NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lehmann HP, Andrews JS, Robinson KA, et al. Management of Acne. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Sep. (Evidence Reports/Technology Assessments, No. 17.)
This publication is provided for historical reference only and the information may be out of date.
Our results fall into two categories: This chapter addresses assessment of the literature and the evidence; the next chapter provides an exhaustive review of the evidence comparing specific pairs of choices of acne therapy. Chapter 5 reports our answers to the specific questions.
Literature Search and Abstract Review
Results from the searches and the abstract review process were maintained in databases developed in ProCite® (Acne Citations Database and Acne Deletions Database). A summary of the search results is provided in Table 1. The bulk of the searching was completed in January and February of 1999, with final searches of PubMed and CENTRAL completed in April 1999. A total of 4,749 citations were identified. Of these, 1,558 were uniquely identified; that is, not previously included in the Acne Citations Database. From the results of the initial PubMed and CENTRAL searches, the search director removed citations dealing only with excluded topics (e.g., rosacea) and, because resources did not allow for translation of articles, citations that were published in languages other than English. A listing of these removed citations was reviewed by one of the principal investigators. Subsequent search results were limited to English language publications and were reviewed by the principal investigators to identify citations for inclusion in the abstract review. Through these processes 829 citations were identified to be included in the abstract review process.
We were unable to review 7 citations as they did not have abstracts and we were unable to retrieve the full articles. Of the 822 citations we could review, 460 (56 percent) were classified as eligible for full article review while 362 (44 percent) were determined to be ineligible for full article review. The majority of abstracts were excluded as not addressing management of acne (291 [80 percent]); the next most common exclusion was no original data (75 [21 percent]). Much smaller numbers of abstracts were excluded as not in English language (6), human data not included (4), only addressing excluded topics (5), and as duplicate publications not caught during searching and retrieving process (4).
Article Review
From the abstract review process, 460 citations were identified for inclusion in the article review phase. Because of this large number, and with consensus from the technical experts, it was decided to focus on the controlled trials. Eligible trials were those where the individuals followed in the trial were definitely or possibly assigned prospectively to one of two or more treatment arms using either random allocation or a quasi-random method of allocation (e.g., date of birth). Of the 460 citations, 331 were identified as reports of controlled trials by the Cochrane Collaboration (citations included in CENTRAL) or by one of the abstract reviewers (for citations from MEDLINE® and other sources).
We were unable to retrieve, and, therefore, unable to complete article review of 12 articles. Of the remaining 319 articles reviewed, 23 (7.2 percent) contained descriptions of more than one trial. Each trial was quality assessed and abstracted separately. There were 351 trials for which a review was completed.
A total of 77 trials (21.9 percent of trials) were excluded during the article review process. The reasons for exclusion are presented in Table 2. The final review thus included 274 trials from 250 articles. (See Appendix H for included and excluded trials.)
As the article review process progressed, we realized that many studies had multiple time points of outcome assessment (e.g., 2, 4, 6, 8, 10, 12, 14, and 16 weeks in a single trial). It was not feasible to continue collection, or data entry, in this manner nor did it seem likely to add to the value of the evidence synthesis. We conferred with our technical experts on an ad hoc basis and included a proposal for dealing with the time points in a formal request for feedback. Based on this, abstraction of outcome data was collected for the time points corresponding to the following time periods:
- Short term -- Longest time interval up to and including 6 weeks (< 6 weeks);
- Mid term -- Longest time interval up to and including 12 weeks (>6 to 12 weeks);
- Long term -- Longest time point reported by study, if greater than 12 weeks; and
- Post-therapy -- Any time period reported after stopping of treatment.
Description of the Acne Literature
General Trial Information
Of the 319 articles reviewed, 296 presented single trials while 23 articles reported multiple trials: 18 articles presented two trials; three articles presented three trials; one article presented four trials; and one article presented six trials.
Figure 2 shows the count of the 274 trials included by publication year. As illustrated in the graph, the number of controlled trials published has generally increased since the mid-1970s.
Study validity is often divided into two parts: external validity and internal validity. Internal validity is the extent to which the methods of the study lead one to trust the results of the study. External validity is the extent to which one may generalize the results beyond the study.
Our method of summarizing methodological strengths and weaknesses is presented in Volume 2 (see Description of the Evidence Tables). Trials that had only strengths numbered 45 (16 percent). Trials that had only weaknesses numbered 106 (39 percent). Trials that had a mix of strengths and weaknesses numbered 107 (37 percent). The remaining 22 (8 percent) were of intermediate quality or did not provide enough information to make a determination.
Funding source is important for assessing bias and in generalizing trial results. Explicit mention of funding source was made in 145 of the trials (53 percent). Of these, 8 were federally funded, 10 reported some other source of funding, and 127 out of 274 trials (46 percent) were drug-company sponsored. We were able to determine additional types of support by drug companies in 113 of the trials, including those where funding source was not clear. Twelve (4 percent of the trials) had a drug-company employee as first author, 38 (14 percent of the trials) had a drug-company employee as co-author, 38 (14 percent of trials) supported the trial by providing medication and one trial (0.4 percent of trials) noted analytic support.
Generalizability also depends on the extent to which results are replicated in multiple locations. The trials spanned the world: Of the 274 included trials for which ata are available, continental Europe accounted for 74 (27 percent); United Kingdom, 56 (20 percent); North America,124 (45 percent -- includes United States, 118 [43 percent], Canada, 5 [2 percent], and Mexico, 1 [0.4 percent]); Asia, 10 (4 percent); Middle East, 2 (1 percent); Oceania, 5 (2 percent); Africa, 1 (0.4 percent); and Iceland, 1 (0.4 percent) for a total of 31 different countries (and one unknown). Although the whole world is represented, patients from American/British Commonwealth countries comprise two-thirds of the trials.
Generalizability is influenced by replication in multiple locations within a trial. Most trials were performed at one study site. Of the 251 trials for which data are available, 177 (71 percent) were single-site, 65 (26 percent) were multi-center, single-country, and 9 (4 percent) were multi-country.
