Pharmacoeconomic Report: Emicizumab (Hemlibra)
Indication: Bleeding prevention, Hemophilia A
CADTH Common Drug Review
CADTH undertook reanalysis to address limitations in the sponsor’s submission, including adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting ages and weights to better reflect the HAVEN 3 patient population. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per quality-adjusted life-year (QALY) in patients with severe hemophilia A without inhibitors. In the CADTH base-case reanalysis, emicizumab was associated with an incremental cost-effectiveness ratio (ICER) of $5.53 million per QALY gained compared to factor VIII (FVIII) prophylaxis. The model was highly sensitive to the price of emicizumab and its comparators. To achieve an ICER of $50,000 per QALY, the price of emicizumab would need to be reduced by at least 89%. However, a greater price reduction may be required if the prices of FVIII products are lower than those published by the Patented Medicine Prices Review Board (PMPRB).
While the evidence for bleeding efficacy is robust for the use of emicizumab in Canadian patients not receiving treatment with prophylactic FVIII, the evidence directly comparing emicizumab to prophylactic FVIII is limited. In patients with severe hemophilia A without inhibitors, emicizumab demonstrated statistically and clinically significant improvements in bleeding outcomes (i.e., annualized bleeding ratio for treated bleeds, all bleeds, treated joint bleeds, and treated spontaneous bleeds) compared to on-demand FVIII treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis, although current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison).
The Health Canada indication for emicizumab includes patients with hemophilia A regardless of their disease severity, while the sponsor’s submitted reimbursement request includes only severe patients and mild and moderate patients who meet specific eligibility criteria. The modelled population only investigated patients with severe hemophilia A, as per HAVEN 3’s eligibility criteria. Given that the sponsor’s reimbursement request does not align with the modelled population, uncertainty remains regarding the cost-effectiveness of emicizumab in both the reimbursement-requested population and the full Health Canada indication.
Abbreviations
- ABTR
annualized treated bleed rate
- BDD
B-domain deleted
- BIA
budget impact analysis
- FVIII
factor VIII
- ICER
incremental cost-effectiveness ratio
- NIS
non-interventional study
- PMPRB
Patented Medicine Prices Review Board
- QALY
quality-adjusted life-year
- VWF
von Willebrand factor
Executive Summary
The executive summary is comprised of 2 tables (Table 1: Background and Table 2: Economic Evaluation) and a conclusion.
Table 1
Submitted for Review.
Table 2
Summary of Economic Evaluation.
Conclusions
CADTH undertook reanalysis to address limitations in the sponsor’s submission, including adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting ages and weights to better reflect the HAVEN 3 patient population. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per quality-adjusted life-year (QALY) in patients with severe hemophilia A without inhibitors. In the CADTH base-case reanalysis, emicizumab was associated with an incremental cost-effectiveness ratio (ICER) of $5.53 million per QALY gained compared to factor VIII (FVIII) prophylaxis. The model was highly sensitive to the price of emicizumab and its comparators. To achieve an ICER of $50,000 per QALY, the price of emicizumab would need to be reduced by at least 89%. However, a greater price reduction may be required if the prices of FVIII products are lower than those published by the Patented Medicine Prices Review Board (PMPRB).
While the evidence for bleeding efficacy is robust for the use of emicizumab in Canadian patients not receiving treatment with prophylactic FVIII, the evidence directly comparing emicizumab to prophylactic FVIII is limited. In patients with severe hemophilia A without inhibitors, emicizumab demonstrated statistically and clinically significant improvements in bleeding outcomes (i.e., annualized bleeding ratio for treated bleeds, all bleeds, treated joint bleeds, and treated spontaneous bleeds) compared to on-demand FVIII treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis, although current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison).
The Health Canada indication for emicizumab includes patients with hemophilia A regardless of their disease severity, while the sponsor’s submitted reimbursement request includes only severe patients and mild and moderate patients who meet specific eligibility criteria. The modelled population only investigated patients with severe hemophilia A, as per HAVEN 3’s eligibility criteria. Given that the sponsor’s reimbursement request does not align with the modelled population, uncertainty remains regarding the cost-effectiveness of emicizumab in both the reimbursement-requested population and the full Health Canada indication.
Stakeholder Input Relevant to the Economic Review
This section is a summary of the feedback received from the patient groups, registered clinicians, and drug plans that participated in the CADTH review process.
Patient input was received from the Canadian Hemophilia Society. According to this input, hemophilia A affects patients’ lives negatively on physical, psychological, and financial levels. The key concerns raised by patients are breakthrough bleeds, venous access challenges, and adherence difficulties due to the complex treatment regimen. Many patients were concerned that the standard treatment for hemophilia A without inhibitors, FVIII replacement therapy, provides insufficient protection given that breakthrough bleeds still occur, leading to a risk of chronic joint damage. The most common challenge reported by patients is difficult venous access, which is particularly challenging in infants or children. A common challenge faced by patients and caregivers is the need to travel long distances to treatment centres for check-ups, treatments, or to pick up factor supplies for home use. This can make it difficult for patients to adhere to the treatment regimen and affect caregivers’ employability. A separate survey of hemophilia A health care providers with patients who have been prescribed emicizumab reported dramatic improvements in their patients’ health outcomes and quality of life. These health care providers stated that patients required fewer treatment administrations, experienced less joint pain and discomfort, and had fewer hospital visits and greater treatment adherence.
In Canada, access to emicizumab is currently restricted to individuals with hemophilia A with inhibitors. Approximately 15 people with hemophilia A without inhibitors were granted compassionate access starting in autumn 2019.
Several of the following concerns were addressed in the sponsor’s model:
- Since the health care payer perspective was adopted in this economic submission, patient-borne costs, such as those related to travel and lost potential income, were not considered. The sponsor included a scenario analysis adopting a societal perspective that considered the costs of productivity loss.
- Patients receiving emicizumab are expected to require fewer hospitalizations than those treated with FVIII prophylaxis, according to the health care providers surveyed. These costs have been included in the model, with fewer annual days of hospitalization associated with emicizumab compared to FVIII prophylaxis. No feedback was received on how the experience of patients receiving on-demand FVIII may differ from the experiences of those receiving emicizumab.
- Difficulties with venous access for patients receiving FVIII were captured indirectly by applying a utility improvement that was associated with subcutaneous administration (0.1112).
