NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Berkman ND, Chang E, Seibert J, et al. Management of High-Need, High-Cost Patients: A “Best Fit” Framework Synthesis, Realist Review, and Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Oct. (Comparative Effectiveness Review, No. 246.)

Management of High-Need, High-Cost Patients: A “Best Fit” Framework Synthesis, Realist Review, and Systematic Review [Internet].
Show detailsResults of Literature Searches
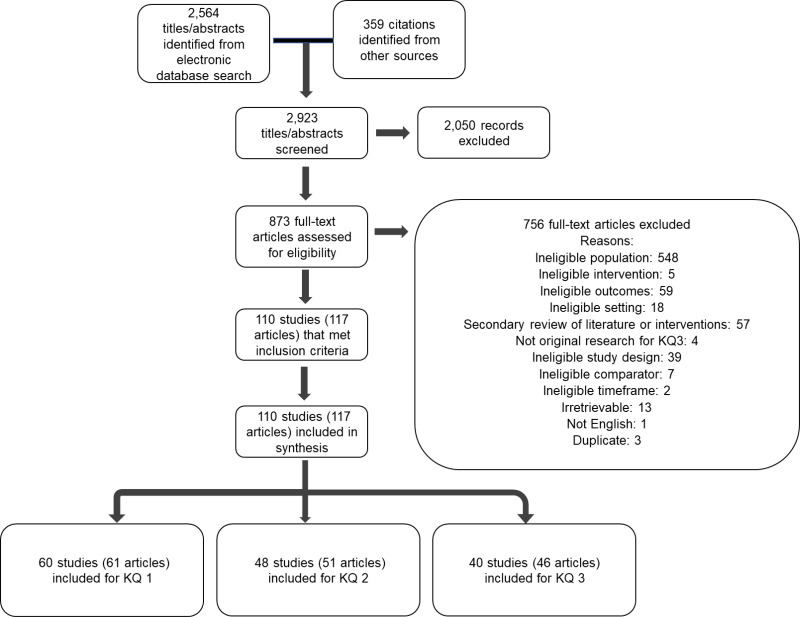
The electronic search, grey literature, and reference mining identified 2,923 citations. After title and abstract screening, 873 citations were retrieved for full-text review. A total of 110 studies (117 articles) met eligibility criteria. A total of 110 studies (117 articles) were included in the analyses.
Description of Included Studies
For KQ 1, we identified 60 studies (61 articles), of which 33 were cross sectional, 10 latent class, 11 predictive, and 6 qualitative.16–76
For KQ 2, we identified we identified 48 studies (51 articles).12, 17, 22, 27–29, 31, 49, 53, 55, 58, 61, 62, 77–114 As for unique KQ 2 includes, we identified 10 studies (10 articles).12, 78, 91, 93, 94, 104–106, 113, 114
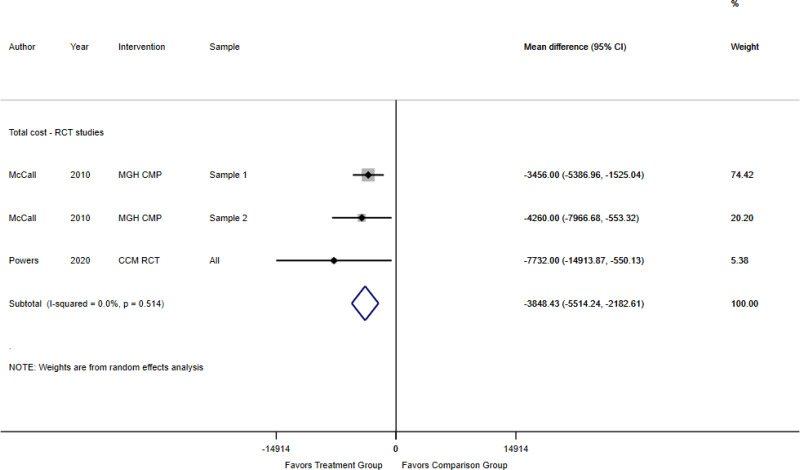
For KQ 3, we identified 19 trials and 21 observational studies (46 articles). Five RCTs were assessed as having low risk of bias, and 14 RCTs (15 articles) were assessed as having some concerns for bias,79–82, 84, 86, 87, 90, 96, 97, 99, 108–112, 115–118 No observational studies were assessed as having low risk of bias, 13 observational studies (17 articles) were assessed as having some concerns for bias, and eight observational studies (9 articles) were assessed as having high risk of bias.83, 85, 88, 92, 95, 98, 100–103, 107, 119–133
List of Excluded Studies
- X1: Ineligible Population
- X2: Ineligible Intervention
- X3: Ineligible Comparator
- X4: Ineligible Outcome
- X5: Ineligible Time Frame
- X6: Setting/Country
- X7: Secondary Review of Literature or Interventions
- X8: Not Original Research for KQ 3
- X9: Study Design
- X10: Not English
- X11: Irretrievable
- X12: Duplicate
- 1.
- Magellan initiative targets high utilizers in private plans. Mental Health Weekly. 2000;10(32):1. PMID: 3493535. Exclusion Code: X1.
- 2.
- Hand-held devices ease burden of behavioral health assessment. Dis Manag Advis. 2002;8(10):152–6, 45. Exclusion Code: X1. [PubMed: 12422804]
- 3.
- Medicaid best buys: improving care management for high-need, high-cost beneficiaries. Hamilton, N.J.: Center for Health Care Strategies, Inc.; 2008. Exclusion Code: X4.
- 4.
- Medicaid best buys: critical strategies to focus on high-need, high-cost beneficiaries. Hamilton, N.J.: Center for Health Care Strategies, Inc.; 2010. Exclusion Code: X1.
- 5.
- Building the national care service. Norwich: Great Britain Stationary Office; 2010. Exclusion Code: X1.
- 6.
- ED diversion: multidisciplinary approach engages high utilizers, helps them better navigate the health care system. ED management: the monthly update on emergency department management. 2011;23(11):127–30. Exclusion Code: X1. [PubMed: 22043590]
- 7.
- CM program keeps high utilizers out of hospital. Hosp Case Manag. 2012;20(7):108–9. PMID: 104470969. Language: English. Entry Date: 20120711. Revision Date: 20150711. Publication Type: Journal Article. Journal Subset: Nursing. Exclusion Code: X1. [PubMed: 22808541]
- 8.
- Hospitals collaborate to reduce ED overuse. Hosp Case Manag. 2012 Oct;20(10):151–3. PMID: 23084509. Exclusion Code: X1. [PubMed: 23091842]
- 9.
- Patient navigation for Medicaid frequent ED users. Yale University. 2013. https://www
.cochranelibrary .com/central/doi/10 .1002/central/CN-01579091/full. PMID: CN-01579091. Exclusion Code: X4. - 10.
- Identifying high utilizers of surgical care after colectomy. J Surg Res. 2014;186(2):495-. doi: 10.1016/j.jss.2013.11.036. PMID: 93484679. Exclusion Code: X1. [CrossRef]
- 11.
- Programs focusing on high-need, high-cost populations. [Trenton, N.J.]: Center for Health Care Strategies, Inc.; 2016. Exclusion Code: X1.
- 12.
- Understanding the needs of different types of high-need patients and their caregivers. Commonwealth Fund; 2019. Exclusion Code: X1.
- 13.
- Community Care For High-Need Patients. Health Aff (Millwood). 2019 Jun;38(6):892–3. doi: 10.1377/hlthaff.2019.00473. PMID: 31454082. Exclusion Code: X8. [PubMed: 31158014] [CrossRef]
- 14.
- Evaluation of a Multidisciplinary Care Coordination Program for Frequent Users of the Emergency Department. Prof Case Manag. 2019 Sep/Oct;24(5):E1–e2. doi: 10.1097/ncm.0000000000000388. PMID: 30593912. Exclusion Code: X1. [PubMed: 31369485] [CrossRef]
- 15.
- Extreme consumers of health care: patterns of care utilization in patients with multiple chronic conditions admitted to a novel integrated clinic. Journal of multidisciplinary healthcare. 2019;12:1075–83. doi: 10.2147/JMDH.S214770. PMID: CN-02136481. Exclusion Code: X6. [PMC free article: PMC6935286] [PubMed: 31920324] [CrossRef]
- 16.
- Assertive Community Treatment for Alcohol Misuse Disorder Patients Who Are High Utilizers of Emergency Department Services (ARFA). Khoo Teck Puat Hospital. Jun 25, 2020. Exclusion Code: X11.
- 17.
- Ackroyd-Stolarz S, Read Guernsey J, Mackinnon NJ, et al. The association between a prolonged stay in the emergency department and adverse events in older patients admitted to hospital: a retrospective cohort study. BMJ Qual Saf. 2011 Jul;20(7):564–9. doi: 10.1136/bmjqs.2009.034926. PMID: 21776300. Exclusion Code: X1. [PubMed: 21209130] [CrossRef]
- 18.
- Acosta AM, Lima MA. Frequent users of emergency services: associated factors and reasons for seeking care. Rev Lat Am Enfermagem. 2015 Feb–Apr;23(2):337–44. doi: 10.1590/0104-1169.0072.2560. PMID: 26240244. Exclusion Code: X1. [PMC free article: PMC4459009] [PubMed: 26039306] [CrossRef]
- 19.
- Adler-Milstein J. Assessing the impact of Medicare advantage on high-cost, high-need beneficiaries and exploring an intervention to improve outcomes. Michigan: Commonwealth Fund; 2017. Exclusion Code: X1.
- 20.
- Agarwal G, Lee J, McLeod B, et al. Social factors in frequent callers: a description of isolation, poverty and quality of life in those calling emergency medical services frequently. BMC Public Health. 2019 Jun 3;19(1):684. doi: 10.1186/s12889-019-6964-1. PMID: 31277151. Exclusion Code: X1. [PMC free article: PMC6547509] [PubMed: 31159766] [CrossRef]
- 21.
- Agarwal G, McDonough B, Angeles R, et al. Rationale and methods of a multicentre randomised controlled trial of the effectiveness of a Community Health Assessment Programme with Emergency Medical Services (CHAP-EMS) implemented on residents aged 55 years and older in subsidised seniors’ housing buildings in Ontario, Canada. BMJ Open. 2015 Jun 11;5(6):e008110. doi: 10.1136/bmjopen-2015-008110. PMID: 26206441. Exclusion Code: X1. [PMC free article: PMC4466604] [PubMed: 26068514] [CrossRef]
- 22.
- Agterberg J, Zhong F, Crabb R, et al. Cluster analysis application to identify groups of individuals with high health expenditures. Health Services & Outcomes Research Methodology. 2020;20(2/3):140–82. doi: 10.1007/s10742-020-00214-8. PMID: 145263707. Exclusion Code: X4. [CrossRef]
- 23.
- Aird P, Hansford P, O’Brien R, et al. The impact of frequent users of OOH services. Br J Gen Pract. 2001 Jun;51(467):494–5. PMID: 15094029. Exclusion Code: X1. [PMC free article: PMC1314038] [PubMed: 11407062]
- 24.
- al Yousif N, Hussain HY, El Din Mhakluf MM. Health care services utilization and satisfaction among elderly in Dubai, UAE and some associated determinants. Middle East Journal of Age & Ageing. 2014;11(3):25–33. PMID: 96513555. Exclusion Code: X1.
- 25.
- Alderwick H, Hood-Ronick CM, Gottlieb LM. Medicaid Investments To Address Social Needs In Oregon And California. Health Aff (Millwood). 2019 May;38(5):774–81. doi: 10.1377/hlthaff.2018.05171. PMID: 31059356. Exclusion Code: X1. [PubMed: 31059356] [CrossRef]
- 26.
- Alexander Billioux KV, Susan Anthony, Dawn Alley. Standardized Screening for Health-Related Social Needs in Clinical Settings The Accountable Health Communities Screening Tool. 2017. Exclusion Code: X2.
- 27.
- Alghanim SA, Alomar BA. Frequent use of emergency departments in Saudi public hospitals: implications for primary health care services. Asia Pac J Public Health. 2015 Mar;27(2):Np2521–30. doi: 10.1177/1010539511431603. PMID: 25547107. Exclusion Code: X1. [PubMed: 22186384] [CrossRef]
- 28.
- Allen CG, Escoffery C, Satsangi A, et al. Strategies to improve the integration of community health workers into health care teams: “a little fish in a big pond”. Prev Chronic Dis. 2015 Sep 17;12:E154. doi: 10.5888/pcd12.150199. PMID: 26875022. Exclusion Code: X1. [PMC free article: PMC4576500] [PubMed: 26378900] [CrossRef]
- 29.
- Althaus F, Paroz S, Hugli O, et al. Effectiveness of interventions targeting frequent users of emergency departments: a systematic review. Ann Emerg Med. 2011 Jul;58(1):41–52 e42. doi: 10.1016/j.annemergmed.2011.03.007. PMID: 21689565. Exclusion Code: X1. [PubMed: 21689565] [CrossRef]
- 30.
- Althaus F, Stucki S, Guyot S, et al. Characteristics of highly frequent users of a Swiss academic emergency department: a retrospective consecutive case series. Eur J Emerg Med. 2013 Dec;20(6):413–9. doi: 10.1097/MEJ.0b013e32835e078e. PMID: 25139183. Exclusion Code: X1. [PubMed: 23337095] [CrossRef]
- 31.
- Altman D. A small group of patients account for a whole lot of spending. Axios. 2019. https://www
.axios.com /drug-prices-health-care-costs-spending-employers-63a65abc-0148-4f98-bd39-b30e4d3c9caf.html. Exclusion Code: X7. - 32.
- Alvarado LP. Mobile psychiatric services decrease readmission rates among high utilizers of emergency treatment services: ProQuest Information & Learning; 2021. Exclusion Code: X3.
- 33.
- Amarasingham R, Xie B, Karam A, et al. Using community partnerships to integrate health and social services for high-need, high-cost patients. Commonwealth Fund. 2018. https://www
.commonwealthfund .org/publications /issue-briefs/2018 /jan/using-community-partnerships-integrate-health-and-social. Exclusion Code: X1. [PubMed: 29320140] - 34.
- Anderson DR, Mangen DJ, Grossmeier JJ, et al. Comparing alternative methods of targeting potential high-cost individuals for chronic condition management. J Occup Environ Med. 2010 Jun;52(6):635–46. doi: 10.1097/JOM.0b013e3181e31792. PMID: 15175823. Exclusion Code: X9. [PubMed: 20523235] [CrossRef]
- 35.
- Anderson GM. Program delivery for high-needs, high-cost populations: international perspectives on the way forward, phase I. University of Toronto, Faculty of Medicine, Institute of Health Policy, Management and Evaluation 2017. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191229. Exclusion Code: X7. - 36.
- Andrews G, Sunderland M. Telephone case management reduces both distress and psychiatric hospitalization. Aust N Z J Psychiatry. 2009 Sep;43(9):809–11. doi: 10.1080/00048670903107617. PMID: 17515738. Exclusion Code: X1. [PubMed: 19670053] [CrossRef]
- 37.
- Angelelli J, Gifford D, Intrator O, et al. Access to postacute nursing home care before and after the BBA (Balanced Budget Act). Health Aff (Millwood). 2002 Sep–Oct;21(5):254–64. doi: 10.1377/hlthaff.21.5.254. PMID: 12450957. Exclusion Code: X1. [PubMed: 12224890] [CrossRef]
- 38.
- Armstrong G, De Marchis E, Mix R, et al. Using Social Needs Screening and Patient Feedback for Complex Care. National Center for Complex Health and Social Needs; 2020. alcomplex
.care/research-policy /resources /webinars/using-social-needs-screening-and-patient-feedback-for-complex-care /2020. Exclusion Code: X1. - 39.
- Avila J, Jupiter D, Chavez-MacGregor M, et al. High-Cost Hospitalizations Among Elderly Patients With Cancer. J Oncol Pract. 2019 May;15(5):e447–e57. doi: 10.1200/jop.18.00706. PMID: 30354887. Exclusion Code: X1. [PubMed: 30946640] [CrossRef]
- 40.
- Azogil-López LM, Pérez-Lázaro JJ, Ávila-Pecci P, et al. Effectiveness of a new model of telephone derivation shared between primary care and hospital care. Atencion primaria / Sociedad Espanola de Medicina de Familia y Comunitaria. 2019;51(5):278–84. doi: 10.1016/j.aprim.2018.02.006. PMID: CN-02133656. Exclusion Code: X10. [PMC free article: PMC6836997] [PubMed: 29699717] [CrossRef]
- 41.
- Bagnall AM, South J, Forshaw MJ, et al. Self-care in primary care: findings from a longitudinal comparison study. Prim Health Care Res Dev. 2013 Jan;14(1):29–39. doi: 10.1017/s1463423612000199. PMID: 24568286. Exclusion Code: X1. [PubMed: 22717510] [CrossRef]
- 42.
- Bailey JE, Surbhi S, Wan JY, et al. Effect of Intensive Interdisciplinary Transitional Care for High-Need, High-Cost Patients on Quality, Outcomes, and Costs: a Quasi-Experimental Study. J Gen Intern Med. 2019 Sep;34(9):1815–24. doi: 10.1007/s11606-019-05082-8. PMID: 32245460. Exclusion Code: X1. [PMC free article: PMC6712187] [PubMed: 31270786] [CrossRef]
- 43.
- Baker JM, Grant RW, Gopalan A. A systematic review of care management interventions targeting multimorbidity and high care utilization. BMC Health Serv Res. 2018 Jan 30;18(1):65. doi: 10.1186/s12913-018-2881-8. PMID: 29382327. Exclusion Code: X1. [PMC free article: PMC5791200] [PubMed: 29382327] [CrossRef]
- 44.
- Balaban RB, Zhang F, Vialle-Valentin CE, et al. Impact of a Patient Navigator Program on Hospital-Based and Outpatient Utilization Over 180 Days in a Safety-Net Health System. J Gen Intern Med. 2017 Sep;32(9):981–9. doi: 10.1007/s11606-017-4074-2. PMID: 28523476. Exclusion Code: X1. [PMC free article: PMC5570741] [PubMed: 28523476] [CrossRef]
- 45.
- Balfour ME, Zinn TE, Cason K, et al. Provider-payer partnerships as an engine for continuous quality improvement. Psychiatr Serv. 2018;69(6):623–5. doi: 10.1176/appi.ps.201700533. PMID: 129890973. Language: English. Entry Date: In Process. Revision Date: 20180609. Publication Type: Article. Journal Subset: Biomedical. Exclusion Code: X1. [PubMed: 29493418] [CrossRef]
- 46.
- Barker SL, Maguire NJ, Das S, et al. Values-Based Interventions in Patient Engagement for Those with Complex Needs. Popul Health Manag. 2020;23(2):140–5. doi: 10.1089/pop.2019.0084. PMID: 142272277. Language: English. Entry Date: 20200320. Revision Date: 20200324. Publication Type: Article. Exclusion Code: X1. [PubMed: 31503526] [CrossRef]
- 47.
- Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28(11):1396–404. doi: 10.1007/s11606-013-2392-6. PMID: CN-00909578. Exclusion Code: X1. [PMC free article: PMC3797340] [PubMed: 23494213] [CrossRef]
- 48.
- Bauman CA, Fillingham J, Keely-Dyck E, et al. An approach to interprofessional management of complex patients: a case report. Journal of the Canadian Chiropractic Association. 2019;63(2):119–25. PMID: 138594442. Language: English. Entry Date: 20190916. Revision Date: 20190926. Publication Type: Article. Exclusion Code: X6. [PMC free article: PMC6743649] [PubMed: 31564750]
- 49.
- Bayliss EA, Ellis JL, Powers JD, et al. Using self-reported data to segment older adult populations with complex care needs. EGEMS (Wash DC). 2019 Apr 12;7(1):12. doi: 10.5334/egems.275. PMID: 31065556. Exclusion Code: X1. [PMC free article: PMC6484372] [PubMed: 31065556] [CrossRef]
- 50.
- Beach SR, Schulz R, Friedman EM, et al. Adverse Consequences of Unmet Needs for Care in High-Need/High-Cost Older Adults. J Gerontol B Psychol Sci Soc Sci. 2020 Jan 14;75(2):459–70. doi: 10.1093/geronb/gby021. PMID: 30522388. Exclusion Code: X3. [PubMed: 29471360] [CrossRef]
- 51.
- Becker T, Steffen S, Puschner B. Discharge planning, an intervention study - and where to go next? Eur Psychiatry. 2011;26. doi: 10.1016/S0924-9338(11)73744-0. PMID: CN-01782093. Exclusion Code: X1. [CrossRef]
- 52.
- Bedoya P, Neuhausen K, Dow AW, et al. Student Hotspotting: Teaching the Interprofessional Care of Complex Patients. Acad Med. 2018 Jan;93(1):56–9. doi: 10.1097/acm.0000000000001822. PMID: 28700461. Exclusion Code: X1. [PubMed: 28700461] [CrossRef]
- 53.
- Beesley VL, Smithers BM, O’Rourke P, et al. Variations in supportive care needs of patients after diagnosis of localised cutaneous melanoma: a 2-year follow-up study. Support Care Cancer. 2017 Jan;25(1):93–102. doi: 10.1007/s00520-016-3378-9. PMID: 26389982. Exclusion Code: X1. [PubMed: 27562298] [CrossRef]
- 54.
- Beima-Sofie K, Begnel ER, Golden MR, et al. “It’s Me as a Person, Not Me the Disease”: Patient Perceptions of an HIV Care Model Designed to Engage Persons with Complex Needs. AIDS Patient Care STDS. 2020 Jun;34(6):267–74. doi: 10.1089/apc.2019.0310. PMID: 31670693. Exclusion Code: X1. [PMC free article: PMC7262637] [PubMed: 32484744] [CrossRef]
- 55.
- Bélanger E, McHugh J, Meyers DJ, et al. Characteristics of Top-Performing Hospitals Caring for High-Need Medicare Beneficiaries. Popul Health Manag. 2020;23(4):313–8. doi: 10.1089/pop.2019.0145. PMID: 144824249. Language: English. Entry Date: 20200813. Revision Date: 20200813. Publication Type: Article. Exclusion Code: X1. [PubMed: 31816254] [CrossRef]
- 56.
- Bellon JA, Rodriguez-Bayon A, Luna JD, et al. Successful GP intervention with frequent attenders in primary care: randomised controlled trial. Br J Gen Pract. 2008 May;58(550):324–30. doi: 10.3399/bjgp08X280182. PMID: WOS:000256654700006. Exclusion Code: X1. [PMC free article: PMC2435670] [PubMed: 18482486] [CrossRef]
- 57.
- Bergenstal TD, Reitsema J, Heppner P, et al. Personalized Care Plans: Are They Effective in Decreasing ED Visits and Health Care Expenditure Among Adult Super-Utilizers? J Emerg Nurs. 2020 Jan;46(1):83–90. doi: 10.1016/j.jen.2019.09.001. PMID: 32750132. Exclusion Code: X1. [PubMed: 31685338] [CrossRef]
- 58.
- Bergeron P, Courteau J, Vanasse A. Proximity and emergency department use: multilevel analysis using administrative data from patients with cardiovascular risk factors. Can Fam Physician. 2015 Aug;61(8):e391–7. PMID: 26747456. Exclusion Code: X1. [PMC free article: PMC4541449] [PubMed: 26505061]
- 59.
- Bergh H, Marklund B. Characteristics of frequent attenders in different age and sex groups in primary health care. Scand J Prim Health Care. 2003 Sep;21(3):171–7. doi: 10.1080/02813430310001149. PMID: WOS:000185314100010. Exclusion Code: X1. [PubMed: 14531510] [CrossRef]
- 60.
- Berghofer A, Hartwich A, Bauer M, et al. Efficacy of a systematic depression management program in high utilizers of primary care: a randomized trial. BMC Health Serv Res. 2012 Sep 3;12:298. doi: 10.1186/1472-6963-12-298. PMID: 25527472. Exclusion Code: X1. [PMC free article: PMC3489593] [PubMed: 22943609] [CrossRef]
- 61.
- Berghöfer A, Roll S, Bauer M, et al. Screening for depression and high utilization of health care resources among patients in primary care. Community Ment Health J. 2014;50(7):753–8. doi: 10.1007/s10597-014-9700-4. PMID: 103896118. Language: English. Entry Date: 20140924. Revision Date: 20151001. Publication Type: Journal Article. Exclusion Code: X1. [PubMed: 24449430] [CrossRef]
- 62.
- Berkowitz SA. Capsule Commentary on Schickedanz et al., Impact of Social Needs Navigation on Utilization Among High-Utilizers in a Large Integrated Health System: a Quasi-Experimental Study. JGIM: Journal of General Internal Medicine. 2019;34(11):2582-. doi: 10.1007/s11606-019-05296-w. PMID: 139599661. Exclusion Code: X8. [PMC free article: PMC6848514] [PubMed: 31452028] [CrossRef]
- 63.
- Bertoli-Avella AM, Haagsma JA, Van Tiel S, et al. Frequent users of the emergency department services in the largest academic hospital in the Netherlands: a 5-year report. Eur J Emerg Med. 2017 Apr;24(2):130–5. doi: 10.1097/mej.0000000000000314. PMID: 28863030. Exclusion Code: X1. [PubMed: 26287805] [CrossRef]
- 64.
- Bieler G, Paroz S, Faouzi M, et al. Social and medical vulnerability factors of emergency department frequent users in a universal health insurance system. Acad Emerg Med. 2012 Jan;19(1):63–8. doi: 10.1111/j.1553-2712.2011.01246.x. PMID: 21701913. Exclusion Code: X1. [PubMed: 22221292] [CrossRef]
- 65.
- Bilazarian A. High-need high-cost patients: A Concept Analysis. Nurs Forum. 2020. doi: 10.1111/nuf.12500. Exclusion Code: X1. [PubMed: 32851669] [CrossRef]
- 66.
- Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008 Sep;94(9):1194–9. doi: 10.1136/hrt.2007.122671. PMID: 17610000. Exclusion Code: X1. [PubMed: 17646191] [CrossRef]
- 67.
- Bischoff RJ, Hollist CS, Patterson J, et al. Providers’ perspectives on troublesome overusers of medical services. Families, Systems, & Health. 2007;25(4):392–403. doi: 10.1037/1091-7527.25.4.392. PMID: 2007-19994-004. Exclusion Code: X1. [CrossRef]
- 68.
- Blair M. Organizations provide $8.7 million to launch a national center to improve care for complex patients. Robert Wood Johnson Foundation. 2016. https://www
.rwjf.org /en/library/articles-and-news /2016/03/organizations-provide-8-7-million-to-launch-national-center .html. Exclusion Code: X7. - 69.
- Blakey R, Court G, Peaker A, et al. High service users: does the clinical psychologist have a role? Health Bull (Edinb). 2000 May;58(3):203–9. PMID: 11860388. Exclusion Code: X1. [PubMed: 12813826]
- 70.
- Blank FS, Li H, Henneman PL, et al. A descriptive study of heavy emergency department users at an academic emergency department reveals heavy ED users have better access to care than average users. J Emerg Nurs. 2005 Apr;31(2):139–44. doi: 10.1016/j.jen.2005.02.008. PMID: 15047073. Exclusion Code: X4. [PubMed: 15834378] [CrossRef]
- 71.
- Bledsoe SE. Adult health care services and psychiatric disorders: the use and costs of health care services in a low income, minority population coming to an urban primary care clinic: ProQuest Information & Learning; 2007. Exclusion Code: X1.
- 72.
- Bleich SN, Sherrod C, Chiang A, et al. Systematic review of programs treating high-need and high-cost people with multiple chronic diseases or disabilities in the United States, 2008–2014. Prev Chronic Dis. 2015 Nov 12;12:E197. doi: 10.5888/pcd12.150275. Exclusion Code: X7. [PMC free article: PMC4651160] [PubMed: 26564013] [CrossRef]
- 73.
- Blumenthal D, Abrams MK. Tailoring complex care management for high-need, high-cost patients. JAMA. 2016 Oct 25;316(16):1657–8. doi: 10.1001/jama.2016.12388. Exclusion Code: X1. [PubMed: 27669168] [CrossRef]
- 74.
- Blumenthal D, Chernof B, Fulmer T, et al. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016 Sep 8;375(10):909–11. doi: 10.1056/NEJMp1608511. Exclusion Code: X1. [PubMed: 27602661] [CrossRef]
- 75.
- Bobo WV, Hoge CW, Messina MA, et al. Characteristics of repeat users of an inpatient psychiatry service at a large military tertiary care hospital. Mil Med. 2004 Aug;169(8):648–53. doi: 10.7205/milmed.169.8.648. PMID: 15576522. Exclusion Code: X1. [PubMed: 15379078] [CrossRef]
- 76.
- Bodenmann P, Baggio S, Iglesias K, et al. Characterizing the vulnerability of frequent emergency department users by applying a conceptual framework: a controlled, cross-sectional study. Int J Equity Health. 2015 Dec 9;14:146. doi: 10.1186/s12939-015-0277-5. PMID: 27142996. Exclusion Code: X1. [PMC free article: PMC4673736] [PubMed: 26645272] [CrossRef]
- 77.
- Bodenmann P, Velonaki VS, Griffin JL, et al. Case management may reduce emergency department frequent use in a universal health coverage system: a randomized controlled trial. J Gen Intern Med. 2017 May;32(5):508–15. doi: 10.1007/s11606-016-3789-9. PMID: 27578689. Exclusion Code: X1. [PMC free article: PMC5400747] [PubMed: 27400922] [CrossRef]
- 78.
- Boehmer KR, Abu Dabrh AM, Gionfriddo MR, et al. Does the chronic care model meet the emerging needs of people living with multimorbidity? A systematic review and thematic synthesis. PLoS One. 2018;13(2):e0190852. doi: 10.1371/journal.pone.0190852. PMID: 29420543. Exclusion Code: X7. [PMC free article: PMC5805171] [PubMed: 29420543] [CrossRef]
- 79.
- Boling PA, Leff B. Comprehensive longitudinal health care in the home for high-cost beneficiaries: a critical strategy for population health management. J Am Geriatr Soc. 2014 Oct;62(10):1974–6. doi: 10.1111/jgs.13049. PMID: 25960393. Exclusion Code: X9. [PubMed: 25294407] [CrossRef]
- 80.
- Bornstein D. The 5 percent: solutions reporting on care for the nation’s sickest. Solutions Journalism Network, Inc.: Commonwealth Fund; 2016. Exclusion Code: X7.
- 81.
- Bradley EH. Identifying effective strategies for coordinating health care and social services for high-need, high-cost patients. Yale University, Yale School of Public Health 2015. https://www
.commonwealthfund .org/grants/identifying-effective-strategies-coordinating-health-care-and-social-services-high-need-high. Exclusion Code: X7. - 82.
- Brannon E, Wang T, Lapedis J, et al. Towards a learning health system to reduce emergency department visits at a population level. AMIA Symposium; 2018. Annual Symposium proceedings; 2018. pp. 295–304. Exclusion Code: X1. [PMC free article: PMC6371247] [PubMed: 30815068]
- 83.
- Braveman P, Dekker M, Egerter S, et al. How does housing affect health? An examination of the many ways in which housing can influence health and strategies to improve health through emphasis on healthier homes. 2011. Exclusion Code: X1.
- 84.
- Breland JY, Chee CP, Zulman DM. Racial differences in chronic conditions and sociodemographic characteristics among high-utilizing veterans. J Racial Ethn Health Disparities. 2015 Jun;2(2):167–75. doi: 10.1007/s40615-014-0060-0. PMID: 26147126. Exclusion Code: X4. [PMC free article: PMC6200449] [PubMed: 26863335] [CrossRef]
- 85.
- Brenner JC. Jeffrey C Brenner: on driving down the cost of care. Healthc Financ Manage. 2013 Jan;67(1):72–5. PMID: 23360057. Exclusion Code: X1. [PubMed: 23360057]
- 86.
- Brewster AL, Brault MA, Tan AX, et al. Patterns of collaboration among health care and social services providers in communities with lower health care utilization and costs. Health Serv Res. 2018 Aug;53 Suppl 1:2892–909. doi: 10.1111/1475-6773.12775. Exclusion Code: X1. [PMC free article: PMC6056597] [PubMed: 28925041] [CrossRef]
- 87.
- Brian W. Powers SP, Nupur Mehta. Impact of Complex Care Management on Spending and Utilization for High-Need, High-Cost Medicaid Patients. The American Journal of Managed Care. 2020. Exclusion Code: X12. [PubMed: 32059101]
- 88.
- Bronsky ES, McGraw C, Johnson R, et al. CARES: a community-wide collaboration identifies super-utilizers and reduces their 9-1-1 call, emergency department, and hospital visit rates. Prehosp Emerg Care. 2017 Nov–Dec;21(6):693–9. doi: 10.1080/10903127.2017.1335820. PMID: 25469750. Exclusion Code: X1. [PubMed: 28657819] [CrossRef]
- 89.
- Brooks EM, Winship JM, Kuzel AJ. A “Behind-the-Scenes” Look at Interprofessional Care Coordination: How Person-Centered Care in Safety-Net Health System Complex Care Clinics Produce Better Outcomes. International Journal of Integrated Care. 2020 Apr–Jun;20(2):10. doi: 10.5334/ijic.4734. PMID: WOS:000550985100007. Exclusion Code: X1. [PMC free article: PMC7207252] [PubMed: 32405282] [CrossRef]
- 90.
- Brown AF, Behforouz H, Shah A, et al. The care connections program: A randomized trial of community health workers to improve care for medically and socially complex patients. J Gen Intern Med. 2020;35(SUPPL 1):S288. doi: 10.1007/s11606-020-05890-3. Exclusion Code: X1. [CrossRef]
- 91.
- Brown KE, Fiellin DA, Chawarski MC, et al. A randomized trial of primary intensive care to reduce hospital admissions in high utilizers. J Gen Intern Med. 2002 Apr;17:121-. PMID: WOS:000175158200432. Exclusion Code: X1.
- 92.
- Brown KE, Levine JM, Fiellin DA, et al. Primary intensive care: pilot study of a primary care-based intervention for high-utilizing patients. Dis Manag. 2005 Jun;8(3):169–77. doi: 10.1089/dis.2005.8.169. PMID: 16359548. Exclusion Code: X1. [PubMed: 15966782] [CrossRef]
- 93.
- Broyles J. Strategies to promulgate advanced illness care models that work. Coalition to Transform Advanced Care. Washington, DC: 2013. Exclusion Code: X1.
- 94.
- Bryk J, Fischer GS, Lyons A, et al. Improvement in quality metrics by the UPMC enhanced care program: a novel super-utilizer program. Popul Health Manag. 2018 Jun;21(3):217–21. doi: 10.1089/pop.2017.0064. PMID: 27612609. Exclusion Code: X1. [PubMed: 28945512] [CrossRef]
- 95.
- Buchanan D, Doblin B, Sai T, et al. The effects of respite care for homeless patients: a cohort study. Am J Public Health. 2006 Jul;96(7):1278–81. doi: 10.2105/ajph.2005.067850. PMID: 16735635. Exclusion Code: X1. [PMC free article: PMC1483848] [PubMed: 16735635] [CrossRef]
- 96.
- Buck JA, Teich JL, Miller K. Use of mental health and substance abuse services among high-cost Medicaid enrollees. Adm Policy Ment Health. 2003 Sep;31(1):3–14. doi: 10.1023/a:1026089422101. PMID: 26850904. Exclusion Code: X9. [PubMed: 14650645] [CrossRef]
- 97.
- Buja A, Claus M, Perin L, et al. Multimorbidity patterns in high-need, high-cost elderly patients. PLoS One. 2018;13(12):e0208875. doi: 10.1371/journal.pone.0208875. PMID: 29560049. Exclusion Code: X1. [PMC free article: PMC6296662] [PubMed: 30557384] [CrossRef]
- 98.
- Buja A, Rivera M, De Battisti E, et al. Multimorbidity and Hospital Admissions in High-Need, High-Cost Elderly Patients. J Aging Health. 2020 Jun/Jul;32(5–6):259–68. doi: 10.1177/0898264318817091. PMID: 31682499. Exclusion Code: X6. [PubMed: 30522388] [CrossRef]
- 99.
- Burns C, Wang NE, Goldstein BA, et al. Characterization of young adult emergency department users: evidence to guide policy. J Adolesc Health. 2016 Dec;59(6):654–61. doi: 10.1016/j.jadohealth.2016.07.011. PMID: 27605647. Exclusion Code: X4. [PubMed: 27613220] [CrossRef]
- 100.
- Burns J. Treating Super Utilizers in Rural Pennsylvania. Robert Wood Johnson Foundation. Princeton, NJ: 2013. https://www
.rwjf.org /en/library/articles-and-news /2013/09/treating-superusers-in-rural-pennsylvania .html. Exclusion Code: X1. - 101.
- Burns J. Cleveland Medical Center aims to improve care for super utilizers. Robert Wood Johnson Foundation. Princeton, NJ: 2013. https://www
.rwjf.org /en/library/articles-and-news /2013/09/cleveland-improves-care-for-super-utilizers.html. Exclusion Code: X1. - 102.
- Bush H. Caring for the costliest. (Cover story). H&HN: Hospitals & Health Networks. 2012;86(11):24–9. PMID: 83779325. Exclusion Code: X1. [PubMed: 23214040]
- 103.
- Bush H. Tackling the high cost of chronic disease. Hosp Health Netw. 2012 Oct;86(10):34–6, 8, 40. PMID: 26454353. Exclusion Code: X1. [PubMed: 23163172]
- 104.
- Bynum JPW. Measuring what matters most to people with complex needs. Health Aff (Millwood). Bethesda, MD: Health Affairs; 2018. Exclusion Code: X1.
- 105.
- Bynum JPW, Austin A, Carmichael D, et al. High-Cost Dual Eligibles’ Service Use Demonstrates The Need For Supportive And Palliative Models Of Care. Health Aff (Millwood). 2017 Jul 1;36(7):1309–17. doi: 10.1377/hlthaff.2017.0157. PMID: 31029256. Exclusion Code: X1. [PMC free article: PMC5633373] [PubMed: 28679819] [CrossRef]
- 106.
- California Health Care Foundation. Data symposium and webinar on high utilizers of Medi-Cal Services. Oakland, CA: California Health Care Foundation; 2015. https://www
.chcf.org /event/data-symposium-and-webinar-on-high-utilizers-of-medi-cal-services/2019. Exclusion Code: X1. - 107.
- California Health Care Foundation. Integrated clinics for high utilizers conference. Oakland, CA: California Health Care Foundation; 2015. https://www
.chcf.org /event/integrated-clinics-for-high-utilizers-conference/2019. Exclusion Code: X1. - 108.
- California Health Care Foundation. Care integration for patients with complex needs. California Health Care Foundation. Oakland, CA: 2017. https://www
.chcf.org /project/care-integration-for-patients-with-complex-needs/. Exclusion Code: X1. - 109.
- California Health Care Foundation. CIN Webinar Series — The ROI for addressing social needs in health care (#2). Oakland, CA: California Health Care Foundation; 2018. https://www
.chcf.org /event/cin-webinar-series-roi-addressing-social-needs-health-care-2/2019. Exclusion Code: X1. - 110.
- Capp R, Rosenthal MS, Desai MM, et al. Characteristics of Medicaid enrollees with frequent ED use. Am J Emerg Med. 2013 Sep;31(9):1333–7. doi: 10.1016/j.ajem.2013.05.050. PMID: 23850143. Exclusion Code: X4. [PubMed: 23850143] [CrossRef]
- 111.
- Capp R, Zane R. Using emergency department community health workers as a bridge to ongoing care for frequent ED users. Value and Quality Innovations in Acute and Emergency Care. 2017:209–14. Exclusion Code: X1.
- 112.
- Carney KJL. PRACTITIONER APPLICATION: An Evaluation of Interprofessional Patient Navigation Services in High Utilizers at a County Tertiary Teaching Health System. J Healthc Manag. 2020 Jan–Feb;65(1):71–2. doi: 10.1097/jhm-d-19-00237. PMID: WOS:000561759100012. Exclusion Code: X8. [PubMed: 31913242] [CrossRef]
- 113.
- Carpiac-Claver M, Guzman JS, Castle SC. The Comprehensive Care Clinic. Health Soc Work. 2007 Aug;32(3):219–23. doi: 10.1093/hsw/32.3.219. PMID: 17896679. Exclusion Code: X1. [PubMed: 17896679] [CrossRef]
- 114.
- Carroll CP, Carlton Jr H, Fagan P, et al. The course and correlates of high hospital utilization in sickle cell disease: evidence from a large, urban Medicaid managed care organization. Am J Hematol. 2009;84(10):666–70. doi: 10.1002/ajh.21515. Exclusion Code: X1. [PMC free article: PMC2783233] [PubMed: 19743465] [CrossRef]
- 115.
- Castillo EM, Brennan JJ, Killeen JP, et al. Identifying frequent users of emergency department resources. J Emerg Med. 2014 Sep;47(3):343–7. doi: 10.1016/j.jemermed.2014.03.014. PMID: 27604932. Exclusion Code: X4. [PubMed: 24813059] [CrossRef]
- 116.
- Center for Health Care Strategies. Medicaid-financed services in supportive housing for high-need homeless beneficiaries. 2012. Exclusion Code: X1.
- 117.
- . The Super-Utilizer Summit: resources to support emerging programs. Super-Utilizer Summit; 2013; Alexandra, Virginia. 2442: Center for Health Care Strategies. Exclusion Code: X1.
- 118.
- Center for Integrative Medicine. Webinar — new online resources for complex care. Oakland, CA: California Health Care Foundation; 2015. https://www
.chcf.org /event/webinar-new-online-resources-for-complex-care/2019. Exclusion Code: X1. - 119.
- Chambers C, Chiu S, Katic M, et al. High utilizers of emergency health services in a population-based cohort of homeless adults. Am J Public Health. 2013;103(S2):S302–10. doi: 10.2105/AJPH.2013.301397. PMID: 104161876. Language: English. Entry Date: 20131126. Revision Date: 20150711. Publication Type: Journal Article. Exclusion Code: X1. [PMC free article: PMC3969147] [PubMed: 24148033] [CrossRef]
- 120.
- Chan B, Edwards ST, Devoe M, et al. The SUMMIT ambulatory-ICU primary care model for medically and socially complex patients in an urban federally qualified health center: study design and rationale. Addict Sci Clin Pract. 2018;13(1):27. doi: 10.1186/s13722-018-0128-y. PMID: CN-01925167. Exclusion Code: X1. [PMC free article: PMC6295087] [PubMed: 30547847] [CrossRef]
- 121.
- Chan B, Edwards ST, Mitchell M, et al. Can an intensive ambulatory care intervention improve the experience of high-utilizing patients?: 6-month outcomes of the summit randomized controlled trial. J Gen Intern Med. 2019;34(2):S151–. doi: 10.1007/11606.1525-1497. PMID: CN-01976812. Exclusion Code: X1. [CrossRef]
- 122.
- Chan B, Mitchell M, Edwards ST, et al. Implementation and evaluation of summit, an ambulatory intensive care (A-ICU) model of primary care for high-utilizersata healthcare for the homeless site. J Gen Intern Med. 2018;33(2):791–2. PMID: CN-01606174. Exclusion Code: X1.
- 123.
- Chan BT, Ovens HJ. Frequent users of emergency departments. Do they also use family physicians’ services? Can Fam Physician. 2002 Oct;48:1654–60. PMID: 12870409. Exclusion Code: X1. [PMC free article: PMC2213944] [PubMed: 12449550]
- 124.
- Chandler D, Spicer G. Capitated assertive community treatment program savings: system implications. Adm Policy Ment Health. 2002;30(1):3–19. PMID: CN-00412904. Exclusion Code: X1. [PubMed: 12546253]
- 125.
- Chang E. Intensive Primary Care via Patient Aligned Care Teams (PACT) Intensive Management @ VA. 2019. Exclusion Code: X1.
- 126.
- Chang ET, Raja PV, Stockdale SE, et al. What are the key elements for implementing intensive primary care? A multisite Veterans Health Administration case study. Healthc (Amst). 2018 Dec;6(4):231–7. doi: 10.1016/j.hjdsi.2017.10.001. PMID: 29102480. Exclusion Code: X1. [PubMed: 29102480] [CrossRef]
- 127.
- Chang ET, Zulman DM, Asch SM, et al. An operations-partnered evaluation of care redesign for high-risk patients in the Veterans Health Administration (VHA): study protocol for the PACT Intensive Management (PIM) randomized quality improvement evaluation. Contemp Clin Trials. 2018 Jun;69:65–75. doi: 10.1016/j.cct.2018.04.008. PMID: 29698772. Exclusion Code: X1. [PubMed: 29698772] [CrossRef]
- 128.
- Chang HY, Boyd CM, Leff B, et al. Identifying Consistent High-cost Users in a Health Plan: Comparison of Alternative Prediction Models. Med Care. 2016 Sep;54(9):852–9. doi: 10.1097/mlr.0000000000000566. PMID: 27521041. Exclusion Code: X9. [PubMed: 27326548] [CrossRef]
- 129.
- Chapman H, Farndon L, Matthews R, et al. Okay to Stay? A new plan to help people with long-term conditions remain in their own homes. Primary Health Care Research & Development (Cambridge University Press / UK). 2019;20:N.PAG-N.PAG. doi: 10.1017/S1463423618000786. PMID: 134961288. Language: English. Entry Date: 20190302. Revision Date: 20190304. Publication Type: Article. Exclusion Code: X6. [PMC free article: PMC6476400] [PubMed: 30428937] [CrossRef]
- 130.
- Chaput YJ, Lebel MJ. An examination of the temporal and geographical patterns of psychiatric emergency service use by multiple visit patients as a means for their early detection. BMC Psychiatry. 2007 Oct 29;7:60. doi: 10.1186/1471-244x-7-60. PMID: 17493977. Exclusion Code: X1. [PMC free article: PMC2190759] [PubMed: 17963530] [CrossRef]
- 131.
- Chaput YJ, Lebel MJ. Demographic and clinical profiles of patients who make multiple visits to psychiatric emergency services. Psychiatr Serv. 2007 Mar;58(3):335–41. doi: 10.1176/ps.2007.58.3.335. PMID: 16859808. Exclusion Code: X1. [PubMed: 17325106] [CrossRef]
- 132.
- Chen BK, Cheng X, Bennett K, et al. Travel distances, socioeconomic characteristics, and health disparities in nonurgent and frequent use of hospital emergency departments in South Carolina: a population-based observational study. BMC Health Serv Res. 2015 May 16;15:203. doi: 10.1186/s12913-015-0864-6. PMID: 25970845. Exclusion Code: X4. [PMC free article: PMC4448557] [PubMed: 25982735] [CrossRef]
- 133.
- Cherner R, Ecker J, Louw A, et al. Lessons learned from piloting a pain assessment program for high frequency emergency department users. Scand J Pain. 2019 Jul 26;19(3):545–52. doi: 10.1515/sjpain-2018-0128. PMID: 30834969. Exclusion Code: X6. [PubMed: 31031261] [CrossRef]
- 134.
- Chernof B, McClellan M. Form follows funding: opportunities for advancing outcomes for complex care patients using alternative payment methods. Health Affairs Blog. Bethesda, MD: Health Affairs; 2018. Exclusion Code: X1.
- 135.
- Chhabra M, Spector E, Demuynck S, et al. Assessing the relationship between housing and health among medically complex, chronically homeless individuals experiencing frequent hospital use in the United States. Health Soc Care Community. 2020 Jan;28(1):91–9. doi: 10.1111/hsc.12843. PMID: 30629016. Exclusion Code: X1. [PubMed: 31476092] [CrossRef]
- 136.
- Chiu Y, Racine-Hemmings F, Dufour I, et al. Statistical tools used for analyses of frequent users of emergency department: a scoping review. Bmj Open. 2019 May;9(5). doi: 10.1136/bmjopen-2018-027750. PMID: WOS:000471192800303. Exclusion Code: X1. [PMC free article: PMC6537981] [PubMed: 31129592] [CrossRef]
- 137.
- Chiu YM, Vanasse A, Courteau J, et al. Persistent frequent emergency department users with chronic conditions: A population-based cohort study. PLoS One. 2020;15(2):e0229022. doi: 10.1371/journal.pone.0229022. PMID: 30780151. Exclusion Code: X6. [PMC free article: PMC7015381] [PubMed: 32050010] [CrossRef]
- 138.
- Chuang E, Pourat N, Haley LA, et al. Integrating Health And Human Services In California’s Whole Person Care Medicaid 1115 Waiver Demonstration. Health Aff (Millwood). 2020 Apr;39(4):639–48. doi: 10.1377/hlthaff.2019.01617. PMID: WOS:000523599600015. Exclusion Code: X1. [PubMed: 32250689] [CrossRef]
- 139.
- Chukmaitov AS, Tang A, Carretta HJ, et al. Characteristics of all, occasional, and frequent emergency department visits due to ambulatory care-sensitive conditions in Florida. J Ambul Care Manage. 2012 Apr–Jun;35(2):149–58. doi: 10.1097/JAC.0b013e318244d222. PMID: 23091842. Exclusion Code: X3. [PubMed: 22415289] [CrossRef]
- 140.
- Claxton G, Rae M, Levitt L. A look at people who have persistently high spending on health care. Kaiser Family Foundation. 2019. https://www
.kff.org/health-costs /issue-brief /a-look-at-people-who-have-persistently-high-spending-on-health-care/. Exclusion Code: X7. - 141.
- ClinicalTrials
.gov. Contra Costa Health Services Whole Person Care (CommunityConnect) Program Evaluation. https: //clinicaltrials .gov/show/NCT04000074. 2019. PMID: CN-01952848. Exclusion Code: X9. - 142.
- ClinicalTrials
.gov. High Risk Outpatient Intern-led Care (HeROIC) Clinic Initiative. https: //clinicaltrials .gov/show/NCT04375189. 2020. PMID: CN-02103718. Exclusion Code: X11. - 143.
- CMS. Integrated care resource center available to all states. Resources available to all states to coordinate care for high-cost, high-need beneficiaries. Baltimore, MD: U.S. Centers for Medicare & Medicaid Services; 2017. Exclusion Code: X1.
- 144.
- Cohen D, Wodchis WP, Calzavara A. Can high-cost spending in the community signal admission to hospital? A dynamic modeling study for urgent and elective cardiovascular patients. BMC Health Serv Res. 2018 Nov 15;18(1):861. doi: 10.1186/s12913-018-3639-z. PMID: 23297608. Exclusion Code: X1. [PMC free article: PMC6238318] [PubMed: 30442140] [CrossRef]
- 145.
- Coles S. Strategies to Improve Corporate Financial Investment in Care Coordination Programs. Walden University, ProQuest Dissertations Publishing; 2018. Exclusion Code: X1.
- 146.
- Colla C. Evaluating the formation and performance of accountable care organizations: focus on high-need, high-cost populations. Dartmouth College, Dartmouth Institute for Health Policy and Clinical Practice. 2014. Exclusion Code: X7.
- 147.
- Colla C, Yang WD, Mainor AJ, et al. Organizational integration, practice capabilities, and outcomes in clinically complex medicare beneficiaries. Health Serv Res. 2020 Dec;55:1085–97. doi: 10.1111/1475-6773.13580. PMID: WOS:000583295700001. Exclusion Code: X1. [PMC free article: PMC7720705] [PubMed: 33104254] [CrossRef]
- 148.
- Collins L, Sicks S, Hass RW, et al. Self-efficacy and empathy development through interprofessional student hotspotting. Journal of Interprofessional Care.4. doi: 10.1080/13561820.2020.1712337. PMID: WOS:000523149400001. Exclusion Code: X1. [PubMed: 32233896] [CrossRef]
- 149.
- Connors JDN, Binkley BL, Graff JC, et al. How patient experience informed the SafeMed Program: Lessons learned during a Health Care Innovation Award to improve care for super-utilizers. Healthcare-the Journal of Delivery Science and Innovation. 2019 Mar;7(1):13–21. doi: 10.1016/j.hjdsi.2018.02.002. PMID: WOS:000461053700004. Exclusion Code: X1. [PubMed: 29477610] [CrossRef]
- 150.
- Considine JT. A Feasibility Study for an Advanced Practice Registered Nurse Led Community Engagement Program. Shepherd University, ProQuest Dissertations Publishing; 2018. Exclusion Code: X4.
- 151.
- Cook LJ, Knight S, Junkins EP, Jr., et al. Repeat patients to the emergency department in a statewide database. Acad Emerg Med. 2004 Mar;11(3):256–63. PMID: 11827601. Exclusion Code: X4. [PubMed: 15001405]
- 152.
- Cornell PY, Halladay CW, Ader J, et al. Embedding Social Workers In Veterans Health Administration Primary Care Teams Reduces Emergency Department Visits. Health Aff (Millwood). 2020 Apr;39(4):603–12. doi: 10.1377/hlthaff.2019.01589. PMID: WOS:000523599600011. Exclusion Code: X1. [PubMed: 32250673] [CrossRef]
- 153.
- Costi S, Pellegrini M, Cavuto S, et al. Occupational therapy in rehabilitation of complex patients: protocol for a superiority randomized controlled trial. Journal of interprofessional care. 2020:1–8. doi: 10.1080/13561820.2020.1711720. PMID: CN-02086974. Exclusion Code: X11. [PubMed: 32013621] [CrossRef]
- 154.
- Coughlin TA, Long SK. Health care spending and service use among high-cost Medicaid beneficiaries, 2002–2004. Inquiry. 2009 Winter;46(4):405–17. doi: 10.5034/inquiryjrnl_46.4.405. PMID: 28723691. Exclusion Code: X9. [PubMed: 20184167] [CrossRef]
- 155.
- Couture EM, Chouinard MC, Fortin M, et al. The relationship between health literacy and quality of life among frequent users of health care services: a cross-sectional study. Health Qual Life Outcomes. 2017 Jul 6;15(1):137. doi: 10.1186/s12955-017-0716-7. PMID: 27862572. Exclusion Code: X1. [PMC free article: PMC5500997] [PubMed: 28683743] [CrossRef]
- 156.
- Couture EM, Chouinard MC, Fortin M, et al. The relationship between health literacy and patient activation among frequent users of healthcare services: a cross-sectional study. BMC Fam Pract. 2018 Mar 9;19(1):38. doi: 10.1186/s12875-018-0724-7. PMID: 30118627. Exclusion Code: X1. [PMC free article: PMC5845227] [PubMed: 29523095] [CrossRef]
- 157.
- Cox WK, Penny LC, Statham RP, et al. Admission intervention team: medical center based intensive case management of the seriously mentally ill. Care Manag J. 2003 Winter;4(4):178–84. PMID: 15763120. Exclusion Code: X1. [PubMed: 15628650]
- 158.
- Craig C, Stiefel M, Carr E, et al. WIHI: when everyone knows your name: identifying patients with complex needs. Institute for Healthcare Improvement; 2015. Exclusion Code: X7.
- 159.
- Craig C ED, Whittington J. Care Coordination Model: Better Care at Lower Cost for People with Multiple Health and Social Needs. IHI. 2011. Exclusion Code: X11.
- 160.
- Crane D, Christenson J. The medical offset effect: patterns in outpatient services reduction for high utilizers of health care. Contemporary Family Therapy: An International Journal. 2008;30(2):127–38. doi: 10.1007/s10591-008-9058-2. PMID: 31368504. Exclusion Code: X1. [CrossRef]
- 161.
- Crane M, Warnes AM. Primary health care services for single homeless people: defects and opportunities. Fam Pract. 2001 Jun;18(3):272–6. doi: 10.1093/fampra/18.3.272. PMID: 12195545. Exclusion Code: X1. [PubMed: 11356733] [CrossRef]
- 162.
- Cruwys T, Wakefield JRH, Sani F, et al. Social isolation predicts frequent attendance in primary care. Ann Behav Med. 2018 Oct;52(10):817–29. doi: 10.1093/abm/kax054. PMID: WOS:000449052300001. Exclusion Code: X1. [PubMed: 30212847] [CrossRef]
- 163.
- Cullen W, O’Brien S, O’Carroll A, et al. Chronic illness and multimorbidity among problem drug users: a comparative cross sectional pilot study in primary care. BMC Fam Pract. 2009 Apr 21;10:25. doi: 10.1186/1471-2296-10-25. PMID: 17466465. Exclusion Code: X1. [PMC free article: PMC2678984] [PubMed: 19383141] [CrossRef]
- 164.
- Cunningham PJ. Predicting high-cost privately insured patients based on self-reported health and utilization data. Am J Manag Care. 2017 Jul 1;23(7):e215–e22. PMID: 27914968. Exclusion Code: X9. [PubMed: 28850789]
- 165.
- Curtin CM, Suarez PA, Di Ponio LA, et al. Who are the women and men in Veterans Health Administration’s current spinal cord injury population? J Rehabil Res Dev. 2012;49(3):351–60. PMID: 22606057. Exclusion Code: X1. [PubMed: 22773195]
- 166.
- Dang S, Ruiz DI, Klepac L, et al. Key Characteristics for Successful Adoption and Implementation of Home Telehealth Technology in Veterans Affairs Home-Based Primary Care: An Exploratory Study. Telemed J E Health. 2019 Apr;25(4):309–18. doi: 10.1089/tmj.2018.0009. PMID: 30887432. Exclusion Code: X11. [PubMed: 29969381] [CrossRef]
- 167.
- Darke S, Havard A, Ross J, et al. Changes in the use of medical services and prescription drugs among heroin users over two years. Drug Alcohol Rev. 2007 Mar;26(2):153–9. doi: 10.1080/09595230601146660. PMID: 17325106. Exclusion Code: X1. [PubMed: 17364850] [CrossRef]
- 168.
- Das LT, Abramson EL, Kaushal R. High-need, high-cost patients offer solutions for improving their care and reducing costs. NEJM Catal. 2019;2019. Exclusion Code: X7.
- 169.
- Das LT, Kaushal R, Garrison K, et al. Drivers of preventable high health care utilization: a qualitative study of patient, physician and health system leader perspectives. J Health Serv Res Policy. 2020;25(4):220–8. doi: 10.1177/1355819619873685. PMID: 145701247. Language: English. Entry Date: 20200922. Revision Date: 20200922. Publication Type: Article. Exclusion Code: X12. [PubMed: 31505976] [CrossRef]
- 170.
- Davis A, Osuji T, Chen A, et al. Data-driven decision making for interventions with complex needs’ patients: Identifying the right target population. Health Serv Res. 2020;55(SUPPL 1):75. doi: 10.1111/1475-6773.13433. Exclusion Code: X7. [CrossRef]
- 171.
- Davis AC, Osuji TA, Chen J, et al. Identifying Populations with Complex Needs: Variation in Approaches Used to Select Complex Patient Populations. Popul Health Manag. 2020. doi: 10.1089/pop.2020.0153. Exclusion Code: X7. [PubMed: 32941105] [CrossRef]
- 172.
- Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J. 2011 Oct;70(10):209–13. PMID: 20106475. Exclusion Code: X1. [PMC free article: PMC3215980] [PubMed: 22162595]
- 173.
- Davis R, Nuamah A. Identifying “Rising Risk” Populations: Early Lessons from the Complex Care Innovation Lab. Center for Health Care Strategies; 2020. Exclusion Code: X2.
- 174.
- Davis R, Somers SA. A collective national approach to fostering innovation in complex care. Healthc (Amst). 2018 Mar;6(1):1–3. doi: 10.1016/j.hjdsi.2017.05.003. PMID: 28673816. Exclusion Code: X7. [PubMed: 28673816] [CrossRef]
- 175.
- de Miguel-Diez J, Lopez-de-Andres A, Herandez-Barrera V, et al. Effect of the economic crisis on the use of health and home care services among Spanish COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:725–39. doi: 10.2147/copd.S150308. PMID: 28374978. Exclusion Code: X1. [PMC free article: PMC5836665] [PubMed: 29535513] [CrossRef]
- 176.
- Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22(2):344–50. doi: 10.1016/j.janxdis.2007.03.004. PMID: 19197621. Exclusion Code: X1. [PubMed: 17420113] [CrossRef]
- 177.
- Delaney RK, Sisco-Taylor B, Fagerlin A, et al. A systematic review of intensive outpatient care programs for high-need, high-cost patients. Transl Behav Med. 2020;10(5):1187–99. doi: 10.1093/tbm/ibaa017. Exclusion Code: X7. [PubMed: 33044534] [CrossRef]
- 178.
- Delcher C, Yang C, Ranka S, et al. Variation in outpatient emergency department utilization in Texas Medicaid: a state-level framework for finding “superutilizers”. Int J Emerg Med. 2017 Dec 4;10(1):31. doi: 10.1186/s12245-017-0157-4. PMID: 29204728. Exclusion Code: X9. [PMC free article: PMC5714939] [PubMed: 29204728] [CrossRef]
- 179.
- Dent A, Hunter G, Webster AP. The impact of frequent attenders on a UK emergency department. Eur J Emerg Med. 2010 Dec;17(6):332–6. doi: 10.1097/MEJ.0b013e328335623d. PMID: 20136800. Exclusion Code: X1. [PubMed: 20038842] [CrossRef]
- 180.
- Dent AW, Phillips GA, Chenhall AJ, et al. The heaviest repeat users of an inner city emergency department are not general practice patients. Emerg Med (Fremantle). 2003 Aug;15(4):322–9. PMID: 11502954. Exclusion Code: X1. [PubMed: 14631698]
- 181.
- Dew MA, Goycoolea JM, Harris RC, et al. An internet-based intervention to improve psychosocial outcomes in heart transplant recipients and family caregivers: development and evaluation. J Heart Lung Transplant. 2004 Jun;23(6):745–58. PMID: 15182240. Exclusion Code: X1. [PubMed: 15366436]
- 182.
- Di Lorenzo R, Sagona M, Landi G, et al. The revolving door phenomenon in an Italian acute psychiatric ward: a 5-year retrospective analysis of the potential risk factors. J Nerv Ment Dis. 2016;204(9):686–92. doi: 10.1097/NMD.0000000000000540. PMID: 118071724. Language: English. Entry Date: 20180723. Revision Date: 20171231. Publication Type: journal article. Journal Subset: Biomedical. Exclusion Code: X1. [PubMed: 27227558] [CrossRef]
- 183.
- Di Mauro R, Di Silvio V, Bosco P, et al. Case management programs in emergency department to reduce frequent user visits: a systematic review. Acta Biomed. 2019 Jul 8;90(6-s):34–40. doi: 10.23750/abm.v90i6-S.8390. PMID: 31059373. Exclusion Code: X7. [PMC free article: PMC6776176] [PubMed: 31292413] [CrossRef]
- 184.
- Diaz T. In Memoriam. Patient Educ Couns. 2020 Jun;103(6):1258–9. doi: 10.1016/j.pec.2019.11.027. PMID: WOS:000553018800024. Exclusion Code: X8. [CrossRef]
- 185.
- DiPietro BY. Medicaid accountable care organizations: a case study with Hennepin Health. 2018 May 2018. Exclusion Code: X1.
- 186.
- DiPietro BY, Kindermann D, Schenkel SM. Ill, itinerant, and insured: the top 20 users of emergency departments in Baltimore city. ScientificWorldJournal. 2012;2012:726568. doi: 10.1100/2012/726568. PMID: 22162595. Exclusion Code: X4. [PMC free article: PMC3345528] [PubMed: 22606057] [CrossRef]
- 187.
- Doheny M, Agerholm J, Orsini N, et al. Socio-demographic differences in the frequent use of emergency department care by older persons: a population-based study in Stockholm County. BMC Health Serv Res. 2019 Mar 29;19(1):202. doi: 10.1186/s12913-019-4029-x. PMID: 29620961. Exclusion Code: X1. [PMC free article: PMC6440084] [PubMed: 30922354] [CrossRef]
- 188.
- Dollard J, Harvey G, Dent E, et al. Older people who are frequent users of acute care: a symptom of fragmented care? A case series report on patients’ pathways of care. J Frailty Aging. 2018;7(3):193–5. doi: 10.14283/10.14283/jfa.2018.12. PMID: 29305202. Exclusion Code: X1. [PubMed: 30095151] [CrossRef]
- 189.
- Dombrowski JC, Ramchandani M, Dhanireddy S, et al. The Max Clinic: medical care designed to engage the hardest-to-reach persons living with HIV in Seattle and King County, Washington. AIDS Patient Care STDS. 2018 Apr;32(4):149–56. doi: 10.1089/apc.2017.0313. PMID: 30670044. Exclusion Code: X1. [PMC free article: PMC5905858] [PubMed: 29630852] [CrossRef]
- 190.
- Domenech-Briz V, Romero RG, de Miguel-Montoya I, et al. Results of Nurse Case Management in Primary Heath Care: Bibliographic Review. Int J Environ Res Public Health. 2020 Dec;17(24):17. doi: 10.3390/ijerph17249541. PMID: WOS:000602841900001. Exclusion Code: X7. [PMC free article: PMC7766905] [PubMed: 33419267] [CrossRef]
- 191.
- Donate-Martinez A, Rodenas F, Garces J. Impact of a primary-based telemonitoring programme in HRQOL, satisfaction and usefulness in a sample of older adults with chronic diseases in Valencia (Spain). Arch Gerontol Geriatr. 2016 Jan–Feb;62:169–75. doi: 10.1016/j.archger.2015.09.008. PMID: 27432036. Exclusion Code: X1. [PubMed: 26446784] [CrossRef]
- 192.
- Doran KM, Vashi AA, Platis S, et al. Navigating the boundaries of emergency department care: addressing the medical and social needs of patients who are homeless. Am J Public Health. 2013 Dec;103 Suppl 2:S355–60. doi: 10.2105/ajph.2013.301540. PMID: 24148054. Exclusion Code: X1. [PMC free article: PMC3969133] [PubMed: 24148054] [CrossRef]
- 193.
- Doupe MB, Palatnick W, Day S, et al. Frequent users of emergency departments: developing standard definitions and defining prominent risk factors. Ann Emerg Med. 2012 Jul;60(1):24–32. doi: 10.1016/j.annemergmed.2011.11.036. PMID: 22221292. Exclusion Code: X1. [PubMed: 22305330] [CrossRef]
- 194.
- Dowd BE, Swenson T, Parashuram S, et al. PQRS participation, inappropriate utilization of health care services, and Medicare expenditures. Med Care Res Rev. 2016 Feb;73(1):106–23. doi: 10.1177/1077558715597846. PMID: 27166469. Exclusion Code: X1. [PubMed: 26324510] [CrossRef]
- 195.
- Downey LV, Zun LS. Reasons for readmissions: what are the reasons for 90-day readmissions of psychiatric patients from the ED? Am J Emerg Med. 2015 Oct;33(10):1489–91. doi: 10.1016/j.ajem.2015.06.056. PMID: 26206441. Exclusion Code: X1. [PubMed: 26164411] [CrossRef]
- 196.
- Dreyfus KS. Adult attachment theory and diabetes mellitus: an examination of healthcare utilization and biopsychosocial health: ProQuest Information & Learning; 2015. Exclusion Code: X1.
- 197.
- Dryden EM, King K, Touw S. Facilitators and Barriers to Successful Engagement in a Complex Care Management Program: Patient and Care Manager Perspectives. J Health Care Poor Underserved. 2019 May;30(2):789–805. doi: 10.1353/hpu.2019.0056. PMID: WOS:000468495900026. Exclusion Code: X1. [PubMed: 31130551] [CrossRef]
- 198.
- Duenas M, Sow S, Poveda C, et al. Engaging the disengaged: outcomes of recruitment and enrollment of high-risk, high-utilizers into an intensive outpatient primary care program for complex care management. J Gen Intern Med. 2018;33(2):783–4. Exclusion Code: X1.
- 199.
