Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population: Adults with chronic obstructive pulmonary disease (COPD).
Objectives:
- Provide a framework for management of chronic COPD and for the treatment of mild to moderate acute exacerbations.
- Improve symptoms, quality of life and lung function while reducing morbidity and mortality for patients with COPD.
Key points
COPD is underdiagnosed and misdiagnosed. See Table 1 for an overview of diagnosis and management of COPD.
Table 1
Overview of Diagnosis and Management of Patients with COPD.
Do not perform population-wide screening for COPD. [III-C]
Appropriate comprehensive treatment can improve symptoms and quality of life. [I-A]
Diagnosis
Consider COPD in any patient with dyspnea, chronic cough or sputum production. [I-C] Consider early diagnostic case finding in persons with a history of inhalation exposures known to be risk factors for COPD. [I-D]
Pulmonary function testing with post-bronchodilator assessment demonstrating a reduced FEV1/FVC ratio is required for diagnosis. [I-C]
Assess COPD severity by determining extent of airflow limitation (spirometry), symptom severity, and exacerbation history (Table 5). [I-C]
Table 5
Aspects of COPD Severity.
Treatment
Smoking cessation is the single most important intervention to slow the rate of lung function decline, regardless of disease severity. [I-C]
Chronic medication management includes:
- Inhaled corticosteroids – consider adding to bronchodilators for patients with frequent exacerbations despite bronchodilator therapy [I-A], or with an eosinophil count ≥ 300 cells/μL [I-B], or with features suggestive of asthma-COPD overlap. [II-D]
- Supplemental oxygen if resting oxygen saturation ≤ 88% or PaO2 ≤ 55 mm Hg. [I-A]
Table 7
Medications Commonly Used in Chronic Obstructive Pulmonary Disease.
Acute exacerbation medication management includes bronchodilators (beta-2 agonists and anticholinergics) [I-C], systemic corticosteroid therapy [I-A], and antibiotics [II-A] based on clinical indications (Table 9). Empiric antibiotics are recommended for patients with increased sputum purulence plus either increased dyspnea or increased sputum volume. [I-A] Sputum culture is not routinely recommended. [III-D]
Table 9
Acute Exacerbation: Commonly Used Medications and Doses.
Pulmonary rehabilitation should be considered for all patients with functional impairment. [I-A]
Surgical and minimally invasive options include bullectomy, lung volume reduction procedures, and lung transplantation. [II-B] Life expectancy should be incorporated into shared decision making regarding the potential benefits of surgery. [II-D] Pulmonary consultation is recommended prior to consideration of invasive options. [I-D]
Palliative care should be discussed with patients with advanced COPD. Doing so may help limit unnecessary and burdensome personal and societal costs and invasive approaches. [I-C]
Clinical Background and Rationale for Recommendations
Epidemiology and Impact
COPD is the third-leading cause of death in the United States, accounting for over 147,000 deaths8 and 700,000 hospitalizations annually.9 COPD can have a long pre-symptomatic phase. About 15.7 million Americans have been diagnosed with COPD, but an estimated 50% of those with COPD are undiagnosed, so the actual number affected is probably much higher.10
Etiology
The pulmonary manifestations of COPD include an abnormal inflammatory response to noxious inhaled particles or gases.9 The most common causal agent is cigarette smoke, and cigarette smoking is the single largest risk factor for COPD.11 Second-hand smoke is also a recognized risk factor,12 as are environmental and occupational air pollutants.9,13 Deficiency of alpha-1 antitrypsin is a treatable cause of abnormal inflammatory response. While uncommon, it can be an important etiologic factor in early onset and severe disease.14
Prognosis
COPD is a chronic disease characterized by acute exacerbations. While the disease is heterogeneous with a variable course, airflow limitation is usually progressive, and life expectancy falls as disease progresses. Continued smoking predicts worse prognosis.15 Persons with frequent exacerbations (two or more per year) experience worse quality of life, more rapid loss of lung function, more frequent hospitalization, and increased mortality.16,17
While FEV1 (the forced expiratory volume in the first second) is commonly used to assess airflow limitation, FEV1 does not always directly correlate with symptom burden, functional status, or quality of life, and it is imperfect for predicting prognosis. Multiple dimensions of disease severity (airflow limitation, symptom severity, exacerbation risk) should help guide management.
Management Issues
Both physicians and patients under-recognize the potential benefits of appropriate disease management for COPD. The lack of a large FEV1 response to bronchodilation may contribute to a sense of skepticism regarding the benefits of treatment. However, COPD is a chronic inflammatory disease with systemic manifestations that affect patient function, quality of life, rate of lung function decline and the development of comorbidities. FEV1 is not the sole measure of disease response. COPD is responsive to multiple treatments. Appropriate comprehensive treatment can improve patients’ quality of life and prognosis.
Screening for COPD
Recommendation
Do not perform population-wide screening for COPD.
Although about half of the individuals with COPD in the United States are undiagnosed,8 no direct evidence quantifies the benefits and harms of COPD screening with questionnaires or handheld spirometry, nor does evidence exist to estimate the treatment benefits in screen-detected populations. Screening in older populations may lead to over-diagnosis in “never” smokers. The United States Preventive Services Task Force recommends against population-based screening.18
Diagnosis of COPD (Table 1)
Risk Factors and Clinical History
Recommendation
Consider a diagnosis of COPD in any patient who has dyspnea, chronic cough or sputum production.
Consider early diagnostic case finding in any patient with risk factors for the disease.
While routine population screening cannot be recommended, early diagnostic case finding is encouraged for persons at risk.18 Risk factors for COPD include exposure to chronic tobacco smoke,19 occupational dusts and chemicals,20 and smoke from home cooking and heating fuels (Table 2).21 The likelihood of a COPD diagnosis also increases with age.
Table 2
Symptoms and Signs Suggesting COPD.
Early diagnosis is encouraged, as the most effective therapy for COPD, in terms of slowing the decline of lung function, is smoking cessation.19,22 Also, case finding may improve smoking cessation rates.23
Recent evidence suggests that explaining a patient’s pulmonary function to them in terms of relative “lung-age” enhances smoking cessation rates.23
Differential diagnosis considerations for patients with chronic cough and dyspnea can be found in Tables 3 and 4.
Table 3
Alternative Diagnoses for Chronic Cough and Dyspnea.
Table 4
Factors Differentiating Asthma and COPD.
Symptoms and Signs on Physical Exam (Table 2)
Recommendation
Dyspnea, often preceded by a chronic cough with sputum production, may lead patients to seek care. Physical exam is typically helpful only for patients with more severe disease.
Dyspnea is the symptom that most frequently leads patients to seek medical attention, and dyspnea worsens with increasing disease severity.24 With severe disease, dyspnea can be debilitating. Chronic cough, often accompanied by sputum production, may precede the onset of dyspnea. Dyspnea frequently begins as an intermittent symptom, but later becomes persistent. Wheezing and chest tightness are nonspecific symptoms that may or may not be present. In patients with more severe disease, anorexia and anxiety may also develop.
Physical examination is typically unhelpful in the diagnosis of COPD.25 Early airflow limitation is typically detectable via spirometry before it is evident on physical exam. In patients with more severe disease, the physical exam may reveal decreased breath sounds, decreased air movement, wheezing, and rhonchi. Hyperinflation, as indicated by a “barrel” chest, accessory muscle use and weight loss, typically indicates more advanced disease.
Spirometry
Recommendation
Perform spirometry to diagnose COPD.
Spirometry is the diagnostic “gold standard” because it is the most reproducible, standardized, and objective way of measuring airflow limitation.26 Spirometry should be ordered with bronchodilator, and the post-bronchodilator values should be used to assess both the presence of airflow obstruction and its severity. A post-bronchodilator FEV1/FVC < 0.70 confirms the presence of airflow limitation that is not fully reversible (FVC is the forced vital capacity). The severity of post-bronchodilator airflow obstruction is defined by the FEV1 (Table 5).27
While reversibility with bronchodilator (defined as an increase in FEV1 of ≥ 200 mL and ≥ 12% absolute value) is commonly associated with asthma as opposed to COPD, post-bronchodilator FEV1 improvement of 12% can also be seen in COPD. In such cases of COPD with FEV1 bronchodilation response, the post-bronchodilator FEV1/FVC by definition remains < 0.70 due to persistent airflow limitation. Therefore, FEV1 response to bronchodilator should not be solely relied upon to distinguish between asthma and COPD (Table 4).
