Obsessive-compulsive disorder
A summary of selected new evidence relevant to NICE clinical guideline 31 ‘Obsessive-compulsive disorder: core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder’ (2005)
Evidence Update, No. 47
Introduction
This Evidence Update identifies new evidence that is relevant to, and may have a potential impact on, the following reference guidance:
- 1Obsessive-compulsive disorder. NICE clinical guideline 31 (2005).
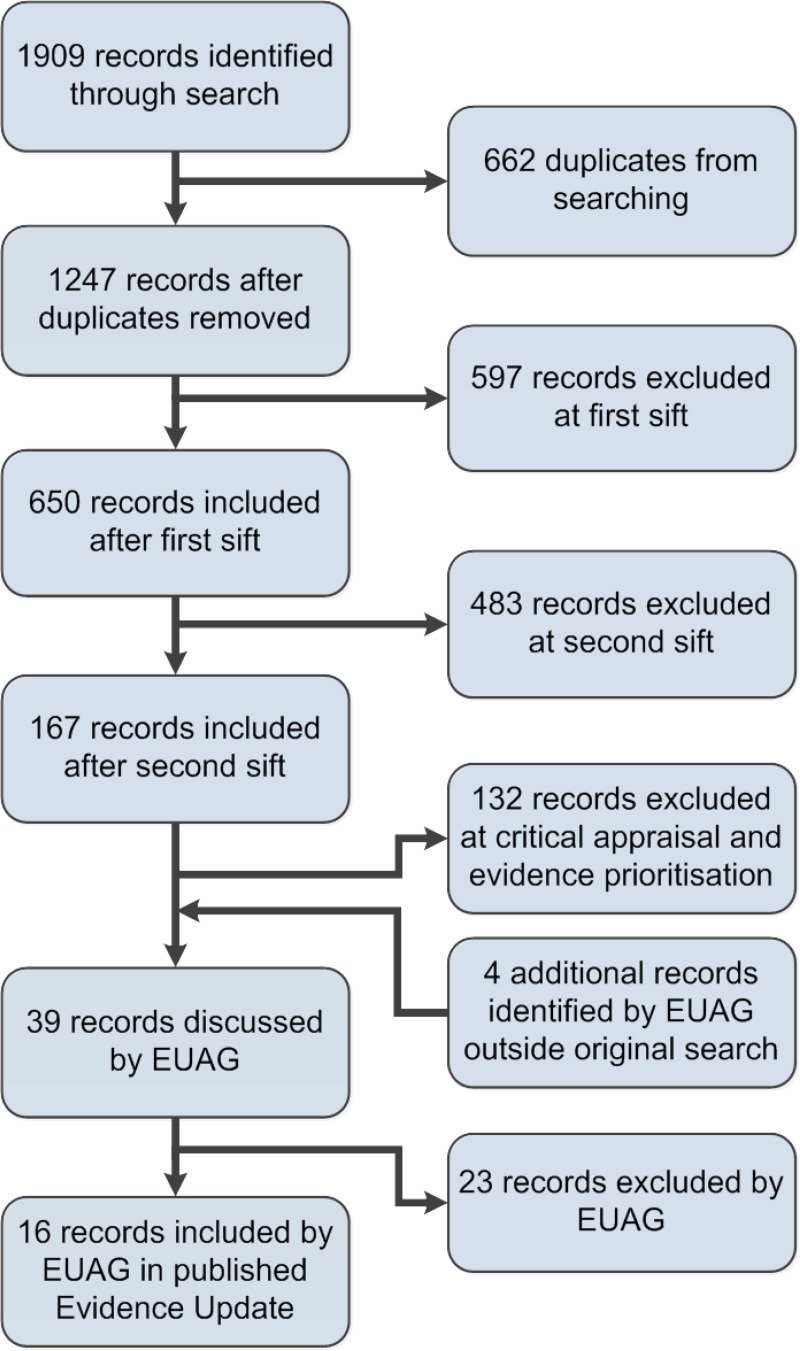
A search was conducted for new evidence from 30 October 2003 to 2 April 2013. A total of 1909 pieces of evidence were initially identified. Following removal of duplicates and a series of automated and manual sifts, 16 items were selected for the Evidence Update (see Appendix A for details of the evidence search and selection process). An Evidence Update Advisory Group, comprising topic experts, reviewed the prioritised evidence and provided a commentary.
Although the process of updating NICE guidance is distinct from the process of an Evidence Update, the relevant NICE guidance development centres have been made aware of the new evidence, which will be considered when guidance is reviewed.
NICE Pathways
- Obsessive-compulsive disorder and body dysmorphic disorder. NICE Pathway.
Feedback
If you have any comments you would like to make on this Evidence Update, please email ku.shn.ecnedive@sutcatnoc
Key points
The following table summarises what the Evidence Update Advisory Group (EUAG) decided were the key points for this Evidence Update. It also indicates the EUAG’s opinion on whether the new evidence may have a potential impact on the current guidance listed in the introduction. For further details of the evidence behind these key points, please see the full commentaries.
The section headings used in the table below are taken from the guidance.
Evidence Updates do not replace current accredited guidance and do not provide formal practice recommendations.
1. Commentary on new evidence
These commentaries analyse the key references identified specifically for the Evidence Update. The commentaries focus on the ‘key references’ (those identified through the search process and prioritised by the EUAG for inclusion in the Evidence Update), which are identified in bold text. Supporting references provide context or additional information to the commentary. Section headings are taken from the guidance.
1.1 . Principles of care for all people with obsessive-compulsive disorder (OCD) or body dysmorphic disorder (BDD) and their families or carers
No new key evidence was found for this section.
1.2 . Stepped care for adults, young people and children with OCD or BDD
No new key evidence was found for this section.
