Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient Population. Adults with acute or chronic pain, including cancer patients, without progressive or terminal disease, treated in an outpatient setting, excluding hospice and end-of-life care.
Objectives. Provide a framework for comprehensive pain evaluation and individualized multimodal treatment. Improve quality of life and function in patients experiencing pain, while reducing the morbidity and mortality associated with pain treatments, particularly opioid analgesics.
Key Points
Acute Pain
Pain resolution. Acute pain is associated with tissue damage. As tissue heals, pain should resolve.
Limit opioid therapy. Avoid opioids for mild to moderate acute pain [IC]. Consider opioids for moderately-severe to severe acute or procedural pain [IIC], but if used, limit dose and duration. Do not prescribe opioids for sprains, lacerations, skin biopsies, or simple dental extractions [IIIE].
Chronic Pain
Chronic pain differs from acute pain. Chronic pain is not acute pain that failed to resolve. It is a distinct condition that is better understood as a disease process than as a symptom. Use a biopsychosocial approach in assessment and management.
Diagnosis
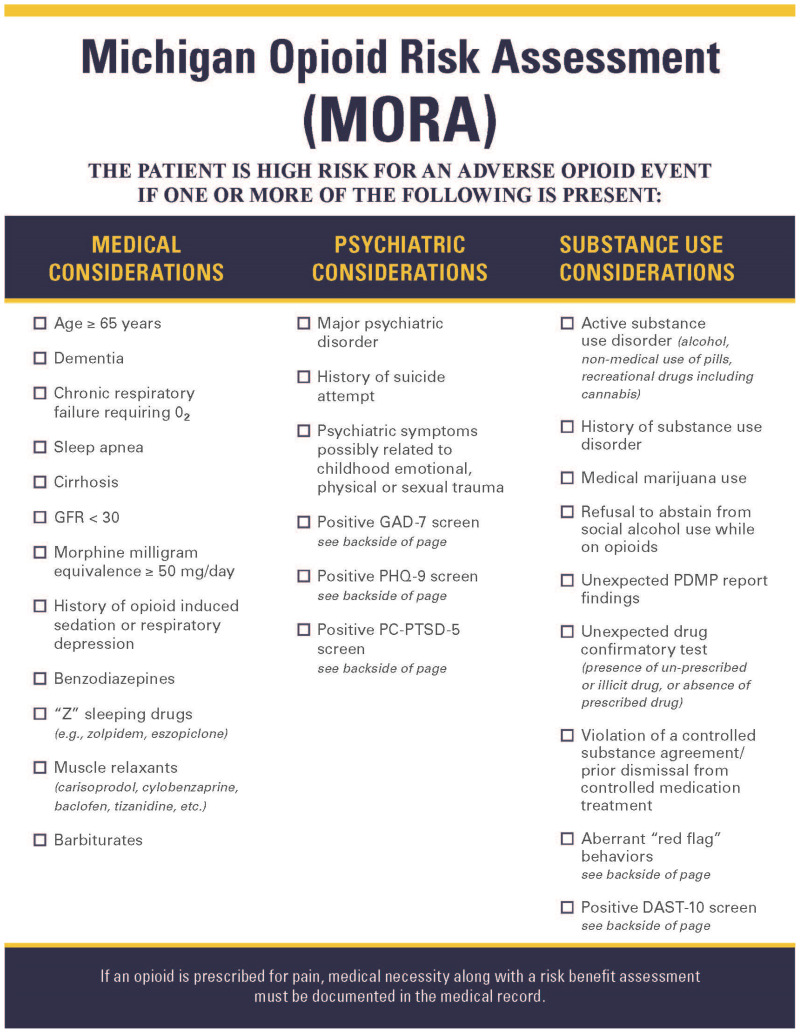
Chronic pain assessment. Perform a history and physical examination. Assess pain characteristics, pain treatment history, quality of life and functional impact, pain beliefs, and psychosocial factors. Assess comorbid conditions, including medical and psychiatric conditions, substance use, pain beliefs and expectations, and suicidality (Table 3) [IC]. Review any pertinent diagnostic studies [IC].
Table 3
Initial Evaluation of Chronic Pain.
Mechanism. Classify chronic pain as primary or secondary. Determine the underlying neurobiologic mechanism of pain: nociceptive, neuropathic, central (nociplastic). Assign the diagnosis of an underlying chronic pain syndrome, when applicable. (Table 2) [IC].
Table 2
Classification of Chronic Pain Syndromes and Relationship to Neurobiologic Mechanism of Pain.
Treatment
Create an individualized treatment plan (Table 4) utilizing multiple modalities, including non-pharmacologic (Tables 5-6) and non-opioid pharmacologic (Table 7) interventions [IC]. Use shared decision-making. Emphasize interventions with the lowest risk [IC].
Table 4
Creating an Individualized Pain Treatment Plan.
Table 5
Non-pharmacological Pain Treatment Options.
Table 6
Herbal Supplements Used in Chronic Pain.
Table 7
Non-Opioid Medications for Pain.
Assess response, address barriers to implementation and adjust the treatment plan [IC].
Generally, avoid opioid therapy. Opioids are not indicated for most patients with chronic pain (Figure 1) [IB]. If considering starting or continuing an opioid, thoroughly assess the risk of harm before proceeding [IB], and perform a full evaluation, including record review, urine comprehensive drug screen, and review of the state prescription drug monitoring program report (MAPS in Michigan). Potential benefits of opioid use must clearly outweigh risks [IE].
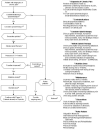
Figure 1
Prescribing Opioids for Chronic Pain in Opioid Naïve Patients (Not Including Active Cancer).
Obtain informed consent when prescribing opioids. Use the Start Talking Form and Controlled Substance Agreement. Provide opioid education. Discuss benefits and harms [IE].
Opioid Management (Figure 2)
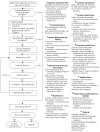
Figure 2
Prescribing Opioids for Chronic Pain in Patients Already on Opioids (Not Including Active Cancer).
Regular visits/assess patients on chronic opioid therapy regularly, at least every 2-3 months (Table 9) [IE]. With each prescription, review benefits versus risks of therapy [IE]. Titrate (adjust) the dose to clinical effect and consider whether taper is indicated.
Table 9
Visit Checklist for Patients on Chronic Opioids.
Monitor closely. Review the state prescription drug monitoring program (PDMP) report with each prescription [IE]. Calculate and monitor morphine milligram equivalents per day (MME/day) (Appendix C). Perform a urine drug screen at least once per year, and more often for patients who are at more than minimal risk [IE] (Appendix D). Watch for red flag behaviors (Table 10).
Table 10
Red Flag Behaviors That May Indicate Addiction or Diversion.
Indications for opioid discontinuation. If functional goals have not been met, adverse effects occur, or medication misuse is present (Table 10), consider opioid dose reduction, discontinuation, or conversion to buprenorphine [IIE]. In cases of opioid diversion, discontinue opioids [IE] and contact local law enforcement. In less urgent situations, discontinue using a rapid or slow taper [IE] (see Appendix F).
Screen for opioid use disorder. Assess for opioid use disorder, and consider complex persistent dependence [IE]. When present, refer to a specialist or offer treatment, including buprenorphine [IE].
Footnotes
- *
Strength of recommendation: I = generally perform; II = may be reasonable to perform; III = generally do not perform.
Level of evidence supporting a diagnostic method or an intervention: A = Systematic review of randomized controlled trials; B = randomized controlled trials; C = systematic review of nonrandomized controlled trials, nonrandomized controlled trials, group observation studies; D = Individual observation descriptive study; E = expert opinion.
Clinical Problem and Current Dilemma
Pain is often undertreated or incorrectly treated.
Chronic pain affects 50-80 million Americans.
Primary care clinicians manage the majority of patients with chronic pain.
The nationwide opioid epidemic adds complexity to the management of chronic pain.
Pain is the most common reason for which individuals seek health care. Effective pain management is a core responsibility of all clinicians, and is a growing priority among clinicians, patients, and regulators. Despite increased attention, many patients’ pain remains under-treated or incorrectly treated.
The prevalence of chronic pain in the US is difficult to estimate, but its impact is profound. Fifty to eighty million Americans experience daily pain symptoms. The cost of pain management is approximately $90 billion annually. Chronic pain is the leading cause of long-term disability in the US. These numbers will only increase as our population ages, amplifying the need for effective, accessible interventions to manage chronic pain and preserve function.
While multidisciplinary subspecialty pain services are increasingly available, primary care clinicians will continue to manage the majority of patients with chronic pain. This care can be challenging and resource-intensive, and many clinicians are reluctant or ill-equipped to provide it.
The current nation-wide opioid epidemic adds another layer of complexity in the management of chronic pain. Opioids carry substantial risk for harm, and are not recommended for the majority of patients with chronic pain. However, due to high rates of opioid prescribing over the last 20-30 years, there are still many patients who remain on chronic opioid therapy. With the widespread adoption of the CDC opioid-prescribing guidelines in 201611, rates of opioid prescriptions have decreased. In some cases, inflexible application of these guidelines has led to patient abandonment and poor outcomes. Prescribers need training, resources, and support to manage patients taking opioid medications in a compassionate and safe manner. There is also a need for better patient access to non-opioid pain management services and treatment for opioid use disorder.
This guideline is intended to support clinicians in evaluating and managing patients with pain and in navigating the complex issues involved with the use of opioids for pain management.
Acute and Subacute Pain – Overview
Definitions
Acute pain is associated with tissue damage and inflammation, with pain resolving as tissue heals.
Subacute pain, a subset of acute pain, may be present for 6 weeks to 3 months as tissue heals.
Chronic pain is a different medical condition involving abnormal peripheral or central neural function.
Acute pain is always associated with tissue damage; as tissue heals, pain should resolve. The definition of acute pain in the Michigan health code focuses on the cause and limited duration: “pain that is the normal, predicted physiological response to a noxious chemical, or a thermal or mechanical stimulus, and is typically associated with invasive procedures, trauma, and disease and usually lasts for a limited amount of time.” The International Association for the Study of Pain (IASP) further emphasizes the time limit for acute pain: it is pain lasting less than 3 months.
Subacute pain is a subset of acute pain: pain that has been present for at least 6 weeks but less than 3 months. This definition reflects the process of tissue healing. The worst of the acute pain phase and inflammation is no longer present, but ongoing tissue healing is required for full resolution.
Chronic pain has little in common with acute pain and should be considered as a separate medical condition. Some differences are:
| Acute Pain | Chronic Pain |
|---|---|
| Is a symptom | Is a diagnosis |
| Is associated with tissue damage | May or may not be associated with tissue damage |
| Lasts a limited time | Does not resolve quickly |
| May respond to opioid therapy for a limited time | Opioid therapy is generally not indicated |
| Has an inflammatory component | May or may not involve inflammation |
The differing pathophysiology for acute pain and chronic pain requires different approaches to their diagnosis and treatment. Effective acute pain management has been shown to improve both patient satisfaction and treatment outcomes, and reduce the risk of developing chronic pain.
Diagnosis and Treatment
Recommendations
Diagnose the cause of acute pain.
- Identify the medical or surgical condition for which acute pain is a symptom.
- Determine whether underlying cause is acute nociceptive pain or acute neuropathic pain.
- Assess the degree of functional impairment to help determine the urgency for addressing the acute pain issue.
Treating acute pain
- Consider the degree of tissue trauma, the patient’s situation, and unique patient factors.
- Select a treatment appropriate for the underlying source of pain (nociceptive or neuropathic).
- Adjust the treatment plan if reinjury or pain exacerbation occurs during the subacute phase.
Diagnosis. Identify the medical or surgical condition for which acute pain is a symptom (see Table 1). Often the cause is obvious or revealed by the history. If the diagnosis is not immediately clear, history, physical examination, laboratory tests, and imaging may all be employed to arrive at the diagnosis.
Table 1
Acute Pain Overview by Severity and Cause.
Determine whether this is acute nociceptive pain (signaled to the brain via normally functioning afferent neural pathways) or acute neuropathic pain (dysfunctional neural functioning). Nociceptive and neuropathic pain are described in more detail below, under “General Approach to Chronic Pain”. This classification helps guide the treatment plan and medications to prescribe.
In some cases, the cause is not immediately obvious, but the category of pain is. For example, burning pain starting in the neck and radiating into the fingers could be associated with acute cervical radiculopathy or may evolve to reveal zoster. Both are types of acute neuropathic pain. Strategies would include reducing inflammation, quieting of nerves. and further diagnostic work up to determine the exact cause. Weakness may point towards radiculopathy, while the presence of a rash points towards zoster.
Assess the degree of functional impairment to help determine the urgency for addressing the acute pain issue. For example, weakness may require a more aggressive strategy with early intervention, such as advanced imaging. If a patient is no longer able to carry on a usual routine or activities of daily living due to acute pain, an aggressive diagnostic workup is needed. An aggressive workup is also required in patients with a history of malignancy or immunosuppression.
Treatment. In the treatment plan, address both the underlying cause and the associated acute pain. In developing a treatment plan for the acute pain, consider the degree of tissue trauma, the patient’s situation, and any unique patient factors. A patient in the immediate postoperative period after a major surgery will likely have more complex needs than a patient presenting for an ambulatory encounter.
The hallmark of acute pain is tissue inflammation. Acute pain can be nociceptive or neuropathic. Accordingly, measures to reduce inflammation are helpful when developing a treatment plan for acute pain conditions. Some treatments to consider for acute pain include those listed in the table below:
| Nociceptive | Neuropathic |
|---|---|
| NSAIDS Acetaminophen Steroids | Gabapentinoid anticonvulsants (gabapentin, pregabalin) |
| Topical anesthetics | |
| Duloxetine Nortriptyline/amitriptyline | |
| Nerve blocks | Nerve blocks |
| Ice, rest, elevation | Capsaicin |
| Distraction, TENS unit | TENS unit |
| Physical therapy, stretching | Desensitization therapy |
| Opioid based medications | |
Muscle relaxants
|
Plan for treatment of reinjury or exacerbation during the subacute pain phase. Often subacute pain occurs with increase in activity before tissue is completely restored to health. Have a plan to escalate analgesic needs for this well-defined occurrence. For example, anticipate how pain with physical therapy should be treated.
General Approach to Chronic Pain
Chronic pain is best understood as a disease process rather than a symptom.
Use a biopsychosocial approach when assessing and managing chronic pain.
Underlying mechanisms for chronic pain are:
- Nociceptive – tissue damage
- Neuropathic – sensory nervous system damage
- Central – heightened pain sensitivity in the central nervous system
Chronic pain has significant cognitive, affective, and interpersonal components.
Effective chronic pain management is focused on maximizing function and limiting disability, not just on reducing pain.
A chronic primary pain syndrome represents a disease that cannot be accounted for by another pain condition.
A chronic secondary pain syndrome initially manifests as a symptom of another disease and then continues after successful treatment of the disease.15
Biological and Psychosocial Factors
Chronic pain – pain that lasts or recurs for longer than 3 months – is not merely acute pain that does not resolve. Increasingly, chronic pain is recognized as a disease entity in and of itself, rather than as a symptom of another disease. Historically, pain has been viewed in a biomedical model, with a focus on identifying a specific pathologic cause of pain which can be treated through pharmacologic or interventional means. However, chronic pain is better understood by applying a biopsychosocial model. Chronic pain is a complex multi-dimensional condition, driven by the interplay of neurobiologic processes with psychosocial factors that may increase vulnerability or resilience to disease.16 A biopsychosocial approach allows the focus to move from the source of the pain to the management of its impact.
Neural mechanisms of Pain. Understanding the basic neurobiological mechanisms in chronic pain pathophysiology is important, since treatment approaches vary depending on these factors. There are three main subtypes of pain pathophysiology: nociceptive, neuropathic, and central sensitization. They are summarized below, with more detail regarding classification in Table 2.
Nociceptive pain is caused by tissue damage due to injury or inflammation, rather than harm to the central or peripheral nervous system. This is the primary type of pain involved in patients with arthritis, musculoskeletal inflammatory disorders (tendinosis, bursitis), or structural spine pain.
Neuropathic pain results from damage to the sensory nervous system. Patients typically describe electric, burning, or tingling sensations. Examples of neuropathic pain include post-herpetic neuralgia, diabetic neuropathy, and trigeminal neuralgia.
Central sensitization occurs when there is heightened pain sensitivity in the central nervous system that is not due to a peripheral pain signal generated by an injury or disease state. Central pain is driven by molecular and structural changes that occur in the central nervous system. It is the primary mechanism in conditions such as fibromyalgia, phantom limb syndrome, and chronic pelvic pain.
Psychosocial factors. Chronic pain has significant cognitive, affective and interpersonal components. Patients with chronic pain are more likely to report depression, anxiety, poor quality of life, and financial stress. They are five times more likely to use health care resources than patients without chronic pain. Pain beliefs and the individual and family response to chronic pain are also important factors.
Chronic Primary and Secondary Pain Syndromes
A classification system for chronic pain syndromes has been devised by the International Association for the Study of Pain (IASP), as outlined in Table 2.
Chronic primary pain syndromes. These syndromes represent a disease itself. A chronic primary pain syndrome is defined as pain in one or more anatomical regions that persists or recurs for longer than 3 months and is associated with significant emotional distress or functional disability (interference with activities of daily life and participation in social roles) and that cannot be better accounted for by another chronic pain condition.17
Chronic primary pain syndromes include:
- Fibromyalgia
- Complex regional pain syndrome
- Chronic primary headache and orofacial pain
- Chronic primary visceral pain
- Chronic primary musculoskeletal pain
Chronic secondary pain syndromes
Each of these syndromes initially manifests as a symptom of another disease. After healing or successful treatment, chronic pain may sometimes continue and hence the chronic secondary pain diagnoses may remain and continue to guide treatment (Table 2).15
Chronic secondary pain syndromes include:
- Cancer-related pain (eg, from tumor mass or treatment)
- Chronic postsurgical or posttraumatic pain
- Chronic neuropathic pain
- Chronic secondary headache or orofacial pain
- Chronic secondary visceral pain
- Chronic secondary musculoskeletal pain
Establishing the diagnosis of a specific chronic pain syndrome can be an important first step in providing clarity for the care team, psychoeducation for patients, and direction for treatment considerations. In order to arrive at a diagnosis, perform a thorough biopsychosocial assessment.
Designing an Individualized Pain Treatment Plan
Recommendations
Use shared decision-making to develop an Individualized Pain Treatment Plan that promotes patient self-management.
