NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Buscemi N, Vandermeer B, Friesen C, et al. Manifestations and Management of Chronic Insomnia in Adults. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Jun. (Evidence Reports/Technology Assessments, No. 125.)
This publication is provided for historical reference only and the information may be out of date.
Literature Review
The database searches resulted in 16,991 references of potentially relevant articles. One thousand two hundred studies were evaluated for inclusion in the review; 528 studies were potentially relevant to prevalence, natural history, incidence, risk factors and consequences of chronic insomnia, and 672 studies were potentially relevant to efficacy and safety of treatments used in the management of chronic insomnia. The application of inclusion criteria resulted in 79 studies included and 449 studies excluded for the questions on manifestations of chronic insomnia, and 116 studies included and 556 studies excluded for the question on management of chronic insomnia.
The primary reasons for exclusion of studies potentially relevant to manifestations of chronic insomnia were as follows: (1) the study was reported in a language other than English (n=9), (2) the report was a review (n=38), (3) the study was not relevant to the review topic (n=71), (4) the study was a case report (n=9), (5) the study did not have a control group (n=47), (6) the study did not examine an adult population (n=8), (7) the study population did not have chronic insomnia as defined in this report (n=208), (8) the study did not report on any of the outcomes of this review (n=58), and (9) data relevant to the study outcomes were not adequately reported (n=1). The primary reasons for exclusion of studies potentially relevant to the management of chronic insomnia were as follows: (1) the study was reported in a language other than English (n=27), (2) the report was a review/commentary/practice parameter (n=32), (3) the study report was a duplicate publication (n=3), (4) the study did not examine an adult population (n=17), (5) the study population did not suffer from chronic insomnia as defined in this report (n=221), (6) the study was not a randomized controlled trial (n=160), (7) the study did not have a placebo arm (n=48), (8) the study was not double-blind (n=15), (9) the study did not report on any of the outcomes of this review (n=16), and (10) data relevant to the study outcomes were not adequately reported (n=15).
The rate of disagreement between reviewers for inclusion/exclusion of studies was 61/528 (11.6 percent) for the questions on manifestations of chronic insomnia and 53/672 (7.9 percent) for the question on management of chronic insomnia. The primary reason for disagreement between reviewers was oversight of study details, such that reviewers erred on the side of over-inclusion. Therefore, consensus often resulted in exclusion of studies: for the questions on manifestations of chronic insomnia, 18 disagreements resulted in inclusion and 43 disagreements resulted in exclusion, and for the question on management of chronic insomnia, eight disagreements resulted in inclusion and 45 disagreements resulted in exclusion.
Flow Diagram 2 outlines study retrieval and selection for the review.
Data Synthesis
How is chronic insomnia defined, diagnosed and classified, and what is known about its etiology?
There is lack of consensus regarding the “ideal” definition of insomnia and what constitutes chronic insomnia. The threshold of clinically significant sleep disturbance is not established, nor has the morbidity resulting from insomnia been well studied. It is a matter of debate as to which definition of insomnia encompasses the problem as it would appear in a clinical and/or research setting, and whether the definition of chronic insomnia should be distinct. As a result, many variations in the definition of insomnia exist, especially for research purposes. We reviewed multiple sources to define, diagnose and classify chronic insomnia, including the Principles and Practice of Sleep Medicine textbook,15 diagnostic manuals (International Classification of Sleep Disorders-Revised (ICSD-R) and Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV)),37–38 and standards of practice published by the American Academy of Sleep Medicine.1;39
Definition of chronic insomnia
The International Classification of Sleep Disorders Manual. The International Classification of Sleep Disorders Manual is a comprehensive diagnostic manual, which is used as a reference among sleep researchers and physicians for sleep disorders in adults. The manual outlines a highly specific system for diagnosis and classification of insomnia, and includes over 40 diagnoses that may involve a complaint of insomnia. The International Classification of Sleep Disorders defines insomnia as difficulty in initiating and/or maintaining sleep or non-restorative sleep after a habitual sleep episode.37 The ICSD-R further differentiates insomnia based on severity of symptoms that impact daytime functioning. Mild insomnia is often associated with a feeling of restlessness, irritability, mild anxiety, daytime fatigue and tiredness, without evidence of social or occupational impairment. In contrast, moderate insomnia is accompanied by either mild or moderate impairment of social and occupational functioning. Moderate insomnia is always associated with feelings of restlessness, irritability, anxiety, daytime fatigue and tiredness. Severe insomnia is associated with symptoms similar to moderate insomnia, with severe impairment of social and/or occupational functioning. The duration of the insomnia is usually classified as acute (< 4 weeks), sub-acute (> 4 weeks but < 6 months) or chronic (> 6 months). Investigators have not consistently adhered to this classification scheme to determine severity and duration of insomnia in study populations, thus the definition of insomnia across studies varies. This classification scheme has coding for insomnia secondary to psychiatric conditions, substance abuse as well as medical and sleep disorders. The ICSD-R has been revised, and another edition of the ICSD (ICSD2) is in press for publication. This revised coding manual will replace the current ICSD-R.
International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). The World Health Organization-supported definition for nonorganic insomnia is a condition of unsatisfactory quantity and/or quality of sleep, which persists for a considerable period of time, including difficulty falling asleep, difficulty staying asleep, or early final wakening. It also states that insomnia is a common symptom of many mental and physical disorders, and should be classified here in addition to the basic disorder only if it dominates the clinical picture. The duration of insomnia is not specified.
Diagnostic and Statistical Manual of Mental Disorders. In contrast to the definition of insomnia in the ICSD-R, in the DSM-IV, insomnia is not subcategorized, but rather referred to as primary insomnia,38 implying that insomnia is not caused or significantly influenced by a psychiatric disorder. Insomnia caused or associated with psychiatric illness is classified separately. The duration of insomnia is listed as being longer than one month. Chronic insomnia is not defined in the DSM nosology.
American Academy of Sleep Medicine: Standards of Practice. The standards of practice published by the American Academy of Sleep Medicine defines insomnia as a complaint of unsatisfactory sleep, which may involve difficulty initiating sleep, frequent or lengthy awakenings, early awakening, inadequate total sleep time or poor quality of sleep, impacting daytime functioning.1 The daytime dysfunction may include any of the following: change in alertness, energy, cognitive function, behavior or emotional state. This definition of insomnia allows for subjective diagnosis in a clinical setting based on the patients' history, without the aid of polysomnography. Although short-term insomnia is generally considered to last less than three months, the time frame for chronic insomnia is not explicitly stated in the standards of practice statement.
Classification of insomnia
The International Classification of Sleep Disorders Manual. A clinically relevant classification of insomnia is outlined in the ICSD-R, 2001.37 According to the ICSD-R, the different categories of insomnia include: psychophysiological insomnia, sleep state misperception, idiopathic insomnia, as well as insomnia secondary to other medical conditions or sleep disorders. Acute and chronic insomnia are not classified separately; however, insomnia is considered to be chronic if the symptoms last for more than six months. A brief overview of the ICSD-R categories follows:
- Psychophysiological insomnia, also known as conditioned or learned insomnia, is a disorder of somatized tension and learned sleep preventing associations that result in a complaint of insomnia and associated decreased functioning during wakefulness.37 Continued problems in somatized tension and maladaptive learned sleep-preventing associations can worsen insomnia, creating a vicious cycle by perpetuating the initial problem. One must search for precipitating, predisposing and perpetuating factors for insomnia. A hallmark of this diagnosis is the individual's fixation with his/her sleep problem. This diagnosis cannot be made in the context of other medical or psychiatric disorders. Associated features include perceived decrement in daytime mood and functioning, without overt sleepiness. The true prevalence of psychophysiological insomnia in the general population is not known, although approximately 15 percent of patients referred to a sleep disorders clinic suffer from this type of insomnia.37 Diagnostic criteria for psychophysiological insomnia include:
- a) A combination of a complaint of insomnia and a complaint of decreased functioning during wakefulness.
