NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
ECRI Health Technology Assessment Group. Diagnosis and Treatment of Swallowing Disorders (Dysphagia) in Acute-Care Stroke Patients. Rockville (MD): Agency for Health Care Policy and Research (US); 1999 Jul. (Evidence Reports/Technology Assessments, No. 8.)
This publication is provided for historical reference only and the information may be out of date.

Diagnosis and Treatment of Swallowing Disorders (Dysphagia) in Acute-Care Stroke Patients.
Show detailsFocus and Refinement of Topic
To focus, refine, and arrive at the key questions addressed by this assessment, the research team consulted with the initiator of the request of an evidence report [the Health Care Financing Administration (HCFA)] and a panel of nine experts in the field. Initial discussions clarified that the topic was to cover only swallowing disorders in elderly individuals and that dysphagia resulting from neoplasms or esophageal dysfunction were beyond the scope of the evidence report. Noninvasive therapies and feeding tubes were similarly deemed the only treatments within the scope of this report. Telephone conversations were then held with the experts, eight of whom were recognized experts in the areas of speech-language pathology, gastroenterology, and neurology, and one of whom was a patient representative.
During these conversations, a set of four basic questions was developed that would address the issues most important to HCFA and the technical experts. The research team then developed an evidence model based on these four questions (see the Evidence Model section below for a description of this model and the key questions). A document containing this evidence model and written descriptions of the specific issues depicted in this model (and addressed in the report) were then sent to HCFA and the experts for comment. Upon receipt of their written comments, members of the research team met to ensure that any issues raised by these individuals would be addressed in the evidence report.
Databases and Other Sources Searched
Electronic Database Searches
Several electronic databases were searched with the intention of retrieving both clinical trials for analysis, and review articles for background knowledge. Specific search strategies are shown in Appendix C. The databases searched and general keywords used are listed below:
American Speech-Language-Hearing Association (ASHA) Database (1945 to 1996)
CANCERLIT (through September 4, 1997)
CATLINE (through August 25, 1997)
The Cochrane Database of Systemic Reviews (through 1998, Issue 3)
The Cochrane Registry of Clinical Trials (through 1998, Issue 3)
The Cochrane Review Methodology Database (through 1998, Issue 3)
Combined Health Information Database (CHID) (through July 29, 1998)
Current Contents (through July 1998)
The Database of Reviews of Effectiveness (Cochrane Library) (through 1998, Issue 3)
DIRLINE (through November 1997)
ECRI Health Devices Alerts (1977 through July 1998)
ECRI Health Devices Sourcebase (through July 1998)
ECRI Healthcare Standards Database (1975 through July 1998)
EMBASE (Excerpta Medica) (1974 through February 6, 1998)
Health Care Financing Administration Coverage (HCFA) Manuals CD-ROM (through July 1998)
HealthSTAR (Health Services, Technology, Administration, and Research) (1990 through May 20, 1998)
Incidence and Prevalence Database (1988 through August 25, 1997)
ECRI International Health Technology Assessment (IHTA) Database (1990 through July 1998)
MEDLINE (1964 through July 24, 1998)
NIH Grants Database (through December 12, 1997)
Nursing and Allied Health (NAHL) (1988 through April 30, 1998)
PsycINFO (1967 through September 10, 1997)
Sociological Abstracts (1963 through November 1997)
The search strategies employed a number of free-text keywords, as well as controlled vocabulary terms, including but not limited to:
Diagnostic modalities: barium sulfate; barium swallow; barium; fluoroscopy; cineradiology; videofluoroscopy; FEES (fiberoptic endoscopic evaluation of swallowing); ESE (endoscopic swallowing evaluation); FEED (fiberoptic endoscopic evaluation of dysphagia); FEESST (fiberoptic endoscopic evaluation of swallowing with sensory testing); VEED (videoendoscopic evaluation of dysphagia)
Disorder: deglutition disorders (exploded); deglutition (exploded); dysphagia; swallowing
Epidemiology: epidemiology; research design; epidemiologic study characteristics; epidemiologic methods; epidemiologic studies; evaluation studies; incidence; prevalence; statistics and numbers; aspiration pneumonia; neurodegenerative diseases (exploded); Parkinson disease; silent aspiration; stroke
Etiology: aging; Alzheimer's disease; dementia; multiple sclerosis; Parkinson disease; stroke
Miscellaneous: cachexia; wasting; weight loss; quality of life; QOL; life satisfaction; satisfaction
Treatment: speech therapy; speech-language pathology; electrical stimulation; enteral nutrition; intubation, gastrointestinal; nasogastric; nasointestinal; NG; percutaneous endoscopic gastrostomy; PEG tube feeding; rehabilitation, geriatric; rehabilitation; speech and language; rehabilitation, patients; elder care; mobile health units
In general, the searches were restricted to human. Case reports were excluded.