Preferred therapy is non-pharmacologic or non-opioid pharmacologic and involves multiple modalities.
Avoid long-term opioid prescriptions for chronic pain. Opioids carry substantial risks of harm.
Identify and address clinician and health care system barriers to care.
Steps in creating an Individualized Pain Treatment Plan are outlined in Table 4.
Shared Decisions for Individualized Treatment
A trusting patient-clinician relationship is key to the development of an effective treatment plan for chronic pain. Construct a unique plan for each patient, taking into consideration the individual’s experience, circumstances, and preferences. The treatment plan should involve multimodal interventions, promote self-management, and enlist the involvement of a health care team. Use a shared decision-making approach, where patients and clinicians discuss values and preferences, review risks and benefits, and make a decision congruent with patient goals and preferences.29 Frequently reassess and adjust the plan to address barriers to care.
Key to developing an effective treatment plan is a supportive relationship with an empathetic clinician who acknowledges and empathizes with the patient’s experience. Set expectations regarding the available treatments for chronic pain. Establish realistic treatment goals for functional improvement or maintenance, not analgesia alone. Inform the patient that finding the right approach may take time.30 Facilitate patient self-management and provide pain psychoeducation (see Appendix H). A team-based approach is helpful in this effort, involving other clinical disciplines such as nursing or behavioral health consultants to provide coaching, education, and support.
A logical rationale for an intervention does not ensure the patient’s acceptance and participation in it. A patient’s acceptance of therapy is influenced by several complex factors, including characteristics of illness and identity. Patient preferences often favor physical rather than psychological intervention, but gains of psychological therapies may exceed patient expectations.31
Preferred Interventions
Non-pharmacologic therapy and non-opioid pharmacologic therapy are preferred for the treatment of chronic pain.11 There is insufficient evidence to support the use of long-term opioid use for chronic pain. Opioids carry substantial risks of harm. Use shared decision-making to choose treatment interventions. A single intervention is unlikely to be fully effective for chronic pain, since chronic pain is a complex disease process with multiple contributing biopsychosocial factors. Combining several modalities, and emphasizing self-management is most effective.32 (Figure 1)
Clinician and Health Care System Barriers
When patients with chronic pain feel judged or scorned by health clinicians, this stigma can be a significant barrier to effective care. Similarly, clinicians caring for patients with chronic pain often experience negative emotions such as frustration, lack of appreciation, and guilt.30
Patients and clinicians alike encounter frustration when confronted with barriers within the health care system. Common barriers include difficulty in accessing care, limited time for visits, and inadequate reimbursement for evidence-based treatments.
A team-based approach, adequate consultative support, and training can begin to address some of these barriers. Patients may have individual barriers to accessing care or participating in self-management. Provide them with specific support as needed.
Assess cognitive and verbal ability
For patients with cognitive and/or verbal disability, when analgesic plan involves a caregiver, caregivers should receive additional education on pain assessment. Providers should also carefully assess function and goals with both patient and caregiver.
- Assess fall risk, cognition, respiratory status, and risk for sleep disordered breathing prior to prescribing opioids.
- Reduce the initial dose of opioids by 25-50% and titrate slowly to avoid over sedation.
- Consider using a non-verbal pain scale such as CPOT (Critical Care Pain Observation Tool) or FLACC (Face, Legs, Activity, Cry, and Consolability) to assess efficacy of pain medications.
- Exercise universal precautions for controlled substance prescribing and limit pill count for patients at risk of having their medications diverted
- - Schedule frequent follow up with patients
- - Consider random urine drug screen (UDS)
- - Consider pill counts
- Emphasize the importance of keeping medications secure and locked to care provider/home manager.
- Provide disposal information for unused pills
- Ensure caregiver receives education on appropriate Intranasal Narcan use and administration to the patient if indicated
Health inequity and disparity
Many patient populations are unintentionally marginalized by both health care providers and health systems. This inequity is especially true with regard to pain management amongst non-white Hispanic, black, and other minority populations.33,34 Several factors should be considered when treating these vulnerable patients. It is the provider’s responsibility to recognize that inequity in this area is due in part, but not limited to, systemic barriers and complex influences such as implicit biases unbeknownst to providers. For example, patients with sickle cell disease frequently report difficulties in obtaining adequate pain relief from providers during a vaso-occlusive crisis. In this vulnerable population, studies have shown delays in administering pain medications due to accusations of drug seeking behavior, exaggeration of pain, and uninformed or negative attitudes held by providers concerning sickle cell disease.35
To diminish these inequities surrounding pain management, providers should attempt to remove as much individual discretion from decision making as feasible. When possible, providers should utilize resources such as: checklist, guidelines, or system protocols to avoid the influences of implicit biases on their management. Providers need also recognize access limitations faced by patients and ensure any treatment regimen or follow-up planning is readily accessible. An important consideration is to involve these patients in shared decision making while offering all available treatment options to circumvent and mitigate any healthcare related obstacles these patients may encounter. Alternative options should then be explored based on individual circumstances.
Non-Pharmacologic Treatment
Recommendations
Lifestyle management. For all patients, recommend:
- Regular exercise. Start small, gradually increase to at least 150 minutes/week at moderate intensity. Adjust this goal to the individual’s status.
- Teach good sleep habits. Screen for sleep disturbance. Consider sleep quality, post sleep evaluations, and sleep disordered breathing.
- A Mediterranean pattern of eating to lower inflammation and maintain a healthy weight.
Physical modalities:
- Consider physical therapy when patients have functional deficits or secondary pain generators.
- Consider massage therapy as part of a multimodal treatment plan.
Behavioral health interventions. Evidence-based interventions include mindfulness-based stress reduction, cognitive behavioral therapy, acceptance and commitment therapy, and self-regulatory and psychophysiological approaches (eg, biofeedback, relaxation training, hypnosis). See Appendix H.
- Refer patients with significant psychological issues (eg, comorbid psychiatric condition, previous trauma, challenges in managing and coping) to a psychologist or therapist.
- Consider referring any patient with chronic pain to a psychologist or therapist to address the psychological effects of chronic pain.
Integrative medicine:
- For interested patients, consider combining or coordinating historically non-mainstream practices that are evidence-based (eg, acupuncture, herbal supplements) as part of a multimodal treatment regimen.
- Evidence regarding the benefits and harms of marijuana for chronic pain is insufficient to recommend its use. Limited data support that using cannabidiol (CBD) alone is safe.
Non-pharmacologic options for treating chronic pain are summarized in Table 5.
Lifestyle Management
Exercise. For all patients recommend regular exercise as a component of multimodal treatment. Decrease the patients’ fear of movement. Encourage a progressive aerobic exercise program with a goal of at least 150 minutes of moderate-intensity exercise weekly. Adjust this goal for each individual’s physical status.
Exercise is structured, repetitive, physical activity to improve or maintain physical fitness. In patients with chronic pain, exercise improves both function and chronic pain symptoms, in addition to overall health and quality of life. Forms of exercise that have been studied include aerobic exercise, resistance-based exercise, water-based exercise, and styles of exercise such as yoga (for chronic primary musculoskeletal pain36), tai chi (for chronic primary musculoskeletal pain, osteoarthritis, osteoporosis, neck pain37), and Pilates (for chronic primary musculoskeletal pain neck pain, osteoporosis38). Studies vary considerably in mode of exercise, content of program, frequency, and duration of activity. In most studies, the frequency of exercise was between 1–5 times per week, averaging 2–3 times a week. Another factor was whether activities were performed in supervised sessions or as home exercises, with home exercises potentially increasing the frequency of the activity. Prescribed exercise was generally moderate to moderately-high in intensity. No one type of exercise has been shown to be superior to another in all patient populations.
Sleep. For all patients recommend good sleep habits. Screen for sleep disturbance. Sleep complaints occur in 67–88% of individuals with chronic pain. Sleep and pain are often linked. Sleep disturbances may decrease pain thresholds and contribute to hypersensitivity of neural nociceptive pathways. Conversely, pain may disturb sleep. Nonpharmacologic sleep treatments are associated with improved fatigue and sleep quality. However, the effect on pain is comparatively modest and short-lived.
Diet. Recommend a Mediterranean pattern of eating to lower inflammation and maintain a healthy weight. Although inflammation is part of the nociceptive process, research into the role of diet in modifying inflammation is in its early stages. The Mediterranean pattern of eating, characterized by a high intake of fruits, vegetables, whole grains and an emphasis on omega-3 fatty acids, has been established as a dietary pattern that lowers inflammation especially in the setting of cardiovascular disease.39 Emerging evidence shows connections between the Mediterranean pattern, lowered inflammation, and improvement in pain and function in osteoarthritis.40
Physical Modalities
Physical therapy. If patients have functional deficits or secondary pain generators that directed therapy may improve, refer them to physical therapy.
The goal of physical therapy is to improve function. Therapeutic exercise, other modalities, manual techniques, and patient education are part of a comprehensive treatment program to accomplish this goal.
- Modalities such as hot packs, ice, ultrasound, transcutaneous electrical nerve stimulation (TENS), iontophoresis, and traction may decrease pain and increase tissue extensibility, thereby facilitating stretching and mobilization. Table 4 reviews selected modalities.
- Manual therapy helps optimize proper mobility, alignment and joint biomechanics.
- Therapeutic exercise consisting of stretching, strengthening, conditioning, and muscle re-education is useful in restoring joint range of motion, muscle strength, endurance, and to correct muscle imbalances.
Evidence is limited regarding the long-term benefit of any single individual treatment modality. However, they may be used as part of a multimodal treatment program to improve function, quality of life, and alleviate pain.
The basic components of a physical therapy prescription include:
- Diagnosis for which therapy is being prescribed.
- Therapeutic protocol for treatment, including therapeutic exercise, other modalities, and manual techniques to be employed or tried.
- Duration and frequency of desired therapy.
- Precautions.
When treatment goals have been met or when progress plateaus, formal therapy may be discontinued, but advise patients to continue with a program of independent daily home exercise.
Transcutaneous electrical nerve stimulation (TENS). Consider TENS either along with physical therapy or as an adjunct to multimodal treatment. TENS applies low voltage electrical stimulation using skin contact electrodes. Proposed mechanisms of action include gate control theory, endorphin theory, and augmentation of descending inhibition. Evidence is limited for the efficacy of TENS in pain management.41 However, it is relatively safe, with units relatively available and easy to use.
Do not use TENS near implanted or temporary stimulators (eg, pacemakers, intrathecal pumps, spinal cord stimulators), near sympathetic ganglia or the carotid sinus, near open incisions or abrasions, over thrombosis or thrombophlebitis, or in pregnancy. Use caution with patients with altered sensation, cognitive impairment, burns, malignancy, or open wounds.41
Massage therapy. Consider massage therapy as part of a multimodal treatment plan. Massage therapy is manual manipulation of muscles and connective tissue to enhance physical rehabilitation and improve relaxation. It can reduce pain scores for patients with low back pain,42 knee osteoarthritis,43 juvenile rheumatoid arthritis,43 chronic neck pain,43 and fibromyalgia.42 Not yet determined are the optimal number, duration and frequency of massage sessions for treating pain.
Behavioral Health Approaches
Refer patients with significant psychological issues (eg, comorbid psychiatric condition; previous physical, emotional, or sexual trauma; challenges in managing and coping) to a psychologist or therapist. Consider referring any patient with chronic pain to a psychologist or therapist to address the psychological effects of chronic pain. These interventions can be successful regardless of the patient’s baseline status.
Current psychological interventions for chronic pain are based on recent advances in our understanding of the complexity of pain perception. Pain is influenced by a wide range of psychosocial factors, such as emotions, sociocultural context, and pain-related beliefs, attitudes and expectations.
Chronic pain that persists for months or years often initiates a progressive loss of control over numerous aspects of one’s psychological and behavioral function. A biopsychosocial model is now the prevailing paradigm for interventional strategies designed to treat chronic pain. This model places an emphasis on addressing cognitive-behavioral factors pertinent to the patient’s pain experience.
The strong evidence for the contribution of psychosocial factors in pain experience, particularly in explaining disability attributed to pain, has led to the development of multidisciplinary pain rehabilitation programs (MPRPs) that simultaneously address physical, psychological, and functional aspects of chronic pain disorders. For some patients, referral for individual behavioral and psychological intervention may be all that is required.
Cognitive behavioral therapy (CBT). The way patients think about themselves, others, and the future can have a major impact on their moods, behavior, and physiology. The two main tenants of CBT approaches to chronic pain are:
- The feeling of pain and the emotional, physical, and social impact of pain are interrelated, but can be separated for treatment purposes. Therefore, problems with functioning related to pain can be addressed even if pain is not targeted directly and remains unchanged.
- Psychological factors can influence the experience of pain itself.
Cognitive restructuring involves several steps that help to modify the way in which patients view pain and their ability to cope with pain. Treatment approaches that incorporate these principles can produce significant benefits, such as reduced pain, improved daily functioning, and improved quality of life.44–48
Mindfulness-based stress reduction. Mindfulness is a process of openly attending, with awareness, to one’s present moment experience.49 Mindfulness aims to empower patients to engage in active coping by encouraging them to be aware of the present, where difficult thoughts, feelings, and sensations are acknowledged and accepted without judgement.50
Mindfulness based stress reduction (MBSR) may improve pain function in people with chronic pain. MBSR can provide patients with long-lasting skills effective for managing pain.34 Strong evidence shows that MBSR reduces functional disability and improves pain management for a variety of chronic pain conditions including low back pain,51 fibromyalgia, rheumatoid arthritis, and patients with opioid misuse. The most studied intervention uses an 8-week format of 2-hour/week classes, a 6-hour day in the middle, and daily at-home audio recordings.
The mechanism of action for mindfulness-based strategies is unknown. It seems to be multifactorial, including both physical changes in the stress response system that drive markers of inflammation, as well as psychological mechanisms such as stress resilience and coping.52
Acceptance and commitment therapy (ACT). ACT is a form of CBT. In some cases, trying to control or change pain and thoughts about pain can be counterproductive. ACT is an alternative way to increase acceptance of some of the aspects of chronic pain that may be difficult to alter. Acceptance may free individuals to pursue activities in line with their values.53 The ACT clinical model has six core processes:
- Acceptance of events and your feelings around them.
- Perceiving things as they are.
- Being present and mindful.
- Observing yourself in context.
- Identifying personal values.
- Setting goals based on your values and committing to actions in accordance with those goals.54
Various methods of delivering ACT have been shown to be effective in treating chronic pain55 either as an individual face-to-face intervention,56,57 a group-delivered face-to-face intervention,58–66 via self-help books,67,68 or through an internet-based delivery.69–73 ACT based therapy has been shown to decrease pain, improve function, and improve quality of life.
Self-regulatory and psychophysiological approaches. The experience of chronic pain elicits strong physiological reactions that are often accompanied by cognitive thoughts and processes. Several simple techniques harness the connection between the mind and body to improve awareness of and increase control over both psychological and physiological responses to pain.
Techniques include biofeedback, relaxation training, and hypnosis. Biofeedback provides real-time information about physiological processes (eg, heart rate, respiratory rate, muscle tension) with a goal of increasing voluntary control over them. It is often coupled with relaxation training (deep breathing or conscious focusing on relaxation). Biofeedback can decrease the frequency of pain, improve self-management, and decrease use of analgesic medications in both migraine and tension type headaches in adults and adolescents.74,75 Hypnosis is a state of increased attentional awareness leading to a state of increased relaxation. It has been examined in a variety of pain conditions and found to be effective in decreasing pain most pain conditions, with a reduction in overall pain between 29–45%.76,77 Effects of specific analgesic hypnotic suggestion were strongest in individuals of high to moderate suggestibility. Most people fall within those two categories, indicating a majority of people would benefit.76 A 2020 meta-analysis indicates a possible role for hypnosis in decreasing opioid medication, with hypnosis moderately reducing pain levels coupled with small reductions in opioid dosing.78
Integrative Medicine
For interested patients, consider adding historically non-mainstream practices that are evidence-based as part of a multimodal treatment regimen. As evidence emerges regarding the biological role of these treatments, their utility may change.
Integrative medicine is an approach that combines and coordinates conventional medicine with evidence-informed practices that historically are not mainstream. Emerging evidence suggests a role for many less conventional treatments in the management of chronic pain due to their benefits and safety compared to opioid therapy. In addition to previously noted treatments (massage, yoga, tai chi, mindfulness), accumulating evidence supports the use of acupuncture and herbal supplements. More information may be found at the Center for Complementary and Integrative Health at https://nccih.nih.gov/.
Acupuncture. Acupuncture in traditional Chinese medicine uses the insertion of needles into specific areas to manipulate anatomical energetic meridians. The nature of the psychological effect continues to be debated, but efficacy has been established for many chronic pain conditions.79 The best evidence exists for osteoarthritis, chronic neck and low back pain, fibromyalgia, and headache. Treatment frequency varies, with the most commonly cited being 1–2 times per week for 4–8 weeks. Some studies show effects lasting 6–12 months.80
Herbal supplements. Patients frequently request information about herbal supplements. The evidence for the use of some supplements is growing. Many are safe and may be considered when patients are interested. See Table 6.
Marijuana. Evidence regarding benefits and harms is currently insufficient to recommend using “medical” marijuana for chronic pain. Some data support cannabidiol (CBD) alone as being relatively safe.
With an increasing number of states legalizing marijuana, clinicians and patients are asking about the use of cannabinoids to treat a variety of conditions. The cannabis plant produces many phytocannabinoids, with the highest concentration of these being tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is the molecule with psychoactive properties that appears to be responsible for most adverse effects. This remains a challenging and complex area to address. Regulatory and historical factors have resulted in very limited evidence concerning the endocannabinoid system and cannabis pharmacology.
Systematic reviews have found that cannabinoids may be modestly effective for some chronic pain, primarily neuropathic pain, based on limited evidence,43,44 However, the evidence is largely based on studies of high THC-containing products, which also show high rates of adverse events, such as sedation and psychomotor impairment. In the absence of regulation, the potency and composition of cannabis products are highly variable. Due to these factors, evidence is currently insufficient to recommend using marijuana for relief of chronic pain.
