NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Risk factors for predicting the presence of an abdominal aortic aneurysm
Review question
Which signs, symptoms and risk factors (or combinations of these) are most accurate in predicting the presence of an abdominal aortic aneurysm? What is the effectiveness of available risk assessment tools?
Introduction
National population-based screening programmes target and invite individuals from particular risk groups in communities for screening whilst opportunistic screening strategies are restricted to patients who consult healthcare practitioners for some other purpose. As a result, a different set of criteria may be necessary to guide clinicians on when it is appropriate to perform diagnostic imaging. This review question aims to determine which signs, symptoms, risk factors or assessment tools are accurate in predicting the presence of an abdominal aortic aneurysm (AAA) and could be used by clinicians in the course of opportunistic screening as a prompt to initiate diagnostic imaging.
PICO table
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A single broad search was used to identify all studies that examine the diagnosis, surveillance or monitoring of AAA. This was a ‘bulk’ search that covered multiple review questions. The database was sifted to identify all studies that met the criteria detailed in Table 1. The relevant review protocol can be found in Appendix A.
Table 1
Inclusion criteria.
Initially the review protocol outlined that prospective observational studies that use multivariate logistic regression or Cox regression to explore the association between risk factors and the development of AAA should be considered for inclusion. Following further discussion with the committee, the study design was changed, retrospectively, to include cross-sectional studies because this design was considered more likely to indicate the presence (as opposed of development) of aneurysms in people at risk of AAA. It was agreed that the amendment was needed to ensure that any identified evidence would fall in line with the objectives of this review question. As a result, cross-sectional studies, with sample sizes of more than 500 participants, exploring the association between potential risk factors and the presence of AAA were included.
Studies were excluded if they:
- were cohort studies, case-controls, or case series
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed.
Clinical evidence
Included studies
From a database of 16,274 abstracts, 76 were identified as being potentially relevant to this review question. Following full-text review of these articles, 15 studies (reported in 19 publications) were included.
An update literature search was performed and provided by Cochrane, in December 2017. The search found a total of 2,180 abstracts; of which, 16 full manuscripts were ordered. Upon review of the full manuscripts, 6 studies met inclusion criteria for this review question, and were added.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix G.
Summary of clinical studies included in the evidence review
A summary of the included studies is included in the table below.
Table 2
Summary of included studies.
See Appendix D for full evidence tables.
Quality assessment of clinical studies included in the evidence review
See Appendix E for full GRADE tables, highlighting the quality of evidence from the included studies.
Economic evidence
Included studies
A literature search was conducted jointly for all review questions by applying standard health economic filters to a clinical search for AAA. This search returned a total of 5,173 citations. Following review of all titles and abstracts, no studies were identified as being potentially relevant to risk factors associated with aneurysm expansion or rupture. No full texts were retrieved, and so no studies were included as economic evidence.
An update search was conducted in December 2017, to identify any relevant health economic analyses published during guideline development. The search found 814 abstracts; all of which were not considered relevant to this review question. As a result no additional studies were included.
Excluded studies
No studies were retrieved for full-text review.
Economic model
This review question does not lend itself to economic evaluation, and was not prioritised by the committee for economic modelling. As such, no economic model was developed for this review question.
Resource impact
Not applicable
Evidence statements
Age
- Low- to moderate-quality evidence from 9 studies, including up to 3,083,743 people enrolled in AAA screening programmes, highlighted that odds of AAA increases with increasing age. Similar trends were found in men (3 studies including up to 12,971 men) and women (2 studies including up to 10,012 women).
Sex
- Low-quality evidence from 7 studies, including 3,217,464 people, indicated that men were more likely to have an AAA than women.
BMI/Weight/Obesity
- Very low- to low-quality evidence from 4 studies, including 3,081,087 people, indicated contradictory associations between increasing BMI and the presence of AAA. In relation to sex-specific associations, low-quality evidence from 1, including 6,386 people, could not identify any association between 4kg/m2 incremental increases in BMI and the presence of AAA in men or women.
Smoking
- Low-quality evidence from 7 studies, including 3,341,733 people, indicated that current smokers were more likely have an AAA than people who have never smoked (never smokers). Additionally, moderate-quality evidence from 4 studies, including 3,351,536 people, indicated that ex-smokers were more likely to have an AAA than never smokers. Low-quality evidence from 4 studies, including 10,134 men highlighted similar relationships between current smokers, ex-smokers and never smokers. In women, moderate-quality evidence from 1 study, including 3,424 women, highlighted that current smokers were more likely to have an AAA than people who had never smoked whereas the evidence could not differentiate between AAA rates between ex-smokers and never smokers.
Palpable aorta on abdominal examination
- Low-quality evidence from 1 study, including 5,328 people, indicated that people with palpable aorta on abdominal examination were more likely to have an AAA than people who did not.
Cardiovascular disease
- Low-quality evidence from 5 studies, including up to 3,186,486 people, indicated that people with coronary artery disease or coronary insufficiency were more likely to have an AAA than people who did not have any of these conditions. Moderate-quality evidence from 2 studies, including up to 12,203 men, indicated that men with a history of myocardial infarction or cardiovascular disease (not specified) were more likely to have an AAA than men without a history of these conditions. Low-quality evidence from 1 study, including 10,012 women, indicated that women with a history of myocardial infarction or coronary revascularisation were more likely to have an AAA than men without a history of these conditions.
Peripheral arterial disease, atherosclerosis, and claudication
- Low-quality evidence from 6 studies, including up to 3,095,008 people, indicated that people with peripheral arterial disease, atherosclerosis, or claudication were more likely to have an AAA than people who did not have any of these conditions. Low-quality evidence from 2 studies, including 1,549 men, also indicated that men with peripheral arterial disease were more likely to have an AAA than men without peripheral arterial disease. With regards to claudication as a risk factor in men, low-quality evidence from 1 study, including 5,623 men could not differentiate rates of AAA between men with claudication and those without claudication.
Cerebrovascular disease
- Low-quality evidence from 2 studies, including 3,179,243 people, indicated that people with cerebrovascular disease were more likely to have an AAA than those without cerebrovascular disease. A similar relationship was found in low-quality evidence from 2 studies that included 6,404 men. No evidence was identified specific to women.
Diabetes
- Low-quality evidence from 4 studies, including 6,505,378 people, indicated that people with diabetes were less likely to have an AAA than those without diabetes. A similar relationship was found in low-quality evidence from 3 studies that included 13,752 men; however, the results across the studies were inconsistent.
Chronic obstructive pulmonary disease (COPD)
- Low-quality evidence from 3 studies, including 130,280 people, indicated that people with COPD were more likely to have an AAA than those who did not have COPD. A similar relationship was found in low-quality evidence from 1 study that included 5,623 men. No evidence was identified specific to women.
Hypertension
- Low-quality evidence from 7 studies, including 6,540,694 people, indicated that people with hypertension were more likely to have an AAA than those who did not have hypertension. A similar relationship was found in low-quality evidence from 4 studies, including 16,714 men, and moderate-quality evidence from 1 study including 3,424 women.
Blood pressure thresholds
- Low-quality evidence from 1 study, including 5,363 people, could not differentiate AAA rates between people with systolic blood pressures equal to or above 200 mmHg and those with pressures below 200 mmHg. The same study could not differentiate AAA rates between people with diastolic blood pressures equal to or above 100 mmHg and those with pressures below 100 mmHg.
Dyslipidaemia (including hyperlipidaemia, hypercholesterolemia, and cholesterol thresholds)
- Low- to moderate-quality evidence from 5 studies, including up to 3,319,993 people, indicated that people with hyperlipidaemia or hypercholesterolemia were more likely to have an AAA than those who did not have any dyslipidaemia. Moderate-quality evidence from 1 study, including 12,203 men, indicated that men with dyslipidaemia were more likely to have an AAA than men who did not have dyslipidaemia. No evidence relating to dyslipidaemia was found for women.
