NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
ECRI Health Technology Assessment Group. Treatment of Degenerative Lumbar Spinal Stenosis. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Jun. (Evidence Reports/Technology Assessments, No. 32.)
This publication is provided for historical reference only and the information may be out of date.
Scope and Purpose of This Report
The purpose of this report is to assess, in an evidence-based fashion, the efficacy of methods for the diagnosis and treatment of degenerative lumbar spinal stenosis. Lumbar spinal stenosis is defined as a focal narrowing of the spinal canal, although the precise amount of narrowing that must occur before the canal is considered stenotic differs among individuals (Alvarez and Hardy Jr, 1998; Bueff and Van der Reis, 1996; Fast and Greenbaum, 1995; Herkowitz, Abrahan, and Fischgrund, 1998; Herno, Saari, Suomalainen et al., 1999; Postacchini, 1996; Spivak, 1998) The general term "spinal stenosis" can be applied to three root compression mechanisms alone or in combination: (1) disk protrusion or herniation, (2) osteotic overgrowth into the spinal canal or the foramina through which the roots pass laterally, and (3) vertebral slippage or spondylolisthesis. Although symptoms overlap for these three mechanisms, the second category, osteotic stenosis, is specifically termed spinal stenosis; this category is the focus of this evidence report, with spondylolisthesis also being addressed.
In extreme cases, lumbar stenosis can cause cauda equina syndrome, which is characterized by severe neuromuscular, bladder, and rectal dysfunction, and is considered to require immediate surgery to prevent permanent nerve damage (Gunzburg and Szpalski, 1999). However, because many studies excluded patients with cauda equina syndrome, virtually no evidence related to this syndrome could be evaluated, and, therefore, consideration of cauda equina syndrome is beyond the scope of this evidence report.
This evidence report, therefore, focuses on less extreme manifestations of lumbar spinal stenosis and considers the evidence surrounding all aspects of this condition. This includes evidence concerning the natural history of lumbar stenosis. This is important because it is not firmly established that this condition is progressive, nor has it been firmly established that stenosis per se is responsible for the symptoms experienced by many patients. Among these symptoms are low back pain, radiculopathy, and neurogenic claudication (Alvarez and Hardy Jr, 1998; Bueff and Van der Reis, 1996; Fast and Greenbaum, 1995; Herkowitz, Abrahan, and Fischgrund, 1998; Herno, Saari, Suomalainen et al., 1999; Postacchini, 1996; Spivak, 1998).
This report also considers the methods used to diagnose lumbar spinal stenosis. Among these methods are myelography, computed tomography (CT), and magnetic resonance imaging (MRI). In evaluating these methods, the typical quantities used to gauge test performance (sensitivity, specificity, and positive and negative predictive values) are considered. Also considered is whether these methods can be used to predict which patients may respond to treatment. Evaluating the efficacy of diagnostic methods for spinal stenosis has many difficulties because imaging is often performed after medical management has proved unsuccessful. Therefore, a common use of imaging is for planning surgery.
Finally, treatments for lumbar spinal stenosis are considered. Included are both medical and surgical treatments. Our searches for information about both classes of treatments were comprehensive. In this analysis, particular attention is paid to patient-oriented outcomes (i.e., relief of symptoms). This is because reducing or eliminating the stenosis may not provide a concomitant reduction in the intensity of symptoms.
Epidemiology of Lumbar Spinal Stenosis
Epidemiology data on lumbar spinal stenosis come from several studies. The annual incidence of spinal stenosis observed in a Swedish study that defined spinal stenosis as a canal of 11 mm or less among patients referred to orthopedic departments was approximately 5 per 100,000 inhabitants (Johnsson, 1995).
In the National Low Back Pain Study (Long, BenDebba, Torgerson et al., 1996), records were examined for 2,374 patients with chronic low back pain. These patients sought help from orthopedic surgeons and neurosurgeons at eight academic medical centers across the United States from 1986 to 1991. Of these patients, 45.8 percent were male and 54.2 percent were female, with a mean age of 45.3 years (standard deviation 12.79, range 25 to 75). The proportions and categories diagnosed were 62 percent root compression, 19.6 percent myofascial syndrome, 18.7 percent instability, 2.1 percent postsurgical complications, and 19.1 percent other (see Table 1). Within the broad category of root compression, the final diagnoses were 59.2 percent herniated disk, 22.6 percent spinal stenosis, 19.7 percent lumbar spondylosis, 14.0 percent osteoarthritic root compression, and 9.8 percent nonherniated degenerated disk. In other words, 69 percent of root compressions were disk-related, and 57 percent were osteo-related (26 percent were both, so these percentages sum to 126 percent). Within the broad category of instability, the final diagnoses were 39.1 percent spondylolisthesis, 25.7 percent facet joint arthritis, 19.3 percent lumbar instability, 16.6 percent spondylolysis, 10.2 percent compression fracture, and 2.7 percent spina bifida. For patients with root compression, 54.5 percent were given conservative care, 30.4 percent were given surgery, and 13.3 percent were given no treatment. For those with instability, 70.6 percent were given conservative care, 15.3 percent were given surgery, and 14.1 percent were given no treatment.
Table
Table 1. National Low Back Pain Study Patient Characteristics and Syndromes.
