Acronyms and Abbreviations
- 3TC
lamivudine
- ADV
adefovir dipivoxil
- ALT
alanine aminotransferase
- ANC
antenatal care
- APASL
Asian Pacific Association for the Study of the Liver
- CI
confidence interval
- CK
creatine kinase
- ETV
entecavir
- FTC
emtricitabine
- GHSS
Global Health Sector Strategy (on viral hepatitis)
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immunoglobulin
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HDV
hepatitis D virus
- HIV
human immunodeficiency virus
- ITT
intention to treat
- LdT
telbivudine
- MTCT
mother-to-child transmission
- OR
odds ratio
- PICO
Population, Intervention, Comparison, Outcome
- PMTCT
prevention of mother-to-child transmission
- PPA
per protocol analysis
- RCT
randomized controlled trial
- TAF
tenofovir alafenamide fumarate
- TDF
tenofovir disoproxil fumarate
- ULN
upper limit of normal
- WHO
World Health Organization
Background
Currently, the World Health Organization (WHO) estimates that chronic hepatitis B virus (HBV) infection affects close to 260 million persons and causes an estimated 900 000 deaths annually through manifestations of chronic liver disease, such as cirrhosis and hepatocellular carcinoma (HCC). The regions with the highest prevalence of chronic HBV infection are the Western Pacific and African regions (WHO, 2017a). In 2016, the World Health Assembly endorsed the Global Health Sector Strategy (GHSS) on viral hepatitis, which calls for the elimination of HBV worldwide as a public health threat by 2030, to be accomplished through reducing the incidence of chronic HBV infection by 90%, and its mortality by 65% (WHO, 2016).
Chronic infection is more likely to develop when HBV is acquired early in life, and therefore, perinatal mother-to-child transmission (MTCT) is a major contributor to the incidence of chronic HBV infection (Edmunds et al., 1993). Moreover, the risk of developing chronic liver disease, including HCC, may be higher in those with established chronic HBV infection through MTCT compared to those who ended up with chronic HBV infection through horizontal transmission later in life (Chang 2008; Shimakawa et al., 2013). To decrease the incidence of chronic HBV infection and eventual chronic liver disease, WHO recommends that all infants be vaccinated against the virus, with the first dose being administered within 24 hours of birth (i.e. timely birth dose vaccination) (WHO, 2017b). Since this recommendation made by WHO in 2009, there has been a significant uptake of the HBV birth dose vaccination globally; however, there are many countries, specifically in highly endemic areas in Africa, where coverage of timely administration is very low (Miyahara et al., 2016; WHO, 2009; WHO, 2017a).
The birth dose vaccination is meant not only to prevent perinatal MTCT that usually happens at the time of birth, but also to prevent horizontal transmission during early childhood. However, the birth dose vaccination alone may be inadequate to prevent MTCT in infants born to mothers with high replication of HBV. In some countries, therefore, hepatitis B immunoglobulin (HBIG) is additionally administered to babies born to HBV-infected mothers. However, this combined active and passive immunoprophylaxis does not completely prevent all MTCT (Chen et al., 2012). The risk of immunoprophylaxis failure is closely correlated with hepatitis B e-antigen (HBeAg) positivity as well as an elevated viral load in pregnant women (Keane et al., 2016; Machaira et al., 2015; Wen et al., 2013). Consequently, MTCT remains a significant contributor to HBV incidence in all regions, and supplementary interventions to further decrease this transmission are urgently needed.
AIM
Rationale
To date, the major international guidelines for the management of chronic HBV infection all recommend the administration of antiviral therapy to pregnant women with high HBV DNA levels to prevent MTCT (AASLD 2018; EASL 2017; APASL 2016); all guidelines recommend tenofovir disoproxil fumarate (TDF), and the Asian Pacific Association for the Study of the Liver (APASL) also recommends telbivudine (LdT). The Food and Drug Administration (FDA) of the United States has classified TDF, LdT, and emtricitabine (FTC) as being category B (i.e. no current evidence of a risk to the fetus during pregnancy; however, robust controlled studies are lacking) (see Table 1).
Table 1
International recommendations for prevention of mother-to-child transmission (PMTCT) using antiviral therapy.
Although the WHO HBV treatment guidelines in 2015 contained a systematic review and meta-analysis on the efficacy, safety and cost–effectiveness of antiviral therapy administered during pregnancy for the prevention of MTCT (PMTCT), this review had some limitations, which dissuaded WHO from making a formal recommendation for its use at that time. In addition, only one observational study that examined the efficacy of tenofovir for PMTCT was available for inclusion; tenofovir is considered a key first-line antiviral therapy for chronic HBV infection given its high potency, higher barrier to drug resistance and evidence of safety in pregnancy (WHO, 2015).
An updated systematic review and meta-analysis on this topic is now pertinent for various reasons. First, there have been important new findings with regard to maternal and infant safety of HBV antiviral medications administered during pregnancy; some recent studies have further evaluated the risk of postpartum hepatic flare in the mother after cessation of treatment as well as changes in bone mineral density in the infant (Pan et al., 2016; Kourtis et al., 2018; Jourdain et al., 2018). Second, recent epidemiological and modelling studies have demonstrated the likely inadequacy of the birth dose vaccination with or without HBIG administration, alone, to reduce the incidence of HBV enough in order to achieve the 2030 elimination goals (Nayagam et al., 2016; Hutin et al., 2018). Third, in countries that have achieved a very high uptake of birth dose vaccination, recommendations are now needed for a further reduction in MTCT.
Objectives
The primary objective is to provide an up-to-date summary estimate of the efficacy, and an overview of the safety of antiviral medicines administered during pregnancy for the reduction of MTCT of HBV; this is meant to inform the WHO’s new guidelines on PMTCT of HIV.
Specific objectives included:
- exploration of the sources of between-study heterogeneity in the efficacy of antiviral treatment, done through subgroup analyses in which there is stratification by the following variables:
- -
maternal HBV viral load threshold at inclusion (e.g. >5 log10 IU/mL, >6 log10 IU/mL, >7 log10 IU/mL)
- Note: this refers to the minimum threshold imposed by each individual study protocol and does not guarantee that each woman enrolled in the study has a viral load at that level. This measure, rather than the mean or median viral load of women in each study, was preferred, as this would have a direct implication for practice
- -
maternal hepatitis B e antigen (HBeAg) serostatus
- -
stage of pregnancy
- 1st, 2nd vs 3rd trimester
- median <28 weeks, median 28 weeks (with maximum range of 26–30 weeks), median >28 weeks
- -
coinfection with hepatitis D virus (HDV) or human immunodeficiency virus (HIV);
- -
type of antiviral therapy administered
- -
type of other preventive measures provided (infant hepatitis B vaccines with or without timely administration of birth dose, HBIG and a combination of these)
- -
WHO region;
- providing an updated Grading of Recommendations Assessment, Development and Evaluation (GRADE) review for the use of antiviral medication for reduction of HBV MTCT;
- identifying gaps in research.
Methods
Narrative review question
Are antiviral therapies efficacious and safe at reducing MTCT of HBV if administered during pregnancy in women with chronic HBV infection?
PICO question
Population
Pregnant women with chronic HBV infection
- Chronic HBV infection was defined as HBsAg seropositivity on two occasions at least 6 months apart. However, because new HBV infection in adults is uncommon in highly endemic areas where the vast majority of HBsAg-positive people acquired the infection perinatally or during childhood, HBsAg positivity on only one occasion (at antenatal care [ANC]) in women living in highly prevalent countries was assumed to reflect chronic HBV infection (Evans et al., 1998).
Intervention
Maternal treatment with antiviral therapy during pregnancy with or without infant birth dose vaccination and/or HBIG.
- The following antiviral therapies were considered for inclusion:
- adefovir dipivoxil (ADV)
- emtricitabine (FTC)
- entecavir (ETV)
- lamivudine (3TC/LAM)
- telbivudine (LdT)
- tenofovir alafenamide fumarate (TAF)
- TDF.
Comparison
Table 2
Comparison groups considered in PICO1.
Outcomes
The primary outcome of interest will be MTCT of HBV, as indicated by infant HBsAg positivity at 6–12 months of life.
Further infant outcomes of interest, specified in the study protocol, included:
- infant HBV DNA positivity at 6–12 months of life
Maternal outcomes of interest, specified in the study protocol, included:
- any maternal adverse event, including:
- -
miscarriage (<28 weeks gestational age, [WHO, https://www
.who.int/maternal _child_adolescent /epidemiology/stillbirth/en/]) - -
stillbirth (>=28 weeks gestational age, [WHO, https://www
.who.int/maternal _child_adolescent /epidemiology/stillbirth/en/.]) - -
HBV flare after discontinuation of treatment (e.g. elevated HBV DNA and/or elevated ALT)
- -
postpartum haemorrhage
- antiviral resistance.
Other inclusion and exclusion criteria: study design, languages, dates of publication
Randomized controlled trials (RCTs) and non-randomized comparative studies were considered for this analysis. Case series without a comparison group were excluded. Studies published in any language were considered. Non-RCTs with a high risk of bias (i.e. a score on the Newcastle-Ottawa scale of <=5 were excluded from analysis. Studies published till 28 March 2019 were included. Studies reported as conference abstracts only were not considered.
Search strategy
The search terms employed covered hepatitis B infection AND antiviral therapy, AND pregnancy. The databases searched included: four English-language databases (PubMed, EMBASE, Scopus, and CENTRAL [the Cochrane Library]); and two Chinese-language databases (the China National Knowledge Infrastructure (CNKI) and the Wanfang database). The exact search strategies used are given in Appendix A.
A manual search through the references of included studies, as well as through those of relevant systematic reviews identified through the literature search, was undertaken to identify any further eligible studies.
Conduct of the review
Titles and abstracts for all of the publications identified by the search strategy were independently screened for relevance by two reviewers (ALF and KY). Following selection of potentially eligible studies, a full-text reading and reviewing was independently performed. Finally, the two reviewers discussed the list of eventually eligible studies, and if discrepancies existed that could not be resolved between the two persons, a third person (YS) was consulted in order to make the final decision. For Chinese databases, the same procedure was followed, by two independent Chinese reviewers (YL and TZ).
For all potentially eligible studies, if information was lacking within the full-text article that limited the ability to make a final decision on whether or not the study should be included, the corresponding author of that study was contacted by mail or phone.
The final protocol for this review was registered on the international prospective register of systematic reviews (PROSPERO) with the registration number: CRD42019134614.
Quality appraisal
RCTs were assessed using the Cochrane Collaboration risk of bias tool (Higgins et al., 2011) (see Appendix B). Observational comparative studies that are included were evaluated using the Newcastle–Ottawa Scale (Wells et al., 2014) (see Appendix C). For both RCTs and non-RCTs, each study was independently assessed by two reviewers, with discrepancies being discussed and resolved with the involvement of a third reviewer (YS) when necessary.
Data extraction
The data were extracted from the selected studies by the two independent reviewers using a pre-piloted data extraction form; the information that was extracted can be found in Appendix D. In case of disagreement in the data extracted between the two reviewers, a deliberation that involved a third person (YS), was carried out. During data extraction, articles from the same study sites with overlapping recruitment periods, enrolment criteria, and treatment types were considered as being part of one study. The lead reviewer for both English (AF) and Chinese (YL) articles then followed up with the corresponding author(s) from each of the article groups in order to understand if there was any patient overlap. If authors explicitly stated in their article that there was overlap, or if the authors responded to the email enquiry confirming overlap, or if the author did not respond, then only the data extracted from the most recently published article were used in data analysis. If authors responded negating any patient overlap between articles then data extracted from all articles within the group were used. In the case of a group of articles from the same study where some articles were published in Chinese and some in English, the latest English article was included in the data analysis sheet, unless a direct communication with the study authors directed the reviewers to use a different article in the group.