- DuGoff EH, Boyd C, Anderson G. Complex Patients and Quality of Care in Medicare Advantage. J Am Geriatr Soc. 2020 Feb;68(2):395–402. doi: 10.1111/jgs.16236. PMID: 31054588. Exclusion Code: X1. [PubMed: 31675101] [CrossRef]
- 200.
- DuGoff EH, Buckingham W, Kind AJ, et al. Targeting high-need beneficiaries in Medicare Advantage: opportunities to address medical and social needs. Issue Brief (Commonw Fund). 2019 Feb 1;2019:1–14. PMID: 29554592. Exclusion Code: X1. [PubMed: 30938944]
- 201.
- Durand-Zaleski I. U.S.–France International Meeting on care for high-need, high-cost patients. Assistance Publique-Hôpitaux de Paris: Commonwealth Fund; 2015. Exclusion Code: X7.
- 202.
- Duru OK, Harwood J, Moin T, et al. Evaluation of a National Care Coordination Program to Reduce Utilization Among High-cost, High-need Medicaid Beneficiaries With Diabetes. Med Care. 2020 Jun;58 Suppl 6 Suppl 1:S14–s21. doi: 10.1097/mlr.0000000000001315. PMID: 32818784. Exclusion Code: X1. [PMC free article: PMC10653047] [PubMed: 32412949] [CrossRef]
- 203.
- Eden E, DeKosky A, Chen LW, et al. High utilizers of condition help: demographic and medical characteristics of patients who repeatedly activate rapid response teams. J Gen Intern Med. 2017;32(2):S205–S6. Exclusion Code: X1.
- 204.
- Edwards ST, Saha S, Prentice JC, et al. Preventing hospitalization with Veterans Affairs home-based primary care: which individuals benefit most? J Am Geriatr Soc. 2017 Aug;65(8):1676–83. doi: 10.1111/jgs.14843. PMID: 28410824. Exclusion Code: X1. [PMC free article: PMC8284941] [PubMed: 28323324] [CrossRef]
- 205.
- Englander H, Michaels L, Chan B, et al. The care transitions innovation (C-train) for socioeconomically disadvantaged adults, results of a clustered randomized controlled trial. J Gen Intern Med. 2014;29:S221–. PMID: CN-01010119. Exclusion Code: X1. [PMC free article: PMC4238212] [PubMed: 24913003]
- 206.
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003 Mar;98(3):269–79. PMID: 12945810. Exclusion Code: X1. [PMC free article: PMC2943839] [PubMed: 12603227]
- 207.
- Etemad LR, McCollam PL. Predictors of high-cost managed care patients with acute coronary syndrome. Curr Med Res Opin. 2005 Dec;21(12):1977–84. doi: 10.1185/030079905x74970. PMID: 30355286. Exclusion Code: X4. [PubMed: 16368049] [CrossRef]
- 208.
- Eton DT, Lee MK, St Sauver JL, et al. Known-groups validity and responsiveness to change of the Patient Experience with Treatment and Self-management (PETS vs. 2.0): a patient-reported measure of treatment burden. Qual Life Res. 2020 Nov;29(11):3143–54. doi: 10.1007/s11136-020-02546-x. PMID: WOS:000539517700001. Exclusion Code: X1. [PMC free article: PMC8012109] [PubMed: 32524346] [CrossRef]
- 209.
- Ettner SL, Frank RG, McGuire TG, et al. Risk adjustment alternatives in paying for behavioral health care under Medicaid. Health Serv Res. 2001 Aug;36(4):793–811. PMID: 14727682. Exclusion Code: X1. [PMC free article: PMC1089257] [PubMed: 11508640]
- 210.
- Faresjo A, Grodzinsky E, Foldevi M, et al. Patients with irritable bowel syndrome in primary care appear not to be heavy healthcare utilizers. Aliment Pharmacol Ther. 2006 Mar 15;23(6):807–14. doi: 10.1111/j.1365-2036.2006.02815.x. PMID: 16672680. Exclusion Code: X1. [PubMed: 16556183] [CrossRef]
- 211.
- Feinglass J, Norman G, Golden RL, et al. Integrating Social Services and Home-Based Primary Care for High-Risk Patients. Popul Health Manag. 2018 Apr;21(2):96–101. doi: 10.1089/pop.2017.0026. PMID: 28609187. Exclusion Code: X9. [PubMed: 28609187] [CrossRef]
- 212.
- Ferrari S, Galeazzi GM, Mackinnon A, et al. Frequent attenders in primary care: impact of medical, psychiatric and psychosomatic diagnoses. Psychother Psychosom. 2008;77(5):306–14. doi: 10.1159/000142523. PMID: 16460995. Exclusion Code: X1. [PubMed: 18600036] [CrossRef]
- 213.
- Fertel BS, Hart KW, Lindsell CJ, et al. Patients who use multiple EDs: quantifying the degree of overlap between ED populations. West J Emerg Med. 2015 Mar;16(2):229–33. doi: 10.5811/westjem.2015.1.22838. PMID: 26447915. Exclusion Code: X4. [PMC free article: PMC4380370] [PubMed: 25834661] [CrossRef]
- 214.
- Fertel BS, Podolsky SR, Mark J, et al. Impact of an individual plan of care for frequent and high utilizers in a large healthcare system. Am J Emerg Med. 2019. doi: 10.1016/j.ajem.2019.02.032. Exclusion Code: X1. [PubMed: 30824276] [CrossRef]
- 215.
- Figueroa JF, Joynt Maddox KE, Beaulieu N, et al. Concentration of potentially preventable spending among high-cost Medicare subpopulations: an observational study. Ann Intern Med. 2017 Nov 21;167(10):706–13. doi: 10.7326/m17-0767. Exclusion Code: X4. [PubMed: 29049488] [CrossRef]
- 216.
- Figueroa JF, Lyon Z, Zhou X, et al. Persistence and Drivers of High-Cost Status Among Dual-Eligible Medicare and Medicaid Beneficiaries: An Observational Study. Ann Intern Med. 2018 Oct 16;169(8):528–34. doi: 10.7326/m18-0085. PMID: 30285049. Exclusion Code: X9. [PubMed: 30285049] [CrossRef]
- 217.
- Filippi A, Sessa E, Pecchioli S, et al. Homecare for patients with heart failure in Italy. Ital Heart J. 2005 Jul;6(7):573–7. PMID: 15911164. Exclusion Code: X1. [PubMed: 16274019]
- 218.
- Finkelstein AN. Health care hotspotting: a randomized controlled trial. Abdul Latif Jameel Poverty Action Lab. Cooper University Hospital, Camden, New Jersey: 2014. Exclusion Code: X1.
- 219.
- Fiorentino M, Mulles S, Cue L, et al. High Acuity of Postoperative Consults to Emergency General Surgery at an Urban Safety Net Hospital. J Surg Res. 2021 Jan;257:50–5. doi: 10.1016/j.jss.2020.07.038. PMID: 32008721. Exclusion Code: X1. [PubMed: 32818784] [CrossRef]
- 220.
- Fisher HM, McCabe S. Managing chronic conditions for elderly adults: the VNS CHOICE model. Health Care Financ Rev. 2005 Fall;27(1):33–45. PMID: 16205429. Exclusion Code: X1. [PMC free article: PMC4194907] [PubMed: 17288076]
- 221.
- Fitzpatrick T, Rosella LC, Calzavara A, et al. Looking Beyond Income and Education: Socioeconomic Status Gradients Among Future High-Cost Users of Health Care. Am J Prev Med. 2015 Aug;49(2):161–71. doi: 10.1016/j.amepre.2015.02.018. PMID: 25748258. Exclusion Code: X9. [PubMed: 25960393] [CrossRef]
- 222.
- Flarup L, Moth G, Christensen MB, et al. Chronic-disease patients and their use of out-of-hours primary health care: a cross-sectional study. BMC Fam Pract. 2014 Jun 9;15:114. doi: 10.1186/1471-2296-15-114. PMID: 24801269. Exclusion Code: X1. [PMC free article: PMC4064509] [PubMed: 24912378] [CrossRef]
- 223.
- Fleishman JA, Cohen JW. Using information on clinical conditions to predict high-cost patients. Health Serv Res. 2010 Apr;45(2):532–52. doi: 10.1111/j.1475-6773.2009.01080.x. PMID: 23740662. Exclusion Code: X1. [PMC free article: PMC2838159] [PubMed: 20132341] [CrossRef]
- 224.
- Fleming MD, Shim JK, Yen I, et al. Caring for “Super-utilizers”: Neoliberal Social Assistance in the Safety-net. Med Anthropol Q. 2019 Jun;33(2):173–90. doi: 10.1111/maq.12481. PMID: 30726920. Exclusion Code: X9. [PubMed: 30291726] [CrossRef]
- 225.
- Fleury MJ, Grenier G, Bamvita JM, et al. Professional service utilisation among patients with severe mental disorders. BMC Health Serv Res. 2010 May 27;10:141. doi: 10.1186/1472-6963-10-141. PMID: 20078404. Exclusion Code: X1. [PMC free article: PMC2896947] [PubMed: 20507597] [CrossRef]
- 226.
- Flottemesch TJ, Anderson LH, Solberg LI, et al. Patient-centered medical home cost reductions limited to complex patients. Am J Manag Care. 2012 Nov;18(11):677–86. PMID: 22961756. Exclusion Code: X1. [PubMed: 23198711]
- 227.
- Flowers A, Shade K. Evaluation of a Multidisciplinary Care Coordination Program for Frequent Users of the Emergency Department. Prof Case Manag. 2019 Sep/Oct;24(5):230–9. doi: 10.1097/ncm.0000000000000368. PMID: 29471360. Exclusion Code: X1. [PubMed: 31373952] [CrossRef]
- 228.
- Flynn P, Ridgeway J, Wieland M, et al. Primary care utilization and mental health diagnoses among adult patients requiring interpreters: a retrospective cohort study. J Gen Intern Med. 2013;28(3):386–91. doi: 10.1007/s11606-012-2159-5. PMID: 85715044. Exclusion Code: X4. [PMC free article: PMC3579974] [PubMed: 22782282] [CrossRef]
- 229.
- Foebel AD, Hirdes JP, Heckman GA, et al. A profile of older community-dwelling home care clients with heart failure in Ontario. Chronic Dis Can. 2011 Mar;31(2):49–57. PMID: 21343403. Exclusion Code: X1. [PubMed: 21466754]
- 230.
- Fondow M, Schreiter EZ, Thomas C, et al. Initial examination of characteristics of patients who are high utilizers of an established primary care behavioral health consultation service. Families Systems & Health. 2017 Jun;35(2):184–92. doi: 10.1037/fsh0000266. PMID: WOS:000403442700009. Exclusion Code: X4. [PubMed: 28617019] [CrossRef]
- 231.
- Foo KM, Sundram M, Legido-Quigley H. Facilitators and barriers of managing patients with multiple chronic conditions in the community: a qualitative study. BMC Public Health. 2020 Feb;20(1):15. doi: 10.1186/s12889-020-8375-8. PMID: WOS:000519946500003. Exclusion Code: X6. [PMC free article: PMC7045577] [PubMed: 32106838] [CrossRef]
- 232.
- Ford JD, Trestman RL, Steinberg K, et al. Prospective association of anxiety, depressive, and addictive disorders with high utilization of primary, specialty and emergency medical care. Soc Sci Med. 2004 Jun;58(11):2145–8. doi: 10.1016/j.socscimed.2003.08.017. PMID: 15323413. Exclusion Code: X4. [PubMed: 15047073] [CrossRef]
- 233.
- Ford JD, Trestman RL, Tennen H, et al. Relationship of anxiety, depression and alcohol use disorders to persistent high utilization and potentially problematic under-utilization of primary medical care. Soc Sci Med. 2005 Oct;61(7):1618–25. doi: 10.1016/j.socscimed.2005.03.017. PMID: WOS:000231024400023. Exclusion Code: X1. [PubMed: 16005791] [CrossRef]
- 234.
- Fordyce MA, Doescher MP, Skillman SM. The aging of the rural primary care physician workforce: will some locations be more affected than others? [Seattle, WA]: WWAMI Rural Health Research Center; 2013. Exclusion Code: X1.
- 235.
- Fortin M, Cao Z, Fleury MJ. A typology of satisfaction with mental health services based on Andersen’s behavioral model. Soc Psychiatry Psychiatr Epidemiol. 2018 Jun;53(6):587–95. doi: 10.1007/s00127-018-1498-x. PMID: 27936849. Exclusion Code: X1. [PubMed: 29450599] [CrossRef]
- 236.
- Franco RA, Ashwathnarayan R, Deshpandee A, et al. The high prevalence of restless legs syndrome symptoms in liver disease in an academic-based hepatology practice. J Clin Sleep Med. 2008 Feb 15;4(1):45–9. PMID: 18399957. Exclusion Code: X1. [PMC free article: PMC2276829] [PubMed: 18350962]
- 237.
- Fraze TK, Beidler LB, Briggs ADM, et al. Translating Evidence into Practice: ACOs’ Use of Care Plans for Patients with Complex Health Needs. JGIM: Journal of General Internal Medicine. 2021;36(1):147–53. doi: 10.1007/s11606-020-06122-4. PMID: 148469973. Exclusion Code: X1. [PMC free article: PMC7858720] [PubMed: 33006083] [CrossRef]
- 238.
- Fraze TK, Briggs ADM, Whitcomb EK, et al. Role of Nurse Practitioners in Caring for Patients With Complex Health Needs. Med Care. 2020 Oct;58(10):853–60. doi: 10.1097/mlr.0000000000001364. PMID: WOS:000576505000002. Exclusion Code: X1. [PMC free article: PMC7552908] [PubMed: 32925414] [CrossRef]
- 239.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study using teledermatology to manage high-need patients with psoriasis. Arch Dermatol. 2010 Feb;146(2):200–1. doi: 10.1001/archdermatol.2009.375. PMID: 20825136. Exclusion Code: X1. [PubMed: 20157037] [CrossRef]
- 240.
- Fuda KK, Immekus R. Frequent users of Massachusetts emergency departments: a statewide analysis. Ann Emerg Med. 2006 Jul;48(1):9–16. doi: 10.1016/j.annemergmed.2006.03.001. PMID: 19487595. Exclusion Code: X4. [PubMed: 16781915] [CrossRef]
- 241.
- Galbraith AA, Meyers DJ, Ross-Degnan D, et al. Long-Term Impact of a Postdischarge Community Health Worker Intervention on Health Care Costs in a Safety-Net System. Health Serv Res. 2017 Dec;52(6):2061–78. doi: 10.1111/1475-6773.12790. PMID: 29130267. Exclusion Code: X1. [PMC free article: PMC5682134] [PubMed: 29130267] [CrossRef]
- 242.
- Gallagher NA, Fox D, Dawson C, et al. Improving care transitions: complex high-utilizing patient experiences guide reform. Am J Manag Care. 2017 Oct 1;23(10):e347–e52. PMID: 28829516. Exclusion Code: X1. [PubMed: 29087639]
- 243.
- Gallagher S. Exploring supportive housing’s impact on health and health care quality among high-cost, high-need populations. Corporation for Supportive Housing 2017. https://www
.commonwealthfund .org/grants/exploring-supportive-housings-impact-health-and-health-care-quality-among-high-cost-high. Exclusion Code: X7. - 244.
- Garg T, Young AJ, Kost KA, et al. Burden of multiple chronic conditions among patients with urological cancer. J Urol. 2018 Feb;199(2):543–50. doi: 10.1016/j.juro.2017.08.005. PMID: 29473945. Exclusion Code: X1. [PubMed: 28789948] [CrossRef]
- 245.
- Gastal FL, Andreoli SB, Quintana MI, et al. Predicting the revolving door phenomenon among patients with schizophrenic, affective disorders and non-organic psychoses. Rev Saude Publica. 2000 Jun;34(3):280–5. doi: 10.1590/s0034-89102000000300011. PMID: 10883718. Exclusion Code: X1. [PubMed: 10920451] [CrossRef]
- 246.
- Geisz M. A coalition creates a citywide care management system. Robert Wood Johnson Foundation. Princeton, NJ: 2014. https://www
.rwjf.org /en/library/research /2011/01/a-coalition-creates-a-citywide-care-management-system-.html. Exclusion Code: X1. - 247.
- Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000 Feb;34(6):1273–302. PMID: 10654830. Exclusion Code: X1. [PMC free article: PMC1089079] [PubMed: 10654830]
- 248.
- Geurts J, Palatnick W, Strome T, et al. Frequent users of an inner-city emergency department. Cjem. 2012 Sep;14(5):306–13. PMID: 21491293. Exclusion Code: X1. [PubMed: 22967698]
- 249.
- Giannouchos TV, Kum HC, Foster MJ, et al. Characteristics and predictors of adult frequent emergency department users in the United States: A systematic literature review. J Eval Clin Pract. 2019 Jun;25(3):420–33. doi: 10.1111/jep.13137. PMID: 32589150. Exclusion Code: X7. [PubMed: 31044484] [CrossRef]
- 250.
- Giannouchos TV, Washburn DJ, Kum HC, et al. Predictors of Multiple Emergency Department Utilization Among Frequent Emergency Department Users in 3 States. Med Care. 2020 Feb;58(2):137–45. doi: 10.1097/mlr.0000000000001228. PMID: 31373550. Exclusion Code: X4. [PubMed: 31651740] [CrossRef]
- 251.
- Gingold DB, Pierre-Mathieu R, Cole B, et al. Impact of the Affordable Care Act Medicaid expansion on emergency department high utilizers with ambulatory care sensitive conditions: A cross-sectional study. Am J Emerg Med. 2017 May;35(5):737–42. doi: 10.1016/j.ajem.2017.01.014. PMID: 27008540. Exclusion Code: X1. [PubMed: 28110978] [CrossRef]
- 252.
- Glick M. Systematic approach for treating the medically complex dental patient. Alpha Omegan. 2001 Jul–Aug;94(2):40–3. PMID: 11407062. Exclusion Code: X1. [PubMed: 11480187]
- 253.
- Goldstein KM, Zullig LL, Dedert EA, et al. Telehealth interventions designed for women: an evidence map. J Gen Intern Med. 2018;33(12):2191–200. doi: 10.1007/s11606-018-4655-8. Exclusion Code: X1. [PMC free article: PMC6258612] [PubMed: 30284173] [CrossRef]
- 254.
- Goodell S, Bodenheimer T, Berry-Millett R. Care management of patients with complex health care needs. Robert Wood Johnson Foundation. Princeton, NJ: 2009. https://www
.rwjf.org /en/library/research /2009/12/care-management-of-patients-with-complex-health-care-needs.html. Exclusion Code: X1. [PubMed: 22052205] - 255.
- Goodwin A, Henschen B, Nichols N, et al. Systematic review of interventions targeting high-utilizers of inpatient resources. J Gen Intern Med. 2018;33(2):352. Exclusion Code: X1.
- 256.
- Goodwin A, Henschen BL, O’Dwyer LC, et al. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853–9. doi: 10.12788/jhm.3090. Exclusion Code: X1. [PubMed: 30379144] [CrossRef]
- 257.
- Gore S, Lind A, Somers SA. Profiles of state innovation: roadmap for improving systems of care for dual eligibles. Hamilton, N.J.: Center for Health Care Strategies, Inc.; 2010. Exclusion Code: X1.
- 258.
- Gottlieb LM, Garcia K, Wing H, et al. Clinical interventions addressing nonmedical health determinants in Medicaid managed care. Am J Manag Care. 2016 May;22(5):370–6. PMID: 27266438. Exclusion Code: X7. [PubMed: 27266438]
- 259.
- Grazioli VS, Moullin JC, Kasztura M, et al. Implementing a case management intervention for frequent users of the emergency department (I-CaM): an effectiveness-implementation hybrid trial study protocol. BMC Health Serv Res. 2019 Jan 11;19(1):28. doi: 10.1186/s12913-018-3852-9. PMID: 30212929. Exclusion Code: X1. [PMC free article: PMC6330435] [PubMed: 30634955] [CrossRef]
- 260.
- Greenberg P, Corey-Lisle PK, Birnbaum H, et al. Economic implications of treatment-resistant depression among employees. Pharmacoeconomics. 2004;22(6):363–73. doi: 10.2165/00019053-200422060-00003. PMID: 12716790. Exclusion Code: X1. [PubMed: 15099122] [CrossRef]
- 261.
- Gregori D, Petrinco M, Barbati G, et al. Extreme regression models for characterizing high-cost patients. J Eval Clin Pract. 2009 Feb;15(1):164–71. doi: 10.1111/j.1365-2753.2008.00976.x. PMID: 23055291. Exclusion Code: X9. [PubMed: 19239597] [CrossRef]
- 262.
- Grembowski D, Schaefer J, Johnson KE, et al. A conceptual model of the role of complexity in the care of patients with multiple chronic conditions. Med Care. 2014 Mar;52 Suppl 3:S7–s14. doi: 10.1097/mlr.0000000000000045. PMID: 24561762. Exclusion Code: X4. [PubMed: 24561762] [CrossRef]
- 263.
- Griffin JL, Yersin M, Baggio S, et al. Characteristics and predictors of mortality among frequent users of an emergency department in Switzerland. Eur J Emerg Med. 2018 Apr;25(2):140–6. doi: 10.1097/mej.0000000000000425. PMID: 30358755. Exclusion Code: X1. [PubMed: 27749377] [CrossRef]
- 264.
- Grinspan ZM, Shapiro JS, Abramson EL, et al. Predicting frequent ED use by people with epilepsy with health information exchange data. Neurology. 2015 Sep 22;85(12):1031–8. doi: 10.1212/wnl.0000000000001944. PMID: 26152468. Exclusion Code: X4. [PMC free article: PMC4603600] [PubMed: 26311752] [CrossRef]
- 265.
- Grover CA, Crawford E, Close RJ. The efficacy of case management on emergency department frequent users: an eight-year observational study. J Emerg Med. 2016 Nov;51(5):595–604. doi: 10.1016/j.jemermed.2016.06.002. PMID: 26784515. Exclusion Code: X1. [PubMed: 27595372] [CrossRef]
- 266.
- Grover CA, Sughair J, Stoopes S, et al. Case management reduces length of stay, charges, and testing in emergency department frequent users. West J Emerg Med. 2018 Mar;19(2):238–44. doi: 10.5811/westjem.2017.9.34710. PMID: 30523008. Exclusion Code: X1. [PMC free article: PMC5851494] [PubMed: 29560049] [CrossRef]
- 267.
- Gruneir A, Cigsar C, Wang X, et al. Repeat emergency department visits by nursing home residents: a cohort study using health administrative data. BMC Geriatr. 2018 Jul 5;18(1):157. doi: 10.1186/s12877-018-0854-8. PMID: 28609191. Exclusion Code: X1. [PMC free article: PMC6034297] [PubMed: 29976135] [CrossRef]
- 268.
- Gudleski GD, Satchidanand N, Dunlap LJ, et al. Predictors of medical and mental health care use in patients with irritable bowel syndrome in the United States. Behav Res Ther. 2017 Jan;88:65–75. doi: 10.1016/j.brat.2016.07.006. PMID: 26002407. Exclusion Code: X1. [PMC free article: PMC5300279] [PubMed: 28110677] [CrossRef]
- 269.
- Gundlapalli A, Hanks M, Stevens SM, et al. It takes a village: a multidisciplinary model for the acute illness aftercare of individuals experiencing homelessness. J Health Care Poor Underserved. 2005 May;16(2):257–72. doi: 10.1353/hpu.2005.0033. PMID: 15937390. Exclusion Code: X3. [PubMed: 15937390] [CrossRef]
- 270.
- Gundlapalli AV, Jones AL, Redd A, et al. Characteristics of the highest users of emergency services in Veterans Affairs hospitals: homeless and non-homeless. Stud Health Technol Inform. 2017;238:24–7. Exclusion Code: X4. [PMC free article: PMC6044274] [PubMed: 28679878]
- 271.
- Gunnarsdottir OS, Rafnsson V. Mortality of the users of a hospital emergency department. Emerg Med J. 2006 Apr;23(4):269–73. doi: 10.1136/emj.2005.026690. PMID: 16445478. Exclusion Code: X1. [PMC free article: PMC2579499] [PubMed: 16549571] [CrossRef]
- 272.
- Gupta ND, Greve J. Overweight and obesity and the utilization of primary care physicians. Health Econ. 2011 Sep;20 Suppl 1:53–67. doi: 10.1002/hec.1711. PMID: 20878846. Exclusion Code: X1. [PubMed: 21351183] [CrossRef]
- 273.
- Gusmano MK, Rodwin VG, Weisz D. Medicare Beneficiaries Living In Housing With Supportive Services Experienced Lower Hospital Use Than Others. Health Aff (Millwood). 2018 Oct;37(10):1562–9. doi: 10.1377/hlthaff.2018.0070. PMID: 30273020. Exclusion Code: X1. [PubMed: 30273020] [CrossRef]
- 274.
- Gyurmey T, Kwiatkowski J. Program of All-Inclusive Care for the Elderly (PACE): Integrating Health and Social Care Since 1973. R I Med J (2013). 2019 Jun 4;102(5):30–2. PMID: 31959926. Exclusion Code: X1. [PubMed: 31167525]
- 275.
- Haas LJ, Spendlove DC, Silver MP. Expanding mental health services to older high-utilizing HMO patients: a pilot study. J Clin Psychol Med Settings. 2001;8(3):189–97. doi: 10.1023/A:1011321704324. PMID: CN-00442641. Exclusion Code: X1. [CrossRef]
- 276.
- Hamblin A. Opportunity knocks to improve care for “super utilizers”. CHCS Blog. Hamilton, New Jersey: Center for Health Care Strategies, Inc.; 2015. Exclusion Code: X1.
- 277.
- Hamblin A. Expanding an inventory of programs serving high-need, high-cost populations. Center for Health Care Strategies 2017. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191185. Exclusion Code: X7. - 278.
- Hamblin A, Davis R, Hunt K. Outreach to high-need, high-cost individuals: best practices for New York health homes. New York: New York State Health Foundation; 2014. Exclusion Code: X1.
- 279.
- Hammond S. Staying home after discharge: nurse-led versus physician-led transitional care models. Nurse Leader. 2015 Jun;13(3):66-+. doi: 10.1016/j.mnl.2014.09.012. PMID: WOS:000438196100016. Exclusion Code: X1. [CrossRef]
- 280.
- Hand C, McColl MA, Birtwhistle R, et al. Social isolation in older adults who are frequent users of primary care services. Can Fam Physician. 2014 Jun;60(6):e322, e4–9. PMID: 24628805. Exclusion Code: X1. [PMC free article: PMC4055344] [PubMed: 24925967]
- 281.
- Hansagi H, Olsson M, Hussain A, et al. Is information sharing between the emergency department and primary care useful to the care of frequent emergency department users? Eur J Emerg Med. 2008 Feb;15(1):34–9. doi: 10.1097/MEJ.0b013e3282aa4115. PMID: 18459089. Exclusion Code: X1. [PubMed: 18180664] [CrossRef]
- 282.
- Hansagi H, Olsson M, Sjoberg S, et al. Frequent use of the hospital emergency department is indicative of high use of other health care services. Ann Emerg Med. 2001 Jun;37(6):561–7. doi: 10.1067/mem.2001.111762. PMID: 11240313. Exclusion Code: X1. [PubMed: 11385324] [CrossRef]
- 283.
- Hardie TL, Polek C, Wheeler E, et al. Characterising emergency department high-frequency users in a rural hospital. Emerg Med J. 2015 Jan;32(1):21–5. doi: 10.1136/emermed-2013-202369. PMID: 25564171. Exclusion Code: X4. [PubMed: 24351523] [CrossRef]
- 284.
- Hardin L, Trumbo S, Murray V. What matters most in driving cross-sector partnerships for complex populations.[webinar]. The National Center for Complex Health and Social Needs; 2019. https://www
.nationalcomplex .care/research-policy /resources/webinars /what-matters-most-in-driving-cross-sector-partnerships-for-complex-populations/. Exclusion Code: X1. - 285.
- Haroun D, Smits F, van Etten-Jamaludin F, et al. The effects of interventions on quality of life, morbidity and consultation frequency in frequent attenders in primary care: a systematic review. European Journal of General Practice. 2016;22(2):71–82. doi: 10.3109/13814788.2016.1161751. PMID: WOS:000379260000002. Exclusion Code: X1. [PubMed: 27122125] [CrossRef]
- 286.
- Harrison C, Britt H, Miller G, et al. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open. 2014 Jul 11;4(7):e004694. doi: 10.1136/bmjopen-2013-004694. PMID: 24735803. Exclusion Code: X1. [PMC free article: PMC4120329] [PubMed: 25015470] [CrossRef]
- 287.
- Hart KM. Coordinated care of medically complex individuals in an individual-centered medical home: the surprising case of Mr. and Mrs. W. J Am Geriatr Soc. 2015 Jan;63(1):173–4. doi: 10.1111/jgs.13186. PMID: 26378131. Exclusion Code: X1. [PubMed: 25597566] [CrossRef]
- 288.
- Hawes EM, Smith JN, Pinelli NR, et al. Accountable Care in Transitions (ACTion): a team-based approach to reducing hospital utilization in a patient-centered medical home. J Pharm Pract. 2018 Apr;31(2):175–82. doi: 10.1177/0897190017707118. PMID: 29453868. Exclusion Code: X1. [PubMed: 28468524] [CrossRef]
- 289.
- Hawkins EJ, Lott AMK, Malte CA, et al. Patients’ perspectives on care management services for complex substance use disorders. J Addict Dis. 2017 Jul–Sep;36(3):193–206. doi: 10.1080/10550887.2017.1326654. PMID: 28481144. Exclusion Code: X11. [PMC free article: PMC9870041] [PubMed: 28481144] [CrossRef]
- 290.
- Hayes SL, McCarthy D. Care management plus: strengthening primary care for patients with multiple chronic conditions. Commonwealth Fund. 2016. https://www
.commonwealthfund .org/publications /case-study/2016 /dec/care-management-plus-strengthening-primary-care-patients-multiple. Exclusion Code: X1. - 291.
- Hayes SL, Salzberg CA, McCarthy D, et al. High-need, high-cost patients: who are they and how do they use health care? A population-based comparison of demographics, health care use, and expenditures. Issue Brief (Commonw Fund). 2016 Aug;26:1–14. PMID: 27571600. Exclusion Code: X4. [PubMed: 27571599]
- 292.
- Heincelman M, Schumann SO, Riley J, et al. Identification of high utilization inpatients on internal medicine services. Am J Med Sci. 2016 Jul;352(1):63–70. doi: 10.1016/j.amjms.2016.04.020. PMID: 28609191. Exclusion Code: X1. [PubMed: 27432036] [CrossRef]
- 293.
- Hempstead K, Delia D, Cantor JC, et al. The fragmentation of hospital use among a cohort of high utilizers: implications for emerging care coordination strategies for patients with multiple chronic conditions. Med Care. 2014;52:S67–74. doi: 10.1097/MLR.0000000000000049. PMID: 107888969. Language: English. Entry Date: 20140502. Revision Date: 20150712. Publication Type: Journal Article. Exclusion Code: X1. [PubMed: 24561761] [CrossRef]
- 294.
- Hempstead K, Delia D, Cantor JC, et al. Important study on high utilization released. Hotspot Blog. Camden, NJ: Camden Coalition; 2014. Exclusion Code: X4.
- 295.
- Hempstead K, Delia D, Cantor JC, et al. The fragmentation of hospital use among a cohort of high utilizers: implications for emerging care coordination strategies for patients with multiple chronic conditions. Med Care. 2014 Mar;52 Suppl 3:S67–74. doi: 10.1097/mlr.0000000000000049. PMID: 24561761. Exclusion Code: X4. [PubMed: 24561761] [CrossRef]
- 296.
- Henninger DE, Whitson HE, Cohen HJ, et al. Higher medical morbidity burden is associated with external locus of control. J Am Geriatr Soc. 2012 Apr;60(4):751–5. doi: 10.1111/j.1532-5415.2012.03904.x. PMID: 22897676. Exclusion Code: X1. [PMC free article: PMC3325320] [PubMed: 22458257] [CrossRef]
- 297.
- Henschen BL. Understanding perspectives of frequently-admitted hospital patients (CHAMP). Northwestern Memorial Hospital. Chicago: 2017. Exclusion Code: X1.
- 298.
- Henschen BL, Chapman M, Toms A, et al. The complex high admission management program (CHAMP): development and preliminary impact on hospital utilization. J Gen Intern Med. 2017;32(2):S799–S800. PMID: CN-01362537. Exclusion Code: X1.
- 299.
- Henschen BL, Liss DT, Golden B, et al. The education centered medical home improves primary care training and may mitigate burnout: a randomized medical education trial. J Gen Intern Med. 2019;34(2):S370–. doi: 10.1007/11606.1525-1497. PMID: CN-01980331. Exclusion Code: X1. [CrossRef]
- 300.
- Hensel JM, Taylor VH, Fung K, et al. Unique characteristics of high-cost users of medical care with comorbid mental illness or addiction in a population-based cohort. Psychosomatics. 2018 Mar – Apr;59(2):135–43. doi: 10.1016/j.psym.2017.10.005. PMID: 30305053. Exclusion Code: X1. [PubMed: 29157683] [CrossRef]
- 301.
- Hewner S, Chen CH, Anderson L, et al. Transitional Care Models for High-Need, High-Cost Adults in the United States A Scoping Review and Gap Analysis. Professional Case Management. 2021 Mar–Apr;26(2):82–98. doi: 10.1097/ncm.0000000000000442. PMID: WOS:000613200500005. Exclusion Code: X9. [PMC free article: PMC10576263] [PubMed: 32467513] [CrossRef]
- 302.
- Hibbard JH, Greene J, Sacks R, et al. Adding a measure of patient self-management capability to risk assessment can improve prediction of high costs. Health Aff (Millwood). 2016 Mar;35(3):489–94. doi: 10.1377/hlthaff.2015.1031. PMID: 26953304. Exclusion Code: X1. [PubMed: 26953304] [CrossRef]
- 303.
- Hildebrandt DE, Westfall JM, Nicholas RA, et al. Are frequent callers to family physicians high utilizers? Ann Fam Med. 2004;2(6):546–8. doi: 10.1370/afm.127. Exclusion Code: X1. [PMC free article: PMC1466750] [PubMed: 15576539] [CrossRef]
- 304.
- Hochman M, Asch SM. Disruptive Models in Primary Care: Caring for High-Needs, High-Cost Populations. J Gen Intern Med. 2017 Apr;32(4):392–7. doi: 10.1007/s11606-016-3945-2. PMID: 26344939. Exclusion Code: X1. [PMC free article: PMC5377887] [PubMed: 28243870] [CrossRef]
- 305.
- Horrocks D, Kinzer D, Afzal S, et al. The adequacy of individual hospital data to identify high utilizers and assess community health. JAMA Intern Med. 2016;176(6):856–8. doi: 10.1001/jamainternmed.2016.1248. PMID: 116097739. Language: English. Entry Date: 20180228. Revision Date: 20180413. Publication Type: journal article. Journal Subset: Biomedical. Exclusion Code: X1. [PubMed: 27111673] [CrossRef]
- 306.
- Horvath B, Silberberg M, Landerman LR, et al. Dynamics of patient targeting for care management in medicaid: a case study of the Durham Community Health Network. Care Management Journals. 2006 Fall2006;7(3):107–14. PMID: 106222651. Language: English. Entry Date: 20070126. Revision Date: 20150820. Publication Type: Journal Article. Exclusion Code: X1. [PubMed: 17214243]
- 307.
- Horwood H, Williams MJ, Mandic S. Examining motivations and barriers for attending maintenance community-based cardiac rehabilitation using the health-belief model. Heart Lung Circ. 2015 Oct;24(10):980–7. doi: 10.1016/j.hlc.2015.03.023. PMID: 25795473. Exclusion Code: X1. [PubMed: 25939724] [CrossRef]
- 308.
- Hostetter M, Klein S, McCarthy D. Aging gracefully: the PACE approach to caring for frail elders in the community. [New York, NY]: Commonwealth Fund; 2016. Exclusion Code: X1.
- 309.
- Hostetter M, Klein S, McCarthy D. Project ECHO’s Complex Care Initiative: building capacity to help “superutilizers” in underserved communities. Commonwealth Fund. 2016. https://www
.commonwealthfund .org/publications /case-study/2016 /aug/project-echos-complex-care-initiative-building-capacity-help. Exclusion Code: X1. - 310.
- Hostetter M, Klein S, McCarthy D. CareMore: improving outcomes and controlling health care spending for high-needs patients. Commonwealth Fund. 2017. https://www
.commonwealthfund .org/publications /case-study/2017 /mar/caremore-improving-outcomes-and-controlling-health-care-spending. Exclusion Code: X1. - 311.
- Hostetter M, Klein S, McCarthy D, et al. Guided care: a structured approach to providing comprehensive primary care for complex patients. Commonwealth Fund. 2016. https://www
.commonwealthfund .org/publications /case-study/2016 /oct/guided-care-structured-approach-providing-comprehensive-primary. Exclusion Code: X1. - 312.
- Hsu KY, Slightam C, Shaw JG, et al. High-Need Patients’ Goals and Goal Progress in a Veterans Affairs Intensive Outpatient Care Program. J Gen Intern Med. 2019 Aug;34(8):1564–70. doi: 10.1007/s11606-019-05010-w. PMID: 31283719. Exclusion Code: X1. [PMC free article: PMC6667543] [PubMed: 31140094] [CrossRef]
- 313.
- Hu J, Wang F, Sun J, et al. A healthcare utilization analysis framework for hot spotting and contextual anomaly detection. AMIA. Annual Symposium proceedings / AMIA Symposium. AMIA Symposium. 2012;2012:360–9. Exclusion Code: X1. [PMC free article: PMC3540544] [PubMed: 23304306]
- 314.
- Hu Z, Hao S, Jin B, et al. Online Prediction of Health Care Utilization in the Next Six Months Based on Electronic Health Record Information: A Cohort and Validation Study. J Med Internet Res. 2015 Sep 22;17(9):e219. doi: 10.2196/jmir.4976. PMID: 26395541. Exclusion Code: X1. [PMC free article: PMC4642374] [PubMed: 26395541] [CrossRef]
- 315.
- Huang JA, Lai CS, Tsai WC, et al. Determining factors of patient satisfaction for frequent users of emergency services in a medical center. J Chin Med Assoc. 2004 Aug;67(8):403–10. PMID: 15889019. Exclusion Code: X1. [PubMed: 15553800]
- 316.
- Huang JA, Tsai WC, Chen YC, et al. Factors associated with frequent use of emergency services in a medical center. J Formos Med Assoc. 2003 Apr;102(4):222–8. PMID: 12449444. Exclusion Code: X1. [PubMed: 12833184]
- 317.
- Huang JA, Weng RH, Tsai WC, et al. Analysis of emergency department utilization by elderly patients under National Health Insurance. Kaohsiung J Med Sci. 2003 Mar;19(3):113–20. doi: 10.1016/s1607-551x(09)70458-9. PMID: 12603227. Exclusion Code: X1. [PubMed: 12751871] [CrossRef]
- 318.
- Huang YY, Cheng SH. A community pharmacist home visit project for high utilizers under a universal health system: a preliminary assessment. Health Policy. 2019;123(4):373–8. doi: 10.1016/j.healthpol.2019.01.011. Exclusion Code: X1. [PubMed: 30739818] [CrossRef]
- 319.
- Hudon C, Chouinard MC, Diadiou F, et al. The Chronic Disease Self-Management Program: the experience of frequent users of health care services and peer leaders. Fam Pract. 2016 Apr;33(2):167–71. doi: 10.1093/fampra/cmw007. PMID: 27401827. Exclusion Code: X1. [PMC free article: PMC4793803] [PubMed: 26984994] [CrossRef]
- 320.
- Hudon C, Chouinard MC, Diadiou F, et al. Case management in primary care for frequent users of health care services with chronic diseases: a qualitative study of patient and family experience. Ann Fam Med. 2015 Nov;13(6):523–8. doi: 10.1370/afm.1867. PMID: 27074709. Exclusion Code: X6. [PMC free article: PMC4639377] [PubMed: 26553891] [CrossRef]
- 321.
- Hudon C, Chouinard MC, Dubois MF, et al. Case management in primary care for frequent users of health care services: a mixed methods study. Ann Fam Med. 2018 May;16(3):232–9. doi: 10.1370/afm.2233. PMID: 30759630. Exclusion Code: X1. [PMC free article: PMC5951252] [PubMed: 29760027] [CrossRef]
- 322.
- Huffstetler AN, Phillips RL. Payment Structures That Support Social Care Integration With Clinical Care: Social Deprivation Indices and Novel Payment Models. Am J Prev Med. 2019 Dec;57(6):S82–S8. doi: 10.1016/j.amepre.2019.07.011. PMID: WOS:000496954300010. Exclusion Code: X1. [PubMed: 31753283] [CrossRef]
- 323.
- Hulen E, Laliberte A, Ono S, et al. “Eyes in the Home”: Addressing Social Complexity in Veterans Affairs Home-Based Primary Care. J Gen Intern Med. 2021 Jan 11. doi: 10.1007/s11606-020-06356-2. Exclusion Code: X1. [PMC free article: PMC8042101] [PubMed: 33432431] [CrossRef]
- 324.
- Humowiecki M, Kuruna T, Sax R, et al. Blueprint for complex care: advancing the field of care for individuals with complex health and social needs. National Center for Complex Health and Social Needs: Center for Health Care Strategies, and Institute for Healthcare Improvement; 2018. Exclusion Code: X7.
- 325.
- Hwang AS, Liu SW, Yelibi C, et al. Characteristics of persistent emergency department high utilizers in a large primary care practice-based research network. J Gen Intern Med. 2014 Apr;29:S47–S. PMID: WOS:000340996200116. Exclusion Code: X4.
- 326.
- Hyer JM, Ejaz A, Diaz A, et al. Characterizing and Assessing the Impact of Surgery on Healthcare Spending Among Medicare Enrolled Preoperative Super-utilizers. Ann Surg. 2019 Sep;270(3):554–63. doi: 10.1097/sla.0000000000003426. PMID: 31140094. Exclusion Code: X1. [PubMed: 31305286] [CrossRef]
- 327.
- Hyer JM, Tsilimigras DI, Gani F, et al. Factors associated with switching between low and super utilization in the surgical population: A study in medicare expenditure. Am J Surg. 2020 Jan;219(1):1–7. doi: 10.1016/j.amjsurg.2019.07.042. PMID: 31835473. Exclusion Code: X9. [PubMed: 31405521] [CrossRef]
- 328.
- International R. Evaluation of Medicare care management for high cost beneficiaries (CMHCB) demonstration second annual report.Centers for Medicare & Medicaid Services; 2008. Exclusion Code: X1.
- 329.
- Iovan S, Lantz PM, Allan K, et al. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2019 Apr 26:1077558719845722. doi: 10.1177/1077558719845722. Exclusion Code: X1. [PubMed: 31027455] [CrossRef]
- 330.
- Iovan S, Lantz PM, Allan K, et al. Interventions to decrease use in prehospital and emergency care settings among super-utilizers in the United States: a systematic review. Med Care Res Rev. 2020;77(2):99–111. doi: 10.1177/1077558719845722. Exclusion Code: X7. [PubMed: 31027455] [CrossRef]
- 331.
- Isasi F. Helping states achieve sustainable transformation of their health systems. National Governors Association Center for Best Practices: Commonwealth Fund; 2015. Exclusion Code: X7.
- 332.
- Ismail MS, Dagerman K, Tariot PN, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness- Alzheimer’s Disease (CATIE-AD): baseline characteristics. Curr Alzheimer Res. 2007 Jul;4(3):325–35. PMID: 17490826. Exclusion Code: X1. [PubMed: 17627490]
- 333.
- Jackson TS, Moran TP, Lin J, et al. Homelessness Among Patients in a Southeastern Safety Net Emergency Department. South Med J. 2019 Sep;112(9):476–82. doi: 10.14423/smj.0000000000001016. PMID: 31485585. Exclusion Code: X1. [PubMed: 31485585] [CrossRef]
- 334.
- Jean Scholz Mellum DM, Greer Glazer, Grant Martsolf, Barbara Tobias. A mixed methods study of the experience of older adults with multimorbidity in a Care Coordination Program. International Journal of Care Coordination. 2018. Exclusion Code: X11.
- 335.
- Jiang HJ, Russo A, Barrett M. Nationwide frequency and costs of potentially preventable hospitalizations, 2006. [Rockville, Md.: Agency for Healthcare Research and Quality; 2009. Exclusion Code: X1. [PubMed: 21510033]
- 336.
- Jimenez XF. Attachment in medical care: a review of the interpersonal model in chronic disease management. Chronic Illn. 2017 Mar;13(1):14–27. doi: 10.1177/1742395316653454. PMID: 27132979. Exclusion Code: X1. [PubMed: 27269506] [CrossRef]
- 337.
- Johnson D, Saavedra P, Sun E, et al. Community health workers and medicaid managed care in New Mexico. J Community Health. 2012 Jun;37(3):563–71. doi: 10.1007/s10900-011-9484-1. PMID: 21953498. Exclusion Code: X1. [PMC free article: PMC3343233] [PubMed: 21953498] [CrossRef]
- 338.
- Johnson I. Alcohol problems in old age: a review of recent epidemiological research. Int J Geriatr Psychiatry. 2000 Jul;15(7):575–81. PMID: 10929791. Exclusion Code: X1. [PubMed: 10918336]
- 339.
- Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015 Aug;34(8):1312–9. doi: 10.1377/hlthaff.2014.1186. PMID: 22943609. Exclusion Code: X4. [PubMed: 26240244] [CrossRef]
- 340.
- Johnston KJ, Hockenberry JM, Wadhera RK, et al. Clinicians With High Socially At-Risk Caseloads Received Reduced Merit-Based Incentive Payment System Scores. Health Aff (Millwood). 2020 Sep;39(9):1504–12. doi: 10.1377/hlthaff.2020.00350. Exclusion Code: X1. [PubMed: 32897781] [CrossRef]
- 341.
- Johnston KJ, Wen H, Joynt Maddox KE. Inadequate Risk Adjustment Impacts Geriatricians’ Performance on Medicare Cost and Quality Measures. J Am Geriatr Soc. 2020 Feb;68(2):297–304. doi: 10.1111/jgs.16297. PMID: 32178926. Exclusion Code: X1. [PubMed: 31880310] [CrossRef]
- 342.
- Johnston SS, Ammann EM, Kashyap SR, et al. Body mass index and insulin use as identifiers of high-cost patients with type 2 diabetes: A retrospective analysis of electronic health records linked to insurance claims data. Diabetes Obes Metab. 2019 Jun;21(6):1419–28. doi: 10.1111/dom.13671. PMID: 31752866. Exclusion Code: X1. [PubMed: 30768824] [CrossRef]
- 343.
- Jones AL, Thomas R, Hedayati DO, et al. Patient predictors and utilization of health services within a medical home for homeless persons. Subst Abus. 2018;39(3):354–60. doi: 10.1080/08897077.2018.1437500. PMID: 29412071. Exclusion Code: X11. [PubMed: 29412071] [CrossRef]
- 344.
- Jones IR, Ahmed N, Kelly M, et al. With an attack I associate it more with going into hospital: understandings of asthma and psychosocial stressors; are they related to use of services? Soc Sci Med. 2008 Feb;66(3):765–75. doi: 10.1016/j.socscimed.2007.09.012. PMID: 18384440. Exclusion Code: X1. [PubMed: 18006131] [CrossRef]
- 345.
- Jorgenson DJ, Landry EJ, Lysak KJ. A mixed methods evaluation of a patient care clinic located within a pharmacy school. Int J Clin Pharm. 2016 Aug;38(4):924–30. doi: 10.1007/s11096-016-0313-6. PMID: 27067760. Exclusion Code: X1. [PubMed: 27166829] [CrossRef]
- 346.
- Joynt KE, Gawande AA, Orav EJ, et al. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013 Jun 26;309(24):2572–8. doi: 10.1001/jama.2013.7103. PMID: 22581227. Exclusion Code: X9. [PubMed: 23797716] [CrossRef]
- 347.
- Jung D, Kind A, Robert S, et al. Linking neighborhood context and health in community-dwelling older adults in the Medicare Advantage program. J Am Geriatr Soc. 2018 Jul;66(6):1158–64. doi: 10.1111/jgs.15366. Exclusion Code: X1. [PMC free article: PMC6105383] [PubMed: 29648689] [CrossRef]
- 348.
- Kadakia K, McClellan M, Udadyakumar K, et al. Adapting promising innovations to meet the needs of high-need, high-cost populations. 2019. https://www
.commonwealthfund .org/blog/2019 /adapting-promising-innovations-meet-needs-high-need-high-cost-populations. Exclusion Code: X1. - 349.
- Kang-Lim TJY. Emergency Department: Effectiveness of a Referral Intervention for High Utilizers: George Fox University; 2014. Exclusion Code: X3.
- 350.
- Kaplowitz RA, Scranton RE, Gagnon DR, et al. Health care utilization and receipt of cholesterol testing by veterans with and those without mental illness. Gen Hosp Psychiatry. 2006 Mar–Apr;28(2):137–44. doi: 10.1016/j.genhosppsych.2005.10.002. PMID: 16876547. Exclusion Code: X1. [PubMed: 16516064] [CrossRef]
- 351.
- Kapur N. Frequent attenders in primary care; 2007. Exclusion Code: X1.
- 352.
- Kapur N, Hunt I, MacFarlane G, et al. Childhood experience and health care use in adulthood - nested case-control study. Br J Psychiatry. 2004 Aug;185:134–9. doi: 10.1192/bjp.185.2.134. PMID: WOS:000223206500008. Exclusion Code: X1. [PubMed: 15286064] [CrossRef]
- 353.
- Kaskie B, Wallace N, Kang S, et al. The implementation of managed behavioral healthcare in Colorado and the effects on older Medicaid beneficiaries. J Ment Health Policy Econ. 2006 Mar;9(1):15–24. PMID: 16959131. Exclusion Code: X1. [PubMed: 16733268]
- 354.
- Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002 Oct;53(4):859–63. doi: 10.1016/s0022-3999(02)00313-6. PMID: WOS:000178737100005. Exclusion Code: X1. [PubMed: 12377294] [CrossRef]
- 355.
- Katz A, Martens P, Chateau D, et al. Do primary care physicians coordinate ambulatory care for chronic disease patients in Canada? BMC Fam Pract. 2014 Aug 30;15:148. doi: 10.1186/1471-2296-15-148. PMID: 25047884. Exclusion Code: X1. [PMC free article: PMC4160545] [PubMed: 25175589] [CrossRef]
- 356.
- Kaushal R. Identifying and predicting patients with preventable high utilization. Joan & Sanford I. Weill Medical College of Cornell University. New York: 2017. https://www
.pcori.org /research-results/2016 /identifying-and-predicting-patients-preventable-high-utilization. Exclusion Code: X7. - 357.
- Keeney T, Belanger E, Jones RN, et al. High-Need Phenotypes in Medicare Beneficiaries: Drivers of Variation in Utilization and Outcomes. J Am Geriatr Soc. 2020 Jan;68(1):70–7. doi: 10.1111/jgs.16146. PMID: 31876646. Exclusion Code: X1. [PMC free article: PMC6952536] [PubMed: 31454082] [CrossRef]
- 358.
- Kehusmaa S, Autti-Ramo I, Helenius H, et al. Factors associated with the utilization and costs of health and social services in frail elderly patients. BMC Health Serv Res. 2012 Jul 19;12:204. doi: 10.1186/1472-6963-12-204. PMID: 22486678. Exclusion Code: X1. [PMC free article: PMC3476428] [PubMed: 22812588] [CrossRef]
- 359.
- Ken Coburn CG, Sophia Demuynck, Margaret Hawthorne. Replicating Effective Models Of Complex Care Management For Older Adults. Health Affairs Blog; 2017. Exclusion Code: X1.
- 360.
- Kern L. Developing indicators of care fragmentation that predict service utilization by high-need, high-cost patients. Cornell University, Weill Cornell Medical College, Department of Healthcare Policy and Research 2015. https://www
.ajmc.com /journals/issue/2018 /2018-vol24-n9/fragmented-ambulatory-care-and-subsequent-healthcare-utilization-among-medicare-beneficiaries?p=4. Exclusion Code: X7. - 361.
- Kim JJ, Kwok ESH, Cook OG, et al. Characterizing highly frequent users of a large Canadian urban emergency department. West J Emerg Med. 2018 Nov;19(6):926–33. doi: 10.5811/westjem.2018.9.39369. PMID: 27749377. Exclusion Code: X1. [PMC free article: PMC6225932] [PubMed: 30429923] [CrossRef]
- 362.
- Kim TY, Mortensen K, Eldridge B. Linking uninsured patients treated in the emergency department to primary care shows some promise in Maryland. Health Aff (Millwood). 2015 May;34(5):796–804. doi: 10.1377/hlthaff.2014.1102. PMID: 25639993. Exclusion Code: X1. [PubMed: 25941281] [CrossRef]
- 363.
- Kimmel HJ, Brice YN, Trikalinos TA, et al. Real-time emergency department electronic notifications regarding high-risk patients: a systematic review. Telemedicine and e-Health. 2019;25(7):604–18. doi: 10.1089/tmj.2018.0117. Exclusion Code: X1. [PMC free article: PMC6664825] [PubMed: 30129886] [CrossRef]
- 364.
- Kirk C. Telephone follow-up of older people after hospital admissions. Curr Aging Sci. 2014;7(2):144–53. PMID: 24588292. Exclusion Code: X1. [PubMed: 25101710]
- 365.
- Kirkpatrick S, Agana DFG, Lynch K, et al. Emergency department high utilizers among family medicine patients. J Am Board Fam Med. 2019;32(2):264–8. doi: 10.3122/jabfm.2019.02.180184. Exclusion Code: X4. [PubMed: 30850463] [CrossRef]
- 366.
- Klein S, Hostetter M, McCarthy D. Bringing primary care home: the medical house call program at MedStar Washington Hospital Center. Commonwealth Fund. 2016. https://www
.commonwealthfund .org/publications /case-study/2016 /jul/bringing-primary-care-home-medical-house-call-program-medstar. Exclusion Code: X1. - 367.
- Klein S, Hostetter M, McCarthy D. The “one care” program at Commonwealth Care Alliance. Commonwealth Fund. 2016. https://www
.commonwealthfund .org/publications /case-study/2016 /dec/one-care-program-commonwealth-care-alliance. Exclusion Code: X1. - 368.
- Klein S, Hostetter M, McCarthy D. Caring for high-need, high-cost patients: exemplars from the field. 2016. https://www
.commonwealthfund .org/blog/2016 /caring-high-need-high-cost-patients-exemplars-field. Exclusion Code: X1. - 369.
- Klompstra L, Ekdahl AW, Krevers B, et al. Factors related to health-related quality of life in older people with multimorbidity and high health care consumption over a two-year period. BMC Geriatr. 2019 Jul 5;19(1):187. doi: 10.1186/s12877-019-1194-z. PMID: 30827894. Exclusion Code: X6. [PMC free article: PMC6612189] [PubMed: 31277674] [CrossRef]
- 370.
- Knowlton A, Weir BW, Hughes BS, et al. Patient demographic and health factors associated with frequent use of emergency medical services in a midsized city. Acad Emerg Med. 2013 Nov;20(11):1101–11. doi: 10.1111/acem.12253. PMID: 25287826. Exclusion Code: X4. [PMC free article: PMC4063348] [PubMed: 24238312] [CrossRef]
- 371.
- Knox K, Schneider J, Gatmaitan V, et al. Breaking the cycle: a successful intervention for high utilizers on a general medicine service. J Gen Intern Med. 2017 Apr;32:S738–S9. PMID: WOS:000440259003121. Exclusion Code: X1.
- 372.
- Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology. 2011 May;16(4):617–24. doi: 10.1111/j.1440-1843.2010.01921.x. PMID: 21734226. Exclusion Code: X1. [PubMed: 21199163] [CrossRef]
- 373.
- Ko M, Lee Y, Chen C, et al. Prevalence of and predictors for frequent utilization of emergency department: a population-based study. Medicine (Baltimore). 2015 Jul;94(29):e1205. doi: 10.1097/md.0000000000001205. PMID: 25956826. Exclusion Code: X1. [PMC free article: PMC4603019] [PubMed: 26200638] [CrossRef]
- 374.
- Koball AM, Rasmussen C, Olson-Dorff D, et al. The relationship between adverse childhood experiences, healthcare utilization, cost of care and medical comorbidities. Child Abuse Negl. 2019;90:120–6. doi: 10.1016/j.chiabu.2019.01.021. Exclusion Code: X1. [PubMed: 30776737] [CrossRef]
- 375.
- Koch KL, Karafin MS, Simpson P, et al. Intensive management of high-utilizing adults with sickle cell disease lowers admissions. Am J Hematol. 2015 Mar;90(3):215–9. doi: 10.1002/ajh.23912. PMID: 23058483. Exclusion Code: X4. [PubMed: 25469750] [CrossRef]
- 376.
- KPMG Government Institute. Investing in social services as a core strategy for healthcare organizations: developing the business case. Commonwealth Fund. 2018. https://www
.commonwealthfund .org/publications /other-publication /2018/mar/investing-social-services-core-strategy-healthcare. Exclusion Code: X1. - 377.
- Kramer W. How employers are improving care and lowering costs for complex patients. Commonwealth Fund; 2016. Exclusion Code: X1.
- 378.
- Kreuter MW, Garg R, Li L, et al. How Do Social Needs Cluster Among Low-Income Individuals? Popul Health Manag. 2020 Sep 1. doi: 10.1089/pop.2020.0107. PMID: 32877298. Exclusion Code: X11. [PMC free article: PMC8215422] [PubMed: 32877298] [CrossRef]
- 379.
- Krieg C, Hudon C, Chouinard MC, et al. Individual predictors of frequent emergency department use: a scoping review. BMC Health Serv Res. 2016 Oct 20;16(1):594. doi: 10.1186/s12913-016-1852-1. PMID: 27756416. Exclusion Code: X1. [PMC free article: PMC5072329] [PubMed: 27765045] [CrossRef]
- 380.
- Kromka W, Simpson S. A Narrative Review of Predictors of Adult Mental Health Emergency Department Return Visits and Interventions to Reduce Repeated Use. J Emerg Med. 2019 Nov;57(5):671–82. doi: 10.1016/j.jemermed.2019.08.005. PMID: 31270786. Exclusion Code: X7. [PubMed: 31610908] [CrossRef]
- 381.
- Krupski BJCDMCZA. Predictive Risk Intelligence System (Prism): A Decision-Support Tool for Coordinating Care for Complex Medicaid Client. Comprehensive Care Coordination for Chronically Ill Adults. 2011. Exclusion Code: X11.
- 382.
- Ku BS, Fields JM, Santana A, et al. The urban homeless: super-users of the emergency department. Popul Health Manag. 2014 Dec;17(6):366–71. doi: 10.1089/pop.2013.0118. PMID: 28602485. Exclusion Code: X4. [PubMed: 24865472] [CrossRef]
- 383.
- Kuek BJW, Li H, Yap S, et al. Characteristics of frequent users of emergency medical services in Singapore. Prehosp Emerg Care. 2019 Mar–Apr;23(2):215–24. doi: 10.1080/10903127.2018.1484969. PMID: 30458798. Exclusion Code: X1. [PubMed: 30118627] [CrossRef]
- 384.
- Kujanp T, Ylisaukko-Oja T, Jokelainen J, et al. Prevalence of anxiety disorders among Finnish primary care high utilizers and validation of Finnish translation of GAD-7 and GAD-2 screening tools. Scand J Prim Health Care. 2014;32(2):78–83. doi: 10.3109/02813432.2014.920597. PMID: 103964008. Language: English. Entry Date: 20140620. Revision Date: 20151123. Publication Type: Journal Article. Exclusion Code: X1. [PMC free article: PMC4075021] [PubMed: 24920316] [CrossRef]
- 385.
- Kuluski K, Gandhi S, Diong C, et al. Patterns of community follow-up, subsequent health service use and survival among young and mid-life adults discharged from chronic care hospitals: a retrospective cohort study. BMC Health Serv Res. 2016 Aug 13;16(1):382. doi: 10.1186/s12913-016-1631-z. PMID: 27042746. Exclusion Code: X1. [PMC free article: PMC4983410] [PubMed: 27522347] [CrossRef]
- 386.
- Kumar GS, Klein R. Effectiveness of case management strategies in reducing emergency department visits in frequent user patient populations: a systematic review. J Emerg Med. 2013 Mar;44(3):717–29. doi: 10.1016/j.jemermed.2012.08.035. PMID: WOS:000315946900033. Exclusion Code: X1. [PubMed: 23200765] [CrossRef]
- 387.
- Kurdyak P, Newman A, Segal Z. Impact of mindfulness-based cognitive therapy on health care utilization: a population-based controlled comparison. J Psychosom Res. 2014;77(2):85–9. doi: 10.1016/j.jpsychores.2014.06.009. PMID: CN-00998955. Exclusion Code: X1. [PubMed: 25077847] [CrossRef]
- 388.
- Kurpas D, Szwamel K, Mroczek B. Frequent attenders with chronic respiratory diseases in primary care settings. 2016. p. 17–29. Exclusion Code: X1. [PubMed: 27573642]
- 389.
- Kwakkenbos L, Bluyssen SJ, Vonk MC, et al. Addressing patient health care demands in systemic sclerosis: pre-post assessment of a psycho-educational group programme. Clin Exp Rheumatol. 2011 Mar–Apr;29(2 Suppl 65):S60–5. PMID: 22096551. Exclusion Code: X1. [PubMed: 21586220]
- 390.
- Labby D, Wright B, Broffman L, et al. Drivers of High-cost Medical Complexity in a Medicaid Population. Med Care. 2020 Mar;58(3):208–15. doi: 10.1097/mlr.0000000000001261. PMID: 31584961. Exclusion Code: X9. [PMC free article: PMC7017939] [PubMed: 31876646] [CrossRef]
- 391.
- LaCalle E, Rabin E. Frequent users of emergency departments: the myths, the data, and the policy implications. Ann Emerg Med. 2010 Jul;56(1):42–8. doi: 10.1016/j.annemergmed.2010.01.032. PMID: 20346540. Exclusion Code: X1. [PubMed: 20346540] [CrossRef]
- 392.
- LaFleur J, Fowler MA, McBeth C, et al. Incidence of drug-related problems among Medicaid high utilizers as identified by clinically-trained pharmacist reviewers. Pharmacotherapy. 2005 Mar;25(3):472-. PMID: WOS:000227288900111. Exclusion Code: X1.
- 393.
- LaFleur J, McBeth C, Gunning K, et al. Prevalence of drug-related problems and cost-savings opportunities in Medicaid high utilizers identified by a pharmacist-run drug regimen review center. J Manag Care Pharm. 2006;12(8):677–85. doi: 10.18553/jmcp.2006.12.8.677. Exclusion Code: X1. [PMC free article: PMC10438027] [PubMed: 17269846] [CrossRef]
- 394.
- Lally K, Tuya Fulton A, Ducharme C, et al. Using Nurse Care Managers Trained in the Serious Illness Conversation Guide to Increase Goals-of-Care Conversations in an Accountable Care Organization. J Palliat Med. 2020;23(1):112–5. doi: 10.1089/jpm.2019.0110. Exclusion Code: X1. [PubMed: 31081710] [CrossRef]
- 395.
- Lantz PM. “Super-utilizer” interventions: what they reveal about evaluation research, wishful thinking, and health equity. Milbank Q. 2020 Feb 7. doi: 10.1111/1468-0009.12449. PMID: 32030820. Exclusion Code: X1. [PMC free article: PMC7077764] [PubMed: 32030820] [CrossRef]
- 396.
- Lauffenburger JC, Mahesri M, Choudhry NK. Not there yet: using data-driven methods to predict who becomes costly among low-cost patients with type 2 diabetes. BMC Endocr Disord. 2020;20(1):N.PAG-N.PAG. doi: 10.1186/s12902-020-00609-1. PMID: 145256772. Exclusion Code: X9. [PMC free article: PMC7433196] [PubMed: 32807156] [CrossRef]
- 397.
- Law DD, Crane DR, Berge JM. The influence of individual, marital, and family therapy on high utilizers of health care. J Marital Fam Ther. 2003 Jul;29(3):353–63. PMID: 15241664. Exclusion Code: X1. [PubMed: 12870409]
- 398.
- LaWall E, Wu YY, Fan VY, et al. Living Alone and Homelessness as Predictors of 30-Day Potentially Preventable Hospital Readmission. Prev Chronic Dis. 2019 Feb 7;16:E16. doi: 10.5888/pcd16.180189. PMID: 30730829. Exclusion Code: X1. [PMC free article: PMC6395076] [PubMed: 30730829] [CrossRef]
- 399.
- LeClair AM, Sweeney M, Yoon GH, et al. Patients’ Perspectives on Reasons for Unplanned Readmissions. J Healthc Qual. 2019 Jul/Aug;41(4):237–42. doi: 10.1097/jhq.0000000000000160. PMID: 31651740. Exclusion Code: X1. [PubMed: 30180043] [CrossRef]
- 400.
- Ledoux Y, Minner P. Occasional and frequent repeaters in a psychiatric emergency room. Soc Psychiatry Psychiatr Epidemiol. 2006 Feb;41(2):115–21. doi: 10.1007/s00127-005-0010-6. PMID: 16982382. Exclusion Code: X1. [PubMed: 16508721] [CrossRef]
- 401.
- Lee JY, Muratov S, Tarride JE, et al. Managing High-Cost Healthcare Users: The International Search for Effective Evidence-Supported Strategies. J Am Geriatr Soc. 2018 May;66(5):1002–8. doi: 10.1111/jgs.15257. PMID: 29398469. Exclusion Code: X6. [PubMed: 29427509] [CrossRef]
- 402.
- Lee KH, Davenport L. Can case management interventions reduce the number of emergency department visits by frequent users? Health Care Manag (Frederick). 2006 Apr–Jun;25(2):155–9. PMID: 17150058. Exclusion Code: X1. [PubMed: 16699330]
- 403.
- Legault F, Humbert J, Amos S, et al. Difficulties encountered in collaborative care: logistics trumps desire. J Am Board Fam Med. 2012 Mar–Apr;25(2):168–76. doi: 10.3122/jabfm.2012.02.110153. PMID: 21614451. Exclusion Code: X1. [PubMed: 22403197] [CrossRef]
- 404.
- Legramante JM, Morciano L, Lucaroni F, et al. Frequent use of emergency departments by the elderly population when continuing care is not well established. PLoS One. 2016;11(12):e0165939. doi: 10.1371/journal.pone.0165939. PMID: 25753453. Exclusion Code: X1. [PMC free article: PMC5156362] [PubMed: 27973563] [CrossRef]
- 405.
- Leininger L, Avery K. The capacity of self-reported health measures to predict high-need Medicaid enrollees. Princeton, NJ: Robert Wood Johnson Foundation; 2015. Exclusion Code: X4.
- 406.
- Leininger LJ, Friedsam D, Voskuil K, et al. Predicting high-need cases among new Medicaid enrollees. Am J Manag Care. 2014 Sep 1;20(9):e399–407. PMID: 23636712. Exclusion Code: X1. [PubMed: 25364876]
- 407.
- Lenz O, Sadhu S, Fornoni A, et al. Overutilization of central venous catheters in incident hemodialysis patients: reasons and potential resolution strategies. Semin Dial. 2006 Nov–Dec;19(6):543–50. doi: 10.1111/j.1525-139X.2006.00220.x. PMID: 19265720. Exclusion Code: X1. [PubMed: 17150058] [CrossRef]
- 408.
