This publication is provided for historical reference only and the information may be out of date.
Overview
The Office of Medical Applications of Research (OMAR) at the National Institutes of Health requested that the Agency for Healthcare Research and Quality (AHRQ), through its Evidence-based Practice Center (EPC) program, produce an evidence report for a State-of-the-Science conference on the topic of Management of Cancer Symptoms: Pain, Depression, and Fatigue.
EPCs review relevant scientific literature on assigned clinical care topics and produce evidence reports and technology assessments, conduct research on methodologies and the effectiveness of their implementation, and participate in technical assistance activities. The purpose of an evidence report is to search for and summarize evidence on several key questions on a specific topic. EPCs collaborate with science partners to formulate specific key questions. As specified by AHRQ in the EPC program, evidence reports do not make specific clinical recommendations; however, recommendations for future research are typically provided. Public and private sector organizations may use the reports and assessments as the basis for their own clinical guidelines and other quality improvement activities.
This evidence report summarizes the evidence on the prevalence, methods of assessment, and management of the following symptoms in patients with cancer: pain, depression, and fatigue. The symptoms and key questions were identified by the State-of-the-Science Conference planning committee composed of staff from OMAR, National Cancer Institute, national experts on this topic, as well as the EPC staff.
Symptoms in Cancer Patients
Despite remarkable advances in cancer biology and a widening array of treatment options, cancer continues to cause devastating suffering not only in the hundreds of thousands of patients who die of it each year in the United States, but also in some patients who are successfully treated and become cancer survivors. Pain, depression, and fatigue are prominent contributors to suffering in many of these individuals. Clinical research on these symptoms holds out the hope of relief for suffering through better understanding of these symptoms and the development of new, more effective treatments for them.
Pain, depression, and fatigue are complex subjective experiences. They are not directly measurable. To be studied, they must be defined operationally and estimated using patient self-report instruments. The development of tools that capture the multidimensional aspects of these symptoms has been an important clinical and research advance.
Figure 1 depicts the relationships between various factors that may contribute to the occurrence of cancer symptoms. Heterogeneity of the factors involved in each study is further compounded by heterogeneity of instruments or scales used to assess these symptoms. Studies that employ different designs, rely upon different inclusion and exclusion criteria, and use different assessment tools will likely report different rates of occurrence of symptoms. Hence, the interpretation and comparison of results from such diverse studies is difficult.
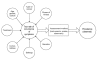
Figure
Figure 1. Relationship between some factors that contribute to the occurrence of cancer symptoms, methods of assessment, and prevalence of symptoms.
Dramatic progress in pain research has resulted from extensive laboratory investigations leading to increasingly detailed models of the fundamental mechanisms of pain. These advances in the understanding of the physiology of pain have led to clinically testable hypotheses regarding pain mechanisms and new treatments. There is, unfortunately, little fundamental research on depression in cancer. Its mechanisms may differ from non-cancer-related depression, but it is reasonable to assume that there is overlap and therefore a rationale for testing interventions found effective outside the context of cancer. Mechanisms of cancer-related fatigue remain generally unexplored despite its prevalence and impact. The dearth of information of the pathophysiology of cancer-related fatigue is reflected in the paucity of treatment trials for this condition.
The studies reviewed in this report provide evidence of progress in a number of other areas of symptom research. Large-scale, population-based studies of symptoms in cancer have been performed, providing estimates of symptom prevalence that are more generalizable than those obtained from small cohort studies. Some of the practical difficulties of accruing and retaining research subjects, who are depressed, fatigued, or in pain, have begun to be addressed. An impressive amount of data has been accumulated and some important insights have been gained about pain, depression, and fatigue in cancer. Using sophisticated assessment tools, investigators have measured the burden of pain, depression, and fatigue, and the factors that correlate with them, in a wide variety of settings: during curative or palliative treatments, in long-term survivors, and near the end of life. Some randomized controlled trials have led to the adoption of new treatments to relieve symptoms and ameliorate suffering. Given the scope and complexity of the problems, much remains to be done.
