NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ratko TA, Douglas GW, de Souza JA, et al. Radiotherapy Treatments for Head and Neck Cancer Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Dec. (Comparative Effectiveness Review, No. 144.)
Overview
In this section, we report our literature search results and PRISMA diagram, which depicts the flow of articles through the review according to our screening and inclusion criteria. We subsequently provide an overview of the design, patients, and study limitations (risk of bias) of all included studies, including relevant studies from CER No. 20. We lay out the results for each Key Question in order, starting with an overview of the relevant current studies, key bulleted points of information, and a synthesis of the evidence when possible. In the results, we did not incorporate formal data synthesis (e.g., meta-analysis) because there was only one randomized trial involving the interventions of interest for treatment of head and neck cancer and the nonrandomized studies were highly heterogeneous and of “poor” quality according to the USPSTF criteria. Finally, we lay out in tabular format the conclusions and evidence base from CER No. 20 and those from this update to qualitatively integrate the findings of both.
Results of Literature Searches
Electronic Search
A medical librarian searched MEDLINE®, EMBASE®, and the Cochrane Controlled Trials Registry for English-language articles. The overall search was performed for a period dating 12 months before the final literature search for CER No. 20 (September 28, 2009) through April 2013. For SBRT, the literature was searched for the period January 1, 1990, through April 2013. The entire search was updated May 1, 2014.
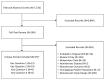
In both searches together, we identified 7,130 unique titles and screened 284 in full text. Of the latter, 15 (N=1,781) met the inclusion criteria, including one RCT (Gupta 2012, N=60).25 In the updated search, we identified a second citation to an RCT (Rathod 2013).26 Because the latter was the same study and included the same patients as the Gupta RCT, it was not double-counted in the total number of patients; however, it reported additional, different outcomes that were reviewed herein and so is counted in that context. Thus, in 14 studies that reported unique data, including the RCT of Rathod,26 3DCRT and IMRT were compared.25,27–39 One study compared 3DCRT and SBRT34; none compared IMRT and SBRT. As in CER No. 20, no evidence was identified on PBT. The flow of articles through the screening and study selection process is shown in the PRISMA diagram (Figure 2).
We note that although CER No. 20 was published in final form in 2010, we had obtained the final data for PARSPORT,40 a key RCT, from the investigators at the time we updated the CER No. 20 literature search. Because the PARSPORT findings appeared in CER No. 20, they were not included in this report.
Gray Literature (Publication Bias)
The study selection criteria for this update stipulate exclusion of abstracts or other non-peer-reviewed or non-full-length studies. Therefore we did not include any information based on comprehensive searches of meeting abstracts. We examined the bibliographies of all papers screened in full text to identify peer-reviewed articles the electronic search may have missed.
We accessed the Web site ClinicalTrials.gov to identify ongoing phase 3 RCTs that would meet the criteria for inclusion based on our protocol. We identified two phase 3 RCTs of conformal RT in head and neck cancer that are recruiting patients. The first trial (National Clinical Trial [NCT] 01893307) is designed to compare IMRT and PBT in the treatment of oropharyngeal cancer. The primary outcome is the incidence of any late-onset (> 90 days) grade 3 toxicity during the 2 years after completion of RT. The second RCT (NCT01216800) is designed to compare the effects of IMRT and 3DCRT on auditory function (hearing) when used as adjuvant therapy in patients who have undergone surgical resection of parotid tumors. A MEDLINE search of the NCT number(s) and title(s) did not yield any published results; it is unknown whether any data have been reported. Examination of a Scientific Information Packet from one manufacturer of RT equipment did not yield published evidence to add to this update.
Description of All Included Studies
We identified 15 reports that met the inclusion criteria for this CER update. All are generally described in this section; other details and results specific to a particular Key Question are considered in the relevant subsections to follow.
Study Limitations
According to the USPSTF criteria for assessing the risk of bias of individual studies, the Gupta RCT was rated “fair,” whereas the 13 nonrandomized studies were rated “poor.” The rationale for the ratings is provided in Table B-1.
