Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population: This guideline applies to the management of indirect hyperbilirubinemia in neonates < 8 days of life and ≥ 35 weeks gestation. This guideline does not include the management of neonatal direct hyperbilirubinemia or hyperbilirubinemia in patients > 8 days of age. This guideline excludes premature neonates born prior to 35 weeks gestation.
Objectives: To create an evidence-based standard for the management of neonates with indirect hyperbilirubinemia across all care settings (newborn nursery, intensive care units, general inpatient service, home care, primary care, and emergency department) that provides appropriate care to patients, reduces unnecessary diagnostic tests and interventions, and improves patient outcomes.
Key points:
Prevention. Feed newborns, starting at birth, at least 8 times per day. Feedings should be based on feeding cues with attempts at least every 3 hours.
- Continue breastfeeding even if feeding difficulties arise. Expressed breast milk or formula supplementation may be warranted in certain circumstances. Discourage discontinuation of breastfeeding, even for diagnostic purposes in the setting of suspected breast-milk jaundice.
- Feeding supplementation is not indicated for sleepy neonates during first 24–48 hours, unless signs of dehydration, or weight loss more than the 95th percentile per the newborn weight tool (NEWT).
- Feeding recommendations are not relevant if a patient is critically ill and enteral feeds are being withheld.
Diagnosis. The approach to diagnosing hyperbilirubinemia will differ depending on whether it is detected via screening during the birth hospitalization (Figure 1) or later in follow-up (Figure 2).
Figure 1
Birth Hospitalization.
History. Assess all newborns for risk factors for developing hyperbilirubinemia (Table 1).
Table 1
Neurotoxicity Risk Factors.
Bilirubin Measurement. A total bilirubin (TSB or TcB) level should be measured on all newborns prior to discharge. [I-C*]
- Choose appropriate test for bilirubin levels (Table 4).
- If TSB is indicated, the first level should be fractionated to rule out direct hyperbilirubinemia. Subsequent measurements can be total bilirubin alone.
- The first measurement should be obtained at 16–24 hours of life. [I-C*]
- Discharge prior to 16 hours of life is strongly discouraged. If extenuating circumstances result in the discharge of a neonate prior to 16 hours of life, appropriate follow-up for evaluation of hyperbilirubinemia should be arranged.
Table 4
Indications for Bilirubin Testing Modalities and Infant Jaundice Studies.
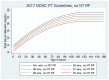
Figure 4
Intensive Phototherapy Thresholds for Infants WITHOUT Neurotoxicity Risk Factors (see Table 1). Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
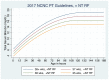
Figure 5
Intensive Phototherapy Thresholds for Infants WITH Neurotoxicity Risk Factors (see Table 1). Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
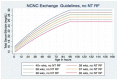
Figure 6
Exchange Transfusion Thresholds for Infants WITHOUT Neurotoxicity Risk Factors (see Table 1). Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
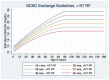
Figure 7
Exchange Transfusion Thresholds for Infants WITH Neurotoxicity Risk Factors (see Table 1). Use total bilirubin. Do not subtract direct reacting or conjugated bilirubin.
Further investigation into underlying etiology. Investigation into rarer causes of hyperbilirubinemia is recommended in certain circumstances (Table 4).
Table 3Circumstances Meriting Consideration of Rare Causes for Hyperbilirubinemia
| Severe hyperbilirubinemia ≥ 25mg/dL |
| Poor response to phototherapy |
| Recurrent admissions for hyperbilirubinemia |
| Direct hyperbilirubinemia |
| Prolonged jaundice (> 14 d for term; > 21 d for preterm) |
| Family history of Glucose-6-phosphate dehydrogenase (G6PD) deficiency or ethnicity suggestive of G6PD deficiency |
| Family history of non-immune hemolytic diseases, such as hereditary spherocytosis |
Risk Stratification. To determine treatment thresholds, note gestational age at birth and determine presence of neurotoxicity risk factors (Table 1). [I-D*]
Treatment. For overview, see Figure 3.
- Decision to admit to the hospital and treat should be based on TSB. [I-D*]
- In the absence of clinical concerns necessitating emergency department evaluation, direct admission to an inpatient service should be facilitated
- Intensive phototherapy can be expected to decrease bilirubin levels by 30–40% in 24 hours, with most being in the first 4–6 hours. Intensive phototherapy should be initiated in the following circumstances:
- - When total bilirubin is at or above the phototherapy treatment threshold based on hour-specific nomograms (Figure 4)
- - When TSB rate of rise > 0.2 mg/dL/hour and TSB is predicted to cross treatment threshold prior to next evaluation. [I-D*]
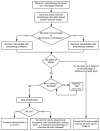
Figure 3
Hyperbilirubinemia Treatment. *Continue to encourage breastfeeding. Consider lactation consultant. If clinically dehydrated, consider oral rehydration versus IVFs. Tx = treatment; TSB = Total Serum Bilirubin; IVF = IV Fluids
There is lack of evidence to support the routine use of home phototherapy when the bilirubin level is at, near, or above the treatment threshold. However, home phototherapy can be considered when bilirubin is 0–2 mg/dL below the treatment threshold at discharge from the birth hospitalization or in the outpatient setting in the following circumstances:
- Neonates who feed well, appear well, and have close follow up arranged.
- Neonates with no neurotoxicity risk factors. [III-C*]
- Neonates without prior history of intensive phototherapy treatment.
Home phototherapy equipment can no longer be obtained in or arranged by Children’s Emergency Services
When bilirubin values are at or near exchange transfusion values:
- Maximize surface area exposed to phototherapy by removing unnecessary clothing (minimal/no diaper)
- Surround the neonate using highly reflective materials to increase surface area exposed and irradiance.
- Use multiple light sources (measure irradiance at various sites).
- Consider adjunctive therapies, including Intravenous immunoglobulin (IVIG) and IV hydration.
- Turning baby from prone to supine in an alternating fashion has not been shown to be efficacious.
For most neonates, routine IV supplementation is not warranted. However, for neonates with severe hyperbilirubinemia, IV fluid administration may be useful and is recommended.
Use of IVIG may be useful in Rh or ABO disease.
- Restrict use to select neonates in the NICU with high bilirubin values or rapid rate of rise (at high risk for exchange transfusion).
- Monitor neonates closely.
- Dose 0.5g/kg over 2 hours, repeat as clinically indicated.
An exchange transfusion should be considered when a serum bilirubin value surpasses the applicable NCNC recommended threshold value (Figures 6 & 7).
Monitoring. Following the initiation of phototherapy, only serum bilirubin (TSB) levels are recommended.
- Stop phototherapy once serum bilirubin has fallen to a level at least 3 mg/dL below the phototherapy threshold.
- Rebound levels at 6 hours are not predictive of subsequent repeat phototherapy.
- Consider repeat TSB 24 hours after discontinuation of phototherapy if treated prior to initial hospitalization discharge (post-delivery). This may be performed as an outpatient.
- Recheck TSB 12–24 hours after discontinuation of phototherapy, if preterm, evidence of hemolysis, or treated after readmission and serum bilirubin is > 14. This may be performed as an outpatient (if bilirubin ≤14, routine repeat TSB is not indicated in all neonates).
