NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Myers ER, Barber MW, Couchman GM, et al. Management of Uterine Fibroids. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Jul. (Evidence Reports/Technology Assessments, No. 34.)
This publication is provided for historical reference only and the information may be out of date.
The results of the data collection and analysis process described in the preceding chapter are presented below. Results are reported separately for each key question using the following general format:
- Approach: Some questions required either further clarification in consultation with the Agency for Healthcare Research and Quality (AHRQ), the American College of Obstetricians and Gynecologists (ACOG), and the advisory panel or some modification of the approach described in Chapter 2 of this report.
- Results: This section describes the number of studies that provided information relevant to the question and summarizes the results of those studies. In some cases, summaries are provided in tabular form, either for ease of reading (in the case of large numbers of studies) or to highlight particular study characteristics.
- Methodological issues: Methodological issues that affected our ability to draw conclusions from the evidence are highlighted.
- Summary: Data addressing the question are summarized, or alternatively, we note the lack of relevant data.
Question 1: What are the risks and benefits of hysterectomy and myomectomy in the treatment of symptomatic and asymptomatic fibroids?
Approach
In the absence of randomized trials comparing hysterectomy and myomectomy in women with symptomatic fibroids, we reviewed the literature on myomectomy and hysterectomy outcomes separately, as well as the few studies that compared nonrandomized patients undergoing the two procedures.
We did not identify any studies that specifically described outcomes in women undergoing surgery for asymptomatic fibroids. However, because many women may be advised to undergo such surgery -- 11 percent of all of the hysterectomies done for fibroids in the Maine Women's Health Study were because of physician concern about delaying treatment (Carlson, Miller, and Fowler, 1994a) -- we reviewed the potential arguments for and against such surgery and examined the literature on prophylactic myomectomy and hysterectomy (see Evidence Table 1).
Myomectomy for Symptomatic Fibroids
The overwhelming majority of studies reporting outcomes of myomectomy were case series. Table 10 presents results for outcomes other than those related to infertility or pregnancy complications (which are discussed in detail under Question 8). Six of 11 studies of laparoscopic myomectomy, 18 of 22 studies of hysteroscopic myomectomy, 0 of 8 studies of abdominal myomectomy, and 2 of 2 studies of vaginal myomectomy reported changes in preoperative symptoms. All studies that reported such results described improvement, but lack of reported detail and variation in the methods used to assess symptoms and in the length of followup prevented any synthesis of the data.
Transfusion was the most commonly reported short-term complication, with rates varying from 1.2 percent to 18 percent. However, wide variations in practice setting and local practices, as well as time and the availability of autologous blood, probably influenced these rates. Uterine perforation and fluid/electrolyte disturbances are a unique risk with hysteroscopic procedures; again, lack of comparability between studies precluded quantitative estimation of this risk.
Only three studies, all of hysteroscopic procedures, reported long-term adverse outcomes. Two reported the development of intrauterine scars (Asherman's syndrome) in up to 10 percent of patients. This may not be a concern for women not planning future pregnancy, but it would have an adverse effect on women's ability to conceive.
Methodological Issues
Numerous factors prevented us from estimating the overall likelihood of beneficial and adverse outcomes from myomectomy, including:
- Variable and often nonstandardized methods for reporting pre- and postintervention symptoms: The lack of standard definitions for reporting menstrual blood loss or symptoms, as well as variability in the timing of the measurement of these symptoms, prevented any meaningful comparison between studies. Without knowing the severity of the baseline symptoms, measured by a common metric, readers cannot assess the impact on those symptoms of two different interventions or the same intervention performed in different places and settings.
- Variations in surgical technique, expertise, and experience: Adjunctive techniques appear to influence short-term outcomes. Randomized trial data suggest that the use of gonadotropin-releasing hormone (GnRH) agonists prior to surgery (Lethaby, Vollenhoven, and Sowter, 1999) and the use of vasopressin during myomectomy (Fletcher, Frederick, Hardie, et al., 1996; Hutchins, 1996) reduce blood loss. The use of barrier technologies may reduce adhesion formation, which in turn may help subsequent fertility (Farquhar, Vandekerckhove, Watson, et al., 2000). Other details of surgical technique that are not reported could conceivably affect short-term outcomes, especially complications. Surgical volume or experience with the procedure also may influence outcomes -- a correlation between volume and reduced complications has been shown for multiple surgical procedures (Harmon, Tang, Gordon, et al., 1999; Ho, 2000; Yao and Lu-Yao, 1999). This may affect the generalizability of findings for a particular procedure -- outcomes for specialized procedures, such as laparoscopic myomectomy, may not be as good for surgeons with less experience, or less volume, compared with the group reporting their results. In this case, even with the best quality reporting, one can only judge the outcomes of a particular procedure performed in a specific setting by a specific surgical team, not the outcomes of the procedure itself.
- Variable length of followup and variations in reporting length of followup: Again, comparison was prevented by the lack of common times for measuring postintervention outcomes or by imprecise reporting.
Summary
Excess blood loss, possibly requiring transfusion, is a risk with all methods of myomectomy. Randomized trials suggest that the use of GnRH agonists prior to surgery (Lethaby, Vollenhoven, and Sowter, 1999) or vasopressin during surgery (Fletcher, Frederick, Hardie, et al., 1996; Hutchins, 1996) result in statistically significant differences in estimated blood loss; however, the clinical significance of this difference is unclear. Hysteroscopic myomectomy carries the unique risks of uterine perforation and fluid/electrolyte disturbances. There is little evidence on long-term risks of myomectomy. Several peer reviewers pointed out case reports of serious adverse events, such as death from vasoconstriction during use of vasopressin or uterine rupture in pregnancy following laparoscopic myomectomy. One limitation of our search strategy is that such case reports were not included. However, without data that would allow estimation of the frequency of such events (i.e., the number of serious adverse events divided by the total number of procedures), quantification of risk is impossible. Adverse events, including death, are possible with all invasive procedures and with many noninvasive therapies. It is just as important to have high-quality data on the frequency of adverse events as it is to have high-quality data on the effectiveness of procedures in estimating relative benefits and risks.
Case series of laparoscopic and hysteroscopic myomectomy reported consistent improvement in symptoms related to bleeding within the first 6 months of treatment. Although published studies of abdominal myomectomy that met our criteria do not provide data on symptomatic relief, there is no evidence to suggest that results from an abdominal procedure would be different from the results of a laparoscopic procedure in terms of symptoms. Earlier case studies cited in the review of Buttram and Reiter (1981) did report consistent improvement in symptoms. Although recovery time from an abdominal procedure may be longer, there is no evidence to suggest that symptomatic relief would vary; route of surgery had no effect on long-term outcomes of hysterectomy in the Maryland Women's Health Study (Rhodes, Kjerulff, Langenberg, et al., 1999). Comparison of results between studies was prevented by lack of standardization in the reporting of measures.
Hysterectomy for Symptomatic Fibroids
Although there are numerous studies reporting in-hospital outcomes of hysterectomy, we identified only three prospective studies that met our search criteria; all were based in the United States, reported both short-term and longer term outcomes, and included standardized measures for assessing physical and psychological outcomes (Carlson, Miller, and Fowler, 1994a; Kjerulff, Langenberg, Rhodes, et al., 2000; Weber, Walters, Schover, et al., 1999). All three studies showed substantial improvement in symptoms at 1 or 2 years after hysterectomy for most women (Table 11). The severity of women's symptoms prior to hysterectomy was substantial. In the Maine Women's Health Study, scores on standardized measures of symptom severity and quality of life were significantly worse in women undergoing hysterectomy than in those managed nonsurgically (Carlson, Miller, and Fowler, 1994a, 1994b). Of note, the technique of hysterectomy (abdominal, vaginal, or laparoscopically assisted vaginal) was not associated with the likelihood of a good or bad outcome. In the Maryland study, sexual function significantly improved after hysterectomy, although preoperative depression was a marker for developing sexual problems after hysterectomy (Rhodes, Kjerulff, Langenberg, et al., 1999).
Methodological Issues
Although all three studies were prospective and used validated instruments for measuring at least some outcomes, the ability to use these results to predict outcomes for women with fibroids is limited by variable levels of detail in reporting results specifically for women with fibroids. Although differences in results may not have been statistically significant across groups, it is possible that there were clinically important differences.
Bilateral oophorectomy was not a predictor of failure to have improvement in symptoms or development of new symptoms in the Maine study (Carlson, Miller, and Fowler, 1994a, 1994b), but it was in the Maryland study (Rhodes, Kjerulff, Langenberg, et al., 1999), although this effect was seen in Maryland only after 2 years. This may be due to the varying length of followup. Although Kjerulff and colleagues reported that there was no relationship between the self-reported use of hormone replacement therapy and poor outcome in oophorectomized women (Kjerulff, Langenberg, Rhodes et al., 2000), it may be that adherence to hormone replacement therapy decreased over time in some cases. This could have resulted in significant differences in outcomes at 2 years but not at 1 year. These studies all took care to differentiate between procedures involving no oophorectomy, a single oophorectomy, or a bilateral oophorectomy.
Another potential reason for the failure to detect an effect of oophorectomy on outcomes is that hysterectomy alone may lead to changes in ovarian function (such as decreased sex steroid production), which in turn could lead to decreased observed differences in outcomes attributable to changes in ovarian hormone production. The literature concerning the effects of hysterectomy on ovarian function is discussed in detail in a later section.
Summary
In prospective studies, hysterectomy results in improvements in symptoms and quality of life up to 2 years after the procedure in most women with sufficiently severe symptoms. For women with less severe symptoms who do not undergo hysterectomy, as many as 30 percent may have some improvement at 1 year; however, another 20-25 percent will undergo hysterectomy during that time. In the Maine study, women with fibroids who initially did not undergo surgery appeared less likely to have resolution of symptoms than women who had pain or bleeding unrelated to fibroids. Type of hysterectomy or short-term outcomes, such as complications, do not appear to influence the likelihood of a favorable outcome. New symptoms developed in 5-12 percent of women, with menopausal symptoms being most common. Low income and preexisting psychiatric disease appear to increase the risk of a poor outcome (defined as either development of new symptoms or failure to have improvement in preoperative symptoms); bilateral oophorectomy at the time of surgery also may increase the risk of a poor outcome. Race alone did not predict a poor outcome.
Hysterectomy Versus Myomectomy for Symptomatic Fibroids
We identified three studies that directly compared patients undergoing abdominal hysterectomy and myomectomy at the same institution (Ecker, Foster, and Friedman, 1995; Iverson, Chelmow, Strohbehn, et al., 1996; Iverson, Chelmow, Strohbehn, et al., 1999) (Table 12). All three studies identified a correlation between increasing uterine size and number of fibroids and estimated blood loss. Febrile morbidity also was more common after myomectomy compared with hysterectomy. Iverson, Chelmow, Strohbehn, et al. (1996) found an increased risk of intraoperative visceral injury with hysterectomy, although this risk was not adjusted for uterine size and number of fibroids because of limited power (DC Chelmow, personal communication).
Methodological Issues
We were unable to identify any randomized trials comparing hysterectomy with myomectomy for women with symptomatic fibroids. Given strong physician and patient beliefs about the risks and benefits of hysterectomy, recruitment into such trials would likely be difficult. Patient age and desire to retain childbearing potential would also contribute to difficulty in recruitment. Setting aside the issue of the inherent biases of nonrandomized study designs in evaluating therapeutic outcomes, any attempt to synthesize the results of studies of both procedures to address this question is limited by multiple factors:
- Inconsistency in methods used to report symptom type and degree of severity; such data would at least allow comparison of results between prospective studies of hysterectomy outcomes with those for myomectomy.
- Inconsistency in reporting anatomical findings (uterine size, fibroid size, number and location of fibroids).
- Inconsistency in reporting patient characteristics that might affect outcomes, both demographic (race, income) and medical (comorbidities, prior surgical procedures).
- Inconsistency in methods for reporting outcomes, variable duration of followup, and variable accounting for patients lost to followup.
- In studies of hysterectomy, inconsistency in reporting results for patients with fibroids separately from patients with other indications for hysterectomy.
- Lack of power to detect statistically or clinically significant differences in important patient characteristics or outcomes.
- Use of inappropriate statistical techniques (for example, use of parametric tests for ordinal variables such as number of fibroids or parity).
Summary
The choice of hysterectomy versus myomectomy appears to reflect patient and provider preferences; for example, for women desiring to retain fertility, myomectomy is clearly the only option. The relative risks and benefits of the two procedures for other women remain unclear. For myomectomy, there appears to be a rough correlation between the number of fibroids removed and the risk of immediate complications and recurrence of symptoms (see next section). For women with symptoms and no desire to retain fertility, hysterectomy appears to result in significant improvements in symptomatic severity and quality of life; myomectomy may result in a lower risk of intraoperative complications. Data are not sufficient to allow comparison of myomectomy with hysterectomy in terms of long-term outcomes. Evidence on the possible effects of hysterectomy on longer term outcomes, such as ovarian function, is discussed later in this chapter under Question 8.
Hysterectomy or Myomectomy for Asymptomatic Fibroids
We were unable to identify any studies that provided direct evidence on risks or benefits of surgery for women with asymptomatic fibroids. Historic arguments for performing surgery on women with asymptomatic fibroids above a certain size have included the following:
- Prevention of ovarian cancer mortality: Decreased sensitivity of bimanual pelvic examination for detecting adnexal pathology (especially ovarian malignancy) secondary to the large uterus.
- Prevention of uterine cancer mortality: An increased risk of uterine sarcoma in large or rapidly growing fibroids.
- Prevention of fibroid growth after menopause: An increased risk of continued fibroid growth in women using postmenopausal hormone replacement therapy.
- Prevention of injury to other organ systems: An increased risk of compromise of adjacent organs (e.g., ureteral obstruction) secondary to compression by an enlarging uterus.
- Prevention of problems related to infertility or pregnancy complications: An increased risk of problems with either fertility or pregnancy with increasing fibroid size (valid only for myomectomy).
- Prevention of future surgical morbidity: An increased perioperative morbidity associated with interval growth of the uterus (Friedman and Haas, 1993).
In 1993, Friedman and Haas concluded that there was little evidence to support any of these arguments (Friedman and Haas, 1993). There has been no substantial change between 1993 and 2000:
- Prevention of ovarian cancer mortality: There are no data supporting the utility of a bimanual examination in women with normal-sized uteri as a screening tool for reducing ovarian cancer mortality (Grover and Quinn, 1995; Schutter, Kenemans, Sohn, et al., 1994). Therefore, the inability to palpate the ovaries in a woman with an enlarged uterus seems unlikely to have a substantial impact on ovarian cancer mortality.
- Prevention of uterine cancer mortality: Uterine leiomyosarcoma is rare, with a reported incidence of fewer than 1 per 100,000 women (Van Dinh and Woodruff, 1982). Sarcomas were found in 0.5 percent or less of all hysterectomies performed for suspected fibroids in most series (Leibsohn, d'Ablaing, Mishell, et al., 1990; Parker, Fu, and Berek, 1994; Takamizawa, Minakami, Usui, et al., 1999). Even in women for whom the preoperative diagnosis was "rapidly enlarging uterus," the prevalence was 0.27 percent (Parker, Fu, and Berek, 1994). Given the prevalence of fibroids and surgical procedures for fibroids and the low incidence of uterine sarcomas it seems likely that many sarcomas are discovered by serendipity (McNaughton Collins, Ransohoff, and Barry, 1997; Ransohoff and Lang, 1990).
- Prevention of growth after menopause: Although submucous fibroids may increase the risk of abnormal bleeding in women on hormone replacement therapy (Akkad, Habiba, Ismail, et al., 1995), data on growth in women on hormone replacement are limited and suggest that growth may be variable and depend on the regimen used (Sener, Seckin, Ozmen, et al., 1996; Ylostalo, Granberg, Backstrom, et al., 1996). If growth alone is not a compelling reason for surgery in premenopausal women, there are no obvious reasons why asymptomatic growth after menopause would be a reason for surgery.
- Prevention of injury to other organ systems: An enlarged uterus can result in distortions of urinary tract anatomy, including partial or complete ureteral obstruction (Piscitelli, Simel, and Addison, 1987). This may be exacerbated during pregnancy, when partial hydronephrosis is normal and, in rare cases, may result in acute renal injury (Courban, Blank, Harris, et al., 1997). However, there is no evidence to allow estimation of the risk of permanent urinary tract or renal injury associated with asymptomatic uterine growth. In fact, given a reported risk of urinary tract injury at hysterectomy of between 0.1 and 1.5 percent (Aslan, Brooks, Drummond, et al., 1999; Courban, Blank, Harris, et al., 1997; Daly and Higgins, 1988; Harkki-Siren, Sjoberg, and Tiitinen, 1998), the risk of permanent ureteral or renal injury secondary to uterine growth prior to the development of other symptoms would have to be at least as large to justify surgery on the basis of preventing obstructive organ injury.
- Prevention of problems related to infertility or pregnancy complications: Clearly, hysterectomy is inappropriate for any woman wishing to retain the option of future childbearing. The effects of fibroids on women seeking to conceive and on pregnancy outcomes are discussed under Question 8. However, there are no data on the impact of prophylactic myomectomy in asymptomatic women on either fertility or pregnancy outcomes. Given the frequency with which adhesions are found after myomectomy (March, Boyers, Franklin, et al., 1993; Tulandi, Murray, and Guralnick, 1993), it is possible that prophylactic myomectomy would have a harmful effect on fertility.
- Prevention of future operative morbidity: Two studies have specifically addressed the risk of perioperative morbidity and uterine size (Table 13). Reiter, Wagner, and Gambone (1992) reviewed the records of 93 women undergoing hysterectomy for fibroids at the San Diego Naval Medical Center. They found no statistical difference in estimated blood loss or complication rates between women with estimated uterine sizes less than 12 weeks gestational age (about 500 grams) and women with larger uteri. Conversely, Hillis, Marchbanks, and Peterson (1996) reviewed the records of 446 women who were enrolled in a prospective study of women undergoing sterilization procedures. Through both univariate and multivariate analyses they found that morbidity, especially transfusion risk, was increased in women with a uterine weight greater than 500 grams. Given the larger sample size, the prospective collection of data, and the use of more appropriate statistical methods, the findings of Hillis and colleagues (1996) are somewhat stronger.
Other indirect evidence supports an increased risk of perioperative morbidity with increasing uterine size. Ecker and colleagues (Ecker, Foster, and Friedman, 1995) and Iverson and colleagues (Iverson, Chelmow, Strohbehn, et al., 1996) both reported a statistically significant increase in estimated blood loss with increasing uterine size in univariate and multivariate analyses. Data from randomized trials of preoperative GnRH agonists show consistently decreased blood loss and operative times with preoperative GnRH agonists, which may be attributable to decreased uterine size (Lethaby, Vollenhoven, and Sowter, 1999).
Although the evidence suggests that increased uterine size is associated with increased blood loss and possibly an increased risk of other complications, this association does not necessarily lead to the conclusion that surgery on women with fibroids above a certain size will reduce morbidity for several reasons:
- None of the studies specifically reported on outcomes in women with asymptomatic fibroids compared with outcomes in women with symptomatic fibroids. It is possible that the natural history of asymptomatic fibroids -- including the rate of growth, number, and location within the uterus -- is different from that of symptomatic fibroids, and this may result in different perioperative outcomes.
- Use of preoperative GnRH agonists may reduce some risks associated with interval increase in uterine size (Lethaby, Vollenhoven, and Sowter, 1999).
- Without good data on the natural history of fibroids, it is difficult to estimate the likelihood that a woman with asymptomatic fibroids will either develop symptoms or reach an arbitrary size threshold within a given time period.
- Using estimates of a substantially increased relative risk of perioperative morbidity associated with increasing fibroid size will overestimate the benefits and underestimate the risks of immediate surgery if the probability of progression is not considered. For example, if the complication rate for a hysterectomy performed with a uterine size of 10 weeks is 10 percent, a two-fold increase in risk would increase the rate to 20 percent. For immediate surgery to be the preferred strategy in terms of reducing morbidity, the probability that the uterus would grow to a size that would result in twice the risk in the absence of any symptoms would need to be greater than 50 percent. If the probability of progression were 40 percent, then the overall risk of complications would be 0.4 x 0.2, or 8 percent, less than the 10 percent risk with immediate surgery.
Methodological Issues
We were unable to identify any randomized trials comparing hysterectomy with myomectomy for women with asymptomatic fibroids. Given strong physician and patient beliefs about the risks and benefits of hysterectomy, recruitment into such trials would likely be difficult. Given the lack of a compelling argument for such surgery, justifying the trial would be difficult both scientifically and ethically. This question may be one for which modeling is appropriate; however, better data on the natural history of asymptomatic fibroids are required before such modeling could be performed.
Summary
There is no evidence to support performing either myomectomy or hysterectomy in asymptomatic women. Clearly, there are risks involved with both surgical procedures. Although fibroids may be associated with some adverse pregnancy outcomes in fertile women (see Question 8, discussion), there are no data that myomectomy in asymptomatic women with uterine fibroids reduces the risk of such complications.
Question 2: What are the risks associated with single versus multiple myomectomies?
Approach
This is a somewhat ambiguous question, which can be interpreted in two ways:
- What are the risks associated with a primary myomectomy compared with the risks associated with repeat myomectomies?
- What are the risks of a myomectomy that results in the removal of a single fibroid compared with the risks associated with the removal of multiple fibroids?
We clarified this through discussions with the advisory panel. Because Question 5 ("Does additional treatment result in significantly increased morbidity?") addresses the first interpretation, we focused on the second issue. We also determined that the question referred to the comparison between women with a single clinically detectable fibroid and women with multiple fibroids, since it would be highly unlikely that any surgeon would not remove all detectable fibroids. In other words, we assumed that once a decision to operate had been made, no surgeon would deliberately leave additional detectable fibroids in situ unless there were compelling intraoperative events (e.g., significant blood loss or complications with anesthesia that required shortening surgical time). In addition to examining the relevant literature, we analyzed a primary data set that allowed us to compare complication rates for women undergoing removal of a single fibroid with those for women undergoing removal of multiple fibroids. The outcomes we considered were perioperative complications, pregnancy rates, recurrence rates, and need for subsequent surgery.
Literature Search
Table 14 summarizes the results of studies that stratified results by the number of fibroids removed. In these series, removal of a single fibroid was associated with a higher pregnancy rate, lower risk of recurrence, and lower risk of subsequent surgery. Data were not provided on short-term complication rates.
Primary Data
Data from the Duke primary chart abstraction suggest a relationship between the number of fibroids removed and complication risk, but the threshold number of fibroids may be somewhat greater than one. For patients with a single fibroid removed (n = 43), the complication rate was 20.9 percent; for those with two to four fibroids removed (n = 45), the complication rate was 11.1 percent; and for those with five or more fibroids removed (n = 112), the complication rate was 42 percent; this trend was highly significant (p < 0.0001). Unfortunately, data on fibroid location were not provided in the charts or operative notes. One difficulty with retrospective studies in teaching hospital settings is that the potential effects of surgeon experience cannot readily be determined; it is often impossible to discern who performed which portion of a given procedure from an operative report.
