NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | [99mTc]-diethylenetriaminepentaacetic acid-galactosyl human serum albumin |
|
| Abbreviated name: | 99mTc-GSA | |
| Synonym: | Technetium-[99mTc]-galactosyl human serum albumin, 99mTc-galactosyl-neoglycoalbumin, 99mTc-DTPA-GSA, 99mTc-NGA | |
| Agent Category: | Protein | |
| Target: | Asialoglycoprotein | |
| Target Category: | Receptors | |
| Method of detection: | Single photon emission computed tomography (SPECT); planar gamma imaging | |
| Source of signal / contrast: | 99mTc | |
| Activation: | No | |
| Studies: |
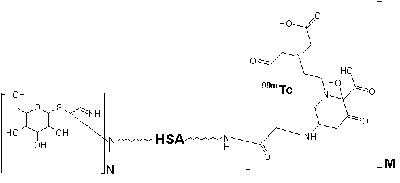
| The structure of 99mTc-GSA. N = number of galactose molecules bound to HSA. M = number of DTPA molecules. The exact coordinates of 99mTc are unknown. |
Background
[PubMed]
The primary function of the liver is to remove toxins from the body through metabolism and excretion, and to synthesize complex biologically active substances such as clotting factors and albumin. Impairment of liver function as a result of hepatitis, infections, jaundice, cirrhosis, or cancer can lead to a variety of gastrointestinal disorders. The evaluation of hepatic function is an important parameter in the determination of the physiological state of the liver, particularly for patients who have been diagnosed with liver disease for which a hepatectomy or liver transplantation is the treatment of choice (1). Therefore, determination of the hepatic function is now considered important before and after a hepatectomy or liver transplantation to predict patient outcome (2).
The asialoglycoprotein receptors (ASGP-R) exist specifically on hepatic cell membranes and are believed to be necessary for intracellular trafficking and endocytic activity in the liver. The number of ASGP-R on the hepatocytes of individuals with liver disease is altered and is thus considered a good indicator for the evaluation of liver function (3, 4). Technetium [99mTc]-diethylenetriaminepentaacetic acid-galactosyl-human serum albumin (99mTc-GSA), originally known as 99mTc-galactosyl-neoglycoalbumin (99mTc-NGA), is an ASGP-R ligand that accumulates specifically in the liver and is used for liver scintigraphy to determine hepatocyte mass and function (1, 5, 6). 99mTc-GSA is available as a commercial kit in Japan (www.nmp.co.jp/eng/about/index.html, in English, and www.nmp.co.jp/index.html, in Japanese). According to Stadalnik and Vera, 99mTc-GSA has been available only at one institution in the United States, the University of California, Davis Medical Center (6).
Synthesis
[PubMed]
The synthesis of 99mTc-GSA in the laboratory was described by Kudo et al. (7). Galactose was derivatized to produce cyanomethyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactose (CNM-thiogalactose). The CNM-thiogalactose was purified by repeated recrystallization from dry methanol. It was then re-dissolved in methanol, and sodium methoxide was added to the solution. This mixture was kept at room temperature for 48 h. The solvent was evaporated in vacuo at 40oC to obtain 2-imino-2-methoxyethyl-1-thio- β-D-galactose (IME-thiogalactose). This reaction step had an estimated yield of 55%.
Human serum albumin (HSA), diluted in borate buffer at pH 8.5, was added to IME-thiogalactose, and the mixture was stirred at 37oC for 1.5 h to yield HSA derivatives with 30–40 moles of galactose coupled per mole of HSA. Subsequently, cyclic diethylenetriaminepentaacetic acid (cDTPA) was slowly added to the reaction mixture, and the coupling was allowed to proceed for 10 min at 37oC with continuous stirring. This reaction yielded HSA derivatives containing 4.5–7.0 moles of DTPA per mole of HSA. The GSA monomers were isolated from the HSA derivative mixture by high-performance liquid chromatography (HPLC) using a NaCl solution as the elution solvent. GSA obtained by this method was >95% pure. The amount of galactose bound to HSA and the degree of DTPA conjugation was determined by methods described by Kudo et al. (7).
To radiolabel GSA with metastable 99mTc, the concentration of HPLC-purified GSA was adjusted to 3 mg/ml at pH 3.1–3.4. The solution was deoxygenated by flushing argon gas through it until the oxygen concentration became <100 parts per billion. Then SnCl2 and ascorbic acid were added to obtain a final concentration of 0.1 and 0.5 µM, respectively. The solution was then dispensed into glass vials (each with a rubber stopper) through a 0.22-µm filter membrane. At this stage the vials may be frozen for the short term or lyophilized for long-term storage. To obtain 99mTc-GSA, a 99mTc solution was added to each vial and mixed gently at room temperature for ~1 min. Purity of 99mTc-GSA was determined to be >98% by thin-layer chromatography. Specific activity of the final product obtained with this method was not provided by the authors.
Another procedure, described by Vera et al., is also available for the preparation of 99mTc-NGA (8).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Using patient biopsies, Kudo et al. (9, 10) concluded that hepatic uptake of 99mTc-NGA could be used to quantify ASGP-R in the liver.
