The relative prevalence of causative organisms for genital ulcer disease varies considerably in different parts of the world and may change dramatically over time. Currently, however, HSV-2 and HSV-1 have become the commonest causative agents of genital ulcer disease in many parts of the world. The other causes frequently identified among people presenting with genital ulcer disease are T. pallidum (syphilis) and C. trachomatis serovars L1–L3, causing lymphogranuloma venereum and, less so, H. ducreyi (chancroid) (49,60,61).
Genital ulceration among people with primary syphilis occurs before serological laboratory tests become positive; thus, laboratory findings are rarely helpful at the initial visit and may even be misleading by being negative in the presence of syphilis infection. Further, in settings with a high prevalence of syphilis, a person with a genital ulcer may have a reactive serological test for syphilis from a previously treated infection, even if HSV-2 is the cause of the current ulcer.
In addition, since the differential diagnosis of genital ulcers using clinical judgement has been shown to be inaccurate in over 50% of cases, even by experienced clinicians, the management of people with genital ulcer disease must be based either on laboratory-based etiological studies or a syndromic approach, guided by periodic evaluation of the causative agents at the local setting.
10.1. Herpes simplex virus
10.1.1. Clinical presentation – symptoms
Although the observation of a cluster of vesicular lesions on the genital area or perianal area is usually used as clinically indicative of genital herpes, other causes of genital ulcers, such as syphilis and chancroid, may have a similar clinical appearance. Clinical manifestations and patterns of genital ulcer disease may also be further altered in the presence of HIV infection.
First-episode genital herpes infections are those in which the person does not have a previous history of genital herpes, and they are often associated with systemic and local symptoms of fever, headache, malaise and myalgia, usually in the first 3–4 days. Locally, there may be pain, itching, dysuria, vaginal or urethral discharge and tender inguinal lymphadenopathy. Among both men and women with primary genital HSV infection, the presentation is with blistering or ulcerative lesions on the external genitalia. The lesions may start as papules (pimples) or vesicles (blisters), which spread rapidly over the genital area. The lesions may last up to 15–20 days until crusting and/or healing. Crusting does not occur on mucosal surfaces.
The first episode can be primary genital herpes in which the person is seronegative for HSV antibodies, occurring after an incubation period of within 5–14 days of sexual contact. Initial episodes of genital herpes refer to individuals who have the lesions for the first time but already have antibodies to HSV-2, indicating past asymptomatic acquisition of HSV-2. Although this would be the person’s first recognized episode, it would not indicate recent acquisition.
Recurrent genital herpes tends to have more localized symptoms of itching, recurrent ulcers and mild pain, and the duration of the episode averages between four and five days but may be as long as up to 12–15 days.
10.1.2. Examination findings – signs
Among both men and women, a cluster of vesicopustular or ulcerative lesions is observed on the external genitalia (penis, urethral meatus, scrotum, pubic area and vulva) or on the anal and perianal areas (anus and buttocks). The patients may describe them as having started as papules or vesicles that spread rapidly. Multiple small vesicular lesions may coalesce into large ulcers. Most people with HSV-2 infection present at later stages of ulceration and hardly show the typical vesicles of early HSV-2 manifestation. However, when a person has a typical appearance of a crop of vesicles or gives a history of recurrent ulcers, a presumptive diagnosis of genital herpes can be made and treatment tailored appropriately.
Among immunosuppressed individuals, these ulcers may persist and continue to expand laterally and superficially for a considerable period of time if not treated. Since the ulceration of herpes is shallow (intraepidermal), residual scarring from these lesions is uncommon.
10.1.3. Molecular testing
Amplified molecular detection by PCR of HSV DNA from swabs of genital lesions is the most sensitive and specific test. Using a combined HSV and T. pallidum PCR, when available, would be of added benefit to implicate or exclude syphilis at the same time. PCR assays have also been developed for HSV-1 and HSV-2.
