Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population. Adults and adolescents 12 years and older with unipolar depressive disorders.
Objectives. (1) Improve early recognition and treatment of unipolar depression. (2) Improve patient understanding of depression and its treatment. (3) Recommend appropriate first-line treatments. (4) Identify when referral is indicated. (5) Explain treatment differences for adolescents, women, and older adults.
Key points
Epidemiology
Common. Depression is common, under-diagnosed, and under-treated (Table 1 and Figures 1, 2).
Table 1
Core Depressive Symptoms.
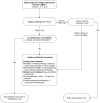
Figure 1
Treatment of Depression: First Visit.
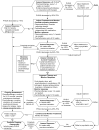
Figure 2
Treatment of Depression: Subsequent Visits.
Recurrent. Depression is frequently a recurrent or chronic disorder, with a 50% recurrence rate after the first episode, 70% after the second, and 90% after the third.
Care provider. Most patients with depression will receive most or all care from their primary care team.
Diagnosis. Screen all patients periodically for depression using a validated questionnaire: PHQ-2, PHQ-9, Edinburgh Postnatal Depression Scale (EPDS), Geriatric Depression Scale. [IA] Somatic complaints are a common presenting feature in depression (Table 2). [C]
Table 2
Common Factors Associated with Depression.
Treatment. Mild episodes of major depressive disorder can be effectively treated with either medication or psychotherapy. Moderate to severe depression may require combining medication and psychotherapy. [IIA*] See Figures 1 and 2 for an overview of treatment.
Frequent initial visits. Initially patients require frequent visits (eg, every 2-4 weeks during the first 12 weeks) to assess response, suicidal ideation, side effects, and psychosocial support systems (Figure 3). [ID]
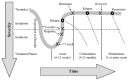
Figure 3
Phases of Treatment for Major Depressive Disorder.
Continuation therapy. Continuing therapy for 9–12 months after acute symptoms resolve decreases the incidence of relapse of major depressive disorder. [IA] Consider long-term maintenance or life-time drug therapy for selected patients with a history of relapse or other clinical features (Figure 3). [IIB]
Psychotherapy. In mild to moderate depression, consider evidence-based psychotherapy alone or with medication. In severe depression, combine psychotherapy with medications (Table 3).
Table 3
General Principles of Evidence-Based Psychotherapy.
Pharmacotherapy. (Table 4). Understand the dosing and potential side effects for at least two first-line antidepressants from different drug classes and two augmentation agents. [IE]
Table 4
Key Medications for Major Depressive Disorder.
First line. 40-50% of patients respond to the first antidepressant [A]. Escitalopram and sertraline are slightly more efficacious and tolerable. [IIA] Achieve therapeutic dose in 2 weeks. If no response to adequate dose at 6-8 weeks, consider switching within class or to another class.
Augmentation. If partial response to medication, consider augmentation with aripiprazole, quetiapine, risperidone, lithium, or brexpiprazole. [IIA]
Monitoring. Monitor patients treated with antidepressants for worsening depression or suicidality (especially in adolescents). [IC] Assess response at week 1 and at week 6. [IE]
Other treatment modalities. Provide patient education and recommend exercise for all. [IA] For seasonal pattern, recommend light therapy. [IA] For patients desiring complementary or alternative treatments, consider St. John’s Wort for mild to moderate symptoms [IIA]; evidence is limited for other options. (See text and Table 6.)
Table 6
Other Modalities and Integrative Treatments for Mild to Moderate Depression.
Integrated behavioral care and collaborative care. For patients with mild to moderate depression, consider referral to collaborative care. [IIA] It is superior to usual care. (Figures 1, 2).
Referral to psychiatry. Refer patients if depression is severe, treatment-resistant, bipolar, failed ≥ 2 medication trials, not responding to collaborative care, requires specialized treatments (page 25), or involves complex situations (Figure 1). [IA]
Special populations
Adolescents. First line is psychotherapy. [IA] For pharmacotherapy, fluoxetine and escitalopram have the most evidence [IIA]; monitor patients weekly for the first 4 weeks of treatment [IIB].
Women. Premenstrual dysphoric disorder: diagnose and treat based on cyclic timing and symptoms (Table 7). [IA] Pregnancy: screen for and treat depression during the first, second, and third trimesters, as well as postpartum (Tables 7 and 8). [IA]
Table 7
Treatment for Major Depressive Disorder in Pregnancy.
Table 8
Treatment of Postpartum Depression during Breastfeeding.
Later life (age ≥ 60 years). With depression diagnosis, consider early dementia. [IIA] When treating depression with medications, start at lower doses and increase slowly to avoid side effects [IA]. Consider non-medication treatments to help avoid polypharmacy [IIC].
Footnotes
- *
Strength of recommendation: I = generally perform; II = may be reasonable to perform; III = generally do not perform.
Level of evidence supporting a diagnostic method or an intervention: A = Systematic review of randomized controlled trials; B = randomized controlled trials; C = systematic review on non-randomized controlled trials, non-randomized controlled trials, group observation studies; D = Individual observation descriptive study, E = expert opinion.
Clinical Background
Prevalence and effects. Depression is common. The lifetime prevalence of major depressive disorder among adults in the US is 20.6%, and the annual prevalence is 10.4%. Women are more commonly affected than men.13 Depression often leads to prolonged impairment in occupational functioning, relationships, and physical health, greatly impacting society.
Patient factors. Many patients receive most or all of their depression care from their primary care physician. Patients with depression frequently present solely with physical complaints and are unaware of interactions of their physical symptoms with depression. Others are unwilling to accept medication or counseling because of the stigma associated with depression. Comorbidities may affect treatment options.
Management barriers. Diagnosing and treating depression in the primary care setting can be challenging. For example, physician-patient encounter time is brief, limiting the physician’s full assessment of the patient for depressive signs and symptoms. Reimbursement restrictions can interfere with comprehensive treatment.
Depressive Disorder Classifications
Table 1 summarizes core depressive symptoms.
DSM-5 modified the classification of depressive disorders.
Common depressive disorders include:
- Major depressive disorder
- Persistent depressive disorder (dysthymia)
- Minor depression
- Premenstrual depressive disorder
- Seasonal affective disorder
- Mood disorder associated with a medical condition
- Bereavement
The term depression reflects several symptoms and specific groupings of symptoms that define diagnostic categories. Core depressive symptoms are presented in Table 1.
Evolving Classifications
The current classifications of depression are based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), published in 2013, and on “Recurrent Depressive Episodes” in the International Classification of Diseases, 10th Revision (ICD-10) classification of mental and behavioral disorders, published in 2018.14,15 Changes from previous categories illustrate the ongoing evolution of diagnostic classification. As examples 16, DSM-5:
- Separated the broad category of mood disorders into more specific categories, such as depressive disorders.
- Removed bereavement as an exclusionary factor for a diagnosis of depression. This change recognized that bereavement may last longer than 2 months and is similar to other stressors or losses that may result in major depressive episodes.
- Added modifiers of anxious distress and mixed features to major depressive disorder.
Common Depressive Disorders
Major depressive disorder (MDD), single or recurrent. Major depressive disorder is a severe form of depression that is often accompanied by significant functional impairment and increased use of health services. Major depressive disorder is the primary focus of this treatment guideline. It is common and has the largest evidence-base for treatment. Both psychotherapy and pharmacotherapy have been shown to be effective treatments for major depressive disorder and may be used together.
Persistent depressive disorder (dysthymia). This is a chronic, smoldering form of depression with fewer, less severe depressive symptoms than MDD, but with functional impairment that can sometimes equal that seen among patients with MDD. Although antidepressants are somewhat less efficacious in dysthymic disorder than in MDD, a substantial proportion of patients will respond to antidepressants or structured psychotherapies. Patients with dysthymic disorder may have periods of time when they also meet criteria of MDD, often called “double depression.”
Minor depression. Minor forms of depression are common. These patients have fewer than 5 depressive symptoms or a duration of symptoms shorter than 2 weeks. Although minor depression produces less functional impairment than MDD or dysthymic disorder, because of its frequency, most work days that are missed in the US are attributable to this milder disorder. Specific treatments such as antidepressants or psychotherapy may not be indicated. Rates of improvement are high for these individuals with watchful waiting. This is not a DSM-5 diagnosis but is often used in research.
Premenstrual dysphoric disorder. Premenstrual dysphoric disorder is a serious form of premenstrual syndrome characterized by intense emotional symptoms occurring in the final week before menses. Typical symptoms include depressed mood, anxiety, mood swings, and irritability.16
Major depressive disorder with seasonal pattern (Seasonal affective disorder). This is a seasonal form of major depression with features similar to MDD, but occurring on a cyclical basis related to ambient light deprivation during winter months. Both light therapy and medications are frequently used.
Mood disorder associated with a general medical condition. This form of depression has features similar to MDD, but is part of the physiological impact of a major medical condition such as cancer, stroke, myocardial infarction, major trauma, or neurodegenerative disorders such as Alzheimer’s disease. A frequent cause of mood disorders in this category is alcohol or other substance use disorder. A less common cause is the use of certain medications.
Appropriate management of primary disorders such as hypothyroidism is essential for patients with depression. Minimizing functional limitations from comorbid medical conditions is also important. However, depression occurring with comorbid conditions should still be treated according to its diagnostic criteria and functional impact, despite the presence of any associated medical illnesses.
Bereavement. Grieving is a normal reaction to a major loss, such as the death of a close relative or friend. Patients may exhibit many symptoms of major depressive disorder following such a loss. However, individuals suffering from bereavement are usually not preoccupied with ideas of worthlessness or guilt and do not experience suicidal ideation. The duration of bereavement varies, but generally symptoms remit or lessen within a few months. Supportive counseling and education usually suffice for treatment, with occasional short-term use of medications for symptom control. In some cases, grief may be more severe and may be prolonged beyond 6 months. Distinguishing it from major depressive disorder can be difficult.
Depression Screening and Diagnosis
Screening
Recommendations
Screen all adults for depression, including pregnant women and postpartum women.
Ensure systems are in place for diagnosis, treatment, and follow-up.
Screen using a validated questionnaire: PHQ-2, PHQ-9, Edinburgh Postnatal Depression Scale (EPDS), Geriatric Depression Scale or other screening tool.
Screen patients periodically based on practical and patient considerations:
- For patients at normal risk, consider screening as part of routine health exams.
- For patients at higher risk, consider screening more frequently.
Population screening. Screen all adults for depression except when patients are already being treated for depression.17 (For patients already being treated for depression, screening tools may be used for monitoring.) Ensure adequate systems are in place for accurate diagnosis, effective treatment, and appropriate follow-up.
Screening tool. Studies have demonstrated that several screening tools can be effective. The two-item PHQ-2 questionnaire is among the simplest to administer and is widely used. A positive answer to either question prompts discussion, with further elaboration by administering the associated nine-item PHQ-9 questionnaire. Another widely used tool is the 16-item Quick Inventory of Depressive Symptomatology Self Report (QIDS-SR 16). Other screening tools often used for specific populations include the Edinburgh Postnatal Depression Scale (EPDS) and the Geriatric Depression Scale. When referring to screening in the general population, we recommend the PHQ-2 and PHQ-9. (See Appendix.)
Patients who directly answering the questions provide better information than when clinician answer for them. Patients may possibly provide false positive and false negative answers. Use the answers to guide a conversation that explores positive answers and negative answers that appear inconsistent with other aspects of the patient’s presentation and circumstances.
Screening frequency. In the absence of evidence concerning optimal frequency for screening, a practical strategy is to:
- Screen all patients periodically, such as with each routine health exam
- Consider screening more frequently for those patients at higher risk for depression. This includes patients with:
- - Chronic medical illness impacting daily life
- - Other psychiatric illness, such as anxiety
- - Substance use
- - Medications that may cause depressive symptoms
- - Childhood trauma
Diagnosis
Recommendations
Use the DSM-5 criteria for major depressive disorder (Table 1) to guide suspicion for and diagnosis of depression.
Look for common presentations of depression and factors associated with it (Table 2).
Obtain a detailed history for diagnosing depression.
- Collect a history that addresses symptoms, their duration and context, previous episodes, any differentiating symptoms and their history, and family history.
- Assess depression severity.
- Check for social and environmental factors, such as partner violence or sexual assault.
- Differentiate depression symptoms from bipolar disorder.
- Screen for suicide risk.
Review medications as potential causes of depression symptoms.
Review comorbid diseases that may cause depression.
Perform a physical exam and laboratory tests as needed to rule out undiagnosed conditions that could cause depression symptoms.
Check for alcohol or substance use disorders, which can cause depression. Patients may use substances to self-treat depression.
The DSM-5 criteria for the diagnosis for major depressive disorder are presented in Table 1. These criteria guide suspicion for depression and ultimately its diagnosis.
Common presentations. Common presentations of depression and factors associated with it are summarized in Table 2. Depression is common in patients with irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, and chronic pain, such as headaches, low back pain, and pelvic pain. Patients with anxiety or depression may deny mood or psychiatric symptoms due to social stigma, because they do not realize that the way they feel is abnormal, or because they truly do not experience mood symptoms. Symptom-sign mismatch suggests a high likelihood of depression or anxiety: the patient may have many seemingly severe symptoms, a negative physical examination, and a long list of normal laboratory tests. It is important to maintain the usual vigilance for undiagnosed medical disease. Check for intimate partner violence, which is associated with depression in pregnancy. Consider intimate partner violence in all women with depression.
History. The patient’s history is extremely important in diagnosing depression.
Symptom history. A history of symptoms for patients with suspected depression should include the following:
- PHQ-9, if not already administered, or other validated questionnaire to elicit symptoms and grade their severity.
- Duration of current symptoms.
- Triggers for symptoms.
- Previous episodes of depression? If so, how many? Severity? What treatments were used? Confirm dose and duration. What was successful and what was not?
- Chronic anxiety?
- Mania or hypomania?
- Psychosis assessment.
- Family history of anxiety, depression, bipolar, or substance use.
Initially many patients may deny any previous history of depression. Further discussion after some treatment may help them recall similar events in the past. Underlying anxiety is common in patients presenting with depression.
Evaluate depression severity. The PHQ-9 will help to establish the severity of the current episode of depression. A PHQ-9 score of 10–15 is often used as a cut-off for mild depression, 16–19 for moderate depression, and > 20 for severe depression. See score interpretation here.
Check for important social and environmental factors. Follow up on any indications of potential contributing social and environmental factors. If not yet addressed, ask about:
- Partner violence – Have you ever been in a relationship where you have been beaten, punched, choked, or hurt in any way? (Women in violent relationships are up to 10 times more likely to report depression.)
- Sexual assault – Have you ever been forced to have sex against your will? As a child or as an adult, did anyone touch you inappropriately sexually? (One-third of all sexual assault victims are depressed.)
Differentiate depression and bipolar disorder. A minority of patients presenting with depression may actually have bipolar disorder. Most patients with bipolar disease present during their episodes of depression, rather than during episodes of mania. Patients with bipolar disorder are likely also to have a history of hypomania; symptoms may include reckless behavior, excessive activity, a decreased need for sleep, racing thoughts, rapid speech, and an elevated mood without psychosis. If bipolar disorder is suspected, the Mood Disorder Questionnaire (MDQ) or Hypomania Checklist (HCL 32) questionnaires are good patient self-rating screening tools which can be easily administered in the office. Links to these two questionnaires are in the Appendix.
Screen for suicide risk. Screen all patients with suspected depression for suicidality and specifically for acute safety risks.18 Suicide and safety screening should occur in primary care clinics as well as mental health clinics. Many patients with suicidal ideation will never present to mental health specialists. However, many patients with suicide attempts will have an interaction with a non-mental health clinician prior to an attempt. Asking about suicidal ideation does not increase the risk of suicide.