As new evidence begins to emerge regarding the possible role of CBD in analgesia and anti-inflammatory pathways, we may see a role for CBD alone or for products with a high CBD: THC ratio in chronic pain.81,82 For patients wishing to use CBD alone, some data support CBD as being relatively safe, although there are some potential cytochrome P450 metabolism interactions that should be reviewed. In 2018 the US Drug Enforcement Administration (DEA) reclassified the CBD-based product Epidiolex as Schedule V, which is the least restrictive schedule; however, it is only approved or studied in the setting of two forms of rare seizure disorder. CBD is not recommended for first-line therapy for the treatment of chronic pain. However, patients who have failed other treatments or are opioid dependent may be started on low (5–10 mg twice daily) doses, with slow increases of dose.81 Of note, these products are not regulated and therefore it is unclear how to determine dose or quality so these products should be considered with caution.
Non-Opioid Pharmacologic Treatment
Recommendations
Consider prescribing systemic or topical non-opioid medications as an adjunct to the non-pharmacologic treatments noted above. Medications often have limited effectiveness, significant interactions or toxicity, and may promote false beliefs about the benefit of medications.
Select medications based on:
- Known effectiveness for specific pain mechanisms (nociceptive, neuropathic, central sensitization)
- Potential to treat comorbid disorders, such as insomnia or mood disorder.
Prescribe an adequate trial of days to weeks of scheduled dosing. Avoid as-needed medication use.
Discontinue all ineffective medications to avoid polypharmacy, minimize toxicity, and limit unrealistic beliefs about the benefit of medications.
Several classes of medications can be part of effective chronic pain management, including acetaminophen, non-steroidal anti-inflammatory medications (NSAIDs), anticonvulsants, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), muscle relaxants, and topical agents. Other non-opioid medications (eg, certain antidepressants and anticonvulsants) may simultaneously treat comorbid problems (eg, mood disorder, insomnia). Classes of non-opioid medications used for chronic pain and their potential benefits and harms are summarized in Table 7.
A successful regimen may combine low doses of different types of pain medications to treat different mechanisms of perceived pain simultaneously, increasing medication effectiveness while limiting the risk of toxicity.
- Primary pain syndromes such as chronic widespread pain (eg, fibromyalgia), headaches, and primary visceral pain (eg, irritable bowel syndrome) may respond to SNRIs, TCAs or anticonvulsants.
- Neuropathic pain may respond to those classes of medications as well as some topical agents.
- Nociceptive pain may respond to acetaminophen, NSAIDs, muscle relaxants, or topical agents.
- Neuropathic pain may respond to SNRIs, TCAs, anticonvulsants, or topical agents.
- Central pain syndromes such as chronic widespread pain (eg, fibromyalgia), headaches, and primary visceral pain (eg, irritable bowel syndrome) may respond to SNRIs, TCAs, or anticonvulsants.
Acetaminophen. Acetaminophen may occasionally be a useful medication to treat mild to moderate chronic pain, whether given as needed (“PRN” dosing), or at scheduled intervals. When combined, an NSAID and acetaminophen can be synergistic and equal to or more effective than acetaminophen plus an opioid.83
For healthy people, avoid total acetaminophen doses > 3 g/day (2 g/day in patients with chronic liver disease). Acetaminophen may cause small increases in the risk for upper gastrointestinal bleeding and small elevations of blood pressure.84
Non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs are among the most widely used medications in the US. Long-term NSAID therapy for chronic pain may benefit some patients, particularly those with defined pain generators and who are at low risk for complications. For low back pain, use of an oral NSAID is somewhat more effective than placebo for analgesia,85 but only slightly for disability. Celecoxib is more effective in low back pain than acetaminophen, but the effect is modest.86
Chronic NSAID use poses significant risks for gastrointestinal bleeding, acute kidney injury or chronic kidney disease, and platelet dysfunction. Older age adds particular risk. Older adults receiving daily NSAIDs for six months or more face a 6-9% risk for upper gastrointestinal bleeding requiring hospitalization. For high risk patients for whom NSAIDs have proved to be the only effective treatment, consider proton-pump inhibitors for upper gastrointestinal prophylaxis.
NSAIDs may also increase risk for exacerbations of hypertension, heart failure, and chronic kidney disease. NSAID use in patients with heart disease or its risk factors increases the overall risk of heart attack or stroke.
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
SNRIs (duloxetine, venlafaxine, or milnacipran) can benefit patients with a variety of pain syndromes, including non-specific low back pain, neuropathic pain of various origins, functional abdominal pain, and central pain syndromes such as fibromyalgia. For low back pain, duloxetine at doses up to 120 mg/day reduced both non-specific and neuropathic symptoms. Its mechanism of action seems to be independent of any antidepressant effect. SNRIs are somewhat more effective for functional abdominal pain than tricyclics.87 Duloxetine is FDA-approved for diabetic neuropathy and fibromyalgia, though it improves pain scores more than function.
SNRIs are generally well-tolerated, but discontinuing an SNRI requires a gradual tapering down of the dose to avoid withdrawal symptoms, which can occasionally be severe.
Anticonvulsants. Anticonvulsant medications such as gabapentin, pregabalin, and topiramate can be effective for treating neuropathic pain. Dosing can be complex. They have significant adverse effects and are often only modestly effective. Additionally, use topiramate with caution in reproductive-aged women because it increases the risk of cleft lip and cleft palate in newborns.
Pregabalin is approved for the treatment of diabetic neuropathy and fibromyalgia, though it improves pain scores more than function. Gabapentin has only minor benefit in chronic daily headache or migraine. It is not effective in chronic non-specific low back pain.61,88 Gabapentin and pregabalin are not effective in acute low back pain.
Older anticonvulsants such as carbamazepine and phenytoin have some efficacy for neuropathic pain, but are associated with frequent adverse effects, drug-drug interactions and potentially severe adverse reactions, such as granulocytopenia and hyponatremia.
Pregabalin is a federal Schedule V controlled substance, and gabapentin has been scheduled in many states. Both of these medications produce an increased addiction risk. When combined with opioids, they have been associated with a small increase in death rate. Advise patients treated with gabapentin or pregabalin about increased appetite and the potential for rapid and marked weight gain.
Topiramate at higher doses has been associated with significant speech and cognitive effects
Tricyclic antidepressants (TCAs). TCAs may be potentially useful in a variety of pain syndromes, particularly in neuropathic pain and headaches. They also may benefit comorbid disorders such as insomnia, anxiety, depression, panic disorder, and even smoking cessation efforts. TCAs may have particular use in neuropathic pain, vascular headache prophylaxis, and centralized pain syndromes such as fibromyalgia. Trial data suggest only a modest benefit in functional abdominal pain and less benefit than SNRIs.89 In chronic low back pain, low dose TCAs resulted in somewhat less disability at 3 months but had less effect at 6 months.90
Doses required for pain treatment are lower than for mood disorders. The lower doses generally avoid problems such as QT prolongation. For patients with sleep initiation problems, taking a TCA at dinnertime rather than bedtime may reduce problems with sleep initiation and with morning fatigue.
When a TCA is used for pain or mood, the time needed for a response can be days to weeks.
TCAs may have adverse effects that can limit their usefulness, such as anticholinergic effects and dysrhythmias. Caution patients about enhanced appetite and the potential for weight gain. Constipation prophylaxis may be needed.
Muscle relaxants. Sedating or non-sedating muscle relaxants are often prescribed for chronic myofascial pain, despite little or no evidence for a long-term benefit.88 Cyclobenzaprine, tizanidine, and metaxalone can cause significant sedation, while methocarbamol is less likely to do so. Benzodiazepines pose a significant risk for long-term dependence and misuse, and they substantially increase the danger of overdose when used together with opioids. Baclofen, while somewhat useful for spasticity, has little role as a muscle relaxant, poses a significant risk for dependence, and should generally be avoided.
Topical agents. Topical NSAIDs and anesthetics are occasionally useful in nociceptive or neuropathic pain syndromes. They can be expensive and are often not covered by insurance.
- Topical lidocaine patches (prescribed or over-the-counter) can be effective. Ointment is less effective and can be messy. Both are expensive and often not covered by insurance. Over the counter 4% lidocaine cream is not expensive, but only marginally effective.
- Capsaicin cream (1%, not 0.25%) can be modestly effective, is available without prescription, but requires care in application to avoid unwanted burning. Compounded capsaicin 8% cream is more effective, but the cost may be prohibitive.
- When other treatments have failed, topical nitroglycerin may have some effect for wound pain, anal fissure pain, vulvodynia, and diabetic neuropathy.
- Compounded topical 5% morphine can provide local wound analgesia and may promote healing. It is only available at compounding pharmacies and can be expensive.
Systemic effects of topical agents are generally minimal. Headache can complicate treatment with nitroglycerin. Avoid nitroglycerin in patients who use phosphodiesterase type 5 inhibitors (avanafil, sildenafil, tadalafil, vardenafil) for erectile dysfunction.
Opioids: Decision Phase
Recommendations
Assess factors that indicate whether opioids may be beneficial.
Consider potential risks of opioids:
- Potential risks of opioid use for all patients include: physical adverse effects; cognitive impairment; social, personal, and family risks; failing urine screening; potential for opioid misuse.
- Special populations – Patients with factors such as older age, pregnancy, lactation, or chronic illness have higher risks associated with opioid use (Table 8).
- Benzodiazepines – Generally do not initiate opioid therapy in patients routinely using benzodiazepine therapy. Both increase sedation and suppress breathing.
- Marijuana – Discourage concomitant use of THC- containing marijuana products and opioids. Marijuana’s adverse effects may compound those of opioids.
Assess the benefits and risks to determine whether an opioid will improve overall chronic pain management.
Decide whether to recommend adding an opioid to treatment.
Considerations for Opioid Use
Deciding whether to prescribe opioids is based on an assessment of benefits and harms. While opioids should never be the main treatment for chronic (or acute) pain, in some circumstances, opioids may complement other therapeutic efforts. Important considerations and branching decisions are illustrated in Figure 1 for opioid naïve patients and Figure 2 for patients already on opioids.
Potential Benefit
Assess factors that indicate whether opioids may be beneficial. Based on pain assessment, characterize the patient’s pain based on:
- Time frame: acute, subacute, or chronic.
- Mechanism: nociceptive, neuropathic, central, or a combination of these. Many pain states are the result of a “mixed picture.”
- Pain generators: list each painful area and determine each pain generator.
- Approximate percentage: establish the percentage of pain each pain generator is contributing to the overall clinical status.
A careful history can indicate the types of pain involved and guide treatment plans. For example, if NSAIDs provide significant relief, an inflammatory component to pain is likely. Note whether other modalities and medications have helped or not, and incorporate that information into the treatment plan. Use past experience to guide the decision to start membrane stabilizers (anticonvulsants) and other non-opioid therapies and to determine initial doses. As with all medical decisions, carefully consider risks and benefits.
Short-term opioid therapy may be appropriate for acute pain management to allow for rehabilitation. For chronic pain, opioid therapy is beneficial if it allows a return to function or maintenance of function with minimal adverse effects. If patients are not meeting functional goals during the course of therapy for nonmalignant pain, opioid therapy has failed and should be discontinued.
Potential Risks
Review the patient’s risks and determine if opioid-based therapy is likely to result in harm.
General risks. Potential risks for all patients include:
- Physical adverse effects. Common opioid adverse effects include nausea, constipation, pruritus, respiratory depression, and hot flashes. Chronic opioid use can alter endocrine function and may also lead to dry mouth and subsequent dental caries.
- Cognitive impairment. Patients new to opioids should not drive a vehicle or operate power equipment or heavy machinery until they see how they are impacted by the therapy.
- Social, personal, and family risks. Being an opioid user carries a risk for social stigma. Additional risks are inherent to possessing opioids, including becoming a target for home invasion. Insecure storage may put other family members and pets at risk for opioid poisoning.
- Failing urine drug screening tests. Some jobs require a negative urine drug screen, and employment may not be compatible with opioid therapy. Patient can be harmed financially and professionally if they screen positive for an opioid, even when prescribed and monitored by a clinician.
- Potential for opioid misuse or opioid use disorder.
- - Patients with depression, anxiety, or a history of substance use disorder are at risk.
- - Patients with active alcohol use disorder, illicit drug use, or a history of these problems are at risk.
Special populations. Older age, pregnancy, lactation, and chronic illness can impact the safety of opioid medications, so use extra caution with these patient populations. Specific considerations are outlined in Table 8.
Table 8
Special Populations for Whom Opioids Increase Risks. Risks of opioid therapy are higher in these populations. Non-pharmacologic and non-opioid pharmacologic therapies are preferred.
Benzodiazepine and opioids – a safety concern. Generally, do not initiate opioid therapy in patients routinely using benzodiazepine therapy. Both drugs are sedating and suppress breathing. Together they can cause a fatal overdose.
In select cases, co-prescribing may be warranted, such as use of a benzodiazepine for an MRI. In those cases, discuss the risks with the patient. Furthermore, consider the kinetics of each drug relative to the timing of procedures. For example, counsel patients taking hydrocodone daily to skip a dose if they need to take a benzodiazepine for an MRI; benzodiazepines and short-acting opioids should not be taken within two hours of each other. When clinicians inherit patients who are co-prescribed sedatives and opioids, carefully review the relative benefit of each medication and prescribe intranasal naloxone. Consider discontinuing one of the medications.
Marijuana, CBD, and opioids. Discourage concomitant use of THC-containing marijuana products and opioids. The adverse effects of cannabis products may compound similar effects with opioids, leading to safety concerns. Evidence about the combined use of cannabis and opioid prescriptions is limited and inconclusive at present.94 If opioids are otherwise indicated, current evidence is insufficient to recommend against prescribing opioids to patients using CBD alone.
Opioid Risk/Benefit Decision
As with all medications, consider the risks and benefits of prescribing an opioid.
Expected functional benefits of opioid use should be clear, with the continuation of opioid therapy dependent on achieving them. While improved sleep and mood are somewhat subjective and should be noted, seek more objective evidence of benefit in order to prescribe and continue opioid therapy. Consider the ability to walk farther, exercise longer, work more, etc. Before initiating opioid therapy, ask patients to identify the functional goals they wish to achieve with opioid therapy, then see if they are meeting these goals at follow up.
Occasionally opioids may have less risk than other pain management medications. Examples include patients vulnerable to gastrointestinal bleeding for whom NSAIDs are contraindicated and patients experiencing cognitive effects from membrane stabilizers.
Opioids: Initiation and Treatment Phase
An overview of prescribing opioids in opioid naïve patients is presented in Figure 1.
Drug Selection and Dosing
Recommendations
In selecting opioids, consider patient factors:
- History with opioids: opioid naïve or opioid tolerant
- Previous opioids used
- Special population factors (eg, older age, pregnancy); see Table 8
- Need for accompanying naloxone prescription.
Dosing:
- For initial daily doses, start with a short-acting opioid, and do not exceed 20 MME/day (oral morphine milligram equivalents per day).
- For up titration over time, do not exceed 50 MME/day.
Assess initial responses frequently. See the patient every 1-4 weeks. Titrate the dose and assess response within 2-6 weeks.
When the benefits of adding an opioid to other therapy outweigh the risks, select the initial drug and dose based on the:
- Patient:
- - Opioid naïve or opioid tolerant (ie, has been on ≥ 60 MME/day or 25 mcg/hour transdermal fentanyl for ≥ 7 days)
- - Patients previously treated with opioids
- - Special populations affecting dosing
- Drug:
- - Duration
- - Potency
- - Delivery mechanism
All opioids are essentially similar regarding effects and adverse effects. True allergy to any of them is very rare. Morphine and codeine may be slightly less well tolerated, but can be used unless adverse effects become intolerable or a medical contraindication is present.
Opioid naïve patients. In these patients, start with a short-acting opioid at a dose of ≤ 20 MME/day. Over time, the dose may be cautiously titrated. Do not exceed a daily dose of 50 MME.
Opioid tolerant patients. Morphine is the default choice, unless contraindicated. Morphine can be prescribed by all routes, unlike oxycodone. It has a straightforward dose calculation with a predictable analgesic interchange and conversion between parenteral and oral dosing. It is available in long-acting preparations. Cost of all forms of oral morphine is lower, and the long-acting form is covered by all insurances, including Medicaid. The maximum daily dose should not exceed 50 MME. For any doses > 90 MME/day, document the medical justification.
Another option for opioid tolerant patients is buprenorphine, transdermal or buccal. Compared to full agonist therapy, buprenorphine has no ceiling on respiratory depression, generally provides good analgesia, gives consistent serum plasma levels, and does not lead to hyperalgesia or tolerance with the same frequency. Transdermal buprenorphine dosed at 5 mcg/hr (one patch per week) is approximately equal to 20 MME/day. Starting doses of buccal buprenorphine would be 75 mcg once or twice/day. Unfortunately, these options may not be covered by some insurances such as Medicaid.
Transdermal buprenorphine takes approximately 12-24 hours to reach a steady state, during which a short-acting oral opioid may be needed for one-half to a full day, and then should be discontinued. Advise patients to rotate patch locations to avoid skin breakdown. If a rash occurs due to contact with the adhesive, minimize this problem by applying a medium-strength topical steroid such as 0.1% triamcinolone cream to the area 2 hours prior to placement of the patch.
Previous opioids used. If a patient is on multiple opioids, convert to a single opioid when possible. For patients treated with short-acting medications, convert to or add a long-acting medication using the equianalgesic dosing (MME/day) and conversion information in Appendix C. Once the patient is on a long-acting opioid, the short-acting opioid should generally be discontinued.
Dosing for special populations. Older age, pregnancy, lactation, and chronic illness impact the safety of opioid medications, opioid choice, and dosing. Specific considerations are outlined in Table 8.
Naloxone indications. Patients with medical conditions impacting the heart, lungs, or central nervous system are candidates for intranasal naloxone as a rescue strategy. Any patient, regardless of medical comorbidity, who is on > 50 MME/day should also have intranasal naloxone prescribed. Educate family and friends on how and under what circumstances to administer the intranasal naloxone. If a patient is taking benzodiazepines or uses other sedating medications, discuss the risks, prescribe intranasal naloxone, and consider tapering down the opioid dose or converting to an alternative analgesic strategy.
Drug duration and conversion to long-acting preparations. Limit short-acting opioid use over time. If pain persists beyond a few weeks and opioid use is thought to be beneficial, or requiring continuation of greater than 20-30 MME/day, consider converting to a long-acting preparation. Long-acting preparations provide more stable serum levels and slow the development of opioid tolerance. Short-acting opioids used over time result in tolerance more rapidly than long-acting opioids.
