NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Balk EM, Adam GP, Cao W, et al. Management of Colonic Diverticulitis [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Oct. (Comparative Effectiveness Review, No. 233.)
Analytic Frameworks
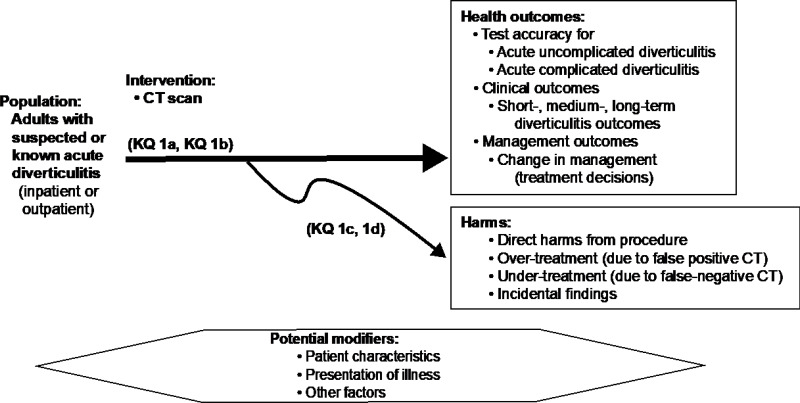
Figure A-1Analytic framework for Key Question 1: Computed tomography for acute diverticulitis
Abbreviations: CT = computed tomography, KQ = Key Question, MRI = magnetic resonance imaging.
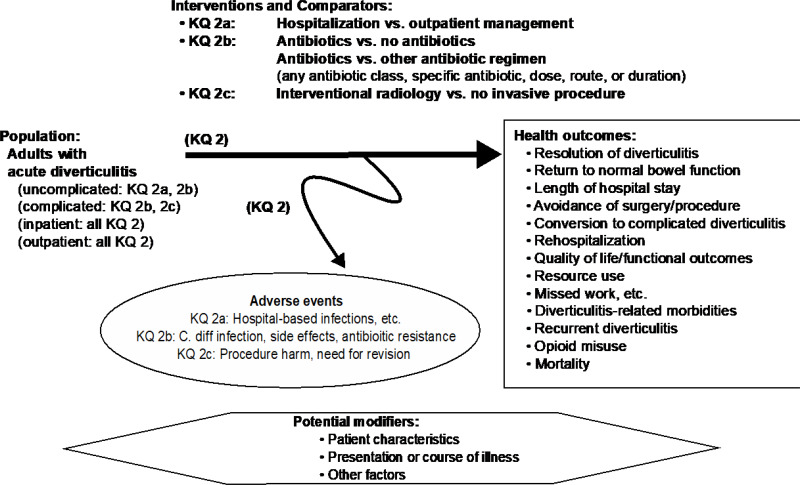
Figure A-2Analytic framework for Key Question 2: Treatment options for acute diverticulitis
Abbreviations: C. diff = Clostridiodes difficile, KQ = Key Question.
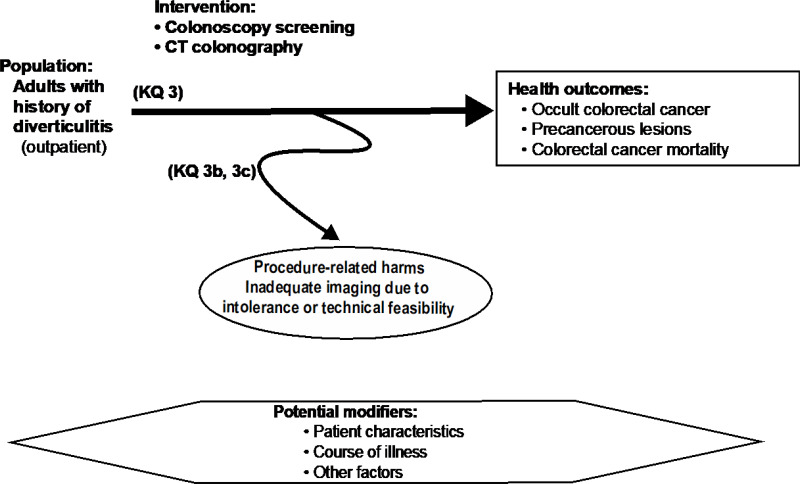
Figure A-3Analytic framework for Key Question 3: Screening for colorectal cancer
Abbreviations: CT = computed tomography, KQ = Key Question.
