Background
WHO estimates that in 2015, 71 million persons were living with chronic hepatitis C virus (HCV) infection worldwide and that 399 000 died from cirrhosis or hepatocellular carcinoma caused by HCV infection. In May 2016, the World Health Assembly endorsed the Global Health Sector Strategy (GHSS) on viral hepatitis, which proposes to eliminate viral hepatitis as a public health threat by 2030 (90% reduction in incidence and 65% reduction in mortality). Elimination of viral hepatitis as a public health threat requires 90% of those infected to be diagnosed and 80% of those diagnosed to be treated.
Rationale
Since the last update to the Guidelines was issued in 2016, three key developments have prompted changes in terms of when to treat and what treatments to use. First, the use of safe and highly effective direct-acting antiviral (DAA) regimens for all persons improves the balance of benefits and harms of treating persons with little or no fibrosis, supporting a strategy of treating all persons with chronic HCV infection, rather than reserving treatment for persons with more advanced disease. Second, since 2016, several new, pangenotypic DAA medicines have been approved by at least one stringent regulatory authority, reducing the need for genotyping to guide treatment decisions. Third, the continued substantial reduction in the price of DAAs has enabled treatment to be rolled out rapidly in a number of low- and middle-income countries.
Scope
These guidelines aim to provide evidence-based recommendations on the care and treatment of persons diagnosed with chronic HCV infection. They update the care and treatment section of the WHO Guidelines for the screening, care and treatment of persons with hepatitis C infection issued in April 2016. The 2017 Guidelines on hepatitis B and C testing update the screening section.
Audience
These guidelines are intended for government officials to use as the basis for developing national hepatitis policies, plans and treatment guidelines. These include country programme managers and health-care providers responsible for planning and implementing hepatitis care and treatment programmes, particularly in low- and middle-income countries.
Methods
WHO developed these guidelines in accordance with procedures established by its Guidelines Review Committee. Systematic reviews were undertaken to assess the safety and efficacy of treatment regimens in adults, to examine the morbidity and mortality from extrahepatic manifestations in persons with HCV infection and to review the literature on cost–effectiveness. In addition, modelling was carried out. A regionally representative and multidisciplinary Guidelines Development Group met in September 2017 to formulate the recommendations, using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. This included an assessment of the quality of evidence (high, moderate, low or very low), consideration of the overall balance of benefits and harms (at individual and population levels), patient/health worker values and preferences, resource use, cost–effectiveness, and consideration of feasibility and effectiveness across a variety of resource-limited settings.
Summary of the new recommendations
When to start treatment in adults and adolescents
What treatment to use for adults and adolescents
Pangenotypic regimens currently available for use in adults 18 years of age or older
For adults without cirrhosis, the following pangenotypic regimens can be used:
- Sofosbuvir/velpatasvir 12 weeks
- Sofosbuvir/daclatasvir 12 weeks
- Glecaprevir/pibrentasvir 8 weeks3
For adults with compensated cirrhosis, the following pangenotypic regimens can be used:
- Sofosbuvir/velpatasvir 12 weeks
- Glecaprevir/pibrentasvir 12 weeks3
- Sofosbuvir/daclatasvir 24 weeks
- Sofosbuvir/daclatasvir 12 weeks4
Treatment of children 0–12 years of age
Clinical considerations
General clinical considerations
- The use of pangenotypic regimens obviates the need for genotyping before treatment initiation.
- In resource-limited settings, WHO recommends that the assessment of liver fibrosis should be performed using non-invasive tests (e.g. aspartate/platelet ratio index (APRI) score or FIB-4 test, see existing recommendations, p. xvii). This can determine if there is cirrhosis before initiation of treatment.
- There are a few contraindications to using pangenotypic DAAs together with other medicines.
- DAAs are well tolerated, with only minor side-effects. Therefore, the frequency of routine laboratory toxicity monitoring can be limited to a blood specimen at the start and end of treatment.
- Following completion of DAA treatment, sustained virological response (SVR) at 12 weeks after the end of treatment is used to determine treatment outcomes (See existing recommendations, p. xvii).
HIV/HCV coinfection
- Persons with HIV/HCV coinfection are at a higher risk for progression of fibrosis and were included in the list of persons prioritized for treatment since the 2014 WHO treatment guidelines. Treatment for HCV infection needs to consider drug–drug interactions with antiretroviral medications.
HBV/HCV coinfection
- Persons with HBV/HCV coinfection are at risk for HBV reactivation during and following HCV treatment. An assessment for HBV treatment eligibility with initiation of HBV treatment for those eligible may prevent HBV reactivation during HCV treatment.
Cirrhosis
- Persons with cirrhosis, including those who have achieved SVR, may be regularly screened for hepatocellular carcinoma (HCC).
Chronic kidney disease
- Data are insufficient on the safety and efficacy of sofosbuvir-based regimens in persons with severe renal impairment. Glecaprevir/pibrentasvir is effective against infection with all six major genotypes in persons with chronic kidney disease.
TB/HCV coinfection
- In persons with TB/HCV coinfection, treatment for active TB is considered before treatment of HCV infection. TB/HCV-coinfected persons treated for TB are at an increased risk of hepatotoxicity.
Retreatment after DAA treatment failure
- Currently, only one pangenotypic DAA regimen, sofosbuvir/velpatasvir/voxilaprevir, is approved by a stringent regulatory authority for the retreatment of persons who have previously failed DAA treatment.
