Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population: Adult patients with suspected or confirmed small bowel obstruction. It does not apply to medical problems that mimic bowel obstruction (eg, scleroderma, Hirschsprung-type disease, opioid induced ileus, etc.).
Objectives: Develop a standard for the diagnosis, triage, and management of small bowel obstruction to improve patient outcomes at Michigan Medicine.
Key points
Clinical Presentation
Patients presenting with abdominal pain, nausea, abdominal distention, vomiting, and/or obstipation/constipation, should be evaluated for a small bowel obstruction (SBO). Clinical signs and symptoms of SBO are in Table 1.
Table 1
Findings Consistent with SBO Without Bowel Compromise.
Definitions
Small bowel obstruction (SBO): An intrinsic, extrinsic, or endoluminal process which narrows the bowel lumen and delays the passage of luminal contents.
Complete small bowel obstruction: Obstruction with no passage of luminal contents beyond the point of obstruction. Clinically recognized when patients exhibit the signs, symptoms, and radiographic findings consistent with SBO (Table 1). Bowel movements and flatus are absent with complete obstruction.
Partial small bowel obstruction (pSBO): Incomplete obstruction with luminal narrowing but some contents continue to pass through the intestine. This is distinguished from complete obstruction by the continued passage of bowel movements and flatus, and a benign abdominal exam.
Ileus: Functional motility disorder characterized by adynamic paralytic bowel, leading to many of the same symptoms of mechanical obstruction, but without a single site of obstruction demonstrable on imaging. Common causes include recent abdominal surgery, medications such as opiates, and electrolyte disturbances.
Small bowel dilatation: Abnormally dilated when it reaches a diameter of >3 cm.
Transition zone: Short segment area between dilated proximal bowel and decompressed distal bowel. Sometimes called a transition point and is radiographic evidence of SBO.
Bowel compromise: Compromised when there is ischemia or injury that has led, or may lead, to necrosis and/or perforation of the bowel wall. There is a high risk of morbidity and mortality if not treated in an expedient manner. Signs and symptoms of bowel compromise are shown in Table 2.
Table 2
Findings Consistent With Small Bowel Compromise.
Diagnosis
Evaluation for SBO is summarized in Figure 1. The diagnosis of SBO is suspected in patients with abdominal pain, distention, nausea and vomiting.
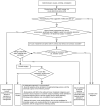
Figure 1
General Approach to Evaluating SBO. Definitions: UA = urinalysis, AKI = acute kidney injury, IBD = Irritable bowel disease, CKD = chronic kidney disease
Diagnosis is further supported by a history, including a complete discussion of prior intra-abdominal surgery.
Majority of stable patients presenting with symptoms consistent with SBO require CT imaging of the abdomen and pelvis with IV but not oral contrast to confirm the diagnosis and determine if urgent surgical intervention is warranted. Risks of intravenous contrast in patients with kidney disease or history of contrast allergy must be considered.
Diagnosis of SBO is highly suspected in patients with appropriate history and physical findings, AND with CT imaging demonstrating a transition zone (Figure 2). Without a transition zone, SBO is unlikely.
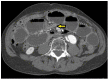
Figure 2
CT Image of Transition Zone. Axial CT of the abdomen demonstrate small bowel dilatation with single abrupt transition zone in the mid abdomen (arrow) consistent with mechanical SBO secondary to adhesions.
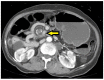
Figure 3
CT Image of Swirl/ Whirl Sign. Axial CT of the abdomen demonstrate swirling of the mesenteric vessels in in the mid abdomen (arrow) consistent with volvulus.
Triage (Figure 1)
Patients with signs of bowel compromise (Table 2), including imaging evidence of free air in the abdomen, pneumatosis, portal venous gas, or clinical evidence of peritoneal signs, and/or signs of sepsis in the context of suspected SBO, require urgent Acute Care Surgery consultation and consideration of urgent surgery.
Patients with complete SBO, as supported by clinical findings consistent with SBO and a transition zone on CT imaging (Figure 2), should be admitted to Acute Care Surgery.
Patients with pSBO as defined by a transition zone on imaging, accompanied by only mild abdominal pain, minimal distention, and the continuing passage of flatus and/or stool can be admitted to a medicine service, as pSBOs are likely to resolve without surgical intervention.
Patients presenting with partial or complete SBO within 30 days following an intra-abdominal surgery should be evaluated by and admitted to a surgery service (ideally, the original operative surgical service).
SBO patients with Inflammatory Bowel Disease (IBD) require gastroenterology and surgery consultation to guide evaluation and treatment. These patients are usually admitted to the gastroenterology service, with the surgery service providing consultation (unless emergent surgery is required).
SBO patients with advanced malignancy require consultation with surgery, and possibly oncology and/or palliative care to guide evaluation and treatment. These patients are usually admitted to a general medicine service, with the surgery service providing consultation (unless emergent surgery is required).
SBO or Partial SBO in patients with a history of Roux-en-Y gastric bypass (RYGB) or Duodenal Switch (DS) always requires emergent surgical evaluation and treatment. A small bowel obstruction in this patient population is almost always a surgical emergency that is treated with urgent or emergent operative intervention.
Treatment
SBO with bowel compromise
Usually require emergent surgical treatment.
SBO with signs of complete obstruction
Most patients are treated with non-operative management initially (Table 4), as most episodes of SBO resolve spontaneously.
Table 4
Non-Operative Medical Management for SBO.
A Gastrografin challenge may be recommended by surgery (usually after 24 - 48 hours of non-operative management) to predict if the obstruction will spontaneously resolve.
Surgical management is reserved for those who fail to resolve their obstruction after Gastrografin challenge, or in those whose exam worsens or, those who develop signs of compromised bowel.
Partial SBO
Most patients are treated with non-operative management (Table 4).
Surgical consultation is warranted for any pSBO patient that does not improve after 24 - 48 hours of non-operative management.
A Gastrografin challenge may be recommended by surgery (usually after 24 - 48 hours of non-operative management) to predict if the obstruction will spontaneously resolve.
Surgical management is reserved for those that fail to resolve the obstruction after 3 - 5 days or after a Gastrografin challenge, those with a worsening exam, or those that develop signs of compromised bowel.
Footnotes
- *
Strength of Recommendation: I = generally should be performed; II = may be reasonable to perform; III = generally should not be performed.
Level of evidence supporting a diagnostic method or an intervention: A = Systematic review of randomized controlled trials; B = Randomized controlled trials, C = Systematic review of non-randomized controlled trials; group observation studies, D = Individual observation descriptive studies, E = Expert opinion.
Clinical Problem and Management Issues
Rationale for Recommendations
The diagnosis and management of small bowel obstruction (SBO) often involves multiple disciplines including emergency medicine, surgery, radiology, internal medicine, and gastroenterology. Quality of care and patient outcomes can be compromised if diagnosis and treatment are delayed. Proper and efficient disposition from the emergency department is essential for appropriate patient management. This guideline was created to help guide clinicians in the diagnosis of SBO, to help determine proper service triage and to guide management. The goal is to enhance consistency in patient management, facilitate interdisciplinary consensus, increase efficiency of care, and improve clinical outcomes. This guideline is not meant to be comprehensive; it is intended to guide the care of most patients with SBO. This guideline does not apply to medical problems that mimic bowel obstruction (eg, scleroderma, Hirschsprung-type disease, opioid induced ileus, etc.).