- Health care providers noted that patients on emicizumab experienced less joint pain and discomfort compared to patients on FVIII prophylaxis. Although the economic model assumed that the same percentage of treated bleeds would be joint bleeds (73%) across treatment options, the ABTRs were lower in patients on emicizumab. Therefore, the model aligned with the clinical experts’ views. The ATBRs in the economic model were informed by HAVEN 3 and non-interventional study (NIS).
Economic Review
The current review is for emicizumab (Hemlibra) for patients with severe hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors as routine prophylaxis.
Economic Evaluation
Summary of Sponsor’s Economic Evaluation
Overview
The sponsor submitted a cost-utility analysis comparing emicizumab to prophylaxis with factor VIII (FVIII) and on-demand (episodic) use of FVIII. In both cases, recombinant FVIII products included: Antihemophilic Factor (Recombinant) B-domain deleted (BDD), Fc Fusion Protein (Eloctate); Antihemophilic Factor (Recombinant) PEGylated (Adynovate); Antihemophilic Factor (Recombinant) (Kovaltry); Antihemophilic Factor (Recombinant) BDD simoctocog alfa (Nuwiq); and Antihemophilic Factor (Recombinant) BDD recombinant FVIII (Xyntha). The modelled patient population reflected patients recruited in the HAVEN 3 trial, although the model start age was set to 2. This did not align with the Health Canada–indicated population nor the reimbursement-requested population. The sponsor requested reimbursement of emicizumab in eligible patients with severe hemophilia A without FVIII inhibitors, as per the HAVEN 3 trial, and in patients who were candidates for routine prophylaxis if they had limited ability to receive regular IV therapy due to factors such as venous access challenges or geographical treatment access restrictions. It also included patients who were candidates for routine prophylaxis if they were at significant risk of increased bleeding rates due to factors leading to poor adherence or persistence. No scenario analyses were provided to address the populations described by the Health Canada indication or the sponsor’s reimbursement request.
The dosage regimen recommended by Health Canada for emicizumab is subcutaneous administration, with a loading dose of 3.0 mg/kg for the first 4 weeks.3 This is followed by a maintenance regimen that is age- and weight-specific. Adolescent and adult patients weighing more than 40 kg have the option of 1.5 mg/kg once weekly, 3.0 mg/kg every 2 weeks, or 6.0 mg/kg every 4 weeks. In pediatric patients, and in any patients weighing less than 40 kg, the recommended maintenance regimen is either 1.5 mg/kg once weekly or 3.0 mg/kg every 2 weeks.3 This aligns with the dose used in the economic model, which was based on 3.0 mg/kg as a loading dose for the first 4 weeks of treatment and 1.5 mg/kg once weekly as a maintenance dose for all ages. At the sponsor’s submitted price for emicizumab of $122.05 per mg, the cost for each single-use vial was $3,661.52 (for 30.0 mg/mL), $7,323.04 (for 60.0 mg/ 0.4mL), $12,815.31 (for 105.0 mg/0.7 mL), and $18,307.59 (for 150.0 mg/mL). For an adult patient (70 kg), the sponsor estimated the first-year annual acquisition cost of emicizumab to be $719,946; for subsequent years, $668,685. For FVIII prophylaxis, the annual acquisition cost was calculated by weighting the annual costs for each product by their estimated market shares. This produced an estimate of $472,731 for an adult patient. The sponsor did not factor drug wastage in emicizumab or the FVIII comparators, assuming treatment would be dispensed to the nearest milligram.
The primary clinical outcomes of interest in the model were QALYs. The economic analysis was conducted from the Canadian public payer perspective over a lifetime time horizon (98 years). Both outcomes and costs accrued beyond the first year of the model were discounted at a rate of 1.5%, as per CADTH guidelines.
Model Structure
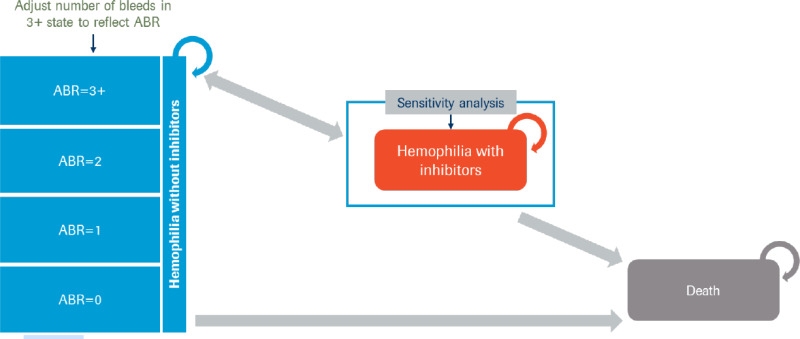
A Markov state-transition model was submitted based on 2 health states: alive with hemophilia A, and death, with the model cycle length defined as 1 year and in which half-cycle corrections were applied (Figure 1 in Appendix 3). All patients entered the alive health state without inhibitor development. Patients had the risk of dying during each model cycle, depending on their treatment and their age. Alive patients could experience bleed events. Bleed events were stratified by the number of events experienced per year (i.e., 0, 1, 2, or 3 or more bleeds). Patients were at risk of experiencing treatment-related adverse clinical events during the first year of treatment. In addition, the model assumed no patients being treated with emicizumab or with FVIII prophylaxis would require arthroplasty, while patients being treated with on-demand FVIII would require 2 arthroplasties over the course of their lifetimes.
Model Inputs
Patients entered the model at the age of 2 years. Patients’ weight was sourced from the UK population. Pediatric patients were assumed to weigh, on average, 12.5 kg from ages 1 to 4, 28.6 kg from ages 5 to 13, and 60.8 kg from ages 14 to 18. Adults (greater than 18 years of age) were assumed to weigh 75.2 kg on average.
