadily increased for many years,2 but it is now decreasing.3–6 Burton and colleagues4 found a 49.6-percent decrease in the overall incidence of MRSA central line–associated bloodstream infection in U.S. intensive care units (ICUs) from 1997 to 2007. In a study of nine U.S. metropolitan areas, Kallen and colleagues6 found a reduction in the incidence rate of hospital-onset invasive MRSA infections of 9.4 percent per year from 2005 to 2008 (95% confidence interval [CI], 14.7 to 3.8%; p=0.005).
While the decrease in the incidence of MRSA infection may be due to efforts to screen for MRSA carriage, it may also be due to secular trends (such as efforts to improve patient safety) and to confounders (such as efforts to improve the appropriate use of antibiotics and to decrease healthcare-associated infections in general, including catheter-associated bloodstream infection, ventilator-associated pneumonia, and SSI). Although not all studies concur, a number of analyses suggest that MRSA infections are associated with increased mortality and cost of care when compared with those due to strains that are susceptible to methicillin. Even the availability of newer pharmaceutical agents with specific activity against MRSA has not ameliorated the challenge of caring for patients with MRSA. The widespread use of these agents has been limited, in part due to toxicity, cost, and uncertainty as to optimal indications.3
The management and control of MRSA have been further complicated by dramatic changes in the epidemiology of transmission and infection observed over the past two decades. Specifically, S. aureus strains resistant to methicillin, once exclusively linked to hospital care, have increasingly been detected among patients in the community who lack conventional risk factors for MRSA infection.5,7 Community-acquired MRSA has been linked to outbreaks of infection in hospitals and health care facilities.8
Conventional strategies for the control of MRSA (whether hospital or community associated) have focused on the prevention of spread from patient to patient (horizontal transmission). The effectiveness of hand hygiene in preventing the spread of MRSA has been demonstrated in observational studies in which hand hygiene promotion campaigns were associated with subsequent reductions in the incidence of MRSA among hospitalized patients.9 While hand hygiene remains important in the effort to control MRSA transmission, the continued spread of the pathogen after its initial introduction in most facilities has prompted efforts to identify additional strategies. The use of contact isolation—including the donning of gowns and gloves when interacting with patients colonized or infected with MRSA and the assignment of such patients to single rooms or to a room with a group of affected patients—has been widely promoted and adopted. Such isolation precautions now are the centerpiece of most authoritative guidelines for MRSA control.10 Despite the broad consensus associated with the use of contact isolation for MRSA prevention, the specific evidence in support of this practice remains limited and indirect.
Given the continued dissemination of MRSA at most U.S. hospitals, it is clear that these measures, as presently deployed, have been insufficient to check the spread of MRSA and other antibiotic-resistant pathogens.
A further limitation of these approaches—and, specifically, the use of isolation precautions—is the potential negative consequences of these measures. A series of studies have associated isolation precautions with worsened outcomes in terms of safety and patient satisfaction.11 In addition, questions have been raised about specific performance measures, such as the frequency with which patients on isolation precautions are visited by treating physicians and the timely recording of vital signs. While the methodology employed in some of these studies has been questioned, no rigorous definitive analysis has been completed to exonerate isolation precautions.12
Based on the failure of conventional strategies (hand hygiene, barrier precautions, and isolation) to adequately control MRSA, more aggressive measures have been promoted in an effort to check the spread of this particularly virulent pathogen. In some European countries, an aggressive containment program identifies contacts of colonized and infected patients in an effort to intercede to prevent dissemination.13 While such measures have not been widely adopted in most settings, some clinicians and scientists, and increasing numbers of public advocates and legislators have raised the call for more intensive efforts at MRSA control in the United States. Particular attention has been given to the potential value of active surveillance screening for MRSA. Because routine clinical cultures may identify as few as 18 percent of patients with asymptomatic carriage of antibiotic-resistant organisms such as MRSA, there exists a large reservoir of patients who are silent carriers of these organisms. These individuals may serve as a reservoir for further transmission. With active surveillance, microbiological samples are obtained from at-risk patients in the absence of signs or symptoms of infection in an effort to identify the underlying population of colonized individuals. By detecting the larger population of colonized individuals, conventional precautions, at the very least, can be implemented in a broader and more timely manner so as to interrupt horizontal transmission of MRSA. Detection of colonized patients also permits consideration of more aggressive interventions, including attempts at microbiological eradication or decolonization.
The specific evidence in support of active surveillance for MRSA has been promising, although a number of questions remain about the effectiveness of active surveillance for MRSA carriage and whether screening should be applied to all patient populations (universal screening) or to selected populations such as patients in the ICU or those undergoing surgical procedures (targeted screening). In addition, knowing which patients are colonized with MRSA is not expected to affect the frequency of spread if adherence to transmission-control strategies remains inadequate. Moreover, other efforts (such as attempts at decolonization or eradication, as well as programs to decrease healthcare-associated infections in general) may dramatically affect the impact of a MRSA-screening program. Therefore, trying to determine the impact of a screening program without detailed information about the deployment of decolonization measures is an important limitation to the available studies and has engendered considerable confusion among clinicians and policymakers.
Thus, a systematic review of the evidence is both justified and timely. The importance of gaining a better understanding of the evidence is also highlighted by the increasing demand for better control of MRSA and a higher standard for prevention of hospital-acquired infections in general.
Objective
The objective of this systematic review was to synthesize comparative studies that examined the benefits or harms of screening for MRSA carriage in the inpatient or outpatient settings. The review examined MRSA-screening strategies applied to all hospitalized or ambulatory patients (universal screening), as well as screening strategies applied to selected inpatient or outpatient populations (e.g., patients admitted to the ICU, patients admitted for a surgical procedure, or patients at high risk of MRSA colonization or infection), and compared them with no screening or with screening of selected patient populations (targeted screening). The review evaluated MRSA-screening strategies that included screening with or without isolation and with or without attempted eradication/decolonization. The patient population included all ambulatory patients (outpatients) and hospitalized patients (inpatients).