World Wide Web Searches
Searches of the World Wide Web were also conducted using various search engines including, but not limited to, AltaVista, Hotbot, and Yahoo. Pertinent websites include:
Dysphagia
American Academy of Private Practice in Speech Pathology and Audiology (AAPPSPA) http://www.aappspa.org
American Dietetic Association (ADA) http://www.eatright.org
American Physical Therapy Association http://www.apta.org
American Speech-Language-Hearing Association http://www.asha.org
Atlanta Voice and Swallowing Center http://www.mindspring.com/~newvoice/
Otolaryngology for the Primary Care Practitioner [continuing education] http://cpmcnet.columbia.edu/dept/cme/cont0013.html
Dysphagia Research Society http://www.als.uiuc.edu/drs/
Evaluation and Interpretation of Acute Dysphagia Disorders and Dysphagia Rehabilitation/EMG
Biofeedback Assisted Treatment http://www.speechpaths.com/pms9.htm
Ivanhoe's Medical Breakthroughs-Electrical Swallowing #1057 http://www.ivanhoe.com/docs/backissues/electricalswallowing.html
NIH Speech Pathology http://www.cc.nih.gov/rm/sp
NYEEI: Otolaryngology: FAQs about Swallowing Disorders http://www.nyee.edu/otolaryn/dysphfaq.htm
Swallowing Disorders http://www.webpages.marshall.edu/~lynch4/swallow.html
Swallowing Disorders Program http://www.stjosephs.org/iv_k_6.htm
Aging
AgeInfo http://www.cpa.org.uk/ageinfo/html
GoldenAge.Net http://www.elo.mediasrv.swt.edu/goldenage/script.htm
National Aging Information Center http://www.aoa.dhhs.gov/naic/
Netherlands Institute of Gerontology: GeronLine http://www.nig.nl/
Statistical Information on the Aging - Online Data http://www.aoa.dhhs.gov/aoa/stats/statlink.html
Diseases
American Heart Association 1997 Statistical Supplement http://www.amhrt.org/1997/stats/Stroke.html
Ask NOAH About: Aging and Alzheimer's Disease http://www.noah.cuny.edu/aging/aging.html
The National Multiple Sclerosis Society http://www.nmss.org
National Stroke Association http://www.stroke.org
NINDS Stroke Information Guide http://www.ninds.nih.gov/healinfo/disorder/stroke/strokehp.htm
Hand Searches of Journal and Nonjournal Literature
In addition to searching Current Contents - Clinical Medicine on a weekly basis - more than 1,600 journals and supplements maintained in ECRI's collections were routinely reviewed. A hand search of the Cumulated Index Medicus (1960-1964) was conducted for the terms deglutition: deglutition disorders, speech therapy, and speech disorders. Nonjournal publications and conference proceedings from professional organizations, private agencies, and government agencies were also screened.
Other Mechanisms
Other mechanisms were used to retrieve additional relevant information, including review of bibliographies/reference lists from peer-reviewed and gray literature. (Gray literature includes reports, studies, etc., produced by local government agencies, private organizations, educational facilities, corporations, etc., that do not commonly appear in the published peer-reviewed journal literature.) Published and unpublished information was also solicited from a panel of experts in the field.
