NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau J, Zucker D, Engels EA, et al. Diagnosis and Treatment of Acute Bacterial Rhinosinusitis. Rockville (MD): Agency for Health Care Policy and Research (US); 1999 Mar. (Evidence Reports/Technology Assessments, No. 9.)
This publication is provided for historical reference only and the information may be out of date.
Attachment A: Article Screening and Abstraction Forms


Subgroup data
available:
Yes No
If YES, list subgroups:
Outcome
Measure:
Comments on Outcomes:

Attachment B: Meta-Analysis of Diagnostic Test Studies
Sinus puncture with culture is the acknowledged diagnostic reference standard for diagnosing acute bacterial rhinosinusitis. However, sinus puncture is invasive, and few patients are willing to undergo this procedure. Also, it is costly and impractical in the primary care setting. The aim of this meta-analysis is to assess the accuracy of other noninvasive procedures such as sinus radiography and ultrasonography, as well as the use of clinical criteria to guide treatment. Additional details of the methodology are described in relevant methods sections in Chapter 2.
Description of Studies and Populations of Subjects
Fourteen studies were included, five of which provided comparisons of more than two tests; these studies are described in Evidence Tables 1-8. Twelve studies were conducted in Europe, including all of those utilizing sinus puncture as a reference test. Only two studies were conducted in the United States (Rohr, Spector, Siegel, et al., 1986; Williams, Simel, Roberts, et al., 1992).
Among 12 studies that described the age range of subjects, eight studies examined only adolescents and/or adults, and four studies included children or examined only children. Six studies provided a description of symptoms that had to be present for subjects to be included in the study (typically nasal symptoms or headache); the remainder of studies included patients as study subjects when they or their physicians suspected sinusitis. Only four studies limited how long subjects could have symptoms before evaluation: two studies limited duration of symptoms to 30 days, and two studies limited duration of symptoms to 90 days.
Five studies used the patient as the unit of analysis for comparing diagnostic tests (Berg and Carenfelt, 1985, 1988; Jannert, Andreasson, Helin, et al., 1982; van Duijn, Brouwer, and Lamberts, 1992; Williams, Simel, Roberts, et al., 1992). In the remaining nine studies, the unit of analysis was the sinus.
Four studies that used sinus puncture as a reference test provided estimates of the prevalence of sinusitis in the populations. Two studies that included subjects from an office practice provided prevalence estimates of 49 percent and 51 percent (Laine, Maättä, Varonen, et al., 1998;van Buchem, Peeters, Beaumont, et al., 1995). A study that included only subjects from an otolaryngology clinic found a higher prevalence of 83 percent (Savolainen, Pietola, Kiukaanniemi, et al., 1997). A Finnish study observed a prevalence of 53 percent (Kuusela, Kurri, and Sirola, 1982).
Only four studies stated that interpretation of both the reference test and test of interest occurred under blinded conditions (Jensen and von Sydow, 1987; Rohr, Spector, Siegel et al., 1986; van Buchem, Peeters, Beaumont, et al., 1995; Williams, Simel, Roberts, et al., 1992). Four other studies described blinded interpretation of the test of interest, but investigators interpreting the reference test were not blinded to the results of the test of interest (Berg and Carenfelt, 1988; Laine, Maättä, Varonen, et al., 1998; Revonta, 1980; Savolainen, Pietola, Kiukaanniemi, et al., 1997). In the study by Laine, Maättä, Varonen, et al. (1998), interpretation of sinus aspiration results occurred with knowledge of results of radiography but not ultrasound.
Comparisons of Diagnostic Modalities
Sinus Radiograph Compared with Sinus Puncture
Six studies compared sinus radiographs with sinus puncture (Kuusela, Kurri, and Sirola, 1982; Laine, Maättä, Varonen, et al., 1998; McNeill, 1962; Revonta, 1980; Savolainen, Pietola, Kiukaanniemi, et al., 1997; van Buchem, Peeters, Beaumont, et al., 1995). The studies used a series of three or four radiographs, all including the occipitomental (Water's) view. Because three studies provided more than one comparison of test performance, there were 10 values of sensitivity and specificity available for analysis.
Results of the analysis are shown in Table 7. Figure 5 (Chapter 3) displays the 10 pairs of sensitivity and specificity and the SROC curve derived from the studies. The 10 data points appear to be well described by the curve. The random effects estimates are displayed in Figure 8 (Chapter 3). The area under the weighted (extrapolated) SROC curve was 0.83.
Table
Table 7. Comparison of sinus radiography to sinus puncture (all studies).
Shown in Figure 6 (Chapter 3) as dark gray ellipses are the five observations of sensitivity and specificity using the criterion "sinus fluid or opacity" to define positive radiographs. Shown as light gray ellipses are the three observations based on the criterion "sinus fluid or opacity or mucous membrane thickening" to define positive radiographs. The remaining two estimates are shown as white ellipses: one of these used the criterion "opacification of sinus," and the explicit criterion defining a positive radiograph was not available for one estimate.
Adding "mucous membrane thickening" as one of the criteria for a positive radiograph increased the sensitivity of radiographs and decreased their specificity. The random effects estimates for sensitivity and specificity, using "fluid or opacity" as the definition of a positive radiograph, were 0.76 (95 percent CI, 0.62-0.86) and 0.79 (95 percent CI, 0.63-0.89), respectively. With the definition of positive radiograph "sinus fluid or opacity or mucous membrane thickening," the estimates for sensitivity and specificity were 0.90 (95 percent CI, 0.68-0.97) and 0.61 (95 percent CI, 0.20-0.91), respectively. With positive radiographs restricted to "opacification of sinus," specificity increased only slightly to 0.85 (95 percent CI, 0.76-0.91), but sensitivity decreased dramatically to 0.41 (95 percent CI, 0.33-0.49).
Clinical Examination Compared with Sinus Puncture
A single study compared clinical examination with sinus puncture (Berg and Carenfelt, 1988). This study provided data for clinicians' overall impressions and also for a risk score derived from the number of findings present from the following four-item list: purulent rhinorrhea with unilateral predominance, local pain with unilateral predominance, bilateral purulent rhinorrhea, and presence of pus in the nasal cavity.
Table 8 shows the result of the analysis, and Figure 7 (Chapter 3) plots the five values of sensitivity and specificity derived from this report. The four-item risk score (shown with dark gray ellipses) appears to have better discrimination than the overall clinical impression (white ellipse); the SROC curve, fit only to the risk score thresholds, has an area under the weighted (extrapolated) SROC curve of 0.91.
Table
Table 8. Comparisons of clinical criteria to sinus puncture.
Unfortunately, the characteristics of this study throw into question its internal validity. First, the reference test, sinus puncture and aspiration, is poorly described in the report because it is not clear whether radiography was used in conjunction with aspiration in identifying those with sinusitis. Second, it is unclear how to use both "purulent rhinorrhea with unilateral predominance" and "bilateral purulent rhinorrhea" as independent risk score predictors of sinusitis.
Clinical Examination Compared with Sinus Radiograph
Three studies evaluated clinical examination in comparison with sinus radiographs. The Axelsson and Runze (1976) study compared an otolaryngologist's overall clinical impression with sinus radiography. The study by Jannert, Andreasson, Helin, et al. (1982) evaluated a clinical risk score for children in which individuals could have 0-3 of the following findings: purulent nasal secretions on examination, history of upper respiratory infection during the 2 weeks prior to presenting symptoms, and sinus pain or tenderness. The study by Williams, Simel, Roberts, et al. (1992) evaluated a clinical risk score for adults in which individuals could have 0-5 of the following findings: maxillary toothache, abnormal transillumination, poor response to decongestants, purulent secretions on examination, and colored nasal discharge by history. The Williams study also included data for overall clinical impressions of intermediate" or "high" probability of sinusitis. Therefore, there were 11 values of sensitivity and specificity available for analysis.
Table 9 and Figure 8 (Chapter 3) display these 11 values of sensitivity and specificity and the SROC curve. The data points are well-described by the SROC curve. The risk scores, shown as gray ellipses, appear to have similar discrimination to the overall impressions of clinicians, shown as white ellipses. The area under the (extrapolated) weighted SROC curve was 0.74.
Table
Table 9. Comparison of clinical criteria to sinus radiography.
Ultrasound Compared with Sinus Puncture or Radiograph
Five reports provided data comparing ultrasound of the sinuses with sinus aspiration (Kuusela, Kurri, and Sirola, 1982; Laine, Maättä;, Varonen, et al., 1998; Revonta, 1992; Savolainen, Pietola, Kiukaanniemi, et al., 1997; van Buchem, Peeters, Beaumont, et al., 1995). Reports provided data on more than one set of patients or for more than one diagnostic cutpoint, so there were 10 pairs of sensitivity and specificity data available for analysis (Table 10; Figure 9[Chapter 3]). Of note, these points do not appear well-described by the SROC curve, implying that variability in test performance is present. Ultrasound performance appeared to be poorest in the study by Laine and colleagues (shown as a white ellipse in Figure 9) (Laine, Maättä, Varonen, et al., 1998); this was the only study in which untrained primary care physicians performed and interpreted the ultrasounds.
Table
Table 10. Comparison of sinus ultrasonography to puncture (all studies).
Three reports (Berg and Carenfelt, 1988; Jensen and von Sydow, 1987; Rohr, Spector, Siegel, et al., 1986) provided data for five comparisons of ultrasound to sinus radiograph (Figure 10 [Chapter 3]). It is difficult to interpret these comparisons because the five data points fall close together in the SROC plot, and it is unclear how well the SROC curve describes them or how the SROC curve can be extrapolated.
Clinical Examination Compared with Ultrasound
A single study compared findings on clinical examination with ultrasound (van Duijn, Brouwer, and Lamberts, 1992). Although this study provided data on sensitivity and specificity of individual clinical symptoms and signs, it provided no data for an overall clinical impression or risk score. However, summarizing data from this study, a published meta-analysis reported a sensitivity of 0.36 (95 percent CI, 0.29-0.43) and specificity of 0.90 (95 percent CI, 0.85-0.93) for clinical examination compared with ultrasound (de Bock, Houwing-Duistermaat, Springer, et al., 1994).