The United States Preventive Services Task Force (USPSTF) has concluded that there is insufficient evidence to screen for suicide in the general adolescent, adult, and older adult populations.9
A positive response on question 9 of the PHQ-9 (regarding “Thoughts that you would be better off dead or of hurting yourself in some way”) has been shown to indicate higher risk for suicide attempts.19 Among various risk stratification scales for assessing suicide severity, the Columbia Suicide Severity Rating Scale (C-SSRS) and Suicide Assessment Five-step Evaluation and Triage (SAFE-T) are being widely used. (See Appendix.)
A full explanation of the evaluation of suicide risk is beyond the scope of this guideline. However, suicidal ideation is particularly concerning in depressed patients with psychotic symptoms, such as command auditory hallucinations. Strongly consider referring these patients to psychiatry because they have higher rates of suicide attempts.20 Other factors that increase the risk of suicide include a previous history of suicide attempt, chronic health issues, low job security, past risky behavior, impulsivity, personality disorder, and co-occurring substance use disorder.21–23
A collaborative care management approach has shown promise in lowering suicidal ideation in homebound and geriatric patients with depression, as has telephone monitoring. Consider using collaborative care to help with monitoring patients with suicidal ideation.24,25
Medications. Review the patient’s current medications for any that might cause depressive symptoms. Examples include:
- chronic benzodiazepines, especially in the elderly
- beta-blockers
- hormones (eg, estrogen)
- interferon
- corticosteroids
- anticonvulsants
- varenicline
- isotretinoin
- alcohol
- opioids
Chronic opioid use, whether prescription or illicit, and non-prescription substances such as alcohol, cocaine, and methamphetamine all can cause or exacerbate depression.26 Tapering off these medications may decrease or completely resolve the depressive symptoms.
Comorbid illness and depression. Having a chronic illness significantly raises the risk of having depression. For example, almost 50% of people with chronic neurologic diseases like Parkinson’s disease, or headaches severe enough to require referral to subspecialty care, will also have depression. Acute events like myocardial infarction and stroke are associated with a spike in depression in the subsequent three months. For many diseases such as diabetes, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease (IBD), and chronic pain, having untreated depression is associated with worse control and increased mortality.
Physical exam and lab testing. Look for undiagnosed medical conditions that could cause the patient’s symptoms. No laboratory or radiologic test will prove or disprove depression. Rather, testing is to rule out suspected mimickers of depression, such as thyroid disease as a cause of anxiety or fatigue, or cancer or uncontrolled diabetes as causes of weight loss. Fatigue is a common complaint, so testing for hepatic and renal dysfunction, uncontrolled diabetes, thyroid dysfunction, and sleep apnea may be appropriate. Most antidepressants are metabolized or excreted by the kidneys, so assessing renal function can help guide medication choice and dosing. For many patients it may be sufficient to do tests such as creatinine, glucose, TSH, and CBC, with or without hepatic function tests. The need for further testing depends on specific symptoms.
Substance use disorders. Substance use disorders, including alcohol use disorder, frequently co-occur with depression and can either cause the depressed mood or may represent an attempt to self-medicate for the mood disorder.
If alcohol use disorder and depression are concurrent, address both and consider starting a selective serotonin reuptake inhibitor (SSRI). A period of sobriety will help determine whether use is causing depression. If unable to achieve sobriety, patients with concurrent depression and alcohol use disorder may still be treated with an SSRI.
Suicide rates are higher among depressed patients with alcohol use disorder. Be vigilant in assessing suicide risk. A commonly used 3-item assessment to identify patients who are risky or hazardous drinkers is the AUDIT-C. (See Appendix.)
Treatment of Depression
See Figures 1 and 2 for an overview of the process for monitoring, assessing, and augmenting the treatment of depression.
This section is organized as follows:
- Treatment overview
- Psychotherapy principles
- Pharmacotherapy principles
- Other treatment modalities
- Integrated behavioral care and collaborative care
- Complementary and alternative treatments
- Treatment of comorbid medical and psychiatric illnesses
- Referral
Treatment Overview
Recommendations
Conceptualize treatment in three phases (Figure 3):
- Acute treatment – approximately 12 weeks. Aim for remission of symptoms.
- Continuation treatment – 9–12 months to prevent relapse.
- Maintenance treatment – ongoing if at high risk for relapse.
During the acute treatment phase, monitor frequently.
- Follow up with the patient 1 week after diagnosis, and then every 2–4 weeks during the first 12 weeks.
- Assess symptoms and change (eg, with PHQ-9). If on medications, assess adherence and side effects. Monitor for suicidality. Assess functional status. Rule out comorbid disorders.
- Depending on the patient’s situation and available resources, follow-up may be in person or by phone and may be performed by a physician, advanced practice provider, nurse, or social worker.
During the continuation phase of treatment, conduct office visits on an as-needed basis.
- Assess patients for risk factors for recurrence or relapse.
- Consider lifetime maintenance on antidepressants for those with a high risk of relapse.
In choosing psychotherapy or pharmacotherapy:
- In patients with mild to moderate depression, initial treatment may be with medication, evidence-based psychotherapy or both.
- In patients with moderate to severe depression, medication is frequently the first-line treatment.
- Psychotherapy and pharmacotherapy combined together outperform either psychotherapy alone or pharmacotherapy alone.
Treatment phases. Conceptualize treatment of depression as occurring in three phases (Figure 3). Only the first two phases (acute and continuation therapy) apply to most patients.
- Acute treatment. Aim for remission of all depressive symptoms (eg, PHQ-9 score < 5). Most patients will notice some relief from pharmacotherapy for depression at around 2–4 weeks. The average time to remission, when it occurs, is 6–12 weeks after starting antidepressant medications, but many patients may need more than 12 weeks to reach remission.
- Continuation treatment. To prevent symptom relapse, continue treatment for 9–12 months after symptom relief in most patients. Residual symptoms during this period are highly predictive of relapse, so try to achieve remission using additional treatments as needed. For example, consider adding psychotherapy or pharmacotherapy, exercise, light therapy, or natural products.
- Maintenance treatment. Maintain ongoing treatment for patients who are at high risk for relapse. This includes those with 3 or more episodes of major depressive disorder, a history of severe episodes (psychosis, severe impairment, suicidality), difficult-to-treat depression, residual symptoms, serious medical or psychiatric comorbidity, or severe ongoing stressors.
Ongoing assessment. Follow-up is frequent during the acute treatment phase. Check-in at 1 week to assess:
- Symptoms and severity of depression (eg, repeat PHQ-9) in response to the treatment intervention.
- If a medication was prescribed, ask if medication was filled, whether it was started, and if there have been any side effects.
- Evaluate suicide risk and rule out comorbid disorders.
This follow-up may be in person or by phone, and may be done by a physician, advanced practice provider, nurse, or social worker.
During the first 12 weeks of treatment, follow up about every 2-4 weeks to assess the items immediately above. Monitor:
- Symptom severity (eg, PHQ-9) at each visit, with a treatment goal of remission (PHQ-9 score < 5) or at least reducing PHQ-9 score by 50%.
- Patient’s functional status in response to treatment. Consider using self-report scales like the Sheehan Disability Scale or the Lam Employment Absence and Productivity Scale.16,27,28
During the continuation phase of treatment, conduct office visits on an as-needed basis. Assess risk factors for recurrence or relapse. Consider lifetime maintenance on antidepressants for those at high risk of relapse.
For patients with mild to moderate depression (PHQ-9 < 20), consider referral to a collaborative care model, if available. (See section on Integrated Behavioral Care and Collaborative Care.)
Pharmacotherapy and psychotherapy comparisons. Evidence demonstrates that:
- In patients with mild to moderate depression, initial treatment can be with medication, evidence-based psychotherapy, or both, as guided by patient and physician preferences.
- In patients with moderate to severe depression, medication is frequently the first-line treatment.
Some evidence shows that after taking medications, a course of cognitive behavioral therapy (CBT) or mindfulness-based cognitive therapy (MBCT) can prolong the remission from depression.31
Psychotherapy Principles
Recommendations
If evidence-based psychotherapy is available in the community and the patient is comfortable with it:
- For mild to moderate depression, consider either psychotherapy alone or medication alone.
- For moderate to severe depression, prescribe medication and consider adding psychotherapy.
- For severe or difficult cases of depression, prescribe medication and strongly consider adding psychotherapy.
Several types of psychotherapy are supported by evidence. Eight options and evidence for their use are summarized in the text, with more detailed information presented in Table 3.
Monitor patients being treated with psychotherapy alone.
- If response is insufficient in 12–16 weeks, consider treatment with antidepressant medication.
- If it is unclear whether appropriate evidence-based psychotherapy is being provided, ask the patient about their therapy. See Table 3.
When to offer psychotherapy. Patient preference makes a difference when considering whether to start treatment with psychotherapy or pharmacotherapy. For mild to moderate depression, consider either psychotherapy alone or medication alone. For moderate to severe depression, consider medication with added psychotherapy. For severe or difficult cases of depression, strongly consider using both medication and psychotherapy. Severe and difficult cases include:
- Severe depression (eg, PHQ-9 > 20, hospitalization)
- Chronic depression (lasting > 2 years, or persistent depressive disorder)
- Recurrent depression (three or more episodes)
- History of only partial response to previous trials of medication or psychotherapy
- History of two or more episodes of major depressive disorder with poor interval functioning
- Psychosocial difficulties that interfere with treatment adherence.
Choose an evidence-based psychotherapy that is available in the community and with which the patient is comfortable.
Psychotherapy options. Table 3 describes several evidence-based psychotherapeutic treatments for depression, the evidence for them, and some questions to ask patients to understand appropriate engagement in therapy. In summary, consider:
- Cognitive behavioral therapy (CBT) as a first-line therapy for the acute, continuation, and maintenance phases of depression, even when depression is severe or treatment resistant.
- Interpersonal psychotherapy (IPT) as a first-line treatment for acute depression that is mild to moderate in severity, as a maintenance therapy adjunct to medications, or as a relapse prevention method after remission of depression.
- Behavioral activation (BA) as a first-line treatment for acute mild to moderate depression and in the treatment of depression in the maintenance phase. Behavioral activation can be done in person or by telephone.
- Mindfulness-based cognitive therapy (MBCT) as a first-line treatment for acute depression that is mild to moderate in severity, as a maintenance therapy adjunct to medications, or as a relapse prevention method after remission of depression.
- Problem solving therapy (PST) as a first-line acute treatment for mild to moderate depression in primary care populations, particularly for older adults.
- Acceptance and commitment therapy (ACT) as a first-line acute treatment for mild to moderate depression. It does not have the evidence base of CBT, IPT, PST, or MBCT.
- Cognitive behavioral analysis system of psychotherapy (CBASP) as a second-line treatment for acute and chronic depression, with or without pharmacotherapy. It does not have the evidence base of CBT, IPT, PST, or MBCT.
- Short-term psychodynamic psychotherapy (STPP) as an effective second-line treatment for acute depression in patients who decline either pharmacology treatment or a first-line evidence-based psychotherapy.
Studies comparing group and internet or computer-based psychotherapy, primarily with CBT, generally demonstrate outcomes similar to traditional individualized therapy.32–38
In patients with issues with intimate relationships and mild to moderate depression, consider couples or marital therapy with or without pharmacotherapy. Studies on marital therapy are more difficult to quantify as it is not a specific type of psychotherapy and can use many different modalities.39–41
Monitoring patients treated with psychotherapy alone. These patients should be monitored, although there are no clear guidelines how frequently. Some therapies, such as CBT, IPT, PST, and MBCT show efficacy in as little as 8 sessions. If response is insufficient after 12–16 weeks, consider treatment with antidepressant medication.
As part of monitoring, consider asking patients about their psychotherapy to ascertain whether evidence-based therapy is being provided. Psychotherapy practiced in the community may or may not resemble the standardized psychotherapies proven effective in randomized controlled trials. If patients state that they are doing CBT, it usually includes homework for patients between sessions. Therefore, ask the patient, “What type of homework are you given to work on after your sessions?” Most evidence-based psychotherapies will also have the patient working on goals, so ask, “What specific goals are you and your therapist working on right now?” Questions to ask regarding each type of psychotherapy are listed in Table 3.
Pharmacotherapy Principles
Recommendations
When to prescribe medication:
- For mild to moderate depression, consider medication alone or psychotherapy alone.
- For moderate to severe depression, prescribe medication and consider adding psychotherapy.
- For severe or difficult cases of depression, prescribe medication and strongly consider adding psychotherapy.
When choosing medications:
- Understand the dosing and potential side effects for at least two first-line antidepressants from different drug classes and two augmentation agents.
- Slightly superior medications are escitalopram, and sertraline (efficacy and tolerability).
- Consider using medications that have worked for the patient or an immediate family member in the past.
- See text for additional considerations.
When initiating a trial of a drug:
- Achieve a minimum therapeutic dose in the first 2 weeks of treatment. If necessary, increase the dose over the next 4–6 weeks.
- Assess response in 1 week.
- If the response after 4–6 weeks is only partial and side effects are modest, consider augmentation with aripiprazole, brexiprazole, lithium, quetiapine, or risperidone.
- If the patient shows no response to an antidepressant trial at 6–8 weeks, consider switching either within class or to another class.
- Consider adding stimulants to improve energy, concentration, and functioning.
- Generally, do not augment one antidepressant with another antidepressant.
Monitor symptom response (eg, with PHQ-9) at each follow-up visit.
When remission is achieved:
- If this is a first episode, continue medication for an additional 9–12 months.
- If risk factors for recurrence are present, continue medication longer.
- Schedule office visits as needed.
Manage any medication side effects, such as insomnia, akathisia, weight gain, sexual dysfunction.
To manage refractory depression:
- Use augmentation strategies like dose optimization, adding an augmentation agent, or switching to another medication.
- Consider and address related factors. (See text.)
If medication adherence is a concern, consider:
- Scheduling more frequent visits and supportive contacts, particularly during weeks 1 and 6. Consider referral to a collaborative care program.
- Prescribing long-acting medications to reduce dosing frequency and side effects of missed doses.
- Educating patients about their medications.
Taper most SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) to avoid serotonin discontinuation syndrome.
When to prescribe medication. Consider medication alone for mild to moderate depression. For moderate to severe depression, treat with medication and consider adding psychotherapy. For severe or difficult cases of depression, treat with medication and strongly consider adding psychotherapy. Severe and difficult cases include:
- Severe depression (eg, PHQ-9 > 20, hospitalization)
- Chronic depression (lasting > 2 years, or persistent depressive disorder)
- Recurrent depression (three or more episodes)
- History of only partial response to previous trials of medication or psychotherapy
- History of two or more episodes of major depressive disorder with poor interval functioning
- Psychosocial difficulties that interfere with treatment adherence.
Choice of medications. Prescribing clinicians should understand the dosing and potential side effects for at least two first-line antidepressants from different drug classes and two augmentation agents.
Table 4 lists frequently prescribed medications, dosing strategies, and tips. Consider the following in selecting medications.
- Slightly superior medications. Escitalopram, sertraline, and vortioxetine have the best balance of efficacy and tolerability. Amitriptyline, escitalopram, mirtazapine, paroxetine, venlafaxine, and vortioxetine have shown modest superiority in efficacy in various meta-analyses. However, citalopram, escitalopram, fluoxetine, sertraline, and vortioxetine had the best tolerability.
- Previous success. Use what has worked for the patient in the past.
- Response to a specific medication not predictable. There is no reliable test or biomarker that predicts response to a particular medication.
- Pharmacogenomic testing. Consider pharmacogenomics testing only for patients with multiple antidepressant failures or a history of extreme sensitivity to other medications. Only limited evidence demonstrates that pharmacogenomic testing improves patient outcomes or provides cost-savings.42 Current pharmacogenomic tests cannot identify who will respond to which medication, but may identify which medications will be more problematic in dosing or safety, primarily due to hepatic metabolism.