While diffusion capacity and lung volumes obtained using plethysmography can aid in patient characterization, these results are not required for making the diagnosis of COPD.
Differentiating COPD, Asthma, and Overlap Syndrome
Recommendation
Use the clinical history and spirometry to differentiate COPD, asthma, and overlap syndrome.
Distinguishing between COPD and asthma is important because they differ in first-line therapies and chronic management. A set of clinical factors to help guide clinicians in distinguishing between asthma and COPD can be found in Table 4.
Fixed airflow obstruction must be demonstrated to make a diagnosis of COPD. Many patients with a diagnosis of COPD have not undergone confirmatory testing, suggesting that both under-diagnosis and misdiagnosis are common.2 Unfortunately, no single test can reliably distinguish asthma from COPD.
Some patients have features of both asthma and COPD. The Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) recognize Asthma COPD Overlap (ACO) or Asthma COPD Overlap Syndrome (ACOS) as characterized by “persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.”1
Imaging
Recommendation
Do not image to diagnose early or moderate COPD.
Generally, COPD can be documented by spirometry before it is seen on chest radiograph. A chest radiograph may suggest a diagnosis of COPD, particularly if it demonstrates hyperinflation, but it should not be considered diagnostic of the disease.
Current evidence is not sufficient to recommend routine chest imaging with computed tomography (CT) in early or moderate COPD. In patients with severe disease, high resolution CT is required to evaluate the appropriateness of therapies such as lung volume reduction surgery or transplant. The utility of chest CT in patients with COPD is an area of active investigation.2 However, it should be noted that many individuals with COPD also meet criteria for lung cancer screening with CT, due to their smoking history.18
Alpha-1 Antitrypsin
Recommendation
Perform alpha-1 antitrypsin testing in patients with early onset COPD or a family history of early onset COPD. Consider alpha-1 antitrypsin testing in the presence of prominent basilar lucency, unexplained liver disease, or the absence of other risk factors for COPD.
While alpha-1 antitrypsin deficiency has some association with COPD,28 testing is controversial. Some of the controversy derives from quality of data regarding efficacy of treatment with alpha-1 antitrypsin augmentation therapy.
In the absence of clear empirical data, we recommend following the GOLD Guidelines to test alpha-1 antitrypsin in persons with COPD who:2
- are of Caucasian descent under 45 years of age or
- have a strong family history of COPD.
Consider broader testing based on clinician judgment. The American Thoracic Society and European Respiratory Society joint statement recommends testing for:29
- symptomatic adults with emphysema, COPD, or asthma with airflow obstruction that is incompletely reversible after aggressive treatment with bronchodilators
- adolescents with persistent airflow obstruction
- asymptomatic individuals with persistent airflow obstruction and no risk factors.
Assess COPD Severity
Recommendation
Assess the extent of airflow limitation (using spirometry), symptom severity, and exacerbation risk (Table 5) to monitor patient status and guide therapy.
Separately assessing airflow limitation, symptom severity, and exacerbation risk helps individualize planning for each patient.2 While these dimensions of COPD severity are somewhat interrelated, individuals vary appreciably on their status across these dimensions and on progression along each of these dimensions. All of these dimensions should be considered when assessing disease severity in an individual patient.
The upper part of Table 5 shows levels of airflow limitation (mild to very severe) by spirometry.
The middle part of Table 5 shows two approaches to assessing symptom severity. Patients use the Modified Medical Research Council (mMRC) Dyspnea Scale to rate their shortness of breath.3,30 Patients use the COPD Assessment Test (CAT) to rate their health status impairment in 8 areas likely to be affected by COPD, and then the score is totaled.4,31 Either method of assessing symptom severity can be used, although the mMRC Dyspnea Scale has a narrower focus. The choice can be made based on local preference and likely relevance to a specific patient.
The lower part of Table 5 addresses exacerbation risk and other aspects of severity. Having had ≥ 2 exacerbations in the past year or ≥ 1 exacerbation resulting in hospitalization is associated with increased risk for future exacerbations.17 Other aspects of severity include oxygen therapy and comorbid conditions.
Recognition of Comorbid Diseases
Comorbid Disease
Recommendation
Recognize and diagnose comorbid diseases affecting the management and health of patients with COPD.
Patients with COPD are frequently at increased risk for:
- cardiovascular disease, heart failure, hypertension
- diabetes
- osteoporosis
- cancer
- psychiatric disorders including anxiety and depression32
Cigarette smoking, an established risk factor for COPD, also places patients at risk for other diseases due to its systemic effects.
COPD itself is an independent risk factor for cardiovascular disease, even after controlling for smoking.33 This risk may be related to systemic inflammation.
Cardiovascular Conditions
Recommendation
Beta-blockers necessary for cardiovascular conditions can be prescribed for most patients with comorbid COPD.
Data from several studies demonstrate that beta-blockers necessary for cardiovascular conditions can be safely prescribed for most patients with COPD, particularly beta-1 cardioselective blockers (eg, atenolol, metoprolol) or combined beta and alpha blockers (eg, carvedilol).34,35
Heart Failure
Recommendation
For inpatients with COPD, a low BNP (< 100) can help rule out heart failure and a high BNP (> 500) can help rule in heart failure.
Clinicians may have difficulty distinguishing between heart failure and COPD in certain clinical settings. Baseline BNP measurements may be elevated in COPD patients compared to those without COPD, but are not as high as measurements in patients with heart failure. In patients with COPD, a low BNP (less than 100) can help rule out significant heart failure, while a very high BNP (greater than 500) can help rule in heart failure.36,37 Values between 100 and 500 must be interpreted with caution, keeping in mind the entire clinical picture. For further guidance regarding the management of heart failure, see the University of Michigan Health System (UMHS) Heart Failure Clinical Guideline.
Diabetes and Osteoporosis
Recommendation
Inhaled corticosteroids have only a small effect on serum glucose in diabetics and on bone mineral density related to osteoporosis.
Inhaled corticosteroid use is associated with only a small (approximately 2 mg/dL) dose-dependent increase in serum glucose concentration in diabetic patients. For further guidance regarding diabetes management, see the UMHS Diabetes Clinical Guideline.
While inhaled corticosteroids may carry a theoretical risk for decreasing bone mineral density (BMD), several studies following BMD over 3 years indicate the risk is minimal.38 For further guidance regarding osteoporosis management, see the UMHS Osteoporosis Clinical Guideline.
Psychiatric Disorders
Recommendation
Identify psychiatric disorders in COPD patients and treat with cognitive behavioral therapy, mind-body interventions, and non-sedating therapies when possible.
Depression and anxiety are highly prevalent in COPD patients, and both predict a worse quality of life.39 Cognitive behavioral therapy may improve psychological outcomes, and mind-body interventions (such as mindfulness, yoga, and relaxation) may decrease physical suffering.40 Due to the risk of respiratory suppression, non-selective benzodiazepine anxiolytic sedation should be avoided when possible.41 For further guidance regarding depression management, see the UMHS Depression Clinical Guideline.
Management
Overview of COPD Management
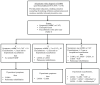
Managing COPD includes patient education, preventive care, medications, pulmonary rehabilitation, and oxygen therapy. Using symptoms and exacerbations as a guide, Figure 1 provides an approach to incorporating all of these management activities except oxygen therapy, which is addressed separately below. The rationale for each recommendation is explained in the relevant sections below.
Patient Education
Recommendation
Provide patient-focused education to support disease self-management including: smoking cessation, avoidance of exacerbations, appropriate inhaler technique, healthy behaviors, and end-of-life planning (Table 6).
Table 6
Patient Education Overview.
While evidence for clinical benefit due to self-management support is lacking,42 patients should understand their disease, risk factors for progression, and their roles in optimizing their health and wellness. Education should include identification and reduction of exposures to inhalant irritants and appropriate use of inhalers.2 Patients should be informed that smoking cessation has the greatest capacity to influence the natural history of COPD. Prospective end-of-life discussions help patients understand advance directives and therapies near the end of life.
Patients enrolled in pulmonary rehabilitation at UMHS receive instruction in self-management skills that enhance self-efficacy and support chronic disease management.