Key Questions
Key Question 1
Among ambulatory or hospitalized patients, what are the effects of a universal screening strategy for MRSA carriage (screen, isolate, eradicate/decolonize) when compared with no screening on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
Key Question 2
Among ambulatory or hospitalized patients, what are the effects of a universal screening strategy for MRSA carriage (screen, isolate, eradicate/decolonize) when compared with screening of selected patient populations (targeted screening) on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
Key Question 3A
Among ambulatory or hospitalized patients, what are the effects of screening ICU patients for MRSA carriage (screen, isolate, eradicate/decolonize) when compared with no screening on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
Key Question 3B
Among ambulatory or hospitalized patients, what are the effects of screening surgical patients for MRSA carriage (screen, isolate, eradicate/decolonize) when compared with no screening on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
Key Question 3C
Among ambulatory or hospitalized patients, what are the effects of screening high-risk patients for MRSA carriage (screen, isolate, eradicate/decolonize) when compared with no screening on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
Key Question 4
Among ambulatory or hospitalized patients, what are the effects of an expanded screening strategy for MRSA carriage (e.g., screen, isolate, eradicate/decolonize a broader group of patients, such as all patients admitted to the medical ward, the surgical ward, or the ICU) when compared with a limited screening strategy (e.g., screen, isolate, eradicate/decolonize a limited group of patients, such as patients admitted to the ICU) on:
- Intermediate outcomes such as MRSA transmission (as measured by new acquisition events)?
- Health outcomes such as the incidence of MRSA infection, morbidity (including complications of MRSA infection), mortality, adverse events (including allergic and nonallergic toxicity [e.g., hypotension], antimicrobial resistance, reduced quality of care, and medical errors), and hospital resource utilization (e.g., length of stay)?
PICOTS (Population Intervention, Comparator, Outcome, Timing, and Setting) for the Key Questions
Population
All ambulatory patients (outpatients) and all hospitalized patients (inpatients). In addition, the following subpopulations were evaluated: (1) patients admitted to an ICU, (2) patients undergoing surgical procedures, and (3) patients at high risk of MRSA colonization or infection (e.g., patients transferred from another health care facility, patients receiving hemodialysis).
Intervention
A MRSA screening strategy applied to all patients in a setting (universal screening) or applied to particular wards, units, or patients (targeted screening) that includes:
- MRSA screening using a testing modality (typically polymerase chain reaction [PCR]) with rapid turnaround (results available on the same day as the testing is performed) or
- MRSA screening using a testing modality with intermediate turnaround (results available next day to 2 days after testing performed) or
- MRSA screening using a testing modality (typically culture) with a longer turnaround time (results available more than 2 days after testing performed)
The screening strategy also may include:
- Isolation and/or
- Eradication/decolonization
Comparator
No screening or screening of selected patient populations (targeted screening).
Outcomes
Healthcare-associated MRSA acquisition; healthcare-associated MRSA infection; morbidity (including complications of MRSA infection); mortality; quality of care for noninfectious conditions; medical errors; adverse effects of screening and treatment, including allergic reactions, nonallergic toxicities, and resistance to antimicrobials; and hospital resource utilization such as length of stay.
Timing
Intervention through followup.
Settings
Inpatient (hospital wards and ICUs) and outpatient (ambulatory clinics, urgent care centers, and emergency departments).
A comprehensive review evaluating the benefits and harms of screening for MRSA carriage will identify areas of certainty and those that require additional prospective research.
Analytic Framework
The analytic framework (Figure A) depicts the effects of screening for MRSA carriage on intermediate outcomes (including MRSA acquisition) and health outcomes (including MRSA infection, morbidity, and mortality). The detailed analytic framework (Figure B) depicts the effects of screening for MRSA carriage in detail. Once screened, patients may or may not be isolated while waiting for screening test results. Once the screening test results are received, patients who screen positive may be isolated; patients who screen negative are not. Eradication/decolonization may be attempted in patients who screen positive. Intermediate outcomes of MRSA screening, including MRSA transmission, are depicted in the figure. Health outcomes, including MRSA infection, morbidity, and mortality, are also depicted. Potential harms of screening include decreased room availability, decreased attention from health care personnel, antibiotic resistance, allergic reactions, and nonallergic toxicity.
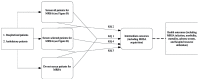
Figure A
Analytic framework for MRSA screening. KQ = Key Question; MRSA = methicillin-resistant Staphylococcus aureus
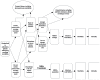
Figure B
Detailed analytic framework for MRSA screening. MRSA = methicillin-resistant Staphylococcus aureus
Methods
Input From Stakeholders
This systematic review was developed by the Evidence-based Practice Center (EPC) with input from stakeholders. Stakeholders were broadly defined as anyone involved with making health care decisions, including patients, clinicians, professional and consumer organizations, and purchasers of health care. Individuals from various stakeholder groups were invited as Key Informants, Technical Experts, and/or Peer Reviewers to guide this systematic review.
Key Informants are end-users of research. A Key Informant panel highlighted the controversies surrounding MRSA screening and the challenges inherent in a review of this topic. The Key Questions were then posted on the Agency for Healthcare Research and Quality (AHRQ) Web site for public commentary. Input from the Key Informants panel and public were incorporated into the scope of the report and the analytic framework (Figures A and B).
The Technical Expert Panel reviewed the research protocol in two phases: (1) initial draft protocol; (2) revised protocol that incorporated the Panel’s comments on the draft and findings of a preliminary literature search.
All potential Key Informants, Technical Experts, and Peer Reviewers were required to disclose any potential conflicts of interest in accordance with AHRQ policy. The AHRQ Task Order Officer and the EPC worked to balance, manage, or mitigate any potential conflicts of interest identified. Individuals who had conflicts of interest that precluded participation as informants, experts, or reviewers were able to submit comments through the public comment mechanism. Writing and editing the report were solely the responsibility of the EPC.
Data Sources and Selection
MEDLINE® was searched from January 1, 1990, through March 30, 2012, for randomized and nonrandomized comparative studies. Embase® was searched from January 1, 1990, to March 30, 2012, for randomized controlled trials (RCTs), nonrandomized comparative studies, and case series using similar search terms. The Cochrane Controlled Trials Register was searched without date restriction using the same search terms utilized for the MEDLINE and EMBASE searches. In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and the Web sites of the National Institute for Clinical Excellence (United Kingdom), the National Guideline Clearinghouse, and the Health Technology Assessment Programme (United Kingdom). The gray literature was also searched, including databases with regulatory information, clinical trial registries, abstracts and conference papers, grants and federally funded research, and manufacturing information.
The titles and abstracts were screened for studies that looked at MRSA acquisition, MRSA infection, morbidity, mortality, harms of screening, and resource utilization when screening for MRSA carriage compared with no screening or with limited screening. A single reviewer made the decision about full-text review. Citations marked as uncertain were reviewed by a second reviewer for consideration of full-text review. A third reviewer was consulted if necessary. We included RCTs and nonrandomized comparative studies.