In an effort to understand the occurrence of hepatic ischemia/reperfusion injury under clinical conditions, 99mTc-GSA was used to characterize ASGP-R in primary cultured rat hepatocytes under hypoxic conditions (5). The maximal binding (Bmax) of 99mTc-GSA to the hepatocyte membranes and ketone body ratio (KBR) that reflects cell injury in the medium was determined for three durations of hypoxia (1, 2, and 3 h). The Bmax for receptor binding and endocytosis decreased (ng/dish) with the increase in hypoxia, although the number of viable cells remained constant. The KBR was also significantly decreased during hypoxic conditions, which indicated that the extent of cell injury depended on duration of hypoxia. The investigators concluded that the hypoxic conditions reduced the number of ASGP-R binding sites involved in endocytosis per hepatocyte and that the cells were irreversibly injured if maintained under these conditions for a prolonged period.
In another study that used cultured primary hepatocytes, Hata and Ishii showed that galactose inhibited the binding to and the internalization of 99mTc-GSA into the hepatocytes (11).
Animal Studies
Rodents
[PubMed]
Toxicological studies with 99mTc-NGA were performed in mice and rabbits (8), and the animals showed no toxicological effects with the treatment. In a biodistribution study of rabbits, the investigators demonstrated that 99mTc-NGA accumulated primarily in the liver of these animals, and almost no radioactivity was accumulated in the other organs.
The biodistribution of 99mTc-GSA has been reported for rats and rabbits (7). Ten minutes after injection of 99mTc-GSA, 92.4% of the injected radioactivity was found in the liver, and 74.8% of the injected dose was excreted through the hepatobiliary route into the feces at 48 h. The investigators performed 99mTc-GSA imaging studies in rabbits and reported no accumulation of radioactivity in the spleen or bone marrow of the animals.
The use of 99mTc-GSA scintigraphy for intraperitoneal (i.p.) imaging of tumors in a nude mouse model was investigated (12). The nude mice were inoculated with either human SHIN-3 (ovarian cancer) or LS 180 (colon cancer) cells to establish the tumors. Radiolabeled GSA was injected into the tumor-bearing mice and the biodistribution of radioactivity was examined. The tumors were clearly visible by scintigraphic imaging and the investigators concluded that 99mTc-GSA imaging was useful for the imaging of i.p. tumors.
99mTc-GSA scintigraphy was suggested to be useful in evaluating hepatic tissue blood flow (13, 14) and fatty liver and ischemia-reperfusion injury (15) in rats.
Human Studies
Virgolini et al. demonstrated that 99mTc-NGA scintigraphy could be used to detect liver disease, including cirrhosis and viral hepatitis, by quantification of the ASGP-R (16). In another study it was shown that 99mTc-NGA scintigraphy could be used for the differential diagnosis of hepatic focal nodular hyperplasia and hepatic tumors (17).
Using 99mTc-GSA for scintigraphy of the liver, Le et al. (18) devised a method to obtain a quantitative index to analyze the regional ASGP-R concentration to assess regional function of the liver. Similarly, Onodera et al. (19) used 99mTc-GSA clearance from the blood pool and its binding to the ASGP-R to devise a liver uptake ratio to assess liver functional reserve. 99mTc-GSA scintigraphy was also used to detect changes in functional volume of individual lobes of the liver and was suggested to be a better method than computed tomography for the detection of morphological changes in the organ (20). Hirai et al. (21) suggested that 99mTc-GSA scintigraphy was a useful technique to evaluate preoperative portal embolism and postoperative liver failure after a hepatectomy.
99mTc-GSA scintigraphy has also been used in humans to establish the prognosis of patients with hepatic cirrhosis (22), predict the clinical outcome of hepatitis C after interferon treatment (23), and to evaluate hepatic regional reserve changes before and after chemolipiodolization in hepatocellular carcinoma patients (24).
References
- 1.
- Clavien P.A., Petrowsky H., DeOliveira M.L., Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–59. [PubMed: 17429086]
- 2.
- Ishikawa M., Yogita S., Miyake H., Fukuda Y., Harada M., Wada D., Tashiro S. Clarification of risk factors for hepatectomy in patients with hepatocellular carcinoma. Hepatogastroenterology. 2002;49(48):1625–31. [PubMed: 12397750]
- 3.
- Stockert R.J. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75(3):591–609. [PubMed: 7624395]
- 4.
- Sawamura T., Nakada H., Hazama H., Shiozaki Y., Sameshima Y., Tashiro Y. Hyperasialoglycoproteinemia in patients with chronic liver diseases and/or liver cell carcinoma. Asialoglycoprotein receptor in cirrhosis and liver cell carcinoma. Gastroenterology. 1984;87(6):1217–21. [PubMed: 6092193]
- 5.
- Kwon A.H., Inoue T., Ha-Kawa S.K. Characterization of the asialoglycoprotein receptor under hypoxic conditions in primary cultured rat hepatocytes. J Nucl Med. 2005;46(2):321–5. [PubMed: 15695793]
- 6.