- Explain potential side effects. Side effects are common. When initiating a medication, explain side effects in detail. Typical side effects include constipation, diarrhea, nausea, dizziness, headache, insomnia, and somnolence. Most are transient and manageable. Sexual adverse effects are particularly common in both women and men (occurring in up to 60%) and can include decreased sexual desire, delayed or absent orgasm, delayed ejaculation, and erectile dysfunction. Bupropion and mirtazapine have lower rates of sexual side effects.
- Special issues with SSRIs and SNRIs.
- - Patients with a seizure disorder. Use SSRIs or SNRIs, rather than bupropion, for patients with a history of seizure disorder. Doses of bupropion higher than 300 mg/day are associated with higher rates of seizure.
- - Less effective with chronic pain. In general, SSRIs may be less helpful for pain. Consider using venlafaxine, duloxetine, or a tricyclic antidepressant.
- - Bone loss. Some evidence suggests that SSRIs may increase bone loss and increase the risk for fragility fractures in older adults. However, no studies show that either no treatment or other antidepressants perform better overall.
- - Upper GI bleed. SSRIs slightly increase the risk for upper gastrointestinal bleeding, mostly in patients who are also on NSAIDs. Consider other medication alternatives for patients at high risk for GI bleed, or discuss risks and benefits with patients.
- - Serotonin syndrome. Serotonergic medications (SSRIs and SNRIs) used in combination with monoamine oxidase inhibitors (MAOIs) increase the risk of serotonin syndrome, with the potential serious effects of altered mental status, agitation, myoclonus, and hyperreflexia. MAOIs include phenelzine, selegiline, and tranylcypromine. Other medications with MAOI activity include the antibiotic linezolid and the dye methylene blue. Very rarely serotonergic antidepressants may produce a serotonin syndrome with the concomitant use of buspirone, dextromethorphan, tramadol, or St. John’s Wort. The clinical risk, however, is extremely low with these combinations.
Trials of drugs. With an adequate trial (4–6 weeks) of first antidepressant, rates are 50% response and 30% remission compared to a 20–40% response rate for placebo. The most common cause of treatment failure is an inadequate medication trial (either medication dosed too low or for too short of a duration).
When initiating a drug trial for a patient, consider the following:
- Initial dosing and monitoring. Achieve a minimum therapeutic dose in the first 2 weeks of treatment (Table 4). If necessary, increase the dose over the next 4–6 weeks.
- Assess response in 1 week. A phone call to the patient by clinic staff about progress or any questions can dramatically increase antidepressant compliance and response rates.
- If the response is partial and side effects modest after 6-8 weeks, consider augmentation with aripiprazole, quetiapine, risperidone, lithium, or brexpiprazole (Table 4).
- If the patient shows no response to an antidepressant trial at 6–8 weeks, consider switching either:
- - within class (ie, SSRI to another SSRI) if a stronger medication exists (eg, fluoxetine to sertraline) OR
- - to another class (eg, SSRI to SNRI).
- Consider adding stimulants (eg, methylphenidate, modafanil) to address problems with energy, concentration, and functioning if not fully resolved after 6-8 weeks of treatment. These medications usually do not improve mood.
- Do not augment one antidepressant with another antidepressant. However, bupropion and mirtazapine are commonly added to SSRIs. Bupropion is less likely than other medications to cause sexual dysfunction. Mirtazapine is helpful in patients with low appetite and poor sleep associated with their depression, but rarely improves the underlying depression.43
Monitoring symptom response. At each follow-up visit, measure response to treatment with a serial measurement tool. Patient self-reports, particularly the PHQ-9 and the QIDS-SR 16, are widely used and validated for monitoring. (See Appendix.)
Some patients treated with antidepressants may experience increased agitation, anxiety, and hostility, particularly in the early stages of treatment, potentially placing them at increased risk of suicidal behavior. Warning patients about this risk and arranging close follow-up after initiation of antidepressants may help mitigate this risk. A black box warning exists regarding potential increased suicide risks among adolescents and young adults. (This warning is controversial. See the section on Depression in Adolescents.)
Approximately 40%–50% of treated patients will show a good response to pharmacotherapy within about 8–12 weeks, although a smaller percentage (approximately 30%) will meet criteria for complete remission. Most patients who show a response at 6–12 weeks will show a partial response to treatment as early as 2–4 weeks.44–46
Continuation phase. When remission is achieved, if this is a first episode, continue medication for an additional 9–12 months from remission. However, if risk factors for recurrence are present, continue medication longer. Discontinuing medication too soon, or decreasing the dosage below that required for treatment response, is associated with a higher rate of relapse.
During the continuation phase of treatment, conduct office visits on an as-needed basis. Assess patients for risk factors for recurrence or relapse. Consider lifetime maintenance on antidepressants for those with a high risk of relapse.
Managing side effects. Insomnia, akathisia (characterized by motor restlessness), weight gain, and sexual dysfunction are side effects commonly associated with the use of antidepressant medications. Consider the following strategies for managing related side effects:
- Insomnia: Add a small dose of trazodone (25–50 mg at bedtime) to an SSRI. Dose sedating antidepressants at bedtime.
- Akathisia: Motor restlessness is most commonly caused by antipsychotics, but it has also been associated with newer antidepressants. Consider adding a small dose of clonazepam (0.5 mg at bedtime) or utilize propranolol.
- Weight gain: No proven remedies exist. Warn patients about this potential side effect and encourage healthy eating, as well as regular exercise for both its weight maintenance effect and its potential to further help with depressive symptoms. Bupropion may be less likely than other antidepressants to result in weight gain.
- Sexual dysfunction: Sexual dysfunction with antidepressants is common in both men and women. Distinguishing between drug effects and symptoms of depression can be difficult. Bupropion is the antidepressant least likely to cause sexual dysfunction and can be used concomitantly with SSRIs or SNRIs. Other less well-proven or studied strategies include the use of sildenafil, cyproheptadine, and gingko biloba.
Augmentation and treatment of refractory depression. Although no single definition of treatment refractory depression exists, patients have often been considered to be treatment refractory if they have been treated with two successive trials of antidepressants:
- in adequate doses
- for adequate periods of time (6–12 weeks).
Approximately 40% of patients with major depressive disorder will not respond to two trials of antidepressant medication.
To manage refractory depression, consider the antidepressant augmentation strategies outlined earlier:
- dose optimization, or
- augmentation, or
- switching to another antidepressant.
For patients whose risk of suicide is manageable and who are agreeable, conduct several trials of antidepressants and augmentation agents prior to psychiatric referral.
For treatment refractory depression, consider:
- Is the diagnosis correct? Could this be bipolar depression, or could it be secondary to another illness?
- Is a comorbid substance use disorder present?
- Has the patient actually been compliant with the medication strategy?
- Has the patient engaged in evidence-based psychotherapy such as CBT?
- Do overwhelming life circumstances require broader social and environmental interventions?
- Are key psychiatric comorbid conditions – perhaps hidden – complicating the presentation, such as obsessive-compulsive disorder, personality disorder, or childhood trauma?
- Are medical comorbid conditions present that affect response, such as untreated obstructive sleep apnea?
- Is additional testing needed, such as thyroid stimulating hormone, CBC, renal function, liver function tests, vitamin B12, or neuroimaging?
Medication adherence. Medication nonadherence is a large problem. Up to 1 in 5 patients are not taking their medications for depression.47
Schedule more frequent visits and supportive contacts to assess and increase adherence. Weeks 1 and 6 are peak times for nonadherence in patients. Some have not filled their prescription, some have not started their medication, and some have stopped it because they are feeling better, feel little effect, or have side effects. Adherence to mental health medication treatment is increased by supportive calls to patients encouraging adherence and answering questions.48 The collaborative care model provides care management phone calls to help with medication adherence, as even simple messages about continuing medications can increase adherence.49
Adverse side effects can cause patients to stop their medications. Prescribing long-acting medications helps increase adherence, both by decreasing frequency of dosing and decreasing side effects from medication withdrawal if a dose is missed.50
Lack of understanding of treatment course or efficacy of medications also can result in patients stopping their medications. Educating patients about their medications is important. However, systematic reviews demonstrate that educating patients on treatment course, medication side effects, efficacy of medications, and involving them in decision making regarding their medications alone does not improve adherence.51–58 Collaborative care management, on the other hand, which also involves educating patients on medications and expected side effects, has consistently been demonstrated to increase compliance with treatment plans through patient monitoring and education.51,52,59
Withdrawal syndrome. Taper most SSRIs and SNRIs to avoid serotonin discontinuation syndrome.
This syndrome can occur when abruptly stopping many serotonergic antidepressants (SSRI, SNRI, TCA), particularly short-acting drugs such as paroxetine or venlafaxine. Common symptoms are dizziness, fatigue, anxiety, headache, and nausea. Less common symptoms can include agitation, dysphoria, insomnia, irritability, myalgias, paresthesias, tremor, rhinorrhea, chills, and diaphoresis. To characterize these symptoms to a patient, consider describing them as a ‘flu-like’ syndrome.
Strategies to decrease the risk of serotonin discontinuation syndrome include:
- Tapering medications
- Cross-tapering off one medication while simultaneously starting another medication
Medication treatment of suicidality. Although antidepressants have not been shown to reliably lower suicidality,67 ketamine has multiple studies demonstrating short term efficacy in lowering acute suicidal ideation.68–72 The use of ketamine in suicidality should be monitored in clinical practice in the future as newer formulations are released.
Other Treatment Modalities
Recommendations
Educate patients about depression at each visit, particularly at week 1 (initial diagnosis and treatment initiation) and at weeks 4–6 (likely time for patients to stop treatment). Key points to discuss:
- Depression is chronic but treatable. Review risk factors and consequences of untreated depression.
- Review evidence-based options for therapy, patient’s preferences, what is involved in the preferred therapy, when to expect results, and misconceptions about antidepressant medications.
- Plan for frequent follow up until there is a clear response (PHQ-9 decrease of 50% initially or clinical improvement). Continue treatment for 9–12 months for a first episode treatment. For recurring depression, treatment may be lifelong.
Recommend exercise for all patients.
- For mild depression, consider exercise as a first-line option.
- For others consider exercise as augmentation treatment.
Light therapy may be beneficial.
- For patients with a seasonal pattern of mild to moderate depression, recommend light therapy as a first-line option.
- For nonseasonal major depressive disorder of mild to moderate severity, consider light therapy as an adjunct treatment.
Specialized treatments with psychiatry consultation.
- MAOIs are highly effective but difficult to use, and referral to psychiatry is appropriate for MAOIs in treatment resistant depression.
- Consider transcranial magnetic stimulation (TMS) for patients not responding to traditional antidepressants or with intolerable medication side effects.
- Consider electroconvulsive therapy (ECT) for patients with severe major depressive disorder.
Consider ketamine (primarily IV ketamine) for treatment-resistant depression.
Educate the patient about depression. At each visit provide and reinforce education about depression, its treatment, and ongoing management. Particularly important times are:
- Week 1 (initial diagnosis and treatment initiation) to establish expectations and understand patient preferences.
- Weeks 4–6 of treatment, as patient is usually in a different emotional state and can focus better on education about depression. Patients experiencing remission at this point may stop taking their medication, believing they no longer need it.
Regarding the disease, explain:
- Major depressive disorder is a chronic, relapsing disease, but is highly treatable. Without treatment, episodes of depression tend to last approximately 6 months, although 20% will become chronic.
- Risk factors: personal history of anxiety or eating disorder, drug or alcohol use, presence of other major medical condition, chronic pain, or family history of depression or anxiety.
- Untreated depression increases burdens on loved ones and can lead to burnout in the spouse, family, and other caregivers.
Regarding treatment, explain and discuss:
- Evidence-based options for therapy and patient’s preferences. For mild depression, several options include psychotherapy, medication, vigorous exercise for 45 minutes three or more times per week, light therapy, and possibly St John’s Wort or omega 3-fatty acids.
- What is involved in preferred therapy (eg, CBT includes homework to build skills).
- Results occur in 3–4 weeks
- Antidepressant misconceptions: not addicting, not tranquilizers, not mood altering.
Regarding ongoing management, explain:
- Frequent follow up (every 1–3 weeks) is needed until a clear response is achieved. Response is often defined as a PHQ-9 decrease of 50% initially or clinical improvement. Follow up can be by phone with a trained staff member, video visit, or in-clinic visit.
- Continue medications for 6–9 months from the point of remission for a first episode of major depressive disorder.
- For recurring depression, continue with lifelong treatment (including medications).
Exercise. Recommend exercise (see Table 6). More than 80% of people with major depressive disorder do not meet guidelines for physical activity. Abundant evidence demonstrates that increasing exercise will improve depressive symptoms, particularly in the short-term (6 weeks). This has been proven across all ages of adults, including those who are healthy and those with chronic medical disease. Evidence is limited regarding long-term benefits of exercise.
When recommending exercise, consider:
- Frequency may be more important than intensity.
- For mild depression, exercise may be a first-line therapy for motivated patients.
- Exercise can be as effective as augmentation treatment.
- Supervised exercise, group exercise, and individual exercise have all demonstrated benefit.
Light therapy. For patients with a seasonal pattern of mild to moderate depression, recommend light therapy as a first-line option (see Table 6). For nonseasonal mild to moderate depression, consider light therapy as an adjunct treatment.
Use a light box delivering 10,000-lux intensity, slanted toward the face, for 30 minutes per day, preferably in the morning. Improvement in depressive symptoms usually occurs within 1–3 weeks. Side effects of light therapy (headaches, eye strain, irritability, insomnia) are usually mild.
Specialized treatments. Patients with severe depression and treatment-resistant depression may benefit from specialized treatments offered by specialists in depression, eg, psychiatry.
Monoamine Oxidase Inhibitors (MAOIs). MAOIs are effective for treatment resistant depression but difficult to use due to the risk of serotonin syndrome and hypertensive crisis with some foods. These are usually prescribed by a psychiatrist and if a patient or PCP is interested in MAOI use, consider referral to psychiatry.
Transcranial magnetic stimulation (TMS). Consider TMS for patients who have not responded to traditional antidepressants or who have had intolerable medication side effects. TMS is FDA-approved for depression and several other psychiatric disorders. It is a form of brain stimulation applied to outpatients daily for 4–6 weeks in specialized TMS clinics. It is delivered to an alert patient and has few side effects besides initial scalp pain during the first week of treatment. Its overall efficacy is similar to antidepressants, but it is superior to using another antidepressant after an initial medication trial. Access and cost are limitations and TMS is usually considered if trial of therapeutic dose of antidepressant is ineffective after 8 weeks. Many insurance companies require trial of 4 different antidepressants (either alone or in combination with augmenting agents) before they approve TMS.
Electroconvulsive therapy (ECT). Consider ECT in patients with severe major depressive disorder. ECT is among the most effective treatments in medicine, with rapid onset and very high response rates, particularly notable in the most severely ill patients. ECT is an option for severely medically ill patients, in pregnancy, and in various treatment-refractory disorders because of its efficacy and ease of use. However, significant side effects (eg, shortly after treatment: head and muscle ache, confusion, nausea, memory loss; long-term memory loss), cost, and societal stigma limit its use.
Ketamine. Consider ketamine (primarily IV ketamine) for treatment-resistant depression. Prior to using ketamine, individuals should have tried several antidepressants (including augmentation agents) and psychotherapy. Although IV ketamine is not FDA-approved as a treatment for major depressive disorder (and may not be covered by insurance), dozens of academic clinical trials summarized in meta-analyses demonstrate high efficacy and good tolerability in treatment-resistant depression. Common side effects include dream-like feeling, blurred/double vision, jerky muscle movements, dizziness, drowsiness, and nausea.
