NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Centre (UK). Cirrhosis in Over 16s: Assessment and Management. London: National Institute for Health and Care Excellence (NICE); 2016 Jul. (NICE Guideline, No. 50.)
N.1. Introduction
Diagnosing cirrhosis in people with liver disease is a crucial point in a patient's disease pathway as it triggers a more intensive clinical path that includes surveillance for the cirrhosis complications of hepatocellular carcinoma (HCC) and oesophageal varices. Failing to detect cirrhosis at an early stage can have detrimental clinical effects for patients. Amongst hepatologists and gastroenterologists, the only commonly agreed reference standard for the diagnosis of advanced fibrosis or cirrhosis is liver biopsy. By nature liver biopsy is an invasive test associated with adverse clinical events and disutility for some people. In addition, it is a resource-intensive procedure, conducted with the guidance of ultrasound, which usually requires a day-case admission and has a considerable cost.
With the rising popularity of blood biomarkers associated with liver function and the increasing use of imaging tests that can stage liver fibrosis, without carrying the disadvantages of liver biopsy, these non-invasive liver tests have found their way into current clinical practice. However, the availability of the tests and the way that these are embedded into clinical practice vary substantially across NHS providers. For these reasons the GDG prioritised original economic analysis to be conducted for the review questions that address objective diagnostic tests for the diagnosis of cirrhosis and who should be offered such a test.
The economic review identified 3 studies (Canavan 2013, Steadman 2013, Stevenson 2012) that reported cost-effectiveness results in patients with different stages of fibrosis. However these studies reported outcomes for mixed populations at different stages of liver disease; none of the studies reported outcomes for only people with cirrhosis. A recently published NIHR HTA was also identified (Crossan 2015) that reported results for a population of people with cirrhosis, but this looked only at a population with mixed liver disease aetiology (including patients with viral hepatitis, alcohol-related liver disease and non-alcoholic fatty liver disease together).
Other areas of uncertainty identified in the clinical review questions were the optimal frequencies of surveillance for HCC and for oesophageal varices in people with cirrhosis, as regular surveillance for these complications is believed to lead to clinical benefits for patients but the best frequencies are unclear. These 2 review questions were hence also examined using the same whole disease pathway model.
N.2. Methods
N.2.1. Model overview
N.2.1.1. Comparators
The NGC liver disease pathway model (LDPM) was developed for this guideline and for the NICE non-alcoholic fatty liver disease guideline. The model is composed of 3 modules, covering steatosis, fibrosis and cirrhosis, and follows the progression of people with liver disease through the course of their lifetime. For this economic analysis the cirrhosis module was used, and was adapted for separate populations with alcohol-related liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), hepatitis B (HBV) and hepatitis C (HCV).
For this analysis, 23 single tests, identified in the relevant clinical review, and 4 combinations of tests, were compared for 1 or more of the 4 populations of interest. These are summarised below. Several tests were not considered for modelling due to the absence of sensitivity and specificity data in the relevant papers (only area under the curve figures reported). For each aetiology population the diagnostic tests are also compared against the reference standard, liver biopsy.
Two further strategies were also considered which did not include any tests:
- no test, monitor all patients in the relevant population assuming they have cirrhosis
- no test, monitor no-one, assuming none have cirrhosis until later clinical presentation.
In the hepatitis C cohort, for modelling purposes, there was an additional no-testing strategy for which in addition to no monitoring there would also be no treatment given for hepatitis C.
N.2.1.1.1. Combinations of more than 1 test
In planning the model structure, the inclusion of combinations of tests was considered. Four algorithms were identified in papers included for the hepatitis C population (at the bottom of Table 52 above) and these were included alongside the single tests. The GDG also considered using 2 of the single tests (excluding liver biopsy) consecutively. The GDG considered that combinations should include 1 blood test and 1 imaging test as these would be likely to give independent results. The most promising combination would be one using a blood test with high sensitivity (to maximise true positives and minimise false negatives) followed by an imaging test with high specificity (to rule out true negatives). However, when viewing the diagnostic accuracy values found in the clinical review (see Section N.2.3.2 below) no such combination could be found. Consequently there was no reason to believe any combination of 2 tests would give more accurate results than the best single tests, but with an increased cost for using 2 tests instead of 1. Therefore no such combinations were modelled.
Table 52
Tests included in the model by disease aetiology.
N.2.1.2. Population
The model considers people aged 50 years at the start of the model with one of the 4 major underlying causes of cirrhosis (hepatitis B, hepatitis C, alcohol-related liver disease, non-alcoholic fatty liver disease) who are therefore at risk of developing cirrhosis. Patients with different aetiologies are treated as separate patient cohorts in the model. Hepatitis B patients are further separated in 2 cohorts (positive or negative hepatitis B e Antigen, HBeAg). Hepatitis C patients are further separated by disease genotype (Genotypes 1–4).
N.2.1.3. Time horizon, perspective, discount rates used
The analysis follows the standard assumptions of the NICE reference case including discounting at 3.5% for costs and health effects, and the perspective of the UK NHS and personal social services. A sensitivity analysis will also be conducted using a discount rate of 1.5% for costs and health benefits. A lifetime horizon has been chosen to fully capture the adverse outcomes derived from incorrect diagnosis.
N.2.2. Approach to modelling
The model is based on 2 phases:
- Decision tree: Using the sensitivity and specificity, combined with data on the prevalence of cirrhosis in each of the target populations, the model identifies the proportion of people who receive a true positive (TP), true negative (TN), false positive (FP) or false negative (FN) diagnosis.
- Markov model: Once the diagnosis is made the people move into the second part of the model which involves a Markov model to fully evaluate long-term health and cost outcomes for people starting with each diagnosis. The model has 6-monthly cycles and continues until death or age 100 years.
Further information and technical details are provided below.
N.2.2.1. Model structure

Figure 199
Graphical depiction of the decision tree.
N.2.2.2. High-level model structure
Initially, a decision tree determines the proportion of people with cirrhosis who receive a correct diagnosis (true positive – TP) and an incorrect diagnosis (false negative – FN); and the proportion of people without cirrhosis who receive a correct diagnosis (true negative – TN) and an incorrect diagnosis (false positive – FP) depending on the diagnostic accuracy of every test. People diagnosed as not having cirrhosis were assumed to have advanced fibrosis (F3 on the METAVIR scale).
It is assumed that 27% of people with cirrhosis will already have medium or large varices at the time when they are first diagnosed with cirrhosis. People will receive endoscopic surveillance for oesophageal varices immediately following a positive diagnosis of cirrhosis. It is assumed that this is 100% successful at identifying medium or large varices.
Consequently, patients enter the Markov model through 6 health states:
- advanced fibrosis with a true negative diagnosis – (F3-TN)
- advanced fibrosis with a false positive diagnosis of cirrhosis– (F3-FP)
- compensated cirrhosis with a true positive diagnosis – (comp-TP)
- compensated cirrhosis with a false negative diagnosis of advanced fibrosis only – (comp-FN)
- compensated cirrhosis with oesophageal varices with a true positive diagnosis, hence immediately receiving prophylactic measures to prevent variceal bleeding – (VarTP-Pr)
- compensated cirrhosis with oesophageal varices with a false negative diagnosis of advanced fibrosis only, and hence not assessed or receiving treatment for varices – (Var-FN)
It is assumed that everyone with cirrhosis at the start of the model has compensated cirrhosis, as decompensated cirrhosis would have previously been identified by a clinician's observations without the need for the diagnostic tests examined here. Under GDG guidance, retesting for those with a negative diagnosis was set at 2 years for all populations. The cost-effectiveness of decreasing the retesting frequency to 1 year was examined in sensitivity analyses for each population.
