NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood vascular tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Notes on Vascular Malformations
While information about vascular malformations is covered at the beginning of this summary, the remainder of this summary focuses on neoplasms, not malformations.
Although not considered neoplasms, many vascular malformations are caused by targetable somatic mutations; this discovery means that pediatric oncologists will be asked to be involved in management of these lesions. Therefore, it is important for oncologists to have some understanding of the biology and clinical management of common vascular malformations.
Vascular malformations are distinguished from vascular tumors by their low cell turnover and lack of invasiveness.[1] They tend to grow in proportion to the child and are generally stable in adulthood. Nonetheless, endothelial cells isolated from vascular malformations have been found in vitro to have some tumor-like behaviors, such as increased growth, migration, and resistance to apoptosis.[2]
In the International Society for the Study of Vascular Anomalies (ISSVA) classification, vascular malformations are subdivided according to vessel type.[3] High-flow lesions, arteriovenous malformations are the most aggressive type, but they are relatively rare. Low-flow lesions may be venous, lymphatic, or mixed. The symptoms in patients with low-flow malformations most often relate to the bulk of the lesion, episodic thrombosis, or bleeding, which causes pain. In patients with large lesions, there is a risk of pulmonary embolism. Capillary malformations include port-wine stains and a number of less common lesions. Treatment for patients with both high-flow and low-flow malformations is usually either surgery, endovascular intervention, or some combination of the two; ideally, a multidisciplinary vascular anomalies team is involved in the treatment of these patients. Only a low level of evidence supports the choice of treatment between these options, and the recurrence rates for large lesions are relatively high.[4]
Patients with low-flow malformations are the most likely to present for oncological treatment; this will normally occur after conventional treatments have failed. Approximately one-third to one-half of venous malformations result from somatic or, rarely, germline mutations in the TEK (or TIE2) gene.[5] Another one-third of venous malformations, and nearly all lymphatic malformations, are caused by somatic mutations in PIK3CA.[6] In most cases, PIK3CA mutations are identical to canonical cancer mutations. Lesions harboring PIK3CA mutations are frequently associated with overgrowth of adjacent tissues, as seen in patients with Klippel-Trénaunay syndrome.[7] Sirolimus has been used to target the phosphatidylinositol 3-kinase (PI3K) pathway in low-flow malformations, leading to symptomatic improvement in many patients. It is unclear whether treatment reduces the size of lesions because there is usually considerable fluctuation in size, and treatment generally begins when lesions are enlarged. The use of sirolimus in venous and lymphatic malformations is supported by level 3 evidence (case series or other observational study designs).[8,9] Both PIK3CA- and TEK-mutated lesions appear to respond equally to treatment with sirolimus. Phase III clinical trials are under way (e.g., NCT02638389 and NCT03987152). A 2018 study reported promising level 3 evidence for the use of the PI3K inhibitor BYL719 to treat patients who have lesions with a PIK3CA mutation.[10]
There is currently no evidence to support the use of targeted therapies in patients with arteriovenous malformations. The finding that most of these malformations appear to be caused by somatic mutations in the mitogen-activated protein (MAP) kinase pathway, including gain of function mutations in MAP2K1, KRAS, and BRAF,[11] along with limited in vitro data, suggests that MEK pathway inhibition may soon have a role in treating patients with these aggressive, highly symptomatic, and sometimes fatal lesions.
References
- Mulliken JB, Glowacki J: Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 69 (3): 412-22, 1982. [PubMed: 7063565]
- Lokmic Z, Mitchell GM, Koh Wee Chong N, et al.: Isolation of human lymphatic malformation endothelial cells, their in vitro characterization and in vivo survival in a mouse xenograft model. Angiogenesis 17 (1): 1-15, 2014. [PubMed: 23884796]
- Wassef M, Blei F, Adams D, et al.: Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 136 (1): e203-14, 2015. [PubMed: 26055853]
- van der Vleuten CJ, Kater A, Wijnen MH, et al.: Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol 37 (4): 977-89, 2014. [PubMed: 24196269]
- Soblet J, Limaye N, Uebelhoer M, et al.: Variable Somatic TIE2 Mutations in Half of Sporadic Venous Malformations. Mol Syndromol 4 (4): 179-83, 2013. [PMC free article: PMC3666452] [PubMed: 23801934]
- Luks VL, Kamitaki N, Vivero MP, et al.: Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr 166 (4): 1048-54.e1-5, 2015. [PMC free article: PMC4498659] [PubMed: 25681199]
- Keppler-Noreuil KM, Rios JJ, Parker VE, et al.: PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A 167A (2): 287-95, 2015. [PMC free article: PMC4480633] [PubMed: 25557259]
- Adams DM, Trenor CC, Hammill AM, et al.: Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 137 (2): e20153257, 2016. [PMC free article: PMC4732362] [PubMed: 26783326]
- Hammer J, Seront E, Duez S, et al.: Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study. Orphanet J Rare Dis 13 (1): 191, 2018. [PMC free article: PMC6206885] [PubMed: 30373605]
- Venot Q, Blanc T, Rabia SH, et al.: Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558 (7711): 540-546, 2018. [PMC free article: PMC7610773] [PubMed: 29899452]
- Couto JA, Huang AY, Konczyk DJ, et al.: Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am J Hum Genet 100 (3): 546-554, 2017. [PMC free article: PMC5339083] [PubMed: 28190454]
General Information About Childhood Vascular Tumors
Vascular anomalies are a spectrum of rare diseases classified as vascular tumors or malformations. An updated classification system was adopted at the General Assembly of the International Society for the Study of Vascular Anomalies (ISSVA, April 2014) and further additions were added in 2018 (ISSVA, May 2018).[1,2] Generally, vascular tumors are proliferative, while malformations enlarge through expansion of a developmental anomaly without underlying proliferation.
Growth and/or expansion of vascular anomalies can cause clinical problems such as disfigurement, chronic pain, recurrent infections, coagulopathies (thrombotic and hemorrhagic), organ dysfunction, and death. Individuals often experience progressive clinical symptoms with worsening quality of life.
In the past, limited treatment options were available and efficacy was not validated in prospective clinical trials. Historically, therapies consisted of interventional and surgical procedures used to palliate symptoms. New drugs such as propranolol and sirolimus are now available for the treatment of patients with complex conditions, and additional targeted therapies are in development. The first prospective clinical trial using propranolol for infantile hemangioma has been published, as well as the first prospective clinical trial that studied the effectiveness of sirolimus for complicated vascular anomalies.[3,4]
With a prevalence of 4% to 5%, infantile hemangiomas are the most common benign tumors of infancy. Other vascular tumors are rare. The classification of these tumors has been difficult, especially in the pediatric population, because of their rarity, unusual morphologic appearance, diverse clinical behavior, and the lack of independent stratification for pediatric tumors. In 2013, The World Health Organization (WHO) updated the classification of soft tissue vascular tumors. Pediatric tumors were not independently stratified and the terminology was mostly left unchanged, but the intermediate category of tumors was divided into locally aggressive and rarely metastasizing. The ISSVA classification of tumors is based on the WHO classification (refer to Tables 1 and 2) but the ISSVA classification uses more precise terminology and phenotypes that have been agreed upon by the members of ISSVA.
Table 1. 2013 World Health Organization Classification of Vascular Tumors
| Category | Vascular Tumor Type |
|---|---|
| Benign | Hemangioma |
| Epithelioid hemangioma | |
| Angiomatosis | |
| Lymphangioma | |
| Intermediate (locally aggressive) | Kaposiform hemangioendothelioma |
| Intermediate (rarely metastasizing) | Retiform hemangioendothelioma |
| Papillary intralymphatic angioendothelioma | |
| Composite hemangioendothelioma | |
| Kaposi sarcoma | |
| Malignant | Epithelioid hemangioendothelioma |
| Angiosarcoma of soft tissue |
aAdapted from Fletcher et al.[5]
Table 2. 2018 International Society for the Study of Vascular Anomalies (ISSVA) Classification of Vascular Tumorsa
| Category | Vascular Tumor Type (Causal Genes) |
|---|---|
| Benign (type 1b) | Infantile hemangioma/hemangioma of infancy |
| Congenital hemangioma (GNAQ/GNA11) | |
| —Rapidly involuting (RICH) | |
| —Non-involuting (NICH) | |
| —Partially-involuting (PICH) | |
| Tufted angiomac | |
| Spindle cell hemangioma (IDH1/IDH2) | |
| Epithelioid hemangioma (FOS) | |
| Pyogenic granuloma (also known as lobular capillary hemangioma) (BRAF/RAS/GNA14) | |
| Others | |
| Locally aggressive or borderline | Kaposiform hemangioendothelioma (KHE) (GNA14) |
| Retiform hemangioendothelioma | |
| Papillary intralymphatic angioendothelioma (PILA), Dabska tumor | |
| Composite hemangioendothelioma | |
| Pseudomyogenic hemangioendothelioma (FOSB) | |
| Polymorphous hemangioendothelioma | |
| Hemangioendothelioma not otherwise specified | |
| Kaposi sarcoma | |
| Others | |
| Malignant | Angiosarcoma (MYC: postradiation therapy) |
| Epithelioid hemangioendothelioma (EHE) (CAMTA1/TFE3) | |
| Others |
aAdapted from ISSVA Classification of Vascular Anomalies. ©2018 International Society for the Study of Vascular Anomalies. Available at "issva
.org/classification." Accessed June 2018.[1] bRefer to the ISSVA classification 2018 for benign vascular tumors 2.[1]
cTufted angioma and kaposiform hemangioendothelioma are a spectrum of the same entity and will be discussed together.
The quality of evidence regarding childhood vascular tumors is limited by retrospective data collection, small sample size, cohort selection and participation bias, and heterogeneity of the disorders.
References
- International Society for the Study of Vascular Anomalies: ISSVA Classification of Vascular Anomalies. Milwaukee, Wi: International Society for the Study of Vascular Anomalies, 2018. Available online. Last accessed June 17, 2020.
- Wassef M, Blei F, Adams D, et al.: Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 136 (1): e203-14, 2015. [PubMed: 26055853]
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al.: A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 372 (8): 735-46, 2015. [PubMed: 25693013]
- Adams DM, Trenor CC, Hammill AM, et al.: Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 137 (2): e20153257, 2016. [PMC free article: PMC4732362] [PubMed: 26783326]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al., eds.: WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press, 2013.
Benign Tumors
Benign vascular tumors include the following:
- Juvenile nasopharyngeal angiofibroma is not included in the World Health Organization classification or the International Society for the Study of Vascular Anomalies classification of vascular tumors. It is included here because growing evidence reveals vascular differentiation and proliferation in these tumors with response to vascular remodeling and antiproliferative agents.
Infantile Hemangioma
Incidence and epidemiology
Infantile hemangiomas (IH) are the most common benign vascular tumor of infancy, occurring in 4% to 5% of infants. The true incidence is unknown.[1] They are not usually present at birth and are diagnosed most commonly at age 3 to 6 weeks.[2-5] The lesion proliferates for an average of 5 months, stabilizes, and then involutes over several years.
Infantile hemangiomas are more common in females, non-Hispanic white patients, and premature infants. Multiple hemangiomas are more common in infants who are the product of multiple gestations.[5] Infantile hemangiomas are associated with advanced maternal age, placenta previa, pre-eclampsia, and other placental anomalies.
Biology
Most infantile hemangiomas occur sporadically. However, they may rarely be caused by an abnormality of chromosome 5 and present in an autosomal dominant pattern.[6] In a study that evaluated inheritance patterns of infantile hemangiomas, 34% of patients had a family history of infantile hemangioma, most commonly in a first-degree relative.[6,7]
The exact mechanism that causes the initial proliferation of blood vessels followed by involution of the vascular component of hemangioma and replacement of fibrofatty tissue is unknown.
Several cell types have been isolated from hemangiomas: progenitor/stem cells (HemSC), endothelial cells (HemEC), and pericytes (HemPericytes).[8] HemSC represent a small percentage of proliferating hemangioma cells and have the ability for self-renewal and multilineage differentiation. These cells differentiate into endothelial cells, adipocytes, and pericytes. When HemSC are implanted into immunodeficient mice, hemangioma-like lesions form and then spontaneously regress, similar to infantile hemangioma.[9] This suggests that infantile hemangioma proliferation occurs during vasculogenesis (the formation of new blood vessels from angioblasts), as opposed to angiogenesis (the formation of new blood vessels from existing blood vessels).[9]
In the proliferative phase, the HemEC are plump and metabolically active, resembling fetal endothelial cells. Evaluation of infantile hemangioma endothelial cells suggest that they are clonal in nature.[9-11]
HemPericytes surround the vasculature, are abundant in the proliferative phase, and express markers of pericytes and smooth muscle cells, such as neural-glial antigen 2 (NG2), platelet-derived growth factor receptor beta (PDGFR-beta), calponin, alpha smooth muscle actin (SMA), and NOTCH3. These cells are proangiogenic, as they express increased vascular endothelial growth factor A (VEGF-A), decreased angiopoietin-1 (ANGPT1), increased proliferation, increased vessel formation in vivo, and decreased ability to suppress proliferation.[12]
Mast cells are found largely in the early involuting phase. They are also found in small numbers in the proliferative phase and at the end of involution. Their role is unknown but they have been shown to play a role in other skin tumors such as basal cell carcinoma, squamous cell carcinoma, and melanoma.[13]
During proliferation, provasculogenic factors are expressed, such as VEGF, fibroblast growth factor (FGF), CD34, CD31, CD133, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), and insulin-like growth factor 2 (IGF-2).[14-17] During involution, infantile hemangiomas express increased apoptosis.[17] During this phase, there are also increased mast cells and levels of metalloproteinase, as well as upregulation of interferon and decreased basic FGF (bFGF).[17-19] Throughout proliferation and involution, endothelial cells in infantile hemangioma express a particular phenotype showing positive staining for GLUT1 and placenta-associated antigens (Fc-gamma receptor II, merosin, Lewis Y antigen). These markers are absent in normal capillaries and in other vascular tumors such as congenital hemangioma and vascular malformations. Placental chorionic villi share these same markers; however, no relationship between hemangiomas and placental chorionic villi has been found.[14]
Hypoxia appears to have a critical role in the pathogenesis of hemangiomas. There is an association of hemangiomas with placental hypoxia, which is increased in prematurity, multiple pregnancies, and placental anomalies.[2,5] Multiple targets of hypoxia [20,21] are demonstrated in proliferating hemangiomas, including VEGF-A, GLUT1, and IGF-2.[14,16,22] The hypothesis suggests that a proliferating hemangioma is an attempt to normalize hypoxic tissue that occurred in utero.
Clinical presentation
Most infantile hemangiomas are not present at birth but precursor lesions such as telangiectasia or faint discoloration of the skin or hypopigmentation can often be seen. The lesion can be mistaken as a bruise from birth trauma or as a capillary malformation (port-wine stain) (refer to Figure 1).[23,24]
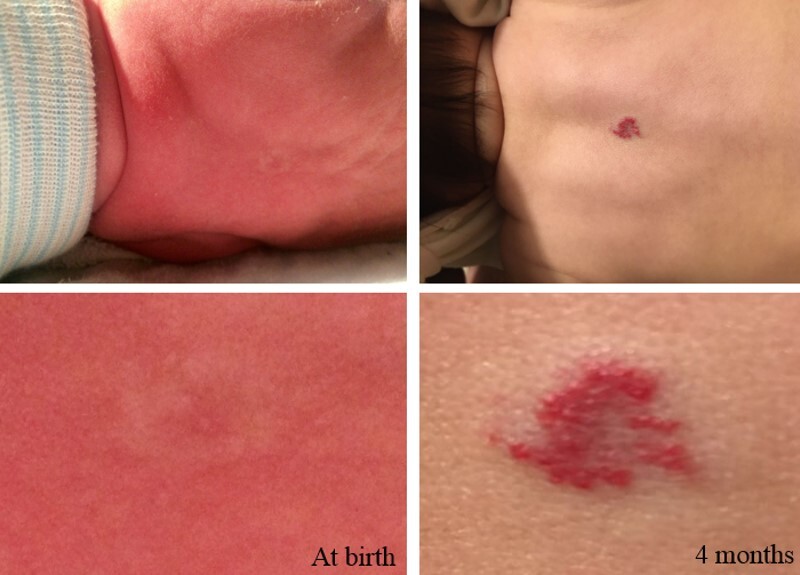
Figure 1. The photos on the left depict the precursor lesion (faint color with halo). The photos on the right depict the hemangioma after proliferation (slightly raised with a brighter central color). Credit: Israel Fernandez-Pineda, M.D.
Infantile hemangiomas can be superficial in the dermis, deep in the subcutaneous tissue, combined, or in the viscera. Combined lesions are common. They are most common in the head and neck but can be anywhere on the body.
Infantile hemangioma can be characterized as follows:
- Local: Most lesions are localized and noted to be in a well-defined area without evidence of a geometric pattern.
- Segmental: Most segmental hemangiomas occur in the head and neck region but can be seen in the genitourinary area, arm, chest, or legs. Diffuse hemangiomas of the face demonstrate defined cutaneous patterns. Several studies have evaluated the distributions of these hemangiomas and found the following four distinct patterns or segments:
- Segment 1 involves the lateral forehead, anterior temporal scalp, and the lateral frontal scalp.
- Segments 2 and 3 are located over the maxillary and mandibular area.
- Segment 4 covers the medial frontal scalp, nose, and philtrum.
Two papers have noted this observation and suggest the involvement of neural crest derivatives in facial hemangioma development.[25,26] Segmental hemangiomas commonly occur in females and are more likely associated with complications and other syndromes.[27,28] (Refer to the Syndromes associated with infantile hemangioma section of this summary for information about PHACE syndrome.) - Multiple: More than one lesion, but noted in the past as greater than five lesions, because of the increased risk of visceral involvement (mostly the liver). Usually characterized by solitary localized lesions.
The cutaneous appearance of infantile hemangiomas is usually red to crimson, firm, and warm in the proliferative phase. The lesion then lightens centrally and becomes less warm and softer; it then flattens and loses its color. The process of involution can take several years and once involution has occurred, regrowth is uncommon. In two patients treated with growth hormone, regrowth after involution was noted.[29] On further investigation, growth hormone receptors were found on the infantile hemangioma cells. Although preliminary, this may advance the research into the etiology of hemangioma growth.
