NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Addendum to Haematological Cancers: improving outcomes (update). London: National Institute for Health and Care Excellence (NICE); 2016 May.
B.1. Methods
Information in this report is drawn from a number of primary sources this section highlights key aspects of relevant methodologies, further details can be found through the reference section.
B.1.1. Definition of included cases and disease groups
Haematological cancers are a very diverse group of malignancies, and traditional disease classification systems (International Classification of Diseases version 10 ICD-10) do not always provide a good match to clinically relevant disease groups. However, the following ICD-10 codes (Table 13) have been used to categorise haematological cancers when national data on incidence, mortality and survival are presented.
Table 13
ICD10 codes for haematological malignancies.
B.1.2. Data sources
New cases of haematological cancers (incidence) in England are registered by the National Cancer Registration Service (NCRS), which is part of Public Health England (PHE).
All deaths in England are certified by a medical professional and then processed by the Office for National Statistics (ONS). The ONS derive a single underlying cause of death – this is what is counted for official statistics.
B.1.3. Age-standardised Rates
To adjust for variation in the age distributions between areas and across time age-standardised rates have been used for measures of incidence and mortality. Rates have been standardised to the European Standard Population (ESP). 1976 ESP weights and ONS mid-year population estimates have been used throughout.
B.1.4. Relative Survival
In a cohort of cancer patients, overall (observed) mortality can be divided into two components: the background mortality, also known as the expected mortality representing all-cause deaths in the general population, and the excess mortality due to cancer. Background mortality is calculated from life tables for England.
Relative survival reflects the excess mortality among cancer patients, over and above the background mortality in the country or region where they live. It is the ratio of the observed survival rate and the expected survival rate of the general population with a similar age/sex structure to the cancer patients in the study.
The survival analyses undertaken in this report use relative survival estimated using the maximum likelihood method for individual records, developed by Estève Et al (1) using the strel2 command in Stata version 13. This method assumes that the hazard is constant within each interval.
B.1.5. Routes to diagnosis
The Routes to Diagnosis study, established by the National Cancer Intelligence Network (NCIN), defines a methodology by which the route the patient follows to the point of diagnosis can be categorised, in order to examine demographic, organisational, service and personal reasons for delayed diagnosis. Administrative Hospital Episode Statistics (HES) data are combined with Cancer Waiting Times (CWT) data, data from the cancer screening programmes and cancer registration data from the National Cancer Data Repository (NCDR). Using these datasets every case of cancer registered in England which was diagnosed in 2006-2013 is categorised into one of eight ‘Routes to Diagnosis’ listed in the table below (Table 14) (Elliss-Brookes et al (2012).
Table 14
Categories of ‘route to diagnosis’.
The methodology is described in detail in the British Journal of Cancer article “Routes to Diagnosis for cancer - Determining the patient journey using multiple routine datasets”.
B.1.6. Patient experience survey
The Cancer Patient Experience Survey 2014 is the fourth iteration of the survey following its successful implementation in 2010, 2012, and 2013. The survey covers all 153 acute and specialist NHS Trusts in England that provide adult acute cancer services.
The 2014 Cancer Patient Experience Survey covered over 118,000 NHS patients who were seen for treatment in hospital in the period 1st September 2013 and 30th November 2013 and who had a primary diagnosis of cancer. More than 70,000 cancer patients responded to the surveya.
B.1.7. National Audit of Cancer Diagnosis in Primary Care
An audit of cancer diagnosis in primary care was undertaken in 2009/10 as part of the National Awareness and Early Diagnosis Initiative (NAEDI) with the intention to better understand and address the reasons for later diagnosis of cancer in England. Information was collected on patient demographics and the nature of the assessment process in primary care, including the time taken from first presentation to referral.
B.2. Key points
Population-based national incidence rates for England (as estimated by cancer registrations) rose over the period 2001-2010 for some haematological cancers: Hodgkin lymphoma, non-Hodgkin lymphoma (NHL) and myeloma. There are no haematological cancers for which incidence rates were in decline over that period.
Registration rates for haematological cancers were subject to change as a consequence of improvements in the ascertainment of new cases and developments in diagnosis and classification of disease; therefore not all observed changes may represent true differences in underlying incidence.
Population-based mortality rates fell over the period 2001-2010 for some haematological cancers: acute lymphoblastic leukaemia, chronic myeloid leukaemia, non-Hodgkin lymphoma and myeloma.
Relative survival improved for individuals in specific age groups who were diagnosed between 2000 and 2010 for a number of haematological cancers: acute lymphoblastic leukaemia (0-14 years males and females; 15-64 years males), acute myeloid leukaemia (15-64 years), chronic myeloid leukaemia, non-Hodgkin lymphoma, and myeloma.
For the most commonly encountered forms of leukaemia in older adult life (65+), acute myeloid leukaemia and chronic lymphocytic leukaemia, there was no evidence of significant change in the outcome for patients diagnosed and registered over this time period.
Although the incidence of haematological malignancy does not generally differ by deprivation, significant differences in the outcomes of patients depending on the level of deprivation in the area in which they live have been noted. For the data examined here, there were some differences observed in incidence by deprivation with higher incidence in the most deprived quintile for acute myeloid leukaemia (AML) and Hodgkin lymphoma. Both Hodgkin lymphoma and NHL showed significantly higher mortality rates in the most deprived quintiles compared to the least deprived; and there were significant differences in relative survival by quintile of deprivation for chronic lymphocytic leukaemia (CLL), chronic myeloid leukaemia (CML) and Hodgkin lymphoma at five years, and myeloma and NHL at one and five years.