Overall, the model attempts to represent the natural history of the disease, from compensated cirrhosis without varices to the development of varices (which may lead to bleeding), HCC and other decompensation events, and finally to a post-liver transplant state or to death.
N.2.2.2.1. Surveillance for hepatocellular carcinoma (HCC)
Patients with cirrhosis run an increased risk of developing hepatocellular carcinoma. It is widely believed that a comprehensive HCC surveillance package can reduce the morbidity and mortality associated with HCC. However, there is a lot of uncertainty around the optimal surveillance frequency.
In the model, most of the health states depicted have a corresponding 5-year HCC state attached to them. Survivors from this cancer tunnel state that do not receive a liver transplant either return to their state of origin or are transferred to their corresponding true positive state in the cases where patients originally received an FN cirrhosis diagnosis. This is because it is assumed that if HCC is detected this would be directly attributed to cirrhosis and therefore patients would immediately receive a positive cirrhosis diagnosis without the need for further diagnostic testing.
As a model base case all patients diagnosed with cirrhosis will be monitored yearly for HCC. This was set after agreement with the GDG that this reflects common current practice in the NHS and in view of the GDG's opinion that having a no-surveillance strategy for HCC would not be appropriate. A 6-monthly surveillance strategy will also be tested for its cost-effectiveness compared with annual surveillance to contribute to the relevant clinical review question.
To apply the clinical benefit of HCC surveillance, figures from 2 different sources, identified by the clinical review (one included in the review: Santi 2010), were combined. A study by Zhang 2004 with a 5-year follow up on 18,816 hepatitis patients reported that 6-monthly surveillance (using alpha-fetoprotein [AFP] blood test plus ultrasound) was associated with a 37% reduction in HCC mortality in comparison to a no-monitor control group. This number was combined with an increased risk of death figure (1.39 hazard ratio) for patients under annual surveillance (AFP blood test plus imaging test) when compared to a 6-monthly surveillance strategy reported by Santi 2010 (649 patients of mixed disease aetiology). Therefore, for use in the model, 6-monthly and yearly surveillance were associated with a risk ratio of 0.63 and 0.88 respectively. These risk ratios were applied to the liver- associated mortality of every true positive HCC health state.
The costs of an AFP blood test and an ultrasound were added accordingly to the model as those tests were considered by the GDG to be the current HCC surveillance practice across the NHS.
Two relevant economic evaluations were identified in our systematic literature review: one that compared annual surveillance and 6-monthly surveillance in people with cirrhosis of mixed aetiology178 and one that compared no surveillance, annual AFP, annual ultrasound, annual AFP plus ultrasound, 6-monthly AFP, 6-monthly ultrasound, and 6-monthly AFP plus ultrasound in people with cirrhosis with either alcohol-related liver disease or hepatitis C.732,733
N.2.2.2.2. Surveillance for oesophageal varices
Variceal bleeding is one the most common complications of cirrhosis and is considered a decompensating event. Endoscopic surveillance for the development and the size estimation of oesophageal varices is believed to have a substantial patient benefit as those identified with medium and large varices receive a band ligation procedure that offers prophylactic benefits against variceal bleeding.
In the model base case, all patients diagnosed with cirrhosis will be monitored every 3 years for varices. This was set after agreement with the GDG that this reflects common current practice in the NHS. A 2-yearly and an annual surveillance strategy will also be tested for their cost-effectiveness compared to a 3-yearly strategy to contribute to the relevant clinical review question.
People who developed medium or large varices whilst in either compensated or decompensated cirrhosis states were represented in separate health states (depicted as Var and dcVar respectively in the model structure, Figure 200). As presented in Figure 201 below, people with cirrhosis are separated between those who have developed varices since their most recent endoscopy and so have not been yet been identified as having varices or offered a prophylactic band ligation (VarTP-Un, dcVar-Un) and those who have received an endoscopy since they have developed varices and so are assumed to have been correctly identified as having varices and consequently protected against bleeding by prophylactic band ligation (VarTP-Pr, dcVar-Pr). Similarly bleeding has been separated from the other decompensating events (ascites, hepatic encephalopathy, jaundice) and is represented as a separate state, which individuals are in for a single Markov cycle, after which if still alive they are transferred to a decompensated state, but with their varices now protected (dcVar-Pr). Prophylactic band ligation was taken to reduce the risk of bleeding by 50%, as found by a literature review provided by the GDG (Berzigotti 2013). The prevalence of varices (of any size) in people diagnosed with cirrhosis (40%) and annual rates of varices development in people with compensated or decompensated cirrhosis but without varices of 6% and 10% respectively were also sourced from this study. Those figures were adjusted accordingly to represent the proportion of people with cirrhosis with medium or large varices, which was set to 67% of the overall cohort of people with cirrhosis with varices of any size (assumption by Stevenson 2012).
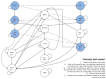
Figure 200
Graphical depiction of the Markov model.
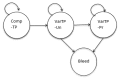
Figure 201
Surveillance for varices structure.
The cost of a diagnostic endoscopy is accordingly added to any Markov cycle during which surveillance for varices is conducted, depending on the frequency chosen. Under GDG guidance it was also assumed that if the endoscopy identified medium to large varices, a band ligation was offered immediately at the same visit. In the described scenario the cost of endoscopy was not applied to avoid double counting as band ligation is conducted endoscopically.
No relevant economic evaluations were identified in our systematic literature review.
N.2.2.3. Population cohorts
N.2.2.3.1. Non-alcoholic fatty liver disease (NAFLD)
This cohort has the simplest representation in the model. As for all populations, people with NAFLD diagnosed with cirrhosis will receive surveillance for HCC and varices. People with NAFLD will be offered lifestyle interventions and pharmacological treatment using pioglitazone or vitamin E regardless of whether they have advanced fibrosis (F3) or cirrhosis, and so diagnosis of cirrhosis will not lead to any change in the treatment for the underlying NAFLD. Baseline probabilities are applied to model the progression of liver disease.
N.2.2.3.2. Alcohol-related liver disease (ALD)
All patients presenting with alcohol-related cirrhosis will need to undergo medically assisted withdrawal from alcohol as specified in NICE CG100 and CG115. Such treatment is not however different depending on whether the patient has cirrhosis or not and therefore is not represented in the current model. Instead, the model examines the effect of a positive cirrhosis test result on a patient's alcohol abstinence. A similar approach was also followed by 2 recently published NIHR HTAs on ALD cohorts (Crossan 2015, Stevenson 2012). Non-invasive liver tests were assumed to have a smaller effect compared to liver biopsy due to the latter's invasive nature. Figures on the abstinence effect of liver biopsy were sourced from Crossan 2015 (authors cite a published abstract) while the abstinence effect of non-invasive liver tests was based on authors' assumptions. Figures are tested in deterministic sensitivity analysis in the current model.
In addition, following assumptions made by the Stevenson 2012 HTA, we attached a different bleeding rate for abstainers and drinkers.
N.2.2.3.3. Hepatitis B (HBV)
Following guidance from the GDG, we assumed that all patients referred for a cirrhosis test are also receiving treatment with antiviral drugs. This was considered a rational assumption as for patients to be suspected for cirrhosis they must have been new referrals and therefore not been appropriately treated for the underlying cirrhosis cause before.