- b) Indications of learned sleep-preventing associations such as trying too hard to sleep, or increased arousal in the bedroom (concern and worry about sleep).
- c) Evidence of somatized tension.
- d) Polysomnography may show increased sleep latency, reduced sleep efficiency and increased number and/or duration of awakenings during the sleep period.
- e) No other medical condition accounts for the sleep disturbance.
- Sleep state misperception, or pseudo insomnia, is a subjective complaint of problems initiating or maintaining sleep without objective findings to support the complaint. There is no psychopathology per se associated with this disorder. The afflicted individual honestly has complaints of insomnia and decreased daytime functioning, without objective data to support the claim. Although the exact prevalence of this disorder is not known, this group accounts for approximately 5 percent of individuals with complaints of insomnia. Diagnostic criteria for sleep state misperception include:
- a) complaint of insomnia.
- b) sleep quality and quantity are normal.
- c) polysomnography shows normal sleep latency, sleep duration and awakenings during the sleep period.
- Idiopathic Insomnia is defined as a life-long inability to obtain adequate sleep and may be related to abnormalities in the neurological systems affecting the sleep-wake cycle. The exact prevalence of this disorder is not known, but it is thought to be rare. Diagnostic criteria for idiopathic insomnia include:
- a) Complaint of insomnia with decreased functioning.
- b) Insomnia is life-long and may begin in early childhood.
- c) Insomnia is relentless and does not vary.
- d) Polysomnography shows increased sleep latency, decreased sleep efficiency and multiple awakenings during the night.
- e) No other medical illness or disease explains the early onset of insomnia.
Proposed classification for insomnia. A more recent article considers a novel method for classifying insomnia for research purposes.40 The authors propose research diagnostic criteria for Insomnia Disorder, Primary Insomnia, Insomnia due to a Mental Disorder, Paradoxical Insomnia and Psychophysiological Insomnia. The main differences between this classification scheme and that of ICDS-R, 2001, is that the criteria are more precise and the duration of symptoms must be more than one month for all categories. This classification scheme does not define a subcategory of chronic insomnia. This classification scheme was developed to allow for clear categorization of insomniacs within a study population, and thus avoid the study of a heterogeneous population. Based on a review of the literature, acute, situational or transient insomnia is considered to be different from chronic insomnia. It is not clear whether there are distinct differences in the nature of insomnia that lasts for more than 1 month, but less than 6 months versus insomnia lasting for more than 6 months.
Diagnosis and assessment of insomnia. Different evaluation methods have evolved to identify individuals with insomnia. Diagnosis of insomnia is made in the context of a clinical history based on any of the aforementioned criteria or definitions. There are semi-structured or structured interviews available for diagnosing insomnia [i.e., Insomnia Interview Schedule and Duke Structured Sleep Inventory (the latter is currently being evaluated in a large-scale study)]. Sleep diaries/logs, sleep histories, actigraphy, ambulatory monitoring, and in-home polysomnography are often used to assess sleep parameters. The most commonly used measure for evaluation of insomnia is self-reported questionnaires. The use of objective tools, such as polysomnography or multiple sleep latency tests for the diagnosis of insomnia are not recommended.41–43 Sleep diaries are essential for identifying sleep onset and sleep maintenance difficulties; however, the reporting of sleep onset latency by diary is subjective. Scientists have tried to evaluate more objective measures for measuring sleep disturbances in patients with insomnia, but currently available tools have limitations (polysomnography and unattended home studies), and are most commonly used to diagnose sleep disorders other than insomnia. Moreover, these methods are cumbersome and costly. Actigraph monitors are small watch-like devices that are worn on the wrist and are used to record movement; they can be useful adjuncts for gathering data from individuals with sleep complaints; however, these devices are not indicated for the routine diagnosis of any sleep disorder.39 There is currently no biomarker of insomnia, which makes objective diagnosis of insomnia more difficult. Research in the area of insomnia has recently been directed towards identifying specific hormones or neurotransmitters that may be involved in this disorder.15 Various research groups are studying the link between specific electroencephalogram findings and insomnia. A less commonly used diagnostic tool for insomnia, position emission tomography imaging, has been used to evaluate brain metabolism and its role in insomnia.44 Insomnia is a clinical diagnosis and the lack of a research model for insomnia makes it difficult to target appropriate therapy for this disorder and evaluate treatment outcomes.
Etiology of insomnia. Despite significant advances in sleep medicine over the past 50 years, much less is known about the cause of insomnia, its natural history, and its consequences than the treatments available for insomnia. Recent studies have demonstrated an increased metabolic rate in patients with insomnia,45 suggesting that sleep difficulties may at least partially have a physiological basis. It is also speculated that patients with insomnia are more aroused than people without insomnia;46 however, this theory is difficult to prove, given that the neurotransmitters involved in arousal are unknown. Advances in molecular genetics have shed light on the potential role of genetics in sleep disorders. Indeed, a familial etiology of this disorder has been postulated. A recent study concluded that more than 33 percent of patients with insomnia had a family history of insomnia.47 A similar study estimated a family history of insomnia among first-degree relatives of people suffering from insomnia to be 48.8 percent, compared to 23.5 percent among first-degree relatives of people who did not suffer from insomnia.48 The familial aggregates of insomnia have led researchers to investigate the genetic basis of insomnia, but no specific gene has been implicated.
Certain populations, including the elderly, psychiatric patients, and those suffering from chronic pain are known to have more chronic sleep maintenance problems.1;49 A strong link has been found between insomnia and depression.50 The directionality of the association has not been fully elucidated, but the association appears to be strong.1
Environmental factors, such as irregular sleep schedules, use of caffeine or other stimulants, co-morbid medical conditions, and/or shift-work may also predispose vulnerable individuals to insomnia.1 Genetic predisposition, in addition to environmental factors, are likely involved in the development and maintenance of insomnia, and differences in the relative exposure to these influences may explain differences in the manifestation of this disorder across affected individuals.
What are the prevalence, natural history, incidence and risk factors for chronic insomnia?
What are the consequences, morbidities, co-morbidities and public health burden associated with chronic insomnia?
Prevalence. A description of key features of studies relevant to prevalence of chronic insomnia is provided in Tables 3a (prevalence in general population), 3b (prevalence in outpatients of general practice) and 3c (prevalence in clinical populations), and additional information on each study is provided in Evidence Table C-1 ♦. The evidence provided in Tables 3a, b and c is summarized here.
Table 3a
Prevalence of chronic insomnia in adults: general population.
Table 3b
Prevalence of chronic insomnia in adults: outpatients of general practice.
Table 3c
Prevalence of chronic insomnia in adults: clinical population.
Forty-one studies provided evidence regarding the prevalence of chronic insomnia in adults; 38/41 studies were cross-sectional studies, one study was a cross-sectional case-control study and compared the prevalence of chronic insomnia in bipolar subjects and healthy controls,51 one study had both cross-sectional and case-control components,52 and one study had both cross-sectional and cohort components.53 Seventeen studies were considered of high quality, 19 studies were considered of moderate quality and five studies were considered of low quality.