Data Extraction and Quality Assessment
Data were abstracted by a team of reviewers and fact-checked by another reviewer. If there were disagreements, they were resolved through discussion among the review team. Categories of data elements were abstracted as follows: quality assessment (number of participants and flow of participants, treatment allocation methods, blinding, and independent outcome assessment); applicability and clinical diversity assessment (patient, diagnostic, and treatment characteristics); outcome assessment (primary and secondary outcomes, response criteria, followup frequency and duration, data analysis details).
Quality of included studies was assessed using the U.S. Preventive Services Task Force framework14 based on the following criteria: assembly and maintenance of comparable groups; loss to followup; measurements (equal, reliable, and valid); clear definition of interventions; consideration of all important outcomes; and analysis (adjustment for potential confounders and intention-to-treat analysis). Three quality categories were used: good, fair, and poor. Quality of the abstracted studies was assessed by at least two independent reviewers, and the final quality rating was assigned by consensus adjudication.
Assessment of individual study quality was greatly informed by whether studies attempted to control for confounding and/or secular trends. Studies that used such analytic techniques are described as CCS studies, while those that did not are called non-CCS studies. Non-CCS studies used simple two-group statistical analyses. Observational studies that do not attempt to control for confounding and/or secular trends do not provide evidence that supports causal inference. The ratings of good, fair, and poor quality are reserved for CCS studies. Comments will be made in the main body of the report about results from non-CCS studies, but they are not included in strength of evidence (SOE) syntheses.
Data Synthesis and Analysis
Evidence was not suitable for quantitative synthesis via meta-analysis; therefore, a qualitative approach to synthesis was pursued.
The overall SOE grade was determined in compliance with the AHRQ “Methods Guide for Effectiveness and Comparative Effectiveness Reviews”15 and is based on a system developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group.16 This system explicitly addressed the following domains: risk of bias, consistency, directness, and precision. The grade of evidence strength was classified into the following four categories: high, moderate, low, and insufficient. Specific outcomes and comparisons were rated depending on the evidence found in the literature. The starting level of strength for a body of evidence differed according to whether it included RCTs or only observational evidence. Bodies of evidence from RCTs would start at high. If evidence was purely observational, the starting level of evidence would be low. However, high risk of bias due to study limitations or publication bias, or lack of consistency, precision, or directness may further decrease the SOE. If observational studies reported large effect sizes, presence of a dose-response association, or plausible confounding that would reduce the observed effect, the SOE could be raised. The grade rating was made by independent reviewers, and disagreements were resolved by consensus adjudication.
Results
Overview
Overall, 48 studies were abstracted for this review. (The complete list of references may be found in the full report.) Three studies reported outcomes that addressed Key Question 1, 2 studies reported outcomes that addressed Key Question 2, 14 studies reported outcomes that addressed Key Question 3A, 18 studies reported outcomes that addressed Key Question 3B, 8 studies reported outcomes that addressed Key Question 3C, and 10 studies reported outcomes that addressed Key Question 4. Healthcare-associated outcomes are the primary outcomes of interest because screening for MRSA carriage in health care facilities is expected to impact healthcare-associated MRSA transmission and infection most proximately.
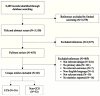
The 16 CCS studies17–32 had the potential to support causal inferences about the impact of MRSA screening on health outcomes and therefore to contribute to the SOE analysis. Because screening for MRSA carriage in the hospital or ambulatory settings is expected to affect healthcare-associated MRSA acquisition, infection, morbidity, and mortality most proximately, healthcare-associated outcomes are the outcomes of interest. The 14 CCS studies17,18,20,21,23–32 that reported a healthcare-associated outcome were included in the SOE analysis across all four Key Questions (Table A). Two of the CCS studies19,22 did not report an outcome that was exclusively healthcare associated and therefore were excluded from the SOE analysis. The remaining 32 non-CCS studies performed simple two-group statistical analyses, which cannot support causal inferences; the non-CCS studies were therefore excluded from the SOE syntheses. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram (Figure C) depicts the flow of search screening and study selection.
Table A
Summary of outcome measures and strength of evidence.
Key Question 1. Universal Screening for MRSA Carriage Compared With No Screening
Three quasi-experimental CCS studies17–19 described universal screening for MRSA carriage compared with no screening. The Robicsek et al. study17 was judged to be of good quality; the Jain et al. study18 and the Reilly et al. study19 were judged to be of poor quality. However, the Reilly study did not contribute to the SOE assessment because it did not report an outcome that was exclusively healthcare associated.
Healthcare-Associated MRSA Acquisition
Only the Jain study18 addressed this outcome. This study showed a statistically significant reduction in healthcare-associated MRSA acquisition in the ICU and non-ICU settings with universal screening for MRSA. The risk of bias was judged to be high, as only one poor-quality observational study addressed this outcome. Because only one study18 evaluated this outcome, the consistency was unknown. The outcome was indirect and findings were precise. Because the evidence base that addressed this outcome consisted of a single observational study, the starting level of SOE was low. SOE was lowered one level based on the high risk of bias. Therefore, the SOE that universal screening for MRSA carriage decreases healthcare-associated MRSA acquisition compared with no screening is insufficient.
Healthcare-Associated MRSA Infection
Both the Robicsek study17 and the Jain study18 addressed this outcome. Both studies found a statistically significant reduction in healthcare-associated MRSA infection with universal screening for MRSA compared with no screening, ranging from a reduction of 45 percent to 70 percent. Because the evidence base that addressed this outcome consisted of two quasi-experimental studies, the starting level for the SOE was low. The results were consistent, the outcome was direct, and the findings were precise. SOE was raised by one level based on the large effect size but lowered one level based on the high risk of bias. Therefore, the SOE that universal screening for MRSA carriage decreases healthcare-associated MRSA infection compared with no screening is low.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE is insufficient to assess the effect of universal screening for MRSA carriage compared with no screening on morbidity, mortality, harms, or resource utilization.
Key Question 2. Universal Screening for MRSA Carriage Compared With Screening of Selected Populations (Targeted Screening)
Two quasi-experimental CCS studies of good quality compared universal screening for MRSA carriage on hospital admission to screening of selected patient populations (targeted screening).17,20
Healthcare-Associated MRSA Acquisition
No studies addressed this outcome. Therefore, the SOE to evaluate the effect of universal screening for MRSA carriage compared with targeted screening on healthcare-associated MRSA acquisition is judged to be insufficient.