Information Retrieved
The use of these search methodologies, as well as personal communications from many technical experts, resulted in the identification of 4,646 items of information in the form of journal articles, book chapters, manuscripts, monographs, Web pages, personal communications, and other miscellaneous items. The titles and abstracts of all electronic search results were independently reviewed by two primary analysts blinded from each other, and articles were ordered using the following inclusion criteria:
- Human studies
- In vivo studies
- English language
- 10 or more subjects
The latter criterion warrants some explanation inasmuch as there are a number of studies in this literature that contain small numbers of patients. The primary reason for excluding such studies is that their results are of uncertain generalizability. It is difficult to determine whether patients chosen for these studies were unique, or whether unique treatments were used in such studies. Hence, it is not clear that the results reported in these studies would be obtained at other sites and in other settings. Another reason for excluding small studies is statistical. The variance (and, hence the confidence intervals) surrounding an effect increases as sample size decreases. Consequently, small studies have larger variances than large ones. This means that the information from small studies is not as "precise" as information from larger ones.
Whenever possible, we used U.S. literature. This was to ensure that the potentially different healthcare systems and practices of other countries did not influence the information obtained. However, when little or no U.S. information was available, we used information collected in other countries.
These inclusion criteria, like our literature search strategies, were chosen to be broad to ensure retrieval of all relevant information. To further this goal, literature requested by each analyst was delivered to both analysts. During an initial review of the retrieved literature, the bibliographies of each article were reviewed for additional information that might not have been found during the database searches.
In certain instances, more specific inclusion criteria were used. These criteria are described in the appropriate sections of this report.
As a result of our searches, a total of 1,808 articles was retrieved. These included 1,467 clinical trials, 183 review articles, and material from 9 World Wide Web sites. In addition, we obtained 32 unpublished articles and received 28 personal communications.
Evidence Model
To organize a discussion on the effects of different diagnostic and treatment strategies for dysphagia management in the elderly, we created an evidence model depicting the relationships among treatments, diagnostics, and patient outcomes. This evidence model is shown in Figure 1. The top box in this model shows the entry of patients age 65 and older into the healthcare system (as related to swallowing disorders). Below this, the model is divided into six sections (indicated by the solid black lines that surround groups of boxes). These sections depict diagnostic tools, symptoms detected by these tools, treatments, short-term outcomes, morbidities, and long-term outcomes. Within each box is a code that consists of a letter followed by a number (e.g., D1). The letters D, S, and T stand for Diagnostic Tools, Symptoms Detected by Diagnostic Methods, and Treatments, respectively. The letter O is used for both Short and Long-Term Outcomes. Any meaningful relationship between two boxes in any given section is noted in the subsequent section.
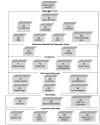
Figure
Figure 1. Evidence Model of Diagnosis and Treatment of Dysphagia in the Elderly.
Typically, lines are drawn between the boxes in an evidence model to indicate the particular relationships being addressed in an evidence report. For example, a line might be drawn between boxes D5 and S1 to indicate the relationship between the modified barium swallow test and aspiration, a relationship that manifests itself as the ability of this test to detect aspiration. As another example, a line between boxes T1 and M1 indicates that the report is addressing the effect of swallow therapy on rates of aspiration pneumonia. These lines, sometimes called links, are not shown in this evidence model because the large number of them that we addressed could not be displayed in an easily readable fashion. Instead, we refer to these links by their code numbers (for example, Link T1-M1 refers to the link, or line, between boxes T1 and M1).
When viewed in this way, the evidence model depicts the kinds of diagnostics and treatments available, and the kinds of patient outcomes that may result from their use. In this respect, the evidence model is a map of available data, and is used to answer the four key questions of this report. These key questions are:
- 1.
How does diagnosis of dysphagia or aspiration affect the subsequent course of treatment and patient outcomes? (A related question, addressed in a Supplemental Analysis, is whether dysphagia diagnosis and treatment programs are cost-effective.)
- 2.
What are the appropriate indications for having patients diagnosed using a full bedside exam (BSE), modified barium swallow (MBS), fiberoptic endoscopy (FE), or another instrumented exam?
- 3.