- Recheck bilirubin q 4–6 hours, depending on the rate of decline, when treating with exchange transfusion.
- A neonate being treated with home phototherapy (fiber optic blanket, Bili Blanket) should have a TSB checked:
- - Every 24–48 hours if the neonate is ≥ 38 weeks gestation.
- - Every 24 hours if the neonate is < 38 weeks gestation.
- Do not delay discharge to obtain a rebound bilirubin level. Check rebound levels as an outpatient when indicated.
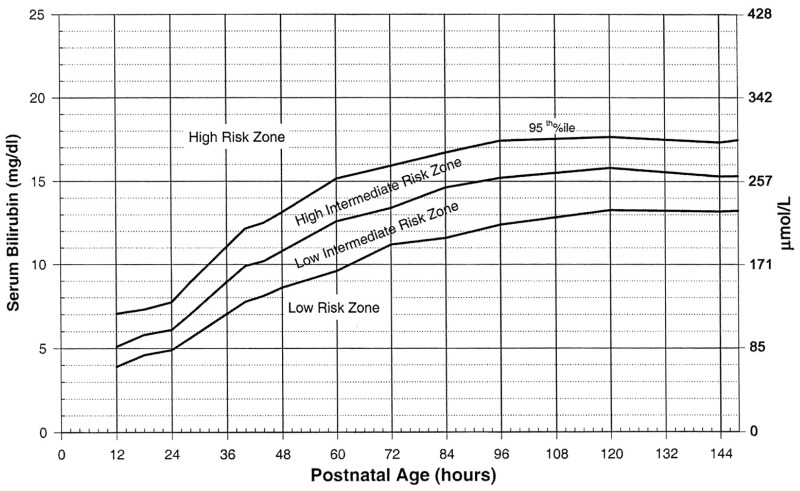
Follow-up. Timing and frequency of follow up after birth hospitalization should be influenced by risk of development of severe hyperbilirubinemia. This is determined by risk factors for development of severe hyperbilirubinemia (Table 2), as well as plotting TcB or TSB on the Bhutani nomogram (Appendix 1).
- Follow up should occur 1 day following birth hospitalization discharge, for those at higher risk.
- PCP follow-up should be arranged within 24 hours, or 48 hours when no serum bilirubin recheck is required (ie, discharge bilirubin <14), after hospital discharge from phototherapy,
Table 2
Risk Factors for the Development of Severe Hyperbilirubinemia.
Phototherapy Techniques. Fiberoptic Phototherapy Blanket: The fiber optic blanket is applied next to the neonate’s skin. The neonate may wear a diaper. The parents should swaddle the neonate with the fiber optic blanket next to the skin to avoid hypothermia. Feeding may continue with the blanket next to the neonate’s skin. If necessary, to optimize feeding, the neonate may be removed from the blanket for up to 30 minutes every 2–3 hours.
Inpatient Phototherapy:
- Babies receiving inpatient phototherapy should receive “intensive” phototherapy. [I-C*]
- For the majority of term neonates, phototherapy using a single overhead LED light source will provide intensive phototherapy and will be sufficient.
- Phototherapy using a single overhead LED light source and fiberoptic blanket may be indicated with the serum bilirubin value:
- - Rising more than 0.5 mg/dL/hour.
- - Within 3 mg/dL below the exchange transfusion threshold.
- - Fails to respond to initial phototherapy.
- Use of 2 angled overhead lights, a fiberoptic blanket, and white sheets as a reflective surface may be indicated when bilirubin is at or above the exchange transfusion threshold. This is done in the NICU.
- - Body surface area exposed and continuity of therapy (i.e. minimizing interruptions) will influence efficacy.
- - Irradiance should be measured regularly.
- - There is a lack of evidence to support the use of fiberoptic blanket alone.
- Phototherapy can be temporarily halted to allow for bonding and breastfeeding, when neonates are not considered high risk for exchange transfusion.
Definitions
- Direct or conjugated hyperbilirubinemia
conjugated bilirubin of 1 mg/dL or more when total bilirubin is less than 5 mg/dL OR 20% of total bilirubin when total bilirubin is over 5 mg/dL.1
- Home phototherapy (Fiberoptic phototherapy Blanket)
phototherapy provided via a fiberoptic blanket in the home setting
- Infant jaundice studies (IJS)
A set of tests including neonatal blood typing for ABO and Rh as well as direct antiglobulin test (DAT), typically performed using cord blood. Note: at UMHS, results regarding maternal ABO status are listed in “comment” area.
- Inpatient phototherapy
All inpatient phototherapy is intensive. The method of delivery (number of light sources) is dependent on the clinical state of the infant.2
- Intensive phototherapy
A light source that provides between 30 microwatts/cm2/nm and 65 microwatts/cm2/nm and delivered to as much of the infant’s surface area as possible.
- Prolonged jaundice
> 14 days for term; > 21 days for preterm.
- Total serum bilirubin (TSB)
A blood test to measure bilirubin levels, both direct bilirubin and indirect bilirubin.
- Transcutaneous bilirubin (TcB)
A non-invasive measure to estimate serum bilirubin levels.
Abbreviations
- AAP
American Academy of Pediatrics
- ABE
Acute bilirubin encephalopathy
- AOD
Admitting officer of the day (Hospitalist taking outside hospital transfer and direct admission calls)
- Ca
Calcium
- CBC
Complete blood count
- CES
Children Emergency Services
- DAT
Direct antiglobulin test
- G6PD
Glucose-6-phosphate dehydrogenase
- HOL
Hours of life
- IJS
Infant (neonate) jaundice studies
- IVIG
Intravenous immunoglobulin
- MVN
Michigan Visiting Nursing
- Na
Sodium
- NCNC
Northern California Neonatology Consortium
- NEWT
Newborn weight tool
- NICU
Neonatal intensive care unit
- PLT
Platelet
- POC
Point of Care
- TcB
Transcutaneous bilirubin
- TSB
Total serum bilirubin
- Tx
Treatment
- UMHS
University of Michigan Health System
Clinical Background and Epidemiology
Between 0.4 and 6.2% of healthy > 35 week newborns receive phototherapy for indirect hyperbilirubinemia,5,6 and severe hyperbilirubinemia occurs in approximately 7–45 neonates per 100,000 live births.7 The definition of severe hyperbilirubinemia varies, but typically refers to bilirubin levels between 25 to 30 mg/dL.
There are several factors that increase a newborn’s risk for development of severe hyperbilirubinemia. A systematic review done in 2010 concluded factors associated with severe hyperbilirubinemia were age < 38 weeks, jaundice within 24 hours of life and intention to breastfeed exclusively. In this review, four out of five studies evaluated family history of jaundice as a risk factor and four found it to be statistically significant.8 Large cohort studies since then confirm some of these findings. Male gender and non-Caucasian race increase the probability of high total serum bilirubin (TSB) as does evidence of cephalohematoma.7 “Dehydration/starvation,” usually measured as a percentage of weight loss since birth, is associated with hyperbilirubinemia. “Exclusive breastfeeding” is often found as a risk factor.9,10
Complications
Unbound and unconjugated (free) bilirubin is neurotoxic.11 Brain damage caused by hyperbilirubinemia requires that a quantity of (free) bilirubin to chemically interact with neurons. The concentration of bilirubin sufficient to cause neuronal cell injury is speculative. The concentration of free bilirubin in the central nervous system and the underlying “susceptibility” of neurons (independent of bilirubin concentration) are key determinants of injury, neither of which is easily measured. The amount of free bilirubin is dependent upon the total serum bilirubin value, the albumin (principle “binder” of bilirubin) concentration, and the chemical kinetics governing the binding of the two. This can be affected by gestational age, concomitant illness and any other compounds competing for bilirubin binding sites. A separate set of kinetics governs the transfer of bilirubin from blood into (and out of) the central nervous system (CNS). These kinetics can be affected by underlying illness.