- Leporatti L, Ameri M, Trinchero C, et al. Targeting frequent users of emergency departments: prominent risk factors and policy implications. Health Policy. 2016 May;120(5):462–70. doi: 10.1016/j.healthpol.2016.03.005. PMID: 27046165. Exclusion Code: X1. [PubMed: 27033015] [CrossRef]
- 409.
- Levin S, Brown D, Hernandez E. Contra Costa Health Services whole person care (CommunityConnect) program evaluation (CCHS-WPC). Contra Costa Health Services. 2019. https:
//clinicaltrials .gov/ct2/show/record /NCT04000074?term=NCT04000074&draw =2&rank=1. Exclusion Code: X7. - 410.
- Levinson CM, Druss BG. Health beliefs and depression in a group of elderly high utilizers of medical services. Gen Hosp Psychiatry. 2005;27(2):97–9. doi: 10.1016/j.genhosppsych.2004.09.010. Exclusion Code: X4. [PubMed: 15763120] [CrossRef]
- 411.
- Levy C, Balogh S, Perkins E. Realizing the potential of rehabilitative care for people with complex health conditions: the time is now. Healthc Q. 2016;19(2):49–54. PMID: 27591400. Exclusion Code: X1. [PubMed: 27700974]
- 412.
- Lewis C, Shah A, Abrams MK, et al. Sick and struggling: high-need adults with financial difficulties have worse access to the quality care they need. 2018. https://www
.commonwealthfund .org/blog/2018 /sick-and-struggling-high-need-adults-financial-difficulties-have-worse-access-quality. Exclusion Code: X4. - 413.
- Lewis C, Shah T, Abrams MK. Sick and alone: high-need, socially isolated adults have more problems, but less support. Commonwealth Fund. 2018. https://www
.commonwealthfund .org/blog/2018 /sick-and-alone-high-need-socially-isolated-adults-have-more-problems-less-support. Exclusion Code: X1. - 414.
- Lewis VA, Larson BK, McClurg AB, et al. The promise and peril of accountable care for vulnerable populations: a framework for overcoming obstacles. Health Aff (Millwood). 2012 Aug;31(8):1777–85. doi: 10.1377/hlthaff.2012.0490. PMID: 22869656. Exclusion Code: X1. [PubMed: 22869656] [CrossRef]
- 415.
- Li DR, Brennan JJ, Kreshak AA, et al. Patients Who Leave the Emergency Department Without Being Seen and Their Follow-Up Behavior: A Retrospective Descriptive Analysis. J Emerg Med. 2019;57(1):106–13. doi: 10.1016/j.jemermed.2019.03.051. Exclusion Code: X9. [PubMed: 31078346] [CrossRef]
- 416.
- Li Z, Newton-Dame R, Tatem K, et al. Homegrown safety net system predictive model for risk stratification: Assessing potential racial bias. Health Serv Res. 2020;55(SUPPL 1):76. doi: 10.1111/1475-6773.13435. Exclusion Code: X1. [CrossRef]
- 417.
- Lichtenberg P, Levinson D, Sharshevsky Y, et al. Clinical case management of revolving door patients - a semi-randomized study. Acta Psychiatr Scand. 2008 Jun;117(6):449–54. doi: 10.1111/j.1600-0447.2008.01170.x. PMID: 18185500. Exclusion Code: X4. [PubMed: 18331577] [CrossRef]
- 418.
- Lim A, Warning W. The role of a pharmacist in a super-utilizer care coordination team. Journal of Interprofessional Education and Practice. 2016;3:23–7. doi: 10.1016/j.xjep.2016.03.007. Exclusion Code: X1. [CrossRef]
- 419.
- Lin S, Zhang Q, Chen F, et al. Smooth Bayesian network model for the prediction of future high-cost patients with COPD. Int J Med Inform. 2019 Jun;126:147–55. doi: 10.1016/j.ijmedinf.2019.03.017. PMID: 29211140. Exclusion Code: X6. [PubMed: 31029256] [CrossRef]
- 420.
- Lin WC, Bharel M, Zhang J, et al. Frequent emergency department visits and hospitalizations among homeless people with Medicaid: implications for Medicaid expansion. Am J Public Health. 2015 Nov;105(Suppl 5):S716–22. doi: 10.2105/ajph.2015.302693. PMID: 26223659. Exclusion Code: X1. [PMC free article: PMC4627525] [PubMed: 26447915] [CrossRef]
- 421.
- Lindsay A, Hibbard JH, Boothroyd DB, et al. Patient activation changes as a potential signal for changes in health care costs: cohort study of US high-cost patients. J Gen Intern Med. 2018;33(12):2106–12. doi: 10.1007/s11606-018-4657-6. Exclusion Code: X9. [PMC free article: PMC6258627] [PubMed: 30291604] [CrossRef]
- 422.
- Lines L, Ash A. Predictors of potentially avoidable emergency department visits: a systematic review. 2012. Exclusion Code: X7.
- 423.
- Lipsitt DR. Characteristics of patient–doctor relationships with somatizing patients. International Congress Series. 2006;1287:374–7. doi: 10.1016/j.ics.2005.12.036. PMID: 20918417. Exclusion Code: X1. [CrossRef]
- 424.
- Liss DT, Schaeffer-Pettigrew C, Finch E, et al. A randomized comparative effectiveness trial of a transitional care clinic: 180-day effects. J Gen Intern Med. 2017;32(2):S100. Exclusion Code: X1. [PMC free article: PMC6712181] [PubMed: 31144279]
- 425.
- Little P, Somerville J, Williamson I, et al. Psychosocial, lifestyle, and health status variables in predicting high attendance among adults. Br J Gen Pract. 2001 Dec;51(473):987–94. PMID: 16274019. Exclusion Code: X1. [PMC free article: PMC1314191] [PubMed: 11766871]
- 426.
- Lloyd J. Opportunities to Advance Complex Care in Rural and Frontier Areas. . Center for Health Care Strategies. 2019. https://www
.chcs.org /resource/opportunities-to-advance-complex-care-in-rural-and-frontier-areas /?utm_source =CHCS+Email+Updates&utm _campaign =9c4f67c292-TCC+Rural+brief+05 %2F09 %2F19&utm_medium =email&utm_term =0_bbced451bf-9c4f67c292-157172209. Exclusion Code: X7. - 427.
- Locke SE, Ford P, McLaughlin T. A comparative cost analysis of participating versus non-participating somatizing patients referred to a behavioral medicine group in a health maintenance organization. Disease Management & Health Outcomes. 2003;11(5):327–35. PMID: 106078697. Language: English. Entry Date: 20070101. Revision Date: 20150711. Publication Type: Journal Article. Journal Subset: Health Services Administration. Exclusion Code: X1.
- 428.
- Locker TE, Baston S, Mason SM, et al. Defining frequent use of an urban emergency department. Emerg Med J. 2007 Jun;24(6):398–401. doi: 10.1136/emj.2006.043844. PMID: 19632544. Exclusion Code: X1. [PMC free article: PMC2658272] [PubMed: 17513534] [CrossRef]
- 429.
- Loeb DF, Monson SP, Lockhart S, et al. Mixed method evaluation of Relational Team Development (RELATED) to improve team-based care for complex patients with mental illness in primary care. BMC Psychiatry. 2019;19. doi: 10.1186/s12888-019-2294-1. PMID: 2019-62385-001. Exclusion Code: X1. [PMC free article: PMC6792180] [PubMed: 31615460] [CrossRef]
- 430.
- Long P. An untapped opportunity for health care progress: redesigning care for high-need patients. Health Affairs BLOG. Bethesda, MD: Health Affairs; 2018. Exclusion Code: X1.
- 431.
- Low LL, Vasanwala FF, Ng LB, et al. Effectiveness of a transitional home care program in reducing acute hospital utilization: a quasi-experimental study. BMC Health Serv Res. 2015 Mar 14;15:100. doi: 10.1186/s12913-015-0750-2. PMID: 25857588. Exclusion Code: X1. [PMC free article: PMC4377016] [PubMed: 25888830] [CrossRef]
- 432.
- Luciano JV, Fernandez A, Pinto-Meza A, et al. Frequent attendance in primary care: comparison and implications of different definitions. Br J Gen Pract. 2010 Feb;60(571). doi: 10.3399/bjgp10X483139. PMID: WOS:000275978500008. Exclusion Code: X1. [PMC free article: PMC2814289] [PubMed: 20132693] [CrossRef]
- 433.
- Lyles JS, Hodges A, Collins C, et al. Using nurse practitioners to implement an intervention in primary care for high-utilizing patients with medically unexplained symptoms. Gen Hosp Psychiatry. 2003 Mar–Apr;25(2):63–73. PMID: 14631698. Exclusion Code: X4. [PubMed: 12676418]
- 434.
- Machlin SR, Cohen SB, Yu W. Health care access and expenditures among non-elderly adults with multiple chronic conditions: variations by insurance coverage status, 2007–08 (average annual). [Rockville, Md.: Agency for Healthcare Research and Quality; 2011. Exclusion Code: X4.
- 435.
- Mackelprang JL, Collins SE, Clifasefi SL. Housing first is associated with reduced use of emergency medical services. Prehosp Emerg Care. 2014;18(4):476–82. doi: 10.3109/10903127.2014.916020. PMID: 103891579. Language: English. Entry Date: 20140925. Revision Date: 20160325. Publication Type: Journal Article. Exclusion Code: X1. [PMC free article: PMC5102506] [PubMed: 24878364] [CrossRef]
- 436.
- Maeng DD, Hao J, Bulger JB. Patterns of multiple emergency department visits: do primary care physicians matter? Perm J. 2017;21:16–063. doi: 10.7812/tpp/16-063. PMID: 28143506. Exclusion Code: X4. [PMC free article: PMC5363893] [PubMed: 28333606] [CrossRef]
- 437.
- Maier T. Psychosocial and psychodynamic factors influencing health care utilisation. Health Care Anal. 2006 Jun;14(2):69–78. doi: 10.1007/s10728-006-0013-9. PMID: WOS:000242461900001. Exclusion Code: X1. [PubMed: 17195575] [CrossRef]
- 438.
- Malmstrom T, Peltokorpi A, Lappalainen M, et al. Constructing a framework to manage high utilizers in health and social care; 2015. Exclusion Code: X1.
- 439.
- Malone DK, Collins SE, Clifasefi SL. Single-site housing first for chronically homeless people. Housing, Care and Support. 2015;18(2):62–6. doi: 10.1108/HCS-05-2015-0007. Exclusion Code: X1. [PMC free article: PMC3969126] [PubMed: 24148063] [CrossRef]
- 440.
- . Identifying Super Utilizers CHCS Super-Utilizer Summit; 2013. 11585. Exclusion Code: X1.
- 441.
- Mancuso D. Supporting Delivery System Transformation Through Data Integration and Analytics. 2016. https://www
.milbank.org /resources/supporting-delivery-system-transformation-data-integration-analytics-2/. Exclusion Code: X4. - 442.
- Mandelberg JH, Kuhn RE, Kohn MA. Epidemiologic analysis of an urban, public emergency department’s frequent users. Acad Emerg Med. 2000 Jun;7(6):637–46. doi: 10.1111/j.1553-2712.2000.tb02037.x. PMID: 10905642. Exclusion Code: X1. [PubMed: 10905642] [CrossRef]
- 443.
- Mantwill S, Schulz PJ. Low health literacy and healthcare utilization among immigrants and non-immigrants in Switzerland. Patient Educ Couns. 2017 Nov;100(11):2020–7. doi: 10.1016/j.pec.2017.05.023. PMID: 28542870. Exclusion Code: X1. [PubMed: 28559092] [CrossRef]
- 444.
- Margaret Trinity MBD, and Michael Bailit, Bailit Health. Integrating Health Care and Social Services: Moving from Concept to Practice. State Health & Value Strategies 2016. Exclusion Code: X7.
- 445.
- Martin BC, Ganguly R, Pannicker S, et al. Utilization patterns and net direct medical cost to Medicaid of irritable bowel syndrome. Curr Med Res Opin. 2003;19(8):771–80. doi: 10.1185/030079903125002540. PMID: 14527870. Exclusion Code: X1. [PubMed: 14687449] [CrossRef]
- 446.
- Martin J, Martin A, Schultz C, et al. Innovations in care delivery: embedding civil legal aid services in care for high-utilizing patients using medical-legal partnership. Health Affairs BLOG. Bethesda, Maryland: Health Affairs; 2015. Exclusion Code: X1.
- 447.
- Master RJ, Eng C. Integrating acute and long-term care for high-cost populations. Health Aff (Millwood). 2001 Nov–Dec;20(6):161–72. doi: 10.1377/hlthaff.20.6.161. PMID: 15338802. Exclusion Code: X9. [PubMed: 11816654] [CrossRef]
- 448.
- Mate K. High-need, high-cost playbook version 3.0. Institute for Healthcare Improvement 2018. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191318. Exclusion Code: X7. - 449.
- Mateo-Abad M, Fullaondo A, Merino M, et al. Impact Assessment of an Innovative Integrated Care Model for Older Complex Patients with Multimorbidity: The CareWell Project. International Journal of Integrated Care. 2020 Apr–Jun;20(2):11. doi: 10.5334/ijic.4711. PMID: WOS:000550985100005. Exclusion Code: X6. [PMC free article: PMC7243835] [PubMed: 32477037] [CrossRef]
- 450.
- Mathews SN, Lamm R, Yang J, et al. Factors associated with repeated health resource utilization in patients with diverticulitis. J Gastrointest Surg. 2017 Jan;21(1):112–20. doi: 10.1007/s11605-016-3245-5. PMID: 27882804. Exclusion Code: X4. [PubMed: 27613732] [CrossRef]
- 451.
- Mautner D, Peterson B, Cunningham A, et al. How Multidimensional Health Locus of Control predicts utilization of emergency and inpatient hospital services. J Health Psychol. 2017 Mar;22(3):314–23. doi: 10.1177/1359105315603468. PMID: 28559092. Exclusion Code: X1. [PubMed: 26430065] [CrossRef]
- 452.
- McCabe BK, Potash D, Omohundro E, et al. Seven-month pilot of an integrated, continuous evaluation, and quality improvement system for a state-based home-visiting program. Matern Child Health J. 2012 Oct;16(7):1401–12. doi: 10.1007/s10995-011-0905-7. PMID: 23086065. Exclusion Code: X1. [PubMed: 22246712] [CrossRef]
- 453.
- McCarthy D, Ryan J, Klein S. Models of care for high-need, high-cost patients: an evidence synthesis. Commonwealth Fund. 2015. https://www
.commonwealthfund .org/publications /issue-briefs/2015 /oct/models-care-high-need-high-cost-patients-evidence-synthesis. Exclusion Code: X1. [PubMed: 26591906] - 454.
- McConville S, Raven MC, Sabbagh SH, et al. Frequent emergency department users: a statewide comparison before and after Affordable Care Act implementation. Health Aff (Millwood). 2018 Jun;37(6):881–9. doi: 10.1377/hlthaff.2017.0784. PMID: 28884248. Exclusion Code: X4. [PubMed: 29863931] [CrossRef]
- 455.
- McGarrahan AG. A comparison of psychosocial and health characteristics of medical high utilizers and medical low utilizers in primary health care: ProQuest Information & Learning; 2001. Exclusion Code: X1.
- 456.
- McHugh J, Belanger E, Manning D, et al. The care of high-need patient populations: Findings from a 2 year study of high-performing hospitals. Implement Sci. 2020 May;15:2. PMID: WOS:000533323500049. Exclusion Code: X9.
- 457.
- McKenzie R, Williamson M, Roberts R. Who uses the ‘after hours GP helpline’? A profile of users of an after-hours primary care helpline. Aust Fam Physician. 2016 May;45(5):313–8. PMID: 26407631. Exclusion Code: X1. [PubMed: 27166469]
- 458.
- McLeod CC. Initial Assessment of High Utilizers of Emergency Department Services. Shepherd University, ProQuest Dissertations Publishing,; 2018. Exclusion Code: X3.
- 459.
- McMahon CG, Power Foley M, Robinson D, et al. High prevalence of frequent attendance in the over 65s. Eur J Emerg Med. 2018 Feb;25(1):53–7. doi: 10.1097/mej.0000000000000406. PMID: 29157447. Exclusion Code: X1. [PubMed: 27139928] [CrossRef]
- 460.
- Mechanic R. Testing strategies to improve healthcare for high-need, high-cost (HNHC) individuals: planning phase. Institute for Accountable Care 2018. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191416. Exclusion Code: X7. - 461.
- Meenan RT, Goodman MJ, Fishman PA, et al. Using risk-adjustment models to identify high-cost risks. Med Care. 2003 Nov;41(11):1301–12. doi: 10.1097/01.Mlr.0000094480.13057.75. PMID: 22264989. Exclusion Code: X1. [PubMed: 14583693] [CrossRef]
- 462.
- Mehl-Madrona LE. Prevalence of psychiatric diagnoses among frequent users of rural emergency medical services. Can J Rural Med. 2008 Winter;13(1):22–30. PMID: 18727695. Exclusion Code: X1. [PubMed: 18208649]
- 463.
- Melanie Bella CS, Karen LLanos, Stephen A. Somers. Purchasing Strategies to Improve Care Management for Complex Populations: A National Scan of State Purchasers. Center for Health Care Strategies: Strategies CfHC; 2008. https://www
.chcs.org /resource/purchasing-strategies-to-improve-care-management-for-complex-populations-a-national-scan-of-state-purchasers/. Exclusion Code: X7. - 464.
- Meng X, Muggli T, Baetz M, et al. Disordered lives: life circumstances and clinical characteristics of very frequent users of emergency departments for primary mental health complaints. Psychiatry Res. 2017 Jun;252:9–15. doi: 10.1016/j.psychres.2017.02.044. PMID: 27734180. Exclusion Code: X1. [PubMed: 28237761] [CrossRef]
- 465.
- Mercer T, Bae J, Kipnes J, et al. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med. 2015;10(7):419–24. doi: 10.1002/jhm.2351. PMID: 109592613. Language: English. Entry Date: 20150923. Revision Date: 20160630. Publication Type: journal article. Journal Subset: Biomedical. Exclusion Code: X1. [PubMed: 25854685] [CrossRef]
- 466.
- Meyers DJ, Belanger E, Joyce N, et al. Analysis of Drivers of Disenrollment and Plan Switching Among Medicare Advantage Beneficiaries. JAMA Intern Med. 2019 Apr 1;179(4):524–32. doi: 10.1001/jamainternmed.2018.7639. PMID: 32330140. Exclusion Code: X1. [PMC free article: PMC6450306] [PubMed: 30801625] [CrossRef]
- 467.
- Middleton A, Woodward A, Gunn J, et al. How do frequent users of crisis helplines differ from other users regarding their reasons for calling? Results from a survey with callers to Lifeline, Australia’s national crisis helpline service. Health Soc Care Community. 2017 May;25(3):1041–9. doi: 10.1111/hsc.12404. PMID: 28495031. Exclusion Code: X1. [PubMed: 27862572] [CrossRef]
- 468.
- Miller JB, Brauer E, Rao H, et al. The most frequent ED patients carry insurance and a significant burden of disease. Am J Emerg Med. 2013 Jan;31(1):16–9. doi: 10.1016/j.ajem.2012.05.001. PMID: 23970594. Exclusion Code: X4. [PubMed: 22795986] [CrossRef]
- 469.
- Minassian A, Vilke GM, Wilson MP. Frequent emergency department visits are more prevalent in psychiatric, alcohol abuse, and dual diagnosis conditions than in chronic viral illnesses such as hepatitis and human immunodeficiency virus. J Emerg Med. 2013 Oct;45(4):520–5. doi: 10.1016/j.jemermed.2013.05.007. PMID: 22569944. Exclusion Code: X4. [PubMed: 23845528] [CrossRef]
- 470.
- Mishra S, Gregson M, Lalumiere ML. Framing effects and risk-sensitive decision making. Br J Psychol. 2012 Feb;103(1):83–97. doi: 10.1111/j.2044-8295.2011.02047.x. PMID: 21213152. Exclusion Code: X1. [PubMed: 22229776] [CrossRef]
- 471.
- Mitton CR, Adair CE, McDougall GM, et al. Continuity of care and health care costs among persons with severe mental illness. Psychiatr Serv. 2005 Sep;56(9):1070–6. doi: 10.1176/appi.ps.56.9.1070. PMID: 11508640. Exclusion Code: X1. [PubMed: 16148319] [CrossRef]
- 472.
- Moe J, Bailey AL, Oland R, et al. Defining, quantifying, and characterizing adult frequent users of a suburban Canadian emergency department. Cjem. 2013 Jul;15(4):214–26. PMID: 23337095. Exclusion Code: X1. [PubMed: 23777993]
- 473.
- Moe J, Kirkland SW, Rawe E, et al. Effectiveness of interventions to decrease emergency department visits by adult frequent users: a systematic review. Acad Emerg Med. 2017 Jan;24(1):40–52. doi: 10.1111/acem.13060. PMID: 27473387. Exclusion Code: X1. [PubMed: 27473387] [CrossRef]
- 474.
- Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006 Apr 5;6:88. doi: 10.1186/1471-2458-6-88. PMID: 16906566. Exclusion Code: X1. [PMC free article: PMC1450274] [PubMed: 16595021] [CrossRef]
- 475.
- Moore DT, Rosenheck RA. Factors Affecting Emergency Department Use by a Chronically Homeless Population. Psychiatr Serv. 2016 Dec 1;67(12):1340–7. doi: 10.1176/appi.ps.201500526. PMID: 27540081. Exclusion Code: X1. [PMC free article: PMC5133170] [PubMed: 27417899] [CrossRef]
- 476.
- Moore G, Gerdtz M, Manias E, et al. Socio-demographic and clinical characteristics of re-presentation to an Australian inner-city emergency department: implications for service delivery. BMC Public Health. 2007 Nov 10;7:320. doi: 10.1186/1471-2458-7-320. PMID: 18281644. Exclusion Code: X1. [PMC free article: PMC2222161] [PubMed: 17996112] [CrossRef]
- 477.
- Moore M, Conrick KM, Reddy A, et al. From Their Perspective: The Connection between Life Stressors and Health Care Service Use Patterns of Homeless Frequent Users of the Emergency Department. Health Soc Work. 2019 May 1;44(2):113–22. doi: 10.1093/hsw/hlz010. PMID: 32798454. Exclusion Code: X11. [PMC free article: PMC6642452] [PubMed: 30855682] [CrossRef]
- 478.
- Moore TA, Miller AL, Lopez L, et al. High utilizers of inpatient state hospital services. Schizophr Bull. 2009 Mar;35:133-. PMID: WOS:000263964700385. Exclusion Code: X1.
- 479.
- Morgan PA, Smith VA, Berkowitz TSZ, et al. Impact Of Physicians, Nurse Practitioners, And Physician Assistants On Utilization And Costs For Complex Patients. Health Aff (Millwood). 2019 Jun;38(6):1028–36. doi: 10.1377/hlthaff.2019.00014. PMID: 31753736. Exclusion Code: X1. [PubMed: 31158006] [CrossRef]
- 480.
- Morgello S, Gensler G, Sherman S, et al. Frailty in medically complex individuals with chronic HIV. AIDS. 2019 Aug 1;33(10):1603–11. doi: 10.1097/qad.0000000000002250. PMID: 31706604. Exclusion Code: X1. [PMC free article: PMC6760300] [PubMed: 31305330] [CrossRef]
- 481.
- Mortensen K. Access to primary and specialty care and emergency department utilization of medicaid enrollees needing specialty care. J Health Care Poor Underserved. 2014 May;25(2):801–13. doi: 10.1353/hpu.2014.0097. PMID: 24576026. Exclusion Code: X4. [PubMed: 24858887] [CrossRef]
- 482.
- Moschetti K, Iglesias K, Baggio S, et al. Health care costs of case management for frequent users of the emergency department: Hospital and insurance perspectives. PLoS One. 2018;13(9):e0199691. doi: 10.1371/journal.pone.0199691. PMID: 30031403. Exclusion Code: X1. [PMC free article: PMC6152853] [PubMed: 30248102] [CrossRef]
- 483.
- Moses K, Hamblin A, Somers S. Supportive housing for chronically homeless Medicaid enrollees: state strategies. [Hamilton, N.J.]: Center for Health Care Strategies; 2016. Exclusion Code: X1.
- 484.
- Mullangi S, Press MJ, Ryan AM. A delicate balance: Accountability for very high-cost patients in new payment models. Healthcare-the Journal of Delivery Science and Innovation. 2019 Dec;7(4):3. doi: 10.1016/j.hjdsi.2019.100366. PMID: WOS:000500712400001. Exclusion Code: X1. [PubMed: 31558367] [CrossRef]
- 485.
- Murphy SM, Neven D. Cost-effective: emergency department care coordination with a regional hospital information system. J Emerg Med. 2014 Aug;47(2):223–31. doi: 10.1016/j.jemermed.2013.11.073. PMID: 24717708. Exclusion Code: X1. [PubMed: 24508115] [CrossRef]
- 486.
- Murtagh Kurowski E, Byczkowski T, Grupp-Phelan JM. Comparison of emergency care delivered to children and young adults with complex chronic conditions between pediatric and general emergency departments. Acad Emerg Med. 2014 Jul;21(7):778–84. doi: 10.1111/acem.12412. PMID: 23182399. Exclusion Code: X1. [PubMed: 25039935] [CrossRef]
- 487.
- Myers LA, Carlson PN, Krantz PW, et al. Development and Implementation of a Community Paramedicine Program in Rural United States. Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health. 2020;21(5):1227–33. doi: 10.5811/westjem.2020.7.44571. PMID: 146108730. Language: English. Entry Date: 20201013. Revision Date: 20201013. Publication Type: Article. Exclusion Code: X1. [PMC free article: PMC7514407] [PubMed: 32970579] [CrossRef]
- 488.
- Naessens JA, Baird MA, Van Houten HK, et al. Predicting persistently high primary care use. Ann Fam Med. 2005 Jul–Aug;3(4):324–30. doi: 10.1370/afm.352. PMID: WOS:000231336100008. Exclusion Code: X1. [PMC free article: PMC1466904] [PubMed: 16046565] [CrossRef]
- 489.
- National Academy for State Health Policy , Urban Institute, RTI International. Financial alignment initiative annual report: Washington health homes MFFS Demonstration Bethesda, MD: Centers for Medicare & Medicaid Services; 2017. https://www
.cms.gov/Medicare-Medicaid-Coordination /Medicare-and-Medicaid-Coordination /Medicare-Medicaid-Coordination-Office /FinancialAlignmentInitiative /Downloads /WAFirstAnnualEvalReport.pdf. Exclusion Code: X1. - 490.
- Nault Connors JD, Binkley BL, Graff JC, et al. How patient experience informed the SafeMed Program: Lessons learned during a Health Care Innovation Award to improve care for super-utilizers. Healthc (Amst). 2019 Mar;7(1):13–21. doi: 10.1016/j.hjdsi.2018.02.002. Exclusion Code: X1. [PubMed: 29477610] [CrossRef]
- 491.
- Navathe A. Spending and outcomes for complex Medicare Advantage patients. University of Pennsylvania. 2016. https:
//clinicaltrials .gov/ct2/show/NCT02922361. Exclusion Code: X7. - 492.
- Navathe AS. Segmenting high-need, high-cost veterans into potentially actionable subgroups. Corporal Michael J. Crescenz VA Medical Center, Center for Health Equity Research and Promotion 2018. https://www
.hsrd.research .va.gov/research/abstracts .cfm?Project_ID=2141706856. Exclusion Code: X7. - 493.
- Neighbors CJ, Yerneni R, O’Grady MA, et al. Recurrent use of inpatient withdrawal management services: characteristics, service use, and cost among Medicaid clients. J Subst Abuse Treat. 2018;92:77–84. doi: 10.1016/j.jsat.2018.06.013. Exclusion Code: X1. [PubMed: 30032948] [CrossRef]
- 494.
- Nejtek VA, Aryal S, Talari D, et al. A pilot mobile integrated healthcare program for frequent utilizers of emergency department services. Am J Emerg Med. 2017 Nov;35(11):1702–5. doi: 10.1016/j.ajem.2017.04.061. PMID: 27473387. Exclusion Code: X1. [PubMed: 28495031] [CrossRef]
- 495.
- Nelson RE, Suo Y, Pettey W, et al. Costs Associated with Health Care Services Accessed through VA and in the Community through Medicare for Veterans Experiencing Homelessness. Health Serv Res. 2018 Dec;53 Suppl 3(Suppl Suppl 3):5352–74. doi: 10.1111/1475-6773.13054. PMID: 30246368. Exclusion Code: X1. [PMC free article: PMC6235814] [PubMed: 30246368] [CrossRef]
- 496.
- Nevola A, Morris ME, Felix HC, et al. Improving quality of life assessments for high-need adult Medicaid service users with mental health conditions. Qual Life Res. 2020. doi: 10.1007/s11136-020-02694-0. Exclusion Code: X9. [PubMed: 33211222] [CrossRef]
- 497.
- Ng SHX, Rahman N, Ang IYH, et al. Characterization of high healthcare utilizer groups using administrative data from an electronic medical record database. BMC Health Serv Res. 2019;19(1):452. doi: 10.1186/s12913-019-4239-2. Exclusion Code: X1. [PMC free article: PMC6612067] [PubMed: 31277649] [CrossRef]
- 498.
- Noble N, Paul C, Carey M, et al. A randomised trial assessing the acceptability and effectiveness of providing generic versus tailored feedback about health risks for a high need primary care sample. BMC Fam Pract. 2015 Aug 5;16:95. doi: 10.1186/s12875-015-0309-7. PMID: 26809051. Exclusion Code: X1. [PMC free article: PMC4525725] [PubMed: 26243144] [CrossRef]
- 499.
- Noel K, Messina C, Hou W, et al. Tele-transitions of care (TTOC): a 12-month, randomized controlled trial evaluating the use of Telehealth to achieve triple aim objectives. BMC Fam Pract. 2020 Feb 7;21(1):27. doi: 10.1186/s12875-020-1094-5. PMID: 30855682. Exclusion Code: X5. [PMC free article: PMC7007639] [PubMed: 32033535] [CrossRef]
- 500.
- Nolan RD, Kirkland C, Johnson A, et al. International systematic review on high utilizers diagnosed with severe mental illness. Health Behavior and Policy Review. 2019 Jan;6(1):3–17. doi: 10.14485/hbpr.6.1.1. PMID: WOS:000456577600001. Exclusion Code: X1. [CrossRef]
- 501.
- Norelli LJ, Coates AD, Kovasznay BM. Cancer risk from diagnostic radiology in a deliberate self-harm patient. Acta Psychiatr Scand. 2010 Nov;122(5):427–30. doi: 10.1111/j.1600-0447.2010.01538.x. PMID: 21039327. Exclusion Code: X1. [PubMed: 20136800] [CrossRef]
- 502.
- Norman GJ, Orton K, Wade A, et al. Operation and challenges of home-based medical practices in the US: findings from six aggregated case studies. BMC Health Serv Res. 2018 Jan 27;18(1):45. doi: 10.1186/s12913-018-2855-x. PMID: 30404766. Exclusion Code: X1. [PMC free article: PMC5787297] [PubMed: 29374478] [CrossRef]
- 503.
- Norman GJ, Wade AJ, Morris AM, et al. Home and community-based services coordination for homebound older adults in home-based primary care. BMC Geriatr. 2018 Oct 11;18(1):241. doi: 10.1186/s12877-018-0931-z. PMID: 29630852. Exclusion Code: X1. [PMC free article: PMC6180527] [PubMed: 30305053] [CrossRef]
- 504.
- Nothelle S. Characterization and effect of co-existence of care management programs for high-need, high-cost older adults. Johns Hopkins Medicine, Department of Medicine. 2018. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191491. Exclusion Code: X7. - 505.
- Novak M, Mucsi I, Shapiro CM, et al. Increased utilization of health services by insomniacs--an epidemiological perspective. J Psychosom Res. 2004 May;56(5):527–36. doi: 10.1016/j.jpsychores.2004.02.007. PMID: 11794737. Exclusion Code: X1. [PubMed: 15172209] [CrossRef]
- 506.
- Nuti SV, Doupe P, Villanueva B, et al. Characterizing subgroups of high-need, high-cost patients based on their clinical conditions: a machine learning-based analysis of Medicaid claims data. J Gen Intern Med. 2019 Aug;34(8):1406–8. doi: 10.1007/s11606-019-04941-8. PMID: 30887432. Exclusion Code: X4. [PMC free article: PMC6667598] [PubMed: 30887432] [CrossRef]
- 507.
- Oderda GM, Gunning K, LaFleur J, et al. Reviews of Utah Medicaid high utilizers to control drug costs. Value Health. 2003 May–Jun;6(3):206-. doi: 10.1016/s1098-3015(10)63869-7. PMID: WOS:000183419000071. Exclusion Code: X1. [CrossRef]
- 508.
- O’Donohue W, Cucciare M. Pathways to Medical utilization. J Clin Psychol Med Settings. 2005;12(2):185–97. doi: 10.1007/s10880-005-3278-5. PMID: 16903251. Exclusion Code: X1. [CrossRef]
- 509.
- O’Donohue W, Cucciare MA. The role of psychological factors in medical presentations. J Clin Psychol Med Settings. 2005 Mar;12(1):13–24. doi: 10.1007/s10880-005-0908-x. PMID: WOS:000226939100002. Exclusion Code: X1. [CrossRef]
- 510.
- Okin RL, Boccellari A, Azocar F, et al. The effects of clinical case management on hospital service use among ED frequent users. Am J Emerg Med. 2000 Sep;18(5):603–8. doi: 10.1053/ajem.2000.9292. PMID: 16383099. Exclusion Code: X1. [PubMed: 10999578] [CrossRef]
- 511.
- Okunogbe A, Meredith LS, Chang ET, et al. Care coordination and provider stress in primary care management of high-risk patients. J Gen Intern Med. 2018 Jan;33(1):65–71. doi: 10.1007/s11606-017-4186-8. PMID: 28971306. Exclusion Code: X1. [PMC free article: PMC5756166] [PubMed: 28971306] [CrossRef]
- 512.
- Okuyemi KS, Frey B. Describing and predicting frequent users of an emergency department. J Assoc Acad Minor Phys. 2001 Mar;12(1–2):119–23. PMID: 11543989. Exclusion Code: X4. [PubMed: 11851196]
- 513.
- O’Leary KJ, Chapman MM, Foster S, et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:E1–e6. doi: 10.12788/jhm.3175. PMID: 30897060. Exclusion Code: X1. [PMC free article: PMC6715053] [PubMed: 30897060] [CrossRef]
- 514.
- Olofsson P, Carlstrom ED, Back-Pettersson S. During and beyond the triage encounter: chronically ill elderly patients’ experiences throughout their emergency department attendances. Int Emerg Nurs. 2012 Oct;20(4):207–13. doi: 10.1016/j.ienj.2012.03.006. PMID: 22948324. Exclusion Code: X1. [PubMed: 23084509] [CrossRef]
- 515.
- Olsson M, Hansagi H. Repeated use of the emergency department: qualitative study of the patient’s perspective. Emerg Med J. 2001 Nov;18(6):430–4. doi: 10.1136/emj.18.6.430. PMID: 11682580. Exclusion Code: X1. [PMC free article: PMC1725740] [PubMed: 11696488] [CrossRef]
- 516.
- O’Malley AS, Rich EC, Sarwar R, et al. How accountable care organizations use population segmentation to care for high-need, high-cost patients. Commonwealth. 2019. https://www
.commonwealthfund .org/publications /issue-briefs/2019 /jan/how-acos-use-segmentation-high-need-high-cost. Exclusion Code: X1. [PubMed: 30645057] - 517.
- Ondler C, Hegde GG, Carlson JN. Resource utilization and health care charges associated with the most frequent ED users. Am J Emerg Med. 2014 Oct;32(10):1215–9. doi: 10.1016/j.ajem.2014.07.013. PMID: 25342825. Exclusion Code: X4. [PubMed: 25154348] [CrossRef]
- 518.
- Oreskovic NM, Maniates J, Weilburg J, et al. Optimizing the use of electronic health records to identify high-risk psychosocial determinants of health. JMIR Med Inform. 2017 Aug 14;5(3):e25. doi: 10.2196/medinform.8240. PMID: 28807893. Exclusion Code: X1. [PMC free article: PMC5575417] [PubMed: 28807893] [CrossRef]
- 519.
- O’Rielly C, Sutherland L, Wong C. The characteristics and effectiveness of interventions targeting chronic pain patients in the emergency department: A systematic review and meta-analysis. Canadian Journal of Emergency Medicine. 2019;21:S98–S9. doi: 10.1017/cem.2019.288. Exclusion Code: X7. [CrossRef]
- 520.
- Orza M. High hopes: public health approaches to reducing the need for health care. Washington, D.C.: National Health Policy Forum; 2010. Exclusion Code: X1.
- 521.
- Osborn R. Israel-United States meeting on caring for high need/high cost patients 2017. Haifa: Commonwealth Fund; 2016. Exclusion Code: X1.
- 522.
- Ostermeyer B, Baweja NU, Schanzer B, et al. Frequent utilizers of emergency departments: characteristics and intervention opportunities. Psychiat Ann. 2018 Jan;48(1):42–50. doi: 10.3928/00485713-20171206-02. PMID: WOS:000425843600007. Exclusion Code: X1. [CrossRef]
- 523.
- Oxman TE, Dietrich AJ, Williams JW, et al. A three-component model for reengineering systems for the treatment of depression in primary care. Psychosomatics. 2002 Nov–Dec;43(6):441–50. doi: 10.1176/appi.psy.43.6.441. PMID: WOS:000179173900002. Exclusion Code: X1. [PubMed: 12444226] [CrossRef]
- 524.
- Oyffe I, Kurs R, Gelkopf M, et al. Revolving-door patients in a public psychiatric hospital in Israel: cross sectional study. Croat Med J. 2009 Dec;50(6):575–82. doi: 10.3325/cmj.2009.50.575. PMID: 19446122. Exclusion Code: X1. [PMC free article: PMC2802091] [PubMed: 20017226] [CrossRef]
- 525.
- Panopalis P, Gillis JZ, Yazdany J, et al. Frequent use of the emergency department among persons with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Mar;62(3):401–8. doi: 10.1002/acr.20107. PMID: 19995099. Exclusion Code: X4. [PMC free article: PMC3759153] [PubMed: 20391487] [CrossRef]
- 526.
- Parikh R, Linn K, Yan J, et al. Identifying distinct subgroups of high-need, high-cost veterans using machine learning clustering methods. J Gen Intern Med. 2020;35(SUPPL 1):S155. doi: 10.1007/s11606-020-05890-3. Exclusion Code: X1. [CrossRef]
- 527.
- Park S, Langellier BA, Burke RE, et al. Association of Medicare Advantage Penetration With Per Capita Spending, Emergency Department Visits, and Readmission Rates Among Fee-for-Service Medicare Beneficiaries With High Comorbidity Burden. Med Care Res Rev.10. doi: 10.1177/1077558720952582. PMID: WOS:000563025900001. Exclusion Code: X9. [PubMed: 32842874] [CrossRef]
- 528.
- Parson P. PBS Newshour partnership on high-need high-cost populations. Greater Washington Educational Telecommunications Association: Commonwealth Fund; 2016. Exclusion Code: X7.
- 529.
- Patten SB, Williams JV, Esposito E, et al. Self-reported thyroid disease and mental disorder prevalence in the general population. Gen Hosp Psychiatry. 2006 Nov–Dec;28(6):503–8. doi: 10.1016/j.genhosppsych.2006.09.001. PMID: 16484820. Exclusion Code: X1. [PubMed: 17088166] [CrossRef]
- 530.
- Peck KA. To what extent are chronic care management processes and programs in place to manage patients with high-need, high-cost chronic illnesses? : Commonwealth Fund; 2018. Exclusion Code: X7.
- 531.
- Peddie S, Richardson S, Salt L, et al. Frequent attenders at emergency departments: research regarding the utility of management plans fails to take into account the natural attrition of attendance. N Z Med J. 2011 Mar 25;124(1331):61–6. PMID: 21597126. Exclusion Code: X1. [PubMed: 21725414]
- 532.
- Peng CJ-D. Health services utilization of older adults with depression and comorbid chronic health conditions: evidence from China: ProQuest Information & Learning; 2018. Exclusion Code: X1.
- 533.
- Perez Jolles M, Lengnick-Hall R, Mittman BS. Core functions and forms of complex health interventions: a patient-centered medical home illustration. J Gen Intern Med. 2019;34(6):1032–8. doi: 10.1007/s11606-018-4818-7. Exclusion Code: X1. [PMC free article: PMC6544719] [PubMed: 30623387] [CrossRef]
- 534.
- Petersen LA, Woodard LD, Henderson LM, et al. Will hypertension performance measures used for pay-for-performance programs penalize those who care for medically complex patients? Circulation. 2009 Jun 16;119(23):2978–85. doi: 10.1161/circulationaha.108.836544. PMID: 19083209. Exclusion Code: X1. [PMC free article: PMC2743388] [PubMed: 19487595] [CrossRef]
- 535.
- Peytremann-Bridevaux I, Voellinger R, Santos-Eggimann B. Healthcare and preventive services utilization of elderly Europeans with depressive symptoms. J Affect Disord. 2008 Jan;105(1–3):247–52. doi: 10.1016/j.jad.2007.04.011. PMID: 20001167. Exclusion Code: X1. [PubMed: 17509695] [CrossRef]
- 536.
- Phipps EJ, Singletary SB, Cooblall CA, et al. Food insecurity in patients with high hospital utilization. Popul Health Manag. 2016 Dec;19(6):414–20. doi: 10.1089/pop.2015.0127. PMID: 26141469. Exclusion Code: X1. [PubMed: 27008540] [CrossRef]
- 537.
- Pietz K, Byrne MM, Petersen LA. A decision-theoretic approach to identifying future high-cost patients. Med Care. 2006 Sep;44(9):842–9. doi: 10.1097/01.mlr.0000220680.19667.da. PMID: 16294676. Exclusion Code: X4. [PubMed: 16932136] [CrossRef]
- 538.
- Placona AM, King R, Wang F. Longitudinal Clustering of High-cost Patients’ Spend Trajectories:Delineating Individual Behaviors from Aggregate Trends. AMIA Annu Symp Proc. 2018;2018:907–15. PMID: 29049488. Exclusion Code: X9. [PMC free article: PMC6371335] [PubMed: 30815133]
- 539.
- Pleticha J, Prakken S, Buchheit T, et al. Financial impact of intensive outpatient pain management of duke health system high utilizers. Anesth Analg. 2017 May;124:786–8. PMID: WOS:000412683000408. Exclusion Code: X1.
- 540.
- Polluste K, Kalda R, Lember M. Satisfaction with the access to the health services of the people with chronic conditions in Estonia. Health Policy. 2007 Jun;82(1):51–61. doi: 10.1016/j.healthpol.2006.08.004. PMID: 18030205. Exclusion Code: X1. [PubMed: 17011063] [CrossRef]
- 541.
- Ponka D. The effectiveness of case management interventions for the homeless, vulnerably housed and persons with lived experience: A systematic review. PLoS One. 2020. Exclusion Code: X7. [PMC free article: PMC7313544] [PubMed: 32271769]
- 542.
- Ponka D, Agbata E, Kendall C, et al. The effectiveness of case management interventions for the homeless, vulnerably housed and persons with lived experience: A systematic review. PLoS One. 2020;15(4):e0230896. doi: 10.1371/journal.pone.0230896. PMID: 32271769. Exclusion Code: X7. [PMC free article: PMC7313544] [PubMed: 32271769] [CrossRef]
- 543.
- Poole S, Grannis S, Shah NH. Predicting Emergency Department Visits. AMIA Jt Summits Transl Sci Proc. 2016;2016:438–45. PMID: 27570684. Exclusion Code: X1. [PMC free article: PMC5001776] [PubMed: 27570684]
- 544.
- Pope D, Fernandes CM, Bouthillette F, et al. Frequent users of the emergency department: a program to improve care and reduce visits. CMAJ. 2000 Apr 4;162(7):1017–20. PMID: 15446621. Exclusion Code: X1. [PMC free article: PMC1232308] [PubMed: 10763402]
- 545.
- Poremski D, Harris DW, Kahan D, et al. Improving continuity of care for frequent users of emergency departments: service user and provider perspectives. Gen Hosp Psychiatry. 2016 May–Jun;40:55–9. doi: 10.1016/j.genhosppsych.2016.01.004. PMID: 27206120. Exclusion Code: X1. [PubMed: 26906469] [CrossRef]
- 546.
- Powers A, Strube MJ, Oltmanns TF. Personality pathology and increased use of medical resources in later adulthood. Am J Geriatr Psychiatry. 2014 Dec;22(12):1478–86. doi: 10.1016/j.jagp.2013.10.009. PMID: 23957900. Exclusion Code: X1. [PMC free article: PMC4000746] [PubMed: 24315559] [CrossRef]
- 547.
- Powers M. Sustainable payment models to support effective care management programs for high-need, high-cost patients. Pacific Business Group on Health 2017. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191027. Exclusion Code: X7. - 548.
- Prados-Torres A, Cura-Gonzalez ID, Prados-Torres JD, et al. MULTIPAP Study: improving healthcare for patients with multimorbidity. Br J Gen Pract. 2020;70. doi: 10.3399/bjgp20X711257. PMID: CN-02142881. Exclusion Code: X6. [PubMed: 32554653] [CrossRef]
- 549.
- Prater L, Sanchez A, Modan G, et al. Electronic Health Record Documentation Patterns of Recorded Primary Care Visits Focused on Complex Communication: A Qualitative Study. Appl Clin Inform. 2019 Mar;10(2):247–53. doi: 10.1055/s-0039-1683986. PMID: 30970382. Exclusion Code: X1. [PMC free article: PMC6458018] [PubMed: 30970382] [CrossRef]
- 550.
- Pruitt Z, Emechebe N, Quast T, et al. Expenditure Reductions Associated with a Social Service Referral Program. Popul Health Manag. 2018 Dec;21(6):469–76. doi: 10.1089/pop.2017.0199. PMID: 29664702. Exclusion Code: X1. [PMC free article: PMC6276598] [PubMed: 29664702] [CrossRef]
- 551.
- Pyne JM, Rost KM, Farahati F, et al. One size fits some: the impact of patient treatment attitudes on the cost-effectiveness of a depression primary-care intervention. Psychol Med. 2005 Jun;35(6):839–54. doi: 10.1017/s0033291704003332. PMID: WOS:000231499900007. Exclusion Code: X1. [PubMed: 15997604] [CrossRef]
- 552.
- Radcliff TA, Côté MJ, Duncan RP. The identification of high-cost patients. Hosp Top. 2005 Summer;83(3):17–24. doi: 10.3200/htps.83.3.17-24. PMID: 15612167. Exclusion Code: X9. [PubMed: 16294676] [CrossRef]
- 553.
- Radley DC, Hayes SL, McCarthy D. Assessing state variation in high-need adult populations and their care experiences. Commonwealth Fund. 2017. https://www
.commonwealthfund .org/publications /issue-briefs/2017 /aug/assessing-state-variation-high-need-adult-populations-and-their. Exclusion Code: X1. - 554.
- Radley DC, Hayes SL, McCarthy D. Percent of high-need adults who went without physician care and prescriptions because of costs varied widely. Commonwealth Fund; 2017. Exclusion Code: X7.
- 555.
- Radley DC, Hayes SL, McCarthy D. High-need adults more likely than adults overall to not see a doctor because of costs, but rates vary. Commonwealth Fund; 2017. Exclusion Code: X7.
- 556.
- Ramage-Morin PL, Gilmour H. Chronic pain at ages 12 to 44. Health Rep. 2010 Dec;21(4):53–61. PMID: 20038842. Exclusion Code: X1. [PubMed: 21269012]
- 557.
- Ramey L, Goldstein R, Zafonte R, et al. Variation in 30-day readmission rates among medically complex patients at inpatient rehabilitation facilities and contributing factors. J Am Med Dir Assoc. 2016 Aug 1;17(8):730–6. doi: 10.1016/j.jamda.2016.03.019. PMID: 26930236. Exclusion Code: X1. [PubMed: 27161849] [CrossRef]
- 558.
- Rapoport R. Surveying high-need, high-cost patients about how they experience health system reform. SSRS. 2016. https://www
.commonwealthfund .org/grants/surveying-high-need-high-cost-patients-about-how-they-experience-health-system-reform. Exclusion Code: X7. - 559.
- Raven MC. Homelessness and the Practice of Emergency Medicine: Challenges, Gaps in Care, and Moral Obligations. Ann Emerg Med. 2019 Nov;74(5s):S33–s7. doi: 10.1016/j.annemergmed.2019.08.440. PMID: 31655672. Exclusion Code: X9. [PubMed: 31655672] [CrossRef]
- 560.
- Raven MC, Doran KM, Kostrowski S, et al. An intervention to improve care and reduce costs for high-risk patients with frequent hospital admissions: a pilot study. BMC Health Serv Res. 2011 Oct 13;11:270. doi: 10.1186/1472-6963-11-270. PMID: 21995329. Exclusion Code: X1. [PMC free article: PMC3212942] [PubMed: 21995329] [CrossRef]
- 561.
- Raven MC, Kotchko SM, Gould DA. Can targeted messaging encourage PCP contact before ED visits? Am J Manag Care. 2013 Jan;19(1):41–5. PMID: 23222127. Exclusion Code: X1. [PubMed: 23379743]
- 562.
- Realmuto L, Walker EA, Weiss L. Struggling to stay on track: participants share benefits and barriers to completing the National Diabetes Prevention Program. New York, NY: New York Academy of Medicine; 2017. Exclusion Code: X1.
- 563.
- . Detecting earlier indicators of homelessness in the free text of medical records. 2014. 2665: 202. Exclusion Code: X1. [PubMed: 25000039]
- 564.
- Reddy A. Understanding the impact of health information exchange-enabled care plans to coordinate care among high-need, high-cost patients. University of Washington, School of Medicine, Department of Medicine, Division of General Internal Medicine. Seattle, Washington: 2018. https://app
.dimensions .ai/details/grant/grant.7825128. Exclusion Code: X1. - 565.
- Regenstein M, Andres E. Reducing hospital readmissions among Medicaid patients: a review of the literature. Qual Manag Health Care. 2014;23(1):20–42. doi: 10.1097/QMH.0000000000000016. PMID: 107883398. Language: English. Entry Date: 20140131. Revision Date: 20150712. Publication Type: Journal Article. Exclusion Code: X1. [PubMed: 24368719] [CrossRef]
- 566.
- Rennemark M, Holst G, Fagerstrom C, et al. Factors related to frequent usage of the primary healthcare services in old age: findings from The Swedish National Study on Aging and Care. Health Soc Care Community. 2009 May;17(3):301–11. doi: 10.1111/j.1365-2524.2008.00829.x. PMID: 19098256. Exclusion Code: X1. [PubMed: 19207603] [CrossRef]
- 567.
- Reschovsky JD, Hadley J, Saiontz-Martinez CB, et al. Following the money: factors associated with the cost of treating high-cost Medicare beneficiaries. Health Serv Res. 2011 Aug;46(4):997–1021. doi: 10.1111/j.1475-6773.2011.01242.x. PMID: 27547951. Exclusion Code: X9. [PMC free article: PMC3165175] [PubMed: 21306368] [CrossRef]
- 568.
- Rivlin AM, Burke SP. Preserving the bipartisan commitment to health care delivery system reform. Commonwealth Fund; 2017. Exclusion Code: X7.
- 569.
- Robst J. Developing Models to Predict Persistent High-Cost Cases in Florida Medicaid. Popul Health Manag. 2015 Dec;18(6):467–76. doi: 10.1089/pop.2014.0174. PMID: 28430557. Exclusion Code: X1. [PubMed: 26102363] [CrossRef]
- 570.
- Rochlin DH, Lee CM, Scheuter C, et al. Health Care Is Failing the Most Vulnerable Patients: Three Underused Solutions. Public Health Rep. 2020 Nov;135(6):711–6. doi: 10.1177/0033354920954496. PMID: WOS:000572772800001. Exclusion Code: X1. [PMC free article: PMC7649988] [PubMed: 32962512] [CrossRef]
- 571.
- Rodríguez-Queraltó O, Formiga F, López-Palop R, et al. FRAIL Scale also Predicts Long-Term Outcomes in Older Patients With Acute Coronary Syndromes. J Am Med Dir Assoc. 2020;21(5):683–7.e1. doi: 10.1016/j.jamda.2019.10.007. Exclusion Code: X6. [PubMed: 31780414] [CrossRef]
- 572.
- Rohrer JE, Takahashi PY, Adamson SC. Age, obesity, and medical visits in family medicine. Popul Health Manag. 2008 Oct;11(5):255–9. doi: 10.1089/pop.2008.0005. PMID: 17269846. Exclusion Code: X1. [PubMed: 18942917] [CrossRef]
- 573.
- Rollman BL, Belnap BH, Reynolds CF, et al. A contemporary protocol to assist primary care physicians in the treatment of panic and generalized anxiety disorders. Gen Hosp Psychiatry. 2003 Mar–Apr;25(2):74–82. doi: 10.1016/s0163-8343(03)00004-5. PMID: WOS:000182046600002. Exclusion Code: X1. [PubMed: 12676419] [CrossRef]
- 574.
- Romaire M, Alterbaum R, Collins A. Medicaid behavioral health homes: Lessons learned and early findings from Maine. Psychiatr Serv. 2020;71(11):1179–87. doi: 10.1176/appi.ps.201900490. PMID: 2020-99636-014. Exclusion Code: X1. [PubMed: 32933410] [CrossRef]
- 575.
- Rosen CS. Telephone case monitoring for veterans with PTSD (post-traumatic stress disorder) VA Palo Alto Health Care System, Palo Alto Division. Palo Alto, California: 2006. https://www
.hsrd.research .va.gov/research/abstracts .cfm?Project_ID=2141695811. Exclusion Code: X1. - 576.
- Rosenberg HJ, Rosenberg SD, Wolford Ii GL, et al. The relationship between trauma, PTSD, and medical utilization in three high risk medical populations. Int J Psychiatry Med. 2000;30(3):247–59. doi: 10.2190/J8M8-YDTE-46CB-GYDK. Exclusion Code: X1. [PubMed: 11209992] [CrossRef]
- 577.
- Rosenberg MA, Farrell PM. Predictive modeling of costs for a chronic disease with acute high-cost episodes. North American Actuarial Journal. 2008;12(1):1–19. doi: 10.1080/10920277.2008.10597497. Exclusion Code: X1. [CrossRef]
- 578.
- Rost K, Pyne JM, Dickinson LM, et al. Cost-effectiveness of enhancing primary care depression management on an ongoing basis. Ann Fam Med. 2005 Jan–Feb;3(1):7–14. doi: 10.1370/afm.256. PMID: WOS:000226922000003. Exclusion Code: X1. [PMC free article: PMC1350977] [PubMed: 15671185] [CrossRef]
- 579.
- Rotenberg J, Kinosian B, Boling P, et al. Home-based primary care: beyond extension of the independence at home demonstration. J Am Geriatr Soc. 2018 Apr;66(4):812–7. doi: 10.1111/jgs.15314. PMID: 28468524. Exclusion Code: X4. [PubMed: 29473945] [CrossRef]
- 580.
- Roudsari B, Caetano R, Field C. Alcohol intoxication/dependence, ethnicity and utilisation of health care resources in a level I trauma center. Injury. 2011 Jan;42(1):66–71. doi: 10.1016/j.injury.2010.01.097. PMID: 21199163. Exclusion Code: X1. [PMC free article: PMC2964390] [PubMed: 20106475] [CrossRef]
- 581.
- Rowe JM, Rizzo VM, Kang SY, et al. Time Contribution of Social Workers in Care Management: Value for Older Adults. Prof Case Manag. 2019 Nov/Dec;24(6):306–16. doi: 10.1097/ncm.0000000000000305. PMID: 31580298. Exclusion Code: X1. [PubMed: 31580298] [CrossRef]
- 582.
- Roy-Byrne PP, Wagner AW, Schraufnagel TJ. Understanding and treating panic disorder in the primary care setting. J Clin Psychiatry. 2005;66:16–22. PMID: WOS:000228902800003. Exclusion Code: X1. [PubMed: 15842183]
- 583.
- Rudin RS, Schneider EC, Predmore Z, et al. Knowledge gaps inhibit health IT development for coordinating complex patients’ care. Am J Manag Care. 2016 Sep 1;22(9):e317–22. Exclusion Code: X1. [PubMed: 27662395]
- 584.
- Ruiz S, Snyder LP, Rotondo C, et al. Innovative Home Visit Models Associated With Reductions In Costs, Hospitalizations, And Emergency Department Use. Health Aff (Millwood). 2017 Mar 1;36(3):425–32. doi: 10.1377/hlthaff.2016.1305. PMID: 28264943. Exclusion Code: X1. [PubMed: 28264943] [CrossRef]
- 585.
- Rutgers Biomedical and Health Sciences (RBHS) Working Group on Medicaid High Utilizers. Analysis and recommendations for Medicaid high utilizers in New Jersey. Hotspot Blog. Camden, NJ: Camden Coalition; 2016. Exclusion Code: X1.
- 586.
- Ryan J, Abrams MK, Doty MM, et al. How high-need patients experience health care in the United States. Findings from the 2016 Commonwealth Fund Survey of High-Need Patients. Issue Brief (Commonw Fund). 2016 Dec;43:1–20. PMID: 29118179. Exclusion Code: X1. [PubMed: 27959480]
- 587.
- Ryan J, Brown M. Listening to those living with chronic conditions. Commonwealth Fund; 2016. Exclusion Code: X7.
- 588.
- Sadarangani T, Missaelides L, Eilertsen E, et al. A Mixed-Methods Evaluation of a Nurse-Led Community-Based Health Home for Ethnically Diverse Older Adults With Multimorbidity in the Adult Day Health Setting. Policy Polit Nurs Pract. 2019 Aug;20(3):131–44. doi: 10.1177/1527154419864301. PMID: 30614105. Exclusion Code: X1. [PMC free article: PMC6827350] [PubMed: 31373878] [CrossRef]
- 589.
- Sadowski LS, Kee RA, VanderWeele TJ, et al. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults: a randomized trial. JAMA. 2009 May 6;301(17):1771–8. doi: 10.1001/jama.2009.561. PMID: 19295391. Exclusion Code: X1. [PubMed: 19417194] [CrossRef]
- 590.
- Salhi BA, White MH, Pitts SR, et al. Homelessness and Emergency Medicine: A Review of the Literature. Acad Emerg Med. 2018 May;25(5):577–93. doi: 10.1111/acem.13358. PMID: 29223132. Exclusion Code: X7. [PubMed: 29223132] [CrossRef]
- 591.
- Salzberg CA, Hayes SL, McCarthy D, et al. Health system performance for the high-need patient: a look at access to care and patient care experiences. Issue Brief (Commonw Fund). 2016 Aug;27:1–12. PMID: 27033015. Exclusion Code: X1. [PubMed: 27571600]
- 592.
- Sambamoorthi U, Walkup J, Olfson M, et al. Antidepressant treatment and health services utilization among HIV-infected Medicaid patients diagnosed with depression. J Gen Intern Med. 2000 May;15(5):311–20. doi: 10.1046/j.1525-1497.2000.06219.x. PMID: WOS:000087185700005. Exclusion Code: X1. [PMC free article: PMC1495453] [PubMed: 10840266] [CrossRef]
- 593.
- Sander LD, Albert M, Okeke N, et al. Building a Medicaid ambulatory complex care program within an urban medical home. Popul Health Manag. 2018 Dec;21(6):446–53. doi: 10.1089/pop.2017.0200. PMID: 29939889. Exclusion Code: X1. [PubMed: 29620961] [CrossRef]
- 594.
- Sanders J, Guse C, Onuoha BC. Pilot study of a new model for managing hypertension in an uninsured population. J Prim Care Community Health. 2013 Jan;4(1):44–9. doi: 10.1177/2150131912451742. PMID: 22511403. Exclusion Code: X1. [PubMed: 23799689] [CrossRef]
- 595.
- Sarabia-Cobo C, Fernandez-Pena R, Taltavull JM, et al. Comparison between attention and experiences of chronic complex patients: A multicentric study. Health Soc Care Community.12. doi: 10.1111/hsc.13269. PMID: WOS:000607935400001. Exclusion Code: X6. [PubMed: 33453131] [CrossRef]
- 596.
- Sarabia-Cobo C, Taltavull-Aparicio JM, Miguélez-Chamorro A, et al. Experiences of caregiving and quality of healthcare among caregivers of patients with complex chronic processes: A qualitative study. Appl Nurs Res. 2020;56:N.PAG-N.PAG. doi: 10.1016/j.apnr.2020.151344. PMID: 147365277. Language: English. Entry Date: 20201209. Revision Date: 20201220. Publication Type: Article. Exclusion Code: X9. [PubMed: 32907769] [CrossRef]
- 597.
- Sarah B. Hunter MH, Brian Briscombe, Matthew Cefalu. Evaluation of Housing for Health Permanent Supportive Housing Program. RAND Corporation 2017. Exclusion Code: X1.
- 598.
- Sarnak DO, Ryan J. How high-need patients experiences the health care system in nine countries. Issue Brief (Commonw Fund). 2016 Jan;1:1–14. PMID: 27595372. Exclusion Code: X1. [PubMed: 26809154]
- 599.
- Sarvepalli S, Parikh MP, Sarvepalli S, et al. High utilizers of inpatient services in patients with esophageal stenosis. Am J Gastroenterol. 2018 Oct;113:S198–S9. PMID: WOS:000464611000346. Exclusion Code: X1.
- 600.
- Saxon L ER, Sobhani M. Health impacts of unlimited access to networked transportation in older adults. The Journal of mHealth. 2019. Exclusion Code: X11.
- 601.
- Schaeffer C, Teter C, Finch EA, et al. A pragmatic randomized comparative effectiveness trial of transitional care for a socioeconomically diverse population: design, rationale and baseline characteristics. Contemp Clin Trials. 2018 Feb;65:53–60. doi: 10.1016/j.cct.2017.12.003. PMID: 28793785. Exclusion Code: X1. [PubMed: 29233720] [CrossRef]
- 602.
- Schaink AK, Kuluski K, Lyons RF, et al. A scoping review and thematic classification of patient complexity: offering a unifying framework. J Comorb. 2012;2:1–9. doi: 10.15256/joc.2012.2.15. PMID: 29090137. Exclusion Code: X1. [PMC free article: PMC5556402] [PubMed: 29090137] [CrossRef]
- 603.
- Schoen C, Solís-Román C, Huober N, et al. On Medicare but at risk: a state-level analysis of beneficiaries who are underinsured or facing high total cost burden. New York: Commonwealth Fund; 2016. Exclusion Code: X1. [PubMed: 27214925]
- 604.
- Schulman M, Thomas-Henkel. Opportunities for complex care programs to address the social determinants of health. Center for Health Care Strategies. 2019. https://www
.chcs.org /media/TCC-SDOH-022119.pdf. Exclusion Code: X1. - 605.
- Seferian EG, Afessa B. Demographic and clinical variation of adult intensive care unit utilization from a geographically defined population. Crit Care Med. 2006 Aug;34(8):2113–9. doi: 10.1097/01.Ccm.0000227652.08185.A4. PMID: 16508721. Exclusion Code: X1. [PubMed: 16763514] [CrossRef]
- 606.
- Sewitch MJ, Blais R, Rahme E, et al. Receiving guideline-concordant pharmacotherapy for major depression: Impact on ambulatory and inpatient health service use. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 2007 Mar;52(3):191–200. doi: 10.1177/070674370705200311. PMID: WOS:000245227900011. Exclusion Code: X1. [PubMed: 17479528] [CrossRef]
- 607.
- Shah T, Brown M, Reed B, et al. High-need patients in their own words. Commonwealth Fund. 2017. https://www
.commonwealthfund .org/blog/2017 /high-need-patients-their-own-words. Exclusion Code: X1. - 608.
- Shah T, Brown M, Reed B, et al. Personas for people with three or more chronic conditions. 2019. https://www
.commonwealthfund .org/publications /2019/jan/personas-people-three-or-more-chronic-conditions. Exclusion Code: X1. - 609.
- Shah T, Lewis C, Tsega M, et al. Quick reference guide to promising care models for patients with complex needs. Commonwealth Fund. 2019. https://www
.commonwealthfund .org/publications /2019/feb/care-models-patients-complex-needs. Exclusion Code: X1. - 610.
- Shah V, Stokes J, Sutton M. Effects of non-medical health coaching on multimorbid patients in primary care: a difference-in-differences analysis. BMC Health Serv Res. 2019;19(1):N.PAG-N.PAG. doi: 10.1186/s12913-019-4367-8. PMID: 138199334. Language: English. Entry Date: In Process. Revision Date: 20191220. Publication Type: journal article. Journal Subset: Biomedical. Exclusion Code: X1. [PMC free article: PMC6704561] [PubMed: 31438937] [CrossRef]
- 611.
- Shannon GR, Wilber KH, Allen D. Reductions in costly healthcare service utilization: findings from the Care Advocate Program. J Am Geriatr Soc. 2006 Jul;54(7):1102–7. doi: 10.1111/j.1532-5415.2006.00799.x. PMID: 16866682. Exclusion Code: X1. [PubMed: 16866682] [CrossRef]
- 612.
- Sharpe M, Burton C, Sawhney A, et al. Is co-morbid depression adequately treated in patients repeatedly referred to specialist medical services with symptoms of a medical condition? J Psychosom Res. 2012 Jun;72(6):419–21. doi: 10.1016/j.jpsychores.2012.03.005. PMID: WOS:000305440700002. Exclusion Code: X1. [PubMed: 22656436] [CrossRef]
- 613.
- Sheehan B, Bass C, Briggs R, et al. Somatization among older primary care attenders. Psychol Med. 2003 Jul;33(5):867–77. doi: 10.1017/s003329170300789x. PMID: WOS:000184362600012. Exclusion Code: X1. [PubMed: 12877401] [CrossRef]
- 614.
- Sheff A, Park ER, Neagle M, et al. The patient perspective: utilizing focus groups to inform care coordination for high-risk medicaid populations. BMC Res Notes. 2017 Jul 25;10(1):315. doi: 10.1186/s13104-017-2638-1. PMID: 28743288. Exclusion Code: X1. [PMC free article: PMC5526300] [PubMed: 28743288] [CrossRef]
- 615.
- Shih SL, Gerrard P, Goldstein R, et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015 Nov;30(11):1688–95. doi: 10.1007/s11606-015-3350-2. PMID: 26841193. Exclusion Code: X1. [PMC free article: PMC4617914] [PubMed: 25956826] [CrossRef]
- 616.
- Shim J, Perry B. A social network analysis of poverty and health care utilization. University of California, San Francisco, School of Nursing San Franciso, California: 2018. https://www
.nsf.gov/awardsearch /showAward?AWD_ID =1756099&HistoricalAwards=false. Exclusion Code: X1. - 617.
- Shippee ND, Shah ND, May CR, et al. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012 Oct;65(10):1041–51. doi: 10.1016/j.jclinepi.2012.05.005. PMID: 22910536. Exclusion Code: X1. [PubMed: 22910536] [CrossRef]
- 618.
- Shortell S. How physician organizations manage care for high-need, high-cost patients. Commonwealth Fund; 2015. Exclusion Code: X1.
- 619.
- Shubeck SP, Thumma JR, Dimick JB, et al. Hot Spotting as a Strategy to Identify High-Cost Surgical Populations. Ann Surg. 2019 Mar;269(3):453–8. doi: 10.1097/sla.0000000000002663. PMID: 31521444. Exclusion Code: X1. [PubMed: 29342019] [CrossRef]
- 620.