Finally, generalizability is greatly affected by how subjects (patients) are enrolled into a trial. The further away from being a randomly chosen patient and the more selected a subject is through the clinical-care process, the less likely the study patient is to represent other patients. Of 274 trials, fully 203 (84 percent) gave no information regarding patient selection. Of those trials providing information (71), 45 (63 percent) stated that patients were "recruited" (although rarely with further details), and 19 (42 percent) stated that patients were selected as consecutive visitors to the clinic. Seven trials (3 percent) mentioned other methods. Whether subjects were recruited through a dermatology practice, a general practice, or from the general population is also an important consideration. It was rarely possible to determine this with any specificity in the trials reviewed.
Within trials, it is important that the arms be similar in arm comparability, judged on the basis of baseline patient characteristics. For 103 (38 percent) of the trials arm comparability could not be determined. In 90 trials (33 percent) the arms were definitely comparable, and 76 (28 percent) were judged as maybe comparable. Only 5 (2 percent) provided enough evidence to conclude that the arms were not comparable. Further details concerning baseline patient characteristics are provided in the next section (Patient Characteristics).
Trial design is important for internal validity as well. Most of the trials used standard, two-arm, parallel trial designs (259 [94 percent]). Only 15 (5 percent) of the trials used a cross-over design, where patients serve as their own controls over time, 30 (11 percent) were parallel trials with split-face design, where patients serve as their own controls spatially; and 22 (8 percent) were parallel trials with matched controls. While both cross-over and split-face design have the potential to control for baseline characteristics in the most patient-specific manner, it is crucial that, for cross-over studies, there be a wash-out period, and that, for split-face studies, the investigator should anticipate contamination of the opposite side of the face. 25 For the most part, we were unable to determine this type of detail from the trials.
Treatment assignment is important for internal validity, with randomized assignment being the current gold standard. Relatively few trials (56, or 20 percent) gave enough information to conclude that the patients were randomly assigned, and 175 (64 percent) were stated to be randomized, with no further details. The rest either used deterministic methods of assignment -- i.e., the treatment was assigned using an attribute known by the investigator, such as date of birth or hospital identification number (9, or 3 percent) -- or gave no information at all (34, or 12 percent).
Treatment blinding is also important for maintaining high internal validity. Only 27 trials (10 percent) gave enough details to convince the abstracters that patient blinding was properly performed; 180 (66 percent) made a statement that blinding was performed, but with no further details; and 45 (16 percent) were explicit that patients were not blinded as to their treatment. Similarly, 41 trials (15 percent) convincingly demonstrated that the enrolling or treating physician was adequately blinded, while 177 (64 percent) only said that they were blinded, and 22 (8 percent) were clearly not blinded.
Compliance of patients with therapy is crucial in determining efficacy or effectiveness, and especially so with topical treatment. Seventy-four trials (27 percent) provided evidence that patients' compliance was addressed prospectively from trial onset. A further 17 (6 percent) discussed compliance retrospectively, 17 (6 percent) made statements suggestive of their concern about compliance, and 8 (3 percent) clearly ignored compliance. Fully 148 trials (54 percent) gave no indication one way or the other of this concern, and for 9 (3 percent), compliance monitoring was not applicable.
Trials varied in mode of treatment administration: 101 trials (37 percent) included systemic therapy, 183 trials (67 percent) included topical facial therapy, and 25 (9 percent) addressed other areas, like the back and chest. (Percent sums to greater than 100 percent because trials could address multiple areas.)
Trials excluded a variety of ancillary therapies used by all trial participants. The excluded ancillary treatments included other medications (110, or 40 percent), soaps (44, or 16 percent), and diet (9, or 3 percent); 129 trials (47 percent) mentioned ancillary therapy, but gave no details. A variety of other therapies were mentioned as being explicitly excluded: radiation therapy, sun bathing, unspecified powder, scalp shampoo, among others. We judged 124 trials (45 percent) as providing evidence that subjects received similar ancillary treatment. Seven trials (3 percent) clearly provided asymmetric ancillary therapy -- that is, the groups did not receive the same reatment, besides the treatment that was the focus of the trial. Fully 119 (43 percent) gave no information at all.
In terms of study execution, 45 trials (16 percent) had major losses to followup, while 107 (39 percent) had minor losses. Ninety-one trials (33 percent) documented virtually no such losses, while 31 (11 percent) did not provide enough data for an assessment. Beyond losses to followup, 7 trials (3 percent) reported protocol departures; 44 (16 percent) had minor such departures but 122 (45 percent) provided too little information to make any assessments. We judged 203 trials (74 percent) to have followed their design in execution and only 4 (1 percent) to definitely not have followed the plan, but this could not be determined for 14 trials (5 percent).
As a summary statement regarding external validity, we judged only 4 trials (1 percent) as being generalizable to the general population; 11 trials (4 percent), to more specific populations; and 9 (3 percent), to local populations. The great majority (152, or 55 percent) do not give enough data, while the remainder can be generalized only to the referral population (22, or 8 percent) or to any group beyond the study participants (76, or 28 percent).
Patient Characteristics
For those patient characteristics determined from exclusion criteria, no study mentioned sexual maturity rating (Tanner stage) and 66 (24 percent) did not explicitly mention any exclusion criteria based on patient characteristics. Table 3 shows the breakdown of other exclusion criteria. Acne severity was the basis of exclusion most of the time: 79 trials excluded patients with "severe" acne, while 82 trials excluded patients with "mild" acne. Other morbidity, pregnancy, and young age were the other most cited exclusion criteria.
Based on the patient characteristics used as exclusion criteria, all studies included adolescents and young adults (see Table 3). Specific ages for participants in trials were also collected. Pooling the studies for which mean and standard deviations are available yields a pooled mean of 21 years and a pooled standard deviation of 5 years. This mean is consistent with the age groups implied by the exclusion criteria. The average range was 15 to 32 years, the overall minimum stated in age ranges was 8 years and the maximum was 72 years.