Sub-anesthetic doses, typically 0.5–1.0 mg/kg per infusion (usually 40–100 mg in a dose), administered over 40 minutes, dramatically improve depression in most patients, some in just 1–2 hours. A course of ketamine typically involves 4–6 infusions given over 2–4 weeks. IV ketamine is a schedule III controlled substance and is generally administered in a special IV ketamine clinic on an outpatient basis.
Intranasal esketamine (an enantiomer of ketamine) is an FDA-approved treatment for depression. Intranasal delivery is likely less effective than IV ketamine, but more effective than oral ketamine. A very small literature shows modest efficacy with oral ketamine for treatment-refractory depression.
Integrated Behavioral Care and Collaborative Care
Recommendations
For patients with mild to moderate major depressive disorder, consider referral to a collaborative care model, when it is locally available. This model has a care manager who meets with patients, discusses care recommendations with a psychiatrist, and coordinates medications and referrals with the primary care physician.
Clinics may be set up for the primary care behavioral health model (PCBH), which involves embedding a behavioral health consultant in clinic for management and support of common psychosocial issues in clinic.
Integrated behavioral care is an umbrella term for behavioral health care in the primary care setting and does not refer to any one model. Collaborative care is one model, as is the primary care behavioral health model.
Collaborative Care Model. For patients with mild to moderate major depressive disorder, consider referral to collaborative care, called Behavioral Health Collaborative Care (BHCC) at Michigan Medicine. Care managers meet with patients and coordinate the psychiatrist’s care recommendations with the patients’ primary care physicians, who prescribe treatment. Patients who need frequent contact and encouragement (provided by care managers) to maintain treatment are most likely to benefit from this model.
Substantial high-quality evidence demonstrates the efficacy of the collaborative care model compared to usual care in treating depression in different patient populations. Collaborative care has evidence of benefit in treating depression and anxiety for up to two years.
A collaborative care model has five essential components:
- Patient-centered team care. Teams consist of care managers, psychiatrists, primary care physicians, and advanced practice providers. The care manager (a health care professional) provides the linking communication. The care manager meets with the patient either in clinic or by phone, holds regular patient reviews with a psychiatrist to discuss care recommendations, and coordinates needed medication changes and referrals with the primary care clinician.
- Population-based care. The care manager maintains a patient registry that tracks patient’s PHQ-9 scores and other measures. Using the registry, even if a patient disengages from treatment, the care team will still provide outreach.
- Measurement-based treatment-to-target. Objective measures (eg, PHQ-9) track patient improvement, with a goal of having at least 50% symptom reduction and/or a PHQ-9 score less than 5. If patients are not improving as expected, medications and therapy can be changed, or the patient can be referred to standard psychiatric care for diagnosis clarification and management.
- Evidence-based care. The collaborative care model itself has evidence for effectiveness. Care managers use brief, evidence-based therapies like behavioral activation, problem solving therapy, and cognitive behavioral therapy.
- Accountable care. Collaborative care improves quality of care in a fiscally responsible manner.
In the collaborative care model, psychiatrists do not meet with patients, so this model is not appropriate for patients:
- With severe mental illness
- Who require diagnostic clarification
- For whom the primary care clinician is not comfortable prescribing medications.
Collaborative care is not long-term psychotherapy, although patients can participate in collaborative care in addition to psychotherapy and medications.
Collaborative care has been reimbursable by Medicare since January 2017. Reimbursement by private insurers and Medicaid programs is on a case-by-case basis.
Other integrated behavioral care. Other models for integrated behavioral may be considered, but current evidence is that they are either less effective than collaborative care or provide no advantage over standard care.
The primary care behavioral health model (PCBH) involves embedding a behavioral health consultant in clinic for management and support of common psychosocial issues in clinic. Evidence demonstrates that this model results in shorter wait times to behavioral health care, attending more visits, and higher rates of treatment engagement.73 This model is widely used in primary care, but the clinical implementation of this practice has outpaced the actual evidence for efficacy of this model.
Models demonstrating no advantage over standard care include: having co-located psychiatrists or psychologists on site and using co-located behavioral health consultants in the clinic 74 and having a psychiatrist do a one-time assessment and recommendations for patients.75
Integrative Treatment
Recommendations (also see Table 6)
Natural products. While evidence is typically limited and of poor quality, for patients desiring to use them with mild to moderate depression:
- St. John’s Wort – consider as a first-line monotherapy option for patients with mild to moderate depression and as an adjunct for non-pharmacologic treatment of moderate depression (only natural product with extensive, high quality evidence).
- Omega-3 fatty acids – consider for treating mild to moderate depression, either as monotherapy or as an adjunct treatment.
- Acetyl-L-carnitine – consider as a monotherapy for depression.
- S-Adenosyl methionine (SAM-e) – consider as an adjunctive treatment for mild to moderate depression.
- Methylfolate – consider as an adjunctive treatment.
- For vitamin D, crocus sativus (saffron), DHEA, and lavandula, evidence shows little to no benefit. However, their use is usually safe.
Yoga – consider as a monotherapy treatment for mild depression, as well as an adjunctive treatment.
Acupuncture – consider as an adjunctive treatment in mild to moderate depression.
Information about integrative treatments is summarized in Table 6.
Natural products. Many natural products have been evaluated for depression. Some have demonstrated usefulness, while some have potential drug interactions with prescription antidepressants or other medications.
St. John’s Wort. Consider this product as a potential first-line monotherapy option for patients with mild to moderate depression and as an adjunct treatment for moderate depression. It has been demonstrated to be effective, although determining the bioavailability of the active ingredient in preparations has been difficult across research studies and will be a problem for assessing benefit in patients.
St. John’s Wort is an inducer of CYP3A4 and P-glycoprotein, so it can decrease the bioavailability of many drugs and can interact with some medications, for instance it can increase the bleeding risk with clopidogrel. It is serotonergic (weak MAOI), so can cause serotonin syndrome when combined with other medications such as SSRIs or SNRIs. It is not recommended at this time in pregnancy or breastfeeding.
Omega-3 fatty acids. Consider these fatty acids for mild to moderate depression, either as monotherapy or as an adjunct treatment. Evidence has demonstrated that they may be beneficial for mild to moderate depression. Omega-3 fatty acids are commonly found in fish and nuts. They have potential function in multiple pathways linked to depression.
Acetyl-L-carnitine. Consider this product for second-line treatment of depression. Limited studies demonstrate efficacy comparable to traditional antidepressants. Additional data may justify use as a first-line treatment. Acetyl-L-carnitine theoretically works by helping with fatty acid transport and energy metabolism, which may help with improving the reduced neuroplasticity seen in depression. It is usually well tolerated.
S-adenosyl methionine (SAM-e). Consider SAM-e as an adjunctive treatment for mild to moderate depression. The low quality of evidence for SAM-e limits consideration for monotherapy. It is a synthetic form of a dietary amino acid that is well tolerated with few adverse effects. However, it may be associated with serotonin syndrome when combined with other serotonergic drugs.
Methylfolate. Consider methylfolate as an adjunctive treatment. Folic acid in the format of L-methylfolate (15 mg/day) showed evidence as an adjunctive treatment to SSRI therapy. However, folic acid itself has mixed evidence, and a 2016 meta-analysis showed no effect compared to placebo.76 Some variation in outcomes of folic acid may be due to genetic characteristics.
Vitamin D, crocus sativus (saffron), DHEA, and lavender. Evidence shows little to no benefit for these treatments. However, their use is usually safe.
Other complementary and alternative pharmacologic treatments do not have a level of evidence to support recommendations for their use at this time.
Yoga. Consider yoga as a monotherapy treatment for mild depression as well as an adjunctive treatment.
Yoga has been studied in multiple small randomized controlled trials, many of which have methodological issues. A systematic review found evidence that yoga improved depression compared to placebo, but could not recommend yoga due to methodological issues.77 A small randomized controlled trial has also looked at yoga vs. placebo in prenatal patients as monotherapy and found benefits for yoga in mild to moderate depression.
Based on the quality of limited available evidence, groups differ in recommendations regarding yoga. The VA/DoD Clinical Practice Guideline for Management of Major Depressive Disorder cited insufficient evidence to recommend yoga, and the Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline recommends yoga as a second-line adjunct treatment for mild to moderate depression.
Risks of yoga are few in most patients. Therefore, in patients with mild depression who are interested in yoga, adding yoga as an adjunctive treatment is reasonable. When discussing yoga, advise patients about the lack of reliable data.
Acupuncture. Consider acupuncture as an adjunctive treatment in mild to moderate depression.
No standard protocol exists for treating depression with acupuncture, so studies are difficult to compare and interpret. Some low-quality evidence supports acupuncture as an adjunctive treatment.78 A randomized controlled trial showed that acupuncture can increase quality of life for patients suffering from depression.79 Evidence for acupuncture as monotherapy is limited, of low quality, and mixed.
Based on the limited evidence, groups differ regarding recommendations. The VA/DOD and CANMAT guidelines recommend against acupuncture as monotherapy for depression. The World Health Organization clinical guidelines recommend acupuncture alone for mild depression and as an adjunct to medications for moderate depression.80
Risks of acupuncture are relatively few and mild, including mild bleeding, bruising at needle sites, and headache. Infection is rare if aseptic technique is used.81,82 With multiple trials demonstrating benefit, for patients who are interested in an integrative method of care for their depression, a reasonable approach is to consider acupuncture as an adjunct treatment, but not as monotherapy. When discussing acupuncture, advise patients about the lack of reliable data.
Treatment of Comorbid Medical and Psychiatric Illnesses
Recommendations
Treat comorbid medical conditions as you would treat them in patients without depression, taking into consideration possible medication interactions.
Patients with major depressive disorder frequently have other psychiatric disorders and special situations (eg, alcohol use disorder, anxiety, dementia). See Table 5 for special treatment considerations for depression in the presence of these disorders.
Comorbid medical conditions. Treat comorbid medical conditions as you would treat them in patients without depression, taking into consideration possible interactions between medications to treat depression and medications to treat other conditions. No specific treatment mode for depression is preferred for specific comorbidities, so follow patient preference when possible.
Collaborative care, especially when focused on behavioral activation, been shown to be effective in improving depression scores in patients with comorbid illness, although the effect is modest, and most studies only look at improving depression, not improving control of the comorbid medical illness, eg, diabetes. See Integrated Behavioral Care below.
Comorbid psychiatric illnesses and other special situations. Patients with major depressive disorder frequently also have other psychiatric disorders. The treatment for depression is influenced by the presence of these comorbid conditions. Table 5 describes common psychiatric comorbidities (alcohol use disorder, anxiety, dementia, and eating disorders) and special treatment considerations for depression.
Table 5
Management of Depression with Comorbid Psychiatric Conditions.
Chronic pain also may be comorbid with depression, as noted in Table 5. In patients with chronic pain, treat any underlying mood disorder whether the patient is on or off of chronic opioids. Consider that patients prescribed narcotics could be using them to self-treat an underlying mood disorder, or that the opioid medication could be the cause of their depression symptoms. For additional information on chronic pain management, see the UMHS clinical guideline Ambulatory Pain Management.
Referral
Recommendations
Consider referral to a depression specialist for the following patients.83,84
- Who have severe mental health issues:
- - Suicidality
- - Psychotic or bipolar depression
- - Severe psychosocial problems
- - Quickly increasing depressive symptoms.
- Who do not respond to adequate trials of treatment:
- - Fail two or more medication trials
- - Do not respond to collaborative care management.
- Who have complex situations:
- - Comorbid substance use disorders
- - Require specialized treatments such as MAOI, TMS, ECT, or ketamine
- - Have an unclear diagnosis or suspected personality disorder.
Special Populations
Depression in Adolescents
Recommendations
More detailed guidelines for depression in adolescents are available. In summary:
In adolescents (12 years and older) with mild to moderate depression:
- Offer evidence-based psychotherapy as first-line treatment. CBT and IPT have the most evidence in adolescents.
- Consider recommending exercise.
In adolescents with moderate to severe depression, or if psychotherapy is not an option for mild-to moderate depression, offer pharmacotherapy as first-line treatment. Fluoxetine has the highest level of evidence in adolescents, followed by escitalopram, followed by sertraline and citalopram.
- When treating adolescents with medications, monitor weekly during the first 4 weeks, then every 2 weeks for a month, and then in 12 weeks. Consider a treatment duration of 6–12 months for a first episode, and longer than 12 months for patients with recurrent episodes.
- If an adolescent fails to respond to an SSRI (PHQ-9 decreases by less than 25%) after 4 weeks, consider another SSRI. If there is a partial response to SSRI, or if the adolescent is treatment resistant, consider adding psychotherapy.
Guidelines for Adolescent Depression in Primary Care (GLAD-PC) offers detailed recommendations for diagnosis and treatment in adolescents that are consistent with care for all patients with depression http://www.gladpc.org/.85 A full discussion of the intricacies of depression management in children and adolescents is beyond the scope of this guideline. Some key aspects of initial treatment and management are summarized below.
Few long-term studies have been performed on adolescents. Information is limited for this age group on prevalence, treatment, and impact on long term prognosis.
Presentation. In adolescence, presentation of a major depressive disorder may include substance use, antisocial behavior, social withdrawal, and academic failure. Suicide attempts and ideation are common.
In children and adolescents, anxiety and depression are commonly comorbid and underestimated. Compared to depression or anxiety alone, comorbid anxiety and depression increase symptom severity and treatment resistance.86
Treatment. While both psychotherapy and pharmacotherapy are effective in children and adolescents, pharmacotherapy has potential for side effects. Therefore, generally offer psychotherapy in mild to moderate depression. CBT and IPT have the most evidence in adolescents. Exercise may also improve mild to moderate depression in children and adolescents. However, evidence is limited and insufficient to determine whether exercise is an option equivalent to psychotherapy or pharmacotherapy, or whether exercise is effective as an adjunct in more severe depression.87,88
In moderate to severe depression or if psychotherapy is not available for patients with mild to moderate depression, offer pharmacotherapy. Fluoxetine has the highest level of evidence, followed by escitalopram. Fluoxetine is FDA-approved for depression in children age eight years and older, while escitalopram is approved for depression in adolescents age 12 years and older. Sertraline and citalopram also have evidence of efficacy in adolescents, but are not FDA-approved.
For adolescents being treated with SSRIs, regular monitoring for suicidality and thoughts of self-harm is important. While controversial, concerns have been raised regarding suicidal ideation and behavior in adolescents on SSRIs. Based on adverse event data, the FDA issued a black box warning for the use of SSRIs in adolescents. However, a new review shows that fluoxetine and escitalopram are effective in treating depression in adolescents, with no increase in suicide between placebo and medication treatment.89
To mitigate any potential increase in suicidality in adolescents, monitor weekly for 4 weeks after starting medications, then every 2 weeks for a month, and then every 12 weeks. This monitoring may be done using an integrated behavioral health model, with behavioral health consultants checking on patients, nurse phone calls, phone call checks by clinicians, video visits, or brief medication checks in the clinic.
For children and adolescents who require medication, extrapolations from adult data suggest a treatment duration of 6–12 months for a first episode, and longer than 12 months for patients with recurrent episodes.90
If an adolescent fails to respond to an SSRI (PHQ-9 decreases by less than 25%) after an adequate trial of 4 weeks, consider changing to another SSRI.91 If there is a partial response to an SSRI, or if the adolescent is treatment resistant, consider adding evidence-based psychotherapy to an SSRI.92
Women: Premenstrual Syndrome and Premenstrual Dysphoric Disorder
Recommendations
Diagnose by cyclic timing and nature of symptoms.