Permanent sequelae, such as telangiectasia, anetodermal skin, redundant skin, and a persistent superficial component, can occur after hemangioma involution (refer to Figure 2). In a retrospective cohort study of 184 hemangiomas, the overall incidence of significant sequelae was 54.9%. Sequelae were more common in combined hemangiomas, hemangiomas with a step or abrupt border, and cobblestone surface hemangiomas. Furthermore, this study revealed that the average age to hemangioma involution was 3.5 years.[30]
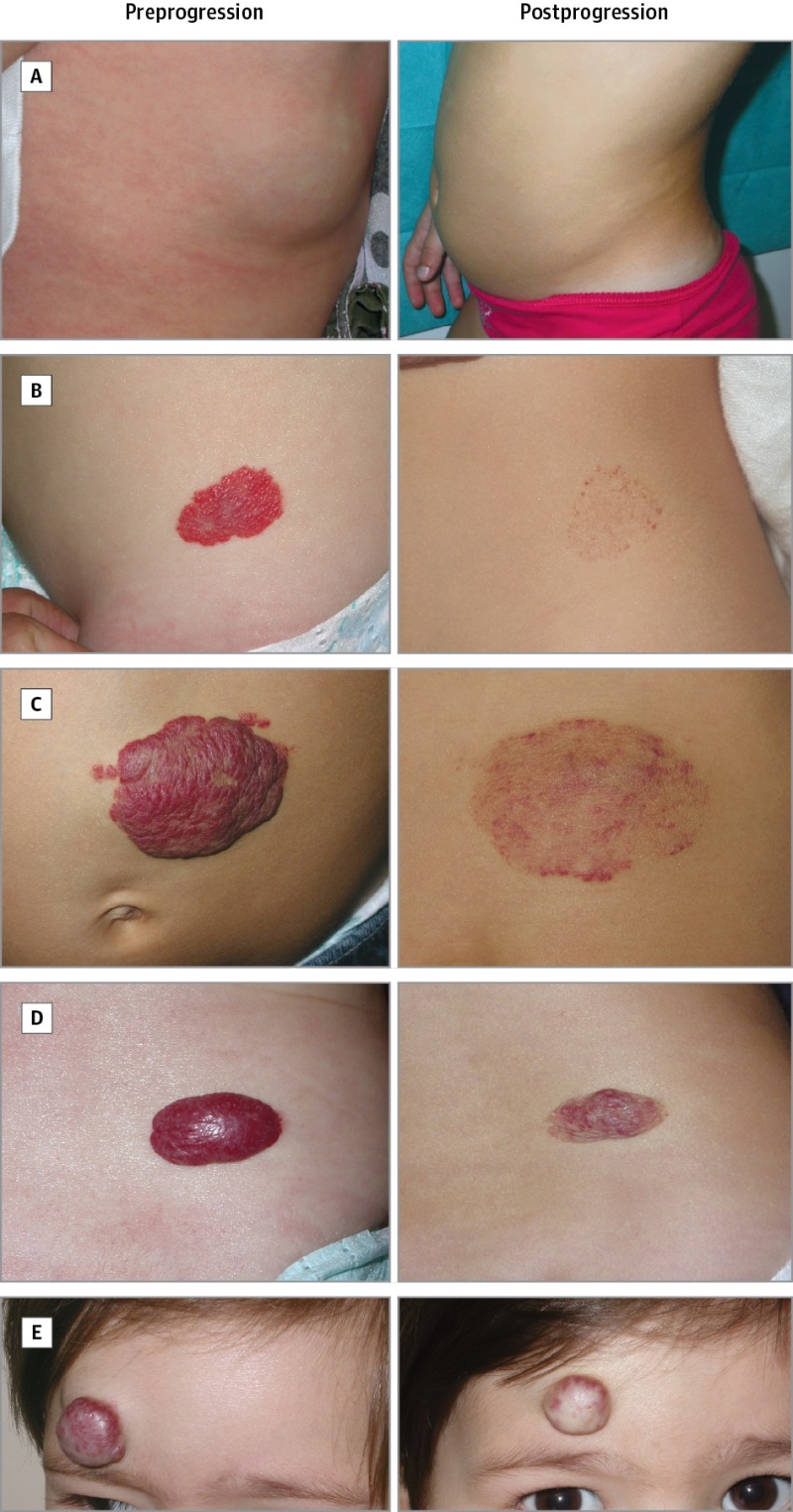
Figure 2. Examples of different types of sequelae. A, deep hemangioma that regressed without sequelae; B, superficial hemangioma that left only telangiectasia; C, mixed hemangioma that left anetodermic skin; D, mixed hemangioma that left redundant skin; and E, mixed hemangioma that left fibrofatty tissue. Reproduced with permission from JAMA Dermatology. 2016. 152 (11): 1239–1243. Copyright © (2016) American Medical Association. All rights reserved.
Diagnostic and staging evaluation
Infantile hemangiomas are usually diagnosed by the history and clinical appearance. Biopsy is rarely needed and performed only if there is an atypical appearance and/or atypical history and presentation. Imaging is not usually necessary, but if there is a deeper lesion without a cutaneous component, ultrasonography is beneficial for diagnosis because it reveals a well-circumscribed, hypoechoic, high flow lesion with a typical Doppler wave characteristic.[31] Additionally, infants with five or more cutaneous hemangiomas should undergo ultrasonography of the liver to scan for hepatic hemangioma.[32]
Infantile hemangioma with minimal or arrested growth
Infantile hemangioma with minimal or arrested growth (IH-MAG) is a variant of hemangioma that can be confused with capillary malformation because of their unusual characteristics. These hemangiomas are mostly fully formed at birth and are characterized by telangiectasia and venules with light and dark areas of skin coloration (refer to Figure 3). They resolve spontaneously and are pathologically GLUT1 positive.[33] They are mainly located on the lower body but can be present in the head and neck area; if they are segmental, they can be associated with PHACE syndrome.[34] Associated soft tissue hypertrophy may persist through childhood.[35]
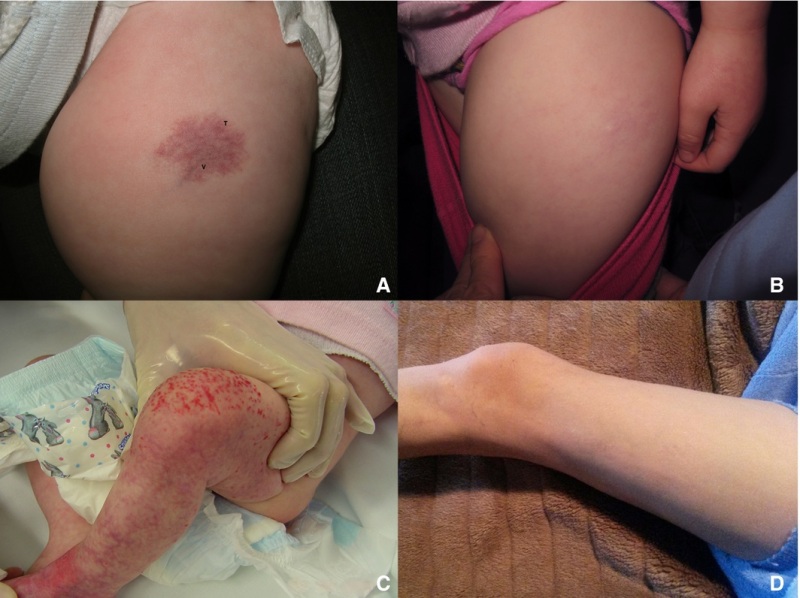
Figure 3. Patient 4 at (A) presentation and (B) resolution. Patient 5 at (C) presentation and (D) resolution. Ma, E. H., Robertson, S. J., Chow, C. W., and Bekhor, P. S. (2017), Infantile Hemangioma with Minimal or Arrested Growth: Further Observations on Clinical and Histopathologic Findings of this Unique but Underrecognized Entity. Pediatr Dermatol, 34: 64–71. doi:10.1111/pde.13022. Used with permission.
Airway infantile hemangioma
Airway infantile hemangiomas are usually associated with segmental hemangiomas in a bearded distribution, which may include all or some of the following—the preauricular skin, mandible, lower lip, chin, or anterior neck. It is important for an otolaryngologist to proactively assess lesions in this distribution before signs of stridor occur. The incidence of an airway infantile hemangioma increases with increased area of bearded involvement.[36] Airway infantile hemangioma can occur without skin lesions. A retrospective study of the Vascular Anomaly Database at the Children's Hospital of Pittsburgh analyzed 761 cases of infantile hemangioma. Thirteen patients (1.7%) had subglottic hemangiomas; of those 13 patients, 4 patients (30%) had bearded distributions, 2 patients (15%) had cutaneous hemangiomas, and 7 patients (55%) had no cutaneous lesions.[37] (Refer to the Propranolol therapy section of this summary for information about the treatment of airway infantile hemangiomas.)
Ophthalmologic involvement of hemangiomas
Periorbital hemangiomas can cause visual compromise.[38] This usually occurs with hemangiomas of the upper medial eyelid but any hemangioma around the eye that is large enough can distort the cornea or obstruct the visual axis. The clinician should be aware of subcutaneous periocular hemangiomas, as these lesions can extend into the orbit, causing exophthalmos or globe displacement with only limited cutaneous manifestations. Issues with these lesions include astigmatism from direct pressure of the growing hemangioma, ptosis, proptosis, and strabismus. One of the leading causes of preventable blindness in children is stimulus-deprivation amblyopia caused by hemangioma obstruction. All periorbital hemangiomas or those with any possibility of potential visual impairment should have an ophthalmologic evaluation.
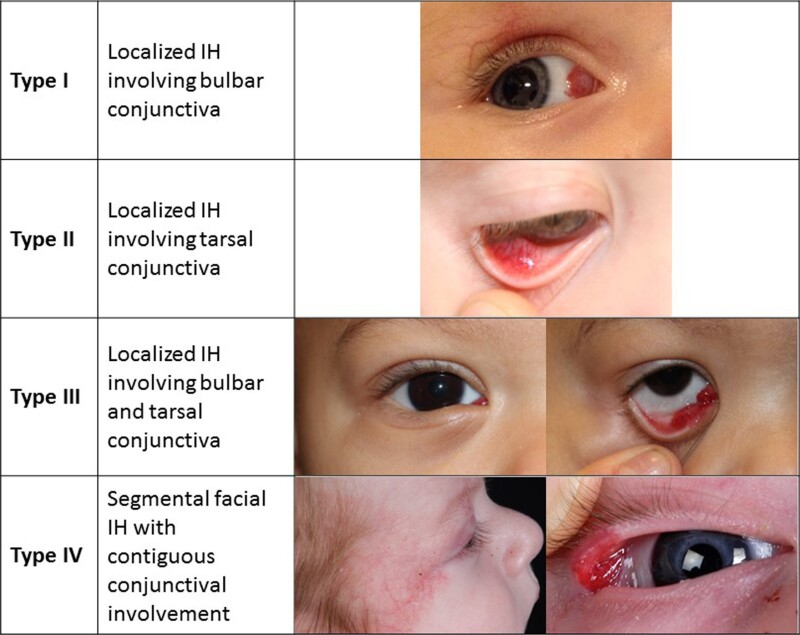
Infantile hemangiomas can occur in the conjunctiva (refer to Figure 4). These hemangiomas can be associated with other ophthalmologic abnormalities and are treated with oral or topical beta-blockers.[39]
Figure 4. Proposed classification of infantile hemangiomas involving the conjunctiva. Theiler M, Baselga E, Gerth-Kahlert C, et al. Infantile hemangiomas with conjunctival involvement: An underreported occurrence. Pediatr Dermatol. 2017;34:681–685. https://doi.org/10.1111/pde.13305 Copyright © 2017 John Wiley & Sons, Inc.
Syndromes associated with infantile hemangioma
Syndromes associated with infantile hemangioma include the following:
- Posterior fossa–brain malformations; Hemangiomas; Arterial, Cardiac, and Eye abnormalities (PHACE) syndrome: PHACE syndrome represents a spectrum of diseases and is defined by the presence of a large segmental infantile hemangioma, usually on the face or head, but can include the neck, chest, or arm, in association with one or more congenital malformations (refer to Figure 5).[40] PHACE syndrome is more common in girls and in full-term, normal birth weight and singleton infants.[28,41-45] The syndrome is not rare among patients with infantile hemangiomas. A prospective study of 108 infants with large facial hemangiomas observed that 31% of patients had PHACE syndrome.[46]
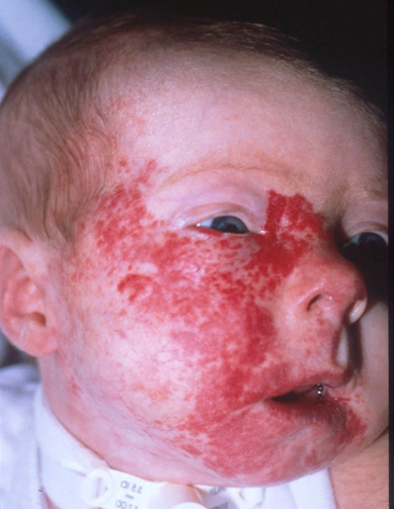
Figure 5. A large segmental infantile hemangioma (plaque-like) in a bearded distribution. This patient has an increased risk of PHACE syndrome, airway infantile hemangioma, and ulceration. A tracheostomy was placed secondary to a very diffuse airway hemangioma. Credit: Denise Adams, M.D. Garzon MC, Epstein LG, Heyer GL, et al.: PHACE Syndrome: Consensus-Derived Diagnosis and Care Recommendations. J Pediatr 178: 24-33.e2, 2016. PMID: 27659028
Consensus criteria for definite and possible PHACE syndrome were updated at an expert panel meeting, as follows:[40]PHACE
- -
Posterior fossa abnormalities. Anomalies include posterior fossa malformations, including Dandy-Walker complex, cerebellar hypoplasia, atrophy, and dysgenesis/agenesis of the vermis. Effects of these anomalies include developmental delays and pituitary dysfunction.[47]
- -
- A large segmental hemangioma over the face or scalp with a surface area of 22 cm2 or greater (5 cm × 4.5 cm).
- One major criterion of PHACE is a large segmental hemangioma of the neck, upper trunk or trunk, and proximal upper extremity.
Infants with two major criteria of PHACE (e.g., supraumbilical raphe and coarctation of the aorta) but lacking cutaneous infantile hemangioma should undergo complete evaluation for PHACE.
- -
Arterial abnormalities. Cerebrovascular anomalies can include carotid artery abnormalities (including tortuosity) and absence, dilation/aneurysm, or narrowing of cerebral vessels. These anomalies, especially the carotid anomalies, can lead to progressive arterial occlusion and even stroke. The risk categories are as follows:[44,45,52-54]
- Low risk: Arterial anomalies frequently seen in a general screening population. It also includes findings that have either no or very minimal clinical impact on patient outcome, even if rarely seen in the general population. Examples are persistent embryonic arteries, anomalous arterial origin or course, and circle of Willis variants.
- Intermediate risk: Includes patients with nonstenotic dysgenesis, including patients with ectatic or segmentally enlarged arteries. It also includes patients with a narrowing or occlusion of arteries proximal to the circle of Willis, with no perceived hemodynamic risk. An evaluation of the patency of the circle of Willis is essential.
- High risk: This category includes patients with one or more of the following:
- -
Significant narrowing (>25%) or occlusion of principal cerebral vessels within or above the circle of Willis that results in an isolated circulation.
- -
Tandem or multiple arterial stenoses associated with complex blood flow that may potentially result in diminished cerebral perfusion. Patients with cerebrovascular stenosis in the setting of coarctation of the aorta are likely at higher risk of transient and permanent neurologic ischemic events.
- -
Imaging findings in the brain parenchyma suggestive of chronic or silent ischemia, or progressive steno-occlusive disease. These parenchymal brain magnetic resonance imaging (MRI) findings include existing infarction, chronic or border zone ischemic changes, and presence of lenticulostriate collateral dilation or pial collaterals.
- -
Cardiac abnormalities.
- Aortic arch anomalies observed in PHACE syndrome are unusually complex, with involvement of the transverse and descending aorta arch. The arch obstruction is most often long-segment. The obstruction is frequently characterized by areas of arch narrowing with adjacent segments of marked aneurysmal dilatation.
- -
Eye abnormalities. Ophthalmologic anomalies can include microphthalmos, retinal vascular abnormalities, persistent fetal retinal vessels, exophthalmos, coloboma, and optic nerve atrophy. These abnormalities are rare and occur in 7% to 10% of patients.[55]
Diagnosis of PHACE requires clinical examination, cardiac evaluation with echocardiogram, ophthalmologic evaluation, and MRI/magnetic resonance angiogram (MRA) of the head and neck. All patients with intermediate-risk and high-risk central nervous system (CNS) findings should be monitored by a neurologist. Coarctation of the aorta requires immediate cardiology consultation and a cardiac MRI/MRA may be warranted. Patients need to be monitored for short-term and long-term effects. There are specific recommendations for follow-up imaging depending on risk category.[40]Other issues include speech and language delay, dysphagia, hearing loss (conductive and sensorineural), early-onset migraines, endocrine abnormalities, dental anomalies, and psychological issues.[56-58]A report of two patients with retro-orbital infantile hemangioma and arteriopathy suggested a possible new presentation of PHACE syndrome.[51] For patients with proptosis, globe deviation, and strabismus, an MRI/MRA is recommended. Further workup for PHACE may be needed on the basis of CNS findings. - LUMBAR/PELVIS/SACRAL syndrome: Infantile hemangiomas located over the lumbar or sacral spine may be associated with genitourinary, anorectal anomalies, or neurological issues such as tethered cord.[59-62] The following criteria have been used to describe segmental infantile hemangioma syndrome in the lumbar, pelvic, and sacral areas. This syndrome has been described in the literature using several acronyms.
LUMBAR
- -
Lower-body hemangioma and other cutaneous defects.
- -
Urogenital anomalies or ulceration.
- -
Myelopathy.
- -
Bony deformities.
- -
Anorectal malformations or arterial anomalies.
- -
Renal anomalies.
PELVIS
- -
Perineal hemangioma.
- -
External genital malformations.
- -
Lipomyelomeningocele.
- -
Vesicorenal abnormalities.
- -
Imperforate anus.
- -
Skin tag.
SACRAL
- -
Spinal dysraphism.
- -
Anogenital.
- -
Cutaneous.
- -
Renal and urologic anomalies Associated with an angioma of Lumbosacral localization.
Multiple hemangiomas
Infants with more than five infantile hemangiomas need to be evaluated for visceral hemangiomas. The most common site of involvement is the liver, in which multiple or diffuse lesions can be noted.[65,66] Often these lesions are asymptomatic, but in a minority of cases, symptoms such as heart failure secondary to large vessel shunts, compartment syndrome, or profound hypothyroidism can occur because of the expression of iodothyronine deiodinase by the hemangioma cells.[67] Multiple or diffuse liver hemangiomas can occur in the absence of skin lesions. (Refer to the Benign Vascular Tumors of the Liver section of this summary for more information.) Other rare potential complications of visceral hemangiomas, dependent on specific organ involvement, include gastrointestinal hemorrhage, obstructive jaundice, and CNS sequelae, caused by mass effects.
Treatment of infantile hemangioma
The decision to treat patients with hemangiomas is based on several factors such as the size of the lesions, type of hemangioma, location, presence or risk of complications, possibility of scarring or disfigurement, the age of the patient, and the stage of growth of the hemangioma. This is individualized among patients, and careful considerations of the risks and benefits of treatment are important.
The American Academy of Pediatrics has published clinical practice guidelines. An early therapeutic intervention was noted to be critical for complicated infantile hemangiomas to prevent medical complications and permanent disfigurement. The timing of interventions was noted to be best in the first 1 to 3 months of age. Photos have been used to triage low-risk versus high-risk infantile hemangiomas,[68] and a scoring system has been used for primary care physicians to encourage early referral to hemangioma specialists.[69] The guidelines specified hemangioma specialists as those practitioners with expertise in the management and care of hemangiomas who have knowledge of risk stratification and treatment options. These providers consisted of experts in the fields of dermatology, hematology/oncology, pediatrics, plastic surgery, general surgery, otolaryngology, and ophthalmology.[70]
Treatment options for infantile hemangioma include the following:
- Pulsed dye laser therapy. Usually reserved for ulcerated infantile hemangiomas and residual lesions, such as telangiectasia after the proliferative period.[71] Pulsed dye laser therapy helps with pain from ulcerative infantile hemangiomas. The use of pulsed dye laser therapy as upfront treatment for infantile hemangiomas is controversial.