Background
Small bowel obstruction is a common reason for hospitalization comprising up to 15% of surgical admissions to United States hospitals, costing the health care system upwards of a billion dollars per year.1,2 SBO causes approximately 300,000 to 350,000 hospitalizations annually and approximately 30,000 deaths in the United States.1,2 Retrospective studies suggest that approximately 65- 75% of SBO is caused by adhesive disease; previous colorectal surgery accounts for the majority of these cases. The next two most common causes of SBO can be attributed to other extra-luminal etiologies such as abdominal wall hernias or malignancy. Less common cases are intrinsic causes such as luminal narrowing from inflammatory diseases or endoluminal impaction.
Mechanical obstruction prevents normal transit of luminal contents causing distention of the bowel proximal to the transition mainly by swallowed air and accumulation of intestinal fluid. As the process progresses, intestinal function is lost. If distention is severe, the bowel may be compromised by decreased intestinal perfusion leading to ischemia (could be reversible), necrosis (irreversible) and perforation.
The diagnosis of SBO is made by history, physical exam and imaging studies. While the majority of patients will have spontaneous resolution of their SBO with non-operative management, there are a substantial number of patients who will require surgical intervention. Although it can be challenging to predict which patients will require surgical intervention, new methods such as Gastrografin studies have shown promise in this area. The diagnosis and management of SBO is largely supported by expert opinion with few randomized control trials supporting current practice.
Diagnosis
The goal of initial evaluation and diagnosis of patients with acute abdominal pain concerning for SBO is to distinguish patients that need urgent surgical intervention from those who can be treated non-operatively. Patients who present with abdominal pain and with peritoneal signs, sepsis, bowel ischemia or compromise require emergent surgical evaluation for possible operative management. Stable patients, without concern for bowel compromise, should undergo thorough evaluation to verify the diagnosis of SBO. The underlying etiology of SBO should be determined since it may affect management.
Clinical Presentation
The clinical presentation of SBO is summarized in Table 1. There are no symptoms that have a high specificity for the diagnosis of SBO, but patients with SBO often present with distention, abdominal pain, nausea and emesis. Patients may not have all of these symptoms.
Depending on the anatomical location of the obstruction, episodes of emesis may be non-bilious or bilious and depending on the time course, can become feculent. Vomiting may be absent especially if the patient’s anatomy has been surgically altered in such a way that cannot produce emesis, such as in gastric bypass procedures, Nissen fundoplication, and esophageal reconstructive surgeries. Most patients will also describe a feeling of abdominal distention or “bloating,” and report decreased, or lack of, passage of flatus and stool. Stool passage may be present early in complete SBO or be evidence of partial SBO.
Past Medical and Surgical History
Recommendation
- Assess risk factors for SBO including prior abdominal surgeries, hernias, cancers, inflammatory bowel disease, radiation exposure, prior intra-abdominal infections, and prior traumas.
Attention to past medical and surgical history is essential to recognizing patients with SBO, as adhesions from prior surgery account for 70% of patients presenting with SBO.3 The incidence of SBO after abdominal surgery is 9%.4 The next most common cause includes hernias which account for 10-15% of SBO. History of bariatric surgery, the most common of which is the Roux-en-Y gastric bypass, requires special attention because of its high association with internal hernias. Given the epidemic of obesity in the United States, these procedures have become more common. Defects in the mesentery that are intentionally created during these procedures may promote formation of internal hernias leading to bowel obstruction. Internal herniation is believed to develop in 2.5% of gastric bypass surgeries and can occur years after surgery.5,6 Malignancy and inflammatory bowel disease are other less frequent causes of SBO but should be considered because they are managed differently than adhesive SBO.1 Some etiologies are associated with prior infections, inflammatory processes, prior radiation, or non-operative treatment of intra-abdominal neoplasms. Rarely, non-operative trauma can cause adhesions. Although uncommon, patients without history of any of these conditions can be found to have congenital adhesions or internal hernias that can cause obstructions.
Medical comorbidities such as heart failure, coronary artery disease, renal failure, COPD and malignancy should be assessed as they may impact surgical candidacy and impact surgical planning. This is particularly true for active medical issues such as acute heart failure, acute coronary syndrome or pulmonary embolism.
Physical Exam
Recommendations
- Perform a complete physical exam, paying particular attention to presence of abdominal scars and hernias.
- Assess for physical manifestations of bowel compromise (Table 2) when SBO is suspected.
- Assess the overlying skin if a hernia is identified:
- - Tense, painful hernias with overlying skin changes should only be reduced by a surgical team.
Physical exam findings of small bowel obstruction often include, manifestations of hypovolemia (ie, orthostasis, decreased skin turgor, dry mucous membranes), abdominal distension, tenderness on palpation, tympany on percussion, high pitched tinkling bowel sounds, and peritoneal signs (such as guarding and rebound tenderness) (Table 1). Gastric decompression with an NG tube often reveals a large volume of gastric contents, which is often bilious.
Assessing for bowel compromise is of upmost importance as a delay in diagnoses can lead to ischemia, perforation, sepsis or septic shock and death. Vital signs are nonspecific but help determine clinical stability. Sinus tachycardia can be found in patients with dehydration, severe anxiety, or pain, or sometimes severe gastric distention. Hypotension can also indicate dehydration, but may also be concerning for sepsis and septic shock, and indicate hemodynamic instability. Abdominal pain accompanied by hypotension, tachycardia, tachypnea, confusion and fever should prompt urgent intervention for septic shock and determination of the source of abdominal pain.
Development of peritonitis is often identified by severe acute abdominal pain, worsened by movement of the peritoneum, rebound tenderness to palpation of the abdomen, a rigid abdomen and abdominal guarding. The presence of fever, tachycardia, tachypnea, confusion and hypotension all support the development of sepsis in the context of peritoneal signs and may indicate bowel ischemia. If there is concern for peritonitis, emergent surgical evaluation is required. These patients often proceed directly to the OR for exploration.
Hernias should be searched for and evaluated. Tense, painful abdominal wall hernias and hernias with overlying skin changes in the setting of SBO are signs of bowel compromise and should only be reduced by the surgical team, particularly if bowel is identified within the hernia. These patients often go directly to the operating room for assessment of bowel health and repair of the hernia. In cases where the surgical team reduces the hernia at bedside, patients require monitoring for delayed bowel ischemia and potential perforation, which is optimally provided by the surgical service.
Laboratory Testing
Recommendations
- Obtain a BMP with magnesium and phosphate, CBC with differential, and lactate.
- - Normal WBC count and/or lactate do not exclude the diagnosis of SBO and/or bowel compromise.
- Obtain a type and screen, PT/PTT, and urine pregnancy test (if appropriate) for operative planning.
There are no laboratory tests that are sensitive or specific for the diagnosis of SBO, or that predict ischemic bowel. Recommended tests in patients with abdominal pain, nausea and vomiting include: basic metabolic panel with magnesium and phosphate, a complete blood count with differential, and if bowel ischemia is suspected, a lactic acid level. A basic metabolic panel can identify electrolyte imbalances and renal dysfunction caused by hypovolemia. Patients with SBO may have hypokalemia, contraction alkalosis or metabolic acidosis. A CBC with differential can assess leukocytosis. Leukocytosis with a left shift is a non-specific indicator of inflammation and/or infection―it does not correlate with disease severity.7 An elevated lactic acid level can be seen with bowel ischemia, but notably, a normal lactate level does not rule out ischemia. An elevated lactic acid level can also be non-specific indicator of inadequate perfusion of any number of organs.