Table 4 supplies the number of trials providing information for each patient characteristic: age, sex, race, skin type, diet, duration of disease, insurance status, prescription plan, source of care, practice setting and phase of care. One trial reported on patients' diets, none reported on insurance status or the availability of a prescription plan. Few studies reported on skin type or disease duration. Most studies reported on the sex of patients, but 70 (26 percent) omitted this essential baseline characteristic.
Eight trials stratified their results. Trials 35, 293, 111, and 277 stratified by gender. Trials 350, 16, 222, stratified by acne severity, and Trial 55 stratified by location.
Acne Severity
As stated above, regarding exclusion criteria, acne severity was the most important patient characteristic, and it is the characteristic our experts raised most often in deciding on individual treatment profiles. In the process of reviewing the literature, 25 different methods of assessment for acne severity were identified. These methods were varied and included lesion counting on all or part of the face, comparison of the patients to a photographic standard, and comparison of the patients to a text description. Many trials did not report their method of assessment. The way in which severity was reported also differed between studies. Many used terminology, "mild, moderate or severe." Others used numerical scores such as 1-4, 0-10, etc. Neither terminology nor score was necessarily consistent across studies; so what was termed "grade 2 acne" in one trial might have been classified as "grade 6" according to a grading system in a different trial.
In our synthesis, an effort was made to standardize the reporting of acne severity so that more accurate comparisons could be made between trials. Terminology used here is the standard "mild, moderate, severe." Each trial was reviewed for the acne severity of the patients included in that study. The reported severity was converted to a "Combined Acne Severity Classification" as follows:
- Trials Reporting Lesion Count
The Combined Acne Severity Classification was derived from the mean lesion counts and standard deviations reported in the studies. The definitions are:- Mild acne -- Fewer than 20 comedones, or fewer than 15 inflammatory lesions, or total lesion count fewer than 30.
- Moderate acne -- 20-100 comedones, or 15-50 inflammatory lesions, or total lesion count 30-125.
- Severe acne -- More than 5 cysts, or total comedo count greater than 100, or total inflammatory count greater than 50, or total lesion count greater than 125.
- Trials Reporting an Acne Severity Grade
Two reviewers evaluated each grading system that was described in detail. These grading systems, a few of which were frequently referenced, were converted to the Combined Acne Severity Classification based on how the grading system compared with the above definitions. This conversion was straightforward in articles that used scoring based on calculations from lesion counts. Some conversions, such as the ones from photographic assessment or from text descriptions, required judgement. - Trials Reporting Both Lesion Count and an Acne Severity Grade
In some instances, studies reported both lesion count and a severity grade. In this case, the lesion count was used to determine the Combined Acne Severity Classification. - Trials With No Reference to Assessment Method
In some trials, severity terminology was used with no reference as to how severity was actually assessed. In these instances, the severity stated by the author was kept as the combined acne severity classification.
When no other information was provided, the exclusion criteria were used to determine the Combined Acne Severity Classification. Patients included were assumed to be all of those not excluded, using the definitions above to categorize the patients.
See Table 5 for comparison of the Combined Acne Severity Classification and frequently cited severity grading systems. See Figure 3 for the distribution of the Combined Acne Severity Classification among the trials included in the review. The trials were distributed as 21 percent (58) in mild/moderate category, 49 (18 percent) in moderate/severe category and 51 (19 percent) in moderate category. Only about 5 percent of the trials had patients classified at either end of the scale, as either mild or severe. We were unable to classify severity in about 22 percent (59) of the trials.
Source of Care
Practice Setting
From our model of acne care (Figure 1), we needed data conditioned on source of care or practice setting. No trial noted self-care or HMO as setting of care, 8 (3 percent) were performed at a health clinic, 21 (8 percent) at a private practice, and 195 (71 percent) of the trials cited study clinic/hospital as practice setting. Miscellaneous sites included schools or student centers and one military clinic.
Care Provider
Eight trials (3 percent) noted generalist as source of care while 68 trials (25 percent) were determined to have care provided by dermatologists. In 157 trials (57 percent) the source of care could not be determined beyond study clinic.
Outcomes
Discrete outcomes are those in which investigators report the proportion of subjects achieving a categorical outcome (i.e., "improved"). Continuous outcomes are based on a quantitative measure of an attribute in each subject (e.g., mean number of lesions). Table 6 shows the distribution of outcomes. 203 (74 percent) of trials had continuous outcomes, and 80 percent (221 trials) had discrete outcomes.
Table 7 shows the distribution of outcomes by outcome type. There were 1,237 outcomes. Of these outcomes, 888 (72 percent) concerned the assessment of acne lesions, and 335 (27 percent) were classified as outcomes of adverse events or side effects. Almost all trials (269, or 98 percent) trials had data on acne outcome, while no trials had data on economic outcomes. Only 2 trials (0.7 percent) had data on psychological outcomes, and only 43 trials (16 percent) provided some data on treatment compliance.
The 19 outcomes shown in Table 8 account for more than half the data. There were 505 acne outcomes, lumping together multiple time points for individual outcomes within a trial. Of these, 142 (28 percent) concerned severity, 211 (42 percent) concerned lesions counts, and 152 (30 percent) were overall assessments. Percentage change with respect to baseline counts accounted for 73 (36 percent) of the lesion count outcomes. Of the 83 trials (31 percent) where location of count was mentioned, 25 (29 percent) simply said "face," 11 (13 percent) stated "entire face," and the remainder used 39 other descriptors, such as "5 cm worst area" or "selected area." Table 9 lists the various descriptors of count location.
Treatments
For purposes of summarizing the data and for organizing the results, the treatments were divided into a number of classes. This classification was completed by consensus of our technical experts. The basis for the division was (1) similarity of pharmacological mechanism, and (2) similarity of perceived severity for which the treatment would be prescribed. For the first reason, anti-bacterials were grouped together, regardless of the specific class of antibiotic. For the second, topical and oral antibiotics were separated because most physicians would treat topically first, before using systemic therapy. Our goal in separating these classes was to find if the literature supported this established use.