Family history of AAA
- Low-quality evidence from 3 studies, including 3,203,875 people, indicated that people with a family history of AAA were more likely to have an AAA than those who did not. Additionally, moderate-quality evidence from 1 study, including 1,350 people, indicated that people with a family history of AAA, Marfan’s syndrome or Ehlers–Danlos syndrome were more likely to have an AAA than those who did not. Low-quality evidence from 2 studies, including 12,984 men, indicated that people with a family history of AAA were more likely to have an AAA than those who did not. Conversely, very low-quality evidence from 1 study, including 10,012 women, could not differentiate rates of AAA between women who had a family history of AAA and women who did not.
Ethnicity
Low-quality evidence from 2 studies, including up to 3,056,455 people, highlighted that Hispanic, black and Asian ethnic groups were individually less likely to have an AAA than white people. In relation to women, very-low quality evidence from 1 study, including 10,012 women, could not differentiate AAA rates between native-American people and white people. No evidence was identified specific to men.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee agreed that the outcomes that matter most were common risk factors for asymptomatic AAAs which could be used in community settings (outside specialist vascular services) to highlight the need for aortic ultrasound imaging.
The quality of the evidence
Since cross-sectional studies were considered the best study design to answer this review question, each cross-sectional study was initially graded as high in quality and was subsequently downgraded if there were any concerns about bias, indirectness, inconsistency, and imprecision. The committee agreed that the quality of evidence ranged from very low to high. Risk of bias was the main reason why some of the identified evidence was downgraded. In these studies the presence of risk factors was not ascertained by clinical examination, laboratory testing or review of medical records. Instead, patients were asked to complete self-administered questionnaires asking whether they had been diagnosed or were receiving medication for clinical risk factors of interest. Another potential bias was related to the way that the data was analysed. In some studies a stepwise approach was not used to input predictor variables into logistic regression models. Instead, investigators only input variables that were found to be significant in univariate analyses into logistic regression models. Although some of the evidence was considered low in quality, the committee agreed that the evidence reflected their clinical experience. Thus, the committee decided that “offer” recommendations were warranted.
It was noted that all but 1 study reported risk factors associated with the presence of an AAA. Pleumeekers et al. (1999) was the only study that assessed a physical sign indicative of the presence of an AAA. This study highlighted that people with a palpable aorta on abdominal examination were more likely to have an AAA than people without a palpable aorta on examination. The committee agreed that a palpable aorta was an important indicator that an aneurysm is present. However, it needed to be explicitly stated that there has to be some suspicion of an aneurysm to prompt abdominal examination.
The committee agreed that there was strong evidence that the risk of AAA increased with age. However, it was noted that various age cut-offs were used across included studies. Expert testimony from the national AAA screening programme (see Appendix H), highlighted that screening strategies focuses on 65-year-old men but there is a chance that older men with AAA are being missed. As a result, the committee agreed that it was important to specifically mention men aged 66 years and older in the recommendations. In relation to women, the committee noted that moderate-quality evidence showed that women aged 70 years or over had an increased risk of AAA when compared with women aged below 70 years. As a result, this age cut-off was used in the recommendations.
In relation to other risk factors associated with AAA, the committee considered that the majority of studies reported similar effect sizes, making it difficult to establish a hierarchy of association. As a result, the remaining risk factors associated with AAA presence were listed as bullet points in the recommendations. The committee agreed that it was more useful to use general terms such as “coronary, cerebrovascular or peripheral vascular disease” than to specify particular diseases.
Although the evidence on diabetes highlighted that the condition was a protective factor, the committee decided not to make any recommendations. This was because the main aim of the review question was to identify factors that would facilitate opportunistic screening (and increase the chances of people receiving abdominal ultrasound imaging to confirm or dismiss the suspicion of an AAA). The committee also decided not to make any recommendation on BMI as a risk factor because they considered that the studies that assessed BMI reported contradictory results.
Benefits and harms
The committee recognised that the national AAA screening programme has the ability to screen and identify a large number of people with AAA in the UK; however, there will always be some people who are missed by the programme. Furthermore, the committee noted that men who do not take up screening often have the highest risk of an AAA. As a result, the committee agreed that focusing recommendations on risk factors that could be used for opportunistic screening would improve detection rates. This would increase the chances that AAAs are identified early (before rupture) and reduce overall AAA-related morbidity and mortality.
The committee noted that there is a small risk of harm (such as unnecessary intervention) associated with population-based screening: evidence from the national screening programme highlighted that approximately 1 in 10,000 men die following intervention indicated by screening. The committee recognised that there may be also be small harms associated with targeted case-finding in men and women. However, it was agreed that the benefits of identifying AAAs early outweighed the risks of intervention-related or rupture-related mortality.
Cost effectiveness and resource use
The committee noted that expert testimony from the national AAA screening programme highlighted that population-based screening of 65-year-old men is cost-effective down to the prevalence of 0.35%. The committee took the view that opportunistic case finding of men 66 years and over as well as women aged 70 years and over was likely to be cost effective, as the recommendations allow for more people with AAAs to be identified early, before complications or rupture arise.
Other factors the committee took into account
The committee considered that the recommendations were primarily intended for general practitioners in order to facilitate diagnosis of AAA in individuals who attend primary care facilities seeking treatment for other conditions. The committee acknowledged that similar considerations could be made in secondary care settings. As a result, no healthcare setting was specified in the guideline recommendations.
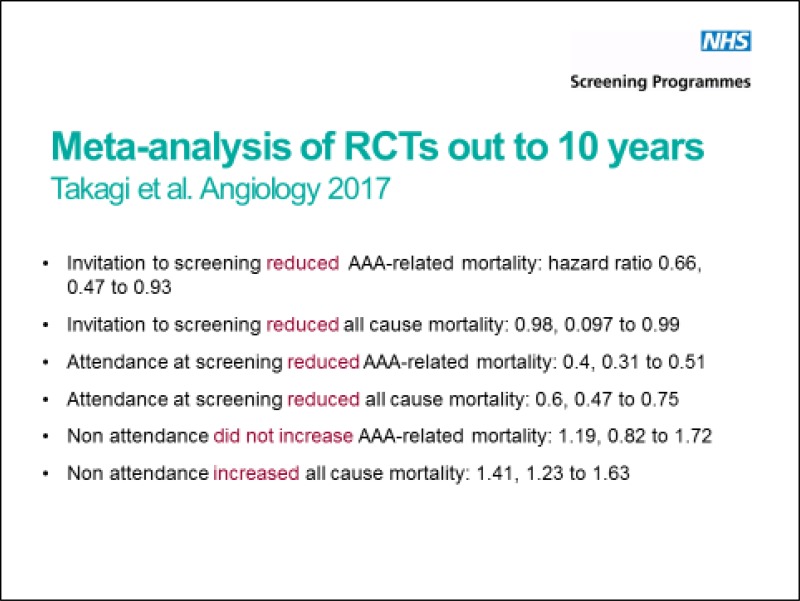
The committee noted the significant advances made by the national AAA screening programme and recognised that population-based screening yields some advantages over opportunistic aortic ultrasound. Notably, invitation to and subsequent attendance at screening reduced all-cause and AAA-related mortality.