Attachment C: Meta-Analysis of Antibiotic Trials
Meta-analyses were conducted to quantify the treatment effect of antibiotics compared with placebo and also the effects of amoxicillin or folate inhibitors compared with newer and more expensive antibiotics. This analysis is based on a meta-analysis (de Ferranti, Ioannidis, Lau et al., 1998) published in the British Medical Journal. The analysis was coauthored by Dr. Lau, (EPC director) and Dr. Barza (EPC technical expert) and was supported in part by an earlier AHCPR grant to Dr. Lau (R01 HS07782). Portions of this publication are used in this evidence report with permission from the British Medical Journal. The published meta-analysis was updated in this evidence report with one study not indexed in MEDLINE (Fiscella and Chow, 1991). Several additional sensitivity analyses were performed. The methodologies of the meta-analysis are described in Chapter 2 of this report.
Description of Studies and Populations of Subjects
Seventy-four randomized clinical trials on the antibiotic treatment of acute sinusitis were found (Evidence Table 9). Forty-eight trials did not evaluate drug comparisons pertinent to the meta-analysis; they mostly compared newer, extended-spectrum antibiotics (such as newer macrolides, quinolones, cephalosporins, or amoxicillin-clavulanate) with each other. Three more trials were rejected because the patients with acute bacterial rhinosinusitis were inextricably mixed with patients with other upper respiratory infections (Alvart, 1992; Falser, Mittermayer, and Weuta, 1988; Soderstrom, Blomberg, Christensen, et al., 1991), and two trials were rejected because they included patients with chronic and/or recurrent rhinosinusitis, without reporting separate outcomes for the acute bacterial rhinosinusitis group (Johnson and Foord, 1972; Podvinec, 1982). The remaining 28 trials (Evidence Tables 11-22) qualified for the meta-analysis: six trials were placebo-controlled (one of the amoxicillin studies also had a placebo arm), and 22 trials compared amoxicillin, trimethoprim-sulfamethoxazole, trimethoprim-sulfamethopyrazine, or brodimoprim with other antibiotics. One additional trial compared penicillin V with azithromycin; penicillin V is not as active in vitro as amoxicillin against Hemophilus influenzae and Moraxella catarrhalis, but since it was the largest of all trials identified (n=438) and of very good quality, we also performed sensitivity analyses including the results along with the amoxicillin comparisons (Bockmeyer, Riebenfeld, and Clasen, 1994). Among the included trials, sample size ranged from 14 to 323 patients. The mean ages of patients in the trials ranged from 25 to 44 years, except for two trials that evaluated pediatric patients exclusively (Wald, Chiponis, and Ledesma-Nedina, 1986; Wald, Reilly, Casselbrant, et al., 1984).
Eleven of the 28 trials were double-blind, six were single-blind (five investigator-blind), and 10 were unmasked. Thirteen of 28 trials used "firm" methods for diagnosing acute bacterial rhinosinusitis. The other 15 trials qualified patients as having acute bacterial rhinosinusitis on clinical grounds. Eight trials specifically required patients to use nasal decongestants, 3 trials allowed but did not require the use of decongestants, and the other 17 did not specify a protocol about the use of over-the-counter medications. The criteria for clinical outcomes were well-specified in 9 of the 28 trials, specified to some extent in 12 trials, and unclear in 7 trials. Bacteriologic evaluation was done using antral puncture only in three trials (Karma, Pukander, Penttila, et al., 1991; Matthews and Suprax/Amoxicillin Clinical Study Team, 1997; Wald,Reilly, Casselbrant, et al., 1984), and antral puncture or nasal swab was done in two trials (Felstead and Daniel, 1991; Mattucci, Levin, and Habib, 1986) included in the amoxicillin analysis. Two folate inhibitor trials and one amoxicillin trial seemingly only cultured nasal discharge, a method that is unreliable.
Some additional methodologic issues were noted. Casiano (1991) excluded all patients infected with resistant organisms from the analysis; the exclusions were not distributed evenly (azithromycin arm n=2; amoxicillin arm n=11). Karma, Pukander, Penttila, et al. (1991) and probably Matthews and Suprax/Amoxicillin Clinical Study Team (1997) also excluded patients with resistant organisms. A fourth trial (Edelstein, Avner, Chow, et al., 1993) changed the treatment to amoxicillin-clavulanate in all patients with resistant organisms but analyzed such patients according to intention-to-treat when they were in the amoxicillin arm, whereas it excluded such patients from the analysis when they were in the cefixime arm. Preferably, all patients should have been analyzed similarly. We performed sensitivity analyses excluding these four trials. Finally, three trials (Matthews and Suprax/Amoxicillin Clinical Study Team, 1997; Rimmer and Suprax/Amoxicillin Clinical Study Team, 1997; von Sydow, Savolainen, and Soderqvist, 1995) used several different approaches for defining the population to be analyzed including "per protocol," "evaluated patients" or "evaluative patients," and a "modified intention-to-treat" analysis (used for the meta-analysis).
Efficacy of Antibiotic Treatment Trials
Six studies compared any antibiotics against placebo (Axelsson, Chidekel, Greblius, et al., 1970; Ganaça and Trabulsi, 1973; Lindbaek, Hjortdahl, and Johnson, 1996a; Stalman, van Essen, van der Graaf, et al., 1997; van Buchem, Knottnerus, Schrijnemackers, et al., 1997; Wald, Chiponis, and Ledesma-Medina, 1986) (Table 11 and Figure 11 [Chapter 3]). Antibiotics were significantly more effective than placebo, reducing treatment failures by almost one-half. However, symptoms improved or were eliminated in 69 percent of patients without any antibiotic treatment (95 percent CI, 57-79) (Table 12). Although the observed heterogeneity did not reach statistical significance, one trial (Stalman, van Essen, van der Graaf, et al., 1997) that included patients simply on the basis of sinusitis-like symptoms without further diagnostic documentation had the highest cure or improvement rates in the placebo group (85 percent at 10 days) and showed absolutely no benefit from antibiotics, whereas trials with more tightly defined patient populations and lower spontaneous improvement rates showed a more clear benefit from antibiotics.
Table
Table 11. Risk ratio of clinical failures of any antibiotic vs. no antibiotic (meta-analysis performed using a random effects model).
Table
Table 12. Meta-analysis of trials with placebo arms: Cures and failures.
Radiographic and bacteriologic data were not available for many trials. Rates of radiographic failures within 48 hours of treatment completion were not significantly different between patients treated with other antibiotics and patients treated with amoxicillin or penicillin or folate inhibitors. Likewise, rates of bacteriologic failure were not significantly different between patients treated with newer antibiotics and those treated with amoxicillin or folate inhibitors, although the majority of samples were obtained using nasal swabs, and the data are therefore not reliable. There was no significant difference between regimens in the rate of withdrawal from treatment, either between other antibiotics and amoxicillin or between other antibiotics and folate inhibitors.
Fourteen trials compared newer antibiotics, most of which have an expanded spectrum of activity, with amoxicillin (Table 13 and Figure 12 [Chapter 3]). The pooled failure rate in patients treated with amoxicillin was low (11 percent; 95 percent CI, 8-14), and the further decrease in clinical failures with broad-spectrum antibiotics was not clinically important. Treating 100 patients with amoxicillin would lead to only 0.85 more failures (95 percent CI, 3.1 more to 1.4 fewer failures).
Table
Table 13. Risk ratio of clinical failures of amoxicillin vs. other antibiotics (meta-analysis performed using a random effects model).
Similar results were obtained when failure data of any antibiotic vs. folate inhibitors were analyzed (Table 14 and Figure 13 [Chapter 3]); treatment failures occurred at an essentially identical rate with other antibiotics as with folate inhibitors. However, data on folate inhibitors were limited and of poor quality. An analysis with respect to cures gave comparable results with risk ratios close to 1, both for any other antibiotic vs. amoxicillin or folate inhibitors (Table 15).
Table
Table 14. Risk ratio of clinical failures of folate inhibitors vs. other antibiotics (meta-analysis performed using a random effects model).
Table
Table 15. Meta-analysis of trials comparing newer, more expensive antibiotics with amoxicillin or folate inhibitors.
The risk differences of clinical cure using amoxicillin or folate inhibitors compared with those of other antibiotics were not clinically important (3.2 percent [95 percent CI, 1.5-7.8 percent], and 1.2 percent [95 percent CI, 10-12.4 percent], respectively). The results were comparable when a trial comparing penicillin with azithromycin was added to the amoxicillin comparisons: risk ratio for clinical cures is 1.05 (95 percent CI, 0.99-1.11); risk ratio for clinical failures is 0.82 (95 percent CI, 0.62-1.11).
There was no heterogeneity of treatment effects in the amoxicillin comparisons; however, there was some evidence of heterogeneity between the studies that compared folate inhibitors with other antibiotics (p=0.09 for clinical cures; p=0.18 for clinical failures), possibly because trimethoprim-sulfamethoxazole seemed less effective than pivampicillin/pivmecillinam in one study (Osman and Menday, 1983).
A number of sensitivity analyses showed that the results were similar when the selection of studies was restricted according to various criteria (Table 16). In all sensitivity analyses, there was a trend for an estimated 11 to 20 percent reduction in clinical failures with other antibiotics over amoxicillin, which did not reach formal statistical significance, possibly because of the limited number of patients. Still, this trend corresponded to a clinically negligible benefit (fewer than 1 failure averted per 100 patients). Sensitivity analyses were less informative for folate inhibitors because the data were very sparse.
Table
Table 16. Sensitivity and subgroup analyses for clinical failures.
Cumulative meta-analyses of studies ordered by decreasing Jadad quality score were also performed to explore the relationships between this quality scale and the magnitude of treatment effect to detect bias in poor quality studies. These analyses are presented in Figures 14, 15, and 16. No apparent trend of bias by studies with a low Jadad score (poor quality) was identified.

Figure
Figure 14. Cumulative meta-analysis of antibiotics vs. placebo.

Figure
Figure 15. Cumulative meta-analysis of amoxicillin vs. other antibiotics.

Figure
Figure 16. Cumulative meta-analysis of folate inhibitors vs. other antibiotics.
Attachment D. Decision and Cost-Effectiveness Analyses
As reported in the meta-analyses of antibiotic treatments for uncomplicated, community-acquired acute bacterial rhinosinusitis, antibiotics provide better clinical outcomes than does symptomatic treatment alone. However, given the uncertainty of bacterial infection in patients with suggestive symptoms, the need to weigh the benefits of treatment against the cost and side effects, and the high cost of many available diagnostic modalities, the management of these patients has been controversial.