In subsequent multivariate analyses, using fibroid number as a continuous variable, increasing the number of fibroids removed was a significant predictor of complications and transfusions. Given the relatively small sample size, we were unable to determine a "threshold" number of fibroids at which risk becomes "unacceptable." However, it seems likely that, given a favorable location, removal of two adjacent fibroids would not result in substantially greater morbidity compared with removal of a single fibroid. Other factors besides the number of fibroids removed -- such as size, location, degree of vascularization, ease of identification of surgical planes -- are likely to play a role in determining surgical difficulty.
Methodological Issues
Many included studies did not stratify results by the number of fibroids removed. Most of those that reported the number of fibroids removed used means as the summary statistic rather than the more appropriate median. The effect of the number of fibroids removed on outcomes was seldom examined in a multivariate analysis. In our analysis of the primary data, we were able to examine only in-hospital and immediate postoperative events.
Summary
In those studies that allowed comparison of results between women undergoing myomectomy for single versus multiple fibroids, there is a consistent pattern of better long-term results for women with single fibroids in terms of pregnancy rates, risk of recurrence, and need for subsequent surgery. Results from our primary data analysis also suggest that women undergoing removal of a single fibroid have fewer complications than women undergoing multiple myomectomies.
The effect of the number of fibroids on immediate short-term surgical outcomes is likely to be related to technical difficulty -- removal of a single fibroid is likely to take less time and result in less blood loss than removal of multiple fibroids. Whether there is a threshold number of fibroids, considering other factors such as size and location, where the perioperative risk of myomectomy becomes unacceptable is not at all clear from the available data. The effect of the number of fibroids on longer term outcomes may be related to technical factors (e.g., failure to remove very small fibroids) or biological factors (e.g., women with multiple fibroids may be more susceptible to fibroid development, or removal of large fibroids may allow smaller, undetected fibroids to grow more rapidly).
Both perioperative outcomes and longer term outcomes appear to be better for women with a single clinically detectable fibroid who undergo myomectomy than for those with multiple fibroids. However, whether there is a threshold number of fibroids above which myomectomy has either an unacceptably high complication risk or an unacceptably low likelihood of long-term benefit cannot be determined from the available data.
Question 3: Who are appropriate candidates for each procedure?
Approach
Although this question was originally intended to focus only on the issue of myomectomy versus hysterectomy, discussions with members of the project's advisory panel led to an expansion to include the other strategies (including no intervention along with other medical and surgical therapies).
The concept of "appropriateness" can have multiple meanings. One frequently used definition is that a procedure is appropriate if a patient undergoing the procedure received all diagnostic evaluations and alternative treatments recommended by a panel of experts (Broder, Kanouse, Mittman, et al., 2000). Alternatively, the most appropriate therapy can be defined as the procedure, treatment, or treatment strategy most likely to result in favorable short- and long-term outcomes, given relevant clinical factors and patient preferences. We discuss the implications of the two definitions below:
- Appropriateness criteria: Broder and colleagues found that 70 percent of hysterectomy cases in Southern California over a 2-year period did not meet the standards of expert panel recommendations, and 76 percent did not meet criteria defined by ACOG (Broder, Kanouse, Mittman, et al., 2000). In devising the RAND appropriateness criteria (Shekelle, Kahan, Bernstein, et al., 1998), the expert panel was hampered by the same lack of high-quality evidence that the we faced in writing this evidence report. Given the lack of evidence supporting many of the criteria in both the expert panel and ACOG recommendations, it is somewhat difficult to conclude that ". . . the care leading to recommendations of hysterectomies in our cohort was suboptimal" (Broder, Kanouse, Mittman, et al., 2000). For example, 9 percent of all cases were "premenopausal women with leiomyomata, bleeding, and pain who ha[d] significant anemia or functional impairment but ha[d] not undergone endometrial sampling," and all of these were judged inappropriate. However, given (a) the low incidence of endometrial carcinoma in premenopausal women; (b) the likelihood that many women with symptomatic fibroids will have undergone ultrasound evaluation, which by itself has acceptable sensitivity and specificity for detecting benign and malignant endometrial changes associated with abnormal bleeding (Tahir, Bigrigg, Browning, et al., 1999); (c) the fact that the presence of fibroids may decrease the ability to obtain adequate tissue on endometrial sampling (Gordon and Westgate, 1999); and (d) the lack of evidence that failure to sample the endometrium in a woman with fibroids associated with bleeding and pain scheduled to undergo hysterectomy would lead to a significant change in outcomes, the "requirement" for endometrial sampling in all women with this indication may be questioned, at least on cost-effectiveness grounds (Dunn, Stamm, Delorit, et al., 2000; Farquhar, Lethaby, Sowter, et al., 1999). Similarly, given the paucity of evidence supporting the effectiveness of medical treatment for symptomatic fibroids, requiring documentation of a trial of such treatment prior to proceeding with hysterectomy as a marker of "optimal" care for all patients seems difficult to justify.
- Choosing the treatment most likely to result in benefit to the patient: This interpretation of "appropriateness" is more clinically relevant and more consistent with the overall purpose of this report. Answering this question depends on the availability of evidence that allows clinicians and patients to estimate the likelihood of benefits and harm over both the short and long term for each procedure or treatment given defined symptoms, pathology, and comorbidities. Unfortunately, the available data do not allow us to answer this question for the majority of fibroid management strategies, using either direct evidence from clinical studies or decision analytic methods (see Question 4), a difficulty faced by other groups as well (Broder, Kanouse, Mittman, et al., 2000). There are some scenarios where preliminary conclusions can be drawn, based either on consistency of evidence, a lack of evidence supporting alternative strategies, or common sense (e.g., hysterectomy is clearly inappropriate for women planning future pregnancies). The discussion of each strategy below summarizes more detailed discussions of the evidence in other sections of this report.
Results
These are discussed in the appropriate sections for each individual treatment option.
Methodological Issues
These are discussed in the appropriate sections for each individual treatment option.
Summary
No Treatment
For women with asymptomatic fibroids, there is no evidence that failure to treat with medical or surgical therapy will result in harm, no evidence that medical or surgical treatment will result in benefits, and for surgical management at least, clear evidence of risks from the treatment itself.
For women with symptomatic fibroids, the likelihood of success with no treatment is likely to be a function of the nature of the patient's symptoms and her proximity to menopause, although data do not allow us to quantify this probability. In the Maine Women's Health Study, 68 percent of women with fibroids were managed with observation alone. At the end of 1 year, there were no significant changes in the amount of bleeding or pain or in the degree to which patients were bothered by these symptoms (Carlson, Miller, and Fowler, 1994b). Twenty-five percent of all women in this cohort eventually underwent hysterectomy during the followup period, although the data do not describe the risk for women with fibroids alone. For women with bleeding as the primary symptom, the probability of worsening of symptoms may well be inversely related to the likelihood of undergoing menopause; the effect of hormone replacement therapy on this is uncertain. For women with noncyclic pain, especially symptoms associated with an enlarged uterus, the degree to which menopause will resolve these symptoms is also uncertain.
Oral Contraceptives, NSAIDs, and Progestins (see under Question 6)
Given the scarcity of data on the effectiveness of these medical treatments in relieving symptoms related to fibroids, it is difficult to identify appropriate candidates for the treatments. In symptomatic women for whom there is no contraindication to their use, there is no evidence to suggest that a trial will result in harm and limited evidence that it may result in an improvement in symptoms.
GnRH Agonists (see under Question 6)
Although there is consistent evidence based on randomized trials that preoperative use of GnRH agonists reduces some morbidity associated with both hysterectomy and myomectomy, especially blood loss (Lethaby, Vollenhoven, and Sowter, 1999), the clinical relevance of the reduction is unclear. Statistically significant reductions in length of surgery (a difference of 6.6 minutes), blood loss (7.8 cc), reduction in vertical incisions, and length of stay after hysterectomy were observed; there was not a significant difference in transfusion rates. Women who are candidates for either hysterectomy or myomectomy may benefit from preoperative use of these agents (e.g., if anemia secondary to heavy menstrual blood loss would increase the likelihood of perioperative morbidity). However, additional study of the cost-effectiveness of routine use of these agents prior to surgery and of the impact of side effects on short-term quality of life, is needed. For perimenopausal women with symptoms, the use of these agents may avoid the need for additional therapy; however, there are no data to allow estimation of which perimenopausal women are most likely to benefit from their use.
Uterine Artery Embolization (see under Question 4)
Uterine artery embolization appears to hold promise as an alternative to other invasive procedures. There are no randomized trial data and only a few series with more than 20 cases (Goodwin, McLucas, Lee, et al., 1999; Hutchins, Worthington-Kirsch, and Berkowitz, 1999; Pelage, Le Dref, Soyer, et al., 2000; Siskin, Stainken, Dowling, et al., 2000; Spies, Scialli, Jha, et al., 1999; Spies, Warren, Mathias, et al., 1999; Worthington-Kirsch, Popky, and Hutchins, 1998). Resolution of bleeding and bulk symptoms range from 80-90 percent in these series. Although postprocedure pain requiring overnight hospitalization and narcotics is common, reported serious complications are rare. In all these studies, followup has been relatively short, with loss to followup in the largest series of close to 50 percent for 1-year outcomes (Hutchins, Worthington-Kirsch, and Berkowitz, 1999). Data on outcomes related to pregnancy are limited; almost every series reports at least one pregnancy, and there has been one series of 12 cases reported (see under Question 8). Notably, there appears to be a serious effort among those performing this procedure to collect prospective data using validated instruments (Spies, Warren, Mathias, et al., 1999) within a registry organized by the Society for Cardiovascular and Interventional Radiologists (J Spies, personal communication). Given the relatively short reported experience, the most appropriate candidates for this procedure would be women willing to participate in research protocols or registries performed at centers with significant experience and technical expertise. This is especially true for women considering future pregnancy.
Myomectomy (see under Questions 1 and 8)
The risks and benefits of myomectomy appear to be partly related to the location and number of fibroids, as well as the presenting symptoms. Women with a single clinically detectable fibroid appear to have fewer perioperative complications and a lower chance of recurrence following myomectomy than women with multiple fibroids. For women with submucous fibroids, the probability of success with hysteroscopic myomectomy appears to be related to the depth of invasion into the myometrium (Nezhat, Roemisch, Nezhat, et al., 1998). The likelihood of benefit from myomectomy appears to be related to presenting symptoms -- women with bleeding and submucous fibroids appear to have favorable short-term responses; pain symptoms may be less likely to resolve with any type of myomectomy (see under Questions 1 and 8). There are no data to support performing myomectomy in asymptomatic women.
Hysterectomy (see under Question 1)
Hysterectomy is clearly inappropriate for women who wish to retain the ability to carry a pregnancy. For women who do not desire to retain this ability, data from two large prospective cohort studies suggest that physical and psychological outcomes at 1 year after surgery in women with symptomatic fibroids are favorable (Carlson, Miller, and Fowler, 1994a; Kjerulff, Langenberg, Rhodes, et al., 2000). Women with preexisting depression or anxiety appear to be more likely to have persistent symptoms or develop new symptoms after hysterectomy for all benign indications (Kjerulff, Langenberg, Seidman, et al., 1996; Rhodes, Kjerulff, Langenberg, et al., 1999), suggesting that in such women, depending on severity and types of symptoms, hysterectomy may be less likely to result in an overall improvement in quality of life compared with women without depression and anxiety. Interestingly, in-hospital complications did not significantly affect the likelihood of having favorable outcomes in the longer term (Kjerulff, Langenberg, Rhodes, et al., 2000). Data supporting suggested rationales for performing hysterectomy in asymptomatic women are lacking (Friedman and Haas, 1993) (see also under Question 1).
Question 4: What is the incidence of need for additional treatment after myomectomy or other uterus-sparing interventions?
Approach
We approached this question by dividing it into four separate clinical scenarios:
- Initial treatment with medical therapy, with additional medical therapy for persistent or recurrent symptoms.
- Initial treatment with invasive therapy, such as embolization or uterus-conserving surgery, with additional medical therapy for persistent or recurrent symptoms.
- Initial treatment with medical therapy, with additional invasive therapy (including hysterectomy) for persistent or recurrent symptoms.
- Initial treatment with invasive therapy, with additional invasive therapy (including hysterectomy) for persistent or recurrent symptoms.
"Recurrence" in the setting of conservative treatment for fibroids can mean several things:
- Regrowth of a fibroid after stopping suppressive therapy, such as GnRH agonists.
- Regrowth of a fibroid after incomplete surgical excision.
- Development of new fibroids.
For the purposes of the review, we considered all three possibilities, since most studies, especially of surgical treatments, did not distinguish between these possibilities.
During our review, we discovered that many studies reported "recurrence" rates after uterus-conserving treatment but did not often distinguish between anatomical recurrence documented by physical or radiological examination and symptomatic recurrence. Therefore, we summarize all recurrences below and, where possible, attempt to distinguish between anatomical and symptomatic recurrence. If possible, we also report on any patient or clinical characteristics that predicted either recurrence or the need for additional therapy.
Results
We did not identify any studies that allowed estimation of the incidence of additional medical treatment after either initial medical treatment or initial invasive therapy. Results for studies that reported either recurrence or the need for additional invasive therapy are summarized below.
No Treatment, Hormonal Therapy, or Treatment with NSAIDs
Incidence of recurrence
The Maine Women's Health Study (Carlson, Miller, and Fowler, 1994b) is the only prospective study we identified that used validated outcome measures and reported on the incidence of additional treatment in women followed expectantly or managed with nonsteroidal or hormonal medication (Table 15). Data on the 1-year incidence of hysterectomy were not reported separately for patients with fibroids, but the authors stated that this risk was not different among women with fibroids than among women with abnormal bleeding or pelvic pain. Data were not reported on additional treatments other than hysterectomy (e.g., the addition of medications to women initially managed expectantly or myomectomy).
Predictors of recurrence
Baseline data on uterine size, number of fibroids, and other anatomical measures were not reported. The association of other potential predictors of hysterectomy -- such as age, race, and parity -- with the occurrence of hysterectomy also was not reported.
GnRH Agonist Therapy
Incidence of recurrence
Although several studies reported on fibroid regrowth after cessation of GnRH agonist therapy (de Aloysio, Altieri, Pretolani, et al., 1995; Donnez, Schrurs, Gillerot, et al., 1989; Palomba, Affinito, Di Carlo, et al., 1999; Serra, Panetta, Colosimo, et al., 1992; van Leusden, 1992), fewer reported on symptomatic recurrence or incidence of surgical treatment (Table 16). However, given both the small number of studies that report data on symptoms and additional treatment and the small size of the studies, accurate estimation of the risk of symptomatic recurrence is difficult. Given that regrowth is an almost universal occurrence after cessation of GnRH agonist therapy in premenopausal women, it is not surprising that recurrent symptoms, in some cases leading to myomectomy or hysterectomy, occur as well. However, it is unclear in what proportion of women symptoms recur, at what level of severity, and whether alternative treatments would be more or less likely to work after treatment with a GnRH agonist.
Predictors of recurrence
In the small series described by van Leusden (van Leusden, 1992), perimenopausal women appeared less likely to undergo surgery after treatment; however, the small size of the study precluded significance testing. None of the other studies reported on factors influencing the likelihood of recurrent symptoms or incidence of subsequent surgery.
Myomectomy
Incidence of recurrence
The reported incidence of recurrence of fibroids -- as defined by anatomical diagnosis, recurrence of symptoms, need for any additional surgery, or hysterectomy after abdominal, hysteroscopic, and laparoscopic myomectomy -- is shown in Table 17.
Given variation in definitions of recurrence and length of followup, estimation of an overall probability of symptomatic recurrence after myomectomy is not possible. Reported incidence of subsequent conservative surgery ranged from 10-12 percent for laparoscopic myomectomy, 4-10 percent for abdominal myomectomy, and 3-76 percent for hysteroscopic myomectomy. Incidence of hysterectomy ranged from 2-6 percent for laparoscopic myomectomy, 3-8 percent for abdominal myomectomy, and 0.5-12 percent for hysteroscopic myomectomy. Given the likely differences in patient characteristics that led to the selection of a particular treatment in these nonrandomized studies, comparison of rates between different approaches to myomectomy is inappropriate.
Predictors of recurrence
Patient characteristics associated with a statistically significant increased risk of recurrence in individual studies included:
- Increasing preoperative uterine size (Emanuel, Wamsteker, Hart, et al., 1999) (hysteroscopic myomectomy only).
- Incomplete excision of fibroid (Emanuel, Wamsteker, Hart, et al., 1999) (hysteroscopic myomectomy only) (Dueholm, Forman, and Ingerslev, 1998).
- Increasing extension into the myometrium of submucous fibroids (Nezhat, Roemisch, Nezhat, et al., 1998) (hysteroscopic myomectomy only) (Emanuel, Wamsteker, Hart, et al., 1999).
Embolization
Incidence of recurrence
Reported experience with uterine artery embolization is limited in terms of both numbers of patients followed and length of followup (Table 18). In addition, in the paper with the largest number of patients (Hutchins, Worthington-Kirsch, and Berkowitz, 1999), loss to followup was substantial, making estimations of true cumulative rates of recurrence, persistence, or need for subsequent therapy difficult.
Predictors of recurrence
None were reported.
Methodological Issues
The available literature does not allow calculation of summary estimates of the need for additional treatment after uterus-sparing interventions. Limitations of the literature that preclude summary estimates include:
- Variability in surgical technique: Even for a given procedure, such as abdominal myomectomy, it is possible that variation in technique might affect recurrence risk.
- Variability in definitions of recurrence: Some studies reported radiological persistence or recurrence without correlation with symptoms; others reported only those patients needing additional surgical treatment. We did not identify any study that addressed the issue of the use of medical treatments such as hormonal or nonsteroidal agents after myomectomy or embolization.
- Variability in methodology used to estimate recurrence risk: Some studies reported only the number of patients requiring additional treatment without accounting for loss to followup or time of additional treatment, while others appropriately used life-table measures.
- Variability in reporting of potential predictors of risk: Considerable variation was observed in the reporting of the size, location, and number of fibroids removed, as well as other variables such as age, race, parity, and educational level.
- Variability in patient populations: Women undergoing uterus-sparing therapy who plan on pregnancy represent a different population than women who have no plans for future pregnancies; the type of additional therapies available are quite different, so "recurrence" as defined by additional treatment may not reflect symptomatic recurrence or persistence.
- Variable length of followup: Variable length of followup makes estimation of the true risk of recurrence or persistence difficult, unless survival analysis techniques are explicitly used to account for this variability.
- Variable loss to followup: Patients lost to followup may be more likely to have recurrent symptoms and seek treatment elsewhere. Although life-table methods may be helpful in accounting for the effects of this loss on estimates of cumulative recurrence, these methods depend on an assumption of nondifferential treatment outcomes between patients who remain in the study and those who are lost to followup.
- "Recurrence" versus new fibroids: Given the relatively high incidence of fibroids, it is possible that at least some fibroids detected after uterus-conserving treatment represent new lesions that would have developed with or without the intervention. Indeed, it is possible that removing fibroids may induce changes, such as increased expression or secretion of cytokines and growth factors that facilitate the growth of new fibroids. Thus, development of fibroids after conservative therapy may not represent failure of the therapy but may simply reflect the natural history of uterine leiomyomata.
Summary
Clearly, all uterus-sparing treatments for symptomatic fibroids leave some risk of recurrence of symptoms, resulting in the need for additional therapy. However, the available literature does not allow quantification of this risk for any of these "conservative" treatments. For myomectomy, factors such as size, number, and location of fibroids appear to increase the risk of recurrence. It is unclear whether this increased risk is related to differences in underlying biology (women with larger, more numerous fibroids may be more likely to develop new fibroids) or the technical difficulty of the surgery. Future estimation of the risk of recurrence and need for additional treatment would be facilitated by standardization in the reporting of pre- and postoperative patient characteristics, definitions of recurrence and persistence, and use of appropriate statistical techniques. Without such standardization, quantitative comparison of recurrence risk between treatments is impossible.
Question 5: Does additional treatment result in significantly increased morbidity?
Approach
As with Question 4, we approached this question by dividing it into four separate clinical scenarios describing the initial treatment. We then considered three scenarios in which increased morbidity might result from additional treatment:
- Initial treatment with medical therapy, with additional medical therapy for persistent or recurrent symptoms; or initial treatment with invasive therapy such as embolization or uterus-conserving surgery, with additional medical therapy for persistent or recurrent symptoms. Theoretically, increased morbidity could result in such circumstances from either side effects specifically related to the additional agents, drug interactions when additional pharmaceutical agents are added to a preexisting drug regimen, or changes in physiology related to prior invasive therapy, such as embolization or surgery, which would lead to either increasing the severity of side effects or new types of side effects.
- Initial treatment with medical therapy, with subsequent invasive management of recurrent or persistent symptoms. Theoretically, morbidity could result from steroid hormones. Estrogens appear to increase the risk of perioperative venous thrombolic events (Anonymous. 1999), and current practice is to discontinue oral contraceptives and other estrogens for at least 4 weeks prior to surgery. It is conceivable that patients using oral contraceptives to treat symptoms related to fibroids who did not discontinue these agents prior to additional surgical therapy might have an increased risk of venous thrombophlebitis or pulmonary embolism. Nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk of perioperative hemorrhage by inhibiting platelet function (Connelly and Panush, 1991; Scher, 1996); again, current practice is to discontinue their use prior to procedures with substantial bleeding risk. Patients using NSAIDs to treat symptoms related to fibroids who did not discontinue these agents prior to additional invasive therapy might have an increased risk of hemorrhagic complications, including hematomas. In the case of changes in underlying severity of illness or development of comorbidity, it is possible that medical therapy that alleviates symptoms without substantially altering the natural history of fibroid growth could result in increased risks of morbidity with subsequent therapy, either by allowing further growth of the fibroids -- resulting in an increased risk of surgical morbidity (Hillis, Marchbanks, and Peterson, 1996) -- or by the development of unrelated conditions (e.g., diabetes, hypertension, or cardiac disease) that would increase the risk of complications.
- Initial treatment with invasive therapy, with additional invasive therapy (including hysterectomy) for persistent or recurrent symptoms might increase the risk of morbidity in several ways. One is through multiple exposure to risk. Even if having undergone a prior procedure does not increase the inherent risk of morbidity associated with the procedure, patients undergoing additional procedures will have an increased likelihood of a complication or adverse outcome simply by being exposed to the same risks on multiple occasions. These multiple exposures increase the cumulative risk of an adverse outcome (although for most serious complications, this cumulative risk is likely to be quantitatively small). Changes in the underlying severity of condition or development of comorbidity also might increase the risk of morbidity. As with medical therapy, it is possible that the interval development of unrelated conditions might increase the risk of subsequent invasive procedures. Increased technical difficulty related to a prior procedure also may increase risk. Any surgical procedure can result in adhesions, and the association of myomectomy with adhesion formation is well-documented (Adamson, 1993; Anonymous. 1992; Diamond, Bieber, Coddington, et al., 1996; Franklin, Haney, Kettel, et al., 1995; Haney, 1997; Mais, Ajossa, Piras, et al., 1995; March, Boyers, Franklin, et al., 1993; Murray and Tulandi, 1996; Tulandi, Murray, and Guralnick, 1993; Ugur, Turan, Mungan, et al., 1996). The presence of adhesions increases the technical difficulty of the procedure and may increase the risk of complications.
