Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population:
Adults and children with a primary or recurrent episode of Clostridioides difficile infection (CDI).
Objectives:
- Provide a brief overview of the epidemiology of, and risk factors for development of CDI.
- Provide guidance regarding which patients should be tested for CDI, summarize merits and limitations of available diagnostic tests, and describe the optimal approach to laboratory diagnosis.
- Review the most effective treatment strategies for patients with CDI including patients with recurrences or complications.
Key Points for Adult Patients:
Diagnosis
Definitive diagnosis of CDI requires either the presence of toxigenic C. difficile in stool with compatible symptoms, or clinical evidence of pseudomembranous colitis (Table 2, Figure 4).
Once identified, CDI should be classified according to severity (Table 3).
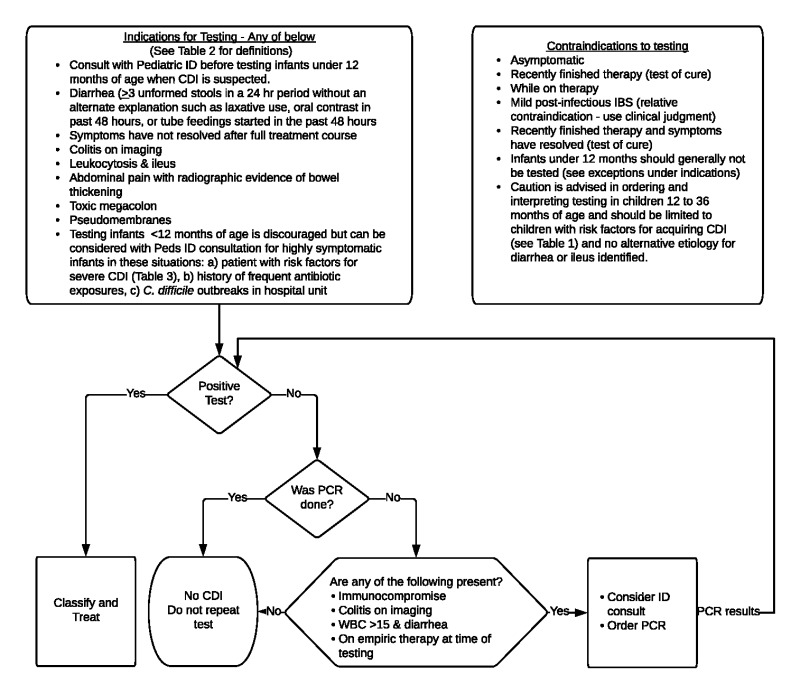
Although risk factors for CDI (Table 1) should guide suspicion for CDI, testing should be ordered only when indicated (Figure 1). Use judgment and consider not testing in patients that have recently started tube feeds, are taking a laxative medication, or have recently received oral radiologic contrast material. [IC]
Choice of test should be guided by a multi-step algorithm for the rapid diagnosis of CDI (Figure 2). [IIC]
Single-step PCR testing (not part of the UMHS algorithm) occurs as part of the new Biofire test panel for gastrointestinal pathogens and should not be used if only CDI is suspected. [IIIB] If C. difficile is detected as part of this panel and the patient’s symptoms are compatible with CDI, then treatment is appropriate and additional testing is unnecessary. [IIC]
Patients who are asymptomatic, actively being treated or completed treatment for CDI with clinical improvement in symptoms, or have post-infectious irritable bowel syndrome after CDI should not undergo testing for CDI. [IIIC]
Treatment:
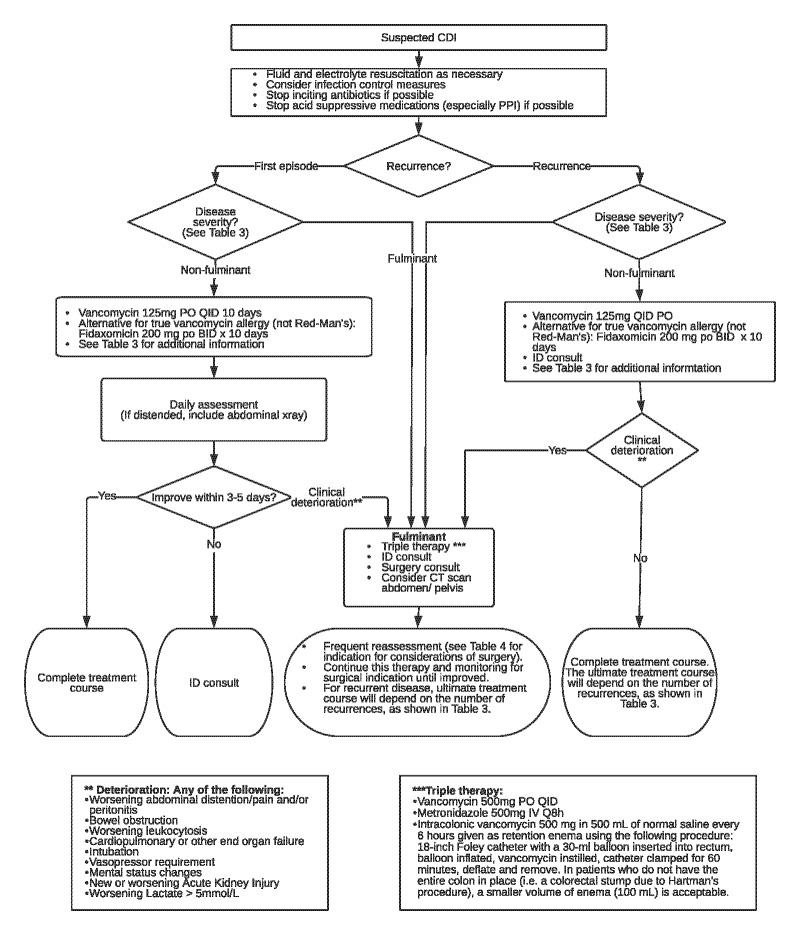
Treatment of CDI is based on classification of the disease by severity (eg, fulminant or non-fulminant) and number of recurrences (eg, first episode, first recurrence, second recurrence, etc.) (Figure 3 and Table 3).
In some cases, surgical consultation is recommended in the management of CDI (Table 4). The surgical management strategy is summarized in Figure 5.
Hospitalized patients with diarrhea should be placed in contact isolation if CDI is likely, as shown in Table 5.
Special considerations for recurrent disease are shown in Table 3.
Key Points for Patients ≤18 Years of Age:
Diagnosis
The decision to test children for CDI is complicated given a high rate of asymptomatic carriage, especially in infants <12 months of age. Although risk factors for CDI should guide suspicion for CDI, testing should be ordered only when indicated (Figure 1). [IC] Indications and contraindications for testing pediatric patients are included in Figure 1, but testing is rarely indicated or recommended for infants <12 months [IIC] and consultation with pediatric ID is strongly recommended. Testing symptomatic infants <12 months of age may be appropriate in certain situations: highly symptomatic infants with Hirschsprung’s disease or other gut motility disorder; history of bowel surgery; or in situations with outbreaks of CDI in a closed unit (eg, NICU). Children from 12 months to 36 months of age may be diagnosed with CDI if no alternative etiology for diarrhea is identified and with positive diagnostic testing. [IIC]
Definitive diagnosis of CDI requires either the presence of toxigenic C. difficile in stool with other symptoms, or clinical evidence of pseudomembranous colitis (Table 2). Choice of test should be guided by a multi-step algorithm for the rapid diagnosis of CDI (Figure 2). Single-step PCR testing (not part of the UMHS algorithm) occurs as part of the new Biofire test panel for gastrointestinal pathogens and should not be used if CDI is suspected. [IIIE] If C. difficile is detected as part of this panel and the patient’s symptoms are compatible with CDI, then treatment is appropriate and additional testing is unnecessary. [IIC] Patients who are asymptomatic, actively being treated or completed treatment for CDI with clinical improvement in symptoms, or have post-infectious irritable bowel syndrome after CDI should not undergo testing for CDI. [IIIC]
CDI should be classified according to severity (Table 3B). Certain pediatric conditions are associated with severe CDI and should be treated as such (see Table 3B). [IIC]
Treatment:
(See Figure 3 for general strategy and Table 3B for pediatric-specific classification and dosing recommendations)
Mild-Moderate CDI: Patients that do not meet criteria for “severe” or “fulminant” CDI are considered to have mild-moderate disease
- Metronidazole 7.5 mg/kg/dose PO QID for 10 days, maximum 500 mg/dose [IIC], OR
- In patients with metronidazole allergy, pregnant, nursing, or on warfarin therapy, or who fail to improve after 3–5 days of PO metronidazole therapy: vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg/dose x 10 days. [IIC]
Severe CDI: Patients with ≥2 lab criteria (WBC ≥15K, Cr ≥1.5x baseline, ANC ≤500, Albumin ≤2.5) OR ANY high-risk condition with Hirschsprung’s Disease or other intestinal dysmotility disorder, neutropenia from leukemia or other malignancy, inflammatory bowel disease, SOT/BMT <100 days, are considered to have severe disease
- vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg/dose x 10 days [IB]
Fulminant CDI: Patients with septic shock, ICU admission within 2 days of CDI diagnosis, surgery related to CDI diagnosis, ileus, toxic megacolon, peritonitis, or bowel perforation are considered to have fulminant disease
- Triple therapy: Vancomycin up to 500 mg PO QID, metronidazole 7.5 mg/kg/dose IV Q6H up to 500 mg/dose, and vancomycin enema 10–20 mL/kg/dose up to a maximum volume 500 mL/dose every 6 hours. For pediatric enemas, use vancomycin 500 mg/L solution. If tolerated, 20 mL/kg/dose, (max 500 mL/dose) every 6 hours is preferred, however in a patient with additional administration considerations, a minimum of 10 mL/kg/dose every 8 hours should be used. Enemas are indicated in patients with ileus, bowel obstruction or toxic megacolon; bowel perforation is a contraindication to enema therapy [IC]
- Consult Pediatric Infectious Diseases
- Consult Pediatric Surgery to assist in management including possible surgical intervention (Table 4)
Recurrent CDI: Recurrent symptoms and positive testing for toxigenic C. difficile within 8 weeks of prior episode.
First recurrence:
- Classify as “mild-moderate,” “severe,” or “fulminant,” and treat similarly to initial episode. If vancomycin was used as initial treatment, then use tapering vancomycin course with vancomycin 10 mg/kg/dose, up to maximum 125 mg PO QID x 14 days, then taper over 5–11 weeks (Table 3B)
- If vancomycin taper cannot be performed, then consider fidaxomicin 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days; Pediatric ID approval is required for use of fidaxomicin
Second or multiple recurrences (third or more episode of CDI):
- Consult Pediatric Infectious Diseases
- Fidaxomicin 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days
- Repeat vancomycin taper, plus a fidaxomicin “chaser” 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days
- Consider fecal microbiota transplant
Table 1.
Risk Factors for CDI
| Past history of CDI |
| Current or recent antibiotic use (highest risk within 3 months of exposure) |
| Advanced age (65 or older) |
| Severe comorbid disease(s) |
| Hospitalization within 30 days |
| Inflammatory bowel disease (IBD |
| Immunosuppressed state and use of immunosuppressive drugs |
| Acid suppressive therapy (especially, proton pump inhibitors) |
| Additional risk factors specific to patients ≤ 18 years of age |
| Patients with history of prematurity, prolonged or frequent hospitalizations, or history of frequent or recent antimicrobial therapy Patients with Hirschsprung’s disease, other intestinal dysmotility disorder, or history of abdominal surgery, including gastrostomy or jejunostomy tubes |
Note: Community-associated CDI1 can be seen in low risk population or in patients without traditional risk factors.
Figure 1.
Indications and Contraindications for CDI Testing
CDI: Clostridioides difficile infection; IBD: inflammatory bowel disease; ID: infectious disease (service); PCR: polymerase chain reaction; WBC: white blood cell count.
Table 2.
Definition of Clostridioides difficile Infection (CDI)
| Requires either of the following: |
| 1. A positive laboratory result for presence of toxigenic C. difficile in the stool |
| PLUS, any of the following symptoms compatible with CDI |
| • Diarrhea (>3 unformed stools in a 24-hour period without an alternate explanation such as laxative use, recent tube feed initiation, or recent administration of oral contrast) |
| • Radiographic evidence of ileus without alternate explanation (especially if leukocytosis present [WBC >15,000 cells/mm3]) |
| • Abdominal pain with radiographic evidence of bowel thickening |
| • Radiographic evidence of toxic megacolon |
| OR |
| 2. Colonoscopic or histopathologic evidence of pseudomembranous colitis. |
CDI: Clostridioides difficile infection; WBC: white blood cell count.
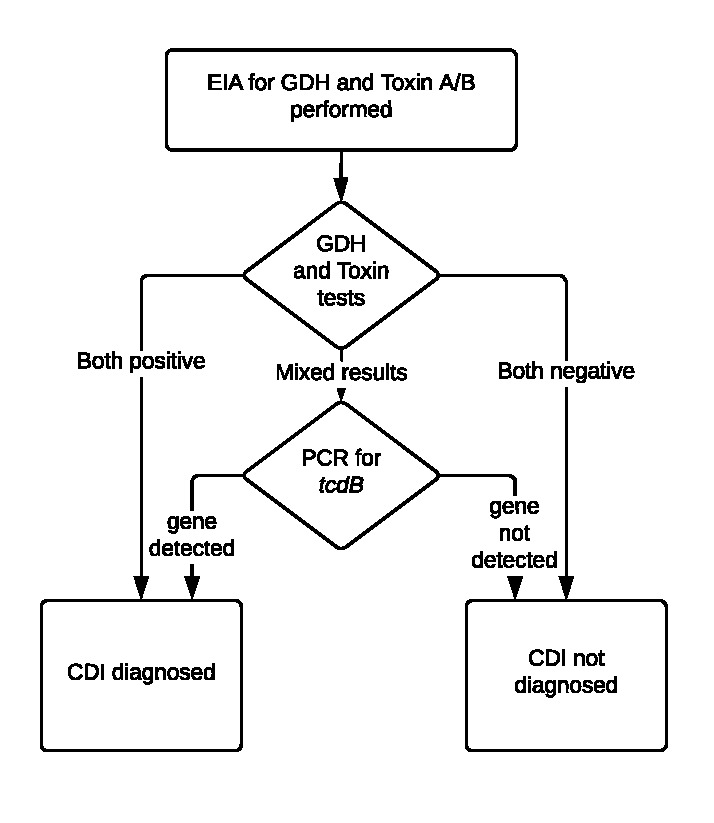
Figure 2.
University of Michigan Health System Multistep Algorithm* for the Rapid Diagnosis of C. difficile Infection
CDI: Clostridioides difficile infection; EIA: enzyme immunoassay; GDH: glutamate dehydrogenase; PCR: polymerase chain reaction.
* Adapted under Creative Commons License from Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridioides difficile Infection. PLoS ONE. 2014;9(3):e92578.
Table 3A.