- Simmons MM, Gabrielian S, Byrne T, et al. A Hybrid III stepped wedge cluster randomized trial testing an implementation strategy to facilitate the use of an evidence-based practice in VA Homeless Primary Care Treatment Programs. Implement Sci. 2017 Apr 4;12(1):46. doi: 10.1186/s13012-017-0563-2. PMID: 28376839. Exclusion Code: X9. [PMC free article: PMC5379611] [PubMed: 28376839] [CrossRef]
- 621.
- Simon GE, Khandker RK, Ichikawa L, et al. Recovery from depression predicts lower health services costs. J Clin Psychiatry. 2006 Aug;67(8):1226–31. doi: 10.4088/JCP.v67n0808. PMID: WOS:000240252400008. Exclusion Code: X1. [PubMed: 16965200] [CrossRef]
- 622.
- Simon GE, Manning WG, Katzelnick DJ, et al. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry. 2001;58(2):181–7. doi: 10.1001/archpsyc.58.2.181. PMID: 2001-03074-009. Exclusion Code: X1. [PubMed: 11177120] [CrossRef]
- 623.
- Simpson GG, Hahn HR, Powel AA, et al. A patient-centered emergency department management strategy for sickle-cell disease super-utilizers. West J Emerg Med. 2017 Apr;18(3):335–9. doi: 10.5811/westjem.2016.11.32273. PMID: 27364978. Exclusion Code: X4. [PMC free article: PMC5391880] [PubMed: 28435481] [CrossRef]
- 624.
- Sinaiko A, Meyers D, Rosenthal MB. Review of medical homes shows reduction in spending for high-risk patients, but design and implementation matter. Commonwealth Fund; 2017. Exclusion Code: X7.
- 625.
- Sisco-Taylor B, Fagerlin A, Weir P, et al. THE IMPACT OF AN INTENSIVE OUTPATIENT CLINIC ON MEDICALLY-COMPLEX PATIENTS’ DECISION MAKING OUTCOMES. Med Decis Making. 2020 Jan;40(1):E153–E. PMID: WOS:000509275600134. Exclusion Code: X9.
- 626.
- Siu HY, Steward N, Peter J, et al. A novel primary-specialist care collaborative demonstration project to improve the access and health care of medically complex patients. Chronic Illn. 2017 Sep;13(3):151–70. doi: 10.1177/1742395316674541. PMID: 28683743. Exclusion Code: X1. [PubMed: 28783974] [CrossRef]
- 627.
- Smeets RGM, Elissen AMJ, Kroese M, et al. Identifying subgroups of high-need, high-cost, chronically ill patients in primary care: A latent class analysis. PLoS One. 2020;15(1):e0228103. doi: 10.1371/journal.pone.0228103. PMID: 30931827. Exclusion Code: X6. [PMC free article: PMC6988945] [PubMed: 31995630] [CrossRef]
- 628.
- Smith BJ, McCall TC, Slaven EM, et al. PAs are a solution to the growing need for clinicians to treat an aging population. Jaapa. 2019 Dec;32(12):46–9. doi: 10.1097/01.Jaa.0000604864.79443.79. PMID: 32391756. Exclusion Code: X1. [PubMed: 31770305] [CrossRef]
- 629.
- Smith GM, Cenzer IS, Covinsky K, et al. Who Becomes a High Utilizer? A Case-Control Study of Older Adults in the USA. JGIM: Journal of General Internal Medicine. 2020;35(2):596–8. doi: 10.1007/s11606-019-05331-w. PMID: 141726521. Exclusion Code: X9. [PMC free article: PMC7018874] [PubMed: 31768905] [CrossRef]
- 630.
- Smith KJ, Handler SM, Kapoor WN, et al. Automated communication tools and computer-based medication reconciliation to decrease hospital discharge medication errors. Am J Med Qual. 2016 Jul;31(4):315–22. doi: 10.1177/1062860615574327. PMID: 26472939. Exclusion Code: X1. [PMC free article: PMC4950978] [PubMed: 25753453] [CrossRef]
- 631.
- Smith KW, Bir A, Freeman NL, et al. Impact Of Health Care Delivery System Innovations On Total Cost Of Care. Health Aff (Millwood). 2017 Mar 1;36(3):509–15. doi: 10.1377/hlthaff.2016.1308. PMID: 28264953. Exclusion Code: X11. [PubMed: 28264953] [CrossRef]
- 632.
- Smith MA, Vaughan-Sarrazin MS, Yu M, et al. The importance of health insurance claims data in creating learning health systems: evaluating care for high-need high-cost patients using the National Patient-Centered Clinical Research Network (PCORNet). J Am Med Inform Assoc. 2019 Nov 1;26(11):1305–13. doi: 10.1093/jamia/ocz097. PMID: 32348251. Exclusion Code: X1. [PMC free article: PMC7647219] [PubMed: 31233126] [CrossRef]
- 633.
- Smith RC, Gardiner JC, Armatti S, et al. Screening for high utilizing somatizing patients using a prediction rule derived from the management information system of an HMO: a preliminary study. Med Care. 2001 Sep;39(9):968–78. doi: 10.1097/00005650-200109000-00007. PMID: 15788554. Exclusion Code: X4. [PubMed: 11502954] [CrossRef]
- 634.
- Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003 Jun;18(6):478–89. doi: 10.1046/j.1525-1497.2003.20815.x. PMID: WOS:000183796100010. Exclusion Code: X1. [PMC free article: PMC1494880] [PubMed: 12823656] [CrossRef]
- 635.
- Smith TE, Appel A, Donahue SA, et al. Determining engagement in services for high-need individuals with serious mental illness. Adm Policy Ment Health. 2014 Sep;41(5):588–97. doi: 10.1007/s10488-013-0497-1. PMID: 24473411. Exclusion Code: X1. [PMC free article: PMC5361220] [PubMed: 23636712] [CrossRef]
- 636.
- Smith TP, Kennedy SL, Smith M, et al. Physiological improvements and health benefits during an exercise-based comprehensive rehabilitation program in medically complex patients. Exerc Immunol Rev. 2006;12:86–96. PMID: 17576592. Exclusion Code: X1. [PubMed: 17201074]
- 637.
- Smith WR, Poses RM, McClish DK, et al. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest. 2002 May;121(5):1610–7. doi: 10.1378/chest.121.5.1610. PMID: 12096148. Exclusion Code: X1. [PubMed: 12006451] [CrossRef]
- 638.
- Smith WR, Sop D, Johnson S, et al. Responsivity of utilization rates to the intensity of case management over time among high-utilizing adults with sickle cell disease. Blood. 2019;134. doi: 10.1182/blood-2019-128259. PMID: CN-02052008. Exclusion Code: X1. [CrossRef]
- 639.
- Smits F, Brouwer HJ, van Weert HCP, et al. Predictability of persistent frequent attendance: a historic 3-year cohort study. Br J Gen Pract. 2009 Feb;59(559):114–9. doi: 10.3399/bjgp09X395120. PMID: WOS:000263896400010. Exclusion Code: X4. [PMC free article: PMC2629841] [PubMed: 19192367] [CrossRef]
- 640.
- Smits FT, Brouwer HJ, Zwinderman AH, et al. Morbidity and doctor characteristics only partly explain the substantial healthcare expenditures of frequent attenders: a record linkage study between patient data and reimbursements data. BMC Fam Pract. 2013;14. doi: 10.1186/1471-2296-14-138. Exclusion Code: X1. [PMC free article: PMC3851974] [PubMed: 24044374] [CrossRef]
- 641.
- Smits FT, Brouwer HJ, Zwinderman AH, et al. Predictability of persistent frequent attendance in primary care: a temporal and geographical validation study. PLoS One. 2013 Sep;8(9). doi: 10.1371/journal.pone.0073125. PMID: WOS:000324481600054. Exclusion Code: X1. [PMC free article: PMC3764153] [PubMed: 24039870] [CrossRef]
- 642.
- Smits FTM, Brouwer HJ, ter Riet G, et al. Epidemiology of frequent attenders: a 3-year historic cohort study comparing attendance, morbidity and prescriptions of one-year and persistent frequent attenders. BMC Public Health. 2009 Jan;9. doi: 10.1186/1471-2458-9-36. PMID: WOS:000264288300001. Exclusion Code: X1. [PMC free article: PMC2649070] [PubMed: 19166622] [CrossRef]
- 643.
- Smits FTM, Mohrs JJ, Beem EE, et al. Defining frequent attendance in general practice. BMC Fam Pract. 2008 Apr;9. doi: 10.1186/1471-2296-9-21. PMID: WOS:000255711400001. Exclusion Code: X1. [PMC free article: PMC2373296] [PubMed: 18412954] [CrossRef]
- 644.
- Smits FTM, Wittkampf KA, Schene AH, et al. Interventions on frequent attenders in primary care. Scand J Prim Health Care. 2008;26(2):111–6. doi: 10.1080/02813430802112997. PMID: WOS:000257081400010. Exclusion Code: X1. [PMC free article: PMC3406646] [PubMed: 18570010] [CrossRef]
- 645.
- So C, Lage DE, Slocum CS, et al. Utility of functional metrics assessed during acute care on hospital outcomes: a systematic review. PM & R : the journal of injury, function, and rehabilitation. 2019;11(5):522–32. doi: 10.1002/pmrj.12013. Exclusion Code: X1. [PMC free article: PMC10108704] [PubMed: 30758920] [CrossRef]
- 646.
- Somers SA, Hamblin A, Verdier JM, et al. Covering low-income childless adults in Medicaid: experiences from selected states. Hamilton, N.J.: Center for Health Care Strategies, Inc.; 2010. Exclusion Code: X1.
- 647.
- Soril LJ, Leggett LE, Lorenzetti DL, et al. Reducing frequent visits to the emergency department: a systematic review of interventions. PLoS One. 2015;10(4):e0123660. doi: 10.1371/journal.pone.0123660. PMID: 25874866. Exclusion Code: X1. [PMC free article: PMC4395429] [PubMed: 25874866] [CrossRef]
- 648.
- Sovso MB, Klojgaard TA, Hansen PA, et al. Repeated ambulance use is associated with chronic diseases - a population-based historic cohort study of patients’ symptoms and diagnoses. Scand J Trauma Resusc Emerg Med. 2019 Apr 16;27(1):46. doi: 10.1186/s13049-019-0624-4. PMID: 30248102. Exclusion Code: X1. [PMC free article: PMC6469091] [PubMed: 30992042] [CrossRef]
- 649.
- Sparling A. Evaluation of a Personalized Care Management Program for High Hospital Utilizers. Atrium Health. Charlotte, NC: January 27, 2021 2021. Exclusion Code: X9.
- 650.
- Springer AM, Condon JR, Li SQ, et al. Frequent use of hospital inpatient services during a nine year period: a retrospective cohort study. BMC Health Serv Res. 2017 May 12;17(1):348. doi: 10.1186/s12913-017-2285-1. PMID: 28376886. Exclusion Code: X1. [PMC free article: PMC5427599] [PubMed: 28499388] [CrossRef]
- 651.
- Sriram V, Wiklund LO, Klein A, et al. Understanding variation in hospitalization trajectory among high cost, high need patients en-rolled in the comprehensive care physician (CCP) program. J Gen Intern Med. 2019;34(2):S393–S4. doi: 10.1007/11606.1525-1497. PMID: CN-01980309. Exclusion Code: X1. [CrossRef]
- 652.
- Stahl JE, Dossett ML, LaJoie AS, et al. Relaxation response and resiliency training and its effect on healthcare resource utilization. PLoS One. 2015;10(10):e0140212. doi: 10.1371/journal.pone.0140212. PMID: 25857588. Exclusion Code: X1. [PMC free article: PMC4603901] [PubMed: 26461184] [CrossRef]
- 653.
- Stark P. Advancing Complex Case Management Competencies in a Health Care System. Prof Case Manag. 2020 Jan/Feb;25(1):19–25. doi: 10.1097/ncm.0000000000000361. PMID: 31157584. Exclusion Code: X2. [PubMed: 31764712] [CrossRef]
- 654.
- Stefanescu Schmidt AC, DeFaria Yeh D, Tabtabai S, et al. National trends in hospitalizations of adults with tetralogy of fallot. Am J Cardiol. 2016 Sep 15;118(6):906–11. doi: 10.1016/j.amjcard.2016.06.034. PMID: 27501487. Exclusion Code: X1. [PMC free article: PMC5349299] [PubMed: 27530825] [CrossRef]
- 655.
- Stergiopoulos V, Gozdzik A, Cohen A, et al. The effect of brief case management on emergency department use of frequent users in mental health: findings of a randomized controlled trial. PLoS One. 2017;12(8):e0182157. doi: 10.1371/journal.pone.0182157. PMID: 26287805. Exclusion Code: X1. [PMC free article: PMC5542632] [PubMed: 28771524] [CrossRef]
- 656.
- Stergiopoulos V, Gozdzik A, Tan de Bibiana J, et al. Brief case management versus usual care for frequent users of emergency departments: the Coordinated Access to Care from Hospital Emergency Departments (CATCH-ED) randomized controlled trial. BMC Health Serv Res. 2016 Aug 24;16(1):432. doi: 10.1186/s12913-016-1666-1. PMID: 26984994. Exclusion Code: X1. [PMC free article: PMC4997752] [PubMed: 27557705] [CrossRef]
- 657.
- Sterk CE, Theall KP, Elifson KW. Health care utilization among drug-using and non-drug-using women. J Urban Health. 2002 Dec;79(4):586–99. doi: 10.1093/jurban/79.4.586. PMID: 12877778. Exclusion Code: X1. [PMC free article: PMC3456726] [PubMed: 12468678] [CrossRef]
- 658.
- Stey AM, Russell MM, Zingmond DS, et al. Using merged clinical and claims registry data to identify high utilizers of surgical inpatient care 1 year after colectomy. J Am Coll Surg. 2015 Aug;221(2):441–51.e1. doi: 10.1016/j.jamcollsurg.2015.03.024. PMID: 27228561. Exclusion Code: X1. [PubMed: 26141469] [CrossRef]
- 659.
- Stirbu I, Kunst AE, Mielck A, et al. Inequalities in utilisation of general practitioner and specialist services in 9 European countries. BMC Health Serv Res. 2011 Oct 31;11:288. doi: 10.1186/1472-6963-11-288. PMID: 21904335. Exclusion Code: X1. [PMC free article: PMC3221557] [PubMed: 22040155] [CrossRef]
- 660.
- Straker JK, Boehle SG, Nelson IM, et al. Common sense for caring organizations: results from a study of high-performing home care agencies and nursing homes. Oxford, Ohio, Columbus, Ohio: Scripps Gerontology Center, Ohio Direct Service Workforce Initiative; 2013. Exclusion Code: X1.
- 661.
- Strategies CfHC. Complex Care Innovation Lab. 2013. https://www
.chcs.org /project/complex-care-innovation-lab/. Exclusion Code: X2. - 662.
- Stremikis K, Connors C, Hoo E. Intensive outpatient care program: a care model for the medically complex piloted by employers. Commonwealth Fund. New York, NY: Commonwealth Fund; 2017. Exclusion Code: X1.
- 663.
- Surbhi S, Munshi KD, Bell PC, et al. Drug therapy problems and medication discrepancies during care transitions in super-utilizers. J Am Pharm Assoc (2003). 2016 Nov – Dec;56(6):633–42.e1. doi: 10.1016/j.japh.2016.07.004. PMID: 28863030. Exclusion Code: X1. [PubMed: 27720595] [CrossRef]
- 664.
- Szalda D, Steinway C, Greenberg A, et al. Developing a hospital-wide transition program for young adults with medical complexity. J Adolesc Health. 2019;65(4):476–82. doi: 10.1016/j.jadohealth.2019.04.008. PMID: 2019-37810-001. Exclusion Code: X1. [PubMed: 31277993] [CrossRef]
- 665.
- Tadros AS, Castillo EM, Chan TC, et al. Effects of an emergency medical services-based resource access program on frequent users of health services. Prehosp Emerg Care. 2012 Oct–Dec;16(4):541–7. doi: 10.3109/10903127.2012.689927. PMID: 21161535. Exclusion Code: X1. [PubMed: 22712694] [CrossRef]
- 666.
- Taylor L. Housing and health: an overview of the literature. Health Affairs/RWJF Health Policy Brief Princeton, NJ: 2018. https://www
.healthaffairs .org/do/10.1377/hpb20180313 .396577/full/. doi: 10.1377/hpb20180313.396577. Exclusion Code: X1. [CrossRef] - 667.
- Taylor LA, Byhoff E. Money Moves the Mare: The Response of Community-Based Organizations to Health Care’s Embrace of Social Determinants. Milbank Q.38. doi: 10.1111/1468-0009.12491. PMID: WOS:000605768700001. Exclusion Code: X1. [PMC free article: PMC7984660] [PubMed: 33420728] [CrossRef]
- 668.
- Taylor WJ, Reitz AC, Coplin M, et al. Patterns of emergency department high utilizers at grady memorial hospital. Ann Emerg Med. 2017;70(4):S32. Exclusion Code: X4.
- 669.
- Teigland C, Pulungan Z. LEVERAGING DATA-DRIVEN INSIGHTS TO SUPPORT DEVELOPMENT OF TARGETED SUPPLEMENTAL BENEFITS UNDER NEWLY EXPANDED FLEXIBILITIES IN MEDICARE ADVANTAGE TO IMPROVE OUTCOMES IN HIGH-COST, HIGH-NEED BENEFICIARIES. Value Health. 2019 May;22:S302–S. doi: 10.1016/j.jval.2019.04.1456. PMID: WOS:000472670103166. Exclusion Code: X7. [CrossRef]
- 670.
- ten Have M, Vollebergh W, Bijl RV, et al. Predictors of incident care service utilisation for mental health problems in the Dutch general population. Soc Psychiatry Psychiatr Epidemiol. 2001 Mar;36(3):141–9. PMID: 16101645. Exclusion Code: X1. [PubMed: 11465786]
- 671.
- Terra SM. What can claims data tell the case manager? Prof Case Manag. 2008 Jul–Aug;13(4):195–208; quiz 9–10. doi: 10.1097/01.Pcama.0000327408.71354.52. PMID: 18586128. Exclusion Code: X1. [PubMed: 18636003] [CrossRef]
- 672.
- Tewarson H. Improving lives and reducing cost of care for high-need, high-cost Medicaid enrollees with mental illness and substance abuse disorders. National Governors Association, Center for Best Practices 2017. https://hsrproject
.nlm .nih.gov/view_hsrproj_record/20191099. Exclusion Code: X7. - 673.
- Thompson T, McQueen A, Croston M, et al. Social Needs and Health-Related Outcomes Among Medicaid Beneficiaries. Health Educ Behav. 2019 Jun;46(3):436–44. doi: 10.1177/1090198118822724. PMID: 30654655. Exclusion Code: X1. [PubMed: 30654655] [CrossRef]
- 674.
- Thornquist L, Biros M, Olander R, et al. Health care utilization of chronic inebriates. Acad Emerg Med. 2002 Apr;9(4):300–8. PMID: 12404942. Exclusion Code: X1. [PubMed: 11927454]
- 675.
- Tompkins R, Khan A. ACHD Care in the United States: Complex Patients, Even More Complex Health Care System. Journal of the American College of Cardiology (JACC). 2020;76(2):183–5. doi: 10.1016/j.jacc.2020.05.030. PMID: 144487246. Language: English. Entry Date: 20200719. Revision Date: 20210110. Publication Type: editorial. Exclusion Code: X1. [PubMed: 32646568] [CrossRef]
- 676.
- Trevena LJ, Simpson JM, Nutbeam D. Soup kitchen consumer perspectives on the quality and frequency of health service interactions. Int J Qual Health Care. 2003 Dec;15(6):495–500. doi: 10.1093/intqhc/mzg065. PMID: 15535509. Exclusion Code: X1. [PubMed: 14660532] [CrossRef]
- 677.
- Trivedi SP, Trawick E, Diuguid-Gerber J, et al. Resource intensive care transitions program impact on 90-day hospital readmissions. J Gen Intern Med. 2019;34(2):S763–. doi: 10.1007/11606.1525-1497. PMID: CN-01981508. Exclusion Code: X5. [CrossRef]
- 678.
- Tsega M, Shah T, Lewis C. The importance of sustainable partnerships for meeting the needs of complex patients: introducing the return-on-investment calculator. Commonwealth Fund. 2019. https://www
.commonwealthfund .org/blog/2019 /importance-sustainable-partnerships-meeting-needs-complex-patients-introducing-return. Exclusion Code: X1. - 679.
- Turbow S, Fakunle O, Okosun IS. Multi- and single-year high-utilizers of inpatient services share many clinical and behavioral characteristics. , <Blank>: Springer Nature; 2018. p. 1614–5. Exclusion Code: X1. [PMC free article: PMC6153227] [PubMed: 29948801]
- 680.
- University of New Mexico Health Sciences Center. Leverage innovative care delivery and coordination model: Project ECHO. New Mexico: CMS; 2019. Exclusion Code: X7.
- 681.
- Ustulin M, Woo J, Woo JT, et al. Characteristics of frequent emergency department users with type 2 diabetes mellitus in Korea. J Diabetes Investig. 2018 Mar;9(2):430–7. doi: 10.1111/jdi.12712. PMID: 30055654. Exclusion Code: X1. [PMC free article: PMC5835454] [PubMed: 28686322] [CrossRef]
- 682.
- Valaitis R, Cleghorn L, Ploeg J, et al. Disconnected relationships between primary care and community-based health and social services and system navigation for older adults: a qualitative descriptive study. BMC Fam Pract. 2020 Apr 23;21(1):69. doi: 10.1186/s12875-020-01143-8. PMID: 32326880. Exclusion Code: X6. [PMC free article: PMC7181491] [PubMed: 32326880] [CrossRef]
- 683.
- van den Bussche H, Kaduszkiewicz H, Schafer I, et al. Overutilization of ambulatory medical care in the elderly German population?--An empirical study based on national insurance claims data and a review of foreign studies. BMC Health Serv Res. 2016 Apr 14;16:129. doi: 10.1186/s12913-016-1357-y. PMID: 26841193. Exclusion Code: X1. [PMC free article: PMC4831189] [PubMed: 27074709] [CrossRef]
- 684.
- van der Feltz-Cornelis CM, van Oppen P, Ader HJ, et al. Randomised controlled trial of a collaborative care model with psychiatric consultation for persistent medically unexplained symptoms in general practice. Psychother Psychosom. 2006;75(5):282–9. doi: 10.1159/000093949. PMID: WOS:000239726600003. Exclusion Code: X1. [PubMed: 16899964] [CrossRef]
- 685.
- Van Orden J. Service design for the complex customer: An empirical analysis of mental health integration at Intermountain Healthcare: The University of Utah; 2010. Exclusion Code: X2.
- 686.
- Vanagas G, Klimaviciute-Gudauskiene R. Factors affecting electronic health information needs in primary care patients. Telemed J E Health. 2012 Dec;18(10):724–8. doi: 10.1089/tmj.2012.0031. PMID: 20623250. Exclusion Code: X1. [PubMed: 23095004] [CrossRef]
- 687.
- Vargiu E, Fernandez JM, Gonzales-Gonzales M, et al. A self-management system for complex chronic patients. International Journal of Integrated Care. 2019;19:2. doi: 10.5334/ijic.s3101. PMID: WOS:000493776000102. Exclusion Code: X1. [CrossRef]
- 688.
- Vedsted P, Fink P, Olesen F, et al. Psychological distress as a predictor of frequent attendance in family practice - A cohort study. Psychosomatics. 2001 Sep–Oct;42(5):416–22. doi: 10.1176/appi.psy.42.5.416. PMID: WOS:000171885100007. Exclusion Code: X1. [PubMed: 11739909] [CrossRef]
- 689.
- Veltman-Verhulst SM, van Rijn BB, Westerveld HE, et al. Polycystic ovary syndrome and early-onset preeclampsia: reproductive manifestations of increased cardiovascular risk. Menopause. 2010 Sep–Oct;17(5):990–6. doi: 10.1097/gme.0b013e3181ddf705. PMID: 20508654. Exclusion Code: X1. [PubMed: 20551845] [CrossRef]
- 690.
- Verdier JM, Byrd V, Stone C. Enhanced primary care case management programs in Medicaid: issues and options for states. Hamilton, N.J.: Center for Health Care Strategies, Inc.; 2009. Exclusion Code: X1.
- 691.
- Vest JR, Grannis SJ, Haut DP, et al. Using structured and unstructured data to identify patients’ need for services that address the social determinants of health. Int J Med Inform. 2017 Nov;107:101–6. doi: 10.1016/j.ijmedinf.2017.09.008. PMID: 28930892. Exclusion Code: X1. [PubMed: 29029685] [CrossRef]
- 692.
- Vickers KS, Ridgeway JL, Hathaway JC, et al. Integration of mental health resources in a primary care setting leads to increased provider satisfaction and patient access. Gen Hosp Psychiatry. 2013 Sep–Oct;35(5):461–7. doi: 10.1016/j.genhosppsych.2013.06.011. PMID: WOS:000324473900004. Exclusion Code: X1. [PubMed: 23910217] [CrossRef]
- 693.
- Vickery KD, Bodurtha P, Winkelman TNA, et al. Cross-Sector Service Use Among High Health Care Utilizers In Minnesota After Medicaid Expansion. Health Aff (Millwood). 2018 Jan;37(1):62–9. doi: 10.1377/hlthaff.2017.0991. PMID: 29309230. Exclusion Code: X9. [PubMed: 29309230] [CrossRef]
- 694.
- Vickery KD, Shippee ND, Guzman-Corrales LM, et al. Changes in quality of life among enrollees in Hennepin Health: a Medicaid expansion ACO. Med Care Res Rev. 2020 Feb;77(1):60–73. doi: 10.1177/1077558718769457. PMID: 29749288. Exclusion Code: X1. [PubMed: 29749288] [CrossRef]
- 695.
- Vickery KD, Shippee ND, Menk J, et al. Integrated, Accountable Care For Medicaid Expansion Enrollees: A Comparative Evaluation of Hennepin Health. Med Care Res Rev. 2020;77(1):46–59. doi: 10.1177/1077558718769481. PMID: 140999606. Language: English. Entry Date: 20201020. Revision Date: 20201020. Publication Type: journal article. Exclusion Code: X12. [PubMed: 29749302] [CrossRef]
- 696.
- Viveiros J. Using health care savings to construct supportive housing in New York. In Housing health: innovations in the field Exclusion Code: X1.
- 697.
- Viveiros J. Addressing housing as a health care treatment. Washington, D.C.: Center for Housing Policy; 2015. Exclusion Code: X1.
- 698.
- Vladutiu CJ, Stringer EM, Kandasamy V, et al. Emergency care utilization among pregnant Medicaid recipients in North Carolina: an analysis using linked claims and birth records. Maternal & Child Health Journal. 2019;23(2):265–76. doi: 10.1007/s10995-018-2651-6. PMID: 134855464. Language: English. Entry Date: 20190225. Revision Date: 20190422. Publication Type: Article. Journal Subset: Continental Europe. Exclusion Code: X1. [PubMed: 30600512] [CrossRef]
- 699.
- Von Korff M, Lin EH, Fenton JJ, et al. Frequency and priority of pain patients’ health care use. Clin J Pain. 2007 Jun;23(5):400–8. doi: 10.1097/AJP.0b013e31804ac020. PMID: 19402853. Exclusion Code: X4. [PubMed: 17515738] [CrossRef]
- 700.
- von Wachter M, Junger S, Renz D, et al. Psychosocial assessment and health care utilization after coronary bypass surgery. Gesundheitswesen. 2000 Aug–Sep;62(8–9):451–6. doi: 10.1055/s-2000-12614. PMID: WOS:000089270900007. Exclusion Code: X1. [PubMed: 11037671] [CrossRef]
- 701.
- Votto JJ, Scalise PJ, Barton RW, et al. An analysis of clinical outcomes and costs of a long term acute care hospital. J Med Econ. 2011;14(2):141–6. doi: 10.3111/13696998.2010.551163. PMID: 21351183. Exclusion Code: X1. [PubMed: 21241209] [CrossRef]
- 702.
- Wajnberg A, Hwang U, Torres L, et al. Characteristics of frequent geriatric users of an urban emergency department. J Emerg Med. 2012 Aug;43(2):376–81. doi: 10.1016/j.jemermed.2011.06.056. PMID: 21999906. Exclusion Code: X4. [PubMed: 22040771] [CrossRef]
- 703.
- Walker BH, McCown JS, Bowser D, et al. An assessment of emergency department use among Mississippi’s Medicaid population. J Miss State Med Assoc. 2015 May;56(5):120–4. PMID: 25886658. Exclusion Code: X1. [PubMed: 26182673]
- 704.
- Walter LJ, Ackerson L, Allen S. Medicaid chemical dependency patients in a commercial health plan: do high medical costs come down over time? J Behav Health Serv Res. 2005 Jul–Sep;32(3):253–63. PMID: 15628650. Exclusion Code: X1. [PubMed: 16010182]
- 705.
- Wammes JJG, van der Wees PJ, Tanke MAC, et al. Systematic review of high-cost patients’ characteristics and healthcare utilisation. Family caregiving: a vision for the future. BMJ Open. 2018 Sep 8; Mar;8(9):e023113. doi: 10.1136/bmjopen-2018-023113; . PMID: 28774786. Exclusion Code: X7. [PMC free article: PMC6129088] [PubMed: 30196269] [CrossRef]
- 706.
- Wang D, Ing C, Blinderman CD, et al. Latent class analysis of specialized palliative care needs in adult intensive care units from a single academic medical center. J Pain Symptom Manage. 2019 Jan;57(1):73–8. doi: 10.1016/j.jpainsymman.2018.10.270. PMID: WOS:000454242100019. Exclusion Code: X1. [PMC free article: PMC6310624] [PubMed: 30315914] [CrossRef]
- 707.
- Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013 Apr;51(4):368–73. doi: 10.1097/MLR.0b013e31827da95a. PMID: 23269113. Exclusion Code: X1. [PubMed: 23269113] [CrossRef]
- 708.
- Washington C, Turbow S. Timing of hospital follow-up does not impact time to readmission among a population of high-utilizers. J Gen Intern Med. 2017 Apr;32:S355–S. PMID: WOS:000440259001131. Exclusion Code: X1.
- 709.
- Wasson JH, Ahles T, Johnson D, et al. Resource planning for patient-centered, collaborative care. J Ambul Care Manage. 2006;29(3):207–14. doi: 10.1097/00004479-200607000-00005. Exclusion Code: X1. [PubMed: 16788353] [CrossRef]
- 710.
- Watase H, Hagiwara Y, Chiba T, et al. Multicentre observational study of adults with asthma exacerbations: who are the frequent users of the emergency department in Japan? BMJ Open. 2015 Apr 28;5(4):e007435. doi: 10.1136/bmjopen-2014-007435. PMID: 25246521. Exclusion Code: X1. [PMC free article: PMC4420980] [PubMed: 25922104] [CrossRef]
- 711.
- Waxmonsky JA, Giese AA, McGinnis GF, et al. Colorado access’ enhanced care management for high-cost, high-need Medicaid members: preliminary outcomes and lessons learned. J Ambul Care Manage. 2011 Apr–Jun;34(2):183–91. doi: 10.1097/JAC.0b013e31820f64be. PMID: 21785095. Exclusion Code: X1. [PubMed: 21415616] [CrossRef]
- 712.
- Webster F, Rice K, Bhattacharyya O, et al. The mismeasurement of complexity: provider narratives of patients with complex needs in primary care settings. International Journal for Equity in Health. 2019 Jul;18:8. doi: 10.1186/s12939-019-1010-6. PMID: WOS:000474558100002. Exclusion Code: X6. [PMC free article: PMC6611020] [PubMed: 31272466] [CrossRef]
- 713.
- Weeks DL, Daratha KB, Towle LA. Diabetes prevalence and influence on resource use in Washington state inpatient rehabilitation facilities, 2001 to 2007. Arch Phys Med Rehabil. 2009 Nov;90(11):1937–43. doi: 10.1016/j.apmr.2009.06.008. PMID: 19851051. Exclusion Code: X1. [PubMed: 19887220] [CrossRef]
- 714.
- Weil AR. Community Care For High-Need Patients. Health Aff (Millwood). 2019 Jun;38(6):891. doi: 10.1377/hlthaff.2019.00553. PMID: 32384483. Exclusion Code: X9. [PubMed: 31158007] [CrossRef]
- 715.
- Weilburg JB, Wong HJ, Sistrom CL, et al. Behavioral Health Factors as Predictors of Emergency Department Use in the High-Risk, High-Cost Medicare Population. Psychiatr Serv. 2018 Dec 1;69(12):1230–7. doi: 10.1176/appi.ps.201800083. PMID: 28679819. Exclusion Code: X9. [PubMed: 30256183] [CrossRef]
- 716.
- Weiner JZ, McCloskey JK, Uratsu CS, et al. Primary Care Physician Stress Driven by Social and Financial Needs of Complex Patients. J Gen Intern Med. 2019 Jun;34(6):818–9. doi: 10.1007/s11606-018-4815-x. Exclusion Code: X1. [PMC free article: PMC6544720] [PubMed: 30623384] [CrossRef]
- 717.
- Weiner S, Schwartz A, Altman L, et al. Evaluation of a Patient-Collected Audio Audit and Feedback Quality Improvement Program on Clinician Attention to Patient Life Context and Health Care Costs in the Veterans Affairs Health Care System. JAMA Netw Open. 2020 Jul 1;3(7):e209644. doi: 10.1001/jamanetworkopen.2020.9644. PMID: 32735338. Exclusion Code: X1. [PMC free article: PMC7395234] [PubMed: 32735338] [CrossRef]
- 718.
- Weir RC, Proser M, Jester M, et al. Collecting Social Determinants of Health Data in the Clinical Setting: Findings from National PRAPARE Implementation. J Health Care Poor Underserved. 2020 May;31(2):1018–35. doi: 10.1353/hpu.2020.0075. PMID: WOS:000539399800039. Exclusion Code: X9. [PubMed: 33410822] [CrossRef]
- 719.
- Weiss Larry P. The identification of emotional disorders among high utilizers of medical care services: Adelphi; 1984. Exclusion Code: X7.
- 720.
- Weiss SJ, Ernst AA, Ong M, et al. Effect of a social services intervention among 911 repeat users. Am J Emerg Med. 2005 Jul;23(4):492–6. doi: 10.1016/j.ajem.2004.11.003. PMID: 15379078. Exclusion Code: X1. [PubMed: 16032618] [CrossRef]
- 721.
- Weissman GE, Kerlin MP, Yuan Y, et al. Potentially Preventable Intensive Care Unit Admissions in the United States, 2006–2015. Ann Am Thorac Soc. 2020 Jan;17(1):81–8. doi: 10.1513/AnnalsATS.201905-366OC. PMID: 30862996. Exclusion Code: X1. [PMC free article: PMC6944341] [PubMed: 31581801] [CrossRef]
- 722.
- Wharam JF, Landon BE, Galbraith AA, et al. Emergency department use and subsequent hospitalizations among members of a high-deductible health plan. JAMA. 2007 Mar 14;297(10):1093–102. doi: 10.1001/jama.297.10.1093. PMID: 17722755. Exclusion Code: X1. [PubMed: 17356030] [CrossRef]
- 723.
- Wherry LR, Burns ME, Leininger LJ. Using self-reported health measures to predict high-need cases among Medicaid-eligible adults. Health Serv Res. 2014 Dec;49 Suppl 2:2147–72. doi: 10.1111/1475-6773.12222. PMID: 24925967. Exclusion Code: X1. [PMC free article: PMC4241135] [PubMed: 25130916] [CrossRef]
- 724.
- Whitson HE, Sanders LL, Pieper CF, et al. Correlation between symptoms and function in older adults with comorbidity. J Am Geriatr Soc. 2009 Apr;57(4):676–82. doi: 10.1111/j.1532-5415.2009.02178.x. PMID: 19282797. Exclusion Code: X1. [PMC free article: PMC2674624] [PubMed: 19392960] [CrossRef]
- 725.
- Whittington JW, Kontz C, Craig C, et al. Bright spots for patients with complex needs. Institute for Healthcare Improvement; 2014. Exclusion Code: X7.
- 726.
- Wier Guilhardi LM. An initial case study of a readmission and emergency department revisit reduction program for high utilizer patients at a large community hospital system in Massachusetts: ProQuest Information & Learning; 2018. Exclusion Code: X1.
- 727.
- Wier Guilhardi LM. An Initial Case Study of a Readmission and Emergency Department Revisit Reduction Program for High Utilizer Patients at a Large Community Hospital System in Massachusetts. Boston University, ProQuest Dissertations Publishin; 2018. Exclusion Code: X1.
- 728.
- Wiler J, Pines JM, Ward MJ. Value and quality innovations in acute and emergency care. Cambridge, United Kingdom: Cambridge University Press; 2017. Exclusion Code: X1.
- 729.
- Williams BC. Limited effects of care management for high utilizers on total healthcare costs. Am J Manag Care. 2015 Apr;21(4):E244–E6. PMID: WOS:000358656900001. Exclusion Code: X1. [PubMed: 26244786]
- 730.
- Williams BC, Paik JL, Haley LL, et al. Centralized care management support for ‘high utilizers’ in primary care practices at an academic medical center. Care Management Journals. 2014;15(1):26–33. doi: 10.1891/1521-0987.15.1.26. PMID: 107897595. Language: English. Entry Date: 20140327. Revision Date: 20150712. Publication Type: Journal Article. Exclusion Code: X1. [PubMed: 24761538] [CrossRef]
- 731.
- Williamson AE, Johnson PC, Mullen K, et al. The disappearance of the “revolving door” patient in Scottish general practice: successful policies. BMC Fam Pract. 2012 Oct 4;13:95. doi: 10.1186/1471-2296-13-95. PMID: 22712694. Exclusion Code: X1. [PMC free article: PMC3528483] [PubMed: 23035887] [CrossRef]
- 732.
- Wilson K, Mottram P, Hussain M. Survival in the community of the very old depressed, discharged from medical inpatient care. Int J Geriatr Psychiatry. 2007 Oct;22(10):974–9. doi: 10.1002/gps.1773. PMID: WOS:000250237800006. Exclusion Code: X1. [PubMed: 17299805] [CrossRef]
- 733.
- Wilson M, Guta A, Waddell K, et al. The impacts of accountable care organizations on patient experience, health outcomes and costs: a rapid review. J Health Serv Res Policy. 2020;25(2):130–8. doi: 10.1177/1355819620913141. Exclusion Code: X1. [PubMed: 32321282] [CrossRef]
- 734.
- Wolff JL, Spillman B. Older adults receiving assistance with physician visits and prescribed medications and their family caregivers: prevalence, characteristics, and hours of care. J Gerontol B Psychol Sci Soc Sci. 2014 Nov;69 Suppl 1:S65–72. doi: 10.1093/geronb/gbu119. PMID: 24749700. Exclusion Code: X1. [PMC free article: PMC4303066] [PubMed: 25342825] [CrossRef]
- 735.
- Wong E, Guo R, Yoon J, et al. Impact of vha’s primary intensive care management program on dual system use. Health Serv Res. 2020;55(SUPPL 1):77–8. doi: 10.1111/1475-6773.13437. Exclusion Code: X1. [PubMed: 32919588] [CrossRef]
- 736.
- Wong ES, Rosland AM, Fihn SD, et al. Patient-Centered Medical Home Implementation in the Veterans Health Administration and Primary Care Use: Differences by Patient Comorbidity Burden. J Gen Intern Med. 2016 Dec;31(12):1467–74. doi: 10.1007/s11606-016-3833-9. PMID: 27503440. Exclusion Code: X1. [PMC free article: PMC5130955] [PubMed: 27503440] [CrossRef]
- 737.
- Woo JH, Grinspan Z, Shapiro J, et al. Frequent users of hospital emergency departments in Korea characterized by claims data from the national health insurance: a cross sectional study. PLoS One. 2016;11(1):e0147450. doi: 10.1371/journal.pone.0147450. PMID: 27376308. Exclusion Code: X1. [PMC free article: PMC4726528] [PubMed: 26809051] [CrossRef]
- 738.
- Woodhouse J, Peterson M, Campbell C, et al. The efficacy of a brief behavioral health intervention for managing high utilization of ED services by chronic pain patients. J Emerg Nurs. 2010 Sep;36(5):399–403. doi: 10.1016/j.jen.2009.02.008. PMID: 20837207. Exclusion Code: X3. [PubMed: 20837207] [CrossRef]
- 739.
- Worthington DC. The role of psychology in integrated primary care for complex patients: Effects on mental health, utilization of medical services, and physiological markers of health: Virginia Commonwealth University; 2015. Exclusion Code: X1.
- 740.
- Wu FM, Slightam CA, Wong AC, et al. Intensive outpatient program effects on high-need patients’ access, continuity, coordination, and engagement. Med Care. 2018 Jan;56(1):19–24. doi: 10.1097/mlr.0000000000000833. PMID: 29560072. Exclusion Code: X1. [PubMed: 29087980] [CrossRef]
- 741.
- Wu J, Grannis SJ, Xu H, et al. A practical method for predicting frequent use of emergency department care using routinely available electronic registration data. BMC Emerg Med. 2016 Feb 9;16:12. doi: 10.1186/s12873-016-0076-3. PMID: 26860825. Exclusion Code: X1. [PMC free article: PMC4748445] [PubMed: 26860825] [CrossRef]
- 742.
- Wyman MF, Liebzeit D, Voils CI, et al.?Hopes and wishes?: Goals of high -need, high -cost older patients and their caregivers. Patient Educ Couns. 2020 Jul;103(7):1428–34. doi: 10.1016/j.pec.2020.02.022. PMID: WOS:000540048500021. Exclusion Code: X1. [PMC free article: PMC7286795] [PubMed: 32098745] [CrossRef]
- 743.
- Wyte-Lake T, Claver M, Johnson-Koenke R, et al. Hurricanes Harvey, Irma, and Maria: Exploring the Role of Home-Based Care Programs. Disaster Med Public Health Prep. 2020 Feb;14(1):119–24. doi: 10.1017/dmp.2019.158. PMID: WOS:000524927400020. Exclusion Code: X1. [PMC free article: PMC7064407] [PubMed: 32014082] [CrossRef]
- 744.
- Xiang X, Zuverink A, Rosenberg W, et al. Social work-based transitional care intervention for super utilizers of medical care: A retrospective analysis of the bridge model for super utilizers. Soc Work Health Care. 2019;58(1):126–41. doi: 10.1080/00981389.2018.1547345. PMID: 2018-59710-001. Exclusion Code: X1. [PubMed: 30424717] [CrossRef]
- 745.
- Xin H. How do high cost-sharing policies for physician care affect inpatient care use and costs among people with chronic disease? J Ambul Care Manage. 2015 Apr–Jun;38(2):100–8. doi: 10.1097/jac.0000000000000050. PMID: 25722302. Exclusion Code: X1. [PubMed: 25748258] [CrossRef]
- 746.
- Xin H. High-cost sharing policies and non-urgent emergency department visits. Int J Health Care Qual Assur. 2018 Aug 13;31(7):735–45. doi: 10.1108/ijhcqa-05-2017-0089. PMID: 31980246. Exclusion Code: X1. [PubMed: 30354887] [CrossRef]
- 747.
- Xin H, Harman JS, Yang Z. How do high cost-sharing policies for physician care affect total care costs among people with chronic disease? J Insur Med. 2014;44(1):38–48. PMID: 16636909. Exclusion Code: X1. [PubMed: 25004597]
- 748.
- Xing J, Goehring C, Mancuso D. Care coordination program for Washington State Medicaid enrollees reduced inpatient hospital costs. Health Aff (Millwood). 2015 Apr;34(4):653–61. doi: 10.1377/hlthaff.2014.0655. PMID: 25847649. Exclusion Code: X1. [PubMed: 25847649] [CrossRef]
- 749.
- Yan J, Linn KA, Powers BW, et al. Applying Machine Learning Algorithms to Segment High-Cost Patient Populations. J Gen Intern Med. 2019 Feb;34(2):211–7. doi: 10.1007/s11606-018-4760-8. PMID: 27087391. Exclusion Code: X4. [PMC free article: PMC6374273] [PubMed: 30543022] [CrossRef]
- 750.
- Yedidia MJ. Competencies for engaging high-needs patients in primary care. Healthc (Amst). 2018 Jun;6(2):122–7. doi: 10.1016/j.hjdsi.2017.06.005. PMID: 27613732. Exclusion Code: X1. [PubMed: 29217389] [CrossRef]
- 751.
- Yip J, Nishita CM, Crimmins EM, et al. High-cost users among dual eligibles in three care settings. J Health Care Poor Underserved. 2007 Nov;18(4):950–65. doi: 10.1353/hpu.2007.0109. PMID: 24215036. Exclusion Code: X1. [PubMed: 17982217] [CrossRef]
- 752.
- Yotani N, Ishiguro A, Sakai H, et al. Factor-associated caregiver burden in medically complex patients with special health-care needs. Pediatr Int. 2014 Oct;56(5):742–7. doi: 10.1111/ped.12339. PMID: 22899484. Exclusion Code: X1. [PubMed: 24628805] [CrossRef]
- 753.
- Zhang YK, Grinspan Z, Khullar D, et al. Developing an actionable patient taxonomy to understand and characterize high-cost Medicare patients. Healthcare-the Journal of Delivery Science and Innovation. 2020 Mar;8(1):6. doi: 10.1016/j.hjdsi.2019.100406. PMID: WOS:000529324400008. Exclusion Code: X9. [PubMed: 31918975] [CrossRef]
- 754.
- Zulman D. Evaluating innovative care models for high-utilizing patients. VA Palo Alto Health Care System, Center for Innovation to Implementation. 2013. https://www
.hsrd.research .va.gov/research/abstracts .cfm?Project_ID=2141703149. Exclusion Code: X1. - 755.
- Zulman DM, Chang ET, Wong A, et al. Effects of intensive primary care on high-need patient experiences: survey findings from a Veterans Affairs randomized quality improvement trial. J Gen Intern Med. 2019 May;34(Suppl 1):75–81. doi: 10.1007/s11606-019-04965-0. PMID: 30789650. Exclusion Code: X9. [PMC free article: PMC6542922] [PubMed: 31098977] [CrossRef]
- 756.
- Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017 Feb 1;177(2):166–75. doi: 10.1001/jamainternmed.2016.8021. PMID: 28783974. Exclusion Code: X1. [PubMed: 28027338] [CrossRef]
Detailed Results Tables
Key Question 1. What criteria identify or predict that patients will be high-need, high-cost (HNHC)?
KQ 1a. How do criteria incorporate patient clinical characteristics?
Table B-1Descriptive multivariate, ED visits outcome (n=13)
| Author, Year | Population | Arthritis | Cancer | aCerebrovascular Disease | aCongestive Heart Failure | aCOPD | aCardiovascular Disease | aDementia | aDiabetes | aHeart Disease | Hep C | Hepatobiliary Disease | HIV Seropositive | Hypertension | Pancreatic Disease | Pulmonary Circulation Disorder | Seizure | Sickle Cell | aVascular Disease | # of Conditions | Charlson Comorbidity Index | Charlson Severity Index | General Health Score | Global Physical Health Score | Health Status | Hierarchic Categorical Condition Score | Morse Fall Score | Quan-Charlson Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buhumaid, 201532 | Hospital/health system | + | ||||||||||||||||||||||||||
| Hasegawa, 201435 | Hospital/health system | + | + | + | ||||||||||||||||||||||||
| Hasegawa, 201436 | Hospital/health system | + | + | + | + | |||||||||||||||||||||||
| Chang, 201440 | Hospital/health system | NS | + | NS | NS | |||||||||||||||||||||||
| Chukmaitov, 2012134 | Hospital/health system | + | ||||||||||||||||||||||||||
| Milbrett and Halm, 200942 | Hospital/health system | NS | ||||||||||||||||||||||||||
| Mandelberg, 200046 | Hospital/health system | + | + | + | + | + | + | |||||||||||||||||||||
| Surbhi, 202069 | Hospital/health system | NS | + | |||||||||||||||||||||||||
| Vinton, 201434 | Population based | + | + | + | + | + | NS | + | ||||||||||||||||||||
| Hunt, 200643 | Population based | + | ||||||||||||||||||||||||||
| Friedman, 200944 | Population based | + | ||||||||||||||||||||||||||
| Zuckerman and Shen, 200447 | Population based | + | ||||||||||||||||||||||||||
| Kanzaria, 201967 | Population based | + | + | + | + | + | + | |||||||||||||||||||||
| Thakarar, 201533 | Single program | + | + | |||||||||||||||||||||||||
| Doran, 201359 | VA | + | + | + | - | + |
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
COPD = chronic obstructive pulmonary disease; ED = emergency department; HIV = human immunodeficiency virus; NS = not statistically significant; n = number; VA = Veterans Health Administration.
Table B-2Predictive, ED visits outcome (n=3)
| Author, Year | Population | Arthritis | aCongestive Heart Failure | aCOPD | aDementia | aDiabetes | Hypertension | Renal Disease | # of Conditions | CCI | Hierarchic Categorical Condition Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Billings and Raven, 201341 | Medicaid | - | |||||||||
| Colligan, 201631 | Medicare | + | + | + | + | + | + | NS | - | + | |
| Brannon, 201877 | Hospital/health system | + |
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not in the model.
CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; ED = emergency department; NS = not statistically significant; n = number.
Table B-3Descriptive multivariate, IP visits outcome (n=2)
| Author, Year | Population | # of Conditions | CCI | Morse Fall Score |
|---|---|---|---|---|
| Bell, 201718 | Hospital/health system | + | ||
| Porter, 201950 | Hospital/health system | + | + | + |
| Surbhi, 202069 | Hospital/health system | + |
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
CCI = Charlson Comorbidity Index ; IP = inpatient; n = number.
Table B-4Predictive, IP visits outcome (n=3)
| Author, Year | Population | Arthritis | Cancer | aChronic Kidney Disease | aCongestive Heart Failure | aCardiovascular Disease | aDiabetes | Hypertension | # of Conditions |
|---|---|---|---|---|---|---|---|---|---|
| Hempstead, 2014135 | Hospital/health system | + | |||||||
| Leininger, 2014136; Leininger, 2015137 | Medicaid | + | |||||||
| Chang, 201954 | VA | + | + | + | + | + | + | + |
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
IP = inpatient; n = number; VA = Veterans Health Administration.
Table B-5Descriptive multivariate, all visits outcome (n=2)
| Author, Year | Population | Charlson Severity Index | Global Physical Health Score |
|---|---|---|---|
| Rohrer, 200820 | Hospital/health system | + | |
| Blumenthal, 201748 | Hospital/health system | + |
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
n = number.
Table B-6Predictive, all visits outcome (n=3)
| Author, Year | Population | aCOPD | aDiabetes | Hypertension | # of Conditions | CCI | Other |
|---|---|---|---|---|---|---|---|
| Meek, 200024 | Commercial (including HMO) | + | + | NS | |||
| Wherry, 2014138; Leininger and Avery, 2015137 | Population based | + | |||||
| Reichard, 201523 | Population based | +/NS | Disability + |
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model. +/NS denotes performance across multiple models within the study, ranging from a positive significant association to no significance.
CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; HMO = health maintenance organization; NS = not statistically significant; n = number.
Table B-7Descriptive multivariate, cost outcome (n=3)
| Author, Year | Population | Cancer | aCerebrovascular Disease | aCardiovascular Disease | aDiabetes | Condition | General Health Score | Quan-Charlson Score | Other |
|---|---|---|---|---|---|---|---|---|---|
| Bayliss, 201656 | Commercial (including HMO) | + | + | ||||||
| Robinson, 201619 | Commercial (including HMO) | NS | NS | + | - | NS | + | ||
| Figueroa, 201971 | Medicare | + | + | + |
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
HMO = health maintenance organization; NS = not statistically significant; n = number.
Table B-8Predictive, cost outcome (n=1)
- a
Complex chronic condition
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
n = number.
KQ 1b. How do criteria incorporate patient demographic, behavioral health, and social risk factors?
Table B-9Multivariate descriptive results
| Author, Year | Outcome | Population | Female | Age | Race/Ethnicity | Depression | Severe Mental Illness | Other Mental Illness | Substance Use | Alcohol Use | Homelessness | Employment | Other Social Risk Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayliss, 201656 | Cost (top 25%) | Commercial (including HMO) | + | Over 55+ | + | ||||||||
| Robinson, 201619 | Cost (top 10% of all-cause total costs) | Commercial (including HMO) | NS | NS | NS | NS/+ | + | NS | NS | ||||
| Sterling, 201821 | Cost (top 20%) | Commercial (including HMO) | + | Any financial burden + | |||||||||
| Walker, 200351 | Cost (adjusted annual cost ratios of median healthcare costs) | Commercial (including HMO) | NR | NR | NR | PTSD+ | |||||||
| Berkowitz, 201870 | Cost (top 10%, 5% & 2%) | Population based | NS | + |
Black: + White: + Hispanic: |
Food insecurity: + Ed: NS Income: NS | |||||||
| Figueroa, 201971 | Cost (top 10%) | Medicare | - | - |
Black: + Hispanic: + | + |
Income: + Dual elig: + | ||||||
| Behr, 201630 | ED visits (2+, 3+, 4+, 5+) | Hospital/health system | + | Black +/NS | + | + | Employed + | ||||||
| Bell, 201718 | Inpatient visits (3+) | Hospital/health system | NR | NR | NS | + | + | + | Community-level income <48k NS | ||||
| Blumenthal, 201748 | All utilization (ED visits, acute hospitalizations) | Hospital/health system | NR | NR | NR | Lowest quartile in Global Mental Health score NS | |||||||
| Buhumaid, 201432 | ED visits (4+) | Hospital/health system | NS | Age (40+) NS | Black + | - | + | + | |||||
| Chang, 201440 | ED visits (4+ in 12 months) | Hospital/health system | NS | NS | Not in model | Personality dx + |
SUD dx NS Positive cocaine screen+ | NS | + | ||||
| Chukmaitov, 2012134 | ED visits (1+, 1–3, 4+) | Hospital/health system | + |
Age (30–39) - (40–49) NS (50–64) + (65–74) + (75–84) + (>85) - |
Black + Hispanic + Other - | ||||||||
| Doran, 201438 | ED visits (3+) | Hospital/health system | NR | NR | NR | ||||||||
| Hasegawa, 201435 | ED visits (3+ ED, 30-day ED revisit, IP visit) | Hospital/health system | - | Age (<74) + |
Black + Hispanic + Other NS | + | + | Quartiles for median household income of patient’s zip code 1 (lowest) and 2 + 3 NS | |||||
| Hasegawa, 201436 | ED visits (3+) | Hospital/health system | - | Over 54+ | +/NS | + | + | + | Lowest quartile median income + | ||||
| Hasegawa, 201437 | ED visits (0, 1–2, 3–5, 6+) | Hospital/health system | NS/- | 40–54 NS/+ | NS | Lowest quartile median income NS | |||||||
| Liu, 201339 | ED visits (4+, 4–7, 8–18, 19+) | Hospital/health system | NR | NR | NR | + | + | + | |||||
| Mandelburg, 200046 | ED visits (5+) | Hospital/health system | NS | Age (30–59) + |
Black + Hispanic - Native American + Asian - Other - | + | + | ||||||
| Surbhi, 202069 | ED visits | Hospital/health system | NS | Black + | + | ||||||||
| Castillo, 201865 | ED visits (>5) vs 1–5 | 326 hospitals in CA | - | 75–84 & 85+ vs 65–73 - |
Black: + Hispanic + Asian/Pacific Islander - | + | + | ||||||
| Millbrett, 200942 | ED visits (>6) | Hospital/health system | - | Nonblack + | NS | Part-time employment retired/unemployed,+ | |||||||
| Porter, 201950 | Inpatient visits (3+) | Hospital/health system | NR | Under 40+ | NR | + | + | ||||||
| Emechebe, 2019 | Inpatient visits (2+) | Medicaid managed care and Medicare Advantage health plan | NR | NR | NR | NR | NR | NR | NR | NR | Housing support need + | Needs: Financial assistance +; food +; transport-ation +, medication assistance + | |
| Surbhi, 202069 | Inpatient visits | Hospital/health system | NS |
Black – Black: NS | + | ||||||||
| Rohrer, 200820 | All utilization (27+ outpatient visits) | Hospital/health system | NS | NS | Not in model | ||||||||
| Ruger, 200445 | ED visits (1, 2, 20+) | Hospital/health system | NR | NR | Not in model | ||||||||
| Kanzaria, 201967 | ED visits (4–7) | Medicaid MCO in one city | - | Older: - |
Black: + Hispanic: NS Asian: - | + | + | + | + | + | + | Interaction with county jail system: + | |
| Thakarar, 201533 | ED visits (2+) | Hospital/health system (single program) | NS | 55+ NS | Minority NS | NS | NS | NS | More than 1 housing change NS | ||||
| Freidman, 200944 | ED visits (4+) | Population based | NS | NS |
Income <$40k + <$90k NS | ||||||||
| Hunt, 200643 | ED visits (1–3, 4+) | Population based | NS | + |
Income by poverty threshold 200–399% + 100–199% + Below threshold + | ||||||||
| Vinton, 201434 | ED visits (0, 1–3, 4–9, 10+) | Population based | + | >44- |
Black + Hispanic - Asian - Other NS | + | NS | Currently employed - | Poverty–income ratio (Ref is ≥4) + | ||||
| Zuckerman, 200447 | ED visits (3+) | Population based |
Black + Hispanic NS Asian NS | Near poor and poor + Single parent + | |||||||||
| Doran, 201359 | ED visits (0, 1, 2–4, 5–10, 11–25, >25)) | VA | - | + | + | + | + | + | + | ||||
| Levinson, 2005139 | All utilization (3+ ED; 2+ specialty clinic visits) | VA | NR | NR | NR |
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
CA = California; dx = diagnosis; Ed = education; ED = emergency department; HMO = health maintenance organization; IP = inpatient; k = thousand; NR = not reported; NS = not statistically significant; PTSD = post-traumatic stress disorder; Ref = reference group; SUD = substance use disorder; VA = Veterans Health Administration; vs = versus.
Table B-10Predictive results
| Author, Year | Outcome | Population | Female | Age | Race/Ethnicity | Depression | Severe Mental Illness | Other Mental Illness | Substance Use | Alcohol Use | Homelessness | Employment | Other Social Risk Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meek, 200024 | All utilization (6+ visits in 6 months) | Commercial (including HMO) | + | Under 40 - | NR | +/NS/- | |||||||
| Brannon, 201877 | ED visits (1+, 2+, 3+, 4+, 5+) | Hospital/health system | Age + but not defined | Zip code+ but not defined | |||||||||
| Hempsted, 2014135 | Inpatient visits (1+, 2, 3+) | Hospital/health system | - |
Age (Ref is 80+) 18–34 + 35–49+ 50–64 + 65–79 + |
Race/ethnicity (Ref is white) Asian/PI - Black - Hispanic - Other + | + | + | HH income < $34.999k + | |||||
| Hwang, 2014140 | ED visits (4+ visits in both years; 4+visits either year; <4 visits in both years) | Hospital/health system | - | Nonwhite+ | NR | NR | Low median household income+ | ||||||
| Billings, 200762 | Inpatient visits (1+ hospital admission in next 12 months) | Medicaid | NR | NR | NR | + | + | + | |||||
| Billings, 201341 | ED visits (3+) | Medicaid | Not in model | ||||||||||
| Leninger, 2014, 2015136, 137 | Inpatient visits (1+ inpatient hospitalization) | Medicaid | NR | NR | NR | + | + | + | |||||
| Raven, 200860 | Inpatient visits (1+ readmission) | Medicaid | |||||||||||
| Yang, 201722, 53 | Cost (persistent top 10% decile) | Medicaid | NR | NR | NR | ||||||||
| Yang, 201825 | Cost (top 10% expenditures in next 12 months) | Medicaid | NR | NR | NR | ||||||||
| Colligan, 201631 | ED visits (0, 1–3, 4+) | Medicare |
≤64 + ≥75 + |
Race (Ref is white) Black + Asian - Hispanic - Native American + Unknown/other - | + | ||||||||
| Kanzaria, 201728 | ED visits (4+ visits in 12 months) | Population based |
Black +/NS Hispanic + Other -/NS | +/NS | -/NS | +/NS | +/NS | +/NS | Poverty indicator NS | ||||
| Reichard, 201523 | All utilization (≥75th percentile) | Population based | NR | NR | NR | ||||||||
| Wherry, 2014137, 138 | All utilization (top decile) | Population based | NS | ||||||||||
| Chang, 201954 | Inpatient visits (persistently high risk, intermittent, or low risk in 2 years) | VA | - | Over 45+ |
Race (ref Hispanic) White - Black - Other - (p<0.05) | + | + (tobacco) | - |
Number of zip code changes + Urban+ |
+ denotes significant positive association, - denotes significant negative association, NS denotes no significance, NR denotes not reported, and a blank cell denotes not included in the model.
ED = emergency department; HH = household; HMO = health maintenance organization; NR = not reported; NS = not statistically significant; PI = Pacific Islander; Ref = reference group; VA = Veterans Health Administration.
Key Question 2. What are the mechanisms that lead to reductions in potentially preventable or modifiable healthcare use and result in improved health outcomes and cost savings in interventions serving HNHC patients?