Results
Additional Medical Treatment after Initial Medical or Invasive Therapy
We were unable to identify any studies that addressed the specific question of increased morbidity associated with additional medical treatment for patients with persistent or recurrent symptoms after conservative surgical or nonsurgical treatment related to new side effects, drug interactions, or changes in anatomy or physiology related to invasive procedures. There is some evidence that uterine artery embolization may result in decreased ovarian hormone production (see under Question 9). Theoretically, this could result in decreases in bone density that would be further exacerbated by additional treatment with GnRH agonists. However, we did not identify any data to support this hypothesis. Also, there are no data to suggest that embolization, myomectomy, or other invasive therapies result in changes in pelvic organ anatomy or physiology that would increase the likelihood of side effects of other pharmacological agents such as steroid hormones or NSAIDs.
Additional Invasive Therapy after Initial Medical Treatment
We did not identify any studies that documented an increased risk of perioperative morbidity related to use of estrogenic compounds, NSAIDs, increasing uterine size, or development of other comorbid conditions.
Additional Invasive Therapy after Initial Surgery or Radiological Procedure
None of the articles that addressed the need for additional surgery or other invasive therapy after an initial procedure (see under Question 4) reported on morbidity related to these additional procedures.
Methodological Issues
Even if additional treatment does result in additional risk (especially for surgical procedures), this additional risk needs to be considered in the context of the likelihood of successful initial treatment when counseling individual patients. For example, consider an initial noninvasive treatment with a likelihood of success of 75 percent with 25 percent of patients requiring a second procedure and a complication rate of 5 percent. The overall risk of a complication for all women undergoing the initial treatment is 5 percent + (25 percent x 5 percent), or 6.25 percent. Therefore, the effect of an increased cumulative risk must be considered in the context of the likelihood of additional therapy.
Summary
There are no data to allow estimation of the degree to which additional therapy, especially additional invasive therapy, increases the overall risk of morbidity for an individual patient or whether any increased risk is statistically or clinically significant.
Question 6: What are the risks and benefits of nonsurgical treatment?
Approach
In this section we consider nonsurgical approaches to managing fibroids, including observation (no treatment), estrogens, progestins, NSAIDs, GnRH agonists, and other drug treatments. These drugs are used either as primary treatments or (in the case of GnRH agonists) as adjuncts to surgical treatment. We did not evaluate possible primary prevention strategies; these are discussed in Chapter 6 (Future Research).
Results
We identified 70 separate studies of medical treatments for uterine fibroids (see Evidence Tables 2 and 3). These included nine studies describing the effects of no treatment (natural history), two studies of NSAIDs, three studies of estrogen-progestin combinations, six studies of progestins, 52 studies of GnRH agonists, and six studies of other hormonal and miscellaneous treatments. All study populations consisted of pre- or perimenopausal women. Nineteen of the GnRH agonist studies were uncontrolled case series; the remainder had control groups, and most of these allocated patients randomly to treatment and control groups. Four of the 20 studies of other drugs were uncontrolled case series.
No Treatment
The results of conservative management (no treatment) were described in the placebo arms of four randomized controlled trials of hormone treatment that followed patients for long periods (more than 6 months) (Friedman, Harrison-Atlas, Barbieri, et al., 1989; Friedman, Hoffman, Comite, et al., 1991; Gregoriou, Vitoratos, Papadias, et al., 1997; Schlaff, Zerhouni, Huth, et al., 1989) and in one cohort study in which the majority of nonsurgically managed patients received observation alone (Carlson, Miller, and Fowler, 1994b).
One of the most useful studies comparing nonsurgical with surgical management of fibroids was a large cohort study (Carlson, Miller, and Fowler, 1994b). A cohort of women with fibroids (uterine size 8 weeks or greater) was assembled from multiple sites in Maine and interviewed prospectively. One hundred and twenty-three women were managed with hysterectomy, and 106 received one of a variety of nonsurgical treatments (observation alone, 68 percent; NSAIDs or iron, 18 percent; hormone therapy, 14 percent). There were significant differences between the two groups at baseline. The patients who were managed without surgery had fewer days of bleeding and dysmenorrhea than those who underwent hysterectomy. In addition, the patients managed without surgery had more positive feelings about their symptoms and better quality of life at baseline than those who underwent surgery. By 1 year, the women who underwent hysterectomy had increased quality-of-life scores and, compared with nonsurgical patients, felt better about their symptoms. Approximately 23 percent of nonsurgical patients underwent hysterectomy in the 1 year followup period.
Baseline differences in the symptoms, attitudes, and quality of life between women managed nonsurgically and those managed surgically suggest that women who were managed nonsurgically were less severely affected by their fibroids. Because of this imbalance, we are limited in our ability to draw inferences about the relative effectiveness of nonsurgical and surgical management from this study. Furthermore, because nonsurgical management included several different treatment approaches and results were not reported separately for the various interventions, comparisons among the various nonsurgical treatments used are impossible.
Long-term observation (at least 1 year) or no treatment was described in the control arm of a trial of the synthetic steroid tibolone, a drug with mixed estrogenic, progestogenic, and androgenic properties (Gregoriou, Vitoratos, Papadias, et al., 1997). In addition, several placebo-controlled studies of the GnRH agonist leuprolide described 6-month outcomes of placebo-treated patients (Friedman, Harrison-Atlas, Barbieri, et al., 1989; Friedman, Hoffman, Comite, et al., 1991; Schlaff, Zerhouni, Huth, et al., 1989) (see Table 19). All of these studies were conducted among women who did not want to have surgery (Friedman, Harrison-Atlas, Barbieri, et al., 1989; Friedman, Hoffman, Comite, et al., 1991; Gregoriou, Vitoratos, Papadias, et al., 1997; Schlaff, Zerhouni, Huth, et al., 1989). Two described hemoglobin or hematocrit decreases over time, in addition to changes in fibroid or uterine size (Friedman, Harrison-Atlas, Barbieri, et al., 1989; Friedman, Hoffman, Comite, et al., 1991). None of the studies reported the number of women who eventually underwent surgery.
Consistent with the Maine Women's Health Study, in which the majority of nonsurgically treated patients received no treatment, these studies showed that "no treatment" resulted in no reduction in uterine size, no alleviation of symptoms, and no resolution of anemia.
Hormone Replacement Therapy with Estrogen and Progestins
Hormone replacement therapy (HRT) with estrogen-progestin was compared with the synthetic steroid tibolone in one study (de Aloysio, Altieri, Penacchioni, et al., 1998). HRT has also been studied as "add-back" therapy during or after treatment with GnRH agonists (Friedman, Daly, Juneau-Norcross, et al., 1994; Maheux and Lemay, 1992).
Tibolone (2.5 mg) was compared with a combination of conjugated equine estrogen (0.625 mg) and medroxyprogesterone acetate (MPA) (5 mg) daily for 1 year (de Aloysio, Altieri, Penacchioni, et al., 1998). Neither group of 25 patients showed a significant change in fibroid size by transvaginal ultrasound, but the proportion of cycles with vaginal bleeding or spotting was significantly lower in the tibolone-treated patients than in the HRT-treated patients (p < 0.02).
Several studies have tested the efficacy of low-dose hormone therapy added to GnRH agonist treatment, referred to as an "add-back" regimen by some authors (Friedman, Daly, Juneau-Norcross, et al., 1993). One study compared an estrogen-progestin with a progestin-only add-back regimen among 51 patients who also were treated with leuprolide acetate for 2 years (Friedman, Daly, Juneau-Norcross, et al., 1994). The add-back regimen was initiated 3 months after the GnRH agonist was started and continued for 21 months. In this study, uterine volume decreased during the 3-month period of GnRH agonist-only therapy by 36-44 percent. Once estrogen-progestin or progestin-only therapy was instituted, uterine volume remained the same in the combined estrogen-progestin group but increased significantly in the progestin-only group. The two groups had similar increases in hemoglobin and hematocrit. The effect on serum high-density lipoprotein (HDL) cholesterol concentration favored the estrogen-progestin group, but there were no between-group differences in bone density of the lumbar spine. The authors of this study concluded that prolonged (more than 6 months) medical treatment with estrogen-progestin combination is safe and effective.
Another study compared sequential versus continuous cyclical low-dose hormone replacement therapy (conjugated equine estrogen and MPA) given as "add-back" therapy along with goserelin depot (Maheux and Lemay, 1992). Mean fibroid volume decreased significantly during the first 3 months of GnRH agonist treatment, but no further changes were evident after hormone replacement therapy was instituted until after GnRH agonist therapy was stopped. Significantly less bleeding was observed in the group receiving continuous rather than sequential hormone replacement therapy. Similarly, hot flashes were less severe in patients receiving continuous HRT than in those receiving sequential HRT.
Our search identified one randomized study of oral contraceptives (Friedman and Thomas, 1995), which was subsequently withdrawn without explanation.
Progestins
MPA has been studied for effectiveness during or following GnRH agonist treatment in six studies (Benagiano, Morini, Aleandri, et al., 1990; Caird, West, Lumsden, et al., 1997; Carr, Marshburn, Weatherall, et al., 1993; Friedman, Barbieri, Doubilet, et al., 1988; Scialli and Jestila, 1995; West, Lumsden, Hillier, et al., 1992) (see Table 20).
Five of the studies of MPA given either concurrently with or following GnRH agonist treatment used doses of MPA ranging from 5 mg to 20 mg daily; however, one study involved treatment with 200 mg daily, tapering to 25 mg daily over 4 months (Benagiano, Morini, Aleandri, et al., 1990). In none of these studies did progestin add-back therapy have an effect on uterine size. Symptoms were reported in markedly different ways among these trials. Vasomotor symptoms, such as hot flashes, were decreased with MPA treatment in both placebo-controlled studies that reported this outcome (Caird, West, Lumsden, et al., 1997; Friedman, Barbieri, Doubilet, et al., 1988), while MPA treatment was associated with increased spotting (irregular bleeding) in two studies (Benagiano, Morini, Aleandri, et al., 1990; Friedman, Barbieri, Doubilet, et al., 1988) and no difference in two studies (Caird, West, Lumsden, et al., 1997; Scialli and Jestila, 1995).
Nonsteroidal Anti-inflammatory Drugs
Two studies described the effects of NSAIDs taken during menstruation to reduce menorrhagia associated with fibroids. The agents studied were indomethacin (Anteby, Yarkoni, and Ever Hadani, 1985) and naproxen (Ylikorkala and Pekonen, 1986). Both studies used a randomized design and included a placebo control. Both included women with menorrhagia from a variety of causes but reported results for the fibroid subgroup separately. One study found statistically significant reductions in days of bleeding (Anteby, Yarkoni, and Ever Hadani, 1985), but there were no differences in mean blood loss during menses in the other study (Ylikorkala and Pekonen, 1986).
GnRH Agonists as Primary Treatment
Fifty-four studies examined the use of GnRH agonists alone. In addition, nine studies examined the effectiveness of combining GnRH agonist treatment with progesterone, estrogen-progestin combinations, or danazol.
Most of these studies described the short-term reduction in uterine or fibroid volume observed with GnRH agonist treatment. The average percentage reduction in uterine size in the 21 studies reporting percentage reduction or pre- and posttreatment values was 46.7 percent (standard deviation [SD] 12.4 percent). Fewer studies measured fibroid volume. Studies that reported both total uterine volume and fibroid volume found the percentage of reductions to be similar (Broekmans, Hompes, Heitbrink, et al., 1996; Costantini, Anserini, Valenzano, et al., 1990; Palomba, Affinito, Di Carlo, et al., 1999), suggesting that shrinkage occurs in fibroid tumors, as well as in the nonmyomatous uterus.
Most of the reduction in fibroid and uterine volume occurred during the first month of treatment with the GnRH agonist, with diminishing reductions in size over subsequent months. The magnitude of change in uterine size during the first month was found to be a significant predictor of the ultimate response (Hackenberg, Gesenhues, Deichert, et al., 1992).
Several studies continued to monitor uterine or fibroid size after GnRH agonist therapy was discontinued (de Aloysio, Altieri, Pretolani, et al., 1995; Donnez, Schrurs, Gillerot, et al., 1989; Palomba, Affinito, Di Carlo, et al., 1999; Serra, Panetta, Colosimo, et al., 1992; van Leusden, 1992). These studies found that after GnRH agonists were stopped, uterine and fibroid sizes returned toward pretreatment values over several months.
Reduction of uterine or fibroid size may be most relevant for preoperative treatment; however, for long-term medical therapy with GnRH agonists, we were more interested in looking at symptoms and adverse effects of treatment. Of the 33 studies of primary GnRH agonist treatment, only nine reported changes in presenting fibroid symptoms as an outcome measure (Coddington, Brzyski, Hansen, et al., 1992; Friedman, Barbieri, Benacerraf, et al., 1987; Friedman, Hoffman, Comite, et al., 1991; Nakamura, Yoshimura, Yamada, et al., 1991; Palomba, Affinito, Di Carlo, et al., 1999; Palomba, Affinito, Tommaselli, et al., 1998; Schlaff, Zerhouni, Huth, et al., 1989; Serra, Panetta, Colosimo, et al., 1992; Vollenhoven, Shekleton, McDonald, et al., 1990) (Table 21). Fibroid-related symptoms responded almost completely to adequate treatment with GnRH agonists in these studies. In one study, intranasally administered leuprolide failed to induce amenorrhea, reduce uterine size, or alleviate fibroid symptoms in most patients (Friedman, Barbieri, Benacerraf, et al., 1987).
Six studies reported data on the number of women with common symptoms but did not relate these to pretreatment symptoms (Abramovici, Dirnfeld, Auslander, et al., 1994; Cirkel, Ochs, Schneider, et al., 1992; Costantini, Anserini, Valenzano, et al., 1990; de Aloysio, Altieri, Pretolani, et al., 1995; Fedele, Bianchi, Raffaelli, et al., 2000; Watanabe, Nakamura, Matsuguchi, et al., 1992). Thirteen studies reported no data on fibroid-related symptom outcomes (Bianchi, Costantini, Anserini, et al., 1989; Broekmans, Hompes, Heitbrink, et al., 1996; Cagnacci, Paoletti, Soldani, et al., 1994; D'Addato, Repinto, and Andreoli, 1992; De Leo, Morgante, Lanzetta, et al., 1997; Donnez, Schrurs, Gillerot, et al., 1989; Felberbaum, Germer, Ludwig, et al., 1998; Friedman, Harrison-Atlas, Barbieri, et al., 1989; Golan, Bukovsky, Schneider et al., 1989; Hackenberg, Gesenhues, Deichert et al., 1992; Kuhlmann, Gartner, Schindler, et al., 1997; Ueki, Okamoto, Tsurunaga, et al., 1995; van Leusden, 1992). Furthermore, the recording and reporting of symptoms and response of symptoms to treatment were inconsistent.
Two studies assessed recurrence of symptoms after GnRH agonist treatment was discontinued (Palomba, Affinito, Di Carlo, et al., 1999; Serra, Panetta, Colosimo, et al., 1992). One study reported only that there was a significant increase in the intensity of myoma-related symptoms during treatment (Palomba, Affinito, Di Carlo, et al., 1999), but the other quantified the proportion of women with recurrent symptoms (Serra, Panetta, Colosimo, et al., 1992). Although all women were symptomatic before treatment, 94 percent had complete resolution or improvement of their symptoms after 4 months of treatment with leuprolide. At 8-12 months after treatment was discontinued, 24 percent of women had recurrent symptoms, 64 percent remained asymptomatic, and 7.3 percent were lost to followup.
Preoperative Use of GnRH Agonists
The most thoroughly studied use of medical treatments for fibroids is short-term treatment with GnRH agonists in preparation for hysterectomy or myomectomy (see Evidence Table 3). Nineteen randomized controlled trials on this topic were described in a recent Cochrane review and meta-analysis (Lethaby, Vollenhoven, and Sowter, 1999). In addition, we identified two randomized controlled trials (Cetin, Vardar, Demir, et al., 1995; Ylikorkala, Tiitinen, Hulkko, et al., 1995) not described in the Cochrane review, one of which was known to the review's authors but not yet assessed (Ylikorkala, Tiitinen, Hulkko, et al., 1995), and three nonrandomized studies with historical or concurrent controls describing the short-term use of GnRH agonists in preparation for definitive surgery (hysterectomy or myomectomy) (Falsetti, Mazzani, Rubessa, et al., 1992; Kiltz, Rutgers, Phillips, et al., 1994; Vercellini, Bocciolone, Colombo, et al., 1993).
Briefly, the Cochrane review found, consistent with the nonsurgical studies, that GnRH analog therapy given for 3-4 months prior to surgery significantly reduced uterine volume and uterine size and improved preoperative hemoglobin and hematocrit. Pelvic symptoms also were reduced, but some adverse events were more likely during GnRH agonist therapy.
Operating time and duration of hospital stay were reduced in GnRH agonist-treated patients. Also, more of the women undergoing hysterectomy were able to have a vaginal rather than an abdominal approach. The intraoperative estimated blood loss (EBL) and rate of vertical incisions were reduced for both myomectomy and hysterectomy. There were no significant differences in transfusion rates. There were no data with which to assess the effects of pretreatment with GnRH agonists on postmyomectomy fertility.
Two randomized controlled trials were not included in the Cochrane review. Ylikorkala, Tiitinen, Hulkko, et al. (1995) described a large trial comparing nafarelin delivered intranasally with a placebo nasal spray. During the 3-month pretreatment period, the nafarelin group experienced a 23.7 percent decrease in uterine size, a 31.4 percent decrease in fibroid size, and a 5.5 mg/dl increase in hemoglobin. However, the study found no significant difference in intraoperative outcomes of estimated blood loss or operating room time. Although the operating room time results are consistent with those reported in the Cochrane review, the EBL data are not. Notably, the change in uterine size in this study was somewhat smaller than that described in other studies and may explain the negative findings.
Cetin, Vardar, Demir, et al. (1995) describe a smaller trial involving 30 women with symptomatic fibroids who were randomized to buserelin intranasally for 3 months prior to myomectomy or immediate myomectomy. The investigators observed a 53 percent decrease in uterine size (p < 0.05) and an increase in hemoglobin from 10.5 to 13.4 (p < 0.05) from pre- to postbuserelin treatment. In this study, EBL was significantly lower in the GnRH agonist group than in the control group (135 cubic centimeters (cc) versus 292 cc; p < 0.05). Operating room time also was significantly lower in the GnRH agonist group than in the control group (87 minutes versus 102 minutes; p < 0.05).
Three nonrandomized studies with historical or concurrent controls examined the short-term use of GnRH agonists in preparation for hysterectomy or myomectomy (Table 22). These studies described the use of goserelin (Falsetti, Mazzani, Rubessa, et al., 1992; Vercellini, Bocciolone, Colombo, et al., 1993) or leuprolide (Kiltz, Rutgers, Phillips, et al., 1994). Statistically significant differences were found in intraoperative blood loss in two of three studies reporting this outcome. However, neither of the two studies comparing operating room time found any difference, even when controlling for single versus multiple myomectomy.
Postoperative Use of GnRH Agonists
One study described the rate of recurrence of fibroids after myomectomy on no treatment or leuprolide (Vavala, Lanzone, Monaco, et al., 1997). Women who agreed to the treatment took leuprolide acetate (LA) depot 3.75 mg subcutaneous (SC) each month for 3 consecutive months each year for 3 years, starting 4 months after surgery. Women who refused postoperative leuprolide treatment were followed as a control group. Regrowth of fibroids (> 2 cm) and uterine size were both statistically significantly less likely in leuprolide-treated than control patients (p < 0.05). There was no assessment of symptom recurrence, fertility, or need for additional surgery, which limits the clinical relevance of the study.
Other Medical Treatments
Several studies described the use of other hormonally active drugs, including danazol (De Leo, la Marca, and Morgante, 1999; De Leo, Morgante, Lanzetta, et al., 1997; Ueki, Okamoto, Tsurunaga, et al., 1995), gestrinone (Coutinho, 1990; Coutinho, Boulanger, and Goncalves, 1986; Coutinho and Goncalves, 1989), tibolone (de Aloysio, Altieri, Penacchioni, et al., 1998; Gregoriou, Vitoratos, Papadias, et al., 1997), and RU-486 (Murphy, Morales, Kettel, et al., 1995). One study described the use of the Chinese herbal medicine kuei-chin-fu-ling-wan (Sakamoto, Yoshino, Shirahata, et al., 1992).
In an uncontrolled trial, De Leo, la Marca, and Morgante, et al. (1999) found that 4 months of treatment with danazol (400 mg per day) reduced uterine size and improved hematocrit. No data on the effect on fibroid symptoms were reported. In an earlier study, De Leo, Morgante, Lanzetta, et al. (1997) found that treatment with danazol (100 mg per day) after 6 months of GnRH agonist treatment reduced regrowth of fibroids by 31 percent compared with historical patients who received no danazol posttreatment (p < 0.001). Ueki, Okamoto, Tsurunaga, et al. (1995) compared change in fibroid size in patients taking danazol (400 mg daily) with patients taking the GnRH agonist buserelin. Although no statistical tests were reported, the study found a large difference in fibroid size reduction between danazol- and buserelin-treated patients (27 percent reduction versus 52 percent reduction, respectively).
Gestrinone, a synthetic steroidal hormone with androgenic, antiestrogenic, and antiprogestogenic properties, was studied in one placebo-controlled study (Coutinho, 1990). Among patients treated with gestrinone (5 mg three times per week), uterine size decreased after 2 months of treatment, and all patients reported alleviation of pretreatment symptoms attributed to fibroids. A high proportion of gestrinone-treated patients reported androgenic side effects, such as hirsutism (25 percent) and acne (87.5 percent). Two other studies tested the effectiveness of lower doses of gestrinone (2.5 mg three times per week) given by mouth or intravaginally (Coutinho, Boulanger, and Goncalves, 1986; Coutinho and Goncalves, 1989). The lower dose and intravaginal route of administration resulted in reductions in uterine size and fibroid-related symptoms similar to those achieved with the oral dose tested in the previously mentioned study.
The synthetic steroid tibolone, which has mixed estrogenic, progestogenic, and androgenic properties, was studied in two controlled trials. Gregoriou, Vitoratos, Papadias, et al. (1997) compared tibolone 2.5 mg daily versus no treatment in 40 women with at least one fibroid more than 20 mm in diameter. After 1 year of treatment, there was no significant change in fibroid volume in either group.
In the other study of tibolone, de Aloysio, Altieri, Penacchioni, et al. (1998) similarly found no change in fibroid size in patients treated for 1 year with 2.5 mg daily of tibolone or a separate group treated with conjugated equine estrogens 0.625 mg daily and medroxyprogesterone acetate 5 mg daily.