Classification and Treatment of Clostridioides difficile Infection in Adults
| Disease Severity | Initial Episode1 | First Recurrence1 | Second Recurrence1 |
|---|---|---|---|
| Non-Fulminant Disease (Disease is considered non-fulminant when none of the criteria for fulminant disease are present) | Consider Infectious Diseases and Surgery consultation in patients with severe disease (defined as WBC ≥15,000, absolute neutrophil count ≤500, or SCr ≥1.5 times premorbid level) Vancomycin 125mg PO QID x 10 days2 True vancomycin allergy (not Red-Man’s): Fidaxomicin 200mg PO BID x 10 days Alternative if Vancomycin and Fidaxomicin cannot be used: Metrondiazole 500 mg po TID x 10 days | Infectious Diseases consultation is recommended If metronidazole was used for the initial episode: Vancomycin 125mg PO QID x 10 days2 If vancomycin was used for the initial episode: Vancomycin 125 mg PO QID x 14 days then taper over 5–11 weeks5 Alternative if vancomycin taper cannot be performed: Fidaxomicin 200mg PO BID x 10 days | Infectious Diseases consultation is strongly recommended If vancomycin taper was NOT used for the first recurrence: Vancomycin 125 mg PO QID x 14 days then taper over 5–11 weeks5. If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases6:
|
| Fulminant Disease (Disease is considered fulminant when any of these criteria are present: hypotension or septic shock, ileus, bowel obstruction, toxic megacolon, bowel perforation or peritonitis) | Surgery and Infectious Diseases consultation are strongly recommended Vancomycin 500mg PO QID + Metronidazole 500mg IV TID3 If ileus, bowel obstruction, or fecal diversion, add Vancomycin by enema every 6 hours4 Duration: Minimum of 14 days of therapy, depending on clinical response | Surgery and Infectious Diseases consultation are strongly recommended Same therapy as for initial episode then taper vancomycin over 5–11 weeks5 Alternative if vancomycin taper cannot be performed: Fidaxomicin 200mg BID x 10 days | Surgery and Infectious Diseases consultation are strongly recommended If vancomycin taper was NOT used for the first recurrence: Repeat primary therapy then taper vancomycin over 5–11 weeks5. If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases6:
|
- 1.
C.difficile colitis recurrence is defined as recurrent symptoms and positive testing (after initial resolution) ≤8 weeks from the start of the original episode
- 2.
Randomized trials have all utilized 10-day durations of therapy. Extension of course to 14 days may be considered in patients who have not had symptom resolution by day 10.
- 3.
Parenteral administration of metronidazole has poor intraluminal penetration and should not be used alone for treatment. Parenteral vancomycin has no significant luminal accumulation and should not be used for C. difficile treatment.
- 4.
Intracolonic vancomycin 500 mg in 500 mL of normal saline every 6 hours given as retention enema using the following procedure: 18-inch Foley catheter with a 30-ml balloon inserted into rectum, balloon inflated, vancomycin instilled, catheter clamped for 60 minutes, deflate and remove. In patients who do not have the entire colon in place (ie a colorectal stump due to Hartman’s procedure), a smaller volume of enema (100 mL) is acceptable.
- 5.
Alternative and/or adjunctive agents:
- Vancomycin tapers should begin after the treatment course is completed. Example of PO vancomycin taper: 125mg PO BID x 7 days, then 125mg PO daily x 7 days, then 125mg PO every other day x 7 days, then 125mg PO every 3 days x 2–8 weeks. Patients on tapered doses of PO vancomycin should continue to be monitored for signs and symptoms of C. difficile disease.Rifaximin ‘chaser’: Vancomycin 125mg PO QID x 10 days followed by Rifaximin 400mg TID x 20 days
- Kefir staggered protocol: Vancomycin 125mg QID x 2 weeks, 375mg Q72h x 2 weeks, 250mg Q72h x 2 weeks, and 125mg Q72h x 2 weeks PLUS kefir (5-oz glass with each meal (at least 3 glasses per day)) for 15 weeks.
- Fecal microbiota transplantation (FMT) is a highly effective option for patients with recurrent CDI. Michigan Medicine uses stool preparations obtained from OpenBiome to perform FMTs in both the inpatient and outpatient settings. Patients with recurrent CDI (defined as having two or more episodes) or CDI not responsive to standard pharmacologic therapies by day 5 may be considered for FMT. Patients with hypotension or shock, ileus, megacolon, severe sepsis, peritonitis, or bowel perforation attributed to CDI are generally not candidates for FMT. For inpatient use, infectious diseases and gastroenterology consultation are required.
- The role of probiotics in prevention and treatment of C. difficile colitis is unclear, and their use is not currently recommended for inpatients. Avoid the use of probiotics in immunocompromised patients (transplant recipients, unintact gut mucosa, neutropenic patients, HIV/AIDS patients, etc.) and patients with severe C. difficile colitis.
- Cholestyramine binds PO vancomycin and may decrease its efficacy. Avoid concomitant use.
Figure 3.
Clostridioides difficile Infection Treatment Overview for Adult Patients
Note: Doses indicated are for adult patients. For pediatric-specific dosing recommendations see Table 3B. BID: twice per day; CDI: Clostridioides difficile infection; CT: computed tomography; ID: infectious disease (service); IV: intravenous; PO: by mouth; QID: four times per day; TID: three times per day.
Table 3B.
Classification and Treatment of C. difficile Infection in Patients ≤18 Years of Age
| Disease Severity | Initial Episode1 | First Recurrence1 | Second Recurrence1 |
|---|---|---|---|
| Mild-Moderate (Disease is considered Mild-Moderate when the criteria for severe or fulminant disease are not met) | Metronidazole 7.5 mg/kg/dose PO QID for 10 days2, maximum 500 mg/dose OR Patients with metronidazole allergy, pregnant, nursing, on warfarin therapy, or who fail to improve after 3–5 days of PO metronidazole: Vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 10 days2 | If metronidazole was used for the initial episode: Vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 10 days2, 5 If vancomycin was used for the initial episode: vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 14 days then taper over 5–11 weeks6. Alternative if vancomycin taper cannot be performed: Fidaxomicin 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days | Infectious Diseases consultation is recommended If vancomycin taper was NOT used for the first recurrence: vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 14 days then taper over 5–11 weeks6 If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases6:
|
| Severe (>2 abnormal lab values OR >1 high-risk condition)
| Vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 10 days2 | Infectious Diseases consultation is recommended If vancomycin was used for the initial episode: vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 14 days then taper over 5–11 weeks6 Alternative if vancomycin taper cannot be performed: Fidaxomicin 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days | Infectious Diseases consultation is strongly recommended If vancomycin taper was NOT used for the first recurrence: vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID x 14 days then taper over 5–11 weeks6 If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases6:
|
| Fulminant Disease (Any of the following criteria are present)
| Surgery and Infectious Diseases consultation are strongly recommended Vancomycin up to maximum 500 mg/dose PO QID + Metronidazole 7.5 mg/kg/dose IV Q6H, up to maximum 500mg per dose IV3 + Vancomycin retention enema for patients with ileus, bowel obstruction, or toxic megacolon.4 Suspected or known bowel perforation is a contraindication for rectal administration. Duration: Minimum of 14 days of therapy, depending on clinical response | Surgery and Infectious Diseases consultation are strongly recommended Repeat primary therapy course, then taper vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID over 5–11 weeks6 Alternative if vancomycin taper cannot be performed: Fidaxomicin 16 mg/kg/dose PO BID, max 200 mg per dose, x 10 days | Surgery and Infectious Diseases consultation are strongly recommended Repeat primary therapy course, then taper vancomycin 10 mg/kg/dose PO QID, up to maximum 125 mg PO QID over 5–11 weeks6. If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases6:
|
- 1.
C.difficile colitis recurrence is defined as recurrent symptoms and positive testing (after initial resolution) ≤8 weeks from the start of the original episode
- 2.
Randomized trials have all utilized 10-day durations of therapy. Extension of course to 14 days may be considered in patients who have not had symptom resolution by day 10.
- 3.
Parenteral administration of metronidazole has poor intraluminal penetration and should not be used alone for treatment. Parenteral vancomycin has no significant luminal accumulation and should not be used for C. difficile treatment.
- 4.
Intracolonic vancomycin, 10–20 mL/kg/dose, up to max 500 mL/dose, of a 500 mg/L solution in normal saline Q6H instilled by appropriately size Foley catheter inserted into rectum with balloon inflated and Foley clamped for 1 hour. Treatment naïve patients should be started at 10 mL/kg/dose Q6H and escalated as tolerated up to the preferred dose of 20 mL/kg/dose Q6H, max 500 mL. In a patient with additional administration considerations, a minimum of 10 mL/kg/dose Q8H should be used.
- 5.
Avoid multiple or prolonged courses of metronidazole in recurrent disease due to the risk for cumulative neurotoxicity.
- 6.
Alternative and/or adjunctive agents:
- 7.
Vancomycin tapers should begin after the treatment course is completed. Example of PO vancomycin taper: 125mg PO BID x 7 days, then 125mg PO daily x 7 days, then 125mg PO every other day x 7 days, then 125mg PO every 3 days x 5–11 weeks. Patients on tapered doses of PO vancomycin should continue to be monitored for signs and symptoms of C. difficile disease.
- 8.
Fecal microbiota transplantation (FMT) is an option for patients with recurrent CDI. Currently, this is only available on an outpatient basis at Michigan Medicine. Consult Pediatric Infectious Diseases if considering outpatient FMT as an option.
- 9.
The role of probiotics in prevention and treatment of C. difficile colitis is unclear, and their use is not currently recommended for inpatients. Avoid the use of probiotics in immunocompromised patients (transplant recipients, unintact gut mucosa, cancer patients, neutropenic patients, HIV/AIDS patients, etc) and patients with severe C. difficile colitis.
- 10.
Cholestyramine binds PO vancomycin and may decrease its efficacy. Avoid concomitant use.

Figure 4.
Imaging of “accordion sign” and “target sign”
Table 4.
| Surgical consultation is appropriate for C. difficile infected patients in these situations: |
| Any patient with fulminant CDI (see Table 3) |
| Any patient with CDI and clinical deterioration attributable to CDI, including the following: |
| • Worsening abdominal distention/pain and/or peritonitis |
| • Bowel obstruction |
| • Intubation |
| • Vasopressor requirement |
| • Mental status changes |
| • New or worsening Acute Kidney Injury |
| • Worsening Lactate > 5mmol/L |
| • Persistent or worsening leukocytosis (WBC ≥35,000 cells/mm3) |
| • Hirschsprung’s disease |
| Any patient with failure to improve with standard therapy within 5 days as determined by resolving symptoms and physical exam, resolving WBC/band count |
CDI: Clostridioides difficile infection; WBC: white blood cell count.
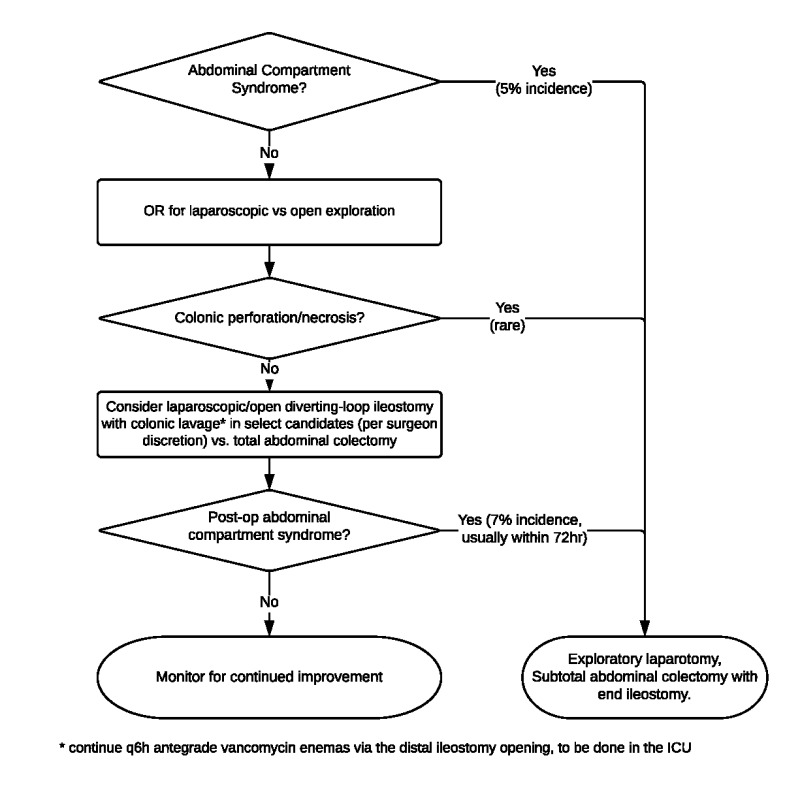
Figure 5.
Operative Management Strategy for CDI
Table 5.
Guidance on Preemptive Isolation for Patients with Diarrhea
| • Preemptive isolation should be considered if patients have diarrhea (3 or more watery stools in 12 to 24 hours) * not caused by laxative use, chemotherapy, enteral feeds or other medical causes AND AT LEAST ONE of the following: |
| • Current or prior antibiotic use (within 30 days) |
| • Significant abdominal pain, not caused by incisional pain, dyspepsia, or nausea |
| • History of C. difficile |
| • Suspect C. difficile |
| Place patient in room with curtain or door (NO HALL BEDS) |
| Place contact precautions-D sign on door/curtain |
- *
1L of colostomy output, >200mL of watery rectal bag output
Clinical Background
Clostridiodes difficile is a Gram-positive bacillus that can asymptomatically colonize the gastrointestinal tract or cause symptomatic disease through the production of cytotoxins TcdA and TcdB.2 Patients usually develop Clostridioides difficile infection (CDI) after exposure to antibiotics, and the severity of CDI can range from self-limited diarrheal illness, to a fulminant, life-threatening colitis.3 Even among those that recover, recurrent disease is common.4 Despite being first identified in 1978 as the causative agent of pseudomembranous colitis5,6 and subsequently garnering significant attention from the medical community, attempts to control C. difficile have not met with much success, especially in hospitals and other acute care settings.
The prior decade saw a significant increase in the incidence of CDI in the United States. In hospitals and nursing homes, there are now at least 450,000 new cases and 29,000 deaths per year.7–9 The increased burden of disease is largely attributable to the emergence of several strains, especially polymerase chain reaction (PCR) ribotype 027, which led to a worldwide epidemic.10 Though CDI occurs in all age groups, infection with ribotype 027, as well as CDI in general, is most common in older adults.11
Thus, as the US population ages, the incidence of CDI and adverse outcomes will likely increase.11 Ninety-two percent of CDI-related deaths occur in adults of age 65 or older, where CDI is the 18th leading cause of mortality.12 The risk of recurrent CDI is 2-fold higher with each decade of life.4 The annual rate of CDI-related hospitalizations in those aged >85 years exceeds those of all other age groups combined.13 All of these epidemiologic trends contribute to an estimated $1.5 billion in excess healthcare costs each year due to CDI.14
Rationale for Recommendations
The increased incidence of CDI and its adverse outcomes among hospitalized patients, coupled with the availability of new therapies has complicated the management of CDI. This underscores the need for guidelines that review the evidence and provide recommendations for the prevention, diagnosis, and treatment of CDI in hospitalized patients.
Clinical Problem and Management Issues
Causes and Risk Factors
Risk factors for CDI are listed in Table 1 and elaborated below.
History of CDI.
As CDI can be recurrent, a history of CDI is predictive of CDI in patients presenting with diarrhea.