Healthcare-Associated MRSA Infection
Two quasi-experimental CCS studies found a reduction in healthcare-associated MRSA infection. Robicsek et al.17 found that the rate of hospital-acquired MRSA infection declined by 52.4 percent (CI, 9.3 to 78.3%) in the universal screening group, while Leonhardt et al.20 showed a 0.12-percent reduction in hospital-acquired infection with universal screening compared with targeted screening (p=0.23; difference in difference p=0.34). The risk of bias was judged to be medium, as two good-quality observational studies addressed this outcome.17,20 The results were consistent, the outcome was direct, and the findings were imprecise. Because the evidence base for this outcome consisted of two observational studies, the starting level for the SOE was low. SOE was lowered by one level based on the medium risk of bias and by one level based on the imprecise results and is therefore insufficient. In summary, the SOE for change in healthcare-associated MRSA infection with universal screening compared with targeted screening for MRSA carriage is insufficient.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE to evaluate the effect of universal screening for MRSA carriage compared with targeted screening on morbidity, mortality, harms, or resource utilization is judged to be insufficient.
Key Question 3A. MRSA Targeted Screening (ICU) Versus No Screening
Seven CCS studies17,21–26 (one cluster RCT, six quasi-experimental studies) reported outcomes that addressed Key Question 3A, screening of ICU patients for MRSA carriage compared with no screening. The Huskins et al. study24 was a good-quality cluster RCT. Of the six quasi-experimental studies, one was good quality,17 one was fair quality,22 and four were poor quality.21,23,25,26 However, the fair-quality study22 did not contribute to the SOE assessment because it did not report an outcome that was exclusively healthcare associated.
Healthcare-Associated MRSA Acquisition
Four CCS studies21,23–25 (one cluster RCT, three quasi-experimental studies) evaluated this outcome. Although the three quasi-experimental studies21,23,25 found statistically significant reductions in healthcare-associated colonization or infection, the good-quality cluster RCT24 found a nonstatistically significant increase in healthcare-associated MRSA colonization or infection with targeted screening. Thus, the results were inconsistent. The outcome was indirect and the findings were imprecise. The evidence base included an RCT of good quality, so the starting level for the SOE was high. However, due to serious concerns about the lack of consistency, the SOE was reduced by two levels. The SOE was further reduced by one level due to lack of precision. In summary, the SOE to evaluate the effect of screening of ICU patients for MRSA carriage on MRSA acquisition is insufficient and lacks precision.
We conducted a sensitivity analysis in which we excluded the cluster RCT24 from the SOE analysis because of criticisms of the lengthy turnaround time of its screening test and the failure to implement contact precautions and/or isolation while awaiting test results.33,34 The three remaining quasi-experimental studies were of poor quality to address this outcome, which would still lead to insufficient SOE to evaluate the effect of screening of ICU patients for MRSA carriage on MRSA acquisition.
Healthcare-Associated MRSA Infection, Irrespective of Site
Two quasi-experimental CCS studies17,26 (one good quality,17 one poor quality26) evaluated this outcome. Both studies found a reduction in healthcare-associated MRSA infection with screening of ICU patients for MRSA carriage compared with no screening, although one of the studies did not find the difference to be statistically significant.17 The risk of bias was judged as high, as the body of evidence that evaluated this outcome included only quasi-experimental studies, only one of which was of good quality. The results were consistent, the outcome was direct, and the findings were imprecise. Because the evidence base for this outcome includes only observational studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias and the lack of precision. In summary, the SOE is insufficient to support or refute the statement that, compared with no screening, screening for MRSA carriage in ICU patients decreases healthcare-associated MRSA infection.
Healthcare-Associated MRSA Bacteremia or Bloodstream Infection
Two quasi-experimental CCS studies17,21 evaluated this outcome. One good-quality study17 found a reduction in the rate of acquired MRSA bloodstream infection with screening for MRSA in the ICU compared with no screening (absolute change in prevalence density, −0.15; 95% CI, −1.14 to 0.85); however, this reduction was not statistically significant. One poor-quality study21 found a statistically significant reduction in the trend of incidence density of hospital-associated MRSA bloodstream infection in the ICU, non-ICU settings, and hospitalwide with screening for MRSA in the ICU. In addition, this study21 found a statistically significant reduction in the trend of incidence of hospital-associated MRSA bloodstream infection hospitalwide with screening for MRSA in the ICU. The risk of bias was deemed to be high, as the body of evidence comprised quasi-experimental studies, only one of which was good quality.17 The results were consistent and the outcome was direct. Because the individual studies did not consistently report statistically significant results, the findings were imprecise. Because the evidence base for this outcome includes only quasi-experimental studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias and the lack of precision. In summary, the SOE is insufficient to support or refute the statement that, compared with no screening, screening for MRSA carriage in ICU patients decreases healthcare-associated MRSA bacteremia or bloodstream infection.
Healthcare-Associated MRSA Surgical Site Infection
One good-quality quasi-experimental CCS study addressed this outcome.17 It found a nonstatistically significant reduction in hospital-associated SSI with screening in the ICU compared with no screening (rate difference, −0.77; 95% CI, −1.85 to 0.30).17 The risk of bias was deemed to be high, as the body of evidence consisted of only a single good-quality observational study. The consistency was unknown, the outcome was direct, and the findings were imprecise. Because the evidence base for this outcome included only one observational study, the starting level for the SOE was low. SOE was lowered by the high risk of bias and lack of precision. In summary, the SOE for the effect of screening of ICU patients on healthcare-associated MRSA SSI is judged to be insufficient.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE to evaluate the effect of screening of ICU patients for MRSA carriage on morbidity, mortality, harms, or resource utilization is judged to be insufficient.
Key Question 3B. MRSA Targeted Screening (Surgical Patients) Versus No Screening
Three CCS studies26–28 described screening of surgical patients for MRSA compared with no screening. The Harbarth et al. study27 was a prospective interventional cohort study with crossover design of good quality. The Muder et al. study26 and the Ellingson et al. study28 were quasi-experimental before/after studies of poor quality.
Healthcare-Associated MRSA Acquisition
Two CCS studies (one good quality,27 one poor quality28) addressed this outcome. Neither study found statistically significant differences in MRSA acquisition with screening surgical patients (rate ratios from 0.78 to 1.1). With screening of surgical patients, the good-quality study found a nonstatistically significant increase in the rate ratio for MRSA acquisition,27 while the Ellingson study28 found nonstatistically significant reductions in the incidence rate ratio as well as in the trend in the incidence of MRSA colonization or infection. The risk of bias was deemed to be high because the body of evidence consisted of quasi-experimental studies, only one of which was good quality. The findings were inconsistent. The outcome was indirect, and the study findings were judged to be imprecise. Because the evidence base for this outcome included only observational studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias, lack of consistency, and lack of precision. In summary, the SOE for the effect of screening of surgical patients on healthcare-associated MRSA acquisition is judged to be insufficient.