Is one diagnostic technology more effective than any other diagnostic?
- 4.
When is noninvasive swallow therapy appropriate? Does it work particularly well or particularly poorly in any particular patient population? Are feeding tubes useful as a primary therapy, or should they be used as a last resort that might be avoided for some patients by dysphagia diagnosis and noninvasive therapy?
Common to the first three questions is a question about the efficacy and appropriate role of the BSE.
In addition to serving as a tool that assists in answering the questions upon which this report is based, the evidence model also serves a second function: to illustrate the approximate clinical pathway that patients follow. Each of these two functions of the evidence model is described in detail in the following sections of this report.
The Evidence Model as a Clinical Pathway
The evidence model approximates the clinical pathway followed by patients. Understanding of this pathway is critical to understanding not only the relationship(s) between the kinds of available evidence, but also for understanding the kinds of future research that might be conducted. Thus, the purpose of this section is to elaborate on the use of the evidence model as an illustration of the clinical pathway.
Section one of evidence model: Diagnostic tools
The first step in patient management is to diagnose the patient as having or not having dysphagia. The patient will likely have a history and physical first, which will identify any outward symptoms that may indicate a problem; alternatively, the physical exam may simply be part of the diagnosis of a disease or disorder with which dysphagia is commonly associated (e.g., stroke). Patients who meet some diagnostic criteria for having dysphagia (or suspected dysphagia) will continue on to a more dysphagia-specific examination (designated as D2 to D7 in the evidence model), which may include one or more of the following diagnostic tests (described in detail in the Introduction to this report):
- 3-ounce water test
- Formal BSE
- Informal BSE
- MBS
- FEESST
- FEES
- Other instrumented exam
Section two of evidence model: Symptoms detected by diagnostic tools
These diagnostic tests will have varying abilities to detect signs and symptoms of dysphagia. One of the main issues of this report is how well these diagnostic tests detect aspiration or risk for aspiration pneumonia. The two important symptoms (designated in the evidence model as S1 and S2) are therefore dysphagia and aspiration.
Section three of evidence model: Treatments
Depending on the symptoms detected, a patient with dysphagia or aspiration may undergo one or more treatments (designated as T1 to T4 in the evidence model). These include:
- Swallow therapy
- Modified diet
- Combined swallow therapy and modified diet
- Feeding tube
The treatment a patient receives depends on the type and severity of the problem detected.
Section four of evidence model: Short-term outcomes
There are many important outcomes reported in the field of medicine. Some of the most common and important ones are complications resulting from treatment and mortality. However, not every outcome of interest to society or patients is relevant to every intervention. It is therefore important to pick outcomes of interest based on relevance to the topic as determined by clinical experience (e.g., the observation of pneumonia in patients with dysphagia) or logical inference (e.g., that dysphagia may lead to malnutrition or dehydration).
It is possible, relatively soon after treatment has begun, to measure certain outcomes that indicate whether the treatment is working. These short-term outcomes are designated O1 to O6 in the evidence model and include:
- Swallow function
- Food intake
- Feeding method
- Weight change
- Aspiration
- Tube complications
Swallow function refers to the physiological changes in the process of swallowing that may occur after certain therapies, such as total swallow time and pharyngeal transit time. Food intake refers to the change in the amount of energy (e.g., kilocalories) ingested by the patient as a result of treatment. Weight change is an important outcome, as many elderly are malnourished, potentially as a result of a swallowing problem. Aspiration indicates whether the therapy of choice has successfully eliminated or reduced a previous aspiration problem. Tube complications, an outcome specific to the feeding tube, indicate that a problem has occurred with the tube, either intraoperatively or postoperatively (e.g., wound infection).