Preventive Care
Preventive care focuses on avoiding irritants that can aggravate COPD. The most common “triggers” are smoking, second-hand smoke, occupational fumes and chemicals, indoor air pollution (eg, cooking with biomass fuels), outdoor air pollution, and infections.
Smoking cessation and second-hand smoke
Recommendation
Advise smokers to quit and assist them in quitting. Assess for exposure to second-hand smoke and counsel its avoidance.
Smoking cessation is the single most important intervention to slow the rate of lung decline and reduce respiratory symptoms, regardless of the severity of the patient’s disease. The beneficial impact of smoking cessation on the natural history of COPD is greater the earlier in the disease that cessation is achieved.22
Smoking cessation should be encouraged at each visit. The combination of pharmacologic and psychosocial treatment for smoking cessation has been shown to be superior to psychosocial treatment alone in patients with COPD.43 Smoking cessation counseling is a billable diagnosis for patients with COPD.44 For further guidance on smoking cessation, see the UMHS Tobacco Treatment Guideline.
Second-hand smoke exposure appears to have an adverse impact on health outcomes in COPD, independent of personal smoking.45 It is a modifiable risk factor.
Occupational fumes and air pollution
Recommendation
Advise patients to identify and avoid occupational fumes and air pollution.
Occupational pulmonary irritant exposure causes 12–17% of COPD cases.46 Therefore, limiting exposure to industrial fumes and dust is recommended. Limiting exposure can help slow the progression of disease and improve symptoms. Occupational exposures include organic and inorganic dusts, chemical agents, and fumes.
Patients with COPD should be counseled to avoid exposure to high air pollution to the extent that avoidance is reasonable. Emergency room visits have been shown to increase among patients with COPD following days of high air pollution. Long-term exposure to air pollution may be associated with increased risk for both COPD hospitalizations and COPD mortality.47
Patients should be counseled to avoid indoor air pollution as well. Use of biomass fuels (eg, wood, crop materials, coal, animal dung) for indoor cooking is a significant risk factor for COPD, especially in developing countries.48
Vaccination
Recommendation
Provide annual influenza vaccination to all COPD patients. Provide 23-valent pneumococcal vaccine at the time of COPD diagnosis. At age 65, provide 13-valent pneumococcal vaccine (at least 1 year after 23-valent vaccination.) Provide 23-valent booster pneumococcal vaccination at least 1 year after 13-valent vaccination and at least 5 years after the initial 23-valent vaccination.
Patients with COPD are at increased risk for complications from pulmonary infections (eg, hospitalization, increased use of antibiotics). Therefore, the CDC Advisory Committee on Immunization Practices recommends all patients receive pneumococcal and influenza vaccines beginning at the time of diagnosis.49
Injectable polyvalent pneumococcal vaccination reduces risk for community acquired pneumonia and disease exacerbation in patients with COPD.50
Inactivated influenza vaccination significantly reduces exacerbations and influenza-related respiratory infections.51 Influenza vaccine reduces serious illness and death from influenza in COPD patients by approximately 50%.52
Medications for Chronic Care
Figure 1 presents an overview of medical management for COPD based on symptom severity and exacerbation risk. It focuses on commonly used medications: short- and long-acting bronchodilators and inhaled corticosteroids. Specific recommendations regarding their use and the use of additional medications are presented below.
Benefits of medication
Recommendation
Medical therapy improves symptoms and functional status. However, no existing medications for COPD have been shown to modify long-term decline in lung function. Oxygen is the only treatment proven to impact mortality.
Medications commonly used in COPD include bronchodilators (both short- and long-acting beta-2 agonists and anticholinergics) and anti-inflammatory agents (inhaled corticosteroid). Detailed dosing and cost information by drug is presented in Table 7.
Beta-2 agonists and anticholinergics
Recommendation
Treatment with bronchodilators provides clinical benefit despite limited change in spirometric measures.
Both beta-2 agonists and anticholinergics are bronchodilators. They are indicated in the treatment of any COPD patient who is symptomatic. Current evidence suggests that the long-acting muscarinic antagonist (LAMA) anticholinergics should be considered first-line agents for baseline bronchodilator control, particularly in patients with severe airflow obstruction.53,54
Dual bronchodilator therapy should be considered for patients who have persistent symptoms despite use of a single bronchodilator, and for patients with frequent exacerbations.2 Combining different types of bronchodilators may increase the degree of bronchodilation with equivalent or fewer side effects.55 Several combinations of long-acting beta agonist with long-acting muscarinic antagonist (LABA/LAMA) bronchodilators are now available. Evidence suggests lung function and quality of life improvements are greater for combination than for single agent bronchodilators.2,56 Dual LABA/LAMA bronchodilators have also been demonstrated to reduce exacerbation frequency to a greater extent than inhaled corticosteroid/long-acting beta agonist (ICS/LABA) combination therapy.
To treat asthma-COPD overlap patients, avoid monotherapy with long-acting beta agonists and consider earlier introduction of inhaled corticosteroid therapy in conjunction with a LABA and/or a LAMA.
Data from several studies demonstrate that beta-blockers necessary for cardiovascular conditions can be safely prescribed for most patients with COPD, particularly beta-1 cardioselective blockers (eg, atenolol, metoprolol) or combined beta and alpha blockers (eg, carvedilol).34,35
Both long-acting beta-2 agonists and anticholinergics have safety concerns.
- Long-acting beta-2 agonists (LABA). An FDA advisory panel recommended that LABAs not be used as single-agent therapy in asthma (see UMHS Asthma Guideline). However, for patients with COPD, LABAs may still be used as single-agent therapy without an inhaled corticosteroid. While LABAs may increase blood pressure and heart rate, data for COPD patients from the TORCH study57 (a three-year trial in COPD patients of fluticasone proprionate and salmeterol combination versus fluticasone alone, salmeterol alone, or placebo), found no increased risk of all-cause death or cardiovascular death in the salmeterol group. These data further underscore the importance of distinguishing asthma from COPD.
- Anticholinergics. Anticholinergic drugs may worsen symptoms and signs associated with narrow-angle glaucoma, prostatic hyperplasia, or bladder-neck obstruction and should be used with caution in patients with any of these conditions. Concerns about cardiovascular effects have diminished. Initially a meta-analysis suggested that inhaled anticholinergics (ipratropium and tiotropium) were associated with significantly increased risk of cardiovascular death, MI, or stroke among patients with COPD.58 However, since then, data from the UPLIFT study (a four-year, placebo controlled trial of tiotropium) found no significant increase in myocardial infarction or stroke in the tiotropium treated group.59
Inhaled corticosteroids
Recommendation
Add an inhaled corticosteroid (ICS) to bronchodilator therapy in patients with features of asthma overlap. Consider adding ICS in patients with an eosinophil count ≥ 300 cells/μL or with frequent (at least annual) severe COPD exacerbations despite maximal bronchodilation.
Inhaled corticosteroids (ICS) should not be used as monotherapy in COPD. However, ICS can provide additive benefit to bronchodilators in reducing the frequency of exacerbations and improving health status.60 Withdrawal from treatment with ICS can lead to a short term increase in exacerbations in some patients.2
Elevated blood eosinophil counts have been shown to predict the efficacy of adding ICS to maintenance bronchodilator therapy in preventing future exacerbations.100–105 Patients with a blood eosinophil count ≥ 300 cells/μL have the highest likelihood of treatment benefit with ICS. Patients with an eosinophil count ≥ 100 cells/μL and who have a history of ≥ 2 moderate exacerbations (or, at least one exacerbation requiring hospitalization) in the past year demonstrated a favorable response to ICS. Patients with an eosinophil count < 100 cells/μL are less likely to receive benefit from ICS.2
An increase in the frequency of pneumonia has been reported in COPD patients using ICS, particularly in patients age 65 and older.2,61,62 The frequency of reported pneumonia appears to be approximately double in several studies comparing ICS/LABA combinations versus placebo in COPD. However, in the largest published mortality study in COPD, no increase in pulmonary related deaths was noted in the ICS/LABA combination therapy group as compared to placebo.63 In patients with COPD being treated with ICS, particularly those age 65 and older, consider the possible increased risk of pneumonia and maintain a lower threshold for considering a diagnosis of pneumonia when patients present with increased symptoms.
ICS may also increase a patient’s risk for cataracts or glaucoma.2 Consider regular eye exams for patients using these medications. Patients using ICS should also be warned about the possibility of oral candidiasis and vocal changes. Rinsing the mouth after administration of ICS should be encouraged.