From the above data, we calculate that of all of these patients seeking treatment for low back problems, 35 percent had osteo-related root compression and were possible candidates for bone-removing surgery (62 percent had root compression, and 57 percent of these had osteo-related compression: 23 percent spinal stenosis, 20 percent lumbar spondylosis, 14 percent osteoarthritic root compression). However, the severity of disease was not reported; thus, the proportion of these patients with disease severe enough to indicate surgery is not known.
The National Ambulatory Medical Care Survey (NAMCS) also provides data on the incidence of lumbar spinal stenosis in the US population (Hart, Deyo, and Cherkin, 1995). The NAMCS is an annual survey of 3,000 general physicians conducted by the National Center for Health Statistics and is intended to be representative of practicing, nongovernmental, office-based physicians in the United States. Over the period 1989 to 1990, the diagnostic cluster for low back pain ranked fifth in frequency among categories and accounted for 2.8 percent of patient visits. Only visits for hypertension, pregnancy, general medical exam, and acute upper respiratory infection ranked higher in frequency of reasons for visiting a physician. The survey estimated that 29,964,894 visits for mechanical back problems were made in the United States during this period. Of these visits, 56.8 percent were classified as nonspecific backache, 11.1 percent as herniated disk, and 3.9 percent as spinal stenosis. For the purposes of analyzing survey data, spinal stenosis was defined as lumbar stenosis or spondylogenic compression of the lumbar spinal cord or nerve roots.
The National Spine Network (NSN) provides another estimate of the prevalence of lumbar spinal stenosis (Fanuele, Birkmeyer, Abdu et al., 2000). Data on 17,774 patients from 25 centers that treat back and neck problems were examined in this study. The average patient age was 47.5 years (SD 15.4, range 17 to 98), 54.7 percent of patients were male, and 84.2 percent of patients were white. Among these patients, 13.1 percent were specifically diagnosed with spinal stenosis, 12.9 percent with degenerative spondylosis due to aging, and 19.2 percent with herniated disks.
A comparison of the data from these three studies indicates that among patients with low back pain who see a specialist, 13 percent to 14 percent may have spinal stenosis. These surveys also indicate that among patients with low back pain who see a general physician, 3 percent to 4 percent may have spinal stenosis (see Table 2). The NAMCS estimate of 3.9 percent of backache patients having lumbar spinal stenosis is probably the more reliable because this patient base comes from office-based physicians in the United States.
Table
Table 2. Comparison of Prevalence Rates for Lumbar Spinal Stenosis Presented in Three Separate Studies.
In regard to spondylolisthesis, in lateral radiographs taken for the longitudinal Framingham Heart Study (Kauppila, Eustace, Kiel et al., 1998), 1 percent (2/219) of men and 1.5 percent (6/400) women already had slippage at the baseline measurement at the mean age of 54 years. Over the following 25 years, 11 percent (23/217) of men and 25 percent (100/400) of women developed degenerative vertebral slippage (see Table 3).
Table
Table 3. Longitudinal Study of Development of Spondylolisthesis in Older Adults.
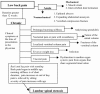
There are many etiologies for chronic low back pain, of which lumbar spinal stenosis is just one. Figure 1 provides a differential diagnosis that separates the typical symptoms of lumbar spinal stenosis from other potential causes of low back pain.
Natural History of Lumbar Spinal Stenosis
Shrinkage and narrowing of disks as well as osteoarthritic changes can lead to both spinal stenosis and spinal instability (Jane, Jane, Helm et al., 1996; Mirkovic, Garfin, Rydevik et al., 1992; Mirkovic, Garfin, Rydevik et al., 1992; Postacchini and Perugia, 1991; Rowe, 1969). Therefore, disk degeneration and osteoarthritis of the spine are discussed in this Natural History section as underlying conditions that can lead to spinal stenosis, even though treatment for disk herniation and rupture and osteoarthritis is outside the scope of our analysis.
Disk Degeneration
Ordinary wear and tear, combined with age-associated changes in the tissue composition of the spinal disks, lead to disk degeneration (see Figure 2). Type II collagen, the more hydrated form, decreases in proportion to type I collagen (Jane, Jane, Helm et al., 1996). Proteoglycan content declines (Mirkovic, Garfin, Rydevik et al., 1992). In the absence of hydrostatic proteoglycans, the water content of the disk declines. As the overall hydration of the disk decreases, the disk becomes less elastic and thus less able to evenly distribute the constantly shifting loads placed upon it (Prescher, 1998). Unevenly distributed stresses may lead to the development of tears and weaknesses in the disk. To a certain extent, this degeneration is an inevitable result of aging (Jane, Jane, Helm et al., 1996). Degeneration begins as early as the second decade in men and the third in women (Mirkovic, Garfin, Rydevik et al., 1992). By the age of 40, 80 percent of males' and 65 percent of females' disks are moderately degenerated (Mirkovic, Garfin, Rydevik et al., 1992).
In susceptible individuals, disk degeneration can lead to pain and damage to the spinal nerves. Whether joint degeneration leads to pathologic consequences depends upon the characteristics of the individual patient (Jane, Jane, Helm et al., 1996).