We performed decision and cost-effectiveness analyses to estimate the effectiveness and cost-effectiveness of various diagnostic and treatment options that a clinician may take in managing a patient who presents with possible acute bacterial rhinosinusitis. The decision analyses are designed from the patients' perspective in that the outcome utilities used relate to the individual patient's quality of life or length of illness. The cost-effectiveness analyses include both the individual patient's perspective and the payer's perspective. The costs are those that the payer, such as a health management organization (HMO) bears in managing the disease. Thus, indirect costs, such as lost days of work, are not included.
Although the number of antibiotic prescriptions written to treat patients are estimated for the various strategies, no estimates could be made as to the direct effect on increasing bacterial antibiotic resistance that each strategy implies. Even though this is an important area of concern, no data are available on antibiotic use causing resistance in rhinosinusitis.
In addition, the question of recurrence of disease after treatment is important. However, few clinical trials report recurrence data. As extrapolation of the models would weaken the conclusions, the models addressed only the clinical management over a 2-week time horizon.
The models include results from meta-analyses conducted in this report, from individual studies, and from expert opinions and consensus. As the preponderance of studies included both pediatric and adult populations and did not report the data separately for the two populations, we designed the models and estimated variable values for the combined population of pediatric and adult patients.
General Methods
We performed two separate analyses. The first compares multiple diagnostic and treatment strategies and uses single time-point estimates of cure. The second focuses on four commonly used strategies and models the development of daily events.
In our first analysis, the multiple-strategies comparison model, we sought to compare all the currently available diagnostic and treatment strategies for acute bacterial rhinosinusitis management. The treatment strategies include symptomatic treatment alone and empiric antibiotic treatment. The diagnostic test strategies use various tests to determine treatment choice. These tests include sinus radiography, ultrasonography, computerized tomography (CT), magnetic resonance imaging (MRI), and the use of a specific set of clinical criteria to diagnose acute bacterial rhinosinusitis. These strategies are also compared with the generally accepted reference standard, sinus puncture and culture. Although sinus puncture is generally impractical and may be ill-advised, it is the only diagnostic method that accurately documents the presence of inflammation and bacteria in the maxillary sinuses.
The majority of studies available reported three possible patient outcomes (cure, improvement without cure, and no improvement) and gave only single time-point estimates (of generally 10 to 14 days) for cure and improvement proportions. Our first model corresponds to the majority of reported data by using these three outcomes, along with serious complications resulting from infection and the single time-point estimate of outcome proportions.
As this first model uses a single time point for clinical outcomes, it fails to consider the possibility that the primary difference in treatment choice is in the duration of symptoms rather than differences in outcome at a predefined time point. We therefore designed a second model, the symptom-duration model, using a Markov process (described below), that used daily cure rates to estimate the number of symptom-free days for each strategy. We focused on the commonly employed strategies of empiric treatment with antibiotics, deferment of antibiotic treatment, and determination of treatment option either by use of a set of clinical criteria or by use of sinus radiography.
We limited the antibiotics studied in our primary analyses to amoxicillin and folate-inhibitors (e.g., trimethoprim-sulfamethoxazole) as our meta-analyses showed that they are equally efficacious as the more costly antibiotics available. However, we also examined the effect on cost of using newer, more expensive antibiotics.
DecisionMaker 7.0TM(Sonnenberg, Pauker, Kassirer, 1990) was used to construct our decision models and MathSoft S-Plus 4.5TM (1997) was used to perform statistical calculations.
The methods and results of the first, multiple-strategies comparisons, model will be presented first, followed by the methods and results of the second, symptom-duration, model.
Multiple-Strategies Comparison Model
Methods/ Model Assumptions
The assumptions used in the model are listed below .
Patient Population/Disease
- Patients are seen by health care providers with symptoms suggestive of uncomplicated community-acquired acute bacterial rhinosinusitis.
- The patient's illness meets criteria for our meta-analyses: they have symptoms for more than 5 days and less than 4 weeks not due to a recurrence of rhinosinusitis. Patients, who are immunocompromised; have a malignancy, cystic fibrosis, or trauma; or had sinus-related surgery are not included.
- A certain percentage of the patients in the model have acute bacterial rhinosinusitis, as determined by the underlying prevalence of the disease; the remainder have a disease process other than bacterial infection such as viral infection or environmental allergies that is not responsive to antibiotic treatment.
- Patients have no known allergy to amoxicillin (penicillins) or folate inhibitors (sulfa drugs).
Treatment
- Patients are given symptomatic treatment only, are given amoxicillin empiricly, or have diagnostic testing done to select the appropriate treatment.
- All patients use over-the-counter and/or prescription symptomatic treatments such as decongestants.
- All the placebo trials allowed use of symptomatic treatment. Thus, the meta-analysis estimate of cure rates on placebo is equivalent to that of symptomatic treatment.
- Amoxicillin is equally efficacious and has a similar aggregate side effect profile (categorized as major and minor) as folate inhibitors and other antibiotics used for acute bacterial rhinosinusitis.
Diagnosis
- The test performances (sensitivity and specificity) for each test used to diagnose acute bacterial rhinosinusitis may be different.
- Patients receiving a sinus puncture have a risk of developing complications due to the procedure independent of whether they have acute bacterial rhinosinusitis. These complications include hemorrhage, orbital trauma, and facial cellulitis.
Antibiotic Side Effects
- Patients receiving amoxicillin assume a given risk of major and minor side effects. A major side effect, such as pruritic or urticarial rash, necessitates changing antibiotics. A minor side effect, such as minor gastrointestinal upset or vaginitis, does not necessitate changing antibiotics.
- Patients who develop a major side effect to amoxicillin are given a folate inhibitor as a replacement antibiotic.
- Patients who are switched to a folate inhibitor because of a side effect to amoxicillin do not develop an additional side effect to the replacement antibiotic. Their cure and improvement rates are determined by the relevant rates for folate inhibitors.
- Patients do not develop a side effect to any adjuvant medications they may take for their rhinosinusitis symptoms (such as decongestants).
Outcomes
- The majority of antibiotic trials reported cure rates at 10 to 14 days. Thus, outcomes are determined at 2 weeks from the decision point (time of initial office visit). This does not imply that antibiotic-treated patients are given a full 14 days of antibiotic treatment.
- Patients with acute bacterial rhinosinusitis may develop a complication due to the infection. These complications include facial osteomyelitis, facial cellulitis, orbital cellulitis and abscesses, subdural empyema, brain abscess, cavernous sinus thrombosis, and meningitis.
- Patients who do not develop an infection complication may be fully cured, improve incompletely, or have no improvement.
- The symptom resolution rate of all patients who do not have acute bacterial rhinosinusitis is the same and is independent of treatment.
- Quality of life over the 2-week period described by the model is affected by the occurrence of unfavorable events and outcomes, including the occurrence of a complication related to sinus puncture, a major or minor side effect, a complication related to disease, and cure, improvement, or no improvement.
- Each event or outcome affects the patient's quality of life.
- Costs of treatment, diagnostic tests, complications, side effects, and necessary followup are those incurred by the payer.
- Costs do not include indirect costs such as loss of income or additional costs of child care.
Model Description
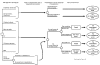
Shown in Figures 17 and 18 is the decision tree that depicts the comparison of the eight management strategies modeled in our analysis. The multiple-strategies comparison model decision tree models management strategies, the risk of complication due to sinus puncture, prevalence of rhinosinusitis, test performance, risk of treatment side effect, risk of disease complication, and probability of cure or improvement.
Figure
Figure 17. Comparison of possible strategies for managing
acute bacterial rhinosinusitis.
Note: CT - computerized tomography; MRI - magnetic
resonance imaging
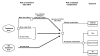
Figure
Figure 18. Subtree depicting treatment side effects, disease complication, and treatment outcomes.
The eight strategies are represented in Figure 17 by the branches off the square decision node to the far left of the diagram. Two of the strategies include no specific diagnostic workup: symptomatic treatment alone without antibiotics and empiric treatment with amoxicillin. The remaining six strategies include the use of diagnostic tests to determine treatment choice. Diagnostic tests include the application of a specific set of clinical criteria, sinus radiography,sinus ultrasonography, sinus CT, sinus MRI, and sinus puncture and culture.
For each strategy there are given probabilities of certain events occurring. These are represented by the circles, or chance nodes, at each subsequent branching. If a given strategy is applied to a cohort of 100 patients, the number of patients who move into each branch at a chance node is determined by the probability of an event occurring at that chance node. For example, if the probability of event A occurring is 15 percent, then 15 patients will move to the branch representing "event A," and the remaining 85 will move to the branch representing "not event A." The 15 patients in the event A branch may incur a cost related to event A and may have their quality of life affected by the occurrence of event A. If there is an "event B" chance node, these 15 patients will again divide into groups depending on the probability of event B occurring. It should be noted that the probability of event B occurring may be determined by whether event A occurred.
In our model, those patients who have the sinus puncture and culture strategy incur a risk of developing a complication due to the sinus puncture. This is represented by the chance node on the "sinus puncture" strategy branch in the lower left of Figure 17.
All patients in all strategies have a given likelihood of having true acute bacterial rhinosinusitis, represented by the prevalence of disease. Thus for all strategies, patients are divided at the "prevalence of acute bacterial rhinosinusitis" chance node into those with and without disease. All patients in the empiric antibiotic and symptomatic treatment strategies move on to the treatment and no treatment arms, shown in Figure 18.
Whether patients in the diagnostic test strategies receive treatment is determined by the outcome of the test (Figure 17). The test outcome, in turn, is determined by the sensitivity (measuring the proportion of patients with disease who have a positive test) and specificity (measuring the proportion of patients without disease who have a negative test) of the given test. Thus, in the model, the sensitivity chance node (which comes off the "acute bacterial rhinosinusitis" branch of the diagnostic tests) separates the true positive tests from the false negative tests. Likewise, the specificity chance node (coming off the "no acute bacterial rhinosinusitis" branch of the diagnostic tests) separates the false positive tests from the true negative tests. All patients with positive test results are treated with amoxicillin. All those with negative test results are not given amoxicillin.
As shown in Figure 18 by the chance node off the treatment arm, all patients who are treated may develop a major or minor side effect to the amoxicillin. If a patient develops a major side effect, the antibiotic is changed to a folate inhibitor. If the patient develops a minor side effect or no side effect, the antibiotic is not changed. Patients who develop a major or minor side effect incur monetary costs due to the side effect, and their quality of life is affected by the side effect. Patients who are not treated with amoxicillin do not develop a side effect to the drug.