ABTRs were sourced from the HAVEN 3 trial, a 24-week, phase III, randomized controlled trial that investigated adult and adolescent patients (aged greater than 12) with severe congenital hemophilia A without FVIII inhibitors.4 Patients who received episodic treatment with FVIII prior to study entry were randomized in a 2:2:1 ratio to the following treatment arms: emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 1.5 mg/kg weekly; emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 3.0 mg/kg every 2 weeks; no prophylaxis (control arm). Patients who received FVIII prophylaxis prior to study entry (derived from the NIS) were enrolled in a separate, non-randomized, single arm where they received treatment with emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 1.5 mg/kg weekly.4 Tracking of patient trajectories in terms of the number of bleeds over time was not directly modelled; rather, the reported proportions from the trial were applied to the sponsor’s economic model. This meant that the proportion of patients by the number of annual bleeding events was the same across all modelled time horizons. The model further derived the overall ATBR to inform costs and utility impacts from treatment (Figure 2, Appendix 3). Rates of clinical adverse events were sourced from the HAVEN 1 trial, a phase III, randomized controlled trial that investigated adult and adolescent patients (aged greater than 12) with hemophilia A with FVIII inhibitors.5 Standardized mortality ratios, from a UK study that looked at patients with hemophilia A or B between 1977 and 1998, were applied to UK life tables for the general public.6 The standardized mortality ratio for mild and moderate hemophilia was applied to patients on emicizumab and FVIII prophylaxis, while the standardized mortality ratio for severe hemophilia was applied to on-demand FVIII patients.6
Health-state utilities were derived from a de novo time trade-off vignette study conducted on the Canadian general public. Baseline utility, by treatment, was derived from this study using 2 random-intercept regression models. Differences in treatment-specific utilities were due to adjustments made according to disutilities associated with the annual number of infusions and treated bleed events expected by treatment (Figure 2) and a utility increment associated with subcutaneous administration. No disutility was captured within the economic model for adverse clinical events.
Drug acquisition costs, hospitalization due to bleeding events, and costs to manage adverse events (including arthroplasty) were considered. Dosing information was obtained from the respective Canadian product monographs, with the cost of emicizumab provided by the sponsor; the unit costs for FVIII products were sourced from the PMPRB.7 Vial wastage and drug administration costs were not included. Costs of arthroplasty were taken from the reported cost per surgery from the Canadian Institute for Health Information. Adverse event and hospitalization costs were obtained from the Ontario Case Costing Initiative.7
Summary of Sponsor’s Economic Evaluation Results
The sponsor’s model reported the mean of their probabilistic results, more than 5,000 model iterations. The model also reported deterministic results, which found results that were similar to the probabilistic analysis.
Base-Case Results
The sponsor’s base-case results are presented in Table 3. The sponsor reported that emicizumab resulted in greater QALYs than FVIII prophylaxis and on-demand treatment. According to the sponsor’s base-case results, emicizumab was associated with 40.37 QALYs, while prophylaxis and on-demand treatment were associated with 33.01 QALYs and 25.39 QALYs, respectively. However, emicizumab was more costly than its comparators, with a total expected cost of $28,750,976 compared with $20,116,294 and $3,907,944 for prophylaxis and on-demand FVIII, respectively. The ICER for emicizumab was $1,657,813 compared to on-demand FVIII. FVIII prophylaxis was subject to extended dominance through on-demand FVIII and emicizumab (combinations of on-demand FVIII and emicizumab are less costly and more effective than FVIII prophylaxis). At a willingness-to-pay threshold of $50,000 per QALY, emicizumab had a 0% probability of being the optimal therapy.
Table 3
Summary of the Sponsor’s Economic Evaluation Results.
Sensitivity and Scenario Analysis Results
The sponsor included a scenario analysis in which patients could develop FVIII inhibitors while undergoing treatment. Allowing patients to develop inhibitors in the model led to a sequential ICER of $1,271,160 per QALY compared to on-demand therapy. This change was driven largely by an increase in expected costs for both comparators. Under this scenario, no patients on emicizumab are at risk of developing inhibitors, while patients receiving the comparators were at risk of developing inhibitors and incurred increased costs from immune tolerance induction and switching to bypassing-drug treatments.
CADTH Appraisal of the Sponsor’s Economic Evaluation
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the economic analysis:
- The model structure does not appropriately capture the clinical disease pathway and the effects of treatment: An appropriate model structure for a given economic evaluation should capture all relevant and meaningful underlying clinical or biological processes.9 The model submitted by the sponsor consisted primarily of 2 health states — alive and dead — and applied transition probabilities to inform the number of patients who would die during each model cycle.7 Although within the alive health state, this was further stratified by ATBRs (i.e., 0,1, 2, or 3 or more bleeds), the sponsor did not derive transition probabilities to describe how patients would move between different bleeding strata over time. Rather, a fixed proportion of patients was applied to each bleed strata at each model cycle.7 This approach is inappropriate for multiple reasons. First, given that transition probabilities were not applied directly to the model, how individuals transition among different bleeding frequency health states over time was not explicitly modelled. The clinical experts consulted by CADTH noted that, in patients with greater ATBRs, they would typically intervene to further optimize their therapy, leading to an expected decline in ATBRs. Without further intervention, these patients would be expected to have an increased risk of bleed-related morbidity and mortality outcomes. Furthermore, patients with higher bleed rates are likely to have bleed-related complications and will be at greater risk of experiencing high frequencies of bleeds in the future. However, the sponsor’s submitted model did not explicitly consider any of these aspects of the condition, the impact of treatment, or its associated costs or utility impacts. Secondly, the sponsor did not incorporate parameter uncertainty. Over each Monte Carlo simulation, the same proportions were applied to the model. This is not appropriate, given that these values were derived from clinical studies and the “true” values remain unknown.
- As this issue relates to the submitted model structure, CADTH was unable to conduct a reanalysis to assess this limitation.
- The target population of the model does not reflect the Health Canada indication under review or the sponsor’s reimbursement population. The submitted Health Canada indication is for patients with hemophilia A (congenital factor VIII deficiency) as routine prophylaxis to prevent bleeding or reduce the frequency of bleeding episodes.3 The sponsor has requested reimbursement in patients with severe hemophilia A (congenital factor VIII deficiency) without FVIII inhibitors, including those who are candidates for routine prophylaxis with FVIII if they are at significant risk of increased bleed rates due to factors leading to poor adherence or persistence, or have limited ability to receive regular IV therapy due to underlying factors. During the review period, the sponsor provided additional information clarifying that the reimbursement-requested indication was for patients with severe, non-inhibitor hemophilia A as well as patients with mild and moderate non-inhibitor hemophilia A who experience geographical treatment access restrictions and venous access challenges.10 The target population in the model considered only patients with severe, non-inhibitor hemophilia A who met HAVEN 3’s eligibility criteria, given that key model parameters were sourced from this trial. As noted in the CADTH clinical review, the inclusion criteria for patients in HAVEN 3 included previously managed by on-demand FVIII and experiencing 5 or more bleeds in the 24 weeks prior to study entry, which is not representative of patients with hemophilia A in Canadian clinical practice. As the study population in HAVEN 3 had greater uncontrolled bleeding than would be expected in Canadian practice, this may overstate the clinical efficacy of emicizumab compared to what would be expected in the clinical setting. The magnitude of the treatment effect in patients with better control (consistent with the Canadian clinical population) compared to those included in the trials remains unknown. Eligibility criteria further excluded patients who had thromboembolic disease, were at high risk for microangiopathy, or had certain autoimmune diseases, thereby decreasing generalizability to the Canadian clinical population. HAVEN 3 further excluded patients under the age of 12. According to clinical experts consulted by CADTH, patients as young as 1 year of age may be prescribed emicizumab.
