Table
Rodents
Background
[PubMed]
Glioblastoma is a common and often lethal malignant form of brain cancer. Although there is a variety of treatments, such as chemotherapy, radiation, a combination of the two, and surgical resection, for patients with glioblastoma, the survival rate after treatment is <20% (1-3). This is primarily caused by recurrence of the disease as a result of incomplete surgical removal or exposure of the glioma tumors to radiation and chemotherapy during therapy. The difficulty in distinguishing the cancerous tissue from the normal surrounding tissue contributes to incomplete removal or treatment of the tumor. In addition, any surviving cancerous cells proliferate and tend to infiltrate other parts of the brain as a result of an inherent nature to migrate. Clear visualization of the tumor and its boundary would help in its complete removal or treatment and lead to improved prognosis for the patient.
Fluorescent dyes have been used for the visualization of glioma tumors, but the contrast between the tumor tissue and the normal tissue is not always clear, especially in the brain (4-6). Intravenous administration of fluorescein-sodium (FLS-Na) in human glioma patients has been used to visualize tumors, but a low contrast was observed between the cancerous tissue and normal tissue, which indicated that FLS-Na was not a very promising agent to visualize tumor boundary (6). Human serum albumin (HSA) can be used as a carrier for anti-cancer drugs and was shown to improve the circulating half-life of molecules conjugated to it (7, 8). In an effort to improve the fluorescence contrast, Ichioka et al. developed and evaluated a FLS-HSA conjugate for the visualization of tumors in a rodent model (3).
Synthesis
[PubMed]
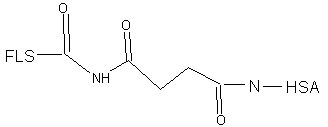
The synthesis of FLS-HSA has been described by Ichioka et al. (3). For this, FLS-Na was dissolved in dimethylsulfoxide, and FLS was activated by dissolving dicyclohexylcarbodiimide and N-hydroxysuccinimide in this solution. This solution was left for 12 h at room temperature. Then HSA was dissolved in phosphate buffer (pH 7.4) and mixed with the activated FLS. The mixture was stirred for 30 min. The FLS-HSA conjugate was purified on a PD-10 column, and the eluted fractions that contained FLS-HSA were concentrated on an ApolloTM concentrator. The final concentration of HSA and FLS was adjusted to 10% and 0.2 mg/ml, respectively. The investigators did not provide data on the yield, purity, and FLS/HSA ratio of the preparation. As a control, FLS-Na was dissolved in lactate Ringer solution at a concentration of 0.2 mg/ml.
Animal Studies
Rodents
[PubMed]
The evaluation of FLS-HSA was performed with SCID mice bearing tumors generated with U251-MG cells, a human malignant glioma cell line (3). Either FLS-Na or FLS-HSA was administered intravenously through the tail vein. The animals were then sacrificed 15, 30, 60, 180, 360, and 720 min after the administration. The tumor nodules were exposed surgically and photographed with a digital camera attached to a fluorescence microscope. For reference, blood glucose analysis glassware filled with FLS-Na was prepared and placed near the tumor nodule when the photographs were taken. The brightness of the tumor nodule, normal tissue, and the reference standard was measured with Photoshop software. Three determinations were made and a mean, calibrated to the reference standard, was calculated. The contrast between the tumor nodule and normal tissue was determined and expressed as a tumor/peripheral brightness (t/p) ratio that was subjected to statistical analysis with ANOVA.