GRADE review process
For each examined treatment comparison, the quality of the evidence studied was evaluated using the GRADE methodology (The GRADE Working Group, 2004). We used this tool to evaluate the risk of bias, inconsistency (high heterogeneity), imprecision (confidence intervals), indirectness (use of surrogate outcomes), reporting and publication bias, and other factors, within each intervention group (i.e. antiviral treatment used as the intervention) from which the evidence was summarized within the review. This eventually gave a score of high (further research is very unlikely to change the effect estimate), moderate, low or very low (all estimates are very uncertain). Decisions for the complex judgements within the GRADE table were made through study group consensus. The study group reviewers were supported in the process of completing this GRADE template through discussion and advice from a WHO-designated methodological expert, Professor Roger Chou (Oregon Health and Science University, USA). For this specific meta-analysis, the following rules were used to determine whether or not a group of studies had no serious, serious or very serious issues with regard to the GRADE criteria:
- -
Limitations – this was rated as “not serious” only in the following circumstances: for RCTs, if >50% of the included studies had “low risk of bias” for the majority of criteria according to the Cochrane Collaboration risk of bias assessment tool; for non-RCTs, if >50% of studies had a “low risk of bias” assessment as per the Newcastle–Ottawa risk of bias assessment tool
- -
Inconsistency – I2<30%= “not serious”, I2>=30% and <60%=“serious”, I2>60%= “very serious”
- -
Indirectness – all studies were considered to have “no serious” issues as this was guaranteed by the PICO question specifications
- -
Imprecision – for odds ratios (ORs), an absolute range in the 95% confidence intervals of 0.5 was considered as “no serious”, a range >=0.5 and <1.0 was considered as “serious”, and a range of >=1 was considered as “very serious”. For risk difference estimates, an absolute range in the 95% confidence intervals of 0.1 was allowed, with the upper range going only as high as 0.05 (indicating a potential harmful effect of treatment in 5% of persons) for a set of studies to be considered as having “no serious” limitations.
- -
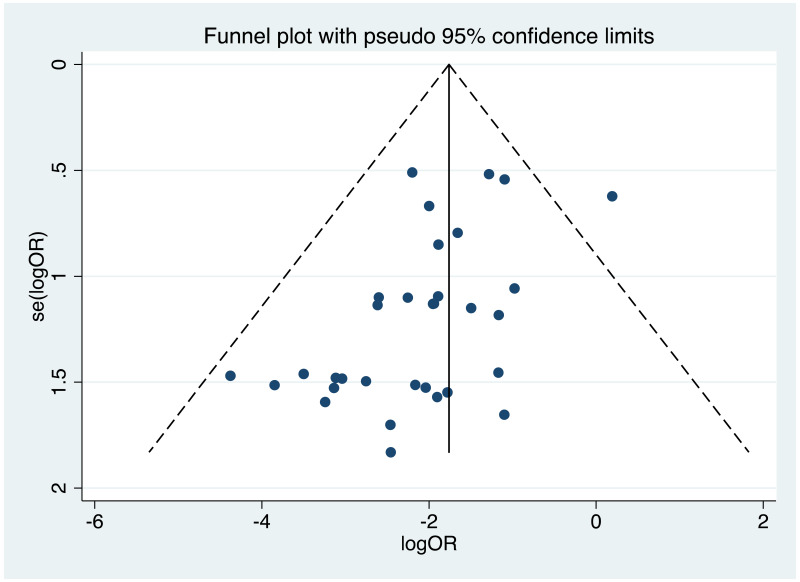
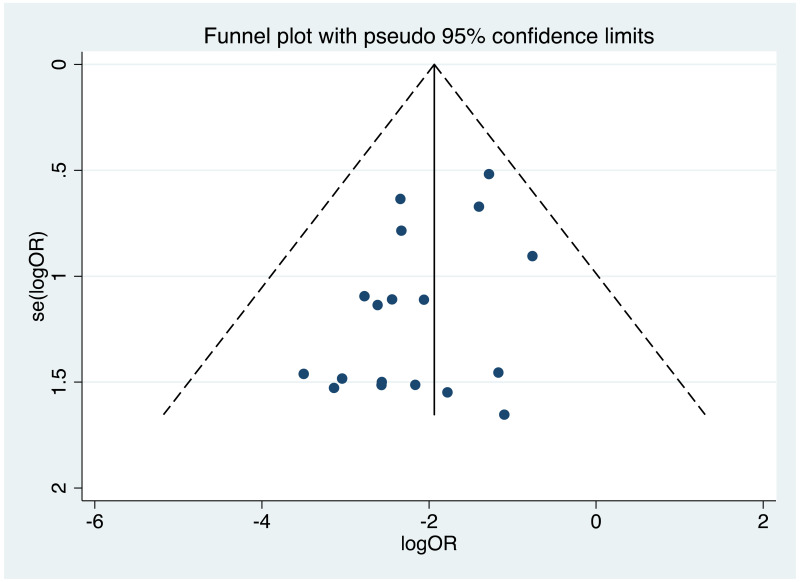
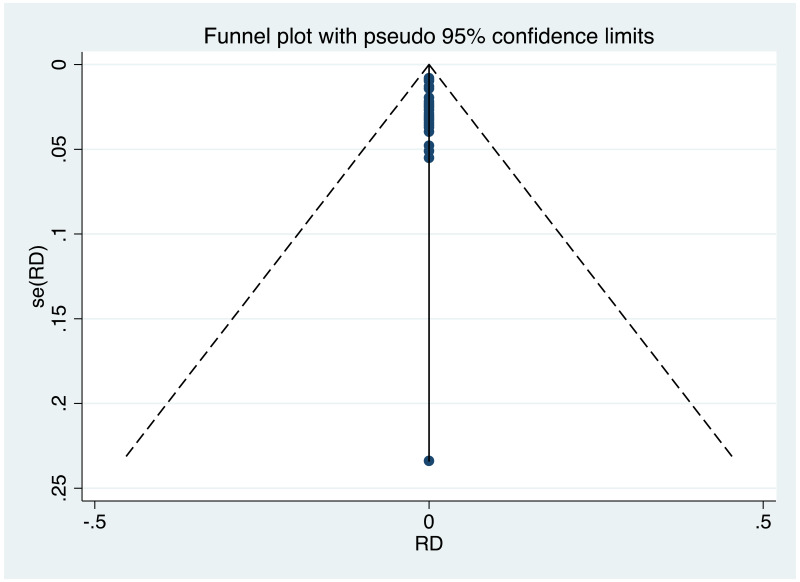
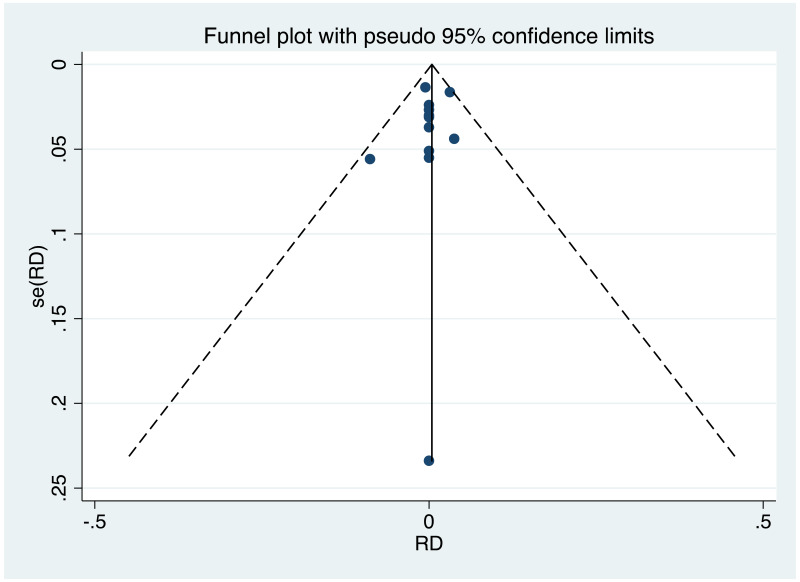
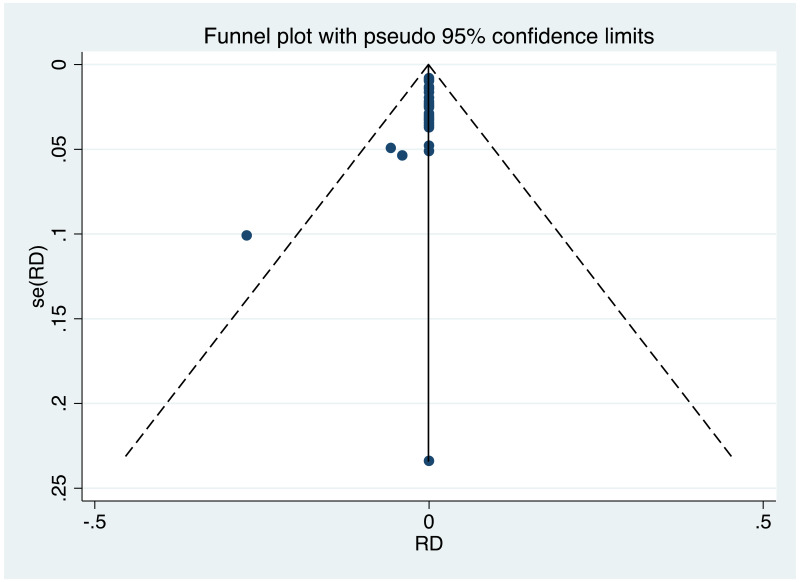
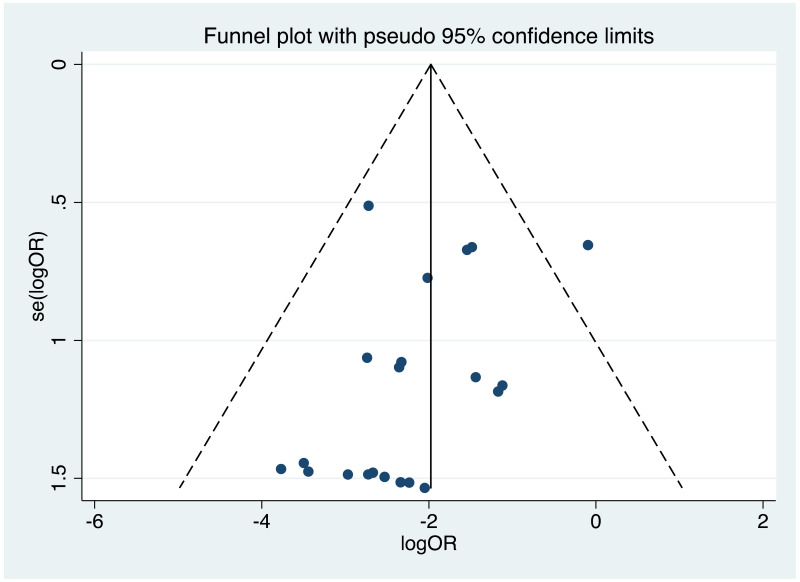
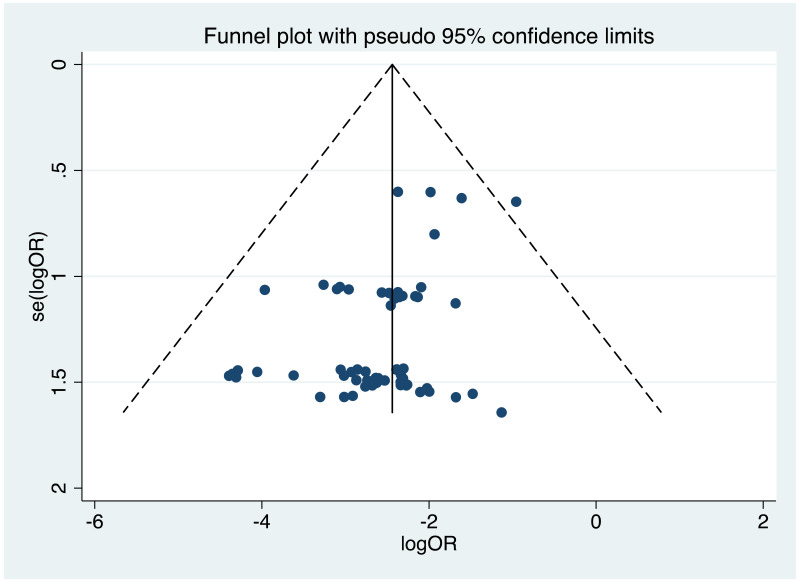
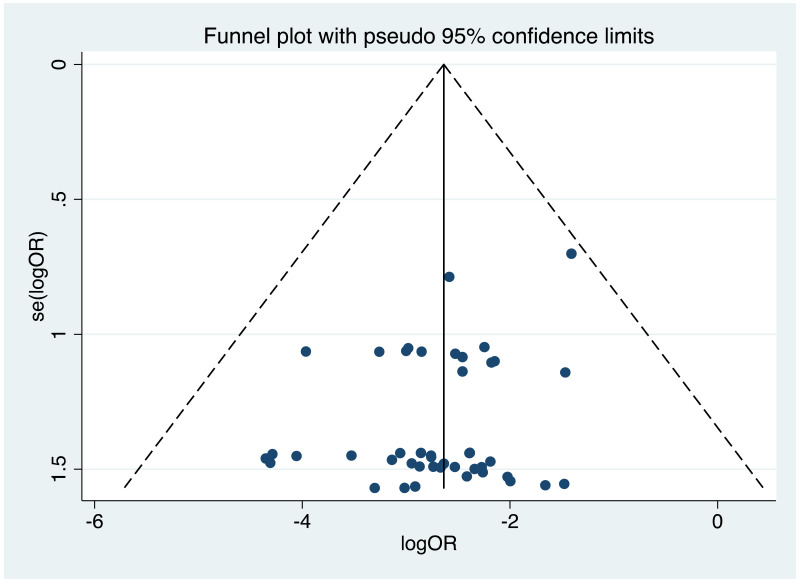
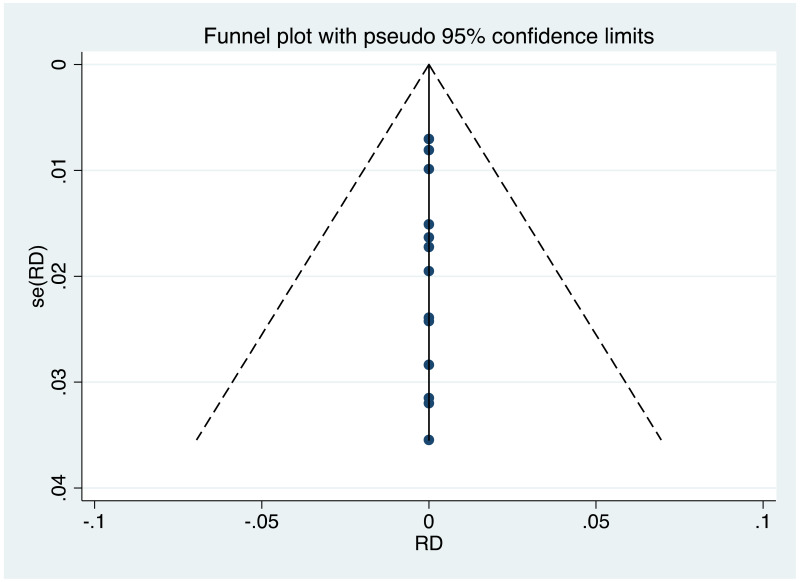
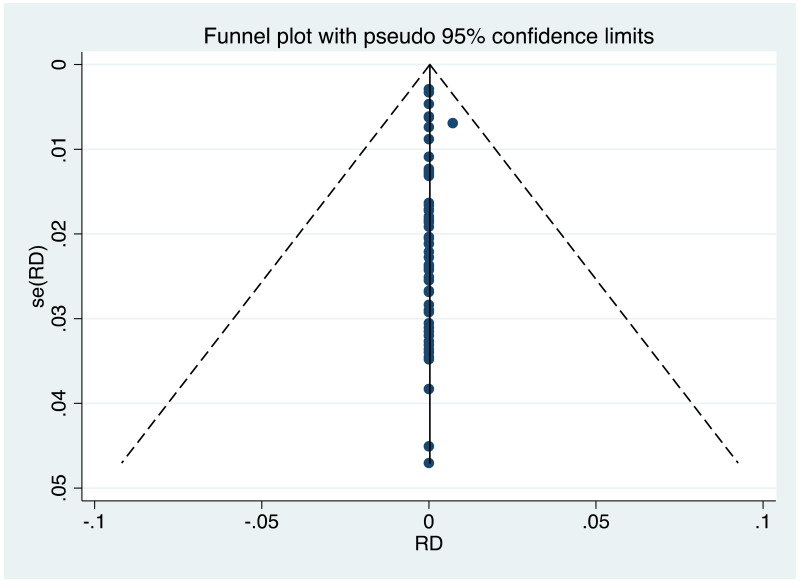
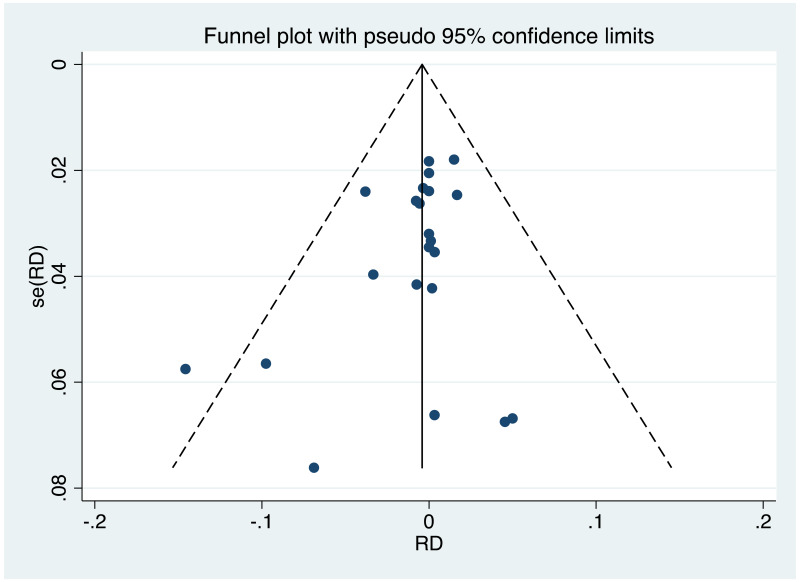
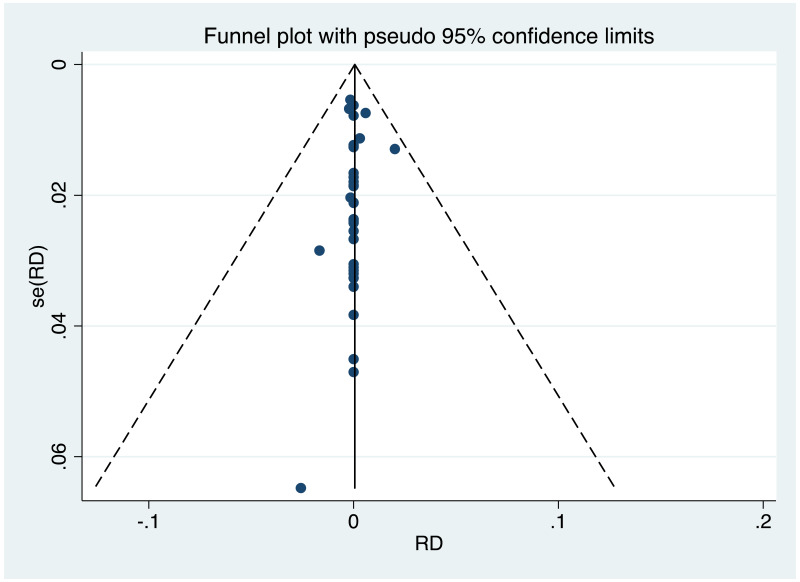
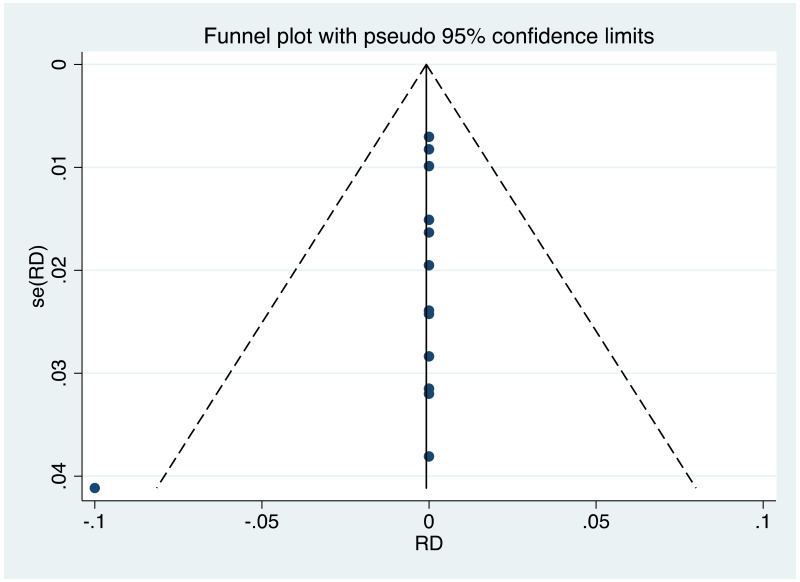
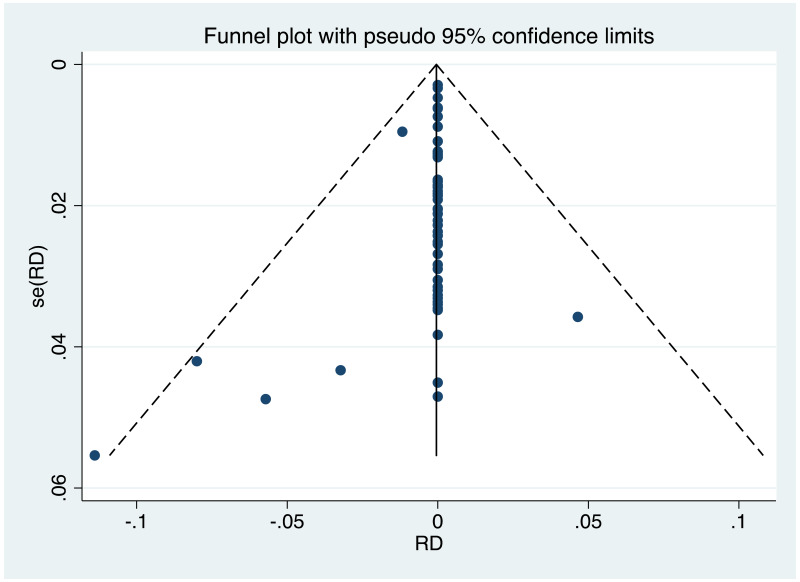
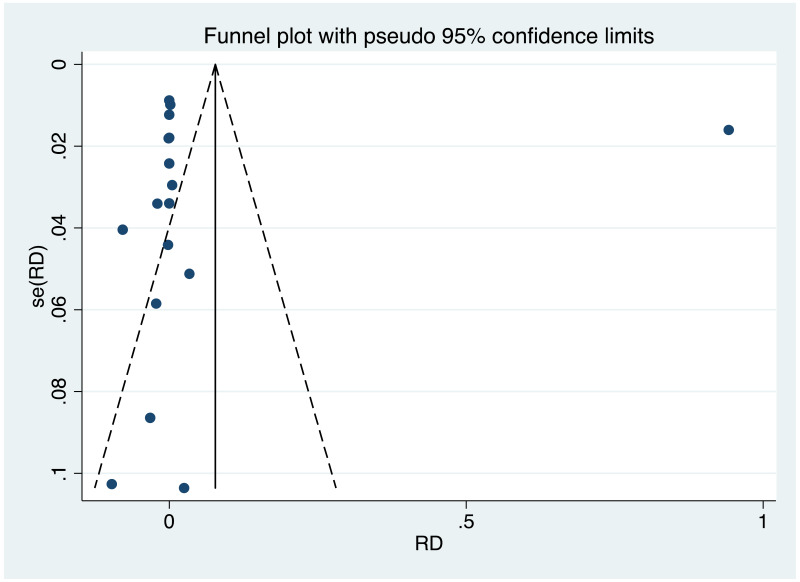
Publication bias – An Egger’s test with p value of <0.05 led to assumption of “possible evidence of publication bias or small study effects” if ORs had been estimated. Where risk difference estimates, only, were estimated, an obviously asymmetrical funnel plot led to the same assumption.
- -
Other – a non-RCT study set could be upgraded for “magnitude of effect” if the protective OR was <0.5 and was not considered as imprecise.
Data synthesis
All statistical analyses were done using STATA version 13 (StataCorp LP, CollegeStation, TX). The pooled OR was generated for the efficacy of antiviral therapy. For safety outcomes, the pooled risk difference was generated. If more than three original studies were eligible for the analysis/subanalysis, then pooling was done using Der Simonian and Laird random-effect models. Where possible, data were analysed according to intention to treat (ITT) – meaning that patients would be included in the group they were initially randomized to, regardless of dropout, loss to follow up or regimen changes. Heterogeneity was estimated using the Mantel–Haenszel model. The amount of overall heterogeneity between studies was measured using the I2 statistic. Where the number of eligible studies (i.e. at least 10 studies, Sterne et al., 2011) and their level of heterogeneity allowed, funnel plots were used to examine the risk of publication bias. When pooled ORs had been estimated, the Egger test was used to assess asymmetry.
Results
Summary of included studies
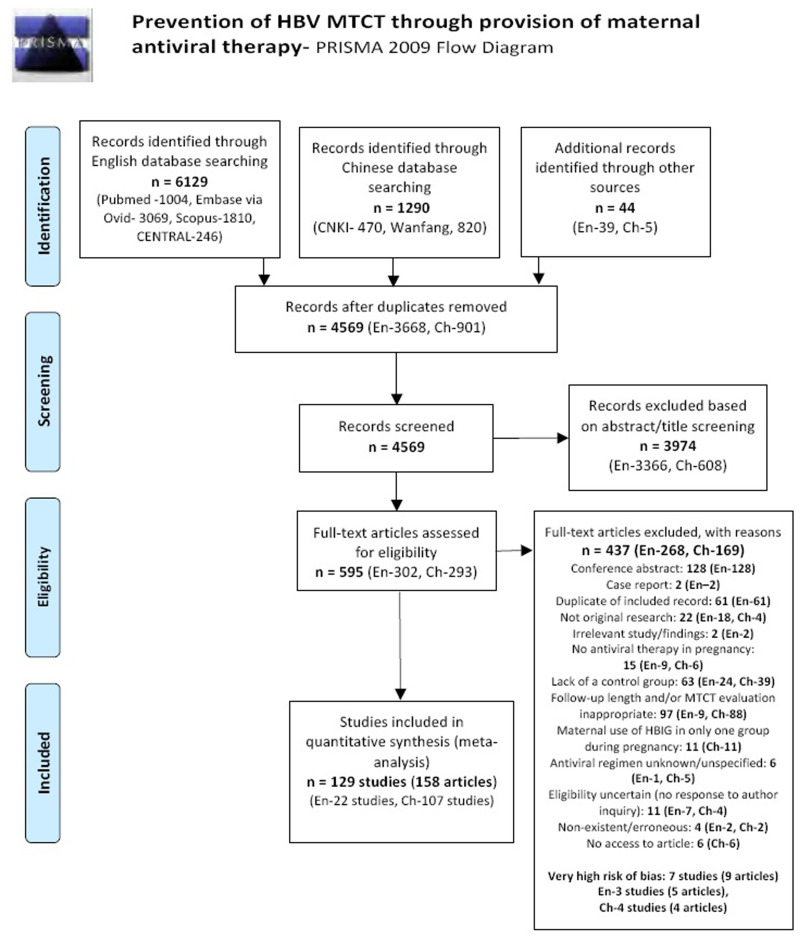
The search strategy identified 7419 papers across English and Chinese databases. An additional 44 articles were manually included. After excluding 2894 articles that were duplicates, 4569 articles were screened and 595 papers were assessed in full text. Finally, 136 original studies were potentially eligible; however, seven of these were deemed at a very high risk of bias and were excluded from the quantitative analysis in this review (see Fig. 1). Although the objectives of this meta-analysis as well as its search strategy included seven different treatments of interest, only studies including TDF 300 mg, LAM 100–150 mg, LdT 100 mg and 600 mg, and ADV 10 mg and 500 mg were found eligible. No studies that investigated any regimens with FTC, ETV, TAF were included (Table 3).
Table 3
Excluded and included studies by treatment, with summary of analyses types presented.
Very few of the RCTs included presented adequate details of loss to follow up (7/33) (Bai HL, 2013; Feng Y, 2018; Jourdain G, 2018; Lin Y, 2018; Pan CQ, 2016; Xu WM, 2009; Zhang LJ, 2009), which limited our ability to perform ITT meta-analysis systematically; therefore, per protocol analysis (PPA) was considered throughout (Table 4).
Table 4
Summary of quantitative/qualitative results presented by the type of treatment.
Fig. 1PRISMA diagram
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097 For more information, visit www.prisma-statement.org. [PMC free article: PMC2707599] [PubMed: 19621072] [CrossRef]
Tenofovir disoproxil fumarate (TDF) 300 mg versus no treatment or placebo
Summary of included studies
There were 20 original studies, including 26 unique treatment arms, eligible for this meta-analysis that used TDF 300 mg. Following risk of bias assessment, one study (with one treatment arm) was excluded (Kochaksaraei et al., 2016). Therefore, 19 original studies with 25 unique treatment arms were included in the analysis. Of the included studies, five were RCTs and 14 were non-randomized trials/observational studies (six prospective and eight retrospective studies).
Risk of bias assessment
- Randomized controlled trials
Of the five RCTs included that investigated TDF, only one study by Jourdain et al., (2018) achieved a “low risk of bias” rating on the main criteria in the Cochrane Collaboration’s Risk of Bias Assessment Tool; only one domain – attrition bias for maternal safety outcomes – was identified as possibly at high risk of bias. Another study, by Pan and colleagues (2016) was deemed at low risk of bias on five of the eight evaluated criteria; however, no allocation concealment was described and blinding was not performed, leading to this study being at a high risk for some selection bias, as well as for performance and detection bias. The other three RCTs were all deemed low risk on the majority of criteria evaluated; the main issues revolved around apparent limited use of blinding and lack of reporting on loss to follow up (Lin Y et al., 2018; Liu MH et al., 2017; Yu CY et al., 2018). The detailed risk of bias assessment for the RCTs investigating TDF can be found in Appendix E.
- Non-randomized controlled trials
The majority of studies (73.3%) were ranked at a score of 6 (high) to 7 (low) on the Newcastle Risk of Bias scale, and only three studies achieved scores of 8–9 on the scale (signifying very low risk of bias). The main weakness of included studies was in reference to loss to follow up – this information was missing in 11 of 15 articles, and was less than adequate (i.e. <80% follow up) in two further studies. The detailed risk of bias assessment for the non-RCTs investigating TDF can be found in Appendix F (Table 5).
Table 5
Risk of bias scores for non-RCTs (prior to exclusion of very high-risk studies).
Publication bias/small study effects assessment
It was possible to examine publication bias for the following outcomes: MTCT indicated by HBsAg positivity at 6–12 months in non-RCTs, neonatal deaths in non-RCTs, and miscarriages and stillbirths in non-RCTs. Of these, there was possible evidence of publication bias only in the first study set (MTCT indicated by HBsAg positivity at 6–12 months in non-RCTs). Funnel plots for TDF 300 mg study sets, as well as results of the Egger test for asymmetry (if examining OR only) can be found in Appendix G.
Characteristics of included studies
Across all included studies (n=19), recruitment took place as early as 2007 and up until 2018. Almost all studies took place in the WHO Western Pacific Region; including China (n=15), Japan (n=1) and Australia (n=1). Additionally, one study took place in the WHO South-East Asia Region (Thailand), and one study in the WHO European Region (Turkey).
HBV genotyping for the entire study population was performed only in three instances. A study from China estimated that the treatment group was 70% genotype B and 30% genotype C, while the control group was 71% B and 29% C (Chen HL et al., 2015). One Chinese study estimated the treatment group as 7% B2 and 93% C2, with the control group being 6% B2 and 94% C2 (Lin Y et al., 2018). In a small study in Japan (n=8), 50% of participants were genotype C and the other 50% had undetermined genotype (Wakano Y et al., 2018).
Most included study arms (i.e. 14/25) started maternal antiviral therapy between 24 and 30 weeks of gestation. The most common HBV DNA level designated for inclusion was >6.0 or >6.3 log10 IU/mL (11 of 25 treatment arms) (table 6).
Table 6
Characteristics of included studies investigating TDF (n=19).
Primary efficacy analysis, narrative descriptions and forest plots
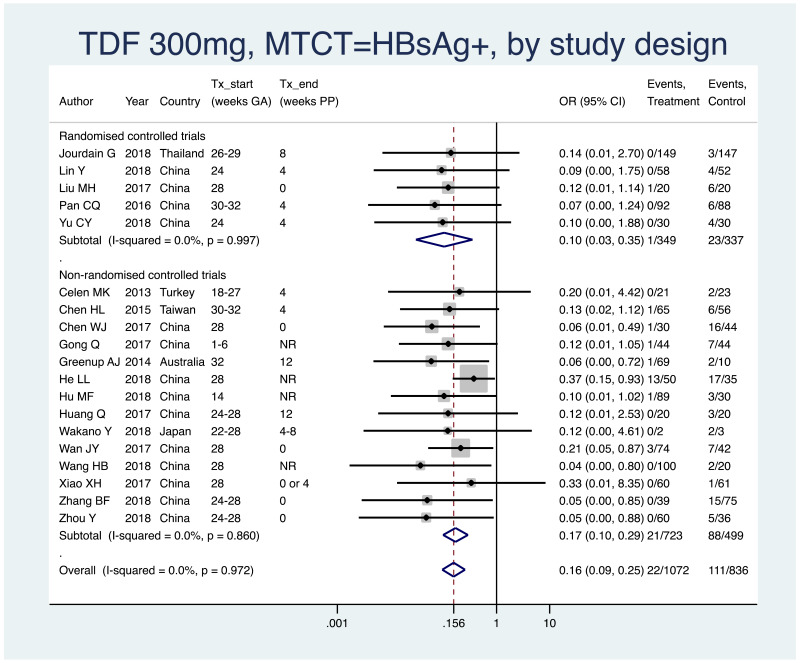
- 1.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT).
- Overall pooled OR=0.16 (95% CI: 0.10–0.26), P<0.001, I2=0%
- RCTs only: pooled OR=0.10 (95% CI: 0.03–0.35), P<0.001, I2=0%
- Non-RCTs only: pooled OR=0.17 (95% CI: 0.10–0.29), P<0.001, I2=0%
- When looking at heterogeneity between RCTs and non-RCTs we arrive at a P value of 0.47, indicating no difference between the estimates.
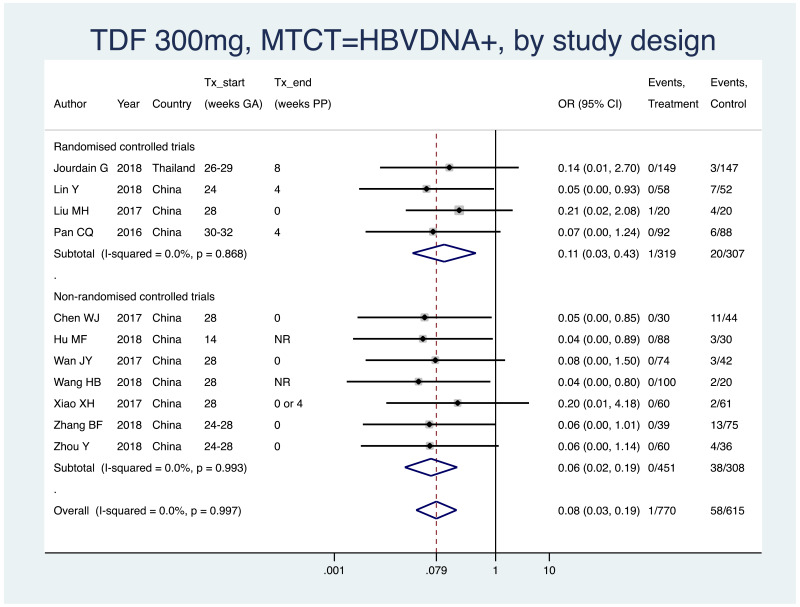
- 2.
PMTCT, as indicated by detection of HBV DNA at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT).
- Overall pooled OR=0.08 (95% CI: 0.03–0.19), P<0.001, I2=0%
- RCTs only: pooled OR=0.11 (95% CI: 0.03–0.43), P=0.001, I2=0%
- Non-RCTs only: pooled OR=0.06 (95% CI: 0.02–0.19), P<0.001, I2=0%
- When looking at heterogeneity between RCTs and non-RCTs we arrive at a P value of 0.52, indicating no difference between the estimates.
Subgroup analysis
In the protocol, it was specified that subgroup analysis would be performed by the following variables: type of antiviral therapy administered, stage of pregnancy at time of treatment start, maternal HBV viral load and HBeAg status, coinfections (e.g. HDV, HIV), other preventive measures provided (i.e. infant immunoprophylaxis), and WHO region where the study was conducted. Finally, all analyses have been presented by treatment type (no “all treatment” analysis was performed), and within that, it was possible to do subgroup analysis by stage of pregnancy, maternal HBV viral load and HBeAg status, and types of other preventive measures provided. It was not possible to do a subgroup analysis by coinfection status, as there were eventually no eligible studies that included coinfected populations. Furthermore, it was not possible to do subgroup analysis by WHO region, as almost all studies came from just one region (i.e. Western Pacific). For TDF, one additional subgroup analysis was presented, which is by timing of treatment end postpartum.
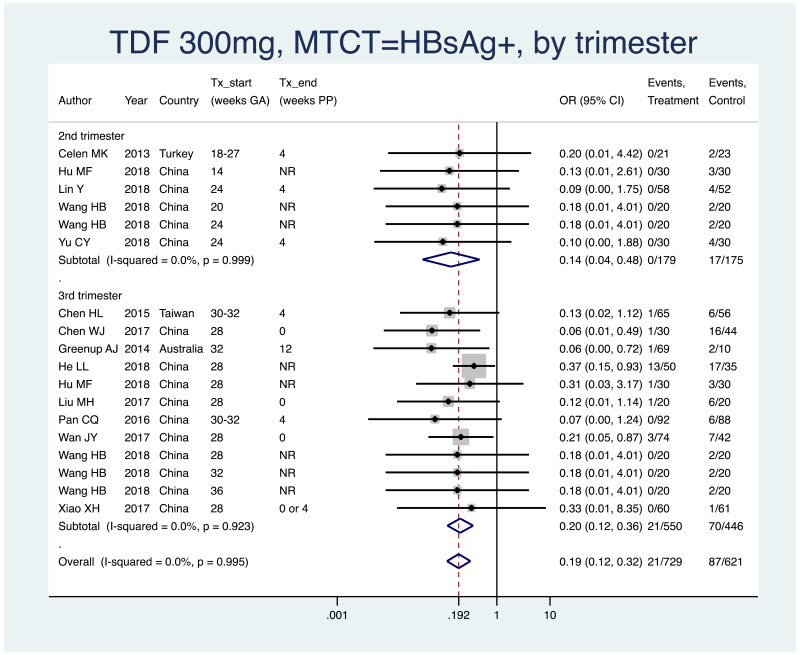
- 1.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by trimester of treatment start
- 1st trimester: not enough studies for meta-analysis (i.e. n<3)
- 2nd trimester: pooled OR=0.14 (95% CI: 0.04–0.48), P=0.002, I2=0.0%
- 3rd trimester: pooled OR=0.21 (95% CI: 0.12–0.36), P<0.001, I2=0.0%
- The P value for heterogeneity between 2nd and 3rd trimester was 0.57.