At present there is no practical way to measure free CNS bilirubin; hence TSB is used as a surrogate. The susceptibility of the CNS is regional in nature; neurons in the basal ganglia and cerebellum appear most susceptible. It is reasonable to assume that underlying cellular “stress” can render certain neurons more vulnerable (e.g. hypoxia, inflammation).
The bottom line is that at any given time for any given baby, there exists a serum bilirubin value that can cause injury, but what that actual value is will always be speculative. Clinical studies in this area focus on values of TSB that are associated with acute bilirubin encephalopathy (ABE) and chronic bilirubin encephalopathy (CBE), and recognizable clinical conditions that are associated with ABE and CBE. These studies seek to define probability estimates (or action threshold probabilities) that trigger clinician behaviors (eg, identify risk, identify TSB threshold for phototherapy, and identify TSB threshold for exchange transfusion).12 In the early phase of ABE, neonates are described as having poor feeding, lethargy, high-pitched cry, increased tone, opisthotonos, or seizures. Later acute encephalopathy is characterized by retrocollis-opisthotonos, shrill cry, no feeding, apnea, fever, deep stupor to coma, sometimes seizures, and death. Sequelae of CBE include: motor delay, sensorineural hearing loss, upward gaze palsy, dental dysplasia, athetoid cerebral palsy, or mental retardation.
The incidence of severe hyperbilirubinemia with subsequent encephalopathy is fairly consistent throughout large epidemiologic studies, at approximately 0.5 to 1.0 cases per 100,000 live births (Canada 2.5/100,000). Most provide no statistical data; the absolute number in each with CBE is very small. A large percentage of neonates with chronic bilirubin encephalopathy (CBE) had additional risk factors, among the most common being hemolysis (isoimmunization, G6PD deficiency, spherocytosis), late prematurity, and infection. Some had multiple risk factors.
The use of “high risk” categorization is justified in that risk factors are used in conjunction with TSB values to determine overall risk of extreme hyperbilirubinemia, a prerequisite for CBE.
Prevention
Exclusive breastfeeding is recommended during first 6 months of life, as all-cause mortality is less in exclusive breast fed neonates.13 Exclusive breastfeeding is strongly associated with an increased risk of hyperbilirubinemia, however, the hyperbilirubinemia appears to be primarily the result of less effective breastfeeding.14–16 To ensure successful breast feeding, lactation support should be initiated in the hospital if any difficulty is suspected, and continued in the physician’s office and at home (e.g. at UMHS, patient educational materials such as Feeding the Baby Born Before 38 Weeks).4
The American Academy of Pediatrics recommends promoting breastfeeding for all term neonates at least eight to 12 times per day during first several days of life to reduce likelihood of dehydration.4 Breastfeeding women whose neonates are jaundiced are at increased risk of early cessation of breastfeeding.17 Mothers should be encouraged to breastfeed, and given lactation support if experiencing difficulty in breastfeeding, unless the level is approaching threshold for exchange transfusion.18 If phototherapy during breast feeding is possible, it can be encouraged.
Supplementation with formula may be considered if the neonate’s intake is inadequate, weight loss is more than the 95th percentile per NEWT, the neonate appears dehydrated, or the jaundice is severe. Signs of adequate intake in breast-fed neonates include four to six thoroughly wet diapers per day, three to four stools per day by the fourth day of life, and a transition to seedy, mustard-colored stools by the third or fourth day of life.4
It is recommend that feeding of all newborns occurs at least eight times per day starting immediately following birth.19 We recommended that babies be kept skin to skin and feed based on feeding cues with attempts at least every three hours. Feeding supplementation is not indicated for sleepy neonates during first 24–48 hours, unless there are signs of dehydration or weight loss is > 95th percentile per NEWT.4 UMHS guidelines for supplementation for difficult to latch term neonate during first 4 days are shown below. This recommendation is based on average intake of colostrum by healthy breast fed neonates.20,21
| Time after birth (Hours) | 12–24 | 24–48 | 48–72 | 72–96 |
| Amount to feed | 5–10ml q2–3hrs | 10–30ml q2–3hrs | 15–30ml q2–3hrs | >30 mL 8 times/day or more |
Diagnostic Procedures and Considerations
A total bilirubin should be measured on all babies prior to discharge from the birth hospitalization.22 Compared with selective testing, universal screening reduces total blood draws and phototherapy rates.23,24 While several clinical risk scores have been developed, none are as predictive of subsequent hyperbilirubinemia as a predischarge bilirubin level.25
Transcutaneous (TcB) levels should be used for screening. Transcutaneous levels are reasonable estimates for serum levels, and can be done rapidly and without a blood draw.3 TcB should be obtained from the sternum in neonates to avoid false negative that may be associated with the neonates face being in the sun.
The predischarge TcB should be obtained between 16 and 24 hours of life. (UMHS guideline regarding measurement of transcutaneous bilirubin.) In cases where a rapid rate of rise of serum bilirubin may occur (eg, hemolysis) TcB can underestimate serum bilirubin values. A serum value may be more accurate.
Predischarge screening bilirubin levels should be used to guide follow-up. The hour-specific Bhutani nomogram can be used to predict the likelihood of subsequent significant hyperbilirubinemia found in Appendix 1.22,26 Significant hyperbilirubinemia in this case is defined as > 95th percentile on the nomogram, and does not necessarily correlate with a need for phototherapy or exchange transfusion, length of stay, or neurologic outcomes. The hour-specific nomogram has been validated for patients with ABO incompatibility and positive direct antiglobulin test, and may be used in this population.27
A serum bilirubin (TSB) is recommended in the circumstances outlined in Table 4.
Infant jaundice studies [IJS; Neonatal blood typing for ABO and Rh as well as direct antiglobulin test (DAT)] should be obtained in circumstances outlined in Table 4.
Infant jaundice studies should not be measured routinely in all babies born to mothers with blood type O.11,28 Only 15% of neonates with ABO-incompatibility and a positive DAT will develop significant hyperbilirubinemia.11 Those that do have ABO-hemolytic disease develop clinically significant jaundice in the first 12–24 hours of life and will be recognized with routine screening.