Appendices
Appendix A. Review protocols
Review protocol for risk factors for predicting presence of an abdominal aortic aneurysm
| Review question 1 | Which signs, symptoms and risk factors (or combinations of these) are most accurate in predicting the presence of an abdominal aortic aneurysm? What is the effectiveness of available risk assessment tools? |
|---|---|
| Objectives | To determine which signs, symptoms, risk factors or assessment tools are accurate in predicting the presence of an AAA and could be used by clinicians in the course of opportunistic screening as a prompt to initiate diagnostic imaging |
| Type of review | Prognostic |
| Language | English |
| Study design | Initially, the following studies designs were included in the review protocol:
|
| Status |
i) Published papers only (full text) No date restrictions ii) Expert witness to present findings from UK registry data |
| Population |
People at risk from abdominal aortic aneurysms Subgroups of interest: by age, sex, comorbidity |
| Index test / factors of interest |
Abdominal pain Back pain Abdominal palpation Pulsatile abdominal mass/pulsation Age Sex Other cardiovascular disease (existing or previous) – other aneurysms, atherosclerotic disease, vascular claudication Inflammatory disease Smoking Blood pressure/hypertension Dislipidaemia Hypercholesterolaemia Family history of abdominal aortic aneurysms, other aneurysms, collagen disorders Ethnicity Diabetes COPD BMI/weight/obesity |
| Endpoint | Radiological diagnosis of abdominal aortic aneurysm |
| Other criteria for inclusion / exclusion of studies |
Exclusion: Non-English language Abstract/non-published Minimum population size of 500 |
| Baseline characteristics to be extracted in evidence tables |
Age Sex Comorbidities |
| Search strategies | See Appendix B |
| Review strategies |
Double-sifting of randomly selected 20%. Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. 20% will be appraised by a second reviewer. Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles and further summarised in evidence statements. |
| Key papers |
Beede SD, Ballard DJ, James EM, Ilstrup DM, Hallet JW Jr. Positive predictive value of clinical suspicion of abdominal aortic aneurysm. Implications for efficient use of abdominal ultrasonography. Arch Intern Med. 1990 Mar;150(3):549–51 [PubMed: 2106847] Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000 Mar 27;160(6):833–6 [PubMed: 10737283] Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999 Jan 6;281(1):77–82 [PubMed: 9892455] Pleumeekers HJ, Hoes AW, Hofman A, van Urk H, van der Does E, Grobbee DE. Selecting subjects for ultrasonographic screening for aneurysms of the abdominal aorta: four different strategies. Int J Epidemiol. 1999 Aug;28(4):682–6 [PubMed: 10480696] |
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 1
|
Medline Strategy, searched 29th September 2016 Database: 1946 to September Week 3 2016 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 Aortic Rupture/ |
| 3 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 4 or/1–3 |
| 5 prognosis.sh. |
| 6 diagnosed.tw. |
| 7 cohort.mp. |
| 8 predictor:.tw. |
| 9 death.tw. |
| 10 exp models, statistical/ |
| 11 or/5–10 |
| 12 (sensitiv: or predictive value:).mp. or accurac:.tw. |
| 13 11 or 12 |
| 14 “signs and symptoms”/ |
| 15 ((sign or signs) adj5 symptom*).tw. |
| 16 Risk Factors/ |
| 17 factor*.tw. |
| 18 predict*.tw. |
| 19 or/14–18 |
| 20 13 or 19 |
| 21 4 and 20 |
| 22 animals/ not humans/ |
| 23 21 not 22 (12444) |
| 24 limit 23 to english language |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Appendix D. Clinical evidence tables
Download PDF (395K)
Appendix E. GRADE tables
Age
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
|
Age: 55–59 60–64 65–69 70–74 75–79 80–84 All vs. <55 (reference) | 1 Kent (2010) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,056,455 |
ORa 2.76 (2.55, 3.00) ORa 5.35 (4.97, 5.76) ORa 9.41 (8.76. 10.12) ORa 14.46 (13.45. 15.55) ORa 20.46 (18.99. 21.99) ORa 28.37 (26.31. 30.59) | Low |
|
Age: 70–74 75–79 All vs. 65–69 (reference) | 1 Vardulaki (2000) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 5,356 |
ORa 1.4 (0.98, 2.1) ORa 1.8 (1.2, 2.7) | Moderate |
|
Age: 66–75 >75 All vs. 55–65 (reference) | 1 Pleumeekers (1999) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 5,328 |
ORa 1.4 (1.1, 1.9) ORa 2.7 (1.8, 4.1) | Low |
| Age: >75 vs. ≤75 | 1 Chun (2014) | Cross-sectional | Serious3 | N/A | Not serious | Not serious | 6,142 | ORa 1.62 (1.33, 1.96) | Moderate |
| Age: >70 vs. ≤75 | 1 Mark-Christensen (2017) | Cross-sectional | Serious2 | N/A | Not serious | Not serious | 24,632 | ORa 1.41 (1.22, 1.63) | Moderate |
| Age: per 7 year increase | 1 Lederle (2000) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 122,788 | ORa 1.58 (1.52, 1.64) | Low |
| Age: per year increase | 3 (De Carvalho, 2012 Corrado 2016, Makrygiannis 2016) | Cross-sectional | Serious1 | Not serious | Not serious | Serious4 | 4,006 |
ORa 1.1 (1.0, 1.2) ORa 1.14 (1.06, 1.22) ORa 1.07 (Not significant; 95% CI not reported) | Low |
| Men only | |||||||||
|
Age: 65–69 70–74 75–84 All vs. 60–64 (reference) | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 2,962 |
ORa 2.18 (1.44, 3.29) ORa 2.29 (1.49, 3.52) ORa 3.31 (1.62, 6.73) | Moderate |
| Age: per year increase | 2 (Le 2007, Bonamigo 2003) | Cross-sectional | Serious1 | Not serious | Not serious | Not serious | 12,971 |
ORa 1.09 (1.07, 1.11) ORa 1.08 (1.022, 1.139) | Moderate |
| Women only | |||||||||
| Age: per year increase | 1 Derubertis (2007) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 10,012 | ORa 1.10 (1.06, 1.14) | Low |
|
Age: 65–69 70–74 75–84 All vs. 60–64 (reference) | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 3,424 |
ORa 1.94 (0.81, 4.65) ORa 4.81 (2.14, 10.84) ORa 4.98 (1.45, 17.07) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors, and covariates adjusted for, was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
It was unclear what people were eligible for screening, downgrade 1 level.
- 4
95% CI crosses the line of no effect (1) in studies with greater weighting (larger populations), downgrade 1 level.
Sex
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Sex: men vs. women | 6 (Kent 2010, Lederle 2000, Vardulaki 2000, Pleumeekers 1999, De Carvalho 2012, Corrado 2016, 1 Mark-Christensen 2017)) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,217464 |
ORa 5.71 (5.57, 5.85) ORa 2.13 (1.45, 3.12) ORa 5.6 (3.7, 8.4) ORa 6.5 (3.8, 11.2) ORa 9.9 (2.0, 50.0) ORa 8.2 (1.79, 37.91) ORa 21.9 (3.07, 156.26) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
BMI/Weight/Obesity
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| BMI: ≥25 kg/m2 vs. <25 kg/ m2 | 1 Kent (2010) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 3,056,455 | ORa 1.20 (1.17, 1.22) | Low |
| BMI: ≥30 kg/m2 vs. <30 kg/ m2 | 2 (Chun 2014 & Mark-Christensen 2017) | Cross-sectional | Very serious2,3 | Serious4 | Not serious | Not serious | 30,744 |
ORa 0.94 (0.77, 1.15) ORa 1.26 (1.06, 1.49) | Very low |
| Weight: per 16 kg | 1 Lederle (2000) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious5 | 122,788 | ORa 1.00 (0.93, 1.07) | Very low |
| Men only | |||||||||
| BMI: per kg/m2 | 1 Le (2007) | Cross-sectional | Serious2 | N/A | Not serious | Not serious | 12,203 | ORa 1.03 (1.01, 1.05) | Moderate |
| BMI: per 4kg/m2 | 1 Singh (2001) | Cross-sectional | Serious2 | N/A | Not serious | Serious5 | 2,962 | ORa 1.14 (0.94, 1.39) | Low |
| Women only | |||||||||
| BMI: per 4kg/m2 | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Serious5 | 3,424 | ORa 0.85 (0.65, 1.11) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
It was unclear what people were eligible for screening, downgrade 1 level.
- 4
Reported findings from included studies highlight inconsistent directions of effect, downgrade 1 level.
- 5
95% CI crosses the line of no effect (1), downgrade 1 level.