Decrease in bone density is a theoretical risk of this class of medication, but available long-term data suggest there is no meaningful association between ICS use and decreased bone mineral density in this patient population.38
Phosphodiesterase-4 inhibitors (roflumilast)
Recommendation
Consider roflumilast to reduce the frequency of acute exacerbations in patients with chronic bronchitis, recognizing that side effects may limit adherence.
Evidence is developing regarding both the role of phosphodiesterase-4 inhibitors in stable COPD management and which patients are most likely to benefit, including patients with a history of chronic bronchitis and frequent or severe exacerbations.2 Roflumilast should be considered in conjunction with consultation with a pulmonologist. Discuss side effects including GI intolerance prior to prescribing.
Antibiotics
Recommendation
Do not routinely use prophylactic antibiotics, but consider referral to a COPD specialist for chronic macrolide therapy in selected patients.
Chronic macrolide therapy with oral azithromycin has been shown to decrease the frequency of COPD exacerbations.64 A follow-up systematic review also suggests an improvement in acute exacerbations and decreased hospitalization with chronic macrolide therapy.65 In patients with persistent exacerbations despite maximal inhaled therapies, consider referral to a specialist in COPD management (eg, pulmonologist) for consideration of additional therapies including macrolide antibiotics.
Leukotriene modifiers
Recommendation
Consider using leukotriene modifiers in patients with asthma/COPD overlap syndrome.
Leukotriene modifiers have not been adequately tested in COPD and cannot be recommended solely for COPD at this time.
Oral corticosteroids
Recommendation
Do not routinely use oral corticosteroids for treatment of chronic COPD. However, they can be used for acute exacerbations of COPD, as discussed below.
Theophylline
Recommendation
Do not routinely use theophylline for control of chronic COPD symptoms.
Theophylline is effective for symptom control in COPD. However, due to its narrow therapeutic window and side effect profile, inhaled bronchodilators are preferred.
Pulmonary Rehabilitation
Recommendation
Consider pulmonary rehabilitation for any patient with COPD who experiences significant dyspnea or exercise limitation, regardless of severity of airflow limitation.
The American Thoracic Society and European Respiratory Society66 define pulmonary rehabilitation as “a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors.”
Pulmonary rehabilitation improves exercise capacity, dyspnea, and quality of life.67 Pulmonary rehabilitation after a recent hospitalization for COPD has been shown to decrease hospital readmissions, decrease mortality, and improve both exercise capacity and health related quality of life.68
Medicare covers pulmonary rehabilitation for patients with COPD who have moderate or greater disease severity, based on FEV1 % predicted. (Table 5.) Medicare patients who continue to smoke must also be enrolled in a smoking cessation program.
Oxygen Therapy
Continuous oxygen
Recommendation
Provide continuous oxygen therapy for COPD patients if resting oxygen saturation is ≤ 88% and confirmed twice over a 3-week period.
The primary goal of oxygen therapy is to maintain vital organ function by ensuring adequate oxygen delivery. This is achieved by increasing the baseline PaO2 to at least 60 mm Hg (or resting oxygen saturation to at least 90%).67,69
In patients with very severe COPD, long-term oxygen therapy has been shown to improve the following outcomes:70–73
- mortality
- quality of life
- cardiovascular morbidity (ie, pulmonary hypertension)
- depression
- cognitive function
- exercise capacity
- frequency of hospitalization
Indications for continuous oxygen use are summarized in Table 8. Once oxygen therapy is initiated, if the clinical picture changes, oxygen requirement may need to be reevaluated. Medicare requires annual recertification of a patient’s continuing need for oxygen therapy.
Table 8
Indications for Continuous Use of Oxygen Therapy for Very Severe COPD .
Currently the Center for Medicare and Medicaid Services covers oxygen use both for patients who meet the evidence-based criteria in Table 8, as well as for individuals with PaO2 ≤ 55 mm Hg (resting oxygen saturation ≤ 88%) either with activity or at night. For these patients with moderate hypoxemia but significant breathlessness despite maximizing other medical therapies, a trial of oxygen therapy may be appropriate.
Exercise and nocturnal oxygen therapy
Recommendation
Oxygen therapy during exercise or during sleep may benefit selected patients.
The Long-term Oxygen Treatment Trial74 randomized COPD subjects who had a moderate exercise-induced desaturation during the six-minute walk test (SpO2 ≥ 80% for ≥ 5 minutes and < 90% for ≥ 10 seconds) or a moderate resting desaturation (pulse oximetry 89–93%) to supplemental oxygen versus no supplemental oxygen. This study found no difference in time to death or first hospitalization, nor in the rates of all hospitalizations or COPD exacerbations. The study also found no consistent between-group differences in measures of quality of life, lung function, or the distance walked in 6 minutes. Patients in the supplemental-oxygen group who had a COPD exacerbation 1–2 months before enrollment, those greater than 71 years at enrollment, and those with lower quality of life at enrollment did experience longer time to death or first hospitalization, although these effects did not remain significant when adjusted for multiple comparisons. However, a lack of evidence for benefit should not be confused with a lack of clinical effectiveness in some patients. A trial of oxygen therapy may still be warranted in selected patients who have moderate exertional hypoxemia and intractable breathlessness despite appropriate evidence-based treatment.
Follow Up Chronic Care
Frequency
Recommendation
Initial chronic care visits should occur at least every 6 months. Visits may be once a year for currently nonsmoking patients with mild disease who are stable on treatment and only rarely have exacerbations.
No consensus exists on the recommended frequency of office visits for chronic COPD care. Frequency of follow-up may be guided by:
- Worsening symptoms not associated with an exacerbation
- Frequency of acute exacerbations
- Smoking status
- Adherence to treatment plan
- Social support systems
- Presence of other comorbid chronic diseases
Factors to Reassess
Recommendation
At follow-up visits, reassess:
- Risk of exposure to pulmonary irritants
- Symptoms: severity, control, new, stable or worsening (eg, sputum production, dyspnea, cough, activities of daily living)
- History of exacerbations and possible causes
- Smoking cessation, if applicable
- Current medications, dosages, adherence, and proper use
- Inhaler technique
- Vaccinations
Evidence regarding comprehensive self-management is limited. One study that compared self-management to routine monitoring did not demonstrate long-term benefits in terms of quality of life or self-efficacy over usual care alone in COPD patients in general practice.75
Spirometry
Recommendation
Monitor symptoms and functional status to guide spirometry use in disease monitoring.
No clear consensus exists on the appropriate frequency of spirometry to guide therapy after the initial diagnosis of COPD. Systematic reviews found insufficient evidence for using spirometry to guide therapy. When patients report symptomatic changes, follow-up spirometry may be warranted to detect clinically significant changes of lung function that may alter clinical therapeutic options.
Medication Management
Recommendation
Adjust medical therapy based on symptom severity as summarized in Figure 1 and as described above under “Medications for Chronic Care.”
If patients remain symptomatic at subsequent follow-up visits despite adherence to medications, intensifying pharmacotherapy should be considered.
Oxygen Therapy Management
Recommendation
Reassess patients who are on oxygen for resting hypoxemia (resting oxygen saturation ≤ 88%).
For patients on long-term oxygen therapy, an annual reevaluation of oxygen saturation is recommended to determine if oxygen is therapeutic and still clinically indicated.
All patients prescribed oxygen must be recertified every 12 months in order to qualify for Medicare and Medicaid coverage. Recertification includes an in-person encounter with the treating physician, a documented exam, and a resting oxygen saturation of < 88%, with improvement of the hypoxemia on oxygen.76
Functional Assessment and Rehabilitation
Recommendation
If not already initiated, consider pulmonary rehabilitation for all patients with significant dyspnea or exercise limitation.
Nutrition
Recommendation
For patients with COPD and malnourishment, consider a referral for nutritional counseling and supplementation.
Insurance coverage for dietician referral for this purpose varies.
Reassessment after Severe Acute Exacerbation
Recommendation
Reassess patients after hospital discharge for an acute COPD exacerbation.
Assessment should include:
- Spirometry about 3 months after the patient’s return to stability. Significant declines in baseline lung function can occur with exacerbations.