Antibiotic exposure.
Antibiotic use is one of the most significant yet modifiable risk factors for CDI. Number (dose dependent risk), class, and duration of antibiotic use (significantly higher risk if >7 days) impact the risk for CDI (See Antibiotic Section under Prevention and Treatment).
Advanced age.
Patients age 65 or older are at several fold higher risk for CDI.15 Older patients are also at higher risk for more severe disease with complications, especially if they have poor preadmission functional status.
Comorbid conditions, severity of disease and hospitalization.
Presence of severe underlying comorbidities has been associated with increased risk of CDI. Severity of comorbid conditions upon admission, and longer hospitalization have all been associated with higher risk for hospital-acquired CDI. Other risk factors for CDI also include gastrointestinal surgery and the use of tube feeds.16
Inflammatory Bowel Disease (IBD).
Patients with inflammatory bowel disease (IBD) are at higher risk for CDI compared to the general population. Thus, all patients with IBD presenting with diarrhea concerning for possible IBD flare should also undergo C. difficile testing, even in the absence of other risk factors for CDI. In a retrospective study, only 61% of IBD patients with CDI had antibiotic exposure.17 The risk in IBD patients is even higher if they have colonic involvement and they are on immunomodulator therapy.17
Immunosuppression.
Immunosuppressed states such as leukemia, lymphoma, HIV, neutropenia, organ transplantation, and use of immunosuppressive drugs significantly increase the risk for CDI. In HIV-infected patients, CDI is the most common cause for bacterial diarrhea and risk of CDI correlates with severity of HIV disease.18,19
Acid Suppressive Therapy.
Acid suppressive therapy, including use of proton pump inhibitors (PPIs), elevates the risk of CDI. (For expanded explanation, see “Proton Pump Inhibitors” section on Page 18).
Pediatric Population Risk Factors (<18 Years of Age)
Risk factors for acquiring CDI in pediatric populations include a history of prematurity, prolonged or frequent hospitalizations, history of antimicrobial therapy, solid organ transplantation, the presence of gastrostomy or jejunostomy tubes, and the use of proton pump inhibitors (see Table 1). Special attention to the importance of Hirschsprung’s disease, neutropenia from leukemia and other malignancies, and inflammatory bowel disease as high-risk factors for severe disease in children should be noted.20–23
Risks in community associated CDI.
Community-associated CDI has a different risk profile, and is less likely to be severe CDI. Community-associated CDI is defined by the Infectious Diseases Society of America as symptom onset occurring in the community or within 48 hours of hospital admission with no prior hospitalization in the past 12 weeks. Community-associated CDI affects populations that were previously thought to be at low risk such as younger adults without the traditional risk factors mentioned above. In a US population-based study involving 385 patients with CDI, 41% of cases met the criteria for community-associated CDI. Patients with community-associated infection were younger (median age of 50 years compared with 72 years), had lower comorbid scores, had less exposure to antibiotics (78% vs 94%), and were less likely to have severe infection.24
Diagnosis
Testing for C. difficile
Patients with diarrhea and risk factors for CDI should undergo testing for C. difficile (Figure 1).
The diagnosis of C. difficile infection (CDI) is based on the combination of both clinical findings (usually diarrhea), as well as laboratory or histopathological findings (Table 2) for definition of CDI. Clinical severity can range from mild diarrhea to severe, fulminant colitis with paralytic ileus and toxic megacolon. Patients can also have asymptomatic carriage or colonization of Clostridioides difficile.
The following signs and symptoms suggest patients who exhibit these features should be tested for the disease. The most common symptom of CDI is diarrhea, defined as 3 or more loose or unformed stool in less than 24 hours, without an alternative cause (eg, laxative use, oral contrast, tube feed initiation). The stool may contain occult blood, but melena and hematochezia are atypical. Fever and abdominal pain are present in about 50% of patients, and can be markers for increasing disease severity. Leukocytosis (WBC >15,000 cells/mm3) occurs frequently and may actually precede diarrhea or other clinical symptoms.
The presence of pseudomembranes on lower endoscopy is essentially pathognomonic for CDI, and occurs in 50% of cases. Endoscopy is not needed for diagnostics, however, as stool studies are readily available and active CDI renders a patient at increased risk for endoscopic complications including perforation.
Less common signs and symptoms of CDI include arthralgias and reactive arthritis, as well as severe protein-wasting diarrhea with resultant hypoalbuminemia, edema and ascites.
Contraindications to Testing for C difficile
Because diarrhea occurs in the majority of patients with CDI, individuals without diarrhea should usually not be tested for CDI (Figure 1). The prevalence of asymptomatic colonization with C. difficile is 10–26%25,26 among hospitalized patients and may approach 50% among patients residing in long-term care facilities. Test of cure should not be performed in patients who have finished treatment for CDI and have experienced clinical improvement in symptoms because they can have persistent shedding of toxin for up to 6 weeks after completing treatment.27 For the same reason, there is no indication for testing while an individual is being actively treated. Testing can be performed if symptoms have not resolved following a full treatment course. Finally, it is important to note that some individuals may take several weeks after completing therapy for stool consistency and frequency to become entirely normal. In addition, some individuals may develop a post-infectious irritable bowel syndrome after CDI, which can be difficult to differentiate from true recurrent CDI and clinicians must use their judgment. In this circumstance, clinicians should refrain from repeat testing for C. difficile.
Laboratory Testing Algorithms
Two- or three-stage testing algorithms are preferable to single-step methods.
The optimal rapid laboratory testing algorithm for presence of toxigenic C. difficile in stool has not been established, but there is evidence that two- or three-stage testing algorithms are preferable to single-step methods due to improved specificity.28
For UMHS testing algorithms see Figure 1 and Figure 2.
When possible, testing should be limited to diarrheal stools unless the suspicion for CDI is high and an ileus is present. In select patients (immunocompromised, ileus, or on empiric therapy) the sensitivity of other rapid tests can be low and ID consultation and PCR-based testing should be considered (Appendix A).
The gold standard for organism detection is cytotoxigenic culture and the corresponding gold standard for toxin detection is the cell cytotoxicity assay.29 Both methods require considerable time and expense and are now rarely performed by clinical laboratories.
An overview of symptoms consistent with CDI is provided in Figure 1. To avoid false-positive results (eg, positive C. difficile testing in the setting of colonization), only diarrheal stools (those that take the shape of the container) should be submitted for testing. Laxatives, tube feed initiation, and oral contrast can lead to loose or liquid stools prompting testing for C. difficile. C. difficile testing when there is an alternative cause of diarrhea may lead to identification of C. difficile colonization rather than true infection, and treatment of colonization is not recommended as it is not helpful and may even increase shedding of spores.
An exception to this is the case of patients with suspected CDI and ileus, in which case stool of any consistency can be considered for testing. If a patient with ileus and suspected CDI is unable to produce stool, a rectal swab for tcdB PCR can be sent for after consultation with ID. If a patient has an ileostomy with higher then baseline output (without an alternative explanation) then a stool specimen can be sent for CDI testing. In patients where there is concern for CDI involving the rectal stump, a rectal swab for direct tcdB PCR may be sent after consultation with ID. (For PCR testing at UMHS, after electronically placing an order for a C. difficile test, phone the lab to request PCR only from the swab. See Appendix A for available diagnostic tests for toxigenic C. difficile).
The recommended UMHS testing algorithm consists of two initial EIA tests (GDH and Toxins A/B), with reflex to a PCR test for tcdB gene for discordant results (Figure 2). Data and recent guidelines support the use of multi-step over single-step testing with EIA for toxigenic C. difficile due to improved test characteristics, though single-step testing via PCR also performed well.28–32 This algorithm has been validated by our clinical laboratory and has a negative predictive value of 99%.33
There are circumstances when false-negative results can occur with EIA testing alone. Immunocompromised patients with symptoms suggestive of CDI (colitis on imaging, ileus with minimal stool production, and/or WBC >15,000 cells/µL with diarrhea) and patients receiving empiric therapy at the time of diagnosis are at risk for a false-negative EIA test.34 In these patients, if PCR testing was not performed, an ID consult and direct PCR for tcdB on stool or via rectal swab should be considered (at UMHS, ordered separately by phone). Finally, single-step PCR testing (not part of the UMHS algorithm) occurs as part of the new BioFire test panel for gastrointestinal pathogens (FilmArray, BioFire Diagnostics Inc., Salt Lake City, UT). This test should not be used if CDI is suspected, and providers should follow the recommendations in Figure 2 and order multi-step testing as indicated. If C. difficile is detected as part of the Biofire panel and the patient’s symptoms are compatible with CDI, then a separate order for multi-step testing is unnecessary and providers should refer to our treatment algorithm (Figure 3) and begin therapy as indicated.29
Imaging.
In patients with abdominal distention and suspicion for CDI, radiologic evaluation may serve as a useful diagnostic adjunct. This is especially true if there is a concern for CDI-induced ileus or toxic megacolon. Plain film abdominal x-rays may show dilated colon or ileus pattern. If free air is present on x-ray imaging, emergent surgical consult is warranted.35,36
In patients who present with abdominal pain, significant abdominal distention, or other signs of fulminant CDI, computed tomography (CT) of the abdomen and pelvis may be considered for further evaluation. Findings on CT may include colonic wall thickening, ascites, megacolon (distension of the colon of >6 cm in transverse width), ileus or perforation.37 Overall CT sensitivity for diagnosis of C. difficile colitis is 52–85%, with specificity of 48–93%.38
The use of enteral, intravenous and rectal contrast is preferred by UMHS Acute Care Surgery group, unless otherwise contraindicated. Some institutions advocate a more rigid adherence to CT scan diagnostic criteria for C. difficile colitis of colon wall thickening of greater than 4 mm combined with any one or more findings of pericolonic stranding, colon wall nodularity, the “accordion” sign (alternating edematous haustral folds separated by transverse mucosal ridges filled with oral contrast material, simulating the appearance of an accordion39), or otherwise unexplained ascites, with a reported sensitivity of 70% and specificity of 93%38 (Figure 4).
Patients who have CT findings concerning for severe or fulminant CDI, or who are critically ill with documented severe CDI warrant early surgical consultation. Specific findings have not been shown to reliably predict the need for surgical intervention.38,40 However, early involvement of a general surgeon may initiate discussions of treatment options, including consideration for diverting loop ileostomy for antegrade colonic irrigation. (Refer to section on surgical management, Figure 5).
Endoscopy.
Colonoscopy may be useful in patients with persistent diarrhea despite negative C. difficile toxin or with toxin-positive CDI refractory to antibiotics. In patients with positive stool testing for CDI, a colonoscopy is not necessary for diagnosis given that pseudomembranes are present only in 50% of patients with toxin-positive CDI.41 Additional indications to perform a colonoscopy in toxin-positive CDI patients include the assessment of CDI severity and the management of severe colonic distension associated with ileus. It is worth noting that a negative flexible sigmoidoscopy does not rule out CDI as sparing of the rectosigmoid colon is common in CDI patients with pseudomembranes on colonoscopy.42
Colonoscopy is contraindicated, especially for diagnostic purposes, in patients with hemodynamic instability or with significant risk for bowel perforation (eg, fulminant colitis, recent bowel surgeries, bowel obstruction).
Differential Diagnosis of Diarrhea.
C. difficile-toxin negative patients with persistent diarrhea should be evaluated further with colonoscopy with random biopsies and esophagogastroduodenoscopy (EGD), with duodenal biopsies for inflammatory and non-inflammatory causes of persistent diarrhea. Inflammatory diarrhea includes inflammatory bowel disease (ulcerative colitis and Crohn’s disease), celiac disease, microscopic colitis (collagenous and lymphocytic colitis), CMV (in immunocompromised hosts), and routine enteric pathogens when patients have exposure history or risk factors. Non-inflammatory causes include, dietary intolerance (lactose, fructose, or rapidly fermentable, short-chain carbohydrates [“FODMAP”43]) or small intestinal bacterial overgrowth in patients with significant abdominal bloating. Functional etiologies (irritable bowel syndrome) should be considered when workup is negative for inflammatory and non-inflammatory diarrhea.
Disease Classification
Criteria for disease classification are summarized in table 3, and discussed below.
Though risk factors for adverse outcomes after CDI have been identified, there is no generally accepted and validated definition for severe CDI. Though the treatment is not different between patients with mild disease and those who have one or more severity criteria, but do not meet criteria for fulminant disease, it is still important to closely monitor them for progression to fulminant disease. It is important to classify the episode as fulminant or non-fulminant prior to initiation of CDI therapy. It is also important to determine whether the CDI episode represents a recurrence, because it influences the approach to therapy. Recurrent CDI often requires a longer duration of therapy (taper/pulse), and consideration of adjunctive treatment such as antimicrobial “chasers,” or fecal microbiota transplantation (FMT).
CDI can range from self-limited disease to severe infection, sometimes resulting in colectomy or death.3 Even among those that recover, recurrent disease is common.4 Although the reasons are incompletely understood, older adults are disproportionately affected by adverse outcomes.12
Despite these epidemiologic links, no robust, validated predictive models for the development of adverse outcomes from CDI or recurrence exist for clinical use. Several prediction models for severe disease outcomes based on initial diagnostic criteria have been proposed with variable sensitivity/specificity. Eight different published scores for severe disease have been compared at diagnosis to assess if they predicted complicated CDI in a validation cohort.36 The scores used a variety of clinical variables including age, medication use, symptoms, vital signs, physical exam findings, laboratory parameters, and abdominal radiographic changes. The agreement between predicted and observed outcomes was variable (Cohen’s κ 0.18–0.69). The “Hines VA” score had the highest κ but was only 73.7% sensitive. The classification criteria for CDI used in this guideline are presented in Table 3. These were drafted after considering published guidelines on CDI,1,29,36,37,44 and several studies on predictors of adverse outcomes and recurrence, and the expert opinion of this guideline committee’s membership.
This guideline’s definition for recurrence follows the CDC definition. Recurrent disease is defined as the presence of recurrent symptoms and positive testing within 8 weeks of initial onset (the index episode), but after the original symptoms resolved.1 There are situations, however, when patients will have another episode of CDI soon after this arbitrary 8-week window has elapsed. In such cases, it may not be unreasonable to classify these episodes as recurrent and treat accordingly (Figure 3). This is especially important in patients with risk factors for further recurrences, including the need for concurrent therapy with antimicrobials to treat condition other than CDI, age 65 or older, and use of proton pump inhibitors.4
Special Populations
Extra-colonic CDI
Small bowel enteritis secondary to C. difficile is rare, but usually occurs in patients with a partial or total colectomy. The literature on this topic consists primarily of case reports,45 making it difficult to recommend specific treatment strategies. However, outcomes in small bowel enteritis from CDI can be poor and, these patients warrant aggressive therapy in most cases (see fulminant CDI arm in Figure 3).