Table B-11Full list of CMO configurations and supporting data for Program Theory 1: Identifying and targeting HNHC patients for inclusion in interventions
Summary: Identifying and targeting HNHC patients for interventions intended to reduce potentially preventable or modifiable healthcare use and costs requires capturing their complexity based on a combination of prior use of healthcare services, chronic disease, nonmedical barriers to accessing care, experience with the healthcare system, clinician judgment, and patient willingness to participate.a
| CMOs | Relevant Data Extracts/Summary Information From Included Literature |
|---|---|
| CMO 1.1. Including historic information obtained from claims and other electronic health records, that can identify prior high cost and/or use of healthcare services [C], adds to intervention designers’ confidence in creating an algorithm [M] that will result in targeting patients who are at the greatest risk of being HNHC in the future [O]. |
Participants were drawn from adult Medicaid patients attributed to CareMore primary care physicians (PCPs). Program eligibility criteria were aimed at identifying patients at risk for poor outcomes and unnecessary spending, as well as those most likely to benefit from complex care management. The criteria drew from analyses suggesting that combining predictive models, historical claims, and clinician judgment is the most effective approach to identifying patients for complex care management. Eligible patients were first required to meet at least 1 of the following criteria: top 5% of total medical expenditures (TME) in the prior 12 months, top 5% of Chronic Illness Intensity Index (CI3) score, or care team member nomination.108 Predicted future health care costs are based on the Predictive Risk Intelligence SysteM (PRISM) medical cost risk score developed and implemented by Washington State Medicaid programs (Court et al. 2011). PRISM combines diagnostic and pharmacy data to predict future expenditures based on grouping algorithms from two risk adjustment models widely used by State Medicaid programs, the Chronic Illness and Disability Payment System (Kronick et al. 2000) and Rx-Medicaid (Gilmer et al. 2001), both of which have predictive accuracy comparable to commercial alternatives. The PRISM risk score is a ratio calculated by dividing the individual’s expected monthly future expenditures by the average monthly future expenditures of all Medicaid SSI recipients. The risk score equals 1.0 if the individual’s expected expenditures equal the average expenditures of the group.112 Rely on more than 1 type of data-because it may be vulnerable to inaccuracies: One program said they use predictive modeling so that they are not “held hostage waiting for claims to come down the road.” Washington State uses predictive modeling to begin to identify the target population. Using its Health Service Encounter algorithm, the state examines 15 months of integrated health care claims to determine future medical costs and inpatient risk scores. The state has found that conditions such as diabetes, cardiovascular disease, MH and substance abuse are common among the superutilizing subset of patients. It uses different approaches to further stratify subgroups for complex care management including identifying individuals with extreme emergency department utilization (e.g., approximately 80 to 130 ED visits in 15 months), high expected future medical costs (predicted by high utilization and costs in the past), high prospective inpatient risk scores, and sign gaps in care and quality indicators.49 The programs represented at the Summit generally use historical claims data as a foundation to understand the size and scope of super-utilization. Claims analysis is an iterative process and includes identifying areas of high cost and high utilization, and/or identifying groups of recipients with a high number of diagnoses. With this initial broad brush information, programs are able to further shape and define the target population. For example, Community Care of North Carolina (CCNC), which includes 14 regional networks that manage the care of Medicaid beneficiaries, will analyze at least 12 months of data in order to understand which chronic illness and mental health indicators are contributing to a high number of ED visits.49 “After an individual’s records were linked across data sets, the process of flagging individuals as ‘‘high utilizers’’ began. Instead of using charges or receipts to define high utilizers, the decision was made to rely on the number of emergency department (ED) and inpatient visits made by an individual over the prior 12-month period.”78 “From the best-fit synthesis we conducted to answer KQ 1, we identified some characteristics that are often available through electronic databases that can be used to help identify patients.” (Discussion section of this report. Also see full results for KQ 1) During the first site visit, physicians at both sites reported that they were initially very enthusiastic about the Health Buddy® program, because it offered a promising way to effectively support patients with chronic disease….. Once the physicians received the list of patients who were eligible for the Health Buddy® program, they reported that they became frustrated with the project because they felt that many of the patients selected would not benefit from participating. Further, physicians reported disappointment that many of the patients they believed could be helped by the program were not eligible to participate in the program because they had not been identified through the claims based algorithm developed by HHN.80 Risk assessments of the TST participant population were conducted to inform the development of individualized care plans and assign participants to one of three risk categories to determine the level of service to be provided to each participant.…TST reported that the high-risk intervention was provided to approximately 5% to 7% of the TST participant population that had depression and/or potentially critical health problems that required immediate attention.…What might explain the lack of success in TST’s demonstration? Ineffective Targeting. One explanation may be the inability to accurately target beneficiaries at greatest risk of intensive, costly, service use (as distinct from the need for general care management). …When TST learned that one of its participants was admitted to the hospital, it reassigned this individual to its high-risk intervention, and when appropriate, a care manager visited the beneficiary in the hospital to determine the cause of the hospitalization and identify any new health or social issues to be addressed. Not surprisingly, TST adopted a strategy of targeting beneficiaries at greatest risk of a hospitalization and higher costs. Their targeting strategy was unsuccessful—and costly. The program was unable to predict future complications with any precision for those with initially stable, less costly, conditions. Lacking direct access to patients’ medical records, the health coaches often began working with beneficiaries with incomplete information.79 There are two key elements to the success of these new efforts to target and improve care for high-cost Medicaid cases. First, it is essential to be able to identify in advance patients who are likely to have high costs in the future. Many high-cost occurrences (such as injury, acute illness, or cancer) might be episodic, and high spending in one year might not mean high spending in subsequent years.62 We conducted a retrospective analysis of secondary data from the Medicare program and other linked sources. We used 3 databases, including the Chronic Condition Data Warehouse, hierarchic categorical condition scores, and timeline files. The Chronic Condition Data Warehouse includes fee-for-service billing history for services reimbursed under Medicare Parts A, B, and D, as well as data about beneficiary demographic characteristics, linked at the beneficiary level with a unique identification number.31 We used data from three health systems to develop, evaluate, and implement a model for the prediction of high ED utilization in Washtenaw and Livingston counties: Michigan Medicine and St. Joseph Mercy Health System (operators of all EDs in Ann Arbor and Livingston County) and Integrated Health Associates (IHA), a multispecialty medical practice with clinical sites in both counties.77 Administrative data from DH’s data warehouse were used to obtain demographic, medical, psychological/behavioral health and service utilization and claims data. The tight administrative and clinical integration among all care settings facilitates data capture across the continuum of care.83 After an individual’s records were linked across data sets, the process of flagging individuals as ‘‘high utilizers’’ began. Instead of using charges or receipts to define high utilizers, the decision was made to rely on the number of emergency department (ED) and inpatient visits made by an individual over the prior 12-month period. This step eliminated potential variability related to differences in treatments and payers. Rather than assigning an artificial cutoff, the nuances of the local population were allowed to set the threshold for what constituted high utilization. The Coalition defined high utilization as ‘‘any individual with total emergency or inpatient visits greater than 1.5 standard deviations above the mean.’’ This definition resulted in any individual with 3 or more inpatient visits, or 6 or more ED visits, being flagged in the database as a high utilizer.78 The site for this study was the Duke Outpatient Clinic (DOC), a large primary care safety net clinic in Durham, North Carolina. Patients at the DOC have a high prevalence of multimorbidity, mental illness, and socioeconomic challenges. Beginning in 2012, the clinic initiated an extensive redesign process to better meet complex population health needs and reduce avoidable utilization of ED and inpatient care. This study was conducted to direct further quality improvement efforts. Multiple methods were applied, including both retrospective quantitative analysis of clinical data and an in-depth chart review. The study team extracted electronic health record data for all patients enrolled at the DOC between July 1, 2014, and June 30, 2015. The team matched these data to ED encounter data for the same year period from 2 local hospitals within the Duke University Health System, where DOC patients receive a vast majority of their emergency and hospital care. Lastly, the team conducted 30 chart reviews for 10 of the highest ED utilizers for each of the 3 leading chief complaints to uncover additional details surrounding their frequent ED use patterns.17 The literature contains varying definitions for super-utilizer. The definition used for this analysis was adapted from the work of Johnson, et al. and defined super-utilizers as adult patients (≥ 18 years of age) who, along with having an admission (analysis index admission) during the requisite timeframe, had at least two other admissions in the year prior, or at least one other admission along with a serious mental health diagnosis… In order to focus the analysis on cost savings that could be linked to our interventions, patients on chemotherapy, patients with orthopedic complications, patients diagnosed with HIV, and patients who had repeated admissions for emergency dialysis were excluded from analysis.83 |
| CMO 1.2 Capturing a patient’s use of services in “real time,” if possible, while the patient is still hospitalized [C], adds to the intervention service provider’s confidence that a patient is identified during a period of urgent need for the services [M], resulting in intervention services being initiated prior to or during a period of high use and not during a later period, when service use may have already declined (regression to the mean) [O]. |
More than half of patients approached in the ED refused to participate, possibly due to competing concerns about their illness or participating in research. Those who enrolled may have been more engaged, and thus, more likely to respond to the intervention, than those who declined. Identifying and approaching patients in real time may be time consuming and resource intensive, but this approach has been found to be more effective in addressing the needs of some populations of high utilizers than using historical claims data to identify and ‘‘segment’’ high utilizers with aligned interventions.110 To be most effective, complex care management programs should target patients at risk of persistently high spending and those whose spending and health outcomes are amenable to complex care planning and engagement. Many complex care management programs use claims data and historical utilization patterns to identify eligible patients. Recent research has highlighted the limitations of this approach—historically high-cost patients often return to normal patterns or spending, or they have drivers of high spending not amenable to complex care management.108 In order to identify chronic frequent users, rather than those with an isolated health event requiring multiple visits, we identified patients with the most ED visits during both the 30-day period and the 12-month period preceding the introduction of the program.111 The Summit participants unanimously agreed that access to real-time information—such as notifications of ED visits or inpatient admissions—and a strong analytics team provide a critical foundation for super-utilizer programs. One leader referred to data as “oxygen for our program.” Programs place a high priority on developing a robust data repository that can be mined to identify groups of patients that might respond well to complex care management.49 Eligible patients were identified through real-time automated methods and recruitment occurred while patients were still hospitalized. Patient intake included an in-depth patient assessment to determine nonmedical barriers to improved health.83 This “real time” approach of using a hospital admission as a triggering event was perceived as useful for two reasons. First, patients with a hospital admission are much more likely to have a subsequent admission in the next twelve months than patients without an admission, which improves the potential case-finding capacity of the algorithm. But, equally important, effective discharge planning is likely to be a critical component of any intervention strategy for high-cost, high risk patients. However, because of limited resources and lag time in acquiring data, our experience in other environments has suggested that some providers and payers are interested in non–“real time,” retrospective analyses. Accordingly, we also examined patients with any claims in 2000–2003, to predict subsequent admissions in 2004 (regardless of whether they had a hospital admission in 2003 or any prior year). This “archival” approach to case finding is somewhat less robust (it finds fewer patients) than the “real time” method and only brief findings for this approach are presented for comparative purposes.62 Risk assessments of the TST participant population were conducted to inform the development of individualized care plans and assign participants to one of three risk categories to determine the level of service to be provided to each participant.…TST reported that the high-risk intervention was provided to approximately 5% to 7% of the TST participant population that had depression and/or potentially critical health problems that required immediate attention.…What might explain the lack of success in TST’s demonstration? Ineffective Targeting. One explanation may be the inability to accurately target beneficiaries at greatest risk of intensive, costly, service use (as distinct from the need for general care management). …When TST learned that one of its participants was admitted to the hospital, it reassigned this individual to its high-risk intervention, and when appropriate, a care manager visited the beneficiary in the hospital to determine the cause of the hospitalization and identify any new health or social issues to be addressed. Not surprisingly, TST adopted a strategy of targeting beneficiaries at greatest risk of a hospitalization and higher costs. Their targeting strategy was unsuccessful—and costly. The program was unable to predict future complications with any precision for those with initially stable, less costly, conditions. Lacking direct access to patients’ medical records, the health coaches often began working with beneficiaries with incomplete information.79 What might explain the lack of success in the Phase II KTBH Demonstration? One explanation may be the targeting of beneficiaries at greatest risk of intensive, costly, service use (as distinct from the need for general care management). Responding to KTBH’s request, CMS staff selected a very costly, complex set of Medicare beneficiaries for their intervention and comparison groups. As a result, the comparison group exhibited substantial regression-to-the-mean (RtoM) effects. While the randomized experimental design should cancel out RtoM effects and isolate a pure intervention effect, the large churning of beneficiaries from lower (higher) to higher (lower) cost groups over time adds considerable statistical noise to the test of savings. Even still, we would have considered the Phase II original intervention to be a success if it had saved 5.4% of costs. Large increases in demonstration period costs in less costly beneficiaries in the base period make it very difficult for intervention staff to target those at highest financial risk. It is much easier to target beneficiaries during the intervention period who actually incur major flare-ups and hospitalizations. Unfortunately, these beneficiaries have already incurred major expenditures by the time they receive intensive disease management services.82 |
| CMO 1.3. Considering patients’ chronic conditions, functional limitations, and clinical severity scores [C] adds to intervention designers’ confidence in identifying HNHC patients [M] who will benefit from intervention services [O1] and who are at risk of having future high healthcare use and cost [O2]. |
Three categories of high-cost users—beneficiaries who had multiple chronic conditions, were hospitalized, or had high total costs—were identified by CBO for study of persistence of Medicare expenditures over time. Beneficiaries that were selected based upon hospitalization or being in the high total cost groups had baseline expenditures that were four times as high as expenditures for a reference group. Beneficiaries selected based upon presence of multiple comorbid conditions had baseline expenditures that were roughly twice as high as expenditures for a reference group. Subsequent years of costs remained higher for all three cohorts than the reference group; however, total expenditures declined the most for those beneficiaries who were identified as high cost due to a hospitalization followed by beneficiaries who had had high total costs in the base year. Subsequent costs were virtually unchanged for beneficiaries with multiple chronic conditions.81 Denver Health reported challenges in using utilization data alone to find patients at chronic high risk of acute care use—but identifying these patients was important for the success of 21st Century Care. That is, Denver Health assumed that 21st Century Care could reduce service use (such as hospitalizations and ED visits) by identifying patients with chronic care needs and then delivering preventive care to preempt higher-cost acute care later on.…Over the course of the award, however, Denver Health learned that many of its highest-cost patients were only temporarily high cost, suggesting that many of them would have returned to moderate- or low-cost status even without intervention. For example, under its risk stratification algorithm, Denver Health identified so-called super utilizers—all of whom were Tier 4—as people with three or more hospital admissions in a 12-month period, or two or more admissions and a mental health diagnosis. These people accounted for about 30 percent of adult facility costs. By analyzing pre-intervention data, however, research staff at Denver Health showed that, even without special intervention, fewer than half of these super utilizers at a single point in time were still in the category seven months later, and only 28 percent were in the category at the end of 12 months (Johnson et al. 2015b). Because of this challenge using utilization data alone to find chronic high-risk patients, Denver Health, as noted previously, added clinical information (in the form of both CRGs and clinical data such as lab results) to its second and third iterations of the risk-stratification algorithm (although lab results were later removed in subsequent algorithm iterations). Denver Health reported that each revision to the algorithm helped to identify patients who would benefit most from 21st Century Care’s intensive services.85 Denver Health recognized that people with exceptionally high service use at one time did not necessarily continue to have exceptional service use in the future. Over the course of the award, Denver Health integrated clinical information into its risk-stratification algorithm to try to better identify patients who would benefit from intervention.85 Key Finding #1: Several vulnerable subpopulations of Medicare FFS beneficiaries were less likely to agree to participate in the CLM demonstration program. Of all CLM intervention beneficiaries, 65% verbally consented to participate in the CMHCB demonstration at some point during the intervention period. We found that Medicaid enrollees and institutionalized beneficiaries were less likely to be participants; both groups are costly and high users of acute care services. In general, participants tended to be healthier than nonparticipants using baseline characteristics including the prospective HCC score. However, beneficiaries with higher concurrent HCC scores based on the first 6 months of the demonstration were more likely to participate than healthier beneficiaries. This suggests that CLM made some inroads into engaging those with acute clinical deterioration. Further, as CLM’s program matured, they appeared to be more successful engaging sicker and more costly beneficiaries based on baseline health status; however, those with Medicare/Medicaid dual enrollment and the institutionalized were still less likely to become participants. These findings suggest alternative recruiting and outreach strategies are needed to reach dual Medicare/Medicaid enrollees and beneficiaries who are institutionalized.84 Key Finding #1: The HBC program was able to engage beneficiaries who were at higher risk of acute clinical deterioration as measured by the concurrent HCC score. Of the HBC original intervention beneficiaries, 45% verbally consented to participate in the CMHCB demonstration at some point during the intervention period; 40% of the refresh population agreed to participate. For the HBC program, we find that beneficiaries with medium and high concurrent HCC scores were more likely to be participants. Beneficiaries with higher prospective HCC scores and baseline Charlson comorbidity scores were less likely to be participants. This suggests that the HBC program was less able to engage the historically sicker Medicare beneficiaries but more able to engage those at higher risk of acute clinical deterioration as measured by the concurrent HCC score.80 While the high-need patient population is diverse, a synthesis of analyses reported in the literature identified three criteria that could form a basis for defining and identifying this population: total accrued health care costs, intensity of care utilized for a given period of time, and functional limitations.12 Medicare FFS beneficiaries with a primary residence in one of five designated counties including Boston, Massachusetts, and surrounding areas, and a high level of disease severity as indicated by Hierarchical Condition Categories (HCC) scores and high health care costs based on Medicare claims filed during calendar year 2005. Beneficiaries with HCC risk scores >=2.0 and annual costs of at least $2,000 or HCC risk scores >=3.0 and a minimum of $1,000 annual medical costs are eligible for the MGH’s CMP.86 Eligible patients were identified by using standard criteria: a risk score in the 90th percentile for 90-day hospitalization from a validated risk-prediction algorithm (13) and a recent hospitalization or emergency department visit.87Although high utilizers differed significantly from other patients in their medical and behavioral health needs, their presenting complaints were not categorically different from those of low utilizers—they simply had more visits for the same types of complaints utilizers were more likely to present to the ED multiple times for the same complaint. However, most high utilizers had 4 unique chief complaints, suggesting that these patients generally have several, rather than a few, reasons for seeking emergency care. No clearly defined pattern of complaints existed for high utilizers. High utilization in such patients is less likely to be caused by clearly defined disease processes and more by a complex mix of multiple chronic medical conditions and psychosocial factors, making it difficult to predict future utilization or identify specific patient needs based on their chief complaint.17 Predictive modeling is a common tool used by super-utilizer programs to identify who might be at risk for super-utilizing in the future. One program said they use predictive modeling so that they are not “held hostage waiting for claims to come down the road.” Washington State uses predictive modeling to begin to identify the target populations. Using its Health Service Encounter algorithm, the state examines 15 months of integrated health care claims to determine future medical costs and inpatient risk scores. The state has found that conditions such as diabetes, cardiovascular disease, mental health and substance abuse are common among the super-utilizing subset of patients. It uses different approaches to further stratify subgroups for complex care management including identifying individuals with extreme ED utilization (e.g., approximately 80 to 130 ED visits in 15 months), high expected future medical costs (predicted by high utilization and costs in the past), high prospective inpatient risk scores, and significant gaps in care and quality indicators.49 |
| CMO 1.4. Considering patients’ behavioral health and social risk factors (e.g., mental health and substance use disorder diagnoses and social needs) [C] adds to intervention designers’ confidence in identifying HNHC patients [M] who will benefit from intervention services [O1] and who are at risk of having future high healthcare use and cost [O2]. |
The screening process includes administrative data screening to determine eligibility by usage criteria, followed by an in-person screening to determine other eligibility criteria and ability to consent. Randomization occurred after consent. A proprietary platform integrates study data with real-time data feeds from multiple sources. Staff screened potential participants based on their use of county-funded services over the prior 1–2 years. Our research team developed an electronic triage tool that uses administrative data to predict the likelihood of future high use of county-funded services. To meet criteria, potential participants must have used various combinations of the ED and psychiatric ED, medical and psychiatric inpatient stays in the County-funded public hospital, and/or jail over the past 1–2 years, at high enough levels to meet a threshold score. We embedded the triage tool into the study database and generated a list of potentially eligible participants with the highest scores, redoing the calculation throughout the enrollment period. All county agencies or service providers could refer individuals they suspected met eligibility criteria, but study staff always used the list generated by the triage tool to confirm initial eligibility. County staff used this list to outreach to the highest using individuals.109 Individuals with disability, transportation challenges, homelessness, mental health conditions, and with substance abuse or chemical addiction may be hard to find and engage when they are not actively in treatment. Partnering with public health agencies and community-based organizations was identified as an approach that allows health care organizations to more successfully identify and engage these hard-to-reach populations, some of whom harbor mistrust of health care professionals. Although such partnerships would ideally build on shared data for surveillance, programs had developed approaches in the absence of a formal data sharing system. For example, the Gatekeeper program in Ohio developed a community referral model to identify high risk individuals in partnership with a network of trained community volunteers such as bank tellers, police officers, paramedics, and pharmacists. Volunteers initiated referrals directly to the Gatekeeper program for older adults who identified as being potentially at risk or who might benefit from community services.113 The data on diagnostic history and characteristics of subsequent admissions may also provide some help in conceptualizing intervention design. The relatively high rates of chronic disease suggest the importance of a comprehensive, multidisciplinary approach to any intervention, using what we already know about improving chronic disease management (such as the chronic care model). But the extraordinarily high levels of substance abuse among high-risk patients and the history of mental illness even among the population without serious and persistent mental illness make clear that any intervention will have to take these factors into account. Whatever is on the shelf from chronic disease management vendors for commercial plans and Medicare will require a serious overhaul for adaptation to these populations.62 There are also other important questions that remain unanswered. From claims records we can say little about the social and personal characteristics of these patients. This is a population living in extreme poverty, and a broad range of factors (educational, behavioral, and coping capacity) likely complicate their lives. We have documented their mental illness and substance abuse problems, and there are also potentially high levels of homelessness and housing instability. Getting more and better information about these issues will require further work, but it is clearly critical to any intervention design. However, the potential impact of solving these problems may also be large, even for the most apparently daunting problems such as the high number of mental illness admissions. For some high-risk patients, an effective, supportive housing environment might be enough to tip the balance, allowing sufficient life stabilization to address previously intractable health and mental health problems. An emerging body of research indicates that these “social service” interventions can have a major impact on the use of health services.62 Our findings also show the importance of including patients with mental health disorders in an intervention program. John Billings and Maria Raven noted that more than a third of high utilizers have at least one claim with a mental health disorder diagnosis. Other studies have However, more than half noted that people with mental health disorders have higher rates of receiving ED and inpatient care.20 Most of the patients enrolled in our study had either depression or anxiety. While B2C did not target people with severe mental health needs (such as those recently hospitalized at a psychiatric facility), to our knowledge, the program is unique in having a behavioral health provider screen every enrollee for mental health disorders—and then address those conditions as appropriate.88 Participating super-utilizer programs reported a high prevalence of behavioral health diagnoses in high-utilizers through claims data. Indeed per capita Medicaid costs increase significantly with the addition of a mental health diagnosis, substance abuse diagnosis, or mental health plus substance abuse diagnosis49 All stakeholders identified poorly managed serious mental illness among HNHC patients as a significant driver of preventable high health care utilization. Patients often had inadequate access to mental-health and substance-abuse resources. This was because outpatient programmes did not exist, were inconveniently located or were not financially feasible to attend. This left patients without any options other than the ED for care. Additionally, several patients acknowledged that feeling depressed negatively impacted their care routines and contributed to missing provider appointments which, over time, compounded the severity of their diseases. Importantly, patients also pointed out that the stigma surrounding mental illness was detrimental to their desire to seek out treatment even if it were available. Some patients also felt that policies such as the Florida Mental Health Act (known as the Baker Act) and its equivalent in New York State (known as Kendra’s Law),20,21 which allow for involuntary institutionalization and examination of an individual with possible mental illness for up to 72 hours, did not adequately address or help mitigate the root causes of substance abuse and mental-health disorders. This increased preventable ED and/or hospital utilization for psychiatric needs.61 “Most high utilizers had ≥4 unique chief complaints, suggesting that these patients generally have several, rather than a few, reasons for seeking emergency care. No clearly defined pattern of complaints existed for high utilizers. High utilization in such patients is less likely to be caused by clearly defined disease processes and more by a complex mix of multiple chronic medical conditions and psychosocial factors, making it difficult to predict future utilization or identify specific patient needs based on their chief complaint.” Most high utilizer ED visits appeared to occur close together in clusters presenting complaints not categorically diff from low utilizers: more visits for same type of complaints. Most common complaint across utilizer groups: abdominal pain, chest pain, and shortness of breath. ” The chart review in this study highlighted the inherent difficulty in determining whether patterns of high utilization for these 3 complaints are related more to medical conditions or social/behavioral factors. Although nearly all of the patients in the chart review had mental illness and/or substance abuse, far fewer visits than expected were clearly linked to these conditions.17 The Camden Coalition conducts a cluster analysis to ID the various subpopulations. This involves sorting cases (usually by patient utilization history) into groups, or clusters, so that the degree of association is strong between people in the same cluster, and weak between members in diff ones. Some programs stratify the typologies by the different social needs faced by the patients such as homelessness, joblessness, and language preference—further indicating what interventions would be the most effective.49 Both the taxonomy developed by the Harvard T.H. Chan School of Public Health and the one developed by The Commonwealth Fund segment high-need individuals based on medical characteristics because this is a feasible starting point for most health care systems. Recognizing that a taxonomy focused on medical characteristics may neglect other factors that are key drivers of need, the taxonomy working group built on these efforts to offer a conceptual starter taxonomy that incorporates functional, social, and behavioral factors into a medically oriented taxonomy, not as independent segments but as factors that influence the care model or care team composition most likely to benefit particular patient segments (Figures S-2 and Table S-1). This starter taxonomy can provide guidance for health system leaders and payers on how to embed social risk factors, behavioral health factors, and functional limitations in a taxonomy for high-need patients. Patients would first be assigned to a clinical segment, with follow-on assessment of behavioral health issues and social services needs to determine the specific type of services that are required. Key behavioral health factors most likely to affect care delivery decisions include substance abuse, serious mental illness, cognitive decline, and chronic toxic stress and key social risk factors include low socioeconomic status, social isolation, community deprivation, and house insecurity.12 In the early stages of the CMHCB demonstration, CMP leadership learned that many high-cost, complex patients have mental health issues that were not effectively addressed by the current model of health care delivery or its pilot program. As a result, the program allocated greater resources to support mental health, hiring a social worker to assess the mental health needs of CMP participants and support them in accessing psychiatric care as needed or provide treatment if appropriate.86 |
| CMO 1.5 Considering patients’ self-assessments of “subjective” characteristics about themselves [C] adds to intervention designers’ confidence in identifying HNHC patients [M] who will benefit from intervention services [O1] and who are at risk of having future high healthcare use and cost [O2]. |
Using the Medicare Health Risk Assessment, we explored two data-driven methods to segment a heterogeneous population of older adults with potentially complex care needs into clinically meaningful subgroups using self-reported information. Input variables for the segmentation analyses were patient-reported variables drawn from the Medicare HRA, a component of the Medicare Annual Wellness Visit designed to identify patient-reported modifiable risk factors and health needs [8]. Required elements include self-assessment of health status, psychosocial risks, depression, behavioral risks, and Activities of Daily Living and Instrumental Activities of Daily Living. Care delivery systems can add additional questions. The Medicare HRA is designed to help clinicians address patient-reported risks for preventable adverse outcomes. Although the HRA is most commonly applied at the point of care, if data are systematically collected, representative, and stored in extractable formats, they can be used to inform program development, population health, and outcomes research. Although content collected through patient-reported outcomes may duplicate content obtainable through more traditional clinical data such as ICD codes, ICD codes alone are unlikely to capture subjective responses to questions about pain, loneliness, and independent activities of daily living (for example). In this project, HRA data revealed meaningful subgroups that might not have been obvious from other electronic clinical data and could inform specific clinical interventions. Important differentiators included function, falls, perceived health status, emotional well-being, pain, and presence or absence of an advance directive. Two large subgroups comprised relatively healthy individuals who could benefit from watchful waiting and routine preventive care plus (for one group) life care planning. Much smaller subgroups could be targeted for more intensive and tailored care management. The size of these subgroups can inform resource allocation within delivery systems.89 As we enrolled patients into the group we found that, despite a broad range of medical and behavioral health problems, the common feature they shared and what ultimately served to bring them together as a group was their status of being “on the fringe,” as they described themselves. Nearly every patient had experienced a number of barriers and frustrations in accessing medical care that the DIGMA team seems to have successfully addressed.98 Traditional electronic data such as diagnostic codes and laboratory values may not capture essential information on factors that drive care needs, including function, personal preferences, and social resources, that can only be reported by individuals themselves. Identifying and characterizing complex needs subpopulations requires patient-reported information to help match care delivery to personal needs. Although newer data from electronic health records (EHRs) such as symptom assessments and ICD-10 codes that capture functional status can improve our ability to identify complex needs subpopulations, subjective information can add a level of specificity unlikely to be captured with objective coding.89 Moreover, recognition that computer-based designations of being at risk for costly care (also decorously called predictive analytics) vary considerably, furnish no specific guidance, and are inaccurate is increasing. Most patients in the small, at-risk subgroup will not use such care, whereas care becomes relatively rationed for most patients not designated as such—including those who may require it.… Nevertheless, health care executives embrace the paradigm of high-risk intensive management despite its flaws.…Meanwhile, many of its shortcomings can be remedied by a few standardized, patient-reported measures that forecast a patient’s risk for costly care in a similar manner to predictive analytics, specifically the risk for direct services. For example, patients may simply indicate that they are only somewhat or not very confident that they can manage and control most of their health problems; have had moderate or severe pain during the past 4 weeks; have been bothered extremely or quite a bit during the past 4 weeks by emotional problems, such as anxiety, irritability, depression, or sadness; believe that the medications they are receiving may be causing illness; and have been prescribed more than 5 medications. Although some payers and providers may disagree with the specific metrics, none should assert that a patient’s standardized self-report is an improper tool for guiding care. They may consider this method too old-fashioned or novel for implementation, but its modesty and low cost are remarkable.87 Dr. Wasson notes the limitations of relying on risk scores derived from electronic data. We agree that patient-reported indices have many advantages. Electronic indices, such as the Veterans Health Administration’s Care Assessment Needs Score (1), also have potential advantages as a screening method in health care systems in which such data can be calculated on a population level. Some high-risk patients identified by the Care Assessment Needs Score did not need or were unlikely to benefit from intensive management, and intensive management teams in our study spent substantial time triaging the heterogeneous populations by reviewing health records, contacting primary care providers, and having telephone or in-person visits. Our results suggest that selecting patients for intervention would ideally combine the use of algorithm-based risk scores with measures focused on such issues as those raised by Dr. Wasson, including whether patients believe that they are activated in managing their health or have difficulty managing their prescriptions.87 Data were collected through computerized administrative databases and HRA questionnaires to measure outcomes for utilization, health risk scores, and self-efficacy.90 Social determinants of health. All stakeholders emphasized the importance of inadequate health literacy, unstable housing conditions, and lack of adequate social support in driving preventable high health care utilization. Low health literacy made it difficult for many HNHC patients to manage complex medical conditions on their own, adversely impacting their ability to follow through with day-to-day self-care regimens. They also felt that for some HNHC patients with unstable housing conditions, being in the ED or an inpatient care setting was desirable, as it was the only avenue, as one HNHC patient put it, to ‘get a meal…have a television… stay overnight’. Finally, health system leaders as well as most physicians felt that the interplay between lack of social support and poor disease control was often a reason for presenting to the ED. Physician: ‘Health literacy and overall education level is probably the biggest impact on the ability to self-manage these conditions’. Unstable housing: Physician: ‘Eventually, [many] of them become homeless or [have] poor living conditions and those patients…arrive because of weather conditions or for other reasons to the ED to seek shelter and respite’. Limited social support Health system leader: ‘[Workable solutions] probably have to do with…social support and reduction of isolation and helping them negotiate the complexities’. Insurance challenges Patient: ‘I have Medicaid and some of the doctors don’t take [it], and so you say to yourself, well, even if I get an appointment, are they going to take me? So just go to the emergency room and let them handle it from there’. Financial burden Patient: ‘A lot of times [we’re] on a fixed income and you need to see a specialist…You may have a co-pay with your specialist. It could add up if you go excessively. It’s easier to go to the [ED] and get what you need’.61 Patient activation refers to an individual’s knowledge, skills, and confidence related to self-management. The construct is commonly measured using the Patient Activation Measure, which is a thirteen-item interval-level scale with strong psychometric properties that generates a score between 0 and 100. A Patient Activation Measure level may be assigned based on the score, from level 1 (least activated) to level 4 (most activated). Studies show that Patient Activation Measure results are predictive of most health behaviors, clinical indicators, and hospital and emergency department (ED) use. Research also shows that less activated patients with chronic illness are more likely to experience care coordination problems, compared to more activated patients. Numerous studies also indicate that compared to more activated patients, less activated ones with chronic disease are less likely to effectively manage their conditions (for example, they are less likely to adhere to medication regimens), have healthy diets and get regular exercise, regularly monitor symptoms and clinical measurements, ask questions in the medical encounter, and report satisfactory care experiences. These findings suggest that less activated patients may benefit more from care coordination and care management services than patients who have equal disease burden but are more proactive about managing their health.104 In this study we used the four Patient Activation Measure levels. Level 1 indicates that a person does not yet understand the important role that patients play in determining their health, and level 4 indicates that a person is proactive about his or her health and engages in many positive health-related behaviors. Compared with lower-risk patients, high-risk patients were twice as likely to be sixty years or older and were somewhat more likely to be lower income (Exhibit 1). High-risk patients were also almost twice as likely to be depressed and more than twice as likely to be at the lowest Patient Activation Measure level. Furthermore, in 2011 high-risk patients were three times more likely to have had an ED visit and fourteen times more likely to have had a hospitalization. Fairview Health Services, a Pioneer ACO, uses the Patient Activation Measure to allocate its resources more efficiently to support patients. For example, Fairview’s care coordinators and health coaches, who manage high-risk patients, use the Patient Activation Measure level to decide how intensely to follow and manage specific patients. Nurses supporting patients during care transitions use a similar approach, in which the Patient Activation Measure score determines the frequency and focus of their posthospital support efforts.104 As we demonstrated in a previous article, although they are very sick, these patients remain surprisingly functional despite their illness(s) (Roberts et al., 2012). Additional important general observations include the following: these individuals are very resilient, highly resourceful, and are extraordinarily patient with the healthcare system. At enrollment, many are overwhelmed; disengaged; nontrusting; and lacking in a feeling of self worth or deserving of services (making it critical to have psychology expertise integrated into the team structure). Furthermore, this patient group wants to be perceived as agreeable (e.g., may know they will be unable to keep their next appointment for some reason but would not offer that unless asked).91 “We found in our case series that trust was a key theme in the relationship between patients and their clinicians or the health system. Lack of trust in individual doctors or institutions, as well as unrealistically high expectations of the same, appeared to be an important driver of higher costs Patient trust seemed to be informed by both patient factors (prior experience, socioeconomic status, activation) and clinician factors (quality of care, communication skills). Among the five patients, trust appeared to mediate the interaction between patient activation and cost: higher activation was associated with lower costs when patients or families had trust in their clinicians or when needed care was low acuity or standard; however, higher activation (in particular, the confidence and ability to advocate for care) was associated with higher costs when trust seemed lacking, particularly when the stakes were high (in critical illness) and the course uncertain.”.… Observations highlight challenges with trust in the setting of increasing medical complexity, specialization, and team-based care. Important to teach docs communication skills that earn trust, particularly around goals of care, and to better match patients to appropriate docs and care managers to ensure trusting relationships.”29 |
| CMO 1.6 Considering patients’ self-assessments of health system-related risk factors and experience [C] adds to intervention designers’ confidence in identifying HNHC patients [M] who will benefit from intervention services [O1] and who are at risk of having future high healthcare use and cost [O2]. |
At enrollment, the concept of self-management is not familiar to most of them. Systems, like the Housing Authority, Medicaid, and health systems, often add to their burden. Examples include applications for benefits are frequently difficult to figure out and time consuming to file, applicants often feel disrespected or treated as if they were helpless, and agency staff are often not adequately sensitive to client issues regarding low/no literacy. In addition, for non-English speaking, translation services can be inadequate, cultural competency is a problem, and mailed annual reapplication notices (such as for Medicaid) are difficult to recognize as something official and may be disregarded.91 Example from CSHP illustrating the program’s theory of action “Patient A in Kansas City has multiple chronic conditions and poly-substance abuse, a history of homelessness, frequent ED visits, and no PCP [primary care provider]. At the initial contact with the care team, the patient stated that he would “never want to conform to the rules.” The care team’s strategy is to first establish firm trust. They accomplished this by identifying opportunities to provide basic help, such as involving family members in explaining the impact on diet of modifying cooking practices, supplying a scale and log to support the modification, organizing and explaining the purpose of medications, arranging for transportation and enabling the patient to do so, scheduling and accompanying patients to medical and social service appointments.85 Long wait times Patient: ‘When I go to the emergency room, they [say], “When you get out of here go see your GI doctor”. But, that’s not the way it works..Last time I called to get in the next day, they told me he had 17 patients, and couldn’t see me. In three months, you don’t know what could happen. So, the next thing is [back to] the ED’.61 Mismatch arises from patients’ social circumstances limiting access to services, behavioral issues interfering with care engagement, and lack of health system flexibility to address these barriers. A staff member reflected on the inability for a patient to receive services due to homelessness: “He was homeless when we made the referral and doesn’t…fit into [the] standard hospice system.… Health care systems are designed for these neat packages of people that are housed, have family support, have access to other resources, are not actively using substances.…The services aren’t really designed for complex folks, so that can be really frustrating and exhausting.” (SUMMIT LCSW)61 A few [stakeholders] even suggested that sometimes it felt easier to take an ambulance to the ED and access different services at the same place and time rather than arrange transport for multiple visits including PCP, specialists, bloodwork, etc.61 Economic determinants of health care. Patients identified insurance-related factors and financial burden of upfront costs (such as co-payments) as reasons for frequent ED visits and hospitalizations. For example, several patients on Medicaid reported knowing that many physicians in their community did not accept their insurance. To avoid losing time by contacting multiple primary care offices, they would go to the ED directly. Also, for underinsured and uninsured patients, the ED was the only health care setting where they could receive health care without having to deal with implications of their insurance status or co-pays right away. Many patients also reported the negative impact of financial burden on their medication adherence as a driver of frequently presenting to the ED. They felt that some of these visits can be prevented if medications and essential medical devices were not so expensive.61 Survey of frequent ED patients while in ED Barriers to care: % agree, could have multiple barriers It is easy for me to make time to get to necessary med appts: 78% I always remember to schedule my annual check-ups, tests, and/or screenings: 65% I feel like I receive better quality health care in the ED than I do in my usual place of care (PCP, clinic): 48% whether certain services would be helpful to the patient, in the event the ED or health system decided to offer the service: After-hours options for minor health issues besides the ED: 63% A nurse to work with you one-on-one to help manage health care needs: 53% Transportation to get to medical appointments on-time: 46% While 42% did not think that a PCP would be helpful, many said that they had one.27 |
| CMO 1.7 An enrollment process that includes an extensive period of outreach and trust building [C] adds to an intervention provider’s confidence [M] in determining a participant’s willingness and ability to participate in the intervention [O]. |
The purpose of community navigators in the Familiar Faces program is to bridge the gaps between their clients and the healthcare and social systems that are often fragmented and difficult to navigate. Furthermore, integration of community navigators into the healthcare system, specifically the information flow offered by EHRs, ensures that community navigators are able to engage with patients during acute episodes of care, when their needs are greatest. In addition to the expertise that community navigators provide to clients in navigating healthcare and social systems, they may build trust between clients and the healthcare system, as they live in the same communities as their Familiar Faces clients. Mistrust of the healthcare system is often high in minority and low socioeconomic populations and may result in delayed medical treatment and use of fewer preventive services.… Because the community navigator was simultaneously a member of the community and the healthcare system, it is possible that they were able to reach community members particularly mistrustful of the healthcare system and start to build a foundation of trust.95 The outreach to ESRD beneficiaries, however, was entirely new in Phase II since ESRD was not part of the clinical focus in Phase I. KTBH program leadership found that when a nurse had an in-person contact with an ESRD beneficiary, the beneficiary was more than twice as likely to enroll in the program. As a result, KTBH program leadership decided to send a nurse to every dialysis facility with more than two eligible beneficiaries. Prior to the visit, the care manager sent a packet to the facility’s administrator, placed a call to the administrator, and tried to make an appointment to conduct an informational breakfast or lunch session with the entire staff to introduce the KTBH program and assuage any concerns about the program. The goal of the informational sessions was to explain to staff that the care managers hoped to accomplish things with the beneficiary that would enable the intervention participants to better manage their condition. They tried to convey to staff that the care managers were not there to make their lives more difficult or to take the place of the existing staff that provided services to beneficiaries. KTBH staff reported receiving the biggest pushback from facility social workers. KTBH staff believed that there was a direct correlation between having the support of social workers and beneficiary participation in that when they received the support of the social workers, prospective participants were more likely to join the KTBH program.82 That’s been the difficult piece, finding people who are appropriate and they want our help at the same time. That’s been the trickiest piece. CCM Nurse94 CCM providers in our study actively looked for positive and negative indicators that patients were willing to engage in care. Providers often found these explicit signs to be inadequate for detecting patients’ desire or readiness to engage in care and therefore looked for more subtle signs and tried to make intuitive assessments.94 The enrollment criteria for the CCM programs included a willingness to engage in care.94 CCM providers looked for indicators that patients were willing to engage during initial patient encounters and enrollment, and looked for signs of successful engagement in ongoing assessments of patients’ communication and actions. When CCM providers first met patients, positive indicators that they were willing to engage included verbally agreeing to take part in the program, returning phone calls to the CCM team or answering the phone when CCM team members call, being receptive to a home visit, and showing up to an initial appointment. CCM providers continued to assess engagement over time by considering how frequently patients missed appointments, how well they adhered to medication and treatment regimes, how much progress they made towards behavioral changes such as reducing substance use or increasing exercise, and how candidly and regularly they communicated with the CCM team. A social worker said that she considers patients likely to engage over time when “we reach out to them by phone and we make an appointment and they show up.”94 We learned that for B2C to reduce the use of acute care, outreach to and enrollment of high utilizers had to happen in real time in the ED.88 Judgment about whether the patient is amenable to management is based on an interview with the patient by the care team and a review of medical records. The assessment of whether the patient is entered into Care One is based on a judgment that the patient has a chronic medical condition, is at high risk for future hospitalization, and is willing to attend outpatient visits and comply with therapy. Other specific exclusions from the Care One program include a single high-cost medical event (e.g., a trauma), residence outside of the hospital’s catchment area, and chronic alcohol or drug abuse.92 Most deep-dive practices indicated that risk-stratification improved the organization and delivery of care. Clinicians and staff continued to report that risk-stratification increased their awareness of high-risk patients’ needs and helped them better allocate staffing resources to different patient populations. For example, in a few practices, patients with a single chronic condition (such as patients with diabetes who needed basic monitoring and health education) received care management from a medical assistant. This enabled the care manager to focus on higher-risk patients (such as patients with poorly controlled diabetes and additional chronic conditions). Risk-stratification continued to help practices identify and prioritize high-risk patients and schedule longer appointments for them as needed. In contrast, respondents in one small deep-dive practice questioned the utility of risk-stratification; they perceived that clinicians knew their patients well enough to determine whether they were high-risk and they believed that the time they spent risk-stratifying patients would be better spent delivering direct patient care.101 Text box III.1. Example from CSHP illustrating the program’s theory of action “Patient A in Kansas City has multiple chronic conditions and poly-substance abuse, a history of homelessness, frequent ED visits, and no PCP [primary care provider]. At the initial contact with the care team, the patient stated that he would “never want to conform to the rules.” The care team’s strategy is to first establish firm trust. They accomplished this by identifying opportunities to provide basic help, such as involving family members in explaining the impact on diet of modifying cooking practices, supplying a scale and log to support the modification, organizing and explaining the purpose of medications, arranging for transportation and enabling the patient to do so, scheduling and accompanying patients to medical and social service appointments.85 |
C = context; M= mechanism; O = outcome
Table B-12Full list of Context-Mechanism-Outcome (CMO) configurations with supporting data for Program Theory 2: Engaging HNHC patients in interventions to improve their management of their chronic conditions, supporting CMO relationships
| Program Theory Issue | CMOs | Relevant Data Extracts From Included Literature |
|---|---|---|
| Patients’ challenges to self-care prior to and during the intervention | CMO 2.1. Past experiences with the healthcare system including encountering barriers accessing medical care and disrespect from providers [C] cause patients to distrust the system and providers [M] which inhibit patients from accepting and seeking appropriate help and medical care [O]. |
More than half of participants (n = 12) related stories of encounters that had upset them; several explicitly mentioned withdrawing from outpatient providers by choosing not to attend appointments with those providers as a result. Over a third of participants switched providers because of dissatisfaction with those relationships. Others who had not switched chose not to follow a given provider’s instructions as a result of these negative interactions. Most of the stories of negative health care encounters focused on feeling disrespect from providers, while others specifically described feeling discriminated against by providers because of race or sex.105 Several participants in this study expressed that they felt that their care sometimes was compromised by perceived disrespect from health care providers, citing race-, sex- or SES [socioeconomic status]-based discrimination.105 When answering questions about trusting their health care providers, almost half (n = 9) of participants stated that they distrusted a particular (usually hospital-based) provider. Respondents generally expressed trust in their primary care providers.105 Nearly every patient had experienced a number of barriers and frustrations in accessing medical care that the DIGMA team seems to have successfully addressed.98 “Patient A in Kansas City has multiple chronic conditions and poly-substance abuse, a history of homelessness, frequent ED [emergency department] visits, and no PCP [primary care provider]. At the initial contact with the care team, the patient stated that he would “never want to conform to the rules.”…His sister reflects, “He used to use the ER [emergency room] for everything.…”85 Challenges associated with accessing health care delivery systems Transportation barriers. Some patients reported that primary care offices were inconveniently located and difficult to access due to transportation barriers. A few even suggested that sometimes it felt easier to take an ambulance to the ED and access different services at the same place and time rather than arrange transportation for multiple visits including primary care, specialists, bloodwork, etc. For patients who could utilize private or public transportation to get to a primary care clinic, the distance often made the trip extremely time-consuming as well as costly. Preventability of ED use appeared contingent upon logistic ease of access to services. Long wait times. All stakeholders identified scheduling challenges at primary care clinics as an important driver. Many patients reported that they were unable to schedule first-time or follow-up appointments quickly (same-day, next day or even in upcoming weeks) and instead had to wait several months. Furthermore, if a disease exacerbation occurred after regular clinic hours or overnight, patients felt that they had no other options but to seek care in the ED.61 |
| CMO 2.2. Previous and current personal life circumstances and characteristics (e.g., mental illnesses, substance abuse, emotional or physical traumas, extreme poverty, and low literacy) [C] results in feelings of stigma in patients [M] that inhibit them from seeking help and medical care [O]. |
A predominance of the participants (n= 12) told stories of childhood instability…Significant subthemes included early life traumas, such as death of a parent or other loved one, and abusive relationships with primary caregivers throughout childhood. Some described state agencies as their primary caregivers. Transiency was noted, often in the context of escape from abusive relationships (n = 5), and often resulted in living on the streets or gang and drug involvement, even as children. Only 2 individuals specifically described how events in childhood affected their health during adulthood. Nevertheless, many participants related stories of how this instability may have manifested in health issues, especially with regard to mental health. For example, one woman described: ‘‘I had sexual and physical abuse from my parents since I was a baby, since I was 3 or 4. Mother has been very abusive over the years. Every time we went somewhere she was hitting me, punching me, scratched me, and I’d cover it up.’’ This individual described ongoing difficulty obtaining effective treatment for the post-traumatic stress disorder that repeatedly sent her to the ED. She reported being frequently ‘‘suicidal’’ and ‘‘in crisis’’ as a specific result of childhood abuse, for which she was ‘‘in and out of hospitals too much.’’ She reported a shared goal with her therapist of accessing consistent community-based mental health care rather than relying solely on the ED. Nearly half of the respondents (n = 8) named their mother or a mother figure as their most important primary caregiver. The other half referred to a variety of other caregivers, such as grandparents, other relatives, or the state, or they declined to answer; these situations were described by some participants as traumatic, and by others as a normal course of events. Of participants who described instability in childhood, half noted familial estrangement in adulthood (n = 6). When asked if there was someone they could depend on now for help, most participants spoke of having only 1 or 2 individuals that they could rely on, if any, suggesting a lack of social support in adulthood for nearly all of these respondents.105 Patients may have a variety of barriers that prevent them from accessing traditional primary care venues, particularly those settings that do not allow patients to walk in at their convenience or patients who may need intensive services during a personal crisis. Patients with difficult life circumstances also may be more likely to not show for an appointment.98 Patients in this quality improvement program tend to be younger than those targeted by previously described care transition models and many have unstable health insurance, a history of substance use, and significant mental illness. Nearly all are from socially disadvantaged communities plagued by poor health status, and low literacy is common. Many are struggling with difficult life circumstances such as an alarming number have been emotionally or physically traumatized;…and many have inadequate, or no, family and social support systems. At enrollment, the concept of self-management is not familiar to most of them.91 There are two key elements to the success of these new efforts to target and improve care for high-cost Medicaid cases. First, it is essential to be able to identify in advance patients who are likely to have high costs in the future. Many high-cost occurrences (such as injury, acute illness, or cancer) might be episodic, and high spending in one year might not mean high spending in subsequent years. Second, and equally critical, is the ability to actually affect the care pathways and outcomes of these patients. Because of the circumstances that define their Medicaid eligibility (extremely low income and medical disability) and other factors that are likely to be associated with their social and personal environment (such as homelessness, substance use, or low educational achievement), these patients will undoubtedly present major challenges.62 All stakeholders identified poorly managed serious mental illness among HNHC patients as a significant driver of preventable high health care utilization. Patients often had inadequate access to mental-health and substance-abuse resources. This was because outpatient programmes did not exist, were inconveniently located or were not financially feasible to attend. This left patients without any options other than the ED for care. Additionally, several patients acknowledged that feeling depressed negatively impacted their care routines and contributed to missing provider appointments which, over time, compounded the severity of their diseases. Importantly, patients also pointed out that the stigma surrounding mental illness was detrimental to their desire to seek out treatment even if it were available. Some patients also felt that policies such as the Florida Mental Health Act (known as the Baker Act) and its equivalent in New York State (known as Kendra’s Law),20,21 which allow for involuntary institutionalization and examination of an individual with possible mental illness for up to 72 hours, did not adequately address or help mitigate the root causes of substance abuse and mental-health disorders. This increased preventable ED and/or hospital utilization for psychiatric needs.61 Low health literacy made it difficult for many HNHC patients to manage complex medical conditions on their own, adversely impacting their ability to follow through with day-to-day self-care regimens.61 Economic determinants of health care. Patients identified insurance-related factors and financial burden of upfront costs (such as co-payments) as reasons for frequent ED visits and hospitalizations. For example, several patients on Medicaid reported knowing that many physicians in their community did not accept their insurance. To avoid losing time by contacting multiple primary care offices, they would go to the ED directly. Also, for underinsured and uninsured patients, the ED was the only health care setting where they could receive health care without having to deal with implications of their insurance status or co-pays right away. Many patients also reported the negative impact of financial burden on their medication adherence as a driver of frequently presenting to the ED. They felt that some of these visits can be prevented if medications and essential medical devices were not so expensive.61 | |
| CMO 2.3. System-level barriers including inadequate systemic support (e.g., Medicaid, translation services, housing) and lack of cultural competency [C] engenders feelings of distrust and marginalization among patients [M] that inhibit their ability to access appropriate healthcare services [O1] and to participate in interventions [O2]. |
…some have no income while others have income that it is insufficient to meet basic survival needs making it challenging to pay even minimal co-pays for prescriptions; many live in unstable housing or in dangerous neighborhoods.…Systems, like the Housing Authority, Medicaid, and health systems, often add to their burden. Examples include applications for benefits are frequently difficult to figure out and time consuming to file, applicants often feel disrespected or treated as if they were helpless, and agency staff are often not adequately sensitive to client issues regarding low/no literacy. In addition, for non-English speaking, translation services can be inadequate, cultural competency is a problem, and mailed annual reapplication notices (such as for Medicaid) are difficult to recognize as something official and may be disregarded.91 Many participants faced a variety of barriers to appropriate care, including lack of stable income, health insurance, legal residency, English language proficiency, knowledge of the health system and chronic disease management, stable housing, social support, and transportation. Many also had issues with cultural barriers, mental illness and substance abuse (despite informal program eligibility criteria that excluded some patients with these conditions), and traumatic experiences that made stabilizing their chronic conditions more difficult.85 Many patients are unable to afford even a minimal copayment that may be expected at time of a nonemergent outpatient visit and may choose to access the ED where a copayment may not be required.98 We found that patients with low health literacy (measured by the REALM-SF) reduced ED utilization to a greater degree than patients with higher health literacy. We hypothesize that patients with lower health literacy may have encountered more barriers to accessing primary care or had greater social needs than those with higher literacy and thus, differentially benefitted from individualized assistance from a patient navigator. These preliminary results suggest that care coordination programs that aim to reduce avoidable ED use and hospital admissions may have a greater impact among patients with lower health literacy.110 In the current study, KCCP Care Managers identified multiple barriers to active participation in the intervention including basic needs for food, shelter, and transportation that took precedence over program participation; depression; not having a phone or being unable to manage a phone due to mental illness or addiction; language or other cultural barriers; and mistrust of the system (Cristofalo et al. unpublished data).112 Social determinants of health. All stakeholders emphasized the importance of inadequate health literacy, unstable housing conditions, and lack of adequate social support in driving preventable high health care utilization.… They also felt that for some HNHC patients with unstable housing conditions, being in the ED or an inpatient care setting was desirable, as it was the only avenue, as one HNHC patient put it, to ‘get a meal…have a television… stay overnight’. Finally, health system leaders as well as most physicians felt that the interplay between lack of social support and poor disease control was often a reason for presenting to the ED.61 | |
| Relationship building with care providers | CMO 2.4. Interventions and care team members initially address patients’ basic needs and explain things in lay terms [C] to establish trust with the patient [M] resulting in building a relationship with their patients [O]. |
Patients generally had positive impressions of their care managers. During semi-structured interviews with a sample of high-risk patients and caregivers from deep-dive practices, patients who reported having regular contact with their care manager or who were open to working with their care manager felt that the care manager was an asset to their team. Patients particularly valued care managers who listened to them and explained things in lay terms, helped to manage medications and chronic conditions, followed up after a hospitalization, and helped to navigate the health care delivery system and community resources.101 “…The patients that have been on SUMMIT [Streamlined Unified Meaningfully Managed Interdisciplinary Team] for a while who have a really solid relationship with us, that makes a huge difference. They are able to call. They are telling us what their needs are. They can make it to appointments and…coordinate all of those needs a little bit better when they know that we’re going to be reliable and [here] is where they can come for help.” (SUMMIT Physician).58 They [the program staff] expected that its program would have the greatest impact by preventing acute health care events among beneficiaries who were initially not having significant health issues; however, case managers found that they spent a lot of time dealing with urgent issues for patients who “spiraled out of control.” Although initially some patients were skeptical about the MGH [Massachusetts General Physicians Organization] CMP [Care Management Program], overall, patients quickly formed relationships with case managers, including several who requested daily contact with their case managers to help them with their numerous issues. The care team’s strategy is to first establish firm trust. They accomplished this by identifying opportunities to provide basic help, such as involving family members in explaining the impact on diet of modifying cooking practices, supplying a scale and log to support the modification, organizing and explaining the purpose of medications, arranging for transportation and enabling the patient to do so, scheduling and accompanying patients to medical and social service appointments. Within weeks, the patient has started scheduling transportation and keeping his appointments independent of the care team, and now states that he cares about his health. His sister reflects, “He used to use the ER [emergency room] for everything. Now he asks when his appointment is.”85 Convenience And Access Our model emphasizes convenience and access, starting with location. Our centers—in the range of 6,500–10,000 square feet—are located in urban areas with a high density of low-to-moderate-income seniors. For our patients’ convenience, we offer a broad set of additional services on site, including dental care, digital x-ray, ultrasound, and acupuncture, as well as five to fifteen high-volume specialists. Our average health maintenance organization (HMO) patient received 86 percent of his or her ambulatory encounters at our centers in 2011, although the most expensive aspects of care occurred outside of our centers— for instance, hospitalizations, surgeries, and imaging. Patients find the one-stop-shop approach to care highly appealing.114 |
| CMO 2.5. When care managers support patients with medical and non-medical problems [C], patients are reassured [M1] and gain confidence [M2] in their ability to manage their own care [O]. |
Care management is a vital piece of the puzzle, pulling together community resources without which recovery would be impossible.…Successful case management also includes assisting with teaching some of these patients basic life skills, for example, not to find housing for them, but rather direct them where to go to get housing assistance. These small, positive steps are then shared with the group, which further reinforces a growing sense of confidence.98 Additionally, efforts to tailor-make health education programs to improve health literacy and numeracy may be warranted for patients to effectively self-manage some of their care needs.61 | |
| CMO 2.6. Patients are more motivated [M] to improve their health behaviors [O] when they feel cared for by their providers and other support groups [C]. |
…participants reported that ‘‘caring’’ providers were particularly important in the trajectories of their illnesses and lives, emphasizing the compassion of the Care Management Team. Providers from the intervention were described as dependable, sensitive, and thoughtful, suggesting that these traits in providers may resonate for individuals whose childhoods lacked caregivers with these qualities.105 Conversely, participants emphasized the importance of caring, trusting, and longitudinal relationships with providers, both on the Care Management Team and with primary care providers. Comorbid mental illness, especially depression, makes managing chronic illnesses such as diabetes more challenging.34–37 Consistent, positive relationships with primary care providers have been shown to decrease rates of hospitalization and ED use for complex patients who struggle with a combination of multiple chronic illnesses, mental illness, and psychosocial challenges.105 Frequent, longer visits built relationships with the care team and other patients. The emotional support provided by the group seemed to be a key factor in assisting patients to find solutions to their health and social problems.98 Half of participants indicated the importance of ‘‘feeling cared for’’ by providers (n = 10). This theme recurred throughout the interviews, especially during descriptions of the Care Management Team. When asked about the best part of the intervention, rather than describing specific services, most participants described the importance of the emotionally supportive interactions they experienced. These participants reported that the experience of feeling cared for was a motivation to improve their own health behaviors (n = 10) (Table 2). Diabetes, depression, and hypertension were the most commonly reported conditions. Despite the natural history of these complex chronic diseases and their tendency to reflect a pattern of deterioration over time, 7 participants reported improvements in their own perceived health status after the intervention. Five of those participants specifically attributed this improvement to the intervention. ‘‘They make you feel like you’re not alone, and they understand you and the things you’re going through. And they actually help explain why you’re going through these things…you don’t feel like just a patient.’’ - 24-year-old African American woman with depression and Type 1 diabetes.105 | |
| CMO 2.7. When intervention care providers build trusting relationships with patients [C], patients have confidence in their providers’ desire to help [M] resulting in patients seeking advice from their intervention care provider before going to the ED/hospital [O] |
During the early months of CLM’s [Care Level Management’s] program implementation, nurse care managers focused on building relationships with the patients during telephone contact between PVP visits, so that patients would be comfortable calling the nurses if health problems arose. Patients at highest risk were to receive calls on a weekly basis, whereas those at moderate and low risk were to receive calls on a monthly or bimonthly basis.…Over the course of the first year of operations, CLM reported …that they reorganized their patient care teams to include more nursing support. CLM believed that this arrangement would allow patients to bond with the nurse care manager over time, whereas CLM had observed that the clinical specialists were not able to forge a sufficient bond as evidenced by the fact that some of their participants were going to the hospital rather than calling the clinical specialists when problems arose.84 In addition to connecting clients to health and social resources in their community, the community navigators focus on building trust between the client and navigator and subsequently with other healthcare entities and social systems in the community.95 The care manager is an experienced, calm, trusted professional patients can call when they are frightened or in crisis between groups visits, which is often the difference between going to the ED to seek immediate care or waiting a day or 2 until the next group visit.98 These participants articulated an appreciation for continuity in relationships with providers, including members of the Care Management Team. A majority of respondents (n = 14) described their preference for office-based primary care with their usual providers, reserving the ED for emergent medical necessity or after-hours needs.105 | |
| Individualized care for HNHC patients | CMO 2.8. Designing flexible interventions that could be tailored and individualized to specific HNHC patient’s needs and circumstances [C] empowers providers [M] to be responsive to each patient’s needs and circumstances. |
Care transitions are normally linear and finite (e.g., from Provider A to Provider B), but in our care coordination programs, the number and nature of care transitions are circular, overlapping, and continual. They involve cross-sectoral care givers including social services, government workers, and church and community members—in addition to medical, social work, and behavioral health providers in one or more health systems—and they take place at multiple locations. Because the interventions need to be tailored to each patient individually, based on their medical and life situations, they are not predictable at the outset, and “model fidelity,” as required by most care transition models, is not feasible.91 Specific interventions were tailored to each patient in collaboration with the patients and their family, reflecting the patient’s unique needs.119 Patient intake at IOC included an in-depth patient assessment to determine non-medical barriers to improved health. Care plans and activity to address needs were individualized.83, 85 The ability to tailor care to patients’ individual needs was another ingredient staff members felt they provided to complex patients. A SUMMIT care coordinator described a strategy to assist patients with attending specialty appointments: “I’ll have appointments with patients just with myself if patients need help with scheduling outside the clinic and scheduling transportation.… If a patient chronically no-shows to a (specialty) appointment…I’ll make an appointment for them to come [see] me and we’ll schedule together and…give them an appointment planner or write up all their appointments for them.”58 Each enrollee gets a tailored 60 day care plan and associated patient services they might need including assistance obtaining housing resources, insurance, disability benefits, refugee services, transportation, coordinating primary and specialty care; and filling prescriptions.88 The housing patterns we found, however, suggest the need for flexibility. Consistent with the experience of many Housing First programs, over two-thirds of the housed intervention participants required rehousing after their first placement did not succeed. The ability to offer a new housing placement is a key component of successful Housing First strategies when working with high complexity populations. With the widespread use of Coordinated Entry that will require that counties place individuals with similar risk profiles into PSH, our findings provide support for the need for flexibility, including the ability to rehouse individuals, in order to serve those at highest risk. Our results offer a measured sense of expected changes in their use of other services.109 Another feature of our program was the flexibility in the range and intensity of services we offered to patients. Some patients required infrequent contact to assist with scheduling and attending primary care appointments. Other patients benefited from more intensive contact, including multiple accompanied clinic visits or home visits.111 |
| CMO 2.9. Having interventions address underlying mental health conditions concurrently or before managing other health conditions [C] helps patients’ ability to cope with their health conditions [M] and allows them to benefit from interventions addressing their chronic conditions [O]. |
Theme 3: Addressing Both Psychosocial and Clinical Needs Participants noted that it wasn’t possible to separate provision of psychosocial support from traditional medical care. This can run counter to what occurs in usual care. “I spent an hour with a patient last week and we didn’t talk about medical problems.… It was a therapeutic session. I’m not a trained therapist, but [that’s] what it was. We didn’t talk about diabetes. We didn’t talk about her foot ulcers.…A lot of times we end up doing the work of social workers, but when you do primary care, you have to do that. It’s not ‘oh hold on,… I’m not getting into that. I’m only here for the medical stuff.’ It all wraps up into one.” (SUMMIT Physician)58 But the extraordinarily high levels of substance abuse among high-risk patients and the history of mental illness even among the population without serious and persistent mental illness make clear that any intervention will have to take these factors into account.62 In the early stages of the CMHCB [Care Management for High Cost Beneficiaries] demonstration, CMP [Care Management Program] leadership learned that many high-cost, complex patients have mental health issues that were not effectively addressed by the current model of health care delivery or its pilot program. As a result, the program allocated greater resources to support mental health, hiring a social worker to assess the mental health needs of CMP participants and support them in accessing psychiatric care as needed or provide treatment if appropriate.86 Many participants faced a variety of barriers to appropriate care, including lack of stable income, health insurance, legal residency, English language proficiency, knowledge of the health system and chronic disease management, stable housing, social support, and transportation. Many also had issues with cultural barriers, mental illness and substance abuse (despite informal program eligibility criteria that excluded some patients with these conditions), and traumatic experiences that made stabilizing their chronic conditions more difficult.85 …the program is unique in having a behavioral health provider screen every enrollee for mental health disorders—and then address those conditions as appropriate.88 We found a significant reduction in use of psychiatric emergency services and a concomitant increase in scheduled mental health visits. Project Welcome Home included Intensive Case Management with a low client-staff ratio led by licensed staff with behavioral health training. Research has shown that experiencing homelessness is one factor that leads to ED visits among psychiatric patients, suggesting an unmet need for mental health care.5,18 Our findings suggest that these visits are amenable to prevention by providing housing with associated low-barriers mental health services.109 | |