The t/p ratio was almost constant at 1.6 with FLS-Na between 15 and 180 min after administration. The fluorescence brightness diminished in both the tumor and the normal tissue 60 min after administration, and by 180 min the brightness was difficult to distinguish under the microscope. At this point the observations with FLS-Na were stopped. With FLS-HSA, the brightness was highest 15 min after administration of the reagent, but the t/p ratio of the tumor to normal tissue was >2.5 between 60 and 360 min after injection. The t/p ratios of FLS-HSA at 60, 180, and 360 min, and the peak t/p ratio of FLS-Na at 30 min, showed a significant statistical difference by ANOVA (P < 0.01). The investigators suggested that the low specificity shown by FLS-Na was probably because it can be passively transported and brightness can be observed at places where it may have accumulated as a result of unknown reasons. Intravenously administered HSA was shown to accumulate in tumor tissue (9), as observed in this study. Because HSA has a longer circulating half-life, a higher amount of FLS-HSA accumulated in the tumors and generated a brighter contrast compared to FLS-Na. The investigators concluded that although FLS-HSA has a distinct advantage over FLS-Na as a fluorescent tumor indicator, more work is required to determine its usefulness for human glioma surgery using an animal intracranial transplantation model. The investigators also mentioned that a limitation of this animal model was that the model lacked the blood–brain barrier present in the human brain as well as the infiltrating tumor cells of the human glioma that can exit into adjacent tissue of the patient’s brain.
References
- 1.
- Nakada M. , Nakada S. , Demuth T. , Tran N.L. , Hoelzinger D.B. , Berens M.E. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007; 64 (4):458–78. [PubMed: 17260089]
- 2.
- Shrieve D.C. , Alexander E. , Black P.M. , Wen P.Y. , Fine H.A. , Kooy H.M. , Loeffler J.S. Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long-term outcome. J Neurosurg. 1999; 90 (1):72–7. [PubMed: 10413158]
- 3.
- Ichioka T. , Miyatake S. , Asai N. , Kajimoto Y. , Nakagawa T. , Hayashi H. , Kuroiwa T. Enhanced detection of malignant glioma xenograft by fluorescein-human serum albumin conjugate. J Neurooncol. 2004; 67 (1-2):47–52. [PubMed: 15072447]
- 4.
- Haglund M.M. , Hochman D.W. , Spence A.M. , Berger M.S. Enhanced optical imaging of rat gliomas and tumor margins. Neurosurgery. 1994; 35 (5):930–40. [PubMed: 7838344]
- 5.
- Poon W.S. , Schomacker K.T. , Deutsch T.F. , Martuza R.L. Laser-induced fluorescence: experimental intraoperative delineation of tumor resection margins. J Neurosurg. 1992; 76 (4):679–86. [PubMed: 1545262]
- 6.
- Kuroiwa T. , Kajimoto Y. , Ohta T. Development of a fluorescein operative microscope for use during malignant glioma surgery: a technical note and preliminary report. Surg Neurol. 1998; 50 (1):41–8. [PubMed: 9657492]
- 7.
- Wunder A. , Stehle G. , Schrenk H.H. , Hartung G. , Heene D.L. , Maier-Borst W. , Sinn H. Antitumor activity of methotrexate-albumin conjugates in rats bearing a Walker-256 carcinoma. Int J Cancer. 1998; 76 (6):884–90. [PubMed: 9626357]
- 8.
- Stehle G. , Wunder A. , Sinn H. , Schrenk H.H. , Schutt S. , Frei E. , Hartung G. , Maier-Borst W. , Heene D.L. Pharmacokinetics of methotrexate-albumin conjugates in tumor-bearing rats. Anticancer Drugs. 1997; 8 (9):835–44. [PubMed: 9402310]
- 9.
- Leppala J. , Kallio M. , Nikula T. , Nikkinen P. , Liewendahl K. , Jaaskelainen J. , Savolainen S. , Gylling H. , Hiltunen J. , Callaway J. et al. Accumulation of 99mTc-low-density lipoprotein in human malignant glioma. Br J Cancer. 1995; 71 (2):383–7. [PMC free article: PMC2033577] [PubMed: 7841057]
Publication Details
Author Information and Affiliations
Publication History
Created: July 26, 2007; Last Update: August 30, 2007.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
The MICAD Research Team. Fluorescein-conjugated human serum albumin. 2007 Jul 26 [Updated 2007 Aug 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 Rodents
Rodents