- Ability to cope in their home environment
- Inhaler technique and understanding of treatment regimen
- Need for oxygen
- Medical regimen optimization for prevention of future exacerbations
Acute Exacerbation in the Outpatient Setting
This guideline focuses on management of an exacerbation treated in the outpatient setting. For information regarding exacerbation in the inpatient setting, see Care of the Hospitalized Patient with Acute Exacerbation of COPD.
Diagnosis
Recommendation
Diagnose an acute exacerbation of COPD based on clinical findings. Consider causes of COPD exacerbation and alternate diagnoses.
No single definition of acute COPD exacerbation is universally accepted. However, it can reasonably be described as an acute change in a patient’s baseline dyspnea, cough, or sputum that is beyond the normal day-to-day variability and is sufficient to warrant a change in medication.
Causes of acute COPD exacerbation include:2,77
- Infections (both viral and bacterial)
- Environmental conditions
- Air pollution
- Lack of compliance with long-term oxygen therapy
- Unknown
Alternate diagnoses need to be considered, including:
- Pneumonia
- Congestive heart failure
- Pneumothorax
- Pleural effusion
- Pulmonary embolism
- Cardiac arrhythmia
Assessment
Recommendation
Assess the patient’s clinical history, physical exam, and oxygen saturation.
Do not routinely perform a chest radiograph or a sputum gram stain and culture.
Outpatient assessment for an acute exacerbation of COPD starts with the clinical history and physical examination. Obtaining an oxygen saturation via pulse oximetry is recommended.
Ordering a chest radiograph is generally not recommended. However, it may be reasonable for patients who are age 65 or older, use inhaled steroids, or have fever.
The utility of sputum gram stain and culture in the outpatient setting is limited given the length of time before results are available as well as the low reliability of the results.2 Patients who have recently been on antibiotics or who are not responding to therapy may be exceptions.
Spirometry, arterial blood gas, and electrocardiogram are generally not recommended in the outpatient assessment of a patient with an acute COPD exacerbation.
Pharmacotherapy
Recommendation
Outpatient management of acute COPD exacerbation involves treatment with bronchodilators, systemic corticosteroids, and antibiotics.
Specific drugs and dosing are described in Table 9. Their use is summarized below.
Bronchodilators
Recommendation
Use bronchodilators as first-line therapy.
Bronchodilators improve respiratory symptoms and FEV1 during acute COPD exacerbations. The dose and/or frequency of short-acting beta-2 agonists, either via inhaler or nebulizer, should be increased. The inhaled anticholinergic ipratropium, which is a short-acting muscarinic antagonist (SAMA), can be added if not already used, although the effectiveness of combination therapy in the setting of acute exacerbation is questionable.78 The clinical response to medication administration via MDI with spacer is similar to that for inhalation via nebulizer for persons who can adequately demonstrate bronchodilator technique (even while dyspneic).79
Systemic corticosteroids
Recommendation
Provide oral corticosteroids to reduce recovery time, improve lung function (FEV1), and improve hypoxemia (PaO2).
Prednisone 40 mg orally daily is recommended for not more than 5–7 days,2,80 particularly in patients with an FEV1 < 50% predicted.
Antibiotics
Recommendation
Treat selected patients with empiric antibiotic therapy.
The benefit of antibiotics for patients with severe COPD exacerbations requiring mechanical ventilation is well established. The role of antibiotics for the treatment of moderate exacerbations in the outpatient setting is more controversial. A few studies have shown that antibiotics reduce treatment failure rate and may increase the time to the next exacerbation.2,81 Some studies suggest that patients who present with purulent sputum may benefit most from antibiotic treatment. COPD guideline documents vary in their recommendations.
For treatment of outpatients with a COPD exacerbation, we recommend using antibiotics in patients with increased sputum purulence and an increase in at least one of the following:
- Dyspnea
- Sputum volume
Antibiotic choice should be selected for presumptive therapy based on local resistance patterns. Sputum cultures are generally not recommended unless the patient has recently been taking antibiotics. For patients who meet the above criteria for antibiotics, the selection of a specific antibiotic depends on risk factors for poor outcomes with narrower spectrum antibiotics (eg, age ≥ 65, FEV1 < 50% predicted, ≥ 2 exacerbations/year, presence of comorbid diseases) and unusual circumstances (Table 9).
Indications for hospitalization
Recommendation
Initiate an emergency department evaluation or hospital admission based on clinical judgment of COPD exacerbation severity, lack of response to treatment, history, and contextual factors.
Consider emergency department evaluation or hospital admission based on clinical judgment:
- Marked increase in symptoms such as dyspnea at rest
- Severe underlying COPD
- Frequent exacerbations
- Significant comorbidities (eg, older age, pneumonia, congestive heart failure, diabetes mellitus, renal or liver failure)
- Worsening hypoxemia or hypercapnia
- Changes in mental status
- Inadequate response to outpatient therapy
- Uncertain diagnosis
- Insufficient home support
Noninvasive and Surgical Therapy for Severe Disease
Noninvasive Ventilation
Recommendation
Refer patients with a history of hospitalization for respiratory failure and with severe chronic hypercapnia to a pulmonologist for consideration of noninvasive ventilation.
Long-term noninvasive positive pressure ventilation, delivered nasally or by mask, may decrease mortality and reduce risk for re-hospitalization in selected patients.2,82
Lung Volume Reduction
Recommendation
Refer patients with very severe COPD who are willing to consider invasive therapy to a pulmonologist for consideration of surgical bullectomy, bronchoscopic lung volume reduction procedures, or lung volume reduction surgery.
Bullectomy may be considered for patients with localized giant bullae that are associated with compression of adjacent lung tissue.2 One-way valve endobronchial lung volume reduction has been shown to improve walk distance, but with increased risk of complications.83,84 Bronchoscopic placement of multiple lung volume reduction coils in affected lobes can improve dyspnea and 6 minute walk distance,85 but may increase major complications.86
Lung volume reduction surgery may be considered after consultation with a pulmonary specialist for patients with bilateral upper lobe disease and reduced exercise capacity (as measured by formal cardiopulmonary exercise testing) despite maximal medical therapy and pulmonary rehabilitation.87 Data demonstrate improvements in exercise capacity, dyspnea, and quality of life; survival outcomes are favorable for those with upper lobe emphysema and reduced exercise capacity.87 Cost-effectiveness of this approach is not demonstrated for even the most favorable subgroup (ie, COPD patients with upper lobe emphysema and reduced exercise capacity) unless outcomes are expected to remain favorable for 10 years.88 Therefore, life expectancy should be incorporated into shared decision making regarding the potential benefits of surgery.
Lung Transplantation
Recommendation
Consider lung transplantation evaluation for patients with severe COPD without comorbid conditions that would otherwise limit their lifespans.
Lung transplantation may be considered for patients who have BODE scores from 7–10 (Table 10) without comorbid conditions that would otherwise limit their expected lifespans. Consideration of transplantation potential requires co-management with a pulmonary specialist for detailed assessment of baseline pulmonary physiology and potential contraindications.89
Table 10
BODE (Body-mass index, Obstruction, Dyspnea and Exercise) Score.
Complementary and Alternative Medicine
Recommendation
Yoga, tai chi, qigong, and nutritional supplements have not been demonstrated to be of greater symptomatic benefit than a standard healthy diet, walking, and breathing exercises for persons with COPD.
While complementary and alternative medical therapies have been proposed for the treatment of COPD, little evidence of significant specific clinical benefit exists. For example, yoga, tai chi, and qigong have been investigated as interventions to reduce dyspnea and improve quality of life, but benefits have not been demonstrated to be greater than those of standard nonpharmacologic interventions such as walking or breathing exercise and education.90–92 While usual advice regarding a healthy diet is generally endorsed, specific nutrient supplements have no proven benefit. Pulmonary function testing demonstrated statistically significant worsening immediately following osteopathic manipulative therapy, as measured by FEV1 and residual volume,93 and a systematic review of osteopathic manipulative therapy for COPD revealed no evidence of benefit from a small number of low quality studies.94
Palliative Care
Recommendation
Engage patients in shared decision making regarding goals of therapy, including end-of-life planning and advance directives.
Severe COPD increases risk of respiratory failure and is a leading cause of death. Given the progressive nature of the disease, a palliative focus for care should be discussed with patients desiring less aggressive therapy, avoidance of endotracheal intubation, or comfort care measures (symptomatic care) at the end of life. The discussion may limit unnecessary and burdensome personal and societal costs and invasive approaches.95,96 Therapies with proven effectiveness for management of dyspnea at the end of life include opioids and oxygen.