The GDG agreed that first-line treatment would be pegylated interferon alfa-2a for 1 year. Patients who do not respond to first-line treatment are switched to either tenofovir or entecavir from the second year onwards indefinitely. For modelling purposes we set 75% of the referrals for second-line treatment for tenofovir and the remaining 25% for entecavir as the GDG felt that this reflects current NHS practice. The rates by which patients respond to first-line treatment were different for patients with positive and negative e antigen. Relevant figures were sourced from the NICE Hepatitis B guideline (CG165). The therapeutic effect of the HBV antiviral drugs was applied through a relative risk ratio attached to the patient's mortality. The model also included a different progression rate from advanced fibrosis (F3) to cirrhosis for patients with positive and negative e antigen, an approach also adopted by the Crossan 2015 HTA.
N.2.2.3.4. Hepatitis C (HCV)
A new generation of polymerase inhibitor drugs for hepatitis C has been recently assessed by NICE in technology appraisals and are entering NHS practice. In order for the present economic model to reflect the most up-to-date NICE recommendations, 2 recently published drug combinations (part of TA330 and TA ID742) covering the 4 most prevalent UK HCV genotypes are included in the modelling of the cirrhosis patient pathway. Ombitasvir-paritaprevir-ritonavir is also an option for genotypes 1 and 4. We chose ledipasvir-sofosbuvir as that is at least as effective and with a similar price. Note that the economic results would not be altered by this choice of drug as both effectiveness and cost are very similar. People with HCV without cirrhosis are assumed to receive the appropriate pegylated interferon and ribavirin regimes since polymerase inhibitor drugs are not currently recommended for these patients.
With the introduction of the new antiviral treatments and their inclusion to the present model, the GDG has made a similar assumption as the one described for the HBV model cohort, that for patients to be suspected for cirrhosis they must be new referrals and therefore not appropriately treated before for the underlying cirrhosis cause (since antiviral treatments would dramatically decrease the progression rate to cirrhosis). Therefore all of the patients in the model cohort will be treated with an antiviral agent.
The treatment effectiveness of the antiviral drugs is represented in the model by their sustained viral response (SVR). This figure is the rate of patients who have responded to treatment and therefore were ‘cured’ of the virus. The SVR was consequently applied to the probability of a patient progressing to the next state in the Markov model as patients that are free from the virus are assumed not to progress to more severe liver disease states. They were also assumed to only receive HCC surveillance and not varices surveillance; this was based on GDG guidance that there is still high uncertainty over the risk of HCC in ‘cured’ patients treated with the new drug combinations. SVRs per genotype where sourced from the evidence reports of TA330 and TA ID742.
Table 53
Sustained viral response per genotype.
In addition, under GDG guidance it was assumed that, for patients falsely identified as having cirrhosis, the drug effectiveness is identical as for the correctly diagnosed with cirrhosis patients. For patients falsely diagnosed as negative, the drug effectiveness of the fibrosis-HCV treatment options was adjusted to 50% in order to depict their lower efficacy in patients with cirrhosis.
N.2.2.4. Uncertainty
The model was built probabilistically to take account of the uncertainty around input parameter point estimates. A probability distribution was defined for each model input parameter. When the model was run, a value for each input was randomly selected simultaneously from its respective probability distribution; mean costs and mean QALYs were calculated using these values. The model was run repeatedly – 5,000 times for the base case and 5,000 times for each sensitivity analysis – and results were summarised.
The way in which distributions are defined reflects the nature of the data, so for example utilities were given a beta distribution, which is bounded by 0 and 1, reflecting that a quality of life weighting will not be outside this range. All of the variables that were probabilistic in the model and their distributional parameters are detailed in Table 54 and in the relevant input summary tables in Section N.2.3. Probability distributions in the analysis were parameterised using error estimates from data sources.
Table 54
Description of the type and properties of distributions used in the probabilistic sensitivity analysis.
In addition, various deterministic sensitivity analyses were undertaken to test the robustness of model assumptions. In these, one or more inputs were changed and the analysis rerun to evaluate the impact on results and whether conclusions on which intervention should be recommended would change.
N.2.2.4.1. Deterministic sensitivity analysis
Apart from assigning distributions to most of the model parameters, deterministic sensitivity analysis was also performed for a variety of variables.
Table 55
Summary of parameters tested in DSA.
N.2.3. Model inputs
N.2.3.1. Summary table of model inputs
Model inputs were based on clinical evidence identified in the systematic review undertaken for the guideline, supplemented by additional data sources as required. Model inputs were validated by clinical members of the GDG. A summary of the model inputs used in the base case (primary) analysis is provided in Table 56 and Table 57 below. Health state costs are presented separately in the relevant cost section. More details about sources, calculations and rationales for selection can be found in the sections following this summary table.
Table 56
Summary of base case model inputs.
Table 57
Overview of parameters and parameter distributions used in the model.
N.2.3.2. Diagnostic accuracy
The characteristics of liver biopsy, when serving as a reference standard, were carefully specified in the diagnostic review protocol. Therefore, after agreement with the GDG, only studies reporting a liver biopsy with at least 6 portal tracts and a length of 15 mm or more were considered in the review of the literature. When there were not enough studies (fewer than 3) around the diagnostic accuracy of a specific test for pooled sensitivity and specificity estimates, the corresponding 2×2 diagnostic table was selected from a single study that was believed to represent the best quality evidence. For the ALD cohort and transient elastography at a 11–<13 threshold, to represent the uncertainty around its diagnostic accuracy and because the log-normal distribution could not fit onto a test with a 100% sensitivity, its 2×2 table was adjusted by adding 0.1 patients in each of the four diagnostic outcomes. This brought down its sensitivity from 100 to 99. A similar approach was followed for the NAFLD cohort and transient elastography at a 15 threshold. Selection criteria for the chosen sources are presented in Table 58 below.
Table 58
Source selection when <3 studies identified.
To account for uncertainty around diagnostic accuracies and correlation between sensitivity and specificity a joint distribution was used when making diagnostic accuracies probabilistic. First of all the diagnostic odds ratio (DOR) was calculated for the diagnostic test:
The standard error of the log DOR was calculated using the absolute values for the number of TP, TN, FP and FN:
Using these equations a normal distribution was fitted around the log of the DOR.
Once the DOR is calculated the sensitivity can become a function of the DOR and the specificity:
Finally a beta distribution was fitted around the specificity, therefore when probabilistic sensitivity analysis is conducted the specificity will change in accordance to the overall diagnostic uncertainty and its relationship with the sensitivity.
When reviewers identified more than 2 studies for a specific test, pooled diagnostic accuracy figures were estimated with the use of Bayesian methods. To account for uncertainty around these figures random samples were drawn from the original joint posterior distribution (WinBUGS iterations) for the purposes of probabilistic sensitivity analysis.
Diagnostic accuracy data for the HBV cohort were sourced from the NICE Hepatitis B guideline (CG165).
N.2.3.3. Baseline transition probabilities
Relevant transition rates were sought in the literature and were confirmed by the GDG as appropriate for use in the current model. All transition rates were transformed to 6-monthly transition probabilities.
N.2.3.3.1. Hepatitis B and hepatitis C
Table 59
HBV – 6-monthly transition probabilities.
Table 60
HCV – 6-monthly transition probabilities.
