Clinical Description
Periodontal EDS (pEDS) is characterized by distinct oral manifestations. Early and severe breakdown of the tooth-supporting tissues (i.e., alveolar bone, periodontal ligament, root cementum, and gingival attachment) result in premature loss of teeth. Lack of attached gingiva and thin and fragile gums lead to gingival recession. Connective tissue abnormalities of pEDS typically include easy bruising, pretibial plaques, distal joint hypermobility, hoarse voice, and less commonly manifestations such as organ or vessel rupture (see Table 2).
Since the first descriptions of pEDS in the 1970s [McKusick 1972, Stewart et al 1977], 148 individuals have been described in 34 case reports, seven pedigree analyses, and two cohort studies [Kapferer-Seebacher et al 2017, Wu et al 2018, Kapferer-Seebacher et al 2019, Cortés-Bretón Brinkmann et al 2021, Kapferer-Seebacher et al 2021]. The following description of the phenotypic features associated with this condition is based on these reports. However, in-depth description of non-oral manifestations in newly diagnosed individuals with a molecularly confirmed diagnosis of pEDS will be important to further define the clinical features.
Table 2.
Periodontal Ehlers-Danlos Syndrome: Frequency of Select Features
View in own window
| Feature | % of Persons
with Feature | Comment |
|---|
| Dental | Severe early-onset periodontitis | 99% | Diagnosed in childhood or adolescence (mean onset age 14 yrs) |
| Receding gums | 98% of adults | |
| Generalized lack of attached gingiva | 93% | Identification requires clinical expertise; appears to be pathognomonic for pEDS but not assessed/reported in all persons w/pEDS. |
| Easy bruising | 96% | Mainly on the shins, but also in unusual areas (e.g., cheeks & thighs) |
| Pretibial plaques | 83% | Also reported as pretibial (hemosiderin) discolorations |
| Skin fragility, atrophic scarring, delayed wound healing | 50% | |
| Joint hypermobility | 44% | Previously reported as mainly affecting the small joints |
| Arterial aneurysms/dissections | 8/145 | Abdominal aorta (n=2), carotid artery (2), cerebral artery (3), unknown location (1) |
| Brain white matter abnormality | 8/8 | Reported in all adults who had a brain MRI |
| Organ rupture | 2/145 | Lung, diaphragm, intestine, small bowel, eardrum |
Dental Findings
Periodontitis with early onset is the predominant feature of pEDS.
Periodontitis with early onset was reported in 98.4% of affected adults [Kapferer-Seebacher et al 2017]. To date, detailed descriptions of periodontal and dental characteristics of pEDS are lacking. Single adults with pEDS but without periodontitis were identified in pedigree analyses: they fulfilled the clinical criteria due to generalized lack of attached gingiva, pretibial plaques, and family history of pEDS.
Median age of onset of periodontitis is reported to be 14 years (range: 2-35 years) [Kapferer-Seebacher et al 2016]. This should be regarded as a rough estimate as the data are based either on retrospectively collected dental radiographs or on recollections by affected individuals (in some cases the remembered age of first tooth loss due to periodontal reasons).
Clinical investigations of 12 affected children (ages 8 to 13 years) revealed localized periodontal bone loss of up to 6 mm only in the oldest individual (age 13 years), an observation supporting the notion that onset of periodontitis is predominantly in adolescence [Kapferer-Seebacher et al 2021]. Additionally, premature loss of primary teeth at age three or four years was reported by parents who noted that the "teeth came out with the whole root," which can be attributed to periodontitis of primary teeth.
Although most individuals report frequent gum bleeding when brushing their teeth, frequent gum bleeding (a sign of gingival inflammation) is difficult to evaluate as it is subjective and very frequent in the general population due to insufficient plaque control.
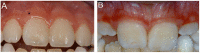
Generalized lack of attached gingiva. Absence or reduced amount of attached gingiva causing oral tissue fragility, first described in 1995 [Cunniff & Williamson-Kruse 1995], appears to be pathognomonic in pEDS.
In healthy individuals, the gums are classified as keratinized attached gingiva and non-keratinized movable mucosa. The keratinized attached gingiva is tightly and unmovably bound to the periosteum via collagen type I fibers as a thick band to provide mechanical protection. The non-keratinized oral mucosa is thin, more fragile, translucent, and only loosely connected to the periosteum. In pEDS, the thick band of gingiva is missing and the fragile mucosa extends to the gingival margin, causing tissue fragility and predisposing to gingival recession.