Overviewed in this chapter are the three cancer-related symptoms identified by the planning committee as the focus for the conference. The prevalence, assessment, and treatment for each of these symptoms are discussed.
Cancer-related Pain
Intractable pain is a complication dreaded by many patients with cancer. "Cancer pain" comprises acute pain, chronic pain, tumor-specific pain, and treatment- (including procedure-) related pain. Pain is a major cause of impaired quality of life (including decreased patient functionality and caregiver burden) in patients with cancer, and intensifies the distress and suffering commonly evoked in patients from diagnosis onwards (Bonica, 1990; Chapman and Garvin, 1999; Loeser and Melzack, 1999). New pain symptoms that prompt the diagnosis of cancer may provoke acute physiological responses and evolve into chronic pain. When a new pain appears in a patient known to have cancer, it may remain noticeable despite analgesic therapy and hence perpetuate fatigue due to interference with sleep, or distress in an anxious or depressed host. Pain caused by poorly controlled underlying or advancing pathophysiology heralds a demoralizing loss of control over one's body that typifies the clinical course of progressive disease. Pain is a reminder of cancer-related mortality, and is experienced within a personal, social, cultural and religious framework (Field and Cassel, 1997).
Inflammatory mediators associated with cancer include prostaglandins, cytokines, tumor necrosis factors, interleukins, growth factors and other tumor-derived algesic molecules such as endothelin (Davar, Hans, Fareed, et al., 1998), each of which can excite nociceptors (Schwei, Honore, Rogers, et al., 1999). Some cancers induce endogenous antibodies and others are treated by therapeutic administration of exogenous antibodies; both types of agents may evoke painful neuropathies (Sorkin, 2000). Preclinical research on bone pain in cancer suggests a distinctive neurochemical and histological "signature" in afferent nerves and their spinal cord connections in animal models of neoplasia (Schwei, Honore, Rogers, et al., 1999). Several elements within a spectrum of possible pain mechanisms may be active in a single patient with cancer pain (Woolf, Bennett, Doherty, et al., 1998). Cancer-related and non-cancer pains may involve neuropathic components, in which the nervous system is damaged (Woolf and Mannion, 1999), and nociceptive components, in which injury to non-neural tissue is conveyed through an undamaged nervous system. Pain may originate from visceral organs due to tumor infiltration or obstruction of a viscus, from pathology involving the surface of the body and conveyed via the somatic nerves that innervate the body surface. Clinicians have delineated a number of cancer pain syndromes. Some of these syndromes are due to tumor-specific patterns of local or distant metastasis, others reflect diffuse neuropathies from tumor products or chemotherapy, and still others involve localized neural damage such as nerve plexus injury from radiation therapy or infiltration by tumor.
The total experience of cancer pain encompasses not just pain intensity but also includes family, spiritual, behavioral, psychosocial and financial dimensions (Ferrell and Ferrell, 1996; Lang and Patt, 1994; McGuire, 1995). For this reason, and also because cancer and cancer treatment produce use cancer and cancer treatment produce a variety of related non-pain sedation, a consensus has emerged that optimal care for patients with cancer employs a multidimensional palliative framework that addresses multiple symptoms and patient concerns are simultaneously.
Prevalence of Cancer-related Pain
Cancer has a profound impact around the world because of its prevalence and associated morbidity and mortality (The World Health Organisation Global Programme For Cancer Control, 1993; Wolff, 1997). Poorly controlled pain is key to the impaired quality of life (QOL) that patients with cancer may suffer. Work in the 1970s and 1980s by Bonica (1985), Twycross (1976), Foley (1985), Daut and Cleeland (1982) and Stjernsward (The World Health Organisation Global Programme For Cancer Control, 1993) established the importance of cancer pain control and showed that about three-quarters of patients with advanced cancer experience pain. Surveillance data gathered in developed countries in order to characterize the incidence, prevalence, and in some studies the nature and severity of pain have enrolled about 60,000 patients. This global experience indicates that one-third to one-half of all patients undergoing active cancer treatment experience pain, and that the likelihood of pain is influenced by type of tumor, stage of disease, and extent of metastases (Bonica, 1985; Daut and Cleeland, 1982).