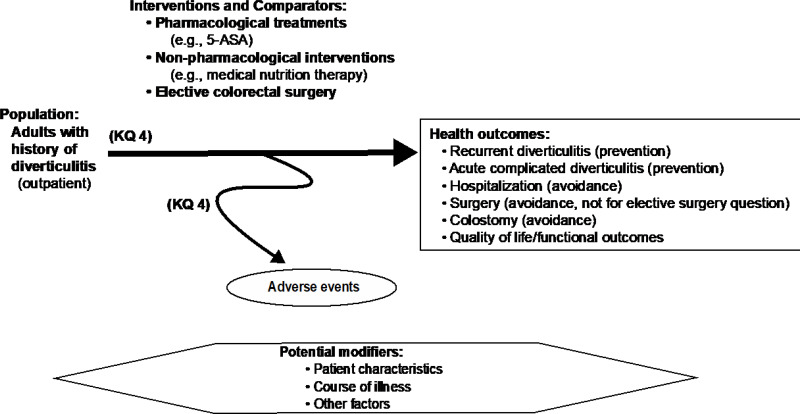
Figure A-4Analytic framework for Key Question 4: Treatments to prevent recurrent diverticulitis
Abbreviations: 5-ASA = 5-aminosalicylic acid (also known as mesalamine or mesalazine), KQ = Key Question.
Study Selection (Details)
We searched for studies and existing systematic reviews in MEDLINE (via PubMed), the Cochrane Register of Clinical Trials, the Cochrane Database of Systematic Reviews, Embase, and CINAHL. Separate, overlapping searches were conducted for each Key Question, then combined. As part of methods project, an independent search was undertaken, which used text-mining software to identify additional relevant keywords and MeSH search terms. This search was also independently peer reviewed. Duplicate citations were removed prior to screening. Searches were restricted to 1990 or later, with no language restriction. (The date restriction was included after discussion with the Key Informants based on important changes in diagnosis and clinical management of diverticulitis based on increased use of computed tomography [CT] imaging.) Search strategies included filters to remove nonhuman studies and articles that were not primary studies, systematic reviews, or clinical practice guidelines.
The searches included MeSH or Emtree terms, along with free-text words, related to diverticulitis, diverticulosis, and diverticular disease (since we have found that numerous articles misname or misclassify diverticulitis as diverticulosis); CT imaging; hospitalization, antibiotics, and interventional radiology for acute diverticulitis; colonoscopy and colonography; treatments to prevent recurrence and elective surgery. We also searched for CT imaging and acute abdomen (regardless of diverticular disease). Searches were independently peer reviewed.
Searches were also conducted in the ClinicalTrials.gov registry for unpublished study protocols, unpublished study results, and ongoing studies. The reference lists of relevant existing systematic reviews were screened for additional eligible studies. A Supplemental Evidence And Data for Systematic review (SEADS) portal was available for this review. Additional articles suggested to us from any source, including peer and public review, were screened applying identical eligibility criteria. Non-English language articles were screened and data extracted either by readers of the relevant languages or after translation via Google Translate (https://translate.google.com/).
Citations from all electronic databases were entered into Abstrackr software (http://abstrackr.cebm.brown.edu/) to enable abstract screening. We compared the search results with the results of our screening from the topic refinement phase (during protocol development). We then prepopulated the software with 753 citations with appropriate labels (accept or reject). The team conducted three rounds of pilot screening, during which each member of the team screen the same 100 abstracts, after which we discussed conflicts, with the goals of training the team in the nuances of the eligibility criteria and refining them as needed. Thereafter, we screened remaining abstracts in duplicate. The Abstrackr software has machine learning capabilities that predict the likelihood of relevance of each citation. Nightly, the list of unscreened abstracts were sorted so that most potentially-relevant articles are presented first the next day. After the software suggested that no remaining unscreened abstracts were likely to be relevant (when the predictor value was <0.40), we single screened an additional 2000 abstracts, none of which were accepted. We then single screened all remaining abstracts. In total 2816 citations were double screened and the remaining 11,233 were single screened (without any accepts). Of note, the number of citations that required double screening was relatively small compared to most projects. This was due to our ability to prepopulate the corpus with the 753 known accepts and rejects.
Potentially relevant citations were retrieved in full text. These articles were entered into an evidence map which captured study design, sample size, start year of study, and which Key Question the study is relevant to. Rejection reasons were captured at this stage. All decisions to include or reject an article were confirmed by at least one additional senior researcher.