Healthcare-Associated MRSA Infection, Irrespective of Site
Two CCS studies (one good quality,27 one poor quality26) reported the effect of screening for MRSA carriage in surgical wards on healthcare-associated infection. The good-quality study27 found a nonstatistically significant increase in rates of MRSA infection with screening surgical patients (1.11/1,000 patient days vs. 0.91/1,000 patient days). However, the poor-quality study26 found that MRSA infection steadily declined in the surgical ward (1.56/1,000 patient days pre, 0.63/1,000 patient days post; p=0.003). The risk of bias was judged to be high because the body of evidence that evaluated this outcome included only quasi-experimental studies, only one of which was of good quality.27 The findings were inconsistent, a direct outcome was measured, and study findings were imprecise. Because the evidence base for this outcome included only observational studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias, lack of consistency, and lack of precision. In summary, the SOE for the effect of screening for MRSA carriage in surgical patients on healthcare-associated MRSA infection is judged to be insufficient.
MRSA Surgical Site Infection
One good quality CCS study27 reported on MRSA SSI. With screening in surgical patients, Harbarth and colleagues27 found a nonstatistically significant increase in MRSA SSI (rate ratio, 1.2; 95% CI, 0.8 to 1.7). The risk of bias was judged to be high because the body of evidence that evaluated this outcome included only a quasi-experimental study.27 With screening in surgical patients, Harbarth and colleagues27 found no reduction in MRSA SSI; in fact, the rate was slightly higher, although not statistically significant. The consistency of the findings is unknown, the outcome is direct, and study findings were imprecise. Because the evidence base for this outcome included only one observational study, the starting level for the SOE was low. The SOE was lowered by the high risk of bias and lack of precision. In summary, the SOE for the effect of screening for MRSA carriage in surgical patients on MRSA SSI is judged to be insufficient.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE to evaluate the effect of screening of surgical patients for MRSA carriage on morbidity, mortality, harms, or resource utilization is judged to be insufficient.
Key Question 3C. MRSA Targeted Screening (High-Risk Patients) Versus No Screening
Three CCS studies29–31 described screening of high-risk patients for MRSA carriage compared with no screening. All of the studies employed a quasi-experimental study design and were of poor quality.
Healthcare-Associated MRSA Acquisition
One CCS study31 evaluated this outcome. This study found a nonstatistically significant decrease in the incidence of MRSA acquisition (−0.065; 95% CI, −0.053 to 0.182). There was a statistically significant reduction in trend in incidence of MRSA acquisition (−0.045; 95% CI, −0.062 to −0.029). The risk of bias for the body of evidence was deemed to be high because only a single poor-quality quasi-experimental study31 evaluated this outcome. The consistency was unknown, the outcome was indirect, and study findings were imprecise. Because the evidence base for this outcome consisted of only one quasi-experimental study, the starting level for the SOE was low. SOE was lowered by the high risk of bias and lack of precision. In summary, the SOE for the effect of screening of high-risk patients on healthcare-associated MRSA acquisition is judged to be insufficient.
Healthcare-Associated MRSA Infection, Irrespective of Site
One30 CCS study evaluated this outcome. This study showed a statistically significant reduction in healthcare-associated MRSA infection with screening of high-risk patients. The risk of bias for the body of evidence was deemed to be high because only one poor-quality quasi-experimental study addressed this outcome. The consistency was unknown, the outcome was direct, and study findings were precise. Because the evidence base for this outcome consisted of only one quasi-experimental study, the SOE was low. SOE was lowered by the high risk of bias. In summary, the SOE for the effect of screening of high-risk patients on healthcare-associated MRSA infection is judged to be insufficient.
Healthcare-Associated MRSA Bacteremia or Bloodstream Infection
Two CCS studies29,31 addressed this outcome. Both studies found statistically significant decreases in MRSA bacteremia. The risk of bias for the body of evidence was determined to be high, as two quasi-experimental studies of poor quality addressed this outcome. The study findings were consistent, the outcomes were direct, and study findings were precise. Because the evidence base for this outcome included only observational studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias. In summary, the SOE for the effect of screening for MRSA carriage in high-risk patients compared with no screening on healthcare-associated MRSA bacteremia or bloodstream infection is judged to be insufficient.
MRSA Surgical Site Infection
One CCS study30 addressed this outcome. The Harbarth30 study showed a statistically significant reduction in MRSA SSI with screening of high-risk patients compared with no screening. The risk of bias for the body of evidence was deemed to be high because only a single poor-quality quasi-experimental study addressed this outcome. The consistency was unknown, the outcome was direct, and study findings were precise. Because the evidence base for this outcome consisted of only one quasi-experimental study, the starting level for the SOE was low. SOE was lowered by the high risk of bias. In summary, the SOE for the effect of screening of high-risk patients on MRSA SSI is judged to be insufficient.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE to evaluate the effect of screening of high-risk patients for MRSA carriage on morbidity, mortality, harms, or resource utilization is judged to be insufficient.
Key Question 4. Screening of a Broader Patient Population for MRSA Carriage (Expanded Screening) Compared With Screening of a Narrower Patient Population (Limited Screening)
Three CCS studies28,31,32 described expanded screening for MRSA carriage compared with limited screening. The study by Rodriguez-Bano and colleagues31 utilized an interrupted time series design, as did the study by Ellingson and colleagues.28 The study by Chaberny and colleagues32 utilized a before/after study design. All three studies were determined to be of poor quality.
Healthcare-Associated MRSA Acquisition
Two CCS studies28,31 evaluated healthcare-associated MRSA infection or colonization. Although both studies found reductions in the incidence and trend of healthcare-associated MRSA colonization or infection with expanded screening, these reductions were not consistently statistically significant. The Rodriguez-Bano study31 showed reductions in the incidence and trend of healthcare-associated MRSA infection or colonization with expanded screening compared with limited screening (change in trend, 0.047; 95% CI, 0.035 to 0.059; change in incidence, 0.077; 95% CI, −0.012 to 0.165). Although the reduction in trend was statistically significant, the reduction in incidence was not.31 The Ellingson study28 showed reductions in the incidence rate ratio for MRSA colonization or infection after the interventions (screening for MRSA carriage in the ICU: incidence rate ratio, 0.913; 95% CI, 0.356 to 2.343; screening for MRSA carriage in all other acute care units: incidence rate ratio, 0.656; 95% CI, 0.440 to 0.979). The reduction was statistically significant for one intervention but not for the other. In addition, the Ellingson study28 showed a reduction in the preintervention to postintervention trends (screening for MRSA carriage in the ICU: incidence rate ratio, 0.971; 95% CI, 0.938 to 1.004; screening for MRSA carriage in all other acute care units: incidence rate ratio, 0.998; 95% CI, 0.982 to 1.014).