| CMO 2.10. Connecting patients and supporting them in navigating services that cross medical sectors (e.g., geriatrics, substance disorder treatment) and non-medical sectors (e.g., employment, housing, transportation) [C] help patients gain the confidence [M] to learn how to navigate multiple systems for themselves [O]. |
Through the CMP, patients are assigned to a personal care manager who assists with access to social and medical resources, helps patients schedule PCP appointments, and helps bridge barriers between patients and the healthcare system. Enrolled patients are assigned to 1 of 3 outpatient primary care clinics. Components of the CMP include: goal creation/assistance in reaching goals, ranging from applying for benefits and receiving stable housing to losing weight and receiving specialty care appointments; assistance with care navigation (schedule appts, follow-up on referrals, and help refill medications); arranging for social services (make personal connections with staff at various agencies in the community and refer patients to appropriate services, including transportation resources, Legal Aid, homeless shelters, faith-based services, and substance abuse resources); care transitions (meet with patients daily while they are admitted and work with discharge planners to assist patients in receiving recommended follow-up care and understanding discharge instructions); and communication with providers (accompanying them to appointments, creating and prioritizing problems lists, coaching patients about questions to ask, and sitting with patients after their visit to explain follow-up instructions).107 After the visit, the patient navigator and patient created a task list based on the provider’s recommendations. For example, if the PCP ordered additional tests or specialist referrals, the navigators assisted in scheduling these additional appointments, phoned patients to remind them, identified and addressed any barriers such as transportation, and encouraged patients to follow PCP recommendations. When needed, navigators helped patients to access medical transportation assistance through the state Medicaid system. If the patient identified social needs such as precarious housing, food insecurity, or insurance questions, they were provided with information to connect with local resources.110 The team (1) conducted home visits, (2) scheduled and accompanied patients to initial primary and specialty care visits to ensure that such appointments are kept and that the patient understands any instructions given during the appointment, (3) coordinated follow-up care and medication management (medication reconciliations), (4) measured blood pressure and blood sugar levels when appropriate, (5) coached patients in disease-specific self care, (6) helped patients apply for social services (e.g. housing support, Social Security (including SSI), Supplemental Nutrition Assistance Program (SNAP), Temporary Aid for Needy Families (TANF), and General Assistance (GA)) and appropriate behavioral health programs. provides disease specific education, coaches the patient in self-care, and works to empower patients to manage their health issues. During subsequent home visits, the team evaluates the patient and team’s progress. The care team works to connect the patient with stable, continuing, and appropriate primary and specialty care. Coalition staff may help schedule further medical appointments as necessary, continue to help organize transportation, accompany patients to medical appointments, check-in after medical appointments to help the patient implement the instructions given by the provider, and continue to organize medications. Home visits in later stages increasingly focus on self-care management skills, health care navigation skills, enhancement of self-efficacy and independence, care plan adjustment and coaching.96 The most frequently used intensive management services were social work and mental health care, highlighting the importance intensive management teams placed on these services on the basis of their comprehensive assessments of patients’ needs. The intensity of services varied greatly among patients assigned to the intervention group; patients who used more services tended to be older and to have more comorbid conditions, higher rates of baseline primary care utilization, and lower rates of substance use disorders and serious mental illnesses. These findings suggest that other models of intensive management may be more appropriate for patients whose mental health and substance use conditions are severe and are likely to prevent effective engagement with the intensive management team. By design, the intensive management programs seem to have facilitated referrals to home-based primary, palliative or hospice, geriatrics telephone, specialty mental health, and telehealth care. Because sites performed comprehensive assessment of patients’ social issues, treatment plans, and care goals, our results suggest that the intensive management programs could identify unmet needs and connect patients to important resources. Home visits seemed to play a key role in patient assessments, because patients with more intensive services had an average of 1.5 home visits.87
| |
| CMO 2.11. Because patients’ burden of coexisting chronic diseases and social and behavioral issues are heterogeneous, allowing the length of the intervention to vary across participants [C] helps patients feel supported [M] by providing them with sufficient time to demonstrate intervention goals (e.g., self-management behaviors) [O]. |
Patients are continually enrolled at different times, resulting in different lengths in the post-enrollment time frame. Patient diagnoses that are driving admissions, and their burden of coexisting chronic diseases, are heterogeneous. The natural history of these common chronic diseases is such that the patients have ever evolving health conditions intermixed with periods of disease decompensation. Length of time in the intensive intervention period is variable and determined by demonstrated need and functionality: socially, medically, and behaviorally. Our intervention is not administered by number of days exposed but instead is administered until the patient demonstrates the behavior criteria we have defined (“graduates”), the patient expires, or transitions. Because the patients are graduated according to demonstration of objective self-management behaviors (Figure 2), the resultant postgraduation time frames are also variable.91 Participants’ issues often took longer to resolve than the intervention’s time line typically allowed.85 | |
| Barriers to HNHC patient change through interventions | CMO 2.12. Despite successful engagement with the intervention and relationships between members of the care team and HNHC patients [C], HNHC patients may continue to prefer seeking primary care at the hospital or ED [M]. Therefore, interventions may not be able to achieve goals such as reducing use of potentially preventable or modifiable healthcare services [O]. |
One challenge for the demonstration was that a sizable minority of beneficiaries and caregivers would prefer to visit the ED [emergency department]—instead of contacting the IAH [Independence at Home] practice—if they were unsure whether symptoms required emergency care (Table III.3). Beneficiaries provided a number of reasons for preferring to go to the ED, including that they or their caregivers thought it was the best place to receive care. Even though three-quarters of beneficiaries reported that the IAH practice visited about as often as the patient wanted them to visit (Appendix C, Table C.8), some beneficiaries’ preference for the ED in uncertain situations might contribute to the demonstration’s lack of an effect on outpatient ED visits.103 Engagement with the program was high (95% of patients had at least three encounters with program staff), and patients received an intensive intervention (averaging 7.6 home visits), but two program goals related to the timing of services — a home visit within 5 days after hospital discharge and a visit to a provider’s office within 7 days after discharge — were achieved less than 30% of the time. Challenges in reaching these goals included patients’ lack of stable housing or a telephone and their behavioral health complexities and providers’ few available appointments. The difficulties that this pioneering, data-driven organization had in achieving rapid assistance for patients may portend difficulties in achieving it at scale.96 Patients randomized to PIM were more likely than patients in PACT to strongly agree that they have a VA healthcare provider whom they trust…Survey findings suggest that the program may have influenced some patients’ experiences with patient-centered care and chronic illness care, and increased the number of patients who reported having a trusted provider, but did not influence satisfaction, perceived access, or most measures of care coordination.97 Though the SUMMIT intervention was developed as a way to address high ED and hospital utilization, staff members did not mention reduced utilization as a marker of success. “We are dealing with a pretty sick population.…These are patients that maybe do need to be in the hospital.… A hospitalization is not necessarily a bad outcome for a lot of these patients.” (SUMMIT Physician)58 |
| CMO 2.13. Improvements in patients’ experiences with their care providers through participation in HNHC patient interventions [C] gradually rebuilds patients’ trust in the health care system [M] that may lead to long-term benefits in health behaviors and clinical outcomes [O] | Furthermore, relationships are at the core of primary care, so this finding suggests that augmenting a medical home with an intensive management program may help fulfill the promise or primary care. In fact, analyses of satisfaction suggest that the program improved patients’ experiences with primary care, but not with other services. Improving primary care processes could potentially have positive long term consequences, including changes in health behaviors and clinical outcomes.97 |
C = context; M= mechanism; O = outcome
Table B-13Full list of Context-Mechanism-Outcome (CMO) configurations with supporting data for Program Theory 3: Care provider engagement in interventions for HNHC patients
| Program Theory Issue | CMOs | Relevant Data Extracts From Included Literature |
|---|---|---|
| Gaining and maintaining support from physicians and other care providers | CMO 3.1. Strong leadership support that facilitates systemic coordination of the intervention and its components smooth the entry of care managers into practices [C] provides credibility of their services to existing practice staff [M], so care managers are more easily incorporated into primary care teams [O]. |
Once MGH [Massachusetts General Physicians Organization] had generated lists of CMP [Care Management Program]-eligible beneficiaries receiving care from each physician, the CMP medical director met with each practice to introduce the program and discuss which patients were at highest risk for acute events and should receive priority for enrollment. The medical director also met with specialty practices such as the oncology, cardiology, emergency, and orthopedics departments to explain the resources available through the program, because case managers would likely interact with these providers as they facilitated patient access to these services.86 At the time of the program launch, strong integration support from MGH leadership afforded the case managers physical entry into the primary care practice settings whereby the case managers were co-located with the primary care physicians ultimately becoming a part of the beneficiaries’ primary health care teams.86 |
| CMO 3.2. Program leaders’ use of tailored strategies and physician champions to explain intervention services [C] helps endorse the intervention [M] and results in physicians participating in the intervention [O]. |
A second round of focus groups was conducted with physician groups to specifically discuss how the CMP could add value to their practices. In addition to providing input about the design of the CMP, the capstone groups provided an opportunity to obtain physician buy-in to the PBCM [practice-based care management] program. Despite the fact that some physician practices already had case managers, CMP management observed that most physician practices were apprehensive about changes such as the introduction of new staff into their practice. CMP leadership used a tailored approach to discuss the project with each practice, offering positive anecdotes from the PBCM pilot project as appropriate. In addition, CMP leadership identified a physician champion for the CMP within each physician practice that had at least 25 or more CMP patients at the start if the project to further ease the transitions involved in the introduction of a case manager into the practice. During program implementation physician champions provided insight about the best way to incorporate case managers into the practice and encourage colleagues to take advantage of services available from the case managers.86 Dr. Fishbane underscored the importance of establishing effective partnerships with the partner nephrologists during [Village Health’s] Phase II and was optimistic about the efforts to secure physician champions, garner enthusiasm and support, and improve physician engagement at the first Medical Advisory Board meeting.82 | |
| CMO 3.3. Face-to-face outreach to physicians and their staff by program leaders and/or nurse care managers [C] effectively garners support of the intervention from existing care providers [O] by helping existing care providers understand the value of the intervention [M]. |
Case managers assigned to each practice met with physicians at the practices to describe the program, the skills that they bring to the physician practice, and their interest in collaborating to support patients in their efforts to manage their medical conditions. Case managers collected information from providers about how they could add value to the medical practice.86 Acquiring buy-in from participating physician practices was viewed as very important. However, it was recognized early on that buy-in was needed on all levels. There was some concern among practice-based nurses, particularly at smaller practices, that there would be a duplication of effort. To obtain buy-in from the nurses, the CMP case managers spent time working with the practice-based nurses to educate them that the goal of the program was to augment and not to replicate their efforts.86 In addition to distributing marketing materials and conducting group presentations, a TST [Texas Senior Trails] nurse with utilization management and provider relations experience visited the offices of the 250 doctors in the Lubbock and Amarillo areas with the highest numbers of CMHCB [Care Management for High Cost Beneficiaries] demonstration-eligible patients. This nurse was largely successful in gaining physician support for the program, often as a result of spending time with physician office staff and administrators who conveyed information about the program to the physicians…Similarly, the TST medical director in Amarillo had so much difficulty obtaining physician support via phone calls to these individuals that he ceased conducting these outreach calls. At the time of our site visit, the TST medical director and managing director were continuing to look for ways to market the program to providers who were not supportive initially. In particular, they were developing messages that conveyed the fact that the program can serve as a resource for physicians, by providing support for patients who are hard to manage because of mental health and/or social issues.79 | |
| CMO 3.4. Using a multi-pronged approach to provide physicians with information about intervention services [C] made it more likely to reach doctors to get their support and engagement [M] in the intervention necessary for the program to succeed [O] |
The program only works well when physicians are highly engaged.86 MGH enlisted physician support to help ensure the success of its CMP in providing high-quality care to patients. Physicians were asked to conduct the following activities: encourage beneficiaries to participate in the program and enroll them in the program when possible; collaborate with case managers to review initial assessment findings and develop care plans for each patient; inform case managers about patient events and refinements to patient care plans during the demonstration period; and discuss advance directives with enrolled patients…MGH physicians received information about the CMP from a variety of sources, including the program’s medical director, the MGH electronic newsletter, and case managers assigned to each practice.86 | |
| CMO 3.5. When an intervention includes an insufficient number of patients the physicians [C], physicians do not fully engage and participate in the program [O] because they feel the intervention is not a good investment in time and resources [M]. |
The staff also suggested implementing a physician referral model to gain physician buy-in and to identify sufficient numbers of patients to make a financially viable care management program. A physician referral model could increase enrollment by more than 10 times, according to one physician’s estimate, with which others agreed. Interviewed physicians and care managers felt that a physician referral model would increase the appropriateness of patients referred for care management services. It was recommended that patient-specific clinical or educational goals accompany an open physician referral model in order to ensure that participants have clearly identified goals against which to measure their progress.80 Although most physicians were supportive of the outreach efforts, they generally only had one or two patients participating in the program. The program had greatest success with offices that had approximately 30 patients participating in the program…To the extent that patients were concentrated with providers, program staff felt that the physicians were better allies and facilitated the clinical interventions. “A couple things we’ve gotten a little bit smarter about—one is the alignment to the provider… One of the things I would definitely do differently is for ESRD [end-stage renal disease] patients, I would do DaVita only and see what kind of change we could drive there. Then if we had a great solution, we could think about how we could scale it. That was probably 70% of the operational hassle that didn’t actually do anything for patients but took a lot of time and energy. The same is true on the CKD [chronic kidney disease] side with the nephrologists.”82 Although the nephrologists were very engaged initially, the program had less of a renal focus than anticipated given that the beneficiary population did not have the extent of CKD that was originally projected. As a result, the program did not maintain as high visibility among physicians during Phase I as the KTBH [Village Health’s Key to Better Health] program leadership would have liked.82 During the first site visit, physicians at both sites reported that they were initially very enthusiastic about the Health Buddy® program, because it offered a promising way to effectively support patients with chronic disease. The Health Buddy® technology coupled with telephonic care management support was viewed as an effective way to maintain and improve patient health and identify symptoms of complications early, so that timely medical intervention could be used to prevent serious problems requiring hospitalization. Once the physicians received the list of patients who were eligible for the Health Buddy® program, they reported that they became frustrated with the project because they felt that many of the patients selected would not benefit from participating. Further, physicians reported disappointment that many of the patients they believed could be helped by the program were not eligible to participate in the program because they had not been identified through the claims based algorithm developed by HHN [Health Hero Network]…Using information gleaned from its early experience with the program, the HBC [Health Buddy Consortium] made a series of changes and enhancements to its operations and as reported to us at our second site visit.80 | |
| CMO 3.6. Developing and implementing a financially supportive system or model for physicians and their practice [C] encourages and motivates physicians and other care providers [M] to spend time with their patients and to continue supporting innovative intervention activities [O]. |
The Care One program provides incentives to primary care providers by valuing a Care One patient as equal to 5 normal primary care patients when adjusting panel size.92 MGH provided physicians with a $150 financial incentive per patient in Year 1 and $50 in Years 2 and 3 to help cover the cost of physician time for these activities.86 Thus, for such team care to be sustainable, time needs to be carved out for the work involved and systems need to support the follow through.102 The staff also suggested implementing a physician referral model to gain physician buy-in and to identify sufficient numbers of patients to make a financially viable care management program. A physician referral model could increase enrollment by more than 10 times, according to one physician’s estimate, with which others agreed.80 Physician: ‘When your hospital is basically saying… ‘Here is 15 minutes for a repeat visit for another patient’, I mean how are you gonna be able to actually provide the kind of care they need?’61 Recent changes in the Medicare Advantage program (nearly all of ChenMed’s patients are enrolled in Medicare Advantage) have created a favorable environment for delivery system innovation. In particular, the 2004 introduction of the Hierarchal Condition Categories risk adjustment model created a mechanism that reduced the financial risk of taking care of high cost patients with multiple chronic conditions. Patients with multiple chronic conditions have higher risk scores and, accordingly, higher reimbursement. Although not perfect, risk adjustment has alleviated participating payers’ and providers’ concerns about attracting sicker and costly patients without receiving commensurate reimbursement.114 In addition, Medicare Advantage’s capitation model is more favorable to delivery system innovation than traditional fee-for-service Medicare because it eliminates the process of negotiating reimbursement for cost-reducing delivery system innovations. Because providers are paid according to the size of their patient panel in a capitated system, they have an incentive to develop and test innovations to determine which ones lower the cost of care without compromising quality—and, ideally, increase it. For those innovations judged to be cost reducing without compromising quality, providers in a capitated system have the flexibility to deploy the innovations across their network. Providers in the fee-for-service system do not have such flexibility because they must negotiate with payers for the reimbursement of care delivery innovations—a step that can delay or even block such efforts.114 Administrative pressures in health care delivery systems. Physicians and health system leaders felt existing payment structures and administrative pressures (such as the impetus to maximize the number of patients seen while minimizing visit time) negatively impacted the way they could interact with patients. Many agreed that when such a limited time frame is allotted for each patient, it barely gives providers time to think, resulting in the delivery of ‘bad care’. This also affected the way physicians communicate with their patients in key situations including discussions of illness, treatment options and care plans. Finally, stakeholders felt that the current care delivery model significantly dis-incentivized physicians from going into primary care, leading to a primary care physician shortage. The underlying sentiment was that if there are fewer primary care doctors overall, then HNHC patients will be at a greater disadvantage to have continuity of care at a primary care site, their diseases will not be well-controlled, leading to more ED visits and inpatient admissions.61 | |
| Staffing arrangements in care management interventions | CMO 3.7. Reducing providers’ workload and responsibilities associated with the implementation of complex intervention activities [C] will reduce provider stress [M] so providers are more satisfied [O1] and more willing and able to engage with their patients and in participate intervention activities [O2] such as attending care team meetings, and carrying out care plans. |
At baseline, members were divided in the anticipated effect of team care on their workload and stress levels. At 3 months, one member noted a decrease in workload, and three perceived an increase. Two indicated that the intervention “increased my stress by adding to my many responsibilities.” Getting to Care Team meetings on time was difficult for about half of the team members.102 Some team members felt their work increased by participating in the team.102 Early on, we determined that certain tasks the HC RNs [Health Coach Registered Nurses] and LCSWs [Licensed Clinical Social Workers] were performing could be offloaded as these did not require their level of licensure, training, and skill. By doing so, we could free up the HC RNs and LCSWs to serve more patients and increase their job satisfaction.91 Respondents from both independent and system-owned practices described turnover that occurred because care managers felt overwhelmed with numerous responsibilities.101 Patient And Physician Time We also emphasize physician and patient time. Our primary care physicians, all internists, have a panel of 350–450 patients. By comparison, physicians at many commercial “concierge” practices, where patients pay sizable out-of-pocket retainers for the additional physician time, have larger panels. Small panel sizes allow our physicians to spend more time with their patients. Our physicians average fewer than eighteen visits a day; in contrast, primary care physicians average nearly thirty.114 Physician: ‘Need more primary care physicians who can manage outpatient things… And so you end up not being able to fill the need, and then we see them in the emergency department.’61 |
| CMO 3.8. Case managers, social workers, and high functioning administrative assistants in turn take on many time-consuming tasks (e.g., medication management, identifying community services, outreach, engagement) to help engage and manage HNHC patients and their paperwork [C] so that providers can focus their efforts [M] on providing continuous, comprehensive care to patients [O]. |
Early on, we determined that certain tasks the HC RNs and LCSWs were performing could be offloaded as these did not require their level of licensure, training, and skill…To address this, we worked with the teams to identify tasks that could be done by high functioning Administrative Assistants (AAs) and we now use AAs for tasks such as maintaining telephone contact with patients to remind them of appointments, check up on them when they have not been heard from, and assist the team members in entering and retrieving data related to the patients they serve. This is effective as long as there are intermittent face to face opportunities for the patients with the HC RNs, LCSWs, and CCLs [Client-Community Liaison].91 “…Case managers take care of things like preauthorization, gathering documentation, medication tracking and other time-consuming issues, allowing PCPs [primary care providers] to focus on the relationship with patients and provide real continuity of care.;…The program does what every PCP needs to be doing but cannot do anymore because of the medicine practice and reimbursement realities and primary care provider shortages.;…Both patients and physicians love the program as case managers take a lot of burden off both sides.;…Key value of the program is in the help they provide PCPs with medication review and management, the most difficult to resolve issue when PCPs do not have any help;…” [Summary from a focus group of multiple physicians]86 The care manager served primarily as an adjunct to the patients’ primary physicians.80 As in 2015, deep-dive practice respondents described approaches to improving support for care managers, to clarify their roles and enhance staffing resources to help them feel less overwhelmed…A few practices were monitoring care managers’ caseloads to determine whether they needed more staff to support high-risk patients, or to reduce (or even eliminate) activities focused on lower-risk patients. These practices brought in social workers to help meet patients’ social needs and medical assistants to assume logistical or administrative tasks.101 Navigators shared information about individual patients utilizing a team-based navigation model that provided flexibility in dividing the workload and providing cross coverage.110 The program was staffed by a multidisciplinary care team consisting of a community health worker (CHW), a social worker (SW), and a PCP [primary care provider]…With guidance and support from the SW and the PCP, the CHW was responsible for patient outreach, engagement, activation, and accompaniment. The SW was responsible for counseling and brief interventions for patients with behavioral health needs and for coordinating referrals to social service agencies and other medical providers. The PCP was responsible for providing comprehensive care for acute and chronic conditions and for coordinating with specialists and inpatient providers.108 The CHW was able to identify unmet social needs contributing to acute care utilization that may not be apparent to busy clinicians and are not readily addressed during a single ED or clinic visit. For example, one patient with chronic restrictive lung disease who was dependent on home oxygen experienced financial insecurity and anxiety related to his inability to make on-time utility payments. The CHW was able to enroll him in a financial assistance program to prevent utility shutoffs, provide a list of local food pantries, and accompany him to primary care appointments where he was connected with the pulmonology clinic social worker who assisted with ongoing needs.111 | |
| CMO 3.9. Providing training for staff members [C] gives them the confidence and skills [M] to function effectively as a care team [O1] and to understand and work with HNHC patients [O2]. |
“…The program has done a remarkable job in training and cultivating case managers who are very good at breaking barriers and making it work for the most difficult patients;…” [Summary from a focus group of multiple physicians]86 …our team members received minimal training in ways to decrease frequent attendance and did not follow a systematic approach in assessing the patient…A more systematic approach, however, would have improved the function of our team.102 Both navigators completed training at the Harold Freeman Institute for Patient Navigation, a 2-day intensive training program that teaches navigators to identify and eliminate barriers to care and serve as a support hub for patients moving through the health care system (22). The PN also completed local training at Gateway Community College, which emphasized needs and resources within the local community (23).110 | |
| CMO 3.10. When care managers have regular opportunities to talk across offices and health care systems [C], they are more emotionally and technically prepared [M] to work with HNHC patients [O]. |
As in 2015, deep-dive practice respondents described approaches to improving support for care managers, to clarify their roles and enhance staffing resources to help them feel less overwhelmed. In some practices affiliated with health systems, respondents described providing opportunities for care managers embedded in practices across the health system to meet regularly, share best practices, and offer one another support.101 CMP leadership also emphasized team support and peer counseling by developing infrastructure that provided opportunities for mutual support among CMP case managers and peer counseling from the members of the mental health team as the emotional toll on staff of working with a highly frail and sick population are substantial.86 | |
| CMO 3.11. Having small care teams [C] helps teams members develop awareness of each HNHC patient’s entire complex care [M] which can improve the coordination of patient care [O]. | In addition, the ability for a team to be small and nimble was seen as a strength as it allowed for increased cohesion. “One of the issues with complex care is [it’s] spread out amongst a bunch of different people.… There’s a learning curve each time the patient meets with a different provider.… With SUMMIT [Streamlined Unified Meaningfully Managed Interdisciplinary Team], it’s a small team.… Everybody knows what’s going on with the patients in terms of their conditions and it really cuts through the confusion.” (Usual Care LCSW)58 | |
| Communication across the care team | CMO 3.12. Leadership-supported, regular communication across all staff [C] builds collaborative feelings among teams [M] that results in job satisfaction for care team members [O], and facilitates implementation success [O]. |
Team communication was important for program implementation, although sites had different levels of success in this area over time. Care teams with a solid supervisory structure and frequent collaboration across all levels of staff experienced greater implementation success and staff satisfaction.85 Due to the complexity of the CMP demonstration population, CMP leadership felt that constant and good communication between all staff within the program was essential.86 |
| CMO 3.13. Having transparent and supportive communication among care team members [C] fosters shared values and commitment [M] that results in stronger, more cohesive care team [O]. |
Team members caring for HNHC patients noted the importance of shared values and commitment, citing mutual respect for other disciplines and appreciation of the need for teamwork. “We respect one another’s clinical view.… We come at this from different backgrounds and feel like we get more out of our patient care experience if we hear what everyone else has to say.… We have a very supportive and inclusive team environment” (SUMMIT Physician) The importance of the team comes through particularly when patients aren’t faring as well as hoped: “They [other team members] really listen and they really care and we all really feel it when someone does fail…or something bad happens. It’s a very empathetic group of people.…” (SUMMIT Nurse)58 Our finding that staff members value a sense of unity and esprit de corps speaks to the value of cohesive multidisciplinary teams doing this work. As prior studies have shown, individual members of multidisciplinary teams may have different conceptualizations of which disciplines are part of a care team—often these are only a team in name.58 | |
| CMO 3.14. Regular, multidisciplinary care team meetings that include physicians and staff [C] gave care team members the openness [M] to discuss patient cases [O1] and the practices’ performance on quality metrics, outcomes, and other performance goals [O2]. |
Our CHAs [Community Health Advocates] provide perspectives in huddles that often enlighten licensed staff and offer a better understanding for the team regarding the unique needs of the patients we serve.91 VPA [Visiting Physicians Association] corporate medical directors conducted weekly company-wide, web-based meetings with all clinicians, and regional managers conducted individual meetings with IAH practices, to review clinicians’ performance on IAH [Independence at Home] quality metrics and outcomes and consider broader implications for all of their patients.103 Common themes and issues from the Virtual Rounds were also presented at bimonthly management meetings. The bi-monthly management meetings were used to review protocols, present resources, provide training, and identify issues and brainstorm solutions.86 In addition to tracking metrics, most practices reported conducting care team meetings. Care team meetings provided a forum for clinical teams and staff to review quality metrics and progress toward performance goals, discuss an individual beneficiary’s case, and receive information on clinical topics.103 We have designed processes and structures that promote a physician culture of collaboration, transparency, and accountability for high-quality care. For example, our primary care physicians meet three times a week to review hospitalized patients and discuss complex cases practice approaches. Specialists and hospitalists join these meetings as well. We use these sessions to conduct traditional morbidity and mortality review as well as to review each hospitalization and ask, “Could this hospitalization have been prevented?” Physicians are prepared to discuss each hospitalized case and explain to their peers the circumstances involved and their clinical thinking.114 The team met weekly throughout the course of the intervention. One- or two-page patient summaries were prepared by the navigators, including a detailed, written summary of the patient’s medical history, prior ED use, barriers to accessing primary care services, life stressors that could be impacting their health, and type of help the patient wished to receive from the program. Each new patient was discussed by the team after the initial enrollment and on an as-needed basis (e.g., emergence of a new or challenging need or a repeat ED visit). The team discussed ways to support the patient’s clinical and social needs, brainstormed specific resources that might be helpful for the patient, and provided guidance to the patient navigators (24).110 We held multidisciplinary team meetings weekly to develop care plans to support patients’ clinical and social needs.110 | |
| CMO 3.15. Having regular care team meetings to discuss HNHC patients [C] may increase provider workloads [M] causing providers’ to be arrive late for meetings [O1] and to not carry out care plans [O2]. | The primary barriers to conducting regular Care Team meetings were the lack of time to meet and carry out the Care Plan and the difficulty of involving the patient…The team met for 40 min on a weekly basis to discuss one or two of the cases. The physicians were the most likely to arrive late and as noted by the chart review, were at times unable to follow through on the Care Plans.102 | |
| CMO 3.16. When providers are given practical, constructive feedback about patient care approaches [C], providers are provided with the clinical knowledge or resources they need [M] to improve the care they provide to their patients [O] |
“[Care team meetings] give us an opportunity to look back upon our encounter with the patient and really be able to gauge, ‘Was there a reason why the hospitalization happened, could it have been prevented, is there something that I missed?’ … It can be a little bit unnerving … but it [has] actually … strengthened my practice quite a bit. Because you learn a lot from that feedback.”103 Clinicians valued receiving performance feedback and appreciated the opportunity to discuss cases with other clinicians and share ideas to improve care.103 The CMP leadership implemented Virtual Rounds, regular e-mail reports that went to all staff, as a mechanism of providing feedback on a weekly basis. Case managers used Virtual Rounds to report on difficult patients and unnecessary admissions, and to describe both positive and negative events. Virtual Rounds were also used for case reviews with forms that staff filled out at the end of the week. These case reviews were then discussed with physicians in weekly face-to-face meetings.86 Physicians in our study acknowledged their frustration in caring for frequent attenders, but also received specific, practical suggestions for changing their approach to care.102 Peer consultation provides much needed perspective, more objective assessment and support for the difficulties of the case.102 | |
| CMO 3.17. Having patients who received care from providers in other healthcare systems or locations [C] creates challenges for care teams [M] to be able to effectively and efficiently communicate with the patient’s providers [O]. |
TST staff reported that most participants had a primary provider that was associated with TTUHSC [Texas Tech University Health Sciences Center]; however, many patients, particularly in Amarillo, received care from additional providers that were not associated with the university. These providers typically operated independent practices, so TST care managers had to establish relationships with a number of different practices.79 The second proposed improvement had to do with excluding beneficiaries from practices outside the care management organizations, if a systematic means of communicating with clinicians from these practices is not established.80 Further, not all intervention beneficiaries had primary care physicians in the two study sites, therefore the care managers had to interact with community-based providers with whom they had little or no prior relationship. During our site visits, the care managers cited several challenges working with these physicians, in particular, because of communication barriers.80 | |
| CMO 3.18. Providing opportunities for face-to-face conversations among care team members (e.g., being co-located, creating spaces that allow for provider conversations) [C] helps build strong working relationships [M] that improve team communication in support of coordination of patient care [O]. |
Our findings speak to the importance of co-located, embedded teams that “hear what everyone else has to say.”58 …the Health Buddy® nurse care managers often were not in direct proximity to their beneficiaries’ primary care physicians, thereby potentially affecting their interactions with the beneficiaries’ primary providers, changing medical care plans, or mitigating deterioration in health status…Interviewed physicians felt that care management would be more effective and efficient if care managers were colocated with primary care physicians.80 Later, they returned patient care coordinators to local practice sites after clinicians and patients expressed dissatisfaction with the centralized system. According to one respondent, locating at the practice enables patient care coordinators to have more inperson contact with clinicians and to build relationships with patients. This change promoted strong working relationships among teams of clinicians, medical assistants, and care coordinators. Those strong working relationships help to address patients’ needs and avoid unnecessary readmissions and hospital and ED [emergency department] visits. Another practice changed where the physicians and other staff on the care team sat in the office. This practice clustered the care team together so they could discuss patients’ concerns and care delivery more easily.103 We have also designed our centers to promote physician collaboration and conversation. They look more like an inpatient setting or intensive care unit than a traditional physician office. There is a large nurses’ station in the middle of the center where specialists do their paperwork, which is a sufficient distance from the patient exam rooms to allow for spontaneous discussions between specialists and primary care physicians after the specialist has seen the patient. In addition, there is a cluster of four to six individual primary care physician workstations away from direct patient view, where private conversations among physicians can readily happen. In the vast majority of cases, a specialist is able to have a brief face-to-face conversation with the patient’s primary care physician after she or he sees the patient. The face-to-face conversation allows for more rapid alignment between primary care physician and specialist than the traditional faxed consult and voice mail.114 | |
| CMO 3.19. Embedding care/case managers into primary care practices [C] makes it more efficient [M1] and convenient [M2] for physicians to use case managers services [O]. |
One improvement proposed was featuring a care management structure that pairs care managers and participants’ primary care physicians in the same physical location.80 CPC [Comprehensive Primary Care] practices greatly increased their use of dedicated care managers who were members of the primary care practice team over time. The number of practice survey respondents from CPC practices who reported that “care managers who were members of the practice team systematically provided care management services to high-risk patients” increased from 20 percent in 2012 to 88 percent in 2014 and 2015, and 89 percent in 2016. In comparison, fewer than half of comparison practices reported in 2016 that care managers who were practice care team members systematically provided these services to high-risk patients101 Most physicians supported the general concept and potential benefits of the program but also expressed frustration with several aspects of the current demonstration design…care managers were not embedded in their physical practice locations.80 At the time of the program launch, strong integration support from MGH leadership afforded the case managers physical entry into the primary care practice settings whereby the case managers were co-located with the primary care physicians ultimately becoming a part of the beneficiaries’ primary health care teams.86 |
C = context; M= mechanism; O = outcome
Key Question 3. Overall, what is the effectiveness and what are the harms of interventions for HNHC patients in reducing potentially preventable or modifiable healthcare use and costs and in improving health outcomes?
Table B-14Summary of strength of evidence by outcome and study model type (by primary setting)
| Outcome Group | Outcome Measure | Population | System Level (N=5) | Telephonic/Mail (N=9) | Community Based (N=9) | ED Based (N=7) | Ambulatory Intensive Caring Unit (N=3) | Primary Care (N=10) | Home Based (N=4) |
|---|---|---|---|---|---|---|---|---|---|
| Utilization outcomes | ED visits, all causea | HNHC patients | I | L-ND | I | M-F | I1 | I | I |
| ED visits at 270 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| ED visits at 180 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| ED visits, ACSC | HNHC patients | I1 | L-ND | - | - | - | I | I | |
| ED visits, outpatient | HNHC patients | I1 | - | - | - | - | - | - | |
| ED visit resulted in inpatient admission | HNHC patients | I1 | - | - | - | - | - | - | |
| ED, any (%) | HNHC patients | - | - | I1 | - | - | I1 | - | |
| Psychiatric emergency visits | HNHC patients | - | - | I1 | I1 | - | - | - | |
| Inpatient admissions, all causea | HNHC patients | I | L-ND | I | L-F | I1 | I | I | |
| Inpatient admissions, any (%) | HNHC patients | - | I | I1 | - | - | L-F | I | |
| Inpatient admissions at 270 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| Inpatient admissions at 180 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| Inpatient admissions, ACSC | HNHC patients | I1 | L-ND | I1 | - | - | I | L-F | |
| Inpatient admissions, any ACSC (%) | HNHC patients | - | I | - | - | - | I | L-F | |
| Acute medical/surgery stays | HNHC patients | - | - | - | - | I1 | - | - | |
| Other inpatient stays | HNHC patients | - | - | - | - | I1 | - | - | |
| Inpatient days | HNHC patients | - | - | I | I1 | - | I | - | |
| Medical inpatient admissions | HNHC patients | - | - | - | I1 | - | - | - | |
| Medical inpatient days | HNHC patients | - | - | - | I1 | - | - | - | |
| Psychiatric inpatient admissions | HNHC patients | - | - | I1 | I1 | - | - | - | |
| Psychiatric inpatient days | HNHC patients | - | - | - | I1 | - | - | - | |
| Total hospital encounters | HNHC patients | - | - | I1 | - | - | - | - | |
| Hospital encounter resulted in discharge to hospital or observation stay | HNHC patients | - | - | I1 | - | - | - | - | |
| Hospital encounter resulted in discharge from ED | HNHC patients | - | - | I1 | - | - | - | - | |
| Outpatient visits | HNHC patients | - | - | I1 | I | - | I | - | |
| Outpatient visits at 6 months | HNHC patients | - | I1 | - | - | - | - | - | |
| Outpatient visits at 12 months | HNHC patients | - | I1 | - | - | - | - | - | |
| Outpatient visits at 30 months | HNHC patients | - | I1 | - | - | - | - | - | |
| Outpatient substance use treatment visits | HNHC patients | - | - | I1 | - | - | - | - | |
| Outpatient mental health visits | HNHC patients | - | - | I1 | - | - | - | - | |
| Outpatient mental health visit, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Primary care visits | HNHC patients | I1b | - | - | L-Fc | I1 | I | - | |
| Primary care visits at 360 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| Primary care visits at 270 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| Primary care visits at 180 days, all cause | HNHC patients | - | - | I1 | - | - | - | - | |
| Total utilization | HNHC patients | - | I1 | - | - | - | I1 | - | |
| 180-day readmission, count | HNHC patients | - | - | I | - | - | - | - | |
| 180-day readmission, any (%) | HNHC patients | - | - | I1 | - | - | - | - | |
| 180-day readmission, ≥2 (%) | HNHC patients | - | - | I1 | - | - | - | - | |
| Specialist visits | HNHC patients | I1 | - | - | - | I1 | I1 | - | |
| Prescription drugs, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Long-term care, any | HNHC patients | - | - | I1 | - | - | - | - | |
| FQHC visits | HNHC patients | I1 | - | - | - | - | - | - | |
| Filled ≥3 antidepressant prescriptions in first 6 months | HNHC patients | - | - | - | - | - | I1 | - | |
| Specialty mental health visit in first 6 months | HNHC patients | - | - | - | - | - | I1 | - | |
| Cancelled visits and/or no shows | HNHC patients | - | - | - | - | - | I1 | - | |
| Intensive care unit visits | HNHC patients | - | - | - | - | - | I1 | - | |
| Dental visits | HNHC patients | - | - | - | - | - | I1 | - | |
| Care center visits | HNHC patients | - | - | - | - | - | I1 | - | |
| Probability of entering institutional long-term care within the demonstration year | HNHC patients | I1 | - | - | - | - | - | - | |
| Long-term institutionalization rate | HNHC patients | - | - | - | - | - | - | I1 | |
| Probability of hospice use | HNHC patients | I1 | - | - | - | - | - | - | |
| Probability of SNF use | HNHC patients | I1 | - | - | - | - | - | - | |
| Probability of home health use | HNHC patients | I1 | - | - | - | - | - | - | |
| Home health days | HNHC patients | I1 | - | - | - | - | - | - | |
| Home health visits | HNHC patients | I1 | - | - | - | - | - | - | |
| Visits in nonacute settings by primary care clinicians | HNHC patients | I1 | - | - | - | - | - | - | |
| Visits in nonacute settings by specialists | HNHC patients | I1 | - | - | - | - | - | - | |
| Care management visits | HNHC patients | - | - | - | - | I1 | - | - | |
| Mental healthcare visits | HNHC patients | - | - | - | - | I1 | - | - | |
| Homeless care visits | HNHC patients | - | - | - | - | I1 | - | - | |
| ED visits at 180 days, all cause | HNHC patients with a mental health diagnosis | - | - | I1 | - | - | - | - | |
| Inpatient admissions at 180 days, all cause | HNHC patients with a mental health diagnosis | - | - | I1 | - | - | - | - | |
| Primary care visits at 180 days, all cause | HNHC patients with a mental health diagnosis | - | - | I1 | - | - | - | - | |
| Outpatient visits at 6 months | HNHC patients with arthritis | - | I1 | - | - | - | - | - | |
| Outpatient visits at 6 months | HNHC patients with high blood pressure | - | I1 | - | - | - | - | - | |
| Outpatient visits at 6 months | HNHC diabetes patients | - | I1 | - | - | - | - | - | |
| Outpatient visits at 12 months | HNHC patients with arthritis | - | I1 | - | - | - | - | - | |
| Outpatient visits at 12 months | HNHC patients with high blood pressure | - | I1 | - | - | - | - | - | |
| Outpatient visits at 12 months | HNHC diabetes patients | - | I1 | - | - | - | - | - | |
| Outpatient visits at 30 months | HNHC patients with arthritis | - | I1 | - | - | - | - | - | |
| Outpatient visits at 30 months | HNHC patients with high blood pressure | - | I1 | - | - | - | - | - | |
| Outpatient visits at 30 months | HNHC diabetes patients | - | I1 | - | - | - | - | - | |
| Total utilization (%) | HNHC patients living in a low income area | - | I1 | - | - | - | - | - | |
| Total utilization (%) | HNHC patients living in a low education area | - | I1 | - | - | - | - | - | |
| Total utilization (%) | HNHC patients with Medicaid | - | I1 | - | - | - | - | - | |
| Cost outcomes | Total costs | HNHC patients | L-ND | L-ND | I | I1 | L-F | L-F | I |
| Inpatient costs | HNHC patients | - | - | I | L-NDc | I1 | I1 | - | |
| ED costs | HNHC patients | - | - | I | L-F | I1 | I1 | - | |
| Hospital costs of care | HNHC patients | - | - | - | I | - | - | - | |
| Hospital charges | HNHC patients | - | - | I1 | - | - | - | - | |
| Hospital payments received | HNHC patients | - | - | I1 | - | - | - | - | |
| Indirect ED costs | HNHC patients | - | - | I1 | - | - | - | - | |
| Medicaid cost | HNHC patients | - | - | - | I1 | - | - | - | |
| Psychiatric emergency costs | HNHC patients | - | - | - | I1 | - | - | - | |
| Psychiatric hospital costs | HNHC patients | - | - | - | I1 | - | - | - | |
| All non-ED case management costs | HNHC patients | - | - | - | I1 | - | - | - | |
| Acute costs | HNHC patients | I1 | - | - | - | - | - | - | |
| Post-acute costs | HNHC patients | - | - | - | - | - | I1 | - | |
| Outpatient department cost | HNHC patients | I1 | - | - | - | - | - | - | |
| Outpatient costs | HNHC patients | I1 | - | I1 | Ic | I1 | I1 | - | |
| Prescription or pharmacy costs | HNHC patients | - | - | I1 | I1 | - | I1 | - | |
| Primary care physician cost | HNHC patients | I1 | - | - | - | - | - | - | |
| Long-term care costs | HNHC patients | - | - | I1 | - | - | - | - | |
| Other costs | HNHC patients | - | - | - | - | - | I1 | - | |
| Total costs | High-cost, high-risk HNHC patients | - | - | - | - | - | - | I | |
| Total costs | High-cost HNHC patients | - | - | - | - | - | - | I | |
| Total costs | HNHC patients with dementia | I1 | - | - | - | - | - | - | |
| Total costs | HNHC patients without dementia | I1 | - | - | - | - | - | - | |
| Clinical and functional outcomes | Mortality rate | HNHC patients | I1 | L-ND | L-ND | I1 | I | I | L-ND |
| Influenza vaccine | HNHC patients | - | I | - | - | - | L-U | L-F | |
| Progression to ESRD | HNHC patients | - | I | - | - | - | - | - | |
| PHC score (physical health) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| MHC score (mental health) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| PQH-2 score (depression) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Number of ADLs difficult to do | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Number ADLs receiving help | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Helping to cope with a chronic condition | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Number of helpful discussion topics | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Discussing treatment choices | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Communicating with providers | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Getting answers to questions quickly | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Multimorbidity Hassles score | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Percent receiving help setting goals | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Percent receiving help making a care plan | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-efficacy: Take all medications | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-efficacy: Plan meals and snacks | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-efficacy: Exercise 2 or 3 times weekly | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-care activities: Prescribed medications taken (mean # of days) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-care activities: Followed healthy eating plan (mean # of days) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Self-care activities: 30 minutes of continuous physical activity (mean # of days) | HNHC patients | - | I1 | - | - | - | I1 | I1 | |
| Patient satisfaction | HNHC patients | - | - | - | I1 | I1 | I1 | - | |
| Psychiatric symptoms (total BSI) | HNHC patients | - | - | - | I1 | - | - | - | |
| Access to care | HNHC patients | - | - | - | - | I1 | - | - | |
| SF-36 Summary Score | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-36 Mental Health Function Score | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-20 subscale: Social Functioning | HNHC patients | - | - | - | - | I1 | - | ||
| SF-20 subscale: Mental Health | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-20 subscale: General Health | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-20 subscale: Physical Functioning | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-20 subscale: Role Functioning | HNHC patients | - | - | - | - | - | I1 | - | |
| SF-20 subscale: Pain Perception | HNHC patients | - | - | - | - | - | I1 | - | |
| Change in HAM-D score | HNHC patients | - | - | - | - | - | I1 | - | |
| In remission (HAM-D < 7) | HNHC patients | - | - | - | - | - | I1 | - | |
| Patient-centered care coordination | HNHC patients | - | - | - | - | I1 | - | - | |
| Relationship with providers: trusted provider | HNHC patients | - | - | - | - | I1 | - | - | |
| Relationship with providers: feel respected by provider | HNHC patients | - | - | - | - | I1 | - | - | |
| Healthcare hassles summary score (challenges in getting care) | HNHC patients | - | - | - | - | I1 | - | - | |
| Patient assessment of chronic illness care (PACIC) Summary Score (receipt of care for chronic illness) | HNHC patients | - | - | - | - | I1 | - | - | |
| Progression to ESRD | HNHC CKD patients | - | I | - | - | - | - | - | |
| Graft or fistula prior to hemodialysis | HNHC CKD patients | - | I | - | - | - | - | - | |
| Graft or fistula prior to hemodialysis | HNHC ESRD patients | - | I | - | - | - | - | - | |
| HbA1c test | HNHC patients, diabetes subgroup | I1 | I | - | - | - | I | I | |
| LDL-C test | HNHC patients, diabetes subgroup | I1 | I | - | - | - | I | I | |
| LDL-C test | HNHC IVD patients | - | I | - | - | - | I | I | |
| Eye exam | HNHC patients, diabetes subgroup | I1 | I | - | - | - | - | - | |
| Nephrology/nephropathy test | HNHC patients, diabetes subgroup | I1 | I | - | - | - | - | - | |
| Lipid test | HNHC patients, IVD subgroup | I1 | I | - | - | - | - | - | |
| Oxygen saturation test | HNHC COPD patients | - | - | - | - | - | - | I | |
| Social risk outcomes | Participation in Supplemental Nutrition Assistance Program, any | HNHC patients | - | - | I1 | - | - | - | - |
| Receipt of temporary assistance for needy families, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Receipt of general assistance, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Ever housed | HNHC patients | - | - | I1 | I1 | - | - | - | |
| Jail stays | HNHC patients | - | - | I1 | - | - | - | - | |
| Criminal convictions, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Criminal convictions | HNHC patients | - | - | I1 | - | - | - | - | |
| Shelter days | HNHC patients | - | - | I1 | - | - | - | - | |
| Drug/alcohol treatment, any | HNHC patients | - | - | I1 | - | - | - | - | |
| Overall wellbeing | HNHC patients | - | - | - | - | - | I1 | - | |
| Problem alcohol use, any | HNHC patients | - | - | - | I1 | - | - | - | |
| Homelessness, any | HNHC patients | - | - | I1 | I1 | - | - | - | |
| Homeless months | HNHC patients | - | - | I1 | - | - | - | - | |
| No health insurance, any | HNHC patients | - | - | - | I1 | - | - | - | |
| No social security income, any | HNHC patients | - | - | - | I1 | - | - | - | |
| Basic financial needs unmet | HNHC patients | - | - | - | I1 | - | - | - |
I1: Insufficient, only one sample reporting on the outcome; I: Insufficient, 2+ samples reporting on the outcome within the model type; L-F: Low strength of evidence for favorable findings for the outcome; L-ND: Low strength of evidence for no difference for the outcome; L-U: Low strength of evidence for unfavorable findings for the outcome; -: No eligible evidence; M-F: Moderate strength of evidence for favorable findings for the outcome.
- a
Includes visits at 12 months.
- b
Defined as evaluation and management primary care visits by Kahn et al.122
- c
Shumway et al. specified the outpatient and inpatient costs as medical outpatient costs and medical hospital costs.116
ACSC = ambulatory care sensitive conditions; ADL = activities of daily living; BSI = brief symptom inventory; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; ED = emergency department; ESRD = end-stage renal disease; FQHC = Federally Qualified Health Center; HAM-D = Hamilton Depression Rating Scale.; HbA1c = hemoglobin A1c; HNHC = high-need, high-cost; IVD = ischemic vascular disease; LDL-C = low-density lipoprotein cholesterol; MHC = mental health composite; PACIC = patient assessment of chronic illness care; PHC = physical health composite; PQH-2 = patient health questionnaire-2; SF = short form; SNF = skilled nursing facility; SOE = strength of evidence.
Table B-15Study characteristics for system-level transformation models
| First Author, Year, Site(s) | Brief Description (Sample Size) | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
Kahn et al., 2016122 503 FQHCs |
Support for FQHCs obtaining PCMH status (HNHC high ED use patient sample N=NR out of 730,353 beneficiaries total) Intervention (N=NR out of 269,364 beneficiaries total) Comparison (N=NR out of 360,989 beneficiaries total) | Observational study (RoB: some concerns) | Utilization of ED visits at baseline: 90th percentile vs. <90th percentile | NA | Medicare FFS beneficiaries; attribution to the practice or clinic responsible for the greatest number of primary care services over the 12-month period preceded the demonstration | NA |
|
National: 14 practices |
Incentive payment (N=42,132) Intervention (N=8,216) Comparison (N=33,916) | Observational study (RoB: some concerns) | Hospitalization and use of acute or subacute rehabilitation services |
2+ chronic conditions 2+ ADLs that require human assistance | Medicare FFS beneficiaries; all IAH-eligible patients of the IAH practices, including those who received home-based care before the demonstration began; not in hospice or long-term care for the entire time they were eligible for the intervention in a given year |
Number of chronic conditions in Year 4 Total <6: 9.8% 6–9: 43.7% >9: 46.6% HCC: 3.90 Depression: 54.3% |
|
Peikes et al., 2018101 AR, CO, NJ, OR, NY, OH, KY, OK: 502 practices and 40 payers |
Primary care model Shared savings: APM (N=1,730,958) Intervention (N=565,674) Comparison (N=1,165,284) Subgroups (N=NR) | Observational study (RoB: some concerns) | 2+ hospitalizations in previous 2 years | 2+ of 13 eligible chronic conditions including congestive heart failure, COPD, acute myocardial infarction, ischemic heart disease, diabetes, any cancer other than skin cancer, stroke, depression, dementia, atrial fibrillation, osteoporosis, rheumatoid arthritis or osteoarthritis, chronic kidney disease | Received care in a CPC practice | NR |
|
AR, CO, HI, KC, KY, MI, MT, NJ, NY (Greater Buffalo); NY (North Hudson-Capital), ND, NE, OH, OK, OR, PA, RI, TN: 1,373 practices |
Primary care model Shared savings: APM Track 1 multiple chronic condition subgroup (N=NR of 5,163,969) Intervention (N=NR of 1,189,438) Comparison (N=NR of 3,974,531) | Observational study (RoB: some concerns) | 1+ hospitalizations in past 1 year | 2+ of 12 eligible chronic conditions including congestive heart failure, COPD, history of acute myocardial infarction, ischemic heart disease, diabetes, severe cancer, history of stroke, depression, dementia, atrial fibrillation, rheumatoid arthritis or osteoarthritis, chronic kidney disease | Enrolled in Medicare Parts A and B, Medicare FFS as primary payer, do not have ESRD and not enrolled in hospice, are not institution-alized or incarcerated, and are not attributed to a PCP for a nonoverlap CMS service | NR |
|
AR, CO, HI, KC, KY, MI, MT, NJ, NY (Greater Buffalo); NY (North Hudson-Capital), ND, NE, OH, OK, OR, PA, RI, TN: 1,515 practices |
Primary care model Shared savings: APM Track 2 multiple chronic condition subgroup (N=NR of 4,804,265) Intervention (N=NR of 1,443,553) Comparison (N=NR of 3,360,712) | Observational study (RoB: some concerns) | 1+ hospitalizations in past 1 year | 2+ of 12 eligible chronic conditions including congestive heart failure, COPD, history of acute myocardial infarction, ischemic heart disease, diabetes, severe cancer, history of stroke, depression, dementia, atrial fibrillation, rheumatoid arthritis or osteoarthritis, and chronic kidney disease | Enrolled in Medicare Parts A and B, Medicare FFS as primary payer, not ESRD and not in hospice, not institution-alized or incarcerated, and are not attributed to a PCP for a nonoverlap CMS service | NR |
ADL = activities of daily living; APM = advanced alternative payment model; AR = Arkansas; CMS = Centers for Medicare & Medicaid Services; CO = Colorado; COPD = chronic obstructive pulmonary disease; CPC = comprehensive primary care; ED = emergency department; ESRD = end-stage renal disease; FFS = fee-for-service; FQHC = Federally Qualified Health Center; HI = Hawaii; HNHC = high-need, high-cost; IAH = Independence at Home; KC = Kansas City; KY = Kentucky; MI = Michigan; MT = Montana; N = number; NA = not applicable; ND = North Dakota; NE = Nebraska; NJ = New Jersey; NR = not reported; NY = New York; OH = Ohio; OK = Oklahoma; OR = Oregon; PA = Pennsylvania; PCMH = patient-centered medical home; PCP = primary care provider; RI = Rhode Island; RoB = risk of bias; TN = Tennessee; vs. = versus.
Table B-16Intervention characteristics for system-level transformation models
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Impact on Clinician Workload/Clinical Practice | Comparison |
|---|---|---|---|---|---|---|---|---|---|---|
|
Kahn et al., 2016122 503 FQHCs | Three intervention components to support FQHC transformation into PCMHs: quarterly care management fee payments, technical assistance (TA), and data and performance feedback reports | 3 years | Periodically received three types of feedback reports: the biannual NCQA RAS report, the quarterly cost and utilization data reports, and the quarterly claims-based beneficiary-level report summarizing cost, utilization, and health. FQHCs were offered TA to prepare documentation for NCQA PCMH recognition through extensive learning systems involving varying partners | Intervention goals based on PCMH principles, which are designed to encourage doctors, hospitals, and other healthcare providers to work together to better coordinate care for patients | NR | PCMHs are physician- or nurse practitioner–directed medical practices | NR | NR | NR | Usual care at comparison FQHCs |
|
Kimmy et al., 2019103 Effect of incentive payment National: 14 practices | Practices may earn an additional payment if their chronically ill, functionally limited patients’ Medicare expenditures are below an estimated spending target and if the practice meets required standards for a set of quality measures | Up to 4 years | Clinicians are available at all hours of the day; carry out individualized care plans; and use electronic health information systems, remote monitoring, and mobile diagnostic technology | Report on other measures, including fall risk assessments and depression screenings, to promote the provision of such care | Some practices added social workers or other staff to coordinate care for their patients with other organizations | Physicians or nurse practitioners. Team may have also included physician assistants, clinical staff, and other health and social services staff | Face-to-face | Clinicians made 3–15 home visits per day, varied by site | ≤1 home visit and no visit from a participating practice in study year | Usual care of Medicare-eligible beneficiaries living in IAH regions |
|
Peikes et al., 2018101 AR, CO, NJ, OR, NY, OH, KY, OK: 502 practices and 40 payers | Comprehensive Primary Care (CPC) Initiative | 51 months | CPC practices received financial support, data feedback, and learning support | Practices required to address access and continuity, planned care for chronic conditions and preventive care, risk-stratified care management, patient and caregiver engagement | Practices required to address coordination of care across the medical neighborhood |
CPC practices, multiple contractors and organizations provided different intervention elements to CPC practices Patients received intervention services from regular practice staff and from specialized staff (e.g., care coordinators, care managers, social workers) | CMS sent reports on practice and patient-level data; learning support was peer-to-peer, didactic, and one-on-one | Reports and financial support were sent quarterly, frequency of learning support varied | Practices perceived that a big benefit of CPC participation was increased capacity to provide care management services to high-risk patients; practices shared with CMS any net savings in healthcare costs beyond amount required to cover their care management fee payments; within practices, care managers were increasingly integrated into clinicians’ work | Usual care at non-CPC practices |
|
Peikes et al., 2019123 AR, CO, HI, KC, KY, MI, MT, NJ, NY (Greater Buffalo); NY (North Hudson-Capital), ND, NE, OH, OK, OR, PA, RI, TN: 2,888 practices | Comprehensive Primary Care Plus (CPC+) | 2 years | Practice-focused intervention; CPC+ practices received financial support, data feedback, health IT support, and learning support; track 2 CPC+ practices received enhanced payments and replacement of some fee-for-service payments with prospective payments |
Required to address access and continuity, care management, comprehensive-ness and coordination, patient and caregiver engagement, and planned care and population health CMS-specified care delivery requirements within each of these functions; they were considered a starting point and practices could choose which care delivery requirements or other changes to adopt first, which personnel would be involved and which tactics they would pursue | Care management, comprehensive-ness and coordination are 2 of the 5 key functions of a CPC+ practice |
CPC+ practices; CMS partnered with 79 public and private payers across 18 CPC+ regions; various contractors and organizations provided intervention elements to CPC+ practices Practices hired new staff to support CPC+ activities: care managers, behavioral health specialists, clinical pharmacists, social workers, data analysts, dietitians, diabetes educators, and QI staff | Practices received data reports; learning was delivered in groups and in-person practice coaching | Reports and financial support were sent quarterly, frequency of learning support NR | NR | Usual care at non-CPC+ practices |
AR = Arkansas; CMS = Centers for Medicare & Medicaid Services; CO = Colorado; CPC = Comprehensive Primary Care; CPC+ = Comprehensive Primary Care Plus; FQHC = Federally Qualified Health Center; HI = Hawaii; IT = information technology; KC = Kansas City; KY = Kentucky; MI = Michigan; MT = Montana; NCQA = National Committee for Quality Assurance; ND = North Dakota; NE = Nebraska; NJ = New Jersey; NR = not reported; NY = New York; OH = Ohio; OK = Oklahoma; OR = Oregon; PA = Pennsylvania; PCMH = patient-centered medical home; QI = quality improvement; RAS = Readiness Assessment Survey; RI = Rhode Island; TA = technical assistance; TN = Tennessee.
Table B-17Healthcare utilization outcomes for system-level transformation model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Inpatient admissions | Observational | NR | NR | FQHC: adjusted year 3 difference in visits: −5.32 (SE: 28.60) (p>0.10)122 |
| Observational | NR | NR | IAH incentive payment 5-year average annual effect DiD=−0.08 (SE: 0.05) (p>0.10)103, 132 | |
| Inpatient admissions, ACSC | Observational | NR | NR | Greater reduction in G1 than G2: IAH incentive payment 5-year average annual effect DiD=−0.04 (SE: 0.02) (p<0.05)103, 132 |
| ED visits | Observational | NR | NR | FQHC: adjusted year 3 difference in visits: −80.75 (SE: 87.65) (p>0.10)122 |
| Observational | NR | NR | Greater reduction in G1 than G2: IAH incentive payment 5-year average annual effect DiD=−0.14 (SE: 0.06) (p<0.01)103, 132 | |
| ED visits, outpatient | Observational | NR | NR | IAH incentive payment 5-year average annual effect DiD=−0.02 (SE: 0.06) (p>0.10)103, 132 |
| ED visits resulted in inpatient admission | Observational | NR | NR | Greater reduction in G1 than G2: IAH incentive payment 5-year average annual effect DiD=−0.11 (SE: 0.05) (p<0.05)103, 132 |
| ED visits, ACSC | Observational | NR | NR | IAH incentive payment 5-year average annual effect DiD=−0.00 (SE: 0.01) (p>0.10)103, 132 |
| Primary care visitsb | Observational | NR | NR | FQHC: adjusted year 3 difference in visits: −132.58 (SE: 84.18) (p>0.10)122 |
| Specialist visits | Observational | NR | NR | FQHC: adjusted year 3 difference in visits: −29.67 (SE: 78.82) (p>0.10)122 |
| FQHC visits | Observational | NR | NR | FQHC: adjusted year 3 difference in visits: −15.90 (SE: 54.65) (p>0.10)122 |
| Probability of entering institutional long-term care within the demonstration year | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=−0.38 (SE: 0.37) (p>0.10)132 |
| Probability of hospice use | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=−0.83 (SE: 0.68) (p>0.10)103 |
| Probability of SNF use | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=0.19 (SE: 0.82) (p>0.10)103 |
| Probability of home health use | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=−0.45 (SE: 0.70) (p>0.10)103 |
| Home health days | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=−0.58 (SE: 5.67) (p>0.10)103 |
| Home health visits | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=0.14 (SE: 2.43) (p>0.10)103 |
| Visits in nonacute settings by primary care clinicians | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=0.59 (SE: 0.57) (p>0.10)103 |
| Visits in nonacute settings by specialists | Observational | NR | NR | IAH incentive payment 4-year average annual effect DiD=−0.39 (SE: 0.32) (p>0.10)103 |
- a
The reported p-value reflect the authors’ adjustment for multiple comparisons.
- b
Defined as Evaluation and Management primary care visits by Kahn et al.122
ACSC = ambulatory care sensitive conditions; DiD = difference-in-difference; ED = emergency department; FQHC = Federally Qualified Health Center; G = group; IAH = Independence at Home; NR = not reported; SE = standard error; SNF = skilled nursing facility.
Table B-18Strength of evidence for system-level transformation model versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | ED visits |
FQHC: adj diff=−80.75 (p>0.10);122 IAH incentive payment: lower use in G1 than G2: DiD=−0.14 (p<0.01)103 | 2 OBSs, N=NRa | Moderate study limitations, inconsistent, direct, imprecise | Insufficient |
| HNHC patients | Inpatient admissions |
FQHC: adj diff=−5.32 (p>0.10);122 IAH incentive payment DiD=−0.08 (SE: 0.05) (p>0.10)103 | 2 OBSs, N=NRa | Moderate study limitations, consistent, direct, imprecise | Insufficient |
| HNHC patients | Total cost |
FQHC: adj diff=$387.23 (p>0.10);122 CPC: DiD=−$45 (p=0.23);101 CPC+ sample 1: DiD=6.2 (p>0.10);133 CPC+ sample 2: DiD=43.0 (p=04);133 IAH incentive payment DiD=−200 (SE: 151) (p>0.10)103 Pooled mean difference: −$5.41 (95% CI, −38.28 to 49.10); 5 observational samples; I2=44.6% | 5 OBSs, N=NRa | Moderate study limitations, inconsistent, direct, imprecise | Low (No difference) |
- a
Note: CPC+ sample 1: HNHC patients in CPC+ practices making less advanced care delivery changes; CPC+ sample 2: HNHC patients in CPC+ practices making more advanced care delivery changes.
adj diff = adjusted difference; CI = confidence interval; DiD = difference-in-difference; ED = emergency department; FQHC = Federally Qualified Health Center; HNHC = high need, high cost; IAH = Independence at Home; N = number; NR = not reported; OBS = observational study; vs. = versus.
Table B-19Cost outcomes for system-level transformation model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost ($) | Observational | NR | NR | FQHC: adjusted difference=387.23 (SE: 494.62) (p>0.10)122 |
| Observational | NR | NR | IAH incentive payment 5-year average annual effect DiD=−200 (SE: 151) (p>0.10)103, 132 | |
| Observational | NR | NR | CPC DiD=−45 (p=0.23)101 | |
| Observational | NR | NR | CPC+ Sample 1 2-year impact DiD=6.2 (SE: 19.2) (p>0.10))123, 133 | |
| Observational | NR | NR | Greater increase in G1 than G2: CPC+ Sample 2 2-year impact DiD=43.0 (SE: 20.5) (p=0.04)123, 133 | |
| Outpatient cost | Observational | NR | NR | FQHC: adjusted differences in visits: 290.07 (SE: 178.26) (p>0.10)122 |
| Acute cost | Observational | NR | NR | FQHC: adjusted for differences in visits: 292.47 (SE: 315.40) (p>0.10)122 |
| OPD cost | Observational | NR | NR | FQHC: adjusted for differences in visits: 218.92 (SE: 150.97) (p>0.10)122 |
| Primary care physician cost | Observational | NR | NR | FQHC: adjusted differences in visits: 18.99 (SE: 26.65) (p>0.10)122 |
Note: CPC+ sample 1: HNHC patients in CPC+ practices making less advanced care delivery changes; CPC+ sample 2: HNHC patients in CPC+ practices making more advanced care delivery changes.
CPC+ = Comprehensive Primary Care Plus; DiD = difference-in-difference;; FQHC = Federally Qualified Health Center; G = group; HNHC = high-need, high-cost; IAH = Independence at Home; NR = not reported; OPD = outpatient department; SE = standard error.
Table B-20Cost outcomes for system-level transformation model studies: Subgroup outcomes
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost (PBPM) | Observational | NR | NR | Beneficiaries with dementia subgroup: IAH incentive payment DiD=−222 (SE: 143)(p=NS)103 |
| Observational | NR | NR | Beneficiaries without dementia subgroup: IAH incentive payment DiD=−347 (SE: 279) (p=NS)103 |
DiD = difference-in-difference; G = group; IAH = Independence at Home; NR = not reported; NS = not statistically significant; PBPM = per beneficiary per month; SE = standard error.
Table B-21Clinical and functional outcomes for system-level transformation model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality rate | Observational | IAH incentive payment 5-year average annual effect DiD: −0.32 (SE: 0.55) (p>0.10)103, 132 |
DiD = difference-in-difference; G = group; IAH = Independence at Home; NR = not reported; SE = standard error.
Table B-22Clinical and functional outcomes for system-level transformation model studies: Subgroup outcomes
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| HbA1c test | Observational | FQHC diabetes subgroup: adjusted year 3 difference in visits: 0.35 (SE: 1.16) (p>0.10)122 |
| LDL test | Observational | FQHC diabetes subgroup: adjusted year 3 difference in visits: −1.17 (SE: 1.37) (p>0.10)122 |
| Eye exam | Observational | FQHC diabetes subgroup: adjusted year 3 difference in visits: 1.09 (SE: 1.53) (p>0.10)122 |
| Nephropathy test | Observational | FQHC diabetes subgroup: adjusted year 3 difference in visits: 2.88 (SE: 1.60) (p>0.10)122 |
| All 4 recommended diabetes tests | Observational | FQHC diabetes subgroup: adjusted year 3 difference in visits: −0.51 (SE: 0.45) (p>0.10)122 |
| Lipid test | Observational | FQHC IVD subgroup: adjusted year 3 difference in visits: −0.81 (SE: 1.80) (p>0.10)122 |
FQHC = Federally Qualified Health Center; G = group; HbA1c = hemoglobin A1c; IVD = ischemic vascular disease; LDL = low-density lipoprotein; NR = not reported; SE = standard error.
Table B-23Study characteristics for telephonic/mail models
| First Author, Year, Site(s) | Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
McCall et al., 201079 48 counties in western TX |
HNHC patients only (N=2,815) Intervention (N=1,986) Comparison (N=1,969) | RCT (RoB: some concerns) | Medicare costs of $6,000 or more in past 1 year | NA | Medicare FFS beneficiaries, majority of visits to TX Tech U Health Sciences Center or Physician Network Services (a PCP) | NR |
|
McCall et al., 201081 Suffolk, Nassau, and Queens, NY: 6 nephrology practices |
Original sample (N=6,996) Intervention (N=4,996) Comparison (N=2,000) Diabetes subgroup (N=2,165) ESRD subgroup (N=331) IVD subgroup (N=2,434) | RCT (RoB: some concerns) | Medicare costs of $5,000 or more in past 1 year | Chronic kidney disease diagnosis on at least one claim, HCC > 1.7 (high risk for future healthcare utilization), excluded ESRD patients | Medicare FFS beneficiaries with primary residence in the intervention county/region | NR |
|
McCall et al., 201081 Suffolk, Nassau, and Queens, NY: 6 nephrology practices |
Refresh sample (N=3,341) Intervention (N=2,385) Comparison (N=956) Diabetes subgroup (N=1,280) ESRD subgroup (N=97) IVD subgroup (N=1,508) | RCT (RoB: some concerns) | Medicare costs of $5,000 or more in past 1 year | CKD diagnosis as evidence by at least one claim, HCC > 1.7 (high risk for future healthcare utilization), excluded ESRD patients | Medicare FFS beneficiary with primary residence in the intervention county/region, excluded patients institutionalized from March to May 2006 (part of the baseline period) | NR |
|
Urato et al., 201382 Sites in NY counties: Nassau, Suffolk, Queens, Kings, Westchester, Richmond, Rockland, and Bronx |
Original sample (N=5,889) Intervention (N=2,945) Comparison (N=2,944) Diabetes subgroup (N=2,284) CKD subgroup (N=3,159) Intervention IVD subgroup (N=2,030) | RCT (RoB: some concerns) | Medicare costs of $5,000 or more for CKD beneficiaries and $12,000 or more for ESRD beneficiaries in past 1 year | Stage 3 CKD diagnosis, as evidence by at least one claim | Medicare FFS beneficiaries with primary residence in the intervention county/region | Mean HCC score: 2.65 |
|
Urato et al., 201382 Sites in NY counties: Nassau, Suffolk, Queens, Kings, Westchester, Richmond, Rockland, and Bronx |
Refresh sample (N=4,467) Intervention (N=2,234) Comparison (N=2,233) Diabetes subgroup (N=2,202) CKD subgroup (N=1,663) IVD subgroup (N=2,061) | RCT (RoB: some concerns) | Medicare costs of $5,000 or more for CKD beneficiaries and $12,000 or more for ESRD beneficiaries in past 1 year | Stage 3 CKD diagnosis, as evidence by at least one claim | Medicare FFS beneficiaries with primary residence in the intervention county/region | NR |
|
Dally et al., 200290 OH |
(N=593) Intervention (N=297) Comparison (N=296) | RCT (RoB: some concerns) | 11+ outpatient visits in 2 years | 1+ visit with a diagnosis of at least 1 of 3 targeted conditions (arthritis, hypertension, diabetes) | Age: 18–64; Kaiser Permanente Ohio patient | |
|
McCall et al., 201180 Central OR and central WA: 2 large multispecialty group practices |
Original HNHC patient sample (N=125) Intervention (N=66) Comparison (N=59) | RCT (RoB: some concerns) | Medicare costs of $6,000 or more in 1 year | HF, diabetes, or COPD diagnosis on at least 1 claim | Medicare FFS beneficiaries; 2+ visits to the HBC medical practices | NR |
|
McCall et al., 201180 Central OR and central WA: 2 large multispecialty group practices |
Refresh HNHC patient sample (N=227) Intervention (N=120) Comparison (N=107) | RCT (RoB: some concerns) | Medicare costs of $6,000 or more in 1 year | HF, diabetes, or COPD diagnosis on inpatient, outpatient hospital, or physician claims only | Medicare FFS beneficiaries; 2+ visits to the HBC medical practices | NR |
|
Schickedanz et al., 2019121 Southern CA: 1 health system including 13 medical centers |
(N=34,225) Intervention (N=7,107) Comparison (N=27,118) |
Observational (RoB: some concerns) | Predicted to be in top 1% of healthcare utilization in health system in the next 1 year | NA | Age: 18+; Kaiser Permanente Southern California patient |
Nonwhite: 50% Cancer: 46% Diabetes: 13% CAD/CHF: 33% Asthma: 6% Charlson Comorbidity score: 7 (3) Depression: 6% Lives in low-income census tract: 14%; Medicare: 60%; Commercial insurance: 25% |
CA = California; CAD = coronary artery disease; CHF = congestive heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; ESRD = end-stage renal disease; FFS = fee-for-service; HF = heart failure; HBC = Health Buddy Consortium; HCC = hierarchical condition category; HNHC = high-need, high-cost; IVD = ischemic vascular disease; N = number; NA = not applicable; NR = not reported; NY = New York; OH = Ohio; OR = Oregon; PCP = primary care practice; RCT = randomized controlled trial; RoB = risk of bias; TX = Texas; U = University; WA = Washington.
Table B-24Intervention characteristics for telephonic/mail models
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
McCall et al., 201079 48 counties in western TX | TX Senior Trails Program: Care Management for High-Cost Beneficiaries Demonstration: TX Tech University Health Sciences Center Shared savings and care management | NR; up to 16 months | Patient education and coaching of chronic conditions and self-management skills | Facilitate communication with providers, care plan compliance, hospital discharge planning, medication adherence, access to clinics; sharing information with physicians | Care team drew on community resources to assist with social issues | Nurse care managers, nurses for telephone support, social workers | Telephone, face-to-face, written: in patient homes, physician offices, or in the hospital, as needed |
Mean contacts per beneficiary: 6 (median: 4) 97% of participants had 1+ contacts with a care manager or physician, 50% had 2–4 contacts, and 25% had 5–9 contacts. Written contact was most frequent, face-to-face was least frequent (20% had face-to-face contact with the care manager) | Usual care: comparison group was not contacted |
|
McCall et al., 201081 Suffolk, Nassau, and Queens, NY: 6 nephrology practices |
Care Management for High-Cost Beneficiaries Demonstration: VillageHealth I Shared savings and one-on-one nurse care manager support | Up to 36 months for original sample, up to 24 months for refresh sample | Initial and continuous risk evaluation, telemonitor health failure patients, 24-hour hotline for assistance requests, develop care plan, renal disease education | Coordinated care, referral to nephrologist when reach stage IV CKD, support from pharmacist, medication therapy management | NR | Care managers (phone and field RNs), pharmacists, dietitians: telephone support and education materials; social workers: telephone psychosocial support (e.g., insurance, transportation); health service assistants provided admin support for patients and providers | Telephone, face-to-face: NR | On average, participants were contacted about every 1.4 months or had 13 contacts over 18 months. Nearly all had a telephone or in-person contact during the last 18 months, mostly by telephone | Usual care: comparison group was not contacted |
|
Urato et al., 201382 Sites in NY counties: Nassau, Suffolk, Queens, Kings, Westchester, Richmond, Rockland, and Bronx |
Extended Medicare Care Management for High Cost Beneficiaries (CMHCB) Demonstration: VillageHealth II Shared savings and disease management/case management through a care management organization | Up to 21 months for original sample, up to 11 months for refresh sample | Provided individualized assessment, including risk stratification, and tailored care plans; education related to self-management activities to decrease risk for acute exacerbations of chronic diseases | Facilitated patient relationships with physicians, helped patients comply with physician care plans, hospital discharge planning support, medication management | Referrals or provision for ancillary services (drugs, community services) | Nurse care managers, nurses for telephone support, registered dietitian, pharmacist, social worker | Face-to-face or telephone contact with nurse care manager, in-person educational classes: in-patient home or over telephone
|
At least monthly contact with care manager; >50% of beneficiaries did not get a call or in-person meeting with a care manager in the last 15 months Telephonic contact was the dominant form of contact (about 70%) | Usual care: comparison group was not contacted |
|
Dally et al., 200290 OH | Mailed health promotion program | 90% remained enrolled for 30 months | Health risk appraisal (HRA) questionnaire, personalized letter and report with feedback after each questionnaire with recommendations to reduce the health risks identified by the questionnaire, and condition-specific health education pamphlets and books | NR | NR | A vendor provided all intervention-related materials | All materials were mailed to participants’ homes | After initial HRA questionnaire, 3 additional HRA questionnaires and individualized feedback letters/reports were delivered approximately every 3 months | Controls also received and completed the baseline HRAs, education materials, and an incentive to complete the final questionnaire |
|
McCall et al., 201180 Central OR and central WA: 2 large multispecialty group practices |
Care Management for High-Cost Beneficiaries Demonstration: Health Buddy Consortium Shared savings and Health Buddy® disease management (Health Buddy® device allowed for daily, routine communication with program staff) | Up to 38 months for original population, up to 26 months for refresh population | Daily Health Buddy questionnaire to assess health condition, followup from nurse CM as appropriate | 13 disease-specific care management programs; triaged and coordinated medical, psychological, or social services | Coordinated social services, as needed | Nurse care managers, physicians | Telephone, over Health Buddy device | Nearly all intervention group members who used the device received at least one call from a care manager during the demo and nearly 60% received >20 contacts during this same period. Over 60% of participants never had a device and scheduled regular telephone calls with a nurse care manager | Usual care: comparison group was not contacted |
|
Schickedanz et al., 2019121 Southern CA: 1 health system including 13 medical centers | Health Leads Program: social needs screening and navigation intervention | NR; up to 14 months (followup time) | Social needs screener, intake assessment | NR | Tailored referral to community-based services (i.e., food banks, housing programs, or other resources to address the identified social need) immediately or during followup calls | Program associates provided screening and navigation | Telephone | Followup calls at minimum every 2 weeks until call were no longer needed or loss to followup | Usual care |
CA = California; CKD = chronic kidney disease; CM = case manager; CMHCB = Care Management for High Cost Beneficiaries; ESRD = end-stage renal disease; HRA = health risk appraisal; NR = not reported; NY = New York; OH = Ohio; OR = Oregon; RN = registered nurse; TX = Texas; WA = Washington.