Smoking
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Current smokers vs. never smokers | 7 (Berger 2013, Chun 2014, Vardulaki 2000, Pleumeekers 1999, De Carvalho 2012, Corrado 2016, Makrygiannis 2016, Mark-Christensen 2017) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,341,7335 |
ORa 1.98 (1.86, 2.03) ORa 1.67 (1.33, 2.10) ORa 2.7 (1.7, 4.4) ORa 3.1 (1.7, 5.1) ORa 6.8 (1.6, 29.4) ORa 4.73 (Significant; (95% CI not reported) ORa 7.61 (5.76, 10.05) | Low |
| Ex-smokers vs. never smokers | 4 (Berger 2013, Vardulaki 2000, Corrado 2016, Mark-Christensen 2017) | Cross-sectional | Serious1 | Not serious | Not serious | Not serious | 3,326,904 |
ORa 2.75 (2.68, 2.82) ORa 1.5 (1.0, 2.3) ORa 2.76 (1.12, 8.94) ORa 3.76 (2.88, 4.93) | Moderate |
| Ever smoked vs. never smoked | 1 Lederle (2000) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 122,788 | ORa 2.97 (2.65, 3.32) | Low |
| Men only | |||||||||
| Current smokers vs. never smokers | 4 (Singh 2001, Hager 2013, Barba 2013, Bonamigo 2003) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 10,134 |
ORa 7.37 (3.70, 14.69) ORa 8.90 (4.2, 18.6) ORa 3.47 (1.67, 7.22) ORa 6.42 (2.18, 18.89) | Low |
| Ex-smokers vs. never smokers | 2 (Singh 2001, Hager 2013) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 8,585 |
ORa 3.60 (1.85, 7.03) ORa 3.30 (1.70, 6.60) | Low |
| Ever smoked vs. never smoked | 1 Le (2007) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 12,203 | ORa 2.04 (1.84, 2.26) | Moderate |
|
Smoking frequency: 10–20 cigarettes/day >20 cigarettes/day All compared with 0 – 20 cigarettes/day (reference) | 1 Salvador-Gonzalez (2016) | Cross-sectional | Serious2 | Not serious | Not serious | Not serious | 651 |
ORa 20.4 (2.6, 162.2) ORa 15.8 (1.7, 146.4) | Moderate |
| Women only | |||||||||
| Current smokers vs never smokers | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 3,424 | ORa 5.82 (2.92, 11.58) | Moderate |
| Ex-smokers vs never smokers | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Serious3 | 3,424 | ORa 1.64 (0.75, 3.58) | Low |
| Tobacco use (greater than or equal to 100 cigarettes in a lifetime) | 1 Derubertis (2007) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 10,012 | ORa 4.02 (2.17, 7.44) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
95% CI crosses the line of no effect (1), downgrade 1 level.
Palpable aorta on abdominal examination
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 1 Pleumeekers (1999) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 5,328 | ORa 7.0 (3.7, 13.2) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
Cardiovascular disease
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Coronary artery disease | 4 (Kent 2010, Lederle 2000, Chun 2014, Makrygiannis 2016) | Cross-sectional | Very serious1,2, | Not serious | Not serious | Not serious | 3,186,486 |
ORa 1.72 (1.69, 1.76) ORa 1.44 (1.34, 1.55) ORa 1.89 (1.59, 2.29) ORa 2.15 (not significant; 95% CI not reported) | Low |
| History of myocardial infarction | 1 Pleumeekers (1999) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious3 | 5,328 | OR 1.5a (0.9, 2.6) | Very low |
| Coronary insufficiency | 1 De Carvalho (2012) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 1,350 | OR 166.7a (25.6, >1,000) | Moderate |
| Men only | |||||||||
| Coronary artery disease | 1 Hager (2013) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious3 | 5,623 | OR 1.7a (1.0, 3.0) | Very low |
| History of myocardial infarction | 1 Salvador-Gonzalez (2016) | Cross-sectional | Serious2 | N/A | Not serious | Not serious | 651 | OR 5.1a (1.4, 18.4) | Moderate |
| History of cardiovascular disease | 1 Le (2007) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 12,203 | OR 1.83a (1.58, 2.12) | Moderate |
| Myocardial disease | 1 Bonamigo (2003) | Cross-sectional | Not serious | N/A | Not serious | Serious3 | 768 | OR 1.66a (0.745, 3.691) | Moderate |
| Women only | |||||||||
| Cardiovascular disease (myocardial infarction or coronary revascularization) | 1 Derubertis (2007) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 10,012 | OR 3.62a (2.08, 6.29) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
95% CI crosses the line of no effect (1), downgrade 1 level.
Peripheral arterial disease
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 6 (Kent 2010, Chun 2014, Pleumeekers 1999, De Carvalho 2012, Makrygiannis 2016 Mark-Christensen 2017) | Cross-sectional | Very serious1,2,3 | Not serious | Not serious | Not serious | 3,095,008 |
ORa 1.59 (1.54, 1.65) ORa 2.28 (1.74, 2.97) ORa 2.1 (1.3, 3.3) ORa 27.0 (5.8, 125.0) ORa 3.29 (Significant; 95% CI not reported) ORa 1.81 (1.51, 2.16) | Low |
| Men only | |||||||||
| Present vs. absent | 2 (Barba 2013, Bonamigo 2003) | Cross-sectional | Serious2 | Serious4 | Not serious | Not serious | 1,549 |
ORa 3.00 (1.16, 7.80) ORa 0.843 (0.281, 2.528) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
It was unclear what people were eligible for screening, downgrade 1 level. 4. Visual inspection of point estimates and 95% CIs across studies indicates inconsistent findings, downgrade 1 level.
- 4
Reported findings from included studies highlight inconsistent directions of effect, downgrade 1 level.
Atherosclerosis
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Atherosclerosis | 1 Lederle (2000) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 122,788 | ORa 1.64 (1.52, 1.78) | Low |
|
Atherosclerotic plaque diameter: 1.5 – 7.7 mm2 7.8 – 12.3 mm2 12.4 – 18.9 mm2 19.0 – 31.1 mm2 31.2 – 246.4 mm2 All vs. no plaque | 1 Johnsen (2010) | Cross-sectional | Not serious | N/A | Not serious | Not serious | 6,142 |
ORa 0.6 (0.3, 1.2) ORa 1.3 (0.8, 2.2) ORa 1.9 (1.2, 2.9) ORa 1.6 (1.0, 2.5) ORa 1.7 (1.1, 2.6) | High |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
It was unclear what people were eligible for screening, downgrade 1 level.
Claudication
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 2 (Lederle 2000, Pleumeekers 1999) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 128,116 |
ORa 1.35 (1.18, 1.53) ORa 1.9 (0.7, 5.0) | Low |
| Men only | |||||||||
| Present vs. absent | 1 Hager (2013) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 5,623 | ORa 2.0 (0.7, 5.6) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant univariate analyses were input into logistic regression models, downgrade 1 level.
Cerebrovascular disease
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 2 (Kent 2010, Lederle 2000) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,179,243 |
ORa 1.18 (1.14, 1.21) ORa 1.28 (1.17, 1.41) | Low |
| Men only | |||||||||
| Present vs. absent | 2 (Hager 2013, Barba 2013) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 6,404 |
ORa 2.0 (1.1, 3.6) ORa 2.37 (0.61, 9.25) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
Diabetes
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 4 (Berger 2013, Kent 2010, Lederle 2000, Chun 2014) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 6,505,378 |
ORa 1.00 (1.00, 1.00) ORa 0.75 (0.73, 0.77) ORa 0.65 (0.59, 0.72) ORa 0.60 (0.47, 0.77) | Low |
| Men only | |||||||||
| Present vs. absent | 3 (Le 2007, Barba 2013, Bonamigo 2003) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Serious3 | 13,752 |
ORa 0.79 (0.63, 0.98) ORa 0.38 (0.11, 1.06) ORa 0.135 (0.002, 1.15) | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
95% CI crosses the line of no effect (1) in studies with greater weighting (larger populations), downgrade 1 level.