Treatment:
- First-line: SSRIs (sertraline, fluoxetine, escitalopram, citalopram), diet, exercise.
- Second-line options: oral contraceptives with drospirenone, venlafaxine (SNRI), or augment SSRI with alprazolam.
- Third-line: GnRH agonist.
- Last resort: surgical oophorectomy.
Prevalence. Premenstrual dysphoric disorder (PMDD), a more severe form of premenstrual syndrome (PMS), is included in the most SM-5. (It was previously called late luteal phase dysphoric disorder.) Premenstrual psychological and somatic symptoms lie on a continuum of severity. Approximately 85% of women experience at least one mild premenstrual symptom, 20–25% experience moderate to severe premenstrual symptoms, and 5% meet DSM-5 diagnostic criteria for PMDD, the most severe form of PMS.14
Diagnosis. Both the timing and nature of symptoms are important.
- Cyclic timing. Symptoms must be:
- - Present the last week before onset of menses and start to improve after the onset of menses
- - Minimal or absent during the weeks after menses
- - Present most cycles of the past year.
- Symptoms. A minimum of five symptoms must be present.
- - At least one core symptom: marked affective lability, irritability, depressed mood, or anxiety.
- - Other potential symptoms: decreased interest in usual activities, concentration difficulty, low energy, changes in sleep or appetite, sense of being overwhelmed and out of control, and physical symptoms.
These symptoms should be associated with significant distress or interference in function.93
PMDD is associated with depressive and anxiety disorders, both concurrently and in the past. A study of 215 women with prospectively confirmed PMDD reported only a minority of women had postpartum depression, but nearly 36% had previous major depressive disorder.94 Impulsivity, anger, affect intensity and lability were significantly associated with PMS and PMDD in a 2016 study of women hospitalized after a suicide attempt.95
Treatment. General treatment principles for PMS and PMDD are to relieve symptoms and improve function. The two main approaches to moderate to severe PMDD are:
- Targeting the serotonin system by increasing central serotonergic transmission with SSRI or SNRI.
- Suppressing the hypothalamic pituitary axis to abolish cyclic changes in gonadal steroids using oral contraceptives or gonadotropin releasing hormone (GnRH).
Other options include surgery and lifestyle modifications.
SSRIs. Recommend SSRIs as the first-line therapy for PMS/PMDD because of their proven effectiveness and safety profile. SSRIs are recommended especially in women with documented impact on their ability to function socially and in the workplace. Sertraline and fluoxetine have both been extensively studied.96 A beneficial effect is expected within the first menstrual cycle. If the response is suboptimal, the dose can be increased in subsequent cycles. The treatment can be either continuous or only during the luteal phase/early menses. Daily treatment is recommended for women with high-level symptoms, symptoms present out of cycle, irregular cycles, or for patient ease. Starting dosage and side effects are similar to SSRI for treatment of depression. While citalopram and escitalopram have been less studied than sertraline and fluoxetine, they appear to be equally effective. Sixty–seven percent of women with PMDD respond to treatment with an SSRI.
Venlafaxine, an SNRI, and clomipramine, a TCA, are both more effective for PMS/PMDD than placebo. However, they are not used as first-line therapy due to need for tapered withdrawal when discontinuing and to side effects.
Treatment augmentation with low-dose alprazolam (0.25 mg twice daily or three times daily) has been successfully used in patients who still have residual symptoms after trying multiple SSRIs with optimal dosing.
Oral contraceptives. Oral contraceptives (OC) are second-line therapy and induce anovulation. Placebo-controlled trials and a meta-analysis suggest that symptoms are effectively treated by OCs containing the progesterone drospirenone, using a pill-free interval of four days rather than a seven days. Typically start with a 3 mg drospirenone/20 mcg ethinyl estradiol OC with a four-day pill-free interval. If symptoms persist or break through bleeding occurs, increase estrogen to 30 mg. If symptoms still persist, use continuous OC administration.
GnRH agonist. For third-line therapy in women with severe symptoms who have not responded to SSRIs or OCs, consider using a GnRH agonist. Leuprolide is a GnRH agonist given as a monthly injection. It produces menopausal symptoms such as hot flashes and loss of bone density. Add-back therapy with estrogen and progesterone in low doses in a continuous fashion can reduce these side effects.
Surgery. A last resort is bilateral oophorectomy, usually with hysterectomy, which is usually successful. Prior to consideration for surgery, the patient should have: confirmation of the diagnosis of PMDD, a six-month trial of GnRH with add back therapy, completed child bearing, and the expected need for several more years of therapy.
Lifestyle modifications.
- A complex carbohydrate diet during the luteal phase (second half of a menstrual cycle) is supported by some evidence.
- Exercise, which stabilizes mood, may be helpful, but evidence is minimal.
- The benefit of cognitive behavioral therapy is similar to that of fluoxetine.97
- The following vitamins and dietary supplements are not consistently better that placebo (30% response rate): vitamin B6, vitamin E, chasteberry, calcium, and magnesium. None of these treatments has been found to be more effective than placebo.
Women: Pregnancy and Depression
Recommendations
When treating depression during any stage of pregnancy, the goals are to maintain maternal mood stability and minimize fetal risk of exposure to medications.
Provide preconception counseling. Assess the patient’s history of depression, and for patients on antidepressants, consider whether or not to decrease or discontinue medications.
Use a validated questionnaire to identify and monitor depression such as PHQ-9 or EPDS in the first, second, and third trimesters and during the postpartum period. (See Appendix.)
Treat depression during pregnancy based on the severity of depression and a sequence of first, second, and third-line options (Table 7).
Treat postpartum depression in breastfeeding mothers based on the severity of depression and a sequence of first, second, and third-line options (Table 8).
For hormonal contraception in breastfeeding mothers, prescribe only progesterone (no estrogen) and consider monitoring for depression.
Perinatal depression is defined as depression occurring at any time during pregnancy and through the first year postpartum. For women diagnosed with perinatal depression, up to 40% of major depressive episodes start during pregnancy. Up to 7.5% of women will have a unipolar major depressive disorder and 6.5% will experience one in the first three months postpartum. This rate increases to 18.4% and 19.2% respectively when including minor and moderate depression.3
Maternal depression, whether antepartum or postpartum, can alter maternal behaviors that have a significant role in child development. Maternal behaviors that affect children include nutrition, smoking, poor medical compliance, and substance use. Poor obstetrical outcomes can result from maternal depression, including small for gestational age infants, NICU admission, impaired maternal infant bonding, infant sleep difficulty, mild developmental delays, and future cognitive, behavioral and emotional problems in offspring. Successful treatment of depression may reduce these risks.
Goals of treating perinatal depression include maintaining mood stability during pregnancy and preventing postpartum decompensation. Unique challenges for treatment include fetal risk of exposure to medication, breastfeeding risks, and the significant risks posed by untreated depression.
Preconception counseling and treatment adjustment. When counseling women with a history of depression who are considering pregnancy, ask about the severity of depression, number and frequency of episodes, and response history.
For women currently on antidepressants, consider the following regarding preconception treatment modification.
- Discontinuing or decreasing antidepressants prior to or early in pregnancy may increase the risk of depression relapse.
- If a woman is responding well to her current medication treatment, continue it unless contraindicated in pregnancy, ie, paroxetine. Use medications with known benefits during pregnancy.
- If decreasing medications, gradually taper the dose (eg, reduce by 25% every 1-2 weeks).
Women who are on medication for depression and become pregnant have a 43% chance of depression relapse. The risk is 25% when medications are continued and 68% when medications are stopped. Consider dose adjustment rather than discontinuation.3 Factors increasing risk include:
- History of depression during prior antepartum or postpartum periods
- Family history of depression
- Psychosocial factors: alcohol, drugs, poor social support, marital instability
- Previous pregnancy loss
- Sleep deprivation
- Pain
- Depression in years prior to or after a previous pregnancy.
Identifying and monitoring depression during pregnancy. Many symptoms of pregnancy are similar to those of depression (eg, fatigue, mood swings).11 Identify any new onset of depression (and monitor patients currently being treated for depression) by systematically screening for depression in the first, second, and third trimesters, and the postpartum period. Use a validated screening tool, such as the PHQ-2 and PHQ-9, or in this population, the Edinburg Postnatal Depression Scale. (See Appendix.)
Treating depression during pregnancy. Recommended treatment depends on depression severity, as shown in Table 7. For example, during pregnancy, first-line treatment for mild to moderate depression is psychotherapy and first-line treatment for severe depression is pharmacotherapy.
Fetuses exposed to selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) in the third trimester are at risk for serotonergic central nervous system adverse effects in the days after delivery. These include jitteriness, irritability, tremor, respiratory distress, and excessive crying. Occurring in 15–30% of exposed infants, these symptoms are time limited (2–14 days) and are not associated with an increased risk of mortality or long-term neurodevelopmental problems. The risk is highest for infants exposed to paroxetine, venlafaxine, and fluoxetine. Limited data also suggest that SSRIs taken late in pregnancy may be associated with an increased risk of persistent pulmonary hypertension of the newborn (PPHN), but this risk is small.
Most antidepressants have not been linked to an increased risk of major congenital malformations.98 The risk of cardiovascular malformations increases with paroxetine and clomipramine, though the majority of these complications resolve spontaneously and pose no significant functional impairment.
Significant evidence has not yet been found for increased fetal risks with other SSRIs, SNRIs, bupropion, mirtazapine, or TCAs (other than clomipramine, which may be associated with cardiovascular malformations, as noted above).
Only limited data exist regarding long-term postnatal effects of fetal in-utero exposure to SSRIs, but no lasting cognitive, language, emotional or behavioral problems have been found in these children. A small number of studies have suggested a risk of autism-spectrum disorder for children exposed in utero to antidepressants, but when the relationship is adjusted for maternal mental illness, the relationship is no longer significant.99
Monoamine oxidase inhibitors (MAOIs) are not recommended in pregnancy given their propensity to interact with certain analgesics. When MAOIs must be used, an anesthesiology consultation is recommended.
After trying first and second-line treatments for perinatal depression (see Table 7), third-line treatment options are non-pharmacologic. Electroconvulsive therapy can be used in pregnancy for severe or treatment-resistant major depressive disorder. A small randomized controlled study of repetitive transcranial magnetic stimulation (TMS) in pregnant women demonstrated a significant decrease in depressive symptoms.100 While larger studies need to be pursued, women who do not tolerate antidepressants or want to avoid medications are good candidates for TMS, even in the absence of treatment resistance. Complementary and alternative treatments such as exercise, yoga, acupuncture (depression-specific placement), and bright light therapy are not recommended as monotherapy for severe major depressive disorder. However, they may be considered third-line treatment.101 The risks and benefits of second generation or atypical antipsychotics in pregnancy are beyond the scope of this guideline and can be considered in consultation with a psychiatrist.
Treating depression during the postpartum period and while breastfeeding. The greatest risk factor for postpartum depression is untreated antenatal depression. Postpartum depression can result in poor mother-infant bonding, as well as cognitive, emotional, and behavioral problems in the child. Suicides in the first year after delivery account for 20% of maternal deaths.102,103
Treatment for postpartum depression during breastfeeding is summarized in Table 8.
All psychotropic medications pass readily into breast milk. Most SSRIs pass at a dose that is less than 10% of the maternal dose. If the infant was exposed to a psychiatric drug in utero and the mother has responded to that drug, continue treating the mother with the drug. Medication doses may need to be adjusted downward as plasma volume decreases and metabolism changes in the postpartum period.104 Consider checking NIH LactMed for drug compatibility with breastfeeding.
Esketamine via nasal spray was FDA-approved in 2019 for adults with treatment-resistant major depressive disorder.104,105 However, until more data are available, repeated doses of esketamine nasal spray should probably be avoided during breastfeeding.106 A 2019 study suggested that prophylactic use of IV ketamine may reduce depressive symptoms in the early postpartum period.107 While limited data suggest that IV ketamine may not affect the breastfed infant or lactation, ketamine should be used with careful monitoring during breastfeeding and alternate agents are preferred.106
Brexanolone, which is a neuroactive steroid and progesterone metabolite (also known as allopregnanolone), was FDA-approved in 2019 as the first drug specifically for use in postpartum depression. It is only available through a restricted program at certified health centers. It works rapidly, within 60 hours, but its disadvantages include its high cost and need for slow infusion, which requires 60 hours’ of inpatient stay. Its levels in milk are not expected to have adverse effects in breastfed infants.106
Hormonal birth control in breastfeeding mothers. For breastfeeding mothers desiring hormonal contraception, prescribe progesterone and consider monitoring for depression. Progesterone is the only hormonal contraception recommended for breastfeeding mothers because estrogen products decrease milk production. Concerns regarding progesterone possibly causing depression arose in 1992. However, as early as 1995 this concern was questioned and has since been studied with conflicting results. A qualitative and narrative assessment of methods of progesterone birth control methods (oral pill, IUD, shot, implant) found that the most robust studies showed no association between depression diagnosis and progestin-only contraception.108 Patients on progestin-only contraception may be monitored for depression as a precaution.
Depression in Later Life
Recommendations
Depression is not a normal part of aging, but it may occur more frequently in individuals age ≥ 60 years as a result of increased likelihood of illness and chronic disease, and may last longer because of increasing debility, loss of friends and family, and financial limitations.
There is a high likelihood of association between depression and dementia:
- When managing a first episode of depression in later life, consider whether the patient may have early dementia.
- In patients with dementia, use special assessment tools to screen for and monitor depression.
- Treat depression first, and then retest cognitive abilities repeatedly over time.
Regularly screen older adults with depression, checking for increased risk for suicide or self-harm.
Consider treatment adjustments in older adults, taking into account possible slower drug metabolism and potential for polypharmacy problems.
- More strongly consider treatments other than or in addition to medications.
- When using medications in older adults, start low and go slow. Allow longer times to see results. When a medication is ineffective, avoid polypharmacy by switching to another medication or augmenting with non-pharmacologic treatment modalities.
Although depression is not a normal part of aging, depression that occurs after age 60 years is more likely to be long-lasting, perhaps because of the effects of life events that often occur in later life (eg, more illnesses, increasing debility, losses of friends and family with subsequent isolation, financial limitations, etc.). Rates of depression in patients with Parkinson’s disease, stroke, and myocardial infarction are particularly high. Having any chronic medical illness, chronic pain, or chronic insomnia substantially raises the risk of depression, and those effects are increased further by having several diseases. Being depressed lowers the quality of a person’s life, leads to increased frailty, and can cause stress and burnout of the family and caregivers.
Screening, depression, and dementia. Individuals in later life should be screened periodically for depression. For older adults with mild to moderate depression, an option to the PHQ-2 and PHQ-9 is the Geriatric Depression Scale.109 (See Appendix.)
Cognitive impairment, even when subclinical, can present with depression. The occurrence of a first episode of depression later in life should prompt vigilance for early dementia.110,111 Common screening tools for depression may be less reliable in patients with dementia. More specialized screening and detection tools such as the Cornell Scale for Depression in Dementia and Hamilton Rating Depression Scale are sufficiently sensitive to identify depression even in the setting of clinical dementia. Further information about the treatment of depression when accompanied by dementia is presented in Table 5.
When patients with dementia also are depressed, treat the depression first, then retest cognitive abilities repeatedly over time.
Screen older adults for suicide risk. A positive indication of risk for suicide or self-harm on depression screening (eg, positive response to the PHQ-9 on item 9) should prompt further evaluation in patients of all ages. Older adults with depression are slightly less likely to attempt suicide, but if they do, they are more likely to die.
Treatment adjustments for late life depression. Decreased metabolic efficiency in drug excretion and increased potential for unintended polypharmacy effects must be considered when treating depression in later life. Adjustments include:
- More strongly consider treatment modalities other than antidepressant medication. Patients over 75 years old have a greater risk of adverse effects from antidepressants, particularly those with anticholinergic effects.