- 2.
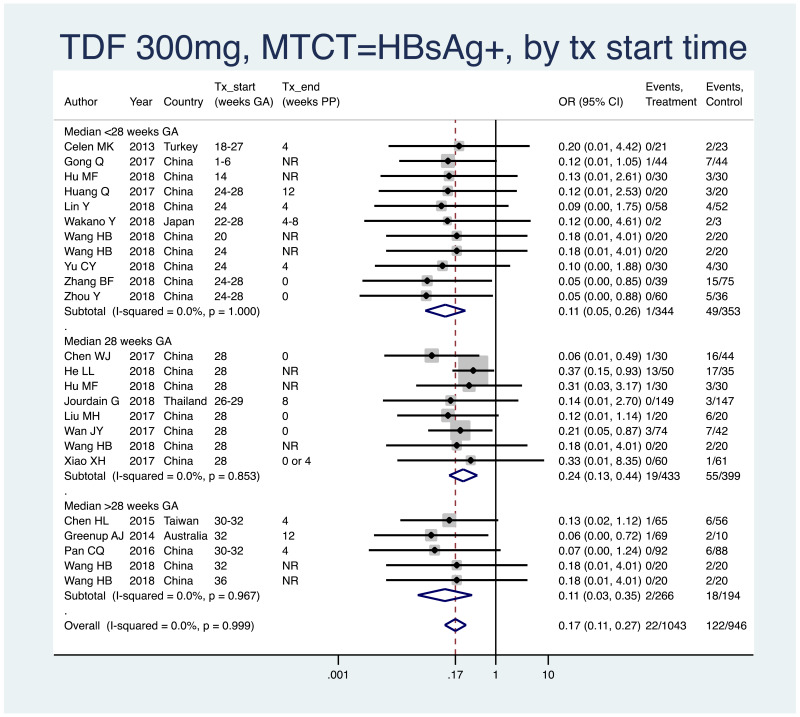
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by median weeks’ gestation at the time of treatment start (<28 weeks, 28 weeks, >28 weeks)
- <28 weeks: pooled OR=0.11 (95% CI: 0.05–0.26), P<0.001, I2=0.0%
- 28 weeks: pooled OR=0.24 (95% CI: 0.13–0.44), P<0.001, I2=0.0%
- >28 weeks: pooled OR=0.11 (95% CI: 0.03–0.35), P<0.001, I2=0.0%
- When looking at heterogeneity across the three subgroups, the P value was 0.26. If comparing <28 weeks median with 28 weeks median, there was no heterogeneity (P=0.15). If comparing <28 weeks median with >28 weeks median, there was no heterogeneity (P=0.98). If comparing 28 weeks median with >28 weeks median, there was no heterogeneity (P=0.24).
- 3.
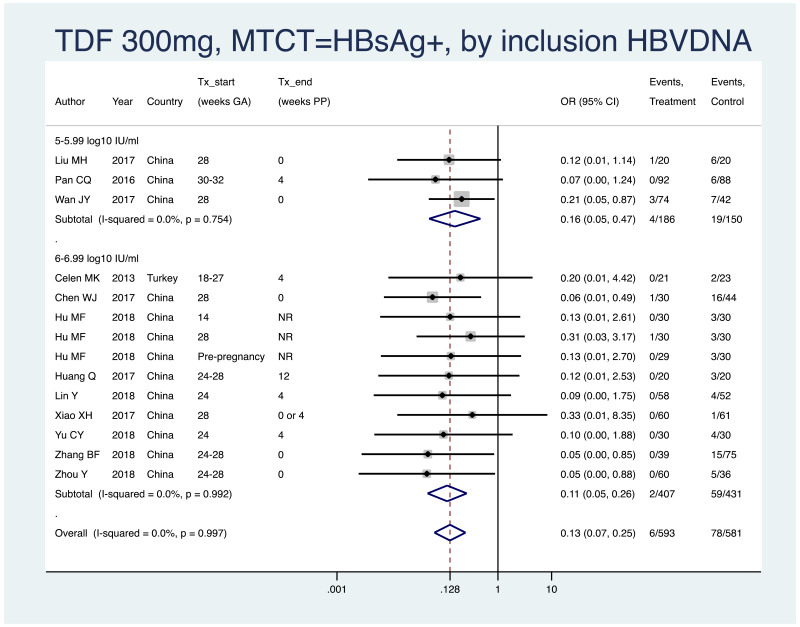
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the minimum HBV DNA level specified in the study inclusion criteria
- >4–4.99 log10 IU/mL: not enough studies (i.e. <3)
- >5–5.99 log10 IU/mL: pooled OR=0.16 (95% CI: 0.05–0.47), P=0.001, I2=0.0%
- >6–6.99 log10 IU/mL: pooled OR=0.11 (95% CI: 0.05–0.26), P<0.001, I2=0.0%
- >7–7.99 log10 IU/mL: not enough studies (i.e. <3)
- When looking at heterogeneity between studies with inclusion criteria of >5–5.99 log10 IU/mL versus >6–6.99 log10 IU/mL, the P value was 0.64.
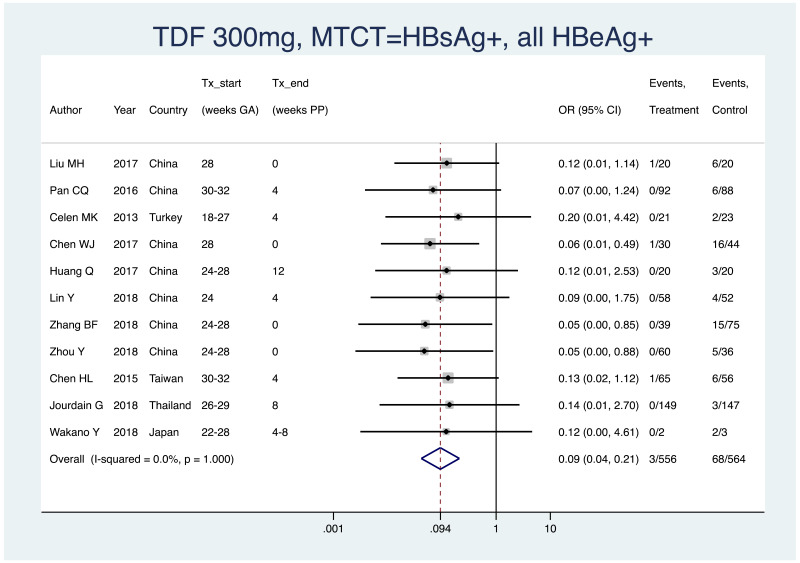
- 4.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), only including studies where all women were confirmed HBeAg positive
- Pooled OR=0.09 (95% CI: 0.04–0.21), P<0.001, I2=0.0%
- 5.
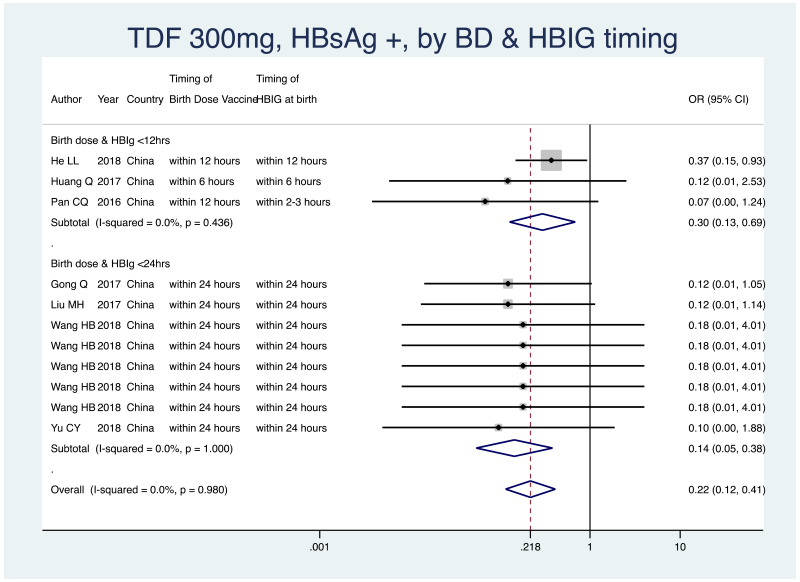
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), by infant immunoprophylaxis regimen (Table 7).
- As most studies provided all of the infant immunoprophylaxis measures (birth dose vaccine, HBIG at birth and subsequent infant vaccinations), stratification by type or combination of infant immunoprophylaxis was not possible in this meta-analysis.
- Therefore, we stratified by whether or not both birth dose vaccine and HBIG were given within 12 hours of life, versus within 24 hours of life.
- -
<12 hours: pooled OR=0.30 (95% CI: 0.13–0.69), P=0.004, I2=0.0%
- -
<24 hours: pooled OR=0.15 (95% CI: 0.06–0.38), P<0.001, I2=0.0%
- -
When looking at heterogeneity between studies that administered both forms of prophylaxis within 12 hours, versus within 24 hours, the P value was 0.28.
Table 7
Infant immunoprophylaxis regimens seen in studies investigating TDF.
- 6.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the timing that treatment was discontinued postpartum
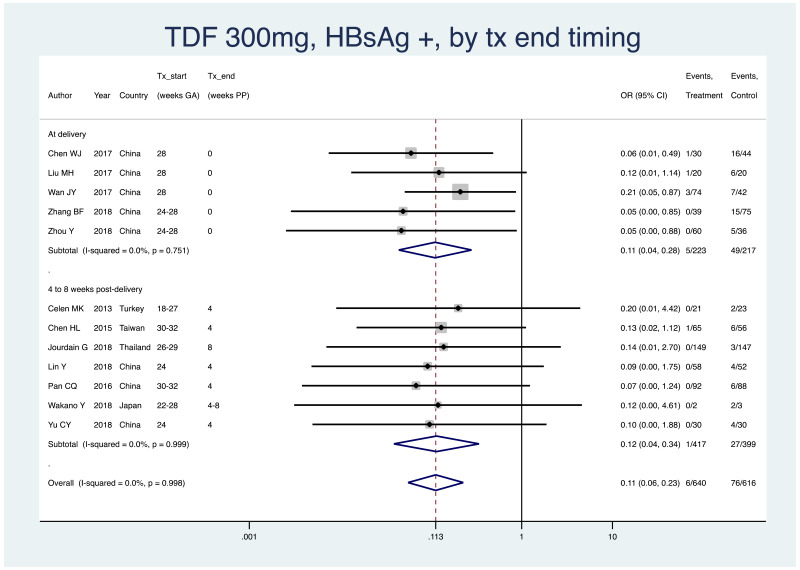
- At delivery: pooled OR=0.11 (95% CI: 0.04–0.28), P<0.001, I2=0.0%
- 4–8 weeks postpartum: pooled OR=0.12 (95% CI: 0.04–0.34), P<0.001, I2=0.0%
- 12 weeks postpartum: not enough studies for subgroup analysis
- 24+ weeks postpartum: no studies within this subgroup
- When looking at heterogeneity across the two subgroups, the P value was 0.96.
Safety analysis, narrative descriptions and selected forest plots
Infant safety outcomes
In the protocol, it was specified that the following safety outcomes for infants would be investigated: neonatal death, prematurity, congenital abnormalities, Apgar score, and bone mineral density. Finally, information on all of these outcomes were collected and results for all of these outcomes, except for the Apgar score, are provided here. The data for Apgar score were not available for the majority of included studies and where it was available the format varied greatly; this led to an inability to combine results in a meaningful way.
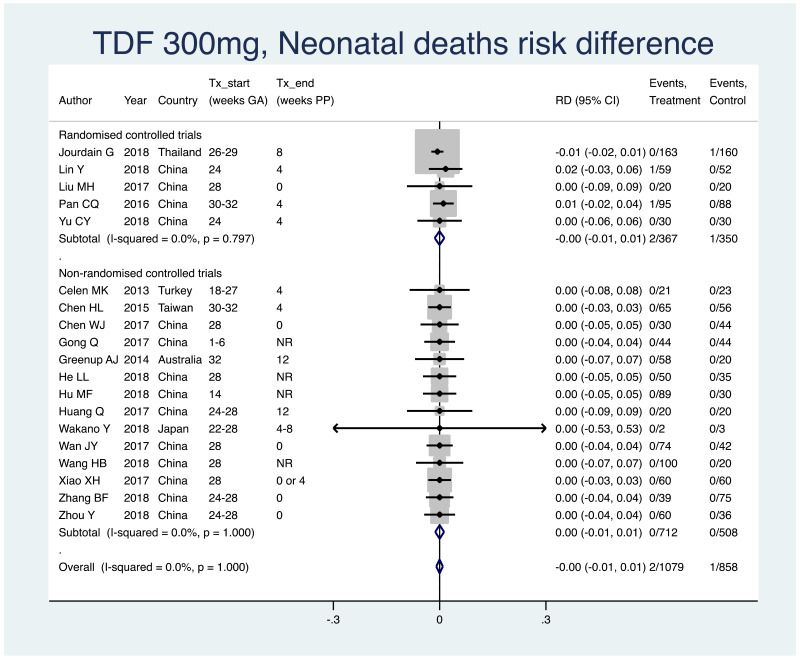
1. Neonatal deaths (death within 28 days of life)
Information on this outcome was available for all studies that administered TDF to mothers. Three neonatal deaths were reported across all study populations. Two deaths (non-weighted average 0.2%; one each from two separate studies) occurred across all treatment groups, out of a total of 1079 infants whose mothers were treated with TDF during pregnancy. One death (non-weighted average 0.1%) occurred in one of the control groups in one study, out of total of 858 infants whose mothers were not treated during pregnancy. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.00 (95% CI: −0.01–0.01). The I2 statistics for the overall pooled OR, as well for RCTs and non-RCTs separately, were all 0%.
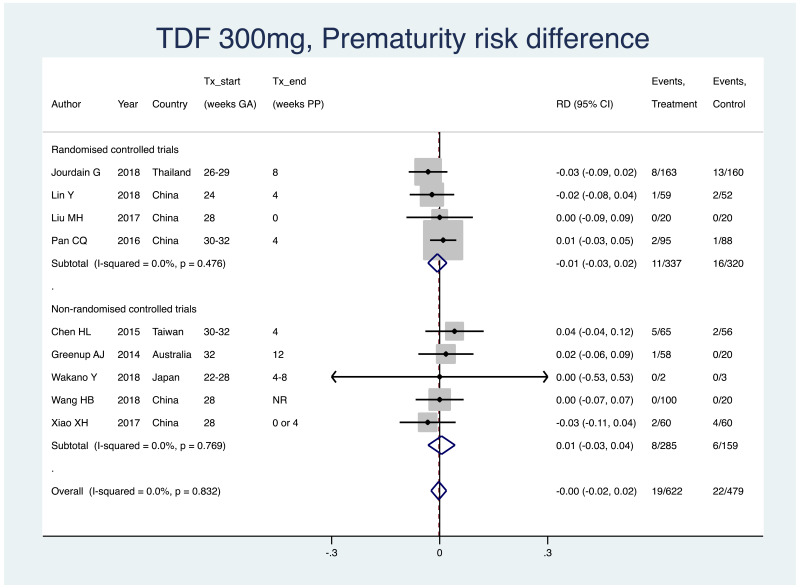
2. Prematurity (typically defined as birth earlier than 37 weeks of gestation)
Information on this outcome was available for nine of the 19 included studies that administered TDF to mothers. Within these studies, 19 of 622 (non-weighted average 3.1%) infants whose mothers were treated with TDF during pregnancy were born prematurely, whereas 22 of 479 (non-weighted average 4.6%) infants whose mothers were not treated during pregnancy were born prematurely. The weighted pooled risk difference for this safety outcome seen following meta-analysis was −0.003 (95% CI: −0.024–0.019). The I2 statistics for the overall pooled OR, as well as for RCTs and non-RCTs separately, were all 0%.
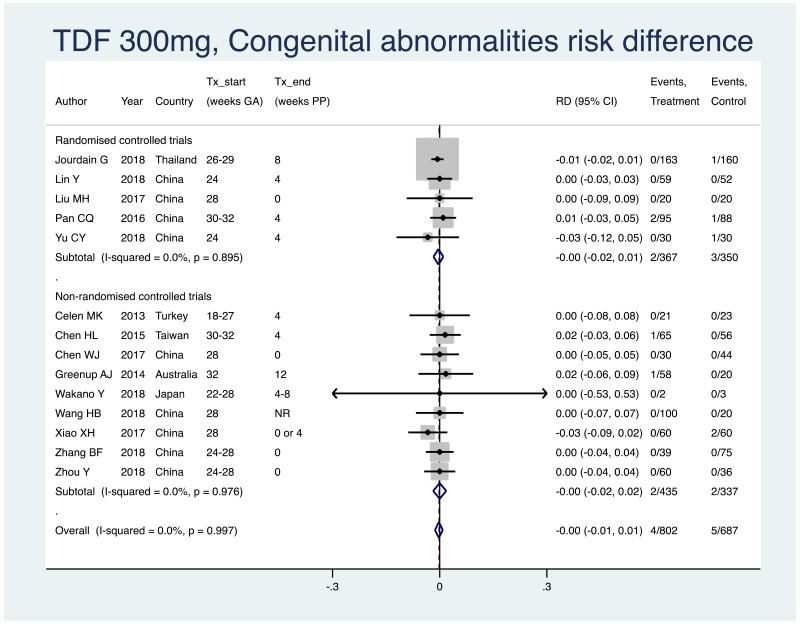
3. Congenital abnormalities
Information on this outcome was available for 14 of the 19 included studies that administered TDF to mothers. Within these studies, four of 802 (non-weighted average 0.5%) infants whose mothers were treated with TDF during pregnancy were noted to have some sort of congenital abnormality, including: torticollis (n=1), umbilical hernia (n=1), congenital unilateral deafness (n=1), polydactyly (n=1). Five of 687 (non-weighted average 0.7%) infants whose mothers were not treated during pregnancy were noted to have some sort of congenital abnormality, including: hypospadias (n=1), “gross abnormalities” (n=1), no detail provided (n=3). The weighted pooled risk difference for this safety outcome seen following meta-analysis was −0.002 (95% CI: −0.013–0.009). The I2 statistics for the overall pooled OR, as well as for RCTs and non-RCTs separately, were all 0%.
4. Bone mineral density
This outcome was investigated only for one of the 19 included studies, an RCT, that administered TDF to mothers. In this study, infant lumbar spine bone mineral density was measured in 62 infants from the treatment group, and 53 infants from the control group at 1 year of age (i.e. not the entire original study population of 163 treatment-exposed infants and 161 controls), with a mean score of 0.324 (SD +/− 0.036), and 0.330 (SD +/− 0.036), respectively. There was no statistically significant difference detected between the two groups (Jourdain et al., 2018; Salvadori et al., 2019).
Maternal safety outcomes
Information was collected and presented on the following maternal safety outcomes: miscarriage/stillbirth, postpartum haemmorhage, antiviral resistance, HBV flare.
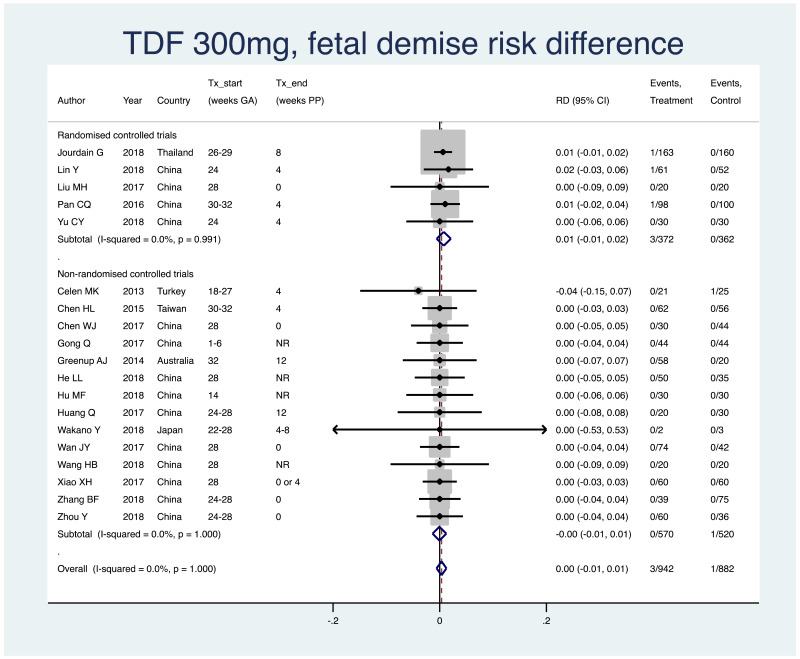
1. Fetal demise (miscarriage [<28 weeks], stillbirth [>=28 weeks])
Information on this outcome was available for all studies that administered TDF to mothers. Four cases of fetal demise were reported across all study populations. Three cases (non-weighted average 0.4%; one each from three separate studies) occurred across all treatment groups, out of a total of 942 mothers/fetuses who were treated with TDF during pregnancy. One case (non-weighted average 0.1%) occurred in one of the control groups in one study, out of a total of 882 mothers/fetuses who were not treated during pregnancy. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.003 (95% CI: −0.006–0.012). The I2 statistics for the overall pooled risk difference estimate, and RCTs and non-RCTs separately, were all 0%.
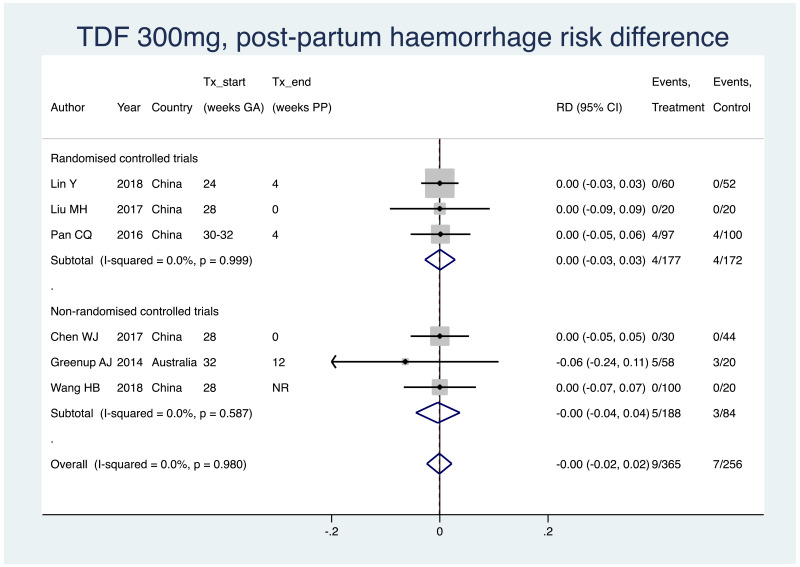
2. Postpartum haemorrhage
Information on this outcome was available for six of the 19 included studies that administered TDF to mothers. Within these studies, nine of 365 (non-weighted average 2.5%) mothers who were treated with TDF during pregnancy experienced postpartum haemorrhage, whereas seven of 256 (2.7%) mothers who were not treated during pregnancy experienced postpartum haemorrhage. The weighted pooled risk difference for this safety outcome seen following meta-analysis was −0.001 (95% CI: −0.024–0.022). The I2 statistics for the overall pooled risk difference estimates, as well as for RCTs and non-RCTs separately, were all 0%.
3. Antiviral resistance
Only one of the 19 studies where mothers were treated with TDF during pregnancy performed antiviral resistance testing for the entire study population. This study, with 120 participants, found no HBV mutations related to antiviral therapy; it was not clearly stated at which time-point this testing was performed (Lin Y et al., 2018). Two further studies reported investigations into antiviral resistance for women defaulting from treatment or where infants were found positive for HBV at 6–12 months, both of these studies reported that no resistance mutations were found (Chen HL et al., 2015; Pan CQ et al., 2016).
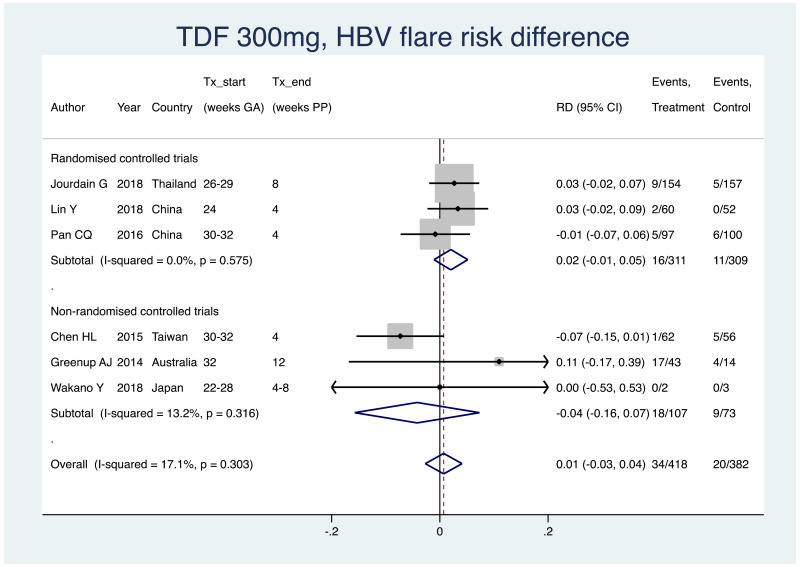
4. HBV flare after treatment discontinuation
Information on this outcome was available for six of the 19 included studies that administered TDF to mothers. Various definitions were used, including: “postpartum flare”, “severe flare”, “ALT >5 ULN”, and others. Within these studies, 34 of 418 (non-weighted average 8.1%) mothers who were treated with TDF during pregnancy experienced a type of HBV flare at the time of treatment discontinuation, whereas 20 of 382 (non-weighted average 5.2%) mothers who were not treated during pregnancy experienced the same type of HBV flare at a matched time-point. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.007 (95% CI: −0.027–0.041). There was no heterogeneity in the RCTs (i.e. I2=0%), however, in non-RCTs and in the overall pooled risk difference estimate, there was an I2 of 13.2% and 17.1%, respectively.