For the majority of haematological malignancies GP referral was the most common route to diagnosis, with the exception of AML and ALL where emergency presentation was the most common route with over half of all presentations being via this route. CML and myeloma had similar proportions of GP referral and emergency presentations. All haematological malignancies with the exception of Hodgkin lymphoma had a significantly higher proportion of emergency presentation than all malignancies combined (23%). Relative survival was significantly poorer for emergency presentations for the majority of haematological malignancies. The exception to this was ALL where one-year relative survival for emergency presentations was similar to all other routes. For some acute haematological malignancies emergency presentation may be the most appropriate route to diagnosis.
The majority of patients included in an audit which asked how many times a patients had consulted their GP prior to diagnosis had consulted their GP once or twice (66%), however a third of myeloma patients (33%) had consulted their GP three or more times, and 14% had consulted their GP five or more times. For lymphoma patients 22% of patients had consulted their GP three times or more, and 8% more than five times.
B.3. Introduction
Haematological malignancies are diseases originating in the bone marrow and lymph nodes and include leukaemias, lymphomas and myeloma. They are a very diverse group of diseases affecting people across the whole life course, but with their greatest incidence among the elderly. The prognosis and responsiveness to treatment of these conditions also varies very widely.
Haematological malignancies accounted for 8.4% of all malignant disease (excluding non-melanoma skin cancer) diagnosed in England in the years 2001 to 2010v.
B.3.1. Data quality and availability
Accurate capture of information on haematological malignancies nationally is an ongoing challenge, although data capture has improved over the period reported. Haematological malignancies are extremely diverse, ranging from highly aggressive forms; to some types so benign that are often only picked up incidentally. Some blood changes which could be classified as chronic leukaemias often producing no symptoms, and incidence of these conditions is therefore dependent on looking at blood samples from these individuals and clinicians' criteria for deciding whether a malignancy exists. Even when it is clear that malignancy exists, identifying the specific type requires sophisticated diagnostic techniques, and the integration of information from clinical and laboratory sources, the results of which are not always available to the registration service (NCIN 2013) leading to some registrations not including sufficient detailed information (NICE 2003) to accurately capture the precise diagnosis. This is particularly true of non-Hodgkin lymphoma (NHL), a large and varied group of conditions, which are often considered as a single group as coding has not been of sufficient quality to distinguish individual types of lymphoma.
Although the National Cancer Registration Service (NCRS) now operates as one national system for England, historically there were eight separate cancer registries, with different practices and levels of ascertainment of haematological malignancies, this led to regional variations in capture of information, and changes in trends in incidence may be due to improved ascertainment in former registries.
As well as the national data, collected by the National Cancer Registration Service (NCRS) within Public Health England, we have also reported on regional data from the Haematological Malignancies Research Network (HMRN), and predictions for the UK based on these data. The HMRN is a collaboration across the former cancer networks of Yorkshire and Humber and Yorkshire Coast, between researchers from the University of York, a clinical network operating across 14 hospitals, and an integrated Haematological Malignancy Diagnostic Service (HMDS) at St James's Hospital in Leeds.
Covering a population of 3.6 million, with a similar socio-demographic profile to the country as a whole, HMRN collects detailed information about all patients diagnosed with a haematological malignancy within the HMRN regionb. Within HMRN, all haematological malignancy diagnoses, are centrally coded using the latest World Health Organization (WHO) classifications by clinical staff at HMDS's laboratory. Following diagnosis, patients are individually tracked, and full details of all treatments, responses and outcomes are collected to clinical trial standardisee.
There is a reasonable expectation that due to the incidence of haematological malignancies not being strongly influenced by social position or deprivation that the incidence observed in the HMRN data for the Yorkshire region is likely to be representative of the national picture. In 2013 the NCIN compared national registration data on haematological malignancies with assumptions about England incidence made using the HMRN data. This analysis found that for 2004-10 the two data sources were largely in agreement for acutely presenting conditions such as ALL, with very little notable variation in recording across the country. However, for conditions for which require integration of information from clinical and laboratory sources there was variation; both between the two data sources and geographically – suggesting that this variation is due to different case ascertainment and coding procedures in different registries.
Clinical networks within the HMRN area apply standard treatment protocols in the management of haematological malignancies and therefore regional outcomes are also of value in estimating likely survival patterns for England as a whole.
B.3.2. What is covered in this report and what is not
This report focuses on presenting English national data on seven main groupings of haematological malignancies, which have been used in previous reports by the National Cancer Intelligence Network (NCIN). These are: Acute Lymphocytic Leukaemia (ALL), Acute Myeloid Leukaemia (AML), Chronic Lymphocytic Leukaemia (CLL), Chronic Myeloid Leukaemia (CML), Myeloma, Hodgkin lymphoma and non-Hodgkin Lymphoma grouped together. These groupings are felt to be the most accurate with the data currently available from NCRS. Due to the data quality issues outlined above these have been defined using ICD10 codes. Any specific known data quality issues with each condition are discussed in the relevant chapter.
Many haematological conditions are not included in this report in detail, but remain important in the picture of haematological malignancies as a whole. These particularly include the myeloproliferative disorders, information on which is not currently collected comprehensively by the NCRS. Where possible information the information presented here is supplemented with available regional data from the HMRN.
B.4. All haematological malignancies
B.4.1. Incidence
When considered overall, age-standardised rates of incidence for haematological malignancies have risen significantly from 2001-2010 in both men and women (Figure 6). Part of this trend is a consequence of improved ascertainment of these cancers particularly from 2008 onwards (NCIN 2010).

