NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Abou-Setta AM, Beaupre LA, Jones CA, et al. Pain Management Interventions for Hip Fracture [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. (Comparative Effectiveness Reviews, No. 30.)
Search Results
All citations generated from electronic or hand searching and expert nominated studies were pooled into a single database (Figure 2).53 Of these 9,357 citations retrieved, 2,241 were duplicates and 7,116 were considered to be unique study reports. Following level I screening, 6,496 were excluded and 620 were further evaluated for inclusion. Of these, 83 primary publications26, 41-43, 54-132 passed level II screening and were included in this Comparative Effectiveness Review. An additional 15 companion publications133-146 were identified and also included. The characteristics of the publications excluded at level II screening are presented in Appendix D. The main exclusion criteria were publication type (e.g., case-report, observational study, review), population characteristics (e.g., average age below 50, fractures other than hip fractures), no details of pain management intervention, and no extractable data related to outcomes of importance to the review (e.g., ongoing studies).

Figure 2
Flow diagram for study retrieval and selection.
Description of Included Studies
Based on the interventions reported in each study, the primary publications were divided into eight groups: systemic analgesia (n = 3),41, 42, 55 anesthesia (n = 30),56-73, 75-85, 145 complementary and alternative medicine (CAM) (n = 2),43, 54 multimodal pain management (n = 2),86, 87 nerve blocks (n = 32),88-119 neurostimulation (n = 2),120, 121 rehabilitation (n = 1),122 and traction (n = 11).26, 123-132 The studies were published between 1990 and 2010 (median = 2003 [interquartile range (IQR): 1998 to 2007]). The majority of the studies were RCTs performed in single university settings in Europe, investigated pre- or intra-operative pain management interventions for hip fracture patients, and were published in peer-reviewed journals (Table 2).
Table 2
Characteristics of included studies.
Methodological Quality of Included Studies
The risk of bias (RoB) of each included randomized and nonrandomized trial was assessed using the RoB tool by two independent reviewers and the consensus ratings are presented in Appendices G and H. The methodological quality of each included cohort study was assessed using the Newcastle Ottawa Scale (NOS) by two independent reviewers and the consensus ratings are presented in Appendix I. A summary of the overall quality trends by study design is presented below.
Randomized and Nonrandomized Controlled Trials
Of the 69 randomized controlled trials (RCTs) and nonrandomized controlled trials (nRCTs), 30 trials26, 54, 56, 60, 64, 68, 77, 88, 90, 92-94, 98, 106, 110, 112, 114, 116, 120-131 were rated as having high risk of bias (RCTs = 24; nRCTs = 5), 37 RCTs41-43, 55, 57-59, 61-63, 65-67, 69-73, 75, 76, 89, 91, 97, 99-105, 107-109, 111, 113, 115, 145 were rated as having an unclear risk of bias, and 2 RCTs95, 96 were considered to have a low risk of bias.
Results of Included Studies
This section is organized by intervention category (i.e., systemic analgesia, anesthesia, etc.). Within each intervention category, the results are presented for the four key questions addressed in this report: KQ1: Acute and chronic pain management; KQ2: Other outcomes; KQ3: Adverse effects; and, KQ4: Effectiveness and safety in differing subpopulations. For each category, we provide a description of the characteristics and findings of the individual trials and cohort studies and a summary of key findings. Appendixes E and F present detailed evidence tables on each of the included studies.
Systemic Analgesia
Overview of Included Studies
Three RCTs41, 42, 55 evaluated the efficacy and/or harms of different types of systemic analgesia, in a total of 214 participants; sample sizes ranged from 30 to 94. See Table E-1 (Appendix E) for details of the study characteristics. Two RCTs41, 42 compared different parenteral analgesics (parecoxib IV vs. diclofenac ± meperidine IM, and intrathecal isotonic clonidine vs. intrathecal hypertonic clonidine, respectively). The third RCT55 compared different oral analgesics (lysine clonixinate vs. metamizole). See Table F-1 (Appendix F) for details of the interventions. The mean age of participants in the trials ranged from 77.3 to 78.5 years. Most were female (74.5 percent). Acute pain was measured using the 10cm Visual Analogue Scale (VAS) and the mean baseline pain measure was 6.5cm. All three trials had an unclear risk of bias (Appendix G). Summary of the evidence from these trials is provided in Table 3.
Table 3
Evidence addressing key questions: Systemic analgesia.
Key Question 1. Acute and chronic pain management
Acute pain (post-treatment means) was reported in all three RCTs41, 42, 55 (Table 4). One RCT41 compared parecoxib intravenous (IV) (n = 35) vs. diclofenac intramuscular (IM) ± meperidine IM (n = 55). There was a statistically significant effect difference in additional pain relief in favor of parecoxib IV (mean difference [MD] -0.70; 95% confidence interval [CI] -1.04, -0.36; p <0.0001). This was not considered clinically significant.
Table 4
Evidence summary table (randomized controlled trials): Systemic analgesia.
The second RCT42 compared intrathecal isotonic clonidine (n = 15) versus intrathecal hypertonic clonidine (n = 15). There was a statistically significant effect difference in additional acute pain relief (post-treatment means) in favor of isotonic clonidine (MD -1.69; 95% CI -2.01, -1.37; p <0.00001). This was not considered clinically significant.
The third RCT55 compared lysine clonixinate (n = 48) versus metamizole (n = 46), but no evidence of a significant effect difference (post-treatment means and at rest) was noted (MD -0.43; 95% CI -1.30, 0.44; p = 0.33).
The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Key Question 2. Other outcomes
Pain medication use. Additional pain medication use was reported in one RCT55 comparing lysine clonixinate (n = 48) versus metamizole (n = 46). There was no statistically significant difference in the number of participants requiring additional pain medication (odds ratio [OR] 3.00; 95% CI 0.30, 29.94; p = 0.35) (Table 4).
Mental status. The incidence of delirium was reported in one RCT55 comparing lysine clonixinate (n = 48) versus metamizole (n = 46). There was no statistically significant difference in the number of participants developing delirium (OR 0.96; 95% CI 0.06, 15.77; p = 0.98) (Table 4). The strength of the evidence was rated as insufficient to make any firm conclusions regarding this intervention.
Key Question 3. Adverse effects
Data on adverse effects associated with the administration of different types of systemic analgesia were available from two RCTs.42, 55 One RCT55 comparing lysine clonixinate (n = 48) versus metamizole (n = 46) reported the number of participants with any adverse event and found a statistically significant difference in the number of patients experiencing any adverse event, in favor of metamizole (OR 3.50; 95% CI 1.04, 11.81; p = 0.04) (Table 4). Similarly, fewer patients in the metamizole group reported any gastrointestinal disturbance (OR 11.84; 95% CI 1.45, 96.75; p = 0.02) (Table 4). The remaining reported adverse effects were from single studies and did not demonstrate any significant statistical differences between the pain management interventions.