The classes shown in Table 10 represent the classes, based on the data available. The classes comprising first-line therapy are Cleanser, eratolytic, Anti-bacterial (topical), and combinations. Second-line therapy includes Retinoid topical), Anti-bacterial (oral), and combinations. Referral treatment includes Retinoid (oral) and Anti-androgen. The class Other includes treatments not classifiable in the previous classes, and Inert refers to placebos, vehicles, and other interventions used as controls for presumed effective acne treatment.
Table 11 lists all of the treatments studied in the trials included in this report. The table includes a brief description, the classification, and the availability of the treatment. Table 12 summarizes the trials as comparisons, grouped by the class assignment of the evaluated treatment: target and comparator. There were 251 comparisons in the two- and three-arm trials.
Figures
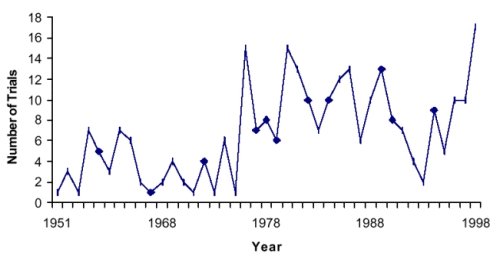
Figure 2. Number of trials published per year
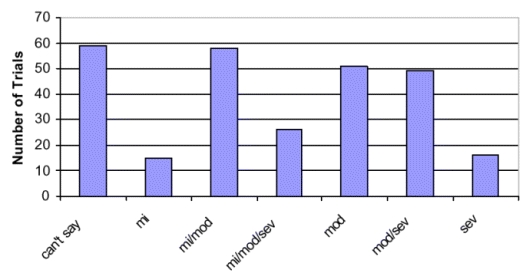
Figure 3. Distribution of trials by combined acne severity classification
Tables
Table 1. Summary of search results
| Source | Date | Retrieved | Unique | Number of citations | Eligible for article review | ||
|---|---|---|---|---|---|---|---|
| Eligible for abstract review | Unable to abstract review | ||||||
| ALL | CTs | ||||||
| CENTRAL Issue 4 1998 | Jan 25, 1999 | 460 | 460 | 375 | 211 | 211 | |
| PubMed core | Jan 27, 1999 | 820 | 312 | 228 | 115 | 55 | |
| OLDMEDLINE | Feb 8, 1999 | 512 | 512a | 126 | 5 | 87 | 39 |
| HealthStar | Feb 25, 1999 | 1,342 | 5a | 0 | 0 | 0 | |
| PsycINFO® | Feb 25, 1999 | 65 | 65a | 25 | 16 | 1 | |
| CINAHL® | Feb 26, 1999 | 125 | 125a | 15 | 0 | 0 | |
| PubMed supplemental search for cost | Feb 26, 1999 | 28 | 25a | 6 | 5 | 0 | |
| PubMed core | Apr 7, 1999 | 835 | 18 | 18 | 10 | 10 | |
| CENTRAL Issue 1 1999 | Apr 27, 1999 | 523 | 30 | 30 | 1 | 13 | 13 |
| Hand Searching reviews | July 1999 | 39 | 6 | 6 | 1 | 3 | 2 |
| TOTAL | 4,749 | 1,558 | 829 | 7 | 460 | 331 | |
- a
Listing reviewed by PIs to identify those to be included in abstract review process
Column Headings:
Source - Name of database searched and, where applicable, version of database and type of search.
Date - Date search completed.
Number of citations: Retrieved - Total number of citations retrieved by search.
Number of citations: Unique - Total number of citations retrieved minus those citations previously retrieved.
Number of citations: Eligible for abstract review - As noted with asterisks, in some cases the PIs reviewed the listing of uniquely retrieved citations to identify those to be included in the abstract review process. The number in this column reflects the citations identified in this manner.
Number of citations: Eligible for article review - Number of citations from abstract review process deemed eligible for article review. This column includes the total number of citations as well as the number of controlled trials (CTs).
Table 2. Reasons for exclusion of trials
| Reason for exclusion | Number of trials | Percent of trials excluded (n=77) | Percent of all trials reviewed (n=351) |
|---|---|---|---|
| Not management of acne | 16 | 20.8 | 4.6 |
| No human data | 1 | 0.1 | 0.3 |
| Excluded topics only: more than 20% of patients have chloracne, rosacea, venanta, fulminans, necroticans, or agminata | 1 | 0.1 | 0.3 |
| Surrogate measures only (e.g., sebum production, P acnes colony counts) | 12 | 15.6 | 12.0 |
| No original data | 7 | 9.1 | 2.0 |
| Not in English | 4 | 5.2 | 1.1 |
| Fewer than 5 patients | 2 | 2.6 | 0.6 |
| Unable to abstract data | 4 | 5.2 | 1.1 |
| Study design not controlled | 30 | 39.0 | 8.5 |
| Total | 77 | ||
Table 3. Exclusion criteria cited by trials
| Patient characteristic cited as exclusion | Number of trials using criterion (percent)a |
|---|---|
| Type of acne | |
| Nodulocystic | 23 (8%) |
| Pustular | 5 (2%) |
| Inflammatory | 5 (2%) |
| Comedonal | 15 (5%) |
| Secondary | 30 (11%) |
| No exclusion based on type | 237 (86%) |
| Severity of acne | |
| Severe | 79 (29%) |
| Moderate | 10 (4%) |
| Mild | 82 (30%) |
| No exclusion based on severity | 124 (45%) |
| Age | |
| < 31 days | 49 (18%) |
| 31 days to 10 years | 39 (18%) |
| 11 to 14 years | 21 (7%) |
| 15 to 18 years | 1 (0.4%) |
| 19 to 34 years | 0 (0%) |
| 35 to 54 years | 28 (10%) |
| > 54 years | 35 (13%) |
| No exclusion based on age | 223 (81%) |
| Sex | |
| Female | 5 (2%) |
| Male | 14 (5%) |
| No exclusion based on sex | 255 (93%) |
| Other conditions | |
| Pregnancy | 63 (23%) |
| Lactation | 39 (14%) |
| Allergy | 45 (16%) |
| Other comorbidity | 68 (25%) |
| No patient characteristic based on exclusion criteria mentioned | 66 (24%) |
- a
A trial may appear several times.