Table B-25Healthcare utilization outcomes for telephonic/mail model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Inpatient admissions, all cause | RCT | ⇧ | ⇧ | Original sample DiD: IRR=0.94 (95% CI, 0.82 to 1.07)81 |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=0.96 (95% CI, 0.80 to 1.15)81 | |
| RCT | ⇧ | ⇧ | Original sample DiD: IRR=1.06 (95% CI, 0.95 to 1.19)82 | |
| RCT | ⇩ | ⇩ | Refresh sample DiD: IRR=0.98 (95% CI, 0.86 to 1.11)82 | |
| Inpatient admissions, ACSC | RCT | ⇧ | ⇧ | Original sample DiD: IRR=1.05 (95% CI, 0.87 to 1.28)82 |
| RCT | ⇧ | ⇩ | Refresh sample DiD: IRR=0.98 (95% CI, 0.80 to 1.20)82 | |
| RCT | ⇧ | ⇧ | Original sample DiD: IRR=0.83 (95% CI, 0.67 to 1.04)81 | |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=1.02 (95% CI, 0.77 to 1.36)81 | |
| Inpatient admissions, any (%) | RCT | ⇧ | ⇧ | Original sample DiD: OR=0.98 (95% CI, 0.82 to 1.18)81 |
| RCT | ⇩ | -- | Refresh sample DiD: OR=0.94 (95% CI, 0.74 to 1.18)81 | |
| Inpatient Admissions, ACSC (%) | RCT | ⇧ | ⇧ | Original sample DiD: OR=0.86 (95% CI, 0.69 to 1.08)81 |
| RCT | ⇧ | ⇧ | Refresh sample DiD: OR=0.93 (95% CI, 0.70 to 1.25)81 | |
| ED visits, all cause | RCT | ⇧ | ⇧ | Original sample DiD: IRR=1.04 (95% CI, 0.91 to 1.19)81 |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=1.01 (95% CI, 0.82 to 1.24)81 | |
| RCT | ⇧ | ⇧ | Original sample DiD: IRR=1.03 (95% CI, 0.90 to 1.17)82 | |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=0.97 (95% CI, 0.85 to 1.10)82 | |
| ED visits, ACSC | RCT | ⇧ | ⇧ | Original sample DiD: IRR=1.09 (95% CI, 0.90 to 1.33)82 |
| RCT | ⇩ | ⇩ | Refresh sample DiD: IRR=1.00 (95% CI, 0.81 to 1.23)82 | |
| RCT | ⇧ | ⇧ | Original sample DiD: IRR=0.87 (95% CI, 0.71 to 1.08)81 | |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=1.07 (95% CI, 0.79 to 1.45)81 | |
| Outpatient visits at 6 months | RCT | NR | NR | Total sample: Poisson coefficient =−0.0328 (p=0.27)90 |
| Outpatient visits at 12 months | RCT | ⇧ | ⇩ | Total sample: Poisson coefficient =−0.0341 (p=0.11)90 |
| Outpatient visits at 30 months | RCT | NR | NR | Total sample: Lower use in G1 than G2: Poisson coefficient =−0.0260 (p=0.04)90 |
| Total Utilization (%) | Observational | NR | NR | Total sample DiD:a −2.2 (95% CI, −4.5 to 0.1)121 |
- a
Total utilization includes a count of all visits in emergency department, outpatient, and/or inpatient settings. The subgroups were based on the census tract estimates of patients’ home address. The low-income subgroup included patients who lived in census tracts where a plurality of residents had income below $34,575. The low-education subgroup included patients who lived in census tracts where a plurality of residents had less than a high school education.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
ACSC = ambulatory care sensitive conditions; CI = confidence interval; DiD = difference-in-difference; ED = emergency department; G = group; IRR = incidence rate ratio; NR = not reported; OR = odds ratio; RCT = randomized controlled trial.
Table B-26Healthcare utilization outcomes for telephonic/mail model studies: Subgroup outcomes
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Outpatient visits at 6 months | RCT | NR | NR | Arthritis subgroup: Poisson coefficient= −0.0710 (p=0.16)90 |
| RCT | NR | NR | Blood pressure subgroup: Poisson coefficient=0.0838 (p=0.15)90 | |
| RCT | NR | NR | Diabetes subgroup: Poisson coefficient= −0.0879 (p=0.07)90 | |
| Outpatient visits at 12 months | RCT | ⬇ | ⬆ | Arthritis subgroup: Lower use in G1 than G2: Poisson coefficient =−0.1130 (p<0.01)90 |
| RCT | ⬇ | ⬇ | Blood pressure subgroup: Higher use in G1 than G2: Poisson coefficient=0.0938 (p=0.02)90 | |
| RCT | ⇩ | ⇧ | Diabetes subgroup: Poisson coefficient= −0.0651 (p=0.06)90 | |
| Outpatient visits at 30 months | RCT | NR | NR | Arthritis subgroup: Lower use in G1 than G2: Poisson coefficient =−0.1004 (p<0.01)90 |
| RCT | NR | NR | Blood pressure subgroup: Poisson coefficient=0.0317 (p=0.21)90 | |
| RCT | NR | NR | Diabetes subgroup: Poisson coefficient= −0.0146 (p=0.50)90 | |
| Total utilization (%) | Observational | NR | NR | Low-income area subgroup DiD:a Greater reduction in G1: −7.0 (95% CI −11.9 to −1.9)121 |
| Observational | NR | NR | Low-education area subgroup DiD:a Greater reduction in G1: −11.5 (95% CI, −17.6 to −5.0)121 | |
| Observational | NR | NR | Medicaid insurance subgroup DiD:a Greater reduction in G1: −12.1 (95% CI, −18.1 to −5.6)121 |
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
CI = confidence interval; DiD = difference-in differences; G = group; NR = not reported; RCT = randomized controlled trial.
Table B-27Strength of evidence for telephonic/mail models versus usual-care outcomesa
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | ED visits, all cause |
VH I: Original sample DiD: IRR=1.04 (95% CI, 0.91 to 1.19)81 VH I: Refresh sample DiD: IRR=1.01 (95% CI, 0.82 to 1.24)81 VH II: Original sample DiD: IRR=1.03 (95% CI, 0.90 to 1.17)82 VH II: Refresh sample DiD: IRR=0.97 (95% CI, 0.85 to 1.10)82 Pooled rate ratio: 1.01 (95% CI, 0.94 to 1.08); 4 RCT samples; I2=0% | 4 RCTs, N=20,693 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | ED visits, ACSC |
VH II: Original sample DiD: IRR=1.09 (95% CI, 0.90 to 1.33)82 VH II: Refresh sample DiD: IRR=1.00 (95% CI, 0.81 to 1.23)82 VH I: Original sample DiD: IRR=0.87 (95% CI, 0.71 to 1.08)81 VH II: Refresh sample DiD: IRR=1.07 (95% CI, 0.79 to 1.45)81 Pooled rate ratio: 0.99 (95% CI, 0.88 to 1.10); 4 RCT samples; I2=0% | 4 RCTs, N=20,693 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Inpatient admissions, all cause |
VH I: Original sample DiD: IRR=0.94 (95% CI, 0.82 to 1.07)81 VH I: Refresh sample DiD: IRR=0.96 (95% CI, 0.80 to 1.15)81 VH II: Original sample DiD: IRR=1.06 (95% CI, 0.95 to 1.19)82 VH II: Refresh sample DiD: IRR=0.98 (95% CI, 0.86 to 1.11)82 Pooled rate ratio: 0.99 (95% CI, 0.92 to 1.06); 4 RCT samples; I2=0% | 4 RCTs, N=20,693 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Inpatient admissions, ACSC |
VH II: Original sample DiD: IRR=1.05 (95% CI, 0.87 to 1.28)82 VH II: Refresh sample DiD: IRR=0.98 (95% CI, 0.80 to 1.20)82 VH I: Original sample DiD: IRR=0.83 (95% CI, 0.67 to 1.04)81 VH I: Refresh sample DiD: IRR=1.02 (95% CI, 0.77 to 1.36)81 Pooled rate ratio: 0.95 (95% CI, 0.85 to 1.06); 4 RCT samples; I2=0% | 4 RCTs, N=20,693 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Inpatient admissions, any (%) |
VH I: Original sample DiD: OR=0.98 (95% CI, 0.82 to 1.18)81 VH I: Refresh sample DiD: OR=0.94 (95% CI, 0.74 to 1.18)81 | 2 RCTs, N=10,337 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient admissions, ACSC (%) |
VH I: Original sample DiD: OR=0.86 (95% CI, 0.69 to 1.08)81 VH I: Refresh sample DiD: OR=0.93 (95% CI, 0.70 to 1.25)81 | 2 RCTs, N=10,337 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Total cost |
TST: DiD=120 (p>0.05)79 HB: Original sample DiD=−308 (p>0.05)80 HB: Refresh sample DiD=178 (p>0.05)80 VH I: Original sample DiD=−111 (p>0.05)81 VH I: Refresh sample DiD=−142 (p>0.05)81 VH II: Original sample DiD=206 (p>0.05)82 VH II: Refresh sample DiD=−99 (p>0.05)82 Pooled mean difference: −$8.52 (95% CI, −130.02 to 112.98); 7 RCT samples; I2=22.4% | 7 RCTs, N=25,000 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Mortality rate |
VH I: Original sample diff=0.8 (p=0.51)81 VH I: Refresh sample diff=−1.1 (p=0.49)81 VH II: Original sample diff=0.6 (p=0.61)82 VH II: Refresh sample diff=0.3 (p=0.76)82 Pooled mean difference: 0.34 (95% CI, −1.06 to 1.74); 4 RCT samples; I2=0% | 4 RCTs, N=20,693 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Influenza vaccine |
VH I: Original sample DiD: OR=1.12 (95% CI, 0.93 to 1.34);81 VH I: Refresh sample DiD: OR=0.91 (95% CI, 0.72 to 1.15)81 | 2 RCTs, N=10,337 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients | Progression to ESRD |
VH I: Original sample diff: −0.75 (95% CI, −1.90 to 0.41)81 VH I: Refresh sample diff: 0.91 (95% CI, −2.23 to 0.41)81 | 2 RCTs, N=10,337 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients CKD subgroup | Progression to ESRD |
VH II: Original sample diff: Greater in G1: 2.92 (95% CI, 0.30 to 5.54)82 VH II: Refresh sample diff: 0.37 (95% CI, −2.53 to 3.28)82 | 2 RCTs, N=4,822 | Moderate study limitations, imprecise, inconsistent, direct | Insufficient |
| HNHC patients CKD subgroup | Graft or fistula prior to hemodialysis |
VH II: Original sample diff: Lower in G1: −3.09 (95% CI, −5.93 to −0.24)82 VH II: Refresh sample diff: −2.05 (95% CI, −6.39 to 2.30)82 | 2 RCTs, N=4,822 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients ESRD subgroup | Graft or fistula prior to hemodialysis |
VH I: Original sample diff: −6.08 (95% CI, −15.75 to 3.59)81 VH I: Refresh sample diff: 2.87 (95% CI, −16.72 to 22.46)81 | 2 RCTs, N=428 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients Diabetes subgroup | HbA1c test |
VH II: Original sample DiD: OR=1.22 (95% CI, 0.73 to 2.03)82 VH II: Refresh sample DiD: OR=0.76 (95% CI, 0.47 to 1.23)82 VH I: Original sample DiD: OR=1.02 (95% CI, 0.68 to 1.54)81 VH I: Refresh sample DiD: OR=0.95 (95% CI, 0.56 to 1.61)81 | 4 RCTs, N=7,931 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
| HNHC patients Diabetes subgroup | LDL test |
VH II: Original sample DiD: OR=1.05 (95% CI, 0.70 to 1.58)82 VH II: Refresh sample DiD: OR=1.15 (95% CI, 0.80 to 1.67)82 VH I: Original sample DiD: OR=1.19 (95% CI, 0.84 to 1.68)81 VH I: Refresh sample DiD: OR=0.72 (95% CI, 0.42 to 1.24)81 | 4 RCTs, N=7,931 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients IVD subgroup | LDL test |
VH I: Original sample DiD: OR=0.98 (95% CI, 0.72 to 1.35)81 VH II: Refresh sample DiD: OR=0.88 (95% CI, 0.54 to 1.43)81 | 2 RCTs, N=3,942 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients Diabetes subgroup | Eye exam |
VH II: Original sample DiD: OR=1.05 (95% CI, 0.81 to 1.137)82 VH II: Refresh sample DiD: OR=1.25 (95% CI, 0.94 to 1.66)82 | 2 RCTs, N=4,486 | Moderate study limitations, imprecise, consistent, direct | Insufficient |
| HNHC patients Diabetes subgroup | Nephrology |
VH II: Original sample DiD: OR=0.92 (95% CI, 0.71 to 1.18)82 VH II: Refresh sample DiD: OR=1.30 (95% CI, 1.01 to 1.67)82 | 2 RCTs, N=4,486 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients IVD subgroup | Lipid panel |
VH II: Original sample DiD: OR=1.10 (95% CI, 0.85 to 1.44)82 VH II: Refresh sample DiD: OR=1.22 (95% CI, 0.17 to 0.92)82 | 2 RCTs, N=4,091 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
- a
Comparison group participants for Dally et al. received baseline education materials and incentives.90
ACSC = ambulatory care sensitive conditions; CI = confidence interval; CKD = chronic kidney disease; DiD = difference-in-difference; diff = difference; ED = emergency department; ESRD = end-stage renal disease; G = group; HB = Health Buddy; HbA1c = hemoglobin A1c; HNHC = high-need, high-cost; IRR = incidence rate ratio; IVD = ischemic vascular disease; LDL = low-density lipoprotein; N = number; OR = odds ratio; RCT = randomized controlled trial; TST = Texas Senior Trails; VH = Village Health; vs. = versus.
Table B-28Cost outcomes for telephonic/mail model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost | RCT | ⇩ | ⇩ | DiD=120 (SE:99.6) (p>0.05)79 |
| RCT | ⇩ | ⇩ | Original sample DiD=−308 (SE: 311.2) (p>0.05)80 | |
| RCT | ⇩ | ⇩ | Refresh sample DiD=178 (SE: 257.5) (p>0.05)80 | |
| RCT | ⇧ | ⇧ | Original sample DiD=−111 (SE: 83.2) (p>0.05)81 | |
| RCT | ⇧ | ⇧ | Refresh sample DiD=−142.1 (SE: 138.7) (p>0.05)81 | |
| RCT | ⇧ | ⇧ | Original sample DiD=206 (SE: 152.2) (p>0.05)82 | |
| RCT | ⇩ | ⇩ | Refresh sample DiD=−99 (SE: 206.6) (p>0.05)82 |
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
DiD = difference-in-difference;; G = group; RCT = randomized controlled trial; SE = standard error.
Table B-29Clinical and functional outcomes for telephonic/mail model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality rate | RCT | Original sample difference=0.8 (p=0.51)81 |
| RCT | Refresh sample difference=−1.1 (p=0.49)81 | |
| RCT | Original sample difference=0.6 (p=0.61)82 | |
| RCT | Refresh sample difference=0.3 (p=0.76)82 | |
| Influenza vaccine | RCT | Original sample DiD: OR=1.12 (95% CI, 0.93 to 1.34)81 |
| RCT | Refresh sample DiD: OR=0.91 (95% CI, 0.72 to 1.15)81 | |
| Progression to ESRD | RCT | Original sample difference=−0.75 (95% CI, −1.90 to 0.41)81 |
| RCT | Refresh sample difference=−0.91 (95% CI, −2.23 to 0.41)81 | |
| PHC score (physical health) | RCT | ANCOVA-adjusted IE=−0.1 (p>0.05)81 |
| MHC score (mental health) | RCT | ANCOVA-adjusted IE=0.0 (p>0.05)81 |
| PHQ-2 (depression, 0 to 6) | RCT | Lower score in G1 than G2:a ANCOVA-adjusted IE=−0.45 (p<0.05)81 |
| Number of ADLs difficult to do (0 to 6) | RCT | ANCOVA-adjusted intervention effect=−0.02 (p>0.05)81 |
| Number of ADLs receiving help (0 to 6) | RCT | ANCOVA-adjusted IE=0.21 (p>0.05)81 |
| Helping to cope with a chronic condition (1 to 5) | RCT | ANCOVA-adjusted IE=0.10, (p>0.05)81 |
| Number of helpful discussion topics (0 to 5) | RCT | ANCOVA-adjusted IE=0.08 (p>0.05)81 |
| Discussing treatment choices (1 to 4) | RCT | Lower score in G1 than G2:b ANCOVA-adjusted IE=−0.19 (p<0.05)81 |
| Communicating with providers (0 to 100) | RCT | ANCOVA-adjusted IE=2.7 (p>0.05)81 |
| Getting answers to questions quickly (0 to 100) | RCT | ANCOVA-adjusted IE=−0.8 (p>0.05)81 |
| Multimorbidity Hassles score (0 to 24) | RCT | ANCOVA-adjusted IE=0.15 (p>0.05)81 |
| Percent receiving help setting goals | RCT | ANCOVA-adjusted IE=9.5 (p>0.05)81 |
| Percent receiving help making a care plan | RCT | ANCOVA-adjusted IE=4.0 (p>0.05)81 |
| Self-efficacy: Take all medications (1 to 5) | RCT | ANCOVA-adjusted IE=0.03 (p>0.05)81 |
| Self-efficacy: Plan meals and snacks (1 to 5) | RCT | ANCOVA-adjusted IE=−0.08 (p>0.05)81 |
| Self-efficacy: Exercise 2 or 3 times weekly (1 to 5) | RCT | ANCOVA-adjusted IE=0.14 (p>0.05)81 |
| Self-care activities: Prescribed medications taken (mean # of days) | RCT | ANCOVA-adjusted IE=−0.15 (p>0.05)81 |
| Self-care activities: Followed healthy eating plan (mean # of days) | RCT | ANCOVA-adjusted IE=−0.03 (p>0.05)81 |
| Self-care activities: 30 minutes of continuous physical activity (mean # of days) | RCT | ANCOVA-adjusted IE=−0.30 (p>0.05)81 |
- a
Lower scores in the PHQ-2 indicate fewer depressive symptoms.
- b
Lower scores indicate worse experience, satisfaction, or self-management.
ADL = activities of daily living; ANCOVA = analysis of covariance; CI = confidence interval; DiD = difference-in-difference; ESRD = end-stage renal disease; G = group; IE = intervention effect; MHC = mental health composite; OR = odds ratio; PHC = physical health composite; PHQ-2 = patient health questionnaire-2; RCT = randomized controlled trial.
Table B-30Clinical and functional outcomes for telephonic/mail model studies: Subgroup outcomes
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Progression to ESRD | RCT | Chronic kidney disease patients subgroup: Original sample difference: Greater in G1: 2.92 (95% CI, 0.30 to 5.54)82 |
| RCT | Chronic kidney disease patients subgroup: Refresh sample difference: 0.37 (95% CI, −2.53 to 3.28)82 | |
| Graft or fistula prior to hemodialysis | RCT | Chronic kidney disease patients subgroup: Original sample difference: Lower in G1: −3.09 (95% CI, −5.93 to −0.24)82 |
| RCT | Chronic kidney disease patients subgroup: Refresh sample difference: −2.05 (95% CI, −6.39 to 2.30)82 | |
| RCT | End-stage renal disease patients subgroup: Original sample difference: −6.08 (95% CI, −15.75 to 3.59)81 | |
| RCT | End-stage renal disease patients subgroup: Refresh sample difference: 2.87 (95% CI, −16.72 to 22.46)81 | |
| HbA1c test | RCT | Diabetes patients subgroup: Original sample DiD: OR=1.22 (95% CI, 0.73 to 2.03)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=0.76 (95% CI, 0.47 to 1.23)82 | |
| RCT | Diabetes patients subgroup: Original sample DiD: OR=1.02 (95% CI, 0.68 to 1.54)81 | |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=0.95 (95% CI, 0.56 to 1.61)81 | |
| LDL-C test | RCT | Diabetes patients subgroup: Original sample DiD: OR=1.05 (95% CI, 0.70 to 1.58)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=1.15 (95% CI, 0.80 to 1.67)82 | |
| RCT | Diabetes patients subgroup: Original sample DiD: OR=1.19 (95% CI, 0.84 to 1.68)81 | |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=0.72 (95% CI, 0.42 to 1.24)81 | |
| RCT | IVD patient subgroup: Original sample DiD: OR=0.98 (95% CI, 0.72 to 1.35)81 | |
| RCT | IVD patient subgroup: Refresh sample DiD: OR=0.88 (95% CI, 0.54 to 1.43)81 | |
| Eye exam | RCT | Diabetes patients subgroup: Original sample DiD: OR=1.05 (95% CI, 0.81 to 1.137)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=1.25 (95% CI, 0.94 to 1.66)82 | |
| Nephrology | RCT | Diabetes patients subgroup: Original sample DiD: OR=0.92 (95% CI, 0.71 to 1.18)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: Greater increase in G1 than G2: OR=1.30 (95% CI, 1.01 to 1.67)82 | |
| All 4 measuresa | RCT | Diabetes patients subgroup: Original sample DiD: OR=1.01 (95% CI, 0.80 to 1.29)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=1.18 (95% CI, 0.93 to 1.52)82 | |
| None of the measuresb | RCT | Diabetes patients subgroup: Original sample DiD: OR=1.74 (95% CI, 0.35 to 8.70)82 |
| RCT | Diabetes patients subgroup: Refresh sample DiD: OR=0.60 (95% CI, 0.16 to 2.33)82 | |
| Lipid panel | RCT | IVD patient subgroup: Original sample DiD: OR=1.10 (95% CI, 0.85 to 1.44)82 |
| RCT | IVD patient subgroup: Refresh sample DiD: greater increase in G1 than G2: OR=1.22 (95% CI, 0.17 to 0.92)82 |
- a
The “All 4 measures” is the rate at which beneficiaries receive all of the following four diabetes measures: rate of annual HbA1c testing, low-density lipoprotein cholesterol (LDL-C) screening, receipt of a retinal eye exam, and medical attention for nephropathy.
- b
The “None of the measures” is the rate at which beneficiaries did not receive any of the four diabetes measures.
CI = confidence interval; DiD = difference-in-difference; ESRD = end-stage renal disease; G = group; HbA1c = hemoglobin A1c; IVD = ischemic vascular disease; LDL-C = low-density lipoprotein cholesterol; OR = odds ratio; RCT = randomized controlled trial.
Table B-31Study characteristics for community-based models
| First Author, Year, Site(s) | Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
Finkelstein et al., 202096 Camden, NJ; 4 local-area hospital systems |
(N=800) Intervention (N=399) Comparison (N=401) | RCT (RoB: Low) | 1 or more IP admissions in past 6 months | 2 or more chronic conditions |
At least 2 of the following traits or conditions constituting medically and socially complex needs: use of 5 or more active outpatient medications, difficulty accessing services, lack of social support, a coexisting MH condition, an active drug habit, and/or homelessness; 18–80 years of age Exclusion criteria: uninsured, cognitively impaired, oncology patient, admitted for a surgical procedure for an acute health problem, for mental healthcare (with no comorbid physical health conditions), or for complications of a progressive chronic disease that has limited treatments |
≥65 years: 28% Nonwhite: 85% Medicare: 48% Medicaid: 45% Hypertension: 80.6%; CHF: 33.9%; diabetes: 53.1% Needs help with mobility: 62% Self-reported health: poor: 54% Depression: 30% Substance abuse: 44% |
|
Raven et al., 2020109 Santa Clara, CA: 1 site |
(N=423) Intervention (N=199) Comparison (N=224) | RCT (ROB: some concerns) | Various combinations of the ED and psychiatric ED, medical and psychiatric inpatient stays in the county-funded public hospital, and/or jail in past 1–2 years at high enough levels to meet a threshold score (threshold score NR) | NR | Met the Federal definition of chronic homelessness (homeless for 1+ years or 4+ episodes in the prior 3 years that last for more than a year total, with a disabling condition), lived in Santa Clara County, not incarcerated, not engaged in another intensive case management program or other permanent supportive housing program, did not require nursing home level care, and did not have metastatic cancer or qualify for hospice care |
Mean age: 51.5 Nonwhite: 35% Medicaid: 35% Medicare: 27% |
|
Bell et al., 2015112 King County, Washington: 1 site |
(N=1,120) Intervention (N=557) Comparison (N=563) | RCT (ROB: low) | Determined to be at risk for high future healthcare costs | Disabled with mental health and/or substance abuse problems | Medicaid beneficiaries |
Mean age: 51 Nonwhite: 43% Medicaid: 100% Medicare: NR Serious mental illness: 50% |
|
Aurora, CO; San Diego, CA; Allentown, PA; Kansas City, MO: 1 site per location |
(N=1,279) Intervention (N=149) Comparison (N=1,130) | Observational study (RoB: some concerns) |
Initial inclusion criteria: 2+ inpatient admissions in 6 months 2 of 4 sites amended criteria: 1 site expanded criteria to also include 3+ inpatient admissions in past 12 months, 1 site expanded criteria to 3+ hospital events (admissions or ED visits) in past 6 months | NA |
Medicare FFS beneficiaries Exclusion: patients whose conditions could not be managed with existing program resources |
Mean age: 59 years Black: 48.4% Hispanic: 8.5% Zip code poverty rate: 25.6% Dual coverage at enrollment: 69% Alzheimer’s: 8.5% Cancer: 5.3% CHF: 56.0% CKD: 63.0% COPD: 56.3% Diabetes: 71.1% |
|
Capp et al., 201788 Colorado: 1 site |
(N=3,802) Intervention (N=406) Comparison (N=3,396) | Observational study (RoB: some concerns) | 2+ ED visits or inpatient admissions in past 180 days | NA |
Age: 18+ years Exclusion criteria: pregnant, primary diagnosis of substance use disorder, active malignancy, or ESRD; have a caregiver or power of attorney who was making primary decisions; psychiatric hospitalization in the previous 180 days; undergone major surgery in the past month |
Nonwhite: 54% ≥56 years: 15% Medicaid or Colorado Indigent Care Program: 69% Medicare: 15% Self-pay: 12% CKD: 3% Congestive heart failure: 5% COPD: 7% Diabetes: 19% Hypertension: 38% Mental health comorbidity: 43% |
|
Weerahandi et al., 2015119 NYC: 1 hospital |
(N=1,158) Intervention (N=579) Comparison (N=579) | Observational study (RoB: some concerns) | At time of index hospitalization: 1 admission in past 30 days or 2 in past 6 months | NA | ≥18 years of age, excluded determined to be too medically acute to benefit from behavioral intervention |
Hypertension: 69% Diabetes without chronic complication: 25% Chronic pulmonary disease: 49% Congestive heart failure: 23% Diabetes w/chronic complication: 8% Depression: 10.5% Drug abuse: 6% Psychoses: 3% Alcohol abuse: 3% Nonwhite: 77% |
|
Shah et al., 2011107 Kern County, CA: 3 sites |
(N=258) Intervention (N=98) Comparison (N=160) | Observational study (RoB: High) | 4+ ED visit or IP admissions OR 3+ IP admissions OR 2+ IP admissions and 1 ED visit in past 1 year | NA | Age: 18–64 years, below 200% FPL, uninsured, not eligible for any public insurance programs |
Charlson comorbidity index: 1.14 nonwhite: 51% |
|
Dallas, TX: 1 study site |
(N=574) Intervention (N=265) Comparison (N=309) | Observational study (RoB: high) | Exceeded average ED visit rate of 1.5 ED visits in past 12 months | NA | Below 200% FPL, uninsured, not eligible for health insurance through local hospital system, not receiving Medicaid or Medicare | Nonwhite: 89.5% |
|
Thompson et al., 201895 Memphis, TN: 1 site |
(N=439) Intervention (N=159) Comparison (N=280) | Observational study (RoB: high) | 11+ hospital encounters originating in the ED in past 1 year | NA | Resident of Memphis, TN, in 38109 zip code |
Moderate or severe Charlson comorbidity index (>3): 13% Nonwhite: 97% None/missing PCP: 21% |
CA = California; CHF = congestive heart failure; CKD = chronic kidney disease; CO = Colorado; COPD = chronic obstructive pulmonary disease; ED = emergency department; ESRD = end-stage renal disease; FFS = fee-for-service; FPL = federal poverty line; IP = inpatient; MH = mental health; MO = Missouri; N = number; NA = not applicable; NJ = New Jersey; NR = not reported; NYC = New York City; PA = Pennsylvania; PCP = primary care provider; RCT = randomized controlled trial; RoB = risk of bias; TN = Tennessee; TX = Texas.
Table B-32Intervention characteristics for community-based models
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
Finkelstein et al., 202096 Camden, NJ; 4 local-area hospital systems | Camden Core Model: hotspotting | Median: 92 days; tailored to patient needs and responsiveness | Disease-specific self-care coaching, patient education | Scheduled and accompanied patients to initial post-IP primary and specialty care visits and followup care, medication reconciliation, medication management | Assistance applying for social services and BH programs | Multidisciplinary: RNs, social workers, LPNs, CHWs, and health coaches |
Face-to-face Enrollment in the hospital; subsequent service delivery through home visits, PCP, and specialty care | Received both a home visit within 14 days and provider visit within 60 days: 75% of participants; further contact tailored to individual | Usual post-discharge care that could include home visits or other outreach |
|
Raven et al., 2020109 Santa Clara, CA: 1 site | Project Welcome Home (PWH), a permanent supportive housing intervention with intensive case management services | Up to 4 years | Voluntary support services included mental health and substance use services; medication support, community living skills, educational and vocational support, and money management | Community-based case management services | Connected participants to temporary housing, permanent supportive housing, and rehousing | Master’s level social behavioral health providers, bachelor’s level case managers, and staff with lived experience (peers) | NR | NR | Usual care |
|
Bell et al., 2015112 King County, Washington: 1 site | Kings County Care Partners (KCCP) Program, a registered nurse-led care management intervention | Up to 24 months | Completed a comprehensive in-person assessment of their medical and social needs with their RN care manager and frequent in-person and phone monitoring | Care manager arranged to join the participant at 1+ clinic appointments, provided participants with chronic disease self-management coaching, and coordinated care across the medical and mental health systems | Care manager connected participants to community resources | Three full-time RNs, two social workers (MSWs) with drug/alcohol treatment training, and a bachelor’s-level chemical dependency counselor | Mixture of face-to-face, telephone, and letter | NR | Usual care |
|
Aurora, CO; San Diego, CA; Allentown, PA; Kansas City, MO: 1 site per location | Health Care Innovation Award: Rutgers Center for State Health Policy (CSHP) community-based care management/care coordination for high-risk patients, replication of Camden Coalition model | Mean: 4.2 months (site means ranged from 2.4 months to 6.3 months) |
Sites received technical assistance to implement the intervention Pt education about the importance of using primary and specialty care instead of, or as a followup to, emergency and hospital care and about managing medical and social needs | Developed individualized care plans, integrated care management services through mobile care teams | Assisted in enrollment in social service and BH service programs | Differing combination of RNs, NPs, social workers, CHWs, peer health coaches, medical assistants, and BH providers | Face-to-face, telephone calls to physicians or other service providers care teams met with participants in their homes or in other community locations (e.g., library) | Mean staff contacts=10.3 per participant per month, almost 6 hours per participant per month | Usual care |
|
Capp et al., 201788 Colorado: 1 site | Bridges to Care (B2C), an ED-initiated, multidisciplinary, community-based program that provides intensive medical, behavioral health, and social care coordination services following an ED visit or hospital discharge | 60 days | Depression screening, behavioral health screening, helping patient learn empowerment skills and using “teach back” opportunities to ensure the patient understands results and next steps | Patient services include coordinating primary and specialty care | Care plan and associated patient services can include assistance with obtaining housing resources, insurance or disability benefits, refugee services, and access to transportation, and filling prescriptions | Primary care provider, care coordinator, health coach, behavioral health evaluator, and community health worker | Face-to-face | First home visit occurs 24–72 hours post-enrollment date; second visit is conducted by PCP within 1 week of ED visit/discharge; third and fourth visits are conducted within 30 days of enrollment. Fifth and sixth visits depend on patient specific needs. Final two visits help patient transition out of program. | Usual care |
|
Weerahandi et al., 2015119 NYC: 1 hospital | Preventable Admissions Care Team (PACT) program: social work transition of care. | 35 days | Comprehensive psychosocial assessment during hospitalization | Facilitated communication with PCP and specialists; collaborated with caregivers; scheduled PCP appointment within 10 days of discharge | NR | MSWs with experience and training working with at-risk, vulnerable populations | Phone; face-to-face: home visits and while attending appointments | NR | Usual care: social worker assistance only during IP |
|
Shah et al., 2011107 Kern County, CA: 3 sites | Care Management Program (CMP) for low-income high utilizers of hospital services | NR | Assisted in goal creation/reaching goals | Care navigation (schedule appts, referral follow up, refill meds), care transitions (assistance in IP and discharge), and communication with providers (accompany to appointments and followup). | Arranged for social services (connect with agency staff, referrals) | Care managers with experience as case workers or medical office assistants | Face-to-face: at appoint-ments, patients’ homes, or resource centers | Met at least monthly | Usual care |
|
DeHaven et al., 2012128 Dallas, TX: 1 study site |
Project Access Dallas (PAD): community faith-health partnership to improve access to care and preventive services to the uninsured, care coordination Participants received $750 a year in pharmacy benefits and were eligible for laboratory tests, ancillary procedures, and IP hospital care. CCC pts: more complex and chronic problems, assigned a CHW Self-care pts: less serious illnesses, access to a telephone help line for medical care questions and CHW services on request | Up to 1 year | Intake interview and HRA to assign to CCC or self-care CCC pts: CHWs developed care coordination plan, taught health education and self-sufficiency | CCC pts: identified and addressed social concerns, identified patients with or at risk of developing type 2 diabetes, identified patients with depression, provided ED and referrals for cancer screening, provided care coordination and other support services (e.g., transportation, translation) | CCW link patients to other service organizations | HRA: community-clinic or hospital-based coordinator Services: CHWs, volunteer PCPs, and specialists |
Face-to-face for CCC pts; phone for self-care pts: in primary care and specialty care offices Volunteer PCPs and specialists met with CHWs monthly to discuss patients | CCC pts: at least monthly self-care: as needed | Usual care |
|
Thompson et al., 201895 Memphis, TN: 1 site | Familiar Faces Program Community navigators (blend of CHWs and patient navigators) to bridge gap between patients and the healthcare and social systems | Up to 1 year | Created a plan for patients’ health behaviors, tailored health information to client needs, and motivated them to make healthy choices | Helped to identify and eliminate barriers to health, coordinated care | Connecting patients to health and social resources in their community | Community navigators employed in the hospital system and received the training | Initial engagement was face-to-face at the ED/hospital. The mode of delivery of other services was not reported | NR | Usual care |
B2C = Bridges to Care; BH = behavioral health; CA = California; CCC = community care coordination; CCW = community care worker; CHW = community health worker; CMP = Care Management Program; CO = Colorado; CSHP = Rutgers Centers for State Health Policy; ED = emergency department; HRA = health risk appraisal; IP = inpatient; KCCP = King County Care Partners; LPN = licensed practical nurse; MO = Missouri; MSW = master of social worker; NJ = New Jersey; NP = nurse practitioner; NR = not reported; NYC = New York City; PA = Pennsylvania; PACT = Preventable Admissions Care Team; PAD = Project Access Dallas; PCP = primary care provider; Pt = patient; RN = registered nurse; TN = Tennessee; TX = Texas.
Table B-33Healthcare utilization outcomes for community-based model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| 180-day readmissions, count | RCT | NR | NR | aBGD=0.01 (95% CI, −0.25 to 0.27)96 |
| Observational | NR | NR | G1 vs. G2: p=0.32119 | |
| 180-day readmissions, any (%) | RCT | NR | NR | aBGD=0.82 (95% CI, −5.97 to 7.61)96 |
| 180-day readmissions, ≥2 (%) | RCT | NR | NR | aBGD=0.27 (95% CI, −6.22 to 6.77)96 |
| ED visits, all causea | RCT | NR | NR | IRR=0.85 (95% CI, 0.67 to 1.08) (p>0.05)109 |
| RCT | NR | NR | DiD=−0.3 (95% CI, −5.0 to 4.5) (p=0.91)112 | |
| Observational | NR | NR | DiD=0.057 (90% CI, −0.194 to 0.309) (p=0.704)85, 124 | |
| Observational | ⬇ | NR | Greater reduction in G1: RR=0.6748 (p<0.0001)107 | |
| Observational | NR | NR | Greater reduction in G1: NR (p<0.01)126, 128 | |
| Observational | NR | NR | Greater reduction in G1 than G2: DiD=−1.623 (p<0.01)88 | |
| ED visits at 270 days, all cause | Observational | NR | NR | Greater reduction in G1 than G2: DiD=−1.091 (p<0.01)88 |
| ED visits at 180 days, all cause | Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD=−1.005 (p<0.01)88 |
| ED visit, any | RCT | NR | NR | DiD=0.96 (95% CI, 0.67 to 1.38) (p=0.82)112 |
| Emergency psychiatric visits | RCT | NR | NR | Greater reduction in G1 than G2: IRR=0.62 (95% CI, 0.43 to 0.91) (p<0.05)109 |
| Inpatient admissions, all causea | RCT | NR | NR | IRR=0.97 (95% CI, 0.70 to 1.35) (p>0.05)109 |
| RCT | NR | NR | DiD=0.3 (95% CI, −1.2 to 1.9) (p=0.68)112 | |
| Observational | NR | NR | DiD: −0.116 (90% CI, −0.252 to 0.020)85, 124 | |
| Observational | ⇩ | NR | RR: 0.8070 (p=0.3771)107 | |
| Observational | NR | NR | Greater reduction in G1 than G2: DiD=−0.906 (p<0.01)88 | |
| Inpatient admissions at 270 days, all cause | Observational | NR | NR | Greater reduction in G1 than G2: DiD=−0.438 (p<0.01)88 |
| Inpatient admissions at 180 days, all cause | Observational | ⇩ | ⇩ | DiD=−0.159 (p<0.1)88 |
| Inpatient admissions, any | RCT | NR | NR | DiD=0.92 (95% CI, 0.66 to 1.27) (p=0.60)112 |
| Inpatient admissions, ACSC | Observational | NR | NR | DiD=−0.027 (90% CI, −0.081 to 0.028)85, 124 |
| Inpatient psychiatric stays | RCT | NR | NR | IRR=0.73 (95% CI, 0.36 to 1.45) (p>0.05)109 |
| Inpatient days | RCT | NR | NR | aBGD=−0.32 (95% CI, −2.17 to 1.53)96 |
| RCT | NR | NR | IRR=1.12 (95% CI, 0.79 to 1.59) (p>0.05)109 | |
| Observational | ⇩ | NR | RR: NR (p=NS)107 | |
| Observational | NR | NR | Greater reduction in G1: NR (p<0.05)126, 128 | |
| Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD: −8 (95% CI, −14 to −2)95 | |
| Total hospital encounters | Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD: −13 (95% CI, −19 to −6)95 |
| Hospital encounter resulted in discharge to hospital or observation stay | Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD: −12 (95% CI, −19 to −5)95 |
| Hospital encounter resulted in discharge from ED | Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD: −12 (95% CI, −19 to −4)95 |
| Outpatient visit | RCT | NR | NR | DiD=−0.11 (95% CI, −0.51 to 0.28) (p=0.58)112 |
| Outpatient substance use treatment visits | RCT | NR | NR | IRR=0.76 (95% CI, 0.46 to 1.24) (p>0.05)109 |
| Outpatient mental health visits | RCT | NR | NR | Greater increase in G1 than G2: IRR=1.84 (95% CI, 1.43 to 2.37) (p<0.01)109 |
| Outpatient mental health visit, any | RCT | NR | NR | Greater increase in G1 than G2: DiD=1.30 (95% CI, 1.07 to 1.58) (p<0.01)112 |
| Primary care visits at 360 days, all cause | Observational | NR | NR | Greater increase in G1 than G2: DiD=1.932 (p<0.01)88 |
| Primary care visits at 270 days, all cause | Observational | NR | NR | Greater increase in G1 than G2: DiD=1.517 (p<0.01)88 |
| Primary care visits at 180 days | Observational | ⬆ | ⬇ | Greater increase in G1 than G2: DiD=1.218 (p<0.01)88 |
| Prescription drugs, any | RCT | NR | NR | DiD=1.99 (95% CI, 0.29 to 13.70) (p=0.49)112 |
| Long-term care, any | RCT | NR | NR | DiD=1.09 (95% CI, 0.88 to 1.35) (p=0.42)112 |
- a
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
aBGD = adjusted between-group difference; ACSC = ambulatory care sensitive conditions; CI = confidence interval; DiD = difference-in-difference; ED = emergency department; G = group; IRR = incidence rate ratio; NR = not reported; NS = not statistically significant; RCT = randomized controlled trial; RR = relative risk.
Table B-34Healthcare utilization outcomes for community-based model studies: Subgroup outcomes
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| ED visits at 180 days, all cause | Observational | ⬇ | ⬇ | Patients with a mental health diagnosis subgroup: Greater reduction in G1 than G2: DiD=−1.377 (p<0.01)88 |
| Inpatient admissions at 180 days, all cause | Observational | ⬇ | ⬆ | Patients with a mental health diagnosis subgroup: Greater reduction in G1 than G2: DiD=−0.417 (p<0.01)88 |
| Primary care visits at 180 days | Observational | ⬆ | ⬇ | Patients with a mental health diagnosis subgroup: Greater increase in G1 than G2: DiD=1.404 (p<0.01)88 |
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
DiD = difference-in-difference; ED = emergency department; G = group.
Table B-35Strength of evidence for community-based models versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | 180-day readmissions |
Camden RCT: aBGD=0.01 (95% CI, −0.25 to 0.27);96 PACT: RR: NR (p=0.32)119 |
1 RCT, N=800 1 OBS, N=1,158 | Moderate study limitations, consistency unknown, imprecise, direct | Insufficient |
| HNHC patients | ED visits, all cause |
PWH: IRR=0.85 (95% CI, 0.67 to 1.08)109 KCCP: DiD=−0.3 (95% CI, −5.0 to 4.5)112 CSHP DiD: 0.057 (90% CI, −0.194 to 0.309) (p=0.704)85, 124 B2C: Greater reduction in G1: DiD=−1.623 (p<0.01)88 CMP: Greater reduction in G1: RR=0.6748 (p<0.0001)107 |
2 RCTs, N=1,543 4 OBSs, N=5,913 | High study limitations (two high RoB OBS studies),107, 126, 128 inconsistent, imprecise, direct | Insufficient (Mixed findings) |
| HNHC patients | Inpatient admissions, all cause |
CSHP DiD: −0.116 (90% CI, −0.252 to 0.020)85, 124 CMP: RR: 0.8070 (p=0.38)107 B2C: Greater reduction in G1: DiD=−0.906 (p<0.01)88 | 3 OBSs, N=5,339 | Moderate study limitations (one high RoB OBS studies),107 consistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient days |
Camden RCT: aBGD=−0.32 (95% CI, −2.17 to 1.53)96 PWH: IRR=1.12 (95% CI, 0.79 to 1.59)109 CMP: RR: NR (p=NS)107 PAD: Greater reduction in G1: NR (p<0.05)126, 128 Familiar Faces: Greater reduction in G1 than G2: DiD: −8% (95% CI, −14% to −2%)95 | 2 RCTs, N=1,223 3 OBSs, N=1,271 | High study limitations (three high RoB studies),95, 107, 126, 128 inconsistent, imprecise, direct | Insufficient (Mixed findings) |
| HNHC patients | Total costs |
KCCP: DiD=51 (95% CI, −242 to 344)112 CSHP DiD: −1405 (90% CI, −3509 to 700) (p=0.268);85, 124 Familiar Faces: DiD: −4903 (95% CI, −$13,579 to $3774)95 |
1 RCT, N=1,120 2 OBSs, N=1,718 | High study limitations (one high RoB OBS studies),95 inconsistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient costs |
KCCP: DiD=−12 (95% CI, −260 to 236)112 |
1 RCT, N=1,120 1 OBS, N=1,279 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | ED costs |
KCCP: DiD=−8 (95% CI, −26 to 10)112 |
1 RCT, N=1,120 1 OBS, N=574 | High study limitations (one high RoB OBS studies),126, 128, consistent, imprecise, direct | Insufficient |
| HNHC patients | Mortality |
Camden RCT: adjusted difference=−1.17 (95% CI, −5.25 to 2.91)96 PWH: Difference=3.9% (P NR)109 KCCP: aOR=0.65 (95% CI, 0.39 to 1.09)112 | 3 RCTs, N=2,343 | Moderate study limitations, consistent, imprecise, direct | Low (No difference) |
aBGD = adjusted between-group difference; aOR = adjusted odds ratio; B2C: Bridges to Care; CI = confidence interval; CMP = Care Management Program; CSHP = Rutgers Center for State Health Policy; DiD = difference-in-difference; ED = emergency department; G = group; HNHC = high-need, high-cost; IRR = incidence rate ratio; KCCP = King County Care Partners; N = number; NR = not reported; NS = not statistically significant; OBS = observational study; PAD = Project Access Dallas; PWH = Project Welcome Home; RCT = randomized controlled trial; RoB = risk of bias; RR = rate ratio; vs. = versus.
Table B-36Cost outcomes for community-based model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Hospital charges | RCT | NR | NR | aBGD=3,722 (95% CI, −23,438 to 30,882)96 |
| Hospital payments received | RCT | NR | NR | aBGD=680 (95% CI, −3,415 to 4,775)96 |
| Total costs | RCT | NR | NR | DiD=51 (95% CI, −242 to 344) (p=0.73)112 |
| Observational | NR | NR | DiD: −1,405 (90% CI, −3,509 to 700) (p=0.268)85, 124 | |
| Observational | ⇩ | ⇩ | DiD: −4,903 (95% CI, −$13,579 to $3774) 95 | |
| Inpatient costs | RCT | NR | NR | DiD=−12 (95% CI, −260 to 236) (p=0.92)112 |
| Observational | NR | NR | DiD: −120 (90% CI, −1,891 to 1,652) (p=0.911)85, 124 | |
| ED costs | RCT | NR | NR | DiD=−8 (95% CI, −26 to 10) (p=0.38)112 |
| Observational | NR | NR | Lower costs in G1:a NR (p=0.01)126, 128 | |
| Indirect ED costs | Observational | NR | NR | Lower costs in G1:b NR (p=0.03)126, 128 |
| Outpatient costs | RCT | NR | NR | DiD=31 (95% CI, −47 to 109) (p=0.43)112 |
| Prescription drug costs | RCT | NR | NR | Greater increase in G1 than G2: DiD=74 (95% CI, 3 to 145) (p=0.048)112 |
| Long-term care costs | RCT | NR | NR | DiD=36 (95% CI, −35 to 107) (p=0.33)112 |
- a
Direct ED costs include costs associated with the delivery of care during an ED visit or inpatient admission in the 12 months following enrollment.
- b
Indirect ED costs are the fixed costs related to building, maintenance, staffing, and utilities in the 12 months following enrollment.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
aBGD = adjusted between-group difference; CI = confidence interval; DiD = difference-in-difference; ED = emergency department; G = group; NR = not reported; RCT = randomized controlled trial.
Table B-37Clinical and functional outcomes for community-based model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality | RCT | Difference=−1.17 (95% CI, −5.25 to 2.91)96 |
| RCT | G1: 18.6% vs. G2: 14.7%, p=NR109 | |
| RCT | aOR=0.65 (95% CI, 0.39 to 1.09) (p=0.10)112 |
aOR = adjusted odds ratio; CI = confidence interval; G = group; NR = not reported; RCT = randomized controlled trial.
Table B-38Social risk outcomes for community-based model studies
| Social Risk Outcomes | Study Design | Difference |
|---|---|---|
| Participation in supplemental nutrition assistance program (%) | RCT | AD=4.59 (95% CI, 0.52 to 8.65)96 |
| Receipt of temporary assistance for needy families (%) | RCT | AD=0.69 (95% CI, −0.34 to 1.71)96 |
| Receipt of general assistance (%) | RCT | AD=0.68 (95% CI, −1.82 to 3.18)96 |
| Ever housed | RCT | Greater increase in G1 than G2: IRR=1.84 (95% CI, 1.43 to 2.37) (p<0.01)109 |
| Any homeless months | RCT | DiD=0.83 (95% CI, 0.60 to 1.17) (p=0.29)112 |
| Homeless months (mean per 1,000 months) | RCT | DiD=−1.5 (95% CI, −4.3 to 1.3) (p=0.29)112 |
| Jail stays | RCT | IRR=1.01 (95% CI, 0.73 to 1.40) (p>0.05)109 |
| Any criminal convictions | RCT | DiD=1.95 (95% CI, 1.10 to 3.44) (p=0.02)112 |
| Criminal convictions (mean per 1,000 months) | RCT | DiD=8.9 (95% CI, −1.5 to 19.3) (p=0.09)112 |
| Shelter days | RCT | Greater reduction in G1 than G2: IRR=0.30 (95% CI, 0.17 to 0.53) (p<0.01)109 |
| Any drug/alcohol treatment | RCT | DiD=0.92 (95% CI, 0.65 to 1.30) (p=0.62)112 |
AD = adjusted difference; CI = confidence interval; DiD = difference-in-difference; G = group; IRR = incidence rate ratio; RCT = randomized controlled trial.
Table B-39Study characteristics of ED-based care model interventions
| First Author, Year, Site(s) | Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
Kelley et al., 2020110 New Haven, CT: 1 site |
(N=100) Intervention (N=49) Comparison (N=51) | RCT (ROB: low) | 4–18 ED visits in past year | NA | 18–62 years, active Medicaid insurance, resident of one of 12 surrounding towns, and English or Spanish speaking |
Mean age: 40 Nonwhite: 82% Medicaid: 100% Medicare: NR |
|
Lin et al., 2017111 Boston, MA: 1 site |
(N=72) Intervention (N=36) Comparison (N=36) | RCT (ROB: some concerns) | Most ED visits during the 30-day period and 12-month period preceding the introduction of the program | NA | NA |
Mean age: 48 Nonwhite: 57% Medicaid: 44% Medicare: 43% |
|
Shumway et al., 2008116 San Francisco, CA: 1 hospital |
(N=252) Intervention (N=167) Comparison (N=85) | RCT (RoB: some concerns) | 5+ ED visits in past 1 year | NA | Age: 18+; psychosocial problems that could be addressed with case management |
Nonwhite: 87%; Homeless: 81% Alcohol problems, alcohol use: 57% Lack health insurance: 67% Charlson Comorbidity Index (Mean [SD]): 1.4 (2.2) Most common diagnosis in prior 1 year: mental disorders, 22% |
|
Seaberg et al., 2017118 Chattanooga, TN metro area: 5 sites |
(N=304) Intervention (N=163) Comparison (N=141) | RCT (RoB: some concerns) | 5+ ED visits in past 1 year | NA | NA | NA |
|
Enard and Ganelin, 2013127 Houston, TX: 1 site |
(N=13,642) Intervention (N=1,905) Comparison (N=11,737) | Observational study (RoB: high) | Frequent use of the ED for primary care, data included visits to any of the 9 EDs in the healthcare system, receiving urgent or primary care in the ED (levels 3, 4, and 5 medical decisions of minimal to moderate complexity but may be considered PCP patients) in past 1 year | NA | Age: 18–64; on Medicaid, uninsured/self-pay, or covered by TX public health benefit |
Nonwhite: 82% Age 18–34: 58.6% Uninsured: 62.7% |
|
McCormack et al., 2013129 New York, NY: 1 site |
(N=60) Intervention (N=20) Prospective controls (N=20) Retrospective controls (N=20) | Observational study (RoB: high) | 5+ ED visits annually for 2 consecutive years and 1 within 6 months | Alcohol dependence | Undomiciled without shelter use for 9 of 24 months |
Mean age: 50.0 +/- 10.0 years Nonwhite: NR Medicaid: NR Medicare: NR |
|
Navratil-Strawn et al., 2014120 National |
(N=14,140) Intervention (N=7,070) Comparison (N=7,070) | Observational study (RoB: some concerns) | 3+ ED visits in past 1 year | NA | Age: 65+; UnitedHealth-Care’s AARP Medigap insurance | Live in area with medium/high Minority status: 38% |
CA = California; CT = Connecticut; ED = emergency department; MA = Massachusetts; N = number; NA = not applicable; NR = not reported; NY = New York; PCP = primary care provider; RCT = randomized controlled trial; RoB = risk of bias; SD = standard deviation; TN = Tennessee; TX = Texas.
Table B-40Intervention characteristics of ED-based care model interventions
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
Kelley et al., 2020110 New Haven, CT: 1 site | Project Access-New Haven: ED-initiated patient navigation program (ED-PN) | 12 months | Completed a detailed questionnaire with the patient (via interview) about demographics, socioeconomic status, health literacy, medical conditions, clinical and social needs, health-related quality of life, utilization of healthcare services, access/barriers to care, and reasons for ED use. Open-ended questions were also asked to elucidate ways in which the patient could most benefit from patient navigator services | Patient navigators connected patients to primary care by meeting face-to-face with patients, offering accompaniment to PCP visit(s), phoning each patient regularly to remind them of medical appointments, and problem-solving to overcome personal barriers such as transportation. Patient navigator and patient created a task list based on the provider’s recommendations including assisting in scheduling any additional appointments recommended by PCP | Connected patients to local resources to address social needs such as precarious housing, food insecurity, or insurance questions | Nurse navigator and a trained patient navigator | Face-to-face or over the phone | Patient navigators scheduled regular phone calls to each patient every 2 weeks during weeks 0–4 and every 4 weeks during weeks 13–52 | Usual care |
|
Lin et al., 2017111 Boston, MA: 1 site | Pilot ED-based care coordination and community health worker program | 7 months | ED physicians performed a detailed chart review for all patients to identify unmet medical and social issues driving frequent ED visits and CHWs reviewed each patient’s chart and called patients to conduct a standardized intake assessment to determine unmet needs | Acute care plans developed in conjunction with the patient’s longitudinal providers including PCPs and uploaded to EHR to be visible to all clinicians and assignment of an ED-based CHW who assisted with care coordination and other tailored needs (e.g., connect with primary care, provide transportation) | CHW addressed unmet social and behavioral needs that contributed to ED utilization (e.g., food banks) | ED physician and physician assistant, ED community health worker, and nurse care coordinator | Face-to-face or phone including home visits | NR | Usual care |
|
Shumway et al., 2008116 San Francisco, CA: 1 hospital | Case management | 24 months | Psychosocial problems and functioning were assessed at study entry and at 6, 12, 18, and 24 months. Psychosocial assessment included homelessness, problem alcohol use, lack of health insurance, lack of Social Security income, unmet basic financial needs, and psychiatric symptoms. | Provided linkage to medical care providers and ongoing assertive community outreach to maintain continuity of care | Assistance in obtaining stable housing, income entitlements, and referral to substance abuse services when needed | Master’s-level psychiatric social workers provided most case management services in collaboration with a nurse practitioner, a primary care physician, and a psychiatrist | Face-to-face individual and group sessions | NR | Usual care |
|
Seaberg et al., 2017118 Chattanooga, TN metro area: 5 sites | Patient navigation for ED patients | 12 months | Reviewed diagnoses and prescriptions | NR | Arranged followup appointments and identified relevant community resources | Patient navigator with hospital case management training | In person and via telephone | Initial ED visit, subsequent ED visits, followup calls within 2 weeks and 12 months of initial ED visit | Usual care |
|
Enard and Ganelin, 2013127 Houston, TX: 1 site | ED-based patient navigation program | ≤10 days | Initial assessment to determine barriers to appropriate primary care use. Educate patients on importance of making and keeping appointments and receiving preventive health care. Help patients identify barriers to primary care and identify local, state, and federal resources appropriate for their needs | Patient navigators maintain relationships with community-based providers to bolster referral relationships ensure that contact information is current | Connect patients with neighborhood providers or clinics and assess their needs for specific types of referrals based on access issues and health conditions. | CHWs as patient navigators | In person and via telephone | Initial ED visit and a followup call 3 to 10 days later | Usual care |
|
McCormack et al., 2013129 New York, NY: 1 site | Coordinated case management and facilitated access to homeless outreach services | 6 months | Social workers approached patients and, if authorized, faxed intake and medical release forms to the outreach team. On a subsequent visit, the outreach team came to the ED to confirm eligibility and complete enrollment | Social worker and outreach team met with participants to relocate patients into supportive settings, coordinate multidisciplinary care, and update plans based on participants’ medical, psychosocial, and housing needs | Care plans to offer shelter on discharge | Social worker and outreach team | Face-to-face | Frequency based on number of visits to the ED | Usual care |
|
Navratil-Strawn et al., 2014120 National | Emergency Room Decision-Support (ERDS) program | NR; up to 12 months (followup time) | Assessment of health needs and treatment options | Make appointments with providers, refer to care coordination programs | Provide connections with health resources | Nurse | Telephone | NR | Usual care |
CA = California; CHW = community health worker; ED = emergency department; ED-PN = ED-initiated Patient Navigation; EHR = electronic health record; ERDS = Emergency Room Decision-Support; MA = Massachusetts; NR = not reported; NY = New York; PCP = primary care provider; TN = Tennessee; TX = Texas.
Table B-41Healthcare utilization outcomes for ED-based care model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| ED visits | RCT | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD=−1.37 (95% CI, −2.40 to −0.34) (p=0.01)110 |
| RCT | NR | NR | Between group difference (G1–G2): −35% (p=0.10)111 | |
| RCT | ⬇ | ⬇ | Lower use in G1 than G2a (p<0.01)116 | |
| RCT | ⬇ | ⬇ | Greater reduction in G1 than G2 (p<0.0001)118 | |
| Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD=−178 (p=0.033)120 | |
| Observational | NR | NR |
Greater reduction in G1 than G2: DiD (prospective controls): −12.1 (95% CI, −22.1 to −2.0) (p=0.02) DiD (retrospective controls): −12.8 (95% CI, −26.1 to 0.6) (p=0.06)129 | |
| ED visits, any | Observational | ⬇ | ⬇ |
Lower use in G1 than G2 among patients with PCR-ED visits in prior 24 months ≥1: OR=0.55 (95% CI, 0.47 to 0.63); ≥2: OR=0.46 (95% CI, 0.37 to 0.57); ≥3: OR=0.32 (95% CI, 0.24 to 0.44); ≥4: OR=0.29 (95% CI, 0.19 to 0.44); or ≥5: OR=0.31 (95% CI, 0.17 to 0.54)127 |
| Observational | ⬇ | ⬇ |
G1 vs. G2 among patients with PCR-ED visits in prior 12 months ≥1: OR=0.83 (95% CI, 0.71 to 0.98); ≥2: OR=0.72 (95% CI, 0.57 to 0.93); ≥3: OR=0.90 (95% CI, 0.60 to 1.3); ≥4: OR=0.98 (95% CI, 0.52 to 1.8); or ≥5: OR=0.96 (95% CI, 0.39 to 2.3)127 | |
| Inpatient admissions, all cause | RCT | ⬇ | ⬆ | Greater reduction in G1 than G2: DiD=−0.97 (95% CI, −1.56 to −0.38) (p=0.001)110 |
| Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: DiD=−53 (p=0.002)120 | |
| Inpatient days | Observational | NR | NR |
DiD (prospective controls): −8.5 (95% CI, −22.8 to 5.8) (p=0.24) DiD (retrospective controls): −19.0 (95% CI, −34.3 to −3.6) (p=0.06)129 |
| Medical inpatient admissions | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Medical inpatient days | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Psychiatric emergency visits | RCT | ⇩ | -- | G1 vs. G2a (p=NS)116 |
| Psychiatric inpatient admissions | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Psychiatric inpatient days | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Outpatient visits | RCT | ⇧ | ⇧ | DiD: 0.60 (95% CI, −0.43 to 1.63) (p=0.25)110 |
| RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 | |
| Primary care visits | RCT | ⬆ | ⬇ | Greater use in G1 than G2 (p=0.001)118 |
| Observational | ⬇ | ⬇ | Smaller reduction in G1 than G2: DiD=897 (p<0.001)120 |
- a
Interaction between level of prior ED use (5 to 11 or ≥12 visits in prior 12 months) and group: p=NS.
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
CI = confidence interval; DiD = difference-in differences; ED = emergency department; G = group; NR = not reported; NS = not statistically significant; OR = odds ratio; PCR-ED = primary care-related emergency department; RCT = randomized controlled trial; vs. = versus.
Table B-42Strength of evidence for ED-based care models versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | ED visits |
ED-PN RCT: greater reduction in G1 than G2: DiD=−1.37 (95% CI, −2.40 to −0.34)110 CHW RCT: between-group difference: −35% (p=0.10)111 Case management RCT: lower use in G1 than G2 (p<0.01);116 Navigation RCT: greater reduction in G1 than G2 (p<0.0001);118 ERDS: greater reduction in G1 than G2: DiD=−178 (p=0.033)120 ED and housing: greater reduction in G1 than G2: DiD (prospective controls): −12.1 (95% CI, −22.1 to −2.0); DiD (retrospective controls): −12.8 (95% CI, −26.1 to 0.6)129 |
4 RCTs, N=728 2 OBSs, N=14,200 | Moderate study limitations (2 high RoB OBS studies),129 consistent, precise, direct | Moderate (Favorable) |
| HNHC patients | Inpatient admissions, all cause |
ED-PN RCT: greater reduction in G1 than G2: DiD=−0.97 (95% CI, −1.56 to −0.38) (p=0.001)110 ERDS: greater reduction in G1 than G2: DiD=−53 (p=0.002)120 |
1 RCT, N=100 1 OBS, N=14,140 | Moderate study limitations, consistent, precise, direct | Low (Favorable) |
| HNHC patients | Primary care visits |
Navigation RCT: greater use in G1 than G2 (p=0.001)118 ERDS: smaller reduction in G1 than G2: DiD=897 (p<0.001)120 |
1 RCT, N=304 1 OBS, N=14,140 | Moderate study limitations, consistent, precise, direct | Low (Favorable) |
| HNHC patients | Outpatient visits |
ED-PN RCT: DiD: 0.60 (95% CI, −0.43 to 1.63) (p=0.25)110 Case management RCT: G1 vs. G2 (p=NS)116 | 2 RCTs, N=352 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient (Mixed findings) |
| HNHC patients | ED costs |
CHW RCT: between-group difference=−15% (p=0.20)111 Case management RCT: lower in G1 than G2a (p<0.01)116 Navigation RCT: greater reduction in G1 than G2 (p<0.0001)118 ERDS: DiD=−21 (p=0.140)120 |
3 RCTs, N=628 1 OBS, N=14,140 | Moderate study limitations; consistent, precise, direct | Low (Favorable) |
| HNHC patients | Inpatient costs |
CHW RCT: between-group difference=−8% (p=0.10)111 Case management RCT: G1 vs. G2 (p=NS)116 ERDS: DiD=−59 (p=0.080)120 |
2 RCTs, N=324 1 OBS, N=14,140 | Moderate study limitations, inconsistent, imprecise, direct | Low (No difference) |
| HNHC patients | Hospital costs of care |
ED-PN RCT: DiD=−10,202 (95% CI, −22,464 to 2,062)110 Case management RCT: G1 vs. G2 (p=NS)116 | 2 RCTs, N=352 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients | Outpatient costs |
Case management RCT: G1 vs. G2 (p=NS)116 ERDS: DiD=10 (p=0.828)120 |
1 RCT, N=252 1 OBS, N=14,140 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
CHW = community health worker; CI = confidence interval: DiD = difference-in differences; ED = emergency department; ED-PN = ED-initiated Patient Navigation; ERDS = Emergency Room Decision-Support; G = group; HNHC = high-need, high-cost; N = number; NS = not significant; OBS = observational study;; RCT = randomized controlled trial; RoB = risk of bias; vs. = versus.
Table B-43Cost outcomes for ED-based care model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| ED costs | RCT | NR | NR | Between-group difference (G1–G2) =−15% (p=0.20)111 |
| RCT | ⬇ | ⬇ | Lower in G1 than G2a (p<0.01)116 | |
| RCT | ⬇ | ⬇ | Greater reduction in G1 than G2 (p<0.0001)118 | |
| Observational | ⇩ | ⇩ | DiD=−21 (p=0.140)120 | |
| Inpatient costs | RCT | ⇩ | ⇩ | G1 vs. G2a,b (p=NS)116 |
| RCT | NR | NR | Between group difference (G1–G2) =−8% (p=0.10)111 | |
| Observational | ⇩ | ⇩ | DiD=−59 (p=0.080)120 | |
| Medicaid cost (payment) | RCT | ⇩ | ⇧ | DiD=−5,765 (95% CI, −15,883 to 4,353) (p=0.26)110 |
| Psychiatric emergency costs | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Psychiatric hospital costs | RCT | ⇩ | ⇧ | G1 vs. G2a (p=NS)116 |
| Hospital costs of care | RCT | ⇩ | ⇧ | DiD=−10,202 (95% CI, −22,464 to 2,062) (p=0.10)110 |
| RCT | ⇩ | ⇩ | G1 vs. G2a,c (p=NS)116 | |
| All non-ED case management costs | RCT | ⇩ | ⇩ | G1 vs. G2a (p=NS)116 |
| Total costs ($) | Observational | ⇩ | ⇩ | DiD=−40 (p=0.502)120 |
| Outpatient costs | RCT | ⇩ | -- | G1 vs. G2a,b (p=NS)116 |
| Observational | ⇩ | ⇩ | DiD=10 (p=0.828)120 | |
| Prescription costs ($) | Observational | ⇧ | ⇧ | DiD=9 (p=0.201)120 |
- a
Interaction between level of prior ED use (5 to 11 or ≥12 visits in prior 12 months) and group: p=NS.
- b
Shumway et al. specified the outpatient and inpatient costs as medical outpatient costs and medical hospital costs.116
- c
Shumway et al. included the costs for the ED case management intervention in all hospital costs.116
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
CI = confidence interval; DiD = difference-in differences; ED = emergency department; G = group; NR = not reported; NS = not statistically significant; RCT = randomized controlled trial; vs. = versus.
Table B-44Clinical and functional outcomes for ED-based care model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality | RCT | G1: 0/20 vs. prospective G2: 2/20 and retrospective G2: 7/20 (p=NR)129 |
| Satisfaction (4-point Likert scale) | RCT | G1 vs. G2 (p=NS)118 |
| Psychiatric symptoms (total BSI) | RCT | G1 vs. G2a (p=NS)116 |
- a
Interaction between level of prior ED use (5 to 11 or ≥12 visits in prior 12 months) and group: p=NS.
BSI = brief symptom inventory; ED = emergency department; G = group; NR = not reported; NS = not statistically significant; RCT = randomized controlled trial; vs. = versus.