Breakthrough Pain. During dose titration, short-acting medication may be provided for breakthrough pain, but should soon be discontinued. In general, when long-acting opioid preparations are prescribed, use of a short-acting opioid should be a few times per month or not at all. Breakthrough dosing should not occur in multiple daily doses. The only exception is during the first few days of titration, when the long-acting medication is being adjusted to a proper steady state dose. This generally takes 3-5 half-lives of the medication.
Frequent initial assessments. Initially see the patient frequently (every 1-4 weeks) to assess their response to the opioid treatment, monitor for adverse effects, assure compliance, and assess for any inappropriate use or behavior. Reminders of the terms of the treatment agreement are useful in this stage.
Reassess the plan in as soon as 2-6 weeks. Keep the dose titration phase relatively short. If after 2-6 weeks, the patient has not achieved satisfactory pain control with a stable dose of medication, refer the patient to a pain management specialist. It is also reasonable to consider discontinuation of opioids at this point, assuming that adequate dosing was given. Opioids do not effectively treat all patients.
Methadone, Buprenorphine, and Fentanyl
Recommendations
Methadone
- Only clinicians with experience with methadone should prescribe it.
- Do not use methadone as first-line treatment for chronic pain.
- Consider methadone for its prolonged duration of effect, which is useful for longer term therapy and minimizes euphoria with low doses.
- Avoid prescribing methadone in combination with other controlled substances.
Buprenorphine
- Consider buprenorphine when a safer, lower side-effect profile medication is preferred over full agonist opioids or for patients with tolerance to other opioids.
- Consider its higher expense.
- Be familiar with transdermal and buccal buprenorphine. Sublingual buprenorphine should be initiated only by prescribers trained in its use. It can provoke acute opioid withdrawal if not done correctly.
- Buprenorphine can be prescribed for pain without an XDEA waiver, but the waiver is required to prescribe medication-assisted therapy for opioid use disorder.
Fentanyl
- Do NOT consider fentanyl for opioid naïve patients.
- Consider prescribing fentanyl in only a few unusual situations (see text).
- Do NOT use transdermal fentanyl over a long period because opioid tolerance develops quickly.
These three drugs have special properties and uses deserving special description.
Methadone. Do not use methadone as first-line treatment for chronic pain. Before a clinician prescribes methadone, the clinician should have gained experience monitoring and prescribing it, or should consult a pain specialist.
Special safety hazard and unique advantages. Methadone is unique among opioids, with both increased safety concerns and advantages in long-term therapy. The safe use of methadone requires knowledge of its particular pharmacologic properties. Methadone’s duration of adverse effects far exceeds its analgesic half-life, making it dangerous when combined inappropriately with other controlled substances. Methadone may be useful for patients who require prolonged opioid therapy because it does not tend to require increasingly large doses over time (tolerance). Methadone is also available at relatively low cost.
Many patients are aware that methadone is often associated with opioid addiction therapy. Patients may need additional counseling that methadone is an effective analgesic, not merely a treatment for opioid addiction.
Longer duration affects dose titration. Methadone has a prolonged terminal half-life, so the degree of potential adverse effects can increase over several days after an initial dose or a change of dosage. The duration of methadone analgesia upon initiation may be only 6-8 hours. However, with repeated use, daily to three times daily dosing is effective.
Be cautious when converting from another opioid to methadone (Appendix C).95,96 As the MME/day rises, the methadone/morphine conversion ratio declines until methadone is approximately twenty times as potent as oral morphine (daily doses of morphine above 500 mg). Refer patients requiring high dose conversions to or from methadone to a specialist in pain management who has experience with methadone dosing.
During the first few days of methadone use, supplemental short-acting opioids may be used to manage inadequate analgesia, then discontinued. Educate the patient about the delayed response of both therapeutic and adverse effects for methadone. For this reason, avoid prescribing benzodiazepines or other sedatives along with methadone. For opioid-naïve patients, initiate methadone at very low doses (< 10 mg/day) divided into twice daily or three times daily dosing. For opioid-tolerant patients, initiate methadone using proper rotation ratios (Appendix C). Starting doses of methadone should not exceed 30 mg/day, even in opioid tolerant patients. Higher dose conversions may be indicated for some patients, but should prompt consultation with a pain management specialist. Regardless of starting dose, titrate (adjust) methadone doses in small increments (max 10-15% of total daily dose) not more often than once every 7 days. Typical methadone dosing for pain is in the range of 5-30 mg/day in divided doses. Higher doses enter the range of opioid addiction treatment.
Effect on QT interval. Methadone can prolong QT interval, especially at higher doses (≥ 100 mg/day) or when used in combination with other medications that prolong QT, including several classes of common antibiotics (eg, macrolides and quinolones). Perform periodic EKG monitoring for patients on higher doses of methadone, and for those being considered for methadone therapy if they are using other QT-prolonging medications (list available at www.qtdrugs.org).
Methadone testing. Methadone, like other opioid analgesics, is associated with a substantial risk for diversion. Mere confirmation of its presence on GC/MS, LC/MS or specific EIA testing (the “opioid” screening test misses methadone) may not be adequate. Prescribers should have a low threshold for periodic testing of serum levels. Specimens should be drawn knowing the variables of patient weight (kg), time since last dose taken (hours), and the total daily methadone dose (mg). Also, be aware of drug interactions that may affect an individual’s methadone clearance. To estimate the expected serum trough level in ng/mL: 263 x total daily dose divided by the patient’s weight. Methadone serum level peaks approximately two hours after dosing and fades over 5-6 hours. A peak level would be approximately double a trough level.
Buprenorphine. Buprenorphine is a partial agonist opioid that is potent and long-acting. Consider prescribing it when a safer, lower adverse effect profile is preferred over full agonist opioids, or for patients who have developed tolerance to other opioids.
Advantages of buprenorphine include its effectiveness, and lack of development of tolerance to it. As a Schedule III drug, it may be written with refills for up to 6 months. Disadvantages include occasional problems with rash from transdermal patch use, and greater expense.
Transdermal buprenorphine (Butrans and generic) is FDA-approved for treating pain. It does not require an XDEA number or training to prescribe. The transdermal form is a good alternative for patients who have developed tolerance to other opioids, had a benefit from opioid treatment but wish to escalate treatment, and are taking ≤ 80 MME/day. Start with a 5 or 10 mcg patch (changed weekly), and discontinue other opioids.
Buccal buprenorphine (Belbuca) is also FDA-approved for pain treatment. It is given twice daily in patients who have previously been treated with opioid up to 160 MME/day. As with transdermal buprenorphine, it is effective, its misuse risk is low and its pharmacokinetics are not complicated. Cost can be a limiting factor.
Sublingual buprenorphine (Suboxone, Subutex and generic) may be prescribed off-label for pain with a regular DEA number. Sublingual buprenorphine has an evolving role, particularly in patients already treated with high dose opioid therapy who continue to complain of uncontrolled pain, and who may or may not have opioid use disorder. It offers a safer, effective option to full agonist opioids, has a lower risk for misuse, produces less opioid tolerance, causes fewer adverse effects, and can enhance mood. Unfortunately, its higher cost and lack of clinician knowledge of its proper use have so far limited its use as a pain treatment.
Initiation of sublingual buprenorphine can provoke acute opioid withdrawal if not done correctly. Therefore, only prescribers trained in its use and in possession of an XDEA number (or working under guidance of such a prescriber) should initiate sublingual buprenorphine/naloxone. Once a patient is on it and stable, primary prescribers may take over chronic management.
Fentanyl. Do not prescribe fentanyl for opioid naïve patients. Only consider prescribing fentanyl in a few unusual situations. Possible examples include: transdermal when gut mu receptors should be avoided; in head and neck cancer when oral intake is challenging; end of life care; intravenous in a patient with intrathecal “pain pump”; buccal and sublingual for episodic and breakthrough end-stage cancer pain.
Transdermal fentanyl (Duragesic and generic) has limited use for treatment of chronic pain. Transdermal fentanyl is a short-acting opioid packaged in a long-acting delivery system, making patients on it especially prone to development of opioid tolerance.
Transdermal fentanyl has a black box warning for opioid naïve patients. It should only be considered, even at low doses, for patients who are tolerant to opioids. Plasma levels of transdermal fentanyl are erratic and are influenced by several factors, including patient temperature, ambient humidity and temperature, skin thickness, presence of adipose tissue, and location of patch. The patch should never be placed on an open wound or mucous membrane. An expired transdermal patch still has a significant amount of fentanyl in it and must be discarded properly.
Dosing of transdermal fentanyl can be complicated; however, a general rule is that the dose of the patch in micrograms x 2 is roughly equivalent to the oral MME/day. For example, a patient on a 50 mcg/hr patch (with a new patch every 3 days) is receiving approximately 100 MME/day (50 x 2 = 100), and a patient on a 100 mcg/hr patch is receiving approximately 200 MME/day, etc. Transdermal fentanyl is commonly available in 12, 25, 50, 75, and 100 mcg/hr patches.
Buccal and lozenge fentanyl. Fentanyl “lollipops” (Actiq) are rapid-acting forms of fentanyl indicated for episodic and breakthrough end-stage cancer pain and generally, should not be prescribed. There is a black box warning on this formulation for using it only in opioid tolerant patients with a cancer-related diagnosis. Unfortunately, it has been used off label with alarming frequency in the last decade.
Fentanyl testing. Fentanyl is a synthetic opioid and its metabolites are often missed in urine drug screens. GC/MS or LCMS are relatively good at detecting it and are reasonable confirmatory tests.
Adverse Effects of Opioid Analgesics
Recommendations
In opioid naïve patients:
- Start opioids at low doses to avoid respiratory depression, which is most likely to occur in the first 24 hours. Use extra caution in patients with COPD or obstructive sleep apnea.
- Provide constipation prophylaxis.
- Consider anti-nausea medication.
When increasing opioid doses:
- Inform patients that temporary cognitive impairment may occur.
- If dosing increases to > 50 MME/day, prescribe naloxone to use if an overdose occurs.
With prolonged use of opioids, particularly with high doses:
- Consider that increased sensitivity to pain (opioid-induced hyperalgesia) may develop.
- Detoxification may be required.
Nearly 80% of patients using opioids experience adverse effects.97 The most common adverse effects are sedation, nausea, headache, pruritus, and constipation. Other effects can be confusion, hallucinations, nightmares, urinary retention, dizziness, and headache. Tolerance and regression of most adverse effects often occur quickly. Constipation and urinary retention (smooth muscle inhibitory effects) are more persistent.
The most serious potential adverse effect is respiratory depression accompanied by symptoms of sedation and confusion. It may occur with high dose administration in opioid naïve patients. Opioids, at therapeutic doses, depress respiratory rate and tidal volume. As CO2 rises, central chemoreceptors cause a compensatory increase in respiratory rate. Patients with impaired ventilatory reserve (COPD, asthma) are at greater risk of clinically significant respiratory depression. Tolerance to respiratory depression develops within just a few days.
In general, all opioids have similar adverse effects, including constipation, nausea, and rash, though constipation may be somewhat worse with oxycodone. For constipation, when initiating opioids, begin constipation prophylaxis using senna or polyethylene glycol 3350. Do not use docusate. During the first few days of treatment, consider antinausea medication. Nausea generally resolves after a few days and may be somewhat more common with codeine. Rash may be more common with morphine.
After an increase in dose, temporary cognitive impairment may occur. Tolerance to adverse cognitive effects usually develops quickly. Cognitive function, including the ability to drive, is preserved when on stable, moderate doses of opioids.
If opioid dosing is > 50 MME/day, prescribe naloxone. Higher opioid dosing increases the potential for overdosing.
With long-term use of opioids, an increased sensitivity to pain (called opioid-induced hyperalgesia) may develop, particularly when doses above those typically prescribed for pain are used. This may be one cause of apparent opioid tolerance, along with true pharmacologic tolerance and disease progression. Fortunately, tolerance to the analgesic effect, when it does occur, develops much more slowly than tolerance to these adverse effects.
Detoxification will likely be required in patients with continued uncontrolled pain on high doses of opioids. Often detoxification can be accomplished by conversion to buprenorphine.
Patient’s Informed Consent and Controlled Substance Agreement
Recommendations
Use a Controlled Substance Agreement to assure that patients are informed of:
- The potential benefits, limitations, and specific risks of opioid treatment and alternative treatments.
- The conditions under which controlled substances will be prescribed, continued, tapered down, converted, or discontinued.
In Michigan, review the Start Talking Form and obtain the patient’s signature verifying that state-mandated opioid education has been provided.
Before starting therapy, establish treatment goals. Focus on small measurable goals that emphasize function. Also discuss the discontinuation plan or “exit strategy” before prescribing. Educate the patient that if the above goals are not being met, then this would be a reason to discontinue opioid based therapy. This discussion is best done during an initial face-to-face visit and can be reiterated on follow up visits.
Prior to prescribing a controlled substance, review the Controlled Substance Agreement (CSA) with the patient. During the review, educate the patient about potential benefits, limitations, and significant risks of the treatment and alternative treatments. Patients must acknowledge that risks exist, that they accept taking those risks, and that they understand what is expected of them if treatment is to be continued. Standards of care for their safety include their submitting urine for toxicology testing and bringing their medication for counting. A CSA does not require a signature. Patients rarely object to a CSA. Any objection is a potential red flag for future problematic behavior. A sample CSA may be found in Appendix B.
In Michigan, laws regarding opioid prescribing require the patient to sign a Start Talking Form, in which they acknowledge in writing that they have been educated about the risks of opioid treatment. This is not the same as informed consent; the Start Talking Form does not meet the legal definition of consent. Instead, it is a document that verifies that education related to the harm of an opioid has been provided to the patient. However, reviewing risks, benefits, and alternatives is a good medical practice.
Advise patients to avoid alcohol while using an opioid. For patients who are pregnant or may become pregnant, discuss the risk of neonatal abstinence syndrome.
Safety Considerations
Recommendations
Prescribe intranasal naloxone for patients at risk of overdose:
- History of overdose or substance use disorder
- Opioid dose > 50 MME/day
- Comorbidities, factors, or medications predisposing to sedation and suppressed breathing.
Educate patients, family, and friends about when and how to use intranasal naloxone and steps after administration.
Advise patients to store:
- Opioids in a secure location, preferably locked.
- Naloxone where it can be easily found and accessed.
Advise patients how to dispose of unused opioid medications safely and securely.
When to prescribe naloxone for opioid reversal. When opioid therapy is determined to be appropriate, consider prescribing intranasal naloxone as a safety strategy for opioid reversal. Consider naloxone for patients with:
- History of overdose
- History of substance use disorder
- Opioid dose > 50 MME/day
- Comorbidities or factors predisposing to sedation and suppressed breathing (eg, obstructive sleep apnea, significant pulmonary disease, cardiac disease, advanced age)
- Other drugs predisposing to sedation and suppressed breathing (eg, benzodiazepines).
Educate patients, family, and friends. When intranasal naloxone is prescribed, educate the patient and the patient’s family and friends about when and how to use intranasal naloxone and steps after administration. Make sure they all know where naloxone is kept. For very vulnerable patients, consider a medical alert bracelet. Consider advising patients to label for others the location of naloxone in their home, such as a sign on the refrigerator or medicine cabinet.
Storage. Advise patients to store opioid medications in a secure location, preferably locked, that is away from household traffic. Opioids are a common reason for home invasion. Accidental ingestion by children and pets is also a concern.
Advise patients to store naloxone in a location where it can be easily found and accessed by the patient and others in an emergency. Store naloxone in a stable temperature environment in a highly visible and easy to access location. Most preparations of intranasal naloxone have a shelf life of 18 months. Instruct patients and families to check the expiration date frequently.
Disposal. Advise patients how to dispose of unused opioid medications safely and securely. Many options for disposal exist. Having unneeded opioids in the home is a vulnerability for patients and their families. Several disposal locations are available in pharmacies, law enforcement locations, hospital drop boxes, and at community take back events. Local pharmacies may sell over the counter tamper proof drug deactivation bags that can be placed in usual household trash.
Legal Considerations
Recommendations
Prescribers must follow state and federal legal requirements when prescribing opioids and other controlled substances.
Do not prescribe opioids to treat opioid use disorder without the proper XDEA training.
Adhere to recommended guidelines and carefully document medical decision-making when prescribing opioids.
State and federal laws. Each prescriber must be aware of state and federal laws governing the prescription of opioids and other controlled substances. In Michigan, the law requires several actions by the prescriber when a controlled substance is prescribed.
Occasionally, clinicians may be asked, or are tempted to prescribe opioid analgesics as therapy for opioid use disorder (illicit or prescription). This is illegal. Only prescribers with a XDEA may prescribe buprenorphine for the purpose of treating opioid use disorder. Methadone prescribed for opioid use disorder must be dispensed at a facility licensed by the DEA.
For clinicians interested in obtaining an XDEA number (waiver for buprenorphine prescribing) in the context of treating substance use disorder, contact the Michigan Opioid Collaborative (734-764-0231 or toll free 1-800-525-5188) or consult their website for information on waiver training.
Medicolegal risk. A 2017 review of malpractice claims involving the use of opioids for chronic pain found that a variety of patient and clinician factors contribute to poor outcomes and litigation. Medical comorbidities such as obstructive sleep apnea and cardiopulmonary disease, when combined with a long-acting opioid prescription, was identified as a particularly dangerous combination.98 The authors advise that clinicians educate patients about the risks, benefits and alternatives of opioid therapy, perform adherence monitoring, and address aberrant behaviors. Careful documentation and adherence to guidelines are proposed to improve patient safety and minimize legal risk.
Opioids: Maintenance Phase
Monitoring Visits
Recommendations
Frequency:
- After initiating an opioid, see the patient within 1-2 weeks. Then see them at least monthly until they reach a stable opioid dose with improvement in pain and function.
- When the opioid dose is stable, see the patient at regular intervals, but at least every 3 months.
Evaluation:
- Follow Checklist (Table 9).
- Check the state’s prescription drug monitoring program report (called MAPS in Michigan).
- Obtain a urine drug screen at least once per year and any time when concerns arise for inappropriate use, the use of other substances, or diversion.
- Perform pill counts in high risk patients.
Medical Decision-Making:
- Reevaluate the risks and benefits of opioid analgesics for each patient.
- Do not exceed 50 MME/day unless clear evidence of benefit outweighs the risk. Avoid prescribing more than 90 MME/day.