Key Question 4. Efficacy, effectiveness, and safety in subpopulations
No data were reported on subpopulations.
Anesthesia
Overview of Included Studies
Twenty-one RCTs56-73, 75, 76, 145 and one nRCT77 evaluated the efficacy and/or harms of anesthesia including neuraxial (i.e., continuous or single administration spinal or epidural anesthesia) or neuraxial anesthesia versus general anesthesia in a total of 1,062 participants; study sample sizes ranged from 20 to 90. Additionally, eight cohort studies78-85 provided data on spinal anesthesia versus general anesthesia or other modes of administration of spinal anesthesia in 3,086 participants; study sample sizes ranged from 25 to 1,333. The mean age of participants ranged from 69.8 to 86.0 years. Most were female (range = 38.9 to 100 percent). Acute pain was measured using different scales (numeric rating score [NRS] [1-5] and 10cm VAS). The average baseline VAS pain score was 4.7. See Tables E-2 and F-2 (Appendices E and F) for details of the study characteristics and the interventions.
Four RCTs56, 60, 64, 68 and one nRCT77 had a high risk of bias, while the other 17 RCTs57-59, 61-63, 65-67, 69-76 had an unclear risk of bias (Appendix G). The cohort studies were of moderate quality (median = 8) (Appendix I). Summary of the evidence from these trials is provided in Table 5.
Table 5
Evidence addressing key questions: Anesthesia.
Based on the primary interventions and comparison groups, the studies were grouped as follows:
Key Question 1. Acute and chronic pain management
Spinal vs. General Anesthesia
One RCT60 comparing spinal anesthesia (n = 15) vs. general anesthesia (n = 15) reported a statistically significant difference of additional pain relief in favor of spinal anesthesia (MD = -0.86; 95% CI -1.30, -0.42; p = 0.0001) (Table 6-B). This was not considered clinically significant. The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 6-B
Spinal versus general anesthesia: (RCT/nRCT).
Table 6-AEpidural (continuous) versus spinal anesthesia (continuous): (RCT/nRCT)
| Outcome or Subgroup | Studies (N) | Participants (N) | Statistical Method | Effect Estimate | I2 | |
|---|---|---|---|---|---|---|
| KQ3 | Damage to surrounding structures65 | 1 | 40 | NE | NA |
KQ = key question; NA = not applicable; NE = not estimable; RCT/nRCT = randomized and nonrandomized controlled trials
Neuraxial Anesthesia: Addition of Clonidine, Fentanyl, Meperidine, Morphine, or Sufentanil
Acute pain (post-treatment means) was reported in three RCTs66, 69, 73 comparing additional fentanyl (n = 20) vs. standard spinal anesthesia (n = 20),69 additional morphine (n = 20) versus standard spinal anesthesia (n = 20),66 and additional sufentanil (n = 25) versus standard spinal anesthesia (n = 25).73 In the studies comparing the addition of fentanyl or sufentanil, no patients reported feeling pain following the procedure. In the study comparing the addition of morphine, there was no significant difference in pain relief versus standard spinal anesthesia (MD = -0.36; 95% CI -1.11, 0.39; p = 0.35) (Table 6-G).
Table 6-G
Spinal (single) anesthesia (addition of morphine): RCT/nRCT.
Acute pain on day 1 was reported in one RCT69 and one nRCT77 comparing additional fentanyl (n = 40) versus standard spinal anesthesia (n = 40). There was no significant difference in pain on day 1 following the addition of fentanyl (OR 1.24; 95% CI 0.34, 4.48; p = 0.75) (Table 6-E and Figure 3). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 6-E
Spinal (single) anesthesia (addition of fentanyl): RCT/nRCT.
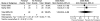
Figure 3
Neuraxial anesthesia: Addition of fentanyl—acute pain (day 1).
Key Question 2. Other outcomes
Spinal vs. General Anesthesia or Spinal vs. Epidural Anesthesia
Mortality (30-day). Thirty-day mortality was reported in two RCTs56, 64 (n = 99 participants). There was no significant difference in mortality rates following spinal anesthesia versus general anesthesia (10/53 vs. 5/46; OR 1.73; 95% CI 0.53, 5.68; p = 0.36) (Table 6-B).
Additionally, 30-day mortality was reported in five cohort studies78, 81, 82, 84, 85 (n = 2960 participants) (Table 7-A). There was no significant difference in mortality rates following spinal anesthesia vs. general anesthesia (78/1259 vs. 117/1701; OR 0.87; 95% CI 0.45, 1.67; p = 0.68. Subgroup analyses according to the mode of administration of spinal anesthesia revealed a statistically significant difference in the incidence of 30-day mortality for participants receiving continuous spinal anesthesia compared with general anesthesia (8/182 vs. 4/28; OR 0.28; 95% CI 0.08, 0.99; P = 0.05) favoring spinal anesthesia. There was no significant difference in mortality rates following single dose spinal versus general anesthesia (70/1077 vs. 113/1673; OR 1.08; 95% CI 0.58, 2.01; p = 0.80). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 7-A
Spinal versus general anesthesia: Cohort studies.
Table 7-CSpinal (single) anesthesia (lateral vs. supine position): Cohort studies
| Outcome or Subgroup | Studies (N) | Participants (N) | Statistical Method | Effect Estimate | I2 | |
|---|---|---|---|---|---|---|
| KQ3 | Bradycardia83 | 1 | 41 | OR (95% CI) | 0.55 (0.15, 1.98) | NA |
| Hypotension83 | 1 | 41 | OR (95% CI) | 0.22 (0.06, 0.86)* | NA |
CI = confidence intervals; KQ = key question; NA = not applicable; NE = not estimable; OR = odds ratio
- *
= statistically significant
Mental status. Delirium measured with the Mini Mental State Examination (MMSE) was reported in one RCT60 comparing spinal anesthesia (n = 15) vs. general anesthesia (n = 15) (Table 6-B). There was no significant difference between the two groups (8/15 vs. 9/15; OR 0.76; 95% CI 0.18, 3.24; p = 0.71). Additionally, delirium was reported in two cohort studies78, 84 There was no significant difference in the incidence of delirium comparing spinal versus general anesthesia (12/448 vs. 11/529; OR 0.79; 95% CI 0.04, 14.13; p = 0.87). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Health services utilization. Length of stay (LOS) for acute hospitalization was reported in two RCTs56, 64 comparing spinal anesthesia (n = 53) vs. general anesthesia (n = 46) (Table 6-B). The LOS was significantly less in the general anesthesia group (MD 1.69; 95% CI 0.38, 3.01; p = 0.01). The variance for one trial64 was imputed from the reported p-value, while the variance for the second trial56 was imputed from the first trial,64 as no measure of variance was reported. LOS for acute hospitalization was also reported in one cohort study85 comparing single spinal anesthesia (n = 383) to general anesthesia (n = 950) but the difference could not be estimated as no measure of variance was reported.