The antiprogestin agent RU-486 was tested in a prospective nonrandomized clinical trial comparing three doses (Murphy, Morales, Kettel, et al., 1995). Limited data showed that although all groups became amenorrheic, there was a smaller reduction in uterine size in the lowest dose group (5 mg daily) compared with the higher (25 mg or 50 mg daily) dose groups (25 percent reduction versus 50 percent and 55 percent reduction; no p-value reported).
A single study reported the results of treatment with the Chinese herbal medicine kuei-chin-fu-ling-wan (Sakamoto, Yoshino, Shirahata, et al., 1992). Kuei-chin-fu-ling-wan, known as keishi-bukuryo-gan (KBG) in Japan, contains five components: bark of Cinnamomum cassia Bl. (Lauraceae), root of Paeonia lactiflora Pall. (Paeoniaceae), seed of Prunus persica Batsch. or P. persiba Batsch.var.davidiana Maxim. (Rosaceae), carpophores of Poria cocos Wolf. (Polyporaceae), and root bark of Paeonia suffruticosa Andr. (Paeoniaceae). It has been reported to act as a luteinizing hormone-releasing hormone (LHRH) antagonist and a weak antiestrogen on uterine DNA synthesis in immature rats (Sakamoto, Kudo, Kawasaki, et al., 1988). Only women with uterine size 10 cm or smaller were included in the study. In addition to fibroid size, investigators assessed symptoms of menorrhagia (57 percent of patients) and dysmenorrhea (46 percent of patients). Fibroids showed complete resolution in 19 percent of patients, decreased in size in 43 percent, showed no change in 34 percent, and increased in 4 percent of patients. Menorrhagia improved in 95 percent of patients, with 46 percent having bleeding "reduced to normal." Similarly, symptoms of dysmenorrhea improved in 94 percent of patients with this symptom and were completely relieved in 51 percent. Fifteen of the 110 patients underwent hysterectomy during followup.
Methodological Issues
Symptom outcomes were poorly measured and reported in these trials. Nearly all studies required subjects to have symptoms associated with fibroids, principally menorrhagia and pelvic pain. When reporting the alleviation of symptoms, many studies either did not differentiate among various symptoms or did not report the number of women reporting certain types of symptoms. Because women may present with multiple symptoms, a combined-symptom measure would be preferable to enumerating the response for each symptom separately. None of the studies used any kind of systematic symptom inventory or disease-specific quality-of-life measure.
One article on oral contraceptives was subsequently withdrawn from publication without explanation (Friedman and Thomas, 1995). Without more explanation, it is unclear whether this retraction should affect the assessment of the validity of other publications by the same authors.
Summary
GnRH agonist treatment is associated with reduction of fibroid and uterine size, control of bleeding, and reduction of some symptoms; however, these drugs cause menopausal symptoms and, with long-term use, bone loss. Followup studies show that after treatment with GnRH agonists is stopped, there is regrowth of both the fibroid tumors and the uterus to near pretreatment size, which is often associated with the return of fibroid-related symptoms.
Concurrent treatment with GnRH agonists and low doses of progestins or estrogen-progestin combinations tends to ameliorate vasomotor menopausal symptoms (hot flashes) associated with GnRH agonists; however, this add-back treatment was associated with irregular bleeding in some studies. Androgen treatment following GnRH treatment may be effective at reducing regrowth of fibroids shrunk by GnRH treatment; also, GnRH agonist therapy may reduce regrowth of fibroids following myomectomy; however, the effect on fibroid-related symptomatology is less clear.
Used in the short term prior to myomectomy, GnRH agonists can correct anemia from menorrhagia (which may permit autologous blood donation), reduce blood loss during surgery, and allow use of transverse rather than midline incision or vaginal (or, conceivably, laparoscopic) rather than abdominal surgical approach due to reduction in uterine size. The clinical significance of statistically significant differences in estimated blood loss, operating time, or incision type is unclear, especially since long-term data from prospective studies of hysterectomy suggest that short-term complications or technical approach to surgery do not influence long-term outcomes. The effectiveness of using GnRH agonists to facilitate autologous blood donation for intra- or postoperative transfusion was not explicitly studied among patients with fibroids. Data on cost-effectiveness would be useful. There are two additional concerns regarding presurgical treatment with GnRH agonists:
- The observation by some surgeons that fibroids are more difficult to separate from the uterus after GnRH agonist treatment is supported by data from hysterectomy specimens, which suggests that pretreatment with GnRH agonists obliterates the cleavage plane between myometrium and fibroid (Deligdisch, Hirschmann, and Altchek, 1997).
- Pretreatment with GnRH may increase the postmyomectomy fibroid recurrence rate by shrinking some small fibroids so that they inadvertently are not removed at the time of myomectomy. This concern is supported by two studies reporting data on recurrence (as measured by ultrasound) (Fedele, Vercellini, Bianchi, et al., 1990; Friedman, Daly, Juneau-Norcross, et al., 1992).
Although most of the trials considered here were limited to women with symptomatic uterine fibroids, with symptoms usually defined as vaginal bleeding (menorrhagia), constant or cyclic pelvic pain, or constipation or urinary symptoms, the trials less often describe symptomatic outcomes other than uterine or fibroid size changes. Data from a limited number of trials, however, show a strong association between the uterine and fibroid size reduction and alleviation of symptoms.
Control groups on placebo or no treatment for prolonged periods demonstrate that patients with symptomatic fibroids for whom nothing is done (no treatment) remain stable in terms of symptoms or uterine and fibroid size. However, these studies were performed in women with mild symptoms. The prognosis for women with more severe fibroid-related symptoms or larger fibroids may be different.
Hormone replacement therapy with cyclic or noncyclic estrogen-progestin combinations appears to be ineffective in alleviating fibroid symptoms or fibroid growth. Progestins are ineffective alone, when used as add-back therapy during GnRH agonist treatment, or when used following GnRH agonist treatment to maintain fibroid control. However, progestins are effective at eliminating vasomotor symptoms of hot flashes associated with GnRH agonists.
Question 7: What are the costs associated with effective surgical and nonsurgical treatments?
Approach
We attempted to collect data on the costs of all the targeted treatments for fibroids, even though evidence for effectiveness was lacking for many of them. We first identified the types of data that would be needed to provide a comprehensive description and analysis of the costs associated with treatments for fibroids. Other considerations often considered as "costs" -- stresses on family life, effects on sexual function -- are more appropriately considered as quality-of-life measures, to be incorporated into the denominator of a cost-effectiveness ratio. Nonmedical costs include:
- Decreased productivity associated with bleeding or pain, either through actual time away from work or decreased ability to perform work because of symptoms.
- Decreased productivity associated with diagnostic and therapeutic interventions; time lost from work would likely affect other family members as well for some interventions.
- Transportation costs associated with provider visits.
- Child care expenses associated with provider visits.
- Use of nonmedical supplies, such as sanitary napkins.
Medical costs for the interventions considered in this report include the following:
For no treatment (for an asymptomatic patient):
- Costs associated with increased surveillance (more frequent pelvic examinations or radiological studies) after detection.
- Costs associated with treatment once uterine or fibroid size has reached a predefined threshold (we did not identify evidence that would permit estimation of an appropriate threshold).
- Costs associated with increased morbidity if the probability of success or the technical difficulty of treatment has increased; evidence supporting increased morbidity associated with increasing fibroid size is inconclusive (see under Question 1).
For oral contraceptives, progestins, and NSAIDS:
- Cost of medications.
- Cost of managing side effects.
- Cost of provider visits during followup.
- Cost of diagnostic tests during followup.
For GnRH agonists:
- Cost of medication.
- Cost for administration of injection for injectable forms.
- Costs of any medications used to manage side effects.
- If used as an adjunct for surgical procedures, any differences in costs between procedures done with and without adjunctive treatment.
For invasive therapies (embolization, myomectomy, hysterectomy):
- Cost of equipment and supplies used to perform the procedure.
- Fixed costs associated with use of radiology suite.
- Personnel costs.
- Medication costs for management of pain.
- Hospitalization costs for patients admitted after procedure.
- Cost of managing complications.
- Followup outpatient visits and testing, if indicated.
We were unable to identify a data source for estimating nonmedical costs associated with the management of asymptomatic fibroids. For estimating medical costs, we used several sources, including the following:
- 2000 "Red Book" of wholesale drug prices (Medical Economics Company. 2000).
- Published literature on hospital costs for surgical management of fibroids.
- Primary data from the Nationwide Inpatient Sample (NIS).
- Primary data from Duke University Medical Center.
Results
Medical Therapy
Costs for a 3-month course of medical therapy were estimated by:
- Identifying minimum and maximum average wholesale prices for medications (including generics if available) using the 2000 "Red Book" of pharmaceutical prices.
- Estimating monthly pill usage based on potential dosing regimens (e.g., up to 7 days of NSAID therapy, or 10 days of oral medroxyprogesterone acetate).
- Estimating monthly costs based on wholesale prices for a given number of dispensed units (e.g., if 100 pills were dispensed at a given price and 21 would be used during a month, the monthly cost was estimated as 0.21 x cost/100 pills).
Costs are given for 3 months to facilitate comparison with 3 months of GnRH agonist therapy, the maximum time GnRH would be given prior to surgical management.
Results are shown in Table 23. NSAIDs are the least expensive treatment. The figures in Table 23 represent prolonged monthly courses of 7 days; costs for women who respond to shorter courses would be lower. Long-acting medroxyprogesterone acetate is next; unfortunately, there are no data on its effectiveness in women with fibroids. Oral progestins and oral contraceptives are next, at two to five times the cost of NSAIDs. GnRH agonists are the most expensive.
Estimation of cost-effectiveness would require better data on effectiveness, as well as on costs. One analysis of the use of adjunctive GnRH agonists prior to surgery suggested that their use might be cost-effective because of decreased complication rates and increased ability to perform less expensive vaginal hysterectomy (Bradham, Stovall, and Thompson, 1995). Replication of this analysis using more recently published effectiveness and cost data would be worthwhile.
Myomectomy
Estimated mean charges for women 18-44 years of age undergoing myomectomy in the NIS for 1997 were $8,884. Mean total costs for women undergoing the procedure at Duke University Medical Center from 1993 through 1998 (normalized to 1999 dollars) by age are shown in Table 24. The total mean Duke cost was $5,171, consistent with a 50-60 percent cost-to-charge ratio compared with the NIS data. Of these costs, 30.4 percent were attributable to inpatient unit costs, and 54.0 percent were attributable to surgical services.
Hysterectomy
Mean Duke costs by age group are shown in Table 25 (all hysterectomies combined). Overall mean cost was $5,961, with 32.6 percent attributable to inpatient unit costs and 49.0 percent attributable to surgical services.
Methodological Issues
Data are not available to estimate the overall economic costs to society of uterine fibroids. Data also are not available on the costs of outpatient management, other than estimates of the wholesale prices of drugs (which clearly do not reflect actual prices paid by providers or patients). Although relatively detailed data on inpatient resource utilization are available, additional work on clinical factors that predict resource utilization needs to be done.
Even with better data on costs, cost-effectiveness analysis cannot be performed without better data on effectiveness. Even if NSAIDs are the least expensive method of treatment, their cost-effectiveness ratio will be quite high if their effectiveness (the denominator in the ratio) is low.
Summary
Data on nonmedical costs and outpatient costs of managing patients with fibroids are not readily available from easily accessed sources. Although data on inpatient costs are somewhat more detailed, most administrative data sources do not provide sufficient clinical detail to allow comparison between procedures. At one academic medical center, total mean inpatient costs for abdominal myomectomy were approximately $800 less than total costs for hysterectomy.
Question 8: Do risks and benefits differ for women according to race, ethnicity, age, interest in future childbearing, and so forth?
Approach
We approached the question of different benefits and risks of strategies for managing fibroids among subpopulations by focusing on four separate topics:
- Racial and ethnic differences in epidemiology and outcomes.
- Effects of age, especially menopausal status, on epidemiology and outcomes.
- Effects of fibroids on pregnancy outcomes and complications.
- Effects of fibroids on fertility.
Articles that met our screening criteria were searched and subcategorized according to the above topics.
Race and Ethnicity
Ten articles were identified initially as being potentially informative in answering questions about whether racial distinctions exist in the risks and benefits of fibroid management. After closer inspection, only four of these actually proved to be useful for this analysis (Hillis, Marchbanks, and Peterson, 1996; Kjerulff, Guzinski, Langenberg, et al., 1993; Kjerulff, Langenberg, Seidman, et al., 1996; Marshall, Spiegelman, Barbieri, et al., 1997). All four articles focused on a comparison between black and white populations. Two included other racial groups, including Hispanics and Asians, with results statistically indistinguishable from the white population with whom they were compared (Hillis, Marchbanks, and Peterson, 1996; Marshall, Spiegelman, Barbieri, et al., 1997). In both studies, "white" was used as the reference group, with no separate analyses looking at differences within other racial groups. The rest of this discussion will focus on the distinctions between blacks and whites unless otherwise specified.
Epidemiology
Using data from the Nurse's Health Survey, Marshall and colleagues determined that the incidence of uterine fibroids among black women is approximately three times that among whites (Marshall, Spiegelman, Barbieri, et al., 1997). In addition, they found that uterine fibroids are diagnosed earlier in black women than in white women, with the highest incidence of diagnosis being from 35-40 years versus 40-44 years for whites. A study by Kjerulff and colleagues corroborated this finding, reporting a mean age of diagnosis for blacks of 37.5 ± 7.9 versus 41.6 ± 6.6 for whites (Kjerulff, Guzinski, Langenberg, et al., 1993).
Management
Although the time from diagnosis of uterine fibroids to hysterectomy has been shown to be longer for blacks than for whites (3.9 ± 5.0 versus 2.8 ± 3.6 years) (Marshall, Spiegelman, Barbieri, et al., 1997), black women overall undergo hysterectomy at a younger age than white women due to their earlier average age at diagnosis. In one prospective cohort, the average age at hysterectomy for blacks was 41.7 ± 6.1 versus 44.6 ± 5.8 for whites (Kjerulff, Langenberg, Seidman, et al., 1996). In one large epidemiological study of more than 53,000 women, uterine fibroids accounted for 65.4 percent of the hysterectomies in black women versus only 28.5 percent in white women (Kjerulff, Guzinski, Langenberg, et al., 1993). Moreover, at the time of hysterectomy, black women have been shown on average to have both more myomas and a larger uterine size (Hillis, Marchbanks, and Peterson, 1996; Kjerulff, Langenberg, Seidman, et al., 1996). Kjerulff and colleagues found that 30 percent of black women who underwent hysterectomy had uteri greater than 500 grams versus only 15 percent of white women (Kjerulff, Langenberg, Seidman, et al., 1996). This difference has potentially important implications because Hillis and colleagues demonstrated that women undergoing hysterectomy with a uterus of > 500 grams were 1.6 (1.0-4.0) times more likely to develop operative or postoperative complications, particularly cuff cellulitis (relative risk [RR], 2.8 [95% confidence interval (CI), 1.3-6.2]) and transfusion (RR, 2.4 [95% CI, 1.3-4.3]).
At the time just prior to hysterectomy, Kjerulff and colleagues found that black women were more likely to be anemic than white women (56 percent vs. 38 percent) and more likely to complain of severe pelvic pain (59 percent vs. 41 percent ) (Kjerulff, Langenberg, Seidman, et al., 1996). There was no evaluation of postoperative symptomatology in this study, and none of the other studies evaluated symptomatology along racial lines.
We identified no studies that evaluated racial differences in response to, or provision of, medical therapies for uterine fibroids, such as NSAIDs, GnRH agonists, or other hormonal preparations. Similarly, there are no published data evaluating racial distinctions concerning myomectomy, uterine artery embolization, or surgical procedures other than hysterectomy.
Preliminary analysis of data from the 1997 Nationwide Inpatient Sample (NIS, 1997) shows the following: 45,740 women were identified as undergoing either myomectomy (n = 7,749) or hysterectomy (n = 37,991) for fibroids. Figures 2 through 5 show the estimated age- and race-specific incidences of myomectomy and hysterectomy based on 1997 NIS and U.S. Census Bureau estimates, as well as the projected race-specific cumulative incidences of each procedure based on these data. Bivariate analysis revealed that, at the time of their procedure, 45 percent of black women were younger than 40 versus 35 percent of Hispanic women and only 27 percent of white women (p < 0.0001). Data on number of prior children, contraceptive use, or history of sterilization are not available within the NIS data. Hispanics were more likely to have Medicaid/Medicare than their black and white counterparts: the figures were 23 percent, 17 percent, and 17 percent respectively (p < 0.0001). Blacks were more likely than either whites or Hispanics to earn $25,000 or less (41 percent vs. 29 percent vs. 26 percent) and less likely to earn more than $35,000 (24 percent vs. 31 percent vs. 36 percent) (p < 0.0001). Blacks were more likely than either whites or Hispanics to earn $25,000 or less (41 percent vs. 29 percent vs. 26 percent) and less likely to earn more than $35,000 (24 percent vs. 31 percent vs. 36 percent (p < 0.0001).
Thirteen percent of white women were treated with myomectomy versus 22 percent of black women and 17 percent of Hispanic women. Results for both the unadjusted and adjusted models were similar and revealed that, when undergoing a procedure for fibroids, black women are 1.6 (95% CI, 1.5-1.7) times more likely to receive a myomectomy than white women. Similarly, Hispanic women are 1.3 (95% CI, 1.2-1.5) times more likely to receive a myomectomy than white women. These findings were consistent for every age group of women. This suggests that, at the least, minority women are not less likely to be offered conservative surgical therapy than white women. There also may be cultural differences that lead some minority women to be more likely to request conservative therapy.
Preliminary analysis of the primary data abstraction of 239 patients undergoing myomectomy at Duke University Medical Center also shows some interesting findings. Black women were more than twice as likely as white women to have an in-hospital complication (odds ratio [OR], 2.48) or transfusion (OR, 2.2), but this increased risk was eliminated after adjusting for uterine size and number of fibroids (adjusted OR for complications 1.36 [95% CI, 0.56-3.15]; adjusted OR for transfusion 0.9 [95% CI, 0.27-2.76]). Other factors, such as body mass index (BMI) or insurance status, were not independent predictors of complications. This suggests that, for myomectomy, an increased complication rate in black women is largely attributable to differences in the underlying disease pathology. Currently, we are analyzing data on hysterectomy from the same time period to see if a similar pattern is observed.
Summary
Though it seems clear that black women are more likely to develop uterine fibroids than are white women and to have them diagnosed earlier, little else is known concerning racial differences in the natural history of fibroids.
Hysterectomy is the best-studied issue with regard to race and fibroids, yet many questions remain unanswered. Previous work has shown that at the time of hysterectomy, black women are more likely than white women to have severe pain, to have a uterus greater than 500 grams, and to be anemic. They also are more likely to have a longer delay from diagnosis to hysterectomy. The reasons for these observed differences are unclear. Studies examining the role of physician and patient beliefs in explaining this difference are needed, as are studies evaluating patient symptomatology pre- and postsurgery. Even more fundamental is determining whether the higher incidence of fibroids in black women explains their overall higher hysterectomy rates compared with white women (49.5 versus 41.2 per 10,000 women) (Kjerulff, Guzinski, Langenberg, et al., 1993). Our analysis of NIS data suggests that black women also are more likely to undergo myomectomy than white women and, in fact, are more likely to undergo myomectomy than hysterectomy for fibroids at every age group, despite having larger and more numerous fibroids. Further examination of these differences is needed. Finally, though no studies looking at hysterectomy rates for fibroids alone have been performed, black women have been found to be at 1.4 (95% CI, 1.3-1.5) times higher risk for surgical complications than white women. Given that black women appear to have larger uteri at the time of hysterectomy and uterine size is associated with an increased rate of complications, studies examining the relationship between uterine size, other comorbidities, race, and complications are needed. Preliminary data from our primary chart abstraction seem to support this relationship for myomectomies.
As stated above, we were unable to identify studies that examined racial differences and medical or invasive therapies other than hysterectomy for fibroids. Therefore, any studies evaluating epidemiological issues, response rates, side effects, and patient symptomatology in regard to race with these therapies would be a contribution.
Age/Menopausal Status
There were 49 articles identified as being potentially informative in evaluating how the risks and benefits of fibroid management differ by menopausal status. Of these, 34 were found useful in providing information on this topic.
Epidemiology
The rate of uterine fibroid diagnosis in the Nurses Health Study is highest between ages 40-44 (Marshall, Spiegelman, Barbieri, et al., 1997). The highest percentage of hysterectomies for fibroids is performed in the age group 40-49 (Kjerulff, Langenberg, Seidman, et al., 1996). From 1988-1990, the rate of admissions nationally for fibroids as the primary indication was 49.3 (95% CI, 43.6-55.0) per 10,000 women, with the peak age group for those admitted being 25-34 years (Velebil, Wingo, Xia, et al., 1995). Differences in these ages may represent differences in diagnostic methods, differences in patient populations, or differences in data sources used. One study of women complaining of abnormal uterine bleeding found submucous fibroids (by hysteroscopy) in 26 percent of premenopausal women, 24 percent of postmenopausal women on hormone replacement therapy, and 15 percent of postmenopausal women not taking hormones (Akkad, Habiba, Ismail, et al., 1995). In a case control study of more than 1,500 women, no relationship between oral contraceptive use and the development of fibroids was found (Parazzini, Negri, La Vecchia, et al., 1992).
Management
GnRH agonists for the premenopausal patient
Details of studies of GnRH agonists in premenopausal women are provided under Question 6.
GnRH agonists for the perimenopausal patient
Little research has been done on the use of GnRH agonists in perimenopausal patients. The two studies that examined this issue found that women who never resumed menses after GnRH agonist treatment or entered menopause within 3 months of treatment continued with the decrease in uterine size and increase in hemoglobin gained from the therapy (Nakamura and Yoshimura, 1993; van Leusden, 1992).
GnRH agonists for the postmenopausal patient
We identified no studies examining the use of GnRH agonists in postmenopausal women.
Invasive therapy
Few studies of surgical therapies have performed stratified analyses by age or menopausal status. Two series reported subgroup analyses on postmenopausal women (6 of 150 total subjects) (Cravello, D'Ercole, Boubli, et al., 1995), with "control" of post-menopausal bleeding in 50 and 92 percent of subjects, respectively (Loffer, 1990). Uterine artery embolization appears to be more likely to result in amenorrhea in perimenopausal women compared with premenopausal women (Goodwin, McLucas, Lee, et al., 1999; Spies, Scialli, Jha, et al., 1999).
Summary
There is little evidence to allow clear conclusions about the effect of age or menopausal status on the risks and benefits of therapies for symptomatic fibroids. GnRH agonists, myomectomy (especially hysteroscopic myomectomy), and uterine artery embolization all appear more likely to induce prolonged amenorrhea in perimenopausal women compared with premenopausal women, but there are no data on long-term side effects with or without the use of hormone replacement therapy. In addition, there are no data that allow prediction of which perimenopausal women would be most likely to respond to conservative therapy.