Table B-45Social risk outcomes for ED-based care model studies
| Social Risk Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Housed | RCT | G1: 18/20 vs. prospective G2: 0/20 and retrospective G2: 0/20 (p=NR)129 |
| Problem alcohol use (%) | RCT | Lower in G1 than G2a (p=0.04)116 |
| Homelessness (%) | RCT | Lower in G1 than G2a (p<0.01)116 |
| No health insurance (%) | RCT | Lower in G1 than G2a (p=0.02)116 |
| No social security income (%) | RCT | Lower in G1 than G2a (p<0.01)116 |
| Basic financial needs unmet | RCT | Lower in G1 than G2a (p=0.04)116 |
ED = emergency department; G = group; NR = not reported; RCT = randomized controlled trial; vs. = versus.
Table B-46Study characteristics of aICU (ambulatory intensive caring unit) interventions
| First Author, Year, Site(s) | Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other |
|---|---|---|---|---|---|
|
Yoon et al., 201987; Zalman, 201997 GA, OH, WI, NC, CA: 5 sites |
(N=2,210) Intervention (N=1,105) Comparison (N=1,105) (N=1,527) Intervention (N=759) Comparison (N=768) | RCT (RoB: some concerns) | Veterans whose risk of 90-day hospitalization was ≥ 90th percentile based on the VA’s Care Assessment Need (CAN) score and who had experienced a hospitalization or ED visit in past 6 months | NA | Intensive management teams at each site reviewed patient charts to determine whether participants would benefit from intensive services |
|
Durfee et al., 201883 CO: 1 site |
(N=3,636) Intervention (N=1,749) Comparison (N=1,887) | Observational study (RoB: some concerns) | IP admission (analysis index admission) and at least 2 other admissions OR at least one other admission with a serious mental health diagnosis in past 1 year | NA | Age: 19+ |
|
Horn et al., 201692 Albuquerque, NM: 1 academic medical center |
(N=1547) Intervention (N=753) Comparison (N=794) | Observational study (RoB: some concerns) | High cost (top 1%) in past 1 year | Medically complex patients with a chronic medical condition and at high risk for future hospitalization | Participant selection guided by the likelihood of recurrent illness and response to care management, and patient willingness to participate in the program and be monitored and contacted |
aICU = ambulatory intensive caring unit; CA = California; CAN = care assessment need; CO = Colorado; ED = emergency department; GA = Georgia; IP = inpatient; N = number; NA = not applicable; NC = North Carolina; NM = New Mexico; OH = Ohio; RCT = randomized controlled trial; RoB = risk of bias; VA = Department of Veterans Affairs; WI = Wisconsin.
Table B-47Intervention characteristics of aICU (ambulatory intensive caring unit) interventions
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
GA, OH, WI, NC, CA: 5 sites | Augmenting the VA’s PCMH PACT with a PACT-PIM intervention for highest cost patients; locally tailored by site | Up to 15 months | Initial assessment: record review, followed by comprehensive in-person assessment for medical, MH, and social needs; goals assessment; health coaching for patient and caregivers, pharmacist medication reconciliation and adherence monitoring | Care coordination; transitional care management post IP discharge; feedback to PCP, assistance with navigating healthcare services | NR: VA provides many support services in-house | Interdisciplinary team: physician or nurse practitioner, a nurse, pharmacist, rehabilitation therapists, MH and addiction support | Outpatient, home, and phone | Limited: 1–2 encounters or referral to PCP; Full: goal was 3+ encounters in person or by phone from PIM team; received by 44% of participants. Tailored to individual needs. Full-intervention participants received mean of 14 encounters (range: 3–116) | Patient Aligned Care Team (PACT) only |
|
Durfee et al., 201883 CO: 1 sites | IOC in integrated delivery system | NR | In-depth intake assessment included determining medical barriers to improving health, taking into account BH needs, social determinants of health, and patient-identified priorities | Sought to develop comprehensive care plans. More nursing support allowed for medical interventions to be done within the clinic instead of the ED or hospital | NR | Eight existing family practice and internal medicine teams composed of a PCP, medical assistant, and shared nursing and social work resources. Supported by clinical pharmacists, patient navigators, BH clinicians | Additional staffing to existing PCPs and developed IOCs (new, specialized primary care clinics). Face-to-face with care team; navigators and pharmacists primarily by phone | IOC had higher staff-patient ratio than regular PCP clinic, longer visits, walk-in availability. Mean number of encounters per patient: NR | Usual care: historic comparison group who received care prior to implementing the IOC |
|
Horn et al., 201692 Albuquerque, NM: 1 academic medical center | Care One, an intensive chronic care, primary care-oriented program designed to target HNHC patients | NR | Assessment of whether amenable to care management based on interview and medical record review | Provided access to specialty care consultations, assistance from nurse coordinators and social workers, and assistance with unanticipated problems related to access or quality | Assistance with social services, such as transportation to clinical appointments, food stamp applications, etc. | A physician, social worker/case manager, patient care coordinator, and MH therapist. A pharmacist assists with medication management for patients with complex comorbidities | Primary care | NR | Usual care |
aICU = ambulatory intensive caring unit; BH = behavioral health; CA = California; CO = Colorado; ED = emergency department; GA = Georgia; HNHC = high-need, high-cost; IOC = intensive outpatient clinic; IP = inpatient; MH = mental health; NC = North Carolina; NM = New Mexico; NR = not reported; OH = Ohio; PACT = patient aligned care team; PCMH = Patient-Centered Medical Homes; PCP = primary care provider; PIM = PACT-intensive management; VA = Department of Veterans Affairs; WI = Wisconsin.
Table B-48Healthcare utilization outcomes for aICU (ambulatory intensive caring unit) model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Acute medical/surgery inpatient stays | RCT | NR | NR | DiD: 1.13 (95% CI, 0.92 to 1.38)87 |
| Other inpatient staysa | RCT | NR | NR | DiD: 1.04 (95% CI, 0.66 to 1.65)87 |
| Inpatient admissions | Observational | ⬇ | ⬇ | DiD: lower reduction in G1 than G2 (negative finding) (p<0.01)83 |
| ED visits | RCT | NR | NR | DiD: 1.02 (95% CI, 0.93 to 1.13)87 |
| Primary care visits | RCT | NR | NR | Greater use in G1: DiD: 1.40 (95% CI, 1.30 to 1.50)87 |
| Care management visits | RCT | NR | NR | Greater use in G1: DiD: 2.70 (95% CI, 1.77 to 4.12)87 |
| Specialty care visits | RCT | NR | NR | DiD: 1.03 (95% CI, 0.96 to 1.10)87 |
| Mental healthcare visits | RCT | NR | NR | Greater use in G1: DiD: 1.33 (95% CI, 1.17 to 1.52)87 |
| Homeless care visits | RCT | NR | NR | DiD: 1.11 (95% CI, 0.86 to 1.44)87 |
- a
Other inpatient stays included psychiatric, substance use disorders, and rehabilitation stays.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
Table B-49Cost outcomes for aICU (ambulatory intensive caring unit) model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Outpatient costs | RCT | ⬆ | ⬇ | DiD: greater increase in G1 than G2: 2636 (95% CI, 524 to 4748)87 |
| Inpatient costs | RCT | ⇩ | ⇧ | DiD: −2,164 (95% CI, −7,916 to 3,587)87 |
| ED costs | RCT | ⇩ | ⇩ | DiD:−20 (95% CI,−277 to 237)87 |
| Total costs/charges | RCT | ⇩ | ⇩ | DiD: 471 (95% CI,−6,347 to 7,290)87 |
| Observational | ⬇ | ⬇ | DiD: greater reduction in G1 than G2 (p<0.04)83 | |
| Observational | ⬇ | ⬇ | DiD: greater reduction in G1 than G2: −$44,504 (p<0.01)92 |
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
CI = confidence interval; DiD = difference-in-difference; ED = emergency department; G = group; RCT = randomized controlled trial.
Table B-50Strength of evidence for aICU models versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | Total costs |
PIM: DiD: 471 (95% CI,−6,347 to 7,290)87 IOC: DiD: greater reduction in G1 than G2 (p<0.04);83 Care One DiD: −$44,504 (p<0.01)92 |
1 RCT, N=2,210 2 OBSs, N=5,183 | Moderate study limitations, consistent, precise, direct | Low (Favorable) |
| HNHC patients | Mortality 1-year post-randomization |
PIM: (p=0.93)87 IOC: Lower in G1 than G2: (p<0.01)83 |
1 RCT, N=2,210 1 OBS, N=3,636 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
aICU = ambulatory intensive caring unit; CI = confidence interval; DiD = difference-in- difference; G = group; HNHC = high need, high cost; IOC: Intensive Outpatient Clinic; N = number; OBS = observational study; PIM: Patient Aligned Care Team (PACT)-Intensive Management; RCT = randomized controlled trial; vs. = versus.
Appendix Table B-51Clinical and functional outcomes for aICU (ambulatory intensive caring unit) model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality, 1-year post-randomization | RCT | (p=0.93)87 |
| Observational | Lower in G1 than G2: (p<0.01)83 | |
| Patient-centered care coordination | RCT | AOR for all 6 measured dimensions past 6 monthsa (p=NS)97 |
| Access to care | RCT | AOR for all 3 measured dimensionsb (p=NS)97 |
| Patient satisfaction with care | RCT | AOR for all 4 measured dimensionsc (p=NS)97 |
| Relationships with Providers: trusted provider | RCT | Greater in G1 than G2: AOR=1.30 (95% CI, 1.04 to 1.62)97 |
| Relationships with Providers: feel respected by provider | RCT | (p=NS)97 |
| Healthcare hassles summary score (challenges in getting care) | RCT | (p=0.61)97 |
| Patient assessment of chronic illness care (PACIC) summary score (receipt of care for chronic illness) | RCT | Greater in G1 than G2: (p=0.022)97 |
- a
Goals assessed: barriers, medications reviewed, between-visit reminders, primary care informed about specialty care, VA healthcare provider helps coordinate care from different doctors and services, someone talked to them about their health goals, report 10 out of 10 satisfaction with primary care, patient assessment of chronic illness care based on PACIC scale, not significant for medications reviewed in past 6 months, between-visit reminders in past 6 months, primary care informed about specialty care in past 6 months.
- b
Dimensions included access to needed services, access to provider when questions about care arise, and received needed services.
- c
Dimensions included satisfaction with overall care at VA facility, social services, mental healthcare services, and primary care services.
AOR = adjusted odds ratio; CI = confidence interval; G = group; NS = not statistically significant; PACIC = patient assessment of chronic illness care; RCT = randomized controlled trial; VA = Department of Veterans Affairs.
Table B-52Study characteristics of primary care-based interventions
| First Author, Year, Site(s) | Type of Intervention; Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
McCall et al., 201086 5 MA counties (Norfolk, Suffolk, Middlesex, Essex, and Plymouth) |
Primary care model includes home visits pay-for-performance APM (Original N=5,374) Intervention (N=2,619) Comparison (N=2,755) | RCT (RoB: some concerns) | Beneficiaries with HCC risk scores ≥2.0 and annual costs of ≥$2,000 or HCC risk scores ≥3.0 and ≥$1,000 annual medical costs in past 1 year | HCC risk scores ≥2.0 | Medicare FFS beneficiaries; at least 2 visits to MA General Hospital physicians for a selected group of outpatient and ED procedures |
Nonwhite: 13.4% Medicaid: 5.3% |
|
McCall et al., 201086 5 MA counties (Norfolk, Suffolk, Middlesex, Essex, and Plymouth) |
Primary care model includes home visits pay-for-performance APM (Refresh N=1,569) Intervention (N=785) Comparison (N=784) | RCT (RoB: some concerns) | Beneficiaries with HCC risk scores ≥2.0 and annual costs of ≥$2,000 or HCC risk scores ≥3.0 and ≥$1,000 annual medical costs in past 1 year | HCC risk scores ≥2.0 | Medicare FFS beneficiaries; at least 2 visits to MA General Hospital physicians for a selected group of outpatient and ED procedures |
Nonwhite: 12.8% Medicaid: 5.4% |
|
Sledge et al., 2006115 Northeastern U.S.: 1 site |
Primary care model (N=96) Intervention (N=47) Comparison (N=49) | RCT (RoB: low) | 2+ medical or surgical hospital admissions per year, excluded highest cost outliers in 12–18 month period | NA | Age: 18+ |
Nonwhite: 62.5% Medicare/Medicaid: 93% Major depression: 34% 6th grade or lower reading level: 32% of those who spoke English (n=91) |
|
Coleman et al., 2001117 Denver, CO: 19 physician-nurse teams in 8 PCP practices |
Primary care model: (Group visits) (N=295) Intervention (N=146) Comparison (N=149) | RCT (RoB: some concerns) | 11+ outpatient visits in past 18 months | 1+ self-reported chronic condition | Age: 60+ | NA |
|
Katzelnick et al., 200099 WI, WA, MA: 163 physician practices affiliated with 1 of 3 included HMOs |
Primary care model (N=407) Intervention (N=218) Comparison (N=189) | RCT (RoB: some concerns) | Ambulatory visit counts above the 85th percentile (including PCP, medical specialty, and walk-in clinic visits—not MH provider visits) in previous 2 years | Screened positive for current major depression or major depression in partial remission (HAM-D score of 15 or more) | Age: 25–63 | Female: 77% |
|
Powers et al., 2020108 Memphis, TN: 1 site |
Primary care model (N=253) Intervention (N=93) Comparison (N=160) | RCT (RoB: some concerns) | Top 5% of total medical expenditures in past year and 2+ inpatient admissions or 3+ ED visits in past year | Top 5% chronic Illness Intensity Index (CI3) score and 2+ chronic conditions | NA |
Mean age: 45 Nonwhite: NR Medicaid: 100% Medicare: NR |
|
Crane et al., 201298 Hendersonville, NC: 1 site |
Primary care model: Group Visits (N=72) Intervention (N=36) Comparison (N=36) | Observational study (RoB: high) | 6+ ED visits in past 1 year | NA | Below 200% FPL; uninsured |
Median age: 32 The following were only reported in intervention group Chronic pain: 75% Uninsured: 100% Substance abuse: 47% Depression: 36% |
|
Adam et al., 2010102 MN: 1 residency clinic |
Primary care model (N=21) Intervention (N=13) Comparison (N=8) | Observational study (RoB: high) | 8+ clinic visits in past 1 year | NA | Age: 18+ |
Psychiatric diagnosis: 85% Nonwhite: 28% Female: 65% Median age: 49.5 |
|
MN: 1 site |
Primary care model (N=NR of 92,891) Intervention (N=NR of 19,433) Comparison (N=NR of 73,458) | Observational study (RoB: some concerns) | HNHC patient subgroup: More than 4 ED visits or 2 inpatient hospital visits in past 1 year | NA | Whole study population: Adults with 1+ months of enrollment under early Medicaid expansion | NR |
|
Harrison et al., 2020130 Philadelphia, PA: 12 sites |
Primary care model (N=3,048) CBCM enrollee (N=896) Comparison (N=2,152) | Observational (ROB: some concerns) | Top 10% of total costs for the Medicaid MCO in past year | NA | Received primary care at 1 of 12 participating clinical practices, 18+ years, enrolled in Medicaid |
Percentage in age range (years): 18–39: 27% 40–59: 60% ≥60: 12% Nonwhite: 88% Medicaid 2+ years: 82% Medicare: NR |
APM = advanced alternative payment model; CBCM = Community-Based Care Management; CO = Colorado; ED = emergency department; FFS = fee-for-service; FPL = federal poverty line; HAM-D = Hamilton Depression Rating Scale; HCC = hierarchical condition category; HNHC = high-need, high-cost; MA = Massachusetts; MCO = managed care organization; MH = mental health; MN = Minnesota; N = number; NA = not applicable; NC = North Carolina; NR = not reported; PA = Pennsylvania; PCP = primary care provider; RCT = randomized controlled trial; RoB = risk of bias; TN = Tennessee; U.S. = United States; WA = Washington; WI = Wisconsin.
Table B-53Intervention characteristics of primary care-based interventions
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(s) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
McCall et al., 201086 5 MA counties (Norfolk, Suffolk, Middlesex, Essex, and Plymouth) | Provider-based care management program | Up to 36 months for original population, up to 24 months for refresh population | Conducted comprehensive assessment to evaluate the unique needs of each patient; educated patients about resources available and lifestyle changes that could help to prevent exacerbations of disease, to prevent or delay hospitalization and about the purpose of their medications and other treatment interventions; reviewed self-management activities | The role of the case manager is to provide support across patients’ continuum of care | Connect patients to resources to meet medical and psychosocial needs | Nurse case managers supported by case management program project manager, an administrative assistant, a community resource specialist, and a patient financial counselor as well as social workers to help with mental health program and pharmacists | In person at primary care offices, hospitals, and home visits as needed and via telephone | Among the original population, 97% of the intervention group received at least 1 contact. The mean number of contacts per patient was 8. A higher proportion of patients in the HCC risk score group received 10 or more calls during the 36-month period compared with the low HCC risk score group (27% compared to 17%), with 10% of patients in the high HCC risk score group receiving 20+ phone calls compared with 4% in the low HHC risk score group. In the refresh group, 87% received at least 1 contact, and the mean number of contacts was 4 | Usual care |
|
Sledge et al., 2006115 Northeastern U.S.: 1 site | Primary Intensive Care (PIC) | 1 year | Comprehensive interdisciplinary medical and psychosocial assessment, used a patient-centered approach to improve self-care patterns and coping skills, aimed to track and facilitate completion of recommendations made to the PCP based on the assessment | A report and recommendations for care were presented to PCP and subspecialty providers; the recommendations were intended to optimize chronic illness management; case manager used patient-centered approach to improve coordination of care | Offered assistance with referrals and appointments | Psychiatric nurse and team including social worker, psychiatrist, and general internist | In person and via telephone; home visits when necessary | Varied based on patient needs; at minimum included a monthly telephone call; patients were defined by 3 levels of contact: minimum contact, biweekly contact, and weekly or greater contact | Usual care (psychiatric consultation provided only if requested by PCP) |
|
Coleman et al., 2001117 Denver, CO: 19 physician-nurse teams in 8 PCP practices | Patient group visits model of care | 2 years | Education on general and specific health topics, medication management, exercise, and nutrition; assessments included health promotion activities such as blood pressure measurement and need for and delivery of immunizations and medication refills and ongoing chronic disease management and evaluation of acute conditions in 1:1 sessions | Provided active care coordination within the primary care team and between other providers and care settings; promoted continuity of care with the health team | NR | Primary care physician, nurse, pharmacist. Periodic ancillary providers: dietitian, social worker, physical therapist | In person | Group visits were held monthly for 24 months, 120 minutes per session, with an average attendance of 8 to 12 participants per group | Usual care |
|
Katzelnick et al., 200099 WI, WA, MA: 163 physician practices affiliated with 1 of 3 included HMOs | Depression management program (DMP) | 1 year | Assessed patients to confirm diagnosis and appropriateness for medication; patients and PCPs received education about depression; periodic followup with PCP to monitor patient status and telephone monitoring by coordinators | PC was supported by coordinators who reviewed patient prescription refills and office visits and monitored treatment adherence, response, and adverse effects | Study psychiatrists had ongoing contact with PCPs via periodic case reviews and as-needed telephone consultation; psychiatric consultations were encouraged for patients not responding to treatment by 10 weeks and those with more complicated depression | PCPs, psychiatrists, and treatment coordinators with some clinical mental health experience | In person and via telephone | Coordinators made telephone contact at 2 weeks, 10 weeks, 18 weeks, and 30 weeks and have an average of 2.7 contacts per patient; followup visits with the PCP were prescheduled at 1, 3, 6, and 10 weeks, subsequent visits occurred approximately every 10 weeks | Usual care |
|
Powers et al., 2020108 Memphis, TN: 1 site | Complex care management program | 12 months | In-person intake visit to assess patient’s medical, behavioral, and social risk factors. The patient and care team co-developed a tailored care plan that outlined interventions, roles, and responsibilities. CHW called patients at least weekly to assess progress and troubleshoot barriers | CHW responsible for patient outreach, engagement, activation, and accompaniment while the social worker was responsible for coordinating referrals to social service agencies and other medical providers and the PCP was responsible for proving comprehensive care for acute and chronic conditions and for coordinating with specialist and inpatient providers | CHW accompanied patients to specialist, social service, and other appointments as needed. Social worker responsible for counseling and brief interventions for patients with behavioral health needs and for coordinating referrals to social service agencies. | Community health worker, a social worker, and a primary care provider | Telephone or face-to-face at the PCP office | Weekly phone call from CHW and monthly in person followup visit to review and revise the care plan | Usual care |
|
Crane et al., 201298 Hendersonville, NC: 1 site | Care management and drop-in group medical appointments (DIGMAs) | 1 year | Small-group sessions emphasized life skills; group and individual sessions addressed health and behavioral issues identified by the patient | For patients with a PCP, care other than emotional or group support provided in the program was reported to or coordinated with the PCP. All care was documented in an electronic medical record, including all phone calls. These records could be accessed by physicians in the ED as needed. | Program was based at a county free clinic that provided office space for case manager, room for DIGMA, and variety of wrap-around services to intervention group including free prescriptions at onsite pharmacy | The care team consisted of a family physician, 2 behavioral health providers, and a nurse care manager | In person in clinic or via telephone | DIGMA visits were scheduled twice a week for 1 hour; small group sessions with care manager, scheduled twice a week for 1 hour; direct telephone access to RN care manager available Monday-Friday, 8 AM to 5 PM; median number of visits per month per patient was 2 and median number of patient contacts per month was 3.5 | Usual care |
|
Adam et al., 2010102 MN: 1 residency clinic | Family medicine care team | 6 months | Care team met to review the healthcare status of a case patient and develop a care plan based on discussions with the patient’s primary physician. A member of the team called the patient to schedule a free visit to review the care plan | The team was interdisciplinary; providing joint care with consultants was encouraged when indicated, as was engaging family members or other stakeholders in the patient’s care | Care plans included referrals as needed to care such as mental health treatment or medications | Faculty physician, 4 resident physicians, the clinic psychologist, pharmacist, triage nurse, certified medical assistant, and front desk manager | In person at family medicine residency clinic | Care team met 1 time per week to discuss the care and status of a patient | |
|
Vickery et al., 2018100 MN: 1 site | Hennepin Health Accountable Care Organization (HH ACO) | 24 months | Developing patient-specific plans based on need, patient education and goal setting | Connected patients to primary care and other necessary services, increased access to care for mental illness and substance use (behavioral health) including integration within primary care, care coordination and disease management service intensity based on risk | Integrated county services for social and behavioral needs (housing, vocational, social services) with health care, increased access to services to meet social needs, improved access to dental services, high-risk patient referred to a Coordinated Care Center |
Care coordination team: RN care coordinators embedded in primary care clinics, clinical social workers, CHW Coordinated care center team: physician, NP or PA, care coordinator, social worker, psychologist, pharmacists, licensed chemical dependency counselors, and a part-time addiction psychiatrist support | Patients received care at various care settings, program managed by county | NR; based on patient risk | Usual care: enrolled in non-Hennepin Health managed care in Hennepin or Ramsey County |
|
Harrison et al., 2020130 Philadelphia, PA: 12 sites | MCO-led Community-Based Care Management (CBCM) | 12 months | NR | Coordinated care and managed medications | Identified social needs and connected patients to local community resources and partnered with a nonprofit to provide health and substance abuse services for Philadelphia County Medicaid recipients | Community health workers hired by practices and nurse case managers hired by the MCO | NR | Varied by practice site | Usual care |
CBCM = Community-Based Care Management; CHW = community health worker; CO = Colorado; DIGMA = drop-in group medical appointments; DMP = depression management program; ED = emergency department; HCC = hierarchical condition category; HH ACO = Hennepin Health Accountable Care Organization; MA = Massachusetts; MCO = managed care organization; MN = Minnesota; NC = North Carolina; NP = nurse practitioner; NR = not reported; PA = Pennsylvania; PC = primary care; PCP = primary care provider; PIC = primary intensive care; RN = registered nurse; TN = Tennessee; U.S. = United States; WA = Washington; WI = Wisconsin.
Table B-54Healthcare utilization outcomes for primary care–based model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| ED visits, all cause | RCT | ⇩ | ⇩ | Difference in change over time, G1 vs. G2: (p=0.9)115 |
| RCT | ⇧ | ⇧ | Original sample: IRR=0.94 (95% CI, 0.81 to 1.09)86 | |
| RCT | ⬆ | ⬆ | Refresh sample: Lower increase in G1 than G2: IRR=0.75 (95% CI, 0.57 to 0.99)86 | |
| RCT | NR | NR | Lower use in G1 than G2: aMD=−0.42 (95% CI, −0.13 to −0.72)117 | |
| RCT | NR | NR | aMD=−0.02 (95% CI, −0.51 to 0.47) (p=1.00)108 | |
| Observational | NR | NR | Greater use in G1 than G2: aMD=0.18 (95% CI, 0.01 to 0.37)130 | |
| Observational | NR | NR | G1 vs. G2: aMD=34.22 (95% CI, −10.9 to 79.3)100, 125 | |
| Observational | ⬇ | ⬇ | Greater reduction in G1 than G2: (p=0.005)98 | |
| Observational | ⇧ | ⇩ | Difference=0.5 (p=NR)102 | |
| ED, any (%) | RCT | NR | NR | Lower use in G1 than G2: aRR=0.64 (95% CI, 0.44 to 0.86)117 |
| ED visits, ACSC | RCT | ⇧ | ⇧ | Original sample: IRR=0.90 (95% CI, 0.70 to 1.16)86 |
| RCT | ⇧ | ⇧ | Refresh sample: IRR=0.80 (95% CI, 0.51 to 1.25)86 | |
| Inpatient admissions, all cause | RCT | ⇩ | ⇩ | Difference in change over time, G1 vs. G2: (p=0.55)115 |
| RCT | ⬆ | ⬆ | Original sample: lower increase in G1 than G2: IRR=0.81 (95% CI, 0.70 to 0.94)86 | |
| RCT | ⬆ | ⬆ | Refresh sample: lower increase in G1 than G2: IRR: 0.76 (95% CI, 0.58 to 0.99)86 | |
| RCT | NR | NR | Lower use in G1 than G2 (mean # of admissions): 0.44 vs. 0.81 (p=0.04)117 | |
| RCT | ⇧ | ⇩ | G1 vs. G2: (p=0.09)99 | |
| RCT | NR | NR | Greater reduction in G1 than G2: aMD=−0.32 (95% CI, −0.54 to −0.11) (p=0.014)108 | |
| Observational | NR | NR | Greater use in G1 than G2: aMD=0.10 (95% CI, 0.03 to 0.16)130 | |
| Observational | NR | NR | Lower use in G1 vs. G2: aMD=−26.11 (95% CI, −35.9 to −16.3)100, 125 | |
| Observational | -- | -- | Difference=0 (p=NR)102 | |
| Inpatient admissions, any (%) | RCT | ⬆ | ⬆ | Original sample: lower increase in G1 than G2, OR=0.65 (95% CI, 0.55 to 0.78}86 |
| RCT | ⬇ | ⬆ | Refresh sample: greater reduction in G1 than G2, OR=0.66 (95% CI, 0.48 to 0.90)86 | |
| Inpatient admissions, ACSC | RCT | ⇧ | ⇧ | Original sample: IRR=0.87 (95% CI, 0.66 to 1.14)86 |
| RCT | ⇧ | ⇧ | Refresh sample: IRR=0.78 (95% CI, 0.49 to 1.24)86 | |
| Inpatient admissions, any ACSC (%) | RCT | ⬆ | ⬆ | Original sample: lower increase in G1 than G2, OR=0.73 (95 %CI, 0.57 to 0.95)86 |
| RCT | ⇧ | ⇧ | Refresh sample: OR=0.79 (95% CI, 0.51 to 1.21)86 | |
| Inpatient days | RCT | NR | NR | Greater reduction in G1 vs. G2: aMD=−3.46 (95% CI, −4.04 to −2.89) (p<0.001)108 |
| Observational | NR | NR | G1 vs. G2: aMD=−219.7 (95% CI, −826 to 386.6)100, 125 | |
| Primary care visits | RCT | ⇧ | ⇩ | Difference in change over time, G1 vs. G2: (p=0.055)115 |
| RCT | NR | NR | G1 vs. G2: (p=0.20)117 | |
| Observational | NR | NR | Less use in G1 than G2: aMD=−1.83 (95% CI, −2.10 to −1.55)130 | |
| Observational | NR | NR | G1 vs. G2: aMD=6.0 (95% CI: −39.3 to 51.4)100, 125 | |
| Outpatient visits | RCT | ⬆ | ⬇ | Greater increase in G1 than G2: (p=0.02)99 |
| Observational | ⇩ | ⇧ | Difference=4.5 (p=NR)102 | |
| Care center visits | RCT | NR | NR | aMD: 0.47 (95% CI, −0.16 to 1.11) (p=0.576)108 |
| Specialist visits | RCT | NR | NR | Greater reduction in G1 vs. G2: aMD: −1.35 (95% CI, −1.98 to −0.73) (p<0.001)108 |
| Total visits | Observational | NR | NR | Less use in G1 than G2: aMD: −1.55 (95% CI, −1.93 to −1.21)130 |
| Filled ≥3 antidepressant prescriptions in first 6 months | RCT | NR | NR | Greater use in G1 than G2: (p<0.001)99 |
| Specialty mental health visit in first 6 months | RCT | NR | NR | Greater use in G1 than G2: (p=0.03)99 |
| Cancelled visits and/or no shows | Observational | ⇧ | ⇩ | Difference=3.5 (p=NR)102 |
| Intensive care unit visits | Observational | NR | NR | G1 vs. G2: aMD=0.1 (95% CI: −0.7 to 0.9)100, 125 |
| Dental visits | Observational | NR | NR | Greater reduction in G1 vs. G2: aMD=−30.7 (95% CI, −41.0 to −20.3)100, 125 |
- a
Outpatient visits were defined as primary care visits and group intervention visits for the intervention group and as primary care visits only for the comparison group.
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
ACSC = ambulatory care sensitive conditions; aMD = adjusted mean difference; aRR = adjusted risk ratio; CI = confidence interval; ED = emergency department; G = group; IRR = incidence rate ratio; NR = not reported; OR = odds ratio; RCT = randomized controlled trial; vs. = versus.
Table B-55Strength of evidence for primary care–based models versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | ED visits, all cause |
PIC RCT: Diff in change over time (p=0.9)115 MGH CMP original sample: IRR=0.94 (95% CI, 0.81 to 1.09)86 MGH CMP refresh sample: IRR=0.75 (95% CI, 0.57 to 0.99)86 Group visit RCT: aMD=−0.42 (95% CI, −0.13 to −0.72)117 CCM RCT: aMD=−0.02 (95% CI, −0.51 to 0.47)108 CBCM: Greater use in G1 than G2: aMD=0.18 (95% CI, 0.01 to 0.37)130 HH ACO: diff=34.22 (95% CI, −10.9 to 79.3)100, 125 Bridges to Health: Greater reduction in G1 than G2: (p=0.005)98 Interdisciplinary pilot: Diff=0.5 (p=NR)102 |
5 RCTs, N=7,587 4 OBSs, N=NRa | Moderate study limitations (2 high RoB OBS studies),98, 102 inconsistent, imprecise, direct | Insufficient (Mixed findings) |
| HNHC patients | ED visits, ACSC |
MGH CMP original sample: IRR=0.90 (95% CI, 0.70 to 1.16)86 MGH CMP refresh sample: IRR=0.80 (95% CI, 0.51 to 1.25)86 | 2 RCTs, N=6,943 | Moderate study limitations, consistent imprecise, direct | Insufficient |
| HNHC patients | Inpatient admissions, all cause |
PIC RCT: diff in change over time: (p=0.55)115 MGH CMP original sample: IRR=0.81 (95% CI, 0.70 to 0.94)86 MGH CMP refresh sample: IRR: 0.76 (95% CI, 0.58 to 0.99)86 Group visit RCT: less use in G1 than G2: 0.44 vs. 0.81, (p=0.04)117 DMP RCT: G1 vs. G2: (p=0.09)99 CCM RCT: Greater reduction in G1 than G2: aMD=−0.32 (95% CI, −0.54 to −0.11) 108 CBCM: Greater use in G1 than G2=aMD: 0.10 (95% CI, 0.03 to 0.16)130 HH ACO: G1 vs. G2: Diff=−26.11 (95% CI, −35.9 to −16.3)100, 125 Interdisciplinary Pilot: diff=0 (p=NR)102 |
6 RCTs, N=7,994 3 OBS, N=NRa | Moderate study limitations (one high RoB OBS studies),102 consistent, precise, direct | Insufficient (Mixed findings) |
| HNHC patients | Inpatient admissions, any (%) |
MGH CMP: original sample: OR=0.65 (95% CI, 0.55 to 0.78)86 MGH CMP refresh sample: OR=0.66 (95% CI, 0.48 to 0.90)86 | 2 RCTs, N=6,943 | Moderate study limitations, consistent, precise, direct | Low (Favorable) |
| HNHC patients | Inpatient admissions, ACSC |
MGH CMP original sample: IRR=0.87 (95% CI, 0.66 to 1.14)86 MGH CMP refresh sample: IRR=0.78 (95% CI, 0.49 to 1.24)86 | 2 RCTs, N=6,943 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient admissions, any ACSC (%) |
MGH CMP original sample: OR=0.73 (95% CI, 0.57 to 0.95)86 MGH CMP refresh sample: OR=0.79 (95% CI, 0.51 to 1.21)86 | 2 RCTs, N=6,943 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient days |
CCM RCT: Greater reduction in G1 vs. G2: aMD=−3.46 (95% CI, −4.04 to −2.89)108 HH ACO: G1 vs. G2: aMD=−219.7 (95% CI, −826 to 386.6)100, 125 |
1 RCT, N=253 1 OBS, N=NRa | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Primary care visits |
PIC RCT: diff in change over time: (p=0.055);115 RCT group visits: G1 vs. G2: (p=0.20);117 CBCM: Less use in G1 than G2: aMD=−1.83 (95% CI, −2.10 to −1.55);130 |
2 RCTs, N=391 2 OBSs, N=NRa | Moderate study limitations, inconsistent imprecise, direct | Insufficient (Mixed findings) |
| HNHC patients | Outpatient visits |
DMP: greater use in G1 than G2: (p=0.02)99 Interdisciplinary pilot: diff=4.5 (p=NR)102 |
1 RCT, N=407 1 OBS, N=21 | High study limitations (one high RoB OBS studies)102 imprecise, inconsistent, direct | Insufficient |
| HNHC patients | Total cost |
PIC RCT: diff in change over time, G1 vs. G2: (p=0.82)115 MGH CMP: original sample DiD: −288 (p<0.01)86 MGH CMP: refresh sample DiD: −355 (p<0.05)86 CCM RCT: Greater reduction in G1 vs. G2: aMD=−7,732 (95% CI, −14,914 to −550)108 CBCM: aMD=829 (95% CI, −1,279 to 3,098)130 Pooled mean difference: −$3,848.43 (95% CI, −5,514.24 to −2,182.61); 3 RCT samples, N=7,196, I2=0.0% |
4 RCTs, N=7,292 1 OBS, N=3,048 | Moderate study limitations, consistent, imprecise, direct | Low (Favorable) |
| HNHC patients | Mortality rate |
MGH CMP: original sample diff: G1 vs. G2, −1.63 (p=0.19)86 MGH CMP: refresh sample diff: G1 vs. G2: −3.97 (p=0.04)86 | 2 RCTs, N=6,943 | Moderate study limitations, imprecise, inconsistent, direct | Insufficient |
| HNHC patients | Influenza vaccine |
MGH CMP: original sample DiD OR=0.79 (95% CI, 0.66 to 0.95)86 MGH CMP refresh sample DiD: OR=0.64 (95% CI, 0.46 to 0.87)86 | 2 RCTs, N=6,943 | Moderate study limitations, consistent, precise, direct | Low (Unfavorable) |
| HNHC patients Diabetes subgroup | HbA1c test |
MGH CMP, original sample: DiD OR=0.99 (95% CI, 0.56 to 1.76)86 MGH CMP refresh sample: DiD OR=0.70 (95% CI, 0.27 to 1.84)86 | 2 RCTs, N=1,959 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients Diabetes subgroup | LDL-C test |
MGH CMP original sample: DiD OR=0.85 (95% CI, 0.58 to 1.24)86 MGH CMP refresh sample: DiD OR=1.72 (95% CI, 0.86 to 3.42)86 | 2 RCTs, N=1,959 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients IVD subgroup | LDL-C test |
MGH CMP original sample: DiD OR=0.92 (95% CI, 0.63 to 1.33)86 MGH refresh sample: DiD OR=1.40 (95% CI, 0.76 to 2.58)86 | 2 RCTs, N=1,923 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
- a
ACSC = ambulatory care sensitive conditions; aMD = adjusted mean difference; CBCM = Community-Based Care Management; CCM = complex care management; CI = confidence interval; DiD = difference-in-difference; DMP = depression management program; ED = emergency department; G = group; HbA1c = hemoglobin A1c; HH ACO = Hennepin Health Accountable Care Organization; HNHC = high-need, high-cost; IRR = incidence rate ratio; IVD = ischemic vascular disease; LDL-C = low-density lipoprotein cholesterol; MGH CMP = Massachusetts General Hospital, Care Management Program, N = number; NR = not reported; OBS = observational study; OR = odds ratio; PIC = Primary Intensive Care; RCT = randomized controlled trial; RoB = risk of bias; vs. = versus.
Table B-56Cost outcomes for primary care–based model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost | RCT | ⇩ | ⇩ | Difference in change over time, G1 vs. G2: (p=0.82)115 |
| RCT | ⬆ | ⬆ | Original sample DiD: Lower increase in G1 than G2: −288 (82.1), (p<0.01)86 | |
| RCT | ⬆ | ⬆ | Refresh sample DiD: Lower increase in G1 than G2: −355 (157.6), p<0.0586 | |
| RCT | NR | NR | Greater reduction in G1 vs. G2: aMD=−7,732 (95% CI, −14,914 to −550) (p=0.036)108 | |
| Observational | NR | NR | aMD=829 (95% CI, −1,279 to 3,098)130 | |
| Inpatient costs | Observational | NR | NR | aMD=297 (95% CI, −1,150 to 1,729)130 |
| Pharmacy costs | Observational | NR | NR | aMD=110 (95% CI, −875 to 1,109)130 |
| Outpatient costs | Observational | NR | NR | aMD=10 (95% CI, −439 to 492)130 |
| Postacute costs | Observational | NR | NR | aMD=362 (95% CI, 150 to 569)130 |
| ED costs | Observational | NR | NR | aMD=5 (95% CI, −155 to 171)130 |
| Other costs | Observational | NR | NR | aMD=45 (95% CI, −159 to 262)130 |
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
aMD = adjusted mean difference; CI = confidence interval; DiD = difference-in-difference; Ed = emergency department; G = group; NR = not reported; RCT = randomized controlled trial; vs. = versus.
Table B-57Clinical and functional outcomes for primary care–based model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality rate | RCT | Original sample difference: G1 vs. G2, −1.63 (p=0.19)86 |
| RCT | Refresh sample difference: lower in G1 than G2: −3.97 (p=0.04)86 | |
| Influenza vaccine | RCT | Original sample DiD: increased less in G1 than G2: OR=0.79 (95% CI, 0.66 to 0.95)86 |
| RCT | Refresh sample DiD: increased less in G1 than G2: OR=0.64 (95% CI, 0.46 to 0.87)86 | |
| PHC score | RCT | Better in G1 than G2: ANCOVA-adjusted intervention effect=2.3, p<0.0186 |
| MHC score | RCT | ANCOVA-adjusted intervention effect=1.1, p>0.0586 |
| PQH-2 score (depression 0 to 6) | RCT | ANCOVA-adjusted intervention effect=−0.03, p>0.0586 |
| Number of ADLs difficult to do (0 to 6) | RCT | ANCOVA-adjusted intervention effect=−0.28, p>0.0586 |
| Number ADLs receiving help (0 to 6) | RCT | ANCOVA-adjusted intervention effect=−0.21, p>0.0586 |
| Patient satisfaction | RCT | Difference in change over time, G1 vs. G2: p=0.30115 |
| SF-36 Summary Score | RCT | Difference in change over time, G1 vs. G2: p=0.32115 |
| SF-36 Mental Health Function Score | RCT | Difference in change over time, G1 vs. G2: p=0.6115 |
| Change in HAM-D score | RCT | Greater decrease in G1 than G2: p<0.00199 |
| In remission (HAM-D <7) | RCT | Higher proportion in G1 than G2: p<0.00199 |
| SF-20 subscale: Social Functioning | RCT | Better in G1 than G2: p<0.05 (data NR)99 |
| SF-20 subscale: Mental Health | RCT | Better in G1 than G2: p<0.05 (data NR)99 |
| SF-20 subscale: General Health | RCT | Better in G1 than G2: p<0.05 (data NR)99 |
| SF-20 subscale: Physical Functioning | RCT | G1 vs. G2: p=NS (data NR)99 |
| SF-20 subscale: Role Functioning | RCT | G1 vs. G2: p=NS (data NR)99 |
| SF-20 subscale: Pain Perception | RCT | G1 vs. G2: p=NS (data NR)99 |
| Helping to cope with a chronic condition (1 to 5) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=0.16, p>0.0586 |
| Number of helpful discussion topics (0 to 5) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=−0.02, p>0.0586 |
| Discussing treatment choices (1 to 4) | RCT | Better score in G1 than G2: ANCOVA-adjusted intervention effect=0.26, p<0.0186 |
| Communicating with providers (0 to 100) | RCT | Better score in G1 than G2: ANCOVA-adjusted intervention effect=4.5, p<0.0586 |
| Getting answers to questions quickly (0 to 100) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=5.0, p>0.0586 |
| Multimorbidity Hassles score (0 to 24) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=−0.27, p>0.0586 |
| Percentage receiving help setting goals | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=−5.6, p>0.0586 |
| Percentage receiving help making a care plan | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=2.3, p>0.0586 |
| Self-efficacy: Take all medications (1 to 5) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=0.05, p>0.0586 |
| Self-efficacy: Plan meals and snacks (1 to 5) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=0.01, p>0.0586 |
| Self-efficacy: Exercise 2 or 3 times weekly (1 to 5) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=0.11, p>0.0586 |
| Self-care activities: Prescribed medications taken (mean # of days) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=−0.10, p>0.0586 |
| Self-care activities: Followed healthy eating plan (mean # of days) | RCT | G1 vs. G2 (N=590), ANCOVA-adjusted intervention effect=−0.16, p>0.05 |
| Self-care activities: 30 minutes of continuous physical activity (mean # of days) | RCT | G1 vs. G2 (N=590): ANCOVA-adjusted intervention effect=−0.05, p>0.0586 |
ADL = activities of daily living; ANCOVA = analysis of covariance; CI = confidence interval; DiD = difference-in-difference; G = group; HAM-D = Hamilton Depression Rating Scale; G = group; MHC = mental health composite; NR = not reported; NS = not statistically significant; OR = odds ratio; PHC = physical health composite; PHQ-2 = Patient Health Questionnaire-2; RCT = randomized controlled trial; SF = short form; vs. = versus.
Table B-58Clinical and functional outcomes for primary care–based model studies: Subgroup outcomes
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| HbA1c test | RCT | Original sample diabetes subgroup: DiD OR=0.99 (95% CI, 0.56 to 1.76)86 |
| RCT | Refresh sample diabetes subgroup: DiD OR=0.70 (95% CI, 0.27 to 1.84)86 | |
| LDL-C test | RCT | Original sample diabetes subgroup: DiD OR=0.85 (95% CI, 0.58 to 1.24)86 |
| RCT | Refresh sample diabetes subgroup: DiD OR=1.72 (95% CI, 0.86 to 3.42)86 | |
| RCT | Original sample IVD subgroup: DiD OR=0.92 (95% CI, 0.63 to 1.33)86 | |
| RCT | Refresh sample IVD subgroup: DiD OR=1.40 (95% CI, 0.76 to 2.58)86 |
CI = confidence interval; DiD = difference-in-difference; HbA1c = hemoglobin A1c; IVD = ischemic vascular disease LDL-C = low density lipoprotein cholesterol; OR = odds ratio; RCT = randomized controlled trial.
Table B-59Social risk outcomes for primary care–based model studies
| Social Risk Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Overall well-being | Observational | G1 vs. G2: p=NR102 |
G = group; NR = not reported; vs. = versus.
Table B-60Study characteristics for home-based care interventions
| First Author, Year, Site(s) | Sample Size | Study Design (Risk of Bias Assessment) | Patient Selection: High Healthcare Use or Cost; Time Period | Patient Selection: Chronic Conditions | Patient Selection: Other | Additional Selected Patient Characteristics |
|---|---|---|---|---|---|---|
|
McCall et al., 201084 CA, FL, TX |
(Original N=16,077) Intervention (N=11,516) Comparison (N=4,561) (COPD subgroup N=3,344) Intervention (N=2,384) Comparison (N=960) (Diabetes subgroup N=4,502) Intervention (N=3,223) Comparison (N=1,279) (IVD subgroup N=7,356) Intervention (N=5,223) Comparison (N=2,133) (High-cost-only PBPM subgroup N=4,344) Intervention (N=3,105) Comparison (N=1,239) (High-cost and high-risk PBPM subgroup N=6,802) Intervention (N=4,845) Comparison (N=1,957) | RCT (RoB: some concerns) | High costs with top 5% of costs and 2+ hospitalizations in past 1 year | 1+ diagnosis from a list, such as heart failure; HCC score was ≥2.75 OR if had an HCC score <2.75, had a diagnosis of selected clinical conditions including peripheral vascular disease, ischemic heart disease, hypertensive heart and/or kidney disease, heart failure, chronic obstructive pulmonary disease (COPD), and asthma | Medicare FFS beneficiaries |
Nonwhite: 27% Mean HCC: 2.8 |
|
McCall et al., 201084 CA, FL, TX |
(Refresh N=18,344) Intervention (N=13,104) Comparison (N=5,240) (COPD subgroup N=4,735) Intervention (N=3,393) Comparison (N=1,342) (Diabetes subgroup N=5,950) Intervention (N=4,199) Comparison (N=1,751) (IVD subgroup N=7,554) Intervention (N=5,384) Comparison (N=2,170) (High-cost-only PBPM subgroup N=1,414) Intervention (N=1,027) Comparison (N=387) (High-cost and high-risk PBPM subgroup N=8,598) Intervention (N=6,142) Comparison (N=2,456) | RCT (RoB: some concerns) | 2+ hospitalizations in past 1 year | HCC score was >2.749 | Medicare FFS beneficiaries; excluded beneficiaries with drug/alcohol psychosis or dependence, major depressive, bipolar, and paranoid disorders; institutionalized in last 3 months of previous year; had a hospital claims where discharge date was equal to admission date |
Nonwhite: 40% Mean HCC: 3.8 |
|
Kimmy et al., 2019103 National: 14 practices |
(Home-based care N=181,001) Intervention (N=30,324) Comparison (N=150,677) | Observational study (RoB: some concerns) | Hospitalization and use of acute or subacute rehabilitation services; and 1+ home visit from the IAH practice in past 1 year | 2+ chronic conditions 2+ ADLs that require human assistance, new to home-based primary care (2+ E&M visits from a primary care clinician in the home or an assisted living facility during the 6-month period starting with the first home visit), majority of E&M visits from a primary care clinician during the same period must have taken place in the home or assisted living facility | Medicare FFS beneficiaries, new patients receiving home-based primary care who were IAH eligible and lived in an area served by an IAH practice |
Mean HCC : 3.686 Chronically critically ill/medically complex: 29.1% Depression: 31.9% |
|
Valluru et al., 2019131 Philadelphia, PA; Richmond, VA; Washington, D.C.: 3 sites |
(N=1,376) IAH patients at 3 sites (N=721) Comparison with home-based care (N=82) Comparison without home-based care (N=573) | Observational study (ROB: high) | Nonelective hospitalization and post-acute care use, either skilled home care or skilled nursing facility, in past year | 2+ chronic conditions | Enrollment in 1 of 3 sites, FFS Medicare, score of 6+ in JEN Frailty Index |
Percentage in age range: <75: 31% 75–84: 30% ≥85: 39% African American: 62% Medicaid: NR Medicare: 100% Mean HCC: 3.58 |
ADL = activities of daily living; CA = California; COPD = chronic obstructive pulmonary disease; E&M = evaluation and management; FFS = fee-for-service; FL = Florida; HCC = hierarchical condition category; IAH = Independence at Home; IVD = ischemic vascular disease; N = number; NR = not reported; OR = odds ratio; PA = Pennsylvania; PBPM = per beneficiary per month; RCT = randomized controlled trial; RoB = risk of bias; TX = Texas, VA = Virginia.
Table B-61Intervention characteristics for home-based models
| First Author, Year, Site(s) | Intervention: Brief Description | Intervention Duration | Assessment, Education, Skills, Monitoring | Coordination and Continuity of Care | Referral to/Linkages to Community-Based Support Services | Providers | Mode of Delivery Setting(S) | Intensity | Comparison |
|---|---|---|---|---|---|---|---|---|---|
|
McCall et al., 201084 CA, FL, TX | Evaluation of Medicare Care Management for High Cost Beneficiaries (CMHCB) Demonstration: Care Level Management (CLM) home-based primary care for patients with multiple chronic conditions | Up to 29 months for original population, up to 18 months for refresh population | Assessment tool on patient acuity: number of admissions and emergency room visits within the last 6 months, presence of unmet social and emotional needs, expected number of PVP visits needed in the next 30 days, and presence of compliance, psychiatric, or ongoing home health issues | Provided home-based care and 24/7 access to a PVP to patients; care management addressed adherence to treatment regimens, coordination of care services, end-of-life planning, home safety, socioeconomic issues, psychosocial issues, and medication management | Helped selected beneficiaries receive services from community-based ancillary services as needed | PVPs, NPs to support PVPs, nurse care managers as patient advocates and care coordinators (PVPs were adjuncts to patients’ PCP) | Face-to-face and by phone | 12% had no contact, 75% of beneficiaries had one or more physician visits, 22% had 10 or more visits, and 14% had 20 or more visits. 88% of beneficiaries received a telephone call from a nurse or physician, while 24% received 10 or more calls, and 39% of beneficiaries received 20 or more calls | Usual care |
|
Kimmy et al., 2019103 Effect of home-based primary care National: 14 practices | Practices may earn an additional payment if their chronically ill, functionally limited patients’ Medicare expenditures are below an estimated spending target and if the practice meets required standards for a set of quality measures | Up to 4 years | Clinicians are available at all hours of the day; carry out individualized care plans; and use electronic health information systems, remote monitoring, and mobile diagnostic technology | Report on other measures, including fall risk assessments and depression screenings, to promote the provision of such care | Some practices added social workers or other staff to coordinate care for their patients with other organizations | Physicians or nurse practitioners; team may have also included physician assistants, clinical staff, and other health and social services staff | Face-to-face | Clinicians made 3–15 home visits per day, varied by site | Usual care: Patients who did not receive primary care in the home during the 6 months after their index date |
|
Valluru et al., 2019131 Philadelphia, PA; Richmond, VA; Washington, DC: 3 sites | Home-based primary care integrated with long-term service supports (LTSS) | 36 months | NR | Integrated care coordination with community supports including adult day healthcare and home health aide-provided personal care services | Assistance with meals and transportation and social workers who collaborate with various community LTSS resources | Care managers, case managers, or social workers depending on the site | Face-to-face in patient home | NR | Usual care: Home-based care without long-term services and supports or no home-based care |
CA = California; CLM = care level management; CMHCB = Care Management for High Cost Beneficiaries; DC = District of Columbia; FL = Florida; LTSS = long-term services and supports NP = nurse practitioner; NR = not reported; PA = Pennsylvania; PCP = primary care provider; PVP = personal visiting physician; TX = Texas; VA = Virginia.
Table B-62Healthcare utilization outcomes for home-based care model studies
| Utilization Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Inpatient admissions, all cause | RCT | ⇩ | ⇩ | Original sample DiD: IRR=0.94 (95% CI, 0.87 to 1.01)84 |
| RCT | ⇩ | ⇩ | Refresh sample DiD: IRR=0.94 (95% CI, 0.88 to 1.00)84 | |
| Observational | NR | NR | Lower reduction in G1 than G2: home-based care DiD=0.05 (90% CI, 0.01 to 0.09)103 | |
| Inpatient admissions, any all cause (%) | RCT | ⇩ | ⇩ | Original sample DiD: OR=1.05 (95% CI, 0.93 to 1.18)84 |
| RCT | ⇩ | ⇩ | Refresh sample DiD: OR=.93 (95% CI, 0.85 to 1.03)84 | |
| Inpatient admissions, ACSC | RCT | ⬇ | ⬆ | Greater reduction in G1 than G2: original sample DiD: IRR=0.86 (95% CI, 0.76 to 0.97)84 |
| RCT | ⬇ | ⬆ | Greater reduction in G1 than G2: refresh sample DiD: IRR=0.89 (95% CI, 0.81 to 0.99)84 | |
| Observational | NR | NR | Home-based care sample DiD=0.00 (90% CI, −0.02 to 0.02)103 | |
| Inpatient admissions, any ACSC (%) | RCT | ⬇ | ⬇ | Greater reduction in G1 than G2: original sample DiD: OR=0.87 (95% CI, 0.77 to 0.99)84 |
| RCT | ⬇ | ⬇ | Greater reduction in G1 than G2: refresh sample DiD: OR=.90 (95% CI, 0.81 to 1.00)84 | |
| ED visits, all cause | RCT | ⇩ | ⇩ | Original sample DiD: IRR=0.88 (95% CI, 0.69 to 1.12)84 |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=0.95 (95% CI, 0.85 to 1.07)84 | |
| Observational | NR | NR | Home-based care DiD=0.00 (90% CI, −0.04 to 0.05) (p>0.10)103 | |
| ED visits, ACSC | RCT | ⇩ | ⇩ | Original sample DiD: IRR=0.89 (95% CI, 0.66 to 1.18)84 |
| RCT | ⇧ | ⇧ | Refresh sample DiD: IRR=1.06 (95% CI, 0.87 to 1.30)84 | |
| Observational | NR | NR | Home-based care DiD=0.00 (90% CI, −0.02 to 0.01) (p>0.10)103 | |
| Long-term institutionalization rate | Observational | NR | NR | G1: 8.1%, G2 with home-based care: 17.7%, G2 without home-based care: 16.4%: p<0.05131 |
⬆ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⬇ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was statistically significant.
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
ACSC = ambulatory care sensitive conditions; CI = confidence interval; DiD = difference-in-difference; ED = emergency department; G = group; IRR = incidence rate ratio; NR = not reported; OR = odds ratio; RCT = randomized controlled trial.
Table B-63Strength of evidence for home-based care models versus usual-care outcomes
| Population | Outcome | Results | Study Design and Sample Size | Strength of Evidence Domains | Overall Evidence Strength (Direction of Effect) |
|---|---|---|---|---|---|
| HNHC patients | ED visits, all cause |
CLM original sample DiD: IRR=0.88 (95% CI, 0.69 to 1.12)84 CLM refresh sample DiD: IRR=0.95 (95% CI, 0.85 to 1.07)84 IAH home-based care: higher use in G1 than G2: DiD=0.00 (90% CI, −0.04 to 0.05), (p>0.10)103 |
2 RCTs, N=34,421 1 OBS, N=181,246 | Moderate study limitations, imprecise, inconsistent, direct | Insufficient |
| HNHC patients | ED visits, ACSC |
CLM original sample DiD: IRR=0.89 (95% CI, 0.66 to 1.18)84 CLM refresh sample DiD: IRR=1.06 (95% CI, 0.87 to 1.30)84 IAH home-based care sample DiD=0.00 (90% CI, −0.02 to 0.01, p>0.10)103 |
2 RCTs, N=34,421 1 OBS, N=181,246 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients | Inpatient admissions, all cause |
CLM original sample: IRR=0.94 (95% CI, 0.87 to 1.01)84 CLM refresh sample: IRR=0.94 (95% CI, 0.88 to 1.00);84 IAH home-based care: lower reduction in G1 than G2: DiD=0.05 (90% CI, 0.01 to 0.09)103 |
2 RCTs, N=34,421 1 OBS, N=181,246 | Moderate study limitations, imprecise, inconsistent, direct | Insufficient |
| HNHC patients | Inpatient admissions, ACSC |
CLM original sample: DiD: IRR=0.86 (95% CI, 0.76 to 0.97)84 CLM refresh sample: DiD: IRR=0.89 (95% CI, 0.81 to 0.99)84 IAH home-based care sample: DiD=0.00 (90% CI, −0.02 to 0.02)103 |
2 RCTs, N=34,421 1 OBS, N=181,246 | Moderate study limitations, imprecise, consistent, direct | Low (Favorable) |
| HNHC patients | Inpatient admissions, any all cause (%) |
CLM original sample: DiD: OR=1.05 (95% CI, 0.93 to 1.18)84 CLM refresh sample: DiD: OR=0.93 (95% CI, 0.85 to 1.03)84 | 2 RCTs, N=34,421 | Moderate study limitations, imprecise, inconsistent, direct | Insufficient |
| HNHC patients | Inpatient admissions, any ACSC (%) |
CLM original sample: DiD: OR=0.87 (95% CI, 0.77 to 0.99)84 CLM refresh sample: DiD: OR=0.90 (95% CI, 0.81 to 1.00)84 | 2 RCTs, N=34,421 | Moderate study limitations; precise, consistent, direct | Low (Favorable) |
| HNHC patients | Total cost |
CLM original sample DiD=41 (p>0.05);84 CLM refresh sample DiD=−29 (p>0.05)84 IAH home-based care sample DiD=451 (90% CI, 342.4 to 559.6, p<0.10)103 |
2 RCTs, N=34,421 1 OBS, N=181,246 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| High-cost, high risk patient subgroup | Total cost | CLM original sample: DiD=107 (p>0.05)84 CLM refresh sample DiD=−21 (p>0.05)84 | 2 RCTs, N=15,400 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| High-cost only patient subgroup | Total cost | CLM original sample DiD=−170 (p>0.05)84 CLM refresh sample: DiD=−236 (p>0.05)84 | 2 RCTs, N=5,758 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients | Mortality rate |
CLM original sample: diff in mean rate=0.4, (p=0.63)84 CLM refresh sample: Diff in mean rate=0.1 (p=0.88)84 IAH LTSS: G1 vs. G2 with HBC and G2 without HBC=NR (p>0.05)131 |
2 RCTs, N=34,421 1 OBS, N=1,376 | Moderate study limitations, consistent, imprecise, direct | Low (No difference) |
| HNHC patients | Influenza vaccine |
CLM original sample: DiD OR=1.15 (95% CI, 1.02 to 1.30)84 CLM refresh sample: DiD OR=1.15 (95% CI, 1.03 to 1.27)84 | 2 RCTs, N=34,421 | Moderate study limitations, consistent, precise, direct | Low (Favorable) |
| HNHC patients COPD subgroup | Oxygen saturation test |
CLM original sample: DiD OR=1.02 (95% CI, 0.77 to 1.34)84 CLM refresh sample: DiD OR=0.97 (95% CI, 0.77 to 1.22)84 | 2 RCTs, N=8,079 | Moderate study limitations, inconsistent, imprecise, direct | Insufficient |
| HNHC patients Diabetes subgroup | HbA1c test |
CLM original sample: DiD OR=0.91 (95% CI, 0.74 to 1.13)84 CLM refresh: DiD OR=0.98 (95% CI, 0.82 to 1.18)84 | 2 RCTs, N=10,452 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| HNHC patients Diabetes subgroup | LDL-C test |
CLM original sample: DiD OR=0.92 (95% CI, 0.75 to 1.12)84 CLM refresh sample: DiD OR=0.97 (95% CI, 0.77 to 1.22)84 | 2 RCTs, N=10,452 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
| IVD patient Subgroup | LDL-C test |
CLM original sample: DiD OR=0.89 (95% CI, 0.76 to 1.05)84 CLM refresh sample: DiD OR=0.95 (95% CI, 0.81 to 1.11)84 | 2 RCTs, N=14,910 | Moderate study limitations, consistent, imprecise, direct | Insufficient |
ACSC = ambulatory care sensitive conditions; CI = confidence interval; CLM = care level management; COPD = chronic obstructive pulmonary disease; DiD = difference-in-difference; ED = emergency department; G = group; HbA1c = hemoglobin A1c; HBC = home-based care; HNHC = high-need, high-cost; IAH = Independence at Home; IRR = incidence rate ratio; IVD = ischemic vascular disease; LDL-C = low-density lipoprotein cholesterol; LTSS = long-term services and supports; N = number; NR = not reported; OBS = observational study; OR = odds ratio; RCT = randomized controlled trial; vs. = versus.
Table B-64Cost outcomes for home-based care model studies
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost | RCT | ⇩ | ⇩ | Original sample DiD=41 (SE: 67.9) (p>0.05)84 |
| RCT | ⇧ | ⇧ | Refresh sample DiD=−29 (SE: 73.1) (p>0.05)84 | |
| Observational | NR | NR | Greater increase in G1 than G2: home-based care DiD=$451 (SE: 66) (90% CI, 342.4 to 559.6)103 |
⇧ = Increase in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
CI = confidence interval; DiD = difference in difference; G = group; NR = not reported; NS = not statistically significant; RCT = randomized controlled trial; SE = standard error.
Table B-65Cost outcomes for home-based care model studies: Subgroup outcomes
| Cost Measures | Study Design | Direction of Change in Intervention Group (G1) | Direction of Change in Comparison Group (G2) | Difference |
|---|---|---|---|---|
| Total cost | RCT | ⇩ | ⇩ | Original sample high-cost, high-risk subgroup DiD=107 (SE: 127.6) (p>0.05)84 |
| RCT | ⇩ | ⇩ | Refresh sample high-cost, high-risk subgroup DiD=−21 (SE: 121.3) (p>0.05)84 | |
| RCT | ⇩ | ⇩ | Original sample high-cost-only subgroup DiD=−170 (SE: 104.0) (p>0.05)84 | |
| RCT | ⇩ | ⇩ | Refresh sample high-cost-only subgroup DiD=−236 (SE: 197.5) (p>0.05)84 |
⇩ = Reduction in the outcome between the intervention period and the baseline period; the difference between the intervention and comparison groups was not statistically significant.
DiD = difference-in-difference; G = group; RCT = randomized controlled trial; SE = standard error.
Table B-66Clinical and functional outcomes for home-based care model studies
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Mortality rate | RCT | Original sample: difference in mean rates=0.4, (p=0.63)84 |
| RCT | Refresh sample: difference in mean rates=0.1 (p=0.88)84 | |
| OBS | G1: 35.8%, G2 with home-based care: 24.9%, G2 without home-based care: 26.9%: p>0.05131 | |
| Influenza vaccine | RCT | DiD greater in G1 than G2: original sample: DiD OR=1.15 (95% CI, 1.02 to 1.30)84 |
| RCT | DiD greater in G1 than G2: refresh sample: DiD OR=1.15 (95% CI, 1.03 to 1.27)84 | |
| PHC score (physical health) | RCT | Greater in G1 than G2: ANCOVA-adjusted IE=2.1 (p<0.0584) |
| MHC score (mental health) | RCT | ANCOVA-adjusted IE=1.7 (p>0.0584) |
| PHQ-2 score (depression) | RCT | ANCOVA-adjusted IE=0.26 (p>0.0584) |
| Number of activities of daily living (ADLs) difficult to do | RCT | ANCOVA-adjusted IE=0.03 (p>0.0584) |
| Number ADLs receiving help | RCT | ANCOVA-adjusted IE=0.00 (p>0.0584) |
| Helping to cope with a chronic condition | RCT | ANCOVA-adjusted IE=0.19 (p>0.0584) |
| Number of helpful discussion topics | RCT | ANCOVA-adjusted IE=0.20 (p>0.0584) |
| Discussing treatment choices | RCT | Greater in G1 than G2: ANCOVA-adjusted IE=0.23 (p<0.0584) |
| Communicating with providers | RCT | Greater in G1 than G2: ANCOVA-adjusted IE=6.55 (p<0.0184) |
| Getting answers to questions quickly | RCT | ANCOVA-adjusted IE=4.90 (p>0.0584) |
| Multimorbidity Hassles score | RCT | ANCOVA-adjusted IE=−0.44 (p>0.0584) |
| Percentage receiving help setting goals | RCT | ANCOVA-adjusted IE=6.1 (p>0.0584) |
| Percentage receiving help making a care plan | RCT | ANCOVA-adjusted IE=3.9 (p>0.0584) |
| Self-efficacy: Take all medications | RCT | ANCOVA-adjusted IE=0.20 (p>0.0584) |
| Self-efficacy: Plan meals and snacks | RCT | ANCOVA-adjusted IE=0.15 (p>0.0584) |
| Self-efficacy: Exercise 2 or 3 times weekly | RCT | ANCOVA-adjusted IE=0.20 (p>0.0584) |
| Self-care activities: Prescribed medications taken | RCT | ANCOVA-adjusted IE=0.06 (p>0.0584) |
| Self-care activities: Followed healthy eating plan | RCT | ANCOVA-adjusted IE=−0.03 (p>0.0584) |
| Self-care activities: 30 minutes of continuous physical activity | RCT | Greater in G1 than G2: ANCOVA-adjusted IE=0.63 (p<0.0584) |
ADL = activities of daily living; ANCOVA = analysis of covariance; CI = confidence interval; DiD = difference-in-difference; G = group; IE = intervention effect; MHC = mental health composite; OR = odds ratio; PHC = physical health composite; PHQ-2 = patient health questionnaire-2; RCT = randomized controlled trial.
Table B-67Clinical and functional outcomes for home-based care model studies: Subgroup outcomes
| Clinical and Functional Outcomes | Study Design | Difference Between Intervention Group (G1) and Comparison Group (G2) |
|---|---|---|
| Oxygen saturation test | RCT | Original sample COPD subgroup: DiD OR=1.02 (95% CI, 0.77 to 1.34)84 |
| RCT | Refresh sample COPD subgroup: DiD OR=0.97 (95% CI, 0.77 to 1.22)84 | |
| HbA1c test | RCT | Original sample diabetes subgroup: DiD OR=0.91 (95% CI, 0.74 to 1.13)84 |
| RCT | Refresh sample diabetes subgroup: DiD OR=0.98 (95% CI, 0.82 to 1.18)84 | |
| LDL-C test | RCT | Original sample diabetes subgroup: DiD OR=0.92 (95% CI, 0.75 to 1.12)84 |
| RCT | Refresh sample diabetes subgroup: DiD OR=0.97 (95% CI, 0.77 to 1.22)84 | |
| RCT | Original sample IVD subgroup: DiD OR=0.89 (95% CI, 0.76 to 1.05)84 | |
| RCT | Refresh sample IVD subgroup: DiD OR=0.95 (95% CI, 0.81 to 1.11)84 |
CI = confidence interval; COPD = chronic obstructive pulmonary disease; DiD = difference-in-difference; HbA1c = hemoglobin A1c; IVD = ischemic vascular disease; LDL-C = low-density lipoprotein-cholesterol; OR = odds ratio; RCT = randomized controlled trial.
Appendix B. Figures
Footnotes
- a
Theory and guidance from National Academy of Medicine report: “Determining an ideal definition for a high-need patient requires a delicate balance. A highly constrained definition will risk missing people, potentially depriving them of needed resources. On the other hand, casting an overly broad definition might include people who are not high-need and do not need additional resources. Abrams noted that basing identification of high-need patients exclusively on cost will miss many people, and if the focus is exclusively on chronic conditions, a large number of people may be identified whose chronic conditions are under control.”12
- Results - Management of High-Need, High-Cost Patients: A “Best Fit” Framework Sy...Results - Management of High-Need, High-Cost Patients: A “Best Fit” Framework Synthesis, Realist Review, and Systematic Review
Your browsing activity is empty.
Activity recording is turned off.
See more...