We assigned a “fair” USPSTF rating to the Gupta RCT, primarily because the study was not double-blinded, particularly its outcomes assessments. Furthermore, the investigators did not clarify the meaning of their reported “modified intention-to-treat” method. Although this point is for practical purposes moot, because they reported a 97 percent followup rate in each of two study arms, the USPSTF method stipulates assignment of this rating. Gupta reported aggregated survival results in patients with tumors in different sites. However, the distribution of tumor sites and characteristics between arms was similar. Overall, the two study arms were statistically similar and comparable. We note also the report of Rathod and colleagues, a RCT that included the same patients of Gupta but reported additional outcomes. 26 We include evidence from this report but do not double count it in the PRISMA diagram or summations of included evidence. Its risk of bias is identical to that of the Gupta study using the USPSTF system..
The 13 nonrandomized studies were retrospective database analyses, one of which used a historical comparator group. All of the included nonrandomized studies reported results in aggregate, mixing outcomes achieved in heterogeneous groups who may not have received the same treatment(s). Overall, we rated these studies as poor according to the USPSTF criteria.
Study Design and Patient Characteristics
Table 3 provides a high-level view of the studies included in this update. For comparative purposes, Table 3 also depicts the studies from CER No. 20 that compared 3DCRT and IMRT and reported on clinical outcomes covered herein. We address applicable evidence in more detail in the Discussion section, relating the results and conclusions to those of this update.
Table 3
Design and characteristics of studies included in the CER No. 20 Update and CER No. 20.
In total, for the update, 3DCRT and IMRT were compared in 13 studies, including one small (N=60) RCT.25,26 One study compared 3DCRT and SBRT34; none compared IMRT and SBRT. As in CER No. 20, no evidence was identified on PBT. Study details are summarized in Table B-2.
Overall, similar to what we identified for CER No. 20, the body of studies in the update is heterogeneous in terms of tumor site and stage, treatment regimen, and treatment intent (e.g., curative vs. palliative or recurrent). Patients were generally in their mid-fifties, with males predominating across studies. Tumor sites included the hypopharynx, larynx, nasal sinus, nasopharynx, oral cavity, and oropharynx. Four studies involved patients with single tumor sites. The majority of patients across studies had locally advanced (stage III and IV) cancer, although small proportions of patients had stage I or II disease.
Treatment regimens included concurrent chemoradiotherapy (CCRT); RT with or without concurrent chemotherapy (CCT); CCRT with or without surgery; and adjuvant postoperative RT. Where it appears in all tables throughout this update, the term RT ± CCT refers to treatment regimens in which all patients received RT, but not all received CCT. This is distinct from CCRT, in which all patients were reported to have received RT and chemotherapy concurrently. We did not abstract information on specific chemotherapy regimens or surgical procedures; they are beyond the scope of this update. As summarized in Table B-3, ionizing radiation was delivered by 3DCRT or IMRT to a total dose of 60–74 Gy using conventional fractionation schedules, which are typical of 3DCRT and IMRT (30–35 fractions, 2 Gy per fraction for 5–7 weeks); SBRT was delivered in a similar total dose but in five single fractions. We did not abstract or report on RT protocols in detail because they also are beyond the proposed scope of the review.
Key Question 1. Comparative Effectiveness of 3DCRT, IMRT, SBRT, and PBT Regarding Adverse Events and QOL
Overview
Tables 4 and 5 depict key comparative acute (< 90 days post-treatment) and late (> 90 days post-treatment) toxicity outcomes reported by each relevant study; a blank cell in any table means that the toxicity was not reported in that study. Acute and late toxicity outcomes were not collected consistently across studies. Only eight (53%) studies reported acute toxicities.25,27,29,31,33,36–38 Nine (60%) studies reported late toxicities.25,27–29,31,33,34,36,39 Only two studies reported QOL evidence according to RT modality, including the RCT by Rathod.26
Table 4
Summary of key reported acute (<90 days post-treatment) comparative toxicity outcomes.
Table 5
Summary of key reported late (>90 days post-treatment) comparative toxicity outcomes.
Because toxicities were inconsistently reported, we focused this update, as we did CER No. 20, on those toxicities prominently associated with RT in the head and neck: dysphagia, salivary gland function, and xerostomia. We also only consider toxicities of grade 2 or greater according to accepted criteria, such as those of the Radiation Therapy Oncology Group or the NCI Common Terminology Criteria for Adverse Events. Grades greater than 2 are those that have direct impact on patient outcomes and can adversely affect treatment delivery.