Referral to COPD Subspecialist
Recommendation
Consider referring COPD patients with severe asthma or other complicating conditions to a subspecialist for co-management and consideration of invasive treatment options.
As COPD worsens, care considerations and decisions become increasingly complex. Consider referring patients with:
- Severe disease (FEV1 ≤ 50% predicted or BODE score ≥ 5)
- Severe or frequent exacerbations
- Dependence on supplemental oxygen
Also consider referral for the following conditions that may require complex management:
- Alpha-1 antitrypsin deficiency
- Concurrent cardiac disease, suspected asthma, or another pulmonary disease that complicates diagnosis or management
- Suspected upper airway obstruction (eg, upper airway wheezing or stridor) – consider referral to otolaryngology or pulmonary medicine
- Symptoms that are not responsive to optimal therapy or are out of proportion to obstructive findings
- Symptoms that are present in the absence of formal spirometric obstruction criteria
- Frequent (at least twice per year) exacerbations or pneumonia complicate management
- Lung volume reduction surgery or lung transplantation is considered (eg, BODE score 7–10, giant bullae, severe disease). Early referral for monitoring and preparation is recommended if FEV1 < 50% and the patient is likely to be a future candidate.89
- Intensive care pulmonary hospitalization or mechanical ventilation is required
Guideline Development Methodology
Funding
The development of this guideline was funded by UMHS.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- Primary care physicians: Davoren A. Chick, MD, General Medicine, Paul J. Grant, MD, General Internal Medicine, and Amal E. Othman, MD, Family Medicine.
- Specialists in COPD care: MeiLan K. Han, MD, Pulmonary Medicine, Sarah E. Roark, MD, Pulmonary Medicine.
- Guideline development methodologist: R. Van Harrison, PhD, Learning Health Sciences.
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
UMHS endorses the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Contributions of team members with relevant financial relationships are reviewed by team members without relevant financial relationships to assure the information is presented without bias.
Individuals with no relevant personal financial relationships: Davoren A. Chick, MD, Paul J. Grant, MD, R. Van Harrison, PhD, Amal E. Othman, MD, Tami L Remington, PharmD, Sarah E. Roark, MD, and Noah Leja, PharmD.
Individuals with relevant personal financial relationships:
MeiLan K. Han, MD
| Consultant | Novartis, Nycomed |
| Speaker’s bureau | Boehringer Ingelheim, GlaxoSmithKline, CLS Boehring |
| Advisory board | CLS Boehring |
Acknowledgements
The following individuals are acknowledged for their contributions to previous versions of this guideline.
2017: Davoren A Chick, MD, Paul J Grant, MD, MeiLan K Han, MD, MS, R Van Harrison, PhD, Amal Othman, MD, Tami L Remington, PharmD, Sarah E Roark, MD.
2010: Davoren A Chick, MD, Paul J Grant, MD, MeiLan K Han, MD, MS, R Van Harrison, PhD, Elisa B Picken, MD.
Systematic Review of Literature
A detailed description of the systematic search and review of literature upon which this guideline is based is presented in the associated UMHS document “Guideline for Chronic Obstructive Pulmonary Disease, 2017: Literature Review Methods and Results.” The following section highlights major aspects of the literature search and review process.
Literature search. The team began the search of literature by accepting the results of a systematic literature review performed in 2014:
- VA/DOD Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease. The Management of Chronic Obstructive Pulmonary Disease Working Group, Department of Veterans Affairs and Department of Defense. Dec. 2014. (Searched literature from January 2005 through February 2014.)
To update those results, we performed a systematic search of literature on Medline and in the Cochrane Database of Systematic Reviews for the time period 1/1/14 – 9/8/16.
The major search term was chronic obstructive pulmonary disease. The searches were for guidelines, controlled trials (including meta-analyses), and cohort studies, for literature on humans in the English language. Within these parameters individual searches were performed for the following topics:
- Etiology: Smoking, particulate inhalation exposures, alpha-1 antitrypsin deficiency, life expectancy based on FEV1/BODE
- Screening: Questionnaires, pulmonary function testing/spirometry
- Diagnosis: History (risk factors, symptoms), physical exam
- Diagnostic studies: PFTs, alpha-1 antitrypsin level, chest X-ray, 6-minute walk test, chest CT
- Diagnostic classification: GOLD classes, MRC or mMRC dyspnea scale, BODE index
- Definition and diagnosis: Acute exacerbation
- Other diagnoses not included in C–F above
- Comorbid diseases (increased risk)
- Prevention: Smoking cessation, vaccination (influenza, pneumococcus)
- Prevention: Irritant avoidance
- Pharmacologic treatment: Bronchodilators, inhaled corticosteroids
- Treatment: Supplemental oxygen
- Treatment: Pulmonary rehabilitation
- Nutrition
- Treatment: Complementary and alternative medicine
- Treatment: Mental health, psychosocial support
- Treatment: Acute exacerbation – outpatient management, hospitalization
- Referral to pulmonary subspecialist
- Surgical treatment: Lung volume reduction surgery, lung transplantation
- Treatment: Follow up care, monitoring, chronic disease management
- Treatment: Palliative care
- Other “treatments” not in I–U above
- Other not in A–V above
A more formal presentation of the inclusion and exclusion criteria is in Section II of the accompanying Literature Review Methods and Results.
The detailed search strategies are presented in Section III of the accompanying Literature Review Methods and Results.
The search was conducted in components of a formal problem structure (outlined above). The search was supplemented with very recent clinical trials known to expert members of the panel. The search was a single cycle. The number of publications identified is presented in Section IV of the accompanying Literature Review Methods and Results.
Literature review and assessment. Members of the guideline team reviewed the publications identified to be relevant to specific topics in order to select those with best evidence. Criteria to identify overall best evidence included relevance of the study setting and population, study design, sample size, measurement methods (variables, measures, data collection), intervention methods (appropriateness, execution), appropriateness of analyses, and clarity of description.
In considering level of evidence based on study design, the classification was:
- A = systematic reviews of randomized controlled trials with or without meta-analysis
- B = randomized controlled trials
- C = systematic reviews of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control)
- D = individual observation studies (case study or case series)
- E = expert opinion regarding benefits and harm
Beginning with best evidence identified by the VA/DoD systematic literature review, team members checked publications identified in the more recent search (1/1/14 – 9/8/16) to determine whether better evidence was available. Team members also had the option of considering very recent literature (published since 9/8/16) in determining whether even better evidence was available.
The process of review and assessment is described in more detail in Section V of the accompanying Literature Review Methods and Results
Best evidence. The best evidence regarding specific topics was summarized in evidence tables listing articles, study designs, patient populations, main outcome variables, results, and notes regarding methodological issues and harms. The evidence tables are presented in Section VI of the accompanying Literature Review Methods and Results.
Recommendations. The guideline team reviewed the evidence and determined the importance of performing or not performing key aspects of care (listed on the first page of this guideline). In the absence of empirical evidence, the guideline team based recommendations on their expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Review and Endorsement
A draft of this guideline was reviewed in clinical conferences and by distribution for comment within departments and divisions of UMHS to which the content is most relevant: Emergency Medicine, Family Medicine, General Medicine, Geriatric Medicine, Obstetrics & Gynecology (Women’s Health), and Pulmonary & Critical Care Medicine. The draft was revised based on comments from these groups.
The final version of this guideline was endorsed by the Clinical Practice Committee of the University of Michigan Medical Group and by the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
References
- 1.
- 2019 GINA report, global strategy for asthma management and prevention. https://ginasthma
.org /reports/2019-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed March 14, 2017. - 2.
- GOLD 2020 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global initiative for chronic obstructive lung disease (GOLD) 2020.
- 3.
- Fletcher CM. Standardized questionnaire on respiratory symptoms: A statement prepared and approved by the MRC committee on the aetiology of chronic bronchitis (MRC breathlessness score). British Medical Journal. 1960;2:1662.
- 4.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. [PubMed: 19720809]
- 5.