Figure 6
Incidence of all haematological malignancies by sex, all ages, England 2001 – 2010.
The incidence of all haematological malignancies in males is consistently significantly higher than females over this time period.
Table 15
Age-standardised incidence rates for haematological malignancies diagnosed in the period 2008-2010 by diagnostic group for males and females.
B.4.2. Mortality
Figure 7 shows trends in age-standardised mortality from all haematological malignancies by sex for England between 2001 and 2010. Mortality rates have decreased significantly over this time.

Figure 7
Mortality from all haematological malignancies by sex, all ages, England 2001 - 2010.
Mortality information is taken from the cause of death recorded on the death certificate for an individual and recorded by ONS. Therefore, accuracy of mortality recording for some of the more complex haematological malignancies must be interpreted with care.
B.4.3. Survival
B.4.3.1. Place of death
Figure 8 shows the proportion of deaths by location of death for all haematological cancers compared to non-haematological cancers from a report done by NCIN in 2011. It shows that patients with haematological cancers are significantly more likely to die in hospital than patients with other cancers, with less than half (46.7%) of patients with other cancers dying in hospital compared to 68.4% of patients with haematological cancers.

Figure 8
Proportion of deaths by location for haematological cancers compared to non-haematological cancers, 2001 - 2009.
B.4.4. Patient Experience
Results in this section come from the 2014 cancer patient experience surveyc
64% of patients diagnosed with a haematological malignancy saw their GP no more than twice before being referred to hospital.
75% of patients diagnosed with a haematological malignancy said they had a completely understandable explanation of their test results.
83% of patients said they were told they had a haematological malignancy sensitively.
58% of patients said they completely understood what was wrong with them which is lower than the response from breast cancer where 81% of patients understood what was wrong with them. Haematology scored the lowest of all other cancer sites for this question.
70% of patients said their views were taken into consideration when discussing treatment and 72% said that side effects of the treatment were explained and only 51% said they were told of possible future side effects from the treatment they received.
87% of patients were given a named clinical nurse specialist which is lower when compared to breast, lower GI, lung, brain, gynaecological and upper GI cancer patients where between 90% and 93% of patients were given a named clinical nurse specialist.
55% of patients said they were given information on financial help/benefits by staff, this was low for all cancer sites included in the survey.
82% of patients were told they could get free prescriptions.
37% of patients said they taking part in research had been discussed with them, this was low for all cancer sites included in the survey.
59% of patients said they were given enough care/help from health or social services.
64% of patients said their general practice did everything to support them.
B.4.5. HMRN incidence data
As we have previously discussed national NCRS data do not allow a breakdown of all haematological malignancies into separate conditions, and does not include reliable data on conditions including myelodysplastic syndromes. Therefore Table 16 shows HMRN regional data on incidence of haematological cancers, including myelodysplasia, and expected UK cases per year, which have been estimated by applying HMRN age and sex specific rates to 2001 UK population census strata.
Table 16
HMRN regional age-standardised rates for haematological neoplasms, 2004-13, and expected UK cases per yr.
The most common specific types of haematological malignancies are diffuse large B-cell lymphoma (a type of NHL) which accounts for 13.1% of the estimated incidence; monoclonal gammopathy of undetermined significance (MGUS) which accounts for 11.3% and chronic lymphocytic leukaemia and myeloma, which each account for 10.8% and 10.6% respectively. If the non-Hodgkin lymphomas are grouped together, they account for around 27.1% of all haematological malignancies using these data.
These data are not directly comparable to those captured nationally by the NCRS, due to data collection and coding methodological issues discussed earlier. For instance MGUS is not registered as it is an asymptomatic potential precursor to myeloma, which is not easily ascertainable through normal reporting systems.
Table 17 shows five-year relative survival for all individual haematological malignancies derived from the regional HMRN data. It shows that there is significant variation both between types of haematological malignancy and within the different types. Five-year relative survival for all haematological malignancies combined is 68.3%, however this varies from 45.4% for plasma cell neoplasms (including myeloma) to 85.7% for Hodgkin lymphoma.
Table 17
HMRN regional 5-year relative survival estimates (%).
Within types of malignancy there is also significant variation, for instance five-year survival from hairy cell leukaemia is 90.5%, whereas for acute myeloid leukaemia it is 15.2%.
Trends in one- and five-year relative survival for the specific conditions defined in the introduction are discussed in the relevant chapters later on in this report.
B.5. Acute lymphoblastic leukaemia (ALL)
B.5.1. Incidence
Acute lymphoblastic leukaemia (ALL) is most common in children, with a higher incidence in males than females (Figure 9). Over the period of this report the age-standardised incidence has not changed significantly for either sex (Table 18).

