NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Yeh HC, Lau BD, Golden SH, et al. Insulin Delivery and Glucose Monitoring Methods: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 57 [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. (Future Research Needs Papers, No. 32.)
Context
Diabetes mellitus is defined as a group of metabolic diseases characterized by hyperglycemia resulting from: defects in insulin secretion from the pancreatic beta cells; resistance to insulin action at the level of skeletal muscle, liver, and fat; or both. The resultant hyperglycemia, if untreated, can lead to long-term complications, including microvascular complications (e.g., retinopathy, nephropathy, and peripheral and autonomic neuropathy) and macrovascular complications (e.g., coronary heart disease, cerebrovascular disease, and peripheral arterial disease).1 The prevalence of diagnosed diabetes in the U.S. is currently 7.7 percent and is expected to increase to nearly 10 percent by 2050, at which time an estimated 39 million people will have diabetes in the U.S.2–4 Thus, a large segment of the population requires glucose-lowering therapies to maintain normal glucose levels (normoglycemia) and prevent diabetes complications, and this number will likely increase.
Type 1 diabetes, which accounts for 5 to 10 percent of all diabetes cases, is characterized by insulin deficiency and a need for daily insulin administration to sustain life, maintain normoglycemia, and maintain normal body weight and promote normal growth and development in children.1 Type 2 diabetes, which accounts for 90 to 95 percent of diabetes in the U.S., is the result of a combination of insulin resistance and impaired insulin secretion by the beta cells of the endocrine pancreas.1 Although the relative contribution of each of these factors to the course of type 2 diabetes varies by patient, eventually, beta cell failure can lead to insulin deficiency, necessitating insulin therapy.
In current practice, tight glycemic control is achieved through the use of physiological basal and meal-time (prandial) insulin that, when used together, mimic normal pancreatic function (e.g. peakless basal insulin secretion, rapid release of insulin in response to meals, and rapid resolution of the prandial insulin peak). Patients take these medications either as three or more daily injections [multiple daily injections (MDI)], or by external continuous subcutaneous insulin infusion (CSII) via a pump, which provides a more physiological means to deliver insulin.
Following publication of the Diabetes Control and Complications Trial, self-monitoring of blood glucose (SMBG) by finger stick replaced the assessment of glucose by urine dipstick to allow more specific and timely feedback on the degree of hyperglycemia.5 The challenges to use of SMBG are the associated pain, costs, behavioral and technical skills, required motivation, and intrusiveness. These challenges directly affect adherence to this technique and are barriers to tight glycemic control. In response to these issues, the health care industry has developed continuous glucose monitoring (CGM) systems that record blood glucose levels throughout the day and night with a significantly decreased need for fingerstick measurements.
A CGM system, in conjunction with intensive insulin treatment, can be a useful tool to lower blood glucose values in adults who are at least 25 years of age and have type 1 diabetes. Success in lowering blood glucose levels depends on adherence to ongoing use of the device.6
The United States Food and Drug Administration first approved real-time continuous glucose monitoring (rt-CGM) in 2005. rt-CGM differs from conventional (retrospective) CGM in that it provides blood glucose feedback data to the patient while he or she is wearing the device and does not need to be downloaded and evaluated after data collection. This advantage of rt-CGM has resulted in it being the preferred method of CGM in the clinical setting.
CSII is currently recommended for patients with type 1 diabetes who are not achieving glycemic goals despite adherence to a maximum MDI regimen. This is especially true when patients also have wide and erratic glycemic excursions, frequent severe hypoglycemia and/or hypoglycemia unawareness, marked dawn phenomenon (pre-breakfast rise in blood glucose seen when bedtime basal insulin effect diminishes),5,7 or are pregnant or planning to become pregnant. Experts may also recommend CSII for patients with type 1 diabetes who feel that pump therapy may be more suitable to their lifestyle, regardless of the level of glycemic control.5 Experts currently recommend rt-CGM for patients with type 1 diabetes who have hypoglycemia unawareness or frequent hypoglycemia (where hemoglobin A1c [HbA1c] is over the recommended target), have excess glycemic excursions, or are pregnant or plan to be pregnant.8
Given new technologies in insulin delivery and glucose monitoring, clinicians are now faced with determining which patient populations benefit most from the use of CSII and rt-CGM in terms of improved glycemic, clinical, and patient-reported outcomes. Because both technologies are expensive and require extensive training and oversight by health care professionals, it is critical to determine how to select patients for their use. It is also important to point out that the most adherent and engaged patients will likely achieve beneficial outcomes as both forms of intensive insulin therapy (MDI and CSII) and both forms of glucose monitoring (rt-CGM and SMBG) require the patient to partner with his/her health care provider.