Radiographic Diagnosis of SBO
Recommendations
- First line imaging in patients with abdominal pain is an acute abdominal x-ray series (AAS).
- - Signs of bowel ischemia such as pneumatosis, portal venous gas, or pneumoperitoneum should prompt urgent surgical evaluation.
- - Air-fluid levels are not specific to SBO and may be seen in other conditions.
- - Paucity of bowel gas does not exclude SBO (fluid filled dilated bowel loops)
- Stable patients should also undergo CT abdomen AND pelvis with IV contrast.
- - Patients with allergies to IV contrast can undergo rapid IV steroid prep.
- Consider the risk of contrast-induced nephropathy in patients with chronic kidney disease (CKD):
- - CKD stage 3 and below: risk of contrast-induced nephropathy (CIN) is low.
- - CKD stages 4-5: patients who are on dialysis but still make significant volumes of urine, and those with acute kidney injury: discuss risks and benefits of IV contrast with the radiologist or nephrologist, and consider IV saline prophylaxis if contrast is to be given.
- Do not use oral contrast in patients with suspected SBO.
- A transition zone is required for the diagnosis of SBO
Radiographic evaluation can help confirm the diagnosis and detect imaging features that can direct management in patients with suspected SBO based on history and physical exam, who are clinically stable to undergo further testing. Two radiographic tests are generally obtained, an acute abdominal x-ray series followed by CT imaging of the abdomen AND pelvis with IV contrast. MRI and small bowel follow through exams are not indicated in the acute setting because they are time consuming and will cause a delay in diagnosis.
Abdominal Radiographs
An acute abdominal x-ray series is useful as the first imaging study in patients with suspected SBO. This study includes an upright CXR, a supine abdomen/pelvis x-ray and an upright abdomen/pelvis x-ray. If patients cannot sit or stand upright, a decubitus abdominal x-ray can be substituted for the upright view to evaluate for free peritoneal air.
Abdominal radiographs are best to evaluate for gas-filled, dilated bowels. The bowel gas pattern suggestive of SBO is asymmetrically dilated proximal small bowel with decompressed distal small bowel/colon. Small bowel dilatation is defined as luminal distention of >3 cm. Pneumatosis of the bowel wall, mesenteric and/or portal venous gas, and extra-luminal free air are late signs of bowel wall compromise and usually indicate bowel wall necrosis in the setting of SBO.
The reported accuracy of radiography for the diagnosis of SBO is between 67% and 83%.1 Ileus and pseudo-obstruction can have similar radiographic findings of diffuse bowel distension or dilatation.
Dilated, fluid-filled bowel loops can be missed on routine acute abdominal series. In the setting of SBO, routine abdominal x-rays may demonstrate a gasless abdomen or paucity of bowel gas, which are non-specific findings and can also be seen with a lack of ingested gas, excessive vomiting or presence of gastric suctioning.
Although air-fluid levels on abdominal x-rays can be seen with SBO, this finding is not specific and can be seen in asymptomatic patients as well as those with ileus or pseudo-obstruction. Air fluid levels should not be used alone to make a diagnosis of SBO but should instead be correlated with symptoms at the time the x-rays were taken.
While abdominal radiographs can increase clinical suspicion for SBO, they are limited in assessing relevant diagnostic information, including site of transition, cause of transition, and signs of ischemia, which are more clearly assessed with CT imaging.
CT Imaging
Always obtain CT imaging of the abdomen and pelvis when evaluating for SBO. Use of intravenous iodinated contrast delineates intra-abdominal organs to assess the bowel wall enhancement pattern. The sensitivity and specificity of CT in diagnosing obstruction is 94% and 96%, respectively, with an accuracy of 95%, all of which are superior to radiographs.8
IV contrast is essential in the assessment of bowel necrosis, enhancement of mesenteric vessels, and exclusion of vascular thrombosis. In stable patients with history of allergic-like reaction to iodine contrast, a rapid IV steroid prep (5 hours) should be administered.9 There is a standard IV steroid prep order set at Michigan Medicine that includes 2 doses of IV hydrocortisone (200 mg), timed 5 and 1 hour(s) prior to contrast administration, and 1 dose of diphenhydramine (50 mg), timed 1 hour prior to contrast administration. In cases of kidney disease, the decision to administer IV contrast should be individualized to every patient. No level of kidney disease is an absolute contraindication to the administration of IV contrast. In CKD patients stage 3 and below, risk of contrast-induced nephropathy (CIN) is low. In CKD patients stages 1 – 3, risk of contrast-induced acute kidney injury is negligible. In CKD patients stages 4 - 5 not receiving dialysis, patients who are on dialysis but still make significant volumes of urine, and patients with acute kidney injury, the risk of CI-AKI is estimated to be between 0 and 17%. In these patients, we recommend a.) a discussion of risks and benefits of IV contrast with the radiologist or nephrologist, and b.) consideration of IV saline prophylaxis if contrast is to be given and the patient does not have risk for congestive heart failure. In cases where the risks of contrast outweigh the benefits, an unenhanced CT of the abdomen and pelvis may still be helpful.10
Oral contrast administration is NOT recommended since oral contrast does not commonly aid in the diagnosis and may put the patient at risk of aspiration.
The CT finding of a transition zone is required for diagnosis of SBO (partial or complete). A transition zone is defined as an area where the bowel transitions proximally to distally over a relatively short segment from distended (gas- or fluid-filled) to a decompressed bowel. This radiographic transition represents the area of obstruction. Small bowel dilatation is defined as distention of >3 cm.
At times, a transition zone identified on CT imaging may not actually represent complete SBO. Symptoms should be correlated with CT findings before making the diagnosis of small bowel obstruction. Although oral contrast should not be used in the setting of suspected SBO, if oral contrast was given, evidence of it beyond the suspected transition point is indicative of a partial obstruction.
Small bowel feces sign can sometimes be seen in SBO, which occurs when intraluminal contents give the appearance of stool in the small bowel lumen just proximal to a transition zone. It is thought to be caused by undigested food, bacterial overgrowth and partial dehydration of enteric contents due to stagnant flow of intraluminal material.
A swirl sign/whirl sign on CT imaging, demonstrating a mass of soft-tissue and/or twisting of the mesenteric vessels around a central point suggests there may be a volvulus component to the obstruction. This most commonly occurs, in patients with a history of gastric bypass surgery (ie, Roux-en-Y) who are at higher risk of developing internal hernias. Small bowel volvulus and closed loop obstructions also occur with adhesions. Emergent surgical consultation is required even in stable patients when this finding is present. SBOs with a volvulus component seldom resolve without surgery and with rare exception require urgent operative intervention to prevent bowel compromise.
Findings concerning for bowel wall compromise include bowel wall edema or hemorrhage, altered bowel wall enhancement, inter-loop ascites, mesenteric edema/fat stranding, vascular engorgement, and/or vessel occlusion. As in abdominal radiographs, pneumatosis of the bowel wall, mesenteric and/or portal venous gas, and extra-luminal free air are late signs of bowel wall compromise and indicate bowel wall necrosis in the setting of SBO.