Figure 9
age-specific incidence rates by age group for ALL in males and females between 2006-08 in England.
Table 18
Incidence; Acute Lymphoblastic Leukaemia by sex, all ages, England 2001-2003 to 2008-2010. Three year averages
Figure 10 shows the incidence of ALL by deprivation quintile and sex, it shows there is no significant relationship between deprivation and the incidence of ALL.
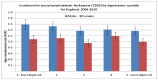
Figure 10
Incidence of ALL by deprivation quintile and sex.
B.5.2. Mortality
Table 19 shows trends in mortality rates from ALL between 2001-03 and 2008-10; there is a small decline in the mortality rates in both sexes.
Table 19
Mortality; Acute Lymphoblastic Leukaemia by sex, all ages, England 2001-2003 to 2008-2010. Three year averages
Figure 11 shows mortality rates for ALL by quintile of deprivation. It shows no significant difference in mortality rates for males by deprivation quintile. For females in the least deprived quintiles the mortality rate is significantly higher than in males, however, in the two most deprived quintiles the mortality rates are more similar to the male mortality rates. The numbers of registered cases are small so patterns should be interpreted with caution.

Figure 11
Mortality for acute lymphoblastic leukaemia (C910) by deprivation quintile for England 2007 - 2011.
B.5.3. Survival
For all ages one-year relative survival for ALL increased between 2000-2003 and 2008-2010, significantly for males from 76.9% (95% CI: 74.5-79.2%) to 83.1% (95% CI:80.4-85.4%) although not significantly for females 77.5% (95% CI: 74.7-80.0%) to 81.0% (95% CI: 77.8-83.7%). Five-year relative survival for ALL increased significantly for both sexes (Figure 12).

Figure 12
Relative 1 and 5 year survival - Acute Lymphoblastic Leukaemia, by sex , diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
The outcome from ALL is strongly influenced by the age at diagnosis, with poorer relative survival in older teenagers and adults.
Over the time period reported here improvements in relative survival are apparent in patients aged 0-14 years, with an increase in relative survival at five years among males and females combined from 83% (95% CI: 81-85%) for individuals diagnosed in 2000-03 to 92% (95% CI: 90- 94%) for those diagnosed in 2008-10 (Figure 13).

Figure 13
Trends in relative survival rates for acute lymphoblastic leukaemia diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 14 shows one- and five-year relative survival for patients with ALL by quintile of deprivation; there is no significant difference in relative survival by quintile of deprivation at either one or five years.

Figure 14
1 and 5-year survival of patients (persons) diagnosed with Acute Lymphoblastic Leukaemia in England, 2000-2007 by deprivation (IMD2004).
B.5.4. Routes to diagnosis – ALL
Figure 15 shows the routes to diagnosis for ALL from 2006-10; the vast majority of admissions for ALL (63.3%) came through the emergency route; this is almost three times the proportion observed for all malignancies combined (23%).
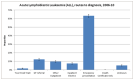
Figure 15
Routes to diagnosis for ALL from 2006-10.
B.5.5. Survival by route
Unlike some other cancer types, where emergency presentations tend to have poorer relative survival, there are no significant differences in survival time by route to diagnosis for ALL (Figure 16). This reflects the fact that diagnosis as an emergency is not an indication of late presentation as is the case for many tumour types.
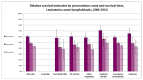
Figure 16
Relative survival estimates by presentation route and survival time, Leukaemia: acute lymphoblastic, 2006-2010.
B.6. Acute Myeloid Leukaemia (AML)
B.6.1. Incidence
Acute myeloid leukaemia is most common in people over the age of 60 and age-standardised incidence is significantly higher in men (Figure 17). Over the period 2001-2010 there was little or no change in the age-standardised incidence of AML (Table 20).

Figure 17
Age-specific incidence rates by age group for acute myeloid leukaemia in males and females in the period 2006-2008 in England.
Table 20
Incidence - Acute Myeloid Leukaemia, by sex, all ages, England 2001-2003 to 2008-2010. Three year averages
Figure 18 shows incidence of AML by quintile of deprivation and sex. There is some evidence of a relationship between deprivation and incidence of AML, with incidence in the most deprived group of males being significantly higher than incidence in the least deprived group (3.8 per 100,000 compared to 4.3). There was no such observable relationship for females.
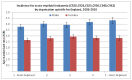
Figure 18
Incidence for acute myeloid leukaemia by deprivation for England. 2006 – 2010)
B.6.2. Mortality
Table 21 presents trends in mortality rates for AML by sex, there has been almost no change in the AML mortality rate for either sex over this time period.
Table 21
Mortality - Acute Myeloid Leukaemia, by sex, England 2001-2003 to 2008-2010. Three year averages.
Figure 19 shows mortality from AML by deprivation quintile and sex, there is no significant differences in mortality rate by quintile of deprivation for AML.
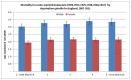
Figure 19
Mortality for acute myeloid leukaemia by deprivation for England, 2007-2011.
B.6.3. Survival
Overall, there was a small, but not significant, increase in one-year relative survival for males and females for AML over the period 2000-2010. There was a significant increase in five-year relative survival for males, but not for females. Female five-year relative survival remained slightly higher than male five-year relative survival (Figure 20).
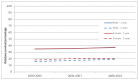
Figure 20
Relative 1 and 5 year survival - Acute Myeloid Leukaemia, by sex, diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
While relative survival among older adults (65+ years) diagnosed with AML was unchanged over this period, a small improvement in outcome was seen in the 25-64 year age range, with an increase in relative survival at five years among males and females combined from 30% (95% CI 28 to 31%) for individuals diagnosed in 2000-03 to 38% (95% CI:36-40%) for those diagnosed in 2008-10 (Figure 21).