TcB can be used in the outpatient setting, including in the clinic or by a visiting nurse, to assess jaundice. This will reduce lab draws by 20–50%.29
Cord blood DAT should not routinely be tested for in a Rh+ neonate of a Rh− mother who was treated with RhoGAM. It has a low positive predictive value for later hyperbilirubinemia and there is a risk of false positive after RhoGAM. [III-C]30 If phototherapy is being considered, DAT should be evaluated via cord blood or blood draw. [I-C]4
We recommend against withholding breast milk for diagnostic purposes in the setting of suspected breast milk jaundice. Significant hyperbilirubinemia is primarily the result of less effective breastfeeding, rather than breast-feeding itself.15 Neonates with jaundice were twice as likely as those without jaundice to have mothers who had stopped breastfeeding by one month (number needed to harm = 4).17
Common causes for hyperbilirubinemia include blood type incompatibility, G6PD deficiency, and infection. No cause was found in 1/3 of cases of neonates discharged healthy and returning with kernicterus in a sample of 125 neonates of ≥ 35 gestational age.9,31 There are several instances when further investigation into underlying etiologies for hyperbilirubinemia are suggested (Table 4).
Suggested testing when investigating underlying etiologies for indirect hyperbilirubinemia include DAT if not previously done, G6PD, hemoglobin/hematocrit, reticulocyte count, peripheral smear (RBC indices), complete blood count with differential, blood cultures and urine cultures.
Risk Stratification
Neonates should be assessed for any neurotoxicity risk factors (Table 1). Using the gestational age at birth and presence or absence of neurotoxicity risk factors, treatment thresholds can be determined using the NCNC nomograms or website [I-D] (Figure 4–7).4
The risk level is used to determine the hour-specific threshold for phototherapy and exchange transfusion.
Do not presume the presence of hemolytic disease, and thus a neurotoxicity risk factor, solely on the basis of maternal blood type O. [III-C]11,28,32 If there is clinical concern for hyperbilirubinemia, infant jaundice studies should be sent to further risk stratify neurotoxicity risk. A positive DAT indicates hemolysis and thus is considered a neurotoxicity risk factor. Of note, there are occasional false negative DAT tests. If there is high clinical concern for hemolytic disease despite a negative DAT, it is an option to risk stratify as having a neurotoxicity risk factor.
Neonates being treated with antibiotics for suspected sepsis should be considered to have a neurotoxicity risk factor [I-C].33–35 In the presence of hyperbilirubinemia, sepsis increases the risk of bilirubin encephalopathy 20-fold or greater. This risk factor may be discounted when blood cultures return negative and antibiotics are discontinued.
Level of Care
Treatment can range from hospital care to home care. Considerations of the various levels of care are listed below.
Transfer from the Newborn Nursery to the Floor
Considerations for transferring newborns with hyperbilirubinemia to the floor will depend on local policies and resource availability. For UMHS, there are UMHS guidelines (Appendix 2) that address when to move a newborn with hyperbilirubinemia from the newborn nursery to the general pediatrics floor.
Triage for Visiting Nursing
If a visiting nurse (RN) has a transcutaneous bilimeter available, and measurement is within 3 mg/dL of treatment threshold, the visiting RN should communicate with the primary care provider (PCP), or discharging physician if PCP relationship has not been established, and unable to begin relationship sooner (ie, same day appointment) for follow-up serum bilirubin. The PCP should facilitate further evaluation and laboratory follow-up. (UMHS MVN TcB policy UMHS internal use only.)
When there is visible jaundice or other concerns such as poor feeding, lethargy, or excessive weight loss, and no transcutaneous bilimeter is available, refer to the PCP (or discharging physician if the PCP relationship has not been established).
Outpatient Assessment (Figure 2)
All neonates discharged prior to 72 hours of life should been seen by a medical provider within 3 days (preferably within 48 hours) to assess for hyperbilirubinemia. Those at higher risk of severe hyperbilirubinemia should be evaluated sooner (preferably the day following birth hospitalization discharge). Risk factors for severe hyperbilirubinemia are listed in Table 2. Bilirubin level can also be plotted on the Bhutani nomogram (Appendix 1) to determine risk of development of severe hyperbilirubinemia and help guide follow up. Outpatient follow up assessments should include: the neonates’ weight, percent change from birth weight (BW), adequacy of feeding, pattern of voiding and stooling, and presence of jaundice. Exclusively breast fed newborns are higher risk for hyperbilirubinemia and dehydration; close follow up until 7–9 days is recommended as 90% of severe hyperbilirubinemia occurs by day 9.31,36 Bilirubin measurement is determined by risk factors for hyperbilirubinemia, neurotoxicity risk factors, and clinical judgment.
The UMHS guideline outlining assessment of bilirubin can be found in Appendix 2. If a baby requires assessment of bilirubin during hours when clinics are closed, the on-call physician may order bilirubin levels and follow up the result. If labs are also closed, these patients can be directed to Children’s Emergency Services (CES) for a phlebotomy lab draw to be followed up by the outpatient, ordering provider. (At UMHS, guidance available at UMHS Department of Pathology - After Hours Phlebotomy Services) If there is concern about the clinical appearance of the patient and the neonate requires assessment by a provider, they may be referred to CES for evaluation. Whenever sending a patient to CES, it is the outpatient and/or ordering provider’s responsibility to provide handoff to the ED physicians.
If repeat bilirubin is needed following initial hospital discharge and before an appointment with the outpatient provider, it is the responsibility of the discharging physician to order and follow up on the bilirubin level.
If the outpatient lab utilizes the Piccolo POC bilirubin, the provider can anticipate it will be up to 10% below the TSB. If the Piccolo total bilirubin is > 11 mg/dL for neonates < 3 days or if the total bilirubin is > 13 mg/dL on infants > 3 days, an automatic sample will be sent to the central lab for a TSB.
If TSB is more than 2 mg/dL below the phototherapy threshold, there is no need to repeat TSB unless worsening jaundice, feeding difficulties, dehydration, sepsis, or other concerns.
If TSB is 0 to 2 mg/dL below phototherapy threshold, the provider should repeat TSB within 24 hours unless bilirubin level is decreasing compared to previous measurement. Consider ordering DAT from cord blood to classify neurotoxicity risk appropriately.
If TSB is at or above phototherapy level of appropriate neurotoxicity risk, repeat TSB within 4 to 6 hours if improvement is expected, or arrange for direct admission of the patient to the hospital. Order DAT from cord blood to classify neurotoxicity risk appropriately.
Admission Triage
The best predictors of admission are elevated bilirubin (TcB or TSB) at 24–48 hours of life and gestational age. [I-C]37
If the bilirubin is at or above the level where intensive phototherapy is recommended [I-C]4 or if rate of rise is > 0.2 mg/dL/hour with a current bilirubin predicted to cross the treatment threshold prior to the next monitoring evaluation, a neonate should be admitted. [I-D]
Consideration for evaluation in Children’s Emergency Services should be given when there is concern for hemodynamic instability, sepsis, or other conditions in the neonate that would require immediate evaluation and resuscitation. In the absence of clinical concerns necessitating emergency department evaluation, direct admission to an inpatient service can be facilitated. For admission at UMHS, when a provider (PCP/newborn service/on-call nurse/other) has determined that a neonate requires hospitalization for management of hyperbilirubinemia, contact the hospitalist on-call (x 2288) for UMHS providers, and on-call admitting officer of the day for Mott hospital (AOD-Mott via transfer operator) for external (non-UMHS) PCPs to arrange for an admission.