Sensitivity and specificity of CT for detecting ischemic bowel is estimated at 83% and 92%, respectively, however standardized criteria for diagnosing ischemia is lacking.1 Patients with little mesenteric fat pose diagnostic challenges because it is difficult to identify transition zones which can be obscured by crowded vessels. Additionally, lack of mesenteric adipose tissue reduces the sensitivity for other concerning findings such as mesenteric edema and inter-loop fluid. CT imaging has limited accuracy in predicting those that will ultimately benefit from operative management vs. those that will not; however, certain imaging findings indicate a high likelihood of needing surgical intervention (eg, volvulus, SBO in setting of Roux-en-Y gastric bypass).3,11 There is no role for ultrasound, fluoroscopy, or magnetic resonance imaging (MRI) in diagnosis of an acute small bowel obstruction in adult patients.
Special Diagnostic Situations
Recurrent small bowel obstruction. There is a small subset of patients who suffer from recurrent uncomplicated adhesive SBO that resolves without surgical intervention and may not require CT imaging for diagnosis. These patients are usually very well known to the surgical service, have received numerous CT scans consistent with findings of simple adhesive bowel obstructions, and do not exhibit any signs concerning for bowel compromise (Table 2). These patients should be discussed with the surgical service to decide if CT imaging is needed.
Inflammatory bowel disease (IBD). Consultation with gastroenterology should determine need for urgent CT enterography or MR enterography imaging.
Classification
The diagnostic process above will allow the patient to be categorized into one of the following 3 groups:
- Partial small bowel obstruction (pSBO): These patients have incomplete obstruction with luminal narrowing, but some contents continue to pass through the intestine. Clinically, this is recognized when patients exhibit the signs, symptoms, and radiographic findings consistent with SBO (Table 1), but exhibit a benign abdominal exam, and continue to pass bowel movements and flatus.
- Complete small bowel obstruction: These patients have obstruction with no passage of luminal contents beyond the point of obstruction. Clinically, this is recognized when patients exhibit the signs, symptoms, and radiographic findings consistent with SBO (Table 1), and are not passing bowel movements or flatus.
- Complete small bowel obstruction with bowel compromise: The bowel is considered compromised when there is ischemia or injury that has led, or may lead, to necrosis and/or perforation of the bowel wall. There is a high risk of morbidity and mortality if compromised bowel is not treated in an expedient manner. Signs, symptoms, and imaging findings of bowel compromise are shown in shown in Table 2.
With these classifications, initial triage and treatment can be established, as discussed below.
Etiology
Once a diagnosis of mechanical SBO is made, the evaluation should then focus on assessing the etiology of the obstruction and whether signs of bowel ischemia or bowel compromise are present. Causes of SBO can be categorized into extrinsic, intrinsic and endoluminal causes (Table 3).
Table 3
Causes of SBO.
Extrinsic causes: The most common extrinsic cause for SBO, and also the most common cause for all SBO, is adhesive disease, accounting for 70% of all cases.3 Other extrinsic causes include surrounding masses, enlarged organs, inflammatory processes external to the small bowel such as an intra-abdominal abscess, and abdominal wall or internal hernias. A closed-loop obstruction is suspected when the dilated small bowel demonstrates radial arrangement with a U- or C-shaped configuration, converging at the site of obstruction effectively preventing inflow and outflow of enteric contents and impeding the arterial and venous blood supply. This can occur when bowel herniates through a hernia defect, when small bowel twists around adhesions or mases, and in cases of congenital malrotation where improper fixing of the bowel allows twisting of the bowel around a narrow mesenteric vascular pedicle. Swirling of mesenteric vessels (“swirl sign” or “whirl sign”) noted on CT imaging indicates there is a volvulus component to the obstruction or may indicate an internal hernia in patients with a history of Roux-en-Y gastric bypass surgery. The presence of swirling requires emergent surgical consultation, as true closed-loop obstructions and internal hernias are treated with urgent operative intervention.
Intrinsic causes (source of pathology is extraluminal component of bowel): There are multiple intrinsic causes for SBO, including strictures of the lumen related to Crohn’s disease, radiation treatment of abdominal and pelvic organs, prior surgical anastomosis sites, resolved ischemic injury, primary small bowel tumors, and sometimes metastasis from distant primary tumors. Additionally, small bowel wall hematomas can narrow the lumen and obstruct flow.
Endoluminal causes (source of pathology is within lumen of bowel): Foreign bodies, bezoars, gallstones (known as a gallstone ileus), and distal small bowel intestinal syndrome (inspissated mucus and stool in patients with cystic fibrosis) can all cause SBO. Tumors, such as lipomas, can grow to a size that can become obstructive, sometimes creating a lead point for intussusception of the bowel.
Small bowel obstruction secondary to IBD requires additional discussion. The etiology of SBO in patient with IBD is diverse. Some patients develop acute SBO from an IBD flare with transmural inflammation leading to luminal narrowing. Others develop stricturing disease over the course of their illness which eventually leads to luminal narrowing and obstruction. Finally, as many as 60% of patients with IBD undergo surgery which puts these patients at risk for the later development of adhesive disease, hernias, or post-operative anastomotic strictures. Small bowel obstruction resulting from a Crohn’s disease flare may resolve with treatment of the inflammatory process. Those with SBO caused by stricturing disease, post-operative anastomotic strictures, or post-operative adhesive disease may not resolve with medical therapy, and sometimes require surgery.
Management
Triage/Admission Guidelines
Recommendations
- Consult Acute Care Surgery for any patient with evidence of complete bowel obstruction, as evidenced by a transition zone on imaging PLUS the absence of bowel movements or flatus, or an abnormal abdominal exam.
- Consult Acute Care Surgery for any suspicion of obstruction occurring within 30 days of abdominal surgery (the primary surgical service should be consulted, if it was performed at Michigan Medicine).
- Urgent Acute Care Surgery consultation is indicated in these situations:
- - All patients with abdominal pain and a history of prior gastric Roux-en-Y bypass procedures.
- - Patients with tense, painful abdominal wall hernias and hernias with overlying skin changes in the setting of possible bowel obstruction (reduction should only be attempted by the surgical team).
- - All patients with evidence of bowel compromise, as per Table 2.
- Patients with a complete SBO should be admitted to a surgical service, except in special circumstances (see “Special Triage Circumstances” below). Patients with complete or partial SBO occurring within 30 days of abdominal surgery should be admitted to a surgical service.
- Patients with a partial SBO, as evidenced by continued passage of bowel movements and flatus and a benign abdominal exam, may be admitted to a Medicine Service, (as long as they are not within 30 days of abdominal surgery).
Note that the primary surgical service (ie, the specific surgical service that performed the recent surgery) should always be consulted for patients being evaluated for SBO within 30 days after abdominal surgery. Any patient requiring hospital admission for suspected partial or complete bowel obstruction within 30 days of abdominal surgery should be admitted to the surgical service.
Complete small bowel obstruction is considered a surgical disease and is best managed on a surgical service.1,12,13 Though most SBO resolves without surgical intervention, SBO can lead to bowel ischemia requiring emergent surgical intervention.