Section five of evidence model: Morbidities
One goal in the diagnosis and treatment of dysphagia is to prevent serious morbidities that can result from aspiration and an inability to ingest adequate amounts of food. The morbidities of interest in this report, designated M1 to M3, are:
- Pneumonia
- Malnutrition/dehydration
- Major tube complications
Pneumonia resulting from dysphagia and aspiration is usually specifically aspiration pneumonia, the result of material passing into the lungs and causing infection. Pneumonia is a major cause of morbidity and mortality in the elderly, and is therefore perhaps the most important consideration. Malnutrition and dehydration are a result of the inability to ingest adequate amounts of food or liquid and are included in this model through logical inference rather than clinical evidence (to be discussed in depth later in this report). Major tube complications can lead to serious systemic morbidity such as gastric fistula, septicemia, peritonitis, or gastric hemorrhage. Treatment effects on the incidence of these morbidities are important to examine.
Section six of evidence model: Long-term outcomes
The ultimate concern in the diagnosis and treatment of any disorder is how the patient is ultimately affected: Does the patient live or die, and if the patient lives, is it a good life? These concerns are addressed in the evidence model by boxes O6 to O9:
- QOL
- Mortality (other)
- Mortality resulting from morbidity
- Mortality resulting from treatment (feeding tube)
QOL specifically addresses a concern about the ability of the elderly person to maintain an active, rewarding life although suffering from dysphagia. The measurement of QOL is a developing field, in which the patient's physical, psychological, and social well-being are assessed. From the patient perspective, this is perhaps the most important outcome to consider because it incorporates all outcomes, including death. Mortality (other) refers to patients who die of their underlying disease rather than from complications related to or resulting from the dysphagia problem. Mortality resulting from morbidity refers to those patients who die directly as a result of the morbidities outlined above: either from malnutrition, pneumonia, or an acute aspiration event. Mortality resulting from treatment refers specifically to those tube-fed patients who experience serious complications with the feeding tube and die as a result; all other treatments covered in this report are noninvasive and therefore do not include any directly related mortality. In this report, the effect of treatment on these long-term outcomes is examined.
Viewing the evidence model as a clinical pathway, and not as six discrete sections, highlights the fact that no single section should be reviewed separately from any other section. Further, to separately measure the effects of diagnosis and treatment of swallowing disorders on patients is not possible (see the section titled The Relationship between Diagnosis and Treatment of Swallowing Problems).
Use of the Evidence Model to Answer the Four Main Questions
In this section, we discuss each of the links addressed in this evidence report as they pertain to the key questions. Noting at the outset that the answers to some of these questions may be of greater interest to patients and society than the answers to others is important to recognize. This suggestion agrees with the evidence hierarchy proposed by Fryback and Thornbury (1991), and we have employed a modification of this scheme for the purposes of the present report. Our scheme, as it pertains to the diagnosis and treatment of swallowing disorders, is depicted in Table 14.
Table
Table 14. Levels of the Key Questions of this Report.
This hierarchy contains six levels. The lowest level (Level 1) addresses the technical efficacy of a technology. This level of evidence is illustrated by the situation often found in radiology in which it is determined that an imaging device can resolve a certain number of lines per centimeter.
Level 2 evidence provides information related to a more general question: how well a given diagnostic can determine whether a patient has (or does not have) a certain disease or condition. Evidence at this level, unlike that at the first level, provides the sensitivity and specificity of the diagnostic.
Level 3 evidence has even greater generality: it addresses how physicians use the results of a diagnostic test. For example, a study providing evidence at this level could measure the percentage of patients whose management was changed as a result of the diagnostic test. The precedence this evidence takes over Level 2 evidence becomes apparent when we consider that even a perfectly sensitive and specific diagnostic test would not benefit patients if its results were not used in patient management. This level of evidence, however, does not specify the treatments given to patients.
At Level 4, there are two types of evidence. The first type is evidence that examines the performance of a diagnostic without regard to treatment efficacy. The second type is evidence that examines the efficacy of treatments without regard to the performance of the diagnostic test. Neither type of evidence is as general as evidence about the efficacy of a program of diagnosis and treatment, and both are more meaningful than the previous levels, which do not provide much information about patient outcomes. An example of the first type of Level 4 evidence is a hypothetical study that specifies that a certain percentage of patients given a MBS exam were instructed to tuck their chins when they swallowed and that another percentage of patients were instructed to turn their heads to one side when they swallowed. Even though the treatments are specified by this type of Level 4 evidence, the efficacy of these treatments (e.g., how many patients died of aspiration pneumonia) is not considered. Rather, this level of evidence specifies the percentages of patients given treatments thought to be effective. Therefore, this type of evidence primarily gives information about the performance of the diagnostic test. An example of the second type of Level 4 evidence would be a study that compared the effectiveness of two treatments given to patients who all had the same results on a diagnostic test. This type of evidence primarily provides information about treatment efficacy and does not consider whether patients are receiving the best possible diagnostic test. As such, information about the outcomes of patients given diagnostic tests thought to be effective is provided.
