NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mulrow CD, Ramirez G, Cornell JE, et al. Defining and Managing Chronic Fatigue Syndrome. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001 Oct. (Evidence Reports/Technology Assessments, No. 42.)
This publication is provided for historical reference only and the information may be out of date.
This section describes methods used to obtain expert input, identify key questions, conduct literature searches, select and abstract relevant studies, analyze data, and obtain peer review.
Expert Input
This evidence report was produced by a multidisciplinary group that included: Cynthia Mulrow, MD, MSc, a general internist, Gilbert Ramirez, DrPH, a research methodologist, and John Cornell, PhD, a psychometrician. Martha Harris, AHIP, MLS, MA, a medical librarian, conducted literature searches and maintained the reference databases. Christine Aguilar, MD, MPH, a physician research associate, and Jodi Sapp, RN, a nurse research associate, abstracted information from the articles; David Mullins developed electronic databases for the project and provided data entry/data validation support. Drs. Ramirez and Cornell calculated effect size statistics for comparing study outcomes. Drs. Mulrow, Ramirez and Aguilar coordinated the project administratively. Karen Stamm edited and formatted the report, and coordinated communication with technical experts and peer reviewers. Drs. Ramirez, Cornell, and Mulrow wrote the report.
An international expert panel provided input regarding: the plan and format for the evidence report; interpretation and presentation of findings; critique of drafts; and future research priorities. Members of the panel from the United States included: Dedra Buchwald, MD, University of Washington-Seattle; Mark Demitrack, MD, Eli Lilly and Company; Sudhir Gupta, MD, PhD, University of California-Irvine; Leonard A. Jason, Ph.D., DePaul University; James F. Jones, MD, National Jewish Medical and Research Center; Nancy G. Klimas, MD, University of Miami; Kathy Rabin, JD, a CFS patient; John H. Renner, MD, National Council for Reliable Health Information (until his death in September 2000); Joan Shaver, PhD, RN, University of Illinois-Chicago; Eng M. Tan, MD, Scripps Research Institute; and Vicki C. Walker, BA, Research and Public Policy Project Manager, The CFIDS Association of America. Non-U.S. members of the technical expert panel included: Susan Abbey, MD, University of Toronto, Canada; Peter Campion, PhD, FRCGP, MRCP (UK), DCCH, University of Hull, United Kingdom; Robert H. Loblay, MD, University of Sydney, Australia; and Peter D. White, MD, St. Bartholomew's and the Royal London School of Medicine and Dentistry, Queen Mary and Westfield College, London, United Kingdom. Ex-officio members included: David Morens, MD, National Institute for Allergy and Infectious Diseases, and William Reeves, MD, MSPH, Centers for Disease Control and Prevention. In addition, the report's penultimate draft was peer-reviewed by 15 individuals selected for their expertise in CFS (see Appendix A for a full list of external peer reviewers).
Questions Addressed in Evidence Report
The agency that nominated the topic initially suggested a broad-ranging list of more than 20 questions for our report to address. We enlisted the help of the technical expert panel to determine which questions we could realistically address, given the enormity of the data, and the limits on time and resources. Using a consensus Delphi process, the technical experts narrowed our initial scope to the following highest priority questions:
- What are the existing case definitions for CFS?
- Which case definitions, if any, have been substantiated and/or validated with reliably discriminating constellations of symptoms in adults?
- What are the prevalence and natural history of CFS in adults?
- Do controlled studies in adults show that particular therapies improve clinical symptoms of CFS, when compared to placebo, no therapy, or each other?
Literature Search and Selection Methods
Sources and Search Methods
English and non-English citations addressing research in humans were identified from: MEDLINE, Cochrane Library, and PsycINFO electronic bibliographic databases (1980 to July 2000); EMBASE (1988-93, 1998-2000); and the Journal of Chronic Fatigue Syndrome (1996-2000). Other sources of citations included: Internet sites addressing clinical guidelines and chronic fatigue syndrome; bibliographical references from pertinent articles and reviews; textbooks; and technical experts. An updated search through October 2000 was conducted using PubMed. Technical panel members and peer reviewers were asked to identify pertinent unpublished literature up to January 2001.
Broad, sensitive search strategies that keyed primarily on the topic chronic fatigue syndrome and its synonyms were used for each electronic database. Subject headings and keyword synonyms are given below.
Subject headings:
chronic fatigue syndrome
neurasthenia
keywords for chronic fatigue:
chronic fatigue disorder(s)
immune dysfunction syndrome
myalgic encephalomyelitis
postviral fatigue
post viral fatigue
infectious mononucleosis like
whiplash syndrome
royal free disease
chronic epstein-barr
(yuppie or yuppy) flu
CFIDS
CFS
ME and chronic
We also conducted specific searches to identify controlled trials of therapy for chronic fatigue syndrome. In these searches we crossed the above subject headings and keywords with the following terms.
comparative study/
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospectiv$ or volunteer$).tw.
clinical trial.pt
exp clinical trials/
(clin$ adj25 trial$).ti,ab.