As presented in the above tables, the majority of the transition probabilities originated from the Wright 2006 UK HTA and an economic evaluation on HBV drugs conducted by Dakin et al 2010. For use in the current model those figures where sourced from the Crossan 2015 HTA. The figures on the prevalence of varices in people with cirrhosis were sourced from a review conducted by Berzigotti 2013 and were adjusted by assuming that two thirds of patients develop medium to large varices; this adjustment was applied to all subgroups evaluated in the model. Bleeding rates were obtained from a prospective study of 321 patients with cirrhosis and varices and no history of bleeding conducted by the North Italian Endoscopic Club (NIEC 1988). The HCC incidence rate was assumed to be constant across all patients with cirrhosis (compensated or decompensated), an approach also followed by the Crossan 2015 HTA. Bleeding mortality was sourced from Stevenson 2012 and it was based on clinical judgement. The decompensation rates were adjusted for people with and without varices with a ±25% adjustment to the baseline rate that was based on GDG expert opinion. This adjustment was considered appropriate by the GDG and was applied to all the subgroups considered in the model.
N.2.3.3.2. NAFLD
Table 61
NAFLD – 6-monthly transition probabilities.
As presented in the above table, for the progression of NAFLD patients to cirrhosis a transition probability was obtained from the Singh 2015 meta-analysis of studies with a paired biopsy study design. The decompensation rate was sourced from Hui 2003, a study observing the long-term outcomes of cirrhosis in non-alcoholic steatohepatitis (NASH) patients. The figures on the prevalence of varices in people with cirrhosis were sourced from a review conducted by Berzigotti 2013 and were adjusted by assuming that two thirds of patients develop medium to large varices. Bleeding rates were obtained from a prospective study of 321 patients with cirrhosis and varices and no history of bleeding conducted by the North Italian Endoscopic Club (NIEC 1988). Bleeding mortality was sourced from Stevenson 2012 and it was based on clinical judgement. The incidence of HCC was obtained from Ascha 2010, a study evaluating the incidence and risk factors of HCC in 195 NASH patients. It was assumed that this rate applied to both compensated and decompensated patients. Due to the lack evidence in the remaining transition probabilities, those from the hepatitis cohorts were used after agreement with the GDG.
N.2.3.3.3. ALD
Table 62
ALD – 6-monthly transition probabilities.
As presented in the table above, progression to cirrhosis was obtained from Pares 1986, a study on the histological course of alcoholic hepatitis. The decompensation rate was sourced from an epidemiologic analysis of patients from the UK General practice research database conducted by Fleming 2010. The figures on the prevalence of varices in people with cirrhosis were sourced from a review conducted by Berzigotti 2013 and were adjusted by assuming that two thirds of patients develop medium to large varices. Bleeding rates were obtained from Stevenson 2012 where separate rates were reported for drinkers and abstainers (for abstainers this was based on clinical judgement). Those were adjusted for decompensated cirrhosis patients according to the proportional increase reported in NIEC 1988 that was used in the HBV, HCV and NAFLD model cohorts. The HCC incidence rate was assumed to be constant across all people with cirrhosis (compensated or decompensated), an approach also followed by the Crossan 2015 HTA. Bleeding mortality was sourced from Stevenson 2012 and it was based on clinical judgement. Due to the lack of evidence in the remaining transition probabilities, the mean between the HBV and HCV cohorts were used after agreement with the GDG.
N.2.3.4. Life expectancy and mortality rates
Life tables for England and Wales, published by the Office of National Statistics (ONS) based on 2011–2013 mortality data were used to establish population mortality rates for men and women for ages 45 to 100 years.561 ONS 2013 mortality statistics for England and Wales by cause of death560 were used to calculate the proportion of deaths for each 5-year age group which were due to liver related or non-liver related causes. These proportions were applied to the mortality rates to give the risk of death due to non-liver related causes for each annual age group for both men and women.
N.2.3.5. Utilities
N.2.3.5.1. Hepatitis B and hepatitis C
Quality of life figures were systematically sought in the literature (details in Appendix G) with priority given to studies in a UK population using EQ-5D with UK weights, in line with the NICE reference case. For both hepatitis B & C cohorts, utilities were sourced from a 2006 NIHR HTA study by Wright et al. on HCV patients. These were obtained through a separate observational study on 355 patients to whom an EQ-5D questionnaire was administered. For the health states of decompensated cirrhosis, HCC and transplant, utilities were sourced from Longworth et al. 2003, a UK transplantation study. Although HBV figures for the later health states were also available for a HBV population in the Longworth study, they were not used as there was a lack of consistency with the utilities reported by Wright 2006 that was highlighted by the GDG.
In addition, our search identified HBV-specific utilities in a non-UK population also used by the NICE Hepatitis B clinical guideline (CG165). They were not used however as they were considered too high for a population with advanced liver disease.
N.2.3.5.2. Alcohol-related liver disease
The systematic literature search identified a lack of quality of life evidence for this population. The GDG noted that this is mainly due to the fact that it is difficult for any quality of life instrument to isolate the effect of liver disease from the other effects of a patient's alcohol dependence.
For this reason the GDG suggested the use of utility values derived from alcohol-dependent patients as a baseline for the quality of life of patients with compensated cirrhosis (since this state is asymptomatic). After a comprehensive literature search, a study from Pettinati et al. 2009 was identified and used as a source for this value. The objective of the study was to quantify the effectiveness of extended-release naltrexone in alcohol-dependent patients through a randomised control trial. The SF-36 values of the trial's control group were transformed into quality of life utilities through the Ara & Brazier mapping algorithm (first regression model).39
To acquire utilities for the remaining model health states, using the baseline value from Pettinati et al. 2009 for the compensated cirrhosis state, we estimated the utilities for the other health states in this subgroup as the product of the baseline value by the proportional difference in utility in the Hepatitis B population for the health state compared to the compensated cirrhosis state. For example, in the Hepatitis B subgroup compensated cirrhosis has a utility of 0.55 while decompensated cirrhosis has a utility of 0.49. Therefore in the ALD subgroup the utility of decompensated cirrhosis is calculated as the utility of compensated cirrhosis in the ALD population (0.52) multiplied by the ratio of the 2 states in the Hepatitis B group (0.52 * 0.49/0.55).
N.2.3.5.3. Non-alcoholic fatty liver disease
The systematic literature review identified a variety of evidence for NAFLD patients. In the majority of this evidence authors did not report quality of life results per liver disease state (fibrosis, compensated cirrhosis, decompensated cirrhosis). In addition, a range of relevant literature could not be used due to the lack of available mapping algorithms for transformation to EQ-5D utilities. A study conducted by David et al. 2009 reported a quality of life estimate specifically on non-NASH NAFLD patients (ADD VALUE), however this was considered too low by the NAFLD GDG and not appropriate to be used in the economic model.
As an alternative, the NAFLD GDG suggested using the utility attributed to patients with obesity as a baseline for quality of life of non-NASH NAFLD patients. This value was obtained from recent NICE public health guidance (PH53) that simulated the relation of BMI with quality of life in two-dimensional tables. To acquire utilities for the remaining model health states the same method used for the ALD subgroup was used (that is, using the proportional increments and decrements from the hepatitis B subgroup).
N.2.3.6. Resource use and cost
N.2.3.6.1. Diagnostic test costs
The majority of the unit costs were sourced from the 2 relevant published HTAs.177,207 The cost of ARFI VTq was built on top of the ultrasound NHS tariff (NHS reference costs 2013–14) assuming an extra kit has to be acquired in order to perform an ARFI examination. The cost of the kit was sourced from the relevant NICE M-Tec assessment.538 A machine lifespan of 5 years with 500 ultrasound or ARFI scans per year was assumed after GDG guidance. Point shear wave elastography cost was assumed to be similar to ARFI due to technology similarities and a lack of available evidence around it.
Table 63
Cirrhosis test unit costs.