- Given the clinical evidence base, CADTH was unable to conduct a reanalysis to adequately assess this limitation. To align with the clinical evidence, the age parameter in the model was revised to reflect the distribution reported in HAVEN 3. A scenario analysis was further conducted in which the patient age was set to 2 years, as assumed in the sponsor’s model. Although evidence on the effects of emicizumab on children without inhibitors is lacking, the efficacy and safety of emicizumab should not be age-dependent, according to the clinical experts consulted by CADTH, given the known mechanism of action of emicizumab and the existing clinical evidence for emicizumab in children with inhibitors.
- Naive comparison for emicizumab versus FVIII prophylaxis: Treatment efficacy in the sponsor’s model was informed by the HAVEN 3 and the NIS.7 The NIS preceded HAVEN 3 and was conducted among patients on FVIII prophylaxis. These patients were subsequently eligible for enrolment in HAVEN 3 in a separate, non-randomized arm, and received emicizumab prophylaxis.4 For the emicizumab and on-demand groups in the economic model, clinical efficacy was informed by the appropriate randomized arms in the HAVEN 3 trial; for FVIII prophylaxis, clinical efficacy was informed by the NIS. It is inappropriate to compare clinical outcomes for FVIII prophylaxis in the NIS with the clinical outcomes for emicizumab in HAVEN 3. As the CADTH clinical report notes, there is no direct comparative evidence to support the efficacy of emicizumab compared to FVIII prophylaxis in patients with severe hemophilia A; the only evidence is limited to an intra-patient analysis (group D in HAVEN 3). The sponsor’s approach reflects a non-randomized, naive comparison; it remains unclear what potential biases are introduced into the economic analysis given this approach. Certain reported baseline characteristics were found to differ between the randomized HAVEN 3 groups and the NIS group: the NIS group was younger and had experienced a lower number of bleeds in the prior 24 weeks. Although the clinical experts consulted by CADTH noted that these baseline differences would be expected in the FVIII prophylaxis population, and that treatment effects are likely independent of patient age, it is unclear whether other biases could have been introduced through this non-randomized comparison. They also commented that while the clinical estimates used in the economic model likely represent the Canadian setting, they may lack precision. The sponsor further commissioned an indirect treatment comparison and incorporated these estimates into a scenario analysis. Although the sponsor’s network meta-analysis suggested that emicizumab prophylaxis was associated with reduced bleed rates compared with FVIII prophylaxis in the treatment of patients with severe hemophilia A without inhibitors, methodological limitations of the indirect treatment comparison affect both the confidence of this finding and the appropriateness of using these estimates to inform the economic model. A small number of trials were included in the analysis, and each trial enrolled a small number of patients. Due to the small evidence base, the results of the analysis were uncertain. A high degree of heterogeneity was further noted across the included studies, including: the severity of hemophilia A; different FVIII products studied; inconsistent or unclear definitions of the bleed outcomes; variable time points for outcome measurement; and differences in study design. Together, these limitations preclude the use of estimates derived from the network meta-analysis to inform the comparative treatment effects of emicizumab relative to FVIII prophylaxis.
- Given the lack of clinical literature comparing FVIII prophylaxis to emicizumab directly, CADTH was unable to conduct a reanalysis to assess this limitation. CADTH conducted a scenario analysis in which FVIII prophylaxis was removed as a comparator.
- Dispensing of treatment does not reflect clinical practice. In the sponsor’s submitted economic model, treatment was dispensed according to the sponsor’s product monographs, with treatment-acquisition costs calculated based on the exact dose (per mg) required. This misaligns with the assumptions stated in the sponsor’s submitted budget impact analysis. The product monograph for emicizumab states that the drug is for single use only and available in pre-set concentrations. According to the CADTH clinical experts, patients on both emicizumab and FVIII would typically have their dose rounded up to the nearest whole vial, with the drug dispensed accordingly to minimize wastage. How treatments are dispensed needs to be accounted for in the cost of treatment. In the sponsor’s approach, the treatment-acquisition costs for both emicizumab and the FVIII comparators were underestimated.
- CADTH considered that dispensed drugs would be rounded up to the nearest whole vial to reflect clinical practice settings.
- Inappropriate modelling of health-utility estimates. The utility estimates were based on a sponsor’s commissioned utility exercise in which a sample of the Canadian public (n = 82) provided utility estimates based on a time trade-off vignette exercise.7 Based on the survey responses, regression models were derived. The first model included treatment administration (subcutaneous versus intravenous) and the number of bleeds per year as predictor variables; using the same dataset, a second regression analysis was modelled specific to prophylaxis treatment with a single predictor: the frequency of infusions per year.The sponsor derived baseline utility for the treatment and comparator arms based on combining the coefficients from both regression analyses. This, in effect, introduces treatment-specific utility values (i.e., emicizumab = 0.908 [based on summing the intercept and the coefficient for subcutaneous treatment from the first regression model]; on-demand FVIII = 0.797 [based on the intercept from the first regression model]; and FVIII prophylaxis = 0.759 [based on the intercept from the first regression model subtracted from the product of the number of infusions and the disutility of infusion from the second regression model]).7 This approach (i.e., combining values from separate regression equations) is inappropriate because these regressions are conceptually different and not compatible given that each regression equation is estimating a different set of estimates. Therefore, this approach lacks credibility. In effect, it applies 2 separate adjustments for IV infusions (i.e., 1 from each regression model) without consideration that the values from the separate regression equations are, in fact, correlated. As per current guidelines for the conduct of economic evaluations,9 utilities should reflect the health states within the model and not be specific to treatment. Although an argument could potentially be made on patient preference for the mode of treatment administration, the sponsor’s estimate would indicate that patients are willing to trade off 1.1 years of perfect health every 10 years to avoid treatments involving IV administration, assuming all else is considered equal. This magnitude is greater than the benefit that a patient with mild anemia achieves with treatment that fully resolves their anemia.11 Of note, the CADTH clinical experts indicated that the effect of treatment on improved quality of life remains inconclusive.
