NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Yeh HC, Lau BD, Golden SH, et al. Insulin Delivery and Glucose Monitoring Methods: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 57 [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. (Future Research Needs Papers, No. 32.)
Background
Diabetes mellitus is defined as a group of metabolic diseases characterized by hyperglycemia resulting from: defects in insulin secretion from the pancreatic beta cells; resistance to insulin action at the level of skeletal muscle, liver, and fat; or both. The resultant hyperglycemia, if untreated, can lead to long-term complications, including microvascular complications (e.g., retinopathy, nephropathy, and peripheral and autonomic neuropathy) and macrovascular complications (e.g., coronary heart disease, cerebrovascular disease, and peripheral arterial disease).1 The prevalence of diagnosed diabetes in the United States (U.S.) is currently 7.7 percent and is expected to increase to nearly 10 percent by 2050, at which time an estimated 39 million people will have diabetes in the U.S.2–4 Thus, a large segment of the population requires glucose-lowering therapies to maintain normal glucose levels (normoglycemia) and prevent diabetes complications, and this number will likely increase.
Type 1 diabetes, which accounts for 5 to 10 percent of all diabetes cases, is characterized by insulin deficiency and a need for daily insulin administration to sustain life, maintain normoglycemia, and maintain normal body weight and promote normal growth and development in children.1 Type 2 diabetes, which accounts for 90 to 95 percent of diabetes in the U.S., is the result of a combination of insulin resistance and impaired insulin secretion by the beta cells of the endocrine pancreas.1 Although the relative contribution of each of these factors to the course of type 2 diabetes varies by patient, eventually beta cell failure can lead to insulin deficiency, necessitating insulin therapy.
In current practice, tight glycemic control is achieved through the use of physiological basal and meal-time (prandial) insulin that, when used together, mimic normal pancreatic function (e.g., peakless basal insulin secretion, rapid release of insulin in response to meals, and rapid resolution of the prandial insulin peak). Patients take these medications either as three or more daily injections [multiple daily injections (MDI)], or by external continuous subcutaneous insulin infusion (CSII) via a pump, which provides a more physiological means to deliver insulin.
Following publication of the Diabetes Control and Complications Trial, self-monitoring of blood glucose (SMBG) by fingerstick replaced the assessment of glucose by urine dipstick to allow more specific and timely feedback on the degree of hyperglycemia.5 The challenges to use of SMBG are the associated pain, costs, behavioral and technical skills, required motivation, and intrusiveness. These challenges directly affect adherence to this technique and are barriers to tight glycemic control. In response to these issues, the health care industry has developed continuous glucose monitoring (CGM) systems that record blood glucose levels throughout the day and night with a significantly decreased need for fingerstick measurements.
A CGM system, in conjunction with intensive insulin treatment, can be a useful tool to lower blood glucose values in adults who are at least 25 years of age and have type 1 diabetes. Success in lowering blood glucose levels depends on adherence to ongoing use of the device.6
The United States Food and Drug Administration first approved real-time continuous glucose monitoring (rt-CGM) in 2005. rt-CGM differs from conventional (retrospective) CGM in that it provides blood glucose feedback data to the patient while he or she is wearing the device and does not need to be downloaded and evaluated after data collection. This advantage of rt-CGM has resulted in it being the preferred method of CGM in the clinical setting.
CSII is currently recommended for patients with type 1 diabetes who are not achieving glycemic goals despite adherence to a maximum MDI regimen. This is especially true when patients also have wide and erratic glycemic excursions, frequent severe hypoglycemia and/or hypoglycemia unawareness, marked dawn phenomenon (pre-breakfast rise in blood glucose seen when bedtime basal insulin effect diminishes),5,7 or are pregnant or planning to become pregnant. Experts may also recommend CSII for patients with type 1 diabetes who feel that pump therapy may be more suitable to their lifestyle, regardless of the level of glycemic control.5 Experts currently recommend rt-CGM for patients with type 1 diabetes who have hypoglycemia unawareness or frequent hypoglycemia (where hemoglobin A1c [HbA1c ] is over the recommended target), have excess glycemic excursions, or are pregnant or plan to be pregnant.8
Given new technologies in insulin delivery and glucose monitoring, clinicians are now faced with determining which patient populations benefit most from the use of CSII and rt-CGM in terms of improved glycemic, clinical, and patient-reported outcomes. Because both technologies are expensive and require extensive training and oversight by health care professionals, it is critical to determine how to select patients for their use. It is also important to point out that the most adherent and engaged patients will likely achieve beneficial outcomes as both forms of intensive insulin therapy (MDI and CSII) and both forms of glucose monitoring (rt-CGM and SMBG) require the patient to partner with his/her health care provider.
