NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Monroe AK, Gudzune KA, Sharma R, et al. Combination Therapy Versus Intensification of Statin Monotherapy: An Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Feb. (Comparative Effectiveness Reviews, No. 132.)
This publication is provided for historical reference only and the information may be out of date.

Combination Therapy Versus Intensification of Statin Monotherapy: An Update [Internet].
Show detailsBackground
Cardiovascular disease (CVD) includes conditions such as coronary heart disease, stroke, heart failure, arrhythmia, heart valve disease, congenital heart disease, and hypertension. The American Heart Association has estimated that CVD affects 83.6 million individuals in the United States, contributes to 32.3 percent of deaths, and is a leading cause of disability.1 Atherosclerosis (hardening of arteries caused by plaque deposition) causes coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease. The American Heart Association estimates that atherosclerotic CVD affects 15.4 million Americans.1 CHD, which includes coronary artery disease, myocardial infarction (MI), unstable angina, and heart failure, is a leading cause of death for both men and women in the United States.2 It is estimated that by 2030, the prevalence of CHD will rise by 16.6 percent and result in more than $106 billion in direct health care costs.3
Abnormal lipoprotein metabolism, especially increased concentrations of apo B-100–containing low-density lipoprotein (LDL-c), predisposes individuals to atherosclerosis. Due to the consistent and robust association of higher LDL-c levels with atherosclerotic CVD across experimental and epidemiologic studies,4,5 therapeutic strategies to decrease risk have focused on LDL-c reduction as a primary goal. In contrast to LDL-c, high-density lipoprotein (HDL-c) has a protective role against atherosclerotic CVD. Epidemiologic studies have demonstrated an inverse association between HDL-c and CVD, where low HDL-c levels are independent predictors of CHD.6
Questions remain as to how best to modify lipid levels with the goal of preventing CHD. The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are the most widely prescribed lipid-lowering agents and are often used as monotherapy. However, some patients do not reach their treatment goals on statin monotherapy or are troubled by side effects, prompting interest in combination therapy as a way to improve lipid levels without having to increase statin dosage or as a way to reduce side effects. Statins can be combined with an additional lipid-modifying medication such as a bile acid sequestrant, cholesterol absorption inhibitor, fibric acid, nicotinic acid, or omega-3 fatty acid. There are potential benefits to treating with multiple agents, as the different mechanisms of action of the other lipid-modifying agents may produce benefits unlikely to be achieved with a statin alone. For example, a fibrate or niacin in combination with a statin may increase HDL-c and decrease triglycerides above what is achieved with statin treatment alone.7 Combination therapy could potentially result in fewer statin-related side effects (e.g., myalgias and elevated liver transaminases), as lower doses of statin could be used. Conversely, a combination of agents could result in an increase in side effects, as patients may experience the side effects common to both drugs.
In 2009, the Agency for Healthcare Research and Quality released an evidence report comparing combinations of these lipid-modifying agents to statin intensification.8,9 However, the authors found insufficient evidence to determine whether combination therapy held benefit over monotherapy. To provide additional information for clinicians treating patients with moderate or high CHD risk, this update reviews the most recent evidence.