COPD
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Present vs. absent | 3 (Lederle 2000, Chun 2014, De Carvalho 2012) | Cross-sectional | Very serious1,2, | Not serious | Not serious | Not serious | 130,280 |
ORa 1.06 (0.97, 1.17) ORa 1.75 (1.41, 2.18) ORa 35.7 (6.3, 200.0) | Low |
| Men only | |||||||||
| Present vs. absent | 1 Hager (2013) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 5,623 | ORa 2.1 (1.1, 3.9) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
Hypertension
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Hypertension (defined as blood pressure measurements or use of antihypertensive drugs) | 7 (Berger 2013, Kent 2010, Lederle 2000, Chun 2014, Vardulaki 2000, Pleumeekers 1999, Mark-Christensen 2017) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 6,540,694 |
ORa 1.24 (1.21, 1.28) ORa 1.25 (1.21, 1.28) ORa 1.23 (1.14, 1.32) ORa 0.92 (0.75, 1.12) ORa 1.7 (1.3, 2.1) ORa 1.8 (1.1, 3.0) ORa 1.66 (1.43, 1.94) | Low |
| Men only | |||||||||
| Hypertension (defined as blood pressure measurements or use of antihypertensive drugs) | 4 (Le 2007, Singh 2001, Bonamigo 2003, Barba 2013) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 16,714 |
ORa 1.47 (1.27, 1.71) ORa 1.61 (1.16, 2.24) ORa 0.71 (0.35, 1.47) ORa 2.43 (1.08, 5.45) | Low |
| Women only | |||||||||
| Hypertension (defined by taking antihypertension meds) | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 3,424 | ORa 2.02 (1.14, 3.57) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
Blood pressure thresholds
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Systolic blood pressure: ≥200 mmHg vs. <200 mmHg | 1 Vardulaki (2000) | Cross-sectional | Serious1 | N/A | Not serious | Serious2 | 5,356 | ORa 1.1 (0.7, 1.8) | Low |
| Diastolic blood pressure: ≥100 mmHg vs. <100 mmHg | 1 Vardulaki (2000) | Cross-sectional | Serious1 | N/A | Not serious | Serious2 | 5,356 | ORa 1.3 (0.8, 2.2) | Low |
| Men only | |||||||||
| Systolic blood pressure: per 1 mmHg | 1 Le (2007) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 12,203 | ORa 0.99 (0.98, 0.99) | Moderate |
| Systolic blood pressure: per 20 mmHg | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Serious2 | 2,962 | ORa 0.97 (0.85, 1.12) | Low |
| Diastolic blood pressure: per 1 mmHg | 1 Le (2007) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 12,203 | ORa 1.03 (1.02, 1.04) | Moderate |
| Women only | |||||||||
| Systolic blood pressure: per 20 mmHg | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 3,424 | ORa 1.39 (1.11, 1.73) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
95% CI crosses the line of no effect (1), downgrade 1 level.
Dyslipidaemia (including hyperlipidaemia, hypercholesterolemia, and cholesterol thresholds)
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Hyperlipidaemia (diagnosis or use of medication) | 1 Berger (2013) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 3,319,993 | ORa 1.45 (1.41, 1.49) | Moderate |
| Hypercholesterolemia (present vs. absent) | 3 (Kent 2010, Lederle 2000, Makrygiannis 2016) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,180,344 |
ORa 1.34 (1.31, 1.37) ORa 1.40 (1.29, 1.52) ORa 4.89 (Significant: 95% CI not reported) | Low |
| Cholesterol levels: ≥200 mg/dL vs. <200 mg/dL | 1 Chun (2014) | Cross-sectional | Serious3 | N/A | Not serious | Not serious | 6,142 | ORa 0.66 (0.49, 0.90) | Moderate |
| Cholesterol levels: ≥6.5 mmol/L vs. <6.5 mmol/L | 1 Pleumeekers (1999) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 5,328 | ORa 1.8 (1.2, 2.7) | Low |
| Men only | |||||||||
| Dyslipidaemia (present vs. absent) | 1 Le (2007) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 12,203 | ORa 1. 42 (1.22, 1.65) | Moderate |
| Hyperlipidaemia (not defined) | 1 Hager (2013) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious4 | 5,623 | ORa 1.2 (0.8, 2.0) | Very low |
| Serum total cholesterol: per 1mmol/L increase | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 2,962 | ORa 1.19 (1.04, 1.35) | Moderate |
| Women only | |||||||||
| Serum total cholesterol: per 1mmol/L increase | 1 Singh (2001) | Cross-sectional | Serious1 | N/A | Not serious | Serious4 | 3,424 | ORa 1.18 (0.96, 1.44) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
It was unclear what people were eligible for screening, downgrade 1 level.
- 4
95% CI crosses the line of no effect (1), downgrade 1 level.
Family history of AAA
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
| Family history of AAA | 3 (Kent 2010, Lederle 2000, Mark-Christensen 2017) | Cross-sectional | Very serious1,2 | Not serious | Not serious | Not serious | 3,203,875 |
ORa 3.80 (3.66, 3.95) ORa 1.93 (1.71, 2.18) ORa 2.17 (1.62, 2.90) | Low |
| Family history of AAA, Marfan syndrome or Ehlers–Danlos syndrome | 1 De Carvalho (2012) | Cross-sectional | Serious1 | N/A | Not serious | Not serious | 1,350 | ORa 500.0 (6.5, >1000) | Moderate |
| Men only | |||||||||
| Family history of AAA | 2 (Le 2007, Barba 2013) | Cross-sectional | Very serious1,1 | N/A | Not serious | Not serious | 12,984 |
ORa 1.88 (1.17, 2.89) ORa 3.17 (0.82, 12.24) | Low |
| Women only | |||||||||
| Family history of AAA | 1 Derubertis (2007) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious3 | 10,012 | ORa 1.95 (0.90, 4.22) | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
95% CI crosses the line of no effect (1), downgrade 1 level.
Ethnicity
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Men and women | |||||||||
|
Ethnicity: Hispanic African American Asian All vs. white (reference) | 1 Kent (2010) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 3,056,455 |
ORa 0.69 (0.62, 0.77) ORa 0.72 (0.66, 0.78) ORa 0.72 (0.59, 0.75) | Low |
|
Ethnicity: Black vs. white | 1 Lederle (2000) | Cross-sectional | Very serious1,2 | N/A | Not serious | Not serious | 122,788 | ORa 0.62 (0.53, 0.73) | Low |
| Women only | |||||||||
|
Ethnicity: Native American vs. white | 1 Derubertis (2007) | Cross-sectional | Very serious1,2 | N/A | Not serious | Serious3 | 10,012 | ORa 1.41 (0.43, 4.63) | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
The presence of risk factors was ascertained by asking participants to complete a self-administered questionnaire, downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models, downgrade 1 level.
- 3
95% CI crosses the line of no effect (1), downgrade 1 level.