Level 5 evidence addresses both the efficacy of the diagnostic test and the efficacy of treatment. Evidence at this level addresses whether patients are receiving both the best available diagnostic and the best available treatment. In other words, the efficacy of dysphagia programs is addressed. As such, information that is of greater interest to patients and society than that provided by the evidence in Levels 1 through 4 is provided.
The highest level of evidence is provided by Level 6, which addresses the cost-effectiveness of any given program. At one extreme are programs that might be highly effective but so expensive that society's finite amount of dollars would save more lives were they spent elsewhere. At the other extreme are inexpensive programs that are not particularly efficacious. Such programs give rise to debates about whether they should be implemented because of the remote possibility they might benefit some patients.
How this evidence hierarchy is used to rank the major questions of this evidence report is explained below, in the discussion of the evidence model links of interest.
Question 1: How does diagnosis of dysphagia affect subsequent course of treatment and outcomes? (Level 5)
This question is a Level 5 question because it calls for assembling evidence or for studies that directly address the efficacy of a swallowing disorder program. As such, this question calls for one to directly link diagnostic methodologies to treatment and then to patient outcomes. Specifically, this question asks: When a particular diagnostic technology is used to direct treatment, how often do patients come down with pneumonia, become malnourished, or require a feeding tube after failed treatment? How many of these patients die or experience decreased quality of life? The ultimate purpose of any diagnostic and treatment program is to avoid such patient outcomes, and thus the focus of this question is of relatively great importance.
The specific links in the evidence model that address this question and the question represented by each link are:
Link D2-D8 to M1: How does the use of each of these diagnostic tests affect the rate of pneumonia in patients with dysphagia?
Link D2-D8 to M2: How does the use of each of these diagnostic tests affect the rate of malnutrition or dehydration in patients with dysphagia?
Link D2-D8 to O7: How does the use of each of these diagnostic tests affect the quality of life of patients with dysphagia?
Link D2-D8 to O9: How does the use of each of these diagnostic tests affect the rate of death due to dysphagia-related morbidities (pneumonia, malnutrition/dehydration)?
Link D2-D8 to T1-T4 to O1-O10: How does the use of each diagnostic to guide treatment affect outcomes after specific treatments?
Link D2-D8 to T1-T4 to M1 to M3: How does the use of each diagnostic to guide treatment affect morbidities after specific treatments?
Link D2-D8 to T1-T4: How are the results of diagnostic tests used to guide treatment?
Question 2: What are the appropriate indications for having patients diagnosed using a full BSE, MBS, or FEES? (Level 2)
This question is a Level 2 question because in asking how to selectively choose patients to undergo more extensive testing for dysphagia and aspiration, the question is about the sensitivities and specificities of a particular noninstrumented exam, and does not consider the efficacy of any treatment(s) that may result from this test or how patient management might be changed by the test results. Of interest in this question is whether particular signs and symptoms are detected during preliminary clinical or noninstrumented examination (general physical exam, preliminary BSE, etc.) that identify patients who would be most or least likely to benefit from further testing (patients who in particular are at risk for morbidities or mortality resulting from dysphagia or aspiration).
The links addressed by this question are:
Link D1-D4 to M1: Do particular signs or symptoms detected during a history and physical or preliminary BSE predict pneumonia?
Link D1-D4 to S1: Do particular signs or symptoms detected during a history and physical or preliminary BSE predict aspiration?