- Yawn BP, Thomashow B, Mannino DM, Han MK, Kalhan R, Rennard S, Cerreta S, Crapo JD, Wise R. The 2017 update to the COPD foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177–185. doi: 10.15326/jcopdf.4.3.2017.0136. 2017;4(3):177–185. [PMC free article: PMC5556909] [PubMed: 28848929] [CrossRef]
- 6.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. [PubMed: 14999112]
- 7.
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. [PubMed: 25359355]
- 8.
- Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. [PubMed: 10847262]
- 9.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. [PMC free article: PMC7172377] [PubMed: 22314182]
- 10.
- Centers for Disease Control and Prevention. Chronic Obstructive Pulmonary Disease (COPD). https://www
.cdc.gov/copd/index.html#4. Accessed September 9, 2017. - 11.
- Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36-2466-11-36. [PMC free article: PMC3128042] [PubMed: 21672193]
- 12.
- Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health. 2015;15:1202. [PMC free article: PMC4667413] [PubMed: 26627181]
- 13.
- Ryu JY, Sunwoo YE, Lee S, et al. Chronic obstructive pulmonary disease (COPD) and vapors, gases, dusts, or fumes (VGDF): A meta-analysis. Copd: Journal of Chronic Obstructive Pulmonary Disease. 2015;12(4):374–380. [PubMed: 25255043]
- 14.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360(26):2749–2757. [PubMed: 19553648]
- 15.
- Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. American Journal of Respiratory & Critical Care Medicine. 2014;189(9):1022–1030. [PubMed: 24552242]
- 16.
- Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14:116-9921-14-116. [PMC free article: PMC3833311] [PubMed: 24168767]
- 17.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. [PubMed: 20843247]
- 18.
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: Evidence report and systematic review for the US preventive services task force. JAMA. 2016;315(13):1378–1393. [PubMed: 27046366]
- 19.
- Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. [PubMed: 19342411]
- 20.
- Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: A study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156(8):738–746. [PubMed: 12370162]
- 21.
- Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural south China. Thorax. 2007;62(10):889–897. [PMC free article: PMC2094241] [PubMed: 17483137]
- 22.
- Dhariwal J, Tennant RC, Hansell DM, et al. Smoking cessation in COPD causes a transient improvement in spirometry and decreases micronodules on high-resolution CT imaging. Chest. 2014;145(5):1006–1015. [PMC free article: PMC4011651] [PubMed: 24522562]
- 23.
- Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: The Step2quit randomised controlled trial. BMJ. 2008;336(7644):598–600. [PMC free article: PMC2267989] [PubMed: 18326503]
- 24.
- Miravitlles M, Worth H, Soler Cataluna JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: Results from the ASSESS study. Respiratory Research. 2014;15:122. [PMC free article: PMC4220061] [PubMed: 25331383]
- 25.
- Holleman DR,Jr, Simel DL. Does the clinical examination predict airflow limitation? JAMA. 1995;273(4):313–319. [PubMed: 7815660]
- 26.
- Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006;28(5):945–952. [PubMed: 16870668]
- 27.
- Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: A prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. [PMC free article: PMC4105297] [PubMed: 24321803]
- 28.
- Rahaghi FF, Sandhaus RA, Brantly ML, et al. The prevalence of alpha-1 antitrypsin deficiency among patients found to have airflow obstruction. COPD. 2012;9(4):352–358. [PubMed: 22506682]
- 29.
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: Standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900. [PubMed: 14522813]
- 30.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434. [PubMed: 12006425]
- 31.
- Karloh M, Fleig Mayer A, Maurici R, Pizzichini MM, Jones PW, Pizzichini E. The COPD assessment test: What do we know so far?: A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149(2):413–425. [PubMed: 26513112]
- 32.
- Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. [PubMed: 18671149]
- 33.
- Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. [PubMed: 15947307]
- 34.
- Etminan M, Jafari S, Carleton B, FitzGerald JM. Beta-blocker use and COPD mortality: A systematic review and meta-analysis. BMC Pulm Med. 2012;12:48-2466-12-48. [PMC free article: PMC3499441] [PubMed: 22947076]
- 35.
- Zeng LH, Hu YX, Liu L, Zhang M, Cui H. Impact of beta2-agonists, beta-blockers, and their combination on cardiac function in elderly male patients with chronic obstructive pulmonary disease. Clin Interv Aging. 2013;8:1157–1165. [PMC free article: PMC3783502] [PubMed: 24072964]
- 36.
- Prosen G, Klemen P, Strnad M, Grmec S. Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit Care. 2011;15(2):R114. [PMC free article: PMC3219397] [PubMed: 21492424]
- 37.
- Zhao SQ, Hu YM, Li Q, et al. The clinical value of rapid assay for plasma B-type natriuretic peptide in differentiating congestive heart failure from pulmonary causes of dyspnoea. Int J Clin Pract. 2008;62(2):214–220. [PubMed: 18081799]
- 38.
- Sarwar G, Bisquera A, Peel R, Hancock S, Grainge C, Attia J. The effect of inhaled corticosteroids on bone mineral density measured by quantitative ultrasonography in an older population. Clin Respir J. 2016. [PubMed: 27805313]
- 39.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: Systematic review and meta-analysis. International Journal of Copd. 2014;9:501–512. [PMC free article: PMC4035108] [PubMed: 24876770]
- 40.
- Farver-Vestergaard I, Jacobsen D, Zachariae R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Psychotherapy & Psychosomatics. 2015;84(1):37–50. [PubMed: 25547641]
- 41.
- Vozoris NT. Do benzodiazepines contribute to respiratory problems? Expert Rev Respir Med. 2014;8(6):661–663. [PubMed: 25193249]
- 42.
- Majothi S, Jolly K, Heneghan NR, et al. Supported self-management for patients with COPD who have recently been discharged from hospital: A systematic review and meta-analysis. International Journal of Copd. 2015;10:853–867. [PMC free article: PMC4425235] [PubMed: 25995625]
- 43.
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. [PMC free article: PMC10042551] [PubMed: 27009521]
- 44.
- Schauer GL, Wheaton AG, Malarcher AM, Croft JB. Health-care provider screening and advice for smoking cessation among smokers with and without COPD: 2009–2010 national adult tobacco survey. Chest. 2016;149(3):676–684. [PubMed: 26291388]
- 45.
- Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4(1):7. [PMC free article: PMC1145187] [PubMed: 15890079]
- 46.
- Bang KM, Syamlal G, Mazurek JM. Prevalence of chronic obstructive pulmonary disease in the U.S. working population: An analysis of data from the 1997–2004 national health interview survey. COPD. 2009;6(5):380–387. [PubMed: 19863367]
- 47.
- Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–727. [PubMed: 23392442]
- 48.
- Romieu I, Riojas-Rodriguez H, Marron-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: Impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180(7):649–656. [PubMed: 19556519]
- 49.
- Kim DK, Bridges CB, Harriman KH, Advisory Committee on Immunization Practices (ACIP), ACIP Adult Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older - United States, 2016. Am J Transplant. 2016;16(6):1930–1932.
- 50.
- Walters JA, Tang JN, Poole P, Wood-Baker R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;1:CD001390. [PMC free article: PMC6422320] [PubMed: 28116747]
- 51.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1)(1):CD002733. [PubMed: 16437444]
- 52.
- Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–1836. [PubMed: 11906772]
- 53.
- Sestini P, Renzoni E, Robinson S, Poole P, Ram FS. Short-acting beta 2 agonists for stable COPD. Cochrane Database Syst Rev. 2000;(3)(3):CD001495. [PubMed: 10908502]
- 54.
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2014;7:009285. [PMC free article: PMC8934583] [PubMed: 25046211]
- 55.
- Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267. [PubMed: 20381630]
- 56.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. [PubMed: 27181606]
- 57.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. [PubMed: 17314337]
- 58.
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA. 2008;300(12):1439–1450. [PubMed: 18812535]
- 59.
- Tashkin D, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease (UPLIFT trial). Rev Port Pneumol. 2009;15(1):137–140. [PubMed: 25965528]
- 60.
- Nannini LJ, Poole P, Milan SJ, Holmes R, Normansell R. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(11):CD003794. doi(11):CD003794. [PMC free article: PMC6485527] [PubMed: 24214176]
- 61.
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991. doi(7):CD002991. [PMC free article: PMC8992433] [PubMed: 22786484]
- 62.
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(12):CD007033. doi(12):CD007033. [PMC free article: PMC6494276] [PubMed: 22161409]
- 63.
- Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. [PubMed: 27203508]
- 64.