Database Search Strategies
PubMed 1946 to June 1, 2020
Key question 1: CT diagnosis
(“Diverticulitis”[Mesh] OR “Diverticulosis, Colonic”[Mesh] OR diverticulitis [tiab] OR diverticulosis [tiab] OR diverticular [tiab] OR “Abdomen, Acute”[Mesh] OR “acute abdomen” OR ((acute or nonspecific OR non-specific OR emergen*) AND (abdome* OR abdomi*) AND pain) OR peritonitis)
AND
(“Tomography, X-Ray Computed”[Mesh] OR CT scan OR “cat scan” OR tomography OR “low dose CT” OR LDCT OR “Spiral CT”)
Key question 2: Treatment of acute diverticulitis
(“Diverticulitis”[Mesh] OR “Diverticulosis, Colonic”[Mesh] OR diverticulitis [tiab] OR diverticulosis [tiab] OR diverticular [tiab])
AND
(Hospital OR hospitals OR hospitalization OR “Hospitalization”[Mesh] OR Inpatient* OR discharge* OR outpatient OR “Ambulatory Care”[Mesh] OR antibiotic* OR “Anti-Bacterial Agents”[Mesh] OR medication* OR medical OR “Radiology, Interventional”[Mesh] OR interventional radiology)
Key question 3: Interval colonoscopy
(“Diverticulitis”[Mesh] OR “Diverticulosis, Colonic”[Mesh] OR diverticulitis [tiab] OR diverticulosis [tiab] OR diverticular [tiab])
AND
(Colonoscopy OR Colonography OR “Colonography, Computed Tomographic”[Mesh] OR “Colonoscopy”[Mesh] OR ((colon OR colorectal) AND (cancer or carcinoma or neoplasm*) AND screen*) OR ((colon OR colorectal) AND “Early Detection of Cancer”[Mesh]) OR (“Colonic Neoplasms”[Mesh] AND screen*))
Key question 4: Prevention of recurrence
(“Diverticulitis”[Mesh] OR “Diverticulosis, Colonic”[Mesh] OR “Diverticulosis, Small Intestinal” [Supplementary Concept] OR diverticulitis[tiab] OR diverticulosis[tiab] OR diverticular[tiab])
AND
(Recur* OR repet* OR repeat OR attacks OR “Elective Surgical Procedures”[Mesh] OR Mesalazine OR Mesalamine OR “Mesalamine”[Mesh] OR 5-ASA OR “5 ASA” OR Aminosalicylic Acid OR Pentacol OR “Diet Therapy”[Mesh] OR diet OR fiber OR fibre OR rifaximin OR “Probiotics”[Mesh] OR probiotic* OR balsalazide OR VSL#3 OR Lactobacillus casei OR ((surger* OR surgic* OR resect* OR operation OR operate) and elective))
Searches combined with OR
NOT
(“addresses”[pt] or “autobiography”[pt] or “bibliography”[pt] or “biography”[pt] or “case reports”[pt] or “comment”[pt] or “congresses”[pt] or “dictionary”[pt] or “directory”[pt] or “festschrift”[pt] or “government publications”[pt] or “historical article”[pt] or “interview”[pt] or “lectures”[pt] or “legal cases”[pt] or “legislation”[pt] or “news”[pt] or “newspaper article”[pt] or “patient education handout”[pt] or “periodical index”[pt] or “comment on” or (“Animals”[Mesh] NOT “Humans”[Mesh]) OR rats[tw] or cow[tw] or cows[tw] or chicken*[tw] or horse[tw] or horses[tw] or mice[tw] or mouse[tw] or bovine[tw] or sheep or ovine or murinae)
Embase 1947 to June 1, 2020
- #30.
(#6 OR #28) AND ([article]/lim OR [article in press]/lim)
- #29.
#6 OR #28
- #28.
#8 AND #27
- #27.
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
- #26.
‘elective surgery’/de
- #25.
‘lactobacillus casei’/de
- #24.
‘balsalazide’/de
- #23.
‘probiotic agent’/de
- #22.
‘rifaximin’/de
- #21.
‘fiber’/de
- #20.
‘diet therapy’/de
- #19.
‘aminosalicylic acid’/de
- #18.
‘mesalazine’/de
- #17.
‘colonography’/de
- #16.
‘colonoscopy’/de
- #15.
‘interventional radiology’/de
- #14.
‘drug therapy’/de
- #13.
‘antibiotic agent’/de
- #12.
‘ambulatory care’/de
- #11.
‘outpatient’/de
- #10.
‘hospital patient’/de
- #9.
‘hospitalization’/de
- #8.
#1 OR #2Diverticulitis
- #7.
#4 AND #5 AND ([article]/lim OR [article in press]/lim)
- #6.
#4 AND #5
- #5.
#1 OR #2 OR #3
- #4.
‘computer assisted tomography’/de
- #3.
‘acute abdomen’/de
- #2.
‘diverticulosis’/de
- #1.