- Stadalnik R.C., Vera D.R. The evolution of (99m)Tc-NGA as a clinically useful receptor-binding radiopharmaceutical. Nucl Med Biol. 2001;28(5):499–503. [PubMed: 11516694]
- 7.
- Kudo M., Washino K., Yamamichi Y., Ikekubo K. Synthesis and radiolabeling of galactosyl human serum albumin. Methods Enzymol. 1994;247:383–94. [PubMed: 7898366]
- 8.
- Vera D.R., Stadalnik R.C., Krohn K.A. Technetium-99m galactosyl-neoglycoalbumin: preparation and preclinical studies. J Nucl Med. 1985;26(10):1157–67. [PubMed: 4045560]
- 9.
- Kudo M., Vera D.R., Trudeau W.L., Stadalnik R.C. Hepatic uptake of [99mTc]galactosyl-neoglycoalbumin is sensitive to receptor quantity. Int J Rad Appl Instrum B. 1991;18(7):663–6. [PubMed: 1787074]
- 10.
- Kudo M., Vera D.R., Trudeau W.L., Stadalnik R.C. Validation of in vivo receptor measurements via in vitro radioassay: technetium-99m-galactosyl-neoglycoalbumin as prototype model. J Nucl Med. 1991;32(6):1177–82. [PubMed: 2045931]
- 11.
- Hata S., Ishii K. Effect of galactose on binding and endocytosis of asialoglycoprotein in cultured rat hepatocytes. Ann Nucl Med. 1998;12(5):255–9. [PubMed: 9839486]
- 12.
- Yao Z., Zhang M., Sakahara H., Saga T., Nakamoto Y., Sato N., Zhao S., Arano Y., Konishi J. Imaging of intraperitoneal tumors with technetium-99m GSA. Ann Nucl Med. 1998;12(2):115–8. [PubMed: 9637283]
- 13.
- Ohno K. , . Hokkaido Igaku Zasshi. 1997;72(3):297–307. [PubMed: 9226469]
- 14.
- Hiraguchi E. , . Kaku Igaku. 1995;32(5):453–63. [PubMed: 7596065]
- 15.
- Kimoto M. , . Nippon Igaku Hoshasen Gakkai Zasshi. 1996;56(5):311–6. [PubMed: 8692657]
- 16.
- Virgolini I., Muller C., Angelberger P., Hobart J., Bergmann H., Sinzinger H. Quantification of human hepatic binding protein (HBP) via 99mTc-galactosyl-neoglycoalbumin (NGA) liver scintigraphy. Wien Klin Wochenschr. 1991;103(15):458–61. [PubMed: 1926872]
- 17.
- Kurtaran A., Muller C., Novacek G., Kaserer K., Mentes M., Raderer M., Pidlich J., Eibenberger K., Angelberger P., Virgolini I. Distinction between hepatic focal nodular hyperplasia and malignant liver lesions using technetium-99m-galactosyl-neoglycoalbumin. J Nucl Med. 1997;38(12):1912–5. [PubMed: 9430468]
- 18.
- Le T.T., Kobayashi H., Takai K., Kato K., Ishigaki T. Quantitative analysis for assessing regional function of liver by using 99mTc-GSA SPECT. Nagoya J Med Sci. 2003;66(1-2):39–43. [PubMed: 12848420]
- 19.
- Onodera Y., Takahashi K., Togashi T., Sugai Y., Tamaki N., Miyasaka K. Clinical assessment of hepatic functional reserve using 99mTc DTPA galactosyl human serum albumin SPECT to prognosticate chronic hepatic diseases--validation of the use of SPECT and a new indicator. Ann Nucl Med. 2003;17(3):181–8. [PubMed: 12846539]
- 20.
- Nanashima A., Yamaguchi H., Shibasaki S., Morino S., Ide N., Takeshita H., Tsuji T., Sawai T., Nakagoe T., Nagayasu T., Ogawa Y. Relationship between CT volumetry and functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization before major hepatectomy: a preliminary study. Dig Dis Sci. 2006;51(7):1190–5. [PubMed: 16944008]
- 21.
- Hirai I., Kimura W., Fuse A., Suto K., Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133(5):495–506. [PubMed: 12773977]
- 22.
- Sasaki N., Shiomi S., Iwata Y., Nishiguchi S., Kuroki T., Kawabe J., Ochi H. Clinical usefulness of scintigraphy with 99mTc-galactosyl-human serum albumin for prognosis of cirrhosis of the liver. J Nucl Med. 1999;40(10):1652–6. [PubMed: 10520705]
- 23.
- Kira T., Tomiguchi S., Takahashi M., Yoshimatsu S., Sagara K., Kurano R. Correlation of 99mTc-GSA hepatic scintigraphy with liver biopsies in patients with chronic active hepatitis type C. Radiat Med. 1999;17(2):125–30. [PubMed: 10399780]
- 24.
- Kira T., Tomiguchi S., Takahashi M. Quantitative evaluation of the regional hepatic reserve by 99mTc-GSA dynamic SPECT before and after chemolipiodolization in patients with hepatocellular carcinoma. Ann Nucl Med. 1998;12(6):369–73. [PubMed: 9972375]
 In vitro
In vitro