In times of high occupancy, when phototherapy will be delayed > 4 hours, the hospitalist on-call or AOD will discuss admission to the NICU for care or consider referring patients who require admission for phototherapy to CES or an alternate location to initiate care. This excludes neonates within 2 mg/dL of exchange transfusion level as they should be admitted directly to the NICU.
Treatment
The goal of treatment is to prevent acute and chronic bilirubin encephalopathy. An additional goal of phototherapy is to prevent the need for exchange transfusion, a therapy that can acutely lower serum bilirubin values but also engenders risk. Phototherapy can also reduce serum bilirubin values but requires time for its therapeutic effect to become apparent. Because of this, risk-averse clinicians are obliged to “over treat” with phototherapy targeting neonates who are at risk for extreme hyperbilirubinemia. Once a neonate has crossed the risk threshold, treatment with a standardized approach to phototherapy is of value.
In the absence of high quality evidence for the thresholds at which hyperbilirubinemia should be treated, we rely on external guidelines. Given evidence of potential harms of treatment and safety of increased treatment thresholds, the Northern California Neonatology Consortium (NCNC) treatment thresholds have been adapted for use. These treatment thresholds are based on prior AAP National guidelines in addition to quality and safety projects in the Kaiser Northern California group showing decreased readmissions and inpatient phototherapy treatment without any increase in harm. [I-D]
Home Phototherapy
If a baby does not have neurotoxicity risk factors, is feeding well, and close follow up is arranged, and when the total bilirubin is 0–2 mg/dL below the treatment level, it is an option to discharge a baby from the birth hospitalization with home phototherapy. [II-D] Home phototherapy should not be used for babies with neurotoxicity risk factors (Table 1), nor those who have required intensive phototherapy. [III-D]
Although efficacy is not well documented, home phototherapy (ie, fiberoptic phototherapy blanket) may be considered in a well-appearing neonate without neurotoxicity risk factors and with a bilirubin that is 0 – 2 mg/dL below the phototherapy level. [II-C]22,38
Home phototherapy should not be used in neonates with neurotoxicity risk factors (Table 1). [III-C]
Inpatient Phototherapy
Neonates at risk for developing significant hyperbilirubinemia should receive phototherapy. Phototherapy is in general effective at decreasing serum bilirubin values (TSB) and preventing the need for exchange transfusion. For the majority of term neonates, phototherapy using a single light source capable of emitting enough energy (irradiance) (30 microwatts/cm2/nm), in the spectrum of 460–490nm will be sufficient. Double light source phototherapy (overhead and fiberoptic blanket) may be indicated when the serum bilirubin values is rising rapidly (more than 0.5 mg/dL/hr), the serum bilirubin is at a level within 3 mg/dL below the threshold for which exchange transfusion is indicated or the bilirubin level fails to respond to initial phototherapy. Use of two angled overhead LED phototherapy lights in combination with a fiberoptic phototherapy blanket and white sheets as a reflective surface may be indicated when the serum bilirubin value is at or above the exchange transfusion threshold. This should be done in the NICU. Irradiance should be measured regularly. Fiberoptic phototherapy alone should not be used for intensive phototherapy.
Where there is hemolysis due to ABO incompatibility (positive DAT), efficacy is less certain. A risk-averse approach would be prudent. With a rapid rate of rise (0.2 mg/dL/hr) of bilirubin values and suspected hemolysis due to blood group incompatibility, consider phototherapy while waiting for confirmation of antibody- mediated hemolysis.
When neonates are not considered high risk for exchange transfusion (≥ 2 mg/dL below exchange level and < 0.2 mg/dL/hr rate of rise), phototherapy can be temporarily halted to allow for bonding and breast feeding.
Likewise, for neonates not considered high risk for exchange transfusion, phototherapy while being held skin-to-skin can be considered. The parent needs to wear eye protection (sunglasses).39
When serum bilirubin values are at or near exchange transfusion values, take steps to insure “maximum” efficacy. Such steps may include: maximizing surface area by removing unnecessary clothing (ie, minimal/no diaper) using multiple light sources (measure irradiance at various sites) using highly reflective materials (ie, white sheets) surrounding a neonate to increase surface area exposed and irradiance consider adjunctive therapies including IVIG and IV “hydration” (see IVIG section, below).
Note: turning baby from prone to supine in an alternating fashion has not been shown to be efficacious.
Risk and Risk Mitigation
There is emerging evidence about the potential risks of phototherapy. Development of melanocytic nevus is a concern. Recent studies have shown the potential for increased risk of seizures in boys who have been treated with phototherapy.40 There is also an increased risk of childhood cancer. The absolute risk is very low, and the number needed to harm is >10,000. In infants with trisomy 21, this risk is further increased given their already higher baseline rates.41 Blue light can induce retinal photoreceptor degeneration in rats and in mammals. To mitigate risk of treatment, consider the following:
- A single overhead light will suffice for most neonates.
- Do not routinely exceed recommendations for maximal irradiance.
- Stop phototherapy when indicated.
- Eye masks to prevent damage to the retina are a simple and prudent prophylactic measure.
IV fluids
For most neonates with hyperbilirubinemia, IV supplementation is not warranted. It is recommended that dehydration in neonates with hyperbilirubinemia be treated similarly to any hospitalized neonate with dehydration.
For neonates with severe hyperbilirubinemia, IV fluid administration is recommended.
Dehydration can theoretically impede bilirubin excretion in urine by decreasing gut motility and increasing enterohepatic recirculation of bilirubin. There are at least 3 randomized controlled trials comparing IV fluid supplementation with no supplementation. Mehta at al, found supplementing with IV fluids for the first 8 hours of phototherapy, followed by enteral supplementation was associated with fewer exchange transfusions.42 Iranpour and colleagues, conducted a similar study in which “dehydrated” neonates were excluded.43 No meaningful differences in outcome were found. Saeidi, et al, performed a randomized, controlled trial in 100 well appearing neonates and found the mean decrease in TCB at 24 hours of phototherapy was greater with IV supplementation.39 Boo et al, studied fluid supplementation provided either entirely enteral or as a combination of enteral and IV fluids.44 No differences in indirect serum bilirubin were appreciated, suggesting either oral/enteral supplementation is as beneficial as IV (or there is no efficacy to either).
IVIG
Use of IVIG may be useful in Rh or ABO disease. Use should be restricted to select neonates in the NICU with high bilirubin values or rapid rate of rise (at high risk for exchange transfusion). Dose 0.5 g/kg over 2 hours, and repeat as clinically indicated.
During the trial of intensive phototherapy, patients with Rh or ABO mediated hemolysis should receive IVIG (0.5 – 1.0 g/kg over 4 hours).
Exchange Transfusion
An exchange transfusion should be considered when a serum bilirubin value surpasses the applicable recommended threshold value (Figures 4–7).
A decision to delay or forgo the transfusion will depend upon the subsequent TSB value, the rate of fall of TSB, and the perceived underlying vulnerability of the neonate. Failure of the TSB value to fall necessitates exchange transfusion.
For the neonate who presents as an outpatient or in whom a trial of phototherapy has not been done, a trial of intense phototherapy is indicated. Exceptions include the neonate who exhibits signs of ABE (including opisthotonos and retrocollis), or in whom intense phototherapy is not likely to be of benefit. The latter includes a neonate who presents with TSB values so high that it is unlikely that a period of phototherapy will lower TSB to < 25 mg/dL, or in whom the rate of rise is predicted to be faster than the expected fall associated with phototherapy.
