Postoperative surveillance after surgical repair of abdominal aortic aneurysms
Evidence review V
NICE Guideline, No. 156
Postoperative surveillance after surgical repair of abdominal aortic aneurysms
Review questions
How frequently should people be monitored for postoperative complications, further aneurysm expansion and aneurysm rupture after EVAR or open repair of an abdominal aortic aneurysm?
Is tailored surveillance more effective than generalised surveillance in monitoring for postoperative complications, further aneurysm expansion and aneurysm rupture after EVAR or open repair of an abdominal aortic aneurysm?
Introduction
Review question 27 aims to determine appropriate intervals for monitoring people who have undergone surgical repair of an abdominal aortic aneurysm (AAA); that is, how frequently people should be monitored to detect complications (endoleak, graft migration, graft kinking, incisional hernia, graft occlusion and aortic neck expansion), further aneurysm expansion and aneurysm rupture.
Review question 29 aims to determine whether tailored surveillance or generalised surveillance is more effective in monitoring for postoperative complications, further aneurysm expansion and aneurysm rupture after EVAR or open repair of an AAA.
PICO tables
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A broad search strategy was used to gather all studies that examine the diagnosis, surveillance or monitoring of AAAs. This was a ‘bulk’ search that covered multiple review questions. The reviewer sifted the database to identify all studies that met the set of criteria outlined in Tables 1 and 2, with the full protocols given in Appendix A.
Table 1
Inclusion criteria for review question 27: frequency of postoperative monitoring.
Table 2
Inclusion criteria for review question 29: tailored or generalised postoperative surveillance.
Study were considered for inclusion if they were systematic reviews, randomised controlled trials or quasi-randomised controlled trials comparing different intervals for monitoring postoperative outcomes of AAA repair or trials comparing tailored and generalised surveillance strategies for monitoring postoperative complications. In the absence of preferred study designs, non-randomised controlled trials and prospective cohort studies with sample sizes of 500 participants, or more, were included.
Studies were excluded if they:
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed
Clinical evidence
Included studies
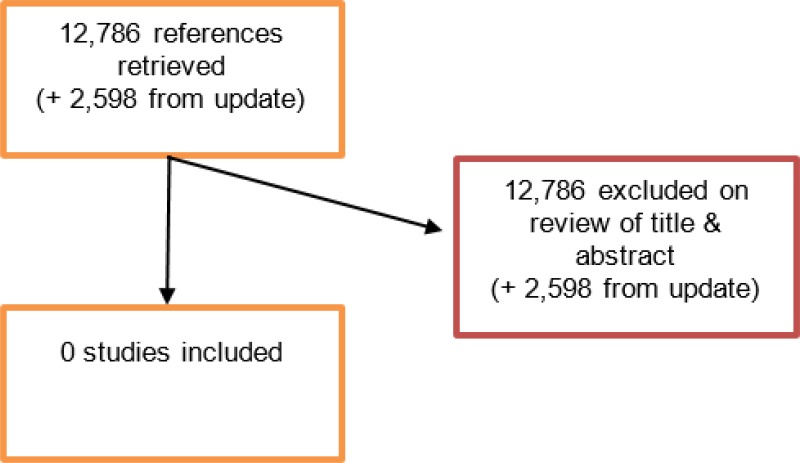
An initial literature search produced a database of 12,786 abstracts. None were identified as being potentially relevant to review question 27 or 29.
An update search was conducted in December 2017, to identify any studies published during guideline development. The search found 2,598 abstracts; all of which were not considered relevant. As a result no additional studies were identified.
Excluded studies
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Economic evidence
Included studies
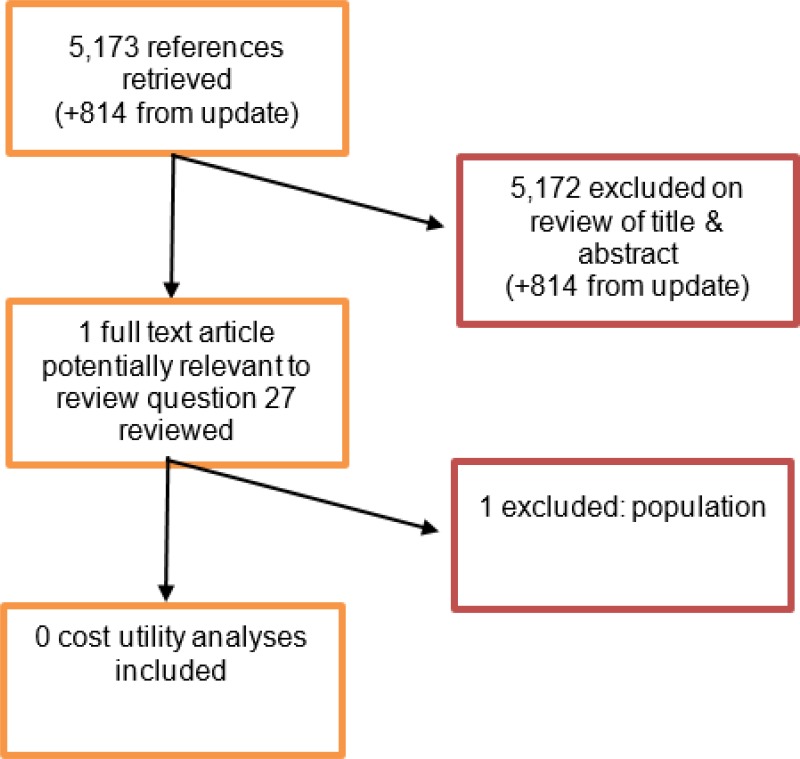
A literature search was conducted jointly for all review questions in this guideline by applying standard health economic filters to a clinical search for AAA. This search returned a total of 5,173 citations. Following review of all titles and abstracts, 1 related to review question 27 was identified as being potentially relevant. Upon examination of the full study manuscript, this study was not considered relevant for inclusion. No studies were identified as being potentially relevant to review question 29.