((singl$ or double or trebl$ or tripl$) adj25 (blind$ or mask$)).tw
single-blind method/
double-blind method/
placebos/
placebo$.tw.
random$.tw.
research design/
Selection Criteria
Based on input from technical experts and feasibility constraints, we used the following criteria to select articles for review. All selected articles addressed research in humans. Unpublished articles that were referred to us from technical experts or peer reviewers were eligible for review if they met all other selection criteria.
Criteria for question 1: existing case definitions
- The case definition must have been developed specifically for chronic fatigue syndrome. (Case definitions developed specifically for conditions such as neurasthenia, fibromyalgia, or myalgic encephalomyelitis were not considered.)
- English language only
Criteria for question 2: substantiation of case definitions
- Studies examining whether individual manifestations listed within specified case definitions occur more frequently in persons with CFS than in other populations, or
- Studies examining whether clusters of manifestations listed within specified case definitions distinguish CFS from other related conditions, and
- Sample size 30 or more participants with CFS, and
- English language only
Criteria for question 3: prevalence and natural history of CFS?
- Prevalence
- Community or primary care setting, and
- Sample size greater than 100 adults, and
- English language only, and
- Specified definition of CFS
- Natural history
- Prospective study, and
- Sample size 30 or more adult participants with CFS, and
- Followup period of at least one year, and
- English language only, and
- Specified definition of CFS
Criteria for question 4: therapy
- Case-control studies or controlled trials (any prospective study that compares patients given an active therapy to patients given placebo, no therapy, or other therapy), and
- Sample size 10 or more adult participants with CFS, and
- English or non-English language, and
- Specified definition of CFS
Selection Process
Figure 1 depicts the selection process. Of 5,221 identified records from the various electronic databases and sources, 1,111 were eliminated because they were duplicate citations cited in more than one database (i.e., same authors, title, journal source information). Two reviewers independently screened the titles and abstracts of the remaining 4,110 records to select articles that were relevant to evidence questions one through four. Of the 4,110 records that were screened, 518 were considered potentially relevant to the specified evidence report questions. Of 124 that addressed case definitions for CFS, 41 met selection criteria. Of 83 articles that addressed prevalence or natural history, 20 met the selection criteria. Of 311 articles that addressed therapies, 38 were controlled trials that met selection criteria.
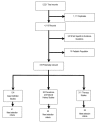
Figure
Figure 1. Literature search results flow chart.
Data Abstraction Process
Two reviewers (physician, psychometrician, methodologist and/or nurse) independently abstracted data from the selected studies. None of the abstractors were blinded either to study title or to author's names. Disagreements in abstractions were uncommon (less than 1% of items) and were resolved by consensus. No formal reliability testing was done.
We critically appraised the quality or rigor of studies by assessing several parameters. For studies addressing prevalence, we assessed: the method and time course of ascertainment, the sampling method, whether the sample was representative, whether an established case definition was used, and the response rate. For studies addressing natural history, we assessed: sampling methods, whether the sample was representative, whether an established case definition was used, methods of followup, and followup rates. For studies addressing substantiation of case definitions, we assessed: incorporation and selection biases, whether the study sample was representative, how the diagnosis was verified and by whom, whether compared groups were equivalent with respect to key sociodemographic or clinical variables that could affect the manifestations being studied, and whether groups were matched on such confounding variables. For studies addressing therapy, we assessed: whether the controlled study was randomized, the adequacy of randomization (method and concealment of assignment); whether the trial was single or double-blind; numbers of dropouts; and appropriateness of analyses methods.
Data Synthesis Process
We synthesized data descriptively, emphasizing methodological characteristics of the studies, such as sources of populations enrolled, CFS case definitions used to select study participants, sample sizes, adequacy of randomization process, interventions and comparisons. We examined relationships between clinical outcomes, participant characteristics, and methodological characteristics in evidence tables and graphical summaries, such as forest plots. We grouped outcomes according to a list that had been developed specifically to assess symptoms in patients with CFS.20
To help compare outcomes across studies, we computed standardized mean differences between treatment and comparison group scores as a measure of effect size for each study. These estimates were adjusted for between-group differences at baseline and for small sample bias.21 We adjusted for baseline differences by calculating an “effect size” at baseline; by definition, it should be zero if study groups were well matched. When we found a non-zero “effect size” at baseline, we adjusted outcome effect sizes were by subtracting the baseline effect size.
- Methodology - Defining and Managing Chronic Fatigue SyndromeMethodology - Defining and Managing Chronic Fatigue Syndrome
Your browsing activity is empty.
Activity recording is turned off.
See more...