Two contextual factors need to be kept in mind while considering the evidence comparing statin intensification to combination therapy. First, guideline recommendations about intensifying statin therapy or adding an additional nonstatin agent to achieve a specific lipid target level have recently changed.10 The National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III provided guidelines on both when to initiate lipid-lowering therapy based on LDL-c level and CHD risk factors and recommended LDL-c targets for optimal CHD risk reduction.11,12 However, the new guidelines for treatment of cholesterol to reduce atherosclerotic CVD, released in November 2013, represent a major change from the ATP III guidelines. No specific LDL-c targets (e.g., LDL-c ≤70 mg/dL) were presented in the new guidelines due to the lack of evidence from randomized controlled trials supporting specific targets. Rather, four “statin benefit groups” were identified: individuals with clinical atherosclerotic CVD, individuals with LDL-c ≥190 mg/dL, people with diabetes aged 40–75, and individuals aged 40–75 with a ≥7.5-percent 10-year atherosclerotic CVD risk. For individuals within these groups, there are recommendations for treatment with moderate- or high-potency statins. The expected response to a moderate-potency statin is an LDL-c reduction of 30 to 50 percent, while the expected response to a high-potency statin is an LDL-c reduction of ≥50 percent. For individuals who do not have the expected response, adherence is assessed. Then the guidelines recommend considering intensification of statin therapy if the patient is not at maximum dose or the addition of a nonstatin agent with proven efficacy in reducing CVD events.10
Second, several large trials, such as ENHANCE (The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression), AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes), and ACCORD (Action to Control Cardiovascular Risk in Diabetes)-Lipid, have compared statin monotherapy to combination therapy with the same statin dose plus another lipid-lowering drug. These trials have demonstrated that “add-on” combination therapy can lead to superior lipid outcomes but fails to reduce atherosclerosis or lead to decreased rates of cardiovascular death, MI, revascularization, or stroke.13 This evidence calls into question previous assumptions that lowering LDL-c or raising HDL-c are always reliable predictors of improved clinical outcomes, as well as increasing the importance of patient-centered clinical outcomes for evaluating the effectiveness of lipid-modifying therapies.7,14
Scope and Key Questions
We aimed to assess the effectiveness, safety, and tolerability of the combination of statin and other lipid-modifying medication compared to intensification of statin monotherapy. Our scope was limited to comparing the combination of statin with other lipid-modifying medication to intensification of statin monotherapy. We did not examine the separate but related question of whether adding another lipid-modifying agent to the same potency statin therapy will improve clinical outcomes (add-on combination therapy). Therefore, a number of high-profile studies that evaluated add-on combination therapy, including ACCORD, AIM-HIGH, HSP-2 THRIVE (Heart Protection Study 2 Treatment of HDL to Reduce the Incidence of Vascular Events), and ENHANCE, are not included in this review. We did not expand our update to evaluate add-on combination therapy for two reasons: (1) the upcoming release of the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International) trial results, which will be critical in characterizing the effect of add-on combination therapy with ezetimibe + statin on clinical outcomes, thereby making a review at this time premature; and (2) resource constraints. Furthermore, we did not include nonstatin monotherapy as a comparison group, given that statins are the first-line treatment for dyslipidemia and the focus of this update is on populations that can tolerate statins at some dose. We aimed to answer the questions below by reviewing trials of adults that compared a higher potency of statin monotherapy to a lower potency statin in combination with another agent (bile acid sequestrant, ezetimibe, fibrate, niacin, or omega-3 fatty acid).
The specific Key Questions (KQs) are:
| KQ 1: | For patients who require intensive lipid-modifying therapy, what are the comparative long-term benefits and rates of serious adverse events of coadministration of different lipid-modifying agents (i.e., a statin plus another lipid-modifying agent) compared with higher dose statin monotherapy? |
| KQ 2: | Do these regimens differ in reaching LDL targets (or other surrogate markers), short-term side effects, tolerability, and/or adherence? |
| KQ 3: | Compared with higher dose statins and with one another, do combination regimens differ in benefits and harms within subgroups of patients? |
The analytic framework for our review is shown in Figure A.
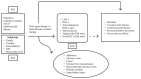
Figure A
Analytic framework for comparative effectiveness of lipid-modifying agents. CVD = cardiovascular disease; DM = diabetes mellitus; HDL-c = high-density lipoprotein; KQ = Key Question; LDL-c = low-density lipoprotein
Methods
Search Strategy, Study Selection, and Data Abstraction
We searched the following databases for primary studies: MEDLINE®, Embase®, and the Cochrane Central Register of Controlled Trials (CENTRAL) from May 2008 through July 2013. We also reviewed relevant review articles. In addition, we requested and reviewed Scientific Information Packets provided by the pharmaceutical manufacturers.
Abstract and full-text screening was performed by two independent reviewers using prespecified eligibility criteria (Table A). All articles included in the prior review were reviewed during the full-text screen. Conflicts were resolved by consensus adjudication.
Table A
Study inclusion and exclusion criteria.
Data abstraction was conducted with a senior reviewer (faculty-level project investigator) abstracting data from articles while having access to the first reviewer's data abstraction. Differences in opinion were resolved through consensus adjudication and, for difficult cases, during team meetings.
Risk-of-Bias Assessment
Risk of bias was assessed independently by two reviewers using the Cochrane Collaboration's tool. For studies included from the prior review, we used the quality assessments from that report, which used the Jadad Score.
