An understanding of the global and regional epidemiology and burden of hepatitis B and C infection with respect to the main routes of transmission, most affected populations, and natural history and time course of serological markers is critical to inform strategies on both who to test and how to test. However, data are limited in many LMICs, particularly in the African region, due to weak surveillance systems with underreporting and therefore unreliable data. The nature of an epidemic within a specific country will determine the appropriate testing strategy and approaches. provides an overview of the risk factors and primary routes of transmission for HBV and HCV infection in populations most affected by HBV and HCV, as well as data on seroprevalence from systematic reviews and other studies.
4.1. Hepatitis B infection
4.1.1. Epidemiology of hepatitis B infection
It is estimated that worldwide, 2 billion people have evidence of past or present infection with HBV, and 248 million are chronic carriers of HBV surface antigen (HBsAg), particularly in LMICs (2). Age-specific HBsAg seroprevalence varies markedly by geographical region, with the highest prevalence (>5%) in sub-Saharan Africa (SSA), east Asia, some parts of the Balkan region, the Pacific Islands and Amazon Basin of South America. Prevalence below 2% is seen in regions such as Central America, North America and Western Europe (2). Overall, almost half of the global population lives in areas of high and intermediate endemicity.
The major complications of CHB are cirrhosis and HCC. Worldwide, it is estimated that around 686 000 people die each year from the complications of CHB (1). Overall, HBV infection accounts for around 45% of cases of HCC and 30% of cirrhosis, with much higher proportions in LMICs (1, 105). In Asia and most other regions, the incidence of HCC and cirrhosis is low before the age of 35–40 years but then rises exponentially (1). However, in some parts of Africa, Alaska and the Amazon, the incidence of HCC is also high in infected children and young adult men (106).
HIV and HBV. There is an estimated global HBsAg prevalence of 7·4% (IQR 5.0–11.2%) in HIV-infected persons, and a burden of 2.73 million (IQR 1.8–3.9 million; IQR 1·3–4·4 million) HIV–HBsAg-coinfected persons (87). The highest burden for HIV–HBV coinfection is in sub-Saharan Africa (SSA) (71% of all cases; 1.96 million).
4.1.2. Transmission of hepatitis B infection
provides an overview of the risk factors and primary routes of transmission for HBV infection in populations most affected by hepatitis B. HBV is spread predominantly by percutaneous or mucosal exposure to infected blood and various body fluids, including saliva and menstrual, vaginal and seminal fluids. Perinatal transmission is the major route of HBV transmission in many parts of the world, and an important factor in maintaining the reservoir of the infection in some regions, particularly in China and South-East Asia (107, 108). Horizontal transmission, including household, interfamilial and especially child to child, is also important (103). Both sexual and oral transmission of hepatitis B may occur, particularly in unvaccinated MSM and heterosexual persons with multiple sex partners or contact with sex workers. Transmission of the virus may also result from accidental inoculation of minute amounts of blood or fluid during medical, surgical and dental procedures, or from razors and similar objects contaminated with infected blood; immunization with inadequately sterilized syringes and needles; injecting drug use; tattooing; body piercing; and acupuncture. Unvaccinated health-care workers are also at risk of accidental transmission of hepatitis B during handling contaminated sharps, body fluids and organs, and medical waste.
4.1.3. Natural history of HBV infection
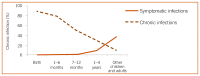
Hepatitis B virus is an enveloped DNA virus, and a member of the family Hepadnaviridae hepatotropic DNA viruses. Hepatitis B virus causes both acute and chronic infection that can range from asymptomatic infection or mild disease to severe or fulminant hepatitis. Acute hepatitis B is usually a self-limiting disease marked by acute inflammation and hepatocellular necrosis, with a case fatality rate of 0.5–1% (109). Chronic hepatitis B (CHB) encompasses a spectrum of disease, and is defined as persistent HBV infection (the presence of detectable HBsAg in the blood or serum for longer than six months), with or without associated active viral replication and evidence of hepatocellular injury and inflammation (109). Age is a key factor in determining the risk of chronic infection. Chronicity is common following acute infection in neonates (90% of neonates born to hepatitis B e antigen [HBeAg]-positive mothers) and in young children under the age of 5 years (20–60%), but occurs less commonly (<5%) when infection is acquired in adulthood (94, 95) (). Worldwide, the majority of persons with CHB were infected at birth or in early childhood.