Pregnancy Outcomes
Epidemiology
Estimates of the prevalence of fibroids during pregnancy range from 1 to 4 percent (Exacoustos and Rosati, 1993; Katz, Dotters, and Droegemeuller, 1989; Kuhlmann, Gartner, Schindler, et al., 1997; Piazze Garnica, Gallo, Marzano, et al., 1995).
Fibroids in Pregnancy
Most fibroids, especially those less than 5 centimeters, appear to remain stable in size during pregnancy. One small prospective cohort of 29 women found that 78 percent of fibroids either remained the same or decreased in size during pregnancy. Further, in the 22 percent of women whose fibroids increased in size, the increase in volume was less than 25 percent (Aharoni, Reiter, Golan, et al., 1988). Another prospective study of 134 women reported that 85 percent of fibroids either remained stable or decreased in size during the second and third trimesters, with 62 percent of fibroids 5 centimeters or less becoming undetectable (Strobelt, Ghidini, Cavallone, et al., 1994). Fibroids greater than 5 centimeters in this study were more likely to increase in size (26.2 percent vs. 9.7 percent, p = 0.03).
There is convincing evidence that the presence of uterine fibroids is associated with pregnancy complications. In one large retrospective cohort of more than 12,000 women, it was estimated that 1/500 pregnancies involved complications due to fibroids, and that 10 percent of women with fibroids experience pregnancy complications (Katz, Dotters, and Droegemeuller, 1989). Unfortunately, no comparisons in complication rates between women with and without fibroids were reported. A population-based series of more than 6,000 singleton live births in Washington state by Coronado and colleagues found that pregnant women with fibroids were 1.9 (95% CI, 1.6-2.2) times more likely to experience antepartum pregnancy complications than women without fibroids (Coronado, Marshall, and Schwartz, 2000). The pregnancy complications that have been found to be associated with the presence of fibroids include pain, first trimester bleeding, polyhydramnios, premature rupture of membranes, and abruption (Coronado, Marshall, and Schwartz, 2000; Exacoustos and Rosati, 1993; Rice, Kay, and Mahony, 1989). In particular, abruption has been associated with submucosal fibroids, fibroids greater than 200 cc, and a placental location that is superimposed over a fibroid (Exacoustos and Rosati, 1993; Rice, Kay, and Mahony, 1989). Pain has been associated with fundal or isthmic location of the fibroids.
Pregnant women with uterine fibroids also are more likely to undergo laparotomy than are pregnant women without fibroids. One large retrospective cohort of more than 120,000 women found that 1.1 pregnant women in 10,000 underwent laparotomy due to fibroids. Although these numbers are small, 28 percent of the women experienced pregnancy loss, with 21 percent undergoing hysterectomy at the time of the procedure (Burton, Grimes, and March, 1989).
Studies have found that fibroids are associated not only with pregnancy complications, but also with complications during labor and delivery. The Coronado study determined that women with fibroids were 1.9 (95% CI, 1.7-2.2) times more likely to have labor complications and also more likely to experience delivery complications than women without fibroids. In particular, they found that these women were more likely to experience dysfunctional labor (OR, 1.9 [95% CI, 1.3-2.7]), breech presentation (OR, 4.0 [95% CI, 3.0-5.2]), and cesarean delivery (OR, 6.4 [95% CI, 5.5-7.5]). In another series, 13 of 492 women with fibroids (2.6 percent) underwent hysterectomy at the time of delivery (no comparison group data were provided) and had higher rates of postpartum sepsis (4 percent vs. 0.4 percent in women without fibroids, p<0.001) (Exacoustos and Rosati, 1993). In an Italian series, the cesarean section (c-section) rate was 76 percent (Piazze Garnica, Gallo, Marzano, et al., 1995), and it was 73 percent in a Malaysian series (Hasan, Arumugam, and Sivanesaratnam, 1991). Consistent associations of fibroids with preterm labor have not been identified.
Only one study has looked at neonatal outcomes in relationship to the presence of fibroids during pregnancy. Coronado and colleagues found that the newborns of women with fibroids were 2.5 (95% CI, 1.5-4.2) times more likely to have a 5-minute Apgar less than 7; 1.9 (95% CI, 1.3-2.3) times more likely to have any malformation; and 2.0 (95% CI, 1.5-2.6) times more likely to have a birthweight below 2500 grams.
These observed associations between fibroids and adverse pregnancy outcomes do not necessarily mean that fibroids are causative of all of these complications. Confounding may play a role. For example, black women are more likely to have fibroids than women from other ethnic groups, and they also are more likely to have pregnancy complications such as preterm labor or low birthweight. Age is another potential confounder; the incidence of fibroids increases with age, as does the incidence of many pregnancy complications. Detection bias also may play a role -- women with pregnancy complications are more likely to undergo multiple ultrasound examinations, and they are more likely to undergo c-section. Therefore, they have more opportunities for detection of fibroids than women with uncomplicated pregnancies. In particular, this may explain some of the observed associations with low Apgar scores, low birthweight, and malformations, since both increased ultrasound surveillance and increased c-section rates would be expected in these circumstances. Physician decisionmaking about operative delivery based on beliefs about risks of fibroids may play a role in higher operative delivery rates in patients with fibroids.
Pregnancy After Invasive Uterus-Conserving Therapy
The studies looking at pregnancy complications postmyomectomy all have been small and descriptive in nature. Loss of clinically identified pregnancies in women postmyomectomy has been reported to range from 12-29 percent (Dubuisson, Chapron, and Levy, 1996; Li, Mortimer, and Cooke, 1999; Sudik, Husch, Steller, et al., 1996). One small case series (n = 46) with 41 months followup noted that "habitual aborters" had a 10 percent live birth rate premyomectomy versus an 87 percent live birth rate postoperatively (Vercellini, Maddalena, De Giorgi, et al., 1999). Several reviewers of this report pointed out case reports of uterine rupture in early pregnancy after laparoscopic myomectomy, but as discussed above, estimation of true risks based on case reports alone is extremely difficult. Although it has been widely believed that women should undergo elective c-section for delivery after a myomectomy, especially when the uterine cavity has been entered, data to support this recommendation are limited. A study by Garnet found that 55 percent of all ruptures during pregnancy had a previous uterine scar; however, fewer than 5 percent of these women had had a myomectomy. Moreover, uterine rupture occurred in less than 1 percent of pregnancies with prior uterine scars, with two-thirds occurring prior to labor onset (Garnet, 1964), suggesting that planned elective cesarean delivery will not eliminate the risk of uterine rupture in women with uterine scars.
The majority of reported series of uterine artery embolization include one or two unplanned, uneventful pregnancies after the procedure. We identified one case series of 12 pregnancies after uterine artery embolization in nine women (Ravina, Vigneron, Aymard, et al., 2000). Although this paper did not meet our usual inclusion criteria, we discuss it here because of intense interest in pregnancy-related outcomes of this procedure. There were no recurrences of fibroids during any of the pregnancies. Five of the 12 pregnancies resulted in early pregnancy loss (all in women over 40). There were two preterm deliveries at 28 weeks (an AIDS patient with sepsis) and 35 weeks (preeclampsia with twins) and five term deliveries. Four deliveries were by cesarean, and three were vaginal. These numbers are too small to allow meaningful conclusions. The relatively advanced age of the women in this study (mean 36.5, with 5 of 9 patients at least 40 years old) makes them a particularly high-risk group for adverse pregnancy outcomes.
Summary
Although the prevalence of fibroids in pregnancy is well established, their behavior during pregnancy and impact on pregnancy outcomes remain to be clarified. Moreover, although pregnant women with fibroids appear to be at a greater risk for complications, the nature and incidence of these complications needs to be more clearly defined through prospective cohorts. For example, the tumor characteristics (such as size, number, and location) associated with each particular complication need to be assessed in a manner similar to that done for abruption. More studies looking at neonatal outcomes in relation to the presence of fibroids in pregnancy are also needed.
The benefit of myomectomy in improving pregnancy complications remains unclear. No randomized studies, or even large cohort or case control studies, have examined the possible benefits and harm resulting from previous myomectomy. We also did not identify any studies comparing pregnancy outcomes pre- and postmyomectomy in the same woman. If fibroids are truly associated with an increased risk of second and third trimester pregnancy complications, then this would justify myomectomy in some asymptomatic women planning pregnancy. However, given the uncertainties surrounding the nature of any risk, its likelihood in a given patient, and the potential adverse effects of myomectomy itself on both the ability to conceive and the ability to undergo labor, additional data are needed before such a strategy can be widely recommended. As a corollary, no studies have provided convincing evidence as to the benefit of c-section after myomectomy. Thus, more studies examining the pregnancy outcomes of women postmyomectomy would be useful. However, since there is a documented risk of uterine rupture during labor in women with prior classical c-sections (an analogous anatomical change), elective cesarean in women with prior myomectomies appears to be a reasonable option.
There are extremely limited data available on pregnancy outcomes after uterine artery embolization. Well-designed prospective studies (possibly including myomectomy patients as a comparison group) would be extremely useful.
Fertility
Epidemiology
Given the increasing incidence of fibroids with age and the fact that many infertility patients present for care at a time in life when fibroid incidence is particularly high, it is not surprising that fibroids are a relatively common finding in women with infertility. Prevalences as high as 13 percent in women with primary infertility have been reported (Valle, 1980). Direct evidence that fibroids may adversely affect fertility comes from studies of women undergoing assisted reproduction, where the presence of uterine fibroids was associated with significantly decreased implantation and pregnancy rates (Eldar-Geva, Meagher, Healy, et al., 1998; Stovall, Parrish, Van Voorhis, et al., 1998). Some of this difference may be related to distortion of the uterine cavity. One small case series (n = 26) noted an implantation rate of 2.7 percent for women with abnormal cavities versus 8.9 percent for those with normal appearing cavities (Farhi, Ashkenazi, Feldberg, et al., 1995). In a larger case series (n = 406), no significant difference in pregnancy rates was noted in women without fibroids (33.5 percent in 367 patients) and those with fibroids that were not intramural or submucosal (38.5 percent in 39 patients) (Ramzy, Sattar, Amin et al., 1998).
Infertility and Treatment of Fibroids
The literature addressing the efficacy of myomectomy for improving fertility comprises many small case series; there are no controlled trials or cohort studies involving comparison groups of infertile women with fibroids who do not undergo myomectomy. Comparing the results of these many case series is difficult because in most cases, "infertility" is not explicitly defined, other causes for infertility are not adjusted for, and in many the followup period is not stated. Of the 11 studies we identified that had at least a 1-year followup period, 26-75 percent of patients previously "infertile" became pregnant, with 75-94 percent of these pregnancies being successfully carried to term (Babaknia, Rock, and Jones, 1978; Berkeley, DeCherney, and Polan, 1983; Chong, Thong, Tan, et al., 1988; Cravello, D'Ercole, Boubli, et al., 1995; Dequesne and Schmidt, 1996; Dubuisson, Chapron, Chavet, et al., 1996; Gehlbach, Sousa, Carpenter, et al., 1993; Phillips, Nathanson, Meltzer, et al., 1995; Starks, 1988; Vercellini, Maddalena, De Giorgi, et al., 1999; Vercellini, Zaina, Yaylayan, et al., 1999). There have been no studies comparing the efficacy of abdominal versus laparoscopic versus hysteroscopic versus laser myomectomy. The reported pregnancy and delivery rates in previously "infertile" patients for all of these procedures, however, are similar (Babaknia, Rock, and Jones, 1978; Berkeley, DeCherney, and Polan, 1983; Chong, Thong, Tan, et al., 1988; Cravello, D'Ercole, Boubli, et al., 1995; Dequesne and Schmidt, 1996; Dubuisson, Chapron, Chavet, et al., 1996; Gehlbach, Sousa, Carpenter, et al., 1993; Phillips, Nathanson, Meltzer, et al., 1995; Starks, 1988; Vercellini, Maddalena, De Giorgi, et al., 1999; Vercellini, Zaina, Yaylayan, et al., 1999). This similarity may be due in part to the wide range of reported rates for each type of procedure, resulting in the failure to detect true differences. In addition, older studies rarely use life-table methods to estimate rates.
Although only case series have been performed, the addition of GnRH agonists prior to myomectomy does not appear to improve pregnancy rates over myomectomy alone (Kuhlmann, Gartner, Schindler, et al., 1997; Narayan, Rajat, and Goswamy, 1994; Sudik, Husch, Steller, et al., 1996). There have been no studies looking at GnRH agonists alone as pretreatment prior to attempting pregnancy in women with infertility.
Two small case series examined the effect of primary versus secondary infertility in relation to myomectomy. In both of these studies, patients with secondary infertility had higher pregnancy rates than those with primary, 37-41 percent versus 45-66 percent (Babaknia, Rock, and Jones, 1978; Darai, Dechaud, Benifla, et al., 1997).
Summary
Infertility is a multifactorial problem. Although fibroids may contribute to fertility problems, it remains unclear how large a role they play. Therefore, research examining the relationship between fibroids and infertility must be meticulous about both noting and accounting for other causes of infertility. Moreover, more investigations examining the effect of fibroid size and location on infertility are needed. A positive effect of myomectomy on fertility is consistently shown in uncontrolled studies, but methodological issues preclude definite conclusions on the overall effectiveness of myomectomy in infertility patients with fibroids. It is difficult to make meaningful comparisons across existing studies because inclusion criteria are unclear, as are the denominators of the pregnancy rates. Thus, future research should explicitly state the criteria being used to define "infertility, pregnancy, conception, and live birth," as well as length of followup.
No studies have been performed comparing the risks and benefits for treatment of infertility of the different types of myomectomies. Research is also needed on the potential role of medical treatment or uterine artery embolization in the treatment of infertility associated with fibroids.
Question 9: What are the effects of surgical management of uterine fibroids, especially hysterectomy, on the aging process?
Approach
The ideal approach to this question would be to compare prospectively collected long-term results in a group of women undergoing surgical management of fibroids with a similar group of women with fibroids who underwent alternative therapies. Unfortunately, such data do not exist. Even without a control group, published prospective studies of women undergoing hysterectomy have, at most, 2 years of followup data, and few report results separately for women undergoing hysterectomy for fibroids.
Because it is performed so frequently, there is a body of literature addressing some of the long-term effects of hysterectomy, although most of these studies have significant methodological limitations, which are described below. Unfortunately, few if any of these studies stratify results based on the indication for the procedure or comorbidities present at the time of surgery. This could result in significant biases. For example, women undergoing hysterectomy primarily for symptoms of prolapse and those who undergo concurrent urinary tract or vaginal surgery at the time of hysterectomy might well be more likely to develop urinary incontinence or pelvic floor dysfunction than women undergoing hysterectomy for bleeding or pain related to fibroids.
Despite these limitations, we elected to review the literature on the effects on the aging process of hysterectomy performed for any benign indication. Our goal was to provide some information for patients and providers to consider when making decisions about therapy and to identify methodological limitations and areas for future research targeted specifically to women with fibroids.
Specifically, after discussion with AHRQ and the technical advisory panel, we attempted to identify studies that addressed the effect of procedures used in the surgical management of fibroids on ovarian function, sexual function, and pelvic floor function, as well as studies that addressed the benefits and risks of prophylactic oophorectomy at the time of hysterectomy for benign disease.
Ovarian Function
After Hysterectomy
We identified 16 studies that addressed the question of subsequent ovarian function in premenopausal women who undergo hysterectomy without removal of the ovaries. This literature is conflicting but suggests that women who have a hysterectomy (with ovarian conservation) may undergo menopause earlier than women who do not. A proposed mechanism for this phenomenon is reduced blood flow to the ovaries following hysterectomy.
Siddle and colleagues demonstrated a significantly lower mean age of menopause in women after hysterectomy compared with controls who did not undergo hysterectomy (45.4 ± 4.0 vs. 49.4 ± 4.0, p < 0.001) in a retrospective study of 316 subjects. Forty-four percent of subjects with a previous hysterectomy developed ovarian failure by age 45 (defined by the presence of vasomotor symptoms, vaginal dryness, and elevated follicle-stimulating hormone [FSH] levels) compared with 13 percent in the control group. One population-based cross-sectional study found that twice as many women with prior hysterectomy visited their physician with menopausal symptoms as age-matched controls (Roos, 1984). Another cross-sectional study of women aged 39-60 from the Netherlands found that women with a previous hysterectomy were significantly more likely to have moderate to severe climacteric complaints, especially vaginal dryness and hot flashes, than age-matched controls who had not undergone hysterectomy (Oldenhave, Jaszmann, Everaerd, et al., 1993). The largest difference was noted in the youngest patient population (age 39-41). In contrast, two prospective studies failed to demonstrate any alteration in ovarian function in women who undergo hysterectomy (Bhattacharya, Mollison, Pinion, et al., 1996; Coppen, Bishop, Beard, et al., 1981). The only randomized trial to examine this issue found no significant difference in the proportion of subjects with hot flashes or elevated FSH levels 2 years after total vaginal hysterectomy compared with endometrial ablation for menorrhagia (Bhattacharya, Mollison, Pinion, et al., 1996). A study of a prospective cohort of women who had hormone level determinations before and 3 years after hysterectomy found no significant change in estrogen, FSH, or luteinizing hormone (LH) levels (Coppen, Bishop, Beard, et al., 1981). These two prospective studies are limited by their relatively short followup, however. Differences between the findings of the prospective and retrospective studies may reflect differences in time between hysterectomy and presentation with symptoms, differences in the indication for hysterectomy, and possible confounding by perimenopause. Women with abnormal bleeding because of perimenopausal changes may be more likely to undergo hysterectomy, and they also may be more likely to experience decreased ovarian steroid production within a short time after undergoing hysterectomy.
The prospect of earlier menopause in women with previous hysterectomy raises concern about the development of osteoporosis and cardiovascular disease in this population. Two cross-sectional studies have demonstrated lower bone density compared with controls in women with prior hysterectomy, despite ovarian conservation (Hreshchyshyn, Hopkins, Zylstra, et al., 1988; Watson, Studd, Garnett, et al., 1995). However, a third and larger cross-sectional study found no difference in bone mineral density and significantly lower FSH levels (p < 0.05) in women who had undergone premenopausal hysterectomy compared with women who had intact uteri (Ravn, Lind, and Nilas, 1995). The effect of premenopausal hysterectomy with ovarian conservation on the risk of subsequent cardiovascular disease is also conflicting. In the Framingham Study, women ages 45 to 54 with premenopausal hysterectomy had a relative odds ratio of new-onset coronary heart disease 2.7 times greater than women of the same age without previous hysterectomy (p < 0.01) (Gordon, Kannel, Hjortland, et al., 1978). In contrast, the Nurse's Health Study demonstrated no increased risk of cardiovascular events in women undergoing hysterectomy alone (RR 0.7 [95% CI, 0.2-1.2]), while women who underwent hysterectomy with bilateral salpingo-oophorectomy (BSO) who did not receive estrogen replacement therapy had an increased risk (RR 2.2 [95% CI, 1.2-4.2]) (Colditz, Willett, Stampfer, et al., 1987).
After Other Invasive Therapies
We identified no studies that evaluated ovarian function after myomectomy. However, studies of uterine artery embolization have shown transient or permanent amenorrhea in some women, suggesting the possibility of altered ovarian function with this technique (Goodwin, McLucas, Lee, et al., 1999; Spies, Scialli, Jha, et al., 1999).
Summary
Some, but not all, retrospective and cross-sectional studies suggest that women who undergo hysterectomy but retain their ovaries may experience menopause earlier than women who do not undergo hysterectomy. Prospective studies with longer followup are necessary to confirm this relationship. There may be some confounding due to perimenopause: perimenopausal women are more likely to have anovulatory bleeding, which may lead to an increased likelihood of hysterectomy. Since, by definition, these women would already have impending ovarian failure, there would be an apparent association between the hysterectomy and more rapid onset of ovarian failure. Preliminary data suggest that uterine artery embolization may alter ovarian function in some women. There currently are no data regarding the effect of myomectomy on ovarian function.
Sexual Function after Hysterectomy
We identified 31 studies that address the effect of hysterectomy in general on sexual functioning, but only one of these (Carlson, Miller, and Fowler, 1994a) provided information that specifically addressed the question of sexual function in women who underwent hysterectomy for uterine fibroids. Furthermore, we were unable to identify any studies that addressed the effect of other invasive therapies for uterine fibroids, such as myomectomy or uterine artery embolization, on sexual function.
Epidemiology
There is no information on the prevalence of sexual dysfunction in women with uterine fibroids. It is unknown if women with uterine fibroids, symptomatic or asymptomatic, have altered sexual function compared with the general population. Furthermore, there is no information available regarding the sexual function of women with symptomatic uterine fibroids compared with women who have similar symptoms but do not have fibroids.
Theoretically, uterine fibroids could affect sexual function through several mechanisms. First, any mass effect caused by uterine fibroids could result in discomfort to a woman or her partner during intercourse. Second, symptoms that result from the presence of uterine fibroids, such as menorrhagia, dysmenorrhea, pelvic pain, or lower urinary tract dysfunction, may affect sexual function through physiological and/or psychological mechanisms. Third, physiological alterations in the uterus that result from the presence of uterine fibroids -- including altered blood flow, alterations in growth factors, and increased prostaglandin production -- may have some as yet undefined impact on sexual response.
Sexual Function after Hysterectomy for Uterine Fibroids
There is very little information regarding the effect on sexual function of hysterectomy performed specifically for symptomatic uterine fibroids. The vast majority of prospective studies that examined sexual function after hysterectomy did not stratify their results by preoperative symptoms or diagnoses. The three randomized trials that examined sexual outcomes after hysterectomy specifically excluded subjects with fibroids (Alexander, Naji, Pinion, et al., 1996; Crosignani, Vercellini, Apolone, et al., 1997; Dwyer, Hutton, and Stirrat, 1993). From 23 to 71 percent of the women in the prospective cohort studies that examined sexual function after hysterectomy had a preoperative diagnosis of uterine fibroids, yet only one study (Carlson, Miller, and Fowler, 1994a) provided results that related specifically to sexual function after hysterectomy for uterine fibroids (Bernhard, 1992; Candiani, Fedele, Parazzini, et al., 1991; Carlson, Miller, and Fowler, 1994a; Clarke, Black, Rowe, et al., 1995; Helstrom, Lundberg, Sorbom, et al., 1993; Lambden, Bellamy, Ogburn-Russell, et al., 1997; Weber, Walters, Schover, et al., 1999). The Maine Women's Health Study, a prospective cohort of 418 women who underwent hysterectomy, demonstrated a significant reduction in dyspareunia and an improvement in sexual interest and sexual enjoyment 1 year after hysterectomy (Carlson, Miller, and Fowler, 1994a). Thirty-five percent of women in this cohort had a diagnosis of uterine fibroids, and subgroup analysis revealed similar improvements in dyspareunia, sexual interest, and sexual enjoyment for this group (p < 0.005 for each). Given the wide variety of symptoms and diagnoses that can serve as indications for hysterectomy, it is unlikely that postoperative sexual function is completely independent of preoperative diagnosis. Extrapolating the results of studies that examine the effect of hysterectomy on sexual function independent of preoperative diagnosis may be hazardous. More studies that look specifically at the effect of hysterectomy for symptomatic uterine fibroids on sexual function will have to be done to confirm the findings of the Maine Women's Health Study.