Of the over one million Americans diagnosed annually with cancer, 1500 die daily from this cause (Landis, Bolden, and Wingo, 1999). Nearly 10 million Americans now have or previously had cancer. During the past 25 years, age-adjusted incidence rates pooled across ages, race, and cancer type have increased significantly (about 20 percent) in the United States (Landis, Bolden, and Wingo, 1999; National Cancer Institute, 1999). In parallel with a global trend throughout developed countries, the population of the United States has on average grown older, and numbers of elderly have risen (U.S. Bureau of the Census, 1999). Although cancer is the second leading cause of death (after accidents) in children younger than 14 years in the United States, 5-year survival rates have improved substantially for many childhood cancers since the 1970s (Landis, Bolden, and Wingo, 1999). Unfortunately, despite calls for assigning a high priority to alleviating cancer pain in children (Schechter, Berde, and Yaster, 1993), many children who die of cancer have substantial suffering at the end of life (Wolfe, Grier, Klar, et al., 2000). The incidence and prevalence and hence impact of cancer increases dramatically with age. The cancer incidence rate is less than 50 cases per 100,000 in those under 25, and rises steadily with age to over 200 cases per 100,000 in the 40 to 44 age group. Pooled data for all ages from birth to 54 yield an incidence of just over 100 cases per 100,000; this rate increases 10-fold to over 1000 cases per 100,000 in the 55 to 64 age group, and further doubles in those over 65 to greater than 2000 cases per 100,000 (National Cancer Institute, 1999). Correspondingly, the cumulative percentage of the US population experiencing invasive cancers during their lifetime increases sharply from below 2 percent in the 0 to 39 age group, to just under 10 percent in the 40 to 59 age group, to about 30 percent in those older than 60 (Landis, Bolden, and Wingo, 1999; National Cancer Institute, 1999).
The aging and overall growth of the population plus the increased incidence and prevalence of cancer in those over 60 ensure that the national disease burden of cancer will increase at least in the near term, and that this burden will continue to fall disproportionately on the elderly (Ferrell BR and Ferrell BA, 1996).
Assessment of Cancer-related Pain
Patients with cancer may experience acute or chronic pain related to their primary diagnosis, from treatment, or from unrelated, even pre-existing disorders. Because of the multiple, often changing origins of pain and the importance of its control, the Joint Commission for the Accreditation of Healthcare Organizations requires that pain be assessed initially and serially thereafter during each clinical encounter in patients at risk for undertreated pain (2000). The subjective nature of pain requires patient participation in its assessment. Therefore the mere act of probing this aspect of a patient's personal, internal experience validates that experience (Morris DB, 1998) and demonstrates a patient-centered point of view (Gerteis, Edgman-Levitan, Daley, et al., 1999). A comprehensive approach to pain assessment in cancer care extends beyond nociceptive evaluation to encompass comorbid medical and psychosocial problems, the meaning and impact of pain upon the patient and significant others, and its effect upon quality of life (The World Health Organisation Global Programme For Cancer Control, 1993).