- If a patient was previously stable on an opioid but requests an increase in dose, assess for tolerance or opioid failure. Consider if tapering down the opioid dose or converting to buprenorphine may be indicated.
An overview of prescribing for patients already taking opioids is presented in Figure 2.
Frequency. After initiating an opioid, see patients within 1-2 weeks, then at least monthly until the patient reaches a stable opioid dose with improvement in pain and function. This allows for close monitoring of opioid adverse effects, adherence and comorbidities.
When the opioid dose is stable with improvement in pain and function, see the patient at regular intervals, but at least every 3 months (CDC guideline).11
Evaluation at each follow up visit. Table 9 provides a checklist of items to accomplish at each visit. Obtain a history and exam to assess the effectiveness of the pain treatment plan as well as the risks and benefits associated with opioid analgesics. Assess pain characteristics, functional status, adverse effects, and adherence to the treatment plan. Review interval diagnostic testing and consults.
Particularly important is information about factors that increase the risk for adverse events or overdose, including decompensation of medical and psychiatric comorbidities, overdose potential, suicidality, as well as active use of other controlled substances, alcohol, marijuana, and illicit drugs.
To facilitate gathering information efficiently, use intake questionnaires or templates within the electronic health record. Consider how to involve clinical team members in the evaluation.
Check the state prescription drug monitoring program report (called MAPS in Michigan) each time a controlled substance is prescribed. Look for multiple prescribers, use of multiple pharmacies, unreported controlled substances, or other red flag behaviors (Table 10).
Urine drug testing. Obtain a urine drug screen (UDS) for all patients on chronic opioid therapy at least once per year, and any time there is a concern for inappropriate use, use of other substances, or diversion.99
Urine drug testing is important for verifying the patient is actually using the prescribed medication, and is not selling it or providing it to others (called “diversion”). Urine drug testing also helps with patient safety, by assuring through testing that other sedating substances or medications are not in use. Interpreting test results requires knowledge and care because of the potential for false positive or negative results.100 When in doubt, consult with your toxicology lab.
Conduct random testing at least yearly and more often if the patient is at additional risk for misuse or diversion for sale. The preferred testing strategy uses a combination of an enzyme linked immunoassay (EIA) for abused illicit substances and gas chromatography/mass spectroscopy (GC/MS) or liquid chromatography/mass spectroscopy (LC/MS). This approach provides the maximum specificity in detecting prescribed or illegally purchased medications that are typically missed by simple screening tests. At Michigan Medicine, order the “controlled medication management panel”.
Three categories of results should raise concern:
- Presence of non-prescribed controlled substances
- Absence of prescribed medications (opioids or other medications)
- Presence of illicit drugs of abuse
Response to these results may include counseling, shortened follow-up intervals and urine testing, pill counts, referral for treatment of substance use disorder, or discontinuation of opioid therapy. See Appendix D for a guide to ordering and interpreting urine drug tests.
Reevaluate the risk/benefit of opioid analgesics. After reviewing the history, exam, and additional data, consider the risks and benefits of continuing opioid therapy. Perform this reevaluation at each visit.
Risk factors may develop during treatment that increase the potential harm of opioid treatment (ie, development of obstructive sleep apnea, new medication interactions, alcohol use, suicidal ideation). Opioids may no longer be resulting in a benefit for pain relief or improvement in functional capacity that warrants the opioid’s risks.
If appropriate, modify opioid dosing. Always use the minimum effective opioid dose, or attempt to taper down the dose. If an increased dose is to be tried, titrate the dose gradually, and do not exceed 50 MME/day unless clear evidence of benefit outweighs the risk. Avoid prescribing more than 90 MME/day (CDC guideline).11 If an increased risk of harm or lack of effectiveness warrants a decrease in opioid dosing, begin a tapering down process. (See section on Discontinuing Opioids below.)
Requests for increases in medication. When patients request increases in opioid medication, perform a full reassessment of any new pain features and changes in psychosocial state. A request for additional opioids could indicate a new or worsened condition, increased tolerance, inappropriate opioid use, diversion, or opioid failure. Check the state prescription drug monitoring program report (called MAPS in Michigan). Perform urine drug testing. Consider doing pill counts. In most cases, avoid escalating opioid dosing for patients who were previously stable on an opioid regimen. A gradual tapering down of the opioid dose or a conversion to buprenorphine may be effective for these patients.
Managing the Prescription of Opioids
Recommendations
Know state and federal regulations regarding controlled substance prescriptions.
Establish personal and office policies, roles, and processes that support and facilitate meeting prescribing requirements.
Understand regulations for prescribing controlled substances. Know state and federal regulations regarding controlled substance prescriptions. Key features include:
- Schedule II controlled substance prescriptions shall be dated the date written, shall be for up to a one-month supply, cannot be phoned in, cannot have any authorized refills, and are valid for up to 60 days. A clinician may write a prescription dated today, but with instructions that the prescription not be filled for up to 60 days. In general, it is preferable to prescribe up to a 4-week supply (not 30 days), to avoiding marching into weekends. This requires becoming accustomed to writing 28, 56, or 84-count prescriptions.
- Schedule III, IV, and V controlled substance prescriptions may be called in, with up to 6 months of refills.
- All prescriptions shall be created and recorded in the medical record and should be readily retrievable. The information should include date prepared, the desired fill date, dose, quantity, and expected duration of use. E-prescribing is preferred and will soon be a requirement in many states, including Michigan.
Manage prescribing and refills. Establish personal and office policies, roles, and processes that support and facilitate meeting prescribing requirements. An example of policy for controlled substance prescription and refills is presented in Appendix G. The prescriber, patient, clinic staff, and covering prescribers should understand expectations and consequences. There should be no differences from in-person visits managing patients virtually. Video visits are preferred over phone visits and permit pill counts. Covering clinicians should NOT manage controlled substance prescriptions at night or on weekends.
Organize office procedures to meet prescribing requirements. See patients who are on a stable Schedule II-III opioid regimen every 2-3 months. Send in prescriptions to last until the next scheduled appointment or beyond to permit pill counts. For example, on one date, electronically send two 4-week prescriptions and specify a future fill date on one of the prescriptions. For patients taking a Schedule II opioid who are seen every 3 months, utilize clinic personnel to monitor prescription dispensing. Clinic staff can follow a protocol to ensure that the patient is up-to-date with appointments and appropriate monitoring. Staff can prepare a prescription for refill. After the clinician has reviewed appropriate information (including reviewing the state prescription drug monitoring program report), the clinician can electronically sign and send the prepared prescription. In general, best practice is to give refills at face-to-face visits, thereby avoiding excess contacts to the office.
Patients on a stable dose of tramadol (Schedule IV) can be seen every 6 months. Refills for up to 6 months can be authorized on Schedule IV medication prescriptions. To avoid early refills, specify the fill dates for each refill in writing on the prescription.
Assess and Respond to Inappropriate Opioid Use
Recommendations
Use established criteria to evaluate inappropriate opioid use by patients who are receiving long-term opioid therapy for chronic pain. Watch for red flag behaviors (Table 10).
Respond to suspicion of opioid misuse or diversion by collecting more information and discussing with the patient.
If criteria are met for the diagnosis of opioid use disorder:
- Initiate treatment for opioid use disorder, including use of medication assisted treatment (MAT).
- Continue to offer multimodal pain management therapies, emphasizing non-opioid strategies
Assess potential misuse of opioids. Use established criteria to evaluate misuse of opioids by chronic pain patients receiving long-term opioid therapy.101 Meeting 3 or more of the following criteria is defined as misuse.
- Focus on opioids. The patient displays an overwhelming focus on opioids during visits. This focus occupies a significant proportion of the clinic visit time and impedes progress on other issues regarding the patient’s pain. This behavior must persist beyond the third clinic treatment session.
- Early refills. The patient demonstrates a pattern of requesting early refills (3 or more) or escalating drug use in the absence of an acute change in his or her medical condition.
- Multiple contacts about opioids. The patient generates multiple telephone calls, visits, or other contacts to the administrative office requesting more opioids or early refills, or for problems associated with the opioid prescription.
- Prescription problems. There is a pattern of prescription problems for a variety of reasons that may include lost, spilled, or stolen medications.
- Multiple sources of opioids. The patient has supplemental sources of opioids obtained from multiple clinicians, emergency rooms, or illegal sources.
- Other substance misuse. Concurrent illicit substance use or alcohol use disorder.
The authors of these criteria reported that 34% of chronic pain patients on opioids met at least one criterion, and 27% met 3 or more. These and other red flag behaviors are listed in Table 10.
Evaluating inappropriate opioid use. Respond to aberrant behaviors by collecting more information and discussing with the patient. Tools such as the drug abuse screening test (DAST-10) or Michigan opioid risk assessment (MORA) can be helpful in this effort. Check the state prescription drug monitoring program report. Perform a urine comprehensive drug screen to check for both the presence of non-prescribed medications or illicit substances and for the presence or absence of prescribed controlled substance medications. If opioid diversion is confirmed, discontinue the opioid prescription immediately. In most cases of opioid misuse, discontinuing opioid prescribing is indicated, using either a rapid or slow taper (see Discontinuing Opioids). Evaluate those patients who misuse opioid prescriptions for the presence of complex persistent dependence or opioid use disorder, and offer treatment.
Complex Persistent Dependence
Recommendations
Consider the presence of complex persistent dependence in patients with high opioid doses (≥ 100 MME/day), impaired function, aberrant opioid use, psychiatric and substance use disorder comorbidities.
Do not escalate opioid doses for patients with complex persistent dependence.
Consider treatment with buprenorphine for patients with complex persistent dependence.
The gray area between dependence and addiction can be challenging for clinicians and patients. A 2012 article by Ballantyne, et.al. proposed a classification of complex persistent dependence that explains the pathophysiology accounting for worsening functional status and pain in patients on long-term opioid therapy.102
When attempting to taper down opioid dosing for a patient with complex persistent dependence, aberrant behaviors and fluctuation in opioid use can occur. The development of protracted abstinence syndrome may lead to worsening pain, declining function, and worsening psychiatric symptoms. Paradoxically, the same symptoms may occur with maintenance of long-term high dose opioid therapy. Pain relief is more complex than analgesia measured by pain scales. Pain relief involves relief in the affective component of the pain experience, as mediated through mesolimbic reward and learning pathways involving the endogenous opioid system. This system is distinct from the pathways involved in nociceptive input. The same endogenous opioid pathways involved in reward are also involved in relief from other experiences including frustration, anger, anxiety, and despair. While analgesics like acetaminophen are thought to have effects on the analgesic pathways involved in nociception, opioids have additional effects on pathways that mediate relief. Opioids have a dual role in mediating direct relief on both analgesic pathways and reward pathways.
Some evidence shows that patients with complex persistent dependence may tolerate transition to buprenorphine better than a tapering down of the opioid dose. When complex persistent dependence is suspected, a more clinically useful approach may be to transition to buprenorphine and then taper down the dose. Start with careful communication with the patient about this strategy, including reassurances that the patient is not being treated “like an addict,” and then refer to a buprenorphine waivered prescriber.
Some evidence exists for methadone use in this population as well. However, it is less promising than buprenorphine.
Opioid Use Disorder in Pain Patients
Recommendations
Use a standard tool such as the drug abuse screening test (DAST-10) to detect risk for medication misuse.
Monitor all patients on controlled substances by checking the state prescription drug monitoring program report with each prescription. Perform periodic urine drug testing. Pill counts are appropriate for the highest risk patients.
Prescribe naloxone to the highest risk patients.
Offer evidence-based treatment for addiction, including medication-assisted therapy
Substance use disorder complicating the treatment of chronic pain. The prevalence of substance use disorder among patients with chronic pain is significant. Studies have repeatedly demonstrated that at least 20% of opioid-treated patients misuse or divert their medication. In one survey 70% of patients reported not taking their opioids as prescribed, often using them up quickly, then going without or obtaining opioids in illicit ways until due for their next refill.103,104
Use drug misuse risk screening tools (such as the DAST-10) to help identify patients for whom risk might be managed by more frequent follow-up visits, checks of the state prescription drug monitoring program report, urine drug testing, or pill counts. However, these measures are imperfect. Risk screening tools can also aid in the decision that opioid risk exceeds the limited benefit for improving a patient’s functional state.
A full discussion of the diagnosis and management of opioid use disorder is beyond the scope of this guideline. However, monitor patients for signs and symptoms of this disorder. Watch for red flag behaviors that may indicate addiction or diversion. Apply the DSM-5 diagnostic criteria to diagnose opioid use disorder, as listed below. (Mild opioid use disorder: 2-3 symptoms; moderate: 4-5 symptoms; severe: 6 or more symptoms):
- Opioids are often taken in larger amounts or over a longer period than intended.
- There is a persistent desire or unsuccessful efforts to cut down or control opioid use.
- A great deal of time is spent in activities necessary to obtain the opioid, use the opioid, or recover from its effects.
- Craving, or a strong desire to use opioids.
- Recurrent opioid use resulting in failure to fulfill major role obligations at work, school, or home.
- Continued opioid use despite persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of opioids.
- Important social, occupational, or recreational activities are given up or reduced because of opioid use.
- Recurrent opioid use in situations in which it is physically hazardous.
- Continued opioid use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by opioids.
- Tolerance, as defined by either of the following: (a) a need for markedly increased amounts of opioids to achieve intoxication or desired effect, or (b) markedly diminished effect with continued use of the same amount of an opioid.
Principles for managing opioid use disorder in pain patients. The treatment of pain patients who exhibit evidence of opioid use disorder requires heightened monitoring, or discontinuation of opioid therapy and initiation of addiction treatment. Successful treatment depends on the clinician understanding that opioids provide more than analgesia, and that loss of control is not a moral failure by the patient, but a known complication of a reinforcing medication.
- Have a frank but supportive discussion with the patient about the fears of a worse lifestyle and risk for overdose. Offer support and addiction treatment.
- Provide support. A patient should not be made to feel judged, scorned, or abandoned by a clinician just because a diagnosis of opioid use disorder is made.
- Consider buprenorphine. For patients with opioid use disorder, conversion from other opioids to buprenorphine can provide a safer alternative while still providing the benefits, if any, of opioid analgesia. This can be done by a prescriber with a XDEA, with input from other specialists as needed.
- Refer when needed. If the complexity of a patient’s management exceeds the capability of the prescriber, refer for formal addiction treatment.
- Do not continue to prescribe full agonist opioids for patients who exhibit loss of control over proper medication use or who use illicit substances. The risks for overdose or diversion outweigh any benefit.
- Prescribe naloxone and instruct the patient, family, and friends on its proper use.
When to Refer to a Pain or Addiction Specialist
Recommendations
Refer chronic pain patients to appropriate specialists for multidisciplinary management of:
- Clinical problems:
- Outcome failure after 6 weeks of treatment
- Ongoing adverse events
- Unexpectedly large doses of opioids required
- Frequent use of breakthrough dosing
- Opioid-induced hyperalgesia
- Opioid overdose
- Need for conversion to sublingual buprenorphine
- Behavioral problems:
- Addiction or diversion suspicion
- Non-adherence with Controlled Substance Agreement
When the management of patients with chronic pain involves difficult clinical or behavioral problems, refer the patient to an appropriate specialist or to multidisciplinary management.
Clinical problems for referral include:
- Outcome failure. Failure to attain adequate pain relief to achieve functional improvement goals after 6 weeks of opioid analgesic dose titration.
- Adverse events. Ongoing adverse effects of opioid therapy.
- Unexpectedly large doses of opioids required.
- - Doses beyond what the primary care clinician is comfortable prescribing or beyond what are considered typically adequate for most pain management.
- - Upper limits typically prompting referral are opioids in combination that exceed a total daily dose > 90 MME/day. (See Appendix C.) Also refer for individual medication doses greater than morphine 90 mg/day, hydrocodone 90 mg/day, oxycodone 60 mg/day, fentanyl 50 mcg/hr, hydromorphone 16 mg/day, methadone 30 mg/day.
- Breakthrough dosing. The patient requires additional dosing for breakthrough pain more than a few times per month.
- Opioid-induced hyperalgesia.
- Opioid overdose
- Need for conversion to sublingual buprenorphine if high opioid doses or insurance do not permit use of transdermal or buccal buprenorphine.
Behavioral problems for referral include:
- Persistent behavior suspicious for addiction or diversion.
- Non-adherence with the Controlled Substance Agreement.
Tapering Down and Discontinuing Opioids
Recommendations
If opioid use is contraindicated, discontinue prescribing immediately, recognizing that withdrawal symptoms will likely occur. Example situations include illegal activity (eg, diversion, prescription forgery or fraud), potential for immediate harm, or threatening behavior toward others.
If a patient is not following appropriate use instructions (eg, use of medication, monitoring visits, contract for refills), discontinue use with a rapid taper to minimize withdrawal symptoms.
If opioid has no benefit or produces clinical harms, discontinue with a slow taper to avoid withdrawal symptoms.
Explain the reasons for discontinuing the opioid medication. Explain the tapering down process.
If simple tapering down is not possible, consider referral to a specialist in addiction medicine.
Reasons for Discontinuing Opioids
Opioids may be discontinued for a variety of reasons, including diversion, prescription forgery or fraud, non-adherence to the treatment plan, lack of benefit, excessive dosing, a need to convert multiple opioids to a single opioid, or hospitalization. Opioid tapering and discontinuation can be challenging. Carefully consider each patient’s medical, psychological, financial, and intellectual factors.
Speed of Discontinuation
The speed with which opioids are stopped depends on the situation. Options include immediate discontinuation, rapid taper, slow taper, buprenorphine conversion, and referral to an addiction specialist. See Appendix F for more information about these actions, along with associated reasons and recommended processes.
Immediate discontinuation. If controlled substance diversion, prescription forgery or fraud is discovered, immediately discontinue opioid prescribing. DEA regulations require that diversion for sale or prescription fraud be reported, so when these illegal activities are suspected, notify local law enforcement. Immediate discontinuation of opioids is also appropriate if there are dangerous behaviors with potential for immediate harm. Example situations include motor vehicle accident or arrest due to opioid or illicit drug or alcohol intoxication, intentional overdose, or suicide attempt. Aggressive or threatening behavior in the clinic can also be grounds for immediate discontinuation of opioids. When immediate discontinuation of opioid therapy is necessary, inpatient detoxification can be offered to help treat withdrawal.