N.2.3.6.2. Surveillance for complications costs
Table 64
Unit costs of surveillance.
N.2.3.6.3. Drugs
Unit costs were sourced from BNF 69. The dosages were either taken from the relevant NICE technology appraisals or were based on GDG guidance.
Table 65
Unit costs of drugs.
N.2.3.6.4. Health states
Health state costs were constructed with GDG guidance so they represent a reference patient pathway. These include staff, test, procedure and drug costs where relevant. When pegylated interferon was used as a drug treatment a more intensive management is assumed according to current clinical protocols. Staff costs were sourced from the NHS reference cost 2013/14 schedules and PSSRU 2014. A multi-speciality staff mix was also agreed with the GDG so that it better represents current care arrangements. Test costs where sourced from a relevant HTA (Donnan 2009). Complication costs related to cirrhosis were sourced from a HTA on HCV patients (Wright 2006) and were assumed to be relevant to all aetiologies. Under GDG guidance, complication costs of patients with ALD were assumed to be 50% higher than those in the other cohorts. Liver transplant costs for hepatitis B or C patients were sourced from Brown 2006 and Wright 2006. An average of those figures was used for the NAFLD and ALD aetiologies.
Table 66
Unit costs of staff.
Table 67
6-monthly health state costs (based on GDG guidance).
N.2.4. Computations
The model was constructed in Microsoft Excel 2010 and was evaluated by cohort simulation. Time dependency was built in by cross referencing the cohort's age as a respective risk factor for other- cause mortality.
Patients start in cycle 0 in an alive health state. Patients moved to the dead health state at the end of each cycle as defined by the mortality transition probabilities.
Where not already available, transition probabilities were calculated using an assumption of a fixed rate across each source-study follow-up.
Rates were converted into transition probabilities for the respective cycle length (6 months) before inputting into the Markov model. The probability of the event over the time horizon specified by the literature was converted into a rate, before being converted into a probability appropriate for the cycle length. The above conversions were done using the following formulae:
|
| Where P=probability of event over time t t=time over which probability occurs (X months) |
|
| Where r=selected rate t=cycle length (6 months) |
Life years for the cohort were computed each cycle. To calculate QALYs for each cycle, Q(t), the time spent in each state of the model (6 months) was weighted by a utility value that is dependent on the time spent in the model and the treatment effect. QALYs were then discounted to reflect time preference (discount rate 3.5%). QALYs during the first cycle were not discounted. The total discounted QALYs were the sum of the discounted QALYs per cycle.
Costs per cycle, C(t), were calculated in the same way as QALYs. Costs were discounted to reflect time preference (discount rate 3.5%) in the same way as QALYs using the following formula:
Discount formula:
|
| Where: r=discount rate per annum n=time (years) |
In the deterministic and probabilistic analyses, the total number of QALYs and resource costs accrued by patients in every health state was recorded. These subtotals were summed across all subgroups to ascertain the total number of patients in the population and the total QALYs and resource costs accrued for the population. The total cost and QALYs accrued by the cohort was divided by the number of patients in the population to calculate a cost per patient and cost per QALY.
N.2.5. Model validation
The model was developed in consultation with the NAFLD and Cirrhosis GDGs; model structures, inputs and results were presented to and discussed with the GDGs for clinical validation and interpretation.
The models were systematically checked by the health economist undertaking the analysis; this included inputting null and extreme values and checking that results were plausible given inputs. The models were peer reviewed by a second experienced health economist from the NGC; this included systematic checking of many of the model calculations.
N.2.6. Estimation of cost-effectiveness
The widely used cost-effectiveness metric is the incremental cost-effectiveness ratio (ICER). This is calculated by dividing the difference in costs associated with 2 alternatives by the difference in QALYs. The decision rule then applied is that if the ICER falls below a given cost per QALY threshold the result is considered to be cost-effective. If both costs are lower and QALYs are higher the option is said to dominate and an ICER is not calculated.
|
| Cost-effective if:
|
When there are more than 2 comparators, as in this analysis, options must be ranked in order of increasing cost then options ruled out by dominance or extended dominance before calculating ICERs excluding these options. An option is said to be dominated, and ruled out, if another intervention is less costly and more effective. An option is said to be extendedly dominated if a combination of 2 other options would prove to be less costly and more effective.
It is also possible, for a particular cost-effectiveness threshold, to re-express cost-effectiveness results in terms of net monetary benefit (NMB). This is calculated by multiplying the total QALYs for a comparator by the threshold cost per QALY value (for example, £20,000) and then subtracting the total costs (formula below). The decision rule then applied is that the comparator with the highest NMB is the most cost-effective option at the specified threshold. That is the option that provides the highest number of QALYs at an acceptable cost.
|
Net Monetary Benefit (X) = (QALYs(X)×λ) − Costs (X) | Cost-effective if:
|
Both methods of determining cost-effectiveness will identify exactly the same optimal strategy. For ease of computation NMB is used in this analysis to identify the optimal strategy. The NMB figure is followed by the test ranking and the 95% confidence intervals of the ranks. An additional figure that represented the percentage of simulations where every test ranked first was also calculated.
Results are also presented graphically where total costs and total QALYs for each diagnostic strategy are shown.
N.2.7. Interpreting results
NICE's report ‘Social value judgements: principles for the development of NICE guidance’539 sets out the principles that GDGs should consider when judging whether an intervention offers good value for money. In general, an intervention was considered to be cost-effective if either of the following criteria applied (given that the estimate was considered plausible):
- The intervention dominated other relevant strategies (that is, it was both less costly in terms of resource use and more clinically effective compared with all the other relevant alternative strategies), or
- The intervention costs less than £20,000 per quality-adjusted life-year (QALY) gained compared with the next best strategy.
As we have several diagnostic tests, we use the NMB to rank the strategies on the basis of their relative cost-effectiveness. The highest NMB identifies the optimal strategy at a willingness to pay of £20,000 per QALY gained. Where the differences in the NMBs between alternative options were considered small, ICERs were calculated to interpret the model results.
N.3. Results
Cost-effectiveness results of the cirrhosis diagnostic tests and the optimal surveillance frequency for HCC and oesophageal varices are presented in separate sections. For ALD, ICERs comparing all strategies to ‘no test – no monitor’ were also calculated due to the high uncertainty depicted in the confidence intervals. For the HCV cohort, diagnostic test results are only presented for genotypes 1 and 3 as those for genotypes 2 and 4 were consistent with these (top 3 test rankings are instead presented for all genotypes). To define the most cost-effective surveillance frequency for HCC and oesophageal varices, ICERs were calculated across the available options. Base case results below were obtained through the probabilistic analysis to take combined parameter uncertainty into account.
Table 68
Definitions of column categories.
N.3.1. Diagnostic tests – base cases
N.3.1.1. People with NAFLD
Table 69
Number of events & time spent in health states.
Table 70
Life years and results.
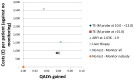
Figure 202
Cost-effectiveness plot: NAFLD.
Across the 6 strategies compared, the non-invasive tests ranked on top with transient elastography at a <15.0 threshold ranking first having an NMB of £165,224. All 3 non-invasive strategies delivered similar QALY figures and slightly differed in the overall mean costs. The confidence intervals in the rankings only excluded liver biopsy and the ‘no-test’ strategies from ranking first, highlighting the uncertainty in the cost-effectiveness of the 3 non-invasive tests. In the probabilistic analysis transient elastography at <15.0 ranked first in 51% of the simulations followed by ARFI and TE at 10.0 < 13.0 (36% and 12% respectively). ICERs comparing all strategies against ‘no test – no monitoring’ ranged from £13,868 to £14,577 for the non-invasive strategies and at £98,051 for liver biopsy.