1.3. Step 1: awareness and recognition
No new key evidence was found for this section.
1.4. Step 2: recognition and assessment
No new key evidence was found for this section.
1.5. Steps 3–5: treatment options for people with OCD or BDD
Initial treatment options – adults
Telemental health and technology interventions
NICE CG31 recommends that in the initial treatment of adults with OCD, low intensity psychological treatments (including exposure and response prevention [ERP]) (up to 10 therapist hours per patient) should be offered if the patient’s degree of functional impairment is mild and/or the patient expresses a preference for a low intensity approach. Low intensity treatments include:
- brief individual cognitive behavioural therapy (CBT) (including ERP) using structured self-help materials
- brief individual CBT (including ERP) by telephone
- group CBT (including ERP) (note, the patient may be receiving more than 10 hours of therapy in this format).
Lovell and Bee (2011) did a systematic review of 13 studies (n=492) of CBT-based treatments that used health or communication technology in adults with OCD. Interventions included self-help manuals and CBT delivered via computer, telephone and video conferencing. Patients had less than 10 hours of therapist time in 11 studies. All studies used the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score as the primary outcome measure. Heterogeneity of populations, interventions and outcomes across the studies prevented meta-analysis.
Self-help manuals were assessed in 5 small, uncontrolled, quasi-experimental studies. This intervention was judged to have promise, with moderate-to-large improvements in OCD symptoms, however the absence of controlled trials means that the generalisability of findings is unclear. Computerised CBT was assessed in 5 studies, 4 of which were of BT-steps (now called OC-Fighter) a computer-guided treatment in which patients were led through selfassessment, given information about, prepared for and guided through ERP self-treatment. The studies reported significant moderate-to-large effects on Y-BOCS score in favour of BT-steps. However all studies were undertaken by the developers of the software so no independent analysis was available. The remaining study was a case series showing no significant difference in Y-BOCS score.
Telephone interventions were assessed in 2 studies and video conferencing was assessed in 1 small study with methodological limitations. Telephone CBT was non-inferior to face-to-face intervention in 1 study, but patients in the telephone group had face-to-face contact with therapists, which may have skewed the results.
The authors concluded that preliminary data support the idea that technology holds promise in treatment for OCD. Nevertheless, definitive conclusions about the relative efficacy of using technology to deliver CBT needs evidence from rigorous randomised controlled trials (RCTs).
Herbst et al. (2012) did a systematic review of 24 studies of telemental health interventions, including computer, internet, telephone, and written self-help material, whether or not therapist contact was also included. Data for around 937 people were analysed by technology used and amount of therapist–patient contact.
The most studied program was BT-steps (7 studies). No meta-analysis was done, but the authors concluded that it was promising for people who do not find an appropriate therapist or do not meet the threshold for psychotherapy. However, the data were limited by high drop-out rates so further studies are needed.
The authors additionally concluded that telemental health interventions do not yet meet criteria for empirically-validated interventions according to criteria from the American Psychological Association.
These studies suggest that telemental health and technology interventions for OCD such as computerised CBT or telephone CBT may have promise but that further studies are needed. This evidence is unlikely to impact NICE CG31, which contained a research recommendation about the efficacy, acceptability and cost effectiveness of different formats for delivering CBT.
Key references
- Herbst N, Voderholzer U, Stelzer N et al. (2012) The potential of telemental health applications for obsessive-compulsive disorder. Clinical Psychology Review 32: 454–66 [PubMed: 22705583]
- Lovell K, Bee P (2011) Optimising treatment resources for OCD: a review of the evidence base for technology-enhanced delivery. Journal of Mental Health 20: 525–42 [PubMed: 22126631]
Acceptance and commitment therapy
NICE CG31 recommends that adults with OCD and moderate functional impairment should be offered either a course of a selective serotonin reuptake inhibitor (SSRI) or more intensive CBT (including ERP) (more than 10 therapist hours per patient).
An RCT (n=79) in the USA reported by Twohig et al. (2010) investigated acceptance and commitment therapy compared with progressive relaxation training in adults with OCD (61% women, mean age=37 years). Participants were recruited after responding to adverts or by referral from health professionals. Participants on psychotropic drugs had to have been on a stable dose for at least 30 days and could not be receiving other psychotherapy or have ceased psychotherapy within the previous 30 days. The primary outcome was Y-BOCS score.
Assessors were blinded and a sample of assessment sessions were scored by a second rater to measure inter-rater reliability.
The acceptance and commitment intervention was based on agreement to participate in behaviour such as playing sport, attending church or spending time with family. The activities were established for specified periods of time irrespective of the nature or intensity of the person’s obsessions or anxiety. The participant would then need to practice therapeutic skills (acceptance, diffusion and present moment awareness) if their OCD interfered with the activity. People in both intervention and progressive relaxation training groups had 8 treatment sessions of 1 hour once a week. At the end of follow-up, participants were offered 8 sessions of the treatment they were not assigned to during the trial.
Between baseline and follow-up, Y-BOCS score decreased by 12.43 points in the acceptance and commitment therapy group and by 9.17 points in the progressive relaxation therapy group (no statistical comparison reported). Hierarchical linear modelling showed significantly improving slopes for both acceptance and commitment therapy (estimate= −1.22, 95% confidence interval [CI] −1.47 to −0.96, p<0.001) and progressive relaxation training (estimate=−0.79, 95% CI −1.06 to −0.52, p<0.001). Acceptance and commitment therapy was associated with a large significant difference in rate of improvement (estimate=0.42, 95% CI 0.05 to 0.80, p=0.026).
