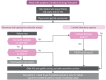
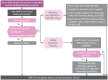
Urethral discharge among men is commonly caused by N. gonorrhoeae and/or C. trachomatis and/or non-gonococcal and non-chlamydial pathogens, such as M. genitalium and T. vaginalis. The prevalence of each of these pathogens varies geographically and by population group. Countries must conduct studies periodically in their settings to determine the most prevalent and important causes of urethral discharge or urethritis in that setting.
7.1. Clinical presentation – symptoms
Characteristically, men with urethritis (inflammation of the urethra) present with urethral discharge with or without dysuria (pain on urination). Occasionally, dysuria or itching at the tip of the urethra may be the only symptoms.
7.2. Examination findings – signs
Most men with urethritis have urethral discharge, which may range in quantity from being scanty to copious and in character from being clear to purulent. Distinguishing between discharge caused by gonorrhoea, chlamydia or any other cause of urethritis is not clinically possible.
7.3. Laboratory diagnosis
7.3.1. Molecular detection
NAAT is the current gold standard for detecting C. trachomatis and N. gonorrhoeae among men and women. NAAT also performs well for pharyngeal and anorectal samples for C. trachomatis and N. gonorrhoeae. For anorectal samples among men who have sex with men, chlamydia genovar testing for lymphogranuloma venereum should be done to guide the appropriate treatment regimen for lymphogranuloma venereum (48).
7.3.1.1. Specimens for N. gonorrhoeae and C. trachomatis for molecular assays
A first-catch urine or a urethral swab can be used for C. trachomatis and N. gonorrhoeae. NAAT for N. gonorrhoeae from anorectal and pharyngeal samples is also good, but there is potential for cross-reactivity with commensal Neisseria spp., especially in the throat.
7.3.1.2. Specimens for M. genitalium
M. genitalium causes urethritis. NAAT offers the best method for detecting M. genitalium from a first-catch urine in men. M. genitalium testing is not yet widely available.
7.3.1.3. Specimens for T. vaginalis
NAAT has the highest sensitivity of all diagnostic methods for detecting T. vaginalis. Urine can be used for some assays, but residual genital swab samples used for diagnosing chlamydia and gonorrhoea using NAAT are also good enough for detecting T. vaginalis nucleic acids.
7.3.2. Culture methods
Culture of N. gonorrhoeae is still the standard method for performing antimicrobial susceptibility testing. However, this organism is not that easy to grow in the laboratory, requiring special training and a special culture medium. For this reason, culture of N. gonorrhoeae is not routinely performed as part of managing people with gonococcal infection in resource-limited settings.
Culture of T. vaginalis was the cornerstone for detecting T. vaginalis before the advent of point-of-care antigen tests and NAAT. Although a culture medium is commercially available, once inoculated into the medium, cultures from men have to be incubated for a full five days while being examined daily using a microscope before being determined to be negative. Further, multiple sites, including semen, urine and urethral swabs, need to be examined before a definitive negative result can be certain. Routine culture methods for detecting T. vaginalis are no longer widely performed.
7.3.3. Microscopy
N. gonorrhoeae can be identified by light microscopy of Gram-stained samples and a presumptive diagnosis of gonorrhoea made if gram-negative diplococci are observed intracellularly in polymorphonuclear leukocytes, best seen when there is a urethral discharge. If carried out by an experienced person, a negative gram stain for intracellular diplococci, in the context of urethral discharge in a man, can be presumed to suggest non-gonococcal urethritis. Microscopy of methylene blue stain of a male urethral sample is an acceptable method for the presumptive diagnosis of gonorrhoea, but it does not allow for the differentiation of gram-negative cocci.
7.4. Recommendations for the management of urethral discharge
7.4.1. Evidence summary (Annex 3)
A systematic review of the accuracy of syndromic approaches for urethral discharge was conducted, including history, risk assessment, examination and microscopy (supplementary material: systematic review urethral discharge). Six studies were found for assessing the accuracy of syndromic management to detect N. gonorrhoeae and C. trachomatis, but the pooled sensitivity and specificity of the approaches did not improve as expected when adding microscopy (low-certainty evidence). In addition, studies show that there is variability in the implementation of the syndromic approaches based on symptoms or laboratory testing (49). Instead, the WHO Guideline Development Group considered that, when available, performing molecular assay tests for N. gonorrhoeae, C. trachomatis, T. vaginalis and/or M. genitalium and basing treatment on these results leads to treating the most people correctly. In the systematic review, the median prevalence of N. gonorrhoeae and/or C. trachomatis was 69% in men with urethral discharge. In a population with 60% prevalence of N. gonorrhoeae and C. trachomatis among those with urethral discharge, if molecular assays are not available, treating everyone for N. gonorrhoeae and C. trachomatis would mean 40% of them would be unnecessarily treated. The Guideline Development Group agreed that this proportion is acceptable and even higher proportions in settings with lower prevalence, because treating everyone would ensure that people infected with N. gonorrhoeae and C. trachomatis are treated, thereby reducing the chance of complications and further transmission. The Guideline Development Group also agreed that simple syndromic treatment based on the presence of urethral discharge would likely improve adherence to the approach and costs a minimal amount more than using history and/or risk assessment with or without examination (but with no missed cases).
7.5. Treatment recommendations for urethral discharge
Based on the recommendations in subsection 7.4, syndromic treatment for urethral discharge combines treatment for gonococcal and chlamydial infections. Other modifications can be made based on the availability of molecular diagnostic tests. Table 3 gives first-line and effective substitutes for treating people with urethral discharge syndrome.
Managing people with recurrent or persistent urethral discharge will require excluding reinfection by taking a thorough sexual history. When that has been done, additional treatment for M. genitalium and T. vaginalis may be considered. WHO guidelines on Neisseria gonorrhoeae (24) give guidance on how to approach apparent treatment failures among people with gonococcal infections.
Table 3
Recommended treatment options for urethral discharge syndrome.
Publication Details
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization (http://www.wipo.int/amc/en/mediation/rules/).
Publisher
World Health Organization, Geneva
NLM Citation
Guidelines for the management of symptomatic sexually transmitted infections [Internet]. Geneva: World Health Organization; 2021 Jun. 7, URETHRAL DISCHARGE SYNDROME.