Neuraxial Anesthesia: Addition of Clonidine, Fentanyl, Meperidine, Morphine, or Sufentanil
Additional pain medication use. Additional pain medication use was reported in sixRCTs58, 65-67, 73, 76 (Table 6D to 6-H). Differences in effect estimates from one RCT65 (n = 40 participants) comparing the addition of clonidine vs. standard spinal anesthesia was not estimable because all participants required additional pain medication. The pooled estimate from three trials58, 67, 76 comparing the addition of fentanyl vs. standard spinal anesthesia (n = 102 participants) showed no statistically significant difference between groups (2/51 vs. 0/51; OR 5.51; 95% CI 0.25, 122.08; p = 0.28).
Table 6-D
Neuraxial anesthesia (addition of clonidine): RCT/nRCT.
Table 6-F
Spinal (continuous) anesthesia (addition of meperidine): RCT/nRCT.
Table 6-H
Spinal (single) anesthesia (addition of sufentanil): RCT/nRCT.
There was no significant difference in additional pain medication use in the RCT66 (n = 40) that compared the addition of morphine to spinal anesthesia vs. standard spinal anesthesia (9/20 vs. 15/20; OR 0.27; 95% CI 0.07, 1.04; p = 0.06). Similarly, there was no difference in reported additional pain medication use between three RCTs67, 73, 76 that compared the addition of sufentanil to spinal anesthesia with standard spinal anesthesia (1/66 vs. 0/ 66; Peto OR 7.39; 95% CI 0.15, 372.38; p = 0.32).
Mental status. Confusion was reported in one RCT66 (n = 40) comparing the addition of morphine versus standard spinal anesthesia (Table 6-G). There was no significant difference in the incidence of postoperative confusion (1/20 vs. 0/20; OR 3.15; 95% CI 0.12, 82.16; p = 0.49). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Neuraxial Anesthesia: Different Doses and Modes of Administration
Spinal Anesthesia (Continuous vs. Single Administration)
Mortality (30-day). Three RCTs62, 64, 71 (n = 163) reported 30-day mortality (Table 6-C). Two of the RCTs62, 71 did not record any events in either group. In the third RCT,64 there was no significant difference between continuous vs. single administration spinal anesthesia (2/14 vs. 4/15; OR 0.46; 95% CI 0.07, 3.02; p = 0.42). Additionally, it should be noted that 30-day mortality was reported in one other cohort study82 (n = 291) (Table 7-B). There was no significant difference between continuous vs. single administration of spinal anesthesia (8/182 vs. 5/109; OR 0.96; 95% CI 0.30, 3.00; p = 0.94). The strength of the evidence was rated as low to make any firm conclusions regarding these interventions.
Table 6-C
Spinal anesthesia (continuous vs. single administration): (RCT/nRCT).
Table 7-B
Spinal anesthesia (continuous vs. single administration): Cohort studies.
Additional pain medication. Additional pain medication use was reported in two RCTs62, 71 (n = 134) (Table 6-C). The OR in additional pain medication use was not estimable as there were no events in either group.
Health services utilization. LOS for acute hospitalization was reported in two RCTs62, 64 (n = 89). There was no significant difference between groups (MD = -0.98; 95% CI -2.06, 0.10; p = 0.07; Table 6-C). The variance for one trial64 was imputed from the reported p-value.
Mental status. Confusion was reported in two RCTs62, 71 (n = 134) (Table 6-C). There was no significant difference between groups in the occurrence of confusion (5/67 vs. 4/67; OR 1.27; 95% CI 0.32, 4.99; p = 0.73). The strength of the evidence was rated as low to make any firm conclusions regarding these interventions.
Spinal Anesthesia (Different Doses)
Delirium. One cohort study80 (n = 60) reported that there was no significant difference in the incidence of delirium between the two groups (2/30 vs. 4/30; OR 0.46; 95% CI 0.08, 2.75; p = 0.40) (Table 7-D). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 7-D
Spinal anesthesia (Different doses): Cohort studies.
Mortality (30-day). One cohort study82 (n = 182) reported that there was no significant difference in 30-day mortality rates between the two groups (4/121 vs. 4/61; OR 0.49; 95% CI 0.12, 2.02; p = 0.32) (Table 7-D). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Pain medication use. Additional pain medication use was reported in one RCT63 (n = 60) (Table 6-I). There was no significant difference between groups following spinal anesthesia at different doses (4 vs. 5mg, 4 vs. 6mg, or 5 vs. 6mg).
Table 6-I
Spinal anesthesia (Different doses): RCT/nRCT.
Key Question 3. Adverse effects
Spinal vs. General Anesthesia or Spinal vs. Epidural Anesthesia
Two RCTs60, 64 (n = 73) and one cohort study82 (n = 333) evaluated the nature and frequency of adverse effects associated with the administration of spinal anesthesia versus general anesthesia (Table 6-B, 7-A). There were no significant differences in the occurrence of hypotension in the RCTs60, 64 (21/44 vs. 21/29; OR 0.36; 95% CI 0.04, 2.92; p = 0.34). The pooled incidence of hypotension from the different arms of the cohort study82 is not reported because of marked heterogeneity among the included cohorts. There was no significant difference in the incidence of hypotension in the continuous spinal anesthesia groups compared with general anesthesia (OR 0.35; 95% CI 0.10, 1.28; p = 0.11). There was a significantly lower incidence of hypotension with single dose spinal anesthesia compared with general anesthesia (OR 0.04; 95% CI 0.01, 0.13; p < 0.00001). The remaining reported adverse effects were from single studies and did not demonstrate any significant statistical differences between the pain management interventions.
Neuraxial Anesthesia: Addition of Clonidine, Fentanyl, Meperidine, Morphine, or Sufentanil
A total of 11 RCTs57, 58, 65-70, 73, 74, 76 and one nRCT77 (n = 490) evaluated the harms of the administration of clonidine, fentanyl, meperidine, morphine, or sufentanil during neuraxaial anesthesia (Table 6-D to 6-H).
Addition of Clonidine
The reported adverse effects were from a single RCT65 and did not demonstrate any significant statistical differences (Table 6-D).
Addition of Fentanyl
Allergic reaction. There was no statistically significant difference in the number of participants reporting an allergic reaction in four trials67-69, 77 (14/81 vs. 5/83; OR 2.68; 95% CI 0.83, 9.80; p = 0.10) (Table 6-E).
Gastrointestinal (GI) symptoms. There were no reports of GI symptoms in three trials69, 74, 77 (n = 140) (Table 6-E).