GRADE summary of findings
Table 8
GRADE evidence profile – TDF 300 mg during pregnancy to prevent HBV mother-to-child transmission (MTCT).
Lamivudine (LAM) 100–150 mg versus no treatment or placebo
Summary of included studies
There were 42 original studies, including 46 unique treatment arms, eligible for this meta-analysis that used LAM 100–150 mg. Following risk of bias assessment, two studies (each with one treatment arm investigating LAM) were excluded (van Zonneveld et al., 2003, Liu CP et al., 2015). Therefore, 40 original studies with 44 unique treatment arms were included in analysis. Of the included studies, eight were RCTs and 32 were non-randomized trials/observational studies (17 prospective and 15 were retrospective studies).
Risk of bias assessment
- Randomized controlled trials
Of the eight RCTs included that investigated LAM, none achieved a “low risk of bias” rating on the majority of the main criteria in the Cochrane Collaboration’s Risk of Bias Assessment Tool. One study, by Xu WM et al. (2009), the only study published in English, had a low risk in selection bias (specifically, allocation concealment), as well as in performance bias and detection bias, but had a high or unclear risk in all other domains. The remaining seven of the eight included RCTs only fulfilled one or two criteria as “low risk of bias”. In these studies, there was always a low risk of selection bias (specifically random sequence generation) and sometimes a low risk of selective reporting; however, no study described loss to follow up and no study described all important adverse outcomes in mothers and infants. The detailed risk of bias assessment for the RCTs investigating LAM can be found in Appendix E.
- Non-randomized controlled trials
Of the original 34 non-RCTs, the majority of studies (67.6%) had low risk of bias scores (i.e. scores of 7, 8, 9) on the Newcastle Risk of Bias scale. The main weakness of included studies was in reference to loss to follow up – this information was missing in 28 of 34 studies, and was less than adequate (i.e. <80% follow up) in three further studies. The detailed risk of bias assessment for the non-RCTs investigating LAM can be found in Appendix F (Table 9).
Table 9
Risk of bias scores for non-RCTs (prior to exclusion of very high-risk studies).
Publication bias/assessment of small study effects
It was possible to examine publication bias for the following outcomes: MTCT indicated by HBsAg positivity at 6–12 months in non-RCTs, MTCT indicated by HBV DNA positivity at 6–12 months in non-RCTs, neonatal deaths in non-RCTs, congenital abnormalities in non-RCTs, and miscarriages and stillbirths in non-RCTs. Of these, there was only possible evidence of publication bias/small study effects in the first study set (MTCT indicated by HBsAg positivity at 6–12 months in non-RCTs. Funnel plots for LAM 100–150 mg study sets, as well as results of the Egger test for asymmetry (if examining OR only) can be found in Appendix G.
Characteristics of included studies
Across all included studies (n=40), recruitment took place as early as 2001 and up to 2016. Almost all studies took place in the WHO Western Pacific Region; including China (n=35), China and the Philippines (n=1), Japan (n=1), and Australia (n=1). Additionally, one study took place in the WHO Eastern Mediterranean Region (i.e. Egypt), and one study took place in the WHO European Region (i.e. Ireland).
HBV genotyping for the entire study population was performed only in three instances. A study from Ireland estimated that the treatment group was 39% genotype B, 33% genotype C, 11% genotype D, 3% genotype E and 14% non-determined (Jackson et al., 2015). One Chinese study estimated the treatment group as 37% genotype B, 63% genotype C, whereas in the control group there were 29% genotype B and 71% genotype C (Shen et al., 2016). In a small study in Japan, all three mothers treated with LAM were genotype C, and in the control group one mother was genotype C and the two other mothers had indeterminable genotype (Wakano et al., 2018).
Most included study arms (i.e. 30/44) started maternal antiviral therapy between 24 and 30 weeks of gestation. The most common HBV DNA level designated for inclusion was >5.3 log10 IU/mL (14 of 44 treatment arms) (Table 10).
Table 10
Characteristics of included studies investigating LAM 100–150 mg.
Primary efficacy analysis, narrative descriptions and forest plots
- 1.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT)
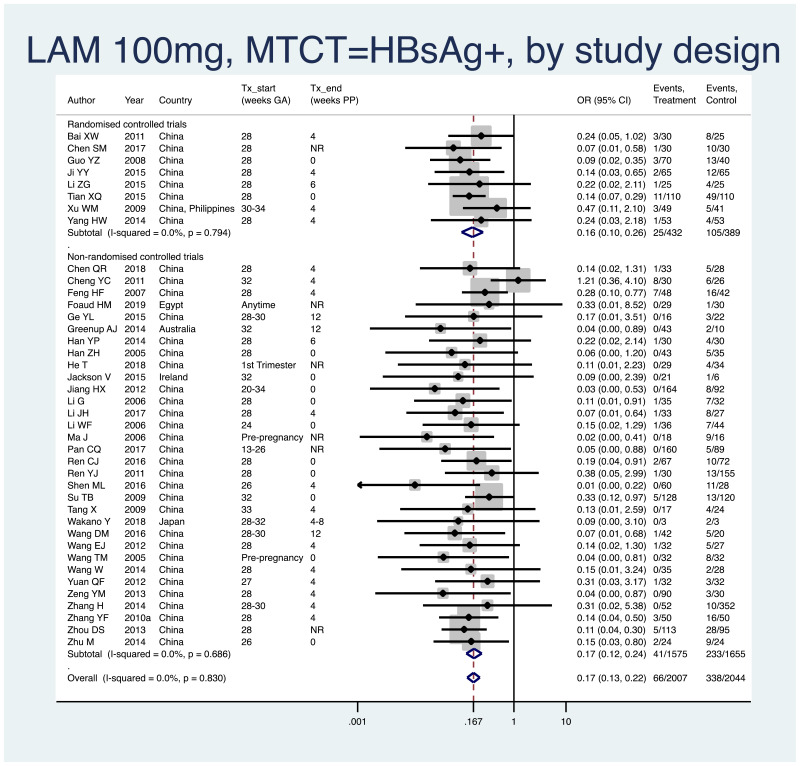
- Overall pooled OR=0.17 (95% CI: 0.13–0.22), P<0.001, I2=0%
- RCTs only: pooled OR=0.16 (95% CI: 0.10–0.26), P<0.001, I2=0%
- Non-RCTs only: pooled OR=0.17 (95% CI: 0.12–0.24), P<0.001, I2=0%
- When looking at heterogeneity between RCTs and non-RCTs, we arrive at a P value of 0.80, indicating no difference between the estimates.
- 2.
PMTCT, as indicated by detection of HBV DNA at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT).
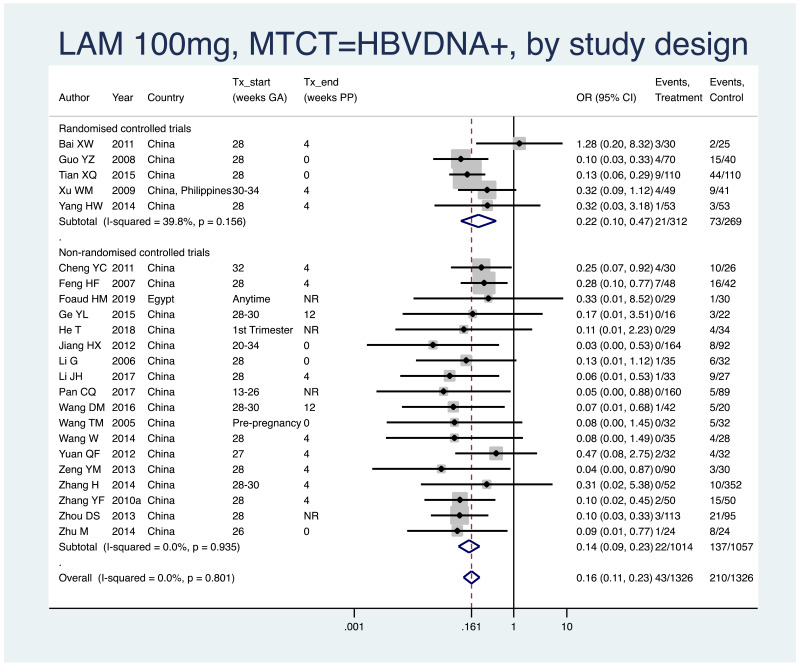
- Overall pooled OR=0.16 (95% CI: 0.11–0.23), P<0.001, I2=0.0%
- RCTs only: pooled OR=0.22 (95% CI: 0.10–0.47), P<0.001, I2=39.8%
- Non-RCTs only: pooled OR=0.14 (95% CI: 0.09–0.23), P<0.001, I2=0%
- When looking at heterogeneity between RCTs and non-RCTs, we arrive at a P value of 0.47.
Subgroup analysis
Of the potential sources of heterogeneity prespecified in the protocol, it was not possible to do a subgroup analysis by coinfection status, as there were eventually no eligible populations who were coinfected. Furthermore, it was not possible to do subgroup analysis by WHO region, as almost all studies came from just one region (i.e. Western Pacific). For LAM, one ad hoc subgroup analysis is presented; timing of treatment being end postpartum.
- 1.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by trimester of treatment start.
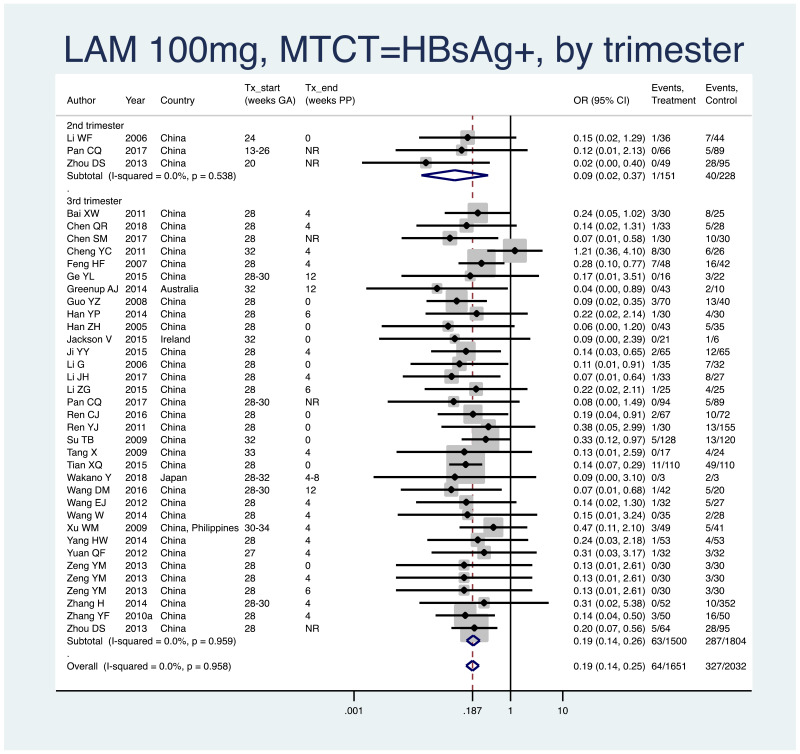
- 1st trimester: not enough studies for meta-analysis (i.e. n<3)
- 2nd trimester: pooled OR=0.09 (95% CI: 0.02–0.37), P=0.001, I2=0.0%
- 3rd trimester: pooled OR=0.19 (95% CI: 0.14–0.25), P<0.001, I2=0.0%
- When looking at heterogeneity between studies where treatment was started in the 2nd versus the 3rd trimester, we arrive at a P value of 0.29, indicating no difference between the estimates.
- 2.
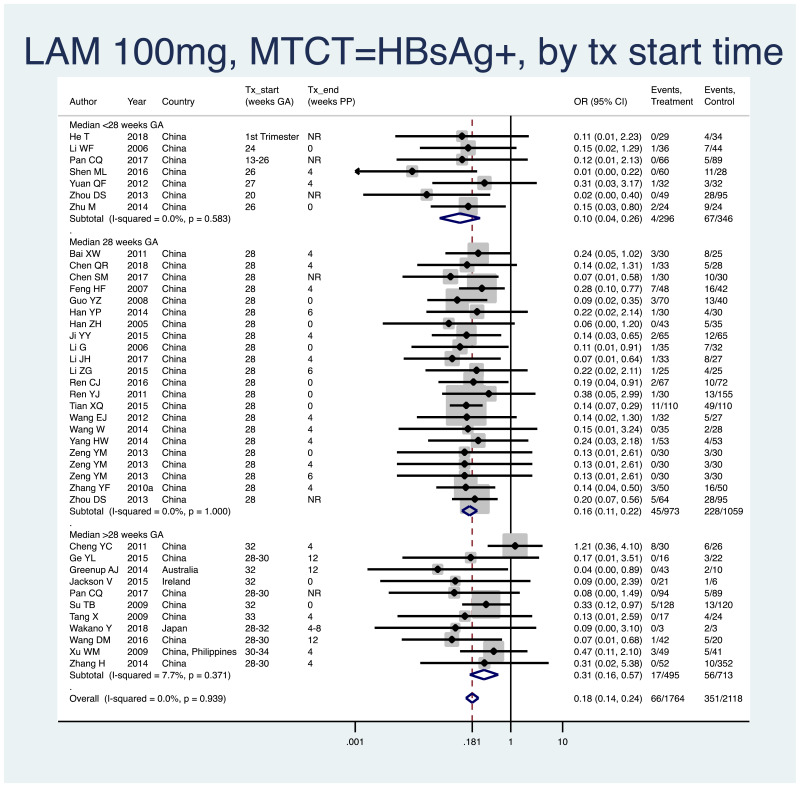
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by median weeks of gestation at the time of start of treatment (<28 weeks, 28 weeks, >28 weeks).
- <28 weeks: pooled OR=0.10 (95% CI: 0.04–0.26), P<0.001, I2=0.0%
- 28 weeks: pooled OR=0.16 (95% CI: 0.11–0.23), P<0.001, I2=0.0%
- >28 weeks: pooled OR=0.31 (95% CI: 0.16–0.57), P<0.001, I2=7.7%
- When looking at heterogeneity across the three subgroups, the P value was 0.06. If comparing <28 weeks median with 28 weeks median, there was no heterogeneity (P=0.38). If comparing <28 weeks median with >28 weeks median, or if comparing 28 weeks median with >28 weeks median, there was evidence of heterogeneity (both with P=0.04); however, because of the mild heterogeneity within the subgroup starting at >28 weeks median, this test may not be valid.
- 3.
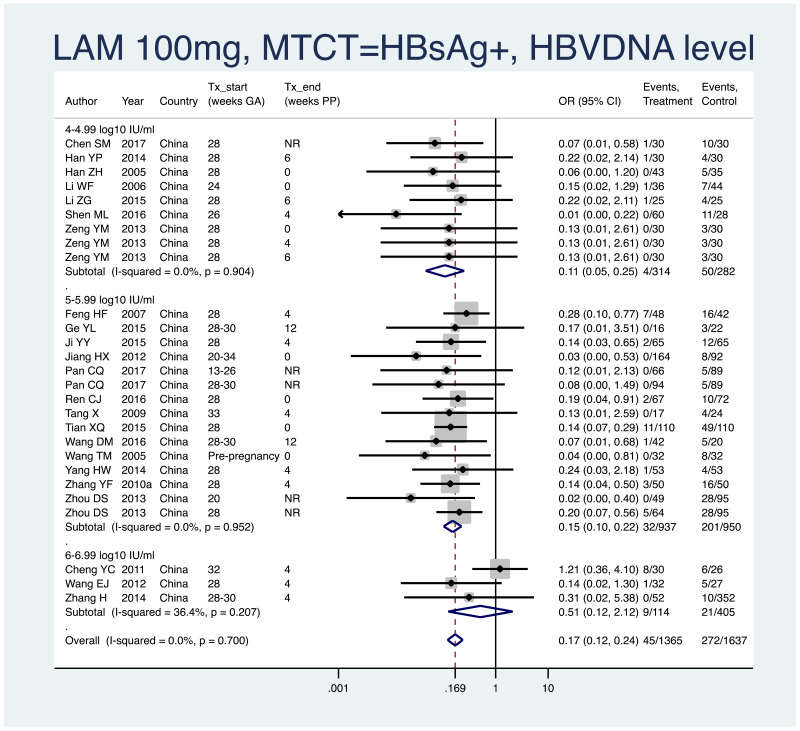
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the minimum HBV DNA level specified in the inclusion criteria of the study.
- >4–4.99 log10 IU/mL: pooled OR=0.11 (95% CI: 0.05–0.25), P<0.001, I2=0.0%
- >5–5.99 log10 IU/mL: pooled OR=0.15 (95% CI: 0.10–0.22), P<0.001, I2=0.0%
- >6–6.99 log10 IU/mL: pooled OR=0.51 (95% CI: 0.12–2.12), P=0.357, I2=36.4%
- >7–7.99 log10 IU/mL: not enough studies (i.e. <3)
- When looking at heterogeneity between studies with inclusion criteria of 4–4.99 log10 IU/mL versus 5–5.99 log10 IU/mL, the P value was 0.48. No comparison was done with 6–6.99 log10 IU/mL, as this OR was both heterogeneous and non-significant.
- 4.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by whether or not all women were HBeAg- positive.
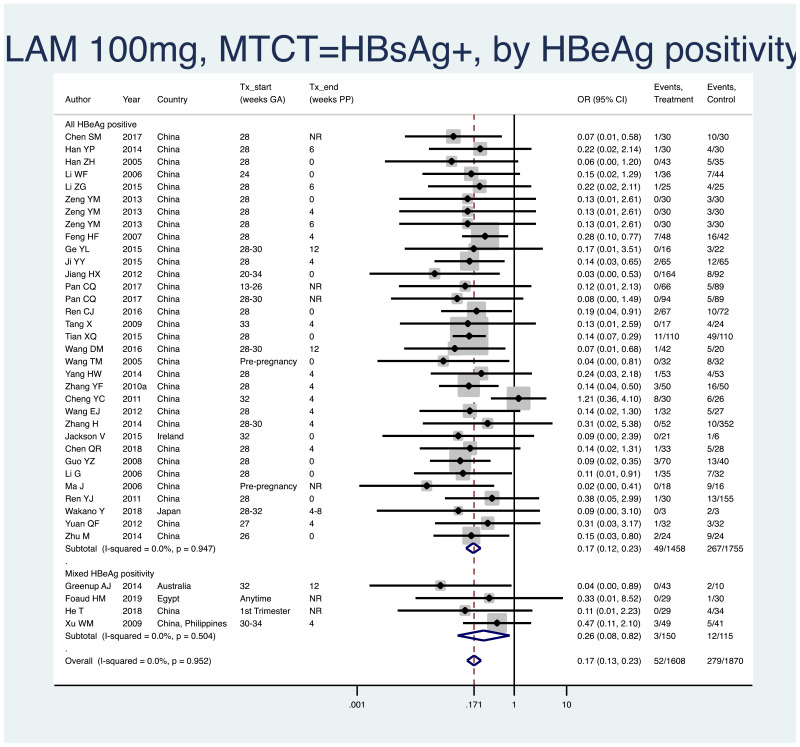
- All HBeAg-positive: pooled OR=0.17 (95% CI: 0.12–0.23), P<0.001, I2=0.0%
- Mixed HBeAg positivity: pooled OR=0.26 (95% CI: 0.08–0.82), P=0.022, I2=0.0%
- When looking at heterogeneity between studies where all women versus only some women were HBeAg positive, we arrive at a P value of 0.46, indicating no difference between the estimates.
- 5.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), by infant immunoprophylaxis regimen (Table 11).
- As most studies provided all of birth dose vaccine, HBIG at birth, and subsequent infant vaccinations, stratification by type or combination of infant immunoprophylaxis was not done in this meta-analysis.
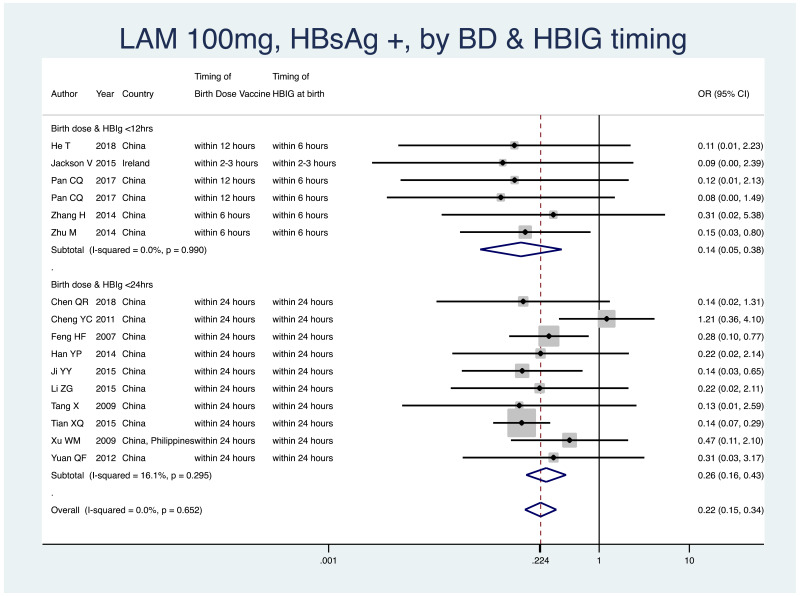
- Therefore, we stratified by whether or not both birth dose vaccine and HBIG were given within 12 hours of life, versus within 24 hours of life.
- <12 hours: pooled OR=0.14 (95% CI: 0.05–0.39), P<0.001, I2=0.0%
- <24 hours: pooled OR=0.26 (95% CI: 0.16–0.43), P<0.001, I2=16.1%
- The P value for heterogeneity between the two subgroups was 0.31.
Table 11
Infant immunoprophylaxis regimens seen in studies investigating LAM.
- 6.
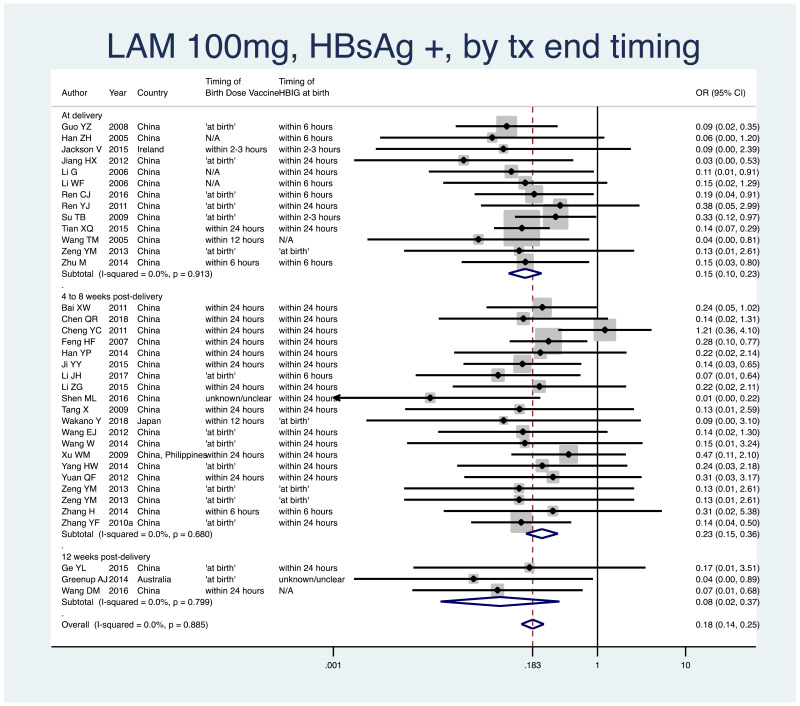
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the timing that treatment was discontinued postpartum.
- At delivery: pooled OR=0.15 (95% CI: 0.10–0.23), P<0.001, I2=0.0%
- 4–8 weeks postpartum: pooled OR=0.23 (95% CI: 0.15–0.36), P<0.001, I2=0.0%
- 12 weeks postpartum: pooled OR=0.08 (95% CI: 0.02–0.37), P=0.001, I2=0.0%
- 24+ weeks postpartum: no studies within this subgroup
- When looking at heterogeneity across the four subgroups, the P value was 0.20.
Safety analysis, narrative descriptions and selected forest plots
Infant safety outcomes
Of the infant safety outcomes prespecified in the protocol, the data for Apgar score were not available for the majority of included studies and where it was available the format varied greatly; this led to an inability to combine results in a meaningful way. None of the included studies for LAM investigated bone mineral density in infants.