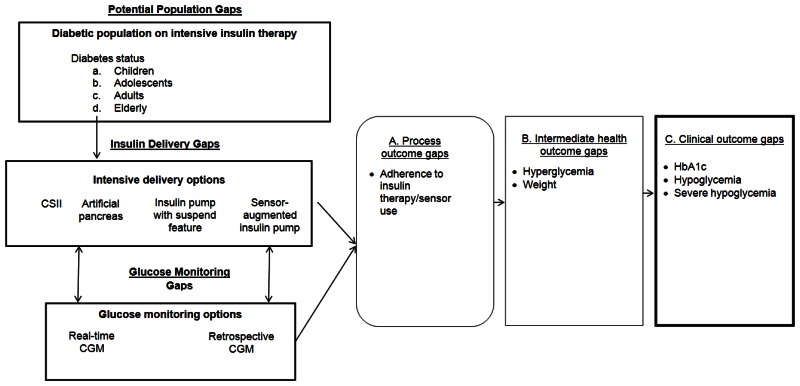
Our recent systematic review examined specific questions about the comparative effectiveness of insulin delivery and glucose monitoring methods (see Table A).9 The review found that intensive insulin therapy delivered either by CSII and MDI is equally effective in lowering glycated hemoglobin (HbA1c) levels in adolescents and adults with type 1 diabetes. Intensive insulin therapy delivered by both methods resulted in similar rates of severe hypoglycemia for adolescents and adults with type 1 diabetes. The review also found evidence that rt-CGM is superior to SMBG in lowering HbA1c, without altering the risk balance of severe hypoglycemia, particularly among those who are compliant with wearing the monitoring device. Even though CSII and MDI without rt-CGM have similar effects on HbA1c, the addition of rt-CGM to CSII is superior to MDI/SMBG in lowering HbA1c. Thus, the addition of this monitoring method to SMBG and intensive insulin therapy can assist in achieving glycemic targets in individuals with type 1 diabetes. However, the review also identified several important gaps in the evidence, as shown in Figure A.9 The objective of this report is to prioritize the needs for research addressing those gaps in the existing literature on management of insulin-requiring diabetes by engaging expert stakeholders using a modified Delphi method.
Methods
Stakeholder Identification
Expert stakeholders for this project were representative of clinicians, researchers, private and federal agencies, and patients. Fourteen experts in the field of insulin delivery and glucose management were identified and invited to serve as expert stakeholders. The stakeholders included one academic physician in the field of pediatric endocrinology, three physicians in the field of adult endocrinology and one patient with diabetes mellitus.