Patients in all studies, except that of Dirix (2010),31 received chemotherapy as part of treatment; those treated by Dirix received postoperative RT. In general, investigators did not adjust results to account for chemotherapy-associated toxicities independent of RT-associated toxicities, which complicates interpretation of toxicity evidence for many adverse events (e.g., mucositis). This is somewhat ameliorated by our focus on studies in which chemotherapy regimens are similar between study arms, thus potentially isolating the effect of the RT modality on such outcomes.
Key Points
- The results of primary interest for this Key Question comprise comparative acute (< 90 days) and late (> 90 days) radiation-associated dysphagia, salivary gland dysfunction, xerostomia, and QOL.
- New comparative evidence assessed in this update strengthens the conclusion from CER No. 20 that the risk of grade 2 or higher late xerostomia is significantly lower in patients treated with IMRT than with 3DCRT.
- Although we identified evidence on other key toxicities (e.g., mucositis, dysphagia, skin toxicities, osteoradionecrosis of the jaw) and QOL, the reported rates compared between modalities were inconsistent. Thus, evidence on adverse events other than late xerostomia remains insufficient to alter conclusions of CER No. 20.
- Post-treatment toxicities were reported inconsistently across studies, precluding comparisons within the body of evidence. We are uncertain whether the limited evidence on RT-associated toxicities overall reflects their absence or that the investigators did not systematically collect or chose not to report them.
- The best quality evidence comprises one small (N=60), fair quality RCT (Gupta, 2012) in which 3DCRT and IMRT were compared in a regimen of CCRT to treat patients with cancer of the hypopharynx, larynx, and oropharynx. Key findings of this study relevant to Key Question 1 pertained to late xerostomia and salivary gland dysfunction.
- One nonrandomized, poor quality study of 3DCRT versus SBRT did not report on primary outcomes for Key Question 1.
- One fair quality study and one poor quality nonrandomized study reported QOL outcomes related to treatment with 3DCRT or IMRT
Qualitative Synthesis
In Table 6, we aggregate new comparative evidence related to Key Question 1 on toxicities actually reported in studies according to the intervention comparison, treatment regimen, and timeframe (acute vs. long-term). We identified no evidence from patients stratified according to tumor site(s), so we did not include tumor information in this table. Although we collected evidence on lesser NCI Common Terminology Criteria for Adverse Events or Radiation Therapy Oncology Group grades, as shown in Tables B-4 and B-5, here we present grade 2 or higher toxicities, which are likely to adversely impact patient management, hospitalization, and survival outcomes. The last two columns of Table 6 show reported proportions for each toxicity and any statistically significant results by study if so achieved.
Table 6
Key Question 1: Qualitative evidence synthesis for key reported comparative grade 2 or higher toxicity outcomes and QOL.
RT-Associated Toxicities
Results from one nonrandomized study show a statistically significant lower rate of acute dysphagia (49 percent vs. 84 percent, respectively, p=0.04) with IMRT compared with 3DCRT in a regimen of CCRT.29 The Gupta25 RCT showed a lower rate of acute dysphagia with 3DCRT (0 percent) than with IMRT (9.5 percent), although the difference was nonsignificant (p=0.21). Significantly reduced rates of late dysphagia were reported in single studies of IMRT compared with 3DCRT in a regimen of CCRT29 or RT with or without CCT.27 Two individual studies showed a reduced rate of acute salivary gland dysfunction with IMRT compared with 3DCRT in a regimen of CCRT25 or postoperative RT,31 respectively.
As shown in Table 6, all three studies of IMRT compared with 3DCRT in a regimen of CCRT showed statistically significant reduction in late xerostomia.25,29,33 The rate of late xerostomia also was significantly lower with IMRT than with 3DCRT in single studies in a regimen of RT with or without CCT,27 or postoperative RT,28 respectively.
RT-Associated QOL
One RCT reported QOL evidence on IMRT versus 3DCRT in a regimen of RT with CCT.26 Rathod et al. reported on mean QOL scores using the European Organization for Research and Treatment of Cancer QOL questionnaire (QLQ-C30) and Head-Neck module (HN-35) validated, self-administered tools at baseline (pretreatment) and periodically on follow-up (3, 6, 12, 18, and 24 months). The study reported global QOL was not significantly affected by RT technique. Treatment with IMRT showed a benefit to some general QOL domains, as well as several head and neck cancer-specific domains, as compared with 3DCRT. General domains toward which IMRT demonstrated a significant benefit included: emotional functioning at 12 months (p=0.008), role functioning at 12 months (p=0.008), and social contact at 24 months (p=0.03). Head and neck cancer-specific symptoms for which IMRT demonstrated a significant benefit (p<0.05) compared with 3DCRT included scales and dry month (6, 12, and 18 months), as well as opening mouth (6 and 24 months). Sticky saliva, pain, swallowing, senses, sexuality, feeling ill, and insomnia tended to be ameliorated by use of IMRT compared with 3DCRT, and were all statistically significant for at least one time point. No QOL domains were worse with IMRT than with 3DCRT at any time point. Among both RT techniques, QOL domains generally experienced maximal deterioration after RT, followed by a trend toward gradual recovery over time.