Limitations recognised by the authors included that follow-up was for only 3 months, but they described their study as preliminary and stated that further research was needed. Also, better matching of treatment and control may have been possible if people in the progressive relaxation therapy group had been instructed to use relaxation techniques in response to obsessive thoughts.
Although the acceptance and commitment therapy studied in this trial involved less than 10 hours of therapist time, the type of face-to-face contact is more like the ‘more intensive’ CBT recommended in NICE CG31. This study suggests that acceptance and commitment therapy may improve symptoms of OCD to a greater extent than progressive relaxation training. This study is unlikely to affect NICE CG31 because the relative effectiveness of acceptance and commitment therapy compared with recommended therapy (CBT with ERP) is unknown.
Key reference
- Twohig MP, Hayes SC, Plumb JC et al. (2010) A randomized clinical trial of acceptance and commitment therapy vs. progressive relaxation training for obsessive compulsive disorder. Journal of Consulting and Clinical Psychology 78: 705–16 [NIH Public Access author manuscript – full text] [PMC free article: PMC2948415] [PubMed: 20873905]
Initial treatment options for adults –SSRIs or group CBT
NICE CG31 recommends that adults with OCD with moderate functional impairment and those with mild functional impairment who are unable to engage in low intensity CBT (including ERP), or for whom low intensity treatment has proved to be inadequate, should be offered the choice of either a course of an SSRI or more intensive CBT (including ERP) (more than 10 therapist hours per patient), because these treatments appear to be comparably efficacious.
In an RCT (n=56) in Brazil, Sousa et al. (2006) compared sertraline 100 mg/day with group CBT over 12 weeks in adults with OCD (77% women, mean age=38.5 years). Participants needed to discontinue use of any drug treatment for OCD for 1 month before starting the trial. Pregnant women and women of childbearing potential who were not using adequate contraception were excluded. People with coexisting Tourette’s disorder, bipolar disorder, psychotic disorder, severe personality disorder, or moderate to severe depression, or substance misuse disorders in the past 6 months, were also excluded.
Group CBT consisted of a 2-hour session once a week with 5–8 participants in the group. In the sertraline group, participants were seen once a week for 20 minutes and no psychoeducational, behavioural or cognitive intervention was given. Response was defined as a reduction in Y-BOCS score of 35% or more, and clinical remission was defined as a Y-BOCS score of 8 or lower at the end of treatment.
No significant differences were noted for response between group CBT (68%) and sertraline (40%, p=0.088). A significantly higher proportion of people assigned to group CBT met criteria for clinical remission (32%) compared with sertraline (4%, p=0.023).
The authors recognised that their sample size may have been too small to detect a difference between groups for some outcomes. The inclusion of people who previously had treatment with SSRIs could have led to a reduced response in the sertraline group. Additionally, a group assessing both group CBT and sertraline, and a placebo group may have been useful.
This study suggests that sertraline or group CBT may result in similar response rates, but that more people may have clinical remission with group CBT than with sertraline. However because of the limitations of the evidence it is unlikely to impact on NICE CG31.
Key reference
- Sousa MB, Isolan LR, Oliveira RR et al. (2006) A randomized clinical trial of cognitive-behavioral group therapy and sertraline in the treatment of obsessive-compulsive disorder. Journal of Clinical Psychiatry 67: 1133–9 [PubMed: 16889458]
Initial treatment options – children and young people
NICE CG31 recommends that children and young people with OCD with moderate to severe functional impairment, and those with OCD with mild functional impairment for whom guided self-help has been ineffective or refused, should be offered CBT (including ERP) that involves the family or carers and is adapted to suit the developmental age of the child as the treatment of choice. Group or individual formats should be offered depending upon the preference of the child or young person and their family or carers.
Family-based CBT
Piacentini et al. (2011) reported an RCT (n=71) in the USA of family-based CBT including individual ERP versus control consisting of psychoeducation about OCD plus progressive relaxation training in children and young people with OCD (63% girls, mean age 12.2 years). Randomisation was done in a 7:3 ratio, with 49 people in the active treatment group and 22 people in the control group. Participants had 12 sessions of their assigned intervention over 14 weeks. The primary outcome was response, defined as a Clinical Global Impression-Improvement score of 1 (very much improved) or 2 (much improved) at the end of treatment.
In the family-based CBT group, the first 2 sessions involved both patients and their parents. Thereafter, the first hour of the session was individual ERP, and the last 30 minutes was family-based CBT attended by at least 1 parent. The family intervention was intended to reduce engagement with the child’s OCD, for example reducing participation in rituals or avoiding changing routines to accommodate the OCD.
The control group followed the same schedule as the ERP plus family-based CBT group, with the first 2 sessions including the family, and subsequent sessions focused on progressive relaxation with 15 minutes at the end spent with parents to review the child’s progress and set homework. Parental instruction in dealing with the child’s OCD symptoms, including exposure, or managing the effect on family functioning was prohibited.
Therapists participated in weekly group supervision and case review. All treatment sessions were videotaped and a sample was reviewed for adherence to the treatment manual and overall quality. Interviewers also had group supervision and a random sample of audiotapes was evaluated for inter-rater reliability.
At the end of treatment, a significantly higher response rate was seen in the family-based CBT group (57.1%) compared with control (27.3%, p<0.05). Clinical remission, defined as total score on the Child version of Y-BOCS (CY-BOCS) of less than 11 was 42.5% for family-based CBT compared with 17.6% for control, but this result was not statistically significant.
The authors recognised that their study design did not allow a controlled assessment of durability of treatment, the possibility of informant bias, and that therapists’ knowledge of family-based CBT may have affected their interactions with the control group. Additionally, the small sample size limited the statistical power for secondary analyses, particularly those looking at predictors of response.
The results of this study suggest that family-based CBT may be associated with higher rates of response to treatment than psychoeducation plus relaxation training, which is consistent with NICE CG31.