Rapid taper. If a patient is not following appropriate use instructions, using a rapid taper to discontinue will prevent opioid withdrawal. The amount of opioid necessary to prevent withdrawal is only 20% of the previous day’s dose (based on rapid detoxification studies). However, a rapid taper that reduces the dose by 25-50% per week over 2-4 weeks is commonly practiced.
Slow taper. If opioid treatment provides no benefit or produces clinical harm, discontinue with a slow taper to avoid withdrawal symptoms. The general rule for a slow taper is similar to initiation of therapy: use adjunct medications and non-pharmacological options (distraction, acupuncture, procedures, etc.) to help facilitate the tapering down process. Tapering down can be challenging, and patients may require frequent visits for reassurance or adjustments. Moreover, some opioid medications only are available in larger doses. Later in the tapering down process, converting to short-acting medications may be desirable to allow smaller dose changes over time. Generally, a slow taper that reduces the dose by 10% per week will prevent withdrawal, but it is important to know that the approach to tapering down must be individualized.
Explain Discontinuation
Prior to discontinuing an opioid, explain the tapering down process and the reasons for discontinuation. Some evidence shows that patients prefer to get this information from their primary care clinician or a clinician with whom they have a close relationship. Often, a patient’s need for an intranasal naloxone rescue strategy can be used to initiate and inform the conversation about why that patient may need to taper down the opioid dose. Providing a written taper schedule may be helpful. Scheduling regular check-in phone calls or telehealth visits can facilitate ongoing communication and increase adherence to a tapering down plan.
Complex Discontinuation
When a simple tapering down of the opioid dose is not feasible, consider referral to a specialist in addiction medicine. It is important to discuss the reason for this with the patient, addressing the social stigma of addiction and the rationale for referral.
Assuming Care for Patients Already on Opioids
Recommendations
When assuming care for a patient already on opioids, perform a full assessment, confirming the diagnosis and need for opioids.
If continuing the use of opioids is appropriate, perform usual maintenance activities for monitoring, evaluation, and management.
If continuing the use of opioids is not appropriate, consider more appropriate treatments. Initiate tapering down and discontinuation of opioids.
When covering for a clinician in your practice, use procedures and precautions that apply to any follow-up visit by a patient on opioids.
When assuming care for a patient already on opioids, treat the patient like a new patient. Perform a full assessment of the patient’s health and pain history. Document medications tried and failed and any prior response to opioids. This review is an opportunity for improvement in clinical care and for patient education.
If continuing the use opioids is deemed appropriate, perform all the usual activities for patients being maintained on opioids:
- Prescription Drug Monitoring Program: Check the state’s prescription drug monitoring program website (called MAPS in Michigan) for controlled substance prescriptions. Look for multiple prescribers, use of multiple pharmacies, unreported controlled substances, and any other red flag behaviors (Table 10).
- Urine drug screen: Screen for both appropriate and inappropriate substances.
- Start Talking Form (in Michigan): Have the patient sign a new form before prescribing.
- Controlled Substance Agreement: Initiate a CSA before prescribing, if one is not already in place.
If continuing the use of opioids is not appropriate, as for any patient who no longer need be on opioids, consider more appropriate treatments. Initiate tapering down and discontinuation of opioids.
If you are covering for a clinician in your practice, the above procedures and precautions still apply, just as for any follow-up visit by a patient on opioids
For every patient on opioids, periodically review the risks, benefits, and alternatives to opioids (eg, use the Start Talking Form). Discuss any concerns about dose and duration, and review indications for intranasal naloxone.
Guideline Development Methodology
Funding
The development of this guideline was funded by UMHS.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- Primary care clinicians: Daniel W. Berland, MD, General Internal Medicine/Addiction Medicine; Jill N. Fenske, MD, Family Medicine.
- Specialists in pain management care: SriKrishna Chandran, MD, Physical Medicine & Rehabilitation, Pain Medicine; Paul E. Hilliard, MD, Pain Medicine, Anesthesiology.
- Consultant specialists: Kimberly C. Bialik, PhD, Psychological Aspects of Pain Management; Daniel J. Clauw, MD, Anesthesiology, Internal Medicine-Rheumatology; Eve D. Losman, MD, Emergency Medicine; Kathleen S. Mehari, MD, Obstetrics & Gynecology; Michael A. Smith, PharmD, College of Pharmacy; and Susan Urba, MD, Hematology/Oncology.
- Guideline development methodologist: R. Van Harrison, PhD, Learning Health Sciences.
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
UMHS endorses the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Contributions of team members with relevant financial relationships are reviewed by team members without relevant financial relationships to assure the information is presented without bias.
None of the team members or consultants have relevant personal financial relationships.
Systematic Review of Literature
A detailed description of the systematic search and review of literature upon which this guideline is based is presented in the associated UMHS document “Ambulatory Pain Management, 2019: Literature Review Methods and Results.” The following section highlights major aspects of the literature search and review process.
Literature search. The team began the search of literature by accepting the results of a systematic literature review performed in 2016:
Institute for Clinical Systems Improvement (ISCI) guideline on Pain: Assessment, Non-Opioid Treatment approaches and Opioid Management. Best evidence for topics: recommendations and key references, pages 9 – 16.
To update those results, we performed a systematic search of literature on Medline and in the Cochrane Database of Systematic Reviews for the time period 1/1/16—10/2/18.
The major search terms were acute and subacute pain, and chronic pain (non-terminal). The searches were for guidelines, controlled trials (including meta-analyses), and cohort studies, for literature on humans in the English language. Within these parameters individual searches were performed for the following topics:
Major Topic: Acute and Subacute Pain
- A. Acute and Subacute Pain: Etiologies of acute/subacute pain, Non-opioid therapies for acute/subacute pain, Use of opioids for acute/subacute pain, Universal precautions for opioid therapy, Acute and subacute pain, not included in A.
Major Topic: Chronic Pain (non-terminal)
- B. Chronic Pain Assessment: Pain characteristics (location, quality, intensity, time course), Pain treatment history, Determination of pain generator (focal pain generator, neuropathic pain, centralized pain syndrome), Quality of life and functional impact, Comorbidities (medical, psychiatric, substance use disorders), Pain beliefs and response to pain, Social determinants (adverse childhood experiences, psychosocial stressors), Chronic pain assessment, not included in B.
- C. Designing an Individualized Pain Treatment Plan: Multimodal interventions, Clinician-patient communication (shared decision-making, team approach), Individualized plan, not included in C.
- D. Non-Pharmacologic Treatments/evidence: Lifestyle management (exercise, sleep hygiene), Physical therapy, Other physical modalities (TENS, massage), Complementary and alternative therapies, Psychological interventions (mindfulness, CBT, etc.), Non-pharmacologic treatment, not included in D.
- E. Non-Opioid Pharmacologic Treatment: Acetaminophen, NSAIDs, Tricyclic, SNRI, Anticonvulsants, Muscle relaxants, Topicals not included in E, Non-opioid pharmacologic treatment, not included in E.
- F. Rational Use of Opioids in Chronic Pain; Decision Phase: Risk-benefit analysis (patient selection, risk analysis, informed consent), Assuming care for patients already on opioids, Special populations (pregnancy/lactation, geriatrics, renal disease, liver disease, pediatrics). (No search for “other”)
- G. Rational Use of Opioids in Chronic Pain: Initiation and Treatment Phase: Drug selection and dosing (general guidance, CDC guidelines [most important example of general guidance]), Methadone, Fentanyl, Buprenorphine, Adverse effects, Controlled substance agreement, Safety considerations (avoid co-prescription with benzodiazepine, naloxone, storage, disposal), State of Michigan controlled substance legislation (no search), Opioids initiation/treatment, not included in G.
- H. Rational Use of Opioids in Chronic Pain: Maintenance Phase: Monitoring (frequency of visits, prescription drug monitoring programs, urine drug screening), Opioid refill management (office procedures), Assessing potential problems with opioid therapy, Response to suspicion for opioid misuse or diversion, Indications for referral to pain/addiction specialist, Legal issues, Medical marijuana, Opioid maintenance, not included in H.
- I. Tapering/Discontinuation of Opioids: Best practice for communication with patients about tapering, General tapering guidelines, Complex persistent dependence, Persistent abstinence syndrome, Opioid tapering/discontinuation, not included in I.
- J. Opioid Use Disorder: Detection, diagnosis, treatment (medication-assisted therapy), referral options, Chronic pain and opioids, not in J, Chronic pain, not included in J.
A more formal presentation of the inclusion and exclusion criteria is in Section II of the accompanying Literature Review Methods and Results.
The detailed search strategies are presented in Section III of the accompanying Literature Review Methods and Results.
The search was conducted in components of a formal problem structure (outlined above). The search was supplemented with very recent clinical trials known to expert members of the panel. The search was a single cycle. The number of publications identified is presented in Section IV of the accompanying Literature Review Methods and Results.
Literature review and assessment. Members of the guideline team reviewed the publications identified to be relevant to specific topics in order to select those with best evidence. Criteria to identify overall best evidence included relevance of the study setting and population, study design, sample size, measurement methods (variables, measures, data collection), intervention methods (appropriateness, execution), appropriateness of analyses, and clarity of description.
In considering level of evidence based on study design, the classification was:
- A = systematic reviews of randomized controlled trials with or without meta-analysis
- B = randomized controlled trials
- C = systematic reviews of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control)
- D = individual observation studies (case study or case series)
- E = expert opinion regarding benefits and harm.
Beginning with best evidence identified by the ISCI systematic literature review, team members checked publications identified in the more recent search (1/1/16—10/2/18) to determine whether better evidence was available. Team members also had the option of considering very recent literature (published since 10/2/18) in determining whether even better evidence was available.
The process of review and assessment is described in more detail in Section V of the accompanying Literature Review Methods and Results.
Best evidence and recommendations. Team members identified articles or other publications with best evidence regarding specific topics.
The guideline team reviewed the evidence and determined the importance of performing or not performing key aspects of care (listed on the first page of this guideline). In the absence of empirical evidence, the guideline team based recommendations on their expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed.
Section VI of the accompanying Literature Review Methods and Results presents a table of each recommendation and the source of best evidence on which the recommendation is based.
Review and Endorsement
A draft of this guideline was reviewed by units within UMHS to which the content is most relevant. Pharmacy Services performed the initial review. Then reviews occurred in clinical conferences or by distribution for comment within the following clinical departments and divisions: General Internal Medicine, Family Medicine, Physical Medicine & Rehabilitation, Pain Medicine, Anesthesiology, Clinical Psychology, Emergency Medicine, Obstetrics & Gynecology, Hematology/Oncology, and the Controlled Substances Quality Improvement Committee. The draft was revised based on comments from these groups.
The final version of this guideline was endorsed by the Clinical Practice Committee of the University of Michigan Medical Group and by the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
Acknowledgements
The following individuals are acknowledged for their contributions to previous versions of this guideline.
2009: Daniel W. Berland, MD; Philip E. Rodgers, MD; Carmen R. Green, MD; R. Van Harrison, PhD; Randy S. Roth, PhD. Consultants: Daniel J. Clauw, MD; Jennifer A. Meddings, MD; Ronald A. Wasserman, MD.
References
- 1.
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19-27. doi:10.1097/j.pain.0000000000001384 [PubMed: 30586067] [CrossRef]
- 2.
- Gagnier JJ, Oltean H, van Tulder MW, Berman BM, Bombardier C, Robbins CB. Herbal Medicine for Low Back Pain: A Cochrane Review. Spine (Phila Pa 1976). 2016;41(2):116-133. doi:https://dx
.doi.org/10 .1097/BRS.0000000000001310 [PubMed: 26630428] - 3.
- Cameron M, Chrubasik S. Oral herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2014;2014(5). doi:10.1002/14651858.CD002947.pub2 [PMC free article: PMC4494689] [PubMed: 24848732] [CrossRef]
- 4.
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic painandan overview of general harms a systematic review. Ann Intern Med. 2017;167(5):319-331. doi:10.7326/M17-0155 [PubMed: 28806817] [CrossRef]
- 5.
- Singh JA, Noorbaloochi S, Macdonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;2017(6). doi:10.1002/14651858.CD005614.pub2 [PMC free article: PMC4881293] [PubMed: 25629804] [CrossRef]
- 6.
- Daily JW, Yang M, Park S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Med Food. 2016;19(8):717-729. doi:10.1089/jmf.2016.3705 [PMC free article: PMC5003001] [PubMed: 27533649] [CrossRef]
- 7.
- Mahnig S, Landmann G, Stockinger L, Opsommer E. Pain assessment according to the International Spinal Cord Injury Pain classification in patients with spinal cord injury referred to a multidisciplinary pain center. Spinal Cord. 2016;54(10):809-815. doi:https://dx
.doi.org/10.1038/sc.2015.219 [PubMed: 26754471] - 8.
- U.S. Food and Drug Administration (FDA). FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Food Drug Adm Drug Saf Commun. 2017:1-9.
- 9.
- Acts P. Michigan Opioid Laws Overview of the Public Acts. 2019:1-18.
- 10.
- Persons AP on PP in O. The Management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2002;50(6 Suppl):205-224. [PubMed: 12067390]
- 11.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:https://dx
.doi.org/10 .1001/jama.2016.1464 [PMC free article: PMC6390846] [PubMed: 26977696] - 12.
- Koncicki HM, Unruh M, Schell JO. Pain Management in CKD: A Guide for Nephrology Providers. Am J Kidney Dis. 2017;69(3):451-460. doi:https://dx
.doi.org/10 .1053/j.ajkd.2016.08.039 [PubMed: 27881247] - 13.
- Chandok N, Watt KDS. Pain management in the cirrhotic patient: The clinical challenge. Mayo Clin Proc. 2010;85(5):451-458. doi:10.4065/mcp.2009.0534 [PMC free article: PMC2861975] [PubMed: 20357277] [CrossRef]
- 14.
- Midland MMH. MidMichigan Opioid Risk Assessment (MORA). 2019.
- 15.
- Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77-82. doi:10.1097/j.pain.0000000000001389 [PubMed: 30586074] [CrossRef]
- 16.
- Clauw DJ, Essex MN, Pitman V, Jones KD. Reframing chronic pain as a disease, not a symptom: rationale and implications for pain management. Postgrad Med. 2019;131(3):185-198. doi:10.1080/00325481.2019.1574403 [PubMed: 30700198] [CrossRef]
- 17.
- Benoliel R, Svensson P, Evers S, et al. The IASP classification of chronic pain for ICD-11. Pain. 2018;160(1):60-68. doi:10.1097/j.pain.0000000000001435 [PubMed: 30586072] [CrossRef]
- 18.
- Krebs EE, Lorenz KA, Bair MJ, et al. Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. J Gen Intern Med. 2009;24(6):733-738. doi:10.1007/s11606-009-0981-1 [PMC free article: PMC2686775] [PubMed: 19418100] [CrossRef]
- 19.
- Van Ryswyk E, Antic NA. Opioids and Sleep-Disordered Breathing. Chest. 2016;150(4):934-944. doi:10.1016/j.chest.2016.05.022 [PubMed: 27262224] [CrossRef]
- 20.
- Lépine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(SUPPL. 1). doi:10.1002/hup.618 [PubMed: 15378670] [CrossRef]
- 21.
- Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain. 2016;17(9 Suppl):T70-92. doi:https://dx
.doi.org/10 .1016/j.jpain.2016.01.001 [PMC free article: PMC5012303] [PubMed: 27586832] - 22.
- Jenewein J, Moergeli H, Wittmann L, Büchi S, Kraemer B, Schnyder U. Development of chronic pain following severe accidental injury. Results of a 3-year follow-up study. J Psychosom Res. 2009;66(2):119-126. doi:10.1016/j.jpsychores.2008.07.011 [PubMed: 19154854] [CrossRef]
- 23.
- J Jenewein L Wittmann H Moergeli J Creutzig U Schnyder. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: A longitudinal study. J Trauma Stress. 2016;29(August):293-300. doi:10.1002/jts [PubMed: 19924822] [CrossRef]
- 24.
- Kongsted A, Bendix T, Qerama E, et al. Acute stress response and recovery after whiplash injuries. A one-year prospective study. Eur J Pain. 2008;12(4):455-463. doi:10.1016/j.ejpain.2007.07.008 [PubMed: 17900949] [CrossRef]
- 25.
- Wuest J, Ford-Gilboe M, Merritt-Gray M, et al. Pathways of chronic pain in survivors of intimate partner violence. J Womens Health (Larchmt). 2010;19(9):1665-1674. doi:10.1089/jwh.2009.1856 [PMC free article: PMC3120089] [PubMed: 20718626] [CrossRef]
- 26.
- Wuest J, Ford-Gilboe M, Merritt-Gray M, et al. Abuse-related injury and symptoms of posttraumatic stress disorder as mechanisms of chronic pain in survivors of intimate partner violence. Pain Med. 2009;10(4):739-747. doi:10.1111/j.1526-4637.2009.00624.x [PubMed: 19453953] [CrossRef]
- 27.
- Petrosky E, Harpaz R, Fowler KA, et al. Chronic Pain Among Suicide Decedents, 2003 to 2014: Findings From the National Violent Death Reporting System. Ann Intern Med. 2018. doi:https://dx
.doi.org/10.7326/M18-0830 [PMC free article: PMC6913029] [PubMed: 30208405] - 28.
- Afari N, Ahumada SM, Wright LJ, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76(1):2-11. doi:10.1097/PSY.0000000000000010 [PMC free article: PMC3894419] [PubMed: 24336429] [CrossRef]
- 29.
- Elwyn G, Cochran NPM. Shared decision making—the importance of diagnosing preferences. JAMA Intern Med. 2017;17:1239-1240. [PubMed: 28692733]
- 30.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care and E. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press; 2011. doi:10.17226/13172 [PubMed: 22553896] [CrossRef]
- 31.
- Bee P, McBeth J, MacFarlane GJ, Lovell K. Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery. BMC Musculoskelet Disord. 2016;17(1):354. doi:https://dx
.doi.org/10 .1186/s12891-016-1194-5 [PMC free article: PMC4994227] [PubMed: 27549811] - 32.
- U.S. Department of Health and Human Services. Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. 2019:116.
- 33.
- Meghani SH, Byun E, Gallagher RM. Time to Take Stock: A Meta-Analysis and Systematic Review of Analgesic Treatment Disparities for Pain in the United States. Pain Med. 2012;13(2):150-174. doi:10.1111/j.1526-4637.2011.01310.x [PubMed: 22239747] [CrossRef]
- 34.