Data Synthesis
We compared lower potency statins in combination therapy with higher potency statin monotherapy, which enabled us to synthesize data across statin type and statin dose. We used specific criteria to determine statin potency (Table B).
Table B
Different dosing of specific statins based on potency to reduce LDL-c.
We calculated and displayed the mean differences with 95-percent confidence intervals for the individual studies grouped by combination therapy agent, statin potency, and population for all comparisons. We considered meta-analysis where there were three or more similar studies. We report qualitative synthesis of data for most outcomes because of the lack of outcomes meeting our criteria for meta-analysis and significant heterogeneity detected when meta-analyses were conducted (I2 >50%).
Strength of the Body of Evidence
We graded the quantity, quality, and consistency of the evidence for the following outcomes: mortality, acute coronary events, revascularization procedures, serious adverse events, LDL-c, and HDL-c. We used an evidence grading scheme recommended by the “Methods Guide for Effectiveness and Comparative Effectiveness Reviews.”15 We created evidence grades for each comparison and outcome by combination agent, statin potency, and population. We used four domains to yield a final evidence grade: risk of bias, consistency, directness and precision.
The final strength-of-evidence (SOE) grades were: (1) “high” grade (indicating high confidence that the evidence reflects the true effect and further research is very unlikely to change our confidence in the estimate of the effect); (2) “moderate” grade (indicating moderate confidence that the evidence reflects the true effect and further research may change our confidence in the estimate of the effect and may change the estimate); (3) “low” grade (indicating low confidence that the evidence reflects the true effect and further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate); and (4) “insufficient” grade (no evidence identified). A comparison-outcome pair with high SOE was one with low risk of bias, directness, consistency, and precision. Moderate SOE indicated that a high risk of bias was noted or that two of the following were observed: a moderate risk of bias, inconsistency, indirectness, or imprecision. Low SOE indicated a high risk of bias and two or more of the following or a moderate risk of bias and three of the following: inconsistency, indirectness, and imprecision.
Investigators writing each section completed the SOE grading, which was then reviewed by the team.
Applicability
We describe the applicability of studies in terms of the degree to which the study population, interventions, outcomes, and settings were relevant to individuals at high CHD risk requiring aggressive lipid-modifying therapy and features that may affect the effectiveness of the intervention.
Results
Results of Literature Searches
Figure B summarizes the search results. The literature search identified 4,293 unique citations. During the title and abstract screening we excluded 3,396 citations; during the article screening we excluded 380 citations (see Appendix D in the full report). Fifty-five studies, reported in 59 articles, were included. All trials were randomized controlled trials.
Overview of Included Trials by Potency and Agent
The SOE was variable across comparisons evaluating the effectiveness and safety of combination therapy versus intensification of statin monotherapy. Evidence for all the clinical outcomes of mortality, acute coronary events, and revascularization procedures was graded as insufficient across all potency comparisons for all combination therapy regimens.
Seven comparisons had moderate SOE for LDL-c and HDL-c outcomes. However, all other comparisons and outcomes had low or insufficient evidence. The interventions and approaches that effectively lowered LDL-c or raised HDL-c are described by combination therapy regimen below. The SOE for the body of evidence is provided in Table C for general populations and Table D for subgroups.
Table C
Summary of the strength of evidence for general populations.
Table D
Summary of the strength of evidence for subgroups.
Combination Therapy Versus Intensification of Statin Monotherapy
Combination Therapy With Bile Acid Sequestrant and Statin
Six randomized trials (410 participants) were identified. Four trials compared low-potency statin in combination with a bile acid sequestrant to mid-potency statin monotherapy (288 participants). Low-potency statin in combination with a bile acid sequestrant lowers LDL-c up to 14 percent more than mid-potency statin monotherapy (SOE: moderate). There was insufficient evidence to evaluate LDL-c outcomes for other potency comparisons or to compare HDL-c outcomes at any statin potency.
We found insufficient evidence to compare combined lipid-modifying therapy with a bile acid sequestrant and statin to intensification of statin monotherapy on the rates of serious adverse events, regardless of statin potency. No study reported on the comparative effectiveness of bile acid sequestrant plus statin on benefits or harms as compared to intensification of statin monotherapy among subgroups.