Appendix G. Excluded studies
Clinical studies
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Xiong Jiang, Wu Zhongyin, Chen Chen, Wei Yingqi, and Guo Wei (2016) Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: A meta-analysis. International journal of cardiology 221, 484–95 [PubMed: 27414727] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 2 | Alcorn H G, Wolfson Jr, S K, Sutton-Tyrrell K, et al. (1996) Risk factors for abdominal aortic aneurysms in older adults enrolled in the Cardiovascular Health Study. Arteriosclerosis, Thrombosis, and and Vascular Biology 16(8), 963–970 [PubMed: 8696960] | Authors reported percentages with adjusted and unadjusted p values. No relative risks, odds ratios or hazard ratios were reported. |
| 3 | Baumgartner I, Hirsch AT, Abola B, et al. (2008) Cardiovascular risk profile and outcome of patients with abdominal aortic aneurysm in out-patients with atherothrombosis: data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Journal of vascular surgery 48(4), 808–14 [PubMed: 18639426] | Wrong study design: case-control. Furthermore, primary aortic imaging was not performed: investigators ascertained the presence of AAA by reviewing documentation by the treating physician. |
| 4 | Beede S D, Ballard D J, James E M, et al. (1990) Positive predictive value of clinical suspicion of abdominal aortic aneurysm. Implications for efficient use of abdominal ultrasonography. Archives of internal medicine 150(3), 549–51 [PubMed: 2106847] | Sample size of less than 500 participants. Furthermore, multivariate analysis was not performed. |
| 5 | Cao H, Hu X, Zhang Q et al. (2014) Homocysteine level and risk of abdominal aortic aneurysm: a meta-analysis. PloS one 9(1), e85831 [PMC free article: PMC3897527] [PubMed: 24465733] | Systematic review and meta-analysis of case controls. |
| 6 | Chabok M, Nicolaides A, Aslam M, Farahmandfar M, Humphries K, Kermani N Z, Coltart J, and Standfield N (2016) Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. The British journal of surgery 103(9), 1132–8 [PubMed: 27332825] | Conference abstract |
| 7 | Chiu HY, Lo PC, Huang WF et al. (2016) Increased risk of aortic aneurysm (AA) in relation to the severity of psoriasis: A national population-based matched-cohort study. Journal of the American Academy of Dermatology 75(4), 747–54 [PubMed: 27473449] | Not specific to AAA: study included a mixed population of people with AAA and thoracic aortic aneurysms. |
| 8 | Cho IJ, Jang SY, Chang HJ et al. (2014) Aortic aneurysm screening in a high-risk population: a non-contrast computed tomography study in korean males with hypertension. Korean circulation journal 44(3), 162–9 [PMC free article: PMC4037638] [PubMed: 24876857] | Not specific to AAA: study included a mixed population of people with AAA and thoracic aortic aneurysms. |
| 9 | Cornuz J, Pinto C S, Tevaearai H, and Egger M (2004) Risk factors for asymptomatic abdominal aortic aneurysm: Sytematic review and meta-analysis of population-based screening studies. European Journal of Public Health 14(4), 343–349 [PubMed: 15542867] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 10 | De Rango , P , Farchioni L, Fiorucci B, and Lenti M (2014) Diabetes and abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 47(3), 243–261 [PubMed: 24447529] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 11 | Duncan JL, Harrild KA, Iversen L et al. (2012) Long term outcomes in men screened for abdominal aortic aneurysm: prospective cohort study. BMJ (Clinical research ed.) 344, e2958 [PMC free article: PMC3344734] [PubMed: 22563092] | Wrong study design: cohort study |
| 12 | Durieux R, Van Damme , H , Labropoulos N et al. (2014) High Prevalence of abdominal aortic aneurysm in patients with three-vessel coronary artery disease. European Journal of Vascular and Endovascular Surgery 47(3), 273–278 [PubMed: 24456737] | Population screening study in which patients undergoing coronary angiography were assessed for the presence of AAA. Authors stated that patients with known AAA or with a history of previous AAA surgery were intentionally included for screening. |
| 13 | Elkalioubie A, Haulon S, Duhamel A et al. (2015) Meta-Analysis of Abdominal Aortic Aneurysm in Patients With Coronary Artery Disease. The American journal of cardiology 116(9), 1451–6 [PubMed: 26347003] | Systematic review of prospective and retrospective observational studies. These study designs were not specified in the review protocol. |
| 14 | Fernandez-Garcia C E, Burillo E, Lindholt J S, Martinez-Lopez D, Pilely K, Mazzeo C, Michel J B, Egido J, Garred P, Blanco-Colio L M, and Martin-Ventura J L (2017) Association of ficolin-3 with abdominal aortic aneurysm presence and progression. Journal of thrombosis and haemostasis : JTH 15(3), 575–585 [PubMed: 28039962] | Out of scope: study assesses the use of a genetic biomarker for indicating the presence/absence of AAA |
| 15 | Fink H A, Lederle F A, Roth C S et al. (2000) The accuracy of physical examination to detect abdominal aortic aneurysm. Archives of Internal Medicine 160(6), 833–836 [PubMed: 10737283] | Wrong study design: case-control. Additionally, investigators did not assess which risk factors were associated with the presence of aneurysms. Finally, the sample size was less than 500 participants. |
| 16 | Forsdahl SH, Singh K, Solberg S et al. (2009) Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation 119(16), 2202–8 [PubMed: 19364978] | Wrong study design: cohort study |
| 17 | Flessenkaemper I H, Loddenkemper R, Roll S, et al. (2015) Screening of COPD patients for abdominal aortic aneurysm. International Journal of COPD 10, 1085–1091 [PMC free article: PMC4468935] [PubMed: 26089658] | Multivariate analysis was not performed. |
| 18 | Goessens B, Visseren FL, Algra A, et al. (2006) Screening for asymptomatic cardiovascular disease with noninvasive imaging in patients at high-risk and low-risk according to the European Guidelines on Cardiovascular Disease Prevention: the SMART study. Journal of vascular surgery 43(3), 525–32 [PubMed: 16520167] | Multivariate analysis was not performed: the prevalence of atherosclerotic risk factors were reported as percentages. |
| 19 | Golledge J, Mallat Z, Tedgui A et al. (2011) Serum secreted phospholipase A2 is associated with abdominal aortic aneurysm presence but not progression. Atherosclerosis 216(2), 458–60 [PubMed: 21382622] | Wrong study design: case control. Men with AAA were identified and their serum secretory phospholipase A levels were compared with those of randomly selected healthy controls. |
| 20 | Golledge J, Clancy P, Yeap BB, et al. (2013) Increased serum angiopoietin-2 is associated with abdominal aortic aneurysm prevalence and cardiovascular mortality in older men. International journal of cardiology 167(4), 1159–63 [PubMed: 22483260] | Wrong study design: case control. Men with AAA were identified and their serum angiopoietin-2 levels were compared with those of randomly selected healthy controls. |
| 21 | Hafez H, Druce P S, and Ashton H A (2008) Abdominal Aortic Aneurysm Development in Men Following a “normal” Aortic Ultrasound Scan. European Journal of Vascular and Endovascular Surgery 36(5), 553–558 [PubMed: 18718773] | Multivariate analysis/regression was not performed. |
| 22 | Harrison Seamus C, Holmes Michael V, Burgess Stephen, Asselbergs Folkert W, Jones Gregory T, Baas Annette F, van ’t Hof, F N, de Bakker , Paul I W, Blankensteijn Jan D, Powell Janet T, Saratzis Athanasios, de Borst , Gert J, Swerdlow Daniel I, van der Graaf , Yolanda , van Rij , Andre M, Carey David J, Elmore James R, Tromp Gerard, Kuivaniemi Helena, Sayers Robert D, Samani Nilesh J, Bown Matthew J, and Humphries Steve E (2017) Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA cardiology [PMC free article: PMC5833524] [PubMed: 29188294] | Out of scope:Genome wide association study assessing the use of a genetic biomarker for indicating the presence/absence of AAA |
| 23 | Henriksen N A, Sorensen L T, Jorgensen L N, and Lindholt J S (2013) Lack of association between inguinal hernia and abdominal aortic aneurysm in a population-based male cohort. The British journal of surgery 100(11), 1478–82 [PubMed: 24037568] | Wrong study design: case-control |