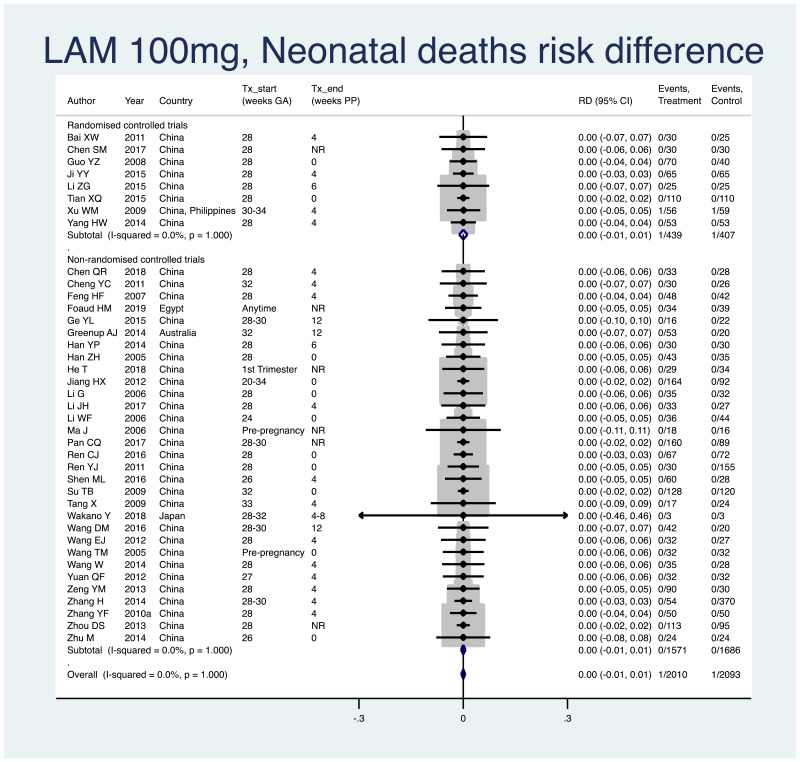
1. Neonatal deaths (death within 28 days of life)
Information on this outcome was available for all except one study that administered LAM to mothers. One death in 2010 infants (non-weighted average 0.05%) was reported across the treatment groups and one death in 2093 infants (non-weighted average 0.05%) was reported across the control groups. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.000 (95% CI: −0.006–0.006). The I2 statistics for the overall pooled risk difference, as well as for RCTs and non-RCTs separately, were all 0.0%.
2. Prematurity (typically defined as birth earlier than 37 weeks of gestation)
Information on this outcome was available for 10 of the 40 included studies that administered LAM to mothers. Within these studies, 14 of 609 (non-weighted average 2.3%) infants whose mothers were treated with LAM during pregnancy were born prematurely, whereas 11 of 399 (non-weighted average 2.8%) infants whose mothers were not treated during pregnancy were born prematurely. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.000 (95% CI: −0.025–0.025). The I2 statistics for the overall pooled risk difference estimated was 43.0%. The I2 statistics for non-RCTs was 55.6%. There were too few RCTs (i.e. <3) to consider the pooled risk difference separately in this subgroup.
3. Congenital abnormalities
Information on this outcome was available for 16 of the 40 included studies that administered LAM to mothers. Within these studies, eight of 845 (non-weighted average 0.9%) infants whose mothers were treated with LAM during pregnancy were noted to have some sort of congenital abnormality, including: atrial septal defect with Ebstein anomaly and pneumothorax (n=1), cleft palate (n=1), polydactyly (n=3), auricular defect (n=1), left ear pinna turn malformation (n=1), and absent ear (n=1). Five of 953 (non-weighted average 0.5%) infants whose mothers were not treated during pregnancy were noted to have some sort of congenital abnormality, including: polydactyly (n=1), talipes equinovarus (n=1), ear accessory (n=1), pulmonary stenosis (n=1), hydrocephalus (n=1). The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.003 (95% CI: −0.007–0.014). The I2 statistics for the overall pooled risk, as well as for RCTs and non-RCTs separately, were all 0%.
Maternal safety outcomes
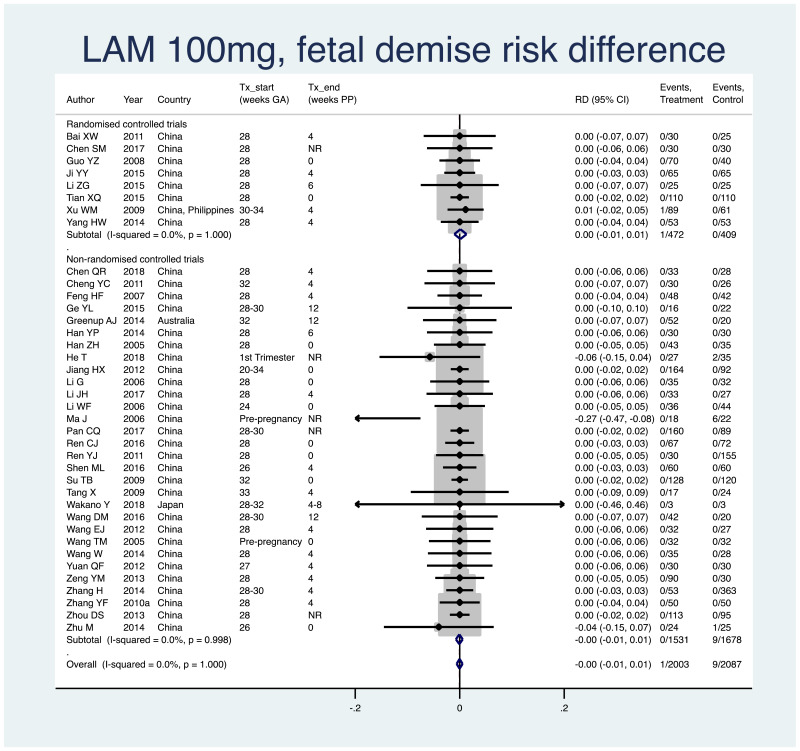
1. Fetal demise (miscarriage [<28 weeks], stillbirth [>=28 weeks])
Information on this outcome was available for 39 of the 40 studies that administered LAM to mothers. Ten cases of fetal demise were reported across all study populations. One case (non-weighted average 0.05%) occurred across 2003 mothers/fetuses who were treated with LAM during pregnancy. Nine cases (non-weighted average 0.4%) occurred across 2087 mothers/fetuses who were not treated during pregnancy. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.000 (95% CI: −0.006–0.005). The I2 statistics for the overall pooled risk difference estimate as well as for RCTs and non-RCTs separately, were all 0%.
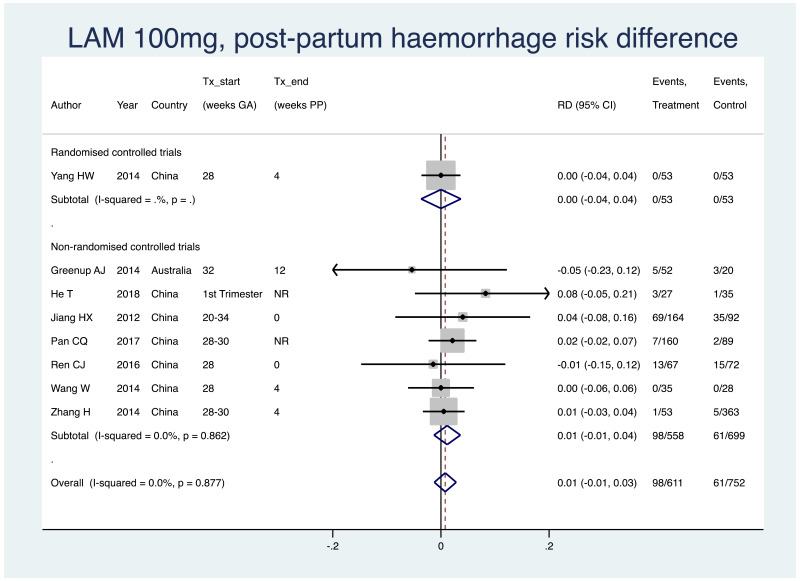
2. Postpartum haemorrhage
Information on this outcome was available for eight of the 40 included studies that administered LAM to mothers. Within these studies, 98 of 611 (non-weighted average 16.0%) mothers who were treated with LAM during pregnancy experienced postpartum haemorrhage, whereas 61 of 752 (8.1%) mothers who were not treated during pregnancy experienced postpartum haemorrhage. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.008 (95% CI: −0.012–0.028). The I2 statistics for the overall pooled OR, as well as for non-RCTs separately were 0%. Not enough RCTs evaluated this safety outcome to consider this subgroup separately.
3. Antiviral resistance
Four studies that treated mothers with LAM during pregnancy reported on some results of antiviral resistance testing. One study from Australia reported the selection of primary resistant variants to LAM in 21 treated women (Greenup AJ et al., 2014). One study from China reported no cases of antiviral resistance in both treated and control groups, with no other details provided (Shen ML et al., 2016). Another Chinese study performed resistance testing in five women with viral breakthrough and found no resistance mutants (Zhang H et al., 2014). Finally, a study from Ireland carried out antiviral resistance testing on 28 of the 36 women treated with LAM during pregnancy and reported identification of wild-type strains in all women (Jackson V et al., 2015).
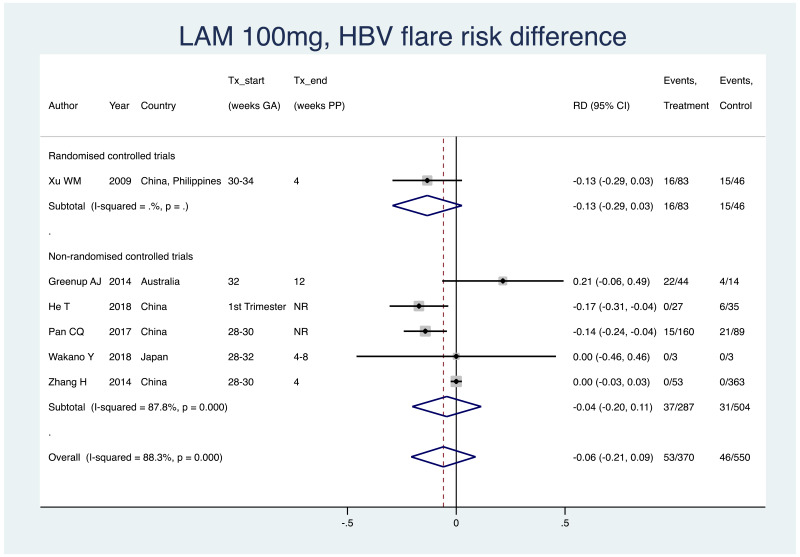
4. HBV flare after treatment discontinuation
Information on this outcome was available for six of the 40 included studies that administered LAM to mothers. Various definitions were used, including: “postpartum ALT elevations”, “postpartum flare”, “grade 3/4 elevation”, as well as no definition in some cases. Within these studies, 53 of 370 (non-weighted average 14.3%) mothers who were treated with LAM during pregnancy experienced a type of HBV flare at the time of treatment discontinuation, whereas 46 of 550 (non-weighted average 8.4%) mothers who were not treated during pregnancy experienced the same type of HBV flare at a matched time-point. The weighted pooled risk difference for this safety outcome seen following meta-analysis was −0.059 (95% CI: −0.207–0.089). Overall, the pooled risk difference had a high level of heterogeneity (I2 of 88.3%), as well as within the non-RCTs only, the I2 was 87.8%. It was not possible to examine the RCTs alone as a subgroup as there was only one study.
GRADE summary of findings
Table 12
GRADE evidence profile: LAM 100–150 mg during pregnancy to prevent HBV mother-to-child transmission (MTCT).
Telbivudine (LdT) 600 mg versus no treatment or placebo
Summary of included studies
There were 87 original studies, including 101 unique treatment arms, eligible for this meta-analysis that used LdT 600 mg. Following risk of bias assessment, four studies (all non-RCTs and each with one treatment arm investigating LdT) were excluded (Chen YL et al., 2014; Liu CP, 2015; Luo DX et al., 2017; Zhang R et al., 2016). Therefore, 83 original studies with 97 unique treatment arms were included in the analysis. Of the included studies, 21 were RCTs and 62 were non-randomized trials/observational studies (39 prospective and 23 retrospective studies).
Risk of bias assessment
- Randomized controlled trials
Of the 21 RCTs included that investigated LdT, none achieved a “low risk of bias” rating on the majority of the main criteria in the Cochrane Collaboration’s Risk of Bias Assessment Tool. All studies had only one or two criteria deemed as “low risk of bias”; in almost all studies there was a low risk of selection bias (specifically random sequence generation) and sometimes a low risk of selective reporting. The remaining criteria for all studies had a high or unclear risk, usually due to a lack of detailed reporting. The detailed risk of bias assessment for the RCTs investigating LdT 600 mg can be found in Appendix E.
- Non-randomized controlled trials
Of the original 66 non-RCTs, the majority of studies (70.0%) had low risk of bias scores (i.e. scores of 7, 8, 9) on the Newcastle Risk of Bias scale. The main weakness of included studies was in reference to loss to follow up – this information was missing in 58 of 66 studies, and was less than adequate (i.e. <80% follow up) in one further study. The detailed risk of bias assessment for the non-RCTs investigating LdT 600 mg can be found in Appendix F (Table 13).
Table 13
Risk of bias scores for non-RCTs (prior to exclusion of very high-risk studies).
Publication bias/assessment of small study effects
It was possible to examine publication bias for most of the outcomes examined. Of these, there was possible evidence of publication bias/small study effects in the three study sets: MTCT indicated by HBsAg positivity at 6–12 months in non-RCTs, MTCT indicated by HBV DNA positivity at 6–12 months in non-RCTs, postpartum haemorrhage in non-RCTs. Funnel plots for LdT 600 mg study sets, as well as results of the Egger test for asymmetry (if examining OR only) can be found in Appendix G.
Characteristics of included studies
Across all included studies, recruitment took place as early as 2000 and up until 2017. All studies took place in the WHO Western Pacific Region, specifically, all studies took place in China (n=83).
HBV genotyping for the entire study population was performed in four instances. One estimated that the treatment group was 44% genotype B, 56% genotype C, whereas the control group was 37% genotype B, 63% genotype C (Hu Y et al., 2018). One study estimated the treatment group as 72% genotype B, 28% genotype C, and the control group was similar with 74% genotype B and 26% genotype C (Liu Y et al., 2016). Another study estimated 40% genotype B, 60% genotype C in the treatment group, compared to 29% genotype B and 71% genotype C in the control group (Shen ML et al., 2016). Finally, one study found 73% genotype B, 26% genotype C, and 1% mixed genotype B/C in the treatment group, compared to 75% genotype B and 25% genotype C in the control group (Wu Q et al., 2015)
Most included study arms (i.e. 59/97) started maternal antiviral therapy between 24 and 30 weeks of gestation. The most common HBV DNA levels designated for inclusion were >5.3 log10 IU/mL (25 of 97 treatment arms) or >6.3 log10 IU/mL (24/97 treatment arms).
Table 14
Characteristics of included studies investigating LdT 600 mg.
Primary efficacy analysis, narrative descriptions and forest plots
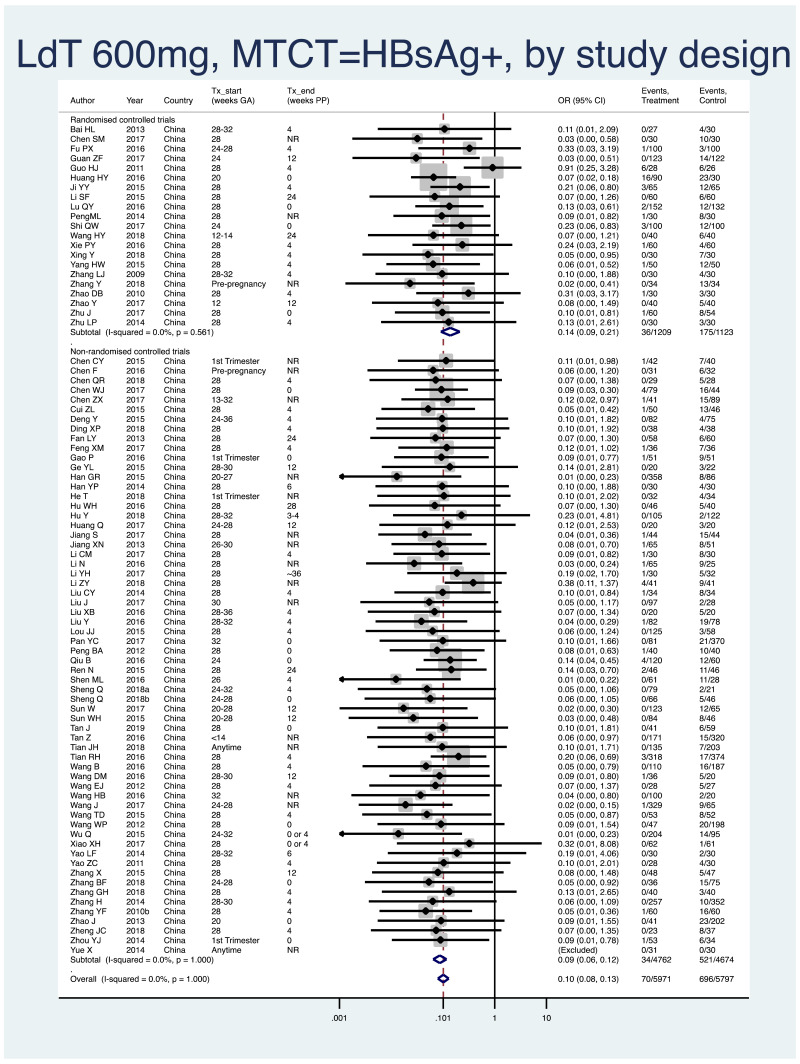
- 1.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT).
- Overall pooled OR=0.10 (95% CI: 0.08–0.13), P<0.001, I2=0%
- RCTs only: pooled OR=0.14 (95% CI: 0.10–0.26), P<0.001, I2=0%
- Non-RCTs only: pooled OR=0.09 (95% CI: 0.07–0.12), P<0.001, I2=0%
- The P value for heterogeneity between RCTs and non-RCTs was 0.08.
- 2.
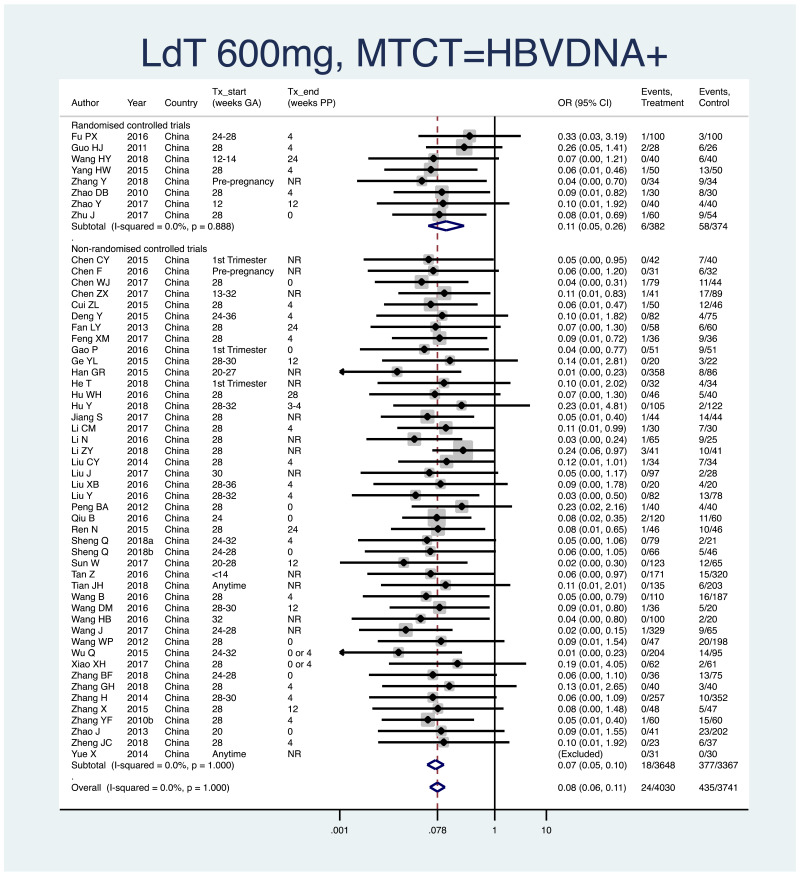
PMTCT, as indicated by detection of HBV DNA at 6–12 months of age, all treatment start times, all HBV DNA levels at inclusion, stratified by study design (RCT and non-RCT).
- Overall pooled OR=0.08 (95% CI: 0.06–0.11), P<0.001, I2=0.0%
- RCTs only: pooled OR=0.12 (95% CI: 0.05–0.26), P<0.001, I2=0%
- Non-RCTs only: pooled OR=0.07 (95% CI: 0.05–0.10), P<0.001, I2=0%
- The P value for heterogeneity between RCTs and non-RCTs was 0.29.
Subgroup analysis
Of the potential sources of heterogeneity predefined in the protocol, it was not possible to do a subgroup analysis by coinfection status, as there were eventually no eligible populations that included those coinfected. Furthermore, it was not possible to do subgroup analysis by WHO region, as almost all studies came from just one region (i.e. Western Pacific). For LdT, one ad hoc subgroup analysis is presented; timing of treatment end postpartum.
- 1.
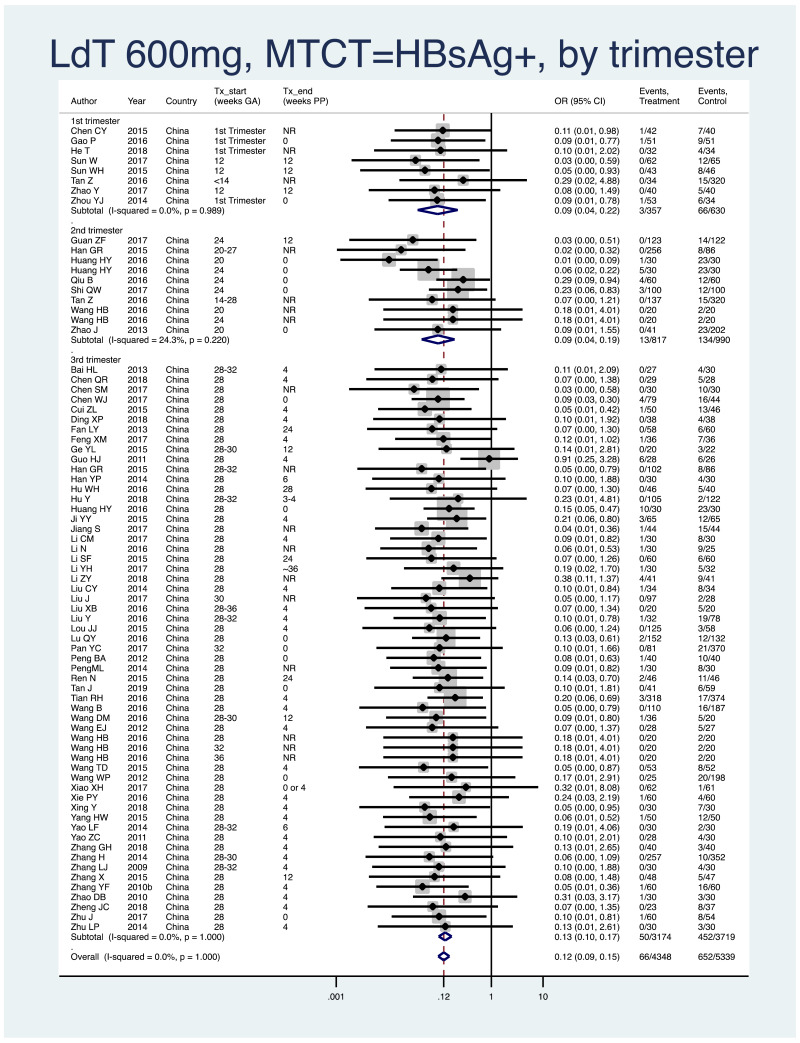
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by trimester of treatment start.
- 1st trimester: pooled OR=0.09 (95% CI: 0.04–0.22), P=0.001, I2=0.0%
- 2nd trimester: pooled OR=0.09 (95% CI: 0.05–0.20), P=0.001, I2=24.3%
- 3rd trimester: pooled OR=0.13 (95% CI: 0.10–0.17), P<0.001, I2=0.0%
- There was no detected heterogeneity between any of the subgroups (i.e. 1st versus 2nd, 2nd versus 3rd, 1st versus 3rd), with P values between 0.49 and 0.80. However, because of the mild heterogeneity seen in the 2nd trimester treatment start subgroup, heterogeneity comparisons with this subgroup may not be valid.
- 2.