Combination Therapy With Ezetimibe and Statin
Forty randomized trials (10,955 participants) were identified, which primarily reported on surrogate outcomes such as LDL-c and HDL-c. Thirteen trials compared low-potency statin in combination with ezetimibe to high-potency statin monotherapy in general populations. Among general populations, low-potency statin in combination with ezetimibe more effectively lowers LDL-c and raises HDL-c than high-potency statin monotherapy (SOE: low).
Eleven trials compared mid-potency statin in combination with ezetimibe to high-potency statin monotherapy in general populations. Mid-potency statin combined with ezetimibe more effectively lowers LDL-c and raises HDL-c than high-potency statin monotherapy among general populations (SOE: moderate and low, respectively).
Six trials compared low-potency statin in combination with ezetimibe to mid-potency statin monotherapy in general populations. Low-potency statin in combination with ezetimibe more effectively lowers LDL-c and raises HDL-c than mid-potency statin monotherapy (SOE: moderate and low, respectively).
Twelve trials among patients with preexisting CHD and four trials among patients with diabetes compared mid-potency statin in combination with ezetimibe to high-potency statin monotherapy. Mid-potency statin combined with ezetimibe more effectively lowers LDL-c than high-potency statin monotherapy among patients with CHD (SOE: moderate); however, there was no difference in HDL-c effects (SOE: low). Mid-potency statin combined with ezetimibe more effectively lowers LDL-c and raises HDL-c than high-potency statin monotherapy among patients with diabetes (SOE: moderate).
Combination Therapy With Fibrate and Statin
Four randomized trials (1,341 participants) were identified. Two trials compared mid-potency statin in combination with fibrate to high-potency statin monotherapy (683 participants). There is insufficient evidence to compare the benefits of combined lipid-modifying therapy with a fibrate and statin to intensification of statin monotherapy on LDL-c, HDL-c, and serious adverse events, regardless of statin potency.
Combination Therapy With Niacin and Statin
Five randomized trials (612 participants) were identified. Three trials compared low-potency statin in combination with niacin to mid-potency statin monotherapy (247 participants). We found inconsistent effects on lowering LDL-c when comparing low-potency statin in combination with niacin to mid-potency statin monotherapy. However, low-potency statin in combination with niacin raised HDL-c 15 percent to 27 percent more than mid-potency statin monotherapy (SOE: moderate).
We found insufficient evidence to compare combined lipid-modifying therapy with niacin and statin to intensification of statin monotherapy on the rates of long-term clinical outcomes and serious adverse events, regardless of statin potency. No study reported on the effectiveness of niacin plus statin compared to intensification of statin monotherapy on benefits or harms among subgroups.
Combination Therapy With Omega-3 Fatty Acid and Statin
No trials were identified that compared combination therapy with omega-3 fatty acid and statin to intensification of statin monotherapy. There is insufficient evidence to compare the benefits of combined lipid-modifying therapy with an omega-3 fatty acid and statin to intensification of statin monotherapy on LDL-c, HDL-c, and serious adverse events, regardless of statin potency.
Discussion
Key Findings
The evidence suggests that some combination therapy regimens may confer benefits with respect to lowering LDL-c, including bile acid sequestrants (up to 14 percent greater LDL-c reduction) and ezetimibe (up to 21 percent greater LDL-c reduction). LDL-c is an important factor in the development of atherosclerotic cardiovascular disease, and higher levels of LDL-c have been associated with greater risk of this disease.4,5 However, there is insufficient evidence to address whether the LDL-c–lowering benefits achieved with these medications translate into decreased rates of atherosclerotic cardiovascular disease. Prior trials comparing combination regimens to statin monotherapy, such as ENHANCE, AIM-HIGH, and ACCORD-Lipid, have demonstrated that combination therapy can lead to superior lipid outcomes but fail to reduce clinical outcomes such as cardiovascular death, MI, revascularization, or stroke.7,13,14
We also found that some combination therapy regimens may confer benefits with respect to raising HDL-c, including ezetimibe (up to 6 percent) and niacin (up to 27 percent). In particular, given that only one prior study has demonstrated the benefit of pharmacologically raising HDL-c with respect to prevention of CVD events,16 the potential long-term clinical benefits of these combination regimens with respect to their HDL-c effects are unclear.