Stakeholder Engagement
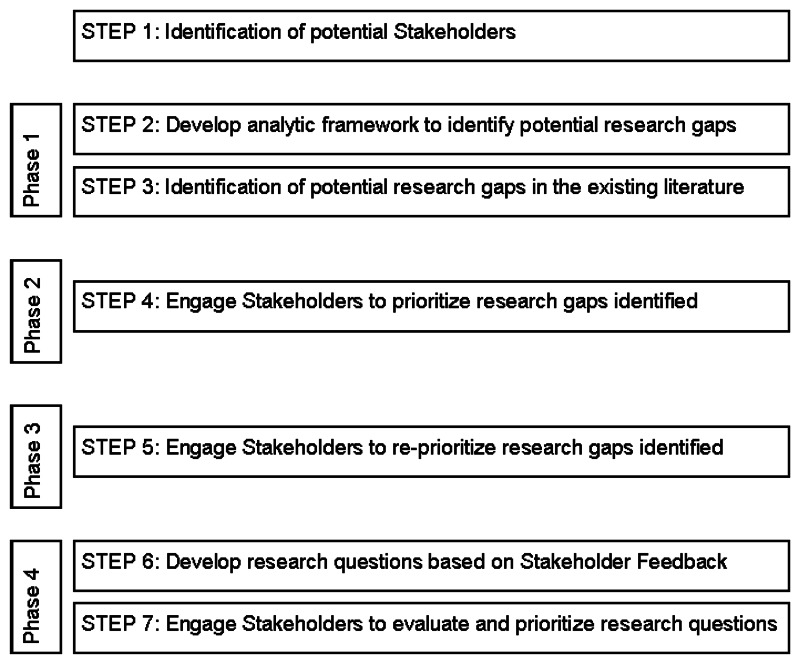
We used a modified Delphi method to identify and prioritize existing gaps in the scope of the systematic review9 as it pertains to insulin delivery and glucose monitoring methods in seven steps across four phases (Figure B). The Delphi method involves iterative rounds of responses by group members, providing aggregated feedback about other members’ responses until consensus is reached. For each round, we used a Web-based assessment tool (SurveyMonkey™, Palo Alto, CA), with the list of the research gaps. Consensus among stakeholders was pre-defined as agreement in responses of 50 percent or higher in three or more options for each category of Future Research Needs. Categories that did not achieve 50% or greater consensus among the stakeholders on three or more options in phase 2 were returned for the stakeholders, with their compiled feedback from phase 2, to reprioritize.
Phase 1
We developed an analytic framework (Figure A) to identify potential populations, delivery and monitoring methods, and outcomes gaps from the 2012 evidence report. As indicated in the analytic framework, we focused on the population of patients having type 1 or type 2 diabetes, with subpopulations defined by age categories. We did not include pregnant women as a separate category in this report because we thought it would require a separate process to adequately assess the needs for future research in pregnant women having type 1 diabetes, type 2 diabetes, or gestational diabetes. In the analytic framework for this study, we included the types of interventions that are currently being evaluated for delivering insulin continuously or for monitoring glucose continuously, even though we did not have any studies about the artificial pancreas or the reactive low glucose suspend pump in the original evidence report.
We then searched the results and discussion sections of the evidence report, using the analytic framework, to identify potential research gaps. A Web-based assessment tool was populated with the identified research gaps. For each research gap category, an optional, free-text field was provided for stakeholders to identify gaps not listed in the assessment tool. Novel stakeholder and/or investigator identified research gaps, including insulin delivery methods, glucose monitoring methods, and outcomes were included for prioritization during phases 2 and 3 and were eligible for inclusion in the final research question development in phase 4.
Phase 2
The Stakeholders were provided with a copy of the Executive Summary of the 2012 evidence report,9 were asked to read the full Executive Summary for familiarization of the findings, and were asked to independently identify the highest priority gaps for future research among individuals with type 1 diabetes and with type 2 diabetes within each of the following categories: populations, insulin delivery methods, glucose monitoring methods and outcomes. First, we asked the stakeholders to rate the three highest priority age-based populations with type 1 diabetes and with type 2 diabetes. Second, we asked stakeholders to rate the highest priority insulin delivery method and the highest priority blood glucose monitoring method for each age stratum of each diabetes type. Finally, we asked the stakeholders to rate the three highest priority outcomes for each age stratum for each diabetes type. Stakeholders were given the opportunity to add insulin delivery methods, glucose monitoring methods, and outcomes not on the list as an ‘other’ free-text option.
Phase 3
Feedback from phase 2 was compiled and analyzed for agreement. Compiled stakeholder feedback for prioritized research gaps that did not achieve consensus were sent back to the stakeholders to re-rate the priorities in an attempt to build consensus.
Phase 4
Research questions were developed based on feedback from stakeholders that achieved consensus during the second and third rounds. The stakeholders were presented with their compiled feedback from the second and third phases along with the research questions developed. They were asked to provide feedback on the clarity, utility, study design feasibility and priority of the research questions.
Results
We identified evidence gaps among populations, insulin delivery and glucose monitoring methods, and outcomes. The gaps varied by diabetes type. Results from the stakeholder prioritization are presented by diabetes type.