Inflammatory Bowel Disease
There is a high incidence of CDI in IBD patients.46–49 The most likely explanation is the shared risk factor of an altered gut microbiota, known as dysbiosis.50 Thus, workup of patients presenting with signs and/or symptoms of IBD flare should include testing for CDI. IBD patients at significant risk for CDI include those living in nursing homes, with recent or ongoing hospitalization, with previous broad-spectrum antibiotic use, and with a surgical pouch.51–54 Other risk factors include increased severity of colitis and immunosuppression (especially corticosteroids with 3-fold risk increase).17,47,55–57
Pediatrics (≤18 Years of Age)
CDI may be associated with significant morbidity and mortality in children.20,58 In general, the diagnosis of CDI in pediatric patients follows the same general diagnostic criteria and algorithms for adult patients as discussed above (Figure 3). The accurate diagnosis of CDI in infants and young children represents a special challenge given a high rate of asymptomatic C. difficile colonization in this population. Treatment decisions in this population should be made in conjunction with ID consultation. Please note the following age-specific recommendations for interpretation of testing results:
- Testing for CDI in infants <12 months of age is generally not recommended. It is recommended to consult Pediatric ID if CDI is suspected in infants <12 months of age. Testing should be limited to those highly symptomatic with frequent diarrhea and/or ileus who meet one or more of the following conditions:
- Have comorbidities associated with severe CDI (Table 3B)
- Have history of multiple antibiotic exposures
- C. difficile outbreak situations
Note: Loose stools in infants <12 months of age can be normal and identifying true diarrhea may be challenging. Infants fed breastmilk may pass six or more loose, seedy stools daily. Formula-fed infants often have fewer stools, typically thicker in consistency like peanut butter. After two months of age, stool output in most infants slows to one or two stools daily. Clues to identifying true diarrhea in infants include a significant change in the baseline quality of stools with increased frequency (especially ≥3 stools in <24 hours), increased looseness, contains blood and/or mucus, or has a strong foul-smell. Alternative etiologies for diarrhea should always be considered even in those infants <12 months of age with a positive test for CDI (eg, viral gastroenteritis, food allergy, carbohydrate malabsorption, immune-mediated condition, etc.). The multiplex nucleic acid amplification assay for GI pathogens (GI-Pan) can be helpful in identifying alternative infectious causes of diarrhea in young children. However, as this test also includes testing for CDI, it should only be sent if the patient is already known to be positive for CDI and testing is being performed to look for an alternative explanation, or in highly symptomatic infants with high risk conditions, in consultation with pediatric ID, for whom CDI treatment would be indicated if present. - Children 12 to 36 months of age may have asymptomatic colonization. A positive test result in a symptomatic patient indicates possible CDI. Alternative etiologies for diarrhea should continue to be considered even in those testing positive for CDI.
- Children >36 months of age who are symptomatic with diarrhea and/or ileus, with positive testing results suggests probable CDI, especially in those with risk factors including history of antibiotic therapy, use of proton pump inhibitors, or comorbidities associated with severe CDI (Table 1 and Table 3B).
Asymptomatic carriage of C. difficile varies significantly by age. A review of multiple studies of carriage in healthy infants and young children determined colonization of neonates up to 1 month of age occurs at an average of 37%, between 1 to 6 months of age at an average of 30%, between 6 to 12 months of age at an average of 14%, and by 36 months of age the carriage rate is similar to that of healthy, non-hospitalized adults at <3%.59 Carriage may be transient, and different strains of C. difficile may colonize an individual over time as new strains are acquired from the environment. Breastfed infants have lower C. difficile carriage rates than do formula-fed infants (14% vs. 30%, respectively), but these differences decrease after 12 months of age.59 Given the frequency of asymptomatic colonization, testing for CDI should only be performed in children with diarrhea and other risk factors for CDI.60
In highly symptomatic infants <12 months of age, conditions such as a history of Hirschsprung’s disease, gut motility disorder, bowel surgery, or possible CDI outbreaks in a closed unit (eg, NICU) could be considered valid indications for CDI testing, in coordination with Pediatric Infectious Disease consultation. Despite high rates of colonization and significant amounts of detectable toxin, clinical illness with CDI is relatively uncommon in children <36 months of age. Hypotheses regarding the lack of clinical illness in infants and young children include the possibility that neonates and infants lack cellular receptors to bind and process C. difficile toxin, preferential colonization by nontoxigenic or less-pathogenic strains, and protective factors in breast milk and the developing microbiota.21,59 Rates of hospitalized infants and young children with positive CDI testing are increasing, but many studies do not adequately distinguish CDI disease from asymptomatic colonization.21,22,61,62 It is critical to consider other etiologies for diarrhea in infants and young children, even in those with positive CDI testing. Consultation with a Pediatric Infectious Diseases specialist may be helpful when considering the need for treatment in young age groups.21
Pediatric Disease Classification (≤18 Years of Age)
In pediatric patients, a diagnosis of CDI may be considered based on a combination of signs and symptoms, generally involving frequent diarrhea, and evidence of C. difficile and toxin present in stool (see age-based interpretation of testing results above).
- In pediatric patients CDI is classified as mild to moderate when lab values are reassuring (eg, WBC <15,000 cells/mm3 and serum creatinine <1.5 times baseline level), similar to adult classification schemes (Table 3B).
- In patients failing to improve after 3 to 5 days of therapy, lab values should be repeated to determine if it is still appropriate to maintain classification as mild to moderate, or if the classification should be escalated to severe.
- In pediatric patients CDI is determined to be severe when ≥2 lab values are abnormal (eg, WBC ≥15,000 cells/mm3, creatinine >1.5 times baseline level, ANC ≤500, or ALB ≤2.5) OR the presence of high-risk patient factors for severe CDI exists (eg, Hirschsprung’s disease, neutropenia from leukemia, IBD). Patients with these high-risk factors should generally be classified as, and treated for, at least severe disease (see Table 3B).
- In pediatric patients CDI is determined to be complicated when evidence exists of sepsis, shock, ileus, toxic megacolon, peritonitis, bowel perforation, or other conditions requiring ICU admission within 2 days of CDI diagnosis are present (see Table 3B).
The majority of CDI in infants and children is of mild to moderate disease severity criteria.22,23,63 Applying the same criteria for adult severe disease to pediatric populations tends to overclassify pediatric disease as severe, hence the need for 2 or more abnormal lab criteria required to make a severe diagnosis (or the presence of a high-risk condition). The frequency of pediatric CDI patients meeting criteria for severe disease is low (~8%), with similar proportions of severe disease noted across all pediatric age groups.63
Prevention
Antibiotics and Prevention
Nearly all antimicrobial classes have been associated with CDI. However, clindamycin and cephalosporins (especially third-generation cephalosporins) have been consistently associated with the highest risk of CDI. After the emergence of the epidemic NAP1/ribotype 027 strain in 2002, fluoroquinolones, have also been associated with a high risk of CDI.64–66
The risk of CDI is highest during antibiotic therapy and in the first month after cessation of antibiotics (7 to 10 fold increased risk compared to patients who did not receive antibiotics). Risk appears to normalize 3 months after cessation of antibiotic therapy.67 The use of >14 Defined Daily Doses (DDDs) of antibiotics in the 3 months prior to CDI had the strongest association with CDI (OR 8.50;95% CI 4.56–15.9). Another study found the risk of CDI increases with cumulative dose and number of antibiotics, as well as days of antibiotic exposure.68 Poor clinical outcomes in patients with CDI were independently associated with concomitant use of non-CDI-related antimicrobials,69 and are associated with a doubling in risk of failure of CDI therapy.70 In addition, use of non-CDI-related antimicrobials within 30 days of an episode of CDI is associated with a 3-fold increase in CDI recurrence.71 Based on the results of these studies, it is imperative that providers stop unnecessary antibiotic therapy to reduce the risk of CDI.
Use of Prophylactic Vancomycin
Recommendations:
- Consider use of vancomycin prophylaxis in patients that recently had a first or greater recurrence of CDI and require antimicrobials for a different infection.
- The dose of prophylactic vancomycin is 125 mg PO BID and the duration of prophylaxis should be at least 50% of the expected duration of antibiotic therapy for the other infection.
There are several retrospective cohort studies evaluating the impact of secondary vancomycin prophylaxis on risk of CDI recurrence in patients with prior CDI and subsequent antibiotic exposure.70,71 One study found that prophylactic vancomycin reduced the risk of recurrent CDI by nearly 50% in patients on non-CDI antibiotics and a history of first or greater CDI recurrence in the 90 days prior to antibiotic exposure.72 Prophylactic vancomycin extending >50% of the non-CDI antibiotic duration was more effective than prophylaxis lasting <50% of the antibiotic duration. Similarly, another study found a reduction in recurrent CDI in patients receiving secondary oral vancomycin prophylaxis (dose range 125–250 mg PO BID). Patients had a CDI episode a mean of 6 months prior to antibiotic exposure and were continued on oral vancomycin up to 7 days after antibiotic discontinuation (mean 0.8 days). Due to insufficient evidence, current guidelines do not provide recommendations for secondary CDI prophylaxis. However, when secondary prophylaxis is utilized, a low doses of oral vancomycin (125 mg QD-BID) should be used while systemic antibiotics are given.1 Potential downsides of this strategy include selection for resistant organisms such as vancomycin-resistant Enterococcus, onset or worsening of antibiotic-associated diarrhea, and additional expense.
Probiotics
Probiotics are not routinely recommended as primary prevention of CDI in hospitalized patients. There is conflicting data on whether probiotics are effective in preventing primary episodes of CDI in patients receiving antibiotics, with the largest RCT to-date including over 2,000 patients failing to replicate possible reductions seen from meta-analyses of smaller, more heterogeneous trials.72 However, a recent meta-analysis, including over 8,000 patients suggests probiotics are effective in patients with a >5% baseline risk of CDI.75 This finding was replicated in a separate meta-analysis that focused only on hospitalized patients receiving antibiotics and included only RCTs with placebo controls.76 There are concerns regarding safety in patients with certain comorbid conditions (central venous catheters, immunocompromised- receiving chemotherapy, recipients of solid organ transplant or bone marrow transplant) which are common among hospitalized patients. The literature has noted invasive infections or poor outcomes with Lactobacillus probiotic use in immunocompromised patients,77–79 Saccharomyces boulardii80,81 probiotics, patients with severe acute pancreatitis,82 and patients with central venous catheters.83 At present, there is insufficient evidence to recommend the routine use of probiotics in primary prevention of CDI in hospitalized patients.
Proton Pump Inhibitors (PPIs)
PPIs and acid suppression may increase the risk of CDI and recurrence of CDI. Unnecessary use of PPIs should be avoided and acid suppression should be minimized, especially in patients with a history or current diagnosis of CDI. A systematic review of case-control and cohort studies has shown an association between PPIs and increased risk of CDI.4 Although a causal relationship is not clear, this finding is similar to prior systemic reviews.84A prospective cohort study showed that increasing levels of acid suppression correlated with increased risk of nosocomial CDI, with the highest risk in patients receiving greater than once daily PPI dosing.85
Infection Control Measures
Isolation.
Patients with diarrhea and a positive C. difficile lab test must be placed in Contact Precautions Diarrheal (CP-D). Patients are placed in either a private room or cohorted with another patient with CDI. CP-D rooms are disinfected with a sporicidal disinfectant on a daily basis and upon discharge. Hydrogen peroxide vapor technology is utilized to terminally disinfect rooms after discharge in most cases. Healthcare personnel must wear an isolation gown and gloves upon entry to the room. Healthcare personnel must remove gowns and gloves upon exiting the room and wash hands with soap and water. Any equipment leaving the room is disinfected with a bleach wipe.
Preemptive Isolation (Initiation of isolation for patients with diarrhea prior to test results)
CP-D is not required, but may be utilized at the discretion of Infection Prevention & Epidemiology (IPE) or the provider. (At UMHS, nursing may initiate this by working through the provider or IPE.) See Table 5 for further guidance on preemptive isolation.
Disinfection and hygiene.
Both the patient’s immediate environment and the broader environment have been implicated in the spread of C. difficile. Quaternary ammonia-based disinfectants have been shown to be ineffective against C. difficile spores. Bleach is sporicidal and has been shown to decrease the bioburden of C. difficile in the healthcare setting.86–89 An approved hospital sporicidal must be used on rooms of patients with CDI as well as any equipment leaving those rooms.
Additionally, patients undergoing inpatient fecal microbiota transplantation (FMT) should have their rooms cleaned with an approved hospital sporicidal, with the same rigor as used in terminal cleaning, prior to them returning to the room from the endoscopy suite. In addition to using the appropriate disinfectant, the importance of good mechanical cleaning with the product should be emphasized to maximize the mechanical removal of spores.
- Alcohol based hand rub is not effective against C. difficile spores.
- Hands are to be cleaned with soap and water upon exit of a CDI room to ensure mechanical removal of the spores.85
Duration of isolation.
Patients are to remain in CP-D for the duration of their hospitalization, as their hospital room will remain contaminated with C. difficile spores as long as they inhabit it. Terminal cleaning and decontamination is not possible with the patient present; spores persist and may continue to be transmitted. Thus, patients must remain in CP-D and the room must be terminally cleaned at discharge. This recommendation for isolation holds even for FMT recipients after their room has been cleaned with bleach following the FMT. In special circumstances, arrangements may be made to move the patient to allow for a terminal clean and the discontinuation of precautions. These are determined on a case-by-case basis with IPE and the clinical team.
Isolation practices for readmission.
When patients with a history of CDI are readmitted, they do not need to be placed in CP-D unless they are readmitted with diarrhea. Patients with a history of CDI that are readmitted with diarrhea should be managed in CP-D until CDI has been ruled out.
Visitor/family recommendations.
For the protection of family members, it is recommended that family and visitors wear an isolation gown and gloves when assisting in the care of a patient with CDI. Family and visitors must wash hands with soap and water upon leaving the patient room. Though family and visitors may move about the facility, they should not utilize the unit nourishment room. There is no published evidence that visitors contribute to the spread of CDI in hospitals. Studies have included interventions such as having visitors comply with wearing cover gowns and gloves in CDI rooms, but no studies have evaluated the sole impact of visitors on CDI transmission.
Isolation of Asymptomatic Carriers.
Except in special circumstances, asymptomatic carriers are not placed in CP-D. At UMHS, the exception to this is the adult bone marrow transplant unit. Adult Bone-Marrow Transplant (BMT) patients are screened for C. difficile upon admission and if positive, placed in CP-D for the duration of their hospitalization. Patients are placed in CP-D, even if they don’t currently have symptoms as many of the treatments for which they are admitted can cause them to become symptomatic. Because BMT patients are such a vulnerable population, this protects non-colonized patients from exposure. Please note this policy does not apply to UMHS Pediatric BMT units given the high rate of asymptomatic colonization among young children.
Medical Treatment of C. difficile Infection
The approach to antibiotic treatment for CDI is shown in Figure 3. Details of the treatment options and considerations are listed below.
Treatment of asymptomatic carriers is not recommended. There is no role for prophylactic CDI therapy in asymptomatic carriers. In a small, randomized placebo controlled trial, only oral vancomycin (not metronidazole) was effective in reducing C. difficile carriage. Oral vancomycin treatment was associated with significantly higher rates of C. difficile carriage 2 months after treatment.90 Further study is needed to assess the role of asymptomatic carriers in C. difficile transmission and the need for isolation precautions. In a prospective study, 29% of cases of hospital acquired CDI were associated with carriers.91
Antimicrobial Treatment Based on Disease Severity
Antimicrobial treatment for CDI is based on the severity of the disease:
- Non-fulminant CDI: vancomycin 125 mg PO QID
- Fulminant CDI: vancomycin 500 mg PO QID, metronidazole 500 mg IV every 8 hours, and if ileus, small bowel obstruction, or toxic megacolon, vancomycin enema every 6 hours.