Hysterectomy and Sexual Function
We identified 17 retrospective and 14 prospective studies that provided information on the effect of hysterectomy on postoperative sexual functioning. From this literature we were able to evaluate the short-term effect of hysterectomy on the following aspects of sexual function: dyspareunia, frequency of intercourse, orgasm, libido/sexual interest, vaginal dryness, and overall sexual function. The long-term effect of hysterectomy on sexual functioning could not be adequately evaluated based on the current literature because there are no prospective studies with followup greater than 2 years after hysterectomy, and none of the retrospective studies had the appropriate design to be informative.
Dyspareunia
All of the prospective studies that examined the effect of hysterectomy on dyspareunia demonstrated either no change or an improvement in this symptom in the majority of women. The Maryland Women's Health Study, a prospective cohort of 1,101 women who underwent hysterectomy, demonstrated a significant decline in the number of women who reported dyspareunia 1 and 2 years after hysterectomy when compared with the preoperative period (preoperative period, 40.8 percent; 1 year, 18.4 percent; 2 years, 14.9 percent; p < 0.001) (Rhodes, Kjerulff, Langenberg, et al., 1999). Eighty-one percent of the women in this study who experienced frequent dyspareunia preoperatively had an improvement in this symptom at 2 years after hysterectomy, while only 1.9 percent of women without dyspareunia preoperatively had developed it by 2 years after surgery. In spite of this, prehysterectomy dyspareunia was found to be the strongest predictor of postoperative dyspareunia in this cohort (OR, 4.47 [95% CI, 2.14-9.33]) (Rhodes, Kjerulff, Langenberg, et al., 1999). In the Maine Women's Health Study, 39 percent of women complained of dyspareunia preoperatively, while only 8 percent had this complaint 1 year after hysterectomy (p < 0.001) (Carlson, Miller, and Fowler, 1994a). Women in this study who were managed nonsurgically showed no decline in the mean frequency of dyspareunia, however (Carlson, Miller, and Fowler, 1994b). In a cohort of 104 women who underwent supracervical hysterectomy, the rate of dyspareunia decreased from 56 percent preoperatively to 10 percent 1 year after surgery (p < 0.001) (Helstrom, Lundberg, Sorbom, et al., 1993). Kilkku (1983) reported a cohort of 105 women who underwent total abdominal hysterectomy and 107 women who underwent supracervical hysterectomy. Subjects reported significantly less dyspareunia 1 year after both procedures, but patients who received the supracervical hysterectomy had a greater decline in dyspareunia than those who received a total abdominal hysterectomy (Kilkku, 1983). Weber and colleagues used the Current Sexual History form to assess sexual function in 43 women before and after total abdominal hysterectomy. Fifteen percent of the subjects complained of dyspareunia preoperatively. Of these, only 3 percent had dyspareunia postoperatively; however, 9 percent of subjects developed dyspareunia as a new symptom after hysterectomy (Weber, Walters, Schover, et al., 1999). Overall, the proportion of women with dyspareunia did not significantly change after hysterectomy in this study.
Frequency of intercourse
Hysterectomy appears to have little impact on the frequency of intercourse. While some retrospective studies suggest decreased sexual frequency, the majority of prospective studies suggest no significant change in sexual frequency after hysterectomy. (Clarke, Black, Rowe, et al., 1995; Coppen, Bishop, Beard, et al., 1981; Helstrom, Lundberg, Sorbom, et al., 1993; Kilkku, 1983; Lambden, Bellamy, Ogburn-Russell, et al., 1997). In the Maryland Women's Health Study, the mean number of instances of sexual intercourse per month increased from 2.3 per month preoperatively to 2.9 per month 2 years after hysterectomy (p < 0.001) (Rhodes, Kjerulff, Langenberg, et al., 1999). Gath and colleagues found that 56 percent of the subjects in their study reported an increase in sexual frequency 18 months after hysterectomy, while 27 percent reported no change, and 17 percent had a decrease in frequency (Gath, Cooper, and Day, 1982).
Orgasm
There is disagreement in the literature on the effect hysterectomy has on postoperative orgasmic function. The Maryland Women's Health Study demonstrated a significant increase in the proportion of women who experienced orgasm after hysterectomy (preoperative, 62.8 percent; 1 year, 72.4 percent; 2 years, 71.5 percent; p < 0.01), as well as an increase in the proportion of women who experienced strong orgasms (preoperative, 44.6 percent; 1 year, 58.4 percent; 2 years, 57.3 percent; p < 0.001) (Rhodes, Kjerulff, Langenberg, et al., 1999). Three smaller prospective cohort studies reported no change in orgasmic function after hysterectomy (Coppen, Bishop, Beard, et al., 1981; Helstrom, Lundberg, Sorbom, et al., 1993; Weber, Walters, Schover, et al., 1999). In the study by Kilkku and colleagues, there was a significant increase in the proportion of women who had orgasmic dysfunction after total abdominal hysterectomy (preoperative, 29.7 percent; 1 year 46.7 percent; p < 0.001), whereas the women who received supracervical hysterectomy demonstrated no significant change (preoperative, 28.6 percent; 1 year, 32 percent; not statistically significant) (p < 0.05 for comparison between groups).
Libido/sexual interest
Most prospective studies that have evaluated libido or sexual interest have demonstrated either no change or an improvement after hysterectomy. In the Maryland Women's Health Study, 70.8 percent of women with low libido preoperatively were improved 1 year after hysterectomy, while only 4.3 percent of women who had normal libido preoperatively developed low libido at 1 year after surgery (Rhodes, Kjerulff, Langenberg, et al., 1999). The presence of low libido prior to hysterectomy significantly predicted posthysterectomy low libido (OR, 5.06 [95% CI, 2.71-9.43]), as did prehysterectomy depression (OR, 2.83 [95% CI, 1.28-6.23]) (Rhodes, Kjerulff, Langenberg, et al., 1999).
In the Maine Women's Health Study, 36 percent of women had decreased sexual interest preoperatively, while 8 percent had this problem 1 year after hysterectomy (Carlson, Miller, and Fowler, 1994a). The proportion of women who developed decreased sexual interest as a new symptom postoperatively was not significantly different from the proportion of women with this complaint in a group that was managed nonsurgically (7 percent vs. 6 percent respectively) (Carlson, Miller, and Fowler, 1994b).
In a randomized trial of hysterectomy versus endometrial ablation/resection for dysfunctional uterine bleeding, there was no significant difference between interventions with regard to postoperative sexual interest (unchanged, 52 percent; increased, 27 percent; decreased, 25 percent for both groups) (Alexander, Naji, Pinion, et al., 1996). The prospective cohort described by Lambden and colleagues demonstrated no change in libido in 59 percent of women, an increase in libido in 29 percent of women, and a decrease in libido in 11 percent of women at 11 months posthysterectomy (Lambden, Bellamy, Ogburn-Russell, et al., 1997). In a study by Virtanen and colleagues, 56 percent of subjects had increased libido after hysterectomy, while only 5 percent had a decrease (Virtanen, Makinen, Tenho, et al., 1993). Kilkku, however, reported no significant change in sexual interest 1 year after either total abdominal or supracervical hystectomy (Kilkku, Gronroos, Hirvonen, et al., 1983).
Vaginal dryness
Only two prospective studies have evaluated the symptom of vaginal dryness after hysterectomy. In the Maryland Women's Health Study, the proportion of women who did not have vaginal dryness increased significantly 1 and 2 years after hysterectomy (preoperative, 37.3 percent; 1 year, 46.8 percent; 2 years, 46.7 percent; p < 0.001) (Rhodes, Kjerulff, Langenberg, et al., 1999). Thirty-five percent of subjects had persistent vaginal dryness after hysterectomy, but only 8.7 percent developed new vaginal dryness postoperatively. Vaginal dryness prior to hysterectomy significantly predicted postoperative vaginal dryness (OR, 5.95 [95% CI, 3.75-9.47]) (Rhodes, Kjerulff, Langenberg, et al., 1999). Interestingly, even after adjusting for menopausal status and posthysterectomy hormone use, BSO was not associated with postoperative vaginal dryness (adjusted OR, 1.30 [95% CI, 0.77-2.20]). Weber and colleagues demonstrated no significant change in vaginal dryness after hysterectomy in their cohort of 43 patients (Weber, Walters, Schover, et al., 1999).
Overall sexual function
Four studies evaluated the effect of hysterectomy on sexuality using a global rating of sexual function. In a randomized controlled trial of vaginal hysterectomy and endometrial resection for menorrhagia, there was no difference in overall sexual function as assessed by the Sabbatsberg Sexual Rating Scale 2 years after surgery (Crosignani, Vercellini, Apolone, et al., 1997). Bernhard administered the Derogatis Sexual Functioning Inventory (DSFI) to 63 women before and 3 months after hysterectomy and found a statistically significant improvement in overall sexual functioning using this instrument (p < 0.0001) (Bernhard, 1992). Weber and colleagues found no significant change in overall sexual functioning using the Current Sexual History Form 1 year after hysterectomy (Weber, Walters, Schover, et al., 1999). Helstrom and colleagues assessed overall sexuality using a semistructured interview in 104 women who underwent supracervical hysterectomy. They found that 50 percent reported an improvement in sexuality 1 year after surgery, and 21 percent reported a deterioration (Helstrom, Sorbom, and Backstrom, 1995). These authors concluded that the best predictors of postsurgical sexuality were prehysterectomy coital frequency, frequency of desire, existence of cyclicity of desire, multiple orgasms, and frequent orgasms. They also found that relief of dysmenorrhea was a strong predictor of postoperative sexuality (Helstrom, Weiner, Sorbom, et al., 1994). Additionally, women who were viewed to have a "good" relationship with their sexual partner before hysterectomy were more likely to have an improved or unchanged sex life after hysterectomy than those with a "poor" relationship (Helstrom, Sorbom, and Backstrom, 1995).
Effect of concurrent oophorectomy on sexual function
The effect on sexual functioning of prophylactic oophorectomy at the time of hysterectomy is largely unknown. Few studies have examined this directly, and those that have looked at it have done so only as part of multiple subgroup analyses. Additionally, confounders such as age, menopausal status, and postoperative hormone replacement therapy are rarely addressed. One study found no significant difference in sexual functioning in women who had their ovaries removed and were not on hormone replacement therapy compared with women who were either on postoperative estrogen replacement therapy or had their ovaries conserved (Weber, Walters, Schover, et al., 1999). The Maine Women's Health Study reported no significant difference in any of the measured outcomes including sexual function, with the exception of new-onset hot flashes, in women who had hysterectomy with BSO compared with those who had only a hysterectomy. However, 91 percent of subjects who received a BSO were taking estrogen replacement therapy postoperatively (Carlson, Miller, and Fowler, 1994a). The Maryland Women's Health Study concluded that BSO was not associated with posthysterectomy dyspareunia, decreased libido, or vaginal dryness. In this cohort, the most important predictor for lack of orgasms postoperatively was lack of orgasms preoperatively (Rhodes, Kjerulff, Langenberg, et al., 1999). When preoperative orgasms and age were included in multivariate analyses, BSO was found to be significantly associated with lack of orgasm 1 year after hysterectomy (adjusted OR, 2.86 [95% CI, 1.10-6.53]).
Given the current data, it is unknown if prophylactic BSO adversely affects sexual function in women after hysterectomy. Furthermore, it also is unclear whether postoperative estrogen replacement therapy ameliorates any adverse effect that may exist.
Supracervical hysterectomy
There is considerable controversy regarding the impact on sexual function of removing the cervix at the time of hysterectomy. Several mechanisms of how cervical removal could adversely affect sexual function have been postulated, including disruption of the parasympathetic and sympathetic nerve fibers that pass from the cervix through the Frankenhauser plexus and pelvic nerves to the second through fourth sacral nerve roots (Hasson, 1993). A number of studies demonstrated improved or unchanged sexual function after supracervical hysterectomy (Helstrom, Weiner, Sorbom, et al., 1994; Kilkku, 1983; Kilkku, Gronroos, Hirvonen, et al., 1983), but only two studies from a single cohort compared the sexual function of women after supracervical hysterectomy with the sexual function of women after total abdominal hysterectomy. Kilkku and colleagues reported on a Finnish cohort of 212 women, 105 of whom received total abdominal hysterectomy, and 107 of whom received a supracervical hysterectomy (Kilkku, 1983; Kilkku, Gronroos, Hirvonen, et al., 1983). The subject's physician determined the type of hysterectomy performed. The investigators found no significant difference between the groups with regard to coital frequency or libido 1 year after surgery. Dyspareunia decreased in both groups but significantly more so in the supracervical hysterectomy group. The frequency of orgasm was significantly reduced 1 year after surgery in the group of women who received total abdominal hysterectomy, but it was unchanged in those who received supracervical hysterectomy (Kilkku, Gronroos, Hirvonen, et al., 1983). The results of these studies should be interpreted with caution, however. First, the choice of operation was determined by the subject's physician, raising the potential for selection bias. In fact, subjects in the supracervical hysterectomy group were significantly more likely to be married than those in the total abdominal hysterectomy group (81 percent vs. 70 percent), a factor that may have affected posthysterectomy sexual functioning. Second, the authors made numerous statistical comparisons in both studies that limited the strength of the results. Currently, there is insufficient evidence to conclude that cervical preservation at the time of hysterectomy affects posthysterectomy sexual functioning either positively or negatively.
Other Invasive Therapies and Sexual Function
There currently are no studies that examine the effect of abdominal, laparoscopic or hysteroscopic myomectomy on sexual function. Additionally, there are no studies that examine sexual outcomes after uterine artery embolization.
Summary
In general, hysterectomy does not appear to adversely affect sexual function in the majority of women in the first 1-2 years after surgery. Women with presurgical dysfunction may experience significant improvement. Only a small proportion of women will have a decline in sexual function after hysterectomy during this time period. The long-term effects of hysterectomy on sexual function are unknown. There are limited data about the effect on sexual function of hysterectomy performed specifically for uterine fibroids. The data that do exist, however, suggest short-term effects similar to hysterectomy in general. There is no substantial evidence that cervical preservation at the time of hysterectomy results in either improved or worsened sexual function compared with total hysterectomy. The effect of prophylactic oophorectomy on sexual function is unknown, as is the effect of postoperative estrogen replacement therapy. There are no studies that examine the effect on sexual function of other invasive therapies for uterine fibroids, such as myomectomy or uterine artery embolization.
Pelvic Floor Dysfunction
For this question, we divided the spectrum of pelvic floor dysfunction into three broad categories: lower urinary tract dysfunction; dysfunction of the colon, rectum, and anus; and pelvic organ prolapse. Forty-one articles were identified that examined the effect of hysterectomy in general on disorders of the pelvic floor. We identified only one article that contained information specifically addressing hysterectomy for fibroids and subsequent pelvic floor dysfunction. Two additional articles were identified that had information on conservative surgery for uterine fibroids and urinary symptoms.
Lower Urinary Tract Dysfunction
Uterine fibroids and urinary tract dysfunction
There is no information on the prevalence of lower urinary tract dysfunction in patients with uterine fibroids. Likewise, there are limited data to suggest that uterine fibroids independently contribute to urinary symptoms. Theoretically, uterine fibroids could affect bladder and urethral function in several ways, including direct pressure/mass effect, alterations in pelvic blood flow, increased local prostaglandin production in the uterus (and subsequent effects on bladder smooth muscle), and alterations in other local cytokines. Two randomized controlled trials in women with both uterine fibroids and lower urinary tract symptoms demonstrated a significant decrease in bladder symptoms, as well as a reduction in fibroid size, among women treated with a GnRH agonist compared with placebo (Friedman, Hoffman, Comite, et al., 1991; Langer, Golan, Neuman, et al., 1990). It is possible (although biologically implausible) that the observed reduction in urinary symptoms in these two studies is a direct result of the hypoestrogenic state induced by the GnRH agonist rather than fibroid shrinkage. One cross-sectional study of 515 45-year-old women found no relationship between an enlarged uterus and urinary incontinence (Hording, Pedersen, Sidenius, et al., 1986).
Hysterectomy and lower urinary tract dysfunction
We identified one randomized controlled trial, 10 prospective cohort studies, four retrospective studies, and seven cross-sectional studies that addressed the relationship between hysterectomy for benign conditions and dysfunction of the lower urinary tract. Studies concerning radical hysterectomy were excluded.
Studies that evaluated the physiology of the lower urinary tract using urodynamics before and after hysterectomy are conflicting. Furthermore, they all are limited by their small size (range 16-72) and short followup (6 months or less). Two studies that prospectively measured vesicourethral function before and after surgery demonstrated a significant increase in vesicourethral dysfunction after hysterectomy (Parys, Haylen, Hutton, et al., 1989; Parys, Haylen, Hutton, et al., 1990). The authors suggest that hysterectomy may damage the autonomic and sensory nerves to the bladder, resulting in bladder dysfunction. In contrast, two other studies demonstrated no significant changes in any urodynamic parameter after hysterectomy (Langer, Neuman, Ron-el, et al., 1989; Stanton, Hilton, Norton, et al., 1982). Wake demonstrated transient changes immediately after hysterectomy, including increased postvoid residual volumes, decreased functional capacity, and increased bladder compliance, but these changes resolved within a week after surgery (Wake, 1980). Vervest and colleagues evaluated 32 women with urodynamic testing before and 12-26 weeks after hysterectomy and demonstrated variable and clinically inconsequential changes in bladder function (Vervest, van Venrooij, Barents, et al., 1989a, 1989b).
The single randomized clinical trial that evaluated the effect of hysterectomy on lower urinary tract function demonstrated no significant difference in either subjective urinary complaints or urodynamic parameters 2 years after simple hysterectomy, when compared with endometrial ablation, for the management of dysfunctional uterine bleeding (difference in urodynamic dysfunction 31 percent in hysterectomy and 34 percent in ablation patients, 95% CI for difference -23 percent to 15 percent) (Bhattacharya, Mollison, Pinion, et al., 1996). Because of its small size, the power of this study to detect a difference was limited. All of the prospective cohort studies that subjectively evaluated lower urinary tract symptoms before and after hysterectomy demonstrated either a significant improvement (Carlson, Miller, and Fowler, 1994a; Carlson, Miller, and Fowler, 1994b; Clarke, Black, Rowe, et al., 1995; Griffith-Jones, Jarvis, and McNamara, 1991; Kjerulff, Langenberg, Rhodes, et al., 2000; Virtanen, Makinen, Tenho, et al., 1993) or no change (Jequier, 1976; Weber, Walters, Schover, et al., 1999) in bladder symptoms 3 months to 2 years after surgery. However, only two of these studies included control groups of women who did not have a hysterectomy (Griffith-Jones, Jarvis, and McNamara, 1991).
One study demonstrated a significant reduction in stress incontinence (p < 0.05) and no change in other urinary symptoms 3 to 21 months after hysterectomy when compared with a similar population of patients who underwent dilation and curettage (Griffith-Jones, Jarvis, and McNamara, 1991). The Maine Women's Health Study demonstrated a significant reduction in urinary incontinence, urinary urgency, and urinary frequency 1 year after hysterectomy. Thirty-five percent of subjects in this study had uterine fibroids, and the changes in this subgroup were similar to the group as a whole (Carlson, Miller, and Fowler, 1994a). In contrast, no significant change in urinary symptoms was seen in the nonsurgically managed population of this cohort (Carlson, Miller, and Fowler, 1994a). Neither of these two studies adjusted for potential confounders such as age, weight, or parity. Even more important, it is unclear in the Maine study whether any of the subjects underwent concomitant procedures for the treatment of urinary incontinence.
Information regarding the long-term effect of hysterectomy on the lower urinary tract is limited to studies that are retrospective or cross-sectional, as no prospective study has followed subjects for more than 2 years. One case-control study found no significant difference in the rate of previous hysterectomy in women with stress urinary incontinence compared with women who did not have stress urinary incontinence (OR, 1.69 [95% CI, 0.58-5.17]) (Skoner, Thompson, and Caron, 1994). A cross-sectional study of 3,896 Swedish women between the ages of 66 and 86 demonstrated a higher prevalence of urinary incontinence in women who had a previous hysterectomy compared with those who had not (20.8 percent vs. 16.4 percent respectively, p < 0.05) (Milsom, Ekelund, Molander, et al., 1993). Similarly, in a cross-sectional study of 7,949 American women over the age of 65, daily urinary incontinence was more common in women who had undergone hysterectomy than those who had not (adjusted OR, 1.4 [95% CI, 1.1-1.6]) (Brown, Seeley, Fong, et al., 1996). The attributable risk proportion associated with hysterectomy in this study was 14 percent, second only to obesity. In a survey of 939 women age 60 or older, univariate analysis demonstrated a greater likelihood of urinary incontinence in women who had received a hysterectomy before the age of 45. However, after adjusting for age and other confounders, this difference was no longer significant (adjusted OR, 1.54 [95% CI, 0.87-2.74]) (Thom, van den Eeden, and Brown, 1997). Diokno and colleagues, in their survey of 1,965 Michigan women over the age of 60, found that women with urinary incontinence were significantly more likely to report previous surgery of the lower genital tract than women without urinary incontinence (64 percent vs. 52.6 percent, respectively; p < 0.0005) (Diokno, Brock, Herzog, et al., 1990). The specific prevalence of hysterectomies in this population is unknown, however. In the only cross-sectional study to examine the relationship between hysterectomy and urinary incontinence in premenopausal women, the prevalence of urinary disorders for women with previous hysterectomy was similar to that of women with previous sterilization (but not hysterectomy) (Iosif, Bekassy, and Rydhstrom, 1988).
These case-control and cross-sectional studies do not report the indications for hysterectomy in their populations. Knowing the proportion of subjects in each study who had hysterectomies that were performed for pelvic organ prolapse or concurrently with procedures to rectify incontinence is necessary to assess the potential for bias, since patients with dysfunction in one compartment of the pelvic floor (prolapse) might be more likely to develop subsequent dysfunction in another (incontinence). Additionally, the heterogeneity of the definitions for urinary incontinence in these studies, ranging from "daily incontinence" in one study to "any incontinence in the last 12 months" in another, makes comparisons difficult.
Supracervical hysterectomy and lower urinary tract dysfunction
Data are limited on the effect of cervical removal/preservation at the time of hysterectomy on lower urinary tract function. One small, randomized controlled trial with limited power found no difference in lower urinary tract function 6 months after surgery in 11 women who underwent supracervical hysterectomy compared with 11 women who received total abdominal hysterectomy (Lalos and Bjerle, 1986). Kilkku and colleagues evaluated urinary symptoms in a Finnish cohort of 212 women before and 1 year after either total abdominal hysterectomy or supracervical hysterectomy. They demonstrated a significant reduction in urinary frequency, nocturia, and dysuria in both groups, but the reduction was significantly greater in those who received a supracervical hysterectomy (Kilkku, Hirvonen, and Gronroos, 1981). The rate of postoperative incontinence was similar, however (Kilkku, 1985). In this study, type of operation was left to the choice of the operating surgeon, raising the potential for selection bias. In a cross-sectional study, Iosif and colleagues demonstrated no difference in the prevalence of urinary disorders in women who underwent total abdominal hysterectomy compared with supracervical hysterectomy (Iosif, Bekassy, and Rydhstrom, 1988).