Osteoarthritis in the Spine
Incremental microdamage in the lower disks of susceptible patients ultimately results in venting and loss of pressure in the disk nucleus (Rowe, 1969). As the disk degenerates, it narrows, decreasing the distance between vertebrae. The distribution of forces in the joint is altered. The ligaments connecting the vertebrae become lax, destabilizing the joint. Instability and altered force distribution lead to mechanical stress, which in turn can cause osteoarthritic changes in the articular processes (Fast and Greenbaum, 1995). Consequently, the vertebral facets become enlarged, the vertebral pedicles thicken, and the ligamentum flavum thickens. Type II collagen replaces elastic tissue (Jane, Jane, Helm et al., 1996; Schrader, Grob, Rahn et al., 1999), and calcium crystals are deposited (Schrader, Grob, Rahn et al., 1999). Hyalinization of the collagen fibers and proliferation of chondrocytes also contribute to the ossification of the ligament. Facet hypertrophy, thickening of the pedicles, and ossification of the ligamentum flavum lead to narrowing of the central spinal canal. Traction spurs may develop. These spurs can also impinge on the spinal canal or the nerve roots.
Eventually, vertebral stability may be regained as scarring occurs across the nuclear compartment (Rowe, 1969). Osteoarthritic changes may also lead to increased stability or even fusion between two vertebrae (Postacchini and Perugia, 1991; Rosenberg, 1975).
In susceptible individuals, the degenerative changes in the facet joints lead to two overlapping pathological and clinical entities: central and lateral stenosis. The two conditions may not be distinguished by their symptoms (Amundsen, Weber, Lilleas et al., 1995). The extent to which the degree and location of stenosis correlates with the nature, intensity, and location of symptoms is unclear. Individuals are frequently observed to have marked stenosis and no symptoms (LaRocca and Macnab, 1969; Nagler and Bodack, 1993; Postacchini and Perugia, 1991; Splithoff, 1953). Among patients with symptoms, long periods of remission are thought, at least by some, to be common (Rosenberg, 1976). However, the incidence and duration of these periods of remission are not well studied.
Degenerative Central Lumbar Stenosis
Lumbar spinal stenosis can be separated into three broad categories, specifically central stenosis, lateral stenosis, and spondylolisthesis. We have used these categories in our analysis to organize the literature and assist in combining evidence. Specific consideration of the etiology of the stenosis is important because different conservative and/or surgical treatments may be more or less effective depending on the nature of the stenosis.
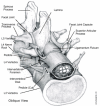
Central stenosis refers to a narrowing of the spinal canal across the anteroposterior diameter, the transverse diameter, or both (Gunzburg and Szpalski, 1999; Postacchini, 1999; Woolsey, 1986). The central canal is enclosed anteriorly by the posterior portion of the vertebral body and the vertebral disk and posteriorly by the lamina and the base of the spinous process (see Figures 3, 5, and 6).

Figure
Figure 3. Top View of a Normal Lumbar Vertebra.
Figure
Figure 5. Oblique View of the Normal L3 to L5 Lumbar Vertebrae.

Figure
Figure 6. Pathology Associated with Lumbar Spinal Stenosis.
Degenerative Lateral Stenosis
Entrapment and compression of the nerve root in its pathway through the spine, referred to as the nerve root canal, is termed lateral stenosis (Gunzburg and Szpalski, 1999; Jenis and An, 2000; Postacchini, 1999; Woolsey, 1986). The nerve root canal begins where the nerve root exits the dura and ends where the nerve root leaves the intervertebral foramen. The nerve root canal is bordered by the pedicle of the vertebra above and the pedicle of the vertebra below. The anterior side of the canal is formed by the vertebral body and vertebral disk. The posterior side of the canal is formed by the facet joint structures of the vertebrae above and below (see Figures 3, 4, and 5). Lateral stenosis occurs when the spinal nerve is compressed within the nerve root canal and/or the vertebral foramina (Fritz, Delitto, Welch et al., 1998). As the disk narrows, the pedicle may move in an inferior direction, narrowing the lateral recess and pinching the spinal nerve (Jane, Jane, Helm et al., 1996; Mirkovic, Garfin, Rydevik et al., 1992). MacNab (1977) originally described this entrapment and compression of the nerve root between a diffuse lateral bulge of the disk and the pedicle above as pedicular kinking. Narrowing of the lateral recess can also be the result of facet hypertrophy or enlargement and ossification of the ligamentum flavum. Radiculopathy, or decreased function of a nerve root, is commonly observed with lateral stenosis. Impingement of the disk into the lateral recess is considered a separate condition (Amundsen, Weber, Lilleas et al., 1995) and is beyond the scope of this evidence report.
Degenerative Spondylolisthesis
Degenerative spondylolisthesis is a slippage of one lumbar (L) vertebra over an adjacent vertebra and commonly occurs between L4-L5 (Woolsey, 1986). When L4 slips forward, the central canal and the nerve roots become entrapped between the posterior body of L5 and the inferior articular facets of lamina of L4 (see Figure 7).
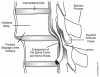
Figure
Figure 7. Spondylolisthesis Showing Entrapment of Spinal Canal and Nerve Roots.
Disk degeneration may lead to spinal instability. Decreased disk height and degenerative changes in the angles of the facet joints contribute to this instability (Mirkovic, Garfin, Rydevik et al., 1992; Postacchini and Perugia, 1991; Rowe, 1969). This can lead to spondylolisthesis, or displacement of the vertebrae. Lumbar vertebrae have a tendency to be displaced forward due to their normal anterior curve and the action of gravity, muscular force, and other forces (Kim and Lee, 1995). As the vertebra slips forward, the medial edge of the superior facet may encroach on the central canal (Postacchini and Perugia, 1991). Distal to the slipping vertebra, the dural sac becomes trapped between the intervertebral disk and the posterosuperior angle of the underlying vertebral body on one side and the advancing neural arch of the slipping vertebra on the other. This is most commonly observed at the L4-5 level (Herkowitz, 1995; Newman, 1976). The anatomical reasons for the greater likelihood of degeneration at L4-5 have been described by MacGibbon and Farfan (MacGibbon and Farfan, 1979). Lateral stenosis may also result from spondylolisthesis when the nerve root becomes compressed between the inferior facet of the superior vertebra and the vertebral body of the inferior vertebra (Newman, 1976).