Twenty-four studies described the sampling frame; most studies used census data, phone lists or patients presenting to a health clinic. Thirty-four studies described the sampling method; 21 studies used a random method, while the majority of the remaining studies used a non-random method, and a minority of studies investigated entire populations. Twenty-seven studies provided a response or follow-up rate. The rate ranged from 25–100 percent; slightly more than half of the studies had a rate greater than 80 percent. All studies except one 54 clearly described the method of data collection; 19 studies used self-reported questionnaires, 12 studies used face-to-face interviews and 10 studies used phone interviews. One of these studies 55 used both self-reported questionnaires and phone interviews. Of the studies that described the method of data collection, slightly more than half used a validated method. The criteria used to establish the duration of chronic insomnia ranged from one month to one year; most of the studies required that participants suffer from insomnia symptoms for at least one month. Most studies reported the gender distribution of the population; the majority of studies used mixed gender populations and a minority of studies used all-female populations. All studies provided an estimate of the age of the population; the age of the populations ranged from 18 to 98 years, based on studies that provided a range.
Twenty-six studies investigated general populations (Table 3a), eight studies investigated populations of outpatients from general practice (Table 3b), and eight studies investigated clinical populations (Table 3c). For high quality studies investigating general populations, the prevalence of chronic insomnia ranged from 5–45 percent, and the median was 17.6 percent. The interquartile range was 8.5–24.3 percent. For moderate quality studies investigating general populations, the prevalence of chronic insomnia ranged from 7.5–42.5 percent, and the median was 15.3. The interquartile range was 11.2–29.2 percent.
We did not identify any high quality studies investigating the prevalence of chronic insomnia in outpatients of general practice. For moderate quality studies investigating this population, the prevalence of chronic insomnia ranged from 11.7–63.7 percent, and the median was 38.4 percent. The interquartile range was 19.8–53.7 percent.
We identified only one high quality study investigating clinical populations, and it compared the prevalence of chronic insomnia in patients with Bipolar I disorder and a non-psychiatric population.56 One hundred percent of participants in the psychiatric group reported a long-standing sleep disturbance, while 21 percent of participants in the non-psychiatric group reported such a disorder. For moderate quality studies investigating clinical populations, the prevalence of chronic insomnia ranged from 26–51.3 percent, with a median of 33.5 percent. The interquartile range was 27.8–43 percent. The disorders of the clinical populations investigated were Parkinson's Disease, brain injury, diabetes, stroke, Bipolar I disorder, migraines, blood disorders and metastatic breast cancer.
Natural history. A description of key features of the study relevant to natural history is provided in Table 4, and additional information on this study is provided in Evidence Table C-1. The evidence provided in Table 4 is summarized here.
Table 4
Natural history of chronic insomnia in adults.
Only one study was identified that provided evidence regarding the natural history of chronic insomnia,53 and it was of moderate quality. In this prospective cohort study, the participants consisted of outpatients from general hospitals with sleep complaints lasting for at least one month. The population was of mixed gender and had an age range of 18–65 years. At a four-month follow-up, the prevalence of chronic insomnia was reduced from 100 percent to 86.9 percent, providing a remission rate of 13.1 percent. The follow-up rate was 42.2 percent.
Incidence. We did not identify any studies that provided evidence on the incidence of chronic insomnia in adults.
Associated factors. The majority of studies identified did not have designs that would support the categorization of an associated factor of chronic insomnia as either a risk factor or consequence of the disorder, such as longitudinal cohort studies. Most studies that examined the risk factors and consequences of interest were either of a cross-sectional or cross-sectional case-control design. Thus, we do not report on risk factors and consequences of chronic insomnia per se, rather we report on associated factors. For simplicity, we separate the results relevant to the various factors according to their categorization in the relevant question of the review, such that potential risk factors are reported separately from potential consequences.
A description of key features of the studies relevant to associated factors is provided in Table 5, and additional information for each study is provided in Evidence Table C-1. The evidence provided in Table 5 is summarized here.
Table 5
Factors associated with chronic insomnia in adults.
Sixty-seven studies provided evidence regarding the association between various factors and chronic insomnia in adults; 30/67 studies were cross-sectional studies and 37/67 studies were cross-sectional case-control studies. The cross-sectional case-control studies compared chronic insomniacs (cases) and normal sleepers (control) on various factors to determine whether these factors are associated with chronic insomnia. Similarly, the cross-sectional studies examined the relationship of various factors and chronic insomnia by comparing chronic insomniacs and normal sleepers within a given population. Twenty-three studies were considered of high quality, 30 studies were considered of moderate quality and 14 studies were considered of low quality.
The criteria for chronic insomnia varied widely across studies and it was explicitly reported for most studies. The duration of sleep disturbance required to meet the criteria for chronic insomnia ranged from one month to 5 years, although the majority of studies required symptoms to be present for one or 6 months. Most studies reported the gender distribution of the population; the majority of populations were of mixed gender, and a minority of populations were all-female. The age of participants was reported for most studies, and ranged from 18 to 98 years, based on studies that provided a range. A number of studies (37/67) did not report the response/follow-up rate for the study. Of those that reported the response/follow-up rate, it ranged from 37.6–100 percent. The majority of studies had a rate of 80 percent or more.
Potential risk factors
Age. Eleven studies found evidence of an association between age and chronic insomnia, while seven studies found no evidence of an association between these variables. Of the studies that found an association, all, except one,57 found evidence that chronic insomnia is associated with older age. Kageyama et al. found evidence that chronic insomnia is associated with age 24 years or less.
Gender. Eleven studies found evidence of an association between gender and chronic insomnia, while seven studies found no evidence of an association between these variables. All of the studies that found evidence of an association between gender and chronic insomnia, found evidence that chronic insomnia is associated with female gender.
Race/ethnicity. Two studies found evidence of an association between ethnicity and chronic insomnia,58–59 while one study found no evidence of an association between these variables.60 Bixler et al. found evidence that chronic insomnia is associated with being a non-Caucasian minority, and Riedel et al. found evidence that chronic insomnia is associated with being White.
Psychiatric illness and psychological problems. Thirty-eight studies found evidence of an association between present or past psychiatric illness or psychological problems and chronic insomnia. Cumulatively, chronic insomnia was found to be associated with anxiety, depression, tension, loneliness, neuroticism, worry, rumination, psychological distress, nervousness, obsessive compulsiveness, maladaptive perfectionism, impulsivity, phobia, paranoid ideation, psychoticism and hypochondrial concerns. Seven studies did not find evidence of an association between one or more of the following conditions and chronic insomnia: neurological problems, anxiety, depression, tension and confusion.
Medical conditions. Twelve studies found evidence of an association between medical conditions or poor general health and chronic insomnia, while one study57 did not find evidence of an association between these variables.
Socioeconomic status. Six studies found evidence of an association between socioeconomic status and chronic insomnia. Cumulatively, chronic insomnia was found to be associated with marital separation, divorce or death of a spouse, unemployment, exposure to poorer working conditions and lower social status. Moreover, chronic insomnia was found to be associated with both lower and higher education. Nine studies did not find evidence of an association between one or more of the following factors and chronic insomnia: education, employment and marital status.
Shift-work. Only two studies provided evidence regarding the relationship between shift-work and chronic insomnia.57–61 The study by Kageyama et al. provided evidence that chronic insomnia is associated with three or less night shifts per month within the preceding three months in hospital nurses. The study by Martikainen et al. found no evidence of an association between shift-work and chronic insomnia.