| 24 | Hernesniemi JA, Vanni V, and Hakala T (2015) The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. Journal of vascular surgery 62(1), 232–240.e3 [PubMed: 26115925] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 25 | Jahangir E, Lipworth L, Edwards T L, Kabagambe E K, Mumma M T, Mensah G A, Fazio S, Blot W J, and Sampson U K (2015) Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. Journal of epidemiology and community health 69(5), 481–488 [PMC free article: PMC4494088] [PubMed: 25563744] | Wrong study design: cohort study |
| 26 | Iribarren C, Darbinian J A, Go A S, et al. (2007) Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Annals of epidemiology 17(9), 669–78 [PubMed: 17512215] | Wrong study design: cohort study |
| 27 | Joergensen T M. M, Houlind K, Green A, and Lindholt J S (2014) Abdominal aortic diameter is increased in males with a family history of abdominal aortic aneurysms: Results from the Danish viva-trial. European Journal of Vascular and Endovascular Surgery 48(6), 669–675 [PubMed: 25443525] | Multivariate analysis was not performed association between risk factors and AAA diagnosis. Instead univariate was performed to assess associations. Linear regression was performed estimate the mean aneurysm diameters in various subgroups of people. |
| 28 | Lederle F A, and Simel D L (1999) Does this patient have abdominal aortic aneurysm?. Journal of the American Medical Association 281(1), 77–82 [PubMed: 9892455] | Systematic review assessing the sensitivity, negative predictive value and positive predictive value of abdominal palpation for detecting abdominal aortic aneurysms. None of the included studies had sample sizes of 500 participants or larger. |
| 29 | Lederle F A, Johnson G R, Wilson S E, Aneurysm Detection, Management Veterans Affairs Cooperative, and Study (2001) Abdominal aortic aneurysm in women. Journal of vascular surgery 34(1), 122–6 [PubMed: 11436084] | Multivariate analysis/regression was not performed: The number of AAAs in women was not large enough to generate valid multivariate models for AAAs in women with all variables included in the questionnaire. |
| 30 | Lederle F A, Nelson D B, and Joseph A M (2003) Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. Journal of vascular surgery 38(2), 329–34 [PubMed: 12891116] | Not specific to AAA. |
| 31 | Lederle F A, Larson J C, Margolis K L, et al. J D (2008) Abdominal aortic aneurysm events in the women’s health initiative: Cohort study. BMJ 337(7677), 1037–1040 | Wrong study design: cohort study |
| 32 | lede A J, Fowkes F G. R, Carson M N, Leng G C, and Allan P L (1997) Smoking, atherosclerosis and risk of abdominal aortic aneurysm. European Heart Journal 18(4), 671–676 [PubMed: 9129900] | Wrong study design: nested case-control. |
| 33 | Lindblad B, Borner G, and Gottsater A (2005) Factors associated with development of large abdominal aortic aneurysm in middle-aged men. European Journal of Vascular and Endovascular Surgery 30(4), 346–352 [PubMed: 15936229] | Wrong study design: nested case-control. |
| 34 | Long A, Bui H T, Barbe C, et al. (2010) Prevalence of abdominal aortic aneurysm and large infrarenal aorta in patients with acute coronary syndrome and proven coronary stenosis: a prospective monocenter study. Annals of vascular surgery 24(5), 602–8 [PubMed: 20371161] | Sample size of less than 500 participants. |
| 35 | Majeed K, Hamer A W, White S C, et al. (2015) Prevalence of abdominal aortic aneurysm in patients referred for transthoracic echocardiography. Internal medicine journal 45(1), 32–9 [PubMed: 25266859] | Investigators included patients with known AAA for screening. Additionally, risk factors (echocardiographic parameters) assessed in this study are not listed in the review protocol. |
| 36 | Mattes E, Davis T M. E, Yang D, et al. (1997) Prevalence of abdominal aortic aneurysms in men with diabetes. Medical Journal of Australia 166(12), 630–633 [PubMed: 9216582] | Sample size of less than 500 participants. |
| 37 | Moxon J V, Jones R E, Norman P E, et al. (2016) Plasma ferritin concentrations are not associated with abdominal aortic aneurysm diagnosis, size or growth. Atherosclerosis 251, 19–24 [PubMed: 27235969] | The risk factor (body iron levels) assessed in this study is not listed in the review protocol. |
| 38 | Ogata T, MacKean G L, Cole C W, et al. (2005) The lifetime prevalence of abdominal aortic aneurysms among siblings of aneurysm patients is eightfold higher than among siblings of spouses: an analysis of 187 aneurysm families in Nova Scotia, Canada. Journal of vascular surgery 42(5), 891–7 [PMC free article: PMC1373672] [PubMed: 16275443] | Sample size of less than 500 participants. Furthermore, multivariate analysis/regression was not performed. |
| 39 | Robson J C, Kiran A, Maskell J, et al. (2013) The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Annals of the Rheumatic Diseases , no pagination [PubMed: 24095936] | Wrong study design: cohort study |
| 40 | Rodin M B, Daviglus M L, Wong G C, et al. (2003) Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension (Dallas, and Tex. : 1979) 42(1), 61–8 [PubMed: 12796281] | Wrong study design: cohort study |
| 41 | Ruff A L, Teng K, Hu B, et al. (2015) Screening for abdominal aortic aneurysms in outpatient primary care clinics. The American journal of medicine 128(3), 283–8 [PubMed: 25446298] | Study did not assess risk factors associated with AAA. Instead, investigators assessed risk factors associated with the decisions to perform ultrasound or computed-tomography imaging. |
| 42 | Sakalihasan N, Defraigne J, Kerstenne MA, et al. (2014) Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: analysis of 618 probands and their families from the Liege AAA Family Study. Annals of vascular surgery 28(4), 787–97 [PMC free article: PMC4082690] [PubMed: 24365082] | The study employed multiple methodological designs. Initially, a case-control design was employed to establish whether people diagnosed with AAA had a family history of AAA. A cross-sectional design was then used to explore the prevalence of aneurysms in family members (n<500) of people diagnosed with AAA. Finally, multivariate analysis was not performed. |
| 43 | Shantikumar S, Ajjan R, Porter K E, et al. (2010) Diabetes and the Abdominal Aortic Aneurysm. European Journal of Vascular and Endovascular Surgery 39(2), 200–207 [PubMed: 19948418] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 44 | Sidloff D A, Stather P W, Choke E, et al. (2014) A systematic review and meta-analysis of the association between markers of hemostasis and abdominal aortic aneurysm presence and size. Journal of vascular surgery 59(2), 528–535.e4 [PubMed: 24461868] | Systematic review of case-controls |
| 45 | Solberg S, Forsdahl S H, Singh K et al. (2010) Diameter of the infrarenal aorta as a risk factor for abdominal aortic aneurysm: the Tromso Study, 1994–2001. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 39(3), 280–4 [PubMed: 19942461] | Wrong study design: cohort study |
| 46 | Stackelberg O, Bjorck M, Sadr-Azodi O, et al. (2013) Obesity and abdominal aortic aneurysm. The British journal of surgery 100(3), 360–6 [PubMed: 23203847] | Wrong study design: cohort study |
| 47 | Stackelberg O, Bjorck M, Larsson S C, Orsini N, and Wolk A (2014) Sex differences in the association between smoking and abdominal aortic aneurysm. The British journal of surgery 101(10), 1230–7 [PubMed: 24916023] | Wrong study design: cohort study |
| 48 | Stackelberg O, Bjorck M, Larsson S C, et al. (2013) Fruit and vegetable consumption with risk of abdominal aortic aneurysm. Circulation 128(8), 795–802 [PubMed: 23960255] | Wrong study design: cohort study |
| 49 | Stackelberg Otto, Wolk Alicja, Eliasson Ken, Hellberg Anders, Bersztel Adam, Larsson Susanna C, Orsini Nicola, Wanhainen Anders, and Bjorck Martin (2017) Lifestyle and Risk of Screening-Detected Abdominal Aortic Aneurysm in Men. Journal of the American Heart Association 6(5), [PMC free article: PMC5524061] [PubMed: 28490522] | Wrong study design: cohort study |
| 50 | Svensjo S, Bjorck M, Gurtelschmid M et al. (2011) Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 124(10), 1118–23 [PubMed: 21844079] | Population screening study in which people identified from a national registry were screened for AAAs. Authors stated that people with previously known AAA or a history of AAA surgery were included in the analysis. |
| 51 | Svensjo S, Bjorck M, and Wanhainen A (2014) Editor’s choice: five-year outcomes in men screened for abdominal aortic aneurysm at 65 years of age: a population-based cohort study. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 47(1), 37–44 [PubMed: 24262320] | Wrong study design: cohort study |