- Excisional surgery. With the advent of new medical treatments, the use of surgery is reserved for ulcerated lesions, residual lesions, large periocular lesions that interfere with vision, and facial lesions with aesthetic impact that do not respond to medical therapy.[72]
Propranolol therapy
Propranolol, a nonselective beta-blocker, is the first-line therapy for infantile hemangiomas. Potential mechanisms of action include vasoconstriction and/or decreased expression of VEGF and bFGF, leading to apoptosis.[73,74] Specific mechanisms of action are under investigation. Two studies suggested that the activity of propranolol on hemangiomas is not secondary to beta blockade but may be related to the ability of the R+ enantiomer of propranolol to inhibit endothelial cell markers through inhibition of SOX18 or the down regulation of other genes, such as ANGPT2.[75,76]
The use of propranolol was first noted in two infants treated for cardiac issues in Europe. A change in color, softening, and decrease in hemangioma size was noted. Since that time, the results of a randomized controlled trial have been reported.[77] In 2014, the U.S. Food and Drug Administration (FDA) approved the drug propranolol hydrochloride for the treatment of proliferating infantile hemangioma.
There are many other published reports about the efficacy and safety of propranolol.[78-82] Lack of response to treatment is rare. Propranolol therapy is usually used during the proliferative phase but has been effective in patients older than 12 months with infantile hemangiomas.[83]
Evidence (propranolol therapy):
- In a large industry-sponsored randomized trial, 456 infants aged 5 weeks to 5 months with a proliferating infantile hemangioma of at least 1.5 cm received either a placebo or propranolol (1 mg/kg per day or 3 mg/kg per day) for 3 or 6 months. After interim analysis of the first 188 patients who completed 24 weeks of trial treatment, the regimen of 3 mg/kg per day for 6 months was selected for the final efficacy analysis.[77][Level of evidence: 1iDiv]
- Of patients who received the selected regimen, 88% showed improvement by week 5, compared with 5% of patients who received the placebo.
- Adverse events occurred infrequently.
- In 635 infants with infantile hemangioma, the overall response rate was 91% after 2 mg/kg per day, with most patients showing regression and only 2% with side effects, none of which were severe.[82][Level of evidence: 3iiiDiv]
- A meta-analysis that evaluated 5,130 patients from 61 studies concluded that propranolol was more effective and safer than were other treatments for infantile hemangioma.[84]
- Airway infantile hemangioma lesions are rare; thus, there are limited prospective studies. A meta-analysis of 61 patients noted a trend of decreased treatment failure with increased dosing strategies, which is consistent with the use of higher doses of propranolol in these patients (3 mg/kg/day). The analysis also suggested that the concurrent use of steroids and propranolol may have reduced efficacy in patients with segmental airway hemangiomas, but previous treatment with steroids had no deleterious effect.[85] Additional prospective studies are needed to validate these findings.
Several expert consensus panel recommendations have been reported including recommendations from the FDA and the European Medicines Agency after a randomized controlled trial of oral propranolol in infantile hemangioma patients led to FDA approval.[86-88] Intralesional administration of propranolol has been used for periorbital lesions in a limited capacity and showed no advantages over oral administration.[89][Level of evidence: 1iiDiv]
A large retrospective multicenter study assessed the safety of outpatient administration of propranolol and evaluated the need for monitoring. In this study, 783 patients with 1,148 office visits were evaluated. No symptomatic bradycardia or hypotension was noted. Blood pressure evaluation was unreliable. The results suggested that outpatient evaluation may not be necessary for standard-risk patients with infantile hemangioma.[90]
Consensus panel recommendations include the following:[86,88,91]
- Initiation of treatment: Treatment should be undertaken in consultation with a pediatric vascular anomaly specialist with expertise in the diagnosis and treatment of pediatric vascular tumors and in the use of propranolol in children. An expert consensus panel suggested that hospitalization for initiation of oral propranolol be considered in the following circumstances:[86]
- -
Infant aged 4 weeks or younger (corrected for gestational age).
- -
Infant of any age with inadequate social support.
- -
Infant of any age with comorbid conditions affecting the cardiovascular or respiratory system, including symptomatic airway infantile hemangiomas.
- -
Infant of any age with conditions affecting blood glucose maintenance.
The pretreatment evaluation (inpatient or outpatient) includes the following:- -
History, with focus on cardiovascular and respiratory abnormalities (e.g., poor feeding, dyspnea, tachypnea, diaphoresis, wheezing, heart murmur) and family history of heart block or arrhythmia.
- -
Physical examination including cardiac and pulmonary assessment and measurement of heart rate.
- -
No need for echocardiogram or electrocardiogram for standard-risk patients. Two studies found no contraindication to beta-blocker therapy in 6.5% to 25% of patients who had electrocardiogram abnormalities.[91,92] Electrocardiogram should be considered in children with heart rate lower than normal for age and history of arrhythmia or arrhythmia detected during examination.
- -
Family history of congenital heart disease or maternal history of connective tissue disease.
- Dosing: The dosing used is generally 1 mg/kg per day to 3 mg/kg per day divided into two or three doses. Starting dose varies depending on risk factors and location of initiation. Outpatients and inpatients are initially started at a dose of 0.5 mg/kg per day to 1 mg/kg per day and increased over time.[87,88,91] Initially, dosing of three times per day is recommended for infants younger than 5 weeks and for patients with PHACE syndrome.[40,86]
- Monitoring: Monitoring varies depending on the institution. However, oral propranolol peaks at 1 to 3 hours after administration and most centers measure heart rate and blood pressure 1 and 2 hours after each dose with initiation and then when the dose is increased by at least 0.5 mg/kg per day. Parent and patient education includes when to hold the medication, signs of hypoglycemia, feeding necessity through the night, and when to call the physician with issues, such as illness, that may interfere with oral intake or lead to dehydration or respiratory problems.
- Contraindications: Propranolol treatment is contraindicated in infants and children with the following:
- -
Sinus bradycardia.
- -
Hypotension.
- -
Heart block greater than first degree.
- -
Heart failure.
- -
Asthma.
- -
Hypersensitivity.
- -
PHACE syndrome. PHACE syndrome with CNS arterial disease and/or coarctation of the aorta may be a relative contraindication. A decision to treat should be made in consultation with neurology and cardiology.
- Adverse effects: Adverse effects of propranolol include the following:[93]
- -
Hypoglycemia.
- -
Hypotension.
- -
Bradycardia.
- -
Sleep disturbance.
- -
Diarrhea/constipation.
- -
Cold extremities.
These complications have been reported in several studies, and severe complications have been rare.[93,94] The risk of these complications is increased in patients with comorbidities and concomitant diseases, including diarrhea, vomiting, and respiratory infections. The need for close monitoring and possible periods of drug discontinuation should be considered during periods of illness.A retrospective review of 1,260 children with infantile hemangiomas who were treated with propranolol identified 26 patients (2.1%) with side effects that required discontinuation of propranolol.[95] Severe sleep disturbance was the most common reason for propranolol cessation, accounting for 65.4% of cases. In total, 23 patients received atenolol and 3 patients received prednisolone as second-line therapy. In the multivariate analysis, only younger age (95% confidence interval [CI], 1.201–2.793; P = .009) and lower body weight (95% CI, 1.036–1.972; P = .014) were associated with intolerable side effects. - Duration of treatment: There are no consensus guidelines for the treatment duration of propranolol. In a prospective, multi-institutional study that assessed efficacy and safety of propranolol in high-risk patients, the administration of propranolol for a minimum of 6 months, up to a maximum of age 12 months, increased treatment success; dosing of propranolol was 3 mg/kg per day. Treatment results were sustained for up to 3 months after discontinuation of therapy. Efficacy and safety of propranolol in this study were similar to those reported in other studies.[96]
- Rebound growth after propranolol therapy: Rebound refers to the growth of infantile hemangiomas after propranolol cessation. A multi-institutional, retrospective review of 997 patients with infantile hemangiomas found a rebound rate of 25.3% in 912 patients with adequate data. On univariate analysis, the factors associated with rebound included discontinuation of treatment before age 9 months, female sex, location on head/neck, segmental pattern, and deep or mixed skin involvement. On multivariate analysis, only deep infantile hemangiomas and female sex were significantly related.[97]
- Late growth of infantile hemangiomas: Growth of a hemangioma can occur in patients older than 3 years, and growth as late as age 8.5 years has been reported. Associated risk factors include segmental morphology, large hemangiomas, PHACE syndrome, and deep cutaneous and subcutaneous lesions in the head and neck.[98,99]
Selective beta-blocker therapy
Because of the nonselective and lipophilic nature of propranolol with the ability to cross the blood-brain barrier, other beta-blockers are being used for the treatment of infantile hemangiomas. In two small comparison studies, there was no difference in efficacy between propranolol and atenolol.[100,101] In a retrospective study using nadolol, similar results were seen.[102] A prospective study of 76 infants treated with atenolol noted efficacy and safety similar to propranolol.[103][Level of evidence: 3iiDiv] Additional studies are needed to assess differences between the toxicities of these agents and the toxicities of propranolol.
There is some suggestion that the more selective beta-blockers have fewer side effects.[104] A study has suggested that the R(+) enantiomer of propranolol, carried over in drug synthesis rather than the anti–beta-adrenergic L(-) enantiomer (commercially available drug is a racemic mixture), may carry the therapeutic anti-infantile hemangioma effect.[75,76]
Corticosteroid therapy
Before propranolol, corticosteroids were the first line of treatment for infantile hemangiomas. They were first used in the late 1950s but were never approved by the U.S. FDA. Corticosteroid therapy has become less popular secondary to the acute and long-term side effects of steroids (gastrointestinal irritability, immunosuppression, adrenocortical suppression, cushingoid features, and growth failure).
Corticosteroids (prednisone or methylprednisolone) are used at times when there is a contraindication to beta-blocker therapy or as initial treatment while a patient is started on beta-blocker therapy.[105]
Topical beta-blocker therapy
Topical beta-blockers are used mainly for the treatment of small, localized, superficial hemangiomas as an alternative to observation. They have also been used in combination with systemic therapy in complicated hemangiomas or to prevent rebound in a hemangioma being tapered off of systemic treatment.[106-108] The same precautions (assessment of comorbidities and family history), as noted previously for propranolol, should be followed for topical beta-blockers. Systemic absorption (plasma and urine) of timolol is variable and prescreening for normal cardiac, pulmonary, and endocrine issues are essential, as well as a recent medical history and physical examination. Cautious administration is necessary for ulcerated and deep hemangiomas because higher plasma concentrations of timolol can be seen.[109,110]
The topical timolol that is used is the ophthalmic gel-forming solution 0.5%. One drop is applied to the hemangioma two times per day until stable response is achieved.
This treatment has limited side effects, but infants with a postmenstrual age of younger than 44 weeks and weight at treatment initiation of less than 2,500 grams may be at risk of adverse events, including bradycardia, hypotension, apnea, and hypothermia.[110,111] Close monitoring of temperature, blood pressure, and heart rate in premature and low birth weight infants with infantile hemangiomas at initiation of and during therapy with topical timolol is necessary.
Evidence (topical timolol therapy):
- In a multicenter, retrospective, cohort study, 731 children with predominantly superficial hemangiomas were treated with topical timolol 0.5% twice daily. Ninety-two percent of patients showed significant improvement in color, and 77% of patients showed improvement in size, extent, and volume. Topical timolol is generally well tolerated. However, data on its safety are limited.[108]
Combined therapy for complicated hemangiomas
Combined therapy is considered either at initiation of treatment in complicated lesions in which there is functional impairment or organ compromise or used at the end of systemic therapy to prevent regrowth of the hemangioma rebound. Further investigation of efficacy and safety is needed for these regimens.
Evidence (combined therapy for complicated hemangiomas):
- A prospective randomized study that compared propranolol and 2 weeks of steroid therapy with propranolol alone revealed a decrease in the size of the hemangioma at 2, 4, and 8 weeks but no statistical difference in the size at 6 months.[112]
- A prospective randomized study that compared timolol and propranolol with propranolol alone reported a decrease in color of the infantile hemangioma in the timolol group but no difference in overall size of the infantile hemangioma between the two treatment groups.[113]
- Topical therapy with timolol combined with oral propranolol has been used.[114,115][Level of evidence: 3iiDiv]
Treatment options under clinical evaluation for infantile hemangiomas
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
In response to the COVID-19 pandemic, the Hemangioma Investigator Group is studying the administration of propranolol for low-risk and standard-risk patients through virtual visits.[116]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Congenital Hemangiomas
Congenital hemangiomas are benign vascular tumors that proliferate in utero. Development of these lesions is complete at birth. Histologically, these lesions are GLUT1 negative, unlike infantile hemangiomas. They are usually cutaneous, but can be found in the viscera. Complications include hemorrhage, transient heart failure, and transient coagulopathy.[117]
To the clinician unfamiliar with these lesions, congenital hemangiomas can be difficult to diagnose. Diagnostic criteria include a purpuric lesion fully formed at birth, frequently with a halo around the lesion, with high flow noted on ultrasound imaging. Essential to the diagnosis is observation of stability or decrease in size over time. These lesions do not enlarge unless there is hemorrhage into the tumor.
Somatic activating mutations of GNAQ and GNA11 have been found to be associated with congenital hemangiomas.[118] Additional research is necessary to assess the significance of these findings, as this may aid in diagnosis and pathophysiology.
Congenital hemangiomas are divided into the following three forms:
- Rapidly involuting congenital hemangiomas (RICH). These lesions are large high-flow lesions that are completely formed at birth but rapidly involute by 12 to 15 months. They can ulcerate and bleed and can cause transient heart failure and mild coagulopathy. After involution, usually some residual changes in the skin are present (refer to Figure 6).[119-122] In a retrospective case series of congenital hemangiomas, several high-risk ultrasound findings were noted for RICH. Venous lakes were associated with cardiac failure, and an increased risk of bleeding was noted with venous lakes and venous ectasia. Infants with RICH should be evaluated with ultrasonography and monitored closely if these high-risk features are noted.[123]
- Partial involuting congenital hemangiomas (PICH). These lesions are completely formed at birth and involute only partially.[124]
Benign Vascular Tumors of the Liver
In the literature, vascular liver tumors are usually classified as liver hemangioendotheliomas, a broad classification no longer in use. These tumors are classified according to their clinical characteristics and radiologic assessment.
Lesions are usually divided into the following three categories:[66]
On MRI, vascular liver tumors are hyperintense on T2 imaging and hypointense on T1 imaging, with postcontrast imaging demonstrating early peripheral enhancement with eventual diffuse enhancement.[66]
Focal vascular lesions (congenital hemangiomas)
Focal lesions of the liver are usually congenital hemangiomas (RICH or NICH) (refer to Figure 7). RICH can present with symptoms of heart failure and mild to moderate coagulopathy, but are typically detected by antenatal ultrasonography or as an asymptomatic mass in the newborn period.
Treatment options for focal vascular lesions include the following:
- Supportive management. Most lesions are asymptomatic and can be monitored through involution using ultrasonography.
- Embolization is considered for severe symptomatic shunting that is unresponsive to treatment for congestive heart failure. These procedures need to be performed by interventional radiologists with expertise in vascular anomalies.[127]
- Surgery. Patients with massive focal symptomatic hepatic congenital hemangioma unresponsive to supportive management or radiological intervention may be surgical candidates for resection. This is a rare circumstance and needs to be evaluated by an interdisciplinary vascular anomaly team. Indication for surgical removal includes rupture, bleeding, and nonresolving coagulopathy. Two patients were reported to require surgical resection after the development of clinically significant ascites as their RICH involuted.[128,129]
No medication has proven to be an effective treatment for these lesions, and infants need to be supported during this initial period until involution begins.[66] These lesions may be diagnosed prenatally. In rare situations, maternal treatment with medications such as steroids appeared to be effective but, more likely, natural involution may have been responsible.[130]
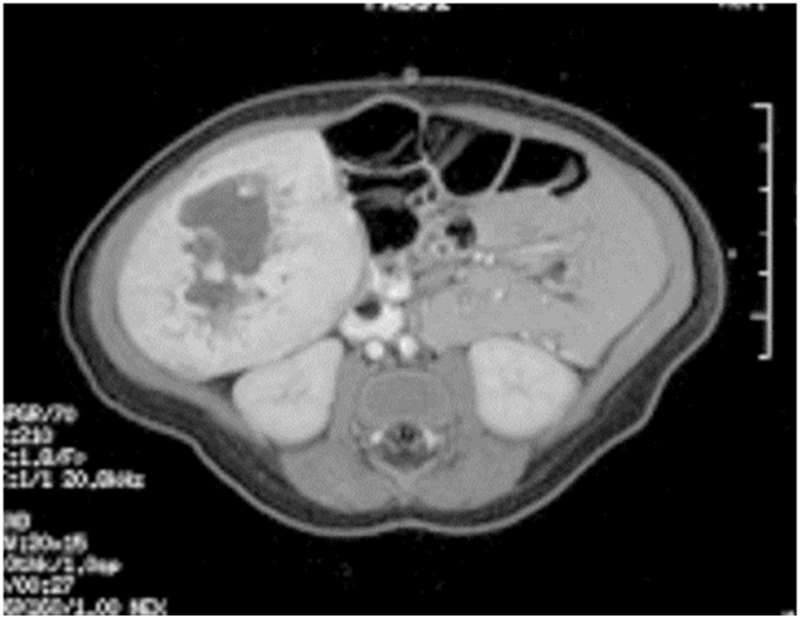
Figure 7. Single liver lesion (intrahepatic congenital hemangioma). MRI image of a congenital hemangioma. Note the central enhancement, which is typical for an intrahepatic congenital hemangioma. Credit: Denise Adams, M.D.
Multiple liver lesions (infantile hemangiomas)
Multifocal hepatic lesions are infantile hemangiomas. Multifocal lesions may not need to be treated if the patient is asymptomatic, and they typically follow the same proliferative and involution course as cutaneous hemangiomas.[66] These lesions are monitored closely and if there is growth, propranolol therapy should be considered. If propranolol is needed, doses of up to 2 mg/kg per day are effective.
Diffuse liver lesions (infantile hemangiomas)
Diffuse liver lesions are very serious (refer to Figure 8). Complications include hypothyroidism caused by the expression of iodothyronine deiodinase, high-output or congestive heart failure, and abdominal compartment syndrome.[65,66,131,132]
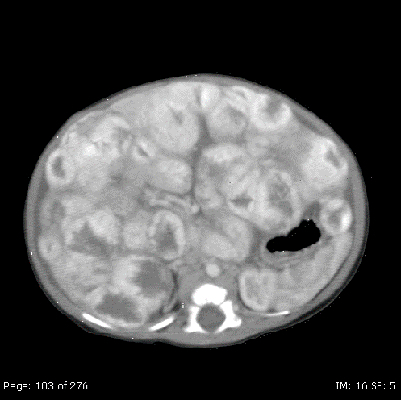
Figure 8. Diffuse liver lesions with classical imaging on CT. Note the peripheral enhancement in early contrast phase. Credit: Denise Adams, M.D.