Potential consequences
Healthcare utilization. Five studies provided evidence of an association between increased healthcare utilization and chronic insomnia. Cumulatively, chronic insomnia was found to be associated with hospitalization, visits to neurology and psychiatric departments and undergoing medical treatment. One study did not find evidence of an association between chronic insomnia and undergoing medical treatment in hospital nurses.57
Absenteeism and work performance. Only two studies provided evidence regarding the relationship between work performance or absenteeism and chronic insomnia;62–63 both studies found evidence of an association between chronic insomnia and absenteeism. The study by Zammit et al. also found evidence of an association between chronic insomnia and impaired work performance.
Quality of life and quality of social relationships. Five studies examined the relationship between either quality of life or quality of social relationships and chronic insomnia. All studies found evidence of an association between chronic insomnia and either lower quality of life or lower quality of social relationships; one of these studies found evidence that both quality of life and quality of social relationships are impaired in chronic insomniacs.64 Lower quality of social relationships was reported as receiving less support from colleagues and conflicts with relatives.
Memory, cognitive function and mood. Fifteen studies found evidence of an association between decrements in memory, mood or cognitive function and chronic insomnia. Cumulatively, the measures of cognitive function were cognitive fatigue, sensory acuity, perceptual/motor skills, reaction time, psychosocial function, concentration, psychomotor function, attention, alertness, mental acuity, reasoning, problem-solving ability and mental reactivity. One study65 found evidence of increased recall of presentations made just before sleep onset in chronic insomniacs. Eleven studies found no evidence of an association between mood, memory or cognitive function and chronic insomnia. Cumulatively, the measures of cognitive function were vigilance, proof-reading, reaction time, motor performance, concentration, divided attention, recent memory, audio/verbal patterns, psychomotor function, words heard and repeated, free recall, alertness and concentration.
We did not identify any studies that provided data relevant to the relationship between accidents or falls in the elderly and chronic insomnia, nor did we find evidence on the direct and indirect costs associated with the disorder.
What treatments are used for the management of chronic insomnia in adults and what is the evidence regarding their safety, efficacy and effectiveness?
One hundred and sixteen studies were relevant to the management of chronic insomnia; 34 studies were considered of high quality, 68 studies were considered of moderate quality and 14 studies were considered of low quality. All studies were described as randomized, while only 91 studies were described as double-blind. Seventy-eight studies provided a description of withdrawals and dropouts. Only nine studies described an appropriate method to generate the sequence of randomization, and 41 studies described an appropriate method of double-blinding. All other studies did not describe the method to generate the sequence of randomization, or the method of double-blinding. Only nine studies had adequate concealment of treatment allocation; for the remaining 107 studies, the adequacy of concealment was unclear. One hundred and four studies reported on sleep onset latency and only 33 studies reported on wakefulness after sleep onset, the outcomes of highest priority in this review. Regarding the other outcomes of interest in this review, 33 studies reported on sleep efficiency, 71 studies reported on total sleep time, 66 studies reported on sleep quality and only one study reported on quality of life.
Only 51 studies provided information on source of funding; 38 studies received private funding, 10 studies received government funding, two studies received academic funding, and one study received foundation funding. The majority of studies did not provide information on the role of the funding organization; however, only two out of 38 studies that reported receiving private funding explicitly stated that the funding organization was involved in data analysis and/or research design and selection of investigators.
The majority of studies had a parallel design. For studies in which there was a discrepancy between the number of participants enrolled and analyzed, it was often unclear whether an intent-to-treat analysis had been conducted. The majority of studies used sleep diary to assess sleep outcomes, and a number used both sleep diary and polysomnography to assess outcomes. Of the 111 studies that reported a treatment length, it ranged from one day to six months, with a median of three weeks. Of the 25 studies that reported a follow-up period, it ranged from one week to three years, with a median of 6 months.
The duration of chronic insomnia suffered by participants ranged from two months to 51 years, based on the 54 studies that provided a range. The age range of the population was 15–95 years, based on the 84 studies that provided a range; only two studies included participants under the age of 18 years old. Of the 111 studies that reported on the gender distribution of the population, 102 studies had a mixed gender population, while five studies had an all-female population and four studies had an all-male population. The inclusion criteria of nine studies were designed to select individuals with a psychiatric illness, including individuals with depression, dysthymic disorder, dementia, schizophrenia, personality disorder, myocolonus, anxiety disorders, Pick's disease, alcoholic psychoses, Huntington chorea, and cerebral laceration and contusion.
The studies were categorized according to intervention: benzodiazepines (n=51), non-benzodiazepines (n=36), antidepressants (n=7), complementary and alternative care (n=14), relaxation therapy (n=15), cognitive/behavioral therapy (n=18), alcohol (n=1), barbiturates (n=2), hormones (n=1) and LEET therapy (n=1). Complementary and alternative care had two studies on L-tryptophan, 8 studies on melatonin and 4 studies on valerian. A number of studies fell under the general category of combination treatment (n=8). A given study could be relevant to more than one category. The number of participants analyzed in the efficacy analysis (sleep onset latency only) was as follows: benzodiazepines (n=1858), non-benzodiazepines (n=4169), antidepressants (n=298), L-tryptophan (n=47), melatonin (n=103), valerian (n=51), relaxation therapy (n=384), cognitive/behavioral therapy (n=276), alcohol (n=11), barbiturates (n=93), hormones (n=49) and LEET therapy (n=97). The number of participants analyzed in the safety analysis was as follows: benzodiazepines (n=3800), non-benzodiazepines (n=5485), antidepressants (n=288), complementary and alternative care (n=87), relaxation therapy (n=0), cognitive/behavioral therapy (n=0), alcohol (n=0), barbiturates (n=48), hormones (n=0) and LEET therapy (n=0).
Benzodiazepines
Sleep onset latency. Meta-analysis of the 32 studies that compared the effects of benzodiazepines and placebo on sleep onset latency (SOL) showed a statistically significant, albeit modest, difference of 16.5 minutes in favour of benzodiazepines (Figure 1). While there was substantial heterogeneity among the studies, all but two studies had a point estimate that favoured benzodiazepines. The heterogeneity was due more to different estimates of how effective the drugs were than as to whether or not they were superior to placebo. The study estimates ranged from about 65 minutes improvement in SOL to no difference.

Figure
Figure 1. Meta graph: Sleep onset latency: benzodiazepines versus placebo.
Table 6 lists all of the sub-group analyses conducted for sleep onset latency. Nine different types of benzodiazepines were examined by the studies. With the exception of nitrazolam (which had a significantly higher estimate than all other drugs except flunitrazepam, flurazepam, and triazolam, but was examined by only one study) all drug types had SOL estimates between 24 and 10 minutes. The four studies that examined patients with a psychiatric illness showed a mean SOL difference of about 26 minutes, 10 minutes more than the remainder of the studies that had patients without a psychiatric illness (not statistically significant). The two studies that had results for long-term treatment showed a nearly identical estimate to those with results for short-term treatment. Similarly, age had little impact on SOL, with a 19-minute difference for elderly patients compared to a 15-minute difference for adult patients. There were only three studies that examined solely male patients and only one study that examined solely female patients. These studies were not very different from each other or the remaining studies. Finally, subdividing the studies by method of measurement of outcomes showed that those studies that used a sleep diary to measure SOL had a significantly greater efficacy estimate than those that used polysomnography (about 18 minutes compared to 7 minutes). The sub-groups of drug type, psychiatric illness, age and method of measurement had a Deeks' chi-square P-value less than 0.05, indicating that heterogeneity was significantly reduced by the sub-group.