The risk of bias for the body of evidence was determined to be high, as two quasi-experimental studies28,31 of poor quality addressed this outcome. The study findings were consistent, the outcome was indirect, and study findings were imprecise. Because the evidence base for this outcome included only quasi-experimental studies, the starting level for the SOE was low. SOE was lowered by the high risk of bias. In summary, the SOE for the effect of expanded screening for MRSA carriage compared with limited screening on healthcare-associated MRSA acquisition is judged to be insufficient.
Healthcare-Associated MRSA Infection, Irrespective of Site
One CCS study32 addressed this outcome. With expanded screening, Chaberny et al.32 found a reduction in the incidence density of healthcare-associated MRSA infection (change in level of −0.122; 95% CI, −0.204 to −0.040; p=0.004). In addition, Chaberny et al.32 found a reduction in the monthly change in incidence density of healthcare-associated MRSA infection (change in slope, −0.008; 95% CI, −0.013 to −0.003; p=0.004). The risk of bias for the body of evidence was determined to be high because only one poor-quality quasi-experimental study addressed this outcome. The consistency was unknown, the outcome was direct, and study findings were precise. Because the evidence base for this outcome consisted of only one quasi-experimental study, the starting level for the SOE was low. SOE was lowered by the high risk of bias. In summary, the SOE for the effect of expanded screening for MRSA carriage compared with limited screening on healthcare-associated MRSA infection is judged to be insufficient.
Healthcare-Associated MRSA Bacteremia or Bloodstream Infection
One CCS study31 addressed this outcome. This study reported a reduction in hospital-acquired MRSA bacteremia with expanded screening compared with limited screening, but the CIs included the null (change in incidence: 0.002; 95% CI, −0.022 to 0.026; change in trend: 0.003; 95% CI, 0.000 to 0.006). The risk of bias was judged to be high because only one poor-quality quasi-experimental study addressed this outcome. The consistency was unknown, the outcome was direct, and study findings were imprecise. Because the evidence base for this outcome consisted of only one quasi-experimental study, the starting level for the SOE was low. SOE was lowered by the high risk of bias and lack of precision. In summary, the SOE for the effect of expanded screening for MRSA carriage compared with limited screening on healthcare-associated MRSA bacteremia is judged to be insufficient.
Morbidity, Mortality, Harms, and Resource Utilization
Because no studies addressed these outcomes, the SOE to evaluate the effect of expanded screening for MRSA carriage compared with limited screening on morbidity, mortality, harms, or resource utilization is judged to be insufficient.
Discussion
This review found a low strength of evidence to support the effectiveness of universal screening for MRSA carriage compared with no screening in reducing healthcare-associated MRSA infection. However, the available evidence is insufficient to reach a conclusion regarding the effectiveness of screening for MRSA carriage for any of the other comparisons and outcomes of interest evaluated.
The bulk of the available literature on the comparative effectiveness of screening for MRSA carriage consists of quasi-experimental studies, largely observational studies with a before/after study design. The sole cluster RCT24 in this literature showed no favorable impact of screening, although concerns about the lengthy turnaround time of the screening modality used and the failure to implement barrier precautions, isolation, and/or decolonization while awaiting screening test results limit the applicability of this study’s findings.
The use of observational studies to determine causal inference requires protection against bias and confounding through features of design, conduct, or analysis. For example, because the incidence of MRSA infection has been decreasing, studies that utilize a before/after study design without adequately controlling for secular trends are unable to distinguish between an effect due to the intervention and an effect due to the persistence of the secular trend itself. Similarly, because other interventions geared toward patient safety, quality improvement, or prevention of healthcare-associated infections may also decrease the incidence of MRSA infection, as may unmonitored efforts at decolonization/eradication or improvements to the physical plant that increase the availability of private hospital rooms, studies that utilize a before/after design and do not adequately control for these and other similar confounders cannot establish whether the effect seen is due to the intervention or to the confounding variable. Therefore, studies that performed simple statistical tests without adequate attempts to control for confounding and/or secular trends had to be excluded from the SOE analysis.
An important limitation of the available evidence regarding MRSA screening relates to heterogeneity in the nature of the interventions performed. By its nature, MRSA screening itself would not be expected to impact the frequency of subsequent transmission or infection. Rather, clinical outcomes are influenced by the application of additional infection-control interventions in response to the detection of colonization, including more rigorous hand hygiene, barrier precautions, environmental cleaning, and antimicrobial decolonization. That these interventions are often deployed as part of a “bundle” further limit the conclusions that can be drawn about the benefit attributable to screening compared with any other component of the intervention.
Many of the included studies provided insufficient information about the full scope of interventions deployed in conjunction with screening for MRSA carriage, especially those measures implemented in response to the new detection of MRSA colonization. For example, while decolonization for MRSA-positive patients may not have been recommended as part of the screening intervention, most studies did not address whether or not decolonization was specifically prohibited. As a result, the measured effect of the screening strategy may have been influenced by the application of uncontrolled and unmeasured interventions targeting MRSA colonization.
In addition, included studies often failed to examine the potential impact of other concurrent infection-prevention efforts on the measured impact of screening for MRSA carriage. Campaigns to reduce the frequency of vascular device infections, initiatives to improve hand hygiene, and interventions to promote an institutional culture of safety have been shown to influence the frequency of many healthcare-associated infections, including those caused by MRSA. Therefore, the omission of this factor may be important.
Findings in Relationship to What Is Already Known
At least two previous systematic reviews have evaluated the impact of screening for MRSA carriage. McGinigle et al.35 concluded that there were significant gaps in the evidence that precluded definitive recommendations about the effectiveness of screening for MRSA carriage. After meta-analysis, Tacconelli et al.36 found a statistically significant reduction in the risk of MRSA bloodstream infection, but not SSI.
The conclusions of the present report are not substantially different from those reached in the previous systematic reviews, although there are some differences in the interpretation of the findings. In all three reports, the paucity of rigorous well-controlled studies employing uniform or even standardized microbiological and infection-control techniques serves as a critical limitation. The present review includes a much larger set of published studies for assessment. In addition, this Comparative Effectiveness Review utilized a more rigorous standard for assessment of study quality than did the prior reviews.
Guidelines and Public Policy
The 2006 Guidelines for the Management of Multidrug-Resistant Organisms in Healthcare Settings published by the Centers for Disease Control and Prevention (CDC) Healthcare Infection Control Practices Advisory Committee (HICPAC)37 include active surveillance screening as a recommended control strategy for multidrug-resistant organisms (MDROs), including MRSA. This document recommends that such interventions be implemented when the frequency of MDRO infections has not decreased despite the use of more routine control measures.