Key reference
- Piacentini J, Bergman RL, Chang S et al. (2011) Controlled comparison of family cognitive behavioural therapy and psychoeducation/relaxation training for childhood obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry 50: 1149–61 [NIH Public Access author manuscript – full text] [PMC free article: PMC3205429] [PubMed: 22024003]
Long-term outcomes after family-based CBT
O’Leary et al. (2009) reported a 7-year follow up of an Australian RCT (Barrett et al. 2004) that compared individual family-based CBT with group family-based CBT and with waiting list. Of the original 77 children and young people who participated, 38 were successfully contacted and agreed to participate in the follow-up study (47% female). Participants who had been in the waiting list group were offered treatment after the treatment period so were included in this analysis.
At follow-up, participants completed a questionnaire and participated in an interview in person or by telephone using the OCD section of the Anxiety Disorders Interview Schedule (ADIS). Participants were also asked about psychotherapy and drug treatment received during the follow-up period.
At follow-up, participants were aged 13–24 years (mean=18 years), 84% reported that they were not receiving any psychological treatment, and 34% were on SSRIs. At 7 years, 87% of participants no longer had a diagnosis of OCD. After a series of analyses on background and outcome variables, the authors concluded that there were no major differences between the group followed-up at 7 years and those who were not followed-up.
The authors noted a lack of statistical power because of the small sample size, and suggested that larger trials are needed. Additionally, reliability checks of ADIS interviews were not possible because most were conducted by telephone; responder bias was a possibility; all data were self-reported by patients; and all participants were white. These factors may be a source of bias or limit generalisability of the findings.
A major limitation of this study is the proportion of people who were followed-up. However, this evidence suggests that family-based CBT may be associated with long-term benefits, for example no longer meeting the criteria for diagnosis of OCD, which is consistent with NICE CG31.
Key reference
- O’Leary EM, Barrett P, Fjermestad KW (2009) Cognitive behavioural family treatment for childhood obsessive-compulsive disorder: a 7-year follow-up study. Journal of Anxiety Disorders 23: 973–8 [PubMed: 19640677]
Supporting reference
- Barrett P, Healy-Farrell L, March JS (2004) Cognitive-behavioral family treatment of childhood obsessive-compulsive disorder: a controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry 43: 46–62 [PubMed: 14691360]
Choice of drug treatment in adults
Switching drug treatments
NICE CG31 recommends that for adults with OCD, the initial pharmacological treatment should be one of the following SSRIs: fluoxetine, fluvoxamine, paroxetine, sertraline or citalopram6. Additionally it notes that serotonin and noradrenaline re-uptake inhibitors (SNRIs), including venlafaxine7 should not normally be used to treat OCD or BDD without comorbidity.
Denys et al. (2004) reported a Dutch double-blind switch study of paroxetine 60 mg/day and venlafaxine 300 mg/day in adults with OCD (56% women, mean age=36 years). This study was the second phase of an RCT in which the first phase evaluated the efficacy of paroxetine compared with venlafaxine after 12 weeks of treatment. In the second phase, participants who had not responded, defined as decrease in Y-BOCS score of less than 25%, were switched to the other treatment. In both phases of the trial, drug doses were gradually increased over 7 weeks, with a 4 week tapering off period before the switch.
Both clinicians and patients were blind to the assigned drug treatment, but not to the fact that non-responders would have a switch in treatment. Of the 150 participants in phase 1 (75 in each group), 16 people originally on paroxetine switched to venlafaxine and 27 people originally on venlafaxine switched to paroxetine.
At the end of the switch period, 3 of 16 (19%) of those switched to venlafaxine and 15 of 27 (56%) of those switched to paroxetine had responded (p=0.01). Almost all participants (98%) reported adverse events, resulting in 1 withdrawal.
The authors noted that differences in response may have origins in the pharmacology of the drugs: paroxetine is a selective serotonin reuptake inhibitor and venlafaxine inhibits serotonin and norepinephrine. Possible limitations to the study included lack of a placebo group and that improvements in the switch phase may have been partly due to having continued drug treatment for 28 weeks.
This evidence suggests that paroxetine may be effective in people whose OCD symptoms do not respond to venlafaxine, but venlafaxine may not be as effective in people whose symptoms have not responded to paroxetine. This evidence is broadly consistent with NICE CG31 which recommends paroxetine, but not venlafaxine, as a treatment option.
Key reference
- Denys D, van Megen HJ, van der Wee N et al. (2004) A double-blind switch study of paroxetine and venlafaxine in obsessive-compulsive disorder. Journal of Clinical Psychiatry 65: 37–43 [PubMed: 14744166]
Maintenance drug treatment
NICE CG31 recommends that if treatment for OCD or BDD with an SSRI is effective, it should be continued for at least 12 months to prevent relapse and allow for further improvements.
Fineberg et al. (2007) reported a 24-week RCT of escitalopram maintenance treatment compared with placebo in adults with OCD after 16 weeks’ open-label treatment with escitalopram 10 mg (increasing to 20 mg if the original dose was ineffective). The study was conducted at 62 centres in 14 countries. The primary outcome was time to relapse, defined as an increase in Y-BOCS score of 5 or more, or lack of efficacy as judged by the investigator.
After the open-label treatment period (n=468), people who responded (n=320), defined as a decrease in Y-BOCS score of at least 25%, were enrolled into a double-blind phase in which people were randomly assigned to continue their dose of escitalopram or to placebo (mean age=36 years, 49% women). People who were on escitalopram 20 mg and subsequently assigned to placebo, had a 1-week tapering down with a 10 mg dose and then received placebo for the remainder of the study.