Figure 21
Trends in Relative survival rates for acute myeloid leukaemia diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 22 shows one- and five-year relative survival for patients diagnosed with AML by quintile of deprivation, there was no significant relationship between deprivation and relative survival for AML.
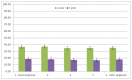
Figure 22
1 and 5-year survival of patients (persons) diagnosed with Acute Myeloid Leukaemia in England, 2000-2007 by deprivation (IMD2004).
B.6.4. Routes to diagnosis
Figure 23 shows a breakdown of routes to diagnosis for AML. The majority of admissions for AML are via the emergency route (52.9%), followed by GP referral (22.6%).The proportion of emergency admissions is almost three times the proportion for all malignancies combined (23%), however, given the acute nature of AML it may be that the emergency route to treatment is entirely appropriate.

Figure 23
Acute myeloid leukaemia (AML) routes to diagnosis, 2006-10.
Figure 24 shows relative survival by route to diagnosis for AML. One-year relative survival is significantly lower for emergency presentations than all other routes. Diagnosis following inpatient elective admissions had significantly better relative survival than all routes combined at one, two and three years.

Figure 24
Relative survival by route to diagnosis for AML.
Figure 25 shows one-year relative survival estimates by age and route to diagnosis. It shows that for the younger age group (0-64) one-year relative survival for emergency admissions (60%) was similar to the overall relative survival (61%), however, one-year relative survival for the 65-84 and 85+ year age groups was significantly worse for emergency admissions than for all other routes combined.
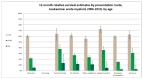
Figure 25
One-year relative survival estimates by age and route to diagnosis.
B.7. Chronic Lymphocytic Leukaemia (CLL)
CLL is a relatively indolent cancer for which histopathology laboratories will not necessarily be involved in diagnosis and where treatment can be delivered in an outpatient setting, factors which combine to reduce the likelihood of notification to cancer registries. There is evidence of wide variation in registration rates at a sub-national level for CLL1 and improvements in ascertainment over time. Therefore, both absolute levels of incidence and trends in incidence should be treated with caution. In addition, as variable levels of ascertainment of CLL may be related to the stage of disease at presentation (with the most indolent cancers probably those least likely to be registered), changes in survival may also be subject to artefact.
B.7.1. Incidence
Chronic lymphocytic leukaemia (CLL) is predominantly a disease of the elderly, with higher age-standardised incidence in males (Figure 26). Table 22 shows the trends in incidence of CLL; there were no marked changes in age-standardised of incidence of CLL over this time period.
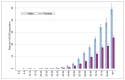
Figure 26
Age-specific incidence rates by age group for Chronic Lymphocytic Leukaemia in males and females in the period 2006-2008 in England.
Table 22
Incidence - Chronic Lymphocytic Leukaemia, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 27 shows the incidence of CLL by quintile of deprivation and sex over the period 2006-2010; there was no relationship between deprivation and the incidence of CLL.

Figure 27
Incidence for chronic lymphocytic leukaemia by deprivation for England, 2006-2010.
B.7.2. Mortality
Table 23 shows trends in mortality from CLL; there were no marked changes across the period reported in the age-standardised mortality of CLL.
Table 23
Mortality- Chronic Lymphocytic Leukaemia, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 28 shows mortality rates for CLL by quintile of deprivation and sex; there was no significant relationship between deprivation and mortality from CLL.
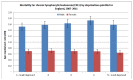
Figure 28
Mortality rates for CLL by quintile of deprivation and sex.
B.7.3. Survival
Figure 29 and 30 show one - and five-year relative survival for males and females with CLL; no statistically significant change in relative survival was observed across this period.
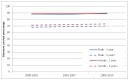
Figure 29
Relative 1 and 5 year survival - Chronic Lymphocytic Leukaemia, by sex, diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.