Table 4. Patient characteristics provided by trials
| Patient characteristic | Number of trials providing data (%) |
|---|---|
| Tanner stage | 0 (0%) |
| Age | 204 (74%) |
| Sex | 200 (73%) |
| Race | 23 (8%) |
| Skin type | 6 (2%) |
| Diet | 1 (0.4%) |
| Disease duration | 40 (14%) |
| Insurance status | 0 (0%) |
| Prescription plan | 0 (0%) |
| Source of care | 222 (81%) |
| Practice setting | 220 (80%) |
| Phase of care | 36 (13%) |
Table 5. Comparison of combined severity grading with frequently cited grading systems
| Combined Acne Severity Classification | Allen 274 | Burke and Cunliffe (Leeds) 275 | Cook 276 | Lucky 230 | Michaelssona209 | Pochi 67 | Pillsbury 10 |
|---|---|---|---|---|---|---|---|
| Mild | 0 | 0 | 0 | None | 0-3 | Mild | 1 |
| Very mild | 4-20 | ||||||
| 2 | 0.25-0.5 | 2 | Mild | ||||
| Moderate | 4 | 0.75-1.5 | 4 | Moderate | 20-30 | Moderate | 2 |
| 6 | 6 | ||||||
| Severe | 8 | 2.0-10.0 | 8 | Severe | >30 | Severe | 3 |
| Very severe | 4 |
- a
Michaelsson severity scores based on Cook's descriptions and using the following assumptions:
Cook 0 = 3 papules
Cook 2 = 20 comedones and 10 papules
Cook 6 = 30 papules
Table 6. Distribution of continuous and discrete outcomes
| Number of trials | Number of outcomes per trial | Total number of outcomes | ||||
|---|---|---|---|---|---|---|
| Average | SD | Median | Range | |||
| Continuous | 203 | 6.3 | 4.5 | 6.0 | 1-21 | 1,282 |
| Discrete | 221 | 2.9 | 3.1 | 2.0 | 1-35 | 637 |
| 1,919 | ||||||
SD - Standard deviation
Table 7. Outcome types
| Outcome type | Total number of outcomes (percentage of outcomes) | Number of trials (percentage of trials) |
|---|---|---|
| Acne | 888 (70%) | 269 (98%) |
| Adverse effects | 335 (26%) | 174 (64%) |
| Psychological effects | 8 (1%) | 2 (1%) |
| Compliance | 43 (3%) | 43 (16%) |
| Cost | 0 (0%) | 0 (0%) |
| TOTAL | 1,274 | 274 |
Table 8. Number of trials employing outcomes accounting for just over half of reported effectiveness outcomes
| Outcome name | Number of trials | Percent of total trials |
|---|---|---|
| Overall change/physician | 51 | 6% |
| Overall change/patient | 34 | 4% |
| Inflammatory (count) | 33 | 4% |
| Inflammatory change (%) | 33 | 4% |
| Pustules (count) | 30 | 3% |
| Papules (count) | 29 | 3% |
| Comedones (count) | 24 | 3% |
| Non-inflammatory change (%) | 23 | 3% |
| Non-inflammatory (count) | 22 | 2% |
| Total lesions change (%) | 22 | 2% |
| Severity change | 16 | 2% |
| Severity (cook) | 14 | 2% |
| Total lesions (count) | 13 | 1% |
| Pustules change (%) | 12 | 1% |
| Papules change (%) | 12 | 1% |
| Severity (leeds) | 12 | 1% |
| Comedones change (%) | 11 | 1% |
| Open comedones (count) | 10 | 1% |
| Closed comedones (count) | 10 | 1% |
| TOTAL | 411 | 46% |
Table 9. Location of lesion counting
| Stated region | Number of trials |
|---|---|
| Face | 25 |
| Entire face | 11 |
| Face unspecified | 4 |
| Face excluding nose | 3 |
| 5 cm diameter | 2 |
| Both sides | 2 |
| Half face | 2 |
| Left side | 2 |
| Symmetric area of face | 2 |
| 2 cm worst | 1 |
| 3 + c face | 1 |
| 5 cm worst area | 1 |
| 5cm diameter-worst | 1 |
| Above jawline | 1 |
| Back | 1 |
| Bilateral cheeks | 1 |
| Both halves (except 1/2 midline overlap) | 1 |
| Butterfly | 1 |
| Chest | 1 |
| Designated area of face, chest and/or back | 1 |
| Each side of face | 1 |
| Entire (with estimate) | 1 |
| Entire face chest & back | 1 |
| Entire face-(photos) | 1 |
| Entire face-photo | 1 |
| Face & chest & back | 1 |
| Face (unspecified) | 1 |
| Face + or - other | 1 |
| Face above jawline | 1 |
| Face/neck | 1 |
| Left cheeks | 1 |
| Neck anterior to sternomastoid muscles and face | 1 |
| One side | 1 |
| Right | 1 |
| Selected area | 1 |
| Side of face | 1 |
| Side of face (spilt face trial) | 1 |
| Well defined regions | 1 |
| Witkowski/simons | 1 |
| Worst area | 1 |
| Worst area/5cm diameter | 1 |
| TOTAL | 85 |
Table 10. Treatment classes
| Treatment | Number of arms |
|---|---|
| Cleanser | 11 |
| Keratolytic | 22 |
| Anti-bacterial (topical) | 83 |
| Anti-bacterial/keratolytic (topical) | 54 |
| Retinoid (topical) | 84 |
| Anti-bacterial/retinoid (topical) | 10 |
| Anti-bacterial (oral) | 98 |
| Anti-bacterial (oral)/keratolytic | 19 |
| Anti-bacterial (oral)/retinoid (topical) | 4 |
| Retinoid (oral) | 26 |
| Anti-androgen | 35 |
| Other | 82 |
| Inert | 130 |
| TOTAL | 659 |
Table 11. Description of treatments in trials reviewed
| Treatment | Description | Category | Currently manufactured | U.S. availability | Number of armsa |
|---|---|---|---|---|---|
| 16-epiestriol-3-allyl ether | Epimer of the estrogenic metabolite estriol | AA | No | No | 1 |
| Acidic syndet bar | A detergent that is not a soap, condensed from synthetic detergent | C | Yes | Yes | 1 |
| Adapalene | A topical retinoid-like compound | R | Yes | Yes | 15 |