All patients from each of the strategies, whether treated or not, and whether they develop a side effect or not, move to the final outcome portion of the tree, represented on the right of Figure 18. All patients with acute bacterial rhinosinusitis have a risk of developing a disease complication. The level of this risk is determined by whether they are being treated with antibiotics. A cost and adjustment to quality of life are incurred by the occurrence of a disease complication. Patients without acute bacterial rhinosinusitis have no risk of developing a disease complication.
All patients who do not develop a complication reach the final chance node to the right of Figure 18. They may be cured, improve, or not improve. The likelihood of a given patient moving into one of the final outcomes is determined by whether he or she has acute bacterial rhinosinusitis, and if so, by whether that patient is being treated with amoxicillin, a folate inhibitor, or no antibiotic. Again, each outcome is associated with a final cost and quality-of-life adjustment.
Data Used in the Analysis
The values used for the variables in the model, the range of values tested in the sensitivity analyses, and the sources of the values used are shown in Table 17. Separate data for pediatric patients are not available; however, the broad range of values used in the sensitivity analyses should include estimates applicable specifically to children.
Table
Table 17. Data for multiple strategies comparison model.
Probabilities
Prevalence
As discussed in meta-analysis of diagnostic test studies, most of the estimates of prevalence of acute bacterial rhinosinusitis in patients seen by providers with sinus symptoms are in the range of 50 percent (Kuusela, Kurri, and Sirola, 1982; Laine, Maättä, Varonen, et al., 1998;van Buchem, Peeters, Beaumont, et al., 1995). We therefore use this prevalence for our baseline estimates. The estimates are tested across the full range of prevalence (0-100 percent).
Complications due to sinus puncture
No data are available as to the complication rate of sinus puncture. We therefore used consensus opinion of our technical experts to arrive at an estimate of a 1 percent complication rate.
Diagnostic tests
We derived estimates of diagnostic test performance from various sources. The values of the sensitivity and specificity of each diagnostic test used are shown in Table 17. For each test we used one set of test performance estimates for the baseline case. For our sensitivity analyses, we tested an available estimate that would bias against the performance of the test (i.e., relatively low sensitivity and specificity, thus increasing the proportion of patients not given the appropriate treatment). We also tested the idealized situation where each test has perfect sensitivity and specificity.
For the clinical criteria strategy we used the approach taken in the one trial that compared clinical signs and symptoms with sinus puncture (Berg and Carenfelt, 1988 ). As described in meta-analysis of diagnostic test studies (clinical examination compared with sinus puncture), Berg's paper provides data from which we were able to derive the sensitivity and specificity for four-item risk scores calculated by the presence of: (1) purulent rhinorrhea with unilateral predominance, (2) local pain with unilateral predominance, (3) bilateral purulent rhinorrhea, and (4) pus in the nasal cavity.
We used a "Berg score" of three or more (three or four of the signs or symptoms are present) which allowed for a moderately high sensitivity and high specificity. For comparison, we used the test performance estimates derived from a study that compared the physician's "overall clinical impression" with sinus radiography (Axelsson and Runze, 1976), which had poorer test performance.
As discussed in meta-analysis of diagnostic test studies, data exist for estimates of sinus radiography sensitivity and specificity for various methods of reading the films. All the methods include a series of three or four views including the occipitomental (Water's) view. However, the definition of a positive radiograph can include "opacification of sinus" only, "sinus fluid or opacity," or "sinus fluid or opacity or mucous membrane thickening." For the baseline decision analysis, we chose the definition of a positive radiograph that yielded the highest sensitivity while allowing for a moderate specificity, namely, "sinus fluid or opacity or mucous membrane thickening." We also performed sensitivity analysis using the lower bounds of the 95 percent confidence interval limits of the random effects pooled results.
We used the random effects pooled estimates of test performance for sinus ultrasonography from our meta-analysis of diagnostic test studies. Most of the available data are from studies in which ultrasonograms were performed by otolaryngologists. The results of the ultrasonography study with the poorest test performance (Laine, Maättä, Varonen, et al., 1998) were used in the sensitivity analysis.
No trials are available that compare either sinus CT or MRI to sinus puncture. Thus, no data are available from the literature of these tests' sensitivity or specificity. We therefore used consensus opinion of our technical experts to arrive at estimates of CT and MRI test performances. The consensus was that CT and MRI have high sensitivity but lower specificity. Lower test performances were also tested in the sensitivity analysis.
Sinus puncture includes endoscopic evaluation of the maxillary sinuses, sampling of any fluid present, and bacteriologic culture and sensitivity of the aspirated fluid. This strategy in conjunction with clinical history and examination is the most reliable method of diagnosing acute bacterial rhinosinusitis. However, because of its invasive nature, it is not routinely used. We included it in our model to provide an assumed perfect reference standard for comparison. Thus, the sensitivity and specificity of sinus puncture and culture are set at 100 percent.
Antibiotic side effects
Reviews of the literature of penicillin allergy generally quote an incidence of reactions or side effects at between 1 and 10 percent (Lin, 1992). The three studies found that discussed allergic reactions to amoxicillin (or ampicillin) (Bigby, Jick, Jick, et al., 1986; Caldwell and Cluff, 1974; Saxon, Beall, Rohr, et al., 1987) all report a rate of cutaneous or more severe drug reactions of approximately 5 percent in hospitalized patients. We used an estimate of 5 percent for severe drug allergies requiring change of antibiotic and tested the estimate in a wide range of 0 to 20 percent.
No data were found estimating the rate of minor side effects, which do not require a change in antibiotic. By consensus expert opinion, we estimated the rate of minor side effect to be somewhat lower than that of major side effect, or 4 percent. This estimate was also tested across a wide range of 0 to 20 percent.
Disease complications
No explicit evidence exists about the rate of complications of community-acquired, acute bacterial rhinosinusitis. However, using Gwaltney's (1996) estimate of approximately 1 billion cases annually of viral rhinosinusitis and a middle estimate that 1 percent of these cases are complicated by acute bacterial infection (Berg, Carenfelt, Rystedt, et al., 1986; Dingle, Badger, and Jordan, 1964), we can estimate 10 million cases of acute bacterial rhinosinusitis annually. The 1994 National Hospital Discharge Survey reported approximately 5,000 or fewer cases of intracranial abcesses. We estimated that approximately 20 percent of these cases were a result of bacterial rhinosinusitis (Bradley and Shaw, 1983; Small and Dale,1984). Extrapolating from these data, we used an estimate of major complication rate due to acute bacterial rhinosinusitis of 1/10,000 cases. To further bias our model toward treatment with antibiotics (specifically to avoid complications), we used this estimate for the complication rate only in patients not receiving antibiotics. We assumed that patients treated with antibiotics are fully protected against complications (their complication rate is 0). Patients who do not in fact have acute bacterial rhinosinusitis cannot develop a complication due to the disease.
Cure rates
Cure and improvement rate estimates were derived from the antibiotic treatment meta-analyses. We derived cure rate estimates for treatment with amoxicillin and folate inhibitors from the meta-analyses of the relevant trials. Cure rate estimates for symptomatic treatment were derived from the meta-analysis of the placebo arms of relevant trials. The cure rates for antibiotic and symptomatic treatments were varied across the 95 percent confidence intervals of the meta-analyses estimates.
We used consensus estimates of 67 percent cure and 17 percent improvement for patients who are seen with symptoms of rhinosinusitis but do not, in fact, have acute bacterial rhinosinusitis.
Outcome utilities
An important issue in the management of acute bacterial rhinosinusitis is the quality of life of the symptomatic patient. We used an arbitrary utility scale from 0 to 1 to assign a quality-of-life value to each of the clinical outcomes. The scale ranged from the value of death (0) to the value of full health (1). Cure without any adverse outcomes at the end of 2 weeks was set to a value of 1. Intermediate health states such as "improvement," "major side effect," or "complication due to disease" were assigned values shown in Table 17. The values were chosen from expert consultation and after patient interviews. In cases where more than one health state applied to a patient (such as "improvement" and "major side effect"), the quality-adjustment values were multiplied together to arrive at the final utility. For example, if a patient had a sinus puncture complication (quality adjustment = 0.6), a minor antibiotic side effect (0.9), and was cured (1.0), that patient's final utility would be 0.6 X 0.9 X 1.0, or 0.54.
In our interviews with patients, we were told that the effect on quality of life of rhinosinusitis symptoms is likely to vary significantly both between patients and in the same patient between episodes of acute bacterial rhinosinusitis. Some episodes are accompanied by more severe symptoms than others thus resulting in lower quality of life. As our baseline estimates of quality adjustments for improvement and no improvement were set for relatively more severe symptoms (which bias the results toward empiric antibiotic treatment), we also tested a scenario where symptoms of disease are relatively mild (milder than antibiotic side-effect symptoms) that would bias the findings away from empiric antibiotic treatment.
Costs
Cost estimates used in our analyses are presented in Table 17. Costs for antibiotics were estimated drug costs (Cardinale, 1997), including the pharmacy handling charges. All patients will have an office visit and will use prescription or over-the-counter symptomatic therapies; no additional costs are accrued because of initial office visit or symptomatic treatment.
Costs of diagnostic tests were derived from maximum allowable reimbursements from a managed care company as of November 1996, including radiologists' fees. Applying clinical criteria as a decision tool for treatment has no additional cost. The cost of a sinus puncture includes the reimbursement cost of obtaining a culture and antimicrobial sensitivities of the fluid sample.
The cost of a major antibiotic side effect includes the reimbursement cost of an additional office visit, the cost of therapy to treat the side effect, and the cost of switching antibiotics. The cost of a minor antibiotic side effect includes the cost of therapy to treat the side effect.
The cost of sinus puncture complication and disease complication includes the total costs of hospitalization including surgery, intravenous antibiotics, and so on. The actual figure used is from consensus expert opinion.
Through consensus expert opinion, we estimated that only 50 percent of patients who improve return for an office visit; the rest either continue symptomatic treatment on their own or speak to their provider without an office visit. The cost of improvement, thus, includes 50 percent of the cost of a return office visit and the cost of additional over-the-counter symptomatic therapy. It is assumed that these patients will not require an additional course of antibiotics.