- CADTH removed treatment-specific utility values by setting the baseline utility to be identical across all treatments. The utility decrements arising from treated bleeds and infusions were incorporated into the model separately. Given the limitations with the structure of the sponsor’s submitted model, utility decrements could not be applied to adverse events.
- Missing comparators. The comparators in the submitted economic model reflected approaches to treating patients with hemophilia A with FVIII products. However, in consultation with the clinical experts, it was noted that plasma-derived von Willebrand factor (VWF) products, such as Antihemophilic Factor/ VWF Complex (Human) (Humate-P) and VWF/Coagulation Factor VIII Complex (Human) (Wilate), may also be used in this patient population. These comparators were not considered in the sponsor’s submitted model.
- Given the lack of comparative clinical effectiveness data presented by the sponsor and the structure of the submitted economic model, CADTH was unable to conduct a reanalysis to assess this limitation.
- Underestimation of adult patient weights. Patient weights used in the model were sourced from UK life tables. Hemophilia A primarily affects men; men are, on average, heavier than women.12 Furthermore, the average weight of patients in the HAVEN 3 trial (79.1kg)4 was greater than the sponsor’s assumption (75.2 kg). This difference in weight would translate to a different vial size for emicizumab and, thereby, a higher treatment-acquisition cost. Setting the weight at 75.2 kg would have underestimated the expected cost of emicizumab.
- CADTH set the average patient weight to reflect that reported in the HAVEN 3 trial.
One additional limitation was identified, but was considered unlikely to change or to affect the analyses significantly. This limitation is outlined next.
- Disutility from arthroplasties was not included in the model. As joint replacement due to severe bleeding events would affect quality of life, a disutility of 0.39 was applied for arthroplasties for the duration across which the model captured their impact.13
Additionally, the following key assumptions were made by the sponsor and have been appraised by CADTH (Table 4).
Table 4
Key Assumptions of the Submitted Economic Evaluation (Not Noted as Limitations to the Submission).
CADTH Reanalyses of the Economic Evaluation
Base-Case Results
While several limitations with the sponsor’s submission could not be addressed (i.e., model structure, patient population, noncomparative clinical outcomes for FVIII prophylaxis, and missing comparators), other limitations could be explored. CADTH undertook a stepped analysis, incorporating each change detailed in Table 5 into the sponsor’s corrected base case to highlight the impact of each change. The summary results of the sponsor’s corrected base case and the CADTH reanalyses are presented in Table 6.
Table 5
CADTH Revisions to the Submitted Economic Evaluation.
Table 6
Summary of the Stepped Analysis of the CADTH Reanalysis Results.
In the CADTH base case, emicizumab was associated with an additional cost of $8,691,419 and 1.57 additional QALYs, for an ICER of 5,530,766 per QALY gained compared to FVIII prophylaxis. Using a willingness-to-pay threshold of $50,000 per QALY, there is a 0% probability that emicizumab would be considered cost-effective in the CADTH base case. This is primarily due to the incorporation of vial wastage and removing treatment-specific utility values.
Detailed results of the CADTH base case are presented in Table 12 of Appendix 4. Of note, the reanalysis is based on publicly available prices of the comparator treatments.
Scenario Analysis Results
Several scenario and sensitivity analyses were conducted on the CADTH base case. These scenario analyses primarily explored changes in starting age, drug prices, and utility. Furthermore, scenario analyses included testing a structural assumption in which patients on FVIII treatments could be at risk of developing inhibitors and a scenario in which FVIII prophylaxis was removed as a possible comparator in light of the noncomparative evidence available. The model interpretations were found to remain robust (Table 13) because no scenario brought the sequential ICER of emicizumab close $50,000 per QALY.
The model was most sensitive to the price of the comparator treatment. The public prices for FVIII, reported by PMPRB are ceiling prices (i.e., they reflect the maximum average potential price). Utilizing these prices in the model, a price reduction of at least 89% is required for emicizumab to be considered cost-effective at a willingness-to-pay threshold of $50,000 per QALY, according to the CADTH base case (Table 7). Given that PMPRB prices may not reflect the actual price of FVIII products in Canada, 2-way price-reduction analyses were further conducted to highlight the influence of price reduction on the cost-effectiveness results for both emicizumab and FVII (Table 8).
Table 7
CADTH Price-Reduction Analyses.
Table 8
CADTH 2-Way Price-Reduction Analyses.
Issues for Consideration
- Emicizumab is administered subcutaneously, whereas FVIII therapies are administered intravenously. According to the clinical experts consulted, both treatments can be administered at home following adequate patient training.
- According to the clinical experts consulted by CADTH and the health care provider input received as part of the patient group’s feedback, adherence is key to reducing breakthrough bleeds that require treatment. The efficacy of treatment is highly dependent on patient adherence. It is plausible that emicizumab may result in better adherence to treatment, given its mode of administration and less frequent administration. Adherence, which would be expected to affect both costs and utilities, was not explicitly captured in the sponsor’s model.
- CADTH was unable to adequately assess the impact of potentially lower prices of the comparators on the cost-effectiveness of emicizumab. Reduced effective prices for comparators, arising from the tendering process by Canadian Blood Services15 (as opposed to PMPRB’s maximum average potential price), may lead to different conclusions than the current analysis, potentially resulting in an even higher ICER for emicizumab.
- The development of neutralizing anti-drug antibodies in patients was not reported in the HAVEN 3 trial, although case reports have been observed in practice.16 The clinical effects of anti-drug antibodies remain unclear, and their impact on both clinical effectiveness and cost-effectiveness remains unknown.
- Emicizumab has been reviewed by other Health Technology Assessment agencies. Quebec’s Institut national d’excellence en santé et services sociaux has not recommended the reimbursement of emicizumab for patients without FVIII inhibitors.17 A draft report from the Institute for Clinical and Economic Review found emicizumab is likely not cost-effective at a threshold of US$200,000 per QALY for patients without FVIII inhibitors.18
- According to the clinical experts consulted by CADTH, there are several emerging treatments for patients with hemophilia that are currently under development or emerging. These include extended half-life factor replacement products, non-factor therapies (e.g., anti-tissue pathway inhibitor antibody), and gene therapies (e.g., valoctocogene roxaparvovec).19
- With respect to desmopressin’s place in therapy, the clinical experts consulted by CADTH noted that it is primarily used to treat patients with mild or moderate hemophilia A.