Recent guidelines44 refer vancomycin over metronidazole for all patients with their first episode of CDI. This recommendation is based on prospective, randomized trials performed in the 2000s that have found vancomycin to be associated with improved clinical success (largely defined as resolution of diarrhea) compared to metronidazole.92,93 Although these trial data have not shown vancomycin to be associated with less recurrence or mortality, readmission, or complications due to CDI, recent guidelines no longer support metronidazole as a first-line option in patients with an initial episode of CDI.44 In patients who cannot tolerate oral vancomycin (due to allergy, for example), fidaxomicin is recommended as alternative therapy. Fidaxomicin is non-inferior to vancomycin for initial cure, but was actually superior to vancomycin for sustained cure, driven by the approximately 50% lower incidence of recurrent CDI compared to vancomycin.94 However, the high cost of fidaxomicin means it is impossible to use in all patients and, thus, is considered an alternative agent that currently requires an ID approval for use.
The duration of therapy in the above trials was 10 days. However, some patients may respond slowly to treatment and IDSA guidelines endorse extending treatment to 14 days in patients who have not had resolution of symptoms by day 10.44 Intravenous metronidazole is less efficacious than oral metronidazole or oral vancomycin in the treatment of CDI, and should only be utilized (in combination with other therapy) when oral or enteral administration is not feasible.
There is no supportive data for the use of oral vancomycin doses ≥125 mg QID in patients with non-fulminant disease. There is an absence of data regarding the optimal treatment of fulminant CDI. Guidelines currently recommend combination therapy with administration of intravenous metronidazole and high-dose oral vancomycin (500mg QID) in patients with fulminant CDI.95,96 Intracolonic administration of vancomycin may be considered in all patients with fulminant CDI, but should be given in patients with ileus, small bowel obstruction, and toxic megacolon.96 In the absence of data, longer durations of therapy (≥14 days) may be recommended in patients with fulminant CDI,29,37,44 and final treatment plan should be formulated in discussion with the Infectious Diseases consult team.97
C. difficile Enteritis (small bowel involvement).
Patient with CDI of the small bowel should be treated with oral vancomycin 125 mg PO QID. Small bowel involvement with C. difficile is rare but has been reported.45 The majority of patients with C. difficile enteritis have either surgically altered intestinal anatomy such as colectomy with ileostomy and/or inflammatory bowel disease. In a review of 56 patients with C. difficile enteritis the majority of patients required ICU management and mortality was high (32.1%).45 In patients with an ileostomy, fever and increased ileostomy output should prompt further evaluation for C. difficile infection. The optimal treatment of C. difficile enteritis is unknown. However, since these patients are often refractory to metronidazole therapy,98 often require ICU management, and have a high mortality,45 vancomycin treatment is recommended.
Recurrent C. difficile Infection.
Patients with recurrent disease should first be prescribed appropriate primary therapy, as described in above. Subsequently, treatment of CDI recurrence depends on how many recurrences the patient has experienced:
- First recurrence:
- - Vancomycin taper: Vancomycin tapers should begin after the treatment course is completed. Example of PO vancomycin taper: 125mg PO BID x 7 days, then 125mg PO daily x 7 days, then 125mg PO every other day x 7 days, then 125mg PO every 3 days x 2–8 weeks.
- - Alternative if vancomycin taper cannot be performed: Fidaxomicin 200mg PO BID x 10 days
- - Second or multiple recurrences (third or more episode of CDI):
- - If vancomycin taper was not used for the first recurrence: vancomycin taper
- - If vancomycin taper was used for the first recurrence, the following options may be considered in consultation with Infectious Diseases:
- - Repeat Vancomycin taper
- - Fidaxomicin 200mg PO BID x 10 days
- - Fecal microbiota transplant
- - Repeat Vancomycin taper followed by Rifaximin ‘chaser’ (Rifaximin 400mg TID x 20 days)
- - Kefir staggered protocol: Vancomycin 125mg QID x 2 weeks, 375mg Q72h x 2 weeks, 250mg Q72h x 2 weeks, and 125mg Q72h x 2 weeks PLUS kefir (5-oz glass with each meal (at least 3 glasses per day)) for 15 weeks,99 addressed in more detail in “Probiotic Treatment” section).
There is a paucity of data regarding the optimal treatment of patients with recurrent CDI. Tapered or pulsed dosing regimens of vancomycin have been shown to reduce the risk of further recurrences compared to placebo in patients with multiple recurrences. Guidelines recommend that vancomycin taper be considered for first recurrence (in patients treated with vancomycin for their primary episode). In patients for whom metronidazole was used for their primary episode, a standard vancomycin course is recommended for treatment of 1st recurrence.29,37,44,100 In patients with multiple recurrences, the dearth of data is even more severe, and as such several options may be considered on a case-by-case basis in consultation with Infectious Diseases. These options include repeating vancomycin taper, fidaxomicin (discussed below), rifaximin ‘chaser’, fecal microbiota transplant (discussed in more detail in the below “Fecal Microbiota Transplant” section), and a Kefir staggered protocol, among others.44,101–104
In a randomized, trial of patients with either a first episode of CDI or first recurrence, fidaxomicin was non-inferior to vancomycin in terms of clinical cure rates.105 Recurrence rates were lower with fidaxomicin, but only in the subset of patients infected with non-NAP1/027 strains. Due to uncertain cost-effectiveness (~$300/day) compared to vancomycin (especially when vancomycin is compounded from the intravenous formulation),106,107 the role of fidaxomicin in CDI therapy remains undefined. At UMHS fidaxomicin requires ID approval and has been reserved for treatment of CDI in patients with documented recurrent disease who have failed a recent vancomycin taper. Fidaxomicin has not been studied in patients with complicated CDI, and should not be utilized in this scenario.
To our knowledge, there is a paucity of data evaluating extension of CDI therapy duration for patients receiving non-CDI antibiotics.108 However, concomitant anti-microbials in the context of CDI are associated with poor clinical outcomes, extended time to resolution of diarrhea, treatment failure, and recurrence of CDI.28,69,70,109 In one study, the use of non-CDI antimicrobials within 30 days of an episode of CDI was associated with a 3-fold increase in CDI recurrence.71 Therefore, in patients being treated for CDI who require concomitant antibiotic therapy beyond the 10–14 days of CDI treatment, vancomycin can be continued for the duration of concomitant antibiotic therapy (at a reduced dose110 of 125 mg PO BID).
Probiotic Treatment
Probiotics as adjunct treatment of CDI is not recommended in hospitalized patients. There are limited data supporting the use of Lactobacillus-containing probiotic preparations in the treatment of CDI, and use is not recommended.
Two randomized, double-blind, placebo-controlled trials have shown that Saccharomyces boulardii, in conjunction with standard treatment for CDI, significantly reduces the number of further episodes of CDI in patients with a history of recurrent infection.111,112 However, a recent meta-analysis only showed a modest but non-significant reduction in recurrent CDI from either S. boulardii or Lactobacillus species.113 Additionally, the use of Saccharomyces probiotic preparations has been associated with invasive infection in patients with central venous catheters, intestinal disease (abdominal surgery, intestinal obstruction, ulcerative colitis, neoplasm, bowel or gastric ulcerations), and critically ill or immunocompromised patients.114 In the opinion of this committee, these safety concerns preclude use of S. boulardii for hospitalized patients with CDI.
In patients with multiple recurrences of CDI, who are not candidates for FMT, a regimen of staggered and tapered oral vancomycin in combination with kefir can be considered. A prospective case series of patients with multiple recurrences of CDI found that daily administration of kefir (a probiotic yogurt drink) in combination with a staggered and tapered oral vancomycin or metronidazole regimen achieved a treatment success rate of 84%.99
Immunotherapy
Intravenous Gamma Globulins (IVIG) can be considered in patients with hypogammaglobulinemia and recurrent CDI, but IVIG in all other patient populations is not recommended. There are no randomized controlled trials on the use of human IVIG. A retrospective analysis of 18 patients with severe CDI treated with IVIG and standard therapy found no difference in clinical outcomes compared with matched controls.115 In patients with recurrent CDI and hypogammaglobulinemia (<450 mg/dL) in the presence of a cancer or immune deficiency disorder, IVIG can be considered on a case by case basis. In a randomized, double-blind, placebo-controlled Phase II trial, addition of two neutralizing monoclonal antibodies against C. difficile toxins A and B to standard therapy did not impact the initial infection course but did significantly reduce infection recurrence. This therapy is currently being evaluated in Phase III trials.29,37,115,116
Two phase-3 studies evaluated the efficacy of two monoclonal antibodies, actoxumab (ACT) and bezlotoxumab (BEZ), with activity against TcdA and TcdB, respectively. The study arm with ACT alone was stopped early due to lack of efficacy in an interim analysis. The pooled analysis of patients that received either ACT + BEZ (n = 773) or BEZ alone (n = 781) observed recurrent CDI in 15.4% and 16.5%, respectively, versus 26.6% in the placebo (n = 773) arm (P <.001). The safety profiles in the interventional arms were similar to the placebo arm.117
Toxin-Binding Polymers and Resins
Therapy with toxin-binding polymers (tolevamer) and resins (cholestyramine and colestipol) is not recommended. In two multinational, randomized, controlled trials, clinical success (defined as resolution of diarrhea and absence of abdominal discomfort) of tolevamer (a non-antibiotic, toxin-binding polymer) was inferior to both metronidazole and vancomycin.92 Regarding toxin-binding resins, colestipol was found to be no more effective than placebo at decreasing fecal excretion of C. difficile toxin.118 Cholestyramine, another resin, binds oral vancomycin, which may lead to decreased concentrations of the antibiotic,119 and thus concomitant cholestyramine and oral vancomycin administration should be avoided in patients with CDI.
Fecal Microbiota Transplantation (FMT)
Recommendations:
- FMT is a good treatment option for multiple recurrences of CDI (after two or more recurrences of CDI within one year) and can be considered in patients with CDI not responsive to standard treatment by day 5, assuming escalation of pharmacologic therapy has already been attempted (Figure 3).
- At UMHS, outpatient FMT is initiated in the outpatient ID clinic and requires a referral. Inpatient FMT is initiated through consultation of the inpatient ID and GI consult services, though GI consult is unnecessary if the patient is already on the GI inpatient service.
- Conventional therapy should be pursued for the treatment of primary CDI or first recurrence. There is insufficient experience with FMT to recommend it as a primary approach. More longitudinal data on patients that have undergone FMT are needed; therefore, judicious use of this treatment modality is warranted.
- Conventional therapy should be pursued first in patients with severe CDI, and FMT is generally not recommended in patients with fulminant CDI because the safety and efficacy of FMT for these patients has not been established.
- - Caution should be exercised with use of FMT in patients with IBD. Those who undergo FMT for CDI may be at increased risk of IBD flare.
- - Either frozen or fresh stool can be used for FMT in the setting of recurrent CDI.
- - Capsule FMT is available only in the ID clinic in an outpatient setting. FMT via G/J tube and sigmoidoscopy/colonoscopy are available for both inpatients and outpatients.
Overview.
The human gut microbiome is a diverse community with thousands of bacterial species120 which likely protects against invasive pathogens.121,122 The pathogenesis of CDI is thought to require disruption of the gut microbiota prior to the onset of symptomatic disease,123 usually through antibiotic exposure.124
Even after recovery from CDI, some patients retain a susceptible microbiome and can have recurrent CDI.125 This can occur either from recrudescence of the original infection or from reinfection with a new C. difficile strain.126 A small minority of patients (<5%) have difficulty achieving clinical cure with conventional antibiotic therapy and will enter a cycle with multiple recurrences,127,128 often relapsing soon after antibiotics are stopped. Restoration of the gut microbiome through fecal microbiota transplantation (FMT) appears to be the most effective treatment strategy for these patients and has gained widespread acceptance in the medical community.129
Indications and summary of evidence.
There is insufficient experience with FMT to recommend it for the treatment of primary CDI or first recurrence vs. standard therapy with either vancomycin or metronidazole.130,131 There is also insufficient evidence for treatment of severe CDI or fulminant CDI with FMT, though several published case reports suggest that it may be effective.132–135 Based on a wealth of data from case reports, systematic reviews, and clinical trials,136–148 FMT appears quite effective for recurrent CDI with cure rates exceeding 85–90% in most studies.
Thus, the UMHS FMT protocol excludes patients with primary CDI, first recurrence of CDI, or complicated disease but other patients become eligible for FMT with two or more recurrences of CDI (especially within 1 year). Additionally, patients are eligible for FMT with CDI not responsive to standard treatment by day 5, assuming escalation of pharmacologic therapy has already been attempted (Figure 3).
Often, donors are family members or close friends. Some studies suggest that related donors are associated with a higher resolution of CDI than unrelated donors, 93% vs. 84%, respectively. However, the results of a meta-analysis indicated that there was no significant difference between outcomes from related and unrelated donors.149
A randomized non-inferiority trial conducted in patients with recurrent CDI found that the use of frozen stool for FMT resulted in a rate of clinical resolution of diarrhea that was no worse than that obtained with fresh stool for FMT (per-protocol analysis revealing, 83.5% vs. 85.1%; difference, −1.6% [95% CI, −10.5% to ∞]; P=0.01).150
Protocol.
The optimal parameters for FMT, including stool preparation, infusion amount, and infusion route, are not known, but FMT appears highly effective regardless of variability in these specifics.136 There are three formulations of stool available at UMHS through the non-profit stool bank OpenBiome (Somerville, MA): oral fecal capsules (outpatient only), upper GI (for G- or J-tubes only; nasogastric or Dobhoff tube routes are not available), and lower GI (sigmoidoscopy- or colonoscopy-delivered, in the medical procedures unit). The upper and lower GI infusions can also utilize stool from a directed donor (related or unrelated), selected by the patient and/or their family.
OpenBiome has a rigorous donor selection process that entails thorough screening questionnaires and testing of donors and donor stool in order to ensure safety. The oral capsule formulation has specific exclusion criteria and lower efficacy compared to lower delivery of FMT.139,146 The efficacy of capsules is 70% with one administration and 94% with multiple administrations.151
Outpatient FMT requires the patient be seen in the infectious diseases (ID) clinic, so the protocol is initiated through a referral to the ID clinic and the ID provider will coordinate the process from that point onward. Inpatient FMT requires consults to be placed to both the ID and gastroenterology (GI) services, if GI is not already the primary service for the patient. The ID/GI inpatient services will coordinate the process from that point onward.
Preparation of the recipient ideally involves cessation of all antibiotics, including those used to treat CDI, 48 hours prior to FMT. In recipients undergoing FMT via colonoscopy a standard colonoscopy bowel preparation usually must also be administered. The ID and GI physicians coordinating the FMT will make any final determinations about exceptions to these criteria or modifications in the FMT preparations.
Safety.