| Alitretinoin | A natural retinoid with actions similar to all-trans-retinoic acid (tretinoin). | R | Yes | Yes | 1 |
| Aluminum chlorhydroxide | Astringent and antiperspirant properties | C | Yes | Yes | 3 |
| Aluminum chloride | Used in manufacturing, antiperspirants, andastringents | C | Yes | Yes | 1 |
| Azelaic acid | Naturally-occurring, saturated, straight-chained dicarboxylic acid | K | Yes | Yes | 8 |
| Bar soap | The sodium or potassium salts of long chain fatty acids | O | Yes | Yes | 1 |
| Benzoyl peroxide | Antibacterial, mildly comedolytic, and sebostatic agent | AB, K | Yes | Yes | 74 |
| Buttoxethyl nicotinate | Used in topical agents as a rubfacient (to cause redness) | O | Yes | Yes | 1 |
| Chloramphenicol | An antimicrobial agent | AB | Yes | Yes | 7 |
| Chlorhydroxyquinoline | Topical antibacterial, antifungal agent | AB | Yes | Yes | 2 |
| Cimetidine | H2-receptor antagonist | O | Yes | Yes | 2 |
| Clindamycin | A lincosamide antimicrobial agent | AB | Yes | Yes | 21 |
| Clindamycin hydrochloride | A lincosamide antimicrobial agent | AB | Yes | Yes | 6 |
| Clindamycin phosphate | A lincosamide antimicrobial agent | AB | Yes | Yes | 27 |
| Clobetasol propionate | The most potent topical corticosteroid available | O | Yes | Yes | 1 |
| Comedone extraction | Office procedure | O | Yes | Yes | 3 |
| Cotrimoxazole | Antibacterial agent comprised of trimethoprim (dihydrofolate reductase inhibitor antimicrobial agent) and sulfamethoxazole (an intermediate-acting sulfonamide) | AB | Yes | Yes | 4 |
| Cyproterone | An antiandrogen with progestogenic activity | AA | Yes | No | 16 |
| Dapsone | A sulfone antibiotic | AB | Yes | Yes | 2 |
| Delta-0.2%-chlormadione | A progestogen structurally related to progesterone | AA | Yes | No | 1 |
| Delta-5%-chlormadione | A progestogen structurally related to progesterone | AA | Yes | No | 1 |
| Demethylchlortetracycline | A bacteriostatic antimicrobial agent | AB | Yes | No | 6 |
| Desogestrel | A progesten used for contraception | AA | Yes | Yes | 6 |
| Doxycycline | A tetracycline antibiotic with a broad spectrum of activity | AB | Yes | Yes | 6 |
| E-solve | Vehicle for erythromycin/clindamycin | O | Yes | Yes | 1 |
| Electrocautery | Minimal burning of lesions via electricity | O | Yes | Yes | 1 |
| Emollient | An agent that softens or soothes the skin or irritation of the skin | O | Yes | Yes | 1 |
| Epiestriol | Epimer of the estrogenic metabolite estriol | AA | No | No | 1 |
| Erythromycin | A macrolide antibiotic | AB | Yes | Yes | 35 |
| Erythromycin acistrate | A macrolide antibiotic | AB | Yes | No | 1 |
| Erythromycin base | A macrolide antibiotic | AB | Yes | Yes | 2 |
| Erythromycin proprionate | A macrolide antibiotic | AB | No | No | 1 |
| Erythromycin stearate | A macrolide antibiotic | AB | No | No | 2 |
| Ethinyl estradiol | An estrogen component of oral contraceptives | AA | Yes | Yes | 28 |
| Etretinate | A derivative of tretinoin | R | Yes | No | 1 |
| Fractionated human dialyzable transfer factor | Leukocyte extract that passively transfers cell mediated immunity | O | Yes | Yes | 1 |
| Fulguration | A form of electrocautery | O | Yes | Yes | 2 |
| Fusidin cream | A steroidal antibiotic, chemically related to cephalosporin P | AB | Yes | No | 1 |
| Fusidic acid | A steroidal antibiotic, chemically related to cephalosporin P | AB | Yes | No | 2 |
| Gamma-globulin | Antibodies derived from human plasma | O | Yes | Yes | 1 |
| Gestodene | A progestogen structurally related to levonorgestrel | AA | Yes | No | 1 |
| Gluconolactone | Available as part of the orphan drug, glucono-delta-lactone and magnesium carbonate | K | No | Yes | 1 |
| Glycolic acid | An organic acid | K | Yes | Yes | 3 |
| Grenz rays | Superficial irradiation | O | No | No | 6 |
| Group therapy | A psychological method | O | Yes | Yes | 1 |
| Gugulipid | The oleoresin of the Indian herb Commiphora mukul or gum guggul. | O | Yes | Yes | 1 |
| Hexachlorophene | A polychlorinated bisphenol, which is an effective inhibitor of gram positive organisms | O | Yes | Yes | 2 |
| Hydrochlorthiazide | A thiazide diuretic | O | Yes | Yes | 2 |
| Hydrocortisone | A corticosteroid with both glucocorticoid and mineralocorticoid activity | O | Yes | Yes | 14 |
| Hydrocortisone F | A corticosteroid with both glucocorticoid and mineralocorticoid activity | O | Yes | Yes | 1 |
| Hydroxyquinoline | Class of agents with antibacterial, antifungal, and deodorant properties | AB | Yes | Yes | 2 |
| Ibuprofen | A non-steroidal anti-inflammatory drug | O | Yes | Yes | 3 |
| Inactive drug | Drug with no therapeutic effect | O | No | No | 1 |
| Inocoterone acetate | A topical, 5-alpha reductase inhibitor anti-androgen | AA | Yes | No | 1 |
| Ionax | Benzalkonium chloride, coal tar, salicylic acid | K | Yes | No | 1 |