- Regarding medication dosing, start low and go slow, especially if the patient is on multiple medications.
- Medication trials may need to last longer (eg, 10–12 weeks), to see a response.
- If a medication is not effective, switch to a different medication rather than adding a second medications. This will reduce the potential for side effects. Another option is to augment treatment with non-pharmacologic modality, such as cognitive behavioral therapy, Life Review therapy (helps older adults find hope, value, and meaning in their lives), or exercise.
First-line treatment options for depression in later life include medications (eg, SSRIs, duloxetine, and mirtazapine) and several other modalities mentioned previously (eg, collaborative care with behavioral activation, evidence-based psychotherapy, exercise, and religious and spiritual interventions), either alone or as adjuncts for neurologically intact patients. Additionally, doing a Life Review, problem-solving therapy, light therapy, and music therapy have all been shown to be at least moderately effective in seniors. Electroconvulsive therapy also has been used in demented patients with severe or resistant depression. Repetitive transcranial magnetic stimulation (TMS) is possibly effective. Vitamin D deficiency does not cause depression and replacement is not an effective treatment.
Treatment continuation. Little evidence exists concerning the benefit of long-term continuation of antidepressants in later life. For antidepressants initiated for older adults in the acute treatment phase, consider individual factors when determining whether and how to prescribe antidepressants into the continuation and maintenance phases of treatment.
Guideline Development Methodology
Funding
The development of this guideline was funded by UMHS.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- Primary care physicians: Barbara C. Soyster, MD, General Internal Medicine; Stephen J. Warnick, Jr., MD, Family Medicine.
- Specialists in obstetrics and gynecology – Amy L. Tremper, MD; specialists in psychiatry – Sagar V. Parikh, MD, FRCPC; Stephen J. Warnick, Jr., MD.
- Guideline development methodologist: R. Van Harrison, PhD, Learning Health Sciences.
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
UMHS endorses the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Contributions of team members with relevant financial relationships are reviewed by team members without relevant financial relationships to assure the information is presented without bias.
Sagar V. Parikh, MD, FRCPC, is a consultant for the pharmaceutical companies Lundbeck, Sunovion, and Takeda, both a consultant and recipient of research funding from the genetic testing company Assurex, and a stock holder in Mensante (online mental health programs).
None of the other team members have relevant personal financial relationships.
The guideline team initiated the update on 8/22/2018.
Systematic Review of Literature
A detailed description of the systematic search and review of literature upon which this guideline is based is presented in the associated UMHS document “Depression, 2020: Literature Review Methods and Results.” The following section highlights major aspects of the literature search and review process.
Literature search. The team began the search of literature by accepting the results of a systematic literature review performed in 2015:
VA/DoD Clinical Practice Guideline for Management of Major Depressive Disorder (MDD). Washington DC: US Department of Veterans Affairs and Department of Defense, 2016. (This guideline searches and synthesizes literature through April 30, 2015.)
To update those results, we performed a systematic search of literature on Medline and in the Cochrane Database of Systematic Reviews for the time period 1/1/2015–11/30/2019.
The major search terms were depression and depressive disorders. The searches were for guidelines, controlled trials (including meta-analyses), and cohort studies, for literature on humans in the English language. Within these parameters individual searches were performed for the following topics:
Diagnosis
- 1. Epidemiology; national cost of treatment
- 2. Spectrum of depression, seasonal affective disorder, dysthymia
- 3. Screening for depression
- 4. Screening for bipolar disorder
- 5. Diagnosis
- 7. Associated medical illnesses
- 8. Associated social and environmental factors (eg, partner violence, sexual assault)
- 9. Suicide risk/assessment
Initiating Treatment
Comorbid depression and complicating circumstances
- 10. Medical comorbidity (eg, myocardial infarction, cerebrovascular accident, Parkinson’s disease, diabetes, chronic pain)
- 11. Alcohol use disorder
- 12. Panic, generalized anxiety disorder, or phobia
- 13. Obsessive compulsive disorder
- 14. Post-traumatic stress disorder
- 15. Eating disorders, anorexia nervosa
- 15a. Dementia (searched past 10 years, not in VA/DoD or CANMET)
General supportive care:
- 16. Patient education
- 17. Exercise
- 18. Pharmacotherapy and psychotherapy: comparisons and in combination
Pharmacotherapy principles
- 19. Pharmacogenomics testing and depression treatment
- 20. Choice of medicine
First-line medicines
- 21. Selective serotonin reuptake inhibitors (paroxetine, sertraline, fluoxetine, citalopram, escitalopram)
- 22. Serotonin norephinephrine reuptake inhibitors (tricyclic antidepressants, venlafaxine, desvenlafaxine, duloxetine)
- 23. Noradrenergic antagonist: mirtazapine [also for augmentation]
- 24. Norephinephrine/dopamine reuptake inhibitor: bupropion [also for augmentation]
- 25. Serotonin-2 antagonist/reuptake inhibitors (trazodone, nefazodone)
- 26. Atypical antidepressants: trintellix, vilazodone
- 27. Drug trial and response rates [results likely overlap with 20. Choice of medicine]
- 28. Pharmacotherapy, not included in 18–27
Psychotherapy:
- 29. Mindfulness based therapy
- 30. Problem solving therapy
- 31. Interpersonal psychotherapy
- 32. Cognitive behavioral therapy
- 33. Short-term or focal psychodynamic psychotherapy
- 34. Behavioral activation
- 35. Marital therapy
- 36. Psychotherapy, not included in 29–35
Other treatment modalities
- 37. Light therapy
- 38. Transcranial magnetic stimulation (TMS)
- 39. Ketamine
Integrated behavioral care
- 40. Collaborative care
- 41. Other integrated behavioral care not in 40 (collaborative care)
Complementary and alternative treatment
- 42. St. John’s Wort (Hypericum perforatum)
- 43. Other supplements: 5-hydroxytryptophan, 5-HTP, methylfolate, deplin, omega 3 fatty acids, S-adenosyl methionine, SAM-e
- 43a. Acupuncture
- 43b. Yoga
Ongoing clinical Assessment and Management
- 44. Assessing response to treatment, frequency of assessment, method (eg, change in PHQ-9)
- 45. Maintenance on pharmacotherapy, duration, phases of care: acute, continuation, maintenance
- 46. Augmentation and treatment resistant depression (Pharmacologic agents for augmentation.) [Note two first-line agents also used for augmentation]
- 47. Buspirone
- 48. Atypical antipsychotics, eg, brexpiprazole, cariprazine, risperidone, quetiapine
- 49. Mood stabilizers: lithium
- 50. Stimulants
- 51. Other agents prescribed for augmentation, eg, cytomel, triiodothyronine
- 52. Managing side effects of medicines
- 53. Withdrawal/discontinuation syndrome (desvenlafaxine, paroxetine, venlafaxine)
- 54. Medication adherence
- 55. Treatment, not included in 10–54
Special Populations
- 56. Adolescents
- 57. Pre-conception counseling
- 58. Pregnancy and pharmacologic treatments; breastfeeding and pharmacologic treatments
- 59. Pregnancy: perinatal and postpartum depression
- 60. Late life depression
- 61. Depression, not in 1–60
A more formal presentation of the inclusion and exclusion criteria is in Section II of the accompanying Literature Review Methods and Results.
The detailed search strategies are presented in Section III of the accompanying Literature Review Methods and Results.
The search was conducted in components of a formal problem structure (outlined above). The search was supplemented with very recent clinical trials known to expert members of the panel. The search was a single cycle. The number of publications identified is presented in Section IV of the accompanying Literature Review Methods and Results.
Literature review and assessment. Members of the guideline team reviewed the publications identified as relevant to specific topics in order to select those with best evidence. Criteria to identify overall best evidence included relevance of the study setting and population, study design, sample size, measurement methods (variables, measures, data collection), intervention methods (appropriateness, execution), appropriateness of analyses, and clarity of description.
In considering level of evidence based on study design, the classification was:
- A = systematic review of randomized controlled trials with or without meta-analysis
- B = randomized controlled trial
- C = systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trial, group observation study (cohort, cross-sectional, case-control)
- D = individual observation descriptive study (case study or case series)
- E = expert opinion
Beginning with best evidence identified by the VA/DoD systematic literature review, team members checked publications identified in the more recent search (1/1/2015—11/30/2018.) to determine whether better evidence was available. Team members also had the option of considering very recent literature (published since 11/30/2018) in determining whether even better evidence was available.
The process of review and assessment is described in more detail in Section V of the accompanying Literature Review Methods and Results
Best evidence and recommendations. Team members identified articles or other publications with best evidence regarding specific topics.
The guideline team reviewed the evidence and determined the importance of key aspects of care (listed on the first page of this guideline). In the absence of empirical evidence, the guideline team based recommendations on their expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Section VI of the accompanying Literature Review Methods and Results presents a table of each recommendation and the source of best evidence on which the recommendation is based.
Review and Endorsement
A draft of this guideline was reviewed by units within UMHS to which the content is most relevant. Pharmacy Services performed the initial review. Then reviews occurred in clinical conferences or by distribution for comment within the following clinical departments and divisions: Family Medicine, General Medicine, General Obstetrics and Gynecology (Women’s Health), Geriatric Medicine, and Psychiatry. The draft was revised based on comments from these groups.
The final version of this guideline was endorsed by the Clinical Practice Committee of the University of Michigan Medical Group and by the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
Acknowledgments
The following individuals are acknowledged for their contributions to previous major versions of this guideline:
- 1998: Thomas L. Schwenk, MD, Family Medicine; Linda B. Terrell, MD, General Internal Medicine; Elizabeth M. Shadigian, MD, Obstetrics and Gynecology; Christopher G. Wise, PhD, Clinical Affairs; Marcia A. Valenstein, MD, Psychiatry. Consultant, David J. Knesper, MD, Psychiatry.
- 2004: Thomas L. Schwenk, MD, Family Medicine; Linda B. Terrell, MD, General Internal Medicine; R. Van Harrison, PhD, Medical Education; Elizabeth M. Shadigian, MD, Obstetrics and Gynecology; Marcia Valenstein, MD, Psychiatry. Consultant, David J. Knesper, MD, Psychiatry.
- 2011: Thomas L. Schwenk, MD, Family Medicine; Linda B. Terrell, MD, General Internal Medicine; R. Van Harrison, PhD, Medical Education; Amy L. Tremper, MD, Obstetrics and Gynecology; Marcia A. Valenstein, MD, Psychiatry. Consultant, Jolene R. Bostwick, PharmD.
References
- 1.
- Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24-31. doi:10.1016/j.genhosppsych.2015.11.005 [PubMed: 26719105] [CrossRef]
- 2.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092 [PubMed: 16717171] [CrossRef]
- 3.
- MacQueen GM, Frey BN, Ismail Z, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 6. Special Populations: Youth, Women, and the Elderly. Can J Psychiatry. 2016;61(9):588-603. doi:https://dx
.doi.org/10 .1177/0706743716659276 [PMC free article: PMC4994788] [PubMed: 27486149] - 4.
- Brnabic A, Lin C, Monkul ES, Dueñas H, Raskin J. Major depressive disorder severity and the frequency of painful physical symptoms: A pooled analysis of observational studies. Curr Med Res Opin. 2012;28(12):1891-1897. doi:10.1185/03007995.2012.748654 [PubMed: 23145858] [CrossRef]
- 5.
- Dahlhamer JM, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. Morb Mortal Wkly Rep. 2018;67(36):1001-1006. doi:10.15585/mmwr.mm6736a2 [PMC free article: PMC6146950] [PubMed: 30212442] [CrossRef]
- 6.
- Gureje O, Von Korff M, Simon GE, Gater R. Persistent Pain and Well-being. Jama. 1998;280(2):147. doi:10.1001/jama.280.2.147 [PubMed: 9669787] [CrossRef]
- 7.
- Novick D, Montgomery WS, Aguado J, Peng X, Brugnoli R, Haro JM. Which somatic symptoms are associated with an unfavorable course in Chinese patients with major depressive disorder?. Asia Pac Psychiatry. 2015;7(4):427-435. doi:https://dx
.doi.org/10.1111/appy.12189 [PubMed: 26047023] - 8.
- Bae J-H, Kim G. Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutr Res. 2018;50:1-9. doi:https://dx
.doi.org/10 .1016/j.nutres.2017.10.013 [PubMed: 29540267] - 9.
- Jahangard L, Sadeghi A, Ahmadpanah M, et al. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - Results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res. 2018;107:48-56. doi:https://dx
.doi.org/10 .1016/j.jpsychires.2018.09.016 [PubMed: 30317101] - 10.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affect Disord. 2018;232:316-328. doi:https://dx
.doi.org/10 .1016/j.jad.2018.01.018 [PubMed: 29501991] - 11.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015;177:7-21. doi:https://dx
.doi.org/10 .1016/j.jad.2015.01.052 [PubMed: 25743368] - 12.
- Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological Treatments. Can J Psychiatry. 2016. doi:10.1177/0706743716659417 [PMC free article: PMC4994790] [PubMed: 27486148] [CrossRef]
- 13.
- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336-346. doi:10.1001/jamapsychiatry.2017.4602 [PMC free article: PMC5875313] [PubMed: 29450462] [CrossRef]
- 14.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- 15.
- Organization WH. ICD-10, International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland: WHO Press; 2010.
- 16.
- Lam RW, Parikh SV, Michalak EE, Dewa CS, Kennedy SH. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27(2):142-149. [PubMed: 25954941]
- 17.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2016;315(4):380-387. doi:10.1001/jama.2015.18392 [PubMed: 26813211] [CrossRef]
- 18.
- (USPSTF) USPSTF. Final Recommendation Statement Suicide Risk in Adolescents, Adults and Older Adults: Screening. https://www
.uspreventiveservicestaskforce .org/Page/Document/RecommendationStatementFinal /suicide-risk-in-adolescents-adults-and-older-adults-screening. Published 2016. - 19.
- Simon GE, Rutter CM, Peterson D, et al. Does Response on the PHQ-9 Depression Questionnaire Predict Subsequent Suicide Attempt or Suicide Death? Psychiatr Serv. 2013;64(12):1195-1202. doi:10.1176/appi.ps.201200587 [PMC free article: PMC4086215] [PubMed: 24036589] [CrossRef]
- 20.
- Gournellis R, Tournikioti K, Touloumi G, et al. Psychotic (delusional) depression and suicidal attempts: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;137(1):18-29. doi:https://dx
.doi.org/10.1111/acps.12826 [PubMed: 29178463] - 21.
- Popovic D, Vieta E, Azorin J-M, et al. Suicide attempts in major depressive episode: evidence from the BRIDGE-II-Mix study. Bipolar Disord. 2015;17(7):795-803. doi:https://dx
.doi.org/10.1111/bdi.12338 [PubMed: 26415692] - 22.
- Weeks M, Colman I. Predictors of Suicidal Behaviors in Canadian Adolescents with No Recent History of Depression. Arch Suicide Res. 2017;21(2):354-364. doi:https://dx
.doi.org/10 .1080/13811118.2016.1193076 [PubMed: 27587262] - 23.
- Kim Y, Kim S-S. Job insecurity and depression among automobile sales workers: A longitudinal study in South Korea. Am J Ind Med. 2018;61(2):140-147. doi:https://dx
.doi.org/10.1002/ajim.22805 [PubMed: 29226347] - 24.
- Lohman MC, Raue PJ, Greenberg RL, Bruce ML. Reducing suicidal ideation in home health care: results from the CAREPATH depression care management trial. Int J Geriatr Psychiatry. 2016;31(7):708-715. doi:https://dx
.doi.org/10.1002/gps.4381 [PMC free article: PMC4861681] [PubMed: 26552852] - 25.