Table 6
Sleep onset latency: benzodiazepines versus placebo.
Analysis of the studies by quality revealed that the high quality studies showed a slightly stronger estimate of SOL difference (19 minutes) than the moderate quality studies (14 minutes) (there were no low quality studies). Although the difference is not statistically significant, Deeks' chi-square shows that this sub-grouping significantly reduced heterogeneity.
Although both Begg's (P-value = 0.81) and Duval's (no studies added) tests indicated no publication bias non-parametrically, Egger's test (p-value < 0.001) showed significant asymmetry in the funnel plot. This finding is also confirmed by a visual inspection of the funnel plot (Figure 2). The finding may indicate that the efficacy estimate given by the meta-analysis may in fact overestimate true efficacy, due to possible unpublished studies with non-significant results.
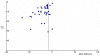
Figure
Figure 2. Funnel Plot: Sleep onset latency: benzodiazepines versus placebo.
Wakefulness after sleep onset. Only eight benzodiazepine studies reported data on wakefulness after sleep onset (WASO). They revealed an average 23-minute improvement in WASO in the benzodiazepine patients as compared to the placebo patients (Figure 3). This result was statistically significant. Although there was substantial heterogeneity in this estimate, seven of the eight studies showed a point estimate that favoured treatment.

Figure
Figure 3. Meta graph: Wakefulness After Sleep Onset: benzodiazepines versus placebo.
Other efficacy outcomes. Three other estimates of efficacy were measured, and the results can be viewed in Table 7. All outcomes showed statistically significant results that favoured benzodiazepines over placebo. Benzodiazepines increased sleep efficiency over placebo by an average of 6.3 percent and increased total sleep time by an average of 39 minutes. Sleep quality also showed a large difference between the groups with the benzodiazepines group having a standard mean difference (SMD) that was 0.8 standard deviations larger than placebo. Heterogeneity was negligible for sleep efficiency, substantial for total sleep time, and moderate for sleep quality.
Table 7
Other outcomes: benzodiazepines versus placebo.
Safety. The benzodiazepines showed a significantly greater proportion of adverse events than did placebo, although the meta-analysis showed substantial heterogeneity (Table 7). The risk difference estimates had a range of (0.01, 0.30) and an interquartile range of (0.08, 0.22) across interventions. The pooled risk-difference of 0.15 translates into a number needed to harm of seven patients (95% CI: 5, 10).
The most commonly reported adverse events of benzodiazepine use were somnolence (n=27 studies), headache (n=18), dizziness (n=16), nausea (n=11) and fatigue (n=11). There were no reports of falls, injury or death.
Non-benzodiazepines
Sleep onset latency. Twenty-nine studies on non-benzodiazepines showed a statistically significant difference of about 18 minutes in sleep onset latency compared to placebo (Figure 4). This difference is similar to the one reported for benzodiazepines. Again, similar to benzodiazepines, there was substantial heterogeneity among the studies, but all studies had a point estimate that favoured non-benzodiazepines. The study estimates ranged from about 67 minutes improvement to 4 minutes improvement in SOL.
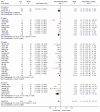
Figure
Figure 4. Meta graph: Sleep Onset Latency: non-benzodiazepines versus placebo.
The results for the sub-group analyses for sleep onset latency can be viewed in Table 8. Four different types of non-benzodiazepines were examined by the studies. The four different drugs examined ranged in their efficacy from a 31-minute improvement (zopiclone) to a 13-minute improvement (zolpidem). Deeks' chi-square test showed that this sub-grouping resulted in a significant reduction in heterogeneity. All of the other sub-groups examined did not show significant reductions in heterogeneity. For psychiatric illness, only one study had patients with such an illness, and that study showed a significantly shorter improvement in SOL. For length of treatment, the short-term and long-term studies had nearly identical improvements, as did the studies for adult and elderly patients. For gender, there was one study that examined an all-male population and one study that examined an all-female population. Although the results differed from the overall average (less effective for males and much more effective for females), there was not enough data to draw any firm conclusions (no statistically significant differences). Finally, the studies that used polysomnography to estimate sleep onset latency were not different from those that used a sleep diary.
Table 8
Sleep onset latency: non-benzodiazepines versus placebo.
The high quality studies had an SOL estimate (30 minutes) that was significantly greater than the estimate for moderate quality studies (14 minutes) (there were no low quality studies), as was the case with the benzodiazepines. However, this sub-grouping did not significantly reduce heterogeneity.
Only Duval's test (no studies added in the trim and fill) showed no evidence of publication bias. Begg's (P-value = 0.01) and Egger's (P-value = 0.01) tests both showed evidence of funnel plot asymmetry, as did a visual examination of the plot (Figure 5). This finding suggests a possible overestimation of the efficacy in terms of SOL of non-benzodiazepines in our meta-analysis.
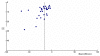
Figure
Figure 5. Funnel Plot: Sleep Onset Latency: non-benzodiazepines versus placebo.
Wakefulness after sleep onset. Nine studies reported on WASO comparing non-benzodiazepines to placebo. The studies found that non-benzodiazepines decreased WASO by an average of about 13 minutes, which was statistically significant. Heterogeneity was substantial, caused mostly by one study, whose estimate was very different from the others (Figure 6).

Figure
Figure 6. Meta graph: Wakefulness After Sleep Onset: non-benzodiazepines versus placebo.
Other efficacy outcomes. Four other estimates of efficacy were measured, and their results can be viewed in Table 9. All outcomes showed statistically significant results that favoured non-benzodiazepines over placebo. Sleep efficiency was increased in non-benzodiazepines by about 6 percent, while total sleep time was increased by about 28 minutes. Both sleep quality and quality of life showed moderate improvements in non-benzodiazepines compared to placebo with SMDs of 0.48 and 0.45, respectively. Heterogeneity was negligible for sleep efficiency, moderate for total sleep time, and substantial for sleep quality.
Table 9
Other outcomes: non-benzodiazepines versus placebo.
Safety. The non-benzodiazepines showed a significantly greater proportion of adverse events than did placebo, although the meta-analysis showed substantial heterogeneity (Table 9). The risk-difference had a range of (0.00, 0.15) and an interquartile range of (0.05, 0.08) across interventions. The pooled risk-difference of 0.05 translates into a number needed to harm of 20 patients (95% CI: 11, 100).
The most commonly reported adverse events of non-benzodiazepine use were headache (n=16 studies), dizziness (n=14), nausea (n=13) and somnolence (n=13). Accidental injury was reported in only one study, although there was no difference in the frequency of this event between experimental and control groups.
Antidepressants
Sleep onset latency. There were six studies that examined the effect of antidepressants (doxepin, pivagabine, trazodone and trimipramine) on sleep onset latency. They showed a small but statistically significant difference of about 7 minutes in sleep onset latency compared to placebo (Figure 7). The heterogeneity in this estimate was minimal and all six studies had an estimate that favoured the drug. The study estimates ranged from about 17 minutes improvement in SOL to no difference.

Figure
Figure 7. Meta graph: Sleep Onset Latency: antidepressants versus placebo.
The results of sub-group analyses for sleep onset latency can be viewed in Table 10. All six studies were of mixed gender and featured only adults, so no sub-group analysis on gender or age was performed. Of the comparisons that were made, none significantly reduced heterogeneity, despite some marked differences in point estimates. This finding was mainly due to the low number of studies: most categories had a sub-group of only one study and method of measurement had a sub-group of only two studies. None of the differences in point estimates were statistically significant.