‘diverticulitis’/de
Cochrane to June 1, 2020
((Diverticulitis OR diverticulosis OR diverticular OR “acute abdomen” OR ((acute or nonspecific OR non-specific OR emergen*) AND (abdome* OR abdomi*) AND pain) OR peritonitis)
AND
(“CT scan” OR “cat scan” OR tomography)) OR (Diverticulitis OR diverticulosis OR diverticular)
CINAHL 1961 to June 1, 2020
((Diverticulitis OR diverticulosis OR diverticular OR “acute abdomen” OR ((acute or nonspecific OR non-specific OR emergen*) AND (abdome* OR abdomi*) AND pain) OR peritonitis) AND (“CT scan” OR “cat scan” OR tomography))
OR
(Diverticulitis OR diverticulosis OR diverticular)
Inclusion/Exclusion Criteria Details
Study Eligibility Criteria for KQ 1 (CT Imaging)
- Population(s):
- KQ 1d: Adults with acute abdominal pain who receive an abdominal CT
- Intervention:
- CT (computed tomography) scan
- With or without IV (intravenous), oral, or rectal contrast
- Comparators:
- No CT scanning (as an explicit comparator)
- MRI (magnetic resonance imaging)
- Ultrasonography
- Other diagnostic interventions
- No comparator (single group studies)
- Outcomes:
- KQ 1a: Diagnostic accuracy (from existing systematic reviews only)
- Acute diverticulitis vs. other condition
- Complicated vs. uncomplicated diverticulitis
- For staging of severity
- KQ 1b: Clinical outcomes
- Short-term (≤1 month)
- ▪
Time to resolution of acute diverticulitis
- ▪
Length of hospital stay
- ▪
Conversion to complicated diverticulitis
- ▪
Diverticulitis-related morbidities (e.g., abscess formation) and mortality
- ▪
Change in management (treatment decisions)
- Medium- (>1 to <12 mo) to long-term (≥1 year)
- ▪
Recurrent diverticulitis
- ▪
Future episode of complicated diverticulitis
- ▪
Diverticulitis-related morbidities (e.g., strictures) and mortality
- KQ 1c: Harms
- Harms of over-treatment (due to false positive findings; e.g., surgery, stress)
- Harms of under-treatment (due to false negative findings; e.g., peritonitis, unnecessary surgery for other condition)
- KQ 1d: Incidental findings
- Sequelae related to incidental findings (e.g., unnecessary liver biopsy)
- Modifiers/Subgroups of interest
- Patient characteristics (e.g., prior history of diverticulitis, age)
- Presentation of illness (e.g., specific signs or symptoms, such as large volume ascites)
- Other factors (e.g., complicated or uncomplicated diverticulitis, hospital setting)
- Timing
- Any
- Setting
- Inpatient, emergency department (or equivalent), outpatient
Study Eligibility Criteria for KQ 2 (Treatment of Acute Diverticulitis)
- Population(s):
- Adults with acute complicated or uncomplicated diverticulitis, whether first or recurrent episode
- Exclude: Complicated diverticulosis, without diverticulitis (e.g., hemorrhagic diverticulosis)
- Exclude: Symptomatic uncomplicated diverticular disease (SUDD)
- Exclude: Meckel’s diverticula (unless concurrent acute diverticulitis)
- Exclude: Non-colonic diverticulitis
- Interventions versus Comparators:
- Hospitalization versus No hospitalization (for patients not requiring surgery)
- Antibiotics versus No antibiotics or versus Alternative antibiotic regimen (for any patient)
- Any class, route, treatment duration, or initiation time, and comparisons among these
- Use of any antibiotics (e.g., at clinician’s discretion) or specific antibiotics
- Interventional radiology procedure versus No procedure (conservative management; for patients with complicated diverticulitis for whom no procedure is an option)
- Any interventional radiology procedure appropriate for the severity and type of complication
- Exclude: Comparison of intervention radiology procedures or techniques
- Outcomes:
- Short-term (≤30 days)
- Resolution of diverticulitis
- Return to normal bowel function
- Length of hospital (or intensive care unit) stay
- Short- and medium-term (<1 year)
- Interventional radiology procedure for diverticulitis (avoidance) (exclude for comparisons of interventional radiology procedure with conservative management)
- Medium- to long term (>1 month)
- Recurrent diverticulitis
- Opioid misuse
- Any duration (short-, medium-, or long-term)
- Conversion to complicated diverticulitis
- Surgery for diverticulitis (avoidance)
- ▪
Including colostomy (avoidance)
- Rehospitalization for diverticulitis or complications
- Quality of life/Functional outcomes
- Resource use
- Missed work, employment, school outcomes, etc.