Further remodeling of the articular processes may eventually limit the vertebral slippage (Postacchini and Perugia, 1991). The degree of slippage usually does not exceed 20 percent to 30 percent of the width of the inferior vertebra (Herkowitz, 1995; Postacchini and Perugia, 1991; Rosenberg, 1975).
Spinal Stenosis and Spondylolisthesis in the Asymptomatic Population
As mentioned above, not all patients with spinal stenosis are symptomatic. Five studies (Boden, Davis, Dina et al., 1990; Healy, Healy, Wong et al., 1996; Jensen, Brant-Zawadzki, Obuchowski et al., 1994; Parkkola, Rytokoski, and Kormano, 1993; Wiesel, Tsourmas, Feffer et al., 1984) have looked at the proportion of the asymptomatic adult population that has spinal stenosis upon imaging (see Table 4). Their results suggest that 3.3 percent to 5 percent of the adult population have central stenosis but is asymptomatic; 7 percent to 16 percent have neural foramen stenosis or narrowing. The proportion of stenotic, asymptomatic individuals was larger in older age groups (Boden, Davis, Dina et al., 1990), 21 percent (3/14) of adults age 60 and over, versus <1 percent (1/53) of adults below age 60.
Table
Table 4. Spinal Stenosis in Asymptomatic Adults.
Although the presence of apparent stenosis in the asymptomatic population raises a question about whether stenosis per se causes symptoms, those with more severe symptoms are more likely to have stenosis. In one study, stenosis was observed by imaging in 50 percent (5/10) of patients with severe low back pain, 11 percent (4/38) of patients with less severe low back pain, and 3.3 percent (2/60) of asymptomatic controls matched to the back pain patients by sex, age, employment, and profession (Parkkola, Rytokoski, and Kormano, 1993).
There is also evidence that not all individuals with vertebral displacement are symptomatic. Based on lateral radiographs taken for the longitudinal Framingham Heart Study (Kauppila, Eustace, Kiel et al., 1998), at the mean age of 79, 19 percent (90/483) of patients without vertebral slippage had back pain or stiffness on most days compared to 32 percent (39/123) of patients with slippage (χ2 = 10.5, p = 0.001). This latter proportion implies that 68 percent of patients with slippage did not have chronic back pain. From this, we were able to calculate that 17.6 percent (84/477) of patients without chronic back pain had degenerative spondylolisthesis (see Table 5).
Table
Table 5. Spondylolisthesis in Asymptomatic Older Adults.
The presence of stenosis and slippage in spinal images of asymptomatic people indicates that treatment must be based on the convergence of symptoms and image evidence rather than on either type of evidence alone. And in spite of the relationship between image evidence and more severe symptoms, the possibility exists that, even in more severe cases, treatment based on the image and symptoms may actually address a coincidental condition without eradicating the actual cause of symptoms.
Burden of Disease
Although there is some literature on the burden of disease of low back pain in general, we could find only one study of the societal burden of disease specifically for spinal stenosis. The 1995 population study of two regions in Sweden (Johnsson, 1995) reported that with a spinal stenosis incidence of about 50 per 100,000, between 42 percent and 58 percent of these patients had claudication (leg pain or weakness upon standing or walking). From these data, the incidence was calculated as about 25 per 100,000 inhabitants for spinal-stenosis-associated claudication. More severe stenosis can result in cauda equina syndrome, which is characterized by the loss of sexual function and urinary and/or fecal incontinence. This same study reported that cauda equina syndrome had an incidence of less than 1 per 100,000.
Review articles (Alvarez and Hardy Jr, 1998; Spivak, 1998; Tatarek and Nancy Elizabeth, 2000) and textbooks (Gunzburg and Szpalski, 1999; Kirkaldy-Willis and Bernard, 1999) provide anecdotal evidence that individual patients with symptomatic spinal stenosis typically have chronic low back pain and pain and weakness in the legs that limits standing and walking to brief durations and short distances. This places limitations on the ability to carry out self-supporting daily activities as well as work, social, and recreational activities. This lack of activity may lead to obesity and general physical deterioration that may eventually result in the onset of cardiovascular and other serious health problems. These activity restrictions may also lead to depression and other psychological problems. More severe stenosis can result in cauda equina syndrome. If untreated, severe spinal stenosis is commonly believed to have the potential to result in severe symptoms that may become permanent and unresponsive to medical or surgical treatment (Dawson and Bernbeck, 1998; Hirsch, 1966; Johnsson, Uden, and Rosen, 1991; Johnsson, Uden, and Rosen, 1992; Kirkaldy-Willis and Bernard, 1999; Nagler and Bodack, 1993; Onel, Sari, and Donmez, 1993; Porchet, Vader, Larequi-Lauber et al., 1999; Postacchini, 1988; Shakil, Vaccaro, Albert et al., 1999; Shapiro S, 2000; Shapiro, Cooper, and Miller, 1993; Wiltse, 1977; Wisneski and Farmer, 1994). However, we could find no data supporting this belief except for acute onset of symptoms with herniated disks (Shapiro S, 2000; Shapiro, Cooper, and Miller, 1993) or postoperative complications (McLaren and Bailey, 1986).