Consider using albumin prior to transfusion (see below).
The exchange transfusion should be a double-volume exchange transfusion and done in the NICU under the direct supervision of a neonatologist. At UMHS, technical guidelines can be found in the Brandon NICU.
Continue phototherapy during the exchange transfusion.
Monitor for potential adverse sequelae including leukopenia, thrombocytopenia, hypocalcemia, hypernatremia, and bacteremia. A post-exchange blood sample looking for chemical abnormalities is recommended (CBC, PLT, Ca, Na and TSB). Calcium need not be administered in the absence of bradycardia.45
Albumin
There is a paucity of evidence surrounding the use of albumin as adjunctive therapy in neonates requiring exchange transfusion and it is used only in the NICU. A single RCT showed the use of albumin priming prior to exchange transfusion (1 mg/kg, 1 hour before exchange transfusion) resulted in lower TBS values at 12 hours and a shorter length of stay for neonates receiving albumin. If used, fluid status and effects of overhydrating should be monitored.
Monitoring
Home Phototherapy
If a patient is being treated with home phototherapy, check total serum bilirubin every 24 hours in a neonate born < 38 weeks gestation and every 24–48 hours in a neonate born ≥ 38 weeks gestation. [I-C]4,22
Inpatient Phototherapy
Total bilirubin should be followed using serum, not transcutaneous, levels while on phototherapy and after discontinuing phototherapy.4 Transcutaneous bilirubin levels are significantly lower compared with serum levels once phototherapy has been initiated.46,47 Phototherapy is expected to decrease total bilirubin levels by 30–40% in the first 24 hours, with most of the decrease in the first 4–6 hours.4
Discontinuation of Intensive Phototherapy
When a neonate is readmitted for hyperbilirubinemia, phototherapy should be stopped when the level is < 13–14 mg/dL.4 If lowered to < 14, there is a significantly decreased risk of requiring repeat phototherapy.48,49 In the absence of hemolysis, a higher level may be used for discontinuation, such as a level 3 mg/dL below current treatment level.50 Discontinuation at a lower level may be appropriate during the birth hospitalization since treatment levels are lower in the first days of life.
Rebound
Hospital discharge should not be delayed to obtain a rebound level. [III-C]4,49 Rebound bilirubin levels 6 hours after discontinuing phototherapy are not predictive of subsequent repeat phototherapy.48 A repeat bilirubin level 12–24 hours after discontinuing phototherapy should be considered in babies for whom phototherapy was required during the birth hospitalization, and for babies with a positive DAT or gestational age < 37 weeks. [I-C]4,51
Follow-up
Schedule outpatient follow-up within 1–3 days (see outpatient assessment) following birth hospitalization discharge to evaluate: neonates’ weight, percent change from the birth weight, adequacy of feeding, pattern of voiding and stooling, and presence of jaundice. Close follow up is recommended after initial evaluation after discharge to ensure adequate feeding in exclusively breast-fed neonates.
Order total serum bilirubin level for evaluation if ongoing feeding difficulties, dehydration, or other concerns are present at outpatient evaluations.
Management of TSB results:
- TSB is less than discharge level, no further measurement is needed.
- Rebound is noted but TSB lower than 2 below treatment threshold (unless concern for weight loss or feeding) no further test is needed.
- Rebound is noted and TSB is within 0–2 below level, recheck TSB in 1–2 days.
- Rebound is noted and TSB is at or above phototherapy level of appropriate neurotoxicity risk, the provider should arrange direct admission to the hospital unless the neonate needs evaluation by ED providers.
Guideline Creation Process and Considerations
Related National Guidelines
The UMHS Clinical Guideline on Management of Indirect Neonatal Hyperbilirubinemia is generally consistent with the following national and international guidelines:
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316.
- National Collaborating Centre for Women’s and Children’s Health. Neonatal jaundice. London. NICE, 2010. https://www.nice.org.uk/guidance/cg98/evidence/full-guideline-245411821. Updated 2010.
- World Health Organization. Guidelines on maternal, newborn, child and adolescent health approved by the WHO guidelines review committee. World Health Organization. 2013.
- Judd WJ, Scientific Section Coordinating Committee of the AABB. Practice guidelines for prenatal and perinatal immunohematology, revisited. Transfusion. 2001;41(11):1445–1452.
Related National Performance Measures
There are no national performance measures associated with hyperbilirubinemia.
Funding
The development of this guideline was funded by the University of Michigan Health System.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- Primary care physicians: Stephanie L Goodson, MD; Sahoko H Little, MD;
- Specialists: Jennifer L Vredeveld, MD; Maria Skoczylas, MD; Robert E Schumacher, MD; Nicole S Sroufe, MD
- Nursing: Linda D Gobeski, RN; Debra K Horvath, RN; Pamela K Hurley, RN; Kelly A McCarley, RN; Michelle Nemshak DNP, RNC-NIC, ACCNS-N; Carolyn M Pawlowski, RN; Rebecca Pehovic, MS, RN, CNS-BC; Deborah R Retzer, RN; Kristin Schuster, RN
- A guideline development methodologist: April Proudlock, RN
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
No relevant personal financial relationships with commercial entities: Linda D Gobeski, RN; Stephanie L Goodson, MD; Debra K Horvath, RN; Pamela K Hurley, RN; Sahoko H Little, MD; Kelly A McCarley, RN; Michelle Nemshak MSN, RNC-NIC, CNS; Carolyn M Pawlowski, RN; Rebecca Pehovic, MS, RN, CNS-BC; Deborah R Retzer, RN; Kristin Schuster, RN; F Jacob Seagull, PhD; Maria S Skoczylas, MD; Nicole S. Sroufe, MD, MPH; Jennifer L Vredeveld, MD
Relevant personal financial relationships with commercial entities: None.
Strategy for Literature Search
Within the Medline (Ovid) database, the following search strategy was used.
- exp *hyperbilirubinemia, neonatal/
- *hyperbilirubinemia/ or *jaundice/ or *kernicterus/
- limit 2 to “all infant (birth to 23 months)”
- (neonatal or neonate* or infant* or newborn*).ti.
- 2 and 4
- exp animals/ not (exp animals/ and humans/)
- 5 not 6
- 1 or 3 or 7
- limit 8 to (english language and yr=“2004 -Current”)
The search was not focused on indirect hyperbilirubinemia because the retrieval was very small. The Main search retrieved 1,213 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follow:
- Neonatal Hyperbilirubinemia -Guidelines, total results were 35
- Neonatal Hyperbilirubinemia -Clinical Trials, total results were 125
- Neonatal Hyperbilirubinemia -Cohort Studies, total results were 262
The MEDLINE In-Process database was also searched using the strategy in the search strategies document. The search retrieved 100 documents. The results with the hedges applied are:
- Guidelines, total results were 2
- Clinical Trials, total results were 11
- Cohort Studies, total results were 21
Within the Cochrane Database of Systematic Reviews, 6 reviews were found using the strategy in the search strategies document.
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. Negative trials were specifically sought. The search was a single cycle.