Question 3: Is there any evidence that one diagnostic technology (full BSE, MBS, etc.) provides more useful information than any other diagnostic? (Level 2)
This question is a Level 2 question because it is restricted to the sensitivity and specificity of each diagnostic modality and compares them with one another in their ability to predict pneumonia or aspiration. This question only addresses the ability of these tests to identify the probability for these problems and does not consider the effect of these technologies on patient outcomes.
The links that represent these issues are:
Link D2-D8 to M1: How well do each of these diagnostic methods predict pneumonia?
Link D2-D7 to S1: How well do each of these diagnostic methods predict chronic aspiration?
Question 4: When is noninvasive swallow therapy appropriate? Does it work particularly well or particularly poorly in any particular patient population? What can the evidence tell us about this therapy? Is PEG useful, or is it a last resort? (Level 4)
This question is a Level 4 question because it addresses the efficacy of different therapies for the treatment of dysphagia and which patients, if any, receive the most benefit from the therapies. It does not, however, address the efficacy of any diagnostic test.
Of interest are both short-term outcomes, such as physiological changes immediately detectable during or after treatment, as well as long-term outcomes, such as rate of morbidity and mortality. The long-term outcomes are more important, and are therefore the primary focus of this question.
Several links in the evidence model are important in answering this question:
Treatments to Short-term Outcomes
Link T1 to 01: How does swallow therapy affect physiological swallow function?
Link T1-T3 to O2: How does noninvasive therapy affect the patient's ability to ingest adequate amounts of food and drink as measured by energy intake and weight change?
Link T1-T3 to O3: How does noninvasive therapy affect the type of feeding (oral versus tube, special textures versus normal diet) the patient is able to safely perform?
Link T1-T3 to O4: How does noninvasive therapy affect the weight of the patient (as an indirect measure of food ingestion)?
Link T1-T3 to O5: How does noninvasive therapy affect the occurrence of aspiration in known aspirators?
Link T4 to O4: How does tube feeding affect the weight of the patient (as an indirect measure of food ingestion)?
Link T4 to O5: How does tube feeding affect the occurrence of aspiration in known aspirators?
Link T4 to O6: How often does the use of a feeding tube result in complications directly related to the tube?
Treatments to Morbidities
Link T1-T3 to M1: How often does pneumonia occur in patients receiving each of these noninvasive therapies compared with those receiving other therapies or no therapy?
Link T1-T3 to M2: How well does each noninvasive therapy prevent or cure malnutrition or dehydration compared with other therapies or no therapy?
Link T4 to M1: How often does pneumonia occur in patients on a feeding tube compared with those receiving other therapies or no therapy?
Link T4 to M2: How well does the use of a feeding tube prevent or cure malnutrition compared with other therapies or no therapy?
Link T4 to M3: How often does the use of a feeding tube result in major tube-related complications?
Treatments to Long-term Outcomes
T1-T3 to O7: How does each noninvasive therapy affect a patient's QOL?
T1-T3 to O8: How often do patients undergoing each noninvasive therapy die of underlying disease?
T1-T3 to O9: How often do patients undergoing each noninvasive therapy die of serious morbidity related to the dysphagia?
T4 to O7: How does tube feeding affect a patient's QOL?
T4 to O8: How often do patients undergoing tube feeding die of their underlying disease?
T4 to O9: How often do patients undergoing tube feeding die of serious morbidity related to dysphagia?
T4 to O10: How often do patients undergoing tube feeding die of causes directly related to the feeding tube itself?
Supplemental analysis: What is the cost-effectiveness of dysphagia diagnosis and treatment programs? (Level 6)
This question addresses the cost-effectiveness of dysphagia programs. Ideally, this sort of analysis is performed from a societal perspective, but such analyses require data that are often difficult to obtain (e.g., costs to family members to drive a patient to a care facility). Even though some data relevant to the strict societal perspective of a Level 6 question have not yet been collected, we will, nevertheless, treat this question as if it were Level 6. It is the only question to take both costs and effectiveness into account, so it is clearly of a higher level than our other questions.