Patterns of Care
The rates of lower back surgery and of surgery for spinal stenosis increased dramatically during the 1980s (Ciol, Deyo, Howell et al., 1996). Data from the National Hospital Discharge Survey showed an age-adjusted 40 percent increase in lumbar spine surgery between 1979 and 1992 from 113 per 100,000 to 132 per 100,000. The increase for older patients was even greater, from 51 per 100,000 to 188 per 100,000, a 3.7-fold increase. This latter increase was attributed mainly to an increase in surgery for spinal stenosis, from 7.8 per 100,000 to 61.4 per 100,000, an almost eight-fold increase. Most of these increases occurred in the early 1980s and appeared to be leveling off by the early 1990s (Davis, 1994); however, we found no published systematic data on back surgery rates during the late 1990s.
According to Medicare records (patients 65 and over), the rate of spinal stenosis surgery in 1989 varied among states by more than four-fold, from 30 per 100,000 in Rhode Island to 132 per 100,000 in Utah (Ciol, Deyo, Howell et al., 1996). The rates were generally highest in Pacific and Mountain states and lowest in New England, Mid-Atlantic, and Southeastern states. While some of this divergence may result from socioeconomic factors, the geographic variations suggest that the divergence is substantially caused by the limited consensus regarding surgical indications for spinal stenosis (Ciol, Deyo, Howell et al., 1996).
Reoperation is an important part of lower back surgeries. A population-based cohort study of patients receiving lower back surgery in 1988 in Washington State reported that within five years there was an 18 percent reoperation rate for patients receiving fusion surgery and a 15 percent reoperation rate for those receiving nonfusion lower back surgery (Malter, McNeney, Loeser et al., 1998).
Ciol and colleagues used the United States Health Care Financing Administration data for the cohort of Medicare beneficiaries who received lumbar spine surgery in 1985 to estimate the rate of reoperation for this type of surgery (Ciol, Deyo, Kreuter et al., 1994). All hospitalizations up to four years after the surgery were examined for repeat surgery. After excluding for malignancy, infection, fracture, trauma, and Medicare eligibility based on end-stage renal disease, 27,111 patients were identified as having lumbar spine surgery related to spinal stenosis (39.3 percent), herniated disk (41.4 percent), degenerative disk disease (11.6 percent), or possible instability (3.3 percent). The average patient age was 71.8 years (range 59 to 97), 57.1 percent were women, and 92.7 percent were white. After four years, the rate of reoperation for lumbar spinal stenosis was approximately 10 percent and the reoperation rate for herniated disks was approximately 12 percent.
Diagnostic Tests
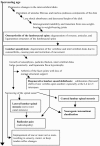
Patients with lumbar spinal stenosis usually undergo a "staged" diagnosis (see Figure 8). The first diagnostic stage is the physician visit, during which the patient receives a physical examination. Results of the physical are combined with information from the patient history in a preliminary diagnosis. Lumbar spinal stenosis is not definitively diagnosed at this stage, so the diagnostic results are described as "consistent with" spinal stenosis or not consistent with spinal stenosis.
Although clinicians consider a combination of results of the history and physical examination and imaging findings to be the most effective means of diagnosing lumbar spinal stenosis, no objective criteria for using the history and physical examination have been reported. In addition, there are no reported clinical trials of the effectiveness of such a composite diagnosis. The only quantitative evidence correlating diagnostic information with outcomes is for the imaging findings. This absence of evidence limits the analysis that can be performed for some of the research questions.
History and Physical Examination
Katz et al. (1995) examined the value of the history and physical examination in the diagnosis of degenerative lumbar spinal stenosis. In this study, 93 patients over 40 years of age with symptoms of low back pain were examined by attending physicians who were then asked the extent to which they were certain the patient had lumbar spinal stenosis. The diagnostic impressions of expert clinicians and imaging, when available, were used as a reference standard to evaluate the attending physician's diagnosis. Severe lower extremity pain, absence of pain when seated, a wide-based gait, thigh pain following 30 seconds of lumbar extension, and neuromuscular deficits were all strongly associated with patients with lumbar spinal stenosis. No pain when seated and wide-based gait had the highest specificity, 93 percent and 97 percent, respectively. The highest sensitivity came from age greater than 65 (77 percent), pain below buttocks (88 percent), and no pain with flexion (79 percent) (Katz, Dalgas, Stucki et al., 1995).