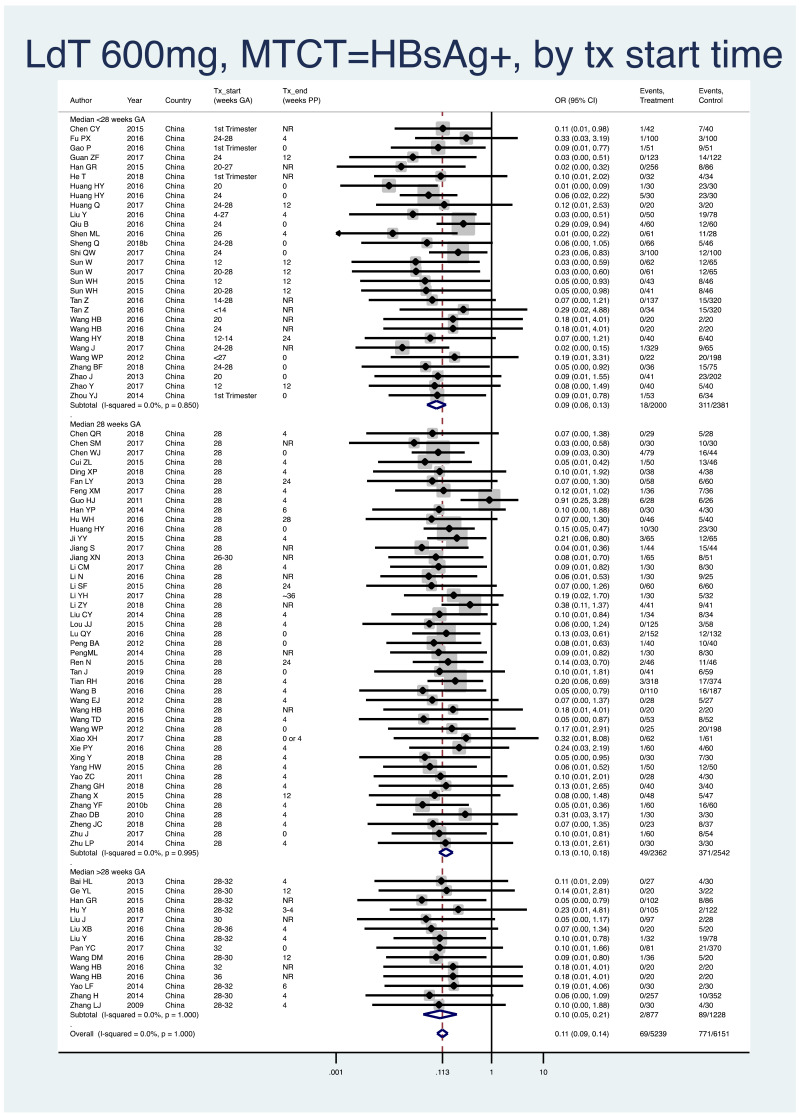
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all HBV DNA levels at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by median weeks of gestation at the time of treatment start (<28 weeks, 28 weeks, >28 weeks).
- <28 weeks: pooled OR=0.09 (95% CI: 0.06–0.13), P<0.001, I2=0.0%
- 28 weeks: pooled OR=0.13 (95% CI: 0.10–0.18), P<0.001, I2=0.0%
- >28 weeks: pooled OR=0.10 (95% CI: 0.05–0.21), P<0.001, I2=0.0%
- When looking at heterogeneity across the three subgroups, the P value was 0.28. If comparing <28 weeks median with 28 weeks median, there was no heterogeneity (p=0.12). If comparing <28 weeks median with >28 weeks median for treatment start, there was no evidence of heterogeneity (P=0.72). If comparing 28 weeks median with >28 weeks median, there was no evidence of heterogeneity (P=0.52).
- 3.
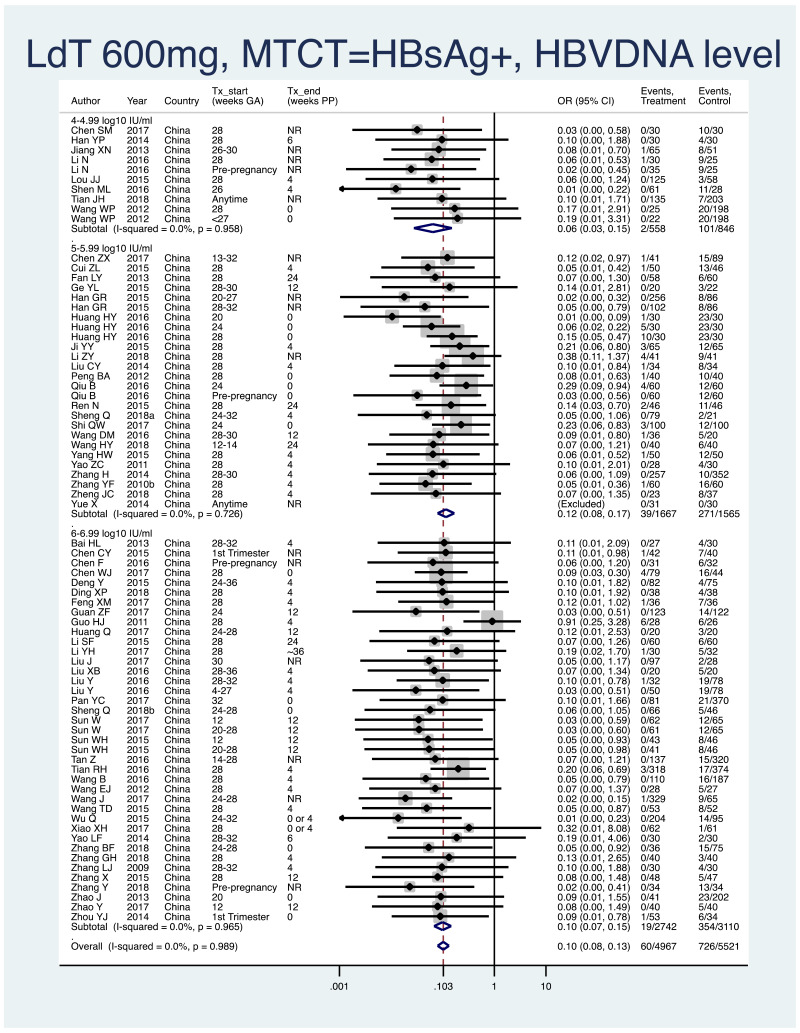
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the minimum HBV DNA level specified in the study inclusion criteria.
- >4–4.99 log10 IU/mL: pooled OR=0.07 (95% CI: 0.03–0.15), P<0.001, I2=0.0%
- >5–5.99 log10 IU/mL: pooled OR=0.12 (95% CI: 0.08–0.17), P<0.001, I2=0.0%
- >6–6.99 log10 IU/mL: pooled OR=0.10 (95% CI: 0.07–0.15), P<0.001, I2=0.0%
- >7–7.99 log10 IU/mL: no studies included
- When looking at heterogeneity across the three HBV DNA level subgroups, the P value was 0.46. If comparing >4–4.99 log10 IU/mL with >5–5.99 log10 IU/mL, there was no heterogeneity (P=0.22). If comparing >5–5.99 log10 IU/mL with >6–6.99 log10 IU/mL, there was no evidence of heterogeneity (P=0.58). If comparing >4–4.99 log10 IU/mL with >6–6.99 log10 IU/mL, there was no evidence of heterogeneity (P=0.36).
- 4.
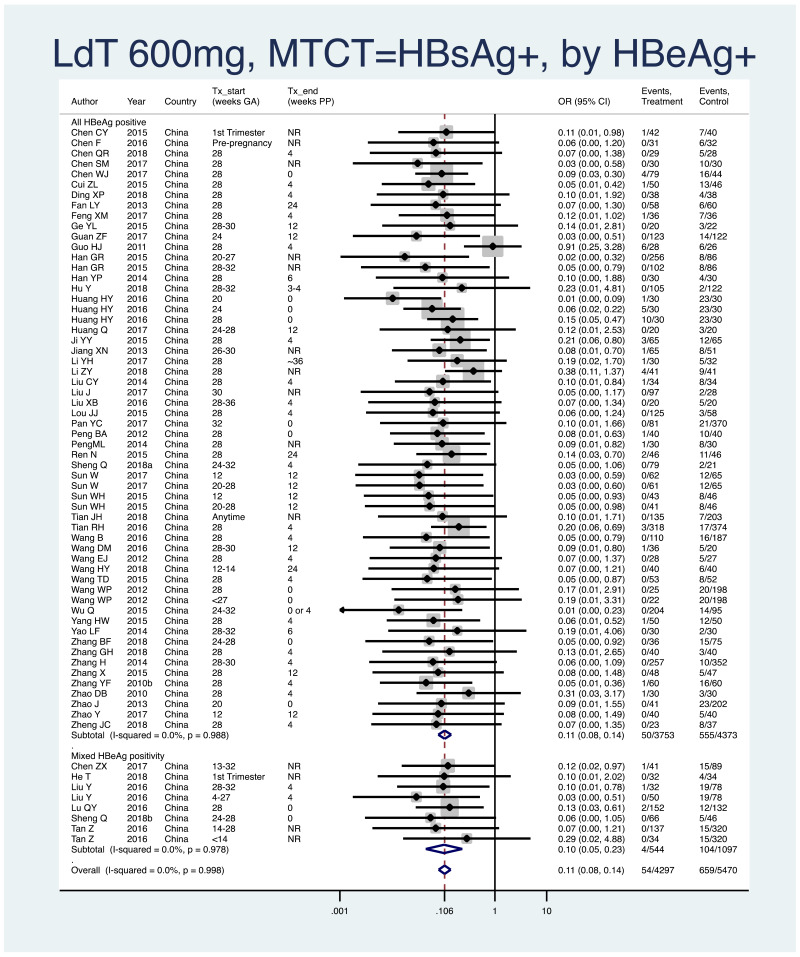
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), stratified by whether or not all women were HBeAg- positive.
- All HBeAg-positive: pooled OR=0.11 (95% CI: 0.08–0.14), P<0.001, I2=0.0%
- Mixed HBeAg-positive: pooled OR=0.10 (95% CI: 0.05–0.23), P<0.001, I2=0.0%
- There was no heterogeneity (P=0.94) between the two subgroups.
- 5.
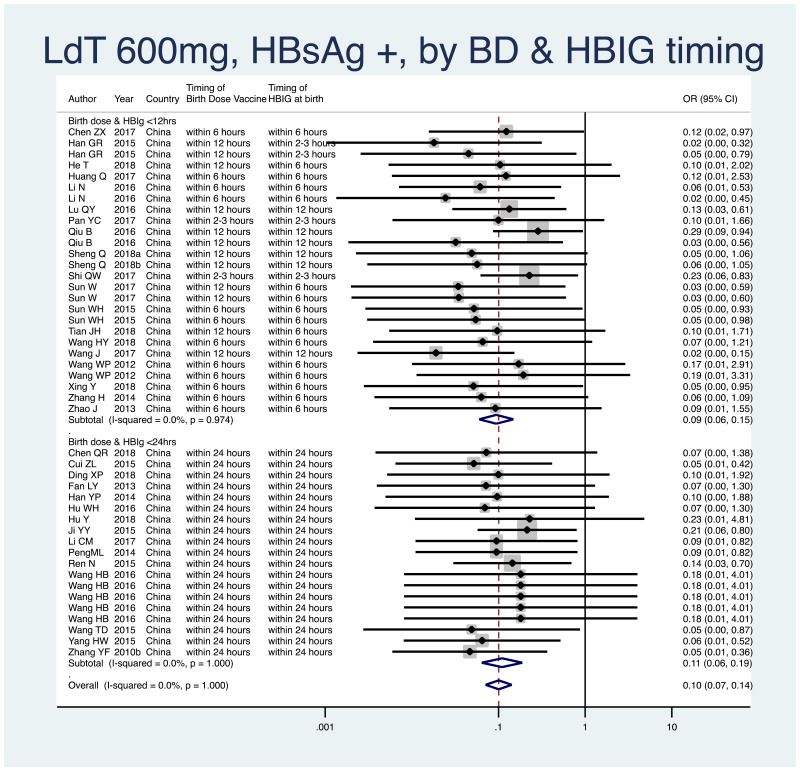
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all HBV DNA levels specified at inclusion, all study designs merged (i.e. RCT and non-RCT), by infant immunoprophylaxis regimen (Table 15).
- As most studies provided all of birth dose vaccines, HBIG at birth, and subsequent infant vaccinations, stratification by type or combination of infant immunoprophylaxis was not done in this meta-analysis.
- Therefore, we stratified by whether or not both birth dose vaccine and HBIG were given within 12 hours of life, versus within 24 hours of life.
- <12 hours: pooled OR=0.09 (95% CI: 0.06–0.15), P<0.001, I2=0.0%
- <24 hours: pooled OR=0.11 (95% CI: 0.06–0.19), P<0.001, I2=0.0%
- The P value for heterogeneity between the two subgroups was 0.67.
Table 15
Infant immunoprophylaxis regimens seen in studies investigating LdT.
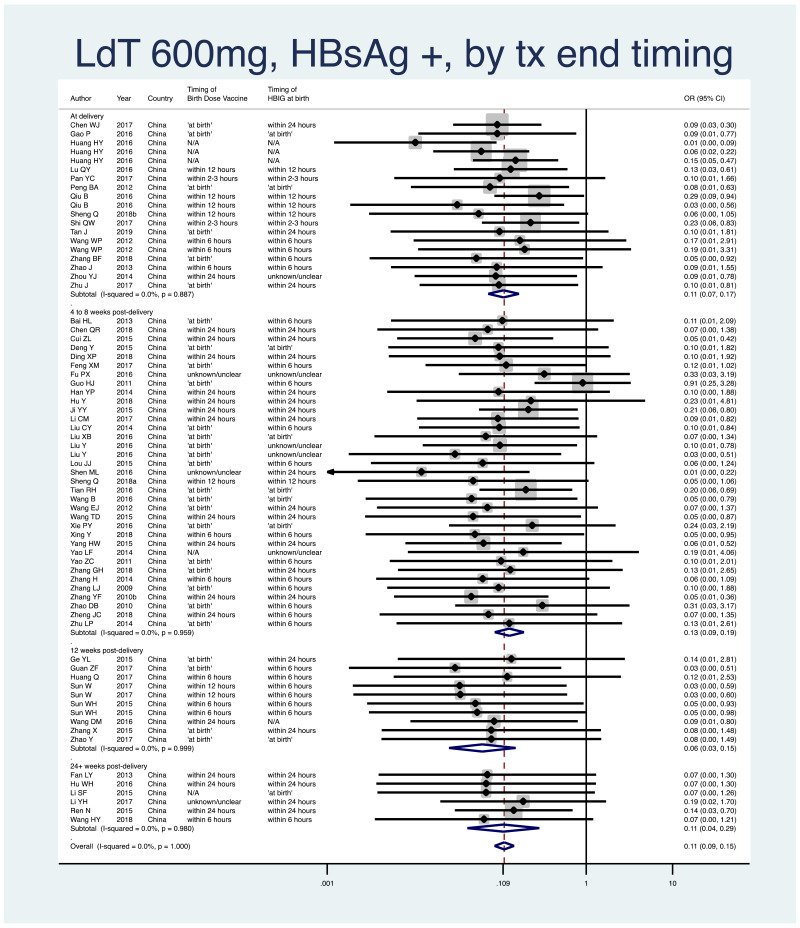
- 6.
PMTCT, as indicated by detection of HBsAg at 6–12 months of age, all treatment start times, all study designs merged (i.e. RCT and non-RCT), stratified by the timing that treatment was discontinued postpartum.
- At delivery: pooled OR=0.11 (95% CI: 0.07–0.17), P<0.001, I2=0.0%
- 4–8 weeks postpartum: pooled OR=0.13 (95% CI: 0.09–0.19), P<0.001, I2=0.0%
- 12 weeks postpartum: pooled OR=0.06 (95% CI: 0.3–0.15), P<0.001, I2=0.0%
- 24+ weeks postpartum: pooled OR=0.11 (95% CI: 0.04–0.29), P<0.001, I2=0.0%
- When looking at heterogeneity across the four subgroups, the P value was 0.55.
Safety analysis, narrative descriptions and selected forest plots
Infant safety outcomes
Of the infant safety outcomes predefined in the protocol, the data for Apgar score were not available for the majority of included studies and where it was available the format varied greatly; this led to an inability to combine results in a meaningful way. None of the included studies for LdT investigated bone mineral density in infants.
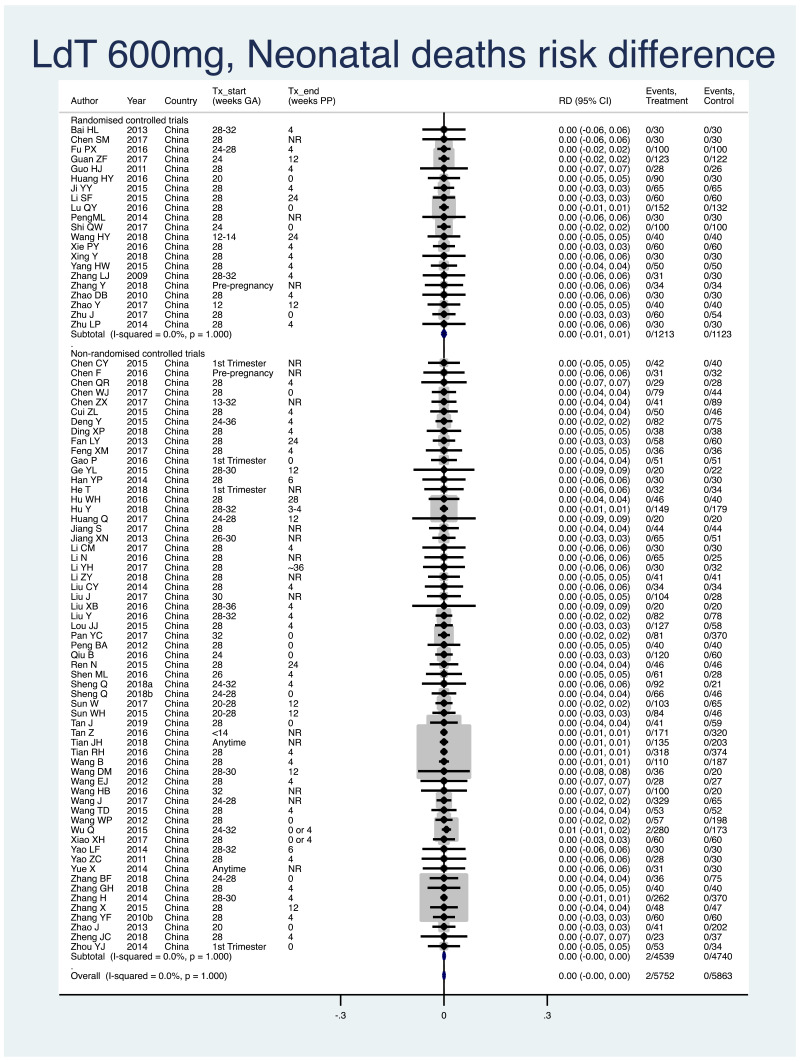
1. Neonatal deaths (death within 28 days of life)
Information on this outcome was available for all except one study that administered LdT to mothers. Two deaths of 5752 infants (non-weighted average 0.03%) were reported across the treatment groups and no deaths in the 5863 infants (0.0%) were reported across the control groups. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.000 (95% CI: −0.002–0.003). The I2 statistics for the overall pooled risk difference, as well as for RCTs and non-RCTs separately, were all 0.0%.
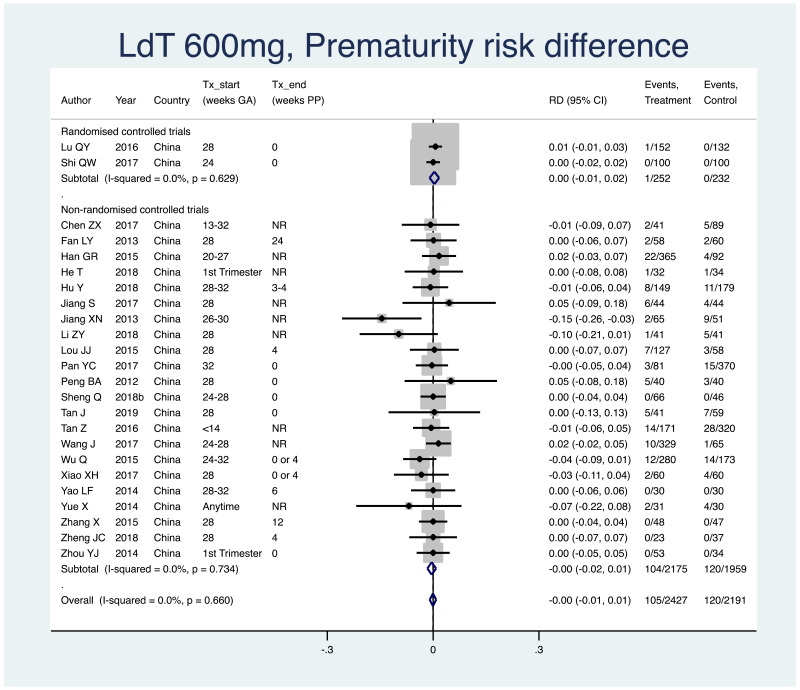
2. Prematurity (typically defined as birth earlier than 37 weeks gestation)
Information on this outcome was available for 24 of the 83 included studies that administered LdT to mothers. Within these studies, 105 of 2427 (non-weighted average 4.3%) infants whose mothers were treated with LdT during pregnancy were born prematurely, whereas 120 of 2191 (non-weighted average 5.5%) infants whose mothers were not treated during pregnancy were born prematurely. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.001 (95% CI: −0.010–0.008). The I2 statistics for the overall pooled risk difference estimated was 0.0%. The I2 statistics for non-RCTs was 0.0%. There were too few RCTs (i.e. <3) to consider the pooled risk difference separately in this subgroup.
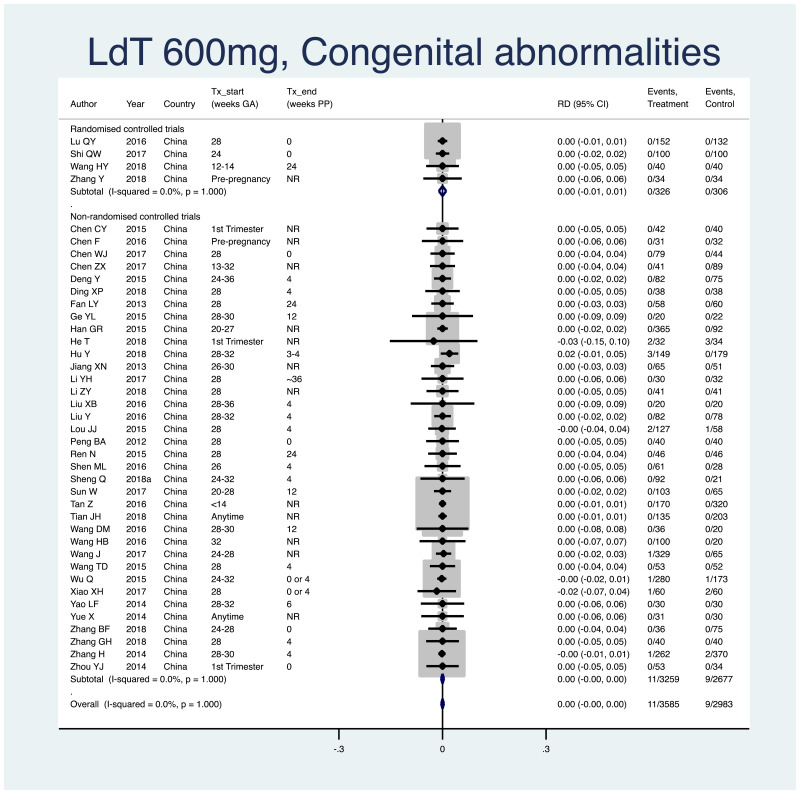
3. Congenital abnormalities
Information on this outcome was available for 40 of the 83 included studies that administered LdT to mothers. Within these studies, 11 of 3585 (non-weighted average 0.3%) infants whose mothers were treated with LdT during pregnancy were noted to have some sort of congenital abnormality, including: anotia (n=1), cerebral palsy (n=1), cinesipathy (n=1), cleft lip and/or palate (n=2), auricular defect (n=1), ear accessory (n=1), no detail provided (n=4). Nine of 2983 (non-weighted average 0.3%) infants whose mothers were not treated during pregnancy were noted to have some sort of congenital abnormality, including: polydactyly (n=1), talipes equinovarus (n=1), ear accessory (n=1), pulmonary stenosis (n=1), hydrocephalus (n=1), congenital ventricular septal defect (n=1), no detail provided (n=3). The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.000 (95% CI: −0.004–0.004). The I2 statistics for the overall pooled risk, as well as for RCTs and non-RCTs separately, were all 0%.