Four studies have evaluated SBO patient outcomes based on admitting service (surgical versus medical). Though each the studies were fairly small, all favored admission to surgical services.14-17 A more recent study evaluating 107,603 admissions for SBO concluded that admission to a medical service was associated with higher healthcare utilization (including overall costs, length of stay, and readmission rates), and worse perioperative outcomes.13,18
Patients with radiographic findings of a transition zone, consistent with diagnosis of a complete SBO, with clinical evidence of a complete SBO (no passage of stool or flatus) should be admitted to a surgical service, unless active medical issues warrant direct monitoring and management by a medical service.
Partial small bowel obstruction (pSBO) usually does not require surgical intervention. These patients can be managed on a medical service.
Special Triage Circumstances
Recommendations
- Partial SBO patients with IBD and no evidence of bowel compromise should be admitted to a medical service, with preference to Medicine GI.
- Complete SBO patients with IBD are typically admitted to the Med GI Service (for trial of intensive medical therapy) with the Surgical Service following closely). Patient should be seen by an attending surgeon and the plan discussed with the GI attending within 12 hours of admission.
- Patients with malignant obstruction (ie, SBO in the setting of a cancer diagnosis with abdominal primary or abdominal metastases) often require a multidisciplinary approach with input from Oncology and Surgery to help determine an individualized treatment plan. These patients are often admitted to a General Medicine Service, with the Surgical Service following closely, to facilitate this approach.
- Patients with a history of recurrent, uncomplicated adhesive SBO may not require CT imaging for diagnosis. Discuss with primary surgical team.
There are two specific situations that involve more complex triage decisions: inflammatory bowel disease (IBD), and malignant bowel obstruction.
Inflammatory bowel disease
SBO patients with IBD require subspecialty consultation from Medicine GI and Colorectal Surgery to determine the optimal therapeutic approach. Early surgical consultation (within 12 hours of admission) is recommended in IBD patients with obstructive signs and symptoms but IBD patients with SBO from an acute disease flare should be admitted to a medical service, with preference to the Medicine GI service.
Malignant small bowel obstruction
SBO secondary to malignancy requires a multidisciplinary approach. Patients’ surgical candidacy often depends on their prognosis related to their malignancy. If the patient is not a surgical candidate (ie, on hospice or electing for comfort care) or does not desire extensive surgical intervention regardless of outcome, they should be admitted to a general medicine or medicine oncology service, with surgical consultation and undergo optimization of non-surgical and/or palliative interventions (which may include surgical procedures). Given the overall mortality rate increase (from less than 10% up to 25%) in the setting of delayed surgical management, patients presenting with malignant SBO deemed to be potential surgical candidates should be seen promptly by surgery, regardless of the ultimate disposition, to determine if surgical intervention is warranted.
Treatment
Complete Small Bowel Obstruction
Between 65 and 80% of SBOs resolve spontaneously with non-operative management.3,14 Therefore, a trial of non-operative management is usually the initial management strategy. In patients with complete SBO that ultimately require operative management, morbidity and mortality risk increases with delay in surgical intervention of >36 hours.1 Because of this, admission to a surgical service with frequent assessment is necessary as a part of the initial non-operative management.
Additionally, some patients with evidence of bowel compromise require early surgical intervention.
Early surgical intervention
Recommendation
- Emergent surgery should be considered for patients with evidence of bowel compromise (Table 2).
Complete SBO may present with clinical signs and laboratory findings concerning for bowel compromise, such as severe abdominal pain, peritoneal signs, tachycardia, fever, leukocytosis and lactic acidosis, which when present, predict bowel ischemia in 40-50% of cases.19,20 Expedient operative management decreases mortality risk in SBO with bowel compromise. Radiographic features on CT imaging that may indicate the need for early operative management include altered bowel wall enhancement, inter-loop ascites, closed loop obstruction, swirl/whirl sign, mesenteric edema/fat stranding, vascular engorgement or occlusion, pneumatosis, portal venous gas, and free intraperitoneal extra-luminal air.
Non-operative management
Recommendations
- Non-operative management of SBO is described in Table 4.
- Non-operative management of SBO without bowel compromise initially includes IV fluid resuscitation, electrolyte monitoring/supplementation, NPO status, nasogastric decompression, serial abdominal exams, and avoidance/minimal use of opiates
- Assess for clinical improvement frequently during the first 3-5 days of initiating non-operative management, as surgical delays in patients who end up requiring operative intervention may increase morbidity and mortality.
- Consider surgical intervention if concern for bowel compromise develops at any time during the trial of non-operative management.
- Administer Gastrografin challenge in appropriate patients, per protocol and ONLY after evaluation and recommendation by surgery.
Because 65-80% of SBOs resolve spontaneously, we recommend a period of non-operative management in patients without signs concerning for bowel compromise. Patients admitted for non-operative management of complete SBO require close monitoring with serial abdominal exams.
The initial management includes NPO (nothing by mouth) status, and placement of a large bore (≥18 F) nasogastric tube to low continuous wall suction. Opioid medications should be avoided/minimized as they decrease bowel motility. Resuscitation with IV fluids and close electrolyte monitoring and supplementation are also key components of SBO non-operative management.
There is debate regarding the appropriate duration of non-operative management before surgical intervention is recommended. Studies indicate that the appropriate time is between 3-5 days. It is thought that delaying required surgical intervention can increase morbidity and mortality.1,3,13,21-23 Surgical treatment must be promptly considered at any time during a period of non-operative management if any signs of bowl compromise develop.
Gastrografin in SBO
Multiple studies have shown the diagnostic utility of Gastrografin to predict which patients with SBO will require surgical intervention versus non-operative managment.24-32 Gastrografin consists of sodium diatrizoate and meglumine diatrizoate and has been used for many years as a water-soluble iodinated oral contrast medium. Use of Gastrografin in SBO to predict the need for operative management has been referred to as the “Gastrografin challenge.” The Gastrografin challenge involves administration of oral Gastrografin contrast and serial abdominal x-rays, with interpretation based on the location and intra-luminal progression of contrast over time. Patients with contrast visualized in the colon are recommended to continue with non-operative management and those in which contrast does not pass into the colon by the end of the protocol should be considered for surgery. The use of Gastrografin challenge protocols has been shown to reduce the number of operations, reduce the time to resolution of SBO, and reduce length of stay for patients with SBO.24-30,32
Observational studies, and recent meta-analyses suggest that Gastrografin may theoretically have some therapeutic benefits in select patients with SBO27. In its undiluted form, Gastrografin has an osmolarity of 1900 mOsm/L which produces an osmotic gradient across the lumen of the intestine, drawing in fluid then transmitting pressure across the obstruction as well as decreasing edema which may help to resolve SBO caused by adhesive disease. Gastrografin also contains polysorbate 80 which is a surfactant and emulsifier. It has been postulated that polysorbate 80 serves as a lubricant for bowel contents aiding in resolution of obstruction.