- Diverticulitis-related morbidities
- Mortality, both diverticulitis-related and all-cause
- All categorical “effectiveness” outcomes include time to outcome
- Harms, adverse events, side effects of interventions (any time frame)
- Hospitalization comparison:
- ▪
Hospital-based infections and other harms
- Antibiotics comparisons:
- ▪
Side effects/adverse events attributable to antibiotics
- ▪
Clostridioides difficile (C diff) infection
- ▪
Antibiotic resistance
- Interventional radiology comparisons:
- ▪
Adverse events related to procedures, including bleeding and catheter infections
- ▪
Need for second procedures or revisions
- Modifiers/Subgroups of interest:
- Patient characteristics (e.g., prior history of diverticulitis, age)
- Presentation or course of illness (e.g., specific symptoms)
- Other factors (e.g., complicated or uncomplicated diverticulitis, hospital setting)
- Timing:
- Minimum duration of follow-up = treatment duration (hospitalization, antibiotic use)
- Setting:
- Inpatient, emergency department (or equivalent), outpatient
- Design:
- Randomized controlled trials (all subquestions)
- N≥10/arm
- Nonrandomized comparative studies
- Antibiotics (all outcomes) or hospitalization and IR comparisons (short- to medium-term outcomes; <1 year)
- ▪
Restrict to studies that use modeling or other analytic methods to minimize selection bias (due to inherent differences between people who receive one or the other intervention), or that restrict study eligibility criteria such that comparisons being made are between patients with similar presentations.
- Long-term outcomes, hospitalization and IR comparisons (long-term outcomes; ≥1 year)
- ▪
Allow crude comparisons of long-term outcomes under the assumption that characteristics during acute diverticulitis that were associated with treatment decision (e.g., older patients being more likely to be hospitalized) would not have a major impact on long-term outcomes.
- Hospitalization and antibiotics: N≥30/arm; Interventional radiology N≥10/arm
- Single group studies
- Only for adverse events
- N>100
- Longitudinal (Exclude: cross-sectional)
- Prospective or retrospective
- Publication since 1990
- Exclude: Case reports (and series of case reports)
Study Eligibility Criteria for KQ 3 (Colonoscopy)
- Population(s)
- Adults with history of (resolved) acute diverticulitis
- Exclude: Active diverticulitis
- Exclude: History of related condition (only), e.g., complicated diverticulosis, SUDD
- Exclude: Meckel’s diverticula (unless concurrent acute diverticulitis)
- Exclude: Non-colonic diverticulitis
- Interventions:
- Elective colonoscopy (full colon)
- Elective CT colonography
- Comparators:
- No colon cancer screening
- Flexible sigmoidoscopy and barium enema
- Limited colonoscopy (e.g., left-sided)
- Virtual colonoscopy
- Stool guaiac testing (etc.)
- Other colon cancer screens (e.g., DNA tests)
- Different intervals, Different initial colonoscopy timing after acute episode
- No comparator
- Outcomes:
- Colorectal cancer death
- Colorectal cancer
- High-risk colonic premalignant lesions
- Adenoma, high grade dysplasia
- Adenoma ≥10 mm
- Adenoma, villous
- Serrated polyp
- Tolerance, feasibility, and completion of procedure; technical adequacy
- Harms, adverse events, and side effects of colonoscopy (e.g. perforation, bleeding)
- Modifiers/Subgroups of interest:
- Patient characteristics (e.g., age, family history)
- Course of illness (e.g., prior complicated vs. uncomplicated diverticulitis)
- Alarm symptoms
- Other factors (e.g., timing since last episode of acute diverticulitis)
- Timing:
- Start of colorectal cancer screening after resolution of acute disease
- Setting:
- Outpatient
- Design:
- Randomized controlled trials
- N≥10/arm
- Nonrandomized comparative studies
- No restriction based on analytic methods
- Including comparisons with healthy (non-diverticulitis) people
- N≥200 (total)
- Single group studies
- N≥200 (receiving colonoscopy or CT colonography)
- Case-control studies
- Including comparisons with healthy (non-diverticulitis) people
- N≥100/arm
- Prospective or retrospective
- Publication since 1990
- Exclude: Case reports (and series of case reports)
Study Eligibility Criteria for KQ 4 (Prevention of Recurrence)
- Population(s):
- Adults with history of (resolved) acute diverticulitis
- Exclude: Ongoing acute diverticulitis
- Exclude: History of related condition (only), e.g., complicated diverticulosis, SUDD
- Exclude: Meckel’s diverticula (unless concurrent acute diverticulitis)
- Exclude: Non-colonic diverticulitis
- Interventions:
- Pharmacological treatments
- Any class, route, regimen, treatment duration, or initiation time
- Non-pharmacological interventions
- Any class/type, route/method, regimen, treatment duration, or initiation time
- Elective surgery
- Laparoscopic, open, robot-assisted, or any other type of colon surgery conducted as an elective (non-emergent) procedure
- Exclude: Natural history or undefined/unspecified intervention or undefined/unspecified comparator
- Comparators:
- Pharmacological and non-pharmacological intervention comparisons:
- Alternative pharmacologic or non-pharmacologic intervention (or regimen)
- ▪
Pharmacologic vs. non-pharmacologic intervention
- ▪
Other class/type
- ▪
Other intervention within class/type
- ▪
Same intervention different treatment duration
- ▪