We assumed that all patients who did not improve would return for an office visit. Those patients who had not been treated initially (because they were in the symptomatic treatment cohort or had a negative diagnostic test) would be given a course of amoxicillin or folate inhibitor. Those who had been treated initially (because they were in the empiric treatment cohort or had a positive diagnostic test) would be given a course of a newer, more expensive antibiotic. The cost of no improvement, thus, includes the costs of a return office visit and the cost of a course of antibiotics. We varied costs of improvement and no improvement, as shown in Table 17, to estimate the effect of using different approaches toward managing patients whose symptom did not resolve.
In addition, we tested the model under the assumption that the first antibiotic given to the patient was a newer, more expensive choice than amoxicillin. However, under this scenario we used the symptom resolution and side effect proportions of amoxicillin.
Results
Base Case
The model estimates the costs and quality-adjusted outcomes at the end of a 10- to 14-day course of treatment of patients with suspected acute bacterial rhinosinusitis. The costs, probability of events, diagnostic test performances, quality-of-life adjustments, and their sources are shown in Table 17. The prevalence of acute bacterial rhinosinusitis in the baseline analysis is set at 50 percent.
The estimates of cost per patient, quality-of-life-adjusted outcome utility, and cost-effectiveness (cost per outcome value of 1 or healthy state) for each strategy are shown in Table 18.
Table
Table 18. Baseline estimates for multiple strategies comparison model.
Quality-Adjusted Outcome Utilities
As we set sinus puncture and culture to be our reference standard, with perfect discriminating ability between acute bacterial rhinosinusitis and other diagnoses, it is expected that puncture-guided treatment will have the greatest quality-adjusted outcome utility (of 0.89; see Table 18). All patients in the sinus puncture cohort were correctly identified as having acute bacterial rhinosinusitis or not. No patients without acute bacterial rhinosinusitis received unneeded antibiotics; thus, no unnecessary side effects occurred. In addition, all patients with acute bacterial rhinosinusitis were treated; thus, all patients responded to treatment as well as possible. The rate of complications due to the sinus puncture procedure was low and thus did not offset the benefit of perfect diagnosis that allowed for the most appropriate therapy for all patients.
As expected, the greater the performance of a given diagnostic test, the greater the quality-adjusted outcome utility. Thus, the order of most effective diagnostic tests (from highest to lowest) at our base prevalence rate of 50 percent was sinus puncture, MRI, CT, radiography, ultrasonography, and clinical criteria. At acute bacterial rhinosinusitis prevalence of 50 percent, empiric treatment with antibiotics was as effective as treatment guided by most of the diagnostic methods. Symptomatic treatment alone of all patients was least effective, as none of the 50 percent of patients with acute bacterial rhinosinusitis was cured as effectively as the patients would have been had they been given antibiotics.
It should be noted, as discussed below, the relative effectiveness of the strategies varies depending on the cohort's prevalence of acute bacterial rhinosinusitis. In addition, all the outcome utilities (as measures of effectiveness) lie within a narrow range of values that is equivalent to being somewhat better than having improved but not been fully cured (utility = 0.8).
Cost per patient
At a 50 percent prevalence of bacterial rhinosinusitis, symptomatic treatment alone is the least costly strategy (see Table 18), with treatment guided by clinical criteria being less than $1.00 more costly. At this prevalence, the cost of treating the majority of patients with rhinosinusitis and some without (as dictated by the clinical criteria) approximately balances the cost of followup for the relatively high percentage of symptomatically treated patients whose symptoms fail to resolve. The additional cost of treating all patients (in the empiric treatment strategy) is greater than the savings of preventing the followup costs of the untreated patients who fail to resolve (in the symptomatic treatment and clinical criteria strategies).
As all the diagnostic procedures are fairly costly, the total cost per patient for each of the diagnostic procedure strategies was significantly more expensive than for the other strategies. The costs per patient were in direct relation to the cost of the diagnostic procedure performed.
Cost-effectiveness
The cost-effectiveness (cost divided by outcome utility, or effectiveness) allows us to determine the value of choosing each strategy, accounting for both the cost and effectiveness of each strategy. For each strategy, the cost (in dollars) per cure (utility = 1) is estimated (see Table 18). Clinical criteria-guided treatment was the most cost-effective, followed closely by symptomatic treatment alone. As the outcome utility for empiric antibiotic treatment was very similar to that of clinical criteria although the cost was greater, empiric antibiotic treatment was less cost effective.
The cost per healthy outcome of all the other diagnostic tests was much greater in direct relation to the cost of the diagnostic test.
Marginal cost-effectiveness
The costs and effectiveness of the strategies compared are graphically represented in Figure 19. The marginal cost-effectiveness of one strategy over another is the additional cost of the first strategy that achieves an additional quality-adjusted outcome of 1 over the second strategy [(Cost Strategy A - Cost Strategy B) / (Effectiveness Strategy A - Effectiveness Strategy B)]. In Figure 19, the more cost-effective strategies will be toward the upper left corner of the figure, having greater outcome utility and lower costs. The marginal cost-effectiveness between any two strategies is the inverse of the slope connecting the two points representing the strategies in the graph above. Only additional costs per additional utility are considered; thus the first strategy being considered must be to the upper right of the second, with greater cost and greater utility. Strategies that are more costly and less effective are "eliminated by strict dominance."

Figure
Figure 19. Treatment cost and effectiveness of each
strategy in the multiple-strategies comparison model.
Note: CT - computerized tomography; MRI -
magnetic resonance imaging
As presented in Table 18, symptomatic treatment is the least costly strategy at a prevalence of 50 percent. The marginal cost-effectiveness of clinical criteria-guided treatment is $18; thus, by using clinical criteria instead of symptomatic treatment, it would cost an additional $18 per patient to achieve one additional healthy outcome (outcome utility of 1). Empiric treatment, however, would require an additional cost of $3,677, and sinus puncture-guided treatment would require $35,865 beyond that to achieve an additional healthy outcome.
Risk profile
Presented in Table 19 are estimates of percentage of patients that would be cured, improve, not improve, and/or suffer a complication due to rhinosinusitis for each of the strategies studied. These estimates are shown for three different prevalences of acute bacterial rhinosinusitis: 25, 50, and 75 percent. In addition, for each prevalence, Table 19 shows the percentage of patients in each cohort given an antibiotic prescription, the number of prescriptions for amoxicillin written per case of real acute bacterial rhinosinusitis, the percentage of all patients who receive a prescription inappropriately (because they do not have acute bacterial rhinosinusitis), and the percentage of all patients who do not receive a prescription inappropriately (because they do have acute bacterial rhinosinusitis). For comparison purposes, the cost per patient of each strategy (including the costs of side effects, complications, and incomplete cures) is shown.
Table
Table 19. Risk profile of multiple strategies comparison model.
Symptomatic treatment alone (withholding antibiotics for all patients) is inferior to all other strategies at all prevalences shown in terms of cure rate, complication rate, and, by definition, percentage of patients with disease who are left untreated. All other strategies have very similar cure and complication rates, and all others but the idealized sinus puncture-guided treatment have similar numbers of prescriptions written per true case of acute bacterial rhinosinusitis and similar numbers of unnecessary prescriptions written for patients without acute bacterial rhinosinusitis.
Sensitivity Analysis of Prevalence
Cure and complication rates for cohorts of patients with different underlying prevalences of acute bacterial rhinosinusitis will differ; for example, those cohorts with a high prevalence of acute bacterial rhinosinusitis will respond much more effectively to antibiotic treatment than those with a low prevalence. To fully evaluate the relative costs, effectiveness, and cost-effectiveness of the various strategies, we performed sensitivity analyses of the prevalence wherein we adjusted the acute bacterial rhinosinusitis prevalence from 0 to 100 percent and determined estimates at various levels.
The results of the prevalence sensitivity analyses for the multiple-strategies comparison model are shown in Figures 20, 21, 22, and 23.

Figure
Figure 20. Expected utilities of strategies in the
multiple-strategies comparison model.
Note: CT - computerized tomography; MRI - magnetic
resonance imaging; TX - treatment

Figure
Figure 21. Average cost per treatment in the
multiple-strategies comparison model.
Note: CT - computerized tomography; MRI - magnetic
resonance imaging; TX - treatment

Figure
Figure 22. Expanding the scale of Figure 21 to highlight strategies of
interest.
Note: TX - treatment

Figure
Figure 23. Cost-effectiveness of strategies in the
multiple-strategies comparison model.
Note: TX - treatment
Effectiveness
The effects of the various strategies across the full range of acute bacterial rhinosinusitis are shown in Figure 20. As it correctly diagnoses acute bacterial rhinosinusitis all the time, sinus puncture and culture avoids unnecessary treatment and maximizes appropriate treatment; it, thus, is always most effective. The effectiveness of the diagnostic test-guided treatment strategies are directly related to the test performance (sensitivity and specificity) of each test. As the test performances modeled are generally fairly high, the effectiveness of the tests is relatively high and stable across the range of prevalence.
Because of the increasing failure rate with symptomatic treatment, its effectiveness falls rapidly with increasing acute bacterial rhinosinusitis prevalence. The effectiveness of empiric antibiotic therapy rises with increasing prevalence, as the proportion of patients benefitting from antibiotic treatment rises. At low prevalence, the value of the empiric antibiotic therapy is low, but the rate of unnecessary side effects is high compared with that of the other strategies. As prevalence increases, the value of empiric treatment rises until it is equal to that of sinus puncture-guided treatment at 100 percent prevalence where all patients are being treated by both strategies.
Cost per treatment
As shown in Figure 21 (average cost per treatment), the costs for strategies involving diagnostic procedures (those lines above $100/treatment) do not vary considerably with acute bacterial rhinosinusitis prevalence. This is a result of the high costs of these procedures relative to the costs of further workup and treatment of the few patients who do not improve with empiric treatment.
Figure 22, an expanded version of Figure 21, reveals that the relative costs of empiric treatment, symptomatic treatment, and treatment guided by a set of clinical criteria differ depending on the underlying prevalence of acute bacterial rhinosinusitis in the cohort of patients. Symptomatic treatment and clinical criteria-guided treatment are very close to each other in cost across the full range of prevalence. Symptomatic treatment is marginally less costly at a prevalence lower than 66 percent; clinical criteria-guided treatment is less costly at a higher prevalence. The cost of empiric treatment is relatively flat across prevalence. Only at very high prevalence (>99 percent) is empiric treatment less costly than clinical criteria.