Fritz et al. (1997) have developed a treadmill test as a clinical diagnostic tool for the differentiation of neurogenic claudication due to lumbar spinal stenosis from other pathologies that may produce similar symptoms. Spinal extension and weight bearing that occur during walking narrow the spinal canal and exacerbate the symptoms of lumbar spinal stenosis. Spinal flexion or nonweight-bearing postures that occur while sitting increase the dimensions of the spinal canal and reduce symptoms. The treadmill test involves having the patient walk on a level surface and an inclined surface. The time until onset of symptoms, total walking time, and time until symptoms return to baseline are recorded for each surface. Walking on an inclined plane produces spinal flexion and may be better tolerated by patients with lumbar spinal stenosis. The treadmill test was evaluated using 45 subjects with low back pain of varying etiologies and self-reported limitations in walking. Diagnostic images with MRI or CT were used as the gold standard for diagnosis. Twenty-six of the subjects were diagnosed by imaging as being stenotic. Self-reported sitting to relieve symptoms was significantly related to diagnosis. The sensitivity of this self-reported measure was 88.5 percent (95 percent confidence interval [CI] of 76.2 to 100), but specificity was 38.9 percent (95 percent CI of 16.4 to 61.4). For the treadmill test, earlier onset of symptoms with level walking, greater total walking time during inclined walking, and prolonged recovery after level walking were significantly related to a diagnosis of lumbar spinal stenosis. The sensitivity and specificity for earlier onset of symptoms with level walking were 68.0 percent (95 percent CI of 49.7 to 86.3) and 83.3 percent (95 percent CI of 66.1 to 100), respectively; for larger total walking time during inclined walking, they were 50.0 percent (95 percent CI of 37.5 to 62.5) and 92.3 percent (95 percent CI of 77.8 to 100), respectively; and for prolonged recovery after level walking, they were 81.8 percent (95 percent CI of 5.7 to 97.9) and 68.4 percent (95 percent CI of 47.5 to 89.3), respectively. The authors concluded that a two-stage treadmill test might be more useful in the differential diagnosis of lumbar spinal stenosis compared to patients' self-reports of posture (Fritz, Erhard, Delitto et al., 1997).
Use of the treadmill-bicycle test for the differential diagnosis of neurogenic claudication was also examined by Tenhula et al. (2000). In this study, 32 patients with documented lumbar spinal stenosis were evaluated before and after surgery. Patients were found to have a significant increase in their symptoms from the start to the end of the treadmill test but fewer patients were found to have significant symptoms on bicycle testing. Two years after surgery, patients had an improvement in their walking ability on treadmill testing, but showed no improvement in their ability to bicycle. The authors believe the treadmill-bicycle test may be a useful tool for the differential diagnosis of neurogenic claudication (Tenhula, Lenke, Bridwell et al., 2000).
Imaging examinations appear to be used primarily in a second diagnostic stage. At this stage, some surgical intervention is usually under consideration, so the imaging examination is as much for surgical planning as it is for confirmation of the preliminary diagnosis. In chiropractic care, a plain x-ray image may initially be obtained to aid in chiropractic therapy even if surgery is not planned (DuPriest, 1993).
In clinical practice, the imaging results help to confirm the diagnosis of spinal stenosis after the history and physical indicate the likelihood of spinal stenosis. Clinical trials interchangeably use myelogram, CT, or MRI results for confirmation of the diagnosis. This is partly because spinal stenosis is defined in terms of the anatomy displayed in the images. Also, there is no other means of verifying the results, short of measurement of the spinal canal during surgery. As discussed in the next section, plain film radiography is not considered a definitive standard for use in diagnosing lumbar spinal stenosis (Widelec, Bacq, and Peetrons, 1999). Because no independent means is available to confirm that imaging results are right or wrong, assessment of the performance of any of the imaging modalities for diagnosis of lumbar spinal stenosis is not possible in the same way we usually assess diagnostics. This is particularly true for negative cases, in which there would not be subsequent surgery on the spine.
Diagnostic Imaging Modalities
Radiography
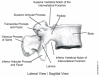
The first imaging modality used to diagnose and evaluate lumbar spinal stenosis was radiography: film-based x-ray imaging, colloquially known as "plain film." The typical lumbar spine examination consists of AP (anteroposterior: front to back), lateral (side to side; see Figure 4), and oblique (diagonal; see Figure 5) views (Widelec, Bacq, and Peetrons, 1999). Film radiography has excellent spatial resolution for displaying small anatomic details but has several characteristics that limit its value in diagnosing spinal stenosis. First, it is a projection method: small anatomic details may be obscured by overlapping structures. Obtaining multiple views can sometimes resolve those structures. Second, the soft-tissue contrast of radiography is relatively low. Bones are depicted very clearly on plain radiographs, so they are frequently used to rule out vertebral fracture, if it is suspected.
Figure
Figure 4. Lateral View of a Normal Lumbar Vertebra.
Plain radiographs depict the spinal canal well enough for measurement of its diameter. The lateral radiograph is also useful for diagnosing spondylolisthesis, forward/backward displacement of a vertebra (Sackett, 1994; Wood, Popp, Transfeldt et al., 1994). For measuring displacement, radiography is considered the gold standard.
Myelography
Intradural contrast-enhanced radiography of the spine is known as myelography. To obtain a myelogram, a radiopaque contrast agent is injected into the spinal canal, and x-ray images are taken. The contrast agent diffuses through the spinal canal, outlining it quite clearly on the myelogram, but if stenosis causes a complete blockage of the spinal canal, no contrast agent will flow below (inferior to) the stenosis. Modern contrast agents for myelography are water soluble and contain iodine. Some patients are sensitive to iodine and may have an allergic reaction to the contrast material. Myelography is contraindicated in these patients. Adverse side effects are common in myelography. About half of patients experience head or neck pain, and 15 percent experience nausea or dizziness. Nearly all patients find myelography uncomfortable and will not be able to resume some normal activities, like driving, until the day after the procedure (American College of Radiology. 1999; Mitchell, 2000; Ramsbacher, Schilling, Wolf et al., 1997).