Table 10
Sleep onset latency: antidepressants versus placebo.
The one high quality study had an SOL estimate (17 minutes) that was higher than the estimate for the moderate quality studies (7 minutes) (there were no low quality studies). This sub-grouping did not significantly reduce heterogeneity, and the difference between estimates was not significant.
Since only six studies were included in this analysis, there were too few studies to perform any meaningful tests of publication bias.
Wakefulness after sleep onset. Three studies reported on WASO comparing antidepressants (doxepin and trazodone) to placebo. The studies found that antidepressants decreased WASO by an average of about 11 minutes, which was statistically significant. Heterogeneity was negligible (Figure 8).

Figure
Figure 8. Meta graph: Wakefulness After Sleep Onset: antidepressants versus placebo.
Other efficacy outcomes. Three other estimates of efficacy were measured (no studies included an analysis of quality of life), and their results can be viewed in Table 11. All outcomes showed statistically significant results that favoured antidepressants over placebo. Sleep efficiency was increased in the antidepressant group by about 13.8 percent, while total sleep time was increased by about 53.1 minutes. Sleep quality showed a moderate increase for antidepressants of about 0.63 on the SMD scale. Heterogeneity was negligible for sleep efficiency and substantial for both total sleep time and sleep quality.
Table 11
Other outcomes: antidepressants versus placebo.
Safety. The antidepressants showed a significantly greater proportion of adverse events than placebo, and the meta-analysis showed negligible heterogeneity (Table 11). The risk-difference had a range of (-0.07, 0.13) and an interquartile range of (0.01, 0.11). The pooled risk-difference of 0.09 translates into a number needed to harm of 11 patients (95% CI: 6, 100).
The most commonly reported adverse events with antidepressant use were somnolence (n=4), headache (n=3), dizziness (n=3), and nausea (n=3). There were no reports of falls, injury or death.
Complementary and alternative care
There were three different types of complementary and alternative therapies observed in our included studies: L-tryptophan, melatonin, and valerian. These three substances were considered too different clinically to combine, and thus their results will be considered separately.
L-tryptophan
Sleep onset latency. Only two studies reported data for l-trytophan versus placebo and the results for sleep onset latency can be seen in Figure 9. The two studies showed an average reduction in SOL of 11 minutes, but the result was not significant, and the heterogeneity between the two studies was substantial. There were too few studies to do any meaningful tests for publication bias. No other outcomes of interest were reported for L-tryptophan (Table 12). The two studies used different methods to measure sleep onset latency. The study that used polysomnography showed a significant effect of L-tryptophan (-20.1 minutes; 95% CI: -33.6, -6.6), while the study that used sleep diary did not (2.9 minutes; 95% CI: -21.6, 27.4). However, the difference between the two studies was not statistically significant.

Figure
Figure 9. Meta graph: Sleep Onset Latency: complementary and alternative care versus placebo.
Table 12
All outcomes: L-trytophan versus placebo.
Melatonin
Sleep onset latency. There were 8 studies on melatonin that examined sleep onset latency. Similar to the antidepressants, this category of intervention showed a small but statistically significant difference of about 8 minutes in sleep onset latency compared to placebo (Figure 9). The heterogeneity in this estimate was moderate, and all but two of the studies had an estimate that favoured the drug. The study estimates ranged from about 20 minutes improvement in SOL to 10 minutes detriment. When the eight studies were grouped by method of measurement, some differences in efficacy estimates were observed among the groups. The estimate for polysomnography (-3.6 minutes; 95% CI: -8.8, 1.6) was significantly different from the estimate for actigraphy (-16.7 minutes; 95% CI: -25.0, -8.3). Neither estimate was significantly different from the estimate for sleep diary (5.1 minutes; 95% CI: -20.0, 30.2).
No publication bias was immediately apparent from the funnel plot (Figure 10), and both Begg's test (P-value = 0.90) and Egger's test (P-value = 0.21) did not show significant asymmetry. However, Duval's trim and fill test did add one study to the meta-analysis and slightly increased the efficacy estimate (-8.7 minutes; 95% CI: -14.9, -2.5).
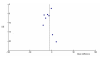
Figure
Figure 10. Funnel Plot: Sleep Onset Latency: melatonin versus placebo.
Wakefulness after sleep onset. Five studies reported on WASO comparing melatonin to placebo. The studies found that melatonin decreased WASO by an average of about 10 minutes, but this difference was not statistically significant. Heterogeneity was substantial, with two studies indicating a significant effect in favour of melatonin, while the other three studies all had estimates on the side of the null favouring placebo (Figure 11).

Figure
Figure 11. Meta graph: Wakefulness After Sleep Onset: complementary and alternative care versus placebo.
Other efficacy outcomes. Three other estimates of efficacy were measured for melatonin versus placebo (no studies included an analysis of quality of life), and their results can be viewed in Table 13. None of the three outcomes showed statistically significant results. The point estimates for sleep efficiency and total sleep time were 3 percent and 6 minutes, respectively. Sleep quality had a small efficacy difference of 0.3 standard deviations. Heterogeneity was substantial for both sleep efficiency and total sleep time, while it was negligible for sleep quality.
Table 13
All outcomes: melatonin versus placebo.
Safety. The melatonin studies did not show any significant difference in number of adverse events versus placebo (Table 13), with an estimated risk difference of 0.09. Heterogeneity among studies was moderate.
Valerian
Sleep onset latency. There were three studies on valerian that examined sleep onset latency. The studies showed a small average difference between valerian and placebo (1 minute), which was not statistically significant (Figure 9). The heterogeneity in this estimate was substantial with two studies favouring valerian and the third being well on the side of placebo. The study estimates ranged from about 17 minutes improvement in SOL to 23 minutes detriment. There were too few studies to do any meaningful tests for publication bias. When the studies were grouped by method of measurement, differences among groups were observed. The estimate for polysomnography (9.5 minutes; -11.3, 30.3) was significantly different from the estimate for sleep diary (-16.0 minutes; 95% CI: -29.5, -2.5).
Wakefulness after sleep onset. Only one study reported on WASO comparing melatonin to placebo, but it did find a difference of 8 minutes between the groups, which favoured valerian and was statistically significant (Figure 11).
Other efficacy outcomes. Three other estimates of efficacy were measured for valerian versus placebo (no studies included an analysis of quality of life), and their results can be viewed in Table 14. None of the three outcomes showed statistically significant results. The point estimates for sleep efficiency and sleep onset latency were very small at 0.1 percent and 1 minute, respectively. Sleep quality had a large efficacy difference of 1.38 standard deviations, but, as mentioned, it was not statistically significant. Heterogeneity was negligible for sleep efficiency and substantial for sleep quality.
Table 14
All outcomes: valerian versus placebo.
Safety. The valerian studies did not show any significant difference in number of adverse events versus placebo (Table 14), with an estimated risk difference of -0.06, which actually favoured valerian. Heterogeneity among studies was substantial.
Relaxation therapy
Sleep onset latency. There were 13 studies on relaxation therapy that examined sleep onset latency. Meta-analysis showed a pooled difference of 15 minutes favouring therapy over placebo (Figure 12). This result was not statistically significant. The heterogeneity in this estimate was extremely high (I2: 96 percent), although all but three of the studies had an estimate that favoured the drug. The study estimates ranged from about 60 minutes improvement in SOL to 14 minutes detriment.