- Okolie C, Dennis M, Simon Thomas E, John A. A systematic review of interventions to prevent suicidal behaviors and reduce suicidal ideation in older people. Int psychogeriatrics. 2017;29(11):1801-1824. doi:https://dx
.doi.org/10 .1017/S1041610217001430 [PubMed: 28766474] - 26.
- MacLean, R.R., Sofuoglu M. Stimulants and mood disorders. Curr Addict Rep. 2018;5:323-329. doi:https://doi
.org/10.1007 /s40429-018-0212-0 - 27.
- Sato S, Yeh TL. Challenges in treating patients with Major depressive disorder: The impact of biological and social factors. CNS Drugs. 2013;27(SUPPL.1):5-10. doi:10.1007/s40263-012-0028-8 [PubMed: 23712795] [CrossRef]
- 28.
- Ishak WW, Greenberg JM, Balayan K, et al. Quality of life: The ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry. 2011;19(5):229-239. doi:10.3109/10673229.2011.614099 [PubMed: 21916825] [CrossRef]
- 29.
- Cuijpers P, Wit LM de, Weitz ES, Andersson G, Huibers MJH. The combination of psychotherapy and pharmacotherapy in the treatment of adult depression: A comprehensive meta-analysis. J Evidence-Based Psychother. 2015;15(2):147-168.
- 30.
- de Maat SM, Dekker J, Schoevers RA, de Jonghe F. Relative efficacy of psychotherapy and combined therapy in the treatment of depression: A meta-analysis. Eur Psychiatry. 2007;22(1):1-8. doi:10.1016/j.eurpsy.2006.10.008 [PubMed: 17194571] [CrossRef]
- 31.
- Guidi J, Fava GA, Fava M, Papakostas GI. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: A preliminary meta-analysis. Psychol Med. 2011;41(2):321-331. doi:10.1017/S0033291710000826 [PubMed: 20444307] [CrossRef]
- 32.
- Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi:https://dx
.doi.org/10.1136/bmj.h6019 [PMC free article: PMC4673103] [PubMed: 26645251] - 33.
- Sztein DM, Koransky CE, Fegan L, Himelhoch S. Efficacy of cognitive behavioural therapy delivered over the Internet for depressive symptoms: A systematic review and meta-analysis. J Telemed Telecare. 2018;24(8):527-539. doi:https://dx
.doi.org/10 .1177/1357633X17717402 [PubMed: 28696153] - 34.
- B Sgletso, Korff M Von. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: A randomized controlled trial. J Am Med Assoc. 2004;292(8):935-942. [PubMed: 15328325]
- 35.
- Conn D, Gajaria A, Madan R. Telepsychiatry: effectiveness and feasibility. Smart Homecare Technol TeleHealth. 2015;3:59. doi:10.2147/SHTT.S45702 [CrossRef]
- 36.
- Chakrabart.pdf.
- 37.
- Khatri N, Marziali E, Tchernikov I, Shepherd N. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: A pilot study. Clin Interv Aging. 2014;9:276-279. doi:10.2147/CIA.S57832 [PMC free article: PMC4019624] [PubMed: 24855345] [CrossRef]
- 38.
- Stubbings DR, Rees CS, Roberts LD, Kane RT. Comparing in-person to videoconference-based cognitive behavioral therapy for mood and anxiety disorders: Randomized controlled trial. J Med Internet Res. 2013;15(11):1-16. doi:10.2196/jmir.2564 [PMC free article: PMC3842436] [PubMed: 24252663] [CrossRef]
- 39.
- Barbato A, D’Avanzo B, Parabiaghi A. Couple therapy for depression. Cochrane database Syst Rev. 2018;6:CD004188. doi:https://dx
.doi.org/10 .1002/14651858.CD004188.pub3 [PMC free article: PMC6513419] [PubMed: 29882960] - 40.
- Barbato A, D’Avanzo B. Efficacy of couple therapy as a treatment for depression: A meta-analysis. Psychiatr Q. 2008;79(2):121-132. doi:10.1007/s11126-008-9068-0 [PubMed: 18259866] [CrossRef]
- 41.
- Cohen S, O’Leary KD, Foran H. A Randomized Clinical Trial of a Brief, Problem-Focused Couple Therapy for Depression. Behav Ther. 2010;41(4):433-446. doi:10.1016/j.beth.2009.11.004 [PMC free article: PMC3536531] [PubMed: 21035609] [CrossRef]
- 42.
- Rosenblat JD, Lee Y, McIntyre RS. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. J Affect Disord. 2018;241:484-491. doi:https://dx
.doi.org/10 .1016/j.jad.2018.08.056 [PubMed: 30149336] - 43.
- Tallon D, Wiles N, Campbell J, et al. Mirtazapine added to selective serotonin reuptake inhibitors for treatment-resistant depression in primary care (MIR trial): study protocol for a randomised controlled trial. Trials. 2016;17:66. doi:https://dx
.doi.org/10 .1186/s13063-016-1199-2 [PMC free article: PMC5526304] [PubMed: 26842107] - 44.
- Kudlow PA, McIntyre RS, Lam RW. Early switching strategies in antidepressant non-responders: Current evidence and future research directions. CNS Drugs. 2014;28(7):601-609. doi:10.1007/s40263-014-0171-5 [PubMed: 24831418] [CrossRef]
- 45.
- Nakajima S, Uchida H, Suzuki T, et al. Is switching antidepressants following early nonresponse more beneficial in acute-phase treatment of depression?: A randomized open-label trial. Prog Neuro-Psychopharmacology Biol Psychiatry. 2011;35(8):1983-1989. doi:10.1016/j.pnpbp.2011.08.008 [PubMed: 21889560] [CrossRef]
- 46.
- Romera I, Pérez V, Menchón JM, et al. Early switch strategy in patients with major depressive disorder: A double-blind, randomized study. J Clin Psychopharmacol. 2012;32(4):479-486. doi:10.1097/JCP.0b013e31825d9958 [PubMed: 22722513] [CrossRef]
- 47.
- Norona JC, Roberson PNE, Welsh DP. Rejection sensitivity and depressive symptoms: Longitudinal actor-partner effects in adolescent romantic relationships. J Adolesc. 2016;51:6-18. doi:https://dx
.doi.org/10 .1016/j.adolescence.2016.05.007 [PubMed: 27254083] - 48.
- Sirey JA, Banerjee S, Marino P, et al. Adherence to Depression Treatment in Primary Care: A Randomized Clinical Trial. JAMA psychiatry. 2017;74(11):1129-1135. doi:https://dx
.doi.org/10 .1001/jamapsychiatry.2017.3047 [PMC free article: PMC5710215] [PubMed: 28973066] - 49.
- Lin EHB, Korff M Von, Katon W, Bush T, Gregory E. The Role of the Primary Care Physician in Patients ‘ Adherence to Antidepressant Therapy Simon, Edward Walker and Patricia Robinson Published by: Lippincott Williams & Wilkins Stable URL: http://www
.jstor.org/stable/3766739 The Role of the Primary Care. 2017;33(1):67-74. [PubMed: 7823648] - 50.
- Nussbaumer B, Morgan LC, Reichenpfader U, et al. Comparative efficacy and risk of harms of immediate- versus extended-release second-generation antidepressants: A systematic review with network meta-analysis. CNS Drugs. 2014;28(8):699-712. doi:10.1007/s40263-014-0169-z [PubMed: 24794101] [CrossRef]
- 51.
- Lin EHb. Adherence with Antidepressant Therapy and Successful Patient Self-Management. CNS Spectr. 2007;12(S13):4-8. doi:10.1017/S1092852900003734 [CrossRef]
- 52.
- Lin EHB, Von Korff M, Ludman EJ, et al. Enhancing adherence to prevent depression relapse in primary care. Gen Hosp Psychiatry. 2003;25(5):303-310. doi:10.1016/S0163-8343(03)00074-4 [PubMed: 12972220] [CrossRef]
- 53.
- Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172. doi:10.1034/j.1600-0447.2002.1r084.x [PubMed: 11939969] [CrossRef]
- 54.
- Pampallona S. BP. TG. KB. MC. Patient adherence in the treatment. Br J Psychiatry. 2002:4-9.
- 55.
- Bull SA, Hu XH, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants: Influence of patient-physician communication. J Am Med Assoc. 2002;288(11):1403-1409. doi:10.1001/jama.288.11.1403 [PubMed: 12234237] [CrossRef]
- 56.
- Kobak KA, Taylor LVH, Katzelnick DJ, Olson N, Clagnaz P, Henk HJ. Antidepressant medication management and health plan employer data information set (HEDIS) criteria: Reasons for nonadherence. J Clin Psychiatry. 2002;63(8):727-732. doi:10.4088/JCP.v63n0811 [PubMed: 12197454] [CrossRef]
- 57.
- Dewé W, Ph D, Boulanger B, et al. Demyttenaere,K 2001 parte1.pdf. 2001;62(suppl 22).
- 58.
- Chong WW1, Aslani PCT. Effectiveness of interventions to improve antidepressant medication adherence: a systematic review. Int J Clin Pr. 2011;65(9):954-975. doi:10.1111/j.1742-1241.2011.02746.x [PubMed: 21849010] [CrossRef]
- 59.
- Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40. doi:https://dx
.doi.org/10 .1176/appi.ajp.2014.14020258 [PMC free article: PMC4301707] [PubMed: 25157500] - 60.
- Fava GA, Gatti A, Belaise C, Guidi J, Offidani E. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: A systematic review. Psychother Psychosom. 2015;84(2):72-81. doi:10.1159/000370338 [PubMed: 25721705] [CrossRef]
- 61.
- MK YKS. Premenstrual disorders. AJOG. 2018;January:68-74. doi:10.1089/acm.2011.0503 [PubMed: 28571724] [CrossRef]
- 62.
- Judge R, Parry MG, Quail D, Jacobson JG. Discontinuation symptoms: Comparison of brief interruption in fluoxetine and paroxetine treatment. Int Clin Psychopharmacol. 2002;17(5):217-225. doi:10.1097/00004850-200209000-00002 [PubMed: 12177584] [CrossRef]
- 63.
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: A randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. doi:10.1016/S0006-3223(98)00126-7 [PubMed: 9646889] [CrossRef]
- 64.
- Michelson D, Fava M, Amsterdam J, et al. Interruption of selective serotonin reuptake inhibitor treatment. Double-blind, placebo-controlled trial. Br J Psychiatry. 2000;176(APR.):363-368. doi:10.1192/bjp.176.4.363 [PubMed: 10827885] [CrossRef]
- 65.
- Hindmarch I, Kimber SCS. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharmacol. 2000;15(6):305-318. [PubMed: 11110006]
- 66.
- Baldwin DS, Cooper JA, Huusom AKT, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. Int Clin Psychopharmacol. 2006;21(3):159-169. doi:10.1097/01.yic.0000194377.88330.1d [PubMed: 16528138] [CrossRef]
- 67.
- Braun C, Bschor T, Franklin J, Baethge C. Suicides and Suicide Attempts during Long-Term Treatment with Antidepressants: A Meta-Analysis of 29 Placebo-Controlled Studies Including 6,934 Patients with Major Depressive Disorder. Psychother Psychosom. 2016;85(3):171-179. doi:https://dx
.doi.org/10.1159/000442293 [PubMed: 27043848] - 68.
- Murrough JW, Soleimani L, DeWilde KE, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45(16):3571-3580. doi:https://dx
.doi.org/10 .1017/S0033291715001506 [PubMed: 26266877] - 69.
- Andrade C. Ketamine for Depression, 6: Effects on Suicidal Ideation and Possible Use as Crisis Intervention in Patients at Suicide Risk. J Clin Psychiatry. 2018;79(2). doi:https://dx
.doi.org/10.4088/JCP.18f12242 [PubMed: 29659211] - 70.
- Wilkinson ST, Ballard ED, Bloch MH, et al. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am J Psychiatry. 2018;175(2):150-158. doi:https://dx
.doi.org/10 .1176/appi.ajp.2017.17040472 [PMC free article: PMC5794524] [PubMed: 28969441] - 71.
- Grunebaum MF, Ellis SP, Keilp JG, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19(3):176-183. doi:https://dx
.doi.org/10.1111/bdi.12487 [PubMed: 28452409] - 72.
- Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am J Psychiatry. 2018;175(7):620-630. doi:https://dx
.doi.org/10 .1176/appi.ajp.2018.17060720 [PubMed: 29656663] - 73.
- Possemato K, Johnson EM, Beehler GP, et al. Patient outcomes associated with primary care behavioral health services: A systematic review. Gen Hosp Psychiatry. 2018;53(April):1-11. doi:10.1016/j.genhosppsych.2018.04.002 [PubMed: 29698902] [CrossRef]
- 74.
- Cape J, Whittington CBP. What is the role of consultation-liaison psychiatry in the management of depression in primary care? A systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32(3):246-254. [PubMed: 20430227]
- 75.
- Linden M, Muschalla B, Noack N, Heintze C, Doepfmer S. Treatment Changes in General Practice Patients With Chronic Mental Disorders Following a Psychiatric–Psychosomatic Consultation. Heal Serv Res Manag Epidemiol. 2018;5:233339281875852. doi:10.1177/2333392818758523 [PMC free article: PMC5858609] [PubMed: 29568790] [CrossRef]
- 76.
- Sarris J, Murphy J, Mischoulon D, et al. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016;173(6):575-587. doi:https://dx
.doi.org/10 .1176/appi.ajp.2016.15091228 [PubMed: 27113121] - 77.
- Cramer H, Anheyer D, Lauche R, Dobos G. A systematic review of yoga for major depressive disorder. J Affect Disord. 2017;213:70-77. doi:https://dx
.doi.org/10 .1016/j.jad.2017.02.006 [PubMed: 28192737] - 78.
- Smith CA, Armour M, Lee MS, Wang L-Q, Hay PJ. Acupuncture for depression. Cochrane database Syst Rev. 2018;3:CD004046. doi:https://dx
.doi.org/10 .1002/14651858.CD004046.pub4 [PMC free article: PMC6494180] [PubMed: 29502347] - 79.
- Fan L, Fu W, Chen Z, et al. Curative effect of acupuncture on quality of life in patient with depression: a clinical randomized single-blind placebo-controlled study. J Tradit Chinese Med = Chung i tsa chih ying wen pan. 2016;36(2):151-159. [PubMed: 27400468]
- 80.
- Zhao B, Li Z, Wang Y, et al. Manual or electroacupuncture as an add-on therapy to SSRIs for depression: A randomized controlled trial. J Psychiatr Res. 2019;114:24-33. doi:https://dx
.doi.org/10 .1016/j.jpsychires.2019.04.005 [PubMed: 31015098] - 81.
- Boutron I, Tubach F, Giraudeau B, Ravaud P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J Clin Epidemiol. 2004;57(6):543-550. doi:10.1016/j.jclinepi.2003.12.010 [PubMed: 15246122] [CrossRef]
- 82.
- Smith CA, Hay PPJ MH. Acupuncture for depression (Review). Cochrane Database Syst Rev. 2010;(1). [PubMed: 20091556]
- 83.
- van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: a systematic review and meta-analysis. Psychol Med. 2015;45(2):231-246. doi:https://dx
.doi.org/10 .1017/S0033291714000701 [PubMed: 25065653] - 84.
- Krahn DD, Bartels SJ, Coakley E, et al. PRISM-E: Comparison of integrated care and enhanced specialty referral models in depression outcomes. Psychiatr Serv. 2006;57(7):946-953. doi:10.1176/ps.2006.57.7.946 [PubMed: 16816278] [CrossRef]
- 85.