Overall Conclusions
Based on the CADTH clinical review of 2 sponsor-submitted trials (i.e., HAVEN 3 and HAVEN 4), emicizumab is associated with a statistically and clinically significantly improvement in bleeding outcomes (i.e., ATBR ratio for treated bleeds, all bleeds, treated joint bleeds, treated spontaneous bleeds) compared to on-demand treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis; however, current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison). No additional studies met the inclusion criteria for the systematic review conducted in the CADTH clinical review, and there is presently no clinical evidence comparing emicizumab to plasma-derived VWF products that could be used in this patient population. These limitations in clinical evidence could not be addressed in the pharmacoeconomic analysis.
Furthermore, CADTH could not address the limitations associated with the model structure and the analysis target population (compared with the intended population). The model target population is specific to patients with severe hemophilia A who meet HAVEN 3’s eligibility criteria. The CADTH reanalysis was able to address only a subset of the limitations in the sponsor’s submission, including: adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting age and weight to better reflect the patient population in HAVEN 3. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per QALY in patients with severe hemophilia A. In the CADTH base-case reanalysis, the ICER for emicizumab was $5,530,766 per QALY compared with FVIII prophylaxis. A price reduction of at least 89% is necessary for emicizumab to be considered cost-effective at a threshold of $50,000 per QALY. However, a greater price reduction may be required if the price of FVIII products is, in fact, lower than the PMPRB published values.
The cost-effectiveness of emicizumab in both the reimbursement-requested population and the broader Health Canada indication remain unknown.
Footnotes
- a
versus reference category of intravenous infusion
Appendix 1. Cost-Comparison Table
The comparators presented in the following table have been to be deemed appropriate based on feedback from clinical experts. Comparators may be recommended (appropriate) practice or actual practice. Existing Product Listing Agreements are not reflected in the table and as such, the table may not represent the actual costs to public drug plans.
Table 9
CADTH Cost-Comparison Table for Prophylaxis of Bleeding in Patients With Hemophilia A Without Factor VIII Inhibitors.
Appendix 2. Submission Quality
Appendix 3. Additional Information on the Submitted Economic Evaluation
Figure 1Model Structure
ABR = annualized (treated) bleed rate. The sponsor used ABR to refer to annualized treated bleed rate in this instance.
Source: Sponsor’s pharmacoeconomic submission.8
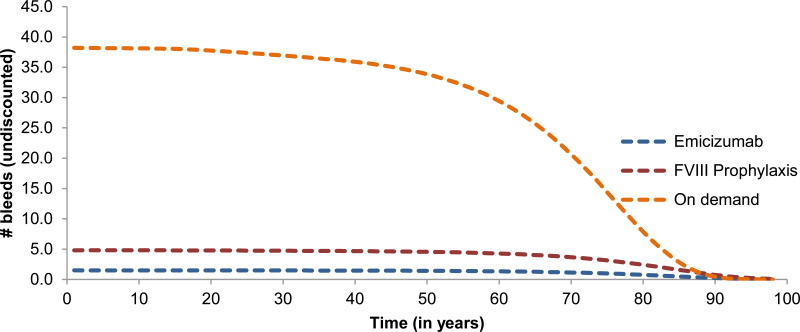
The model estimates that patients receiving on-demand treatment will experience 30 to 40 bleed events, while patients receiving emicizumab will experience nearly 0 bleed events on average (Figure 2).
Figure 2Number of Bleeds per Year
FVIII = factor VIII.
Source: Sponsor’s pharmacoeconomic submission.8
Table 11
Number of Bleeds Experienced in a Year per Treatment Arm.
Appendix 4. Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
Table 12. Disaggregated Summary of CADTH’s Economic Evaluation Results
Appendix 5. Submitted Budget Impact Analysis and CADTH Appraisal
Summary of Sponsor’s Business Impact Analysis
In the submitted BIA, the sponsor assessed the introduction of prophylactic emicizumab for the treatment of patients with hemophilia A without factor VIII (FVIII) inhibitors compared to prophylactic or on-demand treatment with FVIII replacement therapy, consistent with the requested reimbursement criteria (see Table 1). The BIA was undertaken from the perspective of a Canadian public payer over a 3-year time horizon using an epidemiological approach. The sponsor included the acquisition costs associated with plasma protein products, including wastage, but excluded mark-ups and dispensing fees (Table 14). Data for the model were obtained from various sources, including the HAVEN 3 and 4 trials,14,24 the Canadian Blood Disorders Registry,25 published literature, CADTH’s 2015 budget impact analysis for Eloctate,26 and expert opinion.8
Table 14
Summary of Key Model Parameters.
Table 15
Sponsor’s Estimations and Assumptions on Population Size, Disease Severity, and Treatment Regimen (Reference Scenario).
Summary of the Sponsor’s Budget Impact Analysis Results
Results of the sponsor’s base case suggested an incremental cost of $67,028,982 in year 1, $70,861,365 in year 2, and $81,247,668 in year 3, for a total incremental cost of $219,138,016 over the 3-year time horizon when emicizumab is reimbursed for adult patients with severe hemophilia A (congenital FVIII deficiency) without FVIII inhibitors; for adult and pediatric patients with limited ability to receive regular IV therapy due to underlying factors, such as venous access challenges or geographical treatment access restrictions; or for adult and pediatric patients at significant risk for increased bleeding rates due to factors that lead to poor adherence or persistence.
CADTH Appraisal of the Sponsor’s BIA
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the results of the BIA:
- Uncertainty in the cost of FVIII comparators. The price of long- and short-acting FVIII was estimated by the sponsor from the PMPRB, which provides the maximum average potential price of a new patented medicine.20 Public procurement of FVIII products is based on a tendering process in which the reimbursed price is likely to be lower.8 Despite these prices being confidential, using the maximum price for the comparators introduces significant uncertainty because a higher comparator price will favour the adoption of emicizumab.
- To address uncertainty in comparator pricing, CADTH conducted 2-way price-reduction analyses.
- Uncertainty in annualized treated bleed rates for patients receiving on-demand therapy. The sponsor estimated an ATBR of 40 for adult and pediatric patients with severe disease receiving on-demand therapy.26 The clinical experts consulted by CADTH for this review noted that a patient would be unlikely to experience such a high frequency of bleeds without intervention.
- In CADTH reanalyses, the ATBR for severe pediatric and adult patients was changed to 24.1, reflecting the on-demand values reported in patients with severe disease in the PROTEC VIII trial.27
- Inappropriate adult weight used in the model. The sponsor used an adult weight of 72.47 kg.4 CADTH was unable to validate this weight in the cited publication. According to the clinical experts consulted by CADTH for this review, this weight may not be representative of Canadian adults; they noted that the average weight may be higher.