- Lee P, Le Saux M, siegel R et al. Racial and ethnic disparities in the management of acute pain in US emergency departments: Meta-analysis and systematic review. Am J Emerg Med. 2019;37(9):1770-1777. [PubMed: 31186154]
- 35.
- Lattimer L, Haywood C, Lanzkron S, Ratanawongsa N, Bediako SM, Beach MC. Problematic hospital experiences among adult patients with Sickle Cell Disease. J Health Care Poor Underserved. 2010;21(4):1114-1123. doi:10.1353/hpu.2010.0940 [PMC free article: PMC3240938] [PubMed: 21099065] [CrossRef]
- 36.
- Wieland LS, Skoetz N, Pilkington K, Vempati R, D’Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane database Syst Rev. 2017;1:CD010671. doi:https://dx
.doi.org/10 .1002/14651858.CD010671.pub2 [PMC free article: PMC5294833] [PubMed: 28076926] - 37.
- Lauche R, Stumpe C, Fehr J, et al. The Effects of Tai Chi and Neck Exercises in the Treatment of Chronic Nonspecific Neck Pain: A Randomized Controlled Trial. J Pain. 2016;17(9):1013-1027. doi:https://dx
.doi.org/10 .1016/j.jpain.2016.06.004 [PubMed: 27345663] - 38.
- Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for Low Back Pain: Complete Republication of a Cochrane Review. Spine (Phila Pa 1976). 2016;41(12):1013-1021. doi:https://dx
.doi.org/10 .1097/BRS.0000000000001398 [PubMed: 26679894] - 39.
- Giugliano D, Ceriello AEK. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677-685. doi:10.1016/j.jacc.2006.03.052 [PubMed: 16904534] [CrossRef]
- 40.
- Veronese N, Stubbs B, Koyanagi A, et al. Pro-inflammatory dietary pattern is associated with fractures in women: an eight-year longitudinal cohort study. Osteoporos Int. 2018;29(1):143-151. doi:10.1007/s00198-017-4251-5 [PMC free article: PMC5760322] [PubMed: 29018920] [CrossRef]
- 41.
- DXC. Braddom’s Physical Medicine and Rehabilitation 5th Edition. 5th ed. Elsevier; 2015.
- 42.
- Field T. Massage Therapy Research. Complement Ther Clin Pr. 2016;24:19-31. doi:10.1016/B978-0-443-10201-1.X5001-6 [PMC free article: PMC5564319] [PubMed: 27502797] [CrossRef]
- 43.
- Nelson NL, Churilla JR. Massage Therapy for Pain and Function in Patients With Arthritis: A Systematic Review of Randomized Controlled Trials. Am J Phys Med Rehabil. 2017;96(9):665-672. doi:https://dx
.doi.org/10 .1097/PHM.0000000000000712 [PubMed: 28177937] - 44.
- McCracken LMVK. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol. 2014;69(2):178-187. [PubMed: 24547803]
- 45.
- Jensen, Mark P. Turner, Judith A. Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69(4):655-662. [PubMed: 11550731]
- 46.
- Broderick JE, Keefe FJ, Schneider S, et al. Cognitive behavioral therapy for chronic pain is effective, but for whom?. Pain. 2016;157(9):2115-2123. doi:https://dx
.doi.org/10 .1097/j.pain.0000000000000626 [PubMed: 27227692] - 47.
- Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain. 2000;85(1-2):115-125. doi:10.1016/S0304-3959(99)00259-6 [PubMed: 10692610] [CrossRef]
- 48.
- Williams A, Eccleston C, Morley S. Williams et al 2012. Cochrane Review Psychological therapie for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11). doi:10.1002/14651858.CD007407.pub3.www.cochranelibrary.com [PMC free article: PMC6483325] [PubMed: 23152245] [CrossRef]
- 49.
- Creswell JD. Mindfulness Interventions. Annu Rev Psychol. 2017;68:491-516. doi:https://dx
.doi.org/10 .1146/annurev-psych-042716-051139 [PubMed: 27687118] - 50.
- Shapiro SLSG. Intentional systemic mindfulness: an integrative model for self-regulation and health. Adv Mind Body Med. 2000;16(2):128-134. [PubMed: 10835770]
- 51.
- Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA. 2016;315(12):1240-1249. doi:https://dx
.doi.org/10 .1001/jama.2016.2323 [PMC free article: PMC4914381] [PubMed: 27002445] - 52.
- Creswell JD, Lindsay EK, Villalba DK, Chin B. Mindfulness Training and Physical Health: Mechanisms and Outcomes. Psychosom Med. 2019;81(3):224-232. doi:10.1097/PSY.0000000000000675 [PMC free article: PMC6613793] [PubMed: 30806634] [CrossRef]
- 53.
- Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and Commitment Therapy (ACT) for Chronic Pain: A Systematic Review and Meta-Analyses. Clin J Pain. 2017;33(6):552-568. doi:https://dx
.doi.org/10 .1097/AJP.0000000000000425 [PubMed: 27479642] - 54.
- Hayes, SC, Strosahl, K, & Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York: Guilford Press; 1999.
- 55.
- Lin J, Scott W, Carpenter L et al. Acceptance and commitment therapy for chronic pain: protocol of a systematic review and individual participant data meta-analysis. Syst Rev. 2019;8(1):140. doi:10.1186/s13643-019-1044-2 [PMC free article: PMC6570828] [PubMed: 31200768] [CrossRef]
- 56.
- Dahl J, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: A preliminary randomized trial. Behav Ther. 2004;35(4):785-801. doi:10.1016/S0005-7894(04)80020-0 [CrossRef]
- 57.
- Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Eur J Pain (United Kingdom). 2013;17(4):599-611. doi:10.1002/j.1532-2149.2012.00224.x [PubMed: 23090719] [CrossRef]
- 58.
- Kemani MK, Olsson GL, Lekander M, Hesser H, Andersson E, Wicksell RK. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: A Randomized Controlled Trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203 [PubMed: 25585272] [CrossRef]
- 59.
- Luciano JV., Guallar JA, Aguado J, et al. Effectiveness of group acceptance and commitment therapy for fibromyalgia: A 6-month randomized controlled trial (EFFIGACT study). Pain. 2014;155(4):693-702. doi:10.1016/j.pain.2013.12.029 [PubMed: 24378880] [CrossRef]
- 60.
- McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: Pilot outcome and process results. J Pain. 2013;14(11):1398-1406. doi:10.1016/j.jpain.2013.06.011 [PMC free article: PMC3824075] [PubMed: 24035351] [CrossRef]
- 61.
- Atkinson JH, Slater MA, Capparelli EV, et al. A randomized controlled trial of gabapentin for chronic low back pain with and without a radiating component. Pain. 2016;157(7):1499-1507. doi:https://dx
.doi.org/10 .1097/j.pain.0000000000000554 [PMC free article: PMC5001843] [PubMed: 26963844] - 62.
- Plumb Vilardaga JC. Acceptance and commitment therapy for longstanding chronic pain in a community-based outpatient group setting. Diss Abstr Int Sect B Sci Eng. 2013;74(5-B(E)):No-Specified.
- 63.
- Mo’Tamedi H, Rezaiemaram P, Tavallaie A. The effectiveness of a group-based acceptance and commitment additive therapy on rehabilitation of female outpatients with chronic headache: Preliminary findings reducing 3 dimensions of headache impact. Headache. 2012;52(7):1106-1119. doi:10.1111/j.1526-4610.2012.02192.x [PubMed: 22712503] [CrossRef]
- 64.
- Herbert MS, Afari N, Liu L, et al. Telehealth Versus In-Person Acceptance and Commitment Therapy for Chronic Pain: A Randomized Noninferiority Trial. J Pain. 2017;18(2):200-211. doi:https://dx
.doi.org/10 .1016/j.jpain.2016.10.014 [PubMed: 27838498] - 65.
- Alonso-Fernandez M, Lopez-Lopez A, Losada A, Gonzalez JL, Wetherell JL. Acceptance and Commitment Therapy and Selective Optimization with Compensation for Institutionalized Older People with Chronic Pain. Pain Med. 2016;17(2):264-277. [PubMed: 26304771]
- 66.
- Pincus T, Anwar S, McCracken LM, et al. Delivering an Optimised Behavioural Intervention (OBI) to people with low back pain with high psychological risk; results and lessons learnt from a feasibility randomised controlled trial of Contextual Cognitive Behavioural Therapy (CCBT) vs. Physiotherap. BMC Musculoskelet Disord. 2015;16(1):1-11. doi:10.1186/s12891-015-0594-2 [PMC free article: PMC4468803] [PubMed: 26076755] [CrossRef]
- 67.
- Thorsell J, Finnes A, Dahl J, Lundgren T, Gybrant M, Gordh TBM. A Comparative Study of 2 Manual-based Self-Help Interventions, Acceptance and Commitment Therapy and Applied Relaxation, for Persons With Chronic Pain. Clin J Pain. 2011;27(8):716-723. doi:10.1097/AJP.0b013e318219a933. [PubMed: 21540740] [CrossRef]
- 68.
- Johnston M, Foster M, Shennan J, Starkey NJJA. The effectiveness of an Acceptance and Commitment Therapy self-help intervention for chronic pain. Clin J Pain. 2010;26(5):393-402. doi:10.1097/AJP.0b013e3181cf59ce. [PubMed: 20473046] [CrossRef]
- 69.
- Yang SY, Moss-Morris R, McCracken LM. IACT-CEL: A Feasibility Trial of a Face-to-Face and Internet-Based Acceptance and Commitment Therapy Intervention for Chronic Pain in Singapore. Pain Res Treat. 2017;2017. doi:10.1155/2017/6916915 [PMC free article: PMC5343267] [PubMed: 28326196] [CrossRef]
- 70.
- Scott W, Chilcot J, Guildford B, Daly-Eichenhardt A, McCracken LM. Feasibility randomized-controlled trial of online Acceptance and Commitment Therapy for patients with complex chronic pain in the United Kingdom. Eur J Pain (United Kingdom). 2018;22(8):1473-1484. doi:10.1002/ejp.1236 [PubMed: 29704880] [CrossRef]
- 71.
- Lin J, Paganini S, Sander L, et al. An Internet-Based Intervention for Chronic Pain. Dtsch Arztebl Int. 2017;114(41):681-688. doi:https://dx
.doi.org/10 .3238/arztebl.2017.0681 [PMC free article: PMC5672594] [PubMed: 29082858] - 72.
- Buhrman M, Skoglund A, Husell J, et al. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: A randomized controlled trial. Behav Res Ther. 2013;51(6):307-315. doi:10.1016/j.brat.2013.02.010 [PubMed: 23548250] [CrossRef]
- 73.
- Trompetter HR, Bohlmeijer ET, Veehof MM, Schreurs KMG. Internet-based guided self-help intervention for chronic pain based on Acceptance and Commitment Therapy: A randomized controlled trial. J Behav Med. 2014;38(1):66-80. doi:10.1007/s10865-014-9579-0 [PubMed: 24923259] [CrossRef]
- 74.
- Kerns R. Psychological treatments of chronic pain. Annu Rev Clin Psychol. 2011;7(1):411-434. doi:10.1146/annurev-clinpsy-090310-120430 [PubMed: 21128783] [CrossRef]
- 75.
- Kondo K, Noonan KM, Freeman M, Ayers C, Morasco BJ, Kansagara D. Efficacy of Biofeedback for Medical Conditions: an Evidence Map. J Gen Intern Med. 2019;34(12):2883-2893. doi:10.1007/s11606-019-05215-z [PMC free article: PMC6854143] [PubMed: 31414354] [CrossRef]
- 76.
- Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99(February):298-310. doi:10.1016/j.neubiorev.2019.02.013 [PubMed: 30790634] [CrossRef]
- 77.
- Elkins G, Jensen MP, Patterson DR. Hypnotherapy for the management of chronic pain. Int J Clin Exp Hypn. 2007;55(3):275-287. doi:10.1080/00207140701338621 [PMC free article: PMC2752362] [PubMed: 17558718] [CrossRef]
- 78.
- Garland EL, Brintz CE, Hanley AW, et al. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern Med. 2020;180(1):91-105. doi:10.1001/jamainternmed.2019.4917 [PMC free article: PMC6830441] [PubMed: 31682676] [CrossRef]
- 79.
- Hempel S, Taylor SL, Solloway MR et al. Evidence Map of Acupuncture. Washington (DC): Department of Veterans Affairs (US). https://www
.ncbi.nlm .nih.gov/books/NBK185072/?term=NBK185072. Published 2014. [PubMed: 24575449] - 80.
- MacPherson H, Vertosick EA, Foster NE, et al. The persistence of the effects of acupuncture after a course of treatment: a meta-analysis of patients with chronic pain. Pain. 2017;158(5):784-793. doi:https://dx
.doi.org/10 .1097/j.pain.0000000000000747 [PMC free article: PMC5393924] [PubMed: 27764035] - 81.
- Boehnke KF, Clauw DJ. Brief commentary: Cannabinoid dosing for chronic pain management. Ann Intern Med. 2019;170(2):118. doi:10.7326/M18-2972 [PubMed: 30615778] [CrossRef]
- 82.
- MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19. doi:https://dx
.doi.org/10 .1016/j.ejim.2018.01.004 [PubMed: 29307505] - 83.
- Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. JAMA. 2017;318(17):1661-1667. doi:https://dx
.doi.org/10 .1001/jama.2017.16190 [PMC free article: PMC5818795] [PubMed: 29114833] - 84.
- McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol - a review. Br J Clin Pharmacol. 2018;84(10):2218-2230. doi:https://dx
.doi.org/10.1111/bcp.13656 [PMC free article: PMC6138494] [PubMed: 29863746] - 85.
- Poquet N, Lin C-WC, Heymans MW, et al. Back schools for acute and subacute non-specific low-back pain. Cochrane database Syst Rev. 2016;4:CD008325. doi:https://dx
.doi.org/10 .1002/14651858.CD008325.pub2 [PubMed: 27113258] - 86.
- Bedaiwi MK, Sari I, Wallis D, et al. Clinical Efficacy of Celecoxib Compared to Acetaminophen in Chronic Nonspecific Low Back Pain: Results of a Randomized Controlled Trial. Arthritis Care Res (Hoboken). 2016;68(6):845-852. doi:https://dx
.doi.org/10.1002/acr.22753 [PubMed: 26474041] - 87.
- Watson CPN, Gilron I, Sawynok J, Lynch ME. Nontricyclic antidepressant analgesics and pain: Are serotonin norepinephrine reuptake inhibitors (SNRIs) any better? Pain. 2011;152(10):2206-2210. doi:10.1016/j.pain.2011.05.032 [PubMed: 21723037] [CrossRef]
- 88.
- Chou R, Deyo R, Friedly J, et al. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):480-492. doi:https://dx
.doi.org/10.7326/M16-2458 [PubMed: 28192790] - 89.
- Zar-Kessler CAM, Belkind-Gerson J, Bender S, Kuo BM. Treatment of Functional Abdominal Pain With Antidepressants: Benefits, Adverse Effects, and the Gastroenterologist’s Role. J Pediatr Gastroenterol Nutr. 2017;65(1):16-21. doi:https://dx
.doi.org/10 .1097/MPG.0000000000001416 [PubMed: 28644344] - 90.
- Urquhart DM, Wluka AE, Van Tulder M, et al. Efficacy of Low-Dose Amitriptyline for Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(11):1474-1481. doi:10.1001/jamainternmed.2018.4222 [PMC free article: PMC6248203] [PubMed: 30285054] [CrossRef]
- 91.
- Enthoven WTM, Roelofs PDDM, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane database Syst Rev. 2016;2:CD012087. doi:https://dx
.doi.org/10 .1002/14651858.CD012087 [PMC free article: PMC7104791] [PubMed: 26863524] - 92.
- Brutcher RE, Kurihara C, Bicket MC, et al. Compounded topical pain creams to treat localized chronic pain: A randomized controlled trial. Ann Intern Med. 2019;170(5):309-318. doi:10.7326/M18-2736 [PubMed: 30716769] [CrossRef]
- 93.
- Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013;88(2):195-205. doi:10.1016/j.mayocp.2012.11.015 [PubMed: 23374622] [CrossRef]
- 94.
- DiBenedetto DJ, Weed VF, Wawrzyniak KM, et al. The Association Between Cannabis Use and Aberrant Behaviors During Chronic Opioid Therapy for Chronic Pain. Pain Med. 2017. doi:https://dx
.doi.org/10.1093/pm/pnx222 [PubMed: 29947796] - 95.
- Ripamonti C, Groff L, Brunelli C, Polastri D, Stavrakis A, De Conno F. Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio? J Clin Oncol. 1998;16(10):3216-3221. doi:10.1200/JCO.1998.16.10.3216 [PubMed: 9779694] [CrossRef]
- 96.
- Galvagno SM, Correll DJNS. Safe oral equianalgesic opioid dosing for patients with moderate-to severe pain. Resident and Staff Physician. https://www
.mdmag.com /journals/resident-and-staff /2007/2007-04/2007-04_06. Published 2007 - 97.
- Els C, Jackson TD, Kunyk D, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane database Syst Rev. 2017;10:CD012509. doi:https://dx
.doi.org/10 .1002/14651858.CD012509.pub2 [PMC free article: PMC6485910] [PubMed: 29084357] - 98.
- Abrecht CR, Brovman EY, Greenberg P, Song E, Rathmell JP, Urman RD. A Contemporary Medicolegal Analysis of Outpatient Medication Management in Chronic Pain. Anesth Analg. 2017;125(5):1761-1768. doi:https://dx
.doi.org/10 .1213/ANE.0000000000002499 [PubMed: 29049120] - 99.
- Institute for Clinical Systems Improvement. Health Care Guideline: Pain: Assessment, Non-Opioid Treatment Approaches and Opioid Management Care for Adults.; 2019.
- 100.
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66 [PubMed: 18174009] [CrossRef]
- 101.
- Chabal C, Erjavec M, Jacobson LMA. Prescription Opiate Abuse in Chronic Pain Patients: Clinical Criteria, Incidence, and Predictors. Clin J Pain. 1997;13(2):150-155. [PubMed: 9186022]
- 102.
- Ballantyne JC, Sullivan MDKA. Opioid Dependence vs Addiction: A Distinction Without a Difference? Arch Intern Med. 2012;172(17):1342-1343. doi:10.1001/archinternmed.2012.3212 [PubMed: 22892799] [CrossRef]
- 103.