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. [PMC free article: PMC3220999] [PubMed: 21864166]
- 65.
- Yao GY, Ma YL, Zhang MQ, Gao ZC. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: A meta-analysis. Respiration. 2013;86(3):254–260. [PubMed: 23817204]
- 66.
- Garvey C, Bayles MP, Hamm LF, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: Review of selected guidelines: An official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(2):75–83. [PubMed: 26906147]
- 67.
- COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(7):1–64. [PMC free article: PMC3384376] [PubMed: 23074435]
- 68.
- Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305. doi(10):CD005305. [PubMed: 21975749]
- 69.
- Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4)(4):CD001744. [PMC free article: PMC6464709] [PubMed: 16235285]
- 70.
- Nocturnal Oxygen Therapy Trial Group. et al. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980;93(3):391–8. [PubMed: 6776858]
- 71.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;317(8222):681–686. [PubMed: 6110912]
- 72.
- Tanni SE, Vale SA, Lopes PS, Guiotoko MM, Godoy I, Godoy I. Influence of the oxygen delivery system on the quality of life of patients with chronic hypoxemia. J Bras Pneumol. 2007;33(2):161–167. [PubMed: 17724535]
- 73.
- Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176(4):343–349. [PubMed: 17446339]
- 74.
- Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627. [PMC free article: PMC5216457] [PubMed: 27783918]
- 75.
- Bischoff EW, Akkermans R, Bourbeau J, van Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: Randomised controlled trial. BMJ. 2012;345:e7642. [PMC free article: PMC3514071] [PubMed: 23190905]
- 76.
- The Medicare Learning Network®. Home oxygen therapy. https://www
.cms.gov/Outreach-and-Education /Medicare-Learning-Network-MLN /MLNProducts /Downloads/Home-Oxygen-Therapy-Text-Only.pdf. Updated October 2016. Accessed May 2017. - 77.
- Sapey E, Stockley RA. COPD exacerbations. 2: Aetiology. Thorax. 2006;61(3):250–258. [PMC free article: PMC2080749] [PubMed: 16517585]
- 78.
- McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(4)(4):CD003900. [PMC free article: PMC8753782] [PubMed: 12519615]
- 79.
- Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. [PubMed: 15654001]
- 80.
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The REDUCE randomized clinical trial. JAMA. 2013;309(21):2223–2231. [PubMed: 23695200]
- 81.
- Wedzicha JA Ers C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):10.1183/13993003.00791-2016. Print 2017 Mar. [PubMed: 28298398] [CrossRef]
- 82.
- Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: A randomized clinical trial. JAMA. 2017;317(21):2177–2186. [PMC free article: PMC5710342] [PubMed: 28528348]
- 83.
- Klooster K, ten Hacken NHT, Hartman JE, Kerstjens HAM, van Rikxoort EM, Slebos D. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373(24):2325–2335. [PubMed: 26650153]
- 84.
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi trial): Study design and rationale. Thorax. 2015;70(3):288–290. [PMC free article: PMC4345993] [PubMed: 24664535]
- 85.
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: A European multicentre trial. Thorax. 2014;69(11):980–986. [PMC free article: PMC4215297] [PubMed: 24891327]
- 86.
- Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: The RENEW randomized clinical trial. JAMA. 2016;315(20):2178–2189. [PubMed: 27179849]
- 87.
- Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the national emphysema treatment trial research group. Ann Thorac Surg. 2006;82(2):431–443. [PubMed: 16888872]
- 88.
- Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest. 2007;131(3):823–832. [PubMed: 17356099]
- 89.
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. [PubMed: 16818116]
- 90.
- Ding M, Zhang W, Li K, Chen X. Effectiveness of t’ai chi and qigong on chronic obstructive pulmonary disease: A systematic review and meta-analysis. Journal of Alternative & Complementary Medicine. 2014;20(2):79–86. [PMC free article: PMC3924809] [PubMed: 23961940]
- 91.
- Wu W, Liu X, Wang L, Wang Z, Hu J, Yan J. Effects of tai chi on exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. International Journal of Copd. 2014;9:1253–1263. [PMC free article: PMC4230171] [PubMed: 25404855]
- 92.
- Ngai Shirley PC, Jones Alice YM, Tam Wilson Wai S. Tai chi for chronic obstructive pulmonary disease (COPD).. 2016(6). [PMC free article: PMC8504989] [PubMed: 27272131]
- 93.
- Noll DR, Johnson JC, Baer RW, Snider EJ. The immediate effect of individual manipulation techniques on pulmonary function measures in persons with chronic obstructive pulmonary disease. Osteopath Med Prim Care. 2009;3:9-4732-3-9. [PMC free article: PMC2765983] [PubMed: 19814829]
- 94.
- Heneghan NR, Adab P, Balanos GM, Jordan RE. Manual therapy for chronic obstructive airways disease: A systematic review of current evidence. Man Ther. 2012;17(6):507–518. [PubMed: 22703901]
- 95.
- Weber C, Stirnemann J, Herrmann FR, Pautex S, Janssens JP. Can early introduction of specialized palliative care limit intensive care, emergency and hospital admissions in patients with severe and very severe COPD? A randomized study. BMC Palliative Care. 2014;13:47. [PMC free article: PMC4448287] [PubMed: 25927907]
- 96.
- Au DH, Udris EM, Engelberg RA, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest. 2012;141(3):726–735. [PMC free article: PMC3415164] [PubMed: 21940765]
- 97.
- Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894–942. [PMC free article: PMC4388124] [PubMed: 25321320]
- 98.
- US Preventive Services Task Force (USPSTF), Siu AL, Bibbins-Domingo K, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(13):1372–1377. [PubMed: 27046365]
- 99.
- Department of Veterans Affairs Department of Defense. VA/DoD clinical practice guideline for the management of chronic obstructive pulmonary disease. 2014.
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Created: May 2010; Last Update: July 2020.
- Clinical Background and Rationale for Recommendations
- Screening for COPD
- Diagnosis of COPD (Table 1)
- Recognition of Comorbid Diseases
- Management
- Follow Up Chronic Care
- Acute Exacerbation in the Outpatient Setting
- Noninvasive and Surgical Therapy for Severe Disease
- Complementary and Alternative Medicine
- Palliative Care
- Referral to COPD Subspecialist
- Related National Guidelines and Performance Measures
- Guideline Development Methodology
- Acknowledgements
- Systematic Review of Literature
- References
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Guideline for the management of chronic obstructive pulmonary disease--2011 update.[S Afr Med J. 2011]Guideline for the management of chronic obstructive pulmonary disease--2011 update.Abdool-Gaffar MS, Ambaram A, Ainslie GM, Bolliger CT, Feldman C, Geffen L, Irusen EM, Joubert J, Lalloo UG, Mabaso TT, et al. S Afr Med J. 2011 Jan; 101(1 Pt 2):63-73.
- Guideline for the management of chronic obstructive pulmonary disease (COPD): 2004 revision.[S Afr Med J. 2004]Guideline for the management of chronic obstructive pulmonary disease (COPD): 2004 revision.Bateman ED, Feldman C, O'Brien J, Plit M, Joubert JR, COPD Guideline Working Group of the South African Thoracic Society. S Afr Med J. 2004 Jul; 94(7 Pt 2):559-75.
- Review Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: A meta-analysis.[Int J Surg. 2020]Review Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: A meta-analysis.Guo J, Chen Y, Zhang W, Tong S, Dong J. Int J Surg. 2020 Jun; 78:28-35. Epub 2020 Apr 15.
- Review Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD).[Cochrane Database Syst Rev. 2013]Review Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD).Herath SC, Poole P. Cochrane Database Syst Rev. 2013 Nov 28; (11):CD009764. Epub 2013 Nov 28.
- Review Management of chronic obstructive pulmonary disease: A review focusing on exacerbations.[Am J Health Syst Pharm. 2020]Review Management of chronic obstructive pulmonary disease: A review focusing on exacerbations.Bollmeier SG, Hartmann AP. Am J Health Syst Pharm. 2020 Feb 7; 77(4):259-268.
- Chronic Obstructive Pulmonary DiseaseChronic Obstructive Pulmonary Disease
- Antifungal AgentsAntifungal AgentsSubstances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or ...<br/>Year introduced: 1967MeSH
Your browsing activity is empty.
Activity recording is turned off.
See more...