Treatment options for diffuse liver lesions may include the following:
- Propranolol: Beta-blockers are the most common treatment for diffuse and some multifocal infantile hemangiomas of the liver. Treatment doses of 2 to 3 mg/kg per day are indicated.[77]
- Thyroid hormone replacement: Thyroid hormone replacement therapy must be aggressive if hypothyroidism is diagnosed; treatment with higher doses of hormones may be needed because the deficiency is caused by the aggressive consumption of the hormone by the tumor.[67]
- Transplant: If a patient does not respond to medical management, a transplant may be indicated.[135] Transplant is considered only for patients with severe diffuse lesions who have multisystem organ failure and there is insufficient time for effective pharmacologic therapy.
There have been isolated reports of malignancy in patients with diffuse hepatic infantile hemangiomas.[136] It is not clear if all cases were transformation of a benign lesion to a malignant phenotype; however, if the lesion does not respond to standard therapy, biopsy should be considered. Further evaluation and consensus is needed to assess whether these patients need to be monitored over a longer period of time with liver ultrasonography. (Refer to the Angiosarcoma of the Soft Tissue section of this summary for more information.)
The differential diagnosis of vascular liver lesions always includes malignant liver tumors; thus, alpha-fetoprotein (AFP) should be included in the initial lab work. AFP is very high in all newborns, but will rapidly fall to normal levels in several months. AFP levels should rapidly diminish, but failure to do so or a rising trend of AFP should elicit concern for hepatoblastoma. There are no prospective studies investigating AFP elevation in hemangioma.[137,138] Some hypervascular hepatoblastomas in neonates with congestive heart failure have been mistaken for infantile hemangiomas. Other tumors in the differential diagnosis include angiosarcoma, metastatic neuroblastoma, and mesenchymal hamartomas. If there is any question about the diagnosis, a biopsy is recommended, although bleeding is a risk of the procedure.[139]
Spindle Cell Hemangioma
Clinical presentation
Spindle cell hemangiomas, initially called spindle cell hemangioendotheliomas, often occur as superficial (skin and subcutis), painful lesions involving distal extremities in children and adults.[140,141] The tumors appear as red-brown or bluish lesions that can begin as a single nodule and develop into multifocal painful lesions over years. The lesions can be seen in Maffucci syndrome (cutaneous spindle cell hemangiomas occurring with cartilaginous tumors, enchondromas) and Klippel-Trénaunay syndrome (capillary/lymphatic/venous malformations), generalized lymphatic anomalies, lymphedema, and organized thrombus.[142,143] In Maffucci syndrome, spindle cell hemangiomas are associated with IDH1 or IDH2 mutations.[144]
These tumors are well circumscribed, occasionally contain phleboliths, and consist of cavernous blood spaces alternating with areas of nodular spindle cell proliferation. A significant percentage of spindle cell hemangiomas are completely intravascular. The vein containing the tumor is abnormal, as are blood vessels apart from the tumor mass.[142,143]
Epithelioid Hemangioma
Clinical presentation
Epithelioid hemangiomas (EH) are benign lesions that usually occur in the skin and subcutis but can occur in other areas such as the bone, with focal and multifocal lesions.[142,145] Epithelioid hemangiomas may be a reactive process, as they can be associated with local trauma and can develop in pregnancy. Patients usually present with local swelling and pain at the involved site. In the bone, they present as well-defined lytic lesions that involve the metaphysis and diaphysis of long bones.[142,146] They can have a mixed lytic and sclerotic pattern of bone destruction.
On pathologic evaluation, they have small caliber capillaries with eosinophilic, vacuolated cytoplasm and large oval, grooved, and lobulated nuclei. The endothelial cells are plump and are mature, well-formed vessels surrounded by multiple epithelioid endothelial cells within abundant cytoplasm. They lack cellular atypia and mitotic activity.[142,145-147] In 58 cases of epithelioid hemangiomas, 29% were found to have FOS gene rearrangements noted more in cellular epithelioid hemangiomas and intraosseous lesions compared with those in the skin, soft tissue, and head and neck. This genetic abnormality can be helpful in distinguishing epithelioid hemangiomas from other malignant epithelioid vascular tumors.[147]
A single-institution report reviewed 11 patients with epithelioid hemangiomas (median age, 14.4 years) who were diagnosed between 1999 and 2017. Lesions occurred in the lower extremities (five patients), skull (three patients), pelvis (two patients), and spine (one patient). Five patients had multifocal disease. Patients presented with localized pain and neurologic symptoms, including cranial nerve injury. No significant cytologic atypia was noted, and the endothelial cells were positive for CD31 and ERG, and negative for cytokeratin and CAMPTA1. Median follow-up was 1.5 years. Various modalities of treatments were used, including surgery, endovascular embolization, cryoablation, and medical management. One patient received sirolimus, and another patient received interferon; the lesions of both patients shrank within the first year of follow-up. The youngest patient, aged 2.5 years, had multifocal skull lesions that regressed partially by 1 year later without treatment.[148]
Pyogenic Granuloma (Lobular Capillary Hemangioma)
Clinical presentation
Pyogenic granulomas (PG), known as lobular capillary hemangiomas, are benign reactive lesions that can present at any age, including infancy, although it is most common in older children and young adults. They can present as single or multiple lesions.[149-152] These lesions can arise spontaneously, in sites of trauma, or within capillary and arteriovenous malformations. Pyogenic granulomas have also been associated with medications including oral contraceptives and retinoids. Most occur as solitary growths, but multiple (grouped) or rarely disseminated lesions have been described.[153] These lesions appear as small or large, smooth or lobulated vascular nodules that can grow rapidly, sometimes over weeks to months and have a tendency to bleed profusely. These lesions are usually cutaneous, but deep-seated/subcutaneous pyogenic granulomas have been reported and mimic other vascular lesions.[154]
Pyogenic granuloma can be associated with capillary malformations. The pathogenesis of pyogenic granulomas associated with capillary malformations and those that are sporadic are unknown. A study investigated ten patients with pyogenic granulomas arising from a capillary malformation and found eight with BRAF c.1799T>A mutations, one with an NRAS c.182A>G mutation, and one with a GNAQ c.548G>A mutation. This GNAQ mutation was also found in the underlying capillary malformation. In 25 patients with pyogenic granulomas and no capillary malformation, 3 patients had BRAF c.1799T>A mutations and 1 patient had a KRAS c.37G>C mutation. These genetic findings will help with future treatment modalities for this benign vascular tumor.[155]
Histologically, these lesions are composed of capillaries and venules with plump endothelial cells separated into lobules by fibromyxoid stroma. Some untreated lesions eventually atrophy, become fibromatous, and slowly regress.
Treatment of pyogenic granuloma
Full-thickness excision is the treatment with the lowest recurrence rate (around 3%),[156] but curettage, laser photocoagulation, or cryotherapy can also be used.[157] A small case series of four patients with acquired ocular surface pyogenic granulomas were treated with topical timolol 0.5% twice daily for 21 days. In all cases, complete resolution with no recurrence occurred for at least 3 months. More studies are needed to validate these findings.[158] A study of 22 patients with pyogenic granulomas who were treated with topical 1% propranolol ointment with occlusion found that 59% of patients achieved complete responses (mean, 66 days), 18% of patients had stable disease, and 22% of patients did not respond to the treatment.[159] In this study, only skin toxicity was assessed. The authors did not comment on the penetrance of the propranolol formulation or include a safety evaluation of the side effects such as hypoglycemia and the effects on heart rate or blood pressure.
Angiofibroma
Clinical presentation
Angiofibromas are rare, benign neoplasms in the pediatric population. Typically, they are cutaneous lesions associated with tuberous sclerosis, appearing as red papules on the face.
Treatment of angiofibroma
Excision of the tumor, laser treatments, and topical treatments, such as sirolimus, have been used.[160-162]
A prospective, randomized, placebo-controlled trial of sirolimus gel showed significant improvement in 60% of the patients assigned to receive sirolimus.[163] A second prospective, multicenter, randomized, double-blind, vehicle-controlled study with six monthly clinic visits enrolled 179 patients with tuberous sclerosis complex–related facial angiofibromas.[164] This study also showed a statistically and clinically significant improvement in patients treated with a topical formulation containing 0.3 g per 30 g (1%) of rapamycin.
Juvenile Nasopharyngeal Angiofibroma
Clinical presentation
Juvenile nasopharyngeal angiofibromas (JNA) account for 0.5% of all head and neck tumors.[165] While juvenile nasopharyngeal angiofibromas have not classically been included among vascular tumors, histologically, these tumors appear to be vascular tumors, with cells expressing vascular endothelial marker CD31, as well as VEGFA and VEGFR1. Despite their benign-appearing histology, juvenile nasopharyngeal angiofibromas can be locally destructive, spreading from the nasal cavity to the nasopharynx, paranasal sinuses, and orbit skull base, with intracranial extension. Some publications have suggested a hormonal influence on juvenile nasopharyngeal angiofibroma, with emphasis on the molecular mechanisms involved.[166,167]
Treatment of juvenile nasopharyngeal angiofibroma
Surgical excision is the treatment of choice but this can be challenging because of the extent of the lesion. A single-institution retrospective review of juvenile nasopharyngeal angiofibromas identified 37 patients with lateral extension.[168] Anterior lateral extension to the pterygopalatine fossa occurred in 36 patients (97%) and further to the infratemporal fossa in 20 patients (54%). In 16 patients (43%), posterior lateral spread was observed (posterior to the pterygoid process and/or between its plates). The recurrence rate was 29.7% (11 of 37 patients). The recurrence rate in patients with anterior and/or posterior lateral extension was significantly higher than in patients with anterior lateral extension only.
Juvenile nasopharyngeal angiofibromas have also been treated with radiation therapy, chemotherapy, alpha-interferon therapy, and sirolimus.[169-173]
References
- Kilcline C, Frieden IJ: Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol 25 (2): 168-73, 2008 Mar-Apr. [PubMed: 18429772]
- Munden A, Butschek R, Tom WL, et al.: Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol 170 (4): 907-13, 2014. [PMC free article: PMC4410180] [PubMed: 24641194]
- Darrow DH, Greene AK, Mancini AJ, et al.: Diagnosis and Management of Infantile Hemangioma. Pediatrics 136 (4): e1060-104, 2015. [PubMed: 26416931]
- Darrow DH, Greene AK, Mancini AJ, et al.: Diagnosis and Management of Infantile Hemangioma: Executive Summary. Pediatrics 136 (4): 786-91, 2015. [PubMed: 26416928]
- Haggstrom AN, Drolet BA, Baselga E, et al.: Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 150 (3): 291-4, 2007. [PubMed: 17307549]
- Blei F, Walter J, Orlow SJ, et al.: Familial segregation of hemangiomas and vascular malformations as an autosomal dominant trait. Arch Dermatol 134 (6): 718-22, 1998. [PubMed: 9645641]
- Castrén E, Salminen P, Vikkula M, et al.: Inheritance Patterns of Infantile Hemangioma. Pediatrics 138 (5): , 2016. [PubMed: 27940781]
- Greenberger S, Bischoff J: Pathogenesis of infantile haemangioma. Br J Dermatol 169 (1): 12-9, 2013. [PMC free article: PMC3707963] [PubMed: 23668474]
- Khan ZA, Boscolo E, Picard A, et al.: Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest 118 (7): 2592-9, 2008. [PMC free article: PMC2413184] [PubMed: 18535669]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PMC free article: PMC208946] [PubMed: 11254674]
- Yu Y, Flint AF, Mulliken JB, et al.: Endothelial progenitor cells in infantile hemangioma. Blood 103 (4): 1373-5, 2004. [PubMed: 14576053]
- Boscolo E, Mulliken JB, Bischoff J: Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1. Arterioscler Thromb Vasc Biol 33 (3): 501-9, 2013. [PMC free article: PMC3573237] [PubMed: 23288163]
- Biswas A, Richards JE, Massaro J, et al.: Mast cells in cutaneous tumors: innocent bystander or maestro conductor? Int J Dermatol 53 (7): 806-11, 2014. [PubMed: 23621615]
- Barnés CM, Huang S, Kaipainen A, et al.: Evidence by molecular profiling for a placental origin of infantile hemangioma. Proc Natl Acad Sci U S A 102 (52): 19097-102, 2005. [PMC free article: PMC1323205] [PubMed: 16365311]
- Walter JW, North PE, Waner M, et al.: Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer 33 (3): 295-303, 2002. [PubMed: 11807987]
- Ritter MR, Dorrell MI, Edmonds J, et al.: Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci U S A 99 (11): 7455-60, 2002. [PMC free article: PMC124252] [PubMed: 12032304]
- Takahashi K, Mulliken JB, Kozakewich HP, et al.: Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest 93 (6): 2357-64, 1994. [PMC free article: PMC294441] [PubMed: 7911127]
- Ritter MR, Reinisch J, Friedlander SF, et al.: Myeloid cells in infantile hemangioma. Am J Pathol 168 (2): 621-8, 2006. [PMC free article: PMC1606494] [PubMed: 16436675]
- Bielenberg DR, Bucana CD, Sanchez R, et al.: Progressive growth of infantile cutaneous hemangiomas is directly correlated with hyperplasia and angiogenesis of adjacent epidermis and inversely correlated with expression of the endogenous angiogenesis inhibitor, IFN-beta. Int J Oncol 14 (3): 401-8, 1999. [PubMed: 10024670]
- Colonna V, Resta L, Napoli A, et al.: Placental hypoxia and neonatal haemangioma: clinical and histological observations. Br J Dermatol 162 (1): 208-9, 2010. [PubMed: 19863512]
- de Jong S, Itinteang T, Withers AH, et al.: Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res 308 (4): 219-27, 2016. [PubMed: 26940670]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PubMed: 11346333]
- Chang LC, Haggstrom AN, Drolet BA, et al.: Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 122 (2): 360-7, 2008. [PubMed: 18676554]
- Tollefson MM, Frieden IJ: Early growth of infantile hemangiomas: what parents' photographs tell us. Pediatrics 130 (2): e314-20, 2012. [PubMed: 22826568]
- Haggstrom AN, Lammer EJ, Schneider RA, et al.: Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics 117 (3): 698-703, 2006. [PubMed: 16510649]
- Waner M, North PE, Scherer KA, et al.: The nonrandom distribution of facial hemangiomas. Arch Dermatol 139 (7): 869-75, 2003. [PubMed: 12873881]
- Chiller KG, Passaro D, Frieden IJ: Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol 138 (12): 1567-76, 2002. [PubMed: 12472344]
- Metry DW, Garzon MC, Drolet BA, et al.: PHACE syndrome: current knowledge, future directions. Pediatr Dermatol 26 (4): 381-98, 2009 Jul-Aug. [PubMed: 19689512]
- Munabi NC, Tan QK, Garzon MC, et al.: Growth Hormone Induces Recurrence of Infantile Hemangiomas After Apparent Involution: Evidence of Growth Hormone Receptors in Infantile Hemangioma. Pediatr Dermatol 32 (4): 539-43, 2015 Jul-Aug. [PMC free article: PMC5433863] [PubMed: 25690955]
- Baselga E, Roe E, Coulie J, et al.: Risk Factors for Degree and Type of Sequelae After Involution of Untreated Hemangiomas of Infancy. JAMA Dermatol 152 (11): 1239-1243, 2016. [PubMed: 27540637]
- Dubois J, Patriquin HB, Garel L, et al.: Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR Am J Roentgenol 171 (1): 247-52, 1998. [PubMed: 9648798]
- Horii KA, Drolet BA, Frieden IJ, et al.: Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol 28 (3): 245-53, 2011 May-Jun. [PubMed: 21517952]
- Ma EH, Robertson SJ, Chow CW, et al.: Infantile Hemangioma with Minimal or Arrested Growth: Further Observations on Clinical and Histopathologic Findings of this Unique but Underrecognized Entity. Pediatr Dermatol 34 (1): 64-71, 2017. [PubMed: 27873347]
- Valdivielso-Ramos M, Torrelo A, Martin-Santiago A, et al.: Infantile hemangioma with minimal or arrested growth as the skin manifestation of PHACE syndrome. Pediatr Dermatol 35 (5): 622-627, 2018. [PubMed: 29984853]
- Planas-Ciudad S, Roé Crespo E, Sánchez-Carpintero I, et al.: Infantile hemangiomas with minimal or arrested growth associated with soft tissue hypertrophy: a case series of 10 patients. J Eur Acad Dermatol Venereol 31 (11): 1924-1929, 2017. [PubMed: 28681397]
- Elluru RG, Friess MR, Richter GT, et al.: Multicenter Evaluation of the Effectiveness of Systemic Propranolol in the Treatment of Airway Hemangiomas. Otolaryngol Head Neck Surg 153 (3): 452-60, 2015. [PubMed: 26124263]
- McCormick AA, Tarchichi T, Azbell C, et al.: Subglottic hemangioma: Understanding the association with facial segmental hemangioma in a beard distribution. Int J Pediatr Otorhinolaryngol 113: 34-37, 2018. [PubMed: 30174006]
- Xue L, Sun C, Xu DP, et al.: Clinical Outcomes of Infants With Periorbital Hemangiomas Treated With Oral Propranolol. J Oral Maxillofac Surg 74 (11): 2193-2199, 2016. [PubMed: 27235180]
- Theiler M, Baselga E, Gerth-Kahlert C, et al.: Infantile hemangiomas with conjunctival involvement: An underreported occurrence. Pediatr Dermatol 34 (6): 681-685, 2017. [PubMed: 29144051]
- Garzon MC, Epstein LG, Heyer GL, et al.: PHACE Syndrome: Consensus-Derived Diagnosis and Care Recommendations. J Pediatr 178: 24-33.e2, 2016. [PMC free article: PMC6599593] [PubMed: 27659028]
- Frieden IJ, Reese V, Cohen D: PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 132 (3): 307-11, 1996. [PubMed: 8607636]
- Metry D, Heyer G, Hess C, et al.: Consensus Statement on Diagnostic Criteria for PHACE Syndrome. Pediatrics 124 (5): 1447-56, 2009. [PubMed: 19858157]