10.1.4. Culture methods
Culture enables replication of the virus for determining resistance to antiviral therapy and for confirming diagnosis, but results take about 2–4 days, and culture requires appropriate viral transport medium and special expertise to be a viable procedure. In expert hands, culture from vesicles has the highest yield of about 94% rather than from pustules or ulcer base. Crusted lesions give the lowest yield of about 27%.
10.1.5. Serology
Type-specific antibody tests can distinguish between HSV-1 and HSV-2. However, even immunoglobulin G (IgG)-based type-specific testing for HSV-1 and HSV-2 antibodies has limited value in diagnosis. The usefulness of testing is only by demonstrating seroconversion from a negative result at the time of the lesions to a positive result 6–12 weeks later. Although IgM detection can be used in diagnosing a new herpes infection, as many as 35% of the people with recurrent herpes episodes have IgM responses. IgM is therefore a poor marker of new infection and has limited diagnostic value (62).
10.2. Syphilis
Syphilis is a systemic disease caused by the spirochaete T. pallidum. The infection can be classified as congenital or acquired. Congenital syphilis is transmitted from mother to child in utero.
Acquired syphilis is divided into early and late syphilis. Early syphilis comprises the primary, secondary and early latent stages – syphilis of less than two years from acquisition of infection. Late syphilis refers to late latent syphilis, gummatous, nervous system and cardiovascular syphilis.
10.2.1. Clinical presentation – symptoms
Primary syphilis is characterized by an ulcer (syphilitic chancre) at the site of infection that develops after an incubation period of about three weeks from sexual contact but can range from nine to 90 days. The ulcers are usually single lesions and painless. The person with syphilis may miss them if they occur on concealed areas, such as the rectum, the cervix or the pharynx. If not treated, the ulcer will heal without scarring after some 2–10 weeks. The infection may then progress to the secondary stage.
Secondary syphilis sets in about six weeks to six months after infection. In some instances of secondary syphilis, especially among immunosuppressed individuals, the chancre may still be visible at the time secondary manifestation of syphilis occur. At this stage, the spirochaetes enter the blood stream and may cause systemic symptoms of fever, malaise, arthralgia and anorexia. If not treated at this stage, syphilis enters latency which might be followed by the tertiary stage of syphilis. A more detailed account of the natural history of syphilis is available (26).
Below follows a summary of clinical presentations of the different stages of syphilis and some guidance for diagnosis, followed by recommendations for a syndromic approach for managing people with syphilis and a summary of commonly used tests for diagnosing syphilis.
10.2.2. Examination findings – signs and laboratory diagnosis
10.2.2.1. Primary syphilis
Primary syphilis comprises one or more ulcerated lesions called the chancre of syphilis at the site of initial infection. The lesions are minimally tender or nontender and may have characteristic indurated edges with a clean base. Regional lymph nodes may be felt within the first week. The mouth and anus must also be examined for ulcers. Ulcers heal even without treatment in 2–10 weeks.
The following diagnostic tests are used:
- –
darkfield microscopy – syphilitic treponemes from lesions of primary syphilis observed (see section on microscopy); a negative dark-field result does not exclude syphilis;
- –
molecular detection – PCR testing can directly detect T. pallidum from lesion samples; and
- –
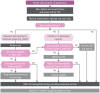
serology – both nontreponemal (such as RPR) and treponemal tests (such as TPHA and rapid syphilis strip test) are negative in the early phase of primary syphilis, taking 1–4 weeks after the chancre appears to become reactive (Fig. 7).
A negative RPR or rapid syphilis test therefore does not exclude syphilis at the primary syphilis stage. Tests should be repeated at four and 12 weeks from the initial testing to be certain if the person does not receive treatment.
10.2.2.2. Secondary syphilis
Secondary syphilis presents with signs of disseminated syphilis, about 3–6 weeks after infection, but this can be as long as six months. The manifestations may include any of the following:
- a generalized maculo-papular rash that is usually asymptomatic or mildly itchy and may also be seen on the palms and plantar surfaces of feet;
- patchy alopecia;
- generalized lymphadenopathy;
- condylomata lata – hypertrophic lesions resembling flat warts in the moist areas, such as the labia and perineum, the folds of the foreskin and around the anus that are teeming with spirochaetes and are therefore highly contagious; and
- painless shallow ulcers of the oral or genital mucous membranes (mucous patches) that are highly contagious.