Outcomes of hepatitis B virus infection by age at infection. Source:
Guidelines for the prevention, care and treatment of persons with hepatitis B infection. Geneva: WHO; 2015 (http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/, accessed (more...)
The natural history of CHB is dynamic and complex, and progresses non-linearly through several recognizable phases (6, 95). The phases are of variable duration, not necessarily sequential, and do not always relate directly to criteria and indications for antiviral therapy (47).
4.1.4. Time course and interpretation of serological markers of HBV infection
A range of HBV markers other than HBsAg, such as anti-HBc total and anti-HBc IgM, HBeAg and antibodies to hepatitis B e and surface antigen (anti-HBe and anti-HBs) and HBV DNA can be used to further characterize HBV infection (see
). When these markers are tested concurrently, a testing profile can be produced to differentiate acute from chronic infection, stage the disease and identify those who may benefit from treatment, monitor disease progression or response to antiviral treatment, as well as those who would benefit from HBV immunization or re-immunization.
Summary of markers of HBV infection.
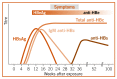
The appearance of HBsAg in the blood is followed by that of HBeAg, which is a marker of high levels of viral replication. In acute HBV infection that resolves by itself, HBeAg seroconverts relatively early to anti-HBe with the disappearance of HBsAg and HBeAg. But in chronic HBV infection, seroconversion to anti-HBe may be delayed for many years, HBeAg may persist, or neither anti-HBe nor HBeAg may be detectable in the presence of HBsAg. Antibodies to hepatitis B core antigen (anti-HBc) may occur relatively early in the infection, often within a week or two after the appearance of HBsAg, and is typified by a profound immunoglobulin (Ig)M anti-HBc response that wanes approximately 6 months later ( and .)
Acute HBV infection with recovery.
CHB is defined as the persistence of HBsAg for more than 6 months. Previous HBV infection is characterized by the presence of antibodies (anti-HBs and anti-HBc). Immunity to HBV infection after vaccination is characterized by the presence of only anti-HBs.
It also needs to be established whether the person is in the HBeAg-positive or HBeAg-negative phase of infection, though both require lifelong monitoring, as the condition may change over time. In persons with CHB, a positive HBeAg result suggests high-level HBV replication and high infectivity. Spontaneous improvement may occur following HBeAg-positive seroconversion (anti-HBe), with a decline in HBV replication, and normalization of alanine aminotransferase (ALT) levels. This confers a good prognosis and does not require treatment.
Further assessment of HBsAg-positive persons is needed to guide management and indicate the need for treatment (6). This generally includes assessment of additional serological markers of HBV infection (HBeAg), measuring aminotransferase levels to help determine liver inflammation, quantification of HBV DNA levels, and stage of liver fibrosis by non-invasive tests (NITs) such as transient elastography or serum biomarker-based tests such as aspartate aminotransferase (AST)-to-platelet ratio index (APRI), and fibrosis-4 (FIB-4).
4.1.5. Preventing hepatitis B infection through vaccination
Vaccination of infants and, in particular, delivery of hepatitis B vaccine within 24 hours of birth is 90–95% effective in preventing infection with HBV as well as in decreasing HBV transmission if followed by at least two other doses. WHO recommends universal hepatitis B vaccination for all infants, and giving the first dose as soon as possible after birth (24). This strategy has resulted in a dramatic decrease in the incidence and prevalence of CHB among young children in regions of the world where universal infant vaccination programmes have been implemented (110, 111). Target groups for catch-up vaccination as well as other preventive strategies include young adolescents, household and sexual contacts of persons who are HBsAg-positive, and persons at risk of acquiring HBV infection, such as PWID, MSM and persons with multiple sex partners.
4.1.6. Treatment of hepatitis B infection
WHO recommends antiviral agents (tenofovir and entecavir) that are active against HBV infection and have been shown to effectively suppress HBV replication, prevent progression to cirrhosis, and reduce the risk of HCC and liver-related deaths (6, 112, 113). However, in the majority of patients, treatment with these drugs does not provide cure (i.e. the person continues to have replicating virus), necessitating potentially lifelong treatment.