Figure 30
Trends in relative survival rates for chronic lymphocytic leukaemia diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 31 shows one- and five-year relative survival of patients with CLL by quintile of deprivation. Despite the lack of any association with incidence of CLL and deprivation, relative survival differs by quintile of deprivation, with poorer relative survival in the more deprived quintiles. This becomes significant at five years with five-year relative survival in the least deprived quintile being significantly better than that in the most deprived (76.3% compared to 66.5%).
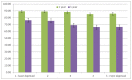
Figure 31
1 and 5 year survival of patients (persons) diagnosed with Chronic Lymphocytic Leukaemia in England, 2000-2007 by deprivation (IMD2004).
B.7.4. Routes to diagnosis
Figure 32 shows a breakdown of the routes to diagnosis for CLL. It shows that the majority of patients were diagnosed via GP referral (43.4%), followed by emergency presentation (24.3%). Fewer patients came in via the two week wait route than for all malignancies combined (11.5% compared to 27.1%).

Figure 32
Routes to diagnosis for CLL.
Figure 33 shows relative survival estimates by route to diagnosis; it shows that emergency presentations had significantly poorer one, two and three year relative survival than all other routes. Patients who came in via the two week wait and GP referral route had significantly better one, two and three year relative survival than all routes combined.

Figure 33
Relative survival estimates by route to diagnosis.
Figure 34 shows relative survival by presentation route and age for CLL. It shows that although one-year relative survival for emergency presentations in the younger age group (0-64) is significantly lower than one-year relative survival for all routes (88% compared to 96%), it is much more comparable than for the older age groups. For the 65-84 year age group one-year relative survival for emergency admissions is 60% compared to 85% for all routes, and for the 85+ group it is 38% compared to 57% for all routes, and 87% in the 2 week wait group.

Figure 34
Relative survival by presentation route and age for CLL.
B.8. Chronic Myeloid Leukaemia (CML)
Cases of CML have been difficult to capture and code accurately as coding systems prior to ICD-10 did not support the required levels of specificity (Caroline's poster). Since the introduction of ICD-10, these cases have been identified using the code C92.1. A recent publication by the National Cancer Intelligence Network (NCIN)v reported significantly poorer CML relative survival rates in the 65+ than the <65 age group which was not consistent with previously reported outcomes by the Haematological Malignancy Research Network (HMRN)2 or with clinical observation. This presented the hypothesis that some cases of CML in the elderly were potentially miscoded.
A recent review of a subset of CML cases by the NCRS team in Northern and Yorkshire confirmed inaccuracies in the final coding, finding that the majority of miscoded CML cases were in the 60+ age groups, confirming the suspicion that some cases of CML, particularly in the elderly, were miscoded. Therefore, interpretation of incidence and relative survival figures for CML presented here should be done with caution.
B.8.1. Incidence
Chronic myeloid leukaemia (CML) is a relatively rare cancer, predominantly affecting people over the age of 60, with higher age-standardised incidence in males (Figure 35). Table 24 shows trends in incidence of CML by sex; there were no changes in the incidence of CML between 2001 and 2010.
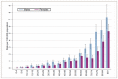
Figure 35
Age-specific incidence rates by age group for chronic myeloid leukaemia in males and females between 2006-2008 in England.
Table 24
Incidence - Chronic Myeloid Leukaemia, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 36 shows incidence of CML by quintile of deprivation and sex; it shows there is no significant relationship between incidence of CML and deprivation.
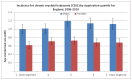
Figure 36
Incidence of CML by quintile of deprivation and sex.
B.8.2. Mortality
Table 25 shows the mortality rates over time for CML by sex; it shows marked changes in the mortality rates over this time, with a significant decrease in mortality for both males (from 0.6 to 0.3 per 100,000) and females (0.4 to 0.2 per 100,000).
Table 25
Mortality - Chronic Myeloid Leukaemia, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 37 shows mortality from CML by quintile of deprivation; it shows that although there is a slighter increasing mortality rate with increasing deprivation for males, this relationship is not statistically significant.

Figure 37
Mortality from CML by quintile of deprivation.
B.8.3. Survival
Figure 38 shows trends in one- and five-year relative survival by sex. It shows a significant improvement for both sexes in both one and five-year relative survival. One-year relative survival has increased from 75.7% to 83.2% for males, and 76.9% to 85.9% in females. Five-year relative survival has increased from 45.8% to 72.2% in males, and 42.8% to 74.3% in females.
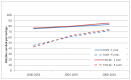
Figure 38
Relative 1 and 5 year survival - Chronic Myeloid Leukaemia, by sex , diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
For patients aged 15-64 years relative survival at five years among males and females combined rose from 59% (95% CI: 56-63%) for individuals diagnosed in 2000-03 to 87% (95% CI: 84-90%) for those diagnosed in 2008-10. Improvement in outcomes has also been observed in older individuals, for patients aged 65 and over relative survival at five years among males and females combined rose from 22% (95% CI 19-26%) for individuals diagnosed in 2000-03 to 44% (95% CI 39-48%) for those diagnosed in 2008-10. However, as discussed above, the observation that reported CML relative survival in older people remains low, may be reflective of miscoding of CML in older patients.

Figure 39
Trends in relative survival rates for chronic myeloid leukaemia diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 40 shows one- and five-year relative survival of patients with CML by quintile of deprivation. It shows that although there is no significant association with deprivation seen with one-year relative survival, at five years relative survival is significantly better in the least deprived quintile than in any other quintile (68.5% in quintile 1 compared to 52.8% in quintile 5).