One nonrandomized study reported QOL evidence on IMRT versus 3DCRT in a regimen of RT with or without CCT. Chen et al. reported on mean QOL scores using the University of Washington Quality of Life validated, self-administered tool.30 In this study, the salivary gland domain was the only specific component of this score wherein significant differences were observed between the IMRT and the 3DCRT groups at both 1 and 2 years (p<0.001 at both points). Other domains (pain, appearance, activity, recreation, swallowing, chewing, speech, shoulder, taste, saliva, mood, anxiety) showed no differences according to RT modality. At 1 year after completion of RT, the global QOL was rated as “very good” or “outstanding” among 51 percent of patients treated with IMRT compared with 41 percent of those treated with 3DCRT (p=0.11). However, at 2 years, the corresponding percentages were 73 percent and 49 percent, respectively (p<0.001), showing a benefit of IMRT. Multivariate analysis showed no effect on QOL scores of age, sex, radiation intent, radiation dose, T stage, primary site, or use of CCT and neck dissection. Use of IMRT was the only variable associated with improved QOL (p<0.01).
Strength of Evidence for Key Question 1
To evaluate the SOE, we used an approach specifically developed by the AHRQ EPC program and referenced in the “Methods Guide.”20 This approach is based on a system initially described by the GRADE Working Group. It explicitly addresses four required domains: risk of bias, directness, consistency, and precision, as outlined in the Methods section.
Table 7 shows the SOE for new evidence on the comparative effects on QOL and toxicities of 3DCRT, IMRT, SBRT, and PBT in the treatment of head and neck cancer patients.
Table 7
SOE for Key Question 1: Adverse effects and QOL.
The evidence we identified for this update supports an SOE rating of “moderate” for the comparison of 3DCRT and IMRT in a regimen of CCRT, showing a benefit of IMRT in significantly reducing the incidence of late grade 2 or higher xerostomia. Two other studies showed a statistically significant reduction in the incidence of late grade 2 or higher xerostomia in two other treatment regimens (RT with or without CCT, postoperative RT). New evidence on any other RT-associated toxicity is insufficient to form conclusions on a benefit or harm of 3DCRT compared with IMRT.
Key Question 2. Comparative Effectiveness of 3DCRT, IMRT, SBRT, and PBT Regarding Tumor Control and Patient Survival
Overview
In this section we summarize evidence on comparative oncologic outcomes for different RT types. As noted in the Methods section, we did not seek evidence from other study designs (e.g., single-arm observational) that may report additional outcomes not captured in the comparative studies.
Table 8 depicts key oncologic outcomes reported by each relevant study; a blank cell in each table means the outcome was not reported in that study. Not all outcomes were collected in each study. Outcomes of primary interest are overall survival, local control (no evidence of primary tumor), or locoregional control (no evidence of primary tumor or regional metastatic spread) among patients treated with IMRT compared with 3DCRT. Other oncologic outcomes were inconsistently reported across the body of studies, as shown in Table 8.
Table 8
Summary of key reported comparative oncologic outcomes.
Key Points
- The results of primary interest for this Key Question comprise overall survival, local control, and locoregional control.
- As we found in CER No. 20, comparative evidence assessed in this update was insufficient to draw relative conclusions on any oncologic outcomes.
- The key oncologic outcomes were not reported universally across studies, so we could not make comparisons across a larger body of evidence.
- The best quality evidence comprises one small (N=60), fair quality RCT (Gupta, 2012) in which 3DCRT and IMRT were compared in a regimen of CCRT to treat patients with cancer of the hypopharynx, larynx and oropharynx.
- Two additional nonrandomized, poor quality studies reported on the key oncologic outcomes with 3DCRT and IMRT in a regimen of CCRT among patients with cancer of the hypopharynx, larynx, oral cavity, nasopharynx, and oropharynx.