Initial evaluation of any patient with cancer-related pain is expected to include the essentials of any symptom history (location, intensity, quality, temporal characteristics, exacerbating and relieving factors, and responses to prior treatments), together with psychosocial assessment, physical examination, and appropriate diagnostic studies (Jacox, Carr, Payne, et al., 1994). Psychosocial assessment addresses the mood of the patient, his or her coping skills, family support structure, signs and symptoms of anxiety or depression, and expectations regarding pain management. Initial evaluation seeks to establish a pathophysiological mechanism for each pain, if possible as a recognized syndrome with well-described key features, natural history, and treatments of choice (Caraceni, Portenoy, and a Working Group of the IASP Task Force on Cancer Pain, 1999; Portenoy and Lesage, 1999). The determination that pain is primarily neuropathic or nociceptive helps guide initial selection of drug or non-drug therapy such as surgery or radiation therapy. Neuropathic pain can result from many potential disorders such as deafferentation, mono- or polyneuropathies, or a complex regional pain syndrome (Woolf and Mannion, 1999). Nociceptive pain may be due to somatic or visceral pathology (Cervero and Laird JMA, 1999).
Failure to assess cancer pain intensity serially using a standard, validated scale makes it difficult to judge the effectiveness of any analgesic regimen, or to compare one regimen with another (Jadad-Bechara AR, 1994; Max, 1996; McQuay and Moore, 1998). The 0-10 visual or verbal analog scales, or variants such as a thermometer, are validated, easy to administer, and widely used. Unless the patient is asked to rate pain at its lowest, average or highest intensity during a specific time interval, analog scale measurements provide only an instantaneous "snapshot" of pain intensity that is an incomplete picture of pain with activity, across days or weeks, or that prevents sleep at night. The Brief Pain Inventory (Daut and Cleeland, 1982) therefore captures data on pain intensity across tie as well as at the moment of completion of this instrument. Recognition that pain may impair other dimensions of quality of life and functionality has prompted inclusion of health-related quality of life (HRQOL) measures into clinical analgesic trials, and some clinics to monitor HRQOL during routine care.
Treatment of Cancer-related Pain
Undertreatment of cancer pain occurs over a third of the time even in wealthy, industrialized nations (Cleeland, Gonin, Hatfield, et al., 1994). The basis for such undertreatment is multifactorial. Inadequacy of clinicians' knowledge of effective pain assessment and management, negative attitudes of patients and clinicians toward the use of drugs for pain relief, regulatory issues that promote healthcare providers' concerns about regulatory scrutiny and unwarranted investigation (Joranson et al., 1998; Joranson et al., 2002) and problems of cost and reimbursement for effective pain management (Cleeland, 1987) each contribute.
Present day practical therapeutic options overlap substantially with those applied to treat noncancer-related pain. A generic approach to pain management involves beginning an NSAID with or without an adjuvant, adding a weak opioid for persistent or unresponsive pain, then exchanging the weak opioid for a strong opioid such as morphine. In this context, adjuvant refers to medications that treat concurrent symptoms that exacerbate pain, such as nausea or insomnia; augments or treats a side effect of opioid analgesia; or can diminish specific types of pain such as neuropathic pain (e.g., an anticonvulsant or a tricyclic antidepressant). This approach, developed and disseminated by the World Health Organization (WHO) as a three-step staircase or ladder whose therapeutic escalation is driven by persistent or unresponsive pain, is widely applied to treat cancer-related and noncancer pain in primary through tertiary care practice settings (Carr, Jacox, Chapman, et al., 1992). Because cancer pain provokes biopsychosocial responses in addition to purely nociceptive reactions, the effective management of cancer pain normally requires a multidisciplinary approach that includes behavioral interventions (similar to the approach to rehabilitation of patients with chronic pain not due to cancer). The "low-tech" WHO approach to pain control relies primarily upon the oral route of drug administration but requires no essential modification when alternate routes such as rectal, sublingual, subcutaneous, topical or transdermal are used for drug delivery (Bruera, Brenneis, Michaud, et al., 1988). Whether or not they suffer from cancer, patients whose pain fails to respond adequately to the WHO method may be offered, in settings with suitable infrastructures, more invasive interventions such as intravenous or intrathecal drug administration. Additional measures to control cancer pain that are rarely if ever used to treat noncancer pain include the use of biphosphonates, radionuclides, and chemical or surgical neurolysis.