N.3.1.2. People with ALD
Table 71
Number of events & time spent in health states.
Table 72
Life years and results.
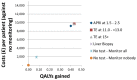
Figure 203
Cost-effectiveness plot: ALD.
In the ALD cohort, testing for cirrhosis was not cost-effective at a £20,000 threshold with the 2 ‘no-test’ strategies ranking higher. The ‘no-monitor’ strategy had the highest NMB value of £68,697 and the ‘monitor-all’ strategy followed with £68,321. The diagnostic test that ranked first was transient elastography at 11.0–<13.0 with an NMB of £67,644. All diagnostic test strategies delivered considerably higher QALY values compared to no testing (up to 0.5 more QALYs) but at increased mean costs. All strategies apart from liver biopsy had wide confidence intervals of their ranks ranging from first or second to fifth and depicting the high uncertainty in the results. ICERs comparing all strategies against ‘no test – no monitoring’ ranged from £22,438 to £22,977 for the non-invasive strategies and at £25,405 for liver biopsy.
N.3.1.3. People with HBV: HBV− antigen
Table 73
Number of events & time spent in health states.
Table 74
Life years and results.
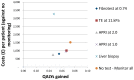
Figure 204
Cost-effectiveness plot: HBV− antigen.
In the HBeAg-negative cohort, APRI at 1.0 ranked first with an NMB of £103,281. Transient elastography at 11.0 kPa and FibroTest at 0.74 followed with NMBs of £103,237 and £103,210 respectively. Transient elastography delivered similar QALYs to APRI at 1.0 but for an incremental cost of £62 per patient. FibroTest was less costly then transient elastography and APRI at 1.0 but less effective too. Liver biopsy ranked lowest across all strategies particularly due to its high overall mean costs. In the confidence intervals of the ranks, transient elastography, FibroTest, APRI at 1.0 and ‘no test – no monitoring’ could all rank first with APRI at 1.0 ranking first in 45% of the simulations followed by FibroTest (23%). ICERs comparing all strategies against ‘no test – no monitoring’ ranged from £14,406 to £16,439 for the non-invasive strategies and at £67,013 for liver biopsy.
N.3.1.4. People with HBV: HBV+ antigen
Table 75
Number of events & time spent in health states.
Table 76
Life years and results.
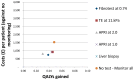
Figure 205
Cost-effectiveness plot: HBV+ antigen.
In the HBeAg-positive cohort, FibroTest at 0.74 ranked first. Transient elastography at 11.0 kPa and APRI at 1.0 followed as second and third options. NMB for FibroTest was £158,366 and for transient elastography at 11.0 kPa and APRI at 1.0 was £158,362 and £158,358 respectively. Liver biopsy ranked lowest across all strategies particularly due to its high mean costs. The top 4 options (transient elastography, FibroTest, APRI at 1.0 and ‘no test – no monitoring’) had NMBs sufficiently close that it is impossible to be sure which of these should be preferred in terms of cost-effectiveness. Each could rank first within the confidence intervals. In the probabilistic analysis, ‘no test – no monitoring’ ranked first in 36% of the simulations with transient elastography, FibroTest, APRI at 1.0 all ranking first in about 20% of the simulations, each highlighting the high uncertainty in the results. ICERs comparing all strategies against ‘no test – no monitoring’ ranged from £19,039 to £25,418 for the non-invasive strategies and at £423,351 for liver biopsy.
N.3.1.5. People with HCV: genotype 1
Table 77
Number of events & time spent in health states.
Table 78
Life years and results.
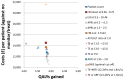
Figure 206
Cost-effectiveness plot: HCV genotype 1.
In the HCV genotype 1 cohort liver biopsy ranked first with a NMB value of £216,472. Transient elastography at 13.0–<15.0 and the Castera algorithm followed with £215,580 and £215,251 respectively. Liver biopsy dominated all the other strategies apart from ‘no test – no monitoring or treatment’ by having the highest QALY value and the second lowest mean costs. Transient elastography at 13.0–< 15.0 delivered slightly lower QALYs for an incremental cost of £656. From all strategies it was only liver biopsy and transient elastography at 13.0–<15.0 that could rank first according to the ranking confidence intervals with liver biopsy ranking first in 90% of the simulations. ICERs comparing liver biopsy and TE at 13.0-<15.0 to ‘no testing – no monitoring or treatment’ were £2,720 and £2,897 respectively.
N.3.1.6. People with HCV: genotype 3
Table 79
Number of events & time spent in health states.
Table 80
Life years and results.
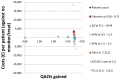
Figure 207
Cost-effectiveness plot: HCV genotype 3.
In the HCV genotype 3 cohort, liver biopsy ranked first with an NMB value of £225,611. Transient elastography at 13.0–<15.0 and the Castera algorithm followed with almost identical NMBs at £223,277 and £223,199 respectively. Liver biopsy dominated the Castera algorithm being more effective and less costly. Transient elastography at 13.0–<15.0 delivered marginally more QALYs but for a considerable incremental cost of £2,575. From all strategies it was only liver biopsy and transient elastography at 13.0–<15.0 that could rank first according to the ranking confidence intervals with liver biopsy ranking first in 97% of the simulations. The ‘no testing – no monitoring or treatment’ strategy was dominated by liver biopsy and TE at 13.0-<15.0 in direct comparisons.
N.3.1.7. People with HCV: all genotypes
Table 81
HCV diagnostic tests – top 3 ranked in every genotype.
N.3.2. Frequency of surveillance
N.3.2.1. Frequency of HCC surveillance
Table 82
ICERs comparing 6-monthly surveillance against annual surveillance.
The cirrhosis test used in each case was that recommended by the GDG following its consideration of the results of Section N.3.1. Where more than 1 test was recommended, the most cost-effective of those tests was used.
Across all aetiologies 6-monthly surveillance for HCC was overall more costly and more effective compared to the annual strategy. At a £20,000 threshold, 6-monthly surveillance was cost-effective only in the HCV genotype 1 cohort (ICER at £18,657). The ICERs in the remaining cohorts ranged between £23,220 and £28,352.
N.3.2.2. Frequency of oesophageal varices surveillance
Table 83
ICERs comparing annual and 2-yearly surveillance against 3-yearly surveillance.
The cirrhosis test used in each case was that recommended by the GDG following its consideration of the results of Section N.3.1. Where more than 1 test was recommended, the most cost-effective of those tests was used.
Surveillance for the presence of oesophageal varices every 2 years was not cost-effective for all cohorts but the two HCV. The ICER comparing 2-yearly with 3-yearly surveillance was below £20,000 for HCV genotype 1, dominating for genotype 3, but not cost-effective for NAFLD, ALD or HBV. Annual surveillance was not cost-effective for any aetiology at a £20,000 threshold.
N.3.3. Sensitivity analyses
N.3.3.1. NAFLD
Table 84
NAFLD model – Cost-effectiveness rank under different scenarios.
Across all scenarios transient elastography at >15.0 ranked first apart from when its unit cost was increased 20% and when its diagnostic accuracy was set at the low CI value. ARFI ranked first in both the aforementioned scenarios showing the amount of uncertainty between the 2 tests. Liver biopsy and the 2 ‘no test’ strategies remained last in all scenarios without a change in their rank.