The strength of evidence is provided for all observed comparisons in general populations in Table C and for subgroups in Table D. Most trials included in this report were of relatively short duration (<3 months). In this limited timeframe, investigators are unlikely to capture any changes in a chronic condition such as atherosclerotic cardiovascular disease, which typically develops and progresses over a number of years. Powering such studies is especially difficult, given that both arms are taking statins, which would reduce the baseline incidence of cardiovascular events. Therefore, currently it is not possible to draw conclusions about the clinical implications of the surrogate marker changes identified. However, until additional data are available, these results may help health care providers tailor lipid-modifying regimens based on individual patient needs and concerns for adverse events.17
Applicability
Many trials that met our inclusion criteria were implemented in patients with hyperlipidemia, and most were designed to evaluate effects on lipid measures and short-term harms. The results of most trials generalize to patients with hyperlipidemia uncomplicated by other major comorbid conditions. Interestingly, we identified fewer trials that were conducted among patients at high risk for CHD, such as those with diabetes or preexisting cardiovascular disease. These patients could benefit the most from improvement in their lipid profiles and are the most likely to be receiving more aggressive lipid-modifying regimens in clinical practice.
Limitations of the Evidence Base and Review Process
The SOE was insufficient for many comparisons and outcomes because of a paucity of studies and poor quality of existing studies. Trials were frequently downgraded in risk-of-bias assessment for lack of blinding by participant and study personnel (performance bias), for not reporting the blinding of outcome assessors (detection bias), or for not accounting for losses to followup or handling of incomplete data (attrition bias). Few studies reported variance estimates for the between-group differences in any outcomes over time. In some instances, the studies did not report a mean difference or point estimate, stating only that there was no significant difference between the groups. In addition, some studies did not report an intention-to-treat analysis and others did not specify the number analyzed in each arm. All of these factors limited our ability to conduct meta-analyses. Where we conducted meta-analyses, substantial heterogeneity was present in most cases.
The evidence base was also limited due to the short duration of most studies. Most trials we identified were of relatively short duration, despite the fact that these medications are currently used in clinical practice as chronic long-term medications. Studies were of insufficient duration to adequately assess long-term clinical outcomes, including mortality, acute coronary events, and revascularization procedures. In addition, losses to followup and medication adherence were often not reported by intervention arm in trials, which may bias our results. While our findings may suggest that one therapeutic option provides a benefit over another, we cannot comment on the tolerability of or persistence with the regimen, given the lack of data and short trial duration. Additional long-term trials are needed to compare the tolerability, side effects, and harms with prolonged use of these combinations.
The review process imposed limitations as well. First, the review focused narrowly on combination therapy compared with statin intensification. As a result, many studies of add-on combination therapy versus the same statin dose or nonstatin monotherapy were excluded because they did not address the Key Questions. Given several previous reviews on dietary modification and reduction of lipids and CVD risk, we did not include these therapies in this review.18,19 Further, we did not examine differences in statin response based on genetic variations.20,21 Second, we excluded non–English-language publications, although we do not believe this introduced significant bias. Third, because this review was conducted prior to the release of the 2013 cholesterol treatment guidelines from the American College of Cardiology/American Heart Association Task Force, we could not define our population eligibility criteria to match their four “statin benefit groups” and our potency categorizations differ slightly from those in the guidelines.10
Future Research Needs
We suggest that most comparisons and outcomes that have low or insufficient evidence are future research needs. In order to answer whether there are long-term benefits with respect to mortality, acute coronary events, and revascularization procedures, future investigators need to make these endpoints the primary outcomes of their trials and ensure that trials are of sufficient duration to actually capture these events (at least 12 months and preferably longer). Short-term trials using surrogate endpoints are of diminishing value at this point.
We further suggest that future studies focus on high-risk CHD populations and populations with greater burden of cardiovascular disease to determine which strategy provides better short-term improvements in lipid profile and long-term clinical benefits. These populations include patients with diabetes and preexisting cardiovascular disease, as well as Black and Native American populations.22 It may be worthwhile to explore differences between men and women, as the ACCORD trial showed benefit of combination therapy with fibrate in men and potential harms with this combination therapy in women.14 Such studies would have tremendous impact on clinical practice, as these patients with greater burden of cardiovascular disease are the most likely to need a more aggressive lipid-modifying regimen.