FMT is generally safe and effective for most patients with recurrent CDI in the short term (1–2 years).97 Among most immunocompromised patients, FMT also appears safe.144 However, patients with IBD may be at increased risk for disease flare, fever, and/or elevation in inflammatory markers following FMT.144,152–154 Though several case reports exist, there is insufficient data on safety or efficacy to support the use of FMT for severe CDI.132–135 Of particular concern is a case of toxic megacolon that resulted in death of a patient that received FMT for severe CDI, though it is undetermined whether FMT itself or withdrawal of antibiotics for CDI following FMT contributed to the outcome.155
There are few data on long-term safety of FMT, but the intestinal microbiota has been associated with colon cancer, diabetes, obesity, and atopic disorders such as asthma.156 One study following patients for 3–68 months (mean 17 months) after FMT157 noted the development of new conditions, including autoimmune disease, ovarian cancer, myocardial infarction, and stroke, though a causal mechanism cannot be inferred from this data. Thus, more longitudinal data on patients that have undergone FMT are needed and at present, judicious use of this treatment modality is warranted.
Avoid Anti-Motility Agents
Anti-motility agents in the initial treatment or treatment adjunct of CDI are not recommended as they can mask symptoms and increase risk of toxic megacolon.44 In a literature review of 55 patients, 31% of patients who received anti-motility agents without initial treatment of C. difficile developed toxic megacolon or colonic dilatation.158 No significant difference in complications was noted in patients who received anti-motility agents along with treatment of C. difficile.158 Further study is needed to assess role of anti-motility agents to control diarrhea in patients with mild-to moderate CDI who are already being treated for CDI.
Treatment Response
Treatment response generally occurs after 3–5 days of therapy and is characterized by a decrease in stool frequency, improvement in stool consistency, and improvement in laboratory, radiologic and physiologic parameters.
For patients with mild to moderate CDI with no improvement or worsening of symptoms after 3–5 days of metronidazole therapy, escalation to vancomycin therapy is indicated.
Recurrence of CDI after the initial episode occurs in is approximately 19–25% of patients.4,136 Approximately 25–64.7% of patients have another recurrence of CDI after first or multiple recurrences.4,136
Surgical Treatment
In patients who have severe, fulminant CDI and clinical deterioration despite maximal medical therapy, surgery may confer mortality benefit However, in advanced fulminant CDI, surgical mortality can be up to 80% (range 19–80%).159,160
The optimal timing for surgical intervention is not well defined, with only expert opinion supporting recommendations. Early surgical involvement may be warranted in patients who are critically ill from CDI.35 While not every patient requires surgical intervention, patients with complicated CDI (Table 4) warrant early surgical consultation.1,35 Indications for surgical consultation and potential considerations for surgery34 are listed in Table 4.
Delayed surgical intervention may result in increased morbidity and mortality.2,161 Prior studies indicate higher mortality rates if surgical intervention occurred after evidence of end-organ failure (eg, intubation for respiratory failure, vasopressor therapy for hemodynamic instability, or acute renal failure). In one study evaluating timing of surgical intervention for fulminant pseudo-membranous colitis, patients who survived received surgical consult at a mean of 3.2 days from admission (or onset of symptoms for those already in the hospital), as compared to the nonsurvivors who received surgical consult at a mean of 5.4 days after admission or symptom onset.162 Likewise, an analysis of the National Inpatient Sample (NIS) database suggested that surgical intervention more than 3 days after admission for CDI may be associated with increased mortality.159
Surgical options include total or subtotal colectomy with end ileostomy (total abdominal colectomy, or TAC).163
- Partial colectomy is contraindicated: in one retrospective analysis, partial colectomy carried a mortality rate of 100% when compared to TAC.164
- In patients with peritonitis, prior abdominal surgeries with adhesions, or frank perforation, exploratory laparotomy with TAC may be warranted.
- Diverting loop ileostomy with antegrade colonic lavage may be associated with reduced mortality.165 Additionally, in one study diverting loop ileostomy allowed the majority of patients to achieve colonic preservation (93%) compared to a historical population of patients undergoing total abdominal colectomy.2 This may also allow for earlier surgical intervention on CDI patients, before the development of end-organ failure and shock progression. However, more research is needed before this can be universally adopted as standard of care.
In patients who are poor surgical candidates, or who are in refractory end-stage septic shock, with continued clinical decline, the risk for morbidity and mortality may be prohibitively high, and a frank discussion with the patient and/or the patient’s family may be warranted regarding goals of care, including the risks and benefits involved with an aggressive attempt at surgery vs. continued nonoperative management with maximal medical therapy.
Special Populations - Pediatric Treatment (Patients ≤ 18 Years of Age)
For pediatric patients, treatment guidelines generally follow those outlined above for adults (Figure 3 and Table 3B). Ideally, discontinuation of antimicrobial agents is the first step in treating CDI and may suffice to resolve symptoms in mild cases. Antiperistaltic medications are not recommended for pediatric patients as these may mask symptoms and precipitate complications, including toxic megacolon.
- For pediatric patients with criteria meeting severe disease (Table 3B), including those with risk factors associated with severe disease (Table 3B), metronidazole allergy, pregnancy/breastfeeding, or without symptom resolution after 3 to 5 days of oral metronidazole, oral vancomycin is the preferred therapy (10 mg/kg/dose QID, up to maximum 125 mg/dose x 10 days).
- Combination therapy with vancomycin, both oral and by retention enema, and intravenous metronidazole, or second-line agents, may be considered in complicated high-risk cases with sepsis, shock, ileus, small bowel obstruction, peritonitis, or toxic megacolon (Triple Therapy in Table 3B). Do not use enema if concern of colonic perforation. Intracolonic vancomycin administered by retention enema (10–20 mL/kg/dose, up to 500 ml max, of a 500 mg/L solution in normal saline q6h, instilled by appropriately-sized Foley catheter inserted into rectum with balloon inflated and Foley clamped for 1 hour. Treatment naïve patients should be initiated at 10 mL/kg/dose and escalated as tolerated to the preferred dose of 20 mL/kg/dose or a maximum of 500 mL. In a patient with additional administration considerations, a minimum of 10 mL/kg/dose every 8 hours should be used, and high-dose oral vancomycin (minimum 10 mg/kg/dose up to 500 mg/dose QID) in combination with IV metronidazole should be administered. For patients receiving combination therapy including PO, IV, and PR vancomycin or in patient at high risk for systemic absorption, serum vancomycin concentrations should be considered to evaluate for elevated serum concentrations.
- For pediatric patients with first recurrence of CDI, that is mild to moderate, oral metronidazole is the preferred therapy.
- For pediatric patients with prolonged or recurrent CDI, metronidazole should not be used for chronic therapy due to possible neurotoxicity. Oral vancomycin is recommended for second or multiple recurrences (10 mg/kg/dose QID, maximum 500 mg/dose, x 10 days), and pulsed or tapered regimens may be beneficial in difficult cases (see adult section above).
- Criteria for optimal use of second-line agents in recurrent CDI, including nitazoxanide, fidaxomicin, and rifaximin, do not exist in children. Consultation with a Pediatric Infectious Diseases specialist is recommended if considering these options for treatment of recurrent CDI, either as alternatives to vancomycin therapy or in “chaser” regimens associated with vancomycin tapers. Pediatric dosing for fidaxomicin is 16 mg/kg/dose BID, max 200 mg per dose; Peds ID approval is required for use of fidaxomicin.
- Fecal microbiota transplantation (FMT) may be considered in children <18 years old as an outpatient to help prevent recurrence of CDI; consultation with a Pediatric Infectious Disease specialist is recommended. There are currently no mechanisms in place to provide FMT to children <18 years old as an inpatient.
- Probiotics are not routinely recommended for either prevention or treatment of CDI in infants and children, due to a lack of sufficient evidence in infants and children and a theoretical risk of bacteremia due to gut translocation.
Summary of evidence.
It should be recognized that oral vancomycin is the only FDA-approved medication for the treatment of CDI in children; however, metronidazole is currently the accepted first-line medication in otherwise healthy infants and children with mild to moderate disease.20,23 Prospective trials evaluating the use of metronidazole or vancomycin for longer than 10 to 14 days in children are lacking.22 Data does not support a clear benefit for combination therapy vs. oral vancomycin monotherapy for treatment of severe CDI.166 Experience with FMT in pediatrics is limited, but growing evidence suggests that FMT is effective and safe for treatment of chronic CDI in most children.167 FMT is not currently recommended for treatment of children with cancer and hematopoietic stem-cell transplantation recipients.168 An FMT program for inpatients <18 years old is currently unavailable at UMHS; consultation with Pediatric Infectious Diseases is suggested if considering FMT as an option as an outpatient procedure to prevent future recurrences. The American Academy of Pediatrics (AAP) does not currently recommend probiotics for prevention or treatment of CDI in infants and children citing insufficient data.21 A recent Cochrane Database review of 23 randomized controlled trials found moderate quality evidence to suggest that short-term use of probiotics in healthy, immunocompetent adults and children was safe and effective in the prevention of CDI.169 UMHS guidelines reflect the recommendation of the AAP and do not recommend probiotic use for prevention or treatment of CDI in this patient population.
Special Populations - Inflammatory Bowel Disease
In IBD patients with concomitant CDI and mild IBD flare, antibiotics for CDI should be started without corticosteroids (patient should be maintained on home immunosuppression medications). This recommendation is based on evidence suggesting worse outcomes in IBD patients with CDI on immunosuppression,145,146 and a reduction of corticosteroids dose is associated with a lower colectomy rate.10 However, with a lack of symptom improvement after 48 hours of CDI therapy or in severe IBD flare with concomitant CDI, antibiotics and corticosteroids co-therapy is advised. This strategy is based on clinical data indicating a more severe IBD course (ie, an increased colectomy rate and mortality) in IBD patients with CDI vs IBD patients without CDI.48,147,148 The recommended practice of IBD experts at UMHS is to use CDI therapy and corticosteroids when IBD patients with concomitant CDI present with a severe IBD flare defined as >6 bloody stools/day and one or more of: Temp >37.5, Pulse >90, Hgb <10.5, ESR >30.
Vancomycin is preferred over metronidazole for the treatment of CDI in IBD patients based on a retrospective study showing fewer readmissions and shorter lengths of stay when IBD patients are treated with vancomycin-containing regimen relative to those treated with metronidazole alone.170
Related National/International Guidelines
The UMHS Clinical Guideline on CDI is generally consistent with other guidelines published nationally and internationally, including:
- Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31(5):431–455.
- Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am J Gastroenterol 2013; 108:478–498.
- European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20 (Suppl. 2): 1–26
Notable differences in recommendations: Our guideline deviates from the above guidelines in the way that it classifies “mild-to-moderate” vs. “severe” vs. “severe and fulminant” disease (Table 3). The change in classification criteria was undertaken to better reflect additional factors we identified in the literature that can predispose patients to developing complicated C. difficile or more severe manifestations of C. difficile.
Guideline Development Methodology
Funding
The development of this guideline was funded by the University of Michigan Health System.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- The medical team: Meghan A Arnold, MD, Pediatric Surgery; Gregory Eschenauer, PharmD, Pharmacy; Tejal N Gandhi, MD, Infectious Diseases; John Y Kao, MD, Gastroenterology; Andrea H Kim, MD, Internal Medicine; Lena M Napolitano, MD Surgery; Krishna Rao, MD, Infectious Diseases; David M. Somand, MD, Emergency Medicine; Daniel H Teitelbaum, MD, Pediatric Surgery; Kathleen B To, MD, Surgery; Alison C Tribble, MD, Pediatric Infectious Diseases; Amanda M Valyko, MPH, CIC, Infection Control; Laraine L Washer, MD Infection Diseases; Michael E Watson, Jr., MD, PhD, Pediatric Infectious Diseases
- A guideline development methodologist: F. Jacob Seagull, PhD, Learning Health Sciences.
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
No relevant personal financial relationships with commercial entities: Meghan A Arnold, Gregory Eschenauer, PharmD, Tejal N Gandhi MD, John Y Kao, MD, Andrea H Kim, MD, Lena M Napolitano, MD, Krishna Rao, MD, F Jacob Seagull, PhD, David M Somand, MD, Daniel H Teitelbaum, MD, Kathleen B To, MD, Alison C Tribble, MD, Amanda M Valyko, Laraine Lynn Washer, MD, Michael E Watson Jr, MD, PhD.
Strategy for Literature Search
Within the Medline (Ovid) database, the following search strategy was used for most of the search topics, except for the searches on suppurative thrombosis, endocarditis, and vascular infection. The search below is identified as Main in the search strategies document. Because the appropriate indexing terms either do not exist or were applied inconsistently, the main search uses keywords in addition to MeSH terms to arrive at the following main strategy.
- exp *Clostridium difficile/ or exp *enterocolitis/ or exp *Clostridium Infections/
- exp *Hirschsprung Disease/
- limit 2 to “all child (0 to 18 years)”
- 1 or 3
- exp animals/ not (exp animals/ and humans/)
- 4 not 5
- limit 6 to (english language and yr=“2013 -Current”)
- remove duplicates from 7. The searches on suppurative thrombosis, endocarditis, and vascular infection used the first 3 searches of the main search strategy. The MEDLINE In-Process search was based entirely on keywords.
Results were limited to: English, and 2013 to present. The Main search retrieved 893 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follow:
- C-Diff -Guidelines, total results were 8
- C-Diff -Clinical Trials, total results were 27
- C-Diff -Cohort Studies, total results were 59
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. Negative trials were specifically sought. The search was a single cycle.
Level of evidence supporting a diagnostic method or an intervention:
- A = systematic reviews of randomized controlled trials with or without meta-analysis,
- B = randomized controlled trials,
- C = systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control),
- D = individual observation studies (case study/case series),
- E = expert opinion regarding benefits and harm
Search details and evidence tables available at http://www.uofmhealth.org/provider/clinical-care-guidelines.
Recommendations
Guideline recommendations were based on prospective randomized controlled trials if available, to the exclusion of other data; if RCTs were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size. The “strength of recommendation” for key aspects of care was determined by expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Medical School to which the content is most relevant: Family Medicine, General Medicine, Department of Surgery, Infectious Diseases Division, Gastroenterology Division. Pediatrics and Communicable Diseases and the Division of Pediatric Infectious Diseases. The Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers endorsed the final version.
References
- 1.
- McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infection control and hospital epidemiology. 2007;28(2):140–145. [PubMed: 17265394]
- 2.
- Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Annals of Surgery. 2011;254(3):423–427; discussion 427–429. [PubMed: 21865943]
- 3.
- Kuijper EJ, Coignard B, Tull P, et al. Emergence of Clostridium difficile-associated disease in North America and Europe. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2006;12 Suppl 6:2–18. [PubMed: 16965399]
- 4.
- Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PloS one. 2014;9(6):e98400. [PMC free article: PMC4045753] [PubMed: 24897375]
- 5.
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. The New England journal of medicine. 1978;298(10):531–534. [PubMed: 625309]
- 6.
- Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75(5):778–782. [PubMed: 700321]
- 7.
- Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infection control and hospital epidemiology. 2009;30(6):526–533. [PubMed: 19419272]
- 8.
- Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infection control and hospital epidemiology. 2013;34(6):588–596. [PubMed: 23651889]
- 9.
- Lessa FC, Winston LG, McDonald LC, Emerging Infections Program CdST. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–2370. [PubMed: 26061850]
- 10.
- He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nature genetics. 2013;45(1):109–113. [PMC free article: PMC3605770] [PubMed: 23222960]
- 11.