Cost-effectiveness
Figure 23 shows the relative cost-effectiveness for the four least costly strategies (symptomatic, clinical criteria-guided, empiric, and radiography-guided treatment). As in the graph of cost across prevalence (Figure 22), symptomatic treatment and clinical criteria-guided treatment are similar across the range of prevalence. Clinical criteria-guided treatment is the most cost-effective strategy when prevalence of acute bacterial rhinosinusitis is between 41 and 95 percent.
Below a prevalence of 41 percent, symptomatic treatment is most cost effective; and at a prevalence greater than 95 percent, empiric treatment is most cost effective. At a low prevalence, most patients' symptoms will respond spontaneously; thus, antibiotic treatment can be deferred and still achieve good average outcome and minimal cost of followup for patients whose symptoms fail to resolve. At very high prevalence, the cost of not treating those patients with acute rhinosinusitis who have negative clinical criteria is greater than the cost of treating everyone, and the outcome is poorer. Therefore, empiric treatment is most cost effective at very high prevalence.
Further Sensitivity Analyses
Since the values of outcome probabilities, test performances, costs, and outcome utilities used in the model are only single estimates of their true values, and in fact, the true values may vary in different settings, a determination of the effect on the findings of using different estimates is necessary. We thus performed sensitivity analyses to determine whether the results are sensitive to (i.e., change substantially with) variation of the values of the estimates used in the original analysis. For the multiple-strategies comparison model, we limited sensitivity analysis to prevalence and test performance. The values tested are shown in Table 17. Further sensitivity analyses are performed in the symptom-duration model below.
We varied the sensitivity and specificity of each diagnostic test to check the sensitivity of the model to different estimates. For each diagnostic test, we tested a low estimate of test performance (as described in the "data used for analysis, diagnostic tests" section, above, and Table 17) and the high estimate of perfect test performance (sensitivity and specificity both equal to 1).
Table 20 shows the effect on cost, effectiveness, and cost-effectiveness of changing the test performances from low sensitivity and specificity to base case sensitivity and from specificity to perfect sensitivity and specificity at an acute bacterial rhinosinusitis prevalence of 50 percent.
Table
Table 20. Sensitivity analysis of diagnostic test performance in multiple strategies comparison model.
For all the diagnostic tests, the effectiveness and total cost of the strategies are fairly stable across the range of test performances tested. The cost-effectiveness of the diagnostic tests with an up-front cost of the test (e.g., radiography) are also fairly stable and at no test performance level approach the cost-effectiveness of the strategies without up-front test costs (symptomatic, empiric, and clinical criteria). The cost-effectiveness of clinical criteria, however, does vary in a range across that of symptomatic treatment.
We, therefore, tested a two-way sensitivity analysis of clinical criteria test performance and rhinosinusitis prevalence to compare the cost-effectiveness of the three low-cost strategies. If clinical criteria had perfect test performance, and thus were perfectly discriminatory between acute bacterial rhinosinusitis and other disease, then clearly this would be the most cost-effective strategy across the range of prevalence. If, however, the test performance of clinical criteria was worse than our base case assumption (with a sensitivity and specificity of 0.70), then the range in which clinical criteria are more cost-effective narrows from 41 to 95 percent (base case) to 66 to 80 percent.
Symptom-Duration Model (Markov Model)
Methods/Model Assumptions
The assumptions used in the model are listed below.
Patient Population/Disease
- Patients are seen by health care providers with symptoms suggestive of uncomplicated possible acute community-acquired bacterial rhinosinusitis.
- The patient's illness meets criteria for our meta-analyses: They have symptoms for more than 5 days and less than 4 weeks and not as a result of a recurrence of rhinosinusitis. Patients who are immunocompromised, have a malignancy, cystic fibrosis, or trauma, or have had sinus-related surgery are not included.
- A proportion of the patients in the model have true acute bacterial rhinosinusitis represented by the prevalence of the disease. The remaining patients have a disease process other than acute bacterial rhinosinusitis such as viral infection or environmental allergies that is not responsive to antibiotic treatment.
- Patients have no known allergy to amoxicillin (penicillins) or folate inhibitors.
Treatment
- Patients are given symptomatic treatment only, are given amoxicillin empirically, have a set of clinical criteria applied to determine treatment choice, or have a sinus radiograph done to determine treatment choice
- All patients use over-the-counter and/or prescription treatment for symptoms such as decongestants.
- All the placebo trials allowed use of symptomatic treatment. Thus, the estimates of cure rate on placebo are equivalent to symptomatic treatment
- Amoxicillin is equally efficacious and has a similar aggregate side effect profile (categorized as major and minor) as folate inhibitors and other antibiotics used for acute bacterial rhinosinusitis.
Diagnosis
Clinical criteria and sinus radiography have a sensitivity and specificity for diagnosing acute bacterial rhinosinusitis obtained from our meta-analyses of diagnostic test studies.
Antibiotic Side Effects
- Patients receiving amoxicillin assume a given risk of all side effects such as dermatitis or gastrointestinal upset. Antibiotic side effects may require change of antibiotics (to a folate inhibitor) but do not alter the cure rate of rhinosinusitis. Our basis for this assumption is that 2-week cure rates with both amoxicillin and folate inhibitors are similar, as shown in Table 17.
- The daily risk of antibiotic side effects remains constant for the 14-day course of treatment.
- Side-effect symptoms last for 2 days only and can occur only once during the 14-day course. Patients who are switched to an alternative antibiotic because of a major side effect do not develop an additional side effect to the replacement antibiotic.
- Side effects minor enough not to necessitate discontinuation of amoxicillin are not included.
- Patients do not develop side effects to any adjuvant medications they may take for their rhinosinusitis symptoms (such as decongestants).
Outcomes
- Patients with acute bacterial rhinosinusitis may develop a complication due to the infection. These complications include facial osteomyelitis, facial cellulitis, orbital cellulitis and abscesses, subdural empyema, brain abscess, cavernous sinus thrombosis, and meningitis.
- On any given day during the 14-day course, patients who are sick may be fully cured or may remain sick. The intermediate state of "improvement" used in the first model is not included. The proportion of patients cured on any given day varies according to data estimated in the literature and from our models.
- Once resolved, a patient's symptoms do not relapse during the 14-day course.
- The cure rate of all patients who do not have acute bacterial rhinosinusitis is the same and is independent of antibiotic treatment.
- The model estimates number of symptom-free days (free of symptoms due to rhinosinusitis or to side effects) and quality-adjusted days (adjusted for severity of rhinosinusitis symptoms) over a 14-day course.
- Measures of effectiveness (symptom-free days or quality-adjusted days) are from the individual patient's perspective.
- Costs are determined by the costs of antibiotic treatment, sinus radiography, treatment of side effects, and followup determined by the final outcome (cured or sick) on day 14 that are incurred by the payer.
- Costs do not include indirect costs such as loss of income or additional costs of child care.
Model Description
Figure 24 and Figure 25 show the symptom-duration model. The model depicts the comparison of four management strategies: withholding antibiotics and treating symptoms of rhinosinusitis only, empiric treatment of all patients with amoxicillin, use of a set of clinical criteria to determine choice of treatment option, and immediate sinus radiograph to determine choice of treatment option. The symptom-duration model uses a Markov decision tree and models the management strategies, prevalence of rhinosinusitis, sinus radiography test performance, risk of disease complication, risk of treatment side effect, and probability of cure.
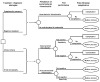
Figure
Figure 24. Markov decision Model.
Note: For Markov process, see Figure 25

Figure
Figure 25. Markov model depicting allowable transition states
per cycle.
Note: w/ - with; SE - antibiotic side effect
Patients with symptoms suggestive of acute bacterial rhinosinusitis are managed by one of the four strategies shown in the left-hand column. The prevalence of acute bacterial rhinosinusitis is modeled in the next column to the right, followed by the test performance for clinical criteria and sinus radiography-guided treatment in the third column. Patients with a positive test result receive antibiotic treatment, whereas those with a negative test result receive symptomatic treatment only. The likelihood of treatment is thus determined by the sensitivity and specificity of the tests.
As illustrated in the right-most column, patients who truly have acute bacterial rhinosinusitis may develop a serious complication due to the disease, such as orbital cellulitis or intracranial abscess. The risk of developing a complication is dependent on whether the patient is given antibiotic treatment. For all patients who do not have a disease complication, the resolution of rhinosinusitis symptoms and the development and resolution of antibiotic side effects are modeled in the Markov process (Figure 25).
Markov Process
The Markov process is a recursive process where patients' health states change during each cycle of the process. Each cycle can represent a given length of time (in our model, a day) and the cycles can be repeated for a set number of times (in our model, for 14 days) or until no further changes in health state occur.
In our model, potential initial states of health for any given day are shown by the ovals in the top row. A cohort of patients enters the model on the first day in the initial state of health (in our model, sick without antibiotic side-effect symptoms). In each cycle of the model, a probability exists of transition from one health state to another (for example, from sick without side-effect symptoms to sick with side-effect symptoms or to cure without side-effect symptoms). The possible health states at the end of each cycle are represented in the bottom row. In our model, the initial and final health states for each cycle are the same. The possible transitions that can be made from initial to final health state in each cycle are represented by the arrows. The proportion of patients moving from a given initial health state to a final health state in each cycle is determined by the probabilities of various events occurring during the cycle (in our model, the probability of cure and of side effect). These probabilities of transitions in health state can vary from cycle to cycle.
For the cohorts of patients who do not develop a disease complication, the possible daily health states in our model, shown in each row of Figure 25, are: sick without side-effect symptoms, sick on the first day of side-effect symptoms, sick on the second day of side-effect symptoms, sick after having had side-effect symptoms, cured without side-effect symptoms, cured on the first day of side-effect symptoms, cured on the second day of side-effect symptoms, and cured after having had side-effect symptoms. The multiple health states involving side-effect symptoms exist to account for the assumptions that side effects last 2 days and can occur only once per course.
Transition possibilities are limited by the model assumptions. The entire cohort of patients start in the "sick, no side effect" state. During the first cycle, they have a probability of remaining sick without antibiotic side effect, remaining sick and starting the first day of side-effect symptoms, being cured with no side effect, and being cured but starting the first day of side-effect symptoms.
In subsequent cycles, the following possibilities can occur:
- Sick patients may be cured.
- Patients who currently have had no side effects may enter the first day of side effects.
- Patients currently in the first day of side effects must enter the second day of side effects (though those who are sick may simultaneously be cured).
- Patients currently in the second day of side effects must enter the sick or cured "post-side-effect" state (in which they no longer have side-effect symptoms).