| Isoconazole nitrate | An imidazole antifungal | AB | Yes | No | 1 |
| Isolutrol | Produced in the liver and gallbladder of sharks | O | Yes | No | 1 |
| Isotretinoin | A synthetic analogue of vitamin A | R | Yes | Yes | 28 |
| Komed® | A multiingredient preparation including salicylic acid, sodium thiosulfate, and isopropyl alcohol. | K | No | No | 2 |
| Meclocycline | A tetracycline antibiotic derived from oxytetracycline | AB | Yes | No | 1 |
| Meclocycline sulfosalicylate | A tetracycline antibiotic derived from oxytetracycline | AB | Yes | No | 3 |
| Methyl prednisolone | A corticosteroid and the methyl derivative of prednisolone | O | Yes | Yes | 5 |
| Metronidazole | A synthetic 5-nitroimidazole | AB | Yes | Yes | 4 |
| Miconazole | An antifungal agent | AB | Yes | Yes | 4 |
| Minocycline | A tetracycline antimicrobial agent | AB | Yes | Yes | 10 |
| Motretinide | A retinoid that inhibits the secretion of sebum | R | Yes | No | 5 |
| Neomycin | An aminoglycoside antimicrobial | AB | Yes | Yes | 10 |
| Nicotinamide | The amide metabolite of niacin | O | Yes | Yes | 1 |
| Norethisterone | A synthetic progestin | AA | Yes | Yes | 1 |
| Norgestimate | A progestogen structurally related to levonorgestrel | AA | Yes | Yes | 2 |
| Ocimum basilicum | Oil of sweet basil herb | O | No | No | 1 |
| Oleandomycin | A macrolide antibiotic | AB | Yes | No | 1 |
| Oxytetracycline | A tetracycline antimicrobial agent | AB | Yes | Yes | 8 |
| Petrolatum | A purified semi-solid mixture of hydrocarbons obtained from petroleum | O | Yes | Yes | 2 |
| Phenethicillin | A phenoxypenicillin | AB | Yes | No | 1 |
| Placebo | An inert substance given as a medicine for its suggestive effect | O | No | No | 98 |
| Polyethylene scrub | An abrasive substance used to unseat comedones and deter formation | O | No | No | 2 |
| Polyoprepolymer-2 | A mixture of glycols labeled according to average molecular weight | O | Yes | Yes | 2 |
| Polythionate | Therapeutically active form of sulfur | K | No | No | 2 |
| Potassium hydroxyquinoline | A mixture of potassium sulfate and quinolin-8-ol sulfate monohydrate with antibacterial, antifungal, and deodorant properties | AB | Yes | No | 2 |
| Povidone-iodine | A broad-spectrum germicidal agent | AB | Yes | Yes | 2 |
| Propylene phenoxetol | A preservative with anti-Pseudomonas activity | AB | Yes | Yes | 1 |
| Proteolytic enzymes | A protein that decomposes other enzymes | O | No | No | 1 |
| Pumice | An abrasive | C | Yes | Yes | 1 |
| Quaternary ammonia complex of polythionates | Therapeutically active form of sulfur | AB | No | No | 1 |
| Quinestradol | A synthetic oestrogen | AA | Yes | No | 1 |
| Relaxation-imagery | A psychological method | O | Yes | Yes | 1 |
| Resorcinol | An aromatic alcohol | O | Yes | Yes | 8 |
| Retinyl beta-glucuronide | Vitamin A metabolite | R | No | No | 2 |
| Roxithromycin | A macrolide antibiotic | AB | Yes | No | 2 |
| Salicylic acid | A topical keratolytic agent | K | Yes | Yes | 6 |
| Saline | Sodium is the principle cation of extracellular fluid and chloride is the principle anion of extracellular fluid | O | Yes | Yes | 1 |
| Serum gonadotrophin | Hormones supports and stimulates the function of the gonads (LH and FSH) | AA | No | No | 1 |
| Shankhabhasma vati | A traditional Indian formulation containing minerals derived from conch shells | O | No | No | 1 |
| Silicic acid | A silicon compound that occurs naturally as opal | K | No | No | 1 |
| Sodium fusidate | A steroidal antibiotic | AB | Yes | No | 2 |
| Sodium novobiocin | An antimicrobial which is structurally related to coumarin | AB | Yes | Yes | 1 |
| Sookshama triphala | A traditional Indian formulation containing dried fruits and minerals | O | No | No | 1 |
| Soybean liposome | No description found) | O | No | No | 2 |
| Spironolactone | A synthetic steroid aldosterone antagonist | AA | Yes | Yes | 7 |
| Staphylococcal toxoid | Detoxified toxins which retain their antigenicity and their immunizing capacity | O | No | No | 1 |
| Sulfadimethoxine | A long-acting sulfonamide | AB | Yes | No | 1 |
| Sulfamethoxazole | An intermediate-acting sulfonamide | AB | Yes | Yes | 2 |
| Sulfur | A pure chemical element with keratoplastic and keratolytic, as well as antifungal, antibacterial, and antiscabies activity | K | Yes | Yes | 24 |
| Sulfur-resorcinol salicylic acid | A combination of a pure chemical element with keratoplastic and keratolytic, as well as antifungal, antibacterial, and antiscabies activity, an aromatic alcohol, and a topical keratolytic agent | K | No | No | 1 |
| Sufur/salicylic acid | A combination of a pure chemical element with keratoplastic and keratolytic, as well as antifungal, antibacterial, and antiscabies activity, and a topical keratolytic agent | K | No | No | 1 |
| Sufurated lime | A mixture containing calcium sulfate and not less than 50% of calcium sulfide | K | Yes | No | 1 |