Type 1 Diabetes
Phase 1
During phase 1 of this study, the stakeholders were presented with gaps identified by the research team (see Tables B, C, D, and E). The stakeholders indicated that additional research was needed for children (age less than 13 years), adolescents (age 13 to 19 years), and adults (age 20–64 years) with type 1 diabetes (see Table B). Potential research needs for insulin delivery methods were related to CSII (i.e. insulin pump), reactive low glucose suspend pumps (i.e. pump that automatically suspends insulin delivery when glucose reaches low threshold), artificial pancreas (i.e. overnight closed loop, senses upper and lower glucose thresholds), and sensor-augmented insulin pumps (see Table C). Potential topics for future research on blood glucose monitoring methods were SMBG, retrospective continuous glucose monitoring, and rt-CGM (see Table D). Outcomes identified for future research were HbA1c, adherence, non-severe hypoglycemia, severe hypoglycemia, and hyperglycemia (see Table E).
Phase 2
During phase 2, three expert stakeholders ranked adolescents as the highest priority group for future research in patients with type 1 diabetes. (see Table B) One stakeholder ranked children and one ranked adults as the highest priority group for future research in patients with type 1 diabetes. Among children with type 1 diabetes, three stakeholders ranked the artificial pancreas as the highest research need for insulin delivery while one stakeholder identified the reactive glucose suspend pump and one rated the sensor-augmented insulin pump as the highest priorities for this population. Among adolescents with type 1 diabetes, three stakeholders also ranked the artificial pancreas as the highest priority for future research while two prioritized the sensor-augmented insulin pump. For adults with type 1 diabetes, two stakeholders identified the artificial pancreas and two identified the sensor-augmented insulin pump as the highest priority for future research. One stakeholder identified the reactive low glucose suspend pump as the highest priority for future research. Four stakeholders ranked the reactive low glucose suspend pump as the highest priority for future research among the elderly with type 1 diabetes and one stakeholder ranked the artificial pancreas as the highest priority in the elderly (see Table C).
For glucose monitoring methods in patients with type 1 diabetes, all Stakeholders agreed that the highest priority was research on rt-CGM (see Table D).
Three Stakeholders ranked adherence as the highest priority outcome for future research in children with type 1 diabetes. For adolescents, two stakeholders rated adherence as highest priority outcome while the other two ranked severe hypoglycemia as highest priority outcome. Among adults, three Stakeholders ranked severe hypoglycemia as the highest priority outcome; and in the elderly, all Stakeholders rated severe hypoglycemia as a high priority outcome in future research (see Table E).
Phase 3
Consensus was achieved during phase 2, negating the need to build consensus in phase 3.
Phase 4
When presented with the research questions developed from feedback in earlier rounds, Stakeholders prioritized glucose monitoring as a higher research need above insulin delivery methods.
Type 2 Diabetes
Phase 1
When the stakeholders focused on insulin-requiring type 2 diabetes in phase 1 of this study, they indicated that additional research was needed in adolescents (age 13 to 19 years), adults (age 20–64 years) and the elderly (age greater than 64) (Table F). For patients with type 2 diabetes, potential research needs for insulin delivery methods identified and/or rated by the stakeholders were related to CSII (i.e., insulin pump), reactive low glucose suspend pumps (i.e., pump that automatically suspends insulin delivery when glucose reaches low threshold), artificial pancreas (i.e., overnight closed loop, senses upper and lower glucose thresholds), and sensor-augmented insulin pumps (see Table G). In this population, potential priorities for research on blood glucose monitoring methods identified and/or rated by the stakeholders were SMBG, retrospective continuous glucose monitoring, and rt-CGM (see Table H). Outcomes rated as priorities for future research in this population were HbA1c, adherence, nonsevere hypoglycemia, severe hypoglycemia, hyperglycemia, and weight (see Table I).
Phase 2
During phase 2, three stakeholders ranked adults as the highest priority group for future research among insulin-requiring type 2 diabetes patients. One stakeholder ranked adolescents and one ranked elderly as the highest priority group for future research in patients with type 2 diabetes (see Table F). Among the patients with insulin-requiring type 2 diabetes, three stakeholders ranked the sensor-augmented insulin pump as the highest research need related to insulin delivery while one stakeholder ranked CSII and one identified the artificial pancreas as the highest priorities for future research on insulin delivery in patients with type 2 diabetes (see Table G).