Figure
Figure 12. Meta graph: Sleep Onset Latency: relaxation therapy versus placebo.
The results for sub-group analyses for sleep onset latency in relaxation therapy can be viewed in Table 15. All 13 studies analysed patients without psychiatric illnesses and used sleep diary to measure SOL, so no sub-group analyses on these variables were performed. The other four sub-groups examined yielded highly significant reductions in heterogeneity. Despite this finding, many of the individual sub-groups had very high heterogeneity. Subdividing by type of relaxation therapy, efficacy estimates ranged from 60 minutes improvement to 5 minutes improvement. Only breathing training, group relaxation, and hypnotic relaxation showed statistically significant efficacy despite each sub-group containing only one trial. The short-term effects of relaxation therapy on SOL proved significantly greater than the long-term effects (22 minutes improvement versus 2 minutes detriment). There was only one study of elderly patients, and it showed no improvement in sleep onset latency compared to the studies on adults, which showed an improvement of 16 minutes. This difference was not significant. Finally, one study had only female participants (there were no studies of all-male populations), and it had a lower efficacy than the remainder of the studies (6 minutes compared to 16 minutes). This difference was non-significant.
Table 15
Sleep onset latency: relaxation therapy versus placebo.
The moderate quality studies had a slightly higher (but not significantly higher) efficacy estimate than the low quality studies (18 minutes compared to 9 minutes) (there were no high quality studies). This sub-grouping significantly reduced heterogeneity.
No publication bias was immediately apparent from the funnel plot (Figure 13). Both Egger's test (P-value = 0.49) and Duval's trim and fill (no studies added) did not show significant asymmetry. However, Begg's test did show some evidence of asymmetry (P-value = 0.004). This finding is somewhat surprising considering that Begg's test is usually the most conservative test (i.e. it is unusual to have a significant Begg P-value and a non-significant Egger P-value).
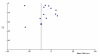
Figure
Figure 13. Funnel Plot: Sleep Onset Latency: relaxation therapy versus placebo.
Wakefulness after sleep onset. Only three studies reported on WASO comparing relaxation therapy to placebo. Their combined efficacy estimate was very small (-2 minutes) and favoured relaxation therapy (Figure 14). The result was not statistically significant. Heterogeneity was minimal.

Figure
Figure 14. Meta graph: Wakefulness After Sleep Onset: relaxation therapy versus placebo.
Other efficacy outcomes. Three other estimates of efficacy were measured for relaxation therapy versus placebo (no studies included an analysis of quality of life), and their results can be viewed in Table 16. Only total sleep time showed a statistically significant result, with an average improvement in the relaxation group of 23 minutes. Sleep efficiency had an estimate of 0.4 percent, while sleep quality showed a small estimate of 0.4 standard deviations. Neither estimate was statistically significant. Heterogeneity was negligible for sleep efficiency and total sleep time, but was substantial for sleep quality.
Table 16
Other outcomes: relaxation therapy versus placebo.
Safety. None of the trials of this category reported on adverse events.
Cognitive/behavioral therapy
Sleep onset latency. There were nine studies on cognitive/behavioral therapy (CBT) that examined sleep onset latency. Meta-analysis showed a pooled difference of 5 minutes favouring therapy over placebo (Figure 15). This result was not statistically significant. The heterogeneity in this estimate was minimal, although three of the nine studies had an estimate that favoured placebo. The study estimates ranged from about 30 minutes improvement in SOL to 19 minutes detriment.
Figure
Figure 15. Meta graph: Sleep Onset Latency: cognitive/behavioral therapy versus placebo.
The results of sub-group analyses for sleep onset latency in CBT can be viewed in Table 17. All nine studies were of mixed gender, analysed patients without psychiatric illnesses and used sleep diary to measure SOL, so no sub-group analyses on gender, psychiatric illness or method of measurement were performed. Of the three sub-groups examined, none showed significant reduction in heterogeneity. Subdividing by type of intervention, stimulus control and thought non-suppression had slightly higher estimates than multi-component CBT, paradoxical intention, and sleep compression, but the differences were not significant. Similarly, the short-term and long- term differences were comparable. There was only one study that examined elderly participants, and its efficacy estimate was not very different from the adult studies.
Table 17
Sleep onset latency: cognitive/behavioral therapy versus placebo.
The low quality studies had a slightly higher efficacy estimate than the moderate quality studies (8 minutes compared to 1 minute), but the difference was not statistically significant (there were no high quality studies). This sub-grouping did not significantly reduce heterogeneity.
There was no evidence of publication bias. The funnel plot did not appear to be asymmetric (Figure 16), and Begg's (P-value = 0.53), Egger's (P-value = 0.37) and Duval's (no studies added) tests all confirmed this finding.

Figure
Figure 16. Funnel Plot: Sleep Onset Latency: cognitive/behavioral therapy versus placebo.
Wakefulness after sleep onset. Eight studies reported on WASO comparing CBT to placebo. Their combined efficacy estimate showed that CBT improved WASO by an average of 18 minutes (Figure 17). The result was statistically significant, although heterogeneity was substantial. Despite the high heterogeneity, all but one study showed a result that favoured CBT.

Figure
Figure 17. Meta graph: Wakefulness After Sleep Onset: cognitive/behavioral therapy versus placebo.
Other efficacy outcomes. Three other estimates of efficacy were measured for CBT versus placebo (no studies included an analysis of quality of life), and their results can be viewed in Table 18. Both sleep efficiency and sleep quality showed statistically significant improvements for CBT over placebo. Sleep efficiency improved by an average of 6 percent, while sleep quality showed an improvement of 0.38 standard deviations. Total sleep time showed no difference. Heterogeneity was negligible for sleep quality, but substantial for both the sleep efficiency and total sleep time estimates.
Table 18
Other outcomes: cognitive/behavioral therapy versus placebo.
Safety. None of the CBT trials reported on adverse events.
Indirect comparisons
Efficacy. Table 19 shows the results of the pair-wise indirect comparisons of sleep onset latency for each of the four pharmacological treatment categories: benzodiazepines, non-benzodiazepines, antidepressants, and complementary and alternative care, the latter divided into L-tryptophan, melatonin, and valerian. Both benzodiazepines and non-benzodiazepines proved significantly more efficacious than antidepressants and melatonin. None of the other comparisons showed significant differences. Comparing the two non-pharmacological treatment categories (relaxation therapy and cognitive/behavioral therapy) also showed no significant difference (-10 min.; 95% CI: -25.7, 5.7).
Table 19
Sleep onset latency: indirect comparisons of main pharmacological treatment categories.
Safety. Table 20 shows the results of indirect comparisons of adverse events for the four main pharmacological treatment categories that provided relevant data: benzodiazepines, non-benzodiazepines, antidepressants and complementary and alternative care, the latter divided into melatonin and valerian (there was no safety data for L-tryptophan). The only significant comparison is that of benzodiazepines and non-benzodiazepines, where the latter treatment category was found to be significantly safer than the former in terms of number of adverse events. Note that despite the fact that valerian had the lowest point estimate, the larger confidence interval prevented a meaningful comparison of its safety relative to the other treatment categories.
Table 20
Adverse events: indirect comparisons of main pharmacological treatment categories.
Other treatments
There were some studies that examined treatments that did not fall under any of the preceding six treatment categories. They are outlined here.
Barbiturates. There were two trials that examined barbiturates versus placebo. Four different types of barbiturates were examined in the trials: glutethimide, methyprylon, phenobarbitol and secobarbitol.