- Louie TJ, Miller MA, Crook DW, et al. Effect of age on treatment outcomes in Clostridium difficile infection. Journal of the American Geriatrics Society. 2013;61(2):222–230. [PubMed: 23379974]
- 12.
- Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55 Suppl 2:S65–70. [PMC free article: PMC3388017] [PubMed: 22752867]
- 13.
- Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clinics in geriatric medicine. 2014;30(1):79–93. [PubMed: 24267604]
- 14.
- Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine. 2013;173(22):2039–2046. [PubMed: 23999949]
- 15.
- Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2004;171(5):466–472. [PMC free article: PMC514643] [PubMed: 15337727]
- 16.
- Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Annals of Internal Medicine. 1998;129(12):1012–1019. [PubMed: 9867755]
- 17.
- Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(3):345–351. [PubMed: 17368234]
- 18.
- Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992–2002. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(11):1621–1627. [PubMed: 16267735]
- 19.
- Haines CF, Moore RD, Bartlett JG, et al. Clostridium difficile in a HIV-infected cohort: incidence, risk factors, and clinical outcomes. AIDS. 2013;27(17):2799–2807. [PMC free article: PMC3880635] [PubMed: 23842125]
- 20.
- Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA pediatrics. 2013;167(6):567–573. [PubMed: 23460123]
- 21.
- Schutze GE, Willoughby RE, Committee on Infectious D, American Academy of P. Clostridium difficile infection in infants and children. Pediatrics. 2013;131(1):196–200. [PubMed: 23277317]
- 22.
- Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics. 2008;122(6):1266–1270. [PubMed: 19047244]
- 23.
- McFarland LV, Ozen M, Dinleyici EC, Goh S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World journal of gastroenterology. 2016;22(11):3078–3104. [PMC free article: PMC4789985] [PubMed: 27003987]
- 24.
- Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clinical Infectious Diseases. 2013;56(10):1401–1406. [PMC free article: PMC3693491] [PubMed: 23408679]
- 25.
- Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. [PubMed: 22047560]
- 26.
- Furuya-Kanamori L, Marquess J, Yakob L, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis. 2015;15:516. [PMC free article: PMC4647607] [PubMed: 26573915]
- 27.
- Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. The American Journal of Medicine. 1989;86(1):15–19. [PubMed: 2910090]
- 28.
- Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. Jama. 2015;313(4):398–408. [PMC free article: PMC6561347] [PubMed: 25626036]
- 29.
- Debast SB, Bauer MP, Kuijper EJ, Committee. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clinical Microbiology & Infection. 2014;20(Suppl 2):1–26. [PubMed: 24118601]
- 30.
- Bignardi GE, Hill K, Berrington A, Settle CD. Two-stage algorithm for Clostridium difficile: glutamate-dehydrogenase-positive toxin-negative enzyme immunoassay results may require further testing. Journal of Hospital Infection. 2013;83(4):347–349. [PubMed: 23399483]
- 31.
- Finch LS, Duncan CM. Molecular test to determine toxigenic capabilities in GDH-positive, toxin-negative samples: evaluation of the Portrait toxigenic C. difficile assay. British journal of biomedical science. 2013;70(2):62–66. [PubMed: 23888607]
- 32.
- Walkty A, Lagace-Wiens PR, Manickam K, et al. Evaluation of an algorithmic approach in comparison with the Illumigene assay for laboratory diagnosis of Clostridium difficile infection. Journal of clinical microbiology. 2013;51(4):1152–1157. [PMC free article: PMC3666773] [PubMed: 23363829]
- 33.
- Brown NA, Lebar WD, Young CL, Hankerd RE, Newton DW. Diagnosis of Clostridium difficile infection: comparison of four methods on specimens collected in Cary-Blair transport medium and tcdB PCR on fresh versus frozen samples. Infectious disease reports. 2011;3(1):e5. [PMC free article: PMC3892603] [PubMed: 24470904]
- 34.
- Sunkesula VC, Kundrapu S, Muganda C, Sethi AK, Donskey CJ. Does empirical Clostridium difficile infection (CDI) therapy result in false-negative CDI diagnostic test results? Clinical Infectious Diseases. 2013;57(4):494–500. [PubMed: 23645849]
- 35.
- To KB, Napolitano LM. Clostridium difficile infection: update on diagnosis, epidemiology, and treatment strategies. Surgical infections. 2014;15(5):490–502. [PubMed: 25314344]
- 36.
- Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infection control and hospital epidemiology. 2011;32(3):220–228. [PubMed: 21460506]
- 37.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. The American Journal of Gastroenterology. 2013;108(4):478–498; quiz 499. [PubMed: 23439232]
- 38.
- Kirkpatrick ID, Greenberg HM. Evaluating the CT diagnosis of Clostridium difficile colitis: should CT guide therapy? AJRAmerican journal of roentgenology. 2001;176(3):635–639. [PubMed: 11222194]
- 39.
- Macari M, Balthazar EJ, Megibow AJ. The accordion sign at CT: a nonspecific finding in patients with colonic edema. Radiology. 1999;211(3):743–746. [PubMed: 10352600]
- 40.
- Ash L, Baker ME, O’Malley CM Jr., Gordon SM, Delaney CP, Obuchowski NA. Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings. AJRAmerican journal of roentgenology. 2006;186(5):1393–1400. [PubMed: 16632736]
- 41.
- Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Archives of Internal Medicine. 1986;146(1):95–100. [PubMed: 3942469]
- 42.
- Tedesco FJ. Antibiotic associated pseudomembranous colitis with negative proctosigmoidoscopy examination. Gastroenterology. 1979;77(2):295–297. [PubMed: 447043]
- 43.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. Journal of gastroenterology and hepatology. 2010;25(2):252–258. [PubMed: 20136989]
- 44.
- Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infection control and hospital epidemiology. 2010;31(5):431–455. [PubMed: 20307191]
- 45.
- Kim JH, Muder RR. Clostridium difficile enteritis: a review and pooled analysis of the cases. Anaerobe. 2011;17(2):52–55. [PubMed: 21334446]
- 46.
- Rodemann JF, Dubberke ER, Reske KA, Seo da H, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(3):339–344. [PubMed: 17368233]
- 47.
- Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Gastroenterology clinics of North America. 2009;38(4):711–728. [PubMed: 19913210]
- 48.
- Ananthakrishnan AN, McGinley EL, Saeian K, Binion DG. Temporal trends in disease outcomes related to Clostridium difficile infection in patients with inflammatory bowel disease. Inflammatory bowel diseases. 2011;17(4):976–983. [PubMed: 20824818]
- 49.
- Kelsen JR, Kim J, Latta D, et al. Recurrence rate of clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflammatory bowel diseases. 2011;17(1):50–55. [PubMed: 20722068]
- 50.
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. [PMC free article: PMC4034132] [PubMed: 24560869]
- 51.
- Li Y, Qian J, Queener E, Shen B. Risk factors and outcome of PCR-detected Clostridium difficile infection in ileal pouch patients. Inflammatory bowel diseases. 2013;19(2):397–403. [PubMed: 23328770]
- 52.
- Shen B, Goldblum JR, Hull TL, Remzi FH, Bennett AE, Fazio VW. Clostridium difficile-associated pouchitis. Digestive diseases and sciences. 2006;51(12):2361–2364. [PubMed: 17103037]
- 53.
- Causey MW, Spencer MP, Steele SR. Clostridium difficile enteritis after colectomy. The American Surgeon. 2009;75(12):1203–1206. [PubMed: 19999913]
- 54.
- Lundeen SJ, Otterson MF, Binion DG, Carman ET, Peppard WJ. Clostridium difficile enteritis: an early postoperative complication in inflammatory bowel disease patients after colectomy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2007;11(2):138–142. [PubMed: 17390162]
- 55.
- Jen MH, Saxena S, Bottle A, Aylin P, Pollok RC. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2011;33(12):1322–1331. [PubMed: 21517920]
- 56.
- Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Digestive diseases and sciences. 2010;55(2):415–420. [PubMed: 19255850]
- 57.
- Schneeweiss S, Korzenik J, Solomon DH, Canning C, Lee J, Bressler B. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Alimentary Pharmacology & Therapeutics. 2009;30(3):253–264. [PubMed: 19438424]
- 58.
- Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clinical Infectious Diseases. 2013;57(1):1–8. [PMC free article: PMC3669523] [PubMed: 23532470]
- 59.
- Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. Journal of pediatric gastroenterology and nutrition. 2010;51(1):2–7. [PubMed: 20512057]
- 60.
- Leibowitz J, Soma VL, Rosen L, Ginocchio CC, Rubin LG. Similar Proportions of Stool Specimens From Hospitalized Children With and Without Diarrhea Test Positive for Clostridium difficile. The Pediatric infectious disease journal. 2015;34(3):261–266. [PubMed: 25247582]
- 61.
- Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Archives of Pediatrics & Adolescent Medicine. 2011;165(5):451–457. [PMC free article: PMC4683604] [PubMed: 21199971]
- 62.
- Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerging infectious diseases. 2010;16(4):604–609. [PMC free article: PMC3363321] [PubMed: 20350373]
- 63.
- Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics. 2014;133(4):651–658. [PubMed: 24590748]
- 64.
- Owens RC Jr., Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46 Suppl 1:S19–31. [PubMed: 18177218]
- 65.
- Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2014;69(4):881–891. [PubMed: 24324224]
- 66.
- Goorhuis A, Debast SB, Dutilh JC, et al. Type-specific risk factors and outcome in an outbreak with 2 different Clostridium difficile types simultaneously in 1 hospital. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(9):860–869. [PubMed: 21914851]
- 67.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. The Journal of antimicrobial chemotherapy. 2012;67(3):742–748. [PubMed: 22146873]
- 68.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(1):42–48. [PubMed: 21653301]
- 69.
- Pop-Vicas A, Shaban E, Letourneau C, Pechie A. Empirical antimicrobial prescriptions in patients with Clostridium difficile infection at hospital admission and impact on clinical outcome. Infection control and hospital epidemiology. 2012;33(11):1101–1106. [PubMed: 23041807]
- 70.
- Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. Journal of clinical gastroenterology. 2006;40(1):49–54. [PubMed: 16340634]
- 71.
- Drekonja DM, Amundson WH, Decarolis DD, Kuskowski MA, Lederle FA, Johnson JR. Antimicrobial use and risk for recurrent Clostridium difficile infection. The American Journal of Medicine. 2011;124(11):1081.e1081–1081.e1087. [PubMed: 21944159]
- 72.
- Carignan A, Poulin S, Martin P, et al. Efficacy of Secondary Prophylaxis With Vancomycin for Preventing Recurrent Clostridium difficile Infections. Am J Gastroenterol. 2016;111(12):1834–1840. [PubMed: 27619835]
- 73.
- Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to Prevent Antibiotic-Associated Diarrhea: A Randomized, Double-Masked, Placebo-Controlled Trial. Open forum infectious diseases. 2016;3(1):ofw011. [PMC free article: PMC4785405] [PubMed: 26973849]
- 74.
- Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37. [PMC free article: PMC4769010] [PubMed: 26955289]
- 75.
- Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095. [PMC free article: PMC6486212] [PubMed: 29257353]
- 76.
- Shen NT, Maw A, Tmanova LL, et al. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. 2017;152(8):1889–1900 e1889. [PubMed: 28192108]
- 77.
- Kato K, Funabashi N, Takaoka H, et al. Lactobacillus paracasei endocarditis in a consumer of probiotics with advanced and severe bicuspid aortic valve stenosis complicated with diffuse left ventricular mid-layer fibrosis. International journal of cardiology. 2016;224:157–161. [PubMed: 27657466]
- 78.
- Naqvi SSB, Nagendra V, Hofmeyr A. Probiotic related Lactobacillus rhamnosus endocarditis in a patient with liver cirrhosis. IDCases. 2018;13:e00439. [PMC free article: PMC6111062] [PubMed: 30155406]
- 79.
- Luong ML, Sareyyupoglu B, Nguyen MH, et al. Lactobacillus probiotic use in cardiothoracic transplant recipients: a link to invasive Lactobacillus infection? Transplant infectious disease : an official journal of the Transplantation Society. 2010;12(6):561–564. [PubMed: 21040283]
- 80.
- Appel-da-Silva MC, Narvaez GA, Perez LRR, Drehmer L, Lewgoy J. Saccharomyces cerevisiae var. boulardii fungemia following probiotic treatment. Med Mycol Case Rep. 2017;18:15–17. [PMC free article: PMC5537395] [PubMed: 28794958]
- 81.
- Herbrecht R, Nivoix Y. Saccharomyces cerevisiae fungemia: an adverse effect of Saccharomyces boulardii probiotic administration. Clin Infect Dis. 2005;40(11):1635–1637. [PubMed: 15889361]
- 82.
- Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. [PubMed: 18279948]
- 83.
- Snydman DR. The safety of probiotics. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46 Suppl 2:S104–111; discussion S144–151. [PubMed: 18181712]
- 84.
- Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(3):225–233. [PubMed: 22019794]
- 85.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Archives of Internal Medicine. 2010;170(9):784–790. [PubMed: 20458086]
- 86.
- Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31(4):995–1000. [PubMed: 11049782]
- 87.
- Hacek DM, Ogle AM, Fisher A, Robicsek A, Peterson LR. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. American Journal of Infection Control. 2010;38(5):350–353. [PubMed: 20123150]
- 88.
- Wilcox MH, Fawley WN, Wigglesworth N, Parnell P, Verity P, Freeman J. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. The Journal of hospital infection. 2003;54(2):109–114. [PubMed: 12818583]
- 89.
- Weber DJ, Anderson DJ, Sexton DJ, Rutala WA. Role of the environment in the transmission of Clostridium difficile in health care facilities. American Journal of Infection Control. 2013;41(5 Suppl):S105–110. [PubMed: 23622740]
- 90.
- Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Annals of Internal Medicine. 1992;117(4):297–302. [PubMed: 1322075]
- 91.
- Curry SR, Muto CA, Schlackman JL, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(8):1094–1102. [PMC free article: PMC3783061] [PubMed: 23881150]
- 92.
- Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(3):345–354. [PubMed: 24799326]
- 93.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(3):302–307. [PubMed: 17599306]
- 94.
- Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55 Suppl 2:S154–161. [PMC free article: PMC3388030] [PubMed: 22752865]
- 95.
- Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27(10):1169–1172. [PMC free article: PMC1433873] [PubMed: 3781329]
- 96.
- Olson MM, Shanholtzer CJ, Lee JT Jr., Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infection control and hospital epidemiology. 1994;15(6):371–381. [PubMed: 7632199]
- 97.
- Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35(6):690–696. [PubMed: 12203166]
- 98.
- Seril DN, Shen B. Clostridium difficile infection in patients with ileal pouches. The American Journal of Gastroenterology. 2014;109(7):941–947. [PubMed: 24989088]
- 99.
- Bakken JS. Staggered and tapered antibiotic withdrawal with administration of kefir for recurrent Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(6):858–861. [PubMed: 24917658]
- 100.
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. The American Journal of Gastroenterology. 2002;97(7):1769–1775. [PubMed: 12135033]
- 101.
- Johnson S, Gerding DN. Fidaxomicin “chaser” regimen following vancomycin for patients with multiple Clostridium difficile recurrences. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(2):309–310. [PubMed: 23024296]
- 102.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44(6):846–848. [PubMed: 17304459]
- 103.