| Sunder vati | A traditional Indian formulation containing dried fruit, bark, and rhizones | O | No | No | 1 |
| Tea tree oil | Obtained by distillation from the leaves of the Australian tea tree, it contains about 50 to 60% of terpenes, cineole (up to 10%), and terpineol | O | Yes | Yes | 1 |
| Tetracycline | A bacteriostatic antimicrobial agent | AB | Yes | Yes | 33 |
| Tetracycline hydrochloride | A bacteriostatic antimicrobial agent | AB | Yes | Yes | 17 |
| Tetracycline phosphate | A bacteriostatic antimicrobial agent | AB | Yes | Yes | 1 |
| Tetracycline-citric acid | A bacteriostatic antimicrobial agent and excipient | AB | Yes | Yes | 1 |
| Tetracycline-novobiocin | Combination of a bacteriostatic antimicrobial agent and a stretomyces antibiotic | AB | Yes | No | 1 |
| Thiostanin | A traditional Indian formulation containing dried fruits, minerals and roots | K | No | No | 1 |
| Tolbutamide | A short-acting sulfonylurea | O | Yes | Yes | 1 |
| Topical treatment | Any traetment applied directly to the skin | O | No | No | 1 |
| Tretinoin | A retinoid derived from naturally occurring all-trans-retinol | R | Yes | Yes | 68 |
| Triacetyl-oleandomycin | An essential trace metal | AB | No | No | 2 |
| Triclosan | An antiseptic used in cosmetics and soaps | AB | Yes | Yes | 3 |
| Trimethoprim | A dihydrofolate reductase inhibitor antimicrobial agent | AB | Yes | Yes | 3 |
| Unfractionated human dialyzable transfer factor | Leukocyte extract that passively transfers cell mediated immunity | O | Yes | Yes | 1 |
| Unknown | Not able to determine what treatment was received | O | Yes | Yes | 1 |
| Untreated | Receiving no treatment | O | Yes | Yes | 3 |
| Urea | An osmotic diuretic, abortifacient, emollient, keratolytic, and diagnostic agent | K | Yes | Yes | 1 |
| Vehicle | A substance without therapeutic action used to administer medication | O | No | No | 78 |
| Vitamin A | A fat-soluble vitamin, it exists within the body as retinol, retinyl esters, retinal, and retinoic acid | R | Yes | Yes | 6 |
| Vitamin A palmitate | A fat-soluble vitamin, it exists within the body as retinol, retinyl esters, retinal, and retinoic acid | R | Yes | Yes | 2 |
| Water avoidance | Avoiding water | O | Yes | Yes | 1 |
| Zinc | An essential trace metal used topically as a drying agent | O | Yes | Yes | 4 |
| Zinc acetate | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 4 |
| Zinc gluconate | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 1 |
| Zinc sulfate | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 10 |
| Zinc sulfate monohydrate | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 1 |
| Zinc sulfate/citrate complex | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 1 |
| Zinc sulfur | Mild astringent with inhibitory effects on copper absorption | O | Yes | Yes | 2 |
| TOTAL | 859 |
- a
Some arms are represented more than once because they employed more than one treatment.
Table 12. Treatment comparisons
| Target class | Comparator class | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inert | Cleanser (topical) | Keratolytic (topical) | Anti-bacterial (topical) | Anti-bacterial/keratolytic (topical) | Retinoid (topical) | Anti-bacterial/retinoid (topical) | Anti-bacterial (oral) | Anti-bacterial (oral)/keratolytic (topical) | Anti-bacterial (oral)/retinoid (topical) | Retinoid (oral) | Anti-androgen | Other | Total | |
| Cleanser (topical) | 2 | 2 | --- | 1 | --- | 1 | --- | --- | --- | --- | --- | --- | --- | 6 |
| Keratolytic (topical) | 7 | 1 | 2 | 1 | 4 | 3 | --- | 1 | --- | --- | --- | --- | 1 | 20 |
| Anti-bacterial (topical) | 10 | 1 | 1 | 7 | 6 | --- | --- | 5 | --- | --- | --- | --- | 3 | 33 |
| Anti-bacterial/keratolytic (topical) | 5 | --- | 5 | 6 | 14 | 2 | --- | 2 | --- | --- | 1 | --- | 6 | 41 |
| Retinoid (topical) | 5 | --- | 3 | --- | 2 | 11 | --- | 1 | --- | --- | --- | --- | 2 | 24 |
| Anti-bacterial/retinoid (topical) | 1 | --- | --- | 1 | --- | 1 | --- | --- | --- | --- | --- | --- | --- | 3 |
| Anti-bacterial (oral) | 12 | --- | 1 | 8 | 2 | 1 | --- | 20 | --- | --- | 1 | 1 | 3 | 49 |
| Anti-bacterial (oral)/keratolytic (topical) | --- | --- | --- | --- | --- | --- | 1 | 1 | --- | 1 | --- | --- | --- | 3 |
| Anti-bacterial (oral)/retinoid (topical) | --- | --- | --- | --- | --- | --- | --- | 2 | 1 | --- | --- | --- | --- | 3 |
| Retinoid (oral) | 2 | --- | --- | --- | --- | --- | --- | 2 | --- | --- | 4 | --- | 1 | 9 |
| Anti-androgen | 10 | --- | --- | --- | --- | --- | --- | 2 | --- | --- | --- | 10 | 1 | 23 |
| Other | 16 | --- | 1 | 2 | 5 | 3 | --- | 3 | --- | --- | --- | --- | 7 | 38 |
| TOTAL | 70 | 4 | 13 | 26 | 33 | 22 | 1 | 39 | 1 | 1 | 6 | 11 | 24 | 250 |
- a
Some arms are represented more than once because they employed more than one treatment.