- Patients currently in a post-side-effect state (sick or cured) remain in a post-side-effect state (sick patients may simultaneously be cured).
- Cured patients remain in a cured health state.
Probabilities
Prevalence
As for the multiple-strategies comparison model, we used a prevalence of 50 percent. We varied the prevalence from 0 to 100 percent in the sensitivity analysis.
Diagnostic Test
Sinus radiograph
We used the same estimates for radiography test performance that were used in the multiple-strategies comparison model. These assumed that a sinus radiograph is read as positive for acute bacterial rhinosinusitis if there is "sinus fluid or opacity of mucous membrane thickening." For the sensitivity analysis, we used the lower bounds of the 95 percent confidence interval limits of the random effects pooled results and the idealized situation of perfect sensitivity and specificity.
Clinical criteria
We used the same estimates for test performance of applying a set of clinical criteria to diagnose acute rhinosinusitis that was used in the multiple-strategies comparison model. These used a "Berg score" of three or more as described above (Berg and Carenfelt, 1988). For sensitivity analysis, we used an estimate of lower test performance from expert opinion.
Disease Complication
As in the first model, we assumed a high disease complication rate of 1/10,000 for patients not treated with antibiotic. To further bias the model toward treatment to prevent complications, we assumed a disease complication rate of 0 for patients treated.
Antibiotic Side Effects
As in the first model, we used an estimate of 5 percent for drug allergies for the entire 14-day course. As we assumed that the risk of side effects was constant during the whole course of treatment, the daily risk of side effect was 0.35 percent. In the sensitivity analysis, the risk of side effect was varied from 0 to 20 percent (or a daily risk of 0 to 1.3 percent).
Cure Rates (Acute Bacterial Rhinosinusitis)
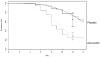
For the Markov process, we required daily cure rates. Only one study (Lindbaek, Hjortdahl, and Johnsen, 1996a) provided near daily data for both amoxicillin and placebo. We fit the available Kaplan-Meier curves to Weibull functions (having the form y = eλ·d α, where y is the proportion remaining sick on day d and λ and α are constants that describe the curvature of the exponential function) to estimate the daily cure rates. The Weibull function is a generalization of the exponential function that is commonly used for failure time data or survival curves (Kalbfleisch and Prentice, 1980). The Weibull function allows for an early period of time when cure rates are low, followed by more rapid cure. The Kaplan-Meier curves for treatment with amoxicillin and placebo from the Lindbaek, Hjortdahl, and Johnsen (1996a) study are shown in Figure 26 with their respective fitted Weibull curves. For comparison, the point estimates and 95 percent confidence intervals for the proportion of patients cured from our meta-analyses of amoxicillin and placebo are included.
To test the stability of the conclusions under varying assumptions, sensitivity analyses of the daily cure rates were performed using Weibull curves fit to the upper and lower 95 percent confidence intervals of the Lindbaek Kaplan-Meier survival curves.
In addition, we ran the model with different assumptions as to the form of the cure-rate curve. We tested a linear function, y = 1-m·d (where m is the slope of the line). From each trial with an amoxicillin or placebo arm, we fit a line to the proportion cured on the final day reported. The slope, m, is the weighted average (by trial size) of the slopes of the fitted lines.
We also tested an exponential curve,y = e -µ·d (where µ describes the slope of the curve). Again, µ is a weighted average of the µ values from fitting an exponential curve to the final day data point from each of the studies.
The values of fitted λ, α, m, and µ are shown in Table 22. Estimates of proportion of patients cured on certain days for each of the fitted curves are presented in Table 21.
Table
Table 22. Coefficient values for symptom duration models.
Cure Rates (Nonacute Bacterial Rhinosinusitis)
From consensus opinion, we used a cure proportion of 50 percent at day 5 and 75 percent at day 10 for our baseline analyses, which is consistent with the estimate of the day-14 proportion of patients cured or improved used in the first model. Estimates of the proportion of patients cured on various days for each of the fitted curves are presented in Table 21. Values of the constants for Weibull, linear and exponential models, derived in the same manner as above, are shown in Table 22.
Outcome Utilities
In the baseline symptom-duration model, all days with symptoms (whether due to rhinosinusitis or to antibiotic side effect) and disease complication were treated equivalently. Each day that a patient was in the cured state without side-effect symptoms has a value of one (1); those who were either sick or had side-effect symptoms on that day had a value of zero (0). Patients who developed a disease complication were assigned a value of zero (0) for the whole 2 weeks. Thus the assumption was made that the quality of life on all days with rhinosinusitis symptoms prior to cure was equally as undesirable as a day with disease complication, rhinosinusitis symptoms, and antibiotic side-effect symptoms or side-effect symptoms alone.
To adjust for the differences in quality of life with varying degree of symptoms, we assigned quality-of-life adjustments to the various symptom days (as shown in Table 21). We calculated quality adjustments for "mild," "moderate," and "severe" symptoms due to rhinosinusitis:
- Mild symptoms reduce quality of life (to a value of 0.75) to a lesser degree than do symptoms of a major antibiotic side effect (0.7).
- Moderate symptoms reduce quality of life to the same degree as having no improvement in the multiple-strategies comparison model (to a value of 0.5).
- Severe symptoms reduce quality of life to a greater degree (to a value of 0.25) but to a lesser degree than disease complication.
- The quality adjustment for side-effect symptoms is that of major side effects (0.7).
- The quality adjustment for disease complication is set at zero (0) to maximally bias the conclusions toward treatment to avoid disease complications.
At the conclusion of each cycle, the percentage of patients in each health state is multiplied by the outcome utility associated with that state and the values are summed across the various health states (e.g., 50% X 0.5 [for Sick No Side Effect] + 5% X 0.5 X 0.7 [for Sick with Side Effect] + 45% X 1 X 0.7 [for Cured No Side Effect] + 0% X 1 X 0.7 [for Cured with Side Effect] = 0.5825). In the model, this number represents the average quality of life of patients on a given day. In the symptom-free-day scenario, it represents the proportion of patients on a given day who are symptom-free. The daily proportions are then summed together across all days. This final outcome utility represents the number of quality-adjusted healthy days or the average number of symptom-free days during the 14 days that the given strategy implies. When symptom-free days are calculated, the average number of days to symptom-free state is simply 14 minus the number of symptom-free days.
Costs
The costs of amoxicillin treatment, symptomatic treatment, and sinus radiograph are the same as described in the multiple-strategies comparison model.
The total cost of an antibiotic side effect is the sum of the costs of treatment for the side effect and of switching to a folate-inhibitor. As the side-effect symptoms are assumed to last for 2 days, the daily cost is one-half the total cost.
The cost of remaining sick at the end of the 14-day course varies depending on the strategy. For patients treated symptomatically, the cost of remaining sick included the cost of a repeat office visit and treatment with amoxicillin. For patients treated empirically, the cost of remaining sick included the cost of a repeat office visit and treatment with a newer, more expensive antibiotic. For patients whose treatment depended on the results of application of clinical criteria or sinus radiography, the costs of remaining sick include the cost of a repeat office visit and treatment with amoxicillin if their diagnostic test had been negative (and thus the patient had been treated symptomatically) or with a newer, more expensive antibiotic if their diagnostic test had been positive (and thus the patient had been treated with amoxicillin). These cost estimates are listed in Table 21.
The cost of disease complication includes the total costs of hospitalization including surgery, intravenous antibiotics, and so on. The actual figure used is from consensus expert opinion.
Results
Base Case
The model estimates the costs and expected number of symptom-free days over a 14-day course after initial evaluation by a health care provider. We also estimated the number of quality-adjusted days over a 14-day course for mild, moderate, and severe rhinosinusitis symptoms. The costs, prevalence of acute bacterial rhinosinusitis, probability of side effect due to antibiotic, the sensitivity and specificity of radiography and clinical criteria, cure rates, and outcome utilities are shown in Table 21.
The estimates of cost per patient, number of symptom-free days, and cost per symptom-free day (cost-effectiveness) at the base prevalence of 50 percent are shown in Table 23 for each strategy. The estimates of cost per patient, number of quality-adjusted days, and cost per quality-adjusted day (cost-effectiveness) at the base prevalence of 50 percent are shown in Table 24 for each strategy and for each of the three rhinosinusitis severity levels tested (mild, moderate, and severe).
Table
Table 23. Baseline estimates for symptom-duration model, symptom-free days.
Table
Table 24. Baseline Estimates for symptom-duration model, quality-adjusted symptom days.
Effectiveness
Whether symptom-free days or quality-adjusted days with mild, moderate, or severe symptoms are estimated, the effectiveness of empiric antibiotic treatment, clinical criteria-guided treatment, and radiography-guided treatment were very similar. These strategies outperform symptomatic treatment because of the latter's high rate of failure to cure. However, in the scenario of mild rhinosinusitis symptoms (where rhinosinusitis symptoms are milder than symptoms of antibiotic side effect) all strategies yield similar estimates of effectiveness.
Cost per patient
At a 50 percent acute bacterial rhinosinusitis prevalence, the cost per patient seen was least for those given symptomatic treatment alone. Because of the cost of amoxicillin for all patients, empiric treatment was somewhat more costly. The cost of clinical criteria-guided treatment was only slightly higher than symptomatic treatment. It was less costly than empiric treatment because the cost of "excess" amoxicillin given to all patients without acute bacterial rhinosinusitis was greater than the cost of the few patients with acute bacterial rhinosinusitis left untreated because of inaccurate clinical criteria. Because of its high cost compared with amoxicillin, sinus radiography-guided treatment is by far the costliest strategy. (Note that the cost per patient is independent of the outcome utility or the severity of disease symptoms.)
Cost-effectiveness
At the given prevalence of 50 percent, clinical criteria-guided treatment is the most cost-effective strategy in all the given scenarios. This strategy incurs no additional up-front cost, yields fewer treatment failures and disease complications than symptomatic treatment alone, yet avoids the additional cost and lessened effectiveness due to additional antibiotic side effects in patients who do not have acute bacterial rhinosinusitis. However, as the severity of a patient's rhinosinusitis symptoms lessen, the cost-effectiveness of symptomatic treatment alone improves, such that with mild and moderate disease symptoms the cost-effectiveness of symptomatic treatment and clinical criteria-guided treatment are very similar.
Marginal cost-effectiveness
The cost and effectiveness for each strategy for the symptom-free-days scenario are shown in Figure 27 and Table 23. The marginal cost-effectiveness for the other scenarios is shown in Table 24.