Hypotension. Seven trials57, 58, 67-69, 76, 77 (n = 284) reported the frequency of hypotension (Figure 4). The pooled results are not reported due to high heterogeneity (I2 = 83 percent) between the included studies, which was not explained by study design (i.e., removal of the nRCT77), risk of bias (i.e., removal of the trials68, 77 with a high risk of bias), or specific intervention details (i.e., type and quantity). No firm conclusion can be made regarding the impact of fentanyl on this outcome.

Figure 4
Neuraxial anesthesia: Addition of fentanyl—hypotension.
Nausea/vomiting. In the five RCTs58, 67-69, 74 (n = 204) that reported the frequency of nausea or vomiting there was no statistically significant difference between the groups (6/111 vs. 3/93; OR 1.10; 95% CI 0.06, 20.73; p = 0.95) (Figure 5).
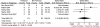
Figure 5
Neuraxial anesthesia: Addition of fentanyl—nausea/vomiting.
Respiratory distress. There were no reports of respiratory distress in three trials67, 68, 77 (n = 124).
Other adverse effects. The remaining reported adverse effects were from single trials and did not demonstrate any statistically significant differences.
Addition of Meperidine
Adverse effects. The reported adverse effects were from a single trial and did not demonstrate any significant statistical differences.
Addition of Morphine
Adverse effects. The reported adverse effects were from a single trial and did not demonstrate any significant statistical differences.
Addition of Sufentanil
Hypotension. Three RCTs67, 73, 76 (n = 132) reported a significantly lower incidence of hypotension in participants receiving sufentanil (8/66 in the group with sufentanil vs. 45/66 in the group with no sulfentantil; OR 0.05; 95% CI 0.01, 0.34; p = 0.002).
Other adverse effects. The remaining reported adverse effects were from single studies and did not demonstrate any significant statistical differences.
Neuraxial Anesthesia: Different Doses and Modes of Administration (i.e., Continuous vs. Single Administration)
Spinal Anesthesia (Continuous vs. Single Administration)
Hypotension. Hypotension was reported for two RCTs64, 71 (n = 103). There was a statistically significant difference between the groups (13/51 vs. 37/52; OR 0.12; 95% CI 0.03, 0.51; p = 0.004). Similarly, one cohort study82 (n = 291) reported a statistically significant difference between groups (26/182 vs. 74/109; OR 0.08; 95% CI 0.04, 0.14; p < 0.00001).
Other adverse effects. The remaining reported adverse effects were from single trials and studies and did not demonstrate any significant statistical differences between the pain management interventions.
Spinal Anesthesia (Different Doses)
Bradycardia. Bradycardia was reported in two RCTs61, 63 (n = 180). There was no significant difference for different doses of spinal anesthesia (bupivacaine: 4 vs. 5mg: 0/30 vs. 0/30; 4 vs. 6mg: 0/30 vs. 0/30; 5 vs. 6 mg: 3/29 vs. 3/31; levobupivacaine: 3/29 vs. 3/31).
Hypotension. Hypotension was reported in four RCTs61, 63, 72, 75 (n = 210). There were statistically significant differences in hypotension following spinal anesthesia with 4mg versus 6mg of bupivacaine (0/30 vs. 10/30; OR 0.03; 95% CI 0.00, 0.58; p = 0.02). The remaining comparisons were not statistically significant.
Three cohort studies79, 80, 82 reported hypotension in 267 participants. There was a statistically significant reduction in hypotension following spinal anesthesia with 2.5mg versus 5mg of bupivacaine (5/121 vs. 21/61; OR 0.08; 95% CI 0.03, 0.23; p <0.00001), 4mg versus 12mg of bupivacaine (3/30 vs. 23/30; OR 0.03; 95% CI 0.01, 0.15) and 0.125% vs. 0.5% of bupivacaine (4/12 vs. 10/13; OR 0.15; 95% CI 0.03, 0.87; p = 0.03).
Nausea/vomiting. There were no reports of nausea or vomiting in two RCTs63, 74 (n = 100).
Other adverse effects. The remaining reported adverse effects were from single RCTs and cohort studies and did not demonstrate any significant statistical differences between the pain management interventions.
Key Question 4. Efficacy, effectiveness, and safety in subpopulations
No data were reported on subpopulations.
Complementary and Alternative Medicine (CAM)
Overview of Included Studies
Two RCTs43, 54 evaluated the efficacy and/or harms of the administration of complementary and alternative medicine (CAM) interventions vs. no intervention or sham intervention (n = 98 participants); sample sizes ranged from 38 to 60. The mean age ranged from 76.8 to 86.3 years. Most were female (81.7 to 86.7 percent). One RCT43 compared acupressure (n = 18 participants) to sham control (n = 20) delivered preoperatively. Acute pain was measured using the VAS and the baseline pain measure was 6.5cm. The second RCT54 compared the Jacobson relaxation technique (n = 30 participants) with no intervention (n = 30). Acute pain was measured using the 10-point verbal “Sensation of Pain and Distress Scale.” Baseline pain measure was not reported for this trial. See Tables E-3 and F-3 (Appendices E and F) for details of the study characteristics and interventions.
One RCT43 had an unclear risk of bias, while the other54 had a high risk of bias (Appendix G). Summary of the evidence from these trials is provided in Table 8.
Table 8
Evidence addressing key questions: Complementary and alternative medicine.
Key Question 1. Acute and chronic pain management
Acute Pain (Post-Treatment Means)
Acupressure reduced pain compared with a sham intervention43 (MD -3.01; 95% CI -4.53, -1.49; p <0.0001; Table 9). It should be noted that the variance was imputed from the reported p value presented in this study. Relaxation also showed a reduction in pain compared with no relaxation (Sensation of Pain Scale (0-10): MD -1.10; 95% CI -1.43, -0.77; p <0.00001) (Table 9). This was not considered clinically significant. The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 9
Evidence summary table (randomized controlled trials): Complementary and alternative medicine.
Key Question 2. Other outcomes
In the RCT54 that compared relaxation versus no intervention, patients in the relaxation group required less additional pain medication (e.g., meperidine (mg) or morphine (mg)) compared with the control group (MD -8.43; 95% CI -15.11, -1.75; p = 0.01; Table 9).
Key Question 3. Adverse effects
No data were reported on adverse effects.
Key Question 4. Efficacy, effectiveness and safety in subpopulations
No data were reported on subpopulations.
Multimodal Pain Management
Overview of Included Studies
Two prospective cohort studies86, 87 evaluated the effectiveness and/or harms of the administration of multimodal pain management versus standard care in 226 participants; sample size ranged from 106 to 120. The mean age was not reported for either study. Most were female (80.8 percent). One study86 compared a formal postoperative protocol of IV and oral tramadol plus acetaminophen versus standard care. The second study87 compared a formal preoperative protocol of skin traction, morphine and acetaminophen versus standard care. See Tables E-4 and F-4 (Appendixes E and F) for details of the study characteristics and interventions.