Same intervention, different initiation time
- No intervention
- ▪
Placebo
- ▪
“Usual care” (needs to be defined)
- Elective surgery comparisons:
- No or deferred elective surgery
- Exclude: Comparisons with other surgical approaches or techniques
- All:
- Exclude: Natural history or undefined/unspecified intervention or comparator
- Outcomes:
- Recurrent diverticulitis
- Acute complicated diverticulitis
- Surgery for diverticulitis (avoidance; except for elective surgery comparisons)
- Including colostomy (avoidance)
- Hospitalization for diverticulitis or diverticulitis-related complications (e.g., fistula, stricture)
- Quality of life/Functional outcomes
- All categorical “effectiveness” outcomes include time to outcome
- Harms, adverse events, or side effects of interventions (e.g., surgical complications)
- From single-group studies of elective surgery, only serious, major, or clinically important adverse events/complications
- Modifiers/Subgroups of interest:
- Patient characteristics (e.g., age)
- Course of illness (e.g., prior complicated vs. uncomplicated diverticulitis)
- Other factors (e.g., time since last episode of diverticulitis)
- Timing:
- No minimum duration of follow-up
- Hospitalization, unit stay, post-hospitalization
- Setting:
- Inpatient, emergency department (or equivalent), outpatient
- Design:
- Randomized controlled trials
- N≥10/arm
- Nonrandomized comparative studies
- Restrict to studies that use modeling or other analytic methods to minimize selection bias (due to inherent differences between people who receive one or the other intervention)
- N≥30/arm
- Single group studies
- Only for adverse events
- Elective surgery
- ▪
N≥500
- Other interventions
- ▪
N≥100
- Longitudinal (Exclude: cross-sectional)
- Prospective or retrospective
- Publication since 1990
- Exclude: Case reports (and series of case reports)
Data Extraction (Details)
For KQ 2 to 4, data were extracted directly into the Systematic Review Data Repository (SRDR) at https://srdr.ahrq.gov/. For KQ 1, data were extracted directly into summary tables, which will be uploaded into SRDR. We created a combined data extraction form for KQ 2 and 4 (on treatments) and, separately a form for KQ 3 (on colonoscopy). We extracted information on study characteristics, eligibility criteria, participant characteristics, intervention and comparator details, outcome definitions, and results (including event numbers, effect sizes, and P values). Study- and outcome-level risk of bias assessment was conducted during data extraction within SRDR.
Risk of Bias Assessment (Details)
We evaluated each study for risk of bias and methodological quality. Because we included a variety of study designs, we incorporated items from three different existing commonly-used tools and tailored the set of items for each study design. The three tools were the Cochrane Risk of Bias Tool,1 the Risk of Bias in Nonrandomized Studies (ROBINS-I) Tool,2 and the National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool.3
For RCTs, we used all the items from the Cochrane Risk of Bias Tool,1 focusing on issues related to randomization and allocation concealment methodology; blinding of patients, study personnel/care providers, objective outcome assessors, and subjective outcome assessors; incomplete outcome data; selective outcome reporting; and other issues that could be related to bias. We also used items from the NHLBI Tool focusing on the adequacy of descriptions of study eligibility criteria, interventions, and outcomes.3
For NRCSs, we used specific sections of the ROBINS-I Tool2 that pertain to confounding and selection bias. ROBINS-I requires the identification of specific confounders of interest for the SR. For the purpose of assessing for the presence of potential confounding in studies, we considered age, severity of headache (or history of headache), and frequency of headache (or history of headache). Because NRCSs, like RCTs, can be impacted by the lack of blinding and by participant loss to followup, we also used the items from the Cochrane Risk of Bias Tool1 that focus on issues related to blinding of patients, study personnel/care providers, objective outcome assessors, and subjective outcome assessors; incomplete outcome data; selective outcome reporting; and other issues that could be related to bias. We also used items from the NHLBI Tool that pertain to the adequacy of descriptions of study eligibility criteria, interventions, and outcomes.3
For single-group studies, we used the items from the Cochrane Risk of Bias Tool1 that pertain to issues of participant loss to followup, specifically, incomplete outcome data, selective outcome reporting, and other issues that could be related bias. We also used items from the NHLBI Tool focusing on the adequacy of descriptions of study eligibility criteria, interventions, and outcomes.3
Data Synthesis and Analysis (Details)
Overall Synthesis
We summarized the evidence both qualitatively and quantitatively. For each set of studies, we provide summary descriptions of their design, characteristics, and included participants. We focus, as pertinent, on demographics and descriptions of participants’ course of diverticulitis such as complications. We also summarize the risk of bias or methodological concerns for each set of studies. With rare exceptions, we do not narratively describe each study.