- Metry DW, Haggstrom AN, Drolet BA, et al.: A prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complications. Am J Med Genet A 140 (9): 975-86, 2006. [PubMed: 16575892]
- Drolet BA, Dohil M, Golomb MR, et al.: Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics 117 (3): 959-64, 2006. [PubMed: 16510684]
- Heyer GL, Dowling MM, Licht DJ, et al.: The cerebral vasculopathy of PHACES syndrome. Stroke 39 (2): 308-16, 2008. [PubMed: 18174492]
- Haggstrom AN, Garzon MC, Baselga E, et al.: Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics 126 (2): e418-26, 2010. [PubMed: 20643720]
- Poindexter G, Metry DW, Barkovich AJ, et al.: PHACE syndrome with intracerebral hemangiomas, heterotopia, and endocrine dysfunction. Pediatr Neurol 36 (6): 402-6, 2007. [PubMed: 17560503]
- Brandon K, Burrows P, Hess C, et al.: Arteriovenous malformation: a rare manifestation of PHACE syndrome. Pediatr Dermatol 28 (2): 180-4, 2011 Mar-Apr. [PubMed: 21504447]
- Chan YC, Eichenfield LF, Malchiodi J, et al.: Small facial haemangioma and supraumbilical raphe--a forme fruste of PHACES syndrome? Br J Dermatol 153 (5): 1053-7, 2005. [PubMed: 16225625]
- Nabatian AS, Milgraum SS, Hess CP, et al.: PHACE without face? Infantile hemangiomas of the upper body region with minimal or absent facial hemangiomas and associated structural malformations. Pediatr Dermatol 28 (3): 235-41, 2011 May-Jun. [PubMed: 21453307]
- Antonov NK, Spence-Shishido A, Marathe KS, et al.: Orbital Hemangioma with Intracranial Vascular Anomalies and Hemangiomas: A New Presentation of PHACE Syndrome? Pediatr Dermatol 32 (6): e267-72, 2015 Nov-Dec. [PubMed: 26446288]
- Burrows PE, Robertson RL, Mulliken JB, et al.: Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: report of eight patients. Radiology 207 (3): 601-7, 1998. [PubMed: 9609880]
- Hess CP, Fullerton HJ, Metry DW, et al.: Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol 31 (10): 1980-6, 2010. [PMC free article: PMC5967888] [PubMed: 20705698]
- Yu J, Siegel DH, Drolet BA, et al.: Prevalence and Clinical Characteristics of Headaches in PHACE Syndrome. J Child Neurol 31 (4): 468-73, 2016. [PMC free article: PMC6457900] [PubMed: 26271792]
- Samuelov L, Kinori M, Mancini AJ, et al.: Ocular Complications in PHACE Syndrome: A True Association or a Coincidence? J Pediatr 204: 214-218.e2, 2019. [PubMed: 30270159]
- Martin KL, Arvedson JC, Bayer ML, et al.: Risk of dysphagia and speech and language delay in PHACE syndrome. Pediatr Dermatol 32 (1): 64-9, 2015 Jan-Feb. [PubMed: 25440893]
- Chiu YE, Siegel DH, Drolet BA, et al.: Tooth enamel hypoplasia in PHACE syndrome. Pediatr Dermatol 31 (4): 455-8, 2014 Jul-Aug. [PubMed: 24916277]
- Duffy KJ, Runge-Samuelson C, Bayer ML, et al.: Association of hearing loss with PHACE syndrome. Arch Dermatol 146 (12): 1391-6, 2010. [PubMed: 20713775]
- Iacobas I, Burrows PE, Frieden IJ, et al.: LUMBAR: association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr 157 (5): 795-801.e1-7, 2010. [PubMed: 20598318]
- Girard C, Bigorre M, Guillot B, et al.: PELVIS Syndrome. Arch Dermatol 142 (7): 884-8, 2006. [PubMed: 16847205]
- Stockman A, Boralevi F, Taïeb A, et al.: SACRAL syndrome: spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology 214 (1): 40-5, 2007. [PubMed: 17191046]
- Anwar T, Malm-Buatsi E: Propranolol as an effective therapy for infantile haemangioma of the urinary bladder. BMJ Case Rep 12 (2): , 2019. [PMC free article: PMC6366799] [PubMed: 30709884]
- Drolet BA, Chamlin SL, Garzon MC, et al.: Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr 157 (5): 789-94, 2010. [PubMed: 20828712]
- Sardana K, Gupta R, Garg VK, et al.: A prospective study of cutaneous manifestations of spinal dysraphism from India. Pediatr Dermatol 26 (6): 688-95, 2009 Nov-Dec. [PubMed: 20199442]
- Rialon KL, Murillo R, Fevurly RD, et al.: Risk factors for mortality in patients with multifocal and diffuse hepatic hemangiomas. J Pediatr Surg 50 (5): 837-41, 2015. [PubMed: 25783331]
- Hsi Dickie B, Fishman SJ, Azizkhan RG: Hepatic vascular tumors. Semin Pediatr Surg 23 (4): 168-72, 2014. [PubMed: 25241093]
- Huang SA, Tu HM, Harney JW, et al.: Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 343 (3): 185-9, 2000. [PubMed: 10900278]
- de Graaf M, Knol MJ, Totté JE, et al.: E-learning enables parents to assess an infantile hemangioma. J Am Acad Dermatol 70 (5): 893-8, 2014. [PubMed: 24524882]
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al.: The Infantile Hemangioma Referral Score: A Validated Tool for Physicians. Pediatrics 145 (4): , 2020. [PubMed: 32161112]
- Krowchuk DP, Frieden IJ, Mancini AJ, et al.: Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 143 (1): , 2019. [PubMed: 30584062]
- Kessels JP, Hamers ET, Ostertag JU: Superficial hemangioma: pulsed dye laser versus wait-and-see. Dermatol Surg 39 (3 Pt 1): 414-21, 2013. [PubMed: 23279058]
- Keller RG, Patel KG: Evidence-Based Medicine in the Treatment of Infantile Hemangiomas. Facial Plast Surg Clin North Am 23 (3): 373-92, 2015. [PubMed: 26208774]
- Sharifpanah F, Saliu F, Bekhite MM, et al.: β-Adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res 358 (2): 443-52, 2014. [PubMed: 25130141]
- Ma X, Zhao T, Ouyang T, et al.: Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int J Clin Exp Pathol 7 (7): 3809-17, 2014. [PMC free article: PMC4128992] [PubMed: 25120757]
- Sasaki M, North PE, Elsey J, et al.: Propranolol exhibits activity against hemangiomas independent of beta blockade. NPJ Precis Oncol 3: 27, 2019. [PMC free article: PMC6825155] [PubMed: 31701018]
- Overman J, Fontaine F, Wylie-Sears J, et al.: R-propranolol is a small molecule inhibitor of the SOX18 transcription factor in a rare vascular syndrome and hemangioma. Elife 8: , 2019. [PMC free article: PMC6667216] [PubMed: 31358114]
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al.: A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 372 (8): 735-46, 2015. [PubMed: 25693013]
- Bauman NM: Propanolol effectively treats significant infantile hemangiomas. J Pediatr 167 (1): 210, 2015. [PubMed: 26117643]
- Chang L, Ye X, Qiu Y, et al.: Is Propranolol Safe and Effective for Outpatient Use for Infantile Hemangioma? A Prospective Study of 679 Cases From One Center in China. Ann Plast Surg 76 (5): 559-63, 2016. [PubMed: 26101993]
- Ames JA, Sykes JM: Current trends in medical management of infantile hemangioma. Curr Opin Otolaryngol Head Neck Surg 23 (4): 286-91, 2015. [PubMed: 26101875]
- Lou Y, Peng WJ, Cao Y, et al.: The effectiveness of propranolol in treating infantile haemangiomas: a meta-analysis including 35 studies. Br J Clin Pharmacol 78 (1): 44-57, 2014. [PMC free article: PMC4168379] [PubMed: 24033819]
- Luo Y, Zeng Y, Zhou B, et al.: A retrospective study of propranolol therapy in 635 infants with infantile hemangioma. Pediatr Dermatol 32 (1): 151-2, 2015 Jan-Feb. [PubMed: 24602103]
- Vivas-Colmenares GV, Bernabeu-Wittel J, Alonso-Arroyo V, et al.: Effectiveness of propranolol in the treatment of infantile hemangioma beyond the proliferation phase. Pediatr Dermatol 32 (3): 348-52, 2015 May-Jun. [PubMed: 25721095]
- Liu X, Qu X, Zheng J, et al.: Effectiveness and Safety of Oral Propranolol versus Other Treatments for Infantile Hemangiomas: A Meta-Analysis. PLoS One 10 (9): e0138100, 2015. [PMC free article: PMC4573957] [PubMed: 26375455]
- Hardison S, Wan W, Dodson KM: The use of propranolol in the treatment of subglottic hemangiomas: A literature review and meta-analysis. Int J Pediatr Otorhinolaryngol 90: 175-180, 2016. [PubMed: 27729127]
- Drolet BA, Frommelt PC, Chamlin SL, et al.: Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 131 (1): 128-40, 2013. [PMC free article: PMC3529954] [PubMed: 23266923]
- Solman L, Glover M, Beattie PE, et al.: Oral propranolol in the treatment of proliferating infantile haemangiomas: British Society for Paediatric Dermatology consensus guidelines. Br J Dermatol 179 (3): 582-589, 2018. [PubMed: 29774538]
- Hoeger PH, Harper JI, Baselga E, et al.: Treatment of infantile haemangiomas: recommendations of a European expert group. Eur J Pediatr 174 (7): 855-65, 2015. [PubMed: 26021855]
- Mehta A, Bajaj MS, Pushker N, et al.: To compare intralesional and oral propranolol for treating periorbital and eyelid capillary hemangiomas. Indian J Ophthalmol 67 (12): 1974-1980, 2019. [PMC free article: PMC6896529] [PubMed: 31755431]
- Püttgen KB, Hansen LM, Lauren C, et al.: Limited Utility of Repeated Vital Sign Monitoring During Initiation of Oral Propranolol for Complicated Infantile Hemangioma. J Am Acad Dermatol : , 2020. [PubMed: 32289387]
- Raphael MF, Breugem CC, Vlasveld FA, et al.: Is cardiovascular evaluation necessary prior to and during beta-blocker therapy for infantile hemangiomas?: A cohort study. J Am Acad Dermatol 72 (3): 465-72, 2015. [PubMed: 25592625]
- Streicher JL, Riley EB, Castelo-Soccio LA: Reevaluating the Need for Electrocardiograms Prior to Initiation of Treatment With Propranolol for Infantile Hemangiomas. JAMA Pediatr 170 (9): 906-7, 2016. [PubMed: 27454424]
- Prey S, Voisard JJ, Delarue A, et al.: Safety of Propranolol Therapy for Severe Infantile Hemangioma. JAMA 315 (4): 413-5, 2016. [PubMed: 26813215]
- Wedgeworth E, Glover M, Irvine AD, et al.: Propranolol in the treatment of infantile haemangiomas: lessons from the European Propranolol In the Treatment of Complicated Haemangiomas (PITCH) Taskforce survey. Br J Dermatol 174 (3): 594-601, 2016. [PubMed: 26473312]
- Ji Y, Chen S, Wang Q, et al.: Intolerable side effects during propranolol therapy for infantile hemangioma: frequency, risk factors and management. Sci Rep 8 (1): 4264, 2018. [PMC free article: PMC5844887] [PubMed: 29523832]
- Baselga E, Dembowska-Baginska B, Przewratil P, et al.: Efficacy of Propranolol Between 6 and 12 Months of Age in High-Risk Infantile Hemangioma. Pediatrics 142 (3): , 2018. [PubMed: 30082451]
- Shah SD, Baselga E, McCuaig C, et al.: Rebound Growth of Infantile Hemangiomas After Propranolol Therapy. Pediatrics 137 (4): , 2016. [PubMed: 26952504]
- O'Brien KF, Shah SD, Pope E, et al.: Late growth of infantile hemangiomas in children >3 years of age: A retrospective study. J Am Acad Dermatol 80 (2): 493-499, 2019. [PubMed: 30293898]
- Phillips RJ, Crock CM, Penington AJ, et al.: Prolonged tumour growth after treatment of infantile haemangioma with propranolol. Med J Aust 206 (3): 131, 2017. [PubMed: 28208045]
- Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, et al.: Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. J Am Acad Dermatol 70 (6): 1045-9, 2014. [PubMed: 24656727]
- Bayart CB, Tamburro JE, Vidimos AT, et al.: Atenolol Versus Propranolol for Treatment of Infantile Hemangiomas During the Proliferative Phase: A Retrospective Noninferiority Study. Pediatr Dermatol 34 (4): 413-421, 2017. [PubMed: 28556385]
- Randhawa HK, Sibbald C, Garcia Romero MT, et al.: Oral Nadolol for the Treatment of Infantile Hemangiomas: A Single-Institution Retrospective Cohort Study. Pediatr Dermatol 32 (5): 690-5, 2015 Sep-Oct. [PubMed: 26215612]
- Ji Y, Wang Q, Chen S, et al.: Oral atenolol therapy for proliferating infantile hemangioma: A prospective study. Medicine (Baltimore) 95 (24): e3908, 2016. [PMC free article: PMC4998480] [PubMed: 27310994]
- Bernabeu-Wittel J, Narváez-Moreno B, de la Torre-García JM, et al.: Oral Nadolol for Children with Infantile Hemangiomas and Sleep Disturbances with Oral Propranolol. Pediatr Dermatol 32 (6): 853-7, 2015 Nov-Dec. [PubMed: 26447831]
- Chinnadurai S, Fonnesbeck C, Snyder KM, et al.: Pharmacologic Interventions for Infantile Hemangioma: A Meta-analysis. Pediatrics 137 (2): e20153896, 2016. [PubMed: 26772662]
- Xu DP, Cao RY, Tong S, et al.: Topical timolol maleate for superficial infantile hemangiomas: an observational study. J Oral Maxillofac Surg 73 (6): 1089-94, 2015. [PubMed: 25843815]
- Tawfik AA, Alsharnoubi J: Topical timolol solution versus laser in treatment of infantile hemangioma: a comparative study. Pediatr Dermatol 32 (3): 369-76, 2015 May-Jun. [PubMed: 25740672]
- Püttgen K, Lucky A, Adams D, et al.: Topical Timolol Maleate Treatment of Infantile Hemangiomas. Pediatrics 138 (3): , 2016. [PubMed: 27527799]
- Drolet BA, Boakye-Agyeman F, Harper B, et al.: Systemic timolol exposure following topical application to infantile hemangiomas. J Am Acad Dermatol 82 (3): 733-736, 2020. [PubMed: 30790601]
- Weibel L, Barysch MJ, Scheer HS, et al.: Topical Timolol for Infantile Hemangiomas: Evidence for Efficacy and Degree of Systemic Absorption. Pediatr Dermatol 33 (2): 184-90, 2016 Mar-Apr. [PubMed: 26840644]
- Frommelt P, Juern A, Siegel D, et al.: Adverse Events in Young and Preterm Infants Receiving Topical Timolol for Infantile Hemangioma. Pediatr Dermatol 33 (4): 405-14, 2016. [PubMed: 27246751]
- Aly MM, Hamza AF, Abdel Kader HM, et al.: Therapeutic superiority of combined propranolol with short steroids course over propranolol monotherapy in infantile hemangioma. Eur J Pediatr 174 (11): 1503-9, 2015. [PubMed: 25982338]
- Li G, Xu DP, Tong S, et al.: Oral Propranolol With Topical Timolol Maleate Therapy for Mixed Infantile Hemangiomas in Oral and Maxillofacial Regions. J Craniofac Surg 27 (1): 56-60, 2016. [PubMed: 26716547]
- Tong S, Xu DP, Liu ZM, et al.: Evaluation of the efficacy and safety of topical timolol maleate combined with oral propranolol treatment for parotid mixed infantile hemangiomas. Oncol Lett 12 (3): 1806-1810, 2016. [PMC free article: PMC4998099] [PubMed: 27588127]
- Ge J, Zheng J, Zhang L, et al.: Oral propranolol combined with topical timolol for compound infantile hemangiomas: a retrospective study. Sci Rep 6: 19765, 2016. [PMC free article: PMC4730155] [PubMed: 26819072]
- Frieden IJ, Püttgen KB, Drolet BA, et al.: Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol 37 (3): 412-418, 2020. [PMC free article: PMC7262142] [PubMed: 32298480]
- Vildy S, Macher J, Abasq-Thomas C, et al.: Life-threatening hemorrhaging in neonatal ulcerated congenital hemangioma: two case reports. JAMA Dermatol 151 (4): 422-5, 2015. [PubMed: 25565634]
- Ayturk UM, Couto JA, Hann S, et al.: Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am J Hum Genet 98 (4): 789-95, 2016. [PMC free article: PMC4833432] [PubMed: 27058448]
- Maguiness S, Uihlein LC, Liang MG, et al.: Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol 32 (3): 321-6, 2015 May-Jun. [PubMed: 25492638]
- Scalise R, Bolton J, Gibbs NF: Rapidly involuting congenital hemangioma (RICH): a brief case report. Dermatol Online J 20 (11): , 2014. [PubMed: 25419759]
- Kumarasamy MT, Castrisios G, Sharma BK: Rapidly involuting congenital haemangioma in a term neonate. BMJ Case Rep 2014: , 2014. [PMC free article: PMC4024566] [PubMed: 24798357]
- Hughes R, McAleer M, Watson R, et al.: Rapidly involuting congenital hemangioma with pustules: two cases. Pediatr Dermatol 31 (3): 398-400, 2014 May-Jun. [PubMed: 24689686]
- Waelti SL, Rypens F, Damphousse A, et al.: Ultrasound findings in rapidly involuting congenital hemangioma (RICH) - beware of venous ectasia and venous lakes. Pediatr Radiol 48 (4): 586-593, 2018. [PubMed: 29362838]
- Nasseri E, Piram M, McCuaig CC, et al.: Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol 70 (1): 75-9, 2014. [PubMed: 24176519]
- Lee PW, Frieden IJ, Streicher JL, et al.: Characteristics of noninvoluting congenital hemangioma: a retrospective review. J Am Acad Dermatol 70 (5): 899-903, 2014. [PubMed: 24630000]
- Enjolras O, Mulliken JB, Boon LM, et al.: Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg 107 (7): 1647-54, 2001. [PubMed: 11391180]
- Kayaalp C, Sabuncuoglu MZ: Embolization of Liver Hemangiomas. Hepat Mon 15 (8): e30334, 2015. [PMC free article: PMC4546810] [PubMed: 26322113]
- Klein M, Chang AK, Vasudevan SA, et al.: Clinically significant ascites as an indication for resection of rapidly involuting congenital hepatic hemangiomas. Pediatr Blood Cancer 65 (8): e27222, 2018. [PMC free article: PMC6019154] [PubMed: 29741223]
- Gourgiotis S, Moustafellos P, Zavos A, et al.: Surgical treatment of hepatic haemangiomas: a 15-year experience. ANZ J Surg 76 (9): 792-5, 2006. [PubMed: 16922900]
- Schmitz R, Heinig J, Klockenbusch W, et al.: Antenatal diagnosis of a giant fetal hepatic hemangioma and treatment with maternal corticosteroid. Ultraschall Med 30 (3): 223-6, 2009. [PubMed: 19507116]
- Rialon KL, Murillo R, Fevurly RD, et al.: Impact of Screening for Hepatic Hemangiomas in Patients with Multiple Cutaneous Infantile Hemangiomas. Pediatr Dermatol 32 (6): 808-12, 2015 Nov-Dec. [PubMed: 26223454]
- Yeh I, Bruckner AL, Sanchez R, et al.: Diffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapy. Pediatr Dermatol 28 (3): 267-75, 2011 May-Jun. [PubMed: 21517953]
- Wasserman JD, Mahant S, Carcao M, et al.: Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics 135 (6): e1501-5, 2015. [PubMed: 25986022]
- Vlahovic A, Simic R, Djokic D, et al.: Diffuse neonatal hemangiomatosis treatment with cyclophosphamide: a case report. J Pediatr Hematol Oncol 31 (11): 858-60, 2009. [PubMed: 19829152]
- Sundar Alagusundaramoorthy S, Vilchez V, Zanni A, et al.: Role of transplantation in the treatment of benign solid tumors of the liver: a review of the United Network of Organ Sharing data set. JAMA Surg 150 (4): 337-42, 2015. [PubMed: 25714928]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PubMed: 24740626]
- Sari N, Yalçin B, Akyüz C, et al.: Infantile hepatic hemangioendothelioma with elevated serum alpha-fetoprotein. Pediatr Hematol Oncol 23 (8): 639-47, 2006. [PubMed: 17065140]
- Seo IS, Min KW, Mirkin LD: Hepatic hemangioendothelioma of infancy associated with elevated alpha fetoprotein and catecholamine by-products. Pediatr Pathol 8 (6): 625-31, 1988. [PubMed: 2469076]
- Langham MR, Furman WL, Fernandez-Pineda I: Current Management of Neonatal Liver Tumors. Curr Pediatr Rev 11 (3): 195-204, 2015. [PubMed: 26168944]
- Perkins P, Weiss SW: Spindle cell hemangioendothelioma. An analysis of 78 cases with reassessment of its pathogenesis and biologic behavior. Am J Surg Pathol 20 (10): 1196-204, 1996. [PubMed: 8827025]
- Fletcher CD, Beham A, Schmid C: Spindle cell haemangioendothelioma: a clinicopathological and immunohistochemical study indicative of a non-neoplastic lesion. Histopathology 18 (4): 291-301, 1991. [PubMed: 2071088]
- Enjolras O, Mulliken JB, Kozakewich HPW: Vascular tumors and tumor-like lesions. In: Mulliken JB, Burrows PE, Fishman SJ, eds.: Mulliken & Young's Vascular Anomalies: Hemangiomas and Malformations. 2nd ed. Oxford University Press, 2013, pp 259-324.