Other invasive therapies
We identified only two studies that examine the effect of laser interstitial therapy and myomectomy on lower urinary tract function (Chapman, 1998; Davies, Hart, and Magos, 1999). Both are uncontrolled case series, with poorly defined urinary symptoms and short followup (less than 6 months). We identified no studies that evaluate the impact of uterine artery embolization on lower urinary tract function.
Summary
In general, the available evidence suggests that simple hysterectomy (as opposed to radical hysterectomy, a procedure performed almost exclusively for invasive cervical cancer) results in either a minimal change or an improvement in lower urinary tract function in most women in the first 1-2 years after surgery. The long-term effect of hysterectomy on bladder and urethral function is largely unknown. Further investigation is necessary to confirm the relationship between hysterectomy and urinary incontinence that is suggested by some epidemiologic studies. There are insufficient data to draw any conclusions regarding the effect of hysterectomy performed specifically for fibroids or other invasive treatments for fibroids on lower urinary tract function.
Dysfunction of the Colon, Rectum, and Anus
Uterine fibroids and bowel dysfunction
There is no information on the prevalence of bowel dysfunction in women with uterine fibroids. Although there are several mechanisms by which uterine fibroids might influence lower gastrointestinal tract function, we identified no studies that specifically address this issue.
Hysterectomy and constipation/defecatory dysfunction
We identified five prospective cohort studies, one case-control study, one cross-sectional study, and three retrospective studies that examined the relationship between hysterectomy and bowel dysfunction. We were unable to identify any study that specifically addressed the question of bowel function in women who underwent hysterectomy for uterine fibroids.
There is disagreement in the literature about the effect of hysterectomy on postoperative bowel dysfunction. One retrospective study of 593 women who had undergone hysterectomy reported that 31 percent of women felt that they had a "severe" deterioration in bowel function (on a scale of "None," Mild," "Moderate," and "Severe," with symptoms consisting primarily of straining and/or incomplete emptying) after their hysterectomy, significantly more than a control group of women who had undergone laparoscopic cholecystectomy (van Dam, Gosselink, Drogendijk, et al., 1997). Conversely, a study of 236 women who had undergone a hysterectomy between 2 and 10 years earlier found that of women who reported bowel symptoms preoperatively, 56 percent had an improvement postoperatively, and 37 percent were unchanged (Schofield, Bennett, Redman, et al., 1991). In a population-based cross-sectional study of 1,058 women, self-reported constipation was significantly more common in subjects with a hysterectomy than women without prior hysterectomy (22 percent vs. 9 percent respectively, p < 0.01), as was straining at defecation and a feeling of incomplete emptying (Heaton, Parker, and Cripps, 1993). A case-control study found that women who had a hysterectomy 2-8 years earlier were significantly more likely to have decreased bowel frequency than controls (Taylor, Smith, and Fulton, 1989).
In contrast to these previous studies, four prospective cohort studies that evaluated women before and 3-12 months after hysterectomy found either no change (Clarke, Black, Rowe, et al., 1995; Prior, Stanley, Smith, et al., 1992; Weber, Walters, Schover, et al., 1999) or an improvement (Prior, Stanley, Smith, et al., 1992) in bowel function postoperatively. In a study of 205 consecutive women who underwent hysterectomy, Prior and colleagues found that 22 percent of the subjects had symptoms consistent with irritable bowel syndrome (IBS), with the constipation predominant subtype noted most frequently (Prior, Stanley, Smith, et al., 1992). One-third of the women had complete resolution of their IBS 6 months after hysterectomy, while 27 percent of women improved, and 20 percent demonstrated worsening of their symptoms. The de novo IBS rate after hysterectomy in this study was 10 percent. The Maine Women's Health Study is the only prospective study to examine this issue and include a control group of women who did not have a hysterectomy. This study found that 9 percent of women who received a hysterectomy developed new-onset constipation by 1 year after surgery, compared with 1 percent of women who were managed nonsurgically (Carlson, Miller, and Fowler, 1994b). No adjustment was made for age, weight, or other potential confounders.
Hysterectomy and fecal incontinence
We found no studies evaluating the effect of hysterectomy on the development of fecal incontinence.
Other invasive therapies
We found no studies evaluating the effect of myomectomy on bowel dysfunction. Similarly, we did not identify any studies on the effects of uterine artery embolization on bowel function.
Summary
The currently available data are insufficient to conclude that hysterectomy affects bowel function either positively or negatively. There is no information available that specifically addresses the effects on bowel function of hysterectomy or other invasive therapies for uterine fibroids.
Pelvic Organ Prolapse
Uterine fibroids and pelvic organ prolapse
We did not identify any evidence that uterine fibroids contribute to the development of pelvic organ prolapse.
Hysterectomy and pelvic organ prolapse
There are limited data on the relationship between hysterectomy and subsequent pelvic organ prolapse. In a consecutive series of 693 patients who presented to the Mayo Clinic for surgical management of posthysterectomy vaginal vault prolapse, the median time from hysterectomy to prolapse repair was 15.8 years (Webb, Aronson, Ferguson, et al., 1998). A retrospective cohort study of 149,554 women aged 20 and older found that, in women who developed pelvic organ prolapse or urinary incontinence, the mean interval between hysterectomy and surgery for prolapse was 19.3 years (Olsen, Smith, Bergstrom, et al., 1997). The Oxford Family Planning Association study followed 17,032 women aged 25 to 39 for an average of 17 years (Mant, Painter, and Vessey, 1997). The annual incidence of surgery for pelvic organ prolapse was 0.162 percent per year. In women who had undergone hysterectomy for reasons other than prolapse, the surgical incidence rate increased to 0.290 percent per year. The cumulative risk of prolapse surgery rose from 1 percent at 3 years after hysterectomy to 5 percent 15 years after hysterectomy. However, a cross-sectional study of 487 Swedish women concluded that previous hysterectomy was not independently associated with the presence of pelvic organ prolapse (Samuelsson, Arne Victor, Tibblin, et al., 1999). This study is limited by the fact that the mean age of respondents was 39, and only 4 percent of the subjects had undergone a hysterectomy.
We identified one study that evaluated surgical techniques used at time of hysterectomy to prevent posthysterectomy prolapse. Cruikshank and Kovac randomized 100 women to either a McCall's type culdoplasty, a Moschcowitz-type culdoplasty, or peritoneal closure at the time of vaginal hysterectomy (Cruikshank and Kovac, 1999). Significantly fewer subjects in the McCall's culdoplasty group developed an enterocele 3 years after surgery than did subjects in either of the two other groups.
Other invasive techniques
We identified no studies that examine the effects of abdominal, laparoscopic, or hysteroscopic myomectomy on the development of pelvic organ prolapse. Similarly, we did not identify any studies that examined the effect of uterine artery embolization on the development of prolapse.
Summary
Hysterectomy may increase the risk of subsequent pelvic organ prolapse; however, the development of symptomatic prolapse appears to occur many years after hysterectomy. Surgical technique at the time of hysterectomy may influence the development of subsequent pelvic organ prolapse. There is no information that specifically addresses the effect of hysterectomy for uterine fibroids or other invasive techniques for fibroids on the development of pelvic organ prolapse.
Prophylactic Oophorectomy
Epidemiology
Elective bilateral oophorectomy is performed in 40-66 percent of hysterectomies in women over the age of 40, primarily as prophylaxis for ovarian cancer (Dicker, Scally, Greenspan, et al., 1982; Lepine, Hillis, Marchbanks, et al., 1997; Pokras and Hufnagel, 1988). Uterine fibroids represent one of the most common indications for hysterectomy in the United States, and women between the ages of 40 and 49 account for the highest proportion of hysterectomies performed for fibroids (Kjerulff, Langenberg, Seidman, et al., 1996). As a result, many hysterectomies for uterine fibroids are accompanied by prophylactic oophorectomy. Factors that may affect the decision to perform a prophylactic oophorectomy at the time of hysterectomy include age, cancer risk, menopausal status, risk of osteoporosis or cardiovascular disease, and the patient's willingness to take estrogen replacement therapy. However, marked variations in physician practice styles exist (Gross, Nicholson, and Powe, 1999). One study demonstrated that women who received abdominal hysterectomy (adjusted OR, 11.42 [95% CI, 9.65-13.51]) or laparoscopically assisted hysterectomy (adjusted OR, 11.34 [95% CI, 8.13-15.81]) were significantly more likely to receive concomitant oophorectomy than women undergoing vaginal hysterectomy (Gross, Nicholson, and Powe, 1999). Significant geographic variations also were noted in this study. It is unlikely that women undergoing procedures other than hysterectomy for uterine fibroids would undergo prophylactic oophorectomy.
Benefits
Prophylactic oophorectomy is performed primarily in women who are peri- or postmenopausal to prevent subsequent ovarian cancer. Although prophylactic oophorectomy greatly reduces cancer risk, primary peritoneal cancer, which behaves much like ovarian cancer, developed in 6 of 324 women from families at high risk for ovarian cancer after ovarian removal (Piver, Jishi, Tsukada et al., 1993). The incidence of this cancer in other women is unknown.
Ovarian cancer is the fourth leading cause of cancer death in American women, with 14,000 deaths annually (Qazi and McGuire, 1995). As the majority of patients with ovarian cancer present in advanced stages of the disease, and there currently is no reliable screening tool, ovarian cancer prevention has been a point of emphasis. One study estimated that approximately 1,000 cases of ovarian cancer could be prevented annually if all women over the age of 40 who underwent hysterectomy (approximately 300,000) also received a prophylactic oophorectomy (Averette and Nguyen, 1994). Another study estimated that 700 prophylactic oophorectomies would have to be performed to prevent one case of ovarian cancer (Schweppe and Beller, 1979). However, the number of ovarian cancers prevented by the current rate of prophylactic oophorectomy is not known. A secondary benefit of elective oophorectomy at the time of hysterectomy is the potential avoidance of future surgery for benign ovarian disease. One retrospective study of 1,200 women who had undergone hysterectomy but retained at least one ovary demonstrated a 4 percent reoperation rate for benign ovarian disease (Plockinger and Kolbl, 1994).
Risks
The major risks associated with prophylactic oophorectomy include increased operative risk and the risks associated with premature estrogen deprivation. One cross-sectional study of 6,227 women found that after adjusting for type of hysterectomy, diagnosis, comorbidities, and age, elective oophorectomy was not associated with increased surgical morbidity or hospital stay (Gross, Nicholson, and Powe, 1999). Two retrospective studies of total vaginal hysterectomy also demonstrated no increased morbidity when prophylactic oophorectomy was performed (Ballard and Walters, 1996; Davies, O'Connor, and Magos, 1996). Women who undergo surgical menopause are more likely to suffer climacteric symptoms, osteoporosis (Ettinger, Genant, and Cann, 1987; Lindsay, Hart, and Clark, 1984), and increased cardiovascular disease (Colditz, Willett, Stampfer, et al., 1987). Several other risks have been suggested, including increased urinary incontinence (Rekers, Drogendijk, Valkenburg, et al., 1992) and alterations in memory function (Phillips and Sherwin, 1992), but these are less well established. Additionally, the Maryland Women's Health Study found that performing a bilateral oophorectomy at the time of hysterectomy was associated with significantly poorer outcomes 2 years after surgery (Kjerulff, Langenberg, Rhodes, et al., 2000). Hormone replacement therapy is effective in treating vasomotor symptoms (Meldrum, Erlik, Lu, et al., 1981) and preventing osteoporosis (Lindsay, Hart, and Clark, 1984; Nachtigall, Nachtigall, Nachtigall, et al., 1979; Paganini-Hill, Ross, Gerkins, et al., 1981). Many studies also suggest a cardiovascular protective effect of estrogen replacement (Anonymous. 1995; Bush, Barrett-Connor, Cowan, et al., 1987; Criqui, Suarez, Barrett-Connor, et al., 1988), but recent clinical trials have questioned this finding in women with preexisting cardiac disease (Hulley, Grady, Bush, et al., 1998). Despite the potential benefits of hormone replacement therapy, patient adherence to this intervention is poor (Ravnikar, 1987). Speroff and colleagues performed a decision analysis using Markov cohort modeling to evaluate prophylactic oophorectomy (Speroff, Dawson, Speroff, et al., 1991). They considered the influence of estrogen on coronary disease, breast cancer, and osteoporotic fracture. When adherence to estrogen replacement therapy was perfect, oophorectomy resulted in increased survival. However, when compliance with hormone replacement therapy was altered to a more realistic rate, retention of the ovaries rather than elective oophorectomy was favored. Preventive measures other than oophorectomy that have been shown to reduce the risk of ovarian cancer include oral contraceptive pills (La Vecchia and Franceschi, 1999), bilateral tubal ligation (Cornelison, Natarajan, Piver, et al., 1997), and hysterectomy without oophorectomy (Whittemore, Harris, and Itnyre, 1992). When assessing the risk and benefits of prophylactic oophorectomy, these alternative options also should be considered.
Summary
Prophylactic oophorectomy is effective in preventing ovarian cancer and appears to add minimal short-term surgical morbidity when performed with a hysterectomy. In women who are postmenopausal, the benefits of prophylactic oophorectomy may outweigh the risks; however, additional data on the potential benefits of ovarian preservation in postmenopausal women are needed. In premenopausal patients, the trade-off between benefit and harm is less clear, especially given the relatively poor adherence to estrogen replacement therapy.
Figures
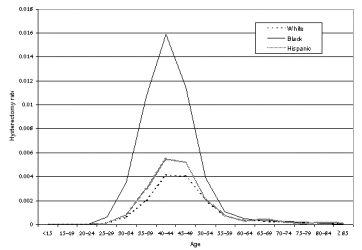
Figure 3. Age- and race-specific incidence of hysterectomy for fibroids, 1997, based on NIS and U.S. Census Bureau estimates
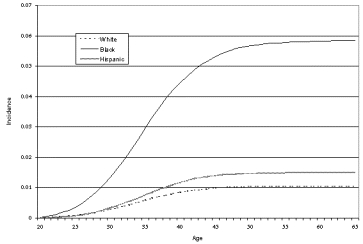
Figure 4. Estimated cumulative incidence of myomectomy based on 1997 NIS and U.S. Census Bureau data
Note: Cumulative incidence may be overestimated, since individual women may undergo the procedure on more than one occasion.
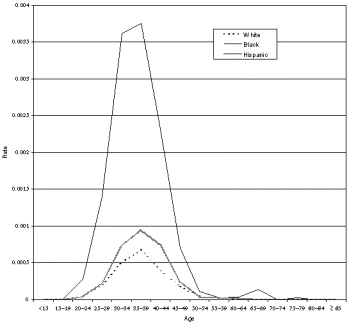
Figure 2. Age- and race-specific incidence of myomectomy, 1997, based on NIS and U.S. Census Bureau estimates
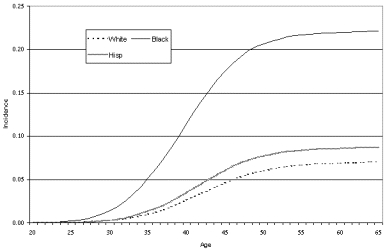
Figure 5. Estimated cumulative incidence of hysterectomy for fibroids based on NIS and U.S. Census Bureau data
Tables
Table 10. Myomectomy for symptomatic fibroids
| Study | No. of patients | Length of followup | Relief of symptoms | Short-term complications | Long-term complications |
|---|---|---|---|---|---|
| Laparoscopic myomectomy | |||||
| Andrei, Crovini, and Rosi, 1999 | 332 | Not reported | Not reported | Transfusion: 1.2% | Not reported |
| Carter and McCarus, 1997 | 28 | 6 months | Bleeding: 100% "satisfied with current bleeding pattern" Pain: 80% "whose chief complaint was pain were satisfied with current levels of pain" | Not reported | Not reported |
| Chapman, 1998 | 300 (laser-induced interstitial thermo-therapy) | 6-72 months | Bleeding: 89% (n = 276) Pain: 85% (n = 156) Dyspareunia: 100% (n = 4) Urinary sx: 100% (n = 24) | Not reported | Not reported |
| Daniell, 1995 | 32 | 6-36 months | Bleeding: 100% (n = 10) Dysmenorrhea: 60% full, 40% partial relief (n = 10) Pelvic pain: 29% full, 54% partial, 14% no relief (n = 7) | Not reported | Not reported |
| Darai, Dechaud, Benifla, et al., 1997 | 143 | Minimum 12 months | Not reported | Transfusion: 1.1% | Not reported |
| Hasson, Rotman, Rana, et al., 1992 | 56 | Mean 9 months | Not reported | Transfusion: 0% Subcutaneous emphysema: 2% | Not reported |
| Nezhat, Nezhat, Bess, et al., 1994 | 57 | Mean 11 months | Menorrhagia: 100% complete relief Pelvic pain: 14/23 complete, 9/23 partial relief | 1 hernia, 1 pneumonia | Not reported |
| Nezhat, Nezhat, Silfen, et al., 1991 | 158 | 3 months-3 years | Not reported | 2.5% major complications | Not reported |
| Nezhat, Roemisch, Nezhat, et al., 1998 | 114 | Mean 37 months | Not reported | Transfusions: 7% | Not reported |
| Rossetti, Paccosi, Sizzi, et al., 1999 | 123 | Not reported | Not reported | 0 | Not reported |
| Seinera, Arisio, Decko, et al., 1997 | 54 | 6 months | Bleeding (n = 20): 90% had "no complaints" | Hemorrhage requiring reoperation: 1.8% | Not reported |
| Stringer, 1996 | 41 | Mean 18 months | 91% "complete or significant relief of symptoms" (type not specified) | Not reported | Not reported |
| Hysteroscopic myomectomy | |||||
| Baggish, Sze, and Morgan, 1989 | 23 | Not reported | 90% "normal menses" | Pulmonary edema: 4% | Not reported |
| Bernard, Darai, Poncelet, et al., 2000 | 31 | Minimum 12 months | Not reported | Uterine perforation: 3.3% Hemorrhage: 3.3% | Not reported |
| Brooks, Loffer, and Serden, 1989 | 62 | Minimum 3 months | Menorrhagia: 46/57 "improved," 4/57 "not improved" | Not reported | Not reported |
| Corson and Brooks, 1991 | 92 | Mean 17 months | Menorrhagia: "Success" 81% Pain: "Success" 85% | Transfusion: 1.1% Uterine perforation: 3.2% Fever: 1.1% | Not reported |
| Cravello, D'Ercole, Boubli, et al., 1995 | 239 | Mean 2.5 years | Bleeding (premenopausal): 81% "satisfied and could lead normal life" | Uterine perforation: 2.3% | Not reported |
| Cravello, Farnarier, De Montgolfier, et al., 1999 | 196 | Mean 73 months | 68.4% "improved" | Not reported | Not reported |
| De Blok, Dijkman, and Hemrika, 1996 | 109 | Mean 2.8 years | 93% "improved" | Not reported | Not reported |
| Derman, Rehnstrom, and Neuwirth, 1991 | 108 | 1-16 years | 75% without recurrent bleeding | Transfusions: 3.7% | Not reported |
| Donnez, Gillerot, Bourgonjon, et al., 1990 | 60 | Not reported | 100% "normal flow" | 0% | Not reported |
| Dubuisson, Chapron, and Levy, 1996 | 213 | Not reported | Not reported | 3.8%, significantly related to increasing fibroid size | Not reported |
| Dueholm, Forman, and Ingerslev, 1998 | 45 | Minimum 3 months | Not reported | Uterine perforation: 4.4% | Not reported |
| Emanuel, Wamsteker, Hart, et al., 1999 | 285 | 16-104 months | Not reported | Uterine perforation: 0.3% Pulmonary edema: 0.3% | Not reported |
| Giatras, Berkeley, Noyes, et al., 1999 | 41 | Not reported | Bleeding: 80% "resolved" | Not reported | Asherman's syndrome: 1/41 |
| Hallez, 1995 | 274 | 5-63 months | 67% free of symptoms after 60 months | Uterine perforation: 0.3% | (At 6 months) Bleeding: 4% Asherman's: 10% |
| Hart, Molnar, and Magos, 1999 | 122 | Mean 2.3 years | Menstrual bleeding 86% "significant
improvement" Dysmenorrhea: 72% "significant improvement" | Not reported | 11% uterine rupture rate during subsequent
pregnancy |
| Indman, 1993 | 38 with concurrent ablation, 13 without | Mean 2.2 years | With ablation: 92% extremely satisfied Without ablation: 60% extremely satisfied | Not reported | Not reported |
| Itzkowic, 1993 | 40 | Mean 14 months | Bleeding: 83% improved | Infection: 2.5% | Not reported |
| Lin, Iwata, and Liu, 1994 | 25 (pre-treated with GnRH) | 9 months | Menorrhagia: 88% improvement | Cervical laceration: 4% | Not reported |
| Loffer, 1990 | 43 | Minimum 12 months | Excessive menstrual bleeding: "controlled in 93%" | Perforation: 1.3% Hyponatremia: 3.8% | Not reported |
| Lomano, 1991 | 33 | Mean 8 months | 88% with heavy/severe menstrual bleeding pre-op; 0% post-op | Not reported | Not reported |