Maternal safety outcomes
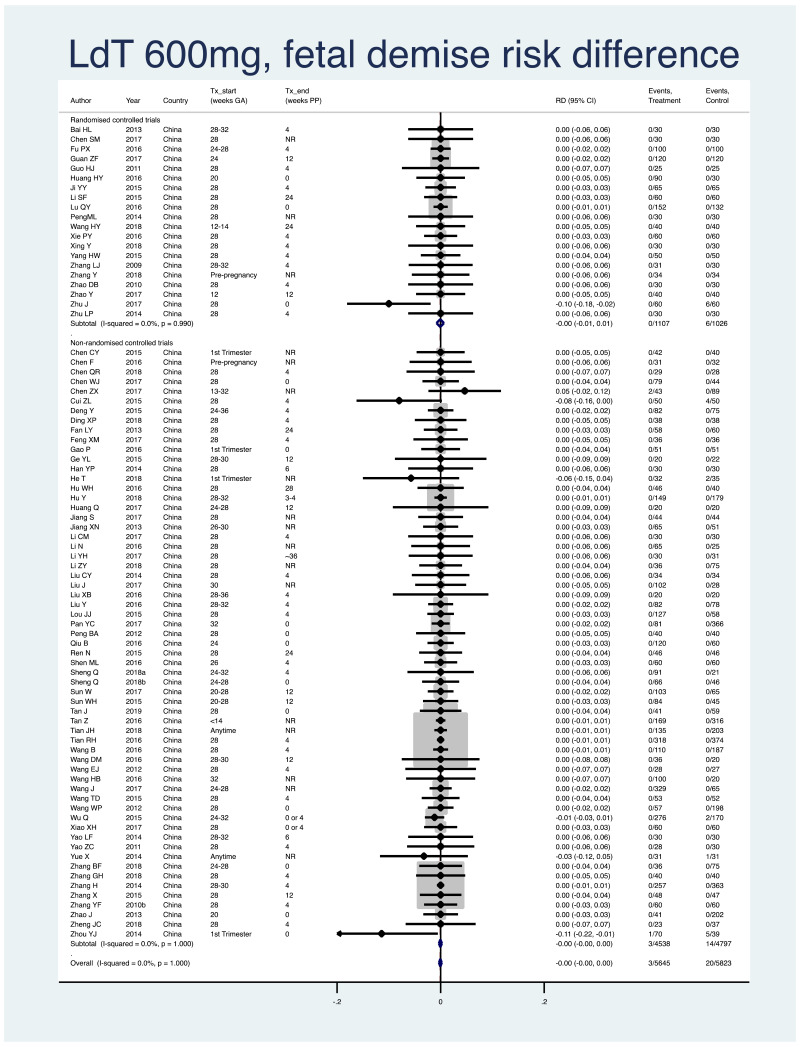
1. Fetal demise (miscarriage [<28 weeks], stillbirth [>=28 weeks])
Information on this outcome was available for 81 of the 83 studies that administered LdT to mothers. Twenty-three cases of fetal demise were reported across all study populations. Three cases (non-weighted average 0.05%) occurred across 5645 mothers/fetuses who were treated with LdT during pregnancy. Twenty cases (non-weighted average 0.3%) occurred across 5823 mothers/fetuses who were not treated during pregnancy. The weighted pooled risk difference for this safety outcome seen following meta-analysis was −0.001 (95% CI: −0.003–0.002). The I2 statistics for the overall pooled risk difference estimate, as well as for RCTs and non-RCTs separately, were all 0%.
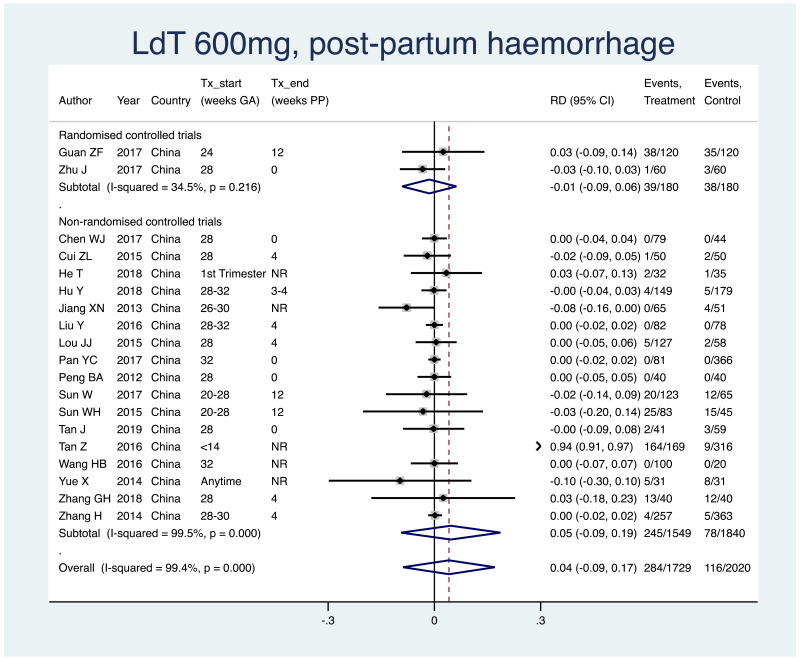
2. Postpartum haemorrhage
Information on this outcome was available for 19 of the 83 included studies that administered LdT to mothers. Within these studies, 284 of 1729 (non-weighted average 16.4%) mothers who were treated with LdT during pregnancy experienced postpartum haemorrhage, whereas 116 of 2020 (5.7%) mothers who were not treated during pregnancy experienced postpartum haemorrhage. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.041 (95% CI: −0.089–0.171). The I2 statistics for the overall pooled risk difference was 99.4%; that for non-RCTs was 99.5%. Not enough RCTs evaluated this safety outcome to consider this subgroup separately.
3. Antiviral resistance
Seven studies that treated mothers with LdT during pregnancy reported on some results of testing for antiviral resistance. One study reported that in 11 of 257 women in the treated group with previous antiviral therapy (LdT or other) no resistance mutations were detected, and that in the entire study, no participant discontinued due to resistance (Han et al., 2015). One study reported that of 78 treatment women, one participant developed an M204I drug-resistance mutation after receiving LdT for 40 weeks (Liu et al., 2016). Another study evaluated drug resistance in all 103 treated participants (timing not clear) and found no evidence of resistance mutations (Sun et al., 2017). Three studies reported antiviral resistance as a quantitative outcome (few details provided), giving case numbers of two in 31 treated women (Chen et al., 2016), one in 35 treated women (Li et al., 2016), and none in 60 treated women (Shen et al., 2016), respectively. Finally, one study evaluated antiviral resistance in seven women (of 105) whose HBV DNA levels did not reduce during treatment, and found no resistance mutations (Hu et al., 2018).
4. HBV flare
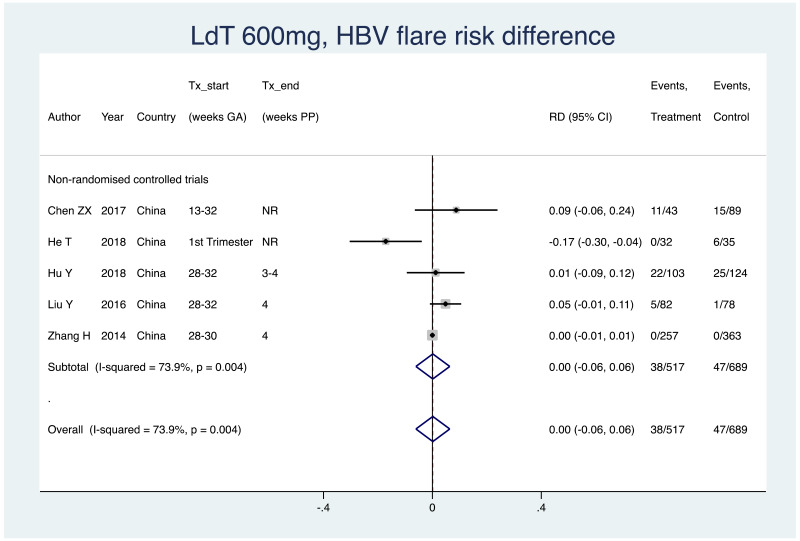
Information on this outcome was available for five of the 83 included studies that administered LdT to mothers. Various definitions were used, including: “ALT >40 U/L”, “ALT >2 times baseline”, “ALT >= 8 times ULN”, “ALT >8 ULN or 5 times baseline”. Within these studies, 38 of 517 (non-weighted average 7.4%) mothers who were treated with LdT during pregnancy experienced a type of HBV flare at the time of treatment discontinuation, whereas 47 of 689 (non-weighted average 6.8%) mothers who were not treated during pregnancy experienced the same type of HBV flare at a matched time-point. The weighted pooled risk difference for this safety outcome seen following meta-analysis was 0.001 (95% CI: −0.061–0.064). Overall, the pooled risk difference (non-RCTs only were included) had a high level of heterogeneity (I2 =73.9%).
GRADE summary of findings
Table 16
GRADE evidence profile: LdT 600 mg during pregnancy to prevent HBV mother-to-child transmission (MTCT).
Other antiviral therapies
Telbivudine (LdT) 100 mg
Three studies were eligible for this meta-analysis that used LdT 100 mg (Ge JQ et al., 2015; Li ZG et al., 2015; Mu YSJ et al., 2018). Of these, one was an RCT and two were non-RCTs. Of the non-RCTs, the risk of bias scores, according to the Newcastle–Ottawa scale, were 5 (high) and 6 (high), respectively (Mu YSJ et al., 2018; Ge JQ et al., 2015); as per protocol, studies with a high risk of bias with scores of 5 or lower were excluded from analysis. Therefore, we describe only the basic details of two studies (one RCT and one non-RCT) here.
One RCT was performed that examined use of LdT 100 mg during pregnancy for the PMTCT of HBV (Li ZG et al., 2015). This study took place in China from 2013 to 2014. Treatment was started at 28 weeks of pregnancy, and stopped after 6 weeks postpartum. Birth dose vaccination and HBIG were given to all infants on the first day of life, and two further vaccinations were given at 1 and 6 months of life. Of 25 infants whose mothers were treated during pregnancy, none were positive for HBsAg at 1 year of life, compared to four of 25 control infants at the same time-point (OR=0.09, 95% CI: 0.01–1.84). Infant and maternal adverse events were not well described in the article.
One non-RCT, specifically a retrospective cohort study, was performed that examined use of LdT 100 mg during pregnancy for the PMTCT of HBV (Ge JQ et al., 2015). This study took place in China from 2012 to 2013. Treatment was started between 28 and 32 weeks of pregnancy, and stopped after 6 weeks postpartum. Birth dose vaccination and HBIG were given to all infants within 12 hours of life, and two further vaccinations were given at 1 and 6 months of life. Of 40 infants whose mothers were treated during pregnancy, one was positive for HBsAg at 12 months of life, compared to 11 of 40 control infants at the same time-point (OR=0.07, 95% CI: 0.01–0.55). Most infant and maternal adverse events were not addressed in the article; however, authors did confirm that there were no congenital abnormalities in either the treated or control group at the time of birth.
Adefovir dipivoxil (ADV) 500 mg
One RCT was performed that examined use of ADV 500 mg during pregnancy for the PMTCT of HBV (Feng Y et al., 2018). This study took place in China in 2017. Treatment was started at 28 weeks of pregnancy, and stopped at the time of delivery. HBIG was given within 24 hours of birth, a vaccination was given at “0 months”, and two further vaccinations were given at 1 and 6 months of life. Of 254 infants whose mothers were treated during pregnancy, six were positive for HBsAg at 1 year of life, compared to 24 of 251 control infants at the same timepoint (OR=0.23, 95% CI: 0.09–0.57). Infant adverse events were not well described in the article. Of maternal adverse events, the authors did report that 5.4% (95% CI: 3.0–8.9) of women in the treated arm had postpartum haemorrhage, whereas this was 10.1% (95% CI: 6.7–14.4) in the control group.
Adefovir dipivoxil (ADV) 10 mg
One non-RCT, specifically a prospective cohort study, was performed that examined uthe se of ADV 10 mg during pregnancy for the PMTCT of HBV (Fang HS et al., 2011). This study took place in China from 2006 to 2008. Treatment with ADV was started prior to pregnancy in all women (end time not reported), and additionally, HBIG was given to women in both the treatment and control groups at 28, 32 and 36 weeks of gestation. Birth dose vaccination was done (timing unclear), and two further vaccinations were given at 1 and 6 months of life. There was no mention of administration of HBIG to infants in the article. Of 42 infants whose mothers were treated during pregnancy, none were positive for HBsAg at 12 months of life, compared to five of 52 control infants at the same time-point (OR=0.10, 95% CI: 0.01–1.89). Most infant and maternal adverse events were not addressed in the article; however, authors did confirm that there were no congenital abnormalities or cases of prematurity in either the treated or control group at the time of birth.
Conclusion
This meta-analysis shows that certain antiviral therapies may be efficacious if used during pregnancy for the PMTCT of HBV, as indicated by the proportion of infants with HBsAg detected at 6–12 months of life. Specifically, meta-analysis of RCTs investigating TDF 300 mg had a protective, pooled OR of 0.10 (95% CI: 0.03–0.35), those investigating LAM 100–150 mg had a protective pooled OR of 0.16 (95% CI: 0.10–0.26), and those investigating LdT 600 mg had a protective pooled OR of 0.14 (95% CI: 0.09–0.21). The GRADE evidence quality for each of these three treatment regimens was “moderate” for RCTs, and “low” for non-RCTs; however, the results for RCTs and non-RCTs were concordant (see Table 17).
Table 17
Meta-analysis odds ratios (OR) for all studies using infant HBsAg as outcome, by study design, by treatment type.
There were almost no differences seen (heterogeneity across pooled OR estimates) for any subgroup analyses for any treatment type included. Only in one subgroup analysis for LAM 100 mg, which examined the difference between treatment starting at a median <28 weeks, 28 weeks or >28 weeks, was heterogeneity observed. In this case, it appeared that starting treatment at median 28 weeks or <28 weeks was significantly more protective than starting treatment at a median >28 weeks.
There was moderate- to low-grade evidence that taking antiviral therapies for PMTCT did not increase the risk of certain infant and maternal safety outcomes, such as neonatal death, congenital abnormalities, fetal demise (miscarriage or stillbirth). However, it is important to note that for some of these outcomes, notably neonatal death and fetal demise, there are important concerns regarding data quality in this review (see Limitations section below). There was always very low evidence with regards to maternal antiviral therapy and the occurrence of HBV flare; few studies presented this information and where it was presented, definitions and time-points varied considerably, limiting our ability to combine these findings in a meaningful way. Across all treatment types, there was very little or no information on antiviral resistance in mothers, and bone mineral density changes in infants; other study designs and evidence should be considered by policy-makers for a better understanding of these risks.
Strengths
This is a thorough and up-to-date review and meta-analysis of the literature on the PMTCT through provision of maternal antiviral therapy. The main strength of this review is its extensive scoping of the Chinese literature; this has not been as exhaustively performed in other recent systematic reviews (Zhou YH, 2016). This led to a large number of studies included for each treatment type when compared to other reviews; for example, two recent meta-analyses with similar objectives as the study we have presented here included 59 and 41 studies, respectively (Song et al., 2019; Tavakolpour et al., 2018). Furthermore, extensive efforts were employed to examine crossover between patient groups from different articles; the inclusion of overlapping patient populations has been criticized in other recent systematic reviews (Zhou YH, 2016).
Limitations
The major limitation of this review is the high risk of bias that defined many of the studies included; only two of 33 (6%) included RCTs could be considered to have a low risk of bias, only seven of 33 (21%) RCTs reported loss to follow up adequately. This limited our ability to perform ITT analysis, which has important implications in terms of attrition bias, and should be considered when interpreting the results for the primary outcomes, as well as for some safety outcomes (e.g. difference in the risk of neonatal death, fetal demise). Furthermore, although non-RCTs with a very high risk of bias were excluded from analysis, 31% of the remaining non-RCTs had a score of 6 (high) on the Newcastle–Ottawa scale (i.e. one point below being “low risk of bias”).
It was not possible to fully examine all important safety outcomes, such as HBV flare, as standardized information was lacking in most papers. Another limitation of this review is that very few studies outside of the Western Pacific Region were included – this limits the ability to generalize our findings to other important regions in terms of prevalence of HBV, such as the African Region.
Implications for research
Due to limited information found in the included studies in this review, some subgroup and safety analyses were not possible. Further research is needed on this PMTCT topic in the following areas:
- -
differences in populations coinfected with HIV, HCV, HDV
- -
differences according to more specific timing/well-defined time periods for starting antiviral therapy (e.g. start of 2nd trimester versus start of 3rd trimester)
- -
differences according to very timely birth dose vaccination – possibly, with prompt delivery of HBIG and birth dose vaccine, the beneficial effect of antiviral therapy during pregnancy diminishes.
Very importantly, no study was included in this meta-analysis that took place in the African Region. There are many differences between Africa and other regions with regard to this topic such as in HBV epidemiology (e.g. high prevalence of genotypes A, D, E in Africa versus higher prevalence of genotypes B and C in Asia) and the natural history of chronic HBV infection. Additionally, the current standard of care varies considerably when comparing Africa and Asia; there is a relatively high coverage of timely birth dose vaccine in Asia as well as some availability of HBIG; however, there is a low coverage of timely birth dose vaccine in Africa as well as a lack of access to HBIG. Along these lines, it is notable that no study included in this review investigated the efficacy of maternal antiviral therapy in the absence of HBIG given to infants at birth even though in resource-limited countries access to HBIG is severely limited.
Finally, no study examined the efficacy of antiviral therapy for PMTCT in HBeAg- negative mothers with a high viral load. There is potentially an important population of women with a high viral load but negative for HBeAg; in the review for the PICO2 question, it was found that 16.4% of women with viraemia ≥5 log10 IU/mL and 2.2% of women with viraemia ≥7 log10 IU/mL are negative for HBeAg.
References
- AASLD, 2018. American Association for the Study of Liver Disease Practice Guidelines. Accessed at: https://www.aasld.org/publications/practice-guidelines. Last accessed on September 2nd, 2019.
- APASL, 2016. Asian Pacific Association for the Study of the Liver Guidelines. Accessed at: http://apasl.info/guidelines/ Last accessed on September 2nd, 2019.
- Celen MK, Mert D, Ay M, Dal T, Kaya S, Yildirim N et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy for the prevention of vertical transmission of HBV infection. World J Gastroenterol 2013 December 28; 19(48): 9377–9382 [PMC free article: PMC3882411] [PubMed: 24409065]
- Chang MH. Natural History and clinical management of chronic hepatitis B virus infection in children. Hepatol Int 2008; 2: S28–S36. [PMC free article: PMC2716838] [PubMed: 19669296]
- Chen CY, Tu X, Cheng Q, Chen F, Dai Y, Gong F, et al. Clinical observation of telbivudine’s antiviral efficacy and protection against mother-to-infant transmission of chronic hepatitis B during the first trimester of pregnancy. Chin J Hepatol 2015; 23 (1): 9–12 [PubMed: 25751379]
- Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM et al. Efficacy of Maternal Tenofovir Disoproxil Fumarate in Interrupting Mother-to-Infant Transmission of Hepatitis B Virus. HEPATOLOGY, Vol. 62. No. 2; 2015, 375–386 [PubMed: 25851052]
- Chen HL, Lin LH, Hu FC, Lee JT, Lin WT, Yang YJ, et al. Effects of Maternal Screening and Universal Immunization to Prevent Mother-to-Infant Transmission of HBV. Gastroenterology 2012; 142: 773–781. [PubMed: 22198276]
- Chen ZX, Gu GF, Bian ZL, Cai WH, Shen Y, Hao YL et al. Clinical course and perinatal transmission of chronic hepatitis B during pregnancy in a real-life setting: A prospective cohort study. J Infect. 2017 Aug;75(2):146–154. doi: 10.1016/j.jinf.2017.05.012 [PubMed: 28551372] [CrossRef]
- Clinical observation on lamivudine preventing mother-to-child transmission of hepatitis B virus. Jiangxi Medical Journal 2009; 44 (3): 250–251
- Deng Y, Wu W, Zhang D, Hu P, Kang J, Yang Y, et al. The safety of telbivudine in preventing mother-to-infant transmission of hepatitis B virus in pregnant women after discontinuation. Chin J Hepatol 2015; 23 (8): 586–589 [PubMed: 26447621]
- EASL. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. (2017) Accessible at: https://easl.eu/wp-content/uploads/2018/10/HepB-English-report.pdf. Last retrieved September 2nd, 2019.
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc. R. Soc. Lond. B 1993; 253:197–201 [PubMed: 8397416]
- Evans AA, O’Connell AP, Pugh JC, Mason WS, Shen F, Chen GC, et al. Geographic variation in viral load among Hepatitis B Carriers with differing risks of hepatocellular carcinoma. Cancer Epidemiology, Biomarkers & Prevention 1998; 7:559–565. [PubMed: 9681522]
- Foaud HM, Maklad S, Gmal El Din A, Mahmoud F. Lamivudine use in pregnant HBsAg-females effectively reduces maternal viremia. Arab J Gastroenterol. 2019 Mar;20(1):8–13. doi: 10.1016/j.ajg.2019.02.003 [PubMed: 30857834] [CrossRef]
- The GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490–4. [PMC free article: PMC428525] [PubMed: 15205295]
- Greenup AJ, Tan PK, Nguyen V, Glass A, Davison S, Chatterjee U et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of Hepatitis B Virus. J Hepatol. 2014 Sep;61(3):502–7. doi: 10.1016/j.jhep.2014.04.038 [PubMed: 24801414] [CrossRef]
- Greenup AJ, Tan PK, Nguyen V, Glass A, Davison S, Chatterjee U et al. Corrigendum to ‘‘Efficacy and safety of tenofovir disoproxil fumarate (TDF) in pregnancy to prevent perinatal transmission of hepatitis B virus’’ [J Hepatol 2014;61:502–507]. Journal of Hepatology 2015 vol. 63: 1054 [PubMed: 24801414]
- Han GR, Jiang HX, Yue X, Ding Y, Wang CM, Wang GJ et al. Efficacy and safety of telbivudine treatment: an open-label, prospective study in pregnant women for the prevention of perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2015 Sep;22(9):754–62. doi: 10.1111/jvh.12379 [PubMed: 25641421] [CrossRef]
- He T, Bai Y, Cai H, Ou X, Liu M, Yi W et al. Safety and efficacy of lamivudine or telbivudine started in early pregnancy for mothers with active chronic hepatitis B. Hepatol Int. 2018 Mar;12(2):118–125. doi: 10.1007/s12072-017-9839-5. [PubMed: 29344772] [CrossRef]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. (www.cochrane-handbook.org. Accessed 13th March 2019).
- Hu Y, Xu C, Xu B, Hu L, Liu Q, Chen J et al. Safety and efficacy of telbivudine in late pregnancy to prevent mother-to-child transmission of hepatitis B virus: A multicenter prospective cohort study. J Viral Hepat. 2018;25:429–437 [PubMed: 29193547]
- Hutin Y, Desai S, Bulterys M. Preventing hepatitis B virus infection: milestones and targets. Bull World Health Organ. 2018; 96(7):443–A. [PMC free article: PMC6022619] [PubMed: 29962544]
- Jackson V, Ferguson W, Kelleher TB, Lawless M, Eogan M, Nusgen U et al. Lamivudine treatment and outcome in pregnant women with high hepatitis B viral loads. Eur J Clin Microbiol Infect Dis. 2015 Mar;34(3):619–23. doi: 10.1007/s10096-014-2270-0. [PubMed: 25381607] [CrossRef]
- Jiang HX, Han G, Wang C, Ji Y. Maternal-fetal outcomes of lamivudine treatment administered during late pregnancy to highly viremic mothers with HBeAg+ chronic hepatitis B. Chin J Hepatol 2012; 20 (12): 888–891 [PubMed: 23522247]
- Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, et al., Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378 (10):911–23. [PMC free article: PMC5895092] [PubMed: 29514030]
- Keane E, Funk A, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Aliment Pharmacol Ther 2016; 44 (10):1005–1017 [PubMed: 27630001]
- Kochaksaraei GS, Castillo E, Osman M, Simmonds K, Scott AN, Oshiomogho JI et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. Journal of Viral Hepatitis, 2016, 23, 15–2 [PubMed: 26192022]
- Kourtis AP, Wiener J, Wang L, Fan B, Shepherd J, Chen L et al., Tenofovir Disoproxil Fumarate Use during Pregnancy and Infant Bone Health: the Tenofovir in Pregnancy Pilot Study. Pediatr Infect Dis J 2018; 37 (11): e264–e268 [PubMed: 30067600]
- Liu CP, Zeng YL, Zhou M, Chen LL, Hu R, Wang L et al. Factors Associated with Mother-to-child Transmission of Hepatitis B Virus Despite Immunoprophylaxis. Intern Med 54: 711–716, 2015 [PubMed: 25832930]
- Liu J, Wang C. Effect of telbivudine in treatment of hyperplasma viremia pregnant women during immune tolerant phase of hepatitis B virus infection and during the second and the third trimesters of pregnancy and observation on the proportion of pregnant women with increased alanine aminotransferase level after drug withdrawal. Maternal and Child Health Care of China 2017; 32 (15): 3477–3480
- Lin Y, Liu Y, Ding G, Touqui L, Wang W, Xu N et al. Efcacy of tenofovir in preventing perinatal transmission of HBV infection in pregnant women with high viral loads. Nature Scientific Reports 2018; 8:15514 [PMC free article: PMC6195597] [PubMed: 30341345]
- Liu Y, Wang M, Yao S, Yuan J, Lu J, Li H et al. Efficacy and safety of telbivudine in different trimesters of pregnancy with high viremia for interrupting perinatal transmission of hepatitis B virus. Hepatology Research 2016; 46: E181–E188 [PubMed: 25869545]
- Machaira M, Papaevangelou V, Vouloumanou EK, Tansarli GS, Falagas ME. Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg− mothers: a systematic review and meta-analysis. J Antimicrob Chemother 2015; 70: 396–404. [PubMed: 25362571]
- Miyahara R, Jasseh M, Gomez P, Shimakawa Y, Greenwood B, Keita K, et al. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine 2016; 34: 3335–3341. [PMC free article: PMC4915601] [PubMed: 27195759]
- Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis 2016; 16(12):1399–408. [PubMed: 27638356]
- Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med 2016;374:2324–34 [PubMed: 27305192]
- Pan CQ, Yi W, Liu M, Wan G, Hu YH, Zhou MF. Lamivudine therapy during the second vs the third trimester for preventing transmission of chronic hepatitis B. J Viral Hepat. 2017 Mar;24(3):246–252. doi: 10.1111/jvh.12640. [PubMed: 28025872] [CrossRef]
PanY,WangC,WenS,WangC,KongF,NiuJ,et al.Clinical effect and short-term safety of telbivudine in blocking mother-to-child transmission of HBV.J Clin Hepatol2017;33(9):1707–1712- Salvadori N, Fan B, Teeyasoontranon W, Ngo-Giang-Huong N, Phanomcheong S, Luvira A et al. Maternal and Infant Bone Mineral Density 1 Year After Delivery in a Randomized, Controlled Trial of Maternal Tenofovir Disoproxil Fumarate to Prevent Mother-to-child Transmission of Hepatitis B Virus. Clin Infect Dis. 2019 Jun 18;69(1):144–146. doi: 10.1093/cid/ciy982. [PMC free article: PMC6579954] [PubMed: 30924492] [CrossRef]
- Sheng Q, Ding Y, Li B, Han C, Li Y, Zhang C et al. Efficacy and safety of nucleos(t)ide analogues to prevent hepatitis B virus mother-to-child transmission in pregnant women with high viremia: real life practice from China. Int. J. Med. Sci. 2018; 15(8): 796–801 [PMC free article: PMC6036077] [PubMed: 30008589]
- Sheng QJ, Wang SJ, Wu YY, Dou XG, Ding Y. Hepatitis B virus serosurvey and awareness of mother-to-child transmission among pregnant women in Shenyang, China: An observational study. Medicine. 2018; 97:22 [PMC free article: PMC6392912] [PubMed: 29851831]
- Shimakawa Y, Hong-Jing Y, Tsuchiya N, BottomLey C, Hall AJ. Association of early age at establishment of Hepatitis B infection with persistent viral replication, liver cirrhosis, and hepatocellular carcinoma: a systematic review. PLOS ONE 2013;8(7):e69430. [PMC free article: PMC3716646] [PubMed: 23894479]
- Song J, Yang F, Wang S, Tikande S, Deng Y et al., Efficacy and safety of antiviral treatment on blocking the mother-to-child transmission of hepatitis B virus: A meta-analysis. Journal of Viral Hepatitis 2019;26:397–406 [PubMed: 30417469]
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, et al., Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002 [PubMed: 21784880]
- Sun WH, Ma L, Hao A, Liu W, Song M, Li M, et al. Predictive value of telbivudine in preventing mother-to-infant transmission of hepatitis B virus in pregnant women with high viremia. Chin J Hepatol 2015; 23(3):180–183 [PubMed: 25938829]
- Sun W, Zhao S, Ma L, Hao A, Zhao B, Zhou L et al. Telbivudine treatment started in early and middle pregnancy completely blocks HBV vertical transmission. BMC Gastroenterology 2017; 17:51 [PMC free article: PMC5390436] [PubMed: 28407735]
- Tan Z, Yin Y, Zhou J, Wu L, Xu C, Hou H. Telbivudine treatment of hepatitis B virus-infected pregnant women at different gestational stages for the prevention of mother-to-child transmission: Outcomes of telbivudine treatment during pregnancy. Medicine 2016; 95:40 [PMC free article: PMC5059039] [PubMed: 27749537]
- Tavakolpour S, Darvishi M, Mirsafaei HS, Ghasemiadl. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B infection during pregnancy: a systematic review. Infectious Diseases, 2018; 50 (2); 95–106. [PubMed: 29020844]
- van Zonneveld M, van Nunen AB, Niesters HGM, de Man RA, Schalm SW, Janssen HLA. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. Journal of Viral Hepatitis, 2003, 10, 294–297 [PubMed: 12823596]
- Wakano Y, Sugiura T, Endo T, Ito K, Suzuki M, Tajiri H et al. Antiviral therapy for hepatitis B virus during second pregnancies. J. Obstet. Gynaecol. Res. Vol. 44, No. 3: 566–569, March 2018 [PubMed: 29227001]
- Wang H, Lu R, Zhong C, Xun S. Clinical studies on telbivudine blocking effect of mother-to-infant transformation of pregnant women with chronic hepatitis B virus. Contemporary Medicine 2018; 24 (10): 70–72
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2014. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Last accessed on March 13, 2019.