Spinal stenosis is traditionally defined by its appearance on a myelogram. Complete blockage is as described above. Partial blockage is a stenosis where the film shows a complete interruption of the column of contrast agent at the point of focal narrowing (i.e., the stenosis), but enough contrast agent gets through the stenosis to opacify the spinal canal below (inferior to) the stenosis. Stenoses of lesser degree are defined by the diameter of the contrast agent column at the stenosis. This is usually measured in the AP dimension, as viewed on the lateral film. The threshold measurement used to define stenosis varies from investigator to investigator (see Table 17 and Question 7 in Chapter 3).
Table
Table 17. Surgical Trials Reporting Imaging Results.
The myelogram has long been considered a gold standard for imaging spinal stenosis and helping to confirm the diagnosis, but it has been supplanted by the cross-sectional modalities CT and MRI. Recent clinical handbooks now recommend MRI rather than plain films or myelography as the primary imaging modality in cases of suspected spinal stenosis. Myelography is now rarely done in routine clinical practice (Eisenberg and Margulis, 2000b; Grossman, Katz, Santelli et al., 1994; Gundry and Heithoff, 1999; Mitchell, 2000).
Computed Tomography
Because it is a three-dimensional modality, CT avoids the overlapping-structure problem of film-based radiography. But CT is still an x-ray modality, and bones will appear more distinct than soft tissue on a CT scan. CT is limited to axial images (cross sections as viewed from the patient's head to toe; see Figure 3), but all currently available CT scanners have tilting gantries that allow the imaging plane to be tilted 20° to 30° in each direction (ECRI. 1999). For many patients, this permits acquisition of images parallel to the disks, so the entire disk is in one image, although the vertebral column is not straight up and down.
CT scans can be acquired following administration of contrast agent, just like radiographs. Because the three-dimensional images resolve overlapping structures, there is less need for contrast agent in CT scans of the spine. Also, smaller quantities of the contrast agent are necessary with CT, but CT can be done following a myelogram with its larger contrast agent dose.
Magnetic Resonance Imaging
While it depicts anatomy in cross-section as CT does, MRI is based on completely different physical principles. A full explanation of how MRI works is beyond the scope of this report. Briefly, MRI derives contrast primarily from differences in the T1 and T2 relaxation times of hydrogen nuclei in the body. Those differences stem from differences in the chemical environment of water in different types of cells. Parameters for magnetic resonance (MR) image acquisition ("pulse sequences") can be varied to emphasize T1 or T2 or to base contrast on some combination of the two. Usually, MR examinations comprise several sets of images using several different pulse sequences. As with all other MRI applications, some pulse sequences are more effective than others for imaging the spine (Ramsbacher, Schilling, Wolf et al., 1997). Because technical characteristics of different MR scanners are different, the pulse sequences that are ideal for one scanner may not be ideal for another scanner.
Besides the advantage of soft-tissue contrast, MRI has the advantage of being able to acquire images in any user-selected plane. Sagittal sections (cross sections as viewed from the patient's left to right; see Figure 4) are particularly useful in spine imaging, and it is quite easy to select axial image planes parallel to the disks. Contrast-enhancement agents are also available for MRI, but instead of containing iodine or other atoms of high atomic number like x-ray contrast agents, MR contrast agents contain paramagnetic ions like gadolinium. Because MR images inherently provide good contrast of the spine and its contents, contrast agents are not usually necessary in MRI of the spine. Studies described as "MR myelography" do not necessarily involve contrast agents (Ramsbacher, Schilling, Wolf et al., 1997).
The chief disadvantage of MRI is that its spatial resolution is not as good as that of film radiographs (Ramsbacher, Schilling, Wolf et al., 1997). MRI can also be susceptible to spatial distortions that would cause errors in quantitative measurements of the vertebrae, but adherence to routine quality assurance procedures at the imaging center will minimize distortions.
Ultrasound
Ultrasound can also generate cross-sectional images, but it is not well suited to imaging of the spine and contents. Ultrasound waves do not penetrate bone, so imaging can only be done through "windows" like the ligamentum flavum (Pai, 1993). Adequate windows cannot be found in some patients, so their spinal canal cannot be visualized with ultrasound. One of the two clinical trials of spinal ultrasound reported that this problem is worse in individuals with spinal stenosis (Pai, 1993). In one-third of the measurements reported in this trial, ultrasound results differed from radiographic results (considered the reference standard) by 2 mm or more. The other published ultrasound trial did not report quantitative measurements (Tait, Charlesworth, and Lemon, 1985). Ultrasound is inexpensive and safe, but it lacks accuracy in quantitative measurement of the spinal canal.
Imaging of the Spine in Typical Practice
Recent textbooks and clinical handbooks of radiology identify an important shift in clinical practice for diagnosis of spinal stenosis, herniation of the disks, and other conditions of the lumbar spine. The cross-sectional modalities, particularly MRI, are now considered the primary imaging tools for diagnosis of spinal stenosis (Eisenberg and Margulis, 2000a; Eisenberg and Margulis, 2000b; Grossman, Katz, Santelli et al., 1994; Gundry and Heithoff, 1999; Kirkaldy-Willis and Bernard Jr, 1999; Mitchell, 2000; Spengler, 2000; Widelec, Bacq, and Peetrons, 1999; Wilmink, 2000). Reasons cited for the shift include multiplanar imaging capabilities, better ability to show bulging or fragmentation of the disks, and the risks and discomfort of myelography. These books should not be considered evidence based. They represent "conventional wisdom" among radiologists, which is shaped in part by clinical trials and other evidence. In fact, Wilmink notes the absence of conclusive evidence that MRI is the most effective modality for diagnosing spinal stenosis (Wilmink, 2000).