Participants had Y-BOCS score of 20 or more before entering the study, duration of OCD of at least 1 year, and stable disease for at least 6 months. People with a primary diagnosis of other mental health, personality or developmental disorders in the past 6 months were excluded. People with suicidal thoughts or who were considered at risk of suicide by investigators were also excluded.
Kaplan-Meier survival analysis showed that time to relapse was significantly longer in people assigned to escitalopram compared with placebo (p<0.001). In the escitalopram group, 23% of patients relapsed, compared with 52% of those on placebo (p<0.001). During the double-blind phase, 13 people in the escitalopram group (8%) and 14 people in the placebo group (9%) withdrew from the study. The overall prevalence of adverse events was 39% in the escitalopram group and 31% in the placebo group.
This study recruited a substantial proportion (data not reported) of participants from general practice, which may have led to increased response to treatment than may be seen in specialist centres, because these sites may see more treatment-resistant cases.
Although the length of this study falls short of the duration of treatment recommended in NICE CG31, this evidence suggests that continuing treatment with escitalopram after initial response may be associated with lower rates of relapse than placebo. This result is generally consistent with NICE CG31, although the evidence does not show whether prevention of relapse is likely to be seen for all SSRIs.
Key reference
- Fineberg NA, Tonnoir B, Lemming O et al. (2007) Escitalopram prevents relapse of obsessive-compulsive disorder. European Neuropsychopharmacology 17: 430–9 [PubMed: 17240120]
Poor response to initial treatment in adults
NICE CG31 recommends that following multidisciplinary review, for adults with OCD if there has been no response to a full trial of at least one SSRI alone, a full trial of combined treatment with CBT (including ERP) and an SSRI, and a full trial of clomipramine alone, the following treatment options should also be considered (at the time of publication, the guidance noted that there was no evidence of the optimal sequence of the options listed below):
- additional CBT (including ERP) or cognitive therapy
- adding an antipsychotic to an SSRI or clomipramine
- combining clomipramine and citalopram.
Add-on treatment with antipsychotics
In a Cochrane review, Komossa et al. (2010) assessed 11 RCTs (n=396) of second-generation antipsychotics as adjunctive treatment in adults with OCD compared with placebo or antidepressants. The primary outcome measure was lack of response, defined by the original trial, or as a reduction in symptoms of at least 25% measured by a validated tool such as Y-BOCS, or a rating of at least ‘much improved’ on the Clinical Global Impression-Improvement.
In 2 trials of olanzapine8 plus antidepressants compared with placebo plus antidepressants (n=70), no significant effect on response was seen (odds ratio [OR]=0.28, 95% CI 0.01 to 6.45, p=0.46). In 1 trial (n=44), olanzapine resulted in significantly more weight gain than placebo (OR=2.30, 95% CI 0.80 to 3.80, p=0.0026).
In 5 trials of quetiapine8 plus antidepressants versus placebo plus antidepressants (n=219), no significant effect on response was seen (OR=0.53, 95% CI 0.27 to 1.05, p=0.068). Quetiapine was associated with more withdrawals due to adverse events (OR=4.48, 95% CI 1.43 to 14.04, p=0.01), weight gain (OR=4.14, 95% CI 1.59 to 10.81, p=0.0037; 2 trials, n=117), and sedation (OR=5.91, 95% CI 2.87 to 12.18, p<0.00001; 4 trials, n=196).
In 3 trials of risperidone8 plus antidepressants compared with placebo plus antidepressants (n=92) risperidone was significantly more likely to result in response (OR=0.17, 95% CI 0.04 to 0.66, p=0.01).
The authors concluded that the available data for olanzapine were too limited to draw any conclusions, and that quetiapine and risperidone had limited data for increased efficacy when added to antidepressants.
Sayyah et al. (2012) conducted an RCT (n=39) in Iran comparing aripiprazole8 10 mg/day (n=18) with placebo (n=21) in adults with OCD whose symptoms had not responded to 3 months of treatment with SSRIs at the maximum tolerated dose (56% women, mean age=38 years). Participants maintained their dose of SSRI throughout the 12-week trial but did not receive other treatments such as CBT. If participants had insomnia, they were given oxazepam 10 mg nightly.
People with other anxiety, personality or mood disorders as the primary diagnosis were excluded. Pregnant and lactating women and those of childbearing potential not using adequate contraception were also excluded. Response was defined as a reduction in Y-BOCS score of at least 25%.
In intention-to-treat analysis using last observation carried forward to account for missing data, Y-BOCS scores were significantly lower at the end of the study in the aripiprazole group (mean=16.12) compared with placebo (mean=24.2, p=0.001). Response was seen in 8 people in the aripiprazole group and 3 people in the placebo group (no statistical analysis reported). Occurrence of adverse events was similar in both groups, with 8 events in the aripiprazole group and 7 in the placebo group.
The method of randomisation was not clearly reported, and the authors acknowledged that the small sample size meant that differences in adverse events between the 2 groups may be difficult to detect. The authors noted the small sample size, possibly using a suboptimum dose of aripiprazole, and continued use of SSRIs as potential limitations of the study.
Evidence for use of antipsychotics added to SSRIs for people whose OCD symptoms have not responded to antidepressants alone is inconclusive. Risperidone and aripiprazole seem to have an effect on symptoms of OCD when added to antidepressants, but quetiapine and olanzapine may have no add-on effects. However, antipsychotics may be associated with increased rates of adverse events. This evidence is unlikely to impact NICE CG31.