Cancer-related Depression
Unfortunately, depression is sometimes viewed as being an "appropriate" symptom in cancer patients. However, it is never appropriate for cancer patients to suffer with significant depression. Cassem, (1997) notes that although massive bleeding is an "appropriate" sequela of a ruptured spleen, it is unthinkable to just stand by and allow a patient bleed to death.
Depression in cancer patients is treatable. Untreated, it can lead to decreased compliance with medical care, prolonged hospital stays, increased morbidity, and perhaps increased mortality (Herrmann, Brand-Driehorst, Kaminsky, et al., 1998; Richardson, Shelton, Krailo, et al., 1990; Spiegel and Kato, 1996). Depressed cancer patients are more likely to request euthanasia or physician-assisted suicide and are more likely to commit suicide (Emanuel, Fairclough, Daniels, et al., 1996; Henderson and Ord, 1997; Chochinov et al., 1999).
Prevalence of Cancer-related Depression
It is estimated that 20 to 25% of all cancer patients will experience depression during the course of their illness (Bottomley, 1998). People with cancer are three times more likely than the general population and almost twice as likely than other medically hospitalized patients to develop depression (Arolt, Fein, Driessen, et al., 1998; Kessler, McGonagle, Zhao, et al., 1994). The prevalence of depression in cancer patients is even higher in those with the greatest disability and distressing physical symptoms, especially uncontrolled pain.
There is great variation in the reported frequencies of depression in cancer populations. These varying estimates of depression in cancer patients may result from differing definitions of "depression," differing assessment tools, and different cancer populations with different significant variables such as timing of the assessment, physical debilitation, and concurrent treatment. Many of the retrieved studies do not employ the criteria for major depression described in the American Psychiatric Association's Diagnostic and Statistical Manual for Mental Disorders and appear instead to be measuring depressive symptoms (American Psychiatric Association, 1994).
Assessment of Cancer-related Depression
Given the seriousness of its impact, it is important for caregivers to recognize and treat depression. Past studies have shown that oncologists and primary care providers have difficulty recognizing depressive symptoms in cancer patients (Newell, Sanson-Fisher, Girgis, et al., 1998; Passik, Dugan, McDonald, et al., 1998). Major depression is a clinical entity with specific signs, symptoms, and treatments. It is more than just sadness.
"Depression" in comparison to pain or fatigue can be a set of symptoms or clinical syndromes. Depressive symptoms are present in several psychiatric disorders, with the most common ones in cancer patients being major depressive disorder, adjustment disorder, and depression secondary to a medical condition. Depressive symptoms can also be present in the absence of a psychiatric disorder.
Depression is more complicated and difficult to distinguish from symptoms of the underlying disease in cancer patients. The psychiatric diagnosis of major depressive disorder is defined by a set of criteria in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994). The DSM defines depression as having at least five of the following symptoms for at least 2 weeks: 1) depressed mood most of the day; 2) loss of interest or pleasure; 3) change in appetite and/or change in weight; 4) insomnia or hypersomnia; 5) psychomotor retardation or agitation; 6) loss of energy; 7) feelings of worthlessness or guilt; 8) poor concentration; and 9) thoughts of death or suicidal ideation. In order to meet the criteria for major depressive disorder, one of the patient's symptoms must be either depressed mood or loss of interest/pleasure, and the individual must also be experiencing distress or impairment in social, occupational, or other areas of functioning. Major depressive disorder is usually distinguished from an adjustment disorder by the degree, duration, or amount of symptoms.
Making the diagnosis of depression can be a challenge with the cancer patient. Many neurovegetative symptoms of depression -- especially loss of energy, loss of appetite, and sleep disturbance -- overlap with common symptoms of cancer or other medical illnesses, and with the side effects of medical treatments used in cancer patients.