Figure 40
1 and 5-year survival of patients (persons) diagnosed with Chronic Myeloid Leukaemia in England, 2000-2007 by deprivation (IMD2004).
B.8.4. Routes to diagnosis
Figure 41 shows a breakdown of the routes to diagnosis for CML. The majority of patients with CML are diagnosed either via GP referral (32.5%) or emergency presentation (34.2%) routes. The proportion of emergency presentations were higher than for all malignancies combined (22.9%).
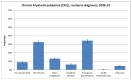
Figure 41
Breakdown of the routes to diagnosis for CML.
Figure 42 shows relative survival estimates by route to diagnosis for CML. Those individuals diagnosed via the emergency route have significantly poorer relative survival than all other routes to diagnosis. Patients who came in via the two week wait, and the inpatient elective route had significantly better one, two and three year relative survival than all routes combined.

Figure 42
Relative survival estimates by route to diagnosis for CML.
B.9. Myeloma
Myeloma is predominantly a disease of older people, with low incidence before the age of 50 years; the incidence is greater in men at all ages.
B.9.1. Incidence
Over the reported period the age-standardised incidence of the disease rose significantly in both males and females, from 6.0 in 2001-03 to 7.0 per 100,000 in 2008-10 in males, and from 3.9 to 4.5 per 100,000 in females over the same time period (Table 26). The rising registration rates for myeloma may in part be due to greater ascertainment of cases, particularly in the elderly.
Table 26
Incidence - Multiple myeloma, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 43 shows incidence of myeloma by quintile of deprivation. There is no relationship seen between deprivation and incidence of myeloma.
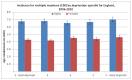
Figure 43
Incidence of myeloma by quintile of deprivation.
B.9.2. Mortality
Table 27 shows trends in the age-standardised mortality rate from myeloma. It shows that mortality for males and females fell slightly over this time, this was significant for males (3.7 to 3.4 per 100,000) but not for females (2.5 to 2.3 per 100,000).
Table 27
Mortality- Multiple myeloma, by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 44 shows age-standardised mortality rates for myeloma by quintile of deprivation. It shows that there is no significant difference in mortality rates between the most and least deprived quintiles for either males or females.

Figure 44
Age-standardised mortality rates for myeloma by quintile of deprivation.
B.9.3. Survival
The time period covered in this report shows an improvement in relative survival in patients with myeloma. There was an increase in relative survival at 5 years among males (all ages) from 30% (95% CI: 29-31%) for individuals diagnosed in 2000-03 to 43% (95% CI: 41-44%) for those diagnosed in 2008-10. Among female patients (all ages) with myeloma there was an increase in relative survival at 5 years from 28% (95% CI: 27-30%) for individuals diagnosed in 2000-03 to 39% (95% CI: 37-40%) for those diagnosed in 2008-10 (Figure 45).
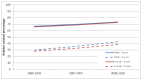
Figure 45
Relative 1 and 5 year survival - Multiple myeloma, by sex , diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
Figure 46 shows one- and five-year relative survival by age group for myeloma. It shows that there have been significant increases in one and five-year relative survival for both the 15-64 year age group, and the 65+ age group.
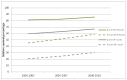
Figure 46
Trends in relative survival rates for myeloma diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 47 shows one and five-year relative survival of patients diagnosed with myeloma by quintile of deprivation. Despite the lack of any observable relationship between incidence of myeloma and deprivation, relative survival shows significant variation by quintile of deprivation, with significantly poorer one-year and five-year relative survival in the most deprived quintile (66.1% one-year relative survival in the most deprived quintile compared to 71.4% in the least, and 30.5% five-year in the most deprived quintile compared to 35.5%).

Figure 47
One and five-year relative survival of patients diagnosed with myeloma by quintile of deprivation.
B.9.4. Routes to diagnosis
Figure 48 shows a breakdown of the routes to diagnosis for myeloma. The highest proportion of diagnoses occur via emergency admission (35.3%), followed by GP referral (33.8%). The proportion of emergency admissions for myeloma is significantly higher than for all malignancies combined (35.3% compared to 22.9%).
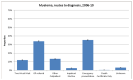
Figure 48
Routes to diagnosis for myeloma.
Figure 49 shows one-, two- and three-year relative survival by presentation route for myeloma. It shows that similarly to other sites, emergency presentations with myeloma had significantly poorer one-, two- and three-year relative survival than all other routes.
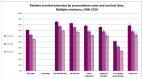
Figure 49
One-, two- and three-year relative survival by presentation route for myeloma.
Figure 50 shows one-year relative survival estimates for myeloma by age and route to diagnosis. It shows that one-year relative survival for emergency presentations is significantly lower than all routes for each age band respectively.
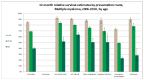
Figure 50
One-year relative survival estimates for myeloma by age and route to diagnosis.
B.9.5. Primary care consultations
As part of the National Audit of Cancer Diagnosis in Primary Care (Royal College of General Practitioners, 2011), participating practices were asked to count all consultations relating to the presenting problem that was associated with the patient's cancer. The majority of patients included in the audit had consulted their GP once or twice (66%), however a third of myeloma patients (33%) had consulted their GP three or more times, and 14% had consulted their GP five or more times (Figure 51).
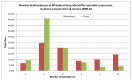
Figure 51
Number of attendances at GP before being referred for specialist assessment.
B.10. Hodgkin lymphoma
B.10.1. Incidence
The age distribution for Hodgkin lymphoma has two peaks, the first in young adults and the second in old age. In the age range 15-24 years the incidence of disease is higher in females, but at all other ages the disease is more common in males (Figure 52). Over the period reported, incidence has risen significantly in both males and females (Table 28).
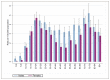
Figure 52
Age-specific incidence rates by age group for Hodgkin lymphoma in males and females between 2006-2008 in England.
Table 28
Incidence - Hodgkin lymphoma by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 53 shows incidence of Hodgkin lymphoma by quintile of deprivation; incidence of Hodgkin lymphoma in males is significantly higher in the most deprived quintile (3.6 per 100,000 population) than in the least deprived quintile (3.0 per 100,000). There was no significant difference in incidence by deprivation for females.