For future research on glucose monitoring methods in patients with insulin-requiring type 2 diabetes, three stakeholders prioritized rt-CGM and two prioritized SMBG. (see Table H)
Two stakeholders prioritized HbA1c, one prioritized severe hypoglycemia and one ranked weight as the highest priority outcome for future research in adolescents with insulin-requiring type 2 diabetes. Three stakeholders prioritized HbA1c, one prioritized adherence and one ranked hyperglycemia as the highest priority outcome for future research among adults with insulin-requiring type 2 diabetes. Three stakeholders prioritized HbA1c, one prioritized severe hypoglycemia and one ranked hyperglycemia as the highest priority outcome for future research among the elderly with insulin-requiring type 2 diabetes (see Table I).
Phase 3
Consensus was also achieved during phase 2 for the research priorities on insulin-requiring type 2 diabetes.
Phase 4
When presented with the research questions developed from feedback in earlier rounds, Stakeholders prioritized glucose monitoring as a higher research need above insulin delivery methods.
Research Questions
Based on stakeholder feedback from phase 2 regarding populations, interventions, comparisons, and outcomes, the following four research questions were identified by our stakeholders as high priorities for future research:
- For adolescents with type 1 diabetes, what is the comparative effectiveness of an artificial pancreas versus other methods of insulin delivery for the outcomes of adherence and severe hypoglycemia?
- For adolescents with type 1 diabetes, what is the comparative effectiveness of rt-CGM versus other methods of glucose monitoring for the outcomes of adherence and severe hypoglycemia?
- For adults with insulin-requiring type 2 diabetes, what is the comparative effectiveness of a sensor-augmented insulin pump versus other methods of insulin delivery for the outcome HbA1c?
- For adults with insulin-requiring type 2 diabetes, what is the comparative effectiveness of rt-CGM versus other methods of glucose monitoring for the outcome HbA1c?
This report reinforces the needs for future research that we outlined in the original evidence report,9 and points to specific types of studies that should have a high priority. In the original report, we identified a need for well-conducted randomized controlled trials (RCTs) of intensive insulin therapy delivered via CSII versus MDI in young children with type 1 diabetes and elderly patients with both type 1 and type 2 diabetes. Based on the input from our stakeholders, higher priority should be given to RCTs of intensive insulin therapy options, including the artificial pancreas, in adolescents with type 1 diabetes. Such studies should be accompanied by efforts to assess the comparative effectiveness of rt-CGM versus other methods of glucose monitoring. At a minimum, the protocols of RCTs of intensive insulin therapy options in adolescents will need to specify the type of glucose monitoring method to be used, so that the effects of differences in insulin therapy can be distinguished from differences in the glucose monitoring methods.
In our original evidence report, we highlighted the need for studies in the elderly. However, for this report on Future Research Needs, the stakeholders made it clear that the entire adult population with type 2 diabetes remains a high priority for future research. For this important population, RCTs are the strongest and most appropriate study design for determining the comparative effectiveness of the sensor augmented pump compared with other insulin delivery methods. As indicated above, such trials should be accompanied by efforts to assess the comparative effectiveness of rt-CGM versus other methods of glucose monitoring, with protocols that clearly specify the type of glucose monitoring method to be used.
Since the cost of long-term RCTs may be prohibitive for addressing all of the outcomes of interest for all of the comparisons of interest, prospective observational studies will continue to have a role. Observational studies will be particularly important in determining the comparative effectiveness of CSII versus MDI and rt-CGM versus SMBG in terms of clinically relevant long-term microvascular and macrovascular outcomes.
Discussion
The majority of stakeholders prioritized adolescents with type 1 diabetes and adults with insulin-requiring type 2 diabetes as the populations in greatest need for future research. For each population, rt-CGM was identified as the highest priority for future research on glucose monitoring methods, while the research priorities on insulin delivery methods and outcomes of interest varied by population. When asked to prioritize the final research question within each category, glucose monitoring methods were universally prioritized above insulin delivery methods.