Because these symptom-based diagnostic criteria may not be specific for depression in the context of medical illness, a set of psychological criteria has been suggested in their place. Endicott suggested substituting the psychological symptoms of self-pity, brooding, crying spells, and pessimism for the neurovegetative symptoms that overlap with cancer (Endicott, 1984). Some clinicians highlight the importance of the cognitive symptoms of depression, such as depressed thoughts, hopelessness about all aspects of their lives, guilt or worthlessness, and suicidal ideation.
Additionally, many medical factors in patients with cancer can contribute to, exacerbate, or even mimic depressive symptoms. These include uncontrolled pain, hypercalcemia, anemia, endocrinologic abnormalities, cancer involvement of the central nervous system, glucocorticoids, interferon, and some other chemotherapeutic agents (Roth and Holland, 1994).
The diagnosis of major depression is further entangled with patients' reactions to being diagnosed with cancer. Holland and Massie have described the natural history of emotions following the diagnosis of cancer (Massie, Speigel, Lederberg, et al., 1995). When confronted with a diagnosis of cancer, most patients experience a period of shock and disbelief. The second phase that follows is characterized by more visible distress: sadness, depressed mood, insomnia, anxiety, anorexia, and irritability that can last up to 2 weeks. During this time there is a sense of sadness and uncertainty about the future, and patients are often preoccupied with thoughts of illness, death, and losses. All of these feelings are within the normal limits of reacting at the time and do not constitute an episode of depression. However, some patients may have difficulty carrying out daily activities and have trouble concentration and processing information. Within a few weeks, however, most patients have adapted to their diagnosis, their depressive symptoms resolve, and treatment begins with some optimism and hope for the future.
Although clinical evaluation specifically for psychiatric symptoms is generally thought to be the best assessment, several instruments, both self-report and clinician-administered are being used to assess depressive symptoms. These instruments include three main types: standard psychiatric assessments (such as the Beck Depression Inventory or Hamilton Depression Inventory); instruments designed to assess symptoms in a medically ill population (such as the Hospital Anxiety and Depression Scale and the Brief Symptom Inventory); and rapid assessment instruments (such as The Distress Thermometer). Some of these instruments are currently being used in clinical settings to screen for depressive symptoms.
Treatment of Cancer-related Depression
Recognizing the importance of psychological symptoms that accompany cancer, the National Comprehensive Cancer Network (NCCN) published its consensus guidelines for the management of psychosocial distress in cancer patients (National Comprehensive Cancer Network, 1999). Depressive symptoms fall under the umbrella of psychosocial distress and are thought to be common in patients with cancer.
The NCCN guidelines contain three main components for management: screening, assessment, and treatment. Part of the assessment process is determining an appropriate treatment or referral. Depending on the severity of symptoms and the presence of suicidal ideation, the NCCN has algorithms for treatment.
As with depression in non-cancer populations, treatment for depression in cancer patients can take the form of psychosocial interventions and psychotherapy, medications, or alternative treatments. Many times patients receive a combination of these treatments.
Much of the research on treatment for depression in cancer patients has been on psychosocial interventions. Support groups, group therapy, psycho-education, cognitive-behavioral therapy, and individual counseling are all psychosocial interventions that are being used clinically and have been studied. In contrast to non-cancer populations and based on the high prevalence rates of depression in cancer patients, there have been many preventative trials of psychosocial interventions on depressive symptoms.
There has been considerably less research on the effects of psychopharmacologic interventions on depression in cancer patients. This may be due at least in part to some of the special challenges that an oncology sample would pose compared to a standard psychiatric sample in a clinical trial. Holland, Romano, Heiligenstein, et al., (1998) have cited some of these challenges: finding treatment groups that are homogeneous enough to obtain meaningful results, recruitment of large numbers of subjects to achieve adequate power to detect differences, and difficulties with retention and completion, often complicated by medical morbidity and mortality.
Increasing general interest in alternative treatments in both oncology and psychiatry has prompted many clinicians and patients to try these therapies. Although there is less data available on these treatments, research in this area is also increasing.