While head-to-head comparisons of a combination regimen to intensification of statin therapy may answer important clinical questions, these trials do not help clinicians decide between different combination therapy options. Once the effectiveness of each combination regimen on long-term clinical outcomes is established, the next step to inform clinical decisionmaking would be to help clinicians determine how to select the most appropriate lipid-modifying regimen from all available options. We suggest that future studies conduct head-to-head comparisons of multiple combination regimens against each other as well as against intensification of statin monotherapy to address this need. Additionally, it would be useful to examine whether it is possible to achieve LDL-c reductions consistent with those from potent statins (50–60%) in patients who are unable to tolerate full-dose statin therapy and what the clinical effects of these reductions would be. Furthermore, it would be useful to determine if LDL-c lowering of 50 percent achieved with a statin and a bile acid sequestrant is as efficacious as similar LDL-c lowering with a statin and ezetimibe, and whether both used together are as efficacious as a potent statin alone. Finally, alternative study designs, such as observational studies using registry data from electronic medical records, may also provide useful data on clinical outcomes.
Conclusions
Although many studies looked at intermediate outcomes, few studies addressed the question of which approach produces better clinical outcomes. Combination of statin with ezetimibe or bile acid sequestrant lowered LDL-c better than intensification of statin monotherapy, but evidence for clinical outcomes (mortality, acute coronary events, and revascularization procedures) was insufficient across all potency comparisons for all combination therapy regimens. Additional studies evaluating long-term clinical benefits and harms are needed to better inform clinical decisionmaking, patient choice, and clinical practice guidelines.
References
- 1.
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. [PMC free article: PMC5408511] [PubMed: 23239837]
- 2.
- Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–54. [PMC free article: PMC3362050] [PubMed: 22064958]
- 3.
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. [PubMed: 21262990]
- 4.
- Pekkanen J, Linn S, Heiss G, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322(24):1700–7. [PubMed: 2342536]
- 5.
- Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161(11):1413–9. [PubMed: 11386890]
- 6.
- Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–10. [PubMed: 17898099]
- 7.
- Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67. [PubMed: 22085343]
- 8.
- Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med. 2009;151(9):622–30. [PubMed: 19884623]
- 9.
- Sharma M, Ansari MT, Soares-Weiser K, et al. Comparative Effectiveness of Lipid-Modifying Agents. Rockville (MD): Agency for Healthcare Research and Quality (US); Sep, 2009. [Internet] Report No. 09-EHC024-EF. AHRQ Comparative Effectiveness Reviews. [PubMed: 20704039]
- 10.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Nov 7 S0735-1097(13)06028-2. [PubMed: 24239923]
- 11.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed: 12485966]
- 12.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–32. [PubMed: 15358046]
- 13.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–43. [PubMed: 18376000]
- 14.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74. [PMC free article: PMC2879499] [PubMed: 20228404]
- 15.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2011. AHRQ Publication No. 10(11)-EHC063-EF. Chapters available at www
.effectivehealthcare.ahrq.gov. - 16.
- Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585–91. [PubMed: 11268266]
- 17.
- Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes. 2012;5(1):2–5. [PubMed: 22253366]
- 18.
- Rees K, Dyakova M, Ward K, et al. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev. 2013;(3):CD002137. [PubMed: 23543514]
- 19.
- Hooper L, Summerbell CD, Thompson R, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2012;(5):CD002137. [PMC free article: PMC6486029] [PubMed: 22592684]
- 20.
- SEARCH Collaborative Group. Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359(8):789–99. [PubMed: 18650507]
- 21.
- Krauss RM, Mangravite LM, Smith JD, et al. Variation in the 3-hydroxyl-3-methylglutaryl coenzyme a reductase gene is associated with racial differences in low-density lipoprotein cholesterol response to simvastatin treatment. Circulation. 2008;117(12):1537–44. [PubMed: 18332269]
- 22.
- Liao Y, Bang D, Cosgrove S, et al. Surveillance of health status in minority communities - Racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) Risk Factor Survey, United States, 2009. MMWR Surveill Summ. 2011;60(6):1–44. [PubMed: 21597458]
- Executive Summary - Combination Therapy Versus Intensification of Statin Monothe...Executive Summary - Combination Therapy Versus Intensification of Statin Monotherapy: An Update
- Executive Summary - Allergen-Specific Immunotherapy for the Treatment of Allergi...Executive Summary - Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review
Your browsing activity is empty.
Activity recording is turned off.
See more...