Key references
- Komossa K, Depping AM, Meyer M et al. (2010) Second-generation antipsychotics for obsessive-compulsive disorder. Cochrane Database of Systematic Reviews issue 12: CD008141 [PubMed: 21154394]
- Sayyah M, Sayyah M, Boostani H et al. (2012) Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double blind clinical trial). Depression and Anxiety 29: 850–4 [PubMed: 22933237]
Add-on treatment with acetylcysteine
Afshar et al. (2012) reported a 12 week RCT (n=48) in Iran of acetylcysteine9 compared with placebo in adults (mean age=31 years, 75% women) with OCD whose symptoms had not responded to 12 weeks of treatment with an SSRI or clomipramine. Women who were pregnant, lactating or planned pregnancy were excluded, as was anyone receiving psychological or behavioural therapy. People with uncontrolled or debilitating medical conditions, substance misuse disorders, convulsive disorders, suicidal thoughts or contraindications to acetylcysteine were also excluded.
Acetylcysteine (or matched placebo) was started at 600 mg/day and could be doubled weekly, depending on Clinical Global Impression-Improvement score to a maximum of 2400 mg/day. Doses of SSRIs or clomipramine were maintained during the study. The primary outcome was change in Y-BOCS score and the rate of response at the end of the trial. Full clinical response was defined as 35% or greater reduction in Y-BOCS score, partial response was 25% or greater reduction in Y-BOCS and no response was less than 25% reduction in Y-BOCS score.
After 12 weeks of treatment, Y-BOCS score decreased significantly more in the acetylcysteine group (mean=−10.87, standard deviation [SD]=2.94) than in the placebo group (mean=−5.73, SD=3.16, p=0.003). Full clinical response was seen in 10 participants in the acetylcysteine group and 3 people in the placebo group (p=0.013).
Acetylcysteine was associated with significantly more nausea and vomiting (8 cases) compared with placebo (2 cases, p=0.03) and with more diarrhoea (4 cases versus 0 cases, p=0.047). Three people in the acetylcysteine group discontinued because of adverse events compared with none in the placebo group.
The authors noted that the dose of acetylcysteine might not have been optimum, and that a smell of sulphur from the acetylcysteine may not have been adequately masked by the flavouring used for both the active and placebo tablets.
This study suggests that acetylcysteine plus SSRIs may result in improvement of symptoms of OCD compared with SSRIs plus placebo, but further research is needed so no impact on NICE CG31 is expected.
Key reference
- Afshar H, Roohafza H, Mohammad-Beigi H et al. (2012) N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology 32: 797–803 [PubMed: 23131885]
Add-on treatment with anticonvulsants
In an Italian double-blind RCT, Bruno et al. (2012) compared lamotrigine10 with placebo as add-on treatment in adults (n=40) with OCD whose symptoms persisted after an adequate trial of an SSRI (60% women, mean age=36 years). People with other major psychiatric disorders, clinically significant medical conditions, history of substance misuse disorders, and intellectual disabilities were excluded, as were pregnant or lactating women and people receiving psychological therapies. Lamotrigine was started at 25 mg/day and increased by 25 mg/day on a weekly basis until the maximum dose of 100 mg/day was reached, and this dose was maintained for the remainder of the 16-week trial. Response was defined as a reduction in Y-BOCS score of at least 25%.
Response was seen in 17 of 20 patients in the lamotrigine group compared with 0 of 20 in the placebo group (no statistical analysis reported, last observation carried forward used to account for drop-outs). Y-BOCS scores reduced significantly more in the lamotrigine group than in the placebo group for obsessions (−4.5 versus 1.0, p<0.0001), compulsions (−4.3 versus −0.6, p<0.0001) and total score (−8.0 versus 0.3, p<0.0001).
Seven people dropped out; 3 in the lamotrigine group (2 because of non-compliance and 1 because of rash) and 4 in the placebo group (2 because of non-compliance and 2 because of perceived inefficacy of treatment). Adverse events occurred in a similar proportion of participants in both groups although no statistical analysis was reported. The authors stated that their results should be interpreted with caution because of the small sample size and short duration of the trial.
Berlin et al. (2011) reported a US 12-week double-blind RCT of topiramate11 compared with placebo in 36 adults with OCD (mean age=40.5 years, 78% women). Participants had OCD for at least 1 year before the study. People with substance misuse problems, bipolar or psychotic disorders, or personality disorders judged likely to interfere with assessment were excluded.
Topiramate (or matched placebo) was titrated up over 8 weeks to 400 mg/day or the maximum tolerated dose, with dose remaining stable for the last 4 weeks. The primary outcomes were change in Y-BOCS total score and obsessions and compulsions subscores.
The mean maximum dose was 206.9 mg/day (SD=126 mg/day) in the topiramate group and 311.1 mg/day (SD=144.1 mg/day) in the placebo group. At the end of treatment a significant effect of topiramate was seen for the Y-BOCS compulsions subscale compared with placebo (p=0.014), but no significant differences were noted for Y-BOCS obsessions subscale (p=0.99) or the total score (p=0.11).
In the topiramate group, 5 people were taken off treatment because of adverse events and in the placebo group 4 people stopped treatment but none of these discontinuations was due to adverse events. Doses were reduced because of adverse events in 7 people in the topiramate group and in 3 people in the placebo group. Individual adverse events were reported by the authors only if they occurred in 15% of participants in either group: 48 such events were reported in the topiramate group and 27 in the placebo group.
The authors described topiramate as not well tolerated in this study and that only 1 of the 3 primary outcome measures was significantly different from placebo.
These studies suggest that the anticonvulsant drugs lamotrigine and topiramate may result in improved OCD symptoms as add-on therapy to SSRIs compared with SSRIs plus placebo, but further research is needed. Topiramate may be associated with increased adverse events. This evidence is unlikely to affect NICE CG31.