Figure
Figure 27. Cost and effectiveness of strategies in
the symptom-duration model.
Note: Effectiveness measured in symptom-free
days.
Although for each scenario symptomatic treatment is the least costly, it is also the least effective. As the cost of clinical criteria-guided treatment is only slightly greater than symptomatic treatment and is more effective, the marginal cost-effectiveness (the additional cost required of the second strategy compared with the first to achieve an additional unit of effectiveness) is small. Empiric antibiotic use, on the other hand, achieves greater effectiveness but at a much greater cost.
As radiography-guided treatment is both less effective than symptomatic treatment and more costly, it is eliminated by strict dominance.
The more severe the rhinosinusitis symptoms, the smaller the marginal cost-effectiveness of both clinical criteria-guided treatment and empiric treatment.
Sensitivity Analysis of Prevalence and Disease Severity
The results of the prevalence sensitivity analysis for the symptom-duration model for the symptom-free-days scenario are shown in Figures 28 to 30.
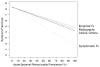
Figure
Figure 28. Symptom-free days over a 14-day course in the
symptom-duration model.
Note: Tx = treatment
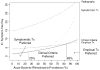
Figure
Figure 30. Cost per symptom-free day of strategies in the
symptom-duration model.
Note: Tx - treatment
Effectiveness
At low acute bacterial rhinosinusitis prevalence, the effectiveness (number of symptom-free or quality-adjusted days over a 14-day course) of all strategies is very similar as the course of symptoms is driven primarily by the cure rate of diseases mimicking acute bacterial rhinosinusitis. The number of symptom-free days estimated across the range of prevalence for the four strategies is shown in Figure 28.
With rising prevalence, symptomatic treatment alone rapidly becomes less effective than the other strategies as no patients with acute bacterial rhinosinusitis are being treated with antibiotics. In the other three strategies (empiric, radiography-guided, and clinical criteria-guided treatment) the relative effectiveness remains similar across the range of prevalence. As more patients with disease are left untreated with clinical criteria-guided treatment than with radiography because of poorer test performance (sensitivity and specificity, see Table 17), the effectiveness of the former is poorer. Likewise, all patients with disease are treated with empiric treatment; thus, the effectiveness of this strategy is always greatest.
The relative effectiveness across prevalence for the symptom severity scenarios is similar to that shown in Figure 28 for mild, moderate, and severe rhinosinusitis symptoms. The milder the symptoms, the closer the relative effectiveness is and the greater the absolute value of the quality-adjusted symptom days.
Cost per treatment
As shown in Figure 29, the cost per patient of radiography-guided treatment rises with increased prevalence, as the proportion of patients being treated (and thus having side effects), and also not being cured and having disease complications rises. At no prevalence is sinus radiography-guided treatment less costly than the other strategies.

Figure
Figure 29. Average cost per treatment of strategies
in the symptom-duration model.
Note: Tx - treatment
The cost of empiric treatment rises only minimally with increasing prevalence as the cost of treatment and side effects are stable and only the proportions of uncured patients and disease complications rise; however, these proportions remain relatively low as all patients are being treated.
The cost of symptomatic treatment rises rapidly with increasing acute bacterial rhinosinusitis prevalence. This is because of the increasing percentage of patients with acute bacterial rhinosinusitis who are not being cured and thus require a return office visit and the patients who are developing disease complications (at a higher rate than if they were treated). The cost of clinical criteria-guided and radiography-guided treatment rises rapidly with increasing prevalence, since at higher prevalence, there is increased use of amoxicillin which includes increased side effects and an increased overall rate of disease complication and treatment failures.
At acute bacterial rhinosinusitis prevalence below 24 percent, symptomatic treatment per patient is least costly. Clinical criteria-guided treatment is least costly from 24 to 91 percent. Thereafter, empiric treatment is least costly. However, the costs of symptomatic and clinical criteria-guided treatment are very similar at lower prevalence, as are the costs of empiric treatment and clinical criteria-guided treatment at high prevalence.
Cost-effectiveness
The cost-effectiveness of the symptom-free-day scenario across the full range of prevalence for the four strategies is represented in Figure 30. For each strategy, as the prevalence of acute bacterial rhinosinusitis rises, the cost increases (as a result of the increased persistence of symptoms requiring followup, the increased rate of disease complications, and the increased use of antibiotics in the test-guided strategies) and the effectiveness decreases (as a result of the longer duration of symptoms and the likelihood of disease complications with acute bacterial rhinosinusitis than without). Thus the value of the cost-effectiveness rises for each strategy with increased prevalence. (Note that the lower the value, in dollars per symptom-free or quality-adjusted day, the more cost effective the strategy.)
In the symptom-free-day scenario, the cost-effectiveness of symptomatic treatment and clinical criteria-guided treatment are similar across the range of prevalence. Symptomatic treatment is more cost effective (fewer dollars per symptom-free day) below a prevalence of 25 percent. Clinical criteria-guided treatment is the most cost effective at a prevalence between 25 and 83 percent. Empiric treatment is most cost effective at prevalence above 83 percent.
The range of cost-effectiveness for each strategy under each scenario (symptom-free and quality-adjusted days) is presented in Table 25 under "base case scenarios." As radiography is never most cost effective, it is left off the table. When rhinosinusitis symptoms are mild, empiric treatment is most cost effective only at prevalence rates higher than when symptoms are more severe. With mild symptoms, empiric treatment is most cost effective only when prevalence exceeds 97 percent; however, with severe symptoms, empiric treatment is most cost effective when prevalence exceeds 90 percent. Likewise, symptomatic treatment is most cost effective at higher prevalence rates when symptoms are less severe. With mild symptoms, symptomatic treatment is most cost effective when prevalence is less than 49 percent, while with severe symptoms, symptomatic treatment is most cost effective only at prevalence rates less than 31 percent.
Table
Table 25. Range of cost-effectiveness in various scenarios.
Further Sensitivity Analyses
In addition to sensitivity analyses on prevalence and symptom severity (utility), we also performed sensitivity analyses on the test performance of clinical criteria, the cure rate of acute bacterial rhinosinusitis with and without treatment with amoxicillin, the rate of antibiotic side effects, and the cost of the initial antibiotic used. The thresholds of the cost-effectiveness of the various strategies under the different scenarios implied by the sensitivity analyses are presented in Table 25. The values used in the sensitivity analyses are presented in Table 21. In each analysis, one value or set of values is changed from the base case scenario.
Clinical criteria test performance
Lowering the test performance (sensitivity and specificity) of the clinical criteria test shrank the range of prevalence in which using clinical criteria to discriminate between those who should be treated symptomatically and those who should be given amoxicillin.
Cure rates with and without amoxicillin
We used the extremes of the 95 percent confidence intervals for the Kaplan-Meier curves of cure rate with and without amoxicillin from the Lindbaek, Hjortdahl, and Johnsen (1996a) study. In one scenario, we assumed amoxicillin is maximally more effective than placebo (or symptomatic treatment) by using the high cure rate estimate for amoxicillin and the low cure rate estimate for placebo. This scenario implies that empiric treatment is most cost effective at lower prevalence than the base case scenario at high prevalence and that symptomatic treatment is most cost effective only at very low prevalence.
The opposite assumption, that the cure rate with amoxicillin is more similar to that of placebo resulted in the conclusion that symptomatic treatment, although not always most effective, is most cost effective across the full range of prevalence, no matter the severity of disease.
To reproduce the effect of antibiotic resistance, we lowered the cure rate with amoxicillin and left that of placebo at its base level. Again, symptomatic treatment was most cost effective across prevalence for disease severity-adjusted outcome. This is because of the lessened effectiveness of the antibiotic. Only if the goal of treatment is the minimization of any symptom is clinical criteria-guided treatment or empiric antibiotic treatment cost effective at very high prevalence.
Antibiotic side-effect rate
If the risk of antibiotic side effects is nil (as opposed to 5 percent in the base case scenario), then use of antibiotics becomes more cost effective as costs (of treating side effects) are lower and effectiveness is higher (as outcome utility is not decreased for any patients because of side effects). The range of prevalence in which both clinical criteria-guided treatment and empiric treatment are most cost effective shift downward somewhat.
If the risk of antibiotic side effect is very high (at 20 percent), then symptomatic treatment is always most cost effective in mild disease (where the symptoms of rhinosinusitis are less severe than those of side effect would be). The range of prevalence in which both clinical criteria-guided treatment and empiric treatment are most cost effective shift upward and, as in all scenarios, the more severe the symptoms (in comparison to the quality of life of having a side effect), the more cost-effective the use of antibiotics becomes.
Cost of initial antibiotic
In our final sensitivity analysis, we tested the scenario in which a newer, more expensive antibiotic is used as initial treatment (either empirically or after applying clinical criteria). As the literature shows that cure rates for newer, more expensive antibiotics are similar to those of amoxicillin and folate inhibitors (see Attachment C), we used the same cure rates estimated for amoxicillin. As a more expensive antibiotic was used initially, the followup costs for those who fail treatment with the medication are similarly more expensive, as shown in Table 21.
Because of the high cost of treatment, symptomatic treatment for mild and moderate disease is most cost effective across the range of prevalence. Even for rhinosinusitis with severe symptoms, the cost-effectiveness of symptomatic treatment is greatest until very high prevalence. Empiric treatment with amoxicillin ranges in cost-effectiveness from approximately $2 to $5 per quality-adjusted day (across the full range of prevalence and in all base case scenarios). Our hypothetical expensive antibiotic meanwhile ranges in cost-effectiveness from $4 to $20 per quality-adjusted day.
Form of the cure-rate function
The model was also tested using linear and exponential functions instead of the Weibull function to describe the change in daily cure rate (as described above). Although there were small quantitative differences in the costs, effectiveness, and cost-effectiveness thresholds, qualitatively the results were the same (data not shown). The Weibull function more closely describes the biology of infectious diseases (Kalbfleisch and Prentice, 1980) and more closely fits the Kaplan-Meier curves from Lindbaek's trial (1996a) than do linear or exponential functions. Therefore, we believe that the results from the Markov model using the Weibull function are more robust, and that the results from models using the other curves can be disregarded.
- Attachments - Diagnosis and Treatment of Acute Bacterial RhinosinusitisAttachments - Diagnosis and Treatment of Acute Bacterial Rhinosinusitis
Your browsing activity is empty.
Activity recording is turned off.
See more...