Study Quality
There are essentially four types of study designs in the literature that we analyzed for an evidence-based appraisal of diagnosis and treatment of swallowing disorders: randomized controlled trials (RCTs), historical prospective case series,1 case-control studies, and case series. In general, we rank data from RCTs higher than data from studies of other design, and assign data from historical prospective case series to the second highest rank. We do not, however, adhere rigidly to this scheme because, as discussed below, some RCTs have flaws serious enough to preclude assigning their data to the highest rank. Similarly, we do not rank case-controlled studies above case series because of certain flaws in the former. These flaws are particularly evident when case controls are used to measure the sensitivity and specificity of diagnostic tests. Such designs artificially set the prevalence and severity of disease, rendering measures of test performance derived from them suspect.
Because no inviolable rule for preferring the data from a study of one design to a study of another design can be constructed for the present report, we do not assign numerical or letter codes to these study designs. Rather, we simply provide the study designs in the evidence tables and the narrative of the report. Also, because of the lack of an inviolable rule, we discuss the quality of individual studies at some length in the following sections. This is done to provide evidence that such rules cannot be rigidly adhered to. Our discussions of studies in the sections below generally follow the order in which studies are typically ranked; it begins with RCTs and ends with case series. As explained in the section entitled Information Retrieved, we did not consider case reports.
Quantitative Methods
Throughout the Results section of this report, we perform numerous original calculations. A list of these original calculations appears in Appendix H. Briefly, however, we computed many of the effect sizes of treatment, computed their 95 percent confidence intervals (C.I.s), conducted statistical tests on the reported effects, and computed most of the sensitivities, specificities, positive predictive values, and negative predictive values discussed below. It is important to note that the 95 percent C.I.s calculated around proportions were computed using a formula that corrects for the distortions observed in these intervals when any given proportion greatly deviates from 0.5. The need to perform this substantial number of original calculations is a reflection of the relatively poor reporting and the relatively poor quality of the literature related to this evidence report. Without these calculations, it would have been difficult to address our four key questions.
In addition to the above-mentioned calculations, we also adjusted for the varying lengths of followup used by different studies, conducted meta-analyses (in the form of summary receiver operating characteristic curves) on the sensitivities and specificities of the 3-ounce water test and the BSE, performed an exploratory meta-analysis (termed exploratory because it employs pooled historical data as the control group data), and conducted a meta-analysis used to illustrate the low statistical power of vote-counting procedures.
We also employed other quantitative methods in our supplemental analysis, which contains two cost-effectiveness analyses. Both of these latter analyses play an important role in our conclusions.
Quality Control Methods
Preparation of this evidence report was monitored by an eight-member ECRI internal review committee. This committee performed several functions. The first was to oversee the abstraction of data from full-length articles. This was accomplished by having the review committee examine the evidence tables into which data were directly entered. Data collection forms were not used in the present report because this method was cost-ineffective. This was because of the large number of links we examined (each of which required unique information) and because of the relative lack of data relevant to some links (preparing an abstraction form for only a few studies is inefficient). Data for the key questions were abstracted independently by two analysts.
The second function of the committee was to critically evaluate the methods, logic, and conclusions of the evidence report. This was accomplished by reviewing the project at several phases. These reviews consisted of examination of all aspects of the report, but particularly the evidence model, the results and conclusions, the supplemental analysis, and all calculations performed in the report. Six full-day review meetings were held in addition to several shorter meetings. As a result of these processes, no one individual had undue influence over the conclusions made in this report.
Footnotes
- 1
Defined, as per Armitage and Berry (1994), as a study in which data from a prospectively selected group of patients are compared with data from historical controls.
- Methodology - Diagnosis and Treatment of Swallowing Disorders (Dysphagia) in Acu...Methodology - Diagnosis and Treatment of Swallowing Disorders (Dysphagia) in Acute-Care Stroke Patients
- GTP Cyclohydrolase 1-Deficient Dopa-Responsive Dystonia - GeneReviews®GTP Cyclohydrolase 1-Deficient Dopa-Responsive Dystonia - GeneReviews®
- K10C9.7 Homeobox domain-containing protein [Caenorhabditis elegans]K10C9.7 Homeobox domain-containing protein [Caenorhabditis elegans]Gene ID:187261Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...