While the stakeholders rated adolescents with type 1 diabetes as the highest priority, the original investigators commented that future studies should focus on populations in which diabetes is growing (i.e., elderly individuals with type 1 and type 2 diabetes, insulin-treated type 2 diabetes, minority populations). This difference may be because the stakeholders took a clinical perspective that focused on treatment while the investigators took a research perspective that focused on the gaps in data. On the other hand, adherence as an outcome was rated high by both stakeholders and the original investigators. It is important to note that stakeholders identified and rated the artificial pancreas as the highest priority for future research, despite the fact that the technology of artificial pancreas is at the developmental stage, not widely used in practice, and was not included in the original Comparative Effectiveness Review for lack of eligible studies. Nonetheless, this consensus reflected the urge to develop a better and more convenient system for diabetes treatment.
Long-term clinical outcomes were not specifically included for prioritization by the stakeholders. While prevention of long-term macrovascular and microvascular complications is the ultimate goal of interventions for type 1 and type 2 diabetes, such trials would need an extremely long time for followup. Comparative effectiveness studies would require very large numbers of patients to be followed for many years to show significant macrovascular and microvascular effects, especially if only small HbA1c differences are seen, something much too costly to do. Supported by strong ratings from the stakeholders, we feel HbA1c is a reasonable surrogate endpoint, and should be used when looking at the comparative effectiveness of rt-CGM and the sensor-augmented insulin pump, versus other interventions.
Our study had several strengths. First, we used an established approach for consensus building. Second, we invited experts from multiple disciplines as stakeholders including practicing endocrinologists, clinical researchers, and a patient, which increased the generalizability. Third, the stakeholders reached consensus with only one round of survey, which reflected high level of consistency.
Nonetheless, this study has some limitations. First, our study was limited to the scope of the original evidence report. The original investigators determined the research gaps based on their own findings. Stakeholders did not independently identify research gaps on the basis of populations, interventions, and outcomes, but rather by the limited options that we provided according to our analytic framework. This limitation is offset by the benefit of keeping the study focused on the populations and interventions that were included in our analytic framework. This study did not specifically address the needs for future research in pregnant women because we thought it would require a separate study to adequately determine the research needs for pregnant women having type 1 diabetes, type 2 diabetes, or gestational diabetes. Another limitation is that the complexity of the concepts in this topic may be a barrier for patient stakeholders to contribute. The decision making process associated with prioritizing clinical interventions could potentially be a daunting task for non-clinicians and non-researchers in the field. Clinicians have a level of standardized education and training in the field. The average patient may or may not have the requisite breadth of knowledge and experience to prioritize interventions for the entire field of insulin delivery and glucose monitoring research. Still, in our study, this was minimized by a patient Stakeholder with a longstanding history of diabetes and who has taken an active role in his care throughout his life. Finally, due to the abundance of outcomes gaps in the literature, it was prohibitive to present all potential outcomes to the stakeholders for prioritization. This limitation was minimized by allowing the stakeholders the option to identify research gaps using a free-text field after reading the Executive Summary of the full Comparative Effectiveness Review.
We feel that the research questions developed by comprehensive feedback from our panel of expert stakeholders represent the highest research priorities in the field of insulin delivery and glucose monitoring methods. We recognize that there are many additional gaps, including insulin delivery and glucose monitoring research for elderly patients with type 1 diabetes; however, the inductive approach to building consensus for research question development resulted in four high priority research needs.
Conclusion
For type 1 diabetes, three expert stakeholders ranked adolescents as the highest priority age group for future research, and three stakeholders ranked the artificial pancreas as the highest priority for future research. For future research on glucose monitoring methods in patients with type 1 diabetes, all Stakeholders identified rt-CGM as the highest priority. For younger populations (children and adolescents) of patients with type 1 diabetes, adherence was ranked as the highest priority outcome for inclusion in future research. For adults and elderly patients with type 1 diabetes, most stakeholders ranked severe hypoglycemia as the highest priority outcome for inclusion in future research. Among insulin-requiring type 2 patients with diabetes, three stakeholders ranked adults as the highest priority age group for future research. For all patients with insulin-requiring type 2 diabetes, three Stakeholders ranked the sensor-augmented insulin pump as the highest priority for research on insulin delivery methods. Likewise, for future research on glucose monitoring methods in patients with insulin-requiring type 2 diabetes, three stakeholders identified rt-CGM as the highest priority. Most stakeholders ranked HbA1c as the highest priority outcome to include in future research on insulin-requiring type 2 diabetes.