Level of evidence supporting a diagnostic method or an intervention:
- A= systematic reviews of randomized controlled trials
- B= randomized controlled trials
- C=systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (e.g., cohort, cross-sectional, case control)
- D= individual observation studies (case or case series)
- E =opinion of expert panel.
Search details and evidence tables available at http://www.uofmhealth.org/provider/clinical-care-guidelines.
Recommendations
Guideline recommendations were based on prospective randomized controlled trials if available, to the exclusion of other data; if RCTs were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size. The “strength of recommendation” for key aspects of care was determined by expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Health System to which the content is most relevant: Family Medicine, Pediatrics, Pediatric Emergency Medicine, Pediatric Internal Medicine. The Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers endorsed the final version.
Acknowledgements
The following individuals are acknowledged for their contributions to previous versions of this guideline.
2017: Michael Levy, MD
References
- 1.
- Feldman AG, Sokol RJ. Neonatal Cholestasis. NeoReviews. 2013;14(2):10.1542/neo.1514-1542-e1563. [PMC free article: PMC3827866] [PubMed: 24244109] [CrossRef]
- 2.
- Stokowski LA. Fundamentals of phototherapy for neonatal jaundice. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2011;11(5 Suppl):S10–21. [PubMed: 22123449]
- 3.
- Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015;135(2):224–231. [PMC free article: PMC4306797] [PubMed: 25601981]
- 4.
- American Academy of Pediatrics Subcommittee on H. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. [PubMed: 15231951]
- 5.
- Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101(6):995–998. [PubMed: 9606225]
- 6.
- Mantagou L, Fouzas S, Skylogianni E, Giannakopoulos I, Karatza A, Varvarigou A. Trends of transcutaneous bilirubin in neonates who develop significant hyperbilirubinemia. Pediatrics. 2012;130(4):e898–904. [PubMed: 22966022]
- 7.
- Manning D, Todd P, Maxwell M, Jane Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F342–346. [PMC free article: PMC2675352] [PubMed: 17074786]
- 8.
- Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134(3):504–509. [PubMed: 25092943]
- 9.
- National Collaborating Centre for Ws, Children’s H. Neonatal Jaundice. London. NICE, 2010. In:2010.
- 10.
- Wickremasinghe AC, Risley RJ, Kuzniewicz MW, et al. Risk of Sensorineural Hearing Loss and Bilirubin Exchange Transfusion Thresholds. Pediatrics. 2015;136(3):505–512. [PubMed: 26283777]
- 11.
- Watchko JF. Identification of neonates at risk for hazardous hyperbilirubinemia: emerging clinical insights. Pediatric clinics of North America. 2009;56(3):671–687. [PubMed: 19501698]
- 12.
- Bhutani VK, Johnson LH, Jeffrey Maisels M, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. Journal of Perinatology. 2004;24(10):650–662. [PubMed: 15254556]
- 13.
- World Health O. Guidelines on Maternal, Newborn, Child and Adolescent Health approved by the WHO Guidelines Review Committee. World Health Organization. 2013.
- 14.
- Gourley GR, Li Z, Kreamer BL, Kosorok MR. A controlled, randomized, double-blind trial of prophylaxis against jaundice among breastfed newborns. Pediatrics. 2005;116(2):385–391. [PubMed: 16061593]
- 15.
- Bertini G, Perugi S, Elia S, Pratesi S, Dani C, Rubaltelli FF. Transepidermal water loss and cerebral hemodynamics in preterm infants: conventional versus LED phototherapy. European journal of pediatrics. 2008;167(1):37–42. [PubMed: 17297614]
- 16.
- Stevenson D, Maisels MJ, Watchko J. The Epidemiology of Neonatal Hyperbilirubinemia. In: Care of the Jaundiced nNeonate. The McGraw-Hill Companies, Inc.; 2012:97–113.
- 17.
- Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84(5):773–778. [PubMed: 2797972]
- 18.
- Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. The Journal of family practice. 2002;51(5):465. [PubMed: 12019058]
- 19.
- Chen YJ, Yeh TF, Chen CM. Effect of breast-feeding frequency on hyperbilirubinemia in breast-fed term neonate. Pediatrics international : official journal of the Japan Pediatric Society. 2015;57(6):1121–1125. [PubMed: 25929838]
- 20.
- Santoro W, Jr., Martinez FE, Ricco RG, Jorge SM. Colostrum ingested during the first day of life by exclusively breastfed healthy newborn infants. The Journal of pediatrics. 2010;156(1):29–32. [PubMed: 19783000]
- 21.
- Casey CE, Neifert MR, Seacat JM, Neville MC. Nutrient intake by breast-fed infants during the first five days after birth. American Journal of Diseases of Children (1960). 1986;140(9):933–936. [PubMed: 3740001]
- 22.
- Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124(4):1193–1198. [PubMed: 19786452]
- 23.
- Wickremasinghe AC, Karon BS, Cook WJ. Accuracy of neonatal transcutaneous bilirubin measurement in the outpatient setting. Clinical pediatrics. 2011;50(12):1144–1149. [PubMed: 22013149]
- 24.
- Wickremasinghe AC, Karon BS, Saenger AK, Cook WJ. Effect of universal neonatal transcutaneous bilirubin screening on blood draws for bilirubin analysis and phototherapy usage. Journal of Perinatology. 2012;32(11):851–855. [PubMed: 22343396]
- 25.
- Keren R, Bhutani VK, Luan X, Nihtianova S, Cnaan A, Schwartz JS. Identifying newborns at risk of significant hyperbilirubinaemia: a comparison of two recommended approaches. Archives of Disease in Childhood. 2005;90(4):415–421. [PMC free article: PMC1720335] [PubMed: 15781937]
- 26.
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103(1):6–14. [PubMed: 9917432]
- 27.
- Schutzman DL, Sekhon R, Hundalani S. Hour-specific bilirubin nomogram in infants with ABO incompatibility and direct Coombs-positive results. Archives of Pediatrics & Adolescent Medicine. 2010;164(12):1158–1164. [PubMed: 21135346]
- 28.
- Madan A, Huntsinger K, Burgos A, Benitz WE. Readmission for newborn jaundice: the value of the Coombs’ test in predicting the need for phototherapy. Clinical pediatrics. 2004;43(1):63–68. [PubMed: 14968894]
- 29.
- Maisels MJ, Kring E. Transcutaneous bilirubin levels in the first 96 hours in a normal newborn population of > or = 35 weeks’ gestation. Pediatrics. 2006;117(4):1169–1173. [PubMed: 16585312]
- 30.
- James RM, McGuire W, Smith DP. The investigation of infants with RhD-negative mothers: can we safely omit the umbilical cord blood direct antiglobulin test? Archives of disease in childhood Fetal and neonatal edition. 2011;96(4):F301–304. [PubMed: 20659940]
- 31.
- Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). Journal of perinatology : official journal of the California Perinatal Association. 2009;29 Suppl 1:S25–45. [PubMed: 19177057]
- 32.
- Judd WJ, Scientific Section Coordinating Committee of the A. Practice guidelines for prenatal and perinatal immunohematology, revisited. Transfusion. 2001;41(11):1445–1452. [PubMed: 11724993]
- 33.
- Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128(4):e925–931. [PMC free article: PMC3182847] [PubMed: 21911352]
- 34.