- Wen WH, Chang MH, Zhao LL, Ni Y, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: Significance of maternal viral load and strategies for intervention. J Hepatol 2013; 59: 24–30. [PubMed: 23485519]
- World Health Organization. Neonatal and perinatal mortality: country, regional and global estimates. WHO library 2006. Available at: https://apps.who.int/iris/bitstream/handle/10665/43444/9241563206_eng.pdf?sequence=1. Last access on March 13, 2019.
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009; 84(40):405–19. [PubMed: 19817017]
- World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. WHO-Geneva 2015. Available at: http://apps.who.int/medicinedocs/documents/s21813en/s21813en.pdf. Last accessed on March 13th 2019.
- World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. WHO-Geneva 2016. Available at: https://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/. Last accessed on March 13th 2019.
- World Health Organization. Global Hepatitis Report 2017. WHO-Geneva 2017a. Available at: https://apps.who.int/iris/rest/bitstreams/1082592/retrieve. Last accessed on March 13th 2019.
- World Health Organization. Hepatitis B vaccines: WHO position paper - July 2017. Wkly Epidemiol Rec 2017b; 27(92): 369–392 [PubMed: 28685564]
- World Health Organization. Preterm Birth. Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth. WHO-Geneva 2018. Last accessed on March 13th, 2019.
- World Health Organization. Maternal, newborn, child and adolescent health. Available at: https://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/. Last accessed on: March 13th, 2019.
- Wu Q, Huang H, Sun X, Pan M, He Y, Tan S et al. Telbivudine Prevents Vertical Transmission of Hepatitis B Virus From Women With High Viral Loads: A Prospective Long-Term Study. Clinical Gastroenterology and Hepatology 2015;13:1170–1176 [PubMed: 25251571]
- Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. Journal of Viral Hepatitis, 2009, 16, 94–103 [PubMed: 19175878]
- Zhang BF, Cheng M, Zhang Q, Zhao X, Yu L, Yang J, et al. Clinical study on blocking mother-to-child transmission of hepatitis B virus with high viral load and HBeAg positivity during pregnancy in Guizhou province. Chin J Hepatol 2018; 26 (12): 945–950 [PubMed: 30669789]
- Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or Lamivudine Use in Late Pregnancy Safely Reduces Perinatal Transmission of Hepatitis B Virus in Real-Life Practice. Hepatology. 2014; 60 (2): 468–476 [PMC free article: PMC4282428] [PubMed: 25187919]
- Zhang L, Wang L. Blocking intrauterine infection by telbivudine in pregnant chronic hepatitis B patients. Chin J Hepatol 2009; 17 (8): 561–563 [PubMed: 19719910]
- Zhou YJ, Zheng J, Pan H, Lu C. Long-term efficacy and safety of telbivudine in the treatment of childbearing patients with chronic hepatitis B. Chin J Hepatol 2014; 22 (8): 573–576 [PubMed: 25243955]
- Zhou YH, Correspondence: Prevention of Mother-to-Child Transmission of Hepatitis BVirus by Treating Mothers With High Viral Loads. Hepatology 2016; 64 (5): 1823–1824. [PubMed: 27118339]
Appendices
APPENDIX A. Search strategies
Database: PubMed
Date searched: 28 March 2019
Search strategy:
Database: Embase Classic + Embase via OvidSP (1947–2019 March 26th)
Date searched: 28 March 2019
Search strategy:
Database: Scopus
Date searched: 28 March 2019
Search strategy:
Database: CENTRAL Database (The Cochrane Library)
Date searched: 28 March 2019
Search strategy:
Database: CNKI
Date searched: 28 March 2019
Search strategy:
SU=‘乙型肝炎‘+’乙肝‘+’乙型肝炎病毒‘+’乙肝病毒‘+’HBV‘+’乙型肝炎表面抗原‘+’乙肝表面抗原’ AND SU=‘抗病毒‘+’抗病毒药物‘+’核苷‘+’核苷酸‘+’核苷类似物‘+’核苷酸 类似物‘+’NAs‘+’阿德福韦酯‘+’hepsera‘+’preveon‘+’bis-POM PMEA‘+’GS 840‘+’ADV‘+’恩曲他滨‘+’emtriva‘+’FTC‘+’恩替卡韦‘+’baraclude‘+’ETV‘+’拉米夫定 ‘+’epivir‘+’3TC‘+’LAM‘+’替比夫定‘+’sebivo‘+’tyzeka‘+’LdT‘+’替诺福韦酯 ‘+’viread‘+’TDF‘+’替诺福韦艾拉酚胺‘+’vemLidy‘+’TAF’ AND SU=‘妊娠‘+’怀孕‘+’孕 妇‘+’孕期‘+’母胎‘+’母亲‘+’胎儿‘+’子代‘+’子女‘+’垂直传播‘+’产前‘+’产时‘+’产间‘+’围产 ‘+’出生前‘+’围生‘+’宫内‘+’跨胎盘‘+’胎盘‘+’母婴传播‘+’预防母婴传播‘+’阻断母婴传播 ‘+’妊娠并发症‘+’产前诊断‘+’先天’
Database: Wanfang
Date searched: 28 March 2019
Search strategy:
主题: (“乙型肝炎“+”乙肝“+”乙型肝炎病毒“+”乙肝病毒“+”HBV“+”乙型肝炎表面抗 原”+”乙肝表面抗原”) and 主题: (“抗病毒“+”抗病毒药物“+”核苷“+”核苷酸“+”核苷类 似物“+”核苷酸类似物“+”NAs“+”阿德福韦酯“+”hepsera“+”preveon“+”bis-POM PMEA“+”GS 840“+”ADV“+”恩曲他滨“+”emtriva“+”FTC“+”恩替卡韦 “+”baraclude“+”ETV“+”拉米夫定“+”epivir“+”3TC“+”LAM“+”替比夫定 “+”sebivo“+”tyzeka“+”LdT“+”替诺福韦酯“+”viread“+”TDF“+”替诺福韦艾拉酚胺 “+”vemlidy“+”TAF”) and 主题: (“妊娠“+”怀孕“+”孕妇“+”孕期“+”母胎“+”母亲“+”胎儿 “+”子代“+”子女“+”垂直传播“+”产前“+”产时“+”产间“+”围产“+”出生前“+”围生“+”宫内 “+”跨胎盘“+”胎盘“+”母婴传播“+”预防母婴传播“+”阻断母婴传播“+”妊娠并发症“+” 产前诊断“+”先天”)
APPENDIX B. Guidance – Cochrane Collaboration’s risk of bias assessment tool
(table taken directly Higgins JPT et al., 2011)
Notes for filling out the table (adapted/made specific for this systematic review and meta-analysis from the Cochrane handbook 2008 and from Higgins JPT et al., 2011):
- -
Within the table, summary descriptions should be provided in order to give an independent reader enough information to see why the specific judgement has been made. For example, if no information on sequence generation can be found in the article or correspondence with the author, you could enter “Comment: no information provided”. If it states that patients were randomly allocated in the article, then you could copy out the phrase directly from the article, e.g. “Quote: “patients were randomly allocated”. In any case, if you have doubts regarding whether or not the study actually did certain things that are mentioned in the article, please include an extra comment describing concern/contradiction in the article.
- -
When providing your judgement as a review author, indicate “low risk” of bias, and “high risk” of bias. If insufficient information is provided, then the judgement should be “unclear” risk of bias.
- See table 8.5c on pages 198–202 in the 2008 Cochrane handbook for systematic reviews of intervention (pages 223–227 of the pdf) for specific guidance on how to make your judgement.
APPENDIX C. Guidance for the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies
(Adapted to PICO1)
Note: The below has been adapted for this specific meta-analysis from the guidance found on the Newcastle–Ottawa quality assessment group website (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Selection
- 1)
Representativeness of the exposed cohort (0 or 1 star)
- a)
Truly representative of the average HBV-infected pregnant women in the community ✵
- ●
Women identified to carry HBsAg at a general antenatal care clinic or general practitioner with or without subsequent referral to the specialist obstetric care centre or hepatologist or infectious disease specialist
- ●
Not part of a special group (e.g. all with recent treatment for hepatocellular carcinoma), then we might assume they reflect/are representative of HBV-infected pregnant women in that community.
- b)
Somewhat representative of the average HBV-infected pregnant women in the community ✵
- ●
e.g. women known to be chronically infected with HBV and have been followed by hepatologist or infectious disease specialist
- c)
Selected group of users
- ●
e.g. Women with severe liver disease (cirrhosis or hepatocellular carcinoma) only, part of a special group (HIV-infected women, intravenous drug users [IVDU]), women working in study centres/hospitals, etc.
- ●
Please provide a comment if you believe that the exposed group does not match well the general community.
- d)
No description of the derivation of the cohort
- 2)
Selection of the non-exposed cohort (0 or 1 star)
- a)
Drawn from the same community as the exposed cohort ✵
- ●
Women presenting at the hospital, pregnant and with HBV (not, most of our studies should fall here in this review)
- b)
Drawn from a different source
- ●
e.g. controls drawn from a historical sample
- ●
Please make a comment if you believe that the controls have been drawn from a different source.
- c)
No description of the derivation of the non-exposed cohort
- 3)
Ascertainment of exposure (exposure = treatment) (0 or 1 star)
- a)
Valid method was used to ascertain adherence to the antiviral therapy✵
- ●
Ideally with some mention of methods to ascertain maternal adherence to treatment (e.g. evaluation of pill count, immunoassay to detect serum/urine metabolite of antiviral agents, or decrease in viral load levels subsequent to the treatment)
- b)
Based on a secure record about adherence✵
- ●
Study staff have recorded good adherence to treatment based on self-report
- ●
Description on the treatment duration supports the confirmation of adherence by study staff
- c)
Data collection through registry
- ●
Care must be taken for a study based on registry data; having started antivirals during pregnancy does not necessarily guarantee that the women adhered to the treatment throughout the intended period.
- d)
No description
- 4)
Demonstration that outcome of interest was not present at start of study (0 or 1 star)
- a)
Yes ✵
- ●
This will always be yes in our case… for this study topic as the outcome of interest is HBV status in infants and infants are born during the course of the study.
- b)
No
Comparability
- 1)
Comparability of cohorts on the basis of the design or analysis (0 or 1 or 2 star(s))
- a)
Study controls/is comparable for both HBV DNA level (within 1 log IU/mL) and HBeAg serostatus (within 10 % points)✵
- ●
The same threshold for HBV DNA level AND same HbeAg serostatus should be used for inclusion of treated and controls and/or the reported mean/median HBV DNA level and HBeAg seroprevalence at baseline should be reported and should be similar.
- ●
If not reported threshold or not reported mean/median and/or not similar then no star. If only one is reported/similar and the other not, then no star.
- b)
Study controls for child immunoprophylaxis at birth (birth dose vaccination, HBIG at birth) ✵
- ●
All have or all don’t have or similar proportions across exposed and unexposed group with a similar timeliness. If not reported at all or very different proportions then no star.
Outcome
- 1)
Assessment of outcome (0 or 1 star)
- a)
Independent blind assessment ✵
- ●
Examiner of infant outcome (e.g. laboratory staff) was blinded to the maternal exposure status.
- b)
Medical records related to outcome were seen and verified by study personnel, or there was record linkage✵
- ●
In the case where testing is done as part of the study, and it is indicated that the same laboratory assays were used to test all infants, then it will be assumed that there was direct verification of test results by study personnel using these medical records.
- c)
No description
- ●
If there is no description of laboratory methods (specifically, specifying which assay was used or indicating that all testing was done by study personnel or records were sent to study personnel) then no star will be given.
- 2)
Was follow up long enough for outcomes to occur (0 or 1 star)
- a)
Yes (at 6–12 months) ✵
- ●
Because we have defined our inclusion criteria for the review as testing needing to be done between 6 and 12 months, all of our studies should fall here.
- b)
No
- ●
This should not be the case for any of our studies. Please provide a detailed comment if you think it is the case.
- 3)
Adequacy of follow up of cohorts (0 or 1 star)
- a)
Complete follow up – all subjects accounted for and lost to follow up reported clearly as 0 ✵
- b)
Subjects lost to follow up unlikely to introduce bias – small number lost – >80% (or description provided of those lost) ✵
- c)
Follow up rate <80% (select an adequate %) and no description of those lost
- d)
No statement about LFU
- ●
If not reporting any LFU, and also not mentioning clearly that “There were no cases of LFU” then we should assume that LFU was not well reported, and this should not be given a star.
APPENDIX D. List of variables present on the data extraction tool
- Publication details
- First author
- Year
- Journal
- Language
- Methods
- Country
- Study design
- Recruitment period
- Recruitment setting (regional details, number of sites)
- Inclusion criteria
- Exclusion criteria
- Intervention arm treatment – including birth dose vaccination and/or HBIG administration if relevant
- Intervention treatment schedule (including birth dose vaccination and/or HBIG administration if relevant) and timing (including hours since birth for birth dose/HBIG)
- Control arm treatment
- Control arm treatment schedule and timing
- Infant treatment 1. Birth dose vaccination (dose, manufacturer)
- Infant treatment 1. Birth dose vaccination (detail the number of hours since birth)
- Infant treatment 2. HBIG (dose, manufacturer)
- Infant treatment 2. HBIG (detail the number of hours since birth)
- Infant treatment 3. Any other treatment (e.g. antiviral therapy in infants)
- Follow-up schedule (mothers)
- Follow-up schedule (infants)
- Number (no.) of participants at enrolment
- No. of women assessed for eligibility
- No. of women who underwent randomization (or included if non-randomized)
- Women’s characteristics in the treatment arm
- Treatment arm: No. of women assigned to treatment (or included if non-randomized)
- Treatment arm: Mean treatment duration
- Treatment arm: Mean or median age
- Treatment arm: No. by ethnicity
- Treatment arm: No. positive for HBeAg
- Treatment arm: HBV DNA threshold used (IU/mL or copies/mL)
- Treatment arm: No. with HBV DNA >threshold
- Treatment arm: No. HDV-positive
- Treatment arm: No. HCV-positive
- Treatment arm: No. HIV-positive
- Treatment arm: No. loss to F/U or regimen change
- Women’s characteristics in control arm
- Control arm: No. of women assigned to control (or included if non-randomized)
- Control arm: Mean treatment duration
- Control arm: Mean or median age
- Control arm: No. by ethnicity
- Control arm: No. positive for HBeAg
- Control arm: HBV DNA threshold used (IU/mL or copies/mL)
- Control arm: No. with HBV DNA >threshold
- Control arm: No. HDV-positive
- Control arm: No. HCV-positive
- Control arm: No. HIV-positive
- Control arm: No. loss to F/U or regimen change
- Infant outcomes at birth in the treatment arm
- No. of infants in treatment arm at birth
- Treatment arm: No. of twins
- Treatment arm: No. of triplets
- Treatment arm: mean gestational age at birth (weeks)
- Treatment arm: mean birthweight (kg)
- Treatment arm: No. male
- Treatment arm: No. by each type of delivery (vaginal or caesarean section)
- Infant outcomes at birth in the control arm
- No. of infants in control arm at birth
- Control arm: No. of twins
- Control arm: No. of triplets
- Control arm: mean gestational age at birth (weeks)
- Control arm: mean birthweight (kg)
- Control arm: No. of male
- Control arm: No. by each type of delivery (vaginal or caesarean section)
- MTCT definition
- MTCT definition used
- HBsAg assay method used to define MTCT
- HBV DNA assay method used to define MTCT
- Exact timing of 6–12 months assessment to define MTCT
- MTCT (intention-to-treat) in the treatment arm
- Denominator for intention-to-treat analysis: mothers assigned to intervention + twin/triplet
- No. of infants completed MTCT evaluation at 6–12 months time-point
- No. of infants with HBsAg at 6–12 months (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- No. of infants with HBV DNA at 6–12 months (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Intention-to-treat MTCT risk (defined by HBsAg)
- Intention-to-treat MTCT risk (defined by HBV DNA)
- MTCT (per protocol) in the treatment arm
- Denominator for per-protocol analysis: mother–infant pairs completed the intervention treatment and completed MTCT evaluation at 6–12 months time-point
- No. of infants with HBsAg at 6–12 months in mother–infant pairs completed the intervention treatment and completed MTCT evaluation at 6–12 months time-point (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- No. of infants with HBV DNA at 6–12 months in mother–infant pairs completed the intervention treatment and completed MTCT evaluation at 6–12 months time-point (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Per-protocol MTCT risk (defined by HBsAg)
- Per-protocol MTCT risk (defined by HBV DNA)
- MTCT (intention-to-treat) in the control arm
- Denominator for intention-to-treat analysis: mothers assigned to control + twins/triplets
- No. of infants completed MTCT evaluation at 6–12 months time-point
- No. of infants with HBsAg at 6–12 months (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- No. of infants with HBV DNA at 6–12 months (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Intention-to-treat MTCT risk (defined by HBsAg)
- Intention-to-treat MTCT risk (defined by HBV DNA)
- MTCT (per protocol) in the control arm
- Denominator for per-protocol analysis: mother–infant pairs completed the control treatment and completed MTCT evaluation at 6–12 months time-point
- No. of infants with HBsAg at 6–12 months in mother–infant pairs completed the control treatment and completed MTCT evaluation at 6–12 months time-point (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- No. of infants with HBV DNA at 6–12 months in mother–infant pairs completed the control treatment and completed MTCT evaluation at 6–12 months time-point (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Per-protocol MTCT risk (defined by HBsAg)
- Per-protocol MTCT risk (defined by HBV DNA)
- No. of infant adverse events in the treatment arm (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Treatment arm: Fetal death
- Treatment arm: Neonatal death (within 28 days)
- Treatment arm: Prematurity (give definition used)
- Treatment arm: Congenital abnormalities #
- Treatment arm: Congenital abnormalities: describe
- Treatment arm: Apgar score at 1 minute is <10
- Treatment arm: Suboptimal bone density (give definition and the age at evaluation)
- Treatment arm: Any other event
- No. of infant adverse events in the control arm (list by maternal HBeAg, HBV DNA, HDV, HIV, where possible)
- Control arm: Fetal death
- Control arm: Neonatal death (within 28 days)
- Control arm: Prematurity (give definition used)
- Control arm: Congenital abnormalities #
- Control arm: Congenital abnormalities: describe
- Control arm: Apgar score at 1 minute is <10
- Control arm: Suboptimal bone density (give definition and the age at evaluation)
- Control arm: Any other event
- HBV flare
- Definition of HBV flare used
- No. of maternal adverse events in the treatment arm (list by maternal HBeAg, HBV DNA, HDV, HIV status where possible)
- Treatment arm: HBV flare after treatment discontinuation
- Treatment arm: Postpartum haemmorhage
- Treatment arm: Antiviral resistance
- Treatment arm: Any other event
- No. of maternal adverse events in the control arm (list by maternal HBeAg, HBV DNA, HDV, HIV status where possible)
- Control arm: HBV flare after treatment discontinuation
- Control arm: Postpartum haemorrhage
- Control arm: Antiviral resistance
- Control arm: Any other event
- Other
- Summary of study conclusions
- Funding by industry
Appendix E. Cochrane Collaboration’s Risk of Bias Assessment Tool for Randomized Controlled Trials
TDF 300 mg
A. English language studies
Table
Low risk Quotes: “Enrollment at each center was performed with the use of blocks and randomized for sample balance. Using a randomization table, we randomly assigned 200 mothers, in a 1:1 ratio”
B. Chinese language studies
Table
Low risk/Unclear Quotes: “60 cases of pregnant women with asymptomatic hepatitis B virus were selected and randomly divided into liver protection group and tenofovir group, with 30 cases in each group”
LAM 100–150 mg
A. English language studies
Table
High risk Comment: Mentions that women were randomly assigned but does not give any indication of method for randomization.
B. Chinese language studies
Table
Low risk/Unclear Quotes: “90 cases of pregnant women chronically infected with HBV were selected and randomly divided into lamivudine group, telbivudine group and control group, with 30 cases in each group”
LDT 600 mg
A. Chinese language studies
Table
Low risk/Unclear Quotes: “80 cases of pregnant women with chronic hepatitis B were randomly divided into experimental group and control group, 40 cases in each group”
APPENDIX F. Newcastle–Ottawa Risk of Bias Assessment Tool
TDF 300 mg
A. English language observational studies
B. Chinese language observational studies
LAM 100–150 mg
A. English language observational studies
B. Chinese language observational studies
APPENDIX G. Publication bias assessment (>=10 studies)
TDF 300 mg
MTCT indicated by HBsAg positivity at 6–12 months, non-RCTs
- Evidence of possible publication bias/small study effects, Egger test P value=0.002
LAM 100–150 mg
MTCT indicated by HBsAg positivity at 6–12 months, non-RCTs
- Evidence of possible publication bias/small study effects, Egger test P value=0.002
MTCT indicated by HBV DNA positivity at 6–12 months, non-RCTs
- No evidence of publication bias, Egger test P value=0.055
LdT 600 mg
MTCT indicated by HBsAg positivity at 6–12 months, RCTs
- No evidence of publication bias, Egger test P value=0.119
MTCT indicated by HBsAg positivity at 6–12 months, non-RCTs
- Evidence of possible publication bias/small study effects, Egger test P value <0.001
MTCT indicated by HBV DNA positivity at 6–12 months, non-RCTs
- Possible evidence of publication bias/small study effects, Egger test P value=0.038
Publication Details
Author Information and Affiliations
Authors
Anna Funk,1 Ying Lu,2 Kyoko Yoshida,3 Tianshuo Zhao,4 Pauline Boucheron,5 and Yusuke Shimakawa6.Affiliations
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization.
Publisher
World Health Organization, Geneva
NLM Citation
Funk A, Lu Y, Yoshida K, et al. Systematic review of the efficacy and safety of antiviral therapy during pregnancy. In: Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Geneva: World Health Organization; 2020 Jul. Web Annex A.