- One study of 3DCRT versus SBRT reported overall survival and local control in a regimen of RT with or without CCRT among patients with nasopharyngeal cancer. However, 22 percent of unidentified patients in the 3DCRT arm received concurrent high-dose rate brachytherapy so the oncologic outcomes are not included in this synthesis.
Qualitative Synthesis
In Table 9, we have aggregated new evidence related to Key Question 2 on comparative oncologic outcomes actually reported in studies according to the intervention comparison, treatment regimen, and timeframe. We identified no evidence from patients stratified according to tumor site(s), so we have not included tumor information in this table. Further, we did not identify any evidence on differences in oncologic outcomes related to the HPV status of patient tumors. The last two columns of Table 9 show reported proportions for each outcome and statistically significant results if attained.
Table 9
Key Question 2: Qualitative evidence synthesis for key reported comparative oncologic outcomes.
In general, evidence on tumor control and survival outcomes is sparse. Table 9 shows that statistically significant differences were inconsistently reported for overall survival, local control, or locoregional control among studies of 3DCRT versus IMRT in any regimen compiled there. All abstracted data are shown in detail in Table B-6.
Strength of Evidence for Key Question 2
To evaluate the SOE, we used an approach specifically developed by the AHRQ EPC program and referenced in the Methods Guide.20 This approach is based on a system initially described by the GRADE Working Group. It explicitly addresses four required domains: risk of bias, directness, consistency, and precision, as outlined in the Methods section.
Table 10 shows the SOE for the comparative effects of 3DCRT, IMRT, SBRT, and PBT on oncologic outcomes in the treatment of head and neck cancer patients. The criteria we used to arrive at the SOE ratings are outlined in the Methods section of the update. Details on how the SOE ratings were determined are summarized in Table 10.
Table 10
SOE for Key Question 2: Oncologic outcomes.
We determined that new evidence, including one “fair” quality RCT (Gupta, 2012),25 is insufficient to support a conclusion on the relative effect of IMRT and 3DCRT on overall survival or locoregional control rates in a regimen of CCRT. New evidence is insufficient to form conclusions on the effect of any other RT modality comparison for any oncologic outcome in any other regimen we identified in this update.
Key Question 3. Comparative Effectiveness of 3DCRT, IMRT, SBRT, or PBT for Specific Patient and Tumor Characteristics
Key Points
- In CER No. 20, no comparative studies addressed these issues. In this update, we did not identify any new evidence that specifically addressed Key Question 3.
- Therefore insufficient evidence exists to form conclusions about the comparative effects or SOE on 3DCRT, IMRT, SBRT, or PBT based on specific patient and tumor characteristics.
Key Question 4. Comparative Effectiveness of 3DCRT, IMRT, SBRT, or PBT Because of Differences in User Experience, Treatment Planning, Treatment Delivery, and Target Volume Delineation
Key Points
- In CER No. 20, no comparative studies addressed these issues. In this update, we did not identify any new evidence that specifically addressed Key Question 4.
- Therefore insufficient evidence exists to form conclusions about the comparative effects or SOE on 3DCRT, IMRT, SBRT, or PBT based on specific patient and tumor characteristics.
- Overview
- Results of Literature Searches
- Study Design and Patient Characteristics
- Comparative Effectiveness of 3DCRT, IMRT, SBRT, and PBT Regarding Adverse Events and QOL
- Comparative Effectiveness of 3DCRT, IMRT, SBRT, and PBT Regarding Tumor Control and Patient Survival
- Comparative Effectiveness of 3DCRT, IMRT, SBRT, or PBT for Specific Patient and Tumor Characteristics
- Comparative Effectiveness of 3DCRT, IMRT, SBRT, or PBT Because of Differences in User Experience, Treatment Planning, Treatment Delivery, and Target Volume Delineation
- Results - Radiotherapy Treatments for Head and Neck Cancer UpdateResults - Radiotherapy Treatments for Head and Neck Cancer Update
- LOC127272475 [Homo sapiens]LOC127272475 [Homo sapiens]Gene ID:127272475Gene
- Tachycardia, SupraventricularTachycardia, SupraventricularA generic expression for any tachycardia that originates above the BUNDLE OF HIS.<br/>Year introduced: 1987MeSH
Your browsing activity is empty.
Activity recording is turned off.
See more...