- Hoeger PH, Colmenero I: Vascular tumours in infants. Part I: benign vascular tumours other than infantile haemangioma. Br J Dermatol 171 (3): 466-73, 2014. [PubMed: 24117053]
- Pansuriya TC, van Eijk R, d'Adamo P, et al.: Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet 43 (12): 1256-61, 2011. [PMC free article: PMC3427908] [PubMed: 22057234]
- Guo R, Gavino AC: Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med 139 (5): 683-6, 2015. [PubMed: 25927152]
- O'Connell JX, Nielsen GP, Rosenberg AE: Epithelioid vascular tumors of bone: a review and proposal of a classification scheme. Adv Anat Pathol 8 (2): 74-82, 2001. [PubMed: 11236956]
- Huang SC, Zhang L, Sung YS, et al.: Frequent FOS Gene Rearrangements in Epithelioid Hemangioma: A Molecular Study of 58 Cases With Morphologic Reappraisal. Am J Surg Pathol 39 (10): 1313-21, 2015. [PMC free article: PMC4567921] [PubMed: 26135557]
- Liu KX, Duggan EM, Al-Ibraheemi A, et al.: Characterization of long-term outcomes for pediatric patients with epithelioid hemangioma. Pediatr Blood Cancer 66 (1): e27451, 2019. [PubMed: 30207085]
- Wassef M, Hunt SF, Santa Cruz DJ: Vascular tumors and vascular malformations. In: Barnhi RL, Crowson AN, Magro CM, et al., eds.: Dermatopathology. 3rd ed. McGraw Hill Medical, 2010, pp 802-56.
- Swerlick RA, Cooper PH: Pyogenic granuloma (lobular capillary hemangioma) within port-wine stains. J Am Acad Dermatol 8 (5): 627-30, 1983. [PubMed: 6863618]
- Campbell JP, Grekin RC, Ellis CN, et al.: Retinoid therapy is associated with excess granulation tissue responses. J Am Acad Dermatol 9 (5): 708-13, 1983. [PubMed: 6227639]
- Mills SE, Cooper PH, Fechner RE: Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol 4 (5): 470-9, 1980. [PubMed: 7435775]
- Browning JC, Eldin KW, Kozakewich HP, et al.: Congenital disseminated pyogenic granuloma. Pediatr Dermatol 26 (3): 323-7, 2009 May-Jun. [PubMed: 19706097]
- Putra J, Rymeski B, Merrow AC, et al.: Four cases of pediatric deep-seated/subcutaneous pyogenic granuloma: Review of literature and differential diagnosis. J Cutan Pathol 44 (6): 516-522, 2017. [PubMed: 28233342]
- Groesser L, Peterhof E, Evert M, et al.: BRAF and RAS Mutations in Sporadic and Secondary Pyogenic Granuloma. J Invest Dermatol 136 (2): 481-6, 2016. [PubMed: 26802240]
- Lee J, Sinno H, Tahiri Y, et al.: Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg 64 (9): 1216-20, 2011. [PubMed: 21316320]
- Patrizi A, Gurioli C, Dika E: Pyogenic granulomas in childhood: New treatment modalities. Dermatol Ther 28 (5): 332, 2015 Sep-Oct. [PubMed: 25818498]
- Oke I, Alkharashi M, Petersen RA, et al.: Treatment of Ocular Pyogenic Granuloma With Topical Timolol. JAMA Ophthalmol 135 (4): 383-385, 2017. [PubMed: 28301661]
- Neri I, Baraldi C, Balestri R, et al.: Topical 1% propranolol ointment with occlusion in treatment of pyogenic granulomas: An open-label study in 22 children. Pediatr Dermatol 35 (1): 117-120, 2018. [PubMed: 29266656]
- Haemel AK, O'Brian AL, Teng JM: Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol 146 (7): 715-8, 2010. [PubMed: 20644030]
- Pignatti M, Spaggiari A, Sala P, et al.: Laser treatment of angiofibromas in tuberous sclerosis. Minerva Pediatr 66 (6): 585-6, 2014. [PubMed: 25336102]
- Lee YI, Lee JH, Kim DY, et al.: Comparative Effects of Topical 0.2% Sirolimus for Angiofibromas in Adults and Pediatric Patients with Tuberous Sclerosis Complex. Dermatology 234 (1-2): 13-22, 2018. [PubMed: 29925060]
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al.: Sirolimus Gel Treatment vs Placebo for Facial Angiofibromas in Patients With Tuberous Sclerosis Complex: A Randomized Clinical Trial. JAMA Dermatol 154 (7): 781-788, 2018. [PMC free article: PMC6128500] [PubMed: 29800026]
- Koenig MK, Bell CS, Hebert AA, et al.: Efficacy and Safety of Topical Rapamycin in Patients With Facial Angiofibromas Secondary to Tuberous Sclerosis Complex: The TREATMENT Randomized Clinical Trial. JAMA Dermatol 154 (7): 773-780, 2018. [PMC free article: PMC6128508] [PubMed: 29800048]
- Coutinho-Camillo CM, Brentani MM, Nagai MA: Genetic alterations in juvenile nasopharyngeal angiofibromas. Head Neck 30 (3): 390-400, 2008. [PubMed: 18228521]
- Riggs S, Orlandi RR: Juvenile nasopharyngeal angiofibroma recurrence associated with exogenous testosterone therapy. Head Neck 32 (6): 812-5, 2010. [PubMed: 19626637]
- Liu Z, Wang J, Wang H, et al.: Hormonal receptors and vascular endothelial growth factor in juvenile nasopharyngeal angiofibroma: immunohistochemical and tissue microarray analysis. Acta Otolaryngol 135 (1): 51-7, 2015. [PubMed: 25384380]
- Szymańska A, Szymański M, Czekajska-Chehab E, et al.: Two types of lateral extension in juvenile nasopharyngeal angiofibroma: diagnostic and therapeutic management. Eur Arch Otorhinolaryngol 272 (1): 159-66, 2015. [PMC free article: PMC4282713] [PubMed: 24599598]
- Samanta D: Topical mTOR (mechanistic target of rapamycin) inhibitor therapy in facial angiofibroma. Indian J Dermatol Venereol Leprol 81 (5): 540-1, 2015 Sep-Oct. [PubMed: 26323682]
- Krakowski AC, Nguyen TA: Inhibition of Angiofibromas in a Tuberous Sclerosis Patient Using Topical Timolol 0.5% Gel. Pediatrics 136 (3): e709-13, 2015. [PubMed: 26304829]
- Mallick S, Benson R, Bhasker S, et al.: Long-term treatment outcomes of juvenile nasopharyngeal angiofibroma treated with radiotherapy. Acta Otorhinolaryngol Ital 35 (2): 75-9, 2015. [PMC free article: PMC4443565] [PubMed: 26019389]
- Peters T, Traboulsi D, Tibbles LA, et al.: Sirolimus: a therapeutic advance for dermatologic disease. Skin Therapy Lett 19 (4): 1-4, 2014 Jul-Aug. [PubMed: 25188522]
- Fernández KS, de Alarcon A, Adams DM, et al.: Sirolimus for the treatment of juvenile nasopharyngeal angiofibroma. Pediatr Blood Cancer 67 (4): e28162, 2020. [PubMed: 31925925]
Intermediate Tumors (Locally Aggressive)
Kaposiform Hemangioendothelioma and Tufted Angioma
Kaposiform hemangioendothelioma (KHE) and tufted angioma are rare vascular tumors that typically occur during infancy or early childhood but have been reported in adults. Both tumors are thought to be a spectrum of the same disease, because both can be locally aggressive and cause Kasabach-Merritt phenomenon, a serious life-threatening coagulopathy characterized by profound thrombocytopenia and hypofibrinogenemia. They are discussed here as a single entity, kaposiform hemangioendothelioma.
Incidence
The exact incidence of kaposiform hemangioendothelioma is unknown but is estimated to be 0.07 cases per 100,000 children per year.[1-3] This lesion affects both sexes equally, with most developing in the neonatal period, one-half presenting at birth, and others presenting during childhood or adulthood.[4]
Pathology
Kaposiform hemangioendothelioma is characterized by sheets of spindle cells with an infiltrative pattern in the dermis, subcutaneous fat, and muscle. There are often areas of fibrosis, with dilated thin-walled vessels infiltrated around the areas of spindle cells. Mixed within these areas are nests of rounded epithelioid cells of vascular origin and aggregates of capillaries with round or irregularly shaped lumens containing platelet-rich fibrin thrombi. There are usually abnormal lymphatic spaces, either within or at the periphery of the lesion. The rate of mitosis is variable but usually low. Tufted angioma is characterized by multiple, discrete lobules of tightly packed capillaries (tufts) scattered in the dermis and sometimes in the subcutis, a so-called cannonball pattern.[5] Mitoses are rare.
The pathogenesis is poorly understood. There is some evidence that kaposiform hemangioendothelioma may be derived from lymphatic endothelium, as the spindle cell expresses the vascular markers CD31 and CD34, the vascular endothelial growth factor receptor-3 (VEGFR-3) (a receptor required for lymphangiogenesis), and the lymphatic markers D2-40 and PROX1.[5-7] There is no evidence of association with human herpesvirus 8 infection as is present in Kaposi sarcoma.[7]
Genomic data are limited. There have been reports of a small number of patients with GNA14 mutations but not in all cases.[8,9]
Angiopoietin-2 (Ang-2): High serum levels of Ang-2 have been found in high-risk patients with kaposiform hemangioendothelioma and kaposiform lymphangiomatosis. The Ang-2 levels have also been noted to decrease in response to therapy with sirolimus and raises the possibility of an effect on the endothelial cells of the kaposiform hemangioendothelioma tumor.[10] Ang-2 is produced and stored in the endothelial cells and acts as a TEK tyrosine kinase antagonist. Ang-2 can promote neovascularization in conjunction with VEGF, and in humans, Ang-2 is greatly increased in vascular remodeling that occurs with sepsis, inflammation, and lymphangiogenesis.[11] These levels have been used for the diagnosis of vascular tumors and assessment of response to therapy.
Clinical presentation
Kaposiform hemangioendothelioma most frequently involves the extremities and less frequently involves the trunk and head and neck area.[3] Most lesions involve the skin (refer to Figure 9). Deeper lesions (retroperitoneum, thoracic cavity, and muscle) can appear as a bluish-purpuric hue on the skin, whereas superficial lesions can be firm, purpuric or ecchymotic, and painful. Lesions are usually unifocal and growth is expansive and contiguous. Local lymph nodes may be involved, but there are no reports of distant metastasis. Rare multifocal presentations have been reported, mostly in the bone.[1-3]
Figure 9. Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon. The lesion is indurated, firm, and warm with petechiae and purpura. Credit: Denise Adams, M.D.
Fifty to seventy percent of patients with kaposiform hemangioendothelioma develop Kasabach-Merritt phenomenon (KMP), which is a life-threatening complication characterized by profound thrombocytopenia (range, 3,000/µL–60,000/µL) and profound hypofibrinogenemia (<1 g/L). D-dimer and fibrin degradation products are elevated. Severe anemia can occur secondary to tumor sequestration. Severe hemorrhage is rare; however, trauma (biopsy, surgical procedure), ulceration, infection, or delay in initiating treatment may induce progression to disseminated intravascular coagulation, serious bleeding, and even death. Aggressive replacement of blood products, especially platelets, can increase the size of the lesion, causing significant pain and should only be considered with active bleeding and under the direction of a vascular anomalies specialist.[3] The risk of developing Kasabach-Merritt phenomenon is highest in patients with congenital lesions, lesions larger than 8 cm, and when kaposiform hemangioendothelioma arises in the retroperitoneum or mediastinum.[3,12] The mortality rate is unclear but it has been reported to be as high as 30%.[3,12]
Diagnostic evaluation
The diagnosis is based on the combination of clinical, histologic, and imaging features. Laboratory evaluation is essential for the diagnosis of Kasabach-Merritt phenomenon. Whenever possible, histologic confirmation should be obtained, because prolonged therapy is often needed. However, if clinical and imaging findings are highly suggestive of the diagnosis, deferring biopsy may be an option but this decision should be reached via an interdisciplinary discussion and approach.
Magnetic resonance imaging (MRI) is the preferred imaging modality, especially for kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon and large lesions. T1-weighted sequences typically show a poorly circumscribed soft tissue mass with soft tissue and dermal thickening and diffuse enhancement with gadolinium. T2-weighted sequences show a diffuse increased signal, with stranding in the subcutaneous fat. Gradient sequences show mildly dilated vessels in and around the soft-tissue mass.[3]
For small and superficial lesions, ultrasonography can be useful for diagnosis and can distinguish tufted angioma from kaposiform hemangioendothelioma. Kaposiform hemangioendothelioma has a more infiltrative pattern, while tufted angioma is more superficial. Tufted angiomas are well defined and hyperechoic, while kaposiform hemangioendotheliomas have ill-defined borders and mixed echogenicity. Kaposiform hemangioendotheliomas also have an increased vascular density than do tufted angiomas.[13]
Treatment of kaposiform hemangioendothelioma and tufted angioma
Treatment varies according to size, location, presence of symptoms, and severity of coagulopathy; there is no evidence-based standard of care. Kaposiform hemangioendothelioma and tufted angioma that are not complicated and are localized can be treated with surgical excision, pulse-dye laser, or topical agents (steroids, sirolimus, or tacrolimus).[14-16] Observation is also an option for patients with low-risk tumors (i.e., no Kasabach-Merritt phenomenon, small tumor size, asymptomatic). Spontaneous regression and/or stability has been noted for kaposiform hemangioendothelioma and tufted angioma.[16]
Surgical excision may be possible for lesions that have failed medical management or are life threatening. Embolization may be performed in conjunction with surgery or medical therapy; usually it is a temporizing measure.[17]
Patients who have Kasabach-Merritt phenomenon and/or functional compromise and are symptomatic need aggressive therapy. An American and Canadian multidisciplinary expert panel published guidelines for the management of complicated kaposiform hemangioendothelioma.[18] A number of treatment therapies have been reported but none have been uniformly effective.[19,20]
The most common treatment option for kaposiform hemangioendothelioma has traditionally been steroid therapy with or without vincristine or other agents;[18-23] however, many institutions are now using the mTOR inhibitor sirolimus, with or without steroid therapy, as primary treatment for high-risk patients.[24-26] Steroid therapy has not been effective as a single agent for complicated kaposiform hemangioendothelioma, even at high doses. Patients treated with steroid therapy have a response rate of 10% to 20% and a significant number of side effects.[18] Steroid therapy is currently being used in combination therapy with vincristine or sirolimus.[27,28]
The following is a summary of treatment options for complicated kaposiform hemangioendothelioma:
Vincristine
Vincristine was shown to have a hematologic response and reduction in tumor volume in patients with high-risk kaposiform hemangioendothelioma.[19] Furthermore, in a retrospective review of 37 children with kaposiform hemangioendothelioma whose lesions did not respond to steroids, 26 of the lesions achieved complete remission, with platelet counts reaching normal levels within 7.6 (± 5.2) weeks after vincristine treatment.[21][Level of evidence: 3iiiDiv] Vincristine monotherapy in other studies has not been shown to be effective.[24,28] In 2013, consensus guidelines for the management of complicated kaposiform hemangioendothelioma proposed, on the basis of available evidence, the use of vincristine with or without steroids as the first-line therapy.[18] Successful management of patients with kaposiform hemangioendothelioma who were treated with vincristine and ticlopidine has also been reported.[29]
Sirolimus
Secondary to promising case reports, case series, and a prospective clinical trial, sirolimus may be considered an alternative first-line therapy for kaposiform hemangioendothelioma.[25,26,30] There are limited studies investigating the effect of sirolimus on kaposiform hemangioendothelioma/tufted angioma without Kasabach-Merritt phenomenon.
Reports that support the use of sirolimus include the following:
- A prospective study that assessed the efficacy and safety of sirolimus for the treatment of complicated vascular anomalies treated 13 patients with kaposiform hemangioendothelioma.[28]
- In patients with kaposiform hemangioendothelioma and Kasabach-Merritt phenomenon, ten of ten patients had partial responses, with normalization of their platelet count and fibrinogen at the end of 6 and 12 courses.
- Of the three patients with kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon, one patient with multifocal bony disease had disease progression, whereas the other two patients experienced partial responses by the end of course 12.
- Side effects were minimal in this group of young patients, and no patient with kaposiform hemangioendothelioma required a dose adjustment or was removed from the study secondary to toxicity of sirolimus.
- A retrospective study of sirolimus therapy in patients who had nearly all received previous other treatments reported a complete response rate of 73%. All patients assessed as having Kasabach-Merritt phenomenon had recovery of platelet counts between 1 day and 3 weeks (mean, 1.3 weeks).[31]
- One death occurred in this study from a respiratory infection in a child with multifocal disease involving the thoracic cavity, with pleural effusion. Biopsy was not routinely used in this study to confirm diagnosis, raising the possibility that this child had an unrecognized complex lymphatic anomaly.
- A multicenter, retrospective cohort study analyzed 52 Chinese patients with progressive kaposiform hemangioendothelioma. Thirty-seven patients (71%) had Kasabach-Merritt phenomenon. Those without Kasabach-Merritt phenomenon received sirolimus alone, and 21 of the patients with Kasabach-Merritt phenomenon received a combination of sirolimus and prednisone.[27]
- Overall, 96% and 98% of patients demonstrated improvement in notable symptoms and/or had improved complications at 6 and 12 months, respectively.
- A single case report of a child with kaposiform hemangioendothelioma who developed recurrence of pain and fibrosis years after initial therapy and was treated with sirolimus for 26 months observed the following:[30]
- The patient's contracture and range of motion improved, the lesion shrank, and the child was well 2 years later.
Dosing of sirolimus: Most high-risk patients (kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon) are treated with sirolimus blood levels of 8 to 10 ng/ml.[27,28,32,33]
Supportive care and close monitoring of infants on sirolimus
A case report described two children with kaposiform hemangioendothelioma and Kasabach-Merritt syndrome who died of pulmonary infections after treatment with sirolimus.[34] Another child who received sirolimus and prednisolone developed Pneumocystis jirovecii pneumonia.[35] P. jirovecii pneumonia prophylaxis and close monitoring of patients on sirolimus (especially infants) is encouraged.