Key references
- Berlin HA, Koran LM, Jenike MA et al. (2011) Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. Journal of Clinical Psychiatry 72: 716–21 [PubMed: 20816027]
- Bruno A, Micò U, Pandolfo G et al. (2012) Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Journal of Psychopharmacology 26: 1456–62 [PubMed: 22351381]
Poor response to initial treatment in children and young people
CBT plus SSRIs
NICE CG31 recommends that for a child or young person with OCD or BDD, if there has not been an adequate response within 12 weeks to a full trial of CBT (including ERP) involving the family or carers, a multidisciplinary review should be carried out. Following multidisciplinary review, for a child (aged 8–11 years) or young person aged 12–18 years) with OCD or BDD with moderate to severe functional impairment, if there has not been an adequate response to CBT (including ERP) involving the family or carers, the addition of an SSRI to ongoing psychological treatment may be considered for children and offered in young people. Careful monitoring should be undertaken, particularly at the beginning of treatment.
Franklin et al. (2011) reported a 3-group RCT (n=124) in the USA in children and young people with OCD (aged 7–17 years, mean age=13.6 years) comparing drug treatment and CBT with drug treatment and instructions in CBT and with drug treatment only. OCD was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV and symptom severity was measured CY-BOCS. The primary outcome measure was response, defined as a reduction in CY-BOCS score of 30% or more. Participants were outpatients who had partial response to a trial of an SSRI. Families and clinicians were not blinded because of the nature of the interventions, but outcomes were assessed by blinded evaluators. Measures to maintain inter-rater reliability were used.
Drug treatment was a maintenance regimen administered by a child and adolescent psychiatrist over 7 visits in 12 weeks. Drug doses could be reduced if adverse events occurred, but dose increases were not permitted. CBT was based on a published manual of established efficacy and consisted of 14 hour-long sessions over 12 weeks. Instructions in CBT were provided by the therapist administering drug treatment and consisted of introducing principles of CBT and planning use of these skills between sessions.
Overall, 101 participants completed the study (81.5%), and analysis suggested that people were significantly more likely to drop out and receive out-of-protocol treatment in the drug treatment only group (p<0.04). Serious adverse events occurred in 2 children: in the drug treatment group, 1 participant attempted suicide; in the instructions in CBT group, 1 participant reported suicidal thoughts.
The proportions of people with at least 30% reduction in CY-BOCS score were:
- 68.6% in the drug treatment plus CBT group
- 34.0% in the drug treatment plus instructions in CBT group
- 30.0% in the drug treatment only group (p=0.002).
Pairwise analysis showed that drug treatment plus CBT was superior to both drug treatment plus instructions in CBT (p=0.002) and drug treatment only (p<0.001). Drug treatment plus CBT instruction was not significantly better than drug treatment only (p=0.72).
The authors noted that instructions in CBT may not be as effective as CBT for several possible reasons including brevity, less time with mental health professionals, and omission of components of CBT such as exposure exercises.
In a UK RCT, Bolton et al. (2011) investigated full CBT (n=36) compared with brief CBT (n=36) and with a waiting list (n=24). Participants were children and young people (aged 10–18 years, mean=14.5 years) who were on a stable dose of drug treatment for at least 6 weeks before trial entry. Changes to drug doses during the study resulted in the participant being withdrawn from the trial.
Full CBT was 12 sessions over 3 months with a therapist and written information explaining the nature of OCD and its treatment by CBT. Brief CBT was 5 sessions with a therapist over 3 months, written information, and detailed workbooks to supplement the therapy sessions. Participants in the waiting list group had no therapist contact or written information for 3 months, after which they were offered treatment.
OCD was diagnosed according to DSM-IV, and the primary outcome measure was total CY-BOCS score. Outcomes were assessed by blinded independent evaluators whose inter-rater reliability was monitored.
The primary outcome was examined by analysis of covariance (ANCOVA), which showed adjusted mean differences in CY-BOCS score of:
- 12.67 for full CBT compared with wait list (p<0.0001)
- 8.98 for brief CBT compared with wait list (p<0.0001).
No significant site or therapist effects were recorded. No significant difference was seen between full or brief CBT methods at the end of treatment or at follow-up 3 months after the end of treatment.
The authors recognised that the inclusion criterion of 6 weeks’ stable drug treatment may be too short to see the effects before starting the trial, so some changes from baseline may be attributed to drug treatment. Additionally, the interventions included some exposure and response prevention, and future studies could compare CBT with exposure and response prevention alone.
The results of these studies suggest that CBT plus drug treatment with SSRIs may result in better outcomes on persistent symptoms of OCD in children than either drug treatment plus low-intensity CBT, or drug treatment alone. Although both studies administered treatments in the opposite order to that recommended in NICE CG31, the findings are consistent with current guidance to use psychological therapies plus drug treatment if response to initial treatment is not adequate.
Key references
- Bolton D, Williams T, Perrin S et al. (2011) Randomized controlled trial of full and brief cognitive-behaviour therapy and wait-list for paediatric obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry 52: 1269–78 [PubMed: 21644984]
- Franklin ME, Sapyta J, Freeman JB et al. (2011) Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the pediatric OCD treatment study II (POTS II) randomized controlled trial. JAMA 306: 1224–32 [PMC free article: PMC3495326] [PubMed: 21934055]
1.6. Step 6: intensive treatment and inpatient services for people with OCD or BDD
No new key evidence was found for this section.
1.7. Discharge after recovery
No new key evidence was found for this section.
Areas not currently covered by NICE CG31
Transcranial magnetic stimulation
NICE CG31 does not contain recommendations about use of transcranial magnetic stimulation in people with OCD.