Based on the NOS, the study quality for both studies was moderate (5 to 7 stars) (Appendix I). Summary of the evidence from these studies is provided in Table 10.
Table 10
Evidence addressing key questions: Multimodal pain management.
Key Question 1. Acute and chronic pain management
There were no data on pain management.
Key Question 2. Other outcomes
Mortality (30-day and one year). Mortality was reported in one prospective cohort study87 (n = 106) (Table 11). There was no significant difference between groups after 30 days (5/55 vs. 8/51; OR 0.54; 95% CI 0.16, 1.77; p = 0.31), or at 1 year (11/55 vs. 15/51; OR 0.60; 95% CI 0.25, 1.47; p = 0.26). The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Table 11
Evidence summary table (cohort studies): Multimodal pain management.
Mental status. Delirium was reported in two prospective cohort studies86, 87 (n = 226) (Table 11). There was no significant difference between groups in the number of patients with delirium (12/60 vs. 14/60; OR 0.82; 95% CI 0.34, 1.96; p = 0.66);86 (1/55 vs. 2/51; OR 0.45; 95% CI 0.04, 5.16; p = 0.52).87 The strength of the evidence was rated as insufficient to make any firm conclusions regarding these interventions.
Key Question 3. Adverse effects
Data on adverse effects were reported in one prospective cohort study87 and were not statistically significant (Table 11).
Key Question 4. Efficacy, effectiveness and safety in subpopulations
No data were reported on subpopulations.
Nerve Blocks
Overview of Included Studies
Twenty-nine RCTs88-116 (n = 1,757) evaluated the efficacy and/or harms of the administration of nerve blocks, including 3-in-1 (neurostimulation [NS]/ultrasound-guided [US]), combined lumbar/sacral plexus, fascia iliaca compartment, femoral, lumbar plexus ± sciatic nerve, posterior lumbar plexus, psoas compartment, obutarator and epidural nerve blocks. These were compared with standard care ± placebo, or a different method of nerve blocks. Sample sizes ranged from 14 to 207 participants. Additionally, three retrospective cohort studies117-119 (n = 696) evaluated 3-in-1, femoral, lumbar plexus plus sciatic nerve blocks versus systemic analgesia, or comparing different analgesic medications in femoral, lumbar plexus plus sciatic blocks. Sample sizes ranged from 62 to 535 participants. The mean age ranged from 59.2 to 85.9 years. Most were female (43.3 to 90.0 percent). Acute pain was measured using different scales (i.e., NRS (0-3, 1-5 and 1-10) and 10cm VAS). Eight studies using the 10cm VAS reported mean baseline pain scores ranging from 1.4cm to 7.3cm. See Tables E-5 and F-5 (Appendices E and F) for details of the study characteristics and interventions.
Two RCTs95, 96 had a low risk of bias, 16 RCTs89, 91, 97, 99-105, 107-109, 111, 113, 115 had an unclear risk of bias, while the remaining 1188, 90, 92-94, 98, 106, 110, 112, 114, 116 had a high risk of bias (Appendix G). Summary of the evidence from these trials is provided in Table 12.
Table 12
Evidence addressing key questions: Nerve blocks.
Based on the primary interventions and comparison groups, the studies were grouped as follows:
- Nerve blocks versus standard care ± placebo
- Nerve blocks versus neuraxial anesthesia
- Nerve blocks: ropivacaine versus bupivacaine
- Nerves blocks: addition of clonidine
- Nerve blocks: US versus NS
Key Question 1. Acute and chronic pain management
Nerve Blocks vs. No Block
Acute pain (post-treatment) was reported in 13 RCTs89, 91, 94, 99, 100, 106-112, 114 (Figure 6 and Table 13-A). The pooled results are not reported due to high heterogeneity (I2 = 92 percent) between the included studies, which was not explained by study design (i.e. all were RCTs) or risk of bias (i.e., removal of the trials with a high risk of bias). Specific intervention details (i.e., type and quantity) could partially explain the heterogeneity with removal of combined nerve blocks groups (e.g. 3-in-1 nerve block group) substantially decreasing the quantified heterogeneity (I2 = 41%). Additionally, another source of identified heterogeneity is the timing of the intervention with postoperative administration of nerve blocks in three RCTs91, 111, 114 showing marked heterogenous results (I2 = 95%), while preoperative administration showed more homogenous results (I2 = 53%) in eight RCTs.89, 94, 99, 100, 106, 108-110 Removal of one of the included RCTs111 decreased the heterogeneity for both the overall results (I2 = 64%) and the subgroup analysis (I2 = 0%) of only postoperative administration of nerve blocks.

Figure 6
Nerve blocks versus no block—acute pain (post-treatment).
Table 13-A
Nerve blocks versus no block: RCT/nRCT.
Day 1 pain. One trial101 (n = 50) reported a statistically significant difference in the frequency of patients who reported postoperative pain on day 1 favoring nerve blocks (7/25 vs. 20/25; OR 0.10; 95% CI 0.03, 0.36; p = 0.0005) (Table 13-A).
Pain on movement. Pain on movement (post-treatment means) was reported in four trials94, 97, 106, 114 (n = 258) (Table 13-A). The pooled results were not reported due to significant heterogeneity (I2 = 95 percent) between the studies (Figure 7). Meta-analysis restricted to two RCTs94, 114 using 3-in-1 nerve block vs. no block showed a significant reduction in pain on movement favoring nerve blocks (SMD -1.02; 95% CI -1.83, -0.21; p = 0.01). One RCT94 investigated preoperative pain relief (numeric rating scale [0-3]) while the other RCT114 investigated postoperative pain (10cm VAS) relief. Both trials had a high risk of bias.
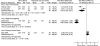
Figure 7
Nerve blocks versus no block—pain on movement (post-treatment).
The third RCT106 examined preoperative epidural analgesia versus no block and showed a significant increase in pain relief (10cm VAS) on movement favoring nerve blocks (MD-2.30; 95% CI -2.92, -1.68; p <0.00001). The trial had a high risk of bias.
The last RCT97 examined preoperative femoral nerve block versus no block and showed no significant difference in pain relief (5-point Verbal Rating Scale) on movement (MD 0.36; 95% CI -0.04, 0.75; p = 0.08).
Pain on rest. Pain on rest (posttreatment) was reported in three trials97, 106, 114 (n = 208) (Table 13-A). The pooled results were not reported due to significant heterogeneity (I2 = 91 percent) between the studies (Figure 8). One RCT114 examined postoperative 3-in-1 nerve block versus standard care and found no significant difference in pain relief (10cm VAS) (MD -0.07; 95% CI -0.41, 0.27; p = 0.69). This study had a high risk of bias. The second RCT106 examined preoperative epidural analgesia versus standard care and found a statistically difference in pain relief in favor of the nerve blocks (10cm VAS) (MD -0.55; 95% CI -0.81, -0.29; p < 0.0001). This study had a high risk of bias.The last RCT97 examined preoperative femoral nerve block versus standard care and reported a statistically significant difference in pain relief in favor of standard care (5-point Verbal Rating Scale) (MD 0.18; 95% CI 0.03, 0.33; p = 0.02). This study had an unclear risk of bias.