| Mints, Radestad, and Rylander, 1998 | 62 | Mean 29 months | 74% of patients with menorrhagia had post-op amenorrhea/hypo-menorrhea | Not reported | Not reported |
| Motashaw, Dave, and Paghdiwalla, 1995 | 73 | Not reported | 83% premenopausal patients "happy with result" | 1% emergent hysterectomy Transfusions:
4% Perforation: 1% | Not reported |
| Phillips, Nathanson, Meltzer, et al., 1995 | 208 (120 with myomectomy alone, 88 with myomectomy + endometrial ablation) | 6 months (n = 208) 6 years (n = 185) | Resection only: 85% with "satisfactory"
results Resection + ablation: 88.5% with "satisfactory" results | Not reported | Not reported |
| Abdominal myomectomy | |||||
| Berkeley, DeCherney, and Polan, 1983 | 50 | Median 50.7 months | Not reported | Transfusion: 18% Fever: 28% Reoperation: 2% | Not reported |
| Brown, Fletcher, Myrie, et al., 1999 | 16 (done at time of c-section) | Not reported | Not reported | Transfusion: 1/16 (not different than c-section without myomectomy) | Not reported |
| Egwuatu, 1989 | 55 | 1-4 years | Not reported | Fever: 71.8% Wound infection: 10.9% Hemorrhage: 2.7% | Not reported |
| Ikpeze and Nwosu, 1998 | 72 | Not reported | Not reported | Transfusion: 15.3% Fever: 17% | Not reported |
| LaMorte, Lalwani, and Diamond, 1993 | 128 | Not reported | Not reported | Transfusion: 20% Fever: 12% Hysterectomy at time of procedure: 1% | Not reported |
| Reilly and Nour, 1998 | 120 | Not reported | Not reported | Autologous transfusions: 4.2% Fever 11.6% | Not reported |
| Sirjusingh, Bassaw, and Roopnarinesingh, 1994 | 114 | 2-10 years | Not reported | Transfusion: 16% | Not reported |
| Vercellini, Maddalena, De Giorgi, et al., 1999 | 466 | 41 months | Not reported | Transfusion: 2% Fever: 18% Reoperation: 2% | Not reported |
| Vaginal myomectomy | |||||
| Ben-Baruch, Schiff, Menashe, et al., 1988 | 46 (all solitary prolapsed, pedun-culated, sub-mucous) | Median 5.5 years | Asymptomatic: 79% | Transfusion: 8.7% pre-op conversion to abdominal procedure: 6.5% | Not reported |
| Davies, Hart, and Magos, 1999 | 35 | Not reported | Menorrhagia: 15/21 "improvement" Pelvic pain: 6/7 "improvement" | Transfusion: 11.4% Hematoma: 11.4% Reoperation or readmission: 8.5% | Not reported |
Table 11. Hysterectomy for symptomatic fibroids
| Study | No. of patients | Length of followup | Relief of symptoms | Short-term complications | Long-term complications |
|---|---|---|---|---|---|
| Carlson, Miller, and Fowler, 1994 | 413 (35% for fibroids) | 12 months | Significant improvement in symptoms in all women with fibroids | In-hospital complications: 7% | New symptoms: Hot flashes: 13% Weight gain: 12% Depression: 8% Anxiety: 7% |
| Kjerulff, Langenberg, Rhodes, et al., 2000 | 1299 (48.1% for fibroids) | 24 months | Results not stratified by indication (not significantly
different by indication, according to authors) All symptoms significantly improved by hysterectomy; quality of life also improved | None: 21.4% Mild: 66.8% Moderate: 11.1% Severe: 0.7% | Problems acquired in up to 12.9% Predictors of poor long-term outcome: Income, baseline depression/anxiety, and bilateral salpingoophorectomy |
| Weber, Walters, Schover, et al., 1999 | 43 (71% for fibroids) | Mean 14.2 ± 3.5 months | Most important benefit of surgery: Relief from pain: 55% Relief from bleeding: 40% 95% satisfied | None: 100% | 4/34 without incontinence pre-operatively developed stress incontinence post-operatively |
Table 12. Studies directly comparing abdominal hysterectomy to abdominal myomectomy
| Reference | No. of patients | Short-term complications | Long-term complications | Comments |
|---|---|---|---|---|
| Ecker, Foster, and Friedman, 1995 | 204 abdominal hysterectomy; 109 abdominal myomectomy | 1) Mean estimated blood loss (EBL) greater in hysterectomy
group 2) EBL correlated with increasing uterine size, number of fibroids removed, and length of procedure 3) Febrile morbidity more common in myomectomy group 4) Myomectomy group more likely to have banked blood, but no difference in transfusion rates | Not reported | Retrospective chart review No multivariate analysis |
| Iverson, Chelmow, Strohbehn, et al., 1996 | 89 abdominal hysterectomy; 103 abdominal myomectomy | 1) EBL greater in hysterectomy group (univariate) 2) Uterine size only significant predictor of EBL in multivariate analysis 3) Increased intraoperative visceral injuries in hysterectomy group (no multivariate analysis) | Not reported | Retrospective chart review |
| Iverson, Chelmow, Strohbehn, et al., 1999 | 160 abdominal hysterectomy; 101 abdominal myomectomy | Myomectomy associated with significantly increased risk of fever compared with hysterectomy (multivariate analysis) | Not reported | None |
Table 13. Studies evaluating the effect of uterine size on hysterectomy complications
| Study | No. of patients | Length of followup | Relief of symptoms | Short-term complications | Long-term complications |
|---|---|---|---|---|---|
| Hillis, Marchbanks, and Peterson, 1996 | 446 | 6 weeks | Not reported | EBL, transfusions, and complications increased for uterine size > 500 g (multivariate analysis) | Not reported |
| Reiter, Wagner, and Gambone, 1992 | 93 | Not reported | Not reported | Estimated blood loss (EBL) and complication rates not significantly different between uterus < 12 weeks size and > 12 weeks size | Not reported |
Table 14. Studies comparing single to multiple myomectomies
| Study | No. of patients | Length of followup | Complications | Pregnancy rate | Recurrence (diagnostic or symptomatic) | Need for subsequent surgery |
|---|---|---|---|---|---|---|
| Acien and Quereda, 1996 | 80 | 10 years | Not reported | 1 fibroid: 79.2% > 2: 37.5% (p < 0.05) | Not reported by number of fibroids | Not reported by number of fibroids |
| Candiani, Fedele, Parazzini, et al., 1991 | 622 | 10 years | Not reported | Not reported | Multivariate relative risk (with 95% confidence
interval): 1 fibroid: 1.0 2-3 fibroids: 1.2 (0.9-1.51) > 4 fibroids: 2.1 (1.7-2.8) | Not reported |
| Fedele, Parazzini, Luchini, et al., 1995 | 145 | 5 years | Not reported | Not reported | Single fibroid: 38% (ultrasound) Multiple: 60% | Not reported |
| Malone, 1969 | 125 | Minimum 5 years | Not reported | 1 fibroid: 59% > 2: 48% | Single: 26.6% Multiple: 58.8% | Single: 11.1% (all hysterectomies) Multiple: 26.3% (22.5% hysterectomies) |
Table 15. No treatment or noninvasive therapy (other than GnRH agonists)
| Study | No. of patients | Length of followup | Diagnostic recurrence (bimanual exam or ultrasound) | Symptomatic recurrence/ persistence | Additional surgery other than hysterectomy | Hysterectomy |
|---|---|---|---|---|---|---|
| Carlson, Miller, and Fowler, 1994 | 106 (68% no treatment, 18% NSAIDs, 14% hormones) | 12 months | Not reported | Bleeding: 83% Pain: 52% Fatigue: 73% New problems: 10% | Not reported | 23% (overall; data not reported separately for patients with fibroids) |
Table 16. Recurrence after GnRH agonist therapy
| Study | No. of patients | Length of followup | Diagnostic recurrence (bimanual exam or ultrasound) | Symptomatic recurrence/ persistence | Additional surgery other than hysterectomy | Hysterectomy |
|---|---|---|---|---|---|---|
| Serra, Panetta, Colosimo, et al., 1992 | 110 | 12 months | Not reported | 24.3% (n = 82, 7% lost to follow-up) | 4 months: 8.4% 12 months: 6.8% | Not reported |
| van Leusden, 1992 | 22 pre-menopaus-al, 6 post-meno-pausal | Up to 42 months | Not reported | Not reported | Premenopausal: 4% Postmenopausal: 0% | Pre-menopausal: 55% Post-menopausal: 0% |
| Vollenhoven, Shekleton, McDonald, et al., 1990 | 40 | Mean 9.6 months | Not reported | Not reported | 22.5% | 0 |
| West, Lumsden, Hillier, et al., 1992 | Buserelin + MPA, either combined (n = 10) or sequential (n = 10) | 24 months | Not reported | Combined: 11% Sequential: 10% | Not reported | Combined: 33% Sequential: 30% |
Table 17. Recurrence after myomectomy
| Study | No. of patients | Length of followup | Diagnostic recurrence/ persistence (bimanual or ultrasound) | Symptomatic recurrence/ persistence | Additional surgery other than hysterectomy | Hysterectomy | |
|---|---|---|---|---|---|---|---|
| Abdominal myomectomy | |||||||
| Berkeley, De Cherney, and Polan, 1983 | 50 | Median 50.7 months | Not stated | Not stated | 8% laparoscopy for infertility | 8% | |
| Egwuatu, 1989 | 55 | 1-4 years | Not reported | Not reported | 10.5% | 3.1% | |
| Fedele, Parazzini, Luchini, et al., 1995 | 145 | 60 months | 5-year cumulative probability: 51% | Not reported | Not reported | Not reported | |
| Gehlbach, Sousa, Carpenter, et al., 1993 | 37 (infertility patients) | Minimum of 12 months | 47% | Not reported | 10.8% | 5.4% | |
| Mais, Ajossa, Guerriero, et al., 1996 | 20 abdominal, 20 laparo-scopic | 6 months | Abdominal: 5%, Laparoscopic: 10% | Not reported | Not reported | Not reported | |
| Sirjusingh, Bassaw, and Roopnarinesingh, 1994 | 114 | 2-10 years | Not reported | 17% | Myomectomy: 4% | Hysterectomy: 8% | |
| Stringer, Walker, and Meyer, 1997 | 49 abdominal, 49 laparo-scopic | Not reported | Not reported | Not reported | Not reported | Abdominal: 6% Laparoscopic: 2% | |
| Abdominal myomectomy with adjunctive GnRH | |||||||
| Fedele, Vercellini, Bianchi, et al., 1990 | GnRH: 8 No GnRH: 16 | Not reported | GnRH: 62.5% No GnRH: 12.5% (OR 10.2; 95% CI, 1.6, 63.3) | Not reported | Not reported | ||
| Friedman, Daly, Juneau-Norcross, et al., 1992 | GnRH: 9 No GnRH: 9 | 27-38 months | GnRH: 67% No GnRH: 56% | GnRH: 22.2% No GnRH: 22.2% | Not reported | Not reported | |
| Sudik, Husch, Steller, et al., 1996 | GnRH: 33 No GnRH: 34 | 12 months | 46.3% | Not reported | Not reported | Not reported | |
| Vavala, Lanzone, Monaco, et al., 1997 | 65: 40 no post-op treatment, 25 post-op GnRH (not
randomized) | 36 months | No GnRH: 22.5% GnRH: 4% | Not reported | Not reported | Not reported | |
| Hysteroscopic myomectomy | |||||||
| Bernard, Darai, Poncelet, et al., 2000 | 31 | Minimum 12 months | Not reported | Not reported | 6.5% repeat myomectomy | Not reported | |
| Corson and Brooks, 1991 | 92 | Mean 17 months | Not reported | Not reported | Overall: 17.3% Hysteroscopic myomectomy: 7.6% Abdominal myomectomy: 2.1% Endometrial ablation: 4.3% | 3.2% | |
| Cravello, D'Ercole, Boubli, et al., 1995 | 239 | Mean 2.5 years | Not reported | Not reported | 16% repeat myomectomy | Not reported | |
| Cravello, Farnarier, De Montgolfier, et al., 1999 | 196 | Mean 73 months | Not reported | 35.7% | Myomectomy: 8.8% Ablation: 6.5% | 14.6% | |
| De Blok, Dijkman, and Hemrika, 1996 | 109 | Mean 2.8 years | Not reported | Not reported | Repeat procedure: 11.9% | 5.5% | |
| Derman, Rehnstrom, and Neuwirth, 1991 | 108 | 1-16 years | Not reported | 25% | Cumulative risk 37% after 11 years | 6.5% | |
| Dueholm, Forman, and Ingerslev, 1998 | 45 (38 with residual tissue at completion of procedure) | 6 months | Not reported separately | 20% | 20% (23.6% with residual tissue) | 2.2% (2.6% with residual tissue) | |
| Emanuel, Wamsteker, Hart, et al., 1999 | 285 | Median 46 months | Not reported separately | Not reported separately | 76.7% at 8 years | 10.8% at 8 years | |
| Hallez, 1995 | 274 | 5-63 months | Not reported | 33% after 60 months | Not reported | Not reported | |
| Hart, Molnar, and Magos, 1999 | 122 | Mean 2.3 years | Not reported | Not reported | 21% first 4 years, none after 4 years | Not reported | |
| Indman, 1993 | 38 with concurrent ablation, 13 without | Mean 2.2 years | Not reported | Not reported | Not reported | With ablation: 0 Without ablation: 8% | |
| Itzkowic, 1993 | 40 | Mean 14 months | Not reported | Not reported | 7.5% | 2.5% converted at time of initial
surgery | |
| Lin, Iwata, and Liu, 1994 | 25 (pretreated with GnRH) | 9 months | Not reported | 12% | 13.6% | Not reported | |
| Loffer, 1990 | 43 | Minimum 12 months | Not reported | Not reported | Repeat hysteroscopy: 9.4% Other: 5.6% | 9.4% | |
| Mints, Radestad, and Rylander, 1998 | 62 | Mean 29 months | Not reported | Not reported | Not reported | 12% | |
| Phillips, Nathanson, Meltzer, et al., 1995 | 208 (120 with myo-mectomy alone, 88 with myo-mectomy + endometrial ablation) | 6 months (n = 208); 6 years (n = 185) | Not reported | Myomectomy: 15% Myomectomy + ablation: 11.5% | Myomectomy: 4.3% Myomectomy + ablation: 3.2% | Myomectomy: 0.5% Myomectomy + ablation: 1.6% | |
| Vercellini, Zaina, Yaylayan, et al., 1999 | 108 | Mean 41 months | 34% (3 years) | 30% (3 years) | Not reported | Not reported | |
| Laparoscopic myomectomy | |||||||
| Chapman, 1998 | 300 | 6-72 months | Not stated | Not stated | Additional laser treatment: 10% | 2% | |
| Nezhat, Roemisch, Nezhat, et al., 1998 | 114 | Mean 37 months | 33.3% Cumulative risk: 1 year 10.6% 3 year 31.7% 5 year 51.4% | Not reported | 12.3% | 6.1% | |
Table 18. Recurrence after uterine artery embolization
| Study | No. of Patients | Length of followup | Ultrasonographic recurrence/ persistence | Symptomatic recurrence/ persistence | Additional surgery/ procedure | Hysterectomy |
|---|---|---|---|---|---|---|
| Goodwin, McLucas, Lee et al., 1999 | 60 | Mean 16.3 months | 12% | 12% | 13% | 10% |
| Hutchins, Worthington-Kirsch, and Berkowitz, 1999 | 305 | Up to 12 months | Not reported | 3 months: 13%, 6 months: 13% 12 months: 14% (crude rate; variable loss to followup, followup time prohibits calculation of cumulative rate) | 4.2% (crude rate; variable loss to followup, followup time prohibits calculation of cumulative rate) | 2.0% (crude rate; variable loss to followup, followup time prohibits calculation of cumulative rate) |
| Pelage, Le Dref, Soyer, et al., 2000 | 80 | 2 years | Not reported | 6% "no improvement" | 6% | 1.2% |
| Spies, Scialli, Jha, et al., 1999 | 61 | Mean 8.7 months | Not reported | 5% ("dissatisfied") | 5% | 3.4% |
Table 19. Natural history of uterine fibroids in prospective controlled studies on no treatment or placebo
| Study | No. of patients | Length of followup | Baseline uterine or fibroid size | Followup uterine or fibroid size | Change |
|---|---|---|---|---|---|
| Friedman, Harrison-Atlas, Barbieri, et al., 1989 | Placebo (n = 20) | 6 months | Uterine size: 426 ± 43 cc | Uterine size: 429 ± 52 cc | No change (p = not significant) |
| Friedman, Hoffman, Comite, et al., 1991 | Placebo (n = 65) | 6 months | Uterine size: 452 ± 51 cc | Uterine size: 5% increase | No change (p = not significant) |
| Gregoriou, Vitoratos, Papadias, et al., 1997 | No treatment (n = 20) | 12 months | Fibroid size: 118.4 cc | Fibroid size: 117.5 cc | No change in fibroid volume |
| Schlaff, Zerhouni, Huth, et al., 1989 | Placebo (n = 6) | 6 months | Uterine size: 457 ± 106 cc Fibroid size: 267 ± 82 cc | Uterine size: 656 ± 208 Fibroid size: 417 ± 169 cc | No change (p = not significant) |
Table 20. Effect of medroxyprogesterone acetate therapy in conjunction with GnRH agonist treatment of uterine fibroids
| Study | Hormonal therapy | GnRH co-intervention (timing) | Baseline uterine volume | Post-treatment uterine volume | Results |
|---|---|---|---|---|---|
| Benagiano, Morini, Aleandri, et al., 1990 | MPA 200 mg tapering to 25 mg daily (n = ?) No treatment (n = ?) | Buserelin (concurrent) | 132 cc 122 cc | 170 cc 198 cc | No difference |
| Caird, West, Lumsden, et al., 1997 | MPA 15 mg qd (n = 12) Placebo (n = 12) | Goserelin (concurrent) | - | - | No difference between groups |
| Carr, Marshburn, Weatherall, et al., 1993 | MPA 20 mg qd 1st 3 mo of GnRH Rx (n = 8) MPA 20 mg qd 2nd 3 mo of GnRH Rx (n = 8) | Leuprolide (concurrent) | 100% 100% | 78% 74% | No difference between groups |
| Friedman, Barbieri, Doubilet, et al., 1988 | MPA 20 mg qd (n = 9) Placebo (n = 7) | Leuprolide (concurrent) | 811 cc 601 cc | 688 cc 294 cc* | |
| Scialli and Jestila, 1995 | MPA 5 mg qd (n = 21) Placebo (n = 20) | Leuprolide (before) | 538 cc 344 cc | 473 cc 510 cc | |
| West, Lumsden, Hillier, et al., 1992 | MPA 15 mg qd (n = 10) MPA 15 mg qd after 3 months (n = 10) | Goserelin (concurrent) | 100% 100% | 82% 77% | No difference |
* P< 0.05 difference from baseline to posttreatment
Table 21. Symptomatic response to GnRH agonist treatment of uterine fibroids
| Study | Year | Initial symptoms | Proportion of patients with resolution of symptoms | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coddington, Brzyski, Hansen, et al., 1992 | 1992 | Menorrhagia (70%), urinary frequency (50%), and tight clothes (50%) | 100% | ||||||
| Friedman, Barbieri, Benacerraf, et al., 1987 | 1987 | Pelvic pain, mass, or menorrhagia | 100% | ||||||
| Friedman, Hoffman, Comite, et al., 1991 | 1991 | Bloating, menorrhagia, pelvic pressure, pelvic pain, constipation, urinary frequency, dyspareunia, menometrorrhagia | Not reported Symptom response was "better" in GnRH than placebo (no test of significance performed) | ||||||
| Nakamura, Yoshimura, Yamada, et al., 1991 | 1991 | Bleeding | Amenorrhea: 24% Improved: 68% Severe bleeding episode: 8% | ||||||
| Dysmenorrhea | 100% resolved | ||||||||
| Pelvic pressure | 100% resolved | ||||||||
| Palomba, Affinito, Di Carlo, et al., 1999 | 1999 | Not reported | Not reported There was a statistically significant reduction in intensity for all myoma related symptoms at sixth month of treatment (p < 0.01); this reduction persisted during the entire course of treatment. | ||||||
| Palomba, Affinito, Tommaselli, et al., 1998 | 1998 | Menorrhagia, pelvic pressure, pelvic pain, constipation, urinary frequency | 100% 94% 89% 100% 84% | ||||||
| Schlaff, Zerhouni, Huth, et al., 1989 | 1989 | Pain | 10/11 resolved, 7/11 persistent response 6 months after cessation | ||||||
| Bleeding | 7/7 resolved, 3/7 persistent response | ||||||||
| Pelvic pressure | 6/8, 3/8 persistent response | ||||||||
| Constipation | 4/6, 3/6 persistent response | ||||||||
| Dyspareunia | 4/5, 2/5 persistent response | ||||||||
| Serra, Panetta, Colosimo, et al., 1992 | 1992 | Not reported | End of 4 months (n = 100):
| ||||||
| Vollenhoven, Shekleton, McDonald, et al., 1990 | 1990 | Not reported | "All" patients reported subjective improvement in symptoms. |
Table 22. Nonrandomized studies of preoperative GnRH agonists on operative outcomes
| Study | Presurgical treatment | Type of surgery | Uterine volume decrease in GnRH-treated patients | Intraoperative blood loss (ml) | Operating room time (minutes) |
|---|---|---|---|---|---|
| Falsetti, Mazzani, Rubessa, et al., 1992 | Goserelin 4 months (n = 30) | Myomectomy | 48% | 205 vs. 310 p < 0.001 | Single: 91 vs. 97 Multiple: 111 vs. 118 p = not significant |
| Kiltz, Rutgers, Phillips, et al., 1994 | Leuprolide for 3 months | Myomectomy | 35% | 680 vs. 722 p = not significant | Not reported |
| Vercellini, Bocciolone, Colombo, et al., 1993 | Goserelin and iron for 6 months | Total abdominal hysterectomy (TAH) or vaginal hysterectomy (VH) | 52% | 186 vs. 351 p < 0.001 | TAH: 102 vs. 104 VH: 83 vs. 77 p = not significant |
Table 23. Average wholesale prices for medical treatments for symptomatic fibroids
| Drug | Minimum price | Maximum price | 3-month minimum | 3-month maximum |
|---|---|---|---|---|
| NSAIDs | ||||
| Ibuprofen | ||||
| 200 mg (100 pills) | $1.75 | $6.18 | $2.94 | $15.57 |
| 400 mg (100 pills) | $17.02 | $26.48 | $14.30 | $22.24 |
| 600 mg (100 pills) | $27.39 | $38.69 | $17.26 | $24.37 |
| 800 mg (100 pills) | $26.21 | $45.34 | $16.51 | $28.56 |
| Naproxen | ||||
| 275 mg (30 pills) | $24.84 | $38.20 | $20.87 | $32.09 |
| 550 mg (30 pills) | $39.21 | $130.69 | $16.47 | $54.89 |
| Mefanamic acid | ||||
| 250 mg (30 pills) | $42.08 | $42.08 | $35.35 | $35.35 |
| Oral contraceptives (6 months) | ||||
| Progestin only | $185.09 | $208.92 | $92.55 | $104.46 |
| Combination | $168.36 | $281.19 | $84.18 | $140.60 |
| Progestins | ||||
| Oral medroxyprogesterone acetate 10 mg (30 pills) | $19.87 | $31.21 | $59.61 | $93.63 |
| Depot medroxyprogesterone acetate | $48.10 | $48.10 | $48.10 | $48.10 |
| GnRH agonist | ||||
| Depot leuprolide Acetate | $478.01 | $518.64 | $1,434.03 | $1,555.92 |
Table 24. Mean total hospital costs for myomectomy, by age
| Age | Mean total cost (n = 239) |
|---|---|
| < 25 | $4,340 ± 663 |
| 25-29 | $4,796 ± 694 |
| 30-34 | $5,105 ± 921 |
| 35-39 | $5,308 ± 2,066 |
| 40-44 | $5,333 ± 1,842 |
| 45-49 | $5,684 ± 1,962 |
| > 50 | $4,490 ± 704 |
Source: Duke University Medical Center, 1992-1998
Table 25. Mean total hospital costs for hysterectomy, by age
| Age | Mean costs (n = 753) |
|---|---|
| < 25 | -- |
| 25-29 | $4,490 ± 704 |
| 30-34 | $5,309 ± 1,413 |
| 35-39 | $5,577 ± 1,908 |
| 40-44 | $5,656 ±1,809 |
| 45-49 | $6,348 ± 2,627 |
| > 50 | $6,222 ± 2,469 |
Source: Duke University Medical Center, 1992-1998