Generalized receding gums, reported in 87.1% of adults with pEDS, are attributable to the lack of attached gingiva and progressive periodontal attachment loss.
Ehlers-Danlos Syndrome Findings
Easy bruising. Almost all individuals with pEDS (96%) reported easy bruising from early childhood, especially in the pretibial area [Kapferer-Seebacher et al 2016]. Although easy bruising is difficult to evaluate as it is subjective and nonspecific, bruising may occur in atypical sites such as the cheeks or the thighs after taking a hot shower or doing sports [Kapferer-Seebacher et al 2019].
Pretibial plaques. Histologic analysis from brownish pretibial skin demonstrated dermal fibrosis and hemosiderin deposition [Ronceray et al 2013]. Affected individuals sometimes report a single pretibial trauma followed by hematomas that never resolve. In contrast to the hemosiderosis of the classic, vascular, and arthrochalastic EDS types, the pretibial plaques of pEDS are often necrobiotic, reflecting more severe connective tissue matrix inflammation and degradation [George et al 2016].
Skin fragility, atrophic scarring, and delayed wound healing. Skin fragility can manifest as easy bruising and "cigarette paper" scars mainly on the shins [George et al 2016]. Chronic leg ulcers may occur within the atrophic pretibial plaques [Ronceray et al 2013]. Single individuals reported open wounds in the pretibial area that took months or even years to heal or even required skin grafting [George et al 2016, Kapferer-Seebacher et al 2016].
Joint hypermobility often only affects the distal joints [Kapferer-Seebacher et al 2016].
Arterial aneurysms/dissections, documented in 8/145 individuals with pEDS (5.5%), may cluster in families. Four clinically affected individuals in one family had diverse cardiovascular events: aortic dissection (age 46 years), sudden death (23 years), hemorrhage of cerebral aneurysm (42 years), and heart attack (43 years) [Kapferer-Seebacher et al 2016]. Cerebral aneurysms were observed in individuals from unrelated families [Cıkla et al 2014, Kapferer-Seebacher et al 2016]. More data over a longer period of time are needed to clarify the risk for arterial aneurysms or dissections. This risk appears lower than in people with vascular EDS, in which 72% of individuals with one or more vascular complications have been reported [Frank et al 2015].
Organ rupture. Multiple organ ruptures were reported in two of 148 affected individuals (1.4%). One individual, age 67 years, had a history of recurrent pneumothoraces, an intestinal perforation, and an inguinal hernia [Kapferer-Seebacher et al 2016]. The other individual, age 57 years, had a rupture of the lung and diaphragm [Kapferer-Seebacher et al 2019].
Other
Acrogeric changes of the hands and feet seen in pEDS [George et al 2016] are reminiscent of those observed in vascular EDS.
Hoarse high-pitched voice has been reported in two individuals with pEDS to date [George et al 2016, Kapferer-Seebacher et al 2019]. Voice abnormalities have occasionally been described in other types of EDS; it has been hypothesized that they may be due to defects in the collagen of the vocal ligament [George et al 2016].
Brain white matter abnormalities. All adults investigated by brain MRI reported to date had brain white matter abnormalities [Spranger et al 1996, Kapferer-Seebacher et al 2019].
The MRI pattern was suggestive of an underlying small-vessel disease that is progressive with age. As observed in other leukoencephalopathies related to microangiopathies, the extent of the white matter changes was disproportionate to the paucity of neurologic features. Medical history revealed recurrent headaches or depression in single cases. Neurologic examination was unremarkable in all individuals except one who had mild cognitive decline, ataxia, and a history of one seizure.
Clinical manifestations in children. All but one child with pEDS reported to date were identified because of a positive family history. Generalized lack of attached gingiva is the only clinical finding that is consistently present in affected children younger than age ten years [Kapferer-Seebacher et al 2021].
Other clinical manifestations of pEDS are mild or absent in affected children, although the parents of all affected children reported easy bruising and gum bleeding. Median age of onset of periodontitis was reported to be 14 years.
Of note, a child with classic EDS, who also had periodontal bone loss in the primary dentition and a gingival phenotype specific for pEDS, had the familial COL5A1 pathogenic variant as well as a de novo
C1R pathogenic variant [Stock et al 2021].


![Figure 2. . Pretibial plaques have been described in 83% of individuals with periodontal EDS [Kapferer-Seebacher et al 2016].](/books/NBK572429/bin/eds-pd-Image002.gif)