Official guidelines developed by professional specialty societies like the American College of Radiology (ACR) and the Canadian Association of Radiologists (CAR) are also based on a mix of scientific evidence and expert opinion. CAR states that "CT and MRI have replaced myelography as the examination of choice for the above [spinal stenosis and other myelographic] indications" (Fontaine, Lee, Maloney et al., 1996). Myelography would still be an appropriate choice if the patient cannot be imaged by CT or MRI due to unavailability of a scanner, metal implants, or patient size or noncompliance. Appropriateness criteria from the ACR do not specifically address diagnosis of spinal stenosis, but for various types of nontraumatic myelopathy, MRI is considered very appropriate (8 on a scale of 1 to 9), and CT and myelography are considered less appropriate (usually 4 on the same scale) (American College of Radiology, 1998).
Plain films still have a role in diagnosis of spinal disorders; they may be all that is necessary to diagnose vertebral fracture and/or spondylolisthesis (Crosby and Brant-Zawadzki, 1994; Grossman, Katz, Santelli et al., 1994), and they are capable of measuring the diameter of the spinal canal (Sackett, 1994). Plain radiographs are considered "appropriate" by ACR in some clinical circumstances (6 or 7 on a 9 point scale) (American College of Radiology, 1998).
All the imaging modalities are adversely affected by surgical hardware and other metal objects implanted in the body. These objects are radiopaque; so they obscure overlapping structures in plain x-rays and myelograms. The effect is heightened in CT scanning, where the metal objects cause artifacts that can affect the entire image. Metal objects also affect MR scans, although most orthopedic devices are nonmagnetic and do not completely contraindicate MRI. Metal causes susceptibility artifacts: there is no signal from the object itself, and portions of the image near the object may be distorted or lose signal. The magnitude of this effect depends on the size and placement of the object and its composition (Fredrickson, 2000). Since diagnosis among patients who already have had surgery is outside the scope of this report, the studies we examined for this technology assessment did not deal with artifacts from metal implants.
All imaging modalities are subject to variation in image quality and diagnostic effectiveness and variation in interpretation by the radiologist. We located one study that addresses how variations in image quality affect the diagnosis of spinal stenosis. Jarvik et al. (2000) examined variations in the quality of lumbar spine MR images, and attempted to correlate them with characteristics like the magnetic field strength of the scanner and the ownership and siting of the scanner. Three readers rated the quality of 69 examinations from 17 centers. Readers were blinded to identifying information on the images, including information that could identify the center or the type of scanner used. The readers graded each examination on a four-point scale for the sharpness with which each of seven clinically important structures was depicted. They also assigned an overall quality score to each examination, using the same scale. The average overall quality rating for each center ranged from 1.96 to 3.56, while the average rating of the seven structures from each center ranged from 2.25 to 3.82 (Jarvik, Robertson, Wessbecher et al., 2000).
Jarvik et al. (2000) found that image quality was significantly decreased by the following characteristics: low magnetic field strength (less than 1.0 tesla), location of the scanner outside a hospital, and for-profit ownership of the scanner. The number of radiologists interpreting images at a center and the percentages of images reviewed by a physician before the patient was dismissed had smaller but still statistically significant effects on study quality. The authors note that they were unable to measure some characteristics that would be expected to affect study quality, such as training and experience of the radiologists. However, the results of this study are consistent with the idea that the quality of diagnostic imaging examinations, especially complicated ones like MRI, should not be taken for granted.
Finally, image quality should not be mistaken for diagnostic effectiveness, although poor image quality can impede effective diagnosis. While one modality may be able to depict structures with more detail and more contrast than another, the modality of lesser "quality" may still depict the anatomy clearly enough to permit a correct diagnosis. For this reason, we base our evaluation of diagnostic imaging modalities on their diagnostic results, not on subjective or objective measurements of image characteristics.
Surgical Planning
Imaging data are also necessary for planning surgical treatment. The surgeon needs to determine the extent of decompression, identify the bony anatomy, and measure the extent of the stenosis (Gunzburg and Szpalski, 1999). Accurate location of the stenosis is essential to avoiding operation at the wrong lumbar level. Identification of vertebral defects, anatomic landmarks, and other bone anatomy is necessary to minimize complications (Bernard and Yong-Hing, 1999). Selection of the correct type, size, and placement of orthopedic hardware is aided by preoperative imaging studies (Visarius, 2000). Both plain x-rays and CT or MRI are often needed in surgical planning (Lazennec, Ramare, Arafati et al., 1999); the specific combination of modalities varies from surgeon to surgeon and from patient to patient. There is no single preoperative imaging plan that is appropriate for all spinal stenosis patients.
Assessment of this indication for spinal imaging is complicated by the absence of any clinical trials measuring the effectiveness of the various imaging modalities in surgical planning.
Summary
Diagnostic imaging data are essential in planning for surgical treatment for lumbar spinal stenosis and related conditions. No one procedure or combination of procedures is right in every case, and multiple imaging modalities are used in most cases. Images obtained at the time the diagnosis of the patient's condition is confirmed are usually sufficient for surgical planning, in part because part of the purpose of those imaging examinations is surgical planning. No clinical evidence is currently available on the effect of any particular imaging modality on surgical outcomes.
- Introduction - Treatment of Degenerative Lumbar Spinal StenosisIntroduction - Treatment of Degenerative Lumbar Spinal Stenosis
Your browsing activity is empty.
Activity recording is turned off.
See more...