N.3.3.2. ALD
Table 85
ALD model – Cost-effectiveness rank under different scenarios.
The ‘no test – no monitor’ strategy remained first in all scenarios. Transient elastography at 11.0–<13.0 remained the diagnostic test ranking first in 9 out of the 10 tested scenarios. In the remaining scenarios transient elastography at >15.0 ranked higher when the diagnostic accuracy of transient elastography at 11.0–<13.0 was set at its low CI.
N.3.3.3. HBeAg-negative
Table 86
HBV- model – Cost-effectiveness rank under different scenarios.
APRI at a 1.0 threshold remained first in 6 out of 10 scenarios and came second in the 5 remaining ones. FibroTest ranked first in 3 scenarios and second or third in the remaining ones. Transient elastography ranked first in 2 scenarios (20% lower fibroscan unit costs or diagnostic accuracy of transient elastography at its high CI) and ranked from second to fourth in the remaining scenarios. No substantial ranking changes are observed in the other test strategies.
N.3.3.4. HCV genotype 3
Table 87
HCV genotype 3 model – Cost-effectiveness rank under different scenarios.
In 13 out of the 15 scenarios liver biopsy remained the first ranked strategy. It came twentieth where HCV treatment was not provided and second where the drug treatment costs were reduced by 50%. The Castera algorithm remained second in 12 out of the 15 scenarios and third, fourth and nineteenth in the remaining 3 ones. Transient elastography at 11.0–<13.0 ranked third in 12 out of the 15 scenarios and first, second and fifth in the remaining 3 ones. Rankings in the remaining test strategies did not differ substantially across the scenarios tested apart from the ‘no HCV treatment’ scenario which seemed to favour the ‘no testing – no monitoring’ strategy.
N.3.3.5. Frequency of cirrhosis testing
Table 88
ICERs comparing 2-yearly testing against annual testing.
Annual testing was not found to be cost-effective at a £20,000 per QALY threshold. ICERs comparing annual and 2-yearly testing ranged from £25,975 to £1,060,920.
N.3.3.6. HCC surveillance frequencies
Table 89
ICERs comparing 6-monthly against annual surveillance.
Lowering the HCC surveillance costs had a moderately small effect on the ICERs with only the HCV cohorts being lower than the 20,000 threshold. Increasing the effectiveness of 6-monthly surveillance had a slightly larger effect still making 6-monthly surveillance cost-effective only in the HCV cohorts.
N.3.3.7. Oesophageal varices surveillance frequencies
Table 90
ICERs compared to 3-year surveillance.
Variation of the surveillance costs and the RR on the bleeding probability had little effect on the overall cost-effectiveness of more frequent surveillance for oesophageal varices. Increasing the frequency to 2 years was only cost-effective for the ALD cohort in 2 out of the 3 tested scenarios.
N.4. Conclusions
N.4.1. Evidence statements
- An original cost-utility analysis that compared 6 strategies to diagnose cirrhosis in people with NAFLD and advanced fibrosis with a retest frequency of 2 years found that transient elastography ranked first compared to the following diagnostic strategies, using relevant thresholds for each test, with reference to a cost-effectiveness threshold of £20,000 per QALY gained:
- ARFI
- transient elastography (lower threshold)
- no test – no surveillance
- no test – surveillance for all
- liver biopsy.
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared 6 strategies to diagnose cirrhosis in people with ALD, with a retest frequency of 2 years, found that:
- The ‘no test – no surveillance’ strategy ranked first compared to the following diagnostic strategies, using relevant thresholds for each test, with reference to a cost-effectiveness threshold of £20,000 per QALY gained:
- –
no test – surveillance for all
- –
transient elastography (low threshold)
- –
transient elastography (high threshold)
- –
APRI
- –
liver biopsy.
- When compared to the ‘no test – no monitor’ strategy, the 3 non-invasive tests had ICERs between £22,438 and £22,977 per QALY gained.
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared 7 strategies to diagnose cirrhosis in people with hepatitis B and HBeAg negative with a retest frequency of 2 years found that APRI ranked first compared to the following diagnostic strategies, using relevant thresholds for each test, with reference to a cost-effectiveness threshold of £20,000 per QALY gained:
- transient elastography
- FibroTest
- APRI (higher threshold)
- no test – no surveillance
- no test – surveillance for all
- liver biopsy.
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared 7 strategies to diagnose cirrhosis in people with hepatitis B and HBeAg positive with a retest frequency of 2 years found that FibroTest ranked first compared to the following diagnostic strategies, using relevant thresholds for each test, with reference to a cost-effectiveness threshold of £20,000 per QALY gained:
- no test – no surveillance
- transient elastography
- APRI (low threshold)
- APRI (high threshold)
- no test – surveillance for all
- liver biopsy.
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared 20 strategies to diagnose cirrhosis in people with hepatitis C with a retest frequency of 2 years found that liver biopsy ranked first compared to the following diagnostic strategies, using relevant thresholds for each test, with reference to a cost-effectiveness threshold of £20,000 per QALY gained:
- Castera algorithm
- transient elastography (medium threshold)
- transient elastography and ARFI
- transient elastography or ARFI
- transient elastography (high threshold)
- SAFE algorithm
- point shear wave elastography
- transient elastography (low threshold)
- ARFI
- platelet count
- APRI
- ELF
- FIB-4
- FibroTest
- APRI
- AST-ALT ratio
- no testing – surveillance for all, treat HCV using medication for people with cirrhosis
- no testing – no surveillance, treat HCV using medication for people with fibrosis
- no testing – no surveillance, no treatment for HCV.
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared 6-monthly with annual surveillance for HCC in people with cirrhosis at a cost-effectiveness threshold of £20,000 per QALY gained found that:
- 6-monthly surveillance was cost-effective compared to annual surveillance for people with HCV genotype 1 (ICER: £18,657 per QALY gained).
- 6-monthly surveillance was not cost-effective compared to annual surveillance for people with NAFLD, ALD, HBV or HCV genotype 1 (ICERs: £20,128–28,352).
This analysis was assessed as directly applicable with minor limitations. - An original cost-utility analysis that compared annual, 2-yearly and 3-yearly surveillance for the detection of varices in people with cirrhosis at a cost-effectiveness threshold of £20,000 per QALY gained found that:
- Annual surveillance was not cost-effective compared to 3-yearly surveillance (ICERs: £48,430–122,413 per QALY gained or dominated).
- 2-yearly surveillance was cost-effective compared to 3-yearly surveillance in people with C (ICERs: £75 per QALY gained or dominant).
- 2-yearly surveillance was not cost-effective compared to 3-yearly surveillance in people with NAFLD and advanced fibrosis, ALD or hepatitis B (ICERs: £36,552–63,167 per QALY gained or dominated).
This analysis was assessed as directly applicable with minor limitations.
N.4.2. Summary of results
N.4.2.1. NAFLD
Transient elastography at a threshold of 15.0 kPa ranked first mainly due to having the highest diagnostic accuracy among the non-invasive tests. ARFI followed second being slightly less accurate but also having lower test unit costs. Transient elastography at 10.0 - <13.0 kPa ranked third having similar specificity to the other 2 tests but lower sensitivity. All 3 non-invasive tests had similarly wide confidence intervals (1 to 4).
In the deterministic sensitivity analysis, rankings were sensitive to increases in the transient elastography and ARFI unit costs and in the decrease of the diagnostic accuracy of transient elastography at 15.0 kPa. Therefore, no safe conclusion can be made over the most cost-effective option among the 3 comparators.
