From: 5, Integration and Decision-Making for Predictive Toxicology

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
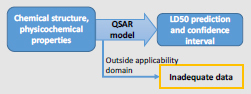
Models and End Points: At Tier 1, a single quantitative structure–activity relationship (QSAR) model is being used to predict rat LD50 values from chemical structure and physicochemical properties. No other models or end points are being considered. To illustrate a simplified approach, chemicals outside a specified applicability domain are placed in the “inadequate data” category for this example. For chemicals inside the applicability domain, the output of the model is an LD50 estimate with a confidence interval that reflects uncertainty.
Integration: Because only a single model and a single end point are being considered, there is no integration of different predictions.
Category Benchmarks: A set of reference chemicals based on DOD interests that have known (possibly more than one) LD50 values are selected to represent the “high toxicity” and “low toxicity” categories from which category benchmarks are derived. For example, the “high toxicity” benchmark could be defined as the highest LD50 of the least toxic “high toxicity” reference chemical. For some end points, generic toxicity benchmarks are available, such as the European Union and Global Harmonized System categories for acute toxicity.
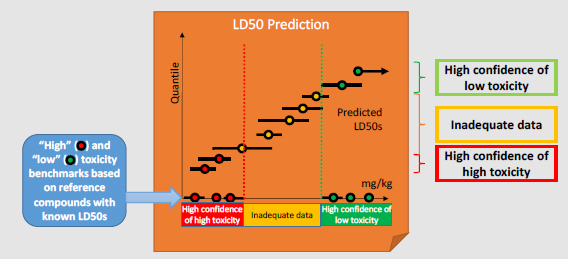
Decision-making: Decisions as to how to place chemicals into categories are based on the confidence bounds for each prediction:
The remaining chemicals are categorized as having “inadequate data.”
From: 5, Integration and Decision-Making for Predictive Toxicology

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.