- Investigations of a failure to achieve SVR with DAA therapy includes re-examination of adherence and of potential drug–drug interactions.
Simplified service delivery models
An eight-point approach to service delivery supports implementation of the clinical recommendations for Treat All and adoption of pangenotypic DAA regimens:
- Comprehensive national planning for the elimination of HCV infection;
- Simple and standardized algorithms across the continuum of care;
- Integration of hepatitis testing, care and treatment with other services;
- Strategies to strengthen linkage from testing to care, treatment and prevention;
- Decentralized services, supported by task-sharing;
- Community engagement and peer support to address stigma and discrimination, and reach vulnerable or disadvantaged communities;
- Efficient procurement and supply management of medicines and diagnostics;
- Data systems to monitor the quality of individual care and the cascade of care.
Public health considerations in specific populations
Five population groups (people who inject drugs [PWID], people in prisons or other closed settings, men who have sex with men, sex workers and indigenous populations) require specific public health approaches because of one or more of the following specific issues: high incidence, high prevalence, stigma, discrimination, criminalization or vulnerability, and difficulties in accessing services.
Summary of the existing WHO recommendations
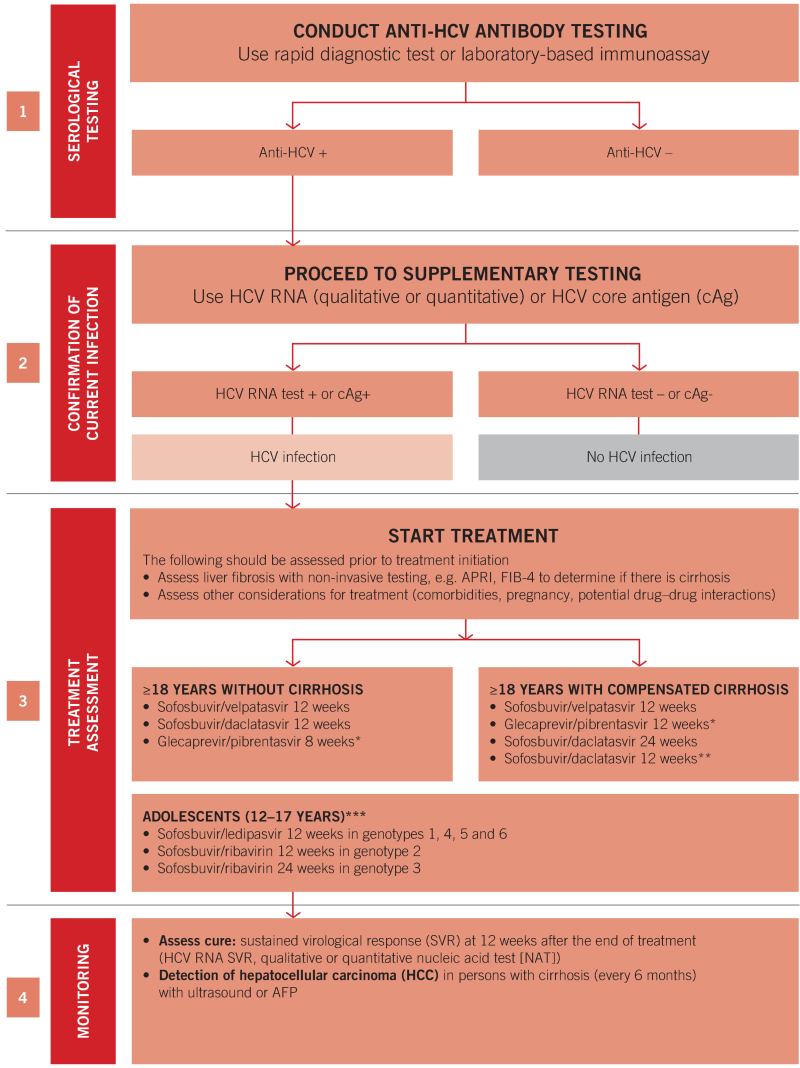
Summary algorithm for the diagnosis, treatment and monitoring of chronic HCV infection in adults and adolescents
* Persons with HCV genotype 3 infection who have received interferon and/or ribavirin in the past should be treated for 16 weeks.
** May be considered in countries where genotype distribution is known and genotype 3 prevalence is <5%.
*** Treatment in adolescents at this time still requires genotyping to identify the appropriate regimen. AFP: alpha fetoprotein, APRI: aspartate-to-platelet ratio index, FIB-4: fibrosis stage
Footnotes
- 1
With the exception of pregnant women
- 2
The Guidelines Development Group defined pangenotypic regimens as those leading to a SVR rate >85% across all six major HCV genotypes.
- 3
Persons with HCV genotype 3 infection who have received interferon and/or ribavirin in the past should be treated for 16 weeks.
- 4
May be considered in countries where genotype distribution is known and genotype 3 prevalence is <5%.
- 5
Prior to approval of DAAs for children aged <12 years of age, exceptional treatment with interferon + ribavirin may be considered for children with genotype 2 or 3 infection and severe liver disease. This may include children at higher risk of progressive disease, such as with HIV coinfection, thalassaemia major and survivors of childhood cancer.
Publication Details
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO license (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this license, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons license. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the license shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization.
Publisher
World Health Organization, Geneva
NLM Citation
Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection [Internet]. Geneva: World Health Organization; 2018 Jul. Executive Summary.