Gastrografin carries the risk of severe and potentially fatal aspiration pneumonitis, particularly in the setting of gastric distention and vomiting. It is not recommend for the treatment of SBO, and only recommend for assessment purposes.33
Patient Selection: Carefully select patients for the Gastrografin challenge. Exclude patients with history of sepsis, hernia, strangulation, peritonitis, abdominal infection, malignancy, incarcerated hernia, history of pelvic radiation, abdominal surgery within 6 months, or those who are pregnant. If signs of bowel compromise develop at any time during the procedure, emergency surgery should be considered. Eligible, patients must also be low risk for aspiration with no history of paraesophageal hernia, hiatal hernia, COPD with home oxygen requirement, or dysphagia. Perform gastric decompression for 12 hours via nasogastric tube before initiating a Gastrografin challenge (provided the patient remains stable after decompression) to decrease the risk of aspiration. Other institutions may recommend a shorter decompression duration (eg, 2 hours) prior to Gastrografin challenge. Although data suggest that these protocols have a low risk of complication, shorter duration of decompression is not supported because the risk of aspiration outweighs the delay incurred by a 12-hour decompression. Only initiate and administer a Gastrografin challenge under direction of the surgical service.
Protocol: In appropriate patients who have undergone 12 hrs NGT decompression, 100 mL of Gastrografin, diluted in 50 mL water is administered via NGT. The NGT is then clamped for 1 hour. If the patient develops nausea or increased abdominal pain, the NGT is placed back to wall suction, and the challenge is over. If the patient remains asymptomatic, abdominal x-rays are obtained 8 hours after Gastrografin administration. If contrast is visualized in the colon at 8 hours, no further imaging is pursued and non-operative management of SBO is continued. If Gastrografin is not visualized in the colon at 8 hours, repeat abdominal x-rays are obtained at 24 hours. If contrast is not visualized in the colon at 24 hours, surgical intervention should be strongly considered.
In studies of Gastrografin used to determine need for surgical intervention, approximately 10% of patients who had Gastrografin in the colon after 24 hours went on to require surgery.30,29 Combine a Gastrografin challenge with clinical judgement.
The concentration of oral Gastrografin that is used in standard CT imaging of the abdomen and pelvis is different than the formulation used for the Gastrografin challenge. It is more dilute, is less readily identified by x-rays, and has not been validated as a way to stratify the need for operative management in patients with SBO. Although oral contrast is not indicated with CT for the evaluation of patients with suspected SBO, if oral contrast happens to have been administered, presence of oral contrast in the colon likely has similar predictive ability as the Gastrografin challenge for identifying patients who may be managed non-operatively.
Surgical intervention should be strongly considered for patients without clinical improvement after 3-5 days of non-operative management.1,3,13,21-23 Clinical improvement is characterized by decreased abdominal distention; improvement in abdominal pain, nausea and vomiting; and decreased NGT output, which indicates return to normal bowel function. If a Gastrografin challenge is performed, progression of contrast to the colon is also a good prognostic marker for SBO resolution. NG tubes can generally be removed when nausea and vomiting cease and NG tube output is decreasing (usually <300-500 mL/ 8 hours). Advanced to the diet of the patient’s choice once they are able to tolerate clear liquids. Discharge may be considered once a patient is tolerating oral intake. Patients should have close outpatient follow-up with primary medical and/or surgical clinics depending on their individual clinical scenario. Patients should be educated that they are at risk for recurrent SBO and should return to the hospital if their symptoms recur.
SBO with transition point at site of hernia
Patients presenting with a transition zone at the site of an abdominal wall hernia require special attention. These obstructions should only be reduced by the surgical team. Assessment for ischemic bowel is required to be performed by a member of a surgical team. If ischemic bowel is suspected (severe pain, tense hernia, overlying skin changes), urgent surgical exploration should be performed. If the surgical team determines there are no concerns for bowel compromise the surgical team admitting the patient may decide to reduce the bowel. They will determine the appropriate amount of time the patient will be monitored post hernia reduction (typically, 24 hours).
Partial Small Bowel Obstruction
Recommendation
- Initiate non-operative management in patients with pSBO without evidence of bowel compromise, as described above in the complete SBO section (Table 4).
The risk of a partial SBO progressing to bowel compromise is extremely low, and symptoms usually resolve quickly. Studies suggest that the vast majority of patients presenting with pSBO have resolution without surgery, with only 3-6% of patients developing bowel ischemia when treated with non-operative management.19
The initial management of pSBO includes NPO (nothing by mouth) status, fluid and electrolyte supplementation. Placement of a large (≥18 F) nasogastric tube (NGT) is recommended when the patient is vomiting, or significant abdominal pain. If an underlying condition causing the partial obstruction is identified, this should be treated. For instance, if the patient is partially obstructed from inflammatory bowel disease, consultation with gastroenterology and medical therapy or endoscopic treatment of this disease may result in resolution of the partial obstruction. Do not to allow a patient to eat solid food with a large bore NGT in place, as the tube will stent open the gastroesophageal junction, increasing the risk of aspiration.
As with SBO, Gastrografin challenge may be useful in assessing need for surgical intervention. There have also been a few low quality randomized controlled trials using oral magnesium oxide, lactobacillus and simethicone in pSBO. Existing evidence does not support the routine use of these agents.34 Exercise caution when recommending probiotics such as lactobacillus, as there have been accounts of blood stream infections in immunocompromised patients.35
No study supports the use of promotility agents, such as Reglan (metoclopramide) in pSBO, and are contraindicated in complete obstruction.
Non-operative management of pSBO is summarized in Table 4. Patients who have resolution of their nausea, vomiting and abdominal pain should have the NG tube removed and their diet advanced. Once tolerating a diet and maintaining hydration, discharge the patient home. Patients who do not have resolution of their symptoms, or experience progression of their obstructive symptoms within 24-48 hours of admission should be promptly evaluated by surgery.
Obstruction Due to Inflammatory Bowel Disease (IBD)
Recommendations
- Manage initially with non-operative management (Table 4).
- Consultation with gastroenterology and colorectal surgery will establish an individualized treatment plan.
Evidence of intestinal obstruction represents one of the defining characteristics for severe/fulminant IBD.36 Although there is not strong evidence in support of specific treatment algorithms on the acute management of intestinal obstruction associated with IBD, a number of evidence-based recommendations have been made. It has been recommended that the treatment plan be based on factors such as the location and length of the stricture, the degree of concomitant inflammation, degree of upstream bowel dilation and accompanying features, such as abscess or phlegmon.37 As such, early cross sectional imaging with an enterography (CTE) protocol is essential, as is the early involvement of both gastroenterology and colorectal surgery. Once imaging has been performed, a colorectal surgeon and a gastroenterologist specializing in the management of IBD should determine early surgery vs. neoadjuvant anti-inflammatory therapy, and the need for antibiotics and/or abscess drainage.
A detailed discussion of the medical management of IBD with small intestinal obstruction is outside of the scope of this document. At Michigan Medicine, review this link: http://www.med.umich.edu/ibd/docs/IBD_PostOp.pdf.
Obstruction Due to Malignancy
Recommendation
- Initial management consists of non-operative management with IV fluid hydration, NPO status, pain control, and consideration of nasogastric decompression (Table 4).
SBO secondary to malignancy imparts a poor prognosis.38-40 The treatment of bowel obstruction due to malignancy remains a challenging clinical scenario, as there is no single, standard approach to decision-making that applies to this diverse group of patients. Patients with known malignancy at high risk for developing bowel obstructions should be counseled about the possibility of developing bowel obstructions as part of the education about their disease. Initial treatment of malignant SBO mirrors non-operative management of SBO as mentioned above.40 Any additional recommendations should be based on the guidance of the above specialties, the clinical situation, and the patient’s goals. (I-E)
Guideline Creation Process and Considerations
Related National Guidelines and Performance Measures
The University of Michigan Health System (UMHS) Clinical Guideline on Small Bowel Obstruction is generally consistent with the Bologna Guidelines for Diagnosis and Management of Adhesive Small Bowel Obstruction (ASBO).