Figure 8
Nerve blocks versus no block – pain on rest (posttreatment).
The strength of the evidence was rated as moderate regarding these interventions.
Nerve Blocks vs. Neuraxial Anesthesia
Acute pain (posttreatment) was reported in three RCTs92, 93, 115 (n = 109) (Table 13-B). There was no statistically significant difference in pain between the two groups (MD -0.35; 95% CI -1.10, 0.39; p = 0.35).
Table 13-B
Nerve blocks versus neuraxial anesthesia: RCT/nRCT.
Key Question 2. Other outcomes
Nerve Blocks vs. No Block
30-day mortality. A total of four RCTs95, 99, 105, 106 evaluated 30-day mortality in a total of 228 participants (Table 13-A). Meta-analysis did not provide evidence of a significant difference in 30-day mortality (2/114 vs. 10/114; OR 0.28; 95% CI 0.07, 1.12; p = 0.07). The strength of the evidence was rated as low to make any firm conclusions regarding these interventions.
1-year mortality. Two RCTs91, 94 evaluated 1-year mortality in a total of 112 participants (Table 13-A). Additionally, one retrospective cohort study119 reported data for 535 participants (Table 14). There was no evidence of a significant difference in mortality in the RCTs (5/45 vs. 9/67; OR 0.82; 95% CI 0.25, 2.72; p = 0.74), or in the cohort study (41/178 vs. 104/357; OR 0.73; 95% CI 0.48, 1.10; p = 0.14).
Table 14
Evidence summary table (cohort studies): Nerve blocks.
Additional pain medication use. Seven RCTs89, 90, 94, 96, 97, 101, 114 evaluated additional pain medication use in a total of 378 participants (Table 13-A). Additionally, one retrospective cohort study117 compared femoral nerve block vs. no block, reporting data for 99 participants (Table 14). Meta-analysis of the seven trials89, 90, 94, 96, 97, 101, 114 resulted in a significant difference in additional pain medication use, favoring nerve blocks (49/197 vs. 68/181; OR 0.32; 95% CI 0.14, 0.72; p = 0.006) (Figure 9). The retrospective cohort study117 reported a statistically significant effect difference favoring nerve blocks (0/49 vs. 14/50; OR 0.03; 95% CI 0.00, 0.44; p = 0.01).
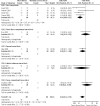
Figure 9
Nerve blocks versus no block – participants requiring additional pain medication.
Mental status. Four RCTs95, 98, 107, 108 (n = 461) and two cohort studies117, 119 (n = 634) reported the occurrence of delirium (Table 13-A, 14-A). Meta-analysis of the trials95, 98, 107, 108 showed a significant difference favoring nerve blocks (11/242 vs. 33/219; OR 0.33; 95% CI 0.16, 0.66; p = 0.002). The pooled results of the cohort studies117, 119 also showed a significant difference in favor of nerve blocks (11/227 vs. 55/407; OR 0.24; 95% CI 0.08, 0.72; p = 0.01). The strength of the evidence was rated as moderate.
Length of stay for acute hospitalization. LOS for acute hospitalization (days) was reported in two retrospective cohort studies117, 119 (n = 634) (Table 14-A). There was significant heterogeneity between the studies and pooled results are not reported. The first study117 was performed using a 3-in-1 nerve block while the second study119 used a femoral nerve block. Both studies showed lower LOS for the nerve blocks with the magnitude larger for the 3-in-1 block.
Quality of sleep. Quality of sleep (10cm VAS) (post-treatment means) was reported in one RCT110 (n = 77) (Table 13-A). There was no significant difference between groups (MD 0.30; 95% CI -0.46, 1.06; p = 0.44).
Nerve Blocks vs. Neuraxial Anesthesia
Additional pain medication use. Additional pain medication use was reported in one RCT115 (n = 30) (Table 13-B). There was no significant difference between the two groups (5/15 vs. 3/15; OR 2.00; 95% CI 0.38, 10.51; p = 0.41).
Mental status. Delirium (MMSE) was reported in one RCT92 (n = 29) (Table 13-B). There was no significant difference between the two groups (6/15 vs. 5/14; OR 1.20; 95% CI 0.27, 5.40; p = 0.81). The strength of the evidence was rated as insufficient to make any firm conclusions.
Nerve Blocks: Ropivacaine vs. Bupivacaine
Additional pain medication use. Additional pain medication use was reported in one cohort study118 (n = 62) (Table 14-B). There was no significant difference between the two groups (10/32 vs. 8/30; OR 1.25; 95% CI 0.42, 3.76; p = 0.69).
Mental status. Delirium (user defined) was reported in one cohort study118 (n = 62) (Table 14-B). There was no significant difference between the two groups (2/32 vs. 1/30; OR 1.93; 95% CI 0.17, 22.50; p = 0.60). The strength of the evidence was rated as insufficient to make any firm conclusions.
Key Question 3. Adverse effects
Nerve Blocks vs. No Block
Any adverse event. Any adverse effects were reported in five RCTs88, 97, 98, 100, 107 (n =392) and there was significant heterogeneity (I2 =94%) (Table 13-A). Two retrospective cohort studies117, 119 (n = 634) found no significant effect difference between the two groups (62/227 vs. 76/407; OR 1.64; 95% CI 0.79, 3.42; p = 0.18) (Table 14-A).
Cardiac complications. Cardiac complications were reported in two RCTs95, 106 (n = 128). There was no significant difference between the two groups (3/64 vs. 8/64; OR 0.35; 95% CI 0.08, 1.44; p = 0.15) (Table 13-A). One retrospective cohort study117 (n = 99) found no significant difference between the two groups (0/49 vs. 1/50; OR 0.33; 95% CI 0.01, 8.38; p = 0.50) (Table 14-A).
Damage to surrounding structures. Damage to surrounding structures was reported in three RCTs88, 97, 116 (n = 224) and found no significant difference between the two groups (3/119 vs. 0/105; OR = 7.44; 95% CI 0.37, 147.92; p = 0.19) (Table 13-A).
Deep venous thrombosis. Deep venous thrombosis was reported in two RCTs94, 99 (n = 100). There was no significant difference between the two groups (4/49 vs. 3/51; OR 1.40; 95% CI 0.29, 6.72; p = 0.67) (Table 13-A).
Infection. There were no reports of infection in two RCTs88, 97 (n = 184) (Table 13-A).