Slotema et al. (2010) did a systematic review and meta-analysis of repetitive transcranial magnetic stimulation compared with control in psychiatric disorders; 3 studies in OCD were included (n=38). The studies were combined and a weighted effect size (Hedges’ g) was calculated. No significant difference between transcranial magnetic stimulation and control was seen (Hedges’ g=0.15, p=0.52). Application of high-frequency transcranial magnetic stimulation to the dorso-lateral prefrontal cortex was associated with headache (n=7), scalp discomfort (n=12), dizziness or fainting (n=3) and tearfulness (n=2).
This evidence suggests that transcranial magnetic stimulation may not be an effective treatment for people with OCD. This evidence is unlikely to affect NICE CG31.
Key reference
- Slotema CW, Blom JD, Hoek HW et al. (2010) Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of the efficacy of rTMS in psychiatric disorders. Journal of Clinical Psychiatry 71: 873–84 [PubMed: 20361902]
2. New evidence uncertainties
During the development of the Evidence Update, the following evidence uncertainties were identified for the UK Database of Uncertainties about the Effects of Treatments (UK DUETs).
Steps 3–5: treatment options for people with OCD or BDD
Further evidence uncertainties for obsessive-compulsive disorder can be found in the UK DUETs database and in the NICE research recommendations database.
UK DUETs was established to publish uncertainties about the effects of treatments that cannot currently be answered by referring to reliable up-to-date systematic reviews of existing research evidence.
Appendix A. Methodology
Scope
The scope of this Evidence Update is taken from the scope of the reference guidance:
- Obsessive-compulsive disorder. NICE clinical guideline 31 (2005).
Observational studies were included in the Evidence Update on expert advice indicating that this type of study is important for this topic.
Searches
The literature was searched to identify studies and reviews relevant to the scope. Searches were conducted of the following databases, covering the dates 30 October 2003 (the end of the search period of NICE clinical guideline 31) to 2 April 2013:
- CDSR (Cochrane Database of Systematic Reviews)
- CENTRAL (Cochrane Central Register of Controlled Trials)
- CINAHL (Cumulative Index to Nursing and Allied Health Literature)
- EMBASE (Excerpta Medica database)
- MEDLINE (Medical Literature Analysis and Retrieval System Online)
- MEDLINE In-Process
- NHS EED (Economic Evaluation Database)
- PsycINFO
Table 1 provides details of the MEDLINE search strategy used (based on the search strategy for the reference guidance), which was adapted to search the other databases listed above. The search strategy was used in conjunction with validated Scottish Intercollegiate Guidelines Network search filters for RCTs and systematic reviews.
Additionally, 4 studies were identified outside of the literature search, of which 1 (Berlin et al. 2011) was included in the Evidence Update. Figure 1 provides details of the evidence selection process. The long list of evidence excluded after review by the Chair of the EUAG, and the full search strategies, are available on request from ku.shn.ecnedive@sutcatnoc
There is more information about how NICE Evidence Updates are developed on the NICE Evidence Services website.
Table 1
MEDLINE search strategy (adapted for individual databases).
Appendix B. The Evidence Update Advisory Group and Evidence Update project team
Evidence Update Advisory Group
The Evidence Update Advisory Group is a group of topic experts who review the prioritised evidence obtained from the literature search and provide the commentary for the Evidence Update.
- Dr David Veale – ChairConsultant Psychiatrist and Visiting Senior Lecturer, South London and Maudsley NHSFoundation Trust and Institute of Psychiatry, King’s College London
- Professor Naomi FinebergConsultant Psychiatrist, Hertfordshire Partnership NHS Foundation Trust
- Tracey FlannaghanCognitive Behavioural Psychotherapist, Leicestershire Partnership NHS Trust
- Dr Isobel HeymanConsultant Child and Adolescent Psychiatrist, Great Ormond Street Hospital for Children
- Dr Ghazanfar KhanGP Registrar, Leeds Teaching Hospitals
- Professor Karina LovellProfessor of Mental Health, University of Manchester
- Professor Roz ShafranProfessor of Translational Psychology, University College London
Evidence Update project team
- Marion SpringAssociate Director
- Chris WeinerConsultant Clinical and Public Health Adviser
- Cath WhiteProgramme Manager
- Fran WilkieProject Manager
- Lynne KincaidMedical Writer
- BazianInformation Specialist support
Footnotes
- 1
Guidance published prior to NICE accreditation
- 2
Venlafaxine is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 3
At the time of publication of this Evidence Update, aripiprazole, olanzapine, quetiapine and risperidone did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented.
- 4
Acetylcysteine is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 5
Lamotrigine and topiramate are not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 6
At the time of publication of this Evidence Update citalopram did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented.
- 7
Venlafaxine is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 8
At the time of publication of this Evidence Update, aripiprazole, olanzapine, quetiapine and risperidone did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented.
- 9
Acetylcysteine is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 10
Lamotrigine is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
- 11
Topiramate is not recommended by current guidance and at the time of publication of this Evidence Update did not have UK marketing authorisation for this indication.
Evidence Updates provide a summary of selected new evidence published since the literature search was last conducted for the accredited guidance they relate to. They reduce the need for individuals, managers and commissioners to search for new evidence. Evidence Updates highlight key points from the new evidence and provide a commentary describing its strengths and weaknesses. They also indicate whether the new evidence may have a potential impact on current guidance. For contextual information, this Evidence Update should be read in conjunction with the relevant clinical guideline, available from the NICE Evidence Services topic page for obsessive-compulsive disorder.
Evidence Updates do not replace current accredited guidance and do not provide formal practice recommendations.
NICE Evidence Services are a suite of services that provide online access to high quality, authoritative evidence and best practice.
National Institute for Health and Care Excellence
Level 1A
City Tower
Piccadilly Plaza
Manchester M1 4BT
All rights reserved. This material may be freely reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the express written permission of NICE.