- Weng YH, Chiu YW. Clinical characteristics of G6PD deficiency in infants with marked hyperbilirubinemia. Journal of Pediatric Hematology/Oncology. 2010;32(1):11–14. [PubMed: 20051781]
- 35.
- Weng YH, Chiu YW, Cheng SW, Hsieh MY. Risk assessment for adverse outcome in term and late preterm neonates with bilirubin values of 20 mg/dL or more. American Journal of Perinatology. 2011;28(5):405–412. [PubMed: 21365530]
- 36.
- Salas AA, Mazzi E. Exchange transfusion in infants with extreme hyperbilirubinemia: an experience from a developing country. Acta Paediatrica. 2008;97(6):754–758. [PubMed: 18422806]
- 37.
- Bhutani VK, Stark AR, Lazzeroni LC, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. Journal of Pediatrics. 2013;162(3):477–482.e471. [PubMed: 23043681]
- 38.
- Malwade US, Jardine LA. Home- versus hospital-based phototherapy for the treatment of non-haemolytic jaundice in infants at more than 37 weeks’ gestation. Cochrane Database of Systematic Reviews. 2014;6:010212. [PubMed: 24913724]
- 39.
- Saeidi R, Heydarian F, Fakehi V. Role of intravenous extra fluid therapy in icteric neonates receiving phototherapy. Saudi medical journal. 2009;30(9):1176–1179. [PubMed: 19750263]
- 40.
- Newman TB, Wu YW, Kuzniewicz MW, Grimes BA, McCulloch CE. Childhood Seizures After Phototherapy. Pediatrics. 2018;142(4). [PubMed: 30249623]
- 41.
- Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal Phototherapy and Infantile Cancer. Pediatrics. 2016;137(6). [PMC free article: PMC9923535] [PubMed: 27217478]
- 42.
- Mehta S, Kumar P, Narang A. A randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. Journal of Pediatrics. 2005;147(6):781–785. [PubMed: 16356431]
- 43.
- Iranpour R NRHI. Effect of Intravenous Fluid Supplementation on Serum Bilirubin Level in Jaundiced Healthy Neonates during Conventional Phototherapy. Journal of Research in Medical Sciences. 2004;4:186–190.
- 44.
- Boo NY, Lee HT. Randomized controlled trial of oral versus intravenous fluid supplementation on serum bilirubin level during phototherapy of term infants with severe hyperbilirubinaemia. Journal of paediatrics and child health. 2002;38(2):151–155. [PubMed: 12030996]
- 45.
- Locham K, Kaur K, Tandon R, Kaur M, Garg R. Exchange Blood Transfusion in Neonatal Hyperbilirubinemia-Role of Calcium. Indian Pediatrics. 2002;39:657–659. [PubMed: 12147892]
- 46.
- Fonseca R, Kyralessa R, Malloy M, Richardson J, Jain SK. Covered skin transcutaneous bilirubin estimation is comparable with serum bilirubin during and after phototherapy. Journal of Perinatology. 2012;32(2):129–131. [PubMed: 21818063]
- 47.
- Grabenhenrich J, Grabenhenrich L, Buhrer C, Berns M. Transcutaneous bilirubin after phototherapy in term and preterm infants. Pediatrics. 2014;134(5):e1324–1329. [PubMed: 25332501]
- 48.
- Berkwitt A, Osborn R, Grossman M. The utility of inpatient rebound bilirubin levels in infants readmitted after birth hospitalization for hyperbilirubinemia. Hospital Pediatrics. 2015;5(2):74–78. [PubMed: 25646199]
- 49.
- Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Archives of Pediatrics & Adolescent Medicine. 2002;156(7):669–672. [PubMed: 12090833]
- 50.
- Barak M, Berger I, Dollberg S, Mimouni FB, Mandel D. When should phototherapy be stopped? A pilot study comparing two targets of serum bilirubin concentration. Acta Paediatrica. 2009;98(2):277–281. [PubMed: 19143666]
- 51.
- Kaplan M, Herschel M, Hammerman C, Hoyer JD, Heller GZ, Stevenson DK. Neonatal hyperbilirubinemia in African American males: the importance of glucose-6-phosphate dehydrogenase deficiency. Journal of Pediatrics. 2006;149(1):83–88. [PubMed: 16860133]
Appendix 1. Bhutani Nomogram
Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics.1999;103 :6–1426
Reproduced with permission from American Academy of Pediatrics Journal, Vol. 103, Page 9, copyright 1999 by AAP
Appendix 2. Internal UMHS Guidelines
Transfer of newborn from well newborn to the floor: http://www.med.umich.edu/i/nursing-birthcenter/docs/pt_placement.pdf
TcB measurement in the newborn nursery: http://cw.i.medicine.umich.edu/sites/default/files/policies/trans_bili.pdf
Visiting nurse TcB measurement: http://hcs.med.umich.edu/procedure/showdoc.ashx?docID=254.054
After hours phlebotomy: http://www.med.umich.edu/i/em/Clinical_Guidelines/Phlebotomy_After_Hours.pdf ; http://inted.sites.uofmhosting.net/sites/default/files/downloads/Phlebotomy_After_Hours.pdf
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Review Management of Indirect Neonatal Hyperbilirubinemia[ 2017]Review Management of Indirect Neonatal HyperbilirubinemiaSroufe NS, Vredeveld JL, Levy M, Little SH, Schumacher RE, Seagull FJ, Skoczylas MS. 2017 Oct
- MANAGEMENT OF NEONATAL HYPERBILIRUBINEMIA.[Clin Obstet Gynecol. 1964]MANAGEMENT OF NEONATAL HYPERBILIRUBINEMIA.BROWN AK. Clin Obstet Gynecol. 1964 Dec; 10:985-1010.
- Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation.[Pediatrics. 2004]Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Pediatrics. 2004 Jul; 114(1):297-316.
- Review Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: analysis of the US Database.[Pediatr Res. 2022]Review Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: analysis of the US Database.Qattea I, Farghaly MAA, Elgendy M, Mohamed MA, Aly H. Pediatr Res. 2022 Jun; 91(7):1662-1668. Epub 2021 Aug 24.
- Rates of Extreme Neonatal Hyperbilirubinemia and Kernicterus in Children and Adherence to National Guidelines for Screening, Diagnosis, and Treatment in Sweden.[JAMA Netw Open. 2019]Rates of Extreme Neonatal Hyperbilirubinemia and Kernicterus in Children and Adherence to National Guidelines for Screening, Diagnosis, and Treatment in Sweden.Alkén J, Håkansson S, Ekéus C, Gustafson P, Norman M. JAMA Netw Open. 2019 Mar 1; 2(3):e190858. Epub 2019 Mar 1.
- Management of Indirect Neonatal HyperbilirubinemiaManagement of Indirect Neonatal Hyperbilirubinemia
- Hyperkalemic metabolic acidosisHyperkalemic metabolic acidosisMedGen
- KRT87P keratin 87, pseudogene [Homo sapiens]KRT87P keratin 87, pseudogene [Homo sapiens]Gene ID:85349Gene
- Nephronophthisis 7Nephronophthisis 7MedGen
Your browsing activity is empty.
Activity recording is turned off.
See more...