- Herpers BL, Vlaminckx B, Burkhardt O, et al. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(12):1732–1735. [PubMed: 19435431]
- 104.
- Musher DM, Logan N, Mehendiratta V, Melgarejo NA, Garud S, Hamill RJ. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. The Journal of antimicrobial chemotherapy. 2007;59(4):705–710. [PubMed: 17337513]
- 105.
- Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55 Suppl 2:S93–103. [PMC free article: PMC3388031] [PubMed: 22752871]
- 106.
- Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(4):555–561. [PMC free article: PMC3719891] [PubMed: 23704121]
- 107.
- Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IA. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. The Journal of antimicrobial chemotherapy. 2014;69(11):2901–2912. [PMC free article: PMC4195473] [PubMed: 25096079]
- 108.
- Rao K, Micic D, Natarajan M, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(2):233–241. [PMC free article: PMC4565993] [PubMed: 25828993]
- 109.
- Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(5):440–447. [PMC free article: PMC3156139] [PubMed: 21844027]
- 110.
- Van Hise NW, Bryant AM, Hennessey EK, Crannage AJ, Khoury JA, Manian FA. Efficacy of Oral Vancomycin in Preventing Recurrent Clostridium difficile Infection in Patients Treated With Systemic Antimicrobial Agents. Clin Infect Dis. 2016;63(5):651–653. [PubMed: 27318333]
- 111.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. Jama. 1994;271(24):1913–1918. [PubMed: 8201735]
- 112.
- Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31(4):1012–1017. [PubMed: 11049785]
- 113.
- McFarland LV. Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics (Basel, Switzerland). 2015;4(2):160–178. [PMC free article: PMC4790329] [PubMed: 27025619]
- 114.
- Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(11):1559–1568. [PubMed: 16267727]
- 115.
- Juang P, Skledar SJ, Zgheib NK, et al. Clinical outcomes of intravenous immune globulin in severe clostridium difficile-associated diarrhea. American Journal of Infection Control. 2007;35(2):131–137. [PubMed: 17327194]
- 116.
- Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. The New England journal of medicine. 2010;362(3):197–205. [PubMed: 20089970]
- 117.
- Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305–317. [PubMed: 28121498]
- 118.
- Mogg GA, George RH, Youngs D, et al. Randomized controlled trial of colestipol in antibiotic-associated colitis. The British journal of surgery. 1982;69(3):137–139. [PubMed: 7039758]
- 119.
- Pantosti A, Luzzi I, Cardines R, Gianfrilli P. Comparison of the in vitro activities of teicoplanin and vancomycin against Clostridium difficile and their interactions with cholestyramine. Antimicrobial Agents and Chemotherapy. 1985;28(6):847–848. [PMC free article: PMC180345] [PubMed: 2935077]
- 120.
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [PMC free article: PMC3376388] [PubMed: 22699611]
- 121.
- van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-v. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. The Journal of hygiene. 1971;69(3):405–411. [PMC free article: PMC2130899] [PubMed: 4999450]
- 122.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrobial Agents and Chemotherapy. 1994;38(3):409–414. [PMC free article: PMC284472] [PubMed: 8203832]
- 123.
- Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547–1553. [PMC free article: PMC3995857] [PubMed: 24503131]
- 124.
- Theriot CM, Koenigsknecht MJ, Carlson PE Jr., et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications. 2014;5:3114. [PMC free article: PMC3950275] [PubMed: 24445449]
- 125.
- Figueroa I, Johnson S, Sambol SP, Goldstein EJ, Citron DM, Gerding DN. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55 Suppl 2:S104–109. [PMC free article: PMC3388025] [PubMed: 22752857]
- 126.
- Eyre DW, Babakhani F, Griffiths D, et al. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for preventing reinfection and relapse of infection with Clostridium difficile. The Journal of infectious diseases. 2014;209(9):1446–1451. [PMC free article: PMC3982846] [PubMed: 24218500]
- 127.
- Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. Journal of clinical gastroenterology. 2004;38(6):475–483. [PubMed: 15220681]
- 128.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289. [PubMed: 19778623]
- 129.
- Bakken JS, Polgreen PM, Beekmann SE, Riedo FX, Streit JA. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24. [PubMed: 24012687]
- 130.
- Lofgren ET, Moehring RW, Anderson DJ, Weber DJ, Fefferman NH. A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infection Control & Hospital Epidemiology. 2014;35(1):18–27. [PMC free article: PMC3977703] [PubMed: 24334794]
- 131.
- Rao K, Young VB, Aronoff DM. Fecal microbiota therapy: ready for prime time? Infection control and hospital epidemiology. 2014;35(1):28–30. [PMC free article: PMC4131295] [PubMed: 24334795]
- 132.
- You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Annals of Internal Medicine. 2008;148(8):632–633. [PubMed: 18413636]
- 133.
- Gallegos-Orozco JF, Paskvan-Gawryletz CD, Gurudu SR, Orenstein R. Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Revista de gastroenterologia de Mexico. 2012;77(1):40–42. [PubMed: 22450020]
- 134.
- Neemann K, Eichele DD, Smith PW, Bociek R, Akhtari M, Freifeld A. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant infectious disease : an official journal of the Transplantation Society. 2012;14(6):E161–165. [PubMed: 23121625]
- 135.
- Trubiano JA, Gardiner B, Kwong JC, Ward P, Testro AG, Charles PG. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. European journal of gastroenterology & hepatology. 2013;25(2):255–257. [PubMed: 23117471]
- 136.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(10):994–1002. [PubMed: 22002980]
- 137.
- Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. The American Journal of Gastroenterology. 2012;107(5):761–767. [PubMed: 22290405]
- 138.
- Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Archives of Internal Medicine. 2012;172(2):191–193. [PubMed: 22271132]
- 139.
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. American Journal of Gastroenterology. 2013;108(4):500–508. [PubMed: 23511459]
- 140.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine. 2013;368(5):407–415. [PubMed: 23323867]
- 141.
- Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(9):1572–1576. [PubMed: 24440222]
- 142.
- Emanuelsson F, Claesson BE, Ljungstrom L, Tvede M, Ung KA. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scandinavian Journal of Infectious Diseases. 2014;46(2):89–97. [PubMed: 24354958]
- 143.
- Friedman-Moraco RJ, Mehta AK, Lyon GM, Kraft CS. Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(2):477–480. [PMC free article: PMC4350815] [PubMed: 24433460]
- 144.
- Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. The American Journal of Gastroenterology. 2014;109(7):1065–1071. [PMC free article: PMC5537742] [PubMed: 24890442]
- 145.
- Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Alimentary Pharmacology & Therapeutics. 2014;39(10):1003–1032. [PubMed: 24641570]
- 146.
- Youngster I, Sauk J, Pindar C, et al. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clinical Infectious Diseases. 2014;58(11):1515–1522. [PMC free article: PMC4017893] [PubMed: 24762631]
- 147.
- Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology & Therapeutics. 2015;41(9):835–843. [PubMed: 25728808]
- 148.
- Lagier JC, Delord M, Million M, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2015. [PubMed: 25947205]
- 149.
- Zanella Terrier MC, Simonet ML, Bichard P, Frossard JL. Recurrent Clostridium difficile infections: the importance of the intestinal microbiota. World journal of gastroenterology. 2014;20(23):7416–7423. [PMC free article: PMC4064086] [PubMed: 24966611]
- 150.
- Lee CH, Steiner T, Petrof EO, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. Jama. 2016;315(2):142–149. [PubMed: 26757463]
- 151.
- Fischer M, Allegretti JR, Smith M, et al. A multi-center, cluster randomized dose-finding study of fecal microbiota transplantation capsules for recurrent Clostridium difficile infection. United European Gastroenterology Week 2015 (oct 24–28, 2015);. 2015;OP054-LB703.
- 152.
- Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. The American Journal of Gastroenterology. 2013;108(10):1620–1630. [PubMed: 24060759]
- 153.
- De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(8):1036–1038. [PubMed: 23669309]
- 154.
- Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflammatory bowel diseases. 2013;19(10):2155–2165. [PubMed: 23899544]
- 155.
- Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered enthusiasm for fecal transplant. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(2):319. [PubMed: 24759832]
- 156.
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiological Reviews. 2010;90(3):859–904. [PubMed: 20664075]
- 157.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. The American Journal of Gastroenterology. 2012;107(7):1079–1087. [PubMed: 22450732]
- 158.
- Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(5):598–605. [PubMed: 19191646]
- 159.
- Ferrada P, Velopulos CG, Sultan S, et al. Timing and type of surgical treatment of Clostridium difficile-associated disease: A practice management guideline from the Eastern Association for the Surgery of Trauma. The Journal of Trauma and Acute Care Surgery. 2014;76(6):1484–1493. [PubMed: 24854320]
- 160.
- Pepin J, Vo TT, Boutros M, et al. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Diseases of the colon and rectum. 2009;52(3):400–405. [PubMed: 19333038]
- 161.
- Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills S. Clostridium difficile colitis in the United States: a decade of trends, outcomes, risk factors for colectomy, and mortality after colectomy. Journal of the American College of Surgeons. 2013;217(5):802–812. [PubMed: 24011436]
- 162.
- Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. The American Surgeon. 2008;74(1):20–26. [PubMed: 18274423]
- 163.
- Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research C. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. The British journal of surgery. 2012;99(11):1501–1513. [PubMed: 22972525]
- 164.
- Lipsett PA, Samantaray DK, Tam ML, Bartlett JG, Lillemoe KD. Pseudomembranous colitis: a surgical disease? Surgery. 1994;116(3):491–496. [PubMed: 8079179]
- 165.
- Ferrada P, Callcut R, Zielinski MD, et al. Loop ileostomy versus total colectomy as surgical treatment for Clostridium difficile-associated disease: An Eastern Association for the Surgery of Trauma multicenter trial. J Trauma Acute Care Surg. 2017;83(1):36–40. [PMC free article: PMC5998809] [PubMed: 28426557]
- 166.
- Bass SN, Bauer SR, Neuner EA, Lam SW. Comparison of treatment outcomes with vancomycin alone versus combination therapy in severe Clostridium difficile infection. Journal of Hospital Infection. 2013;85(1):22–27. [PubMed: 23876778]
- 167.
- Kelly CR, Kahn S, Kashyap P, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149(1):223–237. [PMC free article: PMC4755303] [PubMed: 25982290]
- 168.
- Diorio C, Robinson PD, Ammann RA, et al. Guideline for the Management of Clostridium Difficile Infection in Children and Adolescents With Cancer and Pediatric Hematopoietic Stem-Cell Transplantation Recipients. J Clin Oncol. 2018:JCO1800407. [PMC free article: PMC6209092] [PubMed: 30216124]
- 169.
- Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database of Systematic Reviews. 2013;5:006095. [PubMed: 23728658]
- 170.
- Horton HA, Dezfoli S, Berel D, et al. Antibiotics for Treatment of Clostridium difficile Infection in Hospitalized Patients with Inflammatory Bowel Disease. Antimicrobial Agents and Chemotherapy. 2014;58(9):5054–5059. [PMC free article: PMC4135854] [PubMed: 24913174]
Appendix A. Available Diagnostic Tests for Toxigenic C. difficilea
| Methods | Test Target(s) | Notes on Testing |
|---|---|---|
| Reference Tests | ||
| Cytotoxigenic Culture | Toxigenic C. difficile | Expertise required Takes 24–48 hours Cultures C. difficile and determines if isolate can produce toxin in vitro |
| Cell Cytotoxicity Assay | Toxins A or Ba | Highly sensitive for toxin production Expertise required Takes 24–48 hours |
| Rapid Tests | ||
| EIAc | GDH | Must be paired with a test for toxinb Sensitivity and specificity vary considerably by manufacturer |
| EIAc | Toxins A or Ba | Sufficient for diagnosis alone Sensitivity and specificity vary considerably by manufacturer |
| NAAT | More expensive than EIA Sensitive and specific for presence of toxigenic C. difficile Possible increase in false positive rate if used alone without specimen screening: increases detection of colonization | |
| RT-PCRc | tcdBc or tcdC genes | tcdA and tcdB are the genes for toxins A and B, respectively tcdB presence is necessary for disease tcdC is a regulatory gene for toxin production |
| LAMP | tcdA or tcdB genes | Strains that are tcdA-positive / tcdB-negative do not cause disease; thus false negative results can occur with tcdA testing alone by LAMP |
CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification testing; RT-PCR, real-time polymerase chain reaction.
- a
C. difficile can produce toxin A and/or toxin B. Although both play a role in clinical disease, it is not known if strains producing only toxin A are associated with symptomatic infection in humans.
- b
Non-toxigenic C. difficile strains also exist, do not cause disease, and will be GDH positive.
- c
Test available at University of Michigan (EIA for GDH, EIA for toxin A, and RT-PCR)
Created: December 2016; Last Update: December 2019.
- Patient population:
- Clinical Background
- Rationale for Recommendations
- Clinical Problem and Management Issues
- Diagnosis
- Disease Classification
- Special Populations
- Prevention
- Treatment Response
- Related National/International Guidelines
- Guideline Development Methodology
- Review and Endorsement
- References
- Available Diagnostic Tests for Toxigenic C. difficile
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Review Rapid detection of Clostridium difficile toxins and laboratory diagnosis of Clostridium difficile infections.[Infection. 2017]Review Rapid detection of Clostridium difficile toxins and laboratory diagnosis of Clostridium difficile infections.Chen S, Gu H, Sun C, Wang H, Wang J. Infection. 2017 Jun; 45(3):255-262. Epub 2016 Sep 6.
- Review Updates in the epidemiology and management of candidemia and Clostridioides difficile coinfection.[Expert Rev Anti Infect Ther. 2...]Review Updates in the epidemiology and management of candidemia and Clostridioides difficile coinfection.Falcone M, Tiseo G, Venditti M, Menichetti F. Expert Rev Anti Infect Ther. 2019 May; 17(5):375-382. Epub 2019 May 3.
- The Clinical and Laboratory Impact of Upgrading Clostridioides (formerly Clostridium) difficile Infection Testing from Routine to Molecular Based-Algorithm: an Observational Case-Study from the Eastern Province, Saudi Arabia.[Clin Lab. 2019]The Clinical and Laboratory Impact of Upgrading Clostridioides (formerly Clostridium) difficile Infection Testing from Routine to Molecular Based-Algorithm: an Observational Case-Study from the Eastern Province, Saudi Arabia.Alnimr A, Alwazzeh MJ, Al-Ameer A. Clin Lab. 2019 Aug 1; 65(8).
- Review The changing epidemiology of Clostridium difficile infection.[Curr Opin Gastroenterol. 2014]Review The changing epidemiology of Clostridium difficile infection.Honda H, Dubberke ER. Curr Opin Gastroenterol. 2014 Jan; 30(1):54-62.
- Comparison of Clostridium difficile isolates from individuals with recurrent and single episode of infection.[Anaerobe. 2015]Comparison of Clostridium difficile isolates from individuals with recurrent and single episode of infection.Richardson C, Kim P, Lee C, Bersenas A, Weese JS. Anaerobe. 2015 Jun; 33:105-8. Epub 2015 Mar 11.
- Clostridioides difficile Infection in Adults and ChildrenClostridioides difficile Infection in Adults and Children
Your browsing activity is empty.
Activity recording is turned off.
See more...