Cancer-related Fatigue
Fatigue associated with cancer and cancer treatment appears to be qualitatively and quantitatively distinct from the fatigue that is experienced intermittently by healthy individuals. Although the experience of fatigue probably varies greatly among individual patients, common themes in the subjective accounts of patients with cancer are the persistence of fatigue despite rest and sleep, and its interference with physical and mental function. Cancer-related fatigue probably encompasses a number of distinct syndromes, including muscle weakness, lack of stamina, and loss of ability to concentrate. Imprecision in the terminology of fatigue may sometimes mask important distinctions between these syndromes. Fatigue may be the final common pathway arising from a complex combination of physical syndromes and psychological states that is different in each oncology patient. Nonetheless, the term fatigue is useful because it represents an experience that is universally recognized.
Although fatigue is at least as prevalent and debilitating as pain in many contexts, it may often go unrecognized unless specifically addressed by caregivers. Even when recognized, treatment options are limited unless reversible conditions that contribute to fatigue can be addressed.
Fatigue in cancer patients is both a side effect of treatment and a consequence of the biologic effects of the cancer itself. The direct effects include (but are certainly not limited to) metabolic and nutritional disturbances, endocrine dysfunction, neurologic, psychological, neuromuscular, and cognitive effects. The mechanisms of many of these effects are poorly understood. Fatigue has been ascribed to the abnormal production of inflammatory cytokines in the setting of cancer, but the specific evidence for the role of cytokines in cancer-related fatigue is fragmentary.
Methodological difficulties in the study of the mechanisms of cancer-related fatigue include the ability to determine whether elevated levels of biologic factors such as cytokines cause symptoms or are merely associated with them. Also, the development of animal models of cancer-related fatigue is problematic due to the inherent subjectivity of the symptom and the difficulty of establishing objective, behavioral correlates of fatigue. Voluntary, motivated activity has been proposed as one such correlate in animals (Ottenweller, Natelson, Gause, et al., 1998). In human subjects, patterns of rest and activity as measured continuously by a wrist actigraph were found to correlate with subjective self-reports of fatigue (Berger, 1998).
In addition to the direct effects of cancer and the various modes of cancer treatment, a wide variety of other phenomena contribute to fatigue in cancer patients. The best studied of these (and the most amenable to available interventions) is anemia due to chemotherapy. The impact of depression in cancer is outlined elsewhere in this study, and while depression undoubtedly contributes to fatigue in cancer patients, the interaction of fatigue and depression has not been well studied. A variety of other cancer symptoms, including dyspnea, fever, nausea, and pain probably contribute to fatigue. In addition, sedation is a common side effect of the medications used to treat cancer symptoms, notably the opioid analgesics and anti-emetics, another factor that may contribute to fatigue.
Prevalence of Cancer-related Fatigue
Reported prevalence rates of fatigue in cancer patients and survivors are extremely variable, reflecting both the heterogeneity of the populations studied, the variety of techniques used to measure fatigue, and the absence of consensus on criteria for the definition of fatigue. There is reason to suspect, however, that many studies systematically underestimate the degree of fatigue experienced by cancer patients, since the most fatigued patients may decline to participate in studies or may be unable to attend academic referral centers where most studies were performed. Thus, there are potential biases involved in the use of small, non-random cohorts in studying the prevalence of cancer-related fatigue. There are only two true population-based studies (Cella, Davis, Breitbart, et al., 2001; Vogelzang, Breitbart, Cella, et al., 1997). They assessed the prevalence of fatigue in telephone surveys of cancer patients and survivors recruited from large, representative samples of households in the United States, rather than in non-random or convenience samples as were used in many other studies.
Each study assessed fatigue over a short time period, for example during a course of radiation therapy and shortly after its completion (Irvine, Vincent, Graydon, et al., 1998), or in survivors at one time point after treatment (Woo, Dibble, Piper, et al., 1998). We were unable to identify longitudinal studies of fatigue reporting its prevalence and patterns over the entire course of illness using uniform methodology.