Within the main report we summarize findings either in high-level summary tables that focus on the intervention, sample size, outcome, and results. Further details are included in Appendixes C and D.
Metrics
As pertinent, we calculated event (or findings) rates (i.e., the percentage of participants with the outcome), the odds ratio (OR), or differences between groups. For continuous outcomes other than quality of life (QoL) or related functional outcomes, we estimated mean differences between groups or net mean differences (difference-in-differences) between groups based on reported data. When multivariable metrics (e.g., OR) were reported, we preferentially used those over the unadjusted (crude) metrics.
Notably, with few exceptions (that are called out), from nonrandomized comparative studies (NRCS) we summarized (included) only outcomes for which there were multivariable analyses (or equivalent, such as from matched studies). Since we excluded NRCSs that reported only unadjusted comparisons between inherently different groups, we similarly excluded unadjusted comparisons from articles that reported other multivariable adjusted analyses.
Meta-Analysis
Per protocol, we considered the possibility of conducting network meta-analysis but determined that the evidence base does not contain sufficient data to allow meaningful network meta-analyses for any KQ.
Except as noted below, we conducted meta-analyses when at least three studies (or study groups) were sufficiently similar and reported the same outcome.
For KQ 1 (CT imaging), we drew a summary receiver operating characteristics (ROC) curve for the studies included in the eligible existing systematic reviews. We used the metandi program in Stata 15.1, which conducts a bivariate normal model.
For KQ 2b (antibiotics) and KQ 4a (pharmacologic) we conducted restricted maximum likelihood (REML) model meta-analyses of the OR for outcomes. We used the metaan program in Stata 15.1.
For KQ 3 (colonoscopy), we conducted REML meta-analyses of ORs for comparisons between groups (either study groups or subgroups). In one instance, with very rare events across studies, we estimated the summary Peto OR, also in metaan. To combine estimates of proportions, we used the Freeman-Tukey double arcsine transformation to overcome the nonnormal distribution of proportion estimates (because values are truncated at 0). Proportions were converted to percentages. For this, we used the metaprop program in Stata 15.1.
For KQ 4c (elective surgery), we meta-analyzed all included adverse events, regardless of the clinical heterogeneity between studies (or groups). As an example, we meta-analyzed adverse events from studies that evaluated different types of elective surgery. In addition, we ran meta-analyses of only two studies. The proportions (adverse event rates) were again meta-analyzed with the Freeman-Tukey double arcsine transformation.
Interpretation of Estimates
In determining conclusions based on the estimates, both for individual studies and from meta-analyses, we interpreted estimates based on their precision. While we do not universally highlight statistical significance, we note when conclusions (e.g., evidence of an association) are based on estimates that are not statistically significant. We labeled OR estimates with 95 percent confidence intervals that extend beyond both 0.5 and 2.0 (or close to that) as imprecise. Regardless of the magnitude of the estimate, we do not suggest directionality or effect when the confidence is imprecise.
Grading the Strength of the Body of Evidence (Details)
We evaluated the strength of evidence (SoE) addressing each major conclusion for each KQ (and subquestion). We graded the SoE as per the Agency for Healthcare Research and Quality (AHRQ) Methods Guide.4, 5
For each SoE assessment, we considered the number of studies, the study limitations (i.e., risk of bias and overall methodological quality), the directness of the evidence to the KQs, the consistency of study results, the precision of any estimates of effect, the likelihood of reporting bias, other limitations, and the overall findings across studies. Based on these assessments, we assigned a SoE rating as being either high, moderate, low, or insufficient to estimate an effect. For conclusions that are based on ORs, we deemed the evidence to be imprecise if the nonsignificant lower confidence interval is <0.8 (for estimates >1) or upper confidence interval is >1.25 (for estimates <1).