There are no national performance measures associated with small bowel obstruction.
Funding
The development of this guideline was funded by the University of Michigan Health System.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- The medical team: Gary Vercruysse MD, FACS, Mahmoud Al-Hawary, MD; Jill Cherry-Bukowiec, MD; Derek Dimcheff, MD, PhD; Richard J. Saad, MD, MS, FACG; David Somand, MD; Lauren Wanacata MD; Rebecca Busch MD; Luke Pumiglia, BA
- Guideline development methodologists: Dave Megan Mack, MD; Dave Wesorick, MD; April Proudlock, BBA, RN
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
No relevant personal financial relationships with commercial entities:
Relevant personal financial relationships with commercial entities: None.
Strategy for Literature Search
Three different Main searches were used to search this topic. The comprehensive main search on all intestinal obstruction search retrieved 5,193 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follow:
- Bowel Obstruction -Guidelines, total results were 48
- Bowel Obstruction -Clinical Trials, total results were 316
- Bowel Obstruction -Cohort Studies, total results were 1,041
The retrieval was much smaller for the small bowel and large bowel searches.
- Small Bowel Obstruction -Guidelines, total results were 12
- Small Bowel Obstruction -Clinical Trials, total results were 62
- Small Bowel Obstruction -Cohort Studies, total results were 289
- Large Bowel Obstruction -Guidelines, total results were 11
- Large Bowel Obstruction -Clinical Trials, total results were 115
- Large Bowel Obstruction –Cohort Studies, total results were 394
The MEDLINE In-Process database was also searched using the strategy in the search strategies document. The search retrieved 100 documents. The results with the hedges applied are:
- Guidelines, total results were 6
- Clinical Trials, total results were 12
- Cohort Studies, total results were 40
Within the Cochrane Database of Systematic Reviews, 11 reviews were found using the strategy in the search strategies document.
Level of evidence supporting a diagnostic method or an intervention:
- A = systematic reviews of randomized controlled trials with or without meta-analysis,
- B = randomized controlled trials,
- C = systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control),
- D = individual observation studies (case study/case series),
- E = expert opinion regarding benefits and harm
Search details and evidence tables available at http://www.uofmhealth.org/provider/clinical-care-guidelines.
Recommendations
Guideline recommendations were based on prospective randomized controlled trials if available, to the exclusion of other data; if RCTs were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size. The “strength of recommendation” for key aspects of care was determined by expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Medical School to which the content is most relevant: General Medicine; Department of Emergency Medicine; Department of Radiology; Department of Surgery; Gastroenterology Division. The Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers endorsed the final version.
References
- 1.
- Rami Reddy SR, Cappell MS. A Systematic Review of the Clinical Presentation, Diagnosis, and Treatment f Small Bowel Obstruction. Current gastroenterology reports.2017;19(6):28-017-0566-0569. [PubMed: 28439845]
- 2.
- Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. Journal of the American College of Surgeons.1998;186(1):1-9. [PubMed: 9449594]
- 3.
- Bower KL, Lollar DI, Williams SL, Adkins FC, Luyimbazi DT, Bower CE. Small Bowel Obstruction. The Surgical clinics of North America.2018;98(5):945-971. [PubMed: 30243455]
- 4.
- ten Broek RPG, Issa Y, van Santbrink EJP, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ.2013;347:f5588. [PMC free article: PMC3789584] [PubMed: 24092941]
- 5.
- Eckhauser A, Torquati A, Youssef Y, Kaiser JL, Richards WO. Internal hernia: postoperative complication of roux-en-Y gastric bypass surgery. Am Surg.2006;72(7):581-584; discussion 584-585. [PubMed: 16875078]
- 6.
- Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients--what have we learned? Obes Surg.2000;10(6):509-513. [PubMed: 11175957]
- 7.
- Hernandez MC, Kong VY, Aho JM, et al. Increased anatomic severity in appendicitis is associated with outcomes in a South African population. The journal of trauma and acute care surgery.2017;83(1):175-181. [PMC free article: PMC5478458] [PubMed: 28301395]
- 8.
- Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology.2015;275(2):332-342. [PubMed: 25906301]
- 9.
- Mervak BM, Cohan RH, Ellis JH, Khalatbari S, Davenport MS. Intravenous Corticosteroid Premedication Administered 5 Hours before CT Compared with a Traditional 13-Hour Oral Regimen. Radiology.2017;285(2):425-433. [PubMed: 28745940]
- 10.
- Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology.2020;294(3):660-668. [PubMed: 31961246]
- 11.
- Lazarus DE, Slywotsky C, Bennett GL, Megibow AJ, Macari M. Frequency and relevance of the “small-bowel feces” sign on CT in patients with small-bowel obstruction. AJRAmerican journal of roentgenology.2004;183(5):1361-1366. [PubMed: 15505304]
- 12.
- Aquina CT, Becerra AZ, Probst CP, et al. Patients With Adhesive Small Bowel Obstruction Should Be Primarily Managed by a Surgical Team. Annals of Surgery.2016;264(3):437-447. [PubMed: 27433901]
- 13.
- Aquina CT, Fleming FJ. Who Should Manage Patients with Adhesive Small Bowel Obstruction? Advances in Surgery.2017;51(1):125-140. [PubMed: 28797335]
- 14.
- Bilderback PA, Massman JD, 3rd, Smith RK, La Selva D, Helton WS. Small Bowel Obstruction Is a Surgical Disease: Patients with Adhesive Small Bowel Obstruction Requiring Operation Have More Cost-Effective Care When Admitted to a Surgical Service. Journal of the American College of Surgeons.2015;221(1):7-13. [PubMed: 26095546]
- 15.
- Malangoni MA, Times ML, Kozik D, Merlino JI. Admitting service influences the outcomes of patients with small bowel obstruction. Surgery.2001;130(4):706-711; discussion 711-703. [PubMed: 11602902]
- 16.
- Oyasiji T, Angelo S, Kyriakides TC, Helton SW. Small bowel obstruction: outcome and cost implications of admitting service. American Surgeon.2010;76(7):687-691. [PubMed: 20698371]
- 17.
- Schwab DP, Blackhurst DW, Sticca RP. Operative acute small bowel obstruction: admitting service impacts outcome. The American Surgeon.2001;67(11):1034-1038; discussion 1038-1040. [PubMed: 11730219]
- 18.
- Wahl WL, Wong SL, Sonnenday CJ, et al. Implementation of a small bowel obstruction guideline improves hospital efficiency. Surgery.2012;152(4):626-632; discussion 632-624. [PubMed: 22939746]
- 19.
- Fevang BT, Jensen D, Svanes K, Viste A. Early operation or conservative management of patients with small bowel obstruction? The European journal of surgery = Acta chirurgica.2002;168(8-9):475-481. [PubMed: 12549688]
- 20.