Myocardial infarction. Myocardial infarction was reported in two RCTs106, 110 (n = 145). There was no significant difference between the two groups (1/72 vs. 1/73; OR 1.00; 95% CI 0.06, 16.67; p = 1.00) (Table 13-A). One retrospective cohort study119 (n = 535) found no significant difference between the two groups (1/178 vs. 3/357; Peto OR 0.69; 95% CI 0.09, 5.53; p = 0.72) (Table 14-A).
Nausea/vomiting. Nausea/vomiting was reported in six RCTs91, 96, 97, 107, 113, 114 (n = 421) and found no evidence of a significant difference between the two groups (18/217 vs. 31/204; OR 0.65; 95% CI 0.27, 1.55; p = 0.33) (Table 13-A and Figure 10).

Figure 10
Nerve blocks versus no block—nausea/vomiting.
Pulmonary embolism. Pulmonary embolism was reported in two RCTs95, 106 (n = 128) and found no significant difference between the two groups (2/64 vs. 1/64; OR 1.63; 95% CI 0.19, 13.61; p = 0.65) (Table 13-A).
Respiratory infection. Respiratory infection was reported in five RCTs94, 95, 99, 106, 116 (n = 268) and found no significant difference between the two groups (9/133 vs. 22/135; OR 0.43; 95% CI 0.18, 1.04; p = 0.06) (Table 13-A and Figure 11). One retrospective cohort study119 (n = 535) found a statistically significant difference favoring nerve blocks (9/178 vs. 39/357; OR 0.43; 95% CI 0.21, 0.92; p = 0.03) (Table 14-A).

Figure 11
Nerve blocks versus no block—respiratory infection.
Stroke. Stroke was reported in one RCT99 (n = 50) and found no significant effect between the two groups (1/25 vs. 0/25; OR 3.12; 95% CI 0.12, 80.39; p = 0.49) (Table 13-A). Stroke was also reported in one retrospective cohort study119 (n = 535) and found no significant difference between the two groups (1/178 vs. 8/357; OR 0.25; 95% CI 0.03, 1.99; p = 0.19) (Table 14-A).
Surgical wound infection. Surgical wound infection was reported in two RCTs95, 99 (n = 110) and found no significant difference between the two groups (3/55 vs. 4/55; OR 0.77; 95% CI 0.11, 5.63; p = 0.80) (Table 13-A).
Urinary retention. Urinary retention was reported in two RCTs91, 113 (n = 62) and found no significant difference between the two groups (3/31 vs. 1/31; OR 2.23; 95% CI 0.27, 18.71; p = 0.46) (Table 13-A). One retrospective cohort study119 (n = 535) and found no significant difference between the two groups (4/178 vs. 17/357; OR 0.46; 95% CI 0.15, 1.39; p = 0.17) (Table 14-A).
Urinary tract infection. Urinary tract infection was reported in one RCT99 (n = 50) and found no significant difference between the two groups (4/25 vs. 6/25; OR 0.60; 95% CI 0.15, 2.47; p = 0.48) (Table 13-A). One retrospective cohort study119 (n = 535) found a statistically significant difference favoring nerve blocks (12/178 vs. 63/357; OR 0.34; 95% CI 0.18, 0.64; p = 0.001) (Table 14-A).
Other adverse effects. The remaining reported adverse effects were from single RCTs and cohort studies and did not demonstrate any significant statistical differences between the pain management interventions (Tables 13-A and 14-A).
Nerve Blocks vs. Neuraxial Anesthesia
Adverse effects. The reported adverse effects were from single RCTs and did not demonstrate any significant statistical differences between the pain management interventions (Table 13-B).
Nerve Blocks: Ropivacaine vs. Bupivacaine
Adverse effects. The reported adverse effects were from single RCTs and cohort studies and did not demonstrate any significant statistical differences between the pain management interventions (Tables 13-C, 14-B).
Table 13-C
Nerve blocks (Ropivacaine versus bupivacaine): RCT/nRCT.
Nerve Blocks: Addition of Clonidine
Adverse effects. The reported adverse effects were from single RCTs and did not demonstrate any significant statistical differences between the pain management interventions (Table 13-D).
Table 13-D
Nerve block (addition of clonidine): RCT/nRCT.
Nerve Blocks: US vs. NS
Damage to surrounding structures. Damage to surrounding structures was reported in two RCTs103, 104 (n = 100) (Table 13-E). There was no statistically significant difference between the two groups (0/40 vs. 7/60; OR 0.16; 95% CI 0.02, 1.30; p = 0.09).
Table 13-E
Nerve blocks (US vs. NS): RCT/nRCT.
Other adverse effects. The remaining reported adverse effects were from a single RCT103 and did not demonstrate any significant statistical differences between the pain management interventions.
Key Question 4. Efficacy, effectiveness and safety in subpopulations
One RCT106 only recruited patients with pre-existing heart disease. There was a significant reduction in acute pain (MD -0.98; 95% CI -1.49, -0.48; p <0.0001) favoring nerve blocks. There was no significant difference in 30-day mortality (0/34 vs. 4/34; OR 0.10; 95 % CI 0.01, 1.90; p = 0.12) or adverse effects: participants with any cardiac complications (2/34 vs. 7/34; OR 0.24; 95% CI 0.05, 1.26; p = 0.09); congestive heart failure (1/34 vs. 2/34; OR 0.48; 95% CI 0.04, 5.61; p = 0.56); myocardial infarction (1/34 vs. 1/34; OR 1.00; 95 % CI 0.06, 16.67; p = 1.00); respiratory infection (2/34 vs. 2/34; OR 1.00; 95% CI 0.13, 7.54; p = 1.00); or pulmonary embolism (1/34 vs. 1/34; OR 1.00; 95% CI 0.06, 16.67; p = 1.00).
One RCT95 only recruited participants that were independent prior to their hip fracture. There was no significant difference between nerve blocks versus standard care for 30-day mortality (1/30 vs. 1/30; OR 1.00; 95 % CI 0.06, 16.76; p = 1.00).
Neurostimulation
Overview of Included Studies
Two RCTs120, 121 evaluated the efficacy and/or harms of the administration of transcutaneous electrical neurostimulation (TENS) versus sham control in 123 participants; sample sizes ranged from 60 to 63. One trial administered the TENS preoperatively,121 and the other post-operatively.120 The mean age ranged from 71.2 to 80.5 years. Most were female (66.7 to 92.1 percent). Acute pain was measured using the VAS and the average baseline pain measure 8.8 to 8.9. See Tables E-6 and F-6 (Appendices E and F) for details of the study characteristics and interventions.
Both RCTs had a high risk of bias (Appendix G). Summary of the evidence from these trials is provided in Table 15.
Table 15
Evidence addressing key questions: Neurostimulation.
Key Question 1. Acute and chronic pain management
Acute pain (post-treatment) was reported in both RCTs120, 121 (n = 123) (Table 16). It should be noted that the variance was imputed from the reported p value presented in one of the trials.120 The pooled results showed a significant difference in additional pain relief in favor of TENS (MD -2.79; 95% CI -4.95, -0.64; p = 0.01) (Figure 12). This was not considered clinically significant.