- Zuckerbrot RA, Cheung A, Jensen PS, Stein REK, Laraque D, GROUP G-PS. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): Part I. Practice Preparation, Identification, Assessment, and Initial Management. Levitt A Campo J, Clarke G, Emslie G, Kaufman M, Kelleher KJ, Kutcher S, Malus M, Sacks D, Waslick B, Sarvet B BB, ed. Pediatrics. 2018;141(3). doi:https://dx
.doi.org/10 .1542/peds.2017-4081 [PubMed: 29483200] - 86.
- Melton TH, Croarkin PE, Strawn JR, McClintock SM. Comorbid Anxiety and Depressive Symptoms in Children and Adolescents: A Systematic Review and Analysis. J Psychiatr Pract. 2016;22(2):84-98. doi:https://dx
.doi.org/10 .1097/PRA.0000000000000132 [PMC free article: PMC6267783] [PubMed: 27138077] - 87.
- Carter T, Guo B, Turner D, et al. Preferred intensity exercise for adolescents receiving treatment for depression: a pragmatic randomised controlled trial. BMC Psychiatry. 2015;15:247. doi:https://dx
.doi.org/10 .1186/s12888-015-0638-z [PMC free article: PMC4605143] [PubMed: 26467764] - 88.
- Korczak DJ, Madigan S, Colasanto M. Children’s Physical Activity and Depression: A Meta-analysis. Pediatrics. 2017;139(4). doi:https://dx
.doi.org/10 .1542/peds.2016-2266 [PubMed: 28314824] - 89.
- Ignaszewski MJ, Waslick B. Update on Randomized Placebo-Controlled Trials in the Past Decade for Treatment of Major Depressive Disorder in Child and Adolescent Patients: A Systematic Review. J Child Adolesc Psychopharmacol. 2018. doi:https://dx
.doi.org/10.1089/cap.2017.0174 [PubMed: 30063169] - 90.
- Choe CJ, Emslie GJ MT. Depression. Child Adolesc Psychiatr Clin N Am. 2012;21:807-829. doi:10.1016/j.chc.2012.07.002 [PubMed: 23040903] [CrossRef]
- 91.
- Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418-426. doi:10.1176/appi.ajp.2008.08070976 [PMC free article: PMC3593721] [PubMed: 19223438] [CrossRef]
- 92.
- Lynch FL, Dickerson JF, Clarke G, et al. Incremental cost-effectiveness of combined therapy vs medication only for youth with selective serotonin reuptake inhibitor-resistant depression: Treatment of SSRI-resistant depression in adolescents trial findings. Arch Gen Psychiatry. 2011;68(3):253-262. doi:10.1001/archgenpsychiatry.2011.9 [PMC free article: PMC3679348] [PubMed: 21383263] [CrossRef]
- 93.
- Lanza di Scalea T, Pearlstein T. Premenstrual Dysphoric Disorder. Med Clin North Am. 2019;103(4):613-628. doi:10.1016/j.mcna.2019.02.007 [PubMed: 31078196] [CrossRef]
- 94.
- Kepple AL, Lee EE, Haq N, Rubinow DR, Schmidt PJ. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-20. doi:https://dx
.doi.org/10.4088/JCP.15m09779 [PMC free article: PMC6328311] [PubMed: 27035701] - 95.
- Ducasse D, Jaussent I, Olie E, Guillaume S, Lopez-Castroman J, Courtet P. Personality Traits of Suicidality Are Associated with Premenstrual Syndrome and Premenstrual Dysphoric Disorder in a Suicidal Women Sample. PLoS One. 2016;11(2):e0148653. doi:https://dx
.doi.org/10 .1371/journal.pone.0148653 [PMC free article: PMC4749223] [PubMed: 26863007] - 96.
- Robert F Casper, MD, Kimberly A Yonkers M. Treatment of premenstrual syndrome and premenstrual dysphoric disorder. UpToDate. 2019;July.
- 97.
- Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74. doi:10.1016/j.ajog.2017.05.045 [PubMed: 28571724] [CrossRef]
- 98.
- Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry. 2016;61(9):540-560. doi:https://dx
.doi.org/10 .1177/0706743716659417 [PMC free article: PMC4994790] [PubMed: 27486148] - 99.
- Andrade C. Antidepressant Exposure During Pregnancy and Risk of Autism in the Offspring, 1: Meta-Review of Meta-Analyses. J Clin Psychiatry. 2017;78(8):e1047-e1051. doi:https://dx
.doi.org/10.4088/JCP.17f11903 [PubMed: 28994903] - 100.
- Kim DR, Wang E, McGeehan B, et al. Randomized controlled trial of transcranial magnetic stimulation in pregnant women with major depressive disorder. Brain Stimul. 2018. doi:https://dx
.doi.org/10 .1016/j.brs.2018.09.005 [PMC free article: PMC6301099] [PubMed: 30249416] - 101.
- Ravindran AV, Balneaves LG, Faulkner G, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry. 2016;61(9):576-587. doi:https://dx
.doi.org/10 .1177/0706743716660290 [PMC free article: PMC4994794] [PubMed: 27486153] - 102.
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87. doi:10.1007/s00737-005-0080-1 [PubMed: 15883651] [CrossRef]
- 103.
- Stewart DE, Vigod S. Postpartum Depression. N Engl J Med. 2016;375(22):2177-2186. [PubMed: 27959754]
- 104.
- Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52(December 2018):165-180. doi:10.1016/j.yfrne.2018.12.001 [PMC free article: PMC6370514] [PubMed: 30552910] [CrossRef]
- 105.
- Kraus C, Rabl U, Vanicek T, et al. Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract. 2017;21(1):2-12. doi:https://dx
.doi.org/10 .1080/13651501.2016.1254802 [PubMed: 28097909] - 106.
- Vaughn CJ. Drugs and Lactation Database: LactMed. Journal of Electronic Resources in Medical Libraries. doi:10.1080/15424065.2012.735134 [CrossRef]
- 107.
- Ma JH, Wang SY, Yu HY, et al. Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section✰. Psychiatry Res. 2019;279(November 2018):252-258. doi:10.1016/j.psychres.2019.03.026 [PubMed: 31147085] [CrossRef]
- 108.
- Worly BL, Gur TL, Schaffir J. The relationship between progestin hormonal contraception and depression: a systematic review. Contraception. 2018;97(6):478-489. doi:https://dx
.doi.org/10 .1016/j.contraception.2018.01.010 [PubMed: 29496297] - 109.
- Lach HW, Chang Y-P, Edwards D. Can older adults with dementia accurately report depression using brief forms? Reliability and validity of the Geriatric Depression Scale. J Gerontol Nurs. 2010;36(5):30-37. doi:https://dx
.doi.org/10 .3928/00989134-20100303-01 [PubMed: 20349852] - 110.
- Ismail Z, Malick A, Smith EE, Schweizer T, Fischer C. Depression versus dementia: is this construct still relevant? Neurodegener Dis Manag. 2014;4(2):119-126. doi:10.2217/nmt.14.5 [PubMed: 24832029] [CrossRef]
- 111.
- Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. 2016;12(2):195-202. doi:10.1016/j.jalz.2015.05.017 [PMC free article: PMC4684483] [PubMed: 26096665] [CrossRef]
- 112.
- Cheung AH, Zuckerbrot RA, Jensen PS, Laraque D, Stein REK, GROUP G-PS. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): Part II. Treatment and Ongoing Management. Levitt A Campo J, Clarke G, Emslie G, Kaufman M, Kelleher KJ, Kutcher S, Malus M, Sacks D, Waslick B, Sarvet B BB, ed. Pediatrics. 2018;141(3). doi:https://dx
.doi.org/10 .1542/peds.2017-4082 [PubMed: 29483201] - 113.
- Yonkers KA, Wisner KL, Stewart DE et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obs Gynecol. 2009;114(3):703-713. doi:10.1097/AOG.0b013e3181ba0632 [PMC free article: PMC3103063] [PubMed: 19701065] [CrossRef]
- 114.
- Qaseem A, Barry MJ, Kansagara D, Physicians CGC of the AC of. Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Forciea MA Barry MJ, Boyd C, Chow R, Fitterman N, Harris RP, Humphrey LL, Kansagara D, Manaker S, McLean R, Vijan S, Wilt T DTD, ed. Ann Intern Med. 2016;164(5):350-359. doi:https://dx
.doi.org/10.7326/M15-2570 [PubMed: 26857948] - 115.
- (APA). APA. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Ed. Vol Oct.; 2010.
- 116.
- Parikh SV, Quilty LC, Ravitz P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 2. Psychological treatments. Can J Psychiatry. 2016. doi:10.1177/0706743716659418 [PMC free article: PMC4994791] [PubMed: 27486150] [CrossRef]
- 117.
- Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 4. Neurostimulation Treatments. Can J Psychiatry. 2016;61(9):561-575. doi:https://dx
.doi.org/10 .1177/0706743716660033 [PMC free article: PMC4994792] [PubMed: 27486154] - 118.
- Lam RW, McIntosh D, Wang J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 1. Disease Burden and Principles of Care. Can J Psychiatry. 2016;61(9):510-523. doi:https://dx
.doi.org/10 .1177/0706743716659416 [PMC free article: PMC4994789] [PubMed: 27486151] - 119.
- Siu AL, Force USPST. Screening for Depression in Children and Adolescents: U.S. Preventive Services Task Force Recommendation Statement. Siu AL Bibbins-Domingo K, Grossman DC, Ciofu Baumann L, Davidson KW, Ebell M, Garcia FA, Gillman M, Herzstein J, Kemper AR, Krist AH, Kurth AE, Owens DK, Phillips WR, Phipps MG, Pignone MP PJJ, ed. Ann Intern Med. 2016;164(5):360-366. doi:https://dx
.doi.org/10.7326/M15-2957 [PubMed: 26858097] - 120.
- VA/DoD. VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF MAJOR DEPRESSIVE DISORDER The Management of Major Depressive Disorder Working Group The Office of Quality, Safety and Value, VA, Washington, DC; Office of Evidence Based Practice, U.S. Army Med. 2016.
Appendix. Tools for Screening and Monitoring
| Tool with Link to Source | Description |
|---|---|
| Depression | |
| Patient Health Questionnaire–2 (PHQ-2) | The first two items of the longer PHQ-9. |
| Patient Health Questionnaire–9 (PHQ-9) Instruction Manual | The Patient Health Questionnaire (PHQ-9) is a brief, 9-item self-report screening tool used to help identify symptoms that could relate to depression. The PHQ-9 was developed for use in primary care settings. |
| The Quick Inventory of Depressive Symptomatology (QIDS-SR 16) | A self-report tool to screen for depression and measure changes in the severity of symptoms. It assesses the main criteria for major depressive disorder in the DSM-5. |
| Edinburgh Postnatal Depression Scale (EPDS) | The Edinburgh Postnatal Depression Scale was developed to screen postpartum women for depression in an outpatient setting. It is a 10-question screen that takes about 3–5 minutes to complete and is easy to score. The EPDS asks the patient how she has been feeling over the last seven days. It has been validated for use during both pregnancy and the postpartum period. |
| Geriatric Depression Scale (GDS) | The Geriatric Depression Scale (GDS) is a 30-item self-report assessment used to identify depression in the elderly. A 15-item version is also available. The GDS does not assess somatic symptoms (eg, energy level and appetite) that might be impacted by comorbid physical conditions and the aging process. |
| Lam Employment Absence and Productivity Scale (LEAPS) | Self-report tool to assess functional status in response to treatment. |
| Sheehan Disability Scale | Self-report tool to assess functional status in response to treatment. |
| Other Mental Health Disorders | |
| Alcohol Consumption Questions (AUDIT-C) | The AUDIT-C is scored on a scale of 0-2 (scores of 0 reflect no alcohol use). In men, a score of 4 or more is considered positive; in women, a score of 3 or more is considered positive. Generally, the higher the AUDIT-C score, the more likely it is that the patient’s drinking is affecting their health and safety. |
| Generalized Anxiety Disorder (GAD-7) Instruction Manual | The generalized anxiety disorder seven-item (GAD-7) scale can be used to screen for GAD in primary care. The GAD-7 has been found to be sensitive to change and can be used to monitor symptom severity over time. |
| Hypomania Checklist 32 (HCL-32) | The hypomania checklist-32 (HCL-32) is a widely used questionnaire developed for identifying hypomanic components in patients with a depressive episode. |
| Mood Disorder Questionnaire (MDQ) | The Mood Disorder Questionnaire is a brief, self-report screening instrument that can be used to identify patients most likely to have bipolar disorder. |
| Cornell Scale for Depression in Dementia | Specialized screening tool for depression accompanied by dementia. |
| Hamilton Rating Depression Scale | Specialized screening tool for depression accompanied by dementia. |
| Suicide | |
| Columbia-Suicide Severity Rating Scale (C-SSRS) | The Columbia Protocol, also known as the Columbia-Suicide Severity Rating Scale (C-SSRS), supports suicide risk assessment through a series of simple, plain-language questions that anyone can ask. The answers help users identify whether someone is at risk for suicide, assess the severity and immediacy of that risk, and gauge the level of support that the person needs. |
| Suicide Assessment Five-step Evaluation and Triage (SAFE-T) | This resource gives a brief overview on conducting a suicide assessment using a five-step evaluation and triage plan. The five-step plan involves identifying risk factors and protective factors, conducting a suicide inquiry, determining risk level and interventions, and documenting a treatment plan. |
APPROVALS
| P&T | Date: 9/15/2020 |
| CW Operations Subcommittee | Date: 11/16/2020 |
| CW Executive Committee | Date: 12/7/2020 |
| CPC | Date: 12/4/2020 |
| ECCA | Date: 2/9/2021 |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Created: March 1998; Last Update: September 2020.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients.[Postgrad Med. 2001]The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients.Alexopoulos GS, Katz IR, Reynolds CF 3rd, Carpenter D, Docherty JP, Expert Consensus Panel for Pharmacotherapy of Depressive Disorders in Older Patients. Postgrad Med. 2001 Oct; Spec No Pharmacotherapy:1-86.
- Clinical features of differential diagnosis between unipolar and bipolar depression in a drug-free sample of young adults.[J Affect Disord. 2019]Clinical features of differential diagnosis between unipolar and bipolar depression in a drug-free sample of young adults.Patella AM, Jansen K, Cardoso TA, Souza LDM, Silva RAD, Coelho FMDC. J Affect Disord. 2019 Jan 15; 243:103-107. Epub 2018 Sep 11.
- [Clinical characteristics of unipolar and bipolar depression].[Med Pregl. 1998][Clinical characteristics of unipolar and bipolar depression].Cvjetković-Bosnjak M. Med Pregl. 1998 Jul-Aug; 51(7-8):329-32.
- Review World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 2: Maintenance treatment of major depressive disorder and treatment of chronic depressive disorders and subthreshold depressions.[World J Biol Psychiatry. 2002]Review World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 2: Maintenance treatment of major depressive disorder and treatment of chronic depressive disorders and subthreshold depressions.Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ, World Federation of Societies of Biological Psychiatry (WFSBF) Task Force on Treatment Guidelines for Unipolar Depressive Disorders. World J Biol Psychiatry. 2002 Apr; 3(2):69-86.
- Review Comparison of guidelines for the treatment of unipolar depression: a focus on pharmacotherapy and neurostimulation.[Acta Psychiatr Scand. 2018]Review Comparison of guidelines for the treatment of unipolar depression: a focus on pharmacotherapy and neurostimulation.Bayes AJ, Parker GB. Acta Psychiatr Scand. 2018 Jun; 137(6):459-471. Epub 2018 Mar 25.
- Unipolar DepressionUnipolar Depression
- Drug MisuseDrug Misuse
Your browsing activity is empty.
Activity recording is turned off.
See more...