References
- 1.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33 Suppl 1:S62–9. [PMC free article: PMC2797383] [PubMed: 20042775]
- 2.
- Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009 Feb;32(2):287–94. [PMC free article: PMC2628695] [PubMed: 19017771]
- 3.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004 Jun 1;140(11):945–50. [PubMed: 15172919]
- 4.
- Centers for Disease Control and Prevention (CDC). Crude and age-adjusted incidence of diagnosed diabetes per 1000 population, united states, 1980–2007. Atlanta, GA: Centers for Disease Control and Prevention (CDC);
- 5.
- Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–53. [PMC free article: PMC2637991] [PubMed: 16371630]
- 6.
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–89. [PubMed: 18784090]
- 7.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. the diabetes control and complications trial research group. N Engl J Med. 1993 Sep 30;329(14):977–86. [PubMed: 8366922]
- 8.
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care. 2008 May;31(5):1060–79. [PMC free article: PMC2930883] [PubMed: 18445730]
- 9.
- Golden SH, Brown T, Yeh HC, et al. Methods for Insulin Delivery and Glucose Monitoring: Comparative Effectiveness. Comparative Effectiveness Review No. 57. Rockville, MD: Agency for Healthcare Research and Quality; Jul, 2012. (Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2007-10061-I.) AHRQ Publication No. 12-EHC036-EF. www
.effectivehealthcare .ahrq.gov/reports/final.cfm.
Figures
Tables
Table AKey Questions from Comparative Effectiveness Review
| KQ 1 | In patients receiving intensive insulin therapy, does mode of delivery (MDI vs. CSII) have a differential effect on process measures, intermediate outcomes, and clinical outcomes in patients with diabetes mellitus? |
|---|---|
| KQ 2 | In patients using intensive insulin therapy (MDI or CSII), does the type of glucose monitoring (rt-CGM vs. SMBG) have a differential effect on process measures, intermediate outcomes, and clinical outcomes in patients with diabetes mellitus (i.e., what is the incremental benefit of rt-CGM in patients already using intensive insulin therapy)? |
Abbreviations: KQ = Key Question; CSII = continuous subcutaneous insulin infusion; MDI = multiple daily injections; rt-CGM = Real-time continuous glucose monitoring; SMBG = Self-monitoring of blood glucose
Table BStakeholder identification and prioritization of populations of greatest importance for future research for insulin delivery and blood glucose monitoring methods among patients with type 1 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Populations | |||
| Children (<13 years) | 1 | * | |
| Adolescent (13–19 years) | 3 | * | ‡ |
| Adult (20–64 years) | 1 | * | |
| Elderly (>=65 years) | 0 | * | |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table CStakeholder identification and prioritization of insulin delivery methods of greatest importance for future research among patients with type 1 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Children | |||
| Continuous subcutaneous insulin infusion (insulin pump) | 0 | * | |
| Reactive low glucose suspend pump (automatically suspends insulin delivery when glucose reaches low threshold) | 1 | * | |
| Artificial pancreas (overnight closed loop, sense upper and lower thresholds) | 3 | * | |
| Sensor-augmented insulin pump | 1 | * | |
| Adolescents | |||
| Continuous subcutaneous insulin infusion (insulin pump) | 0 | * | |
| Reactive low glucose suspend pump (automatically suspends insulin delivery when glucose reaches low threshold) | 0 | * | |
| Artificial pancreas (overnight closed loop, sense upper and lower thresholds) | 3 | * | ‡ |
| Sensor-augmented insulin pump | 2 | * | |
| Adults | |||
| Continuous subcutaneous insulin infusion (insulin pump) | 0 | * | |
| Reactive low glucose suspend pump (automatically suspends insulin delivery when glucose reaches low threshold) | 1 | * | |
| Artificial pancreas (overnight closed loop, sense upper and lower thresholds) | 2 | * | |
| Sensor-augmented insulin pump | 2 | * | |
| Elderly | |||
| Continuous subcutaneous insulin infusion (insulin pump) | 0 | * | |
| Reactive low glucose suspend pump (automatically suspends insulin delivery when glucose reaches low threshold) | 4 | * | |
| Artificial pancreas (overnight closed loop, sense upper and lower thresholds) | 1 | * | |