Outcomes with highly imprecise estimates, highly inconsistent findings across studies, or with data from only one study were deemed to have insufficient evidence to allow a conclusion. In this instance, we defined highly imprecise as above, for individual studies, when the OR’s 95 percent confidence intervals extends beyond both 0.5 and 2.0. This overall approach is consistent with the concept that for imprecise evidence “any estimate of effect is very uncertain,” the definition of Very Low quality evidence per GRADE.6
Peer Review and Public Commentary
A preliminary draft version of this report was reviewed from March 17 to April 14, 2020 by invited reviewers, an AHRQ Associate Editor, and AHRQ personnel. A revised version was provided for a public review process from June 2 to 30, 2020. Revisions to the drafts were made to address reviewer comments. The findings and conclusions are those of the authors, who are responsible for the contents of the report.
Glossary of Terms and Abbreviations
Terms
- Acute colonic diverticulitis
An acute bout of inflammation of diverticula in the colon. Usually associated with lower abdominal pain, fever, and gastrointestinal symptoms.
- Clavien Dindo classification
Rating system of the severity of postoperative harms or complications. Briefly,
- No treatment required (e.g., small wound infection)
- Pharmacologic treatment required, including blood transfusion
- Procedure required (e.g., return to operating room)
- Life-threatening, involving one or more organs
- Death
- Complicated diverticulitis
Acute diverticulitis with complications. Complications are mostly caused by perforations to the diverticula. Complications include abscesses, peritonitis, fistulas, and strictures.
- Hinchey classification
A schema that has been modified several times to classify the severity of diverticulitis and complications. Briefly,
- 0.
mild clinical diverticulitis
- 1a.
confined inflammation without obvious abscess
- 1b.
small confined abscess
- II.
distant or large abscesses
- III.
generalized purulent peritonitis
- IV.
fecal peritonitis (free fecal material in the peritoneum
- Meta-analysis
Statistical method to quantitative combine study results
- Strength of evidence
Structured, qualitative method to assess the body of evidence pertaining to each specific conclusion. Rated as high, moderate, and low, or insufficient.
Abbreviations
- 5-ASA
5-aminosalicylic acid, mesalamine
- ACP
American College of Physicians
- AGA
American Gastroenterology Association
- AHRQ
Agency for Healthcare Research and Quality
- CD
Clavien Dindo classification
- CHF
congestive heart failure
- CI
confidence interval (about an estimate)
- COPD
chronic obstructive pulmonary disease
- CRC
colorectal cancer
- CRP
C-reactive protein
- CT
computed tomography imaging test
- ED-5D
EuroQoL 5 dimensions scale of quality of life and function
- EHC
Effective Health Care (program)
- GIQLI
Gastrointestinal Quality of Life Index
- H&S
Hansen & Stock classification system (to grade severity)
- I2
a measure of statistical heterogeneity; the percentage of the differences in study results across studies not attributable to random chance
- IPD MA
individual-patient data meta-analysis
- IV
intravenous
- KQ
Key Question
- NRCS
nonrandomized comparative study
- OR
odds ratio
- Peto OR
an approximation of the summary OR estimated when events are rare in one or both study groups
- RCT
randomized controlled trial
- ROBINS-I
Risk of Bias in Nonrandomized Studies of Interventions
- ROC
receiver operator characteristics (curve)
- SF-12/36
Short Form 12/36 question quality of life scale
- SoE
strength of evidence
- SR
systematic review
- TEP
Technical Expert Panel
- TIQ
Therapy Impact Questionnaire, a measure of physical function
- USPSTF
US Preventive Services Task Force
- VAS
visual analog scale (pain severity scale)
- WBC
white blood cell
References for Appendix A
- 1.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011 Oct 18;343:d5928. doi: 10.1136/bmj.d5928. PMID: 22008217. [PMC free article: PMC3196245] [PubMed: 22008217] [CrossRef]
- 2.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical research ed). 2016 Oct 12;355:i4919. doi: 10.1136/bmj.i4919. PMID: 27733354. [PMC free article: PMC5062054] [PubMed: 27733354] [CrossRef]
- 3.
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools.; 2019. https://www
.nhlbi.nih .gov/health-topics/study-quality-assessment-tools. Accessed on January 23, 2020. - 4.
- Berkman ND, Lohr KN, Ansari M, et al. AHRQ Methods for Effective Health Care Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed: 24404627]
- 5.
- Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. Journal of clinical epidemiology. 2015 Nov;68(11):1312–24. doi: 10.1016/j.jclinepi.2014.11.023. PMID: 25721570. [PubMed: 25721570] [CrossRef]
- 6.
- Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ (Clinical research ed). 2008 May 10;336(7652):1049–51. doi: 10.1136/bmj.39493.646875.AE. PMID: 18467413. [PMC free article: PMC2376019] [PubMed: 18467413] [CrossRef]