Assessment of Cancer-related Fatigue
The NCCN has developed practice guidelines for cancer-related fatigue reflecting currently accepted approaches to assessment and treatment (Atkinson, Barsevick, Cella, et al. 2000). That report emphasizes the high prevalence of fatigue at all stages of cancer and the need for screening and assessment of fatigue as an integral part of cancer care. Since fatigue is subjective, patient self-reports are central to its assessment. Information provided by family members can offer a perspective on the behavioral consequences of fatigue. The medical history, physical examination, and laboratory data may also be useful in the assessment of fatigue. The NCCN guideline has recommended the routine use of a quantitative or semi-quantitative screening device, such as a 0-10 numeric scale. For those with moderate or severe fatigue, a focused evaluation is performed to assess the characteristics of the fatigue and to determine whether contributing factors such as pain, emotional distress, anemia, sleep disturbance or endocrine dysfunction are present. If such factors are not identified a more comprehensive evaluation is recommended, including a review of systems, review of medications, assessment of comorbidities, nutritional and metabolic assessment, and assessment of activity.
The assessment of cancer-related fatigue in the context of clinical research has a somewhat different emphasis than the clinical guidelines promulgated by the NCCN. The foci of assessment in fatigue research have been: 1) to quantify the prevalence, pattern and severity of fatigue in a variety of settings 2) to determine factors that predict or correlate with fatigue, including other symptoms, biological parameters (e.g., cytokine or hormone levels), disease and treatment variables, and demographics, and 3) to assess the response of fatigue to interventions. For these purposes, a number of patient self-report instruments have been developed, and an epidemiology of fatigue is beginning to emerge as described in this report. A number of issues remain to be addressed regarding the assessment of cancer-related fatigue, however. These include the clinical significance of measurements of fatigue, the development of objective, measurable physiologic correlates of fatigue, methods for comparing measurements obtained by different fatigue instruments, and the degree to which cancer-related fatigue can be attributed to specific etiologic factors such as the effects of chemotherapy and radiotherapy, anemia, and depression.
Treatment of Cancer-related Fatigue
There is limited evidence to support specific interventions to ameliorate cancer-related fatigue, with the exception of epoetin alfa for the treatment of anemia and its symptoms during chemotherapy (Littlewood, Bajetta, Nortier, et al., 2001). This is perhaps not surprising given the fragmentary understanding of the fundamental physiology of fatigue, and its probable multifactorial basis in most patients. The initial approach advocated by the NCCN panel is therefore to assess regularly and address the common, potentially remediable factors that may contribute to fatigue, such as anemia, sleep disturbances, medication effects, electrolyte abnormalities and hypothyroidism. The degree to which cancer-related fatigue can be attributed to such reversible causes is unknown, but it is fairly clear that there is an enormous burden of fatigue that is not attributable to such specific causes or persists despite appropriate treatment for them. When specific, reversible causes of fatigue cannot be identified, or fatigue fails to respond to appropriate intervention for them, the NCCN panel of experts advocates a more comprehensive assessment, education and counseling, and consideration of a number of interventions. These include non-pharmacologic approaches such as exercise, stress management, and energy conservation, and pharmacologic approaches such as corticosteroids, psychostimulants, and antidepressants. These recommendations were the result of a consensus based on clinical experience, rather than evidence from randomized clinical trials. As reviewed below, a small number of randomized controlled trials have evaluated psychosocial interventions, exercise programs, and other methods of treatment for fatigue. The data on exercise is promising, but in general unless a specific etiology of fatigue (e.g., anemia or depression) can be identified and treated, there is extremely limited evidence from clinical trials to support any interventions for cancer-related fatigue.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Carr D, Goudas L, Lawrence D, et al. Management of Cancer Symptoms: Pain, Depression, and Fatigue. Rockville (MD): Agency for Healthcare Research and Quality (US); 2002 Jul. (Evidence Reports/Technology Assessments, No. 61.) 1, Introduction.