| Sensor-augmented insulin pump | 0 | * | |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table DStakeholder identification and prioritization of blood glucose monitoring methods of greatest importance for future research among patients with type 1 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Children | |||
| Self-monitored blood glucose | 0 | * | |
| Retrospective continuous glucose monitoring | 0 | * | |
| Real-time continuous glucose monitoring | 5 | * | |
| Adolescents | |||
| Self-monitored blood glucose | 0 | * | |
| Retrospective continuous glucose monitoring | 0 | * | |
| Real-time continuous glucose monitoring | 5 | * | ‡ |
| Adults | |||
| Self-monitored blood glucose | 0 | * | |
| Retrospective continuous glucose monitoring | 0 | * | |
| Real-time continuous glucose monitoring | 5 | * | |
| Elderly | |||
| Self-monitored blood glucose | 0 | * | |
| Retrospective continuous glucose monitoring | 0 | * | |
| Real-time continuous glucose monitoring | 5 | * | |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table EStakeholder identification and prioritization of clinical outcomes of greatest importance for future research of insulin delivery and glucose monitoring methods among patients with type 1 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Children | |||
| HbA1c | 0 | * | |
| Adherence | 3 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 1 | * | |
| Hyperglycemia | 1 | * | |
| Adolescents† | |||
| HbA1c | 0 | * | |
| Adherence | 2 | * | ‡ |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 2 | * | ‡ |
| Hyperglycemia | 0 | * | |
| Adults | |||
| HbA1c | 1 | * | |
| Adherence | 1 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 3 | * | |
| Hyperglycemia | 0 | * | |
| Elderly | |||
| HbA1c | 0 | * | |
| Adherence | 0 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 5 | * | |
| Hyperglycemia | 0 | * | |
Abbreviation: HbA1c = hemoglobin A1c
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
- †
One Stakeholder abstained.
Table FStakeholder identification and prioritization of populations of greatest importance for future research among insulin-requiring patients with type 2 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Adolescent (13–19 years) | 1 | * | |
| Adult (20–64 years) | 3 | * | ‡ |
| Elderly (>=65 years) | 1 | * |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table GStakeholder identification and prioritization of insulin delivery methods of greatest importance for future research among insulin-requiring patients with type 2 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Continuous subcutaneous insulin infusion (insulin pump) | 1 | * | |
| Reactive low glucose suspend pump (automatically suspends insulin delivery when glucose reaches low threshold) | 0 | * | |
| Artificial pancreas (overnight closed loop, sense upper and lower thresholds) | 1 | * | |
| Sensor-augmented insulin pump | 3 | * | ‡ |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table HStakeholder identification and prioritization of blood glucose monitoring methods of greatest importance for future research among insulin-requiring patients with type 2 diabetes
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Self-monitored blood glucose | 2 | * | |
| Retrospective continuous glucose monitoring | 0 | * | |
| Real-time continuous glucose monitoring | 3 | * | ‡ |
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.
Table IStakeholder identification and prioritization of clinical outcomes of greatest importance for future research of insulin delivery and glucose monitoring methods among patients with type 2 diabetes by population
| Phase 1: Identification | Phase 2: Number of Stakeholders Rating Research Gap as Highest Priority (n=5) | Phase 3: Number of Stakeholders Re-rating Gap as Highest Priority (n=5) | Phase 4: High Priority Gap(s) Included as Part of the Final Research Question in Final Research Questions |
|---|---|---|---|
| Adolescents | |||
| HbA1c | 2 | * | |
| Adherence | 1 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 1 | * | |
| Hyperglycemia | 0 | * | |
| Weight | 1 | * | |
| Adults | |||
| HbA1c | 3 | * | ‡ |
| Adherence | 1 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 0 | * | |
| Hyperglycemia | 1 | * | |
| Weight | 0 | * | |
| Elderly | |||
| HbA1c | 3 | * | |
| Adherence | 0 | * | |
| Non-severe hypoglycemia | 0 | * | |
| Severe hypoglycemia | 1 | * | |
| Hyperglycemia | 1 | * | |
| Weight | 0 | * | |
Abbreviation: HbA1c = hemoglobin A1c
- *
Indicates consensus achieved in the previous round; reprioritization not required.
- ‡
Indicates an identified research need.