The following diagnostic tests are used:
- –
dark-field microscopy – syphilitic treponemes can be observed from lesions of secondary syphilis, such as condylomata lata and mucous patches;
- –
molecular detection – T. pallidum can be detected by molecular methods from lesions of secondary syphilis; and
- –
serology – both nontreponemal (such as RPR) and treponemal tests (such as TPHA and a rapid syphilis test) are almost always reactive (positive) in secondary syphilis, and usually in high titre (Fig. 7).
A negative treponemal test at this stage of syphilis can reasonably be used to rule out syphilis. Rarely, some people may have such high levels of antibody that give a false-negative result with nontreponemal tests – a prozone phenomenon. Nontreponemal tests usually have high titres of 1:16 or greater at this stage of infection. Titres decline with adequate treatment and can be used to monitor response to treatment at three-monthly intervals for at least 1 year.
10.2.2.3. Early latent syphilis
As its name implies, latent syphilis has no clinical manifestations. The lesions of primary syphilis and those of secondary syphilis have resolved spontaneously and the infection goes into latency. Early latent syphilis is infection of less than two years in duration, as designated by WHO, based on the infectiousness of syphilis and its response to therapy during this stage of infection.
During the first two years (primary, secondary and early latent) of syphilis infection, the individual is infectious to the sex partner and there is a high risk of transmission to the fetus during pregnancy. In addition, nearly 25% of those with syphilis in the first year of infection will relapse and develop manifestations of secondary syphilis, termed mucocutaneous relapse (63).
10.2.2.4. Late latent syphilis
An infection of more than two years in duration without clinical evidence of treponemal infection is referred to as late latent syphilis. In late latency, the individual with untreated syphilis is less and less likely to transmit the infection to a sex partner and to the fetus during pregnancy as the latency progresses.
Late latent syphilis is diagnosed with serological tests. Nontreponemal and treponemal tests are mostly reactive (positive) in early latent and late latent syphilis, but nontreponemal tests, such as the VDRL, may become negative in late latent syphilis (Fig. 7). A negative treponemal test at this stage of infection can be taken as sufficient to rule out the diagnosis of syphilis. Once the specific treponemal tests are positive, they remain reactive for the person’s lifetime. Therefore, these tests cannot be used for monitoring the response to treatment.
Fig. 7 shows an overview of the reactivity of non-treponemal and treponemal serological tests for syphilis and the effect of successful treatment. Serological tests for syphilis give only a presumptive diagnosis of syphilis, and they must be interpreted together with a good sexual history of the individual, a physical examination and information about any recent treatments with antibiotics, especially for syphilis. A good medical history must also be obtained, since some underlying conditions may cause a false-positive reaction with non-treponemal tests, such as acute febrile illnesses, immunizations, pregnancy and autoimmune disorders, such as rheumatoid arthritis and systemic lupus erythematosus. Such false positives are usually at low titres of less than in 1:8 (64). If possible, positive non-treponemal tests (RPR or VDRL) should be quantified (the titres should be determined).
Non-treponemal tests may be negative in primary syphilis for 1–4 weeks after the appearance of the chancre (4–6 weeks after infection). The tests are reactive almost without exception in secondary syphilis. As the duration of the early and late latent stages of syphilis increases, the antibody titre decreases and may eventually give a negative result in late syphilis (late latent and tertiary stages), even without treatment. With treatment, syphilis serology tests may revert to negative depending on the stage of syphilis when treatment is instituted. This is more likely to happen if the individual is treated during the primary or secondary stage of syphilis. If early syphilis is treated, the non-treponemal test titres will decline and become negative and may thus be used to monitor response to treatment. If the disease is diagnosed at the late syphilis stage, low titres of non-treponemal tests may remain positive for life.

Fig. 7
Reactivity of serological tests by stage of syphilis and effect of treatment.