4.2. Hepatitis C infection
4.2.1. Epidemiology of hepatitis C infection
Recent analyses of the global prevalence of HCV indicate that there may be fewer persons living with hepatitis C infection than previously estimated. A recent systematic review estimated that 110 million persons have a history of HCV infection (i.e. are HCV-antibody positive) and 80 million have chronic viraemic infection (3). Regions estimated to have a high prevalence in the general population (>3.5%) are Central and east Asia, and North Africa/Middle East; those with a moderate prevalence (1.5–3.5%) include South and South-East Asia, Sub-Saharan Africa, Latin America (Andean, central, and southern regions), the Caribbean, Oceania, Australasia, and central, eastern and western Europe; whereas low-prevalence (<1.5%) regions include Asia–Pacific, Latin America, and North America (3). Updated estimates in Africa show a HCV prevalence of 2.98%, with a higher prevalence observed in west Africa and lower in south-east Africa (114).
Despite the declining incidence, a large number of persons who were infected 30–60 years ago are now dying from HCV-related cirrhosis and liver cancer, as these complications often take decades to develop. According to estimates from the Global Burden of Disease study, the number of deaths due to hepatitis C increased from 333 000 in 1990 to 499 000 in 2010 and 704 000 in 2013 (1, 5, 115), and this increase is projected to continue for several more decades, unless treatment is scaled up considerably (116).
HIV and HCV have common routes of transmission, and persons with HIV infection, in particular PWID and MSM, are at increased risk of HCV infection (60, 62, 88–93, 117). In a recent comprehensive systematic review, it is estimated that, globally, 2.3 million persons are coinfected with these two viruses, of whom 1.2 million (interquartile range [IQR] 0.9–1.4 million) are PWID (88). With the widespread use of antiretroviral therapy (ART), which reduces the risk of HIV-associated opportunistic infections, HCV-related liver disease has started to overtake AIDS-defining illnesses as a leading cause of death among people living with HIV in some high-income countries (HICs) (118).
4.2.2. Transmission of hepatitis C infection
provides an overview of the primary routes of transmission for HCV infection and populations most affected. There are four main routes of transmission: healthcare-associated transmission, injecting drug use, mother-to-child transmission (MTCT), and sexual transmission. In LMICs, infection with HCV is most commonly associated with unsafe injection practices, and invasive procedures in health-care facilities with inadequate infection control practices, such as renal dialysis and unscreened (or inadequately screened) blood transfusions (70–74, 77, 78, 119). Persons who received untested blood products prior to the introduction of screening of blood for HCV in (HICs) are also at risk, and WHO reports suggest that there are still 39 countries that do not routinely screen blood transfusions for bloodborne viruses (120). In middle- and high-income countries, most HCV infections occur among people who use unsterile equipment to inject drugs. PWID have a high global prevalence of infection at around 67% (46). Of the estimated 16 million people in 148 countries who actively inject drugs, 10 million have serological evidence of HCV infection (46). There is a moderate risk of MTCT of HCV which is higher in HIV-coinfected mothers (10–20%) (96). The risk of sexual transmission of HCV is also greater in HIV-positive persons, particularly MSM (88), but is low among HIV-uninfected heterosexual couples (102, 121) and MSM (122, 123). Other routes of bloodborne transmission include acquisition by health-care workers, cosmetic procedures (such as tattooing and body piercing), scarification and circumcision (84, 85, 124), and intranasal drug use.
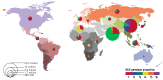
As a result of these different routes of transmission, certain groups are at higher risk of HCV infection (). The relative importance of these risk groups varies substantially, depending on the geographical location and population studied. Persons at risk for HCV infection are also likely to be at risk for infection with other bloodborne viruses, including HBV and HIV. Generally, HCV epidemics around the world are heterogeneous and represent mixtures of three core epidemic components (Box 4.1). However, few countries have epidemics that fall into just one of these categories – most represent some combination of all components.

Global epidemic patterns of HCV infection.
4.2.3. Natural history of hepatitis C infection
HCV is a small, positive-stranded RNA-enveloped virus with multiple genotypes and subgenotypes, and their distribution varies substantially in different parts of the world (). The availability of pangenotypic DAA regimens will increasingly obviate the need for prior genotyping, which will help expand access to HCV treatment.