An update search was conducted in December 2017, to identify any relevant health economic analyses published during guideline development. The search yielded 814 abstracts; all of which were not considered relevant. As a result no additional studies were identified.
Excluded studies
One study was excluded following full text review.
Evidence statements
No evidence was identified.
Research recommendations
RR11. What are the risks, benefits and cost implications of different surveillance protocols in people who have undergone EVAR?
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The outcomes which matter most are all-cause morbidity and mortality, aneurysm-related morbidity and mortality, as well as resource use.
The quality of the evidence
The committee was unable to make recommendations on specific imaging surveillance intervals because there was no evidence from clinical trials. As a result, generic recommendations were made to highlight that it is important for clincians to consider each patient’s level of risk of postoperative complications and adjust surveillance protocols accordingly.
The committee discussed whether a maximum surveillance duration should be specified in the recommendations. It was noted that there is a long-term incidence of complications and reintervention after 5 years, although the incidence is relatively small. Given the lack of evidence the committee decided not to specify a surveillance duration and leave it to the discretion of the clinician, while recommending further research to clarify the issue.
Benefits and harms
The committee discussed whether recommendations were needed if postoperative surveillance is already being performed. However it was highlighted that not enrolling people who had undergone EVAR into a surveillance programme would be considered bad clinical practice. Therefore, the committee thought it was important to make an informal consensus recommendation to ensure that this does not happen.
The committee discussed whether to specify which complications would warrant changing surveillance protocols. It was noted that endoleak was the main complication that clinicians would be mindful of; however, other potential risks included aneurysm neck angle, diameter, shape and length, graft kinks and the potential for graft slippage. The committee also acknowledged that comorbidities could lead to alterations to surveillance protocols. With so many situations in which surveillance protocols could be amended, it was agreed that it is not possible to make extensive recommendations on every possible scenario.
The committee discussed the potential risk of over-treating people who have minor-to-moderate postoperative complications with surgical or endovascular reintervention, which may cause further harm. The committee noted that overtreatment is an potential risk but that, in the absence of clear natural history data for some imaging-identified complications, it is not possible to identify all people who are at risk of overtreatment in advance. In general, it was considered that benefits of treating EVAR-related complications outweigh the risks, so surveillance is justified.
It was noted that postoperative surveillance may impact on the psychological health of people who have undergone EVAR: some people would feel reassured by being entered into a surveillance programme whereas others would feel stressed by a repeated reminder that complications could arise after surgery. As a result, it was considered important to encourage research exploring patients’ attitudes and experiences after EVAR and comparing them with those who receive open surgical repair.
Cost effectiveness and resource use
The committee believed that recommending that postoperative surveillance intervals are amended in accordance with each patient’s perceived level of risk is unlikely to impact on cost effectiveness. This is because the recommendations assert what is generally being done in clinical practice.
Other factors the committee took into account
The committee discussed whether there was a need to make recommendations about tailoring imaging modalities and agreed that it was not necessary as recommendations about postoperative monitoring are covered elsewhere in the guideline (see evidence review W). The committee recognised the general lack of experience dealing with complex aneurysm morphologies but felt unable to recommend specific surveillance frequencies given the wide variation in practice and lack of evidence.
The committee discussed whether different recommendations were appropriate for women compared with men. It was agreed that the same need to monitor postoperative complications applied to both groups. Furthermore, the committee agreed that surveillance intervals for women are just as variable as those for men. As a result, no specific recommendation was made in relation to women.
Appendices
Appendix A. Review protocols
Review protocol for how frequently people should be monitored to detect complications
Table
Systematic reviews of study designs listed below Randomised controlled trials
Review protocol for risk factors for whether tailored surveillance or generalised surveillance is more effective in monitoring for postoperative complications, further aneurysm expansion and aneurysm rupture after EVAR or open repair
Table
Systematic reviews of study designs listed below Randomised controlled trials
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review questions 27 and 29
Table
Medline Strategy, searched 13th April 2016 Database: Ovid MEDLINE(R) 1946 to March Week 5 2016
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
Appendix E. Excluded studies
Clinical studies
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Economic studies
Appendix F. Research recommendations
Appendix G. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.