Table 16
Evidence summary table (randomized controlled trials): Neurostimulation.
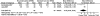
Figure 12
Neurostimulation acute pain (post-treatment).
Pain on movement. Pain on movement (post-treatment means) was reported in one trial120 (n = 60) (Table 16). Neurostimulation provided significantly more pain relief versus sham control (MD -3.90; 95% CI -6.22, -1.58; p = 0.001). The variance was imputed from the reported p value presented in the trial.120
Key Question 2. Other outcomes
One RCT120 comparing TENS (n = 30) versus sham control (n = 30) provided data on health-related quality of lilfe (HRQOL) (10cm VAS) and quality of sleep (10cm VAS) (Table 16). Neurostimulation provided significant improvement in HRQOL versus sham control (MD -4.30; 95% CI -6.86, -1.74; p = 0.001). Similarly neurostimulation provided significant improvement in quality of sleep (MD -3.60; 95% CI -575, -1.45; p = 0.001). The variance was imputed from the reported p value in the trial for both outcomes.120
Key Question 3. Adverse effects
No data were reported on adverse effects.
Key Question 4. Efficiacy, effectiveness, and safety in subpopulations
No data were reported on subpopulations.
Rehabilitation
Overview of Included Studies
One RCT122 evaluated the efficacy and/or harms of the administration of physical therapy (stretching and strengthening of spinal and psoas muscles (n = 18) vs. standard care (n = 19)). The mean age was 67.1 years and all participants were female. Acute pain was measured using the 10cm VAS and the mean baseline pain measure was 7.9cm. See Tables E-7 and F-7 (Appendices E and F) for details of the study characteristics and interventions.
The trial had a high risk of bias (Appendix G). Summary of the evidence from this trial is provided in Table 17.
Table 17
Evidence addressing key questions: Rehabilitation.
Key Question 1. Acute and chronic pain management
Acute Pain (Post-Treatment Means)
There was a statistically significant difference in additional pain relief following stretching-strengthening of spinal and psoas muscles vs. standard care (MD -1.39; 95% CI -2.27, -0.51; p = 0.002) (Table 18). This was not considered clinically significant.
Table 18
Evidence summary table (randomized controlled trials): Rehabilitation.
Key Question 2. Other outcomes
No other outcomes were reported.
Key Question 3. Adverse effects
No data were reported for adverse effects.
Key Question 4. Efficacy, effectiveness and safety in subpopulations
All participants in this trial were female.
Traction
Overview of Included Studies
Six RCTs26, 123-127 and four nRCTs128-131 (n = 1,310) evaluated the efficacy and/or harms of the administration of traction vs. no intervention or other interventions; sample sizes ranged from 64 to 311 participants. Additionally, one prospective cohort study132 (n = 134) provided data. The mean age ranged from 74.0 to 81.0 years. Most were female (66.2 to 84.7 percent). Acute pain was measured using the VAS and the mean baseline pain measure ranged from 0.3 to 6.9. See Tables E-8 and F-8 (Appendices E and F) for details of the study characteristics and interventions.
All the RCTs and nRCTs had a high risk of bias; the cohort study had a moderate score (n = 6 stars) on the NOS (Appendices G, I). Summary of the evidence from these trials is provided in Table 19.
Table 19
Evidence addressing key questions: Traction.
Key Question 1. Acute and chronic pain management
Acute Pain (Post-Treatment Means)
Eight trials26, 124, 125, 127-131 compared skin traction (n = 498) versus no traction (n = 594) (Table 20). There was no significant difference in pain relief between the groups (MD 0.20; 95% CI -0.24, 0.65; p = 0.36) (Figure 13). The variance was imputed for one of the trials127 using the reported p value in the original publication and from the other included trials for four trials.125, 128, 129, 131 The strength of the evidence was rated as insufficient to make any firm conclusions.
Table 20
Evidence summary table (RCT/nRCT): Traction.

Figure 13
Traction—acute pain (post-treatment means).
In the trial126 that compared skin traction (n = 40) vs. skeletal traction (n = 38), there was no significant difference between the two groups (MD 0.10; 95% CI -0.60, 0.80; p = 0.78).
The strength of the evidence was rated as insufficient to make any firm conclusions.
Key Question 2. Other outcomes
Health services utilization. LOS for acute hospitalization was reported in two trials128, 129 comparing skin traction (n = 137) vs. no traction (n = 189) (Table 20). In one trial128 there was no significant difference between the groups (MD 1.20; 95% CI -0.93, 3.33; p = 0.27). The MD was not estimable in the other study129 as no measure of variance was reported; however, the authors reported that the difference was not statistically significant. In order to allow pooling of the two trials, the variance was imputed from the available study variance.128 There was no significant difference in LOS between the two groups (MD 1.08; 95% CI -0.78, 2.95; p = 0.26).
Mortality (30-day). Thirty-day mortality was reported in one trial123 (n = 80) (Table 20). There was no difference in mortality between skin or skeletal traction vs. no traction (0/55 vs. 2/25; OR 0.14; 95% CI 0.01, 1.44; p = 0.10). There were no reports of mortality when comparing skin vs. skeletal traction.
Pain medication use. Additional pain medication use was reported in two trials127, 128 (n = 352) (Table 20). There was no significant difference in pain medication use following skin traction vs. no traction (99/151 vs. 111/201; OR 1.47; 95% CI 0.83, 2.61; p = 0.18).
Key Question 3. Adverse effects
Seven trials124, 126-131 (n = 1,043) evaluated the nature and frequency of adverse effects associated with the administration of skin or skeletal traction vs. no traction (Table 20). Additionally, one cohort study132 (n = 134) compared skeletal traction vs. pillow (Table 21). In two trials126, 131 (n = 389) no adverse effects were reported in either the intervention or control groups. For the following specific adverse effects, there were no significant differences between the study groups: damage to surrounding structures,127 deep venous thrombosis,124 difficult reduction,128, 129 direct skin damage,129, 130 failure to heal,124 peroneal palsy,127, 130 and pressure sores.124
Table 21
Evidence summary table (cohort studies): Traction.
Key Question 4. Efficacy, effectiveness and safety in subpopulations
One trial131 was conducted in Asian participants comparing skin traction (n = 166) versus no traction (n = 145). Acute pain reduction was not significantly different between the two groups (MD -0.04; 95% CI -0.61, 0.53; p = 0.89). No adverse effects were recorded (0/166 vs. 0/145).
- Results - Pain Management Interventions for Hip FractureResults - Pain Management Interventions for Hip Fracture
- DUXAP4 double homeobox A pseudogene 4 [Homo sapiens]DUXAP4 double homeobox A pseudogene 4 [Homo sapiens]Gene ID:503633Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
