From: Virion Proteins
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
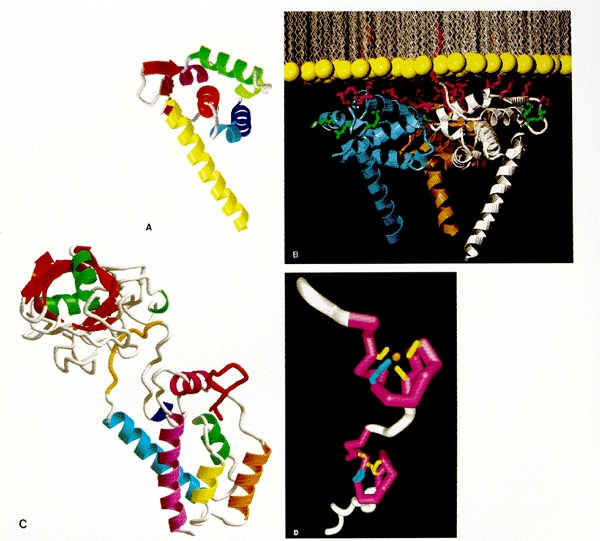
Three-dimensional structures of MA, CA, and NC. (A, B, and C courtesy of Wes Sundquist; D courtesy of Mike Summers.) (A) HIV MA monomer. The drawing shows the polypeptide backbone as observed in the crystal structure of MA purified after expression in E. coli, without amino-terminal myristylation. (B) HIV MA trimer. Hypothetical juxtaposition of HIV MA and viral membrane. The drawing shows the MA trimer as observed in the crystal structure positioned next to a sheet of lipid as might be found in the viral envelope. (Yellow) Lipid head groups; (red) myristyl group from each monomer inserted into the membrane; (purple) positively charged side chains known to be involved in membrane interaction; (green) positively charged side chains not involved in membrane interaction. (C) Amino-terminal portion of HIV CA monomer, as inferred from crystallography. Residues 1 to 151 of CA are shown complexed with cyclophilin A (upper left, with β-strands shown in red and α helixes in green). Residue 1 is at the beginning of the β-hairpin shown in red at the right center of CA; residue 151 is near the bottom. (D) HIV NC monomer. The drawing shows the polypeptide backbone as inferred from NMR studies, with the two Cys-His motifs each binding a Zn++ ion. The amino-terminal and carboxy-terminal portions of the polypeptide projecting away from the Cys-His motifs are shown in an arbitrary orientation, since they are not observed to be structured.
From: Virion Proteins

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.