The 2003 Society for Healthcare Epidemiology of America (SHEA) Guidelines for Preventing Nosocomial Transmission of Multidrug-Resistant Strains of Staphylococcus Aureus and Enterococcus38 recommends that active surveillance cultures and contact precautions be implemented to prevent the spread of epidemiologically significant antibiotic-resistant pathogens. The guidelines further advise that these measures “should be implemented in all types of health care facilities throughout the system.”
A subsequent SHEA position paper39 stepped back from advocating mandatory screening, citing concerns about the importance of institutional risk assessment and possible unintended consequences of mandatory and widespread screening.
Overall, the strength of the available evidence and the findings of this review do not appear to readily support or refute the recommendations adopted by the CDC HICPAC or the SHEA Guidelines.
Applicability
The vast majority of included studies employed a quasi-experimental study design, largely an observational before/after design. The use of historical controls is subject to confounding due to epidemiological trends that contribute to variation in the incidence of infectious diseases over time. Even large studies conducted across multiple geographic sites and clinical settings can be influenced by these secular trends.18 While such changes over time may reflect statistical variation alone, changes in disease incidence also may be due to outbreaks of infection, deviations and departures from best practice, the widespread dissemination of new prevention practices, changes in antibiotic prescribing, seasonal influences, or even the application of other interventions that influence transmission or infection. Unless these epidemiologic trends are identified and accounted for, they may influence the perception of the effectiveness of screening for MRSA carriage.
Implications for Clinical and Policy Decisionmaking
Insufficient evidence is currently available to determine the comparative effectiveness of screening for MRSA carriage on MRSA transmission, MRSA infection, morbidity, mortality, harms, or resource utilization for most comparisons addressed in this review. However, compared with no screening, there is low SOE that universal screening for MRSA carriage decreases healthcare-associated MRSA infection. Unfortunately, we do not have a complete understanding of the health consequences to patients of MRSA screening and the resource utilization tradeoffs for institutions. The lack of evidence to compare the tradeoffs associated with various strategies of MRSA screening precludes conclusions that either support or refute the routine implementation of screening for MRSA carriage as part of organizational infection control in all settings.
Limitations of the Comparative Effectiveness Review Process
Determining the scope of the review posed an important challenge. The decision was made to be inclusive in considering the available literature, in which observational studies were overrepresented. In the same vein, contributors to this review were challenged to negotiate a rational and justifiable framework for presenting the many included observational studies. To this end, the decision was made to recognize the importance of the use of statistical methods to attempt to control for confounding and/or secular trends, as studies using these methods have the potential to support causal inferences about the impact of MRSA screening on health outcomes. The Results section highlights these studies, which also contributed to the SOE assessment.
Limitations of the Evidence Base, Research Gaps, and Future Research Opportunities
The available evidence is limited by inconsistency in the definition, application, and measurement of the interventions commonly bundled together with MRSA screening. Future studies that aim to contribute evidence on the benefits of screening for MRSA carriage must take a more controlled approach to the testing strategy utilized (e.g., PCR vs. culture), test turnaround time, management of patients before screening test results are known, transmission prevention strategy (e.g., contact precautions), and use of decolonization therapy. In addition, future research should quantify and account for the potential bias introduced by temporal trends, as well as the influence of concomitant infection prevention strategies and interventions.
Ideally, future studies will compare the effectiveness of screening strategies that employ different interventions, alone and in combination. In essence, this work will entail examining each element of an intervention bundle in order to accurately determine the benefit or harm that can be attributed to it. For example, it is possible that a single component of an intervention (such as the decolonization of patients found through screening to be MRSA positive) may independently produce a significant clinical benefit.
The cluster RCT is increasingly recognized as the optimal design for testing and evaluating the impact of infection-prevention strategies. In this approach, rather than randomizing individual patients, wards or units are randomized to the intervention or control groups. This approach reduces the bias associated with even large multicenter observational studies. However, cluster RCTs may also face barriers to feasibility due to the large number of institutions needed to achieve balance after randomization. It is also imperative to improve the quality of quasi-experimental studies through: (1) more rigorous study design, (2) controlling for secular trends and confounders, and (3) reporting on the full range of clinically important outcomes.
Precise estimates of the comparative effectiveness of screening for MRSA carriage on morbidity and mortality are lacking. To allow meaningful assessment of these crucial health outcomes, future studies will need to enroll sufficient numbers of patients to be adequately powered to detect any effect. Thus, large multicenter trials will be needed.
Most importantly, to conclusively determine the comparative effectiveness of screening for MRSA carriage, the harms of screening compared with those of not screening or of screening selected patient populations must be clearly delineated. To attempt to measure the favorable impact of screening for MRSA carriage while ignoring its potential risks is to present incomplete and potentially misleading data.
Conclusions
There is low SOE that universal screening of hospital patients decreases MRSA infection. However, there is insufficient evidence on other outcomes of universal MRSA screening, including morbidity, mortality, harms, and resource utilization. There is also insufficient evidence to support or refute the effectiveness of MRSA screening on any outcomes in other settings. The available literature consisted mainly of observational studies with insufficient controls for secular trends and confounding to support causal inference, particularly because other inventions were inconsistently bundled together with MRSA screening. Future research on MRSA screening should use design features and analytic strategies addressing secular trends and confounding. Designs should also permit assessment of effects of specific bundles of screening and infection control interventions and address outcomes, including morbidity, mortality, harms, and resource utilization.
References
- 1.
- Jevons MP. “Celbenin” - resistant Staphylococci. BMJ. 1961;1(5219):124–5.
- 2.
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007 Dec;13(12):1840–6. [PMC free article: PMC2876761] [PubMed: 18258033]
- 3.
- Barton E, MacGowan A. Future treatment options for Gram-positive infections--looking ahead. Clin Microbiol Infect. 2009 Dec;15(Suppl 6):17–25. [PubMed: 19917023]
- 4.
- Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009 Feb 18;301(7):727–36. [PubMed: 19224749]
- 5.
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998 Feb 25;279(8):593–8. [PubMed: 9486753]
- 6.
- Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010 Aug 11;304(6):641–8. [PubMed: 20699455]
- 7.
- Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections--Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52(5):88. [PubMed: 12588006]
- 8.
- McAdams RM, Ellis MW, Trevino S, et al. Spread of methicillin-resistant Staphylococcus aureus USA300 in a neonatal intensive care unit. Pediatr Int. 2008 Dec;50(6):810–5. [PubMed: 19067897]
- 9.
- Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme Lancet. 2000 Oct 14;356(9238):1307–12. [PubMed: 11073019]
- 10.
- Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008 Oct;29(Suppl 1):S12–21. [PubMed: 18840084]
- 11.
- Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003 Oct 8;290(14):1899–905. [PubMed: 14532319]
- 12.
- Weber SG, Perl TM, Cosgrove SE. Quality of care and satisfaction among patients isolated for infection control. JAMA. 2004 Jan 28;291(4):421. author reply 421–2. [PubMed: 14747493]
- 13.
- Vos MC, Behrendt MD, Melles DC, et al. 5 years of experience implementing a methicillin-resistant Staphylococcus aureus search and destroy policy at the largest university medical center in the Netherlands. Infect Control Hosp Epidemiol. 2009 Oct;30(10):977–84. [PubMed: 19712031]
- 14.
- Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001 Apr;20(3 Suppl):21–35. [PubMed: 11306229]
- 15.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2011. AHRQ No. 10(11)-EHC063-EF. Chapters available at www
.effectivehealthcare.ahrq.gov. - 16.
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004 Dec 22;4(1):38. [PMC free article: PMC545647] [PubMed: 15615589]
- 17.
- Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008 Mar 18;148(6):409–18. [PubMed: 18347349]
- 18.
- Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011 Apr 14;364(15):1419–30. [PubMed: 21488764]
- 19.
- Reilly JS, Stewart S, Christie P, et al. Universal screening for meticillin-resistant Staphylococcus aureus in acute care: risk factors and outcome from a multicentre study. J Hosp Infect. 2012 Jan;80(1):31–5. [PubMed: 22104473]
- 20.
- Leonhardt KK, Yakusheva O, Phelan D, et al. Clinical effectiveness and cost benefit of universal versus targeted methicillin-resistant Staphylococcus aureus screening upon admission in hospitals. Infect Control Hosp Epidemiol. 2011;32(8):797–803. [PubMed: 21768764]
- 21.
- Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2006 Oct 15;43(8):971–8. [PubMed: 16983607]
- 22.
- Gould IM, MacKenzie FM, MacLennan G, et al. Topical antimicrobials in combination with admission screening and barrier precautions to control endemic methicillin-resistant Staphylococcus aureus in an Intensive Care Unit. Int J Antimicrob Agents. 2007 May;29(5):536–43. [PubMed: 17337163]
- 23.
- Holzmann-Pazgal G, Monney C, Davis K, et al. Active surveillance culturing impacts methicillin-resistant Staphylococcus aureus acquisition in a pediatric intensive care unit. Pediatr Crit Care Med. 2011;12(4):e171–e5. [PubMed: 20838355]
- 24.
- Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011 Apr 14;364(15):1407–18. [PMC free article: PMC3410743] [PubMed: 21488763]
- 25.
- Raineri E, Crema L, De Silvestri A, et al. Meticillin-resistant Staphylococcus aureus control in an intensive care unit: a 10 year analysis. J Hosp Infect. 2007 Dec;67(4):308–15. [PubMed: 17945395]
- 26.
- Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2008 Aug;29(8):702–8. 7 p following 8. [PubMed: 18624651]
- 27.
- Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299(10):1149–57. [PubMed: 18334690]
- 28.
- Ellingson K, Muder RR, Jain R, et al. Sustained reduction in the clinical incidence of methicillin-resistant Staphylococcus aureus colonization or infection associated with a multifaceted infection control intervention. Infect Control Hosp Epidemiol. 2011 Jan;32(1):1–8. [PubMed: 21133794]
- 29.
- Chowers MY, Paitan Y, Gottesman BS, et al. Hospital-wide methicillin-resistant Staphylococcus aureus control program: a 5-year follow-up. Infect Control Hosp Epidemiol. 2009 Aug;30(8):778–81. [PubMed: 19580437]
- 30.
- Harbarth S, Martin Y, Rohner P, et al. Effect of delayed infection control measures on a hospital outbreak of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2000 Sep;46(1):43–9. [PubMed: 11023722]
- 31.
- Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of endemic hospital-wide methicillin-resistant Staphylococcus aureus (MRSA): the impact of targeted active surveillance for MRSA in patients and healthcare workers. Infect Control Hosp Epidemiol. 2010 Aug;31(8):786–95. [PubMed: 20524852]
- 32.
- Chaberny IF, Schwab F, Ziesing S, et al. Impact of routine surgical ward and intensive care unit admission surveillance cultures on hospital-wide nosocomial methicillin-resistant Staphylococcus aureus infections in a university hospital: an interrupted time-series analysis. J Antimicrob Chemother. 2008 Dec;62(6):1422–9. [PubMed: 18765411]
- 33.
- Enoch DA, Cargill JS, Sismey A, et al. MRSA surveillance in a UK district hospital: measuring clinical isolates with MRSA is more useful than measuring MRSA bacteraemias. J Hosp Infect. 2011 Dec;79(4):287–91. [PubMed: 21978609]
- 34.
- Platt R. Time for a culture change? N Engl J Med. 2011 Apr 14;364(15):1464–5. [PubMed: 21488769]
- 35.
- McGinigle KL, Gourlay ML, Buchanan IB. The use of active surveillance cultures in adult intensive care units to reduce methicillin-resistant Staphylococcus aureus-related morbidity, mortality, and costs: a systematic review. Clin Infect Dis. 2008 Jun 1;46(11):1717–25. [PubMed: 18494098]
- 36.
- Tacconelli E, De Angelis G, de Waure C, et al. Rapid screening tests for meticillin-resistant Staphylococcus aureus at hospital admission: systematic review and meta-analysis. Lancet Infect Dis. 2009 Sep;9(9):546–54. [PubMed: 19695491]
- 37.
- Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007 Dec;35(10 Suppl 2):S165–93. [PubMed: 18068814]
- 38.
- Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003 May;24(5):362–86. [PubMed: 12785411]
- 39.
- Weber SG, Huang SS, Oriola S, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Am J Infect Control. 2007 Mar;35(2):73–85. [PubMed: 17327185]
- Executive Summary - Screening for Methicillin-Resistant Staphylococcus Aureus (M...Executive Summary - Screening for Methicillin-Resistant Staphylococcus Aureus (MRSA)
- References - Management of Colonic DiverticulitisReferences - Management of Colonic Diverticulitis
- Exact Search Strings - Pain Management Interventions for Hip FractureExact Search Strings - Pain Management Interventions for Hip Fracture
- Introduction - Nonsurgical Treatments for Urinary Incontinence in Adult Women: D...Introduction - Nonsurgical Treatments for Urinary Incontinence in Adult Women: Diagnosis and Comparative Effectiveness
Your browsing activity is empty.
Activity recording is turned off.
See more...