- In CADTH reanalyses, the average weight among all patients in HAVEN 3 (79.1 kg) was used to inform the average weight of adults in the BIA.
- Uncertainty regarding the uptake of emicizumab among eligible patients. The BIA investigated the impact of the sponsor’s reimbursement request, which included patients with both severe and non-severe disease who met specific criteria. In the sponsor’s new drug scenario, a proportion of adults with severe disease who are currently receiving both prophylaxis and on-demand therapies were assumed to switch to emicizumab, whereas 0% of pediatric patients who have severe disease were assumed to switch. Separately, the sponsor assumed that ▬% of all patients eligible for emicizumab would switch to emicizumab, given that they would meet the specific sponsor’s requested criteria. However, these patients were redistributed to the adult moderate population and pediatric severe and moderate populations only (Table 14). Estimates of market share are uncertain. Furthermore, this mathematical approach to deriving market share estimates is inconsistent and lacks transparency. The clinical experts consulted by CADTH for this review expected that uptake among adult patients with moderate disease would be similar to adult patients with severe disease. Experts also noted that adult patients receiving on-demand therapy may be less likely to switch to emicizumab than those treated with prophylaxis, which contradicts the sponsor’s assumption that uptake among moderate patients would be equal across both treatment groups. Lastly, the CADTH clinical review report notes that the area of greatest unmet need is in the pediatric population. Therefore, uptake among pediatric patients may be higher than assumed in the sponsor’s base-case analysis.
- Given that uptake of emicizumab is highly uncertain, the impact of alternative uptake rates was explored in 2 scenario analyses.
- Uncertainty in the proportion of patients across current FVIII treatment paradigms. The sponsor estimated the proportions of patients, by age group and severity, who would be currently receiving FVIII prophylaxis versus on-demand therapy based, on clinical expert opinion. These estimates are uncertain, given that the distribution of the current treatment mix is not publicly available. Because experts expect that adult patients currently receiving prophylaxis would be more likely to switch to emicizumab than those receiving on-demand therapy alone, these values in the reference scenario have the potential to influence the BIA results.
- CADTH was unable to address this limitation.
- Comparator product missing. According to the clinical experts consulted by CADTH for this review, some patients may use plasma-derived VWF products for treatment and prophylaxis of bleeds.28
- CADTH was unable to address this limitation. Given that both the price and proportion of patients using VWF is unknown, so too is the direction and magnitude of the effect of their exclusion on the results of the BIA.
CADTH Reanalyses of the Budget Impact Analysis
CADTH revised the sponsor’s submission by changing the ATBR for on-demand patients with severe disease and adjusting the adult weight to align with the HAVEN 3 trial. Table 16 compares the assumption and values used by the sponsor with those used by CADTH in its reanalysis.
Table 16
CADTH Revisions to the Submitted Budget Impact Analysis.
The results of the CADTH step-wise reanalysis are presented in summary format in Table 17. A more detailed breakdown is presented in Table 18. Applying these changes increased the 3-year total budget impact to $239,602,620.
Table 17
Summary of the CADTH Reanalyses of the Budget Impact Analysis.
CADTH also conducted additional scenario analyses to address remaining uncertainties:
- Assume uptake among pediatric patients with severe disease will be equal to that of adults with severe disease, in addition to the ▬% uptake assumed by the sponsor (revised uptake among pediatric patients with severe disease = 40%, 45%, and 50% and 25%, 28%, and 30% for year 1, year 2, and year 3 for pediatric patients on prophylactic and on-demand treatment, respectively).
- Revise uptake such that identical uptake rates were assumed by the approach to management, irrespective of hemophilia A severity and age:
- Assume reimbursement in the population of adults with severe disease only (to align with the population studied in the HAVEN-3 trial and the target population modelled in the cost-effectiveness analysis).
- Revise on-demand ATBR to reflect the sponsor’s pharmacoeconomic submission (38.1).
- Reduce the price of emicizumab to the value at which it would be cost-effective at a threshold of $50,000 per QALY (89%).
The results of CADTH’s scenario analyses demonstrate that the model is highly sensitive to comparator prices and the price of emicizumab. When the price of emicizumab was reduced by 89% (and FVIII comparator pricing remained unchanged), adopting emicizumab became cost-saving. Given that the true price of FVIII products is unknown, a 2-way price-reduction analysis between emicizumab and FVIII was conducted. The results are presented in Table 19.
Table 18
Detailed Breakdown of the CADTH Reanalyses of the Budget Impact Analysis.
Table 19
Two-Way Price-Reduction Analyses: 3-Year Total Budget Impact Analysis.
References
- 1.
- Hemlibra (emicizumab): economic review report (CADTH technology review). Ottawa (ON): CADTH; 2019: https://cadth
.ca/sites /default/files/hta-he /ob0005-emicizumab-eeconomic-report .pdf. Accessed 2020 Sep 25. - 2.
- Launch of Hemlibra (emicizumab injection): customer letter #2019-17. Ottawa (ON): Canadian Blood Services; 2019: https://www
.blood.ca /sites/default/files /2019-08/CL%202019-17 %20Launch%20of%20Hemlibra %20%28emicizumab%20injection%29 .pdf. Accessed 2020 Sep 25. - 3.
- Hemlibra (emicizumab): 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [product monograph]. Mississauga (ON): Hoffmann-La Roche Limited; 2019 Jun 14.
- 4.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–822. [PubMed: 30157389]
- 5.
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. [PubMed: 28691557]
- 6.
- Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. [PubMed: 17446349]
- 7.
- CDR submission: Hemlibra (emicizumab), 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [CONFIDENTIAL sponsor’s submission]. Mississauga (ON): Hoffmann-La Roche Limited; 2020 Jun 30.
- 8.
- Pharmacoeconomic evaluation. In: CDR submission: Hemlibra (emicizumab), 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [CONFIDENTIAL sponsor’s submission]. Mississauga (ON): Hoffmann-La Roche Limited; 2020 Jun 30.
- 9.
- Guidelines for the economic evaluation of health technologies: Canada — 4th Edition. Ottawa (ON): CADTH; 2017: https://www
.cadth.ca /dv/guidelines-economic-evaluation-health-technologies-canada-4th-edition. Accessed 2020 Sep 25. - 10.