| 52 | Takagi H, Umemoto T, and Group Alice (2015) A meta-analysis of circulating homocysteine levels in subjects with versus without abdominal aortic aneurysm. International angiology : a journal of the International Union of Angiology 34(3), 229–37 [PubMed: 24732583] | Systematic review of case-controls. |
| 53 | Takagi H, and Umemoto T (2015) A meta-analysis of the association of obesity with abdominal aortic aneurysm presence. International Angiology 34(4), 383–391 [PubMed: 24945917] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 54 | Takagi H, and Umemoto T (2015) A meta-analysis of the association of primary abdominal wall hernia with abdominal aortic aneurysm. International angiology : a journal of the International Union of Angiology 34(3), 219–28 [PubMed: 24643172] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 55 | Takagi H, and Umemoto T (2015) A contemporary meta-analysis of the association of diabetes with abdominal aortic aneurysm. International Angiology 34(4), 375–382 [PubMed: 24945920] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 56 | Takeuchi Hidemi, Okuyama Michihiro, Uchida Haruhito A, Kakio Yuki, Umebayashi Ryoko, Okuyama Yuka, Fujii Yasuhiro, Ozawa Susumu, Yoshida Masashi, Oshima Yu, Sano Shunji, and Wada Jun (2016) Chronic Kidney Disease Is Positively and Diabetes Mellitus Is Negatively Associated with Abdominal Aortic Aneurysm. PloS one 11(10), e0164015 [PMC free article: PMC5072712] [PubMed: 27764090] | Wrong study design: retrospective case-control |
| 57 | Thompson A R, Golledge J, Cooper J A, et al. (2009) Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. European Journal of Human Genetics 17(3), 391–394 [PMC free article: PMC2986176] [PubMed: 18854858] | Wrong study design: case-control |
| 58 | Tornwall M E, Virtamo J, Haukka J K, et al. (2001) Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology 12(1), 94–100 [PubMed: 11138827] | Wrong study design: cohort study |
| 59 | Ulug P, Powell J T, Sweeting M J, Bown M J, Thompson S G, and Group Swan Collaborative (2016) Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. The British journal of surgery 103(9), 1097–104 [PMC free article: PMC6681422] [PubMed: 27346306] | Conference abstract |
| 60 | van Laarhoven C J, Borstlap A C, van Berge Henegouwen, D P, et al. (1993) Chronic obstructive pulmonary disease and abdominal aortic aneurysms. European journal of vascular surgery 7(4), 386–90 [PubMed: 8359293] | Sample size less than 500 participants |
| 61 | van de Luijtgaarden , Koen M, Rouwet Ellen V, Hoeks Sanne E, Stolker Robert J, Verhagen Hence Jm, and Majoor-Krakauer Danielle (2017) Risk of abdominal aortic aneurysm (AAA) among male and female relatives of AAA patients. Vascular medicine (London, and England) 22(2), 112–118 [PubMed: 28429660] | Study employed multiple study designs. First a case-control study design was used to assess risk factors of people with confirmed AAA. Subsequently, first degree relatives of people with AAA were asked how many relatives they had with AAA. |
| 62 | Van Vlijmen-Van Keulen, C J, Pals G, et al. (2002) Familial abdominal aortic aneurysm: A systematic review of a genetic background. European Journal of Vascular and Endovascular Surgery 24(2), 105–116 [PubMed: 12389231] | Systematic review including studies which employed various study designs (including case-controls, screening studies and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 63 | Wang Lu, Djousse Luc, Song Yiqing, Akinkuolie Akintunde O, Matsumoto Chisa, Manson JoAnn E, Gaziano J Michael, and Sesso Howard D (2017) Associations of Diabetes and Obesity with Risk of Abdominal Aortic Aneurysm in Men. Journal of obesity 2017, 3521649 [PMC free article: PMC5343258] [PubMed: 28326193] | Wrong study design: cohort study in which participants were not screened. Instead investigators ascertained the presence or absence of AAA by asking patients to complete a self-reported questionnaire. |
| 64 | Wang Yunpeng, Shen Guanghui, Wang Haiyang, Yao Ye, Sun Qingfeng, Jing Bao, Liu Gaoyan, Wu Jia, Yuan Chao, Liu Siqi, Liu Xinyu, Li Shiyong, and Li Haocheng (2017) Association of high sensitivity C-reactive protein and abdominal aortic aneurysm: a meta-analysis and systematic review. Current medical research and opinion 33(12), 2145–2152 [PubMed: 28699805] | Systematic review of case-control studies |
| 65 | Wilmink Antonius B. M, Vardulaki Katerina A, Hubbard Catherine S. F, et al. Scott Alan P, and Quick Clive R. G (2002) Are antihypertensive drugs associated with abdominal aortic aneurysms?. Journal of vascular surgery 36(4), 751–7 [PubMed: 12368736] | Wrong study design: nested case-control |
| 66 | Wong DR, Willett WC, and Rimm Eric B (2007) Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. American journal of epidemiology 165(7), 838–45 [PubMed: 17215382] | Wrong study design: cohort study |
| 67 | Wong YYE, Flicker L, Yeap BB, McCaul KA, (2013) Is hypovitaminosis D associated with abdominal aortic aneurysm, and is there a dose-response relationship?. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 45(6), 657–64 [PubMed: 23602862] | Sample size less than 500 participants. Additionally, the risk factor (vitamin D levels) assessed in this study is not listed in the review protocol. |
| 68 | Xiong Jiang, Wu Zhongyin, Chen Chen, Wei Yingqi, and Guo Wei (2016) Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: A meta-analysis. International journal of cardiology 221, 484–95 [PubMed: 27414727] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 69 | Zarrouk M, Keshavarz K, Lindblad B, et al. (2013) APC-PCI complex levels for screening of AAA in patients with peripheral atherosclerosis. Journal of thrombosis and thrombolysis 36(4), 495–500 [PubMed: 23354969] | Multivariate or Cox regression was not performed. Instead, investigators performed linear regression to assess the relationship between activated protein C (APC) - protein C inhibitor (PCI) complex levels and aortic diameter |
Economic studies
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Appendix H. Expert testimony from National Abdominal Aortic Aneurysm Screening Programme
The Clinical Lead of the UK NHS AAA screening programme provided expert testimony to the committee in the form of a presentation. The presentation covered developments since the inception of the screening programme, advantages and disadvantages of screening, challenges faced, and plans for the future. The presentation slides can be found below:
Appendix I. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life-threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Opportunistic screening for abdominal aortic aneurysm.[J Med Screen. 1994]Opportunistic screening for abdominal aortic aneurysm.Derbyshire ND, Lindsell DR, Collin J, Creasy TS. J Med Screen. 1994 Oct; 1(4):220-2.
- Feasibility and Efficiency Study of a Population-Based Abdominal Aortic Aneurysm Screening Program in Men and Women in Spain.[Ann Vasc Surg. 2021]Feasibility and Efficiency Study of a Population-Based Abdominal Aortic Aneurysm Screening Program in Men and Women in Spain.Fite J, Gayarre-Aguado R, Puig T, Zamora S, Escudero JR, Solà Roca J, Bellmunt-Montoya S. Ann Vasc Surg. 2021 May; 73:429-437. Epub 2020 Dec 30.
- Comparison of three targeted approaches to screening for abdominal aortic aneurysm based on cardiovascular risk.[Br J Surg. 2016]Comparison of three targeted approaches to screening for abdominal aortic aneurysm based on cardiovascular risk.Jones GT, Hill BG, Curtis N, Kabir TD, Wong LE, Tilyard MW, Williams MJ, van Rij AM. Br J Surg. 2016 Aug; 103(9):1139-46.
- Review Population screening for abdominal aortic aneurysm: evaluating the evidence against screening criteria.[N Z Med J. 2012]Review Population screening for abdominal aortic aneurysm: evaluating the evidence against screening criteria.Nair N, Sarfati D, Shaw C. N Z Med J. 2012 Feb 24; 125(1350):72-83. Epub 2012 Feb 24.
- Review Abdominal aortic aneurysms.[Dan Med Bull. 2010]Review Abdominal aortic aneurysms.Lindholt JS. Dan Med Bull. 2010 Dec; 57(12):B4219.
- Risk factors for predicting presence of an abdominal aortic aneurysmRisk factors for predicting presence of an abdominal aortic aneurysm
Your browsing activity is empty.
Activity recording is turned off.
See more...