The specific treponemal tests, including TPHA, TPPA and fluorescent treponemal antibody absorption, may become positive earlier than the non-treponemal tests. Once an individual tests positive on a treponemal test, most (85%) remain positive on subsequent treponemal tests even when the infection is successfully treated.
The more recent rapid syphilis tests in circulation are immunochromatographic strips with treponemal antigens. These rapid syphilis tests are therefore equivalent to specific treponemal tests, such as the TPHA and TPPA, and the results produced by such tests should be interpreted as indicated in Fig. 7. A positive rapid syphilis test measures lifetime exposure to treponemal infection and not necessarily active disease that requires treatment. If an RPR-equivalent test is available, it should be performed following a positive rapid syphilis test to determine whether there may be active syphilis infection or not. More detailed guidelines on the use and interpretation of rapid syphilis tests are available (28). For details about the procedures for performing rapid syphilis tests and RPR tests, see the WHO manual (48).
Further, in areas of high syphilis prevalence, a reactive serological test for syphilis may reflect a previous infection and give a misleading picture of the person’s present condition. This is especially important in populations at higher risk of STIs, who may end up being unnecessarily treated repeatedly for syphilis. When available, an RPR test with titration may indicate which people need treatment since false-positive or sero-fast reactions usually have titres of less than 1:8.
10.3. H. ducreyi (chancroid)
10.3.1. Clinical presentation – symptoms
Lesions of chancroid begin as an erythematous papule within hours to days of sexual exposure. Over the following 1–2 days, the papule evolves into a pustule that breaks down and becomes a painful ulcer. People usually seek health care at the stage of the painful ulcer. Among men, the ulcers are usually on the penis (foreskin, shaft and sometimes on the glans), and as many as 50% develop unilateral or bilateral painful inguinal lymph nodes. Large, painful, fluctuant lymph nodes (buboes) may also occur. If not treated, buboes may suppurate and form fistulae or ulcers.
In women, ulcers of chancroid are on the vulva, and anal ulcers from autoinoculation may also occur. Ulcers among women may be asymptomatic, especially when they are internal. Women do not frequently present with inguinal adenopathy because of the different lymphatic drainage.
10.3.2. Examination findings
There are generally single or multiple ulcers on the penile shaft, the foreskin or the glans penis, usually deep with an irregular edge and a red margin. There is usually no induration, and the base is granular or purulent. The ulcers are normally tender when being examined or when walking. Men may have unilateral or bilateral inguinal buboes.
However, chancroid ulcers often have atypical clinical appearances and may dispel suspicion of chancroid, with some small ulcers mimicking infected genital herpes. In HIV infection, the ulcers may be less purulent and resemble syphilitic chancres. Also, people with immunosuppression may have rapidly aggressive and erosive ulcers of chancroid to the point of anatomically destroying the genital organs.
10.3.3. Laboratory diagnosis
H. ducreyi has been characteristically diagnosed by culture methods but only in limited specialized centres because of the fastidious nature of the organism with a sensitivity of 75% compared with M-PCR from genital ulcer swabs. Although multiplex PCR for genital ulcer disease, including H. ducreyi, has been developed, it is found only in research settings and reference centres (65,66). Since H. ducreyi has almost disappeared globally as a cause of genital ulcer disease (67,68), further advances in diagnostic tests for this pathogen are unlikely.
10.4. Recommendations for the management of genital ulcer disease, including anorectal ulcers
10.4.1. Evidence summary (Annex 6)
These recommendations were informed by evidence that was of high and moderate certainty from a systematic review of the sensitivity and specificity of using a clinical diagnosis of an STI pathogen based on the presence of an anogenital ulcer. No studies were found for clinical diagnosis of lymphogranuloma venereum (supplementary material – systematic review genital ulcer disease).