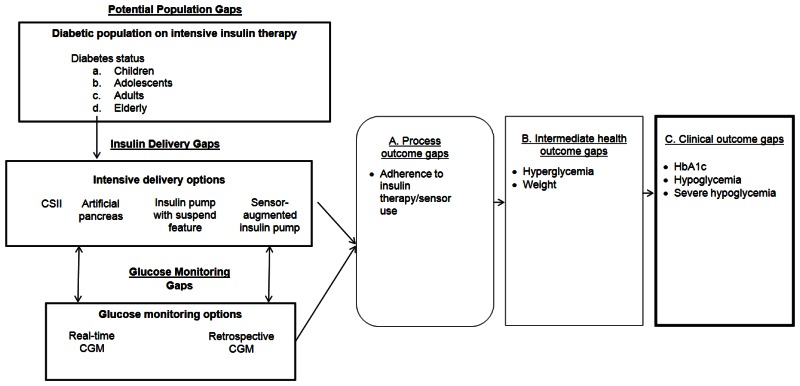
Our recent systematic review examined specific questions about the comparative effectiveness of insulin delivery and glucose monitoring methods (see Table 1).9 The review found that intensive insulin therapy delivered either by CSII and MDI is equally effective in lowering glycated hemoglobin (HbA1c) levels in adolescents and adults with type 1 diabetes. Intensive insulin therapy delivered by both methods resulted in similar rates of severe hypoglycemia for adolescents and adults with type 1 diabetes. The review also found evidence that rt-CGM is superior to SMBG in lowering HbA1c, without altering the risk balance of severe hypoglycemia, particularly among those who are compliant with wearing the monitoring device. Even though CSII and MDI without rt-CGM have similar effects on HbA1c, the addition of rt-CGM to CSII is superior to MDI/SMBG in lowering HbA1c. Thus, the addition of this monitoring method to SMBG and intensive insulin therapy can assist in achieving glycemic targets in individuals with type 1 diabetes. However, the review also identified several important gaps in the evidence, as shown in Figure 1.9 The objective of this report is to prioritize the needs for research addressing those gaps in the existing literature on management of insulin-requiring diabetes by engaging expert stakeholders using a modified Delphi method.
Identification of Evidence Gaps
Populations
There is a need for well-conducted randomized controlled trials (RCTs) of intensive insulin therapy delivered via CSII versus MDI in children with type 1 diabetes and in elderly patients with both type 1 and type 2 diabetes. Studies in the elderly are important as diabetes prevalence increases with age.2 Only a small number of studies in non-adolescent children have compared CSII with MDI on glycemic and non-glycemic outcomes and studies comparing rt-CGM with SMBG have included a mixture of children and adults without stratifications focused exclusively on children.
Future studies should focus on individuals with type 2 diabetes requiring insulin to determine the most effective manner in which to delivery intensive insulin therapy and monitor blood glucose. Given the rise in prevalence of type 2 diabetes in the general population, the number of those individuals requiring insulin therapy will likely rise. Finally, studies of type 2 diabetes should include ethnically diverse populations because type 2 diabetes is more common in blacks than in whites.10
Interventions
Current studies examining the comparative effectiveness of rt-CGM versus SMBG on outcomes have included mixed populations receiving intensive insulin therapy as CSII and/or MDI; however, they have not determined the effect of these two glucose monitoring strategies in individuals treated with only CSII or only MDI. Such a study would help to elucidate whether the observed benefit of sensor-augmented pump compared with MDI/SMBG on glycemic control is secondary to the rt-CGM technology, the mode of intensive insulin delivery, or both.
Study Design
Our report highlights the need for several areas of future research examining the effect of insulin delivery and glucose monitoring devices in the management of diabetes mellitus. To allow cross-comparisons, future RCTs should use a uniform definition of hypoglycemia, preferably that recommended by the American Diabetes Association.11 There is also a need for well-designed prospective, observational studies to determine the comparative effectiveness of CSII versus MDI and rtCGM versus SMBG on clinically relevant long-term micro- and macrovascular outcomes. Such studies would also provide guidance on effect sizes for future power calculations to determine whether it is feasible to undertake RCTs examining these outcomes. Future studies should also seek to identify and use an agreed-upon set of general and diabetes-specific and treatment-related quality of life measures to allow comparisons across studies, including reporting of standard errors and confidence intervals to allow quantitative, pooled assessments. Studies should incorporate measures of adherence to treatment as adherence is important for the effectiveness of any intensive insulin therapy or glucose monitoring system. Our data and other data show that rt-CGM is most effective in those compliant with wearing the sensor at least 60 percent of the time.12,13 Thus, sensor compliance may be a marker for overall treatment adherence and explain the HbA1c reduction, independent of the sensor.
Figures
Tables
Table 1Key Questions from Comparative Effectiveness Review
| KQ 1 | In patients receiving intensive insulin therapy, does mode of delivery (MDI vs. CSII) have a differential effect on process measures, intermediate outcomes, and clinical outcomes in patients with diabetes mellitus? |
|---|---|
| KQ 2 | In patients using intensive insulin therapy (MDI or CSII), does the type of glucose monitoring (rt-CGM vs. SMBG) have a differential effect on process measures, intermediate outcomes, and clinical outcomes in patients with diabetes mellitus (i.e., what is the incremental benefit of rt-CGM in patients already using intensive insulin therapy)? |
Abbreviations: KQ = Key Question; CSII = continuous subcutaneous insulin infusion; MDI = multiple daily injections; rt-CGM = Real-time continuous glucose monitoring; SMBG = Self-monitoring of blood glucose