- Just JM, Bingener L, Bleckwenn M, Schnakenberg R, Weckbecker K. Risk of opioid misuse in chronic non-cancer pain in primary care patients - A cross sectional study. BMC Fam Pract. 2018;19(1):1-5. doi:10.1186/s12875-018-0775-9 [PMC free article: PMC6011396] [PubMed: 29925323] [CrossRef]
- 104.
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP van der GD. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576. [PubMed: 25785523]
Appendices
Appendix A2. PEG Scale Assessing Pain Intensity and Interference (Pain, Enjoyment, General Activity)
Computing the PEG Score
Add the responses to the three questions, then divide by three to get a mean score (out of 10) on overall impact of points.
Using the PEG Score
The score is best used to track an individual’s changes over time. The initiation of therapy should result in the individual’s score decreasing over time.
Source
- Krebs, E. E., Lorenz, K. A., Bair, M. J., Damush, T. M., Wu, J., Sutherland, J. M., Asch S, Kroenke, K. (2009). Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. Journal of General Internal Medicine, 24(6), 733–738. http://doi
.org/10.1007/s11606-009-0981-1 [PMC free article: PMC2686775] [PubMed: 19418100]
Appendix A4. Outline for Follow-up Visits for Patients with Chronic Pain
| Subjective |
|
| Objective |
|
| Assessment |
|
| Plan |
|
Appendix C. Oral Opioid Dose Equivalents and Conversions
Typical oral (every 4 hours) doses of short-acting opioids shown as equivalents to morphine:
| Morphine | 60 mg |
| Hydrocodone (Norco) | 60 mg (equal to morphine potency) |
| Oxycodone | 40 mg (1.5 × morphine potency) |
| Hydromorphone (Dilaudid) | 12 mg (5 × morphine potency) |
| Oxymorphone (Opana) Use of this is generally not recommended due to high expense and abuse rates. | 15 mg (4 × morphine potency) |
| Codeine (Tylenol #3 or #4) | 360 mg (one-sixth morphine potency) |
Dosing Principles
For patients requiring daily opioid therapy for longer than a few days to a few weeks, consider switching from short-acting opioids to long-acting oral therapy. Fentanyl patches are another option, but they are expensive, and it is difficult to titrate the dose. Conversion to methadone is also appropriate for patients requiring opioid use greater than several months, assuming opioids are effective for the patient. Buprenorphine is another option, particularly if opioid use disorder, opioid misuse, or extreme opioid tolerance is a risk.
First, convert any opioid in use to its equivalent amount of morphine in milligrams per day (MME/day). Then, divide into twice daily (or three times daily) Morphine ER doses. Methadone and fentanyl conversions are below.
Morphine to Methadone Conversion
Typical pain doses of methadone are 15-30 mg/day, given in 2-4 divided doses, whereas methadone doses used for treating addiction are higher and may reach 80-120 mg/day. Due to its function through NMDA receptors in addition to mu-receptors as well as its accumulation and excretion into the circulation from the liver, the relative potency of methadone to morphine increases considerably as morphine doses increase. Approximate equivalencies:
| Oral Morphine | Oral Methadone |
|---|---|
| 30-90 mg | One fourth the morphine dose |
| 90-300 mg | One eighth (200 mg/day morphine = 25 mg/day methadone) |
| 300-500 mg | One twelfth the morphine dose |
| > 500 mg | One twentieth the morphine dose |
Morphine to Fentanyl Patch Conversion
Each 2 mg of oral morphine per day is approximately equivalent to 1 mcg/hr fentanyl patch (eg, morphine 100 mg/day is approximately equivalent to a fentanyl 50 mcg/hr patch, applied every 3 days). Use caution in older adults and patients with cachexia; fentanyl is lipid soluble and requires subcutaneous fat for proper absorption.
Tapering Down the Opioid Dose
Slow taper. Reduce the opioid dose every 1-4 weeks by 10% of original dose until 20% remains. Then, taper down the remaining 20% by 5% of the original dose until the opioid has been discontinued, or the patient is at goal.
Rapid taper. Reduce the dose by 25% every 3–7 days, depending upon shorter vs. longer drug half-life.
Appendix D. Ordering and Interpreting Urine Drug Tests
When initiating or monitoring opioid therapy, two tests are often required. The two complimentary tests are the enzyme linked immunoassay (EIA) kit and gas or liquid chromatography/mass spectrometry (GC/MS or LC/MS). They provide different information.
- Illicit drugs: EIA
- Confirm taking prescribed meds (specify meds when order test): GC/MS or LC/MS. (EIA will provide this information if your laboratory runs the test for each med. However, laboratories usually do not. Ask!)
- Use of non-prescribed medication: GC/MS or LC/MS
- Testing for heroin: GC/MS. Check for one of its specific metabolites, eg, 6 monoacetyl morphine (6-AM) duration 2-4 hours only is positive as morphine in 2-3 days
Enzyme linked immunoassay – EIA
- Screening test for illicit substances amphetamine/methamphetamine, marijuana, PCP, cocaine, “opiates” (eg, morphine/codeine)
- Inexpensive, fast, point of care or lab test
- Detects class of substance, not specific medication
- Will be negative for hydrocodone, hydromorphone, oxycodone, methadone, buprenorphine, benzodiazepines (particularly clonazepam) unless a specific test kit for those meds is in use. >Ask your lab!
- High false positive rates caused by numerous prescribed or OTC meds
Gas or liquid chromatography/mass spectrometry – GC/MS or LC/MS. You must tell the laboratory the drugs you are seeking (patient is prescribed).
- More expensive, labor intensive
- Confirming test identifies specific meds and their metabolites. Use to confirm patient is taking prescribed meds and not taking non-prescribed meds
- High sensitivity
- False positives still occur
Interpretation of Results and Possible Causes
Results may be due to several possible causes.
- Illicit substance present: Use by patient; false result related to prescribed or OTC med exposure
- Non-prescribed medication present: Illicit use by patient; false positive testing – cross-reaction or possible known metabolite (hydrocodone can cause a false positive oxycodone test)
- Prescribed medication absent: diversion, or bingeing and running out early; false negative (incorrect use of EIA rather than GC/MS or LC/MS testing); urine adulterated
False positives. Are the results due to illicit use, a false positive on the screen, or a known metabolite of a prescribed medication? In considering prescribed medications, false positives on EIA (and GC/MS or LC/MS where specified) may result from:
- Amphetamines/methamphetamine: bupropion, tricyclic antidepressants, phenothiazines, propranolol, labetalol, OTC cold medications, ranitidine, trazodone. Vicks Nasal Spray can test positive even on GC/MS.
- Barbiturates: phenytoin
- Benzodiazepines: sertraline
- LSD: amitriptyline, doxepin, sertraline, fluoxetine, metoclopramide, haloperidol, risperidone, verapamil
- Opioids
- - EIA testing: quinolones, dextromethorphan, diphenhydramine (Benadryl), verapamil, poppy seeds
- - GC/MS testing
- Morphine: from codeine, heroin (for a few hours), and poppy seeds for 48 hours
- Hydromorphone: from morphine, codeine, hydrocodone, heroin
- Oxycodone: from hydrocodone
- Codeine: from hydrocodone
- Fentanyl: from trazodone
- Methadone: from quetiapine (Seroquel)
- PCP: dextromethorphan, diphenhydramine, NyQuil, tramadol, venlafaxine (Effexor), NSAIDs, imipramine
- Propoxyphene: methadone, cyclobenzaprine (Flexeril), doxylamine (NyQuil), diphenhydramine (Benadryl), imipramine
- Cannabinoids (on EIA not GC/MS): pantoprazole (Protonix), efavirenz (Sustiva, Atripla), NSAIDs
False negatives. Are the results due to the patient running out of medication early, diversion, a tampered specimen, or a threshold issue (eg, workplace testing using a high threshold for reporting a positive test to avoid false positives that require a job intervention)? For EIA (and GC/MS where specified) false negatives may result from:
- Unless bundled (ask your lab!), opioid immunoassays will miss fentanyl, meperidine, methadone, pentazocine (Talwin), oxycodone, tramadol, and often hydrocodone.
- Morphine: GC/MS may miss it unless glucuronide hydrolyzed. Can pick up with a specific test such as a specific qualitative EIA kit such as MSOPIATE. (Ask your lab!)
- Illnesses that cause lactic acidosis can cause false negatives
- Insensitivity of benzodiazepine screen: 40% or less sensitivity for alprazolam, lorazepam, clonazepam all frequently negative on both EIA and GC/MS.
Appendix E. Summary of Michigan Legislation Related to Controlled Substance Prescribing
| Regulation | Pertinent Details |
|---|---|
| PA 0250 Requires health professionals to provide information on substance use treatment services to patients who have experienced an opioid overdose. | Provide a list of substance use disorder services at the time of discharge from care. |
| PA 0247 Requires prescribers to have a bona-fide clinician-patient relationship before prescribing controlled substances and specifies penalties for not meeting these requirements. | Ask and document other controlled substances being taken. Review the patient’s records. Complete a full assessment of the patient’s medical history and current medical condition, either in person or via telehealth. Provide follow-up care to monitor efficacy. |
| PA 0246 Requires prescribers to discuss and provide information about the dangers of opioids and obtain acknowledgement of that information prior to prescribing. | Excludes minors when treated for medical emergencies, surgery, hospice, oncology. Excludes all patients when prescribed for inpatient administration. Extensive content requirements. Requires patient/parent/guardian signature to be stored in the electronic record. |
| PA 0248 and PA 0249 Requires Michigan prescribers to register with MAPS and check MAPS when prescribing more than a three-day supply of a Schedule II - V controlled substance. | Ask and document other controlled substances being taken. Provide follow-up care to monitor efficacy. Excludes hospital/skilled nursing facility administrations. |
| PA 0251 Limits the supply of an opioid that could be prescribed for acute pain to a 7-day supply of an opioid within a 7-day period. | “Acute pain” is typically associated with invasive procedures, trauma, and disease, and usually lasts for a limited amount of time. |
| PA 0554 Amended the Public Health Code to provide for a voluntary nonopioid directive. | The nonopioid directive is a form that can be filled out by the patient (or a person’s legal guardian or patient advocate) directing health professionals and emergency medical services personnel to not administer opioids to the patient. The form is available from the Department of Health and Human Services. Once submitted, the directive must be included in the patient’s medical records. When a patient has this form on file, opioids should not be prescribed. There are exceptions in the law, such as a provision that a prescriber or a nurse under the order of a prescriber may administer an opioid if it is deemed medically necessary for treatment. |
Appendix F. Discontinuing Opioids
| Action | Reasons | Process |
|---|---|---|
| Discontinue Immediately |
| No further prescribing. |
| Rapid Taper |
| Multiple drug conversion. If multiple drugs, first convert all medications to MME/day (Appendix C) and taper down as morphine (using morphine sulfate extended release). If methadone is in use, convert to methadone equivalents. Rapid taper. Taper down by 25% every 3-7 days (shorter interval for short half-life medications). As little as 25% of the preceding dose may be used to avoid severe withdrawal. |
| Slow Taper |
| Multiple drug conversion. If multiple drugs, first convert all medications to MME/day (Appendix C) and taper down as morphine (using morphine sulfate extended release). If methadone in use, convert to methadone equivalents. Slow taper. Taper down by 10% of original dose every week until 20% remains. Taper down the remaining 20% by 5% of original dose each week until off or at goal. |
| Buprenorphine Conversion with Tapering Down (requires XDEA number and experience) |
| Referral for evaluation. Refer to chronic pain service for evaluation and clinic conversion. Evaluation during hospitalization. Evaluate patients with lack of benefit of opioids or with toxicity, who may benefit from conversion to buprenorphine. |
Appendix G. Example Clinical Policy
Clinic Policy Regarding Patients on Long-term Controlled Substances (including opioids, benzodiazepines, and stimulants)
New Patients with a History of Long-term Use of a Controlled Substance
Before a new patient with a history of long-term controlled substance prescription use receives the first prescription from a clinic clinician, our clinic record must contain: the medical records, urine comprehensive drug test results, MAPS search results, and if long-term use is anticipated, a completed Controlled Substance Agreement.
Medical records. Patients must provide medical records documenting the medical workup related to the problem for which the controlled substance was prescribed, and notes from previous clinicians who prescribed these medications.
Obtain relevant medical records from previous clinicians. The patient is responsible for having this information sent. Our clinic staff can provide forms for release of information along with the fax number and mailing address of our clinic. The previous clinician’s office should send the information directly to our clinic. Our clinic will also provide to the patient our clinic phone number so they can verify that the requested medical records have been received and can make appointments.
Use the suggested format outline for the initial clinic note. Include elements of the Past, Family, and Social histories that could put a patient at risk for medication problems. Include a detailed prescription history. Document the last time and date when the controlled substance was taken.
Urine comprehensive drug screen (“DRUG COMP”). DRUG COMP is combined immunoassay screening and gas chromatography/mass spectroscopy that together detect specific synthetic opioids along with morphine/codeine, benzodiazepines and drugs of abuse such as amphetamines, THC, and cocaine. It will also detect many common prescription meds such as tramadol, cyclobenzaprine, and tricyclic antidepressants (TCAs). (A SAMHSA Drug 5 or Drug 6 immunoassay screen is inadequate due to difficulty of interpretation and problems with false positives and negatives.)
Order a DRUG COMP screen for all new patients. To avoid false negatives, inform the lab in the test order if a specific opioid should be present. This is particularly important for methadone, fentanyl, and buprenorphine.
DRUG COMP specimen is collected in the clinic. Patients should not wear coats and other outer clothing nor take purses, bags, or backpacks into the bathroom. The nurse or clinician should confirm promptly that the specimen is appropriately warm and should send it directly to the lab, not give it to the patient to deliver.
Check for consistency between the drug screen results and the patient history. Check that no illicit drugs are present.
Michigan Automated Prescription System (MAPS). Search the state’s online database of prescription fills controlled substances. Look for multiple prescribers or use of multiple pharmacies. Check for consistency between the report and the patient’s history.
(MAPS: https://michigan.pmpaware.net/login for the patient’s filling history. Clinicians should register at https://milogintp.michigan.gov/uisecure/tpselfservice/anonymous/register.)
Controlled Substance Agreement. If long-term use is anticipated, have the patient review and sign a Controlled Substance Agreement. Do this at the visit when the first controlled substance prescription is provided.
Appendix H. Resources and Websites
Information About Chronic Pain

Managing Your Child’s Chronic Pain, by Tonya Palermo is a good example of a book that provides sound scientific knowledge and practical advice for parenting youth who have ongoing pain.
When Your Child Hurts, by Rachel Coakley provides families with basic information about what chronic pain is and how to deal with issues like, “How much do I push my child?”, “Do I still make them go to school?” And other common issues faced by parents.
These books are not designed to replace good cognitive behavioral and family therapy, but can be good additions to the work done in therapy.
The Chronic Pain and Illness Workbook for Teens, by Rachel Zoffness is designed specifically for teens who deal with chronic pain and fatiguing conditions. It takes the best of what we know about CBT and helps the teen apply it to their lives
Several websites designed to provide basic information about chronic pain and its treatment are listed below:
This online program is Cognitive Behavioral Therapy (CBT) based and can be done with or without a pain-trained therapist.
PainBytes
http://www.aci.health.nsw.gov.au/chronic-pain/painbytes
A great option for treatment of Chronic Pain among adolescents is the WebMAP Mobile App. CBT and use of the techniques in the app have been shown to decrease pain, increase functioning and improve quality of life. Get it now in the App Store.
A good general website with resources for helping families cope with chronic pain is the www.MegFoundationforPain.org
A good general explanation of chronic pain: https://www.youtube.com/watch?v=C_3phB93rvI
A good resource for teachers is http://teachpain.wordpress.com/pain-101/
Finding a Local Counselor
ADAA - https://members.adaa.org/page/FATMain
CHRONIC PAIN COPING RESOURCES
Books: Pain is really strange by Stephen Haines
Websites:
Neuroplasticity Transformation: Dr. Moskowitz and Dr. Golden
American Chronic Pain Association
https://mobile.va.gov/appstore/mental-health for various apps
The VA has a page specific to chronic pain https://www.va.gov/PAINMANAGEMENT/Veteran_Public/CHRONIC_PAIN_101.asp
APPROVALS
| P&T | Date: 11/17/2020 |
| Pain Committee | Date: 10/15/2020 |
| CPC | Date: 12/4/2020 |
| ECCA | Date: 1/12/2021 |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Created: May 2021.
- Clinical Problem and Current Dilemma
- Acute and Subacute Pain – Overview
- General Approach to Chronic Pain
- Biopsychosocial Assessment of Chronic Pain
- Designing an Individualized Pain Treatment Plan
- Non-Pharmacologic Treatment
- Non-Opioid Pharmacologic Treatment
- Opioids: Decision Phase
- Opioids: Initiation and Treatment Phase
- Opioids: Maintenance Phase
- Tapering Down and Discontinuing Opioids
- Assuming Care for Patients Already on Opioids
- Related National Guidelines and Performance Measures
- Guideline Development Methodology
- Acknowledgements
- References
- Appendices
- APPROVALS
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Review Urinary Tract Infection[ 2021]Review Urinary Tract InfectionBettcher CM, Campbell E, Petty LA, Rew KT, Zelnik JC, Lane GI, Van Harrison R, Proudlock AL, Rew KT. 2021 May
- Review [National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients].[Urologe A. 2011]Review [National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients].Wagenlehner FM, Schmiemann G, Hoyme U, Fünfstück R, Hummers-Pradier E, Kaase M, Kniehl E, Selbach I, Sester U, Vahlensieck W, et al. Urologe A. 2011 Feb; 50(2):153-69.
- Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection.[Pediatrics. 1999]Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection.. Pediatrics. 1999 Apr; 103(4 Pt 1):843-52.
- Age, nursing home residence, and presentation of urinary tract infection in U.S. emergency departments, 2001-2008.[Acad Emerg Med. 2012]Age, nursing home residence, and presentation of urinary tract infection in U.S. emergency departments, 2001-2008.Caterino JM, Ting SA, Sisbarro SG, Espinola JA, Camargo CA Jr. Acad Emerg Med. 2012 Oct; 19(10):1173-80.
- Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement.[Pediatrics. 1999]Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement.Downs SM. Pediatrics. 1999 Apr; 103(4):e54.
- Pain ManagementPain Management
Your browsing activity is empty.
Activity recording is turned off.
See more...