For detecting syphilis, which typically ranges from 5% to 10% as a cause of anogenital ulcers, if clinical diagnosis was used for 100 people with ulcers (sensitivity 64% and specificity 84%), about 2–4 cases would be missed, and 14–15 people would be falsely identified as having syphilis and unnecessarily treated. Alternatively, if all 100 people with an anogenital ulcer were treated for syphilis, 90–95 would be unnecessarily treated but no cases would be missed. Molecular assays could reduce the number of people unnecessarily treated or missed cases (since they are highly sensitive and specific), but the costs of diagnostics may be high and inaccessible. The WHO Guideline Development Group assessed the long-term consequences of a missed case of syphilis as more important than the number of people unnecessarily treated (false positives) and the cost of unnecessary treatment and therefore suggests treating all ulcers for syphilis when there is limited testing capacity. Other challenges of managing people with syphilis, such as with medications, are likely not linked to cost but to logistical support for procuring and distributing medications.
For detecting herpes, which typically ranges from 30% to 70% as a cause of anogenital ulcers, using a clinical diagnosis for 100 people with ulcers (sensitivity 40% and specificity 88%), about 18–42 cases of herpes would be missed and about 4–8 people with an ulcer would be identified falsely and be unnecessarily treated. Treating all 100 people with an anogenital ulcer for herpes would mean that 30–70 people would be unnecessarily treated, but the cost of treatment may be relatively inexpensive. The WHO Guideline Development Group agreed that it is uncertain that missing a case would lead to serious long-term harm. It likely contributes to increased HIV acquisition or HSV transmission and means discomfort for the people with symptoms (69). Treating everyone with a genital ulcer for herpes was therefore suggested for improving the quality of life when there is limited capacity for laboratory testing. The Guideline Development Group agreed that treating everyone is likely feasible, the costs of treatment would be negligible and be acceptable to all and would likely not have negatively affect equity (in some settings it may increase equitable access to treatment).
The systematic review of the literature found that the prevalence of chancroid has been decreasing in high-income and low- and middle-income countries alike and using a clinical diagnosis to determine treatment of chancroid therefore results in only a trivial number of missed cases and greater unnecessary treatment. Based on the current prevalence of chancroid, the Guideline Development Group therefore agreed to suggest no treatment for chancroid for people with anogenital ulcers, unless surveillance shows reported or emerging cases. Although no evidence was found for lymphogranuloma venereum and the cost-benefit and harm of clinical diagnosis, the Guideline Development Group agreed that performing tests for lymphogranuloma venereum would also depend on the number of emerging cases and suggested no treatment for lymphogranuloma venereum unless a test was positive.
10.5. Treatment of genital ulcer disease, including anorectal ulcers
Table 7 outlines treatment options for the syndromic management of people with genital ulcer disease. Syndromic management should include treatment for syphilis, unless the person has been treated for syphilis within the past three months, and treatment for herpes.
For persons with recurrent ulcers that are too frequent (such as 4–6 episodes or more a year) or with severe symptoms or causing distress, suppressive therapy may be proposed and preferred to episodic treatment (27). People receiving suppressive therapy may be assessed after one year and asked whether they want to continue or to change to episodic therapy. Note that recurrence rates may revert to the period before suppressive therapy started, and patients need to be aware of that.
For people living with HIV and immunosuppressed individuals, dose adjustments are recommended for valaciclovir and famciclovir but not for acyclovir.
- For recurrent episodes, valaciclovir 500 mg is recommended for five days instead of three days, and famciclovir is recommended at a dose of 500 mg twice daily for five days instead of 250 mg.
- For suppressive therapy, valaciclovir is recommended at 500 mg twice daily instead of once daily and famciclovir at 500 mg twice daily instead of 250 mg twice daily.
People who report allergies to penicillin should be treated with the effective alternatives for syphilis, which include doxycycline and erythromycin.
Table 7
Recommended treatment options for genital ulcer disease.
Publication Details
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization (http://www.wipo.int/amc/en/mediation/rules/).
Publisher
World Health Organization, Geneva
NLM Citation
Guidelines for the management of symptomatic sexually transmitted infections [Internet]. Geneva: World Health Organization; 2021 Jun. 10, GENITAL ULCER DISEASE SYNDROME.