- Tsumura H, Ichikawa T, Hiyama E, Murakami Y, Sueda T. Systemic inflammatory response syndrome (SIRS) as a predictor of strangulated small bowel obstruction. Hepato-gastroenterology.2004;51(59):1393-1396. [PubMed: 15362761]
- 21.
- Di Saverio S, Coccolini F, Galati M, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World Journal Of Emergency Surgery.2013;8(1):42. [PMC free article: PMC4124851] [PubMed: 24112637]
- 22.
- Maung AA, Johnson DC, Piper GL, et al. Evaluation and management of small-bowel obstruction: an Eastern Association for the Surgery of Trauma practice management guideline. The Journal of Trauma and Acute Care Surgery.2012;73(5 Suppl 4):S362-369. [PubMed: 23114494]
- 23.
- Schraufnagel D, Rajaee S, Millham FH. How many sunsets? Timing of surgery in adhesive small bowel obstruction: a study of the Nationwide Inpatient Sample. The journal of trauma and acute care surgery.2013;74(1):181-187; discussion 187-189. [PubMed: 23271094]
- 24.
- Abbas S, Bissett Ian P, Parry Bryan R. Oral water soluble contrast for the management of adhesive small bowel obstruction.John Wiley & Sons, Ltd;2007. [PMC free article: PMC6465054] [PubMed: 17636770]
- 25.
- Assalia A, Schein M, Kopelman D, Hirshberg A, Hashmonai M. Therapeutic effect of oral Gastrografin in adhesive, partial small-bowel obstruction: a prospective randomized trial. Surgery.1994;115(4):433-437. [PubMed: 8165534]
- 26.
- Branco BC, Barmparas G, Schnuriger B, Inaba K, Chan LS, Demetriades D. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. The British journal of surgery.2010;97(4):470-478. [PubMed: 20205228]
- 27.
- Ceresoli M, Coccolini F, Catena F, et al. Water-soluble contrast agent in adhesive small bowel obstruction: a systematic review and meta-analysis of diagnostic and therapeutic value. American Journal of Surgery.2016;211(6):1114-1125. [PubMed: 26329902]
- 28.
- Choi HK, Chu KW, Law WL. Therapeutic value of gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Annals of Surgery.2002;236(1):1-6. [PMC free article: PMC1422541] [PubMed: 12131078]
- 29.
- Goussous N, Eiken PW, Bannon MP, Zielinski MD. Enhancement of a small bowel obstruction model using the gastrografin challenge test. Journal of Gastrointestinal Surgery.2013;17(1):110; Jan-116. [PubMed: 22923211]
- 30.
- Haule C, Ongom PA, Kimuli T. Efficacy of Gastrografin((R)) Compared with Standard Conservative Treatment in Management of Adhesive Small Bowel Obstruction at Mulago National Referral Hospital. Journal of clinical trials.2013;3(4):10.4172/2167-0870.1000144. [PMC free article: PMC3982137] [PubMed: 24729947] [CrossRef]
- 31.
- Loftus T, Moore F, VanZant E, et al. A protocol for the management of adhesive small bowel obstruction. The Journal of Trauma and Acute Care Surgery.2015;78(1):13-19. [PMC free article: PMC5125021] [PubMed: 25539198]
- 32.
- Zielinski MD, Eiken PW, Bannon MP, et al. Small bowel obstruction-who needs an operation? A multivariate prediction model. World journal of surgery.2010;34(5):910-919. [PMC free article: PMC4882094] [PubMed: 20217412]
- 33.
- Trulzsch DV, Penmetsa A, Karim A, Evans DA. Gastrografin-induced aspiration pneumonia: a lethal complication of computed tomography. Southern medical journal.1992;85(12):1255-1256. [PubMed: 1470976]
- 34.
- Chen SC, Yen ZS, Lee CC, et al. Nonsurgical management of partial adhesive small-bowel obstruction with oral therapy: a randomized controlled trial. CMAJ Canadian Medical Association Journal.2005;173(10):1165-1169. [PMC free article: PMC1277043] [PubMed: 16275967]
- 35.
- Liong MT. Safety of probiotics: translocation and infection. Nutr Rev.2008;66(4):192-202. [PubMed: 18366533]
- 36.
- Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol.2018;113(4):481-517. [PubMed: 29610508]
- 37.
- Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis.2016;10(8):873-885. [PubMed: 26928961]
- 38.
- Chakraborty A, Selby D, Gardiner K, Myers J, Moravan V, Wright F. Malignant bowel obstruction: natural history of a heterogeneous patient population followed prospectively over two years. Journal of pain and symptom management.2011;41(2):412-420. [PubMed: 21131167]
- 39.
- Mirensky TL, Schuster KM, Ali UA, Reddy V, Schwartz PE, Longo WE. Outcomes of small bowel obstruction in patients with previous gynecologic malignancies. American Journal of Surgery.2012;203(4):472-479. [PubMed: 22172316]
- 40.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. European journal of cancer (Oxford, England: 1990).2008;44(8):1105-1115. [PubMed: 18359221]
APPROVALS
| P&T | Date: 2/16/2021 |
| ECCA | Date: 4/13/2021 |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Created: April 2021.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Value of CT in the diagnosis and management of patients with suspected acute small-bowel obstruction.[AJR Am J Roentgenol. 1995]Value of CT in the diagnosis and management of patients with suspected acute small-bowel obstruction.Taourel PG, Fabre JM, Pradel JA, Seneterre EJ, Megibow AJ, Bruel JM. AJR Am J Roentgenol. 1995 Nov; 165(5):1187-92.
- The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults.[Eur J Radiol. 2004]The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults.Grassi R, Romano S, D'Amario F, Giorgio Rossi A, Romano L, Pinto F, Di Mizio R. Eur J Radiol. 2004 Apr; 50(1):5-14.
- Review Comparative Effectiveness of Imaging Modalities for the Diagnosis of Intestinal Obstruction in Neonates and Infants:: A Critically Appraised Topic.[Acad Radiol. 2016]Review Comparative Effectiveness of Imaging Modalities for the Diagnosis of Intestinal Obstruction in Neonates and Infants:: A Critically Appraised Topic.Carroll AG, Kavanagh RG, Ni Leidhin C, Cullinan NM, Lavelle LP, Malone DE. Acad Radiol. 2016 May; 23(5):559-68. Epub 2016 Feb 5.
- Ileus and intestinal obstruction--comparison between children and adults.[Pol Przegl Chir. 2011]Ileus and intestinal obstruction--comparison between children and adults.Peyvasteh M, Askarpour S, Javaherizadeh H, Taghizadeh S. Pol Przegl Chir. 2011 Jul; 83(7):367-71.
- Review [Ultrasound ileus diagnosis].[Ultraschall Med. 1998]Review [Ultrasound ileus diagnosis].Seitz K, Merz M. Ultraschall Med. 1998 Dec; 19(6):242-9.
- Evaluation and Management of Mechanical Small Bowel Obstruction in AdultsEvaluation and Management of Mechanical Small Bowel Obstruction in Adults
- Plasmodium falciparum strain:3D7Plasmodium falciparum strain:3D7Plasmodium falciparum heterochromatin formationBioProject
- txid4577[orgn] AND "cultivar CML61"[All Fields] (13)PopSet
- Full text in PMC (nucleotide) for Gene (Select 112543429) (24)PMC
Your browsing activity is empty.
Activity recording is turned off.
See more...