- Hoffmann-La Roche Limited response to August 27, 2020 CDR request for additional information regarding Hemlibra (emicizumab) CDR review: clarification on reimbursement criteria [CONFIDENTIAL additional sponsor’s information]. Mississauga (ON). Mississauga (ON): Hoffmann-La Roche Limited; 2020 Aug 27.
- 11.
- Klarenbach S, Manns B, Reiman T, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer. 2010;116(13):3224–3232. [PubMed: 20564645]
- 12.
- Page D, Amesse C, Zereik H. An introduction to hemophilia. All about hemophilia: a guide for families. 2 ed. Montreal (QC): Canadian Hemophilia Society; 2010: https://www
.hemophilia .ca/files/Chapter%2001.pdf. Accessed 2020 Sep 9. - 13.
- Ballal RD, Botteman MF, Foley I, et al. Economic evaluation of major knee surgery with recombinant activated factor VII in hemophilia patients with high titer inhibitors and advanced knee arthropathy: exploratory results via literature-based modeling. Curr Med Res Opin. 2008;24(3):753–768. [PubMed: 18234151]
- 14.
- Clinical Study Report: BO39182 (HAVEN 4). A multicenter, open-label, phase III study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab given every 4 weeks (Q4W) in patients with hemophilia A [CONFIDENTIAL internal sponsor’s report]. Basel (CH); Tokyo (JP): F. Hoffmann-La Roche Ltd., Chugai Pharmaceutical Co. Ltd; 2018 May 30.
- 15.
- The tender process (RFP) for clotting factor concentrates in Canada. Montreal (QC): Canadian Hemophilia Society; date unknown: https://www
.hemophilia .ca/files/What%20is%20an%20RFP.pdf. Accessed 2020 Sep 25. - 16.
- Yada K, Nogami K. Spotlight on emicizumab in the management of hemophilia A: patient selection and special considerations. J Blood Med. 2019;10:171–181. [PMC free article: PMC6613000] [PubMed: 31308776]
- 17.
- Nieminen J, Nshimyumukiza L, Paré A, Saidi R. HemlibraMC (émicizumab) - hémophilie A. Quebec (QC): Institut national d’excellence en santé et en services sociaux (INESSS); 2019: https://www
.inesss.qc .ca/en/publications /publications/publication /hemlibramc-mise-a-jour-de-la-liste-des-produits-du-systeme-du-sang-du-quebec-decembre.html. Accessed 2020 Sep 25. - 18.
- Valoctocogene roxaparvovec and emicizumab for hemophilia A: effectiveness and value [draft evidence report] Boston (MA): Institute for Clinical and Economic Review; 2020: https://icer-review
.org /wp-content/uploads /2019/12/ICER_Hemophilia-A _Draft-Evidence-Report_082620-1 .pdf. Accessed 2020 Sep 25. - 19.
- Arruda VR, Doshi BS, Samelson-Jones BJ. Emerging therapies for hemophilia: controversies and unanswered questions. F1000Res. 2018;7(F1000):Faculty Rev–489.
- 20.
- Patented Medicine Prices Review Board. New patented medicines reported to PMPRB. 2020; http://www
.pmprb-cepmb .gc.ca/pmpMedicines.asp?x=611. Accessed 2020 Sep 16. - 21.
- Nuwiq (antihemophilic factor (recombinant, B-domain deleted)): powder and solvent for solution for intravenous injection 250 IU FViii/vial reconstituted with 2.5 mL of solvent, 500 IU FVIII/vial reconstituted with 2.5 mL of solvent, 1000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 2000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 2500 IU FVIII/vial reconstituted with 2.5 mL of solvent, 3000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 4000 IU FVIII/vial reconstituted with 2.5 mL of solvent [product monograph]. Toronto (ON): Octapharma Canada Inc.; 2018 Dec 14: https://pdf
.hres.ca/dpd_pm/00050487.PDF. Accessed 2020 Sep 22. - 22.
- Xyntha (lyophilized powder for reconstitution in a vial): 250, 500, 1000, or 2000 IU in single-use vials and one pre-filled diluent syringe containing 4 mL 0.9% sodium chloride for reconstitution; Xyntha Solufuse (lyophilized powder for reconstitution in a prefilled dual-chamber syringe): 250, 500, 1000, 2000, or 3000 IU and 4 mL 0.9% sodium chloride solution for reconstitution in a prefilled dula-chamber syringe [product monograph]. Kirkland (QC): T.M. Wyeth LLC, Pfizer Canada Inc., Licensee; 2016 May 11: https://pdf
.hres.ca/dpd_pm/00034847.PDF. Accessed 2020 Sep 22. - 23.
- Saskatchewan Drug Plan: search formulary. 2020; http://formulary
.drugplan .ehealthsask.ca/SearchFormulary. Accessed 2020 Aug 20. - 24.
- Clinical Study Report: BH30071 (HAVEN 3). A randomized, multicenter, open-label, phase III clinical trial to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A patients without inhibitors [CONFIDENTIAL internal sponsor’s report]. Basel (CH), Tokyo (JP): F. Hoffmann-La Roche Ltd., Chugai Pharmaceutical Co. Ltd; 2018 Mar 26.
- 25.
- Canadian Blood Disorders Registry. Factor FVIII deficiency. 2018; https://fhs
.mcmaster .ca/chr/pdf/18/FVIII %20stats%20can%202018.pdf. Accessed 2020 Aug 31. - 26.
- Antihemophilic factor (recombinant BDD) Fc fusion protein (eloctate): treatment cost comparison and budget impact analysis (CADTH technology review). Ottawa (ON): CADTH; 2015: https://cadth
.ca/sites /default/files/pdf /OB0004_Eloctate_Economic_Report.pdf. Accessed 2020 Aug 31. - 27.
- Jivi (antihemophilic factor (recombinant, B-domain deleted, PEGylated)): IV injection 250, 500, 1000, 2000, 3000 IU/vial [product monograph]. Mississauga (ON): Bayer Inc; 2018 Oct 18: https://pdf
.hres.ca/dpd_pm/00047833.PDF. Accessed 2020 Sep 17. - 28.
- Wilate (human von Willebrand factor (VWF) and human coagulation factor VIII (FVIII): 500 IU VWF and 500 IU FVIII reconstituted with 5 mL of diluent, 1000 IU VWF and 1000 IU FVIII reconstituted with 10 mL of diluent [product monograph]. Toronto (ON): Octapharma Canada, Inc; 2018 May 8: https://pdf
.hres.ca/dpd_pm/00045156.PDF. Accessed 2020 Sep 17.
Version: Final
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/