Propranolol therapy
Propranolol therapy has been reported as a treatment option for kaposiform hemangioendothelioma. Its use is based on the positive results of propranolol for other more benign vascular tumors. Results have been mixed, with a report of improved effectiveness using higher doses of propranolol.[36,37] Preliminary results indicate that propranolol should be reserved for patients with kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon and with smaller, less complicated lesions.
Long-term outcomes
Even with therapy, these lesions do not fully regress and can recur; worsened symptomatology (pain, inflammation) can occur with age, especially around the time of puberty.[38]
Long-term effects include chronic pain, lymphedema, heart failure, and orthopedic issues.[17,38] These lesions prove to be a difficult dilemma for the practitioner because they have a varied clinical spectrum and response to therapy.
Treatment options under clinical evaluation for kaposiform hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
References
- Rodriguez V, Lee A, Witman PM, et al.: Kasabach-merritt phenomenon: case series and retrospective review of the mayo clinic experience. J Pediatr Hematol Oncol 31 (7): 522-6, 2009. [PubMed: 19564750]
- Ryan C, Price V, John P, et al.: Kasabach-Merritt phenomenon: a single centre experience. Eur J Haematol 84 (2): 97-104, 2010. [PubMed: 19889011]
- Croteau SE, Liang MG, Kozakewich HP, et al.: Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr 162 (1): 142-7, 2013. [PMC free article: PMC3494787] [PubMed: 22871490]
- Lee B, Chiu M, Soriano T, et al.: Adult-onset tufted angioma: a case report and review of the literature. Cutis 78 (5): 341-5, 2006. [PubMed: 17186794]
- Enjolras O, Soupre V, Picard A: Uncommon benign infantile vascular tumors. Adv Dermatol 24: 105-24, 2008. [PubMed: 19263597]
- Zukerberg LR, Nickoloff BJ, Weiss SW: Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol 17 (4): 321-8, 1993. [PubMed: 8494101]
- Arai E, Kuramochi A, Tsuchida T, et al.: Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol 33 (7): 492-7, 2006. [PubMed: 16872472]
- Lim YH, Bacchiocchi A, Qiu J, et al.: GNA14 Somatic Mutation Causes Congenital and Sporadic Vascular Tumors by MAPK Activation. Am J Hum Genet 99 (2): 443-50, 2016. [PMC free article: PMC4974082] [PubMed: 27476652]
- Lim YH, Fraile C, Antaya RJ, et al.: Tufted angioma with associated Kasabach-Merritt phenomenon caused by somatic mutation in GNA14. Pediatr Dermatol 36 (6): 963-964, 2019. [PMC free article: PMC7039697] [PubMed: 31423605]
- Le Cras TD, Mobberley-Schuman PS, Broering M, et al.: Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis 20 (1): 163-173, 2017. [PubMed: 27990590]
- Saharinen P, Eklund L, Alitalo K: Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov 16 (9): 635-661, 2017. [PubMed: 28529319]
- Ji Y, Yang K, Peng S, et al.: Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol 179 (2): 457-463, 2018. [PubMed: 29603128]
- Gong X, Ying H, Zhang Z, et al.: Ultrasonography and magnetic resonance imaging features of kaposiform hemangioendothelioma and tufted angioma. J Dermatol 46 (10): 835-842, 2019. [PubMed: 31373042]
- Burleigh A, Kanigsberg N, Lam JM: Topical rapamycin (sirolimus) for the treatment of uncomplicated tufted angiomas in two children and review of the literature. Pediatr Dermatol 35 (5): e286-e290, 2018. [PubMed: 30015406]
- Zhang X, Yang K, Chen S, et al.: Tacrolimus ointment for the treatment of superficial kaposiform hemangioendothelioma and tufted angioma. J Dermatol 46 (10): 898-901, 2019. [PubMed: 31373046]
- Osio A, Fraitag S, Hadj-Rabia S, et al.: Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol 146 (7): 758-63, 2010. [PubMed: 20644037]
- Ji Y, Chen S, Yang K, et al.: Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis 15 (1): 39, 2020. [PMC free article: PMC6998257] [PubMed: 32014025]
- Drolet BA, Trenor CC, Brandão LR, et al.: Consensus-derived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr 163 (1): 285-91, 2013. [PubMed: 23796341]
- Haisley-Royster C, Enjolras O, Frieden IJ, et al.: Kasabach-merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol 24 (6): 459-62, 2002 Aug-Sep. [PubMed: 12218593]
- Hauer J, Graubner U, Konstantopoulos N, et al.: Effective treatment of kaposiform hemangioendotheliomas associated with Kasabach-Merritt phenomenon using four-drug regimen. Pediatr Blood Cancer 49 (6): 852-4, 2007. [PubMed: 16411198]
- Wang Z, Li K, Yao W, et al.: Steroid-resistant kaposiform hemangioendothelioma: a retrospective study of 37 patients treated with vincristine and long-term follow-up. Pediatr Blood Cancer 62 (4): 577-80, 2015. [PubMed: 25346262]
- Fernandez-Pineda I, Lopez-Gutierrez JC, Ramirez G, et al.: Vincristine-ticlopidine-aspirin: an effective therapy in children with Kasabach-Merritt phenomenon associated with vascular tumors. Pediatr Hematol Oncol 27 (8): 641-5, 2010. [PubMed: 20863161]
- Fernandez-Pineda I, Lopez-Gutierrez JC, Chocarro G, et al.: Long-term outcome of vincristine-aspirin-ticlopidine (VAT) therapy for vascular tumors associated with Kasabach-Merritt phenomenon. Pediatr Blood Cancer 60 (9): 1478-81, 2013. [PubMed: 23609996]
- Kai L, Wang Z, Yao W, et al.: Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol 140 (3): 471-6, 2014. [PubMed: 24464150]
- Hammill AM, Wentzel M, Gupta A, et al.: Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 57 (6): 1018-24, 2011. [PubMed: 21445948]
- Blatt J, Stavas J, Moats-Staats B, et al.: Treatment of childhood kaposiform hemangioendothelioma with sirolimus. Pediatr Blood Cancer 55 (7): 1396-8, 2010. [PubMed: 20730884]
- Ji Y, Chen S, Xiang B, et al.: Sirolimus for the treatment of progressive kaposiform hemangioendothelioma: A multicenter retrospective study. Int J Cancer 141 (4): 848-855, 2017. [PubMed: 28486787]
- Adams DM, Trenor CC, Hammill AM, et al.: Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 137 (2): e20153257, 2016. [PMC free article: PMC4732362] [PubMed: 26783326]
- López V, Martí N, Pereda C, et al.: Successful management of Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon using vincristine and ticlopidine. Pediatr Dermatol 26 (3): 365-6, 2009 May-Jun. [PubMed: 19706115]
- Oza VS, Mamlouk MD, Hess CP, et al.: Role of Sirolimus in Advanced Kaposiform Hemangioendothelioma. Pediatr Dermatol 33 (2): e88-92, 2016 Mar-Apr. [PubMed: 26864138]
- Wang Z, Yao W, Sun H, et al.: Sirolimus therapy for kaposiform hemangioendothelioma with long-term follow-up. J Dermatol 46 (11): 956-961, 2019. [PubMed: 31489702]
- Zhang G, Chen H, Gao Y, et al.: Sirolimus for treatment of Kaposiform haemangioendothelioma with Kasabach-Merritt phenomenon: a retrospective cohort study. Br J Dermatol 178 (5): 1213-1214, 2018. [PubMed: 29388191]
- Mariani LG, Schmitt IR, Garcia CD, et al.: Low dose sirolimus treatment for refractory tufted angioma and congenital kaposiform hemangioendothelioma, both with Kasabach-Merritt phenomenon. Pediatr Blood Cancer 66 (8): e27810, 2019. [PubMed: 31087627]
- Ying H, Qiao C, Yang X, et al.: A Case Report of 2 Sirolimus-Related Deaths Among Infants With Kaposiform Hemangioendotheliomas. Pediatrics 141 (Suppl 5): S425-S429, 2018. [PubMed: 29610165]
- Russell TB, Rinker EK, Dillingham CS, et al.: Pneumocystis Jirovecii Pneumonia During Sirolimus Therapy for Kaposiform Hemangioendothelioma. Pediatrics 141 (Suppl 5): S421-S424, 2018. [PubMed: 29610164]
- Filippi L, Tamburini A, Berti E, et al.: Successful Propranolol Treatment of a Kaposiform Hemangioendothelioma Apparently Resistant to Propranolol. Pediatr Blood Cancer 63 (7): 1290-2, 2016. [PubMed: 27100060]
- Wang Z, Li K, Dong K, et al.: Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer 61 (8): 1518-9, 2014. [PubMed: 24482015]
- Schaefer BA, Wang D, Merrow AC, et al.: Long-term outcome for kaposiform hemangioendothelioma: A report of two cases. Pediatr Blood Cancer 64 (2): 284-286, 2017. [PubMed: 27701822]
Intermediate Tumors (Rarely Metastasizing)
Intermediate vascular tumors (rarely metastasizing) include the following:
Pseudomyogenic Hemangioendothelioma
Incidence and outcome
Pseudomyogenic hemangioendothelioma is a rare, newly designated, distinct vascular tumor. It is characterized as an intermediate-grade tumor with moderately aggressive local spread and rare distant metastatic disease.
Pathology and biology
Pseudomyogenic hemangioendothelioma is characterized by loose fascicles of plump spindle and epithelioid cells with abundant eosinophils, cytoplasm, and coexpression of keratins and endothelial markers.[1-3] The etiology for this tumor is unclear, although a balanced translocation t(7;19) resulting in the SERPINE1-FOSB fusion gene has been reported.[4]
Clinical presentation and diagnostic evaluation
The tumor usually presents in young men aged 20 to 50 years.[1,2] Multifocal disease occurs in 70% of patients. Sites of involvement include the dermis, subcutis, and bones. Patients usually present with pain or a soft tissue mass.[1,5]
Treatment of pseudomyogenic hemangioendothelioma
Most patients are treated with surgery, including amputation for multifocal bony disease.[1] In reported cases, chemotherapy has produced responses.[6,7] Recently, the mammalian target of rapamycin (mTOR) inhibitors have been considered as treatment options.[7,8] An additional case report noted efficacy of sirolimus with the addition of zoledronic acid in a patient with multifocal bony disease.[9]
Retiform Hemangioendothelioma
Pathology and clinical presentation
Retiform hemangioendotheliomas are slow growing, exophytic, flat tumors found in young adults and occasionally children.[10] They are usually located in the limbs and trunk. Histologically, they are located in the dermis and subcutaneous tissue. Vessels exhibit a pattern resembling the rete testis and are lined by protruding endothelial cells. They do not express lymphatic markers but stain positive for endothelial markers.[11]
Prognostic factors
Local recurrences are common, but distinct metastases are extremely rare.[11]
Papillary Intralymphatic Angioendothelioma
Pathology and clinical presentation
Papillary intralymphatic angioendothelioma, also known as Dabska tumor, can occur in the adult and pediatric population.[16] The lesions occur in the dermis and subcutis on all body parts and there have been some reports of lymph node involvement. They can be large or small raised purplish firm nodules.
Pathologically, they reveal intravascular growth of well-differentiated endothelial cells in a columnar configuration. They have thickened hyaline walls with hobnailed endothelium. Vascular endothelial growth factor receptor type 3, a marker for lymphatic endothelium, is positive in most cases. There is minimal cytologic atypia.[17] Some are associated with vascular malformations.
Treatment of papillary intralymphatic angioendothelioma
Surgical excision is the treatment of choice.[18]
Composite Hemangioendothelioma
Pathology and clinical presentation
Composite hemangioendothelioma is a very rare vascular tumor classified as intermediate because of the combined benign and malignant vascular components. Usually, combined epithelioid and retiform variants are noted but some tumors have three components (epithelioid, retiform, and spindle cell).[19] Angiosarcoma foci have been noted. Pathology reveals positivity for CD31, factor VIII, and vimentin.[19,20] Rarely, D-240 is positive with a Ki-67 index of approximately 20%.[19]
This tumor usually occurs in the dermis and subcutis of the distal extremities but has been found in other areas such as the head, neck, and mediastinum.[19] They have been reported in all age groups.[19]
Kaposi Sarcoma
Pathology and clinical presentation
Kaposi sarcoma (KS) is a rare malignant vascular tumor associated with a viral etiology (human herpesvirus 8).[23] The skin lesions were first described in 1872 by Moritz Kaposi. The incidence has increased worldwide secondary to the HIV-AIDS epidemic. It is an extremely rare diagnosis in children. Epidemic and iatrogenic forms of Kaposi sarcoma in children result from profound acquired T-cell deficiency that results from HIV infection and rare immune disorders.
A retrospective study has investigated the presentation of Kaposi sarcoma in children in endemic areas of Africa. Children usually present with cutaneous lesions, lymphadenopathy, and intrathoracic and oral lesions. Cutaneous lesions initially appear as red, purple, or brown macules, later developing into plaques and then nodules.[24-26]
Kaposi sarcoma is exceedingly rare in the pediatric population and is usually associated with immunocompromised states such as HIV infection or solid organ transplant.
Treatment of Kaposi sarcoma
Children with Kaposi sarcoma have responded to treatment with chemotherapy regimens, including bleomycin, vincristine, and taxanes, although there are no prospective clinical trials. Other treatment options have been based on adult studies (see below).
Because Kaposi sarcoma is rare in the pediatric population, there are no evidence-based studies. Even in adults, the evidence and quality of studies are poor, and it is difficult to recommend particular treatment regimens. Fifty-six Malawian children aged 3 to 12 years with Kaposi sarcoma were treated with six courses of vincristine, bleomycin, and oral etoposide. This was a high-risk population because 48 of the patients (86%) were HIV positive, of whom 36 (77%) were on antiretroviral therapy. Quality of life improved in 45 patients (80%). Eighteen patients (32%) had a complete remission. At 12 months, the overall survival rate was 71%, and the event-free survival rate was 50%.[27][Level of evidence: 3iiA]
In a systematic review of treatment for classic Kaposi sarcoma, 26 articles published from 1980 to 2010 were reviewed; articles describing populations at high risk secondary to previous transplantation and endemic and epidemic Kaposi sarcoma were excluded.[28] All articles had a minimum of five patients per intervention. A greater than 50% decrease in the size of the lesions or lymphedema was considered a response. The quality of the articles was considered poor, primarily because of lack of uniform staging criteria and variable means of assessing response. The following response rates for systemic treatments were noted:
- Pegylated doxorubicin: 71% to 100%.
- Vinca alkaloids: 58% to 90%.
- Etoposide: 74% to 76%.
- Taxanes: 93% to 100%.
- Gemcitabine: 100%.
- Vinblastine and bleomycin: 97%.
- Interferon alfa-2: 71% to 100%.
For local therapies, the following response rates were reported:
- Intralesional vincristine: 62%.
- Intralesional interferon alfa-2: 50% to 90%.
- Imiquimod: 56%.
(Refer to the PDQ summary on Kaposi Sarcoma Treatment for information about the treatment of Kaposi sarcoma in adults.)
References
- Hornick JL, Fletcher CD: Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol 35 (2): 190-201, 2011. [PubMed: 21263239]
- Billings SD, Folpe AL, Weiss SW: Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol 27 (1): 48-57, 2003. [PubMed: 12502927]
- Mirra JM, Kessler S, Bhuta S, et al.: The fibroma-like variant of epithelioid sarcoma. A fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer 69 (6): 1382-95, 1992. [PubMed: 1371711]
- Walther C, Tayebwa J, Lilljebjörn H, et al.: A novel SERPINE1-FOSB fusion gene results in transcriptional up-regulation of FOSB in pseudomyogenic haemangioendothelioma. J Pathol 232 (5): 534-40, 2014. [PubMed: 24374978]
- Amary MF, O'Donnell P, Berisha F, et al.: Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol 42 (7): 947-57, 2013. [PubMed: 23381465]
- Pranteda G, Magri F, Muscianese M, et al.: The management of pseudomyogenic hemangioendothelioma of the foot: A case report and review of the literature. Dermatol Ther 31 (6): e12725, 2018. [PubMed: 30239066]
- Joseph J, Wang WL, Patnana M, et al.: Cytotoxic and targeted therapy for treatment of pseudomyogenic hemangioendothelioma. Clin Sarcoma Res 5: 22, 2015. [PMC free article: PMC4615364] [PubMed: 26500758]
- Ozeki M, Nozawa A, Kanda K, et al.: Everolimus for Treatment of Pseudomyogenic Hemangioendothelioma. J Pediatr Hematol Oncol 39 (6): e328-e331, 2017. [PubMed: 28121744]
- Danforth OM, Tamulonis K, Vavra K, et al.: Effective Use of Sirolimus and Zoledronic Acid for Multiosteotic Pseudomyogenic Hemangioendothelioma of the Bone in a Child: Case Report and Review of Literature. J Pediatr Hematol Oncol 41 (5): 382-387, 2019. [PubMed: 31094908]
- El Darouti M, Marzouk SA, Sobhi RM, et al.: Retiform hemangioendothelioma. Int J Dermatol 39 (5): 365-8, 2000. [PubMed: 10849129]
- Colmenero I, Hoeger PH: Vascular tumours in infants. Part II: vascular tumours of intermediate malignancy [corrected] and malignant tumours. Br J Dermatol 171 (3): 474-84, 2014. [PubMed: 24965196]
- Keiler SA, Honda K, Bordeaux JS: Retiform hemangioendothelioma treated with Mohs micrographic surgery. J Am Acad Dermatol 65 (1): 233-5, 2011. [PubMed: 21679834]
- Hirsh AZ, Yan W, Wei L, et al.: Unresectable retiform hemangioendothelioma treated with external beam radiation therapy and chemotherapy: a case report and review of the literature. Sarcoma 2010: , 2010. [PMC free article: PMC2948930] [PubMed: 20936126]
- Enjolras O, Mulliken JB, Kozakewich HPW: Vascular tumors and tumor-like lesions. In: Mulliken JB, Burrows PE, Fishman SJ, eds.: Mulliken & Young's Vascular Anomalies: Hemangiomas and Malformations. 2nd ed. Oxford University Press, 2013, pp 259-324.
- Tamhankar AS, Vaidya A, Pai P: Retiform hemangioendothelioma over forehead: A rare tumor treated with chemoradiation and a review of literature. J Cancer Res Ther 11 (3): 657, 2015 Jul-Sep. [PubMed: 26458658]
- Dabska M: Malignant endovascular papillary angioendothelioma of the skin in childhood. Clinicopathologic study of 6 cases. Cancer 24 (3): 503-10, 1969. [PubMed: 5343389]
- Fanburr-Smith JC: Papillary intralymphatic angioendothelioma. In: Fletcher CDM, Bridge JA, Hogendoorn P, et al., eds.: WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press, 2013, pp 148.