Only two outcomes (sleep onset latency and adverse events) were analysed in the two trials. The results can be viewed in Table 21. Neither SOL nor number of adverse events differed significantly from placebo in the meta-analyses.
Table 21
All outcomes: barbiturates versus placebo.
Hormones. One trial examined the efficacy of two different hormones (climodein and estradiol) in women with a diagnosis of insomnia related to post-menopausal syndrome. Four outcomes (sleep onset latency, sleep efficiency, total sleep time, and sleep quality) were examined (Table 22). Sleep efficiency and sleep quality showed a statistically significant improvement (5 percent and 22 minutes respectively) with the hormones, while sleep onset latency and total sleep time showed an improvement that was not significant.
Table 22
All outcomes: hormones versus placebo.
Alcohol. One trial examined the efficacy of ethanol versus placebo. The three outcomes examined (sleep onset latency, WASO, and sleep efficiency) had non-significant differences between treatment and placebo (Table 23). All three efficacy estimates favoured placebo, although the differences were not significant.
Table 23
All outcomes: alcohol versus placebo.
Low energy emission therapy. One study compared the effect of low energy emission therapy (LEET) with placebo for insomniacs. The results for the four efficacy (sleep onset latency, WASO, sleep efficiency, and total sleep time) and one safety outcome can be viewed in Table 24. Statistically significant improvements in both sleep efficiency and total sleep time (11 percent and 56 minutes, respectively) were found. The estimates for sleep onset latency and WASO were not significant, but did favour the LEET intervention. There was also no evidence that adverse events were higher for LEET than for placebo.
Table 24
All outcomes: low energy emission therapy versus placebo.
Combination treatments
This section will outline the results of eight trials that employed various combinations of the above treatments. Unlike all other sections of this review, we did not limit ourselves to comparing these treatments to placebo. All comparisons within the trials were examined. Ten different comparisons resulted and are outline below. The combination therapy in each case is always considered to be the “treatment arm.”
Relaxation therapy and cognitive behavioral therapy versus placebo. There were four studies included in a meta-analysis of a combined relaxation therapy and CBT treatment versus placebo. The results for four outcomes (SOL, WASO, total sleep time, and sleep quality) can be viewed in Table 25. Although all estimates favoured treatment, only the result for sleep onset latency proved statistically significant, with an estimated improvement of about 22 minutes. Interestingly, this is nearly identical to the sum of the calculated effect from the meta-analyses of each of these interventions alone.
Table 25
All outcomes: relaxation therapy and cognitive/behavioral therapy versus placebo.
Relaxation therapy and cognitive behavioral therapy versus relaxation therapy. Two studies compared relaxation therapy and CBT treatment with relaxation therapy alone. Table 26 contains the results for the four reported outcomes (SOL, WASO, total sleep time, and sleep quality). None of the results were statistically significant with only sleep onset latency favouring the combined treatment. The other three outcomes showed an efficacy estimate that favoured relaxation alone, although as mentioned, none were significant.
Table 26
All outcomes: relaxation therapy and cognitive/behavioral therapy versus relaxation therapy.
Relaxation therapy and cognitive behavioral therapy versus cognitive behavioral therapy. Two studies compared relaxation therapy and CBT treatment with CBT alone. None of the results for the four outcomes (SOL, WASO, total sleep time, and sleep quality) were statistically significant (Table 27). Two of the outcomes (SOL and sleep quality) favoured the combined treatment, while the other two outcomes (WASO and total sleep time) favoured CBT alone.
Table 27
All outcomes: relaxation therapy and cognitive/behavioral therapy versus cognitive/behavioral therapy.
Relaxation therapy and cognitive behavioral therapy versus benzodiazepine. One study compared relaxation therapy and CBT versus a benzodiazepine. The results for SOL, WASO, and sleep quality can be viewed in Table 28. All three outcomes favoured the benzodiazepine over the combined treatment. The difference was not significant for SOL or WASO, but it was significant for sleep quality (a large difference of about 1.5 SDs).
Table 28
All outcomes: relaxation therapy and cognitive/behavioral therapy versus benzodiazepines.
Benzodiazepine and cognitive behavioral therapy versus placebo. Table 29 lists the results for the four outcomes (SOL, WASO, sleep efficiency, and total sleep time) that were reported in comparisons of benzodiazepine and CBT versus placebo. Two studies were meta-analysed. All four outcomes favoured the combined treatment, and the difference was significant for sleep efficiency (13 percent improvement).
Table 29
All outcomes: benzodiazepine and cognitive/behavioral therapy versus placebo.
Benzodiazepine and cognitive behavioral therapy versus benzodiazepine. One study compared the combined treatment of a benzodiazepine and CBT versus the benzodiazepine alone. The results for three outcomes (WASO, sleep efficiency and total sleep time) can be viewed in Table 30. The result for sleep efficiency was statistically significant in favour of the combined treatment (7 percent improvement). WASO favoured the combined treatment but not significantly. Total sleep time favoured the benzodiazepine alone, but the difference was not significant.
Table 30
All outcomes: benzodiazepine and cognitive/behavioral therapy versus benzodiazepine.
Benzodiazepine and cognitive behavioral therapy versus cognitive behavioral therapy. The comparison of a benzodiazepine and CBT versus CBT alone was available through one study. The results of the three outcomes (WASO, sleep efficiency, and total sleep time) can be viewed in Table 31. None of the results were significant and all had relatively small efficacy estimates.
Table 31
All outcomes: benzodiazepine and cognitive/behavioral therapy versus cognitive/behavioral therapy.
Non-benzodiazepine and cognitive behavioral therapy taken in combination versus the same two treatments taken sequentially. One study examined the difference between the effects of a non-benzodiazepine taken simultaneously with CBT versus the same two treatments taken sequentially. The results for the two outcomes examined (sleep efficiency and total sleep time) can be viewed in Table 32. Neither result was significant.
Table 32
All outcomes: non-benzodiazepine and cognitive/behavioral therapy (in combination) versus non-benzodiazepine and cognitive/behavioral therapy (sequential).
Cognitive behavioral therapy and modafinil versus cognitive behavioral therapy. One study compared CBT combined with the stimulant modafinil versus CBT alone. Results for the three reported outcomes (SOL, WASO, and total sleep time) can be viewed in Table 33. None of the results are significant, and all three efficacy estimates favour CBT alone over the combined treatment.
Table 33
All outcomes: cognitive/behavioral therapy and modafinil versus cognitive/behavioral therapy.
Cognitive behavioral therapy and modafinil versus modafinil. CBT and modafinil was compared to modafinil alone in one study. The results of the three outcomes examined (SOL, WASO, and total sleep time) can be viewed in Table 34. All outcomes favoured the combined treatment, but none of them were significant.
Table 34
All outcomes: cognitive/behavioral therapy and modafinil versus modafinil.
What are the important future directions for insomnia-related research?
The response to this question appears under “Limitations and Future Research” in the Discussion section of the Evidence Report.
Footnotes
- ♦
The Appendices and Evidence Tables cited in this report are provided electronically at http://www
.ahrq.gov/clinic/tp/insomntp .htm.
- Results - Manifestations and Management of Chronic Insomnia in AdultsResults - Manifestations and Management of Chronic Insomnia in Adults
- MRX10 Mrx10p [Saccharomyces cerevisiae S288C]MRX10 Mrx10p [Saccharomyces cerevisiae S288C]Gene ID:851876Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
