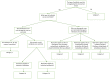
To conduct stratified analysis, interventions needed to be classified based on the type of antibiotic use that was targeted. The following classification scheme was thereby created, with input and feedback from WHO AGISAR:
Of particular note, every study included into the systematic review assessed an intervention that restricted the use of antibiotics. Studies that did not specify the type of antibiotic use or indication that was targeted in this restriction were classified as “Undetermined” (Category 6). This included studies, for example, that compared regions or farms using “more” versus “less” antibiotics with no indication that was specifically targeted or described, or studies that assessed the impact of reducing antibiotic use in a jurisdiction without delineating how this was achieved.
Each category in the classification scheme is mutually exclusive. If a single study included more than one intervention, then each intervention was classified separately based on the above approach. outlines the definitions used by the classification scheme.
illustrates the corresponding decision rules that were followed, to apply the above classification scheme to each study.
A. Results – Classification of study interventions
presents the categorization of interventions by type of antibiotic use being targeted for restriction.
Type of antibiotic use targeted by interventions in 179 animal studies.
Of the 179 animal studies included in the systematic review, 69 restricted all uses of antibiotics, 36 studies restricted use of antibiotics for all non-therapeutic purposes, while 27 restricted the use of antibiotics for growth promotion only. A total of 39 studies could not be classified based on the type of antibiotic use targeted by the intervention. An index of the 179 animal studies, their corresponding references from the original report, and their assigned classifications of interventions is presented in in the Appendix to this supplemental report.
Index of animal studies, reference numbers, and intervention types.
presents the categorization of interventions by type of antibiotic use being targeted for restriction, for human studies.
Type of antibiotic use targeted by interventions in 21 human studies.
Of the 21 human studies, five restricted all uses of antibiotics, two restricted antibiotic use for all non-therapeutic indications, and seven restricted use of antibiotics for growth promotion only. Five studies could not be classified based on the type of antibiotic use targeted by the intervention. An index of the 21 human studies, their corresponding references from the original report, and their assigned classifications of interventions is presented in in the Appendix to this supplemental report.
Index of human studies, reference numbers, and intervention types.
B. Results – Stratified analysis
Similar to the stratified analysis conducted in the original systematic review and meta-analysis, stratified meta-analysis was performed for all studies amenable to meta-analysis, ignoring specific bacterial species, sample types, units of analysis, and antibiotic classes. in the Appendix to this supplemental report lists the individual animal studies amenable to stratified meta-analysis. outlines the results from meta-analysis stratified by the type of antibiotic use targeted by interventions in animal studies.
Stratified meta-analysis for animal studies, by intervention type.
Stratified meta-analysis must be interpreted with some caution, due to the lower numbers of studies that can be included and the overlapping confidence intervals in the pooled estimates across strata. With these caveats in mind, we would propose three high-level observations from the stratified analysis, which we summarize below, followed by further elaboration:
The type of antibiotic use targeted by interventions is not specified in many of the studies identified by our search. This finding underlines the need for better characterization of interventions in future research, and perhaps even more importantly, in the development of future policy and regulations.
There is some suggestion that the interventions that target only specific antibiotic classes or specific antibiotic drugs may have less effect on antibiotic resistance than do antibiotic restrictions covering all classes.
Among antibiotic restriction interventions that target all classes, there does not seem to be any advantage of complete bans preventing any use relative to restrictions that still permit therapeutic and prophylactic use.
In 39 of 179 animal studies and 5 of 21 human studies, the type of antibiotic use that was targeted by interventions could not be determined. For the majority of these studies, antibiotic resistance was compared between groups having “higher” versus “lower” antibiotic use, without further delineation as to how antibiotics were being used or restricted in both groups. Though our stratified analysis suggests that lower overall antibiotic use does seem to be associated with less antibiotic resistance in this “Undetermined” group, the development of policies and regulations on antibiotic use requires more specific information on interventions, to explore whether certain interventions appear to be more or less effective than others. Therefore, future research should focus on better characterizing interventions that restrict antibiotic use.
Secondly, our stratified analysis results suggest that broad interventions that globally restrict the use of all classes of antibiotics may be more effective in reducing antibiotic resistance, compared to interventions that narrowly restrict the use of a few specific antibiotics or antibiotic classes. For example, the absolute risk differences for interventions that broadly restrict antibiotics across different classes range from −0.08 to −0.29. That is, the proportion of bacteria with antibiotic resistance is 8 to 29% lower in intervention versus comparator groups, with such broad interventions. In contrast, the absolute risk differences for interventions that restrict only a single or a few antibiotic(s) or antibiotic class(es) range between −0.02 and 0.04, with confidence intervals overlapping 0, indicating that there is no difference in antibiotic resistance in intervention versus comparator groups. Interventions that restrict specific antibiotics and antibiotic classes may therefore be less beneficial than global restrictions that are not confined to specific antibiotics and antibiotic classes.
Lastly, our results suggest that full restriction of antibiotics (where antibiotics cannot be used for any indication, including non-therapeutic and therapeutic purposes) does not appear to be superior to interventions that do allow for therapeutic use of antibiotics as well as for metaphylaxis and prophylaxis of animals. The absolute risk difference of antibiotic resistance with full antibiotic restriction was −0.18, compared to absolute risk differences between −0.08 and −0.29 for partial restrictions only. Restricted antibiotic use that permits the treatment of diseased animals and/or diseased herds does not seem to undermine efforts to reduce antibiotic resistance.
It is difficult to formulate more precise conclusions beyond the above three observations. For example, the reduction in antibiotic resistance appears to be stronger for interventions that restrict growth promotion only (RD −0.29 [95% CI −0.40, −0.19]), compared with interventions that are even more restrictive, such as those disallowing prophylaxis or metaphylaxis in addition to disallowing growth promotion (RD −0.08 [95% CI −0.11, −0.06]), or disallowing all uses of antibiotics including for therapy (RD −0.18 [95% CI −0.22, −0.14]). However, these differences in the absolute risk reductions of antibiotic resistance across interventions may be artefactual. The pooled prevalence of antibiotic resistance in comparator groups for interventions restricting use of growth promoters is 0.48 (95% 0.23, 0.73), compared to 0.13 (95% CI 0.11, 0.16) in the comparator groups for interventions that restrict all non-therapeutic uses of antibiotics. That is, the pooled “baseline” prevalence in the comparator groups is not the same across all intervention types. There is greater potential for reduction of antibiotic resistance when there is a higher baseline prevalence of resistance, such as in the case for interventions restricting the use of growth promoters. Therefore, differences in pooled effect estimates across different interventions that broadly restrict antibiotics may spurious; we cannot comment on a specific intervention being superior to others, so long as restrictions are not confined to a single class of antibiotics.
Corresponding stratified meta-analysis based on intervention type was also conducted for human studies (). (to be requested upon request) in the Appendix to this supplemental report lists the individual human studies amenable to stratified meta-analysis.
Stratified meta-analysis for human studies, by intervention type.
Due to the relatively small numbers of human studies and the wide and overlapping confidence intervals in subcategories, we caution against attempting to draw specific inferences regarding relative effects of the different intervention types in human studies.