Global distribution of HCV genotypes. Source:
Messina
J.P, Humphreys
I, Flaxman
A, Brown
A, Cooke
GS, Pybus
OG
. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;77–87.25069599
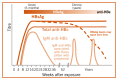
Hepatitis C virus causes both acute and chronic infection. Acute HCV infection is defined as the presence of certain markers of HCV infection within six months of exposure to and infection with HCV, and is characterized by the appearance of HCV RNA, HCV core antigen (p22 Ag), and subsequently HCV antibodies, which may or may not be associated with viral clearance. Antibodies to HCV develop as part of acute infection and persist throughout life. Acute infection is usually clinically silent, and is only very rarely associated with life-threatening disease. Spontaneous clearance of acute HCV infection generally occurs within six months of infection in 15–45% of infected individuals in the absence of treatment, but this varies by region and population (130). Antibodies to HCV develop as part of acute infection and persist throughout life. Almost all the remaining 55–85% of persons who do not clear HCV within six months are defined as having chronic HCV infection. Left untreated, chronic HCV infection can cause liver cirrhosis, liver failure and HCC. Of those with chronic HCV infection, the risk of cirrhosis of the liver is 15–30% within 20 years (131–133). The risk of HCC in persons with cirrhosis is approximately 2–4% per year () (134). Clearance of infection, whether spontaneous or as a result of antiviral treatment, does not provide lasting protection from reinfection.
Approximate Time course of virological and immunological markers of HCV infection with (A) Self-resolving HCV infection, and (B) Chronic HCV infection.
Diagnosis of HCV infection currently consists of initial screening for evidence of past or current HCV infection with a serological assay, followed by NAT for HCV RNA (either quantitative or qualitative) to confirm the presence of HCV viraemia, and therefore chronic HCV infection.
4.2.4. Time course of serological markers for HCV infection
The exact time course of virological and immunological markers of HCV infection is not well defined, particularly during the first months of infection, due to differences in each host (patient) immune response, specific properties of the infecting virus, and sensitivity of assays used to determine the appearance of HCV markers. As illustrated in , following an initial eclipse phase of 1–2 weeks when no virological or serological markers of infection may be detected, the natural course of HCV infection is characterized by the appearance of HCV RNA, then HCV core p22 Ag in the absence of an antibody response for a further 6–10 weeks. During this serological window, it has been shown that free (i.e. not complexed with antibody) HCV core antigen (HCVcAg) can be detected in a proportion of individuals. Following the development of the antibody response, HCVcAg becomes complexed with these antibodies specific for HCV.
Window period. Assays designed solely to detect antibodies to HCV inevitably have a window period of infectivity in early infection, during which antibodies may be undetectable. This window period can be shortened by utilizing assays that also include direct detection of HCVcAg (50–60 days). HCV RNA is typically not used to determine exposure to HCV, in spite of its short window period (1–2 weeks after the onset of acute infection) primarily because of cost (135). There are increasing reports of occult HCV infection, i.e. HCV RNA detectable in the absence of any serological markers (i.e. HCV seronegative) (136–138) which may be due to underlying immunosuppression in, for example, HIV-infected populations.
4.2.5. Prevention of hepatitis C infection
In the absence of a vaccine for hepatitis C, prevention of HCV infection depends upon reducing the risk of exposure to the virus. This is challenging because of the various routes of transmission and the different populations that are affected. Globally, most HCV infections occur in health-care settings as a result of inadequate infection control procedures. WHO has published guidelines with recommendations for preventing health-care-associated HCV infection, and for screening of blood products (20, 30, 139). Universal access to safe blood transfusion requires the implementation of key strategies to ensure access to a safe and sufficient blood supply, including 100% quality-assured testing of donated blood (139). Joint WHO–UNODC guidance recommends a comprehensive package of harm reduction interventions, which comprise nine harm reduction activities specifically for PWID, including the provision of sterile injecting equipment (140), alongside WHO guidance on prevention of viral hepatitis B and C transmission among PWID (28).
4.2.6. Treatment of hepatitis C infection
A new class of medicines, called direct-acting antivirals (DAAs), have transformed the treatment of HCV, with regimens that can be administered for a short duration (as short as eight weeks), resulting in cure rates higher than 90%, but are associated with fewer serious adverse events than the previous interferon-containing regimens. WHO updated its hepatitis C treatment guidelines in 2016 to provide recommendations for the use of new DAAs (5) (see
Web annex 3). There still remains some variation in recommended HCV treatment regimens and duration of therapy by genotype. This requirement to determine a patient's genotype prior to treatment will soon change when antiviral agents that are active against all genotypes (referred to as pangenotypic) are licensed.