- Neves RI, Stevenson J, Hancey MJ, et al.: Endovascular papillary angioendothelioma (Dabska tumor): underrecognized malignant tumor in childhood. J Pediatr Surg 46 (1): e25-8, 2011. [PubMed: 21238627]
- Shang Leen SL, Fisher C, Thway K: Composite hemangioendothelioma: clinical and histologic features of an enigmatic entity. Adv Anat Pathol 22 (4): 254-9, 2015. [PubMed: 26050262]
- Mahmoudizad R, Samrao A, Bentow JJ, et al.: Composite hemangioendothelioma: An unusual presentation of a rare vascular tumor. Am J Clin Pathol 141 (5): 732-6, 2014. [PubMed: 24713748]
- Tateishi J, Saeki H, Ito K, et al.: Cutaneous composite hemangioendothelioma on the nose treated with electron beam. Int J Dermatol 52 (12): 1618-9, 2013. [PubMed: 22998114]
- Soldado F, Fontecha CG, Haddad S, et al.: Composite vascularized fibular epiphyseo-osteo-periosteal transfer for hip reconstruction after proximal femoral tumoral resection in a 4-year-old child. Microsurgery 32 (6): 489-92, 2012. [PubMed: 22511340]
- Jackson CC, Dickson MA, Sadjadi M, et al.: Kaposi Sarcoma of Childhood: Inborn or Acquired Immunodeficiency to Oncogenic HHV-8. Pediatr Blood Cancer 63 (3): 392-7, 2016. [PMC free article: PMC4984265] [PubMed: 26469702]
- Dow DE, Cunningham CK, Buchanan AM: A Review of Human Herpesvirus 8, the Kaposi's Sarcoma-Associated Herpesvirus, in the Pediatric Population. J Pediatric Infect Dis Soc 3 (1): 66-76, 2014. [PMC free article: PMC3933043] [PubMed: 24567845]
- El-Mallawany NK, Kamiyango W, Slone JS, et al.: Clinical Factors Associated with Long-Term Complete Remission versus Poor Response to Chemotherapy in HIV-Infected Children and Adolescents with Kaposi Sarcoma Receiving Bleomycin and Vincristine: A Retrospective Observational Study. PLoS One 11 (4): e0153335, 2016. [PMC free article: PMC4833299] [PubMed: 27082863]
- Rees CA, Keating EM, Lukolyo H, et al.: Mapping the Epidemiology of Kaposi Sarcoma and Non-Hodgkin Lymphoma Among Children in Sub-Saharan Africa: A Review. Pediatr Blood Cancer 63 (8): 1325-31, 2016. [PMC free article: PMC7340190] [PubMed: 27082516]
- Macken M, Dale H, Moyo D, et al.: Triple therapy of vincristine, bleomycin and etoposide for children with Kaposi sarcoma: Results of a study in Malawian children. Pediatr Blood Cancer 65 (2): , 2018. [PubMed: 28988435]
- Régnier-Rosencher E, Guillot B, Dupin N: Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol 68 (2): 313-31, 2013. [PubMed: 22695100]
- Tsao MN, Sinclair E, Assaad D, et al.: Radiation therapy for the treatment of skin Kaposi sarcoma. Ann Palliat Med 5 (4): 298-302, 2016. [PubMed: 27701876]
- Singh NB, Lakier RH, Donde B: Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiother Oncol 88 (2): 211-6, 2008. [PubMed: 18439694]
- Lebbe C, Garbe C, Stratigos AJ, et al.: Diagnosis and treatment of Kaposi's sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur J Cancer 114: 117-127, 2019. [PubMed: 31096150]
Malignant Tumors
Malignant vascular tumors include the following:
Epithelioid Hemangioendothelioma
Incidence and outcome
This tumor was first described in soft tissue by Weiss and Enzinger in 1982. Epithelioid hemangioendotheliomas can occur at younger ages, but the peak incidence is in the fourth and fifth decades of life. The tumors can have an indolent or very aggressive course, with an overall survival rate of 73% at 5 years. There are case reports of patients with untreated multiple lesions who have a very benign course compared with other patients who have a very aggressive course. Some pathologists have tried to stratify patients to evaluate risks and adjust treatment, but more research is needed.[1-7]
A multi-institutional case series reported on 24 patients aged 2 to 26 years with epithelioid hemangioendothelioma.[8][Level of evidence: 3iiiDii] Most patients presented with multiorgan disease. Progression was seen in 63% of patients, with a mean time to progression of 18.4 months (range, 0–72 months).
The presence of effusions, tumor size larger than 3 cm, and a high mitotic index (>3 mitoses/50 high-power fields) have been associated with unfavorable outcomes.[3]
Histopathology and molecular features
A WWTR1-CAMTA1 gene fusion has been found in a large percentage of patients; less commonly, a YAP1-TFE3 gene fusion has been reported.[1] These fusions are not directly targetable with current medicines. Monoclonality has been described in multiple liver lesions, suggesting a metastatic process.
Histologically, these lesions are characterized as epithelioid lesions arranged in nests, strands, and trabecular patterns, with infrequent vascular spaces. Features that may be associated with aggressive clinical behavior include cellular atypia, one or more mitoses per 10 high-power fields, an increased proportion of spindled cells, focal necrosis, and metaplastic bone formation.[3]
The number of pediatric patients reported in the literature is limited.
Clinical presentation and diagnostic evaluation
Common sites of involvement are liver alone (21%), liver plus lung (18%), lung alone (12%), and bone alone (14%).[3,9,10] Clinical presentation depends on the site of involvement, as follows:
- Liver: Hepatic nodules have central vascularity on ultrasound, contrast-enhancing lesions by computed tomography, and low T1 signal and moderate T2 signal on magnetic resonance imaging. These may be incidental findings in asymptomatic patients, but most patients commonly present with signs or symptoms of cholestasis, including pruritus, jaundice, or scleral icterus.
- Lung: Pulmonary epithelioid hemangioendothelioma may be an asymptomatic finding on chest x-ray or be associated with pleuritic pain, hemoptysis, anemia, and fibrosis.
- Bone: Bone metastasis may be associated with pathologic fracture. On x-rays, they are well-defined osteolytic lesions and can be multiple or solitary.
- Soft tissue: Thirty percent of soft tissue cases are associated with metastases, and when present, can have a very aggressive course, with limited response to chemotherapy.
- Skin: Cutaneous lesions can be raised and nodular or can be warm, red-brown plaques.
Treatment of epithelioid hemangioendothelioma
Treatment options for epithelioid hemangioendothelioma include the following:
- Observation.
- Surgery.
- Immunotherapy.
- Targeted therapy.
- Chemotherapy.
- Radiation therapy.
For indolent cases, observation is warranted. For more aggressive cases, multiple medications have been used, including interferon, thalidomide, sorafenib, pazopanib, and sirolimus.[11] The most aggressive cases are treated with angiosarcoma-type chemotherapy. Surgery is performed when resection is possible. Liver transplant has been used with aggressive liver lesions, both with and without metastases.[3,12-15]
A multi-institutional case series reported on 24 patients aged 2 to 26 years with epithelioid hemangioendothelioma.[8][Level of evidence: 3iiiDii] Three patients who were treated with sirolimus achieved stable disease or a partial response for more than 2.5 years.
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment options under clinical evaluation for epithelioid hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT03148275 (Trametinib in Treating Patients with Epithelioid Hemangioendothelioma That Is Metastatic, Locally Advanced, or Cannot Be Removed by Surgery): This is a phase II trial assessing the efficacy of trametinib, with patient-reported outcomes as secondary aims.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Angiosarcoma of the Soft Tissue
Incidence
Angiosarcoma is a rare (accounting for 2% of sarcomas), aggressive, vascular tumor that can arise in any part of the body, but is more common in soft tissues. Angiosarcoma has an estimated incidence of 2 cases per 1 million people; in the United States, it annually affects approximately 600 people who are typically aged 60 to 70 years.[16]
Angiosarcomas are extremely rare in children and it is unclear if the pathophysiology of this tumor is different in the pediatric population. Cases have been reported in neonates and toddlers, with presentation of multiple cutaneous lesions and liver lesions, some of which are GLUT1 positive.[17-20] Most angiosarcomas involve the skin and superficial soft tissue, although the liver, spleen, and lung can be affected; bone is rarely affected.
Risk factors
Established risk factors include the following:[21]
- Vinyl chloride exposure.
- Radiation exposure.
- Chronic lymphedema from any cause, including Stewart-Treves syndrome.
Histopathology and molecular features
Angiosarcomas are largely aneuploid tumors. The rare cases of angiosarcoma that arise from benign lesions such as hemangiomas have a distinct pathway that needs to be investigated. MYC amplification is seen in radiation-induced angiosarcoma. KDR-VEGFR2 mutations and FLT4-VEGFR3 amplifications have been seen with a frequency of less than 50%.[21]
Histopathologic diagnosis can be very difficult because there can be areas of varied atypia. The common feature is an irregular network of channels in a dissective pattern along dermal collagen bundles. There is varied cellular shape, size, mitosis, endothelial multilayering, and papillary formation. Epithelioid cells can also be present. Necrosis and hemorrhage are common. Tumors stain for factor VIII, CD31, and CD34. Some liver lesions can mimic infantile hemangiomas and have focal GLUT1 positivity. Nomenclature of these liver lesions has been difficult and confusing with use of outdated terminology proposed in 1971 (e.g., type I hemangioendothelioma: infantile hemangioma; type II hemangioendothelioma: low-grade angiosarcoma; type III hemangioendothelioma: high-grade angiosarcoma).[18]
Treatment of angiosarcoma of the soft tissue
Treatment options for angiosarcoma of the soft tissue include the following:
- Surgery (localized disease).
- Radiation therapy (localized cutaneous disease in adults).
- Surgery, chemotherapy, and radiation therapy (metastatic disease).
Localized disease can be cured by aggressive surgery. Complete surgical excision appears to be crucial for the long-term survival of patients with angiosarcoma and lymphangiosarcoma despite evidence of tumor shrinkage in some patients who were treated with local or systemic therapy.[19,22-24] A review of 222 patients (median age, 62 years; range, age 15–90 years) showed an overall disease-specific survival (DSS) rate of 38% at 5 years. The 5-year DSS rate was 44% in 138 patients with localized, resected tumors but only 16% in 43 patients with metastases at diagnosis.[24] Data on liver transplant for localized angiosarcomas are limited.[25][Level of evidence: 3iiA]
Localized disease, especially cutaneous angiosarcoma, can be treated with radiation therapy. Most of these reported cases are in adults.[26]
Multimodal treatment with surgery, systemic chemotherapy, and radiation therapy is used for metastatic disease, although it is rarely curative.[27,28] Disease control is the objective in metastatic angiosarcoma, with published progression-free survival between 3 months and 7 months [29] and a median overall survival (OS) of 14 months to 18 months.[30] In both adults and children, 5-year OS rates between 20% and 35% are reported.[19,20,31]
In one child diagnosed with angiosarcoma secondary to malignant transformation from infantile hemangioma, response to treatment with bevacizumab, a monoclonal antibody against vascular endothelial growth factor, combined with systemic chemotherapy, has been reported.[17,27] A report of eight cases of liver angiosarcoma in children highlighted the misuse of the term hemangioendothelioma and the importance of early diagnosis and treatment of these tumors.[32]
Biologic agents that inhibit angiogenesis have shown activity in adults with angiosarcoma.[18,31]
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment options under clinical evaluation for angiosarcoma of the soft tissue
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02834013 (Nivolumab and Ipilimumab in Treating Patients With Rare Tumors): This is a phase II study of nivolumab and ipilimumab to treat patients with rare tumors. Immunotherapy with monoclonal antibodies such as nivolumab and ipilimumab may help the body's immune system attack the cancer and may interfere with the ability of the tumor cells to grow and spread.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Mehrabi A, Kashfi A, Fonouni H, et al.: Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 107 (9): 2108-21, 2006. [PubMed: 17019735]
- Haro A, Saitoh G, Tamiya S, et al.: Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 6 (4): 544-7, 2015. [PMC free article: PMC4511336] [PubMed: 26273413]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al.: Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 8 (2): 259, 2014. [PMC free article: PMC4419652] [PubMed: 25992243]
- Dong K, Wang XX, Feng JL, et al.: Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 8 (9): 11015-23, 2015. [PMC free article: PMC4637634] [PubMed: 26617819]
- Adams DM, Hammill A: Other vascular tumors. Semin Pediatr Surg 23 (4): 173-7, 2014. [PubMed: 25241094]
- Xiao Y, Wang C, Song Y, et al.: Primary epithelioid hemangioendothelioma of the kidney: the first case report in a child and literature review. Urology 82 (4): 925-7, 2013. [PubMed: 23726166]
- Reich S, Ringe H, Uhlenberg B, et al.: Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 32 (4): 274-6, 2010. [PubMed: 20445417]
- Cournoyer E, Al-Ibraheemi A, Engel E, et al.: Clinical characterization and long-term outcomes in pediatric epithelioid hemangioendothelioma. Pediatr Blood Cancer 67 (2): e28045, 2020. [PubMed: 31724797]
- Daller JA, Bueno J, Gutierrez J, et al.: Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 34 (1): 98-105; discussion 105-6, 1999. [PubMed: 10022152]
- Ackermann O, Fabre M, Franchi S, et al.: Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 53 (6): 615-9, 2011. [PubMed: 21832953]
- Stacchiotti S, Provenzano S, Dagrada G, et al.: Sirolimus in Advanced Epithelioid Hemangioendothelioma: A Retrospective Case-Series Analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol 23 (9): 2735-44, 2016. [PubMed: 27334221]
- Semenisty V, Naroditsky I, Keidar Z, et al.: Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15: 402, 2015. [PMC free article: PMC4437555] [PubMed: 25967676]
- Raheja A, Suri A, Singh S, et al.: Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci 22 (9): 1495-8, 2015. [PubMed: 25986183]
- Ahmad N, Adams DM, Wang J, et al.: Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw 12 (9): 1203-7, 2014. [PubMed: 25190690]
- Otte JB, Zimmerman A: The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr Transplant 14 (3): 295-7, 2010. [PubMed: 20331517]
- Cioffi A, Reichert S, Antonescu CR, et al.: Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am 27 (5): 975-88, 2013. [PubMed: 24093171]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PubMed: 24740626]
- Dehner LP, Ishak KG: Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 92 (2): 101-11, 1971. [PubMed: 5559952]
- Ferrari A, Casanova M, Bisogno G, et al.: Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39 (2): 109-14, 2002. [PubMed: 12116058]
- Deyrup AT, Miettinen M, North PE, et al.: Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35 (1): 70-5, 2011. [PubMed: 21164289]
- Elliott P, Kleinschmidt I: Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup Environ Med 54 (1): 14-8, 1997. [PMC free article: PMC1128629] [PubMed: 9072028]
- Lezama-del Valle P, Gerald WL, Tsai J, et al.: Malignant vascular tumors in young patients. Cancer 83 (8): 1634-9, 1998. [PubMed: 9781959]
- Fata F, O'Reilly E, Ilson D, et al.: Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86 (10): 2034-7, 1999. [PubMed: 10570428]
- Lahat G, Dhuka AR, Hallevi H, et al.: Angiosarcoma: clinical and molecular insights. Ann Surg 251 (6): 1098-106, 2010. [PubMed: 20485141]
- Orlando G, Adam R, Mirza D, et al.: Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 95 (6): 872-7, 2013. [PubMed: 23354302]
- Sanada T, Nakayama H, Irisawa R, et al.: Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Mol Clin Oncol 6 (3): 334-340, 2017. [PMC free article: PMC5403362] [PubMed: 28451409]
- Dickson MA, D'Adamo DR, Keohan ML, et al.: Phase II Trial of Gemcitabine and Docetaxel with Bevacizumab in Soft Tissue Sarcoma. Sarcoma 2015: 532478, 2015. [PMC free article: PMC4446476] [PubMed: 26074722]
- Scott MT, Portnow LH, Morris CG, et al.: Radiation therapy for angiosarcoma: the 35-year University of Florida experience. Am J Clin Oncol 36 (2): 174-80, 2013. [PubMed: 22314000]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PubMed: 11346333]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PMC free article: PMC208946] [PubMed: 11254674]
- Ravi V, Patel S: Vascular sarcomas. Curr Oncol Rep 15 (4): 347-55, 2013. [PubMed: 23852636]
- Grassia KL, Peterman CM, Iacobas I, et al.: Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 64 (11): , 2017. [PubMed: 28521077]
Special Considerations for the Treatment of Children With Cancer
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the primary care physician, a surgeon experienced in vascular tumors, a pathologist, radiation oncologists, pediatric oncologists, rehabilitation specialists, pediatric nurse specialists, social workers, and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life. (Refer to the PDQ summaries on Supportive and Palliative Care for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[2] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients and families. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PMC free article: PMC4136455] [PubMed: 24853691]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004. [PubMed: 15173520]
Changes to This Summary (11/24/2020)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Added text to state that most segmental hemangiomas occur in the head and neck region but can be seen in the genitourinary area, arm, chest, or legs. Also revised text to state that diffuse hemangiomas of the face demonstrate defined cutaneous patterns.
Added text to state that intralesional administration of propranolol has been used for periorbital lesions in a limited capacity and showed no advantages over oral administration (cited Mehta et al. as reference 89 and level of evidence 1iiDiv).
Added text to state that in response to the COVID-19 pandemic, the Hemangioma Investigator Group is studying the administration of propranolol for low-risk and standard-risk patients through virtual visits (cited Frieden et al. as reference 116).
Added text to state that indication for surgical removal of focal vascular lesions includes rupture, bleeding, and nonresolving coagulopathy. Two patients were reported to require surgical resection after the development of clinically significant ascites as their rapidly involuting congenital hemangiomas involuted (cited Gourgiotis et al. as reference 129).
Intermediate Tumors (Locally Aggressive)
Added Lim et al. as reference 9.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood vascular tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Vascular Tumors Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Julie Blatt, MD (University of North Carolina)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Holcombe Edwin Grier, MD
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Alberto S. Pappo, MD (St. Jude Children's Research Hospital)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Vascular Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/child-vascular-tumors-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26844334]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
- Notes on Vascular Malformations
- General Information About Childhood Vascular Tumors
- Benign Tumors
- Intermediate Tumors (Locally Aggressive)
- Intermediate Tumors (Rarely Metastasizing)
- Malignant Tumors
- Special Considerations for the Treatment of Children With Cancer
- Changes to This Summary (11/24/2020)
- About This PDQ Summary
- Review Childhood Vascular Tumors Treatment (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Childhood Vascular Tumors Treatment (PDQ®): Patient Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Childhood Liver Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Liver Cancer Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Childhood Soft Tissue Sarcoma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Soft Tissue Sarcoma Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Childhood Extracranial Germ Cell Tumors Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Extracranial Germ Cell Tumors Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Childhood Craniopharyngioma Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Craniopharyngioma Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Childhood Vascular Tumors Treatment (PDQ®) - PDQ Cancer Information SummariesChildhood Vascular Tumors Treatment (PDQ®) - PDQ Cancer Information Summaries
- Pseudomonas sp. 6-CSUMB 16S ribosomal RNA gene, partial sequencePseudomonas sp. 6-CSUMB 16S ribosomal RNA gene, partial sequencegi|608795759|gb|KJ187952.1|Nucleotide
- PhenolphthaleinsPhenolphthaleinsA family of 3,3-bis(p-hydroxyphenyl)phthalides. They are used as CATHARTICS, indicators, and COLORING AGENTS.<br/>MeSH
- ResveratrolResveratrolA stilbene and non-flavonoid polyphenol produced by various plants including grapes and blueberries. It has anti-oxidant, anti-inflammatory, cardioprotective, anti-mutagenic, ...<br/>Year introduced: 2019 (1989)MeSH
Your browsing activity is empty.
Activity recording is turned off.
See more...