N.4.2.2. ALD
Testing people with alcohol-related liver disease for cirrhosis was not cost-effective compared to ‘no test – no monitor’ and ‘no test – monitor all’ at a cost-effectiveness threshold of £20,000 per QALY gained. However, it was cost-effective at a threshold of £30,000 per QALY gained: the ICERs for the 3 non-invasive liver tests were £22,438–£22,977). All 3 non-invasive tests had similarly wide confidence intervals (from first or second to fifth place).
In none of the deterministic sensitivity analysis scenarios did a test strategy rank higher than third. Ranking among the 3 non-invasive liver tests slightly varied across the different scenarios with transient elastography at 11.0–<13.0 remaining third in ranking for 9 out of the 10 tested scenarios.
N.4.2.3. HBV
For the HBeAg negative group, APRI at 1.0 ranked first, most probably due to its low test unit costs and its moderate diagnostic accuracy (second best after transient elastography). Transient elastography and FibroTest ranked second and third. APRI at 2.0 ranked last among the non-invasive liver tests mainly due to its considerably lower sensitivity. All non-invasive liver tests had similarly wide 95% confidence intervals.
In the HBeAg positive group, FibroTest ranked first with transient elastography and APRI at 1.0 ranking second and third. All non-invasive liver tests had similarly wide 95% confidence intervals. In the probabilistic analysis, the 3 tests also shared similar probabilities ranking first (20–23%).
Deterministic sensitivity analysis was only conducted for the HBeAg negative group. Rankings between the deterministic and the probabilistic analyses varied particularly for the FibroTest and transient elastography tests, highlighting how incorporating the uncertainty of the input parameters in the model affects the cost-effectiveness results. APRI at 1.0 ranked first or second in all scenarios. FibroTest and transient elastography followed with alternating first to fourth positions. The cost-effectiveness of APRI at 1.0 was sensitive to the decrease of HBV prevalence, the presence of varices at the point of cirrhosis diagnosis and changes to the cost and accuracy of transient elastography.
N.4.2.4. HCV
For all 4 genotypes, liver biopsy ranked first with substantially higher NMB values compared to the second options. This is mainly attributable to the fact that liver biopsy was assumed to have perfect sensitivity and specificity, and that cirrhosis misdiagnosis is associated with the incorrect administration of the highly costly polymerase inhibitor drugs. This led to the economic model particularly favouring the test with the highest diagnostic accuracy irrespective of its unit cost. In genotypes 1 and 3 where detailed results are presented, liver biopsy ranked first in 90% and 97% of the simulations respectively. Transient elastography at 13.0–<15.0 and the Castera algorithm ranked second and third in genotypes 1–4 and the ‘transient elastography or ARFI’ strategy ranked third in genotype 4.
Deterministic sensitivity analysis was only conducted for the genotype 3 group. Liver biopsy remained first in all but 2 scenarios. These were the ‘no HCV treatment’ and the ‘drug treatment cost - 50% lower’ scenarios, also highlighting how crucial the drug treatment element is for the HCV diagnostic model.
N.4.2.5. Frequency of HCC surveillance
At a cost-effectiveness threshold of £20,000 per QALY gained, 6-monthly surveillance was cost-effective compared to annual surveillance only for the HCV genotype 1 group. Although this group had the fewest liver-related deaths, risk of HCC progression was particularly high in this group compared to other model cohorts, making more frequent surveillance cost-effective at the specified threshold. However, at a cost-effectiveness threshold of £30,000 per QALY gained, 6-monthly surveillance was cost-effective compared to annual surveillance for all groups: ICERs £18,657–28,352. Variation in the ICERs was mainly due to differences in cirrhosis prevalence, risk of progression to HCC, and competing risks of other complications in each aetiology.
In the deterministic sensitivity analysis, changes in the surveillance costs or the 6-monthly surveillance effectiveness reduced the ICERs by up to £2,000 per QALY gained. Such reductions made 6-monthly surveillance cost-effective at a £20,000 per QALY gained threshold only for the 2 HCV cohorts.
N.4.2.6. Frequency of oesophageal varices surveillance
Annual surveillance was not cost-effective compared to 3-yearly surveillance for any of the model cohorts with the ICERs either exceeding £45,000 per QALY gained or showing it being dominated by the 3-year frequency option (more costly and less effective). Two-yearly surveillance was cost-effective compared to 3-yearly surveillance at a cost-effectiveness threshold of £20,000 per QALY in the 2 HCV cohorts. In the deterministic sensitivity analysis, changes in the surveillance costs or the RR applied on the bleeding probability had considerable effect on the ICERs of the higher frequencies. However with the base case ICERs of the deterministic analysis being far beyond the £20,000 threshold, any reductions in the ICERs made 2-yearly surveillance cost-effective only for the ALD cohort.
N.4.3. Comparisons with published studies
N.4.3.1. Cirrhosis diagnostic tests
Three relevant studies identified in our literature review attempted to assess the cost-effectiveness of diagnostic tests for cirrhosis, with contrasting findings.
Canavan 2013119 found TE to have an ICER of £6,557 when compared with no testing in chronic HCV patients. Liver biopsy was dominated by both these strategies. This is in contrast to the result of this model, however, the Canavan evaluation did not include the recently launched HCV treatments which particularly enhance the cost-effectiveness of highly accurate tests (such as liver biopsy) irrespective of their cost due to the very high treatment cost.
Steadman 2013709 concluded that liver biopsy was more costly and more effective compared to TE with a cost per additional correct diagnosis between £1,136 and 3,841 in the HBV, HCV and NAFLD groups. However, no safe conclusions or comparisons can be made based on these figures since important factors such as the follow-up costs and the health-related quality of life following correct or incorrect diagnoses were not included in this economic evaluation.
Stevenson 2012711 compared 6 relevant diagnostic strategies and concluded that only liver biopsy was cost-effective at a cost-effectiveness threshold of £20,000 per QALY gained for people with ALD. This is in contrast with the results of this model which indicated that neither non-invasive liver tests nor liver biopsy were considered cost-effective at the £20,000 threshold. The 2 models followed similar perspectives so result differences are mainly attributable to dissimilarities in the model structure and the input parameters (such as the strict liver biopsy quality criterion for the study selection followed by the present analysis).
N.4.3.2. Frequency of HCC surveillance
Two relevant studies identified in our literature review attempted to assess the cost-effectiveness of HCC surveillance in different frequencies.
Cucchetti 2012178 compared annual versus 6-monthly surveillance and concluded that 6-monthly is not cost-effective for either groups with compensated or decompensated cirrhosis at a cost-effectiveness threshold of £20,000 per QALY gained (ICERs of £21,230 and £40,540 respectively). These figures are similar to the ones in the present analysis, which produced ICERs ranging between £20,000 and £30,000 across the different groups.
Thompson Coon 2008732,733 compared 7 relevant strategies (including annual and 6-monthly frequencies) and concluded that only the ‘no surveillance’ strategy was cost-effective at a cost-effectiveness threshold of £20,000 per QALY gained. ICERs for the non-dominated strategies varied from £25,490 to £83,333. When 6-monthly strategies were directly compared with the annual ones, ICERs were beyond £27,500. The latter results are also in line with those in the present analysis.
N.4.3.3. Frequency of varices surveillance
No relevant studies were identified in the literature.
- Cost-effectiveness analysis: diagnostic tests and surveillance strategies for ci...Cost-effectiveness analysis: diagnostic tests and surveillance strategies for cirrhosis - Cirrhosis in Over 16s
Your browsing activity is empty.
Activity recording is turned off.
See more...