Figure 53
Incidence of Hodgkin lymphoma by quintile of deprivation.
B.10.2. Mortality
Table 29 shows trends in age-standardised mortality rates from Hodgkin lymphoma; mortality from Hodgkin lymphoma did not change significantly between 2001 and 2010 for either males or females.
Table 29
Mortality- Hodgkin lymphoma by sex, England 2001-2003 to 2008-2010. Three year averages
Figure 54 shows age-standardised mortality rates by quintile of deprivation for Hodgkin lymphoma. It shows that the mortality rate for males in the most deprived quintile (0.62 per 100,000) is significantly higher than that in the least deprived (0.39 per 100,000). There is no significant difference in mortality rates by deprivation for females
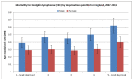
Figure 54
Age-standardised mortality rates by quintile of deprivation for Hodgkin lymphoma.
B.10.3. Survival
Relative survival did not change for females or males in any age group over this time period. Survival is good in children and young adults, but a poorer outcome is seen for elderly patients (Figures 55 and 56).

Figure 55
Relative 1 and 5 year survival - Hodgkin lymphoma, by sex , diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
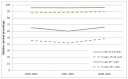
Figure 56
Trends in relative survival rates for Hodgkin lymphoma diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 57 shows one- and five-year relative survival by quintile of deprivation for Hodgkin lymphoma; it shows that five-year relative survival is significantly poorer in the most deprived quintile (80.3%) compared to the least deprived (86.9%), although there is not a clear gradient across deprivation quintiles.

Figure 57
1 and 5-year survival of patients (persons) diagnosed with Hodgkin lymphoma in England, 2000-2007 by deprivation (IMD2004).
B.10.4. Routes to diagnosis
Figure 58 shows the breakdown of routes to diagnosis for Hodgkin lymphoma. It shows that the majority of patients were diagnosed via GP referral (34.9%) and the two week wait route (27.8%). Only 16.4% of patients were diagnosed via an emergency presentation, the lowest of any of the haematological cancers discussed in this report, and significantly lower than for all malignancies combined (22.9%).

Figure 58
Routes to diagnosis for Hodgkin lymphoma.
Figure 59 shows one, two and 3 year relative survival estimates by presentation route for Hodgkin lymphoma. It shows that in common with other tumour sites, patients with emergency presentations have significantly lower relative survival at all intervals than other routes to diagnosis.
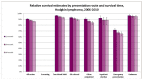
Figure 59
One, two and 3 year relative survival estimates by presentation route for Hodgkin lymphoma.
Figure 60 shows one-year relative survival estimates for Hodgkin lymphoma by age and route to diagnosis. It shows that one-year relative survival was significantly worse in the emergency presentation group for all age groups when compared to all routes combined.

Figure 60
One-year relative survival estimates for Hodgkin lymphoma by age and route to diagnosis.
B.11. Non Hodgkin Lymphoma (NHL)
Non-Hodgkin lymphoma (NHL) is not one but several diseases. For the majority of this report they have been analysed together, but each of the different NHLs has different behaviour, prognosis and treatment, and observed changes in incidence or outcome are unlikely to apply to all forms of NHL.
As discussed in the first section of this report, the quality of coding of the large and varied group of conditions grouped together as NHL, is not currently sufficient to allow disaggregation to the component diseases. However, for some of these analyse we can use HMRN data to give an indication of the breakdown of these individual diseases.
B.11.1. Incidence
The incidence of NHL increases with age, with most cases occurring in the elderly, incidence rates are higher in men at all ages (Figure 61).
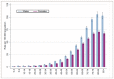
Figure 61
Age-specific incidence rates by age group for Non-Hodgkin lymphoma in males and females between 2006-2008 in England.
Age-standardised incidence rates rose over the period reported in men and women (Table 30). Registration rates for NHL have been rising since the 1970's; it is not clear exactly what the determinants of this apparent increase in incidence are. But it is important to recognise that improvements in the ascertainment of these cancers, with changing thresholds for diagnosis and greater access to diagnostic testing particularly in the elderly, is likely to contribute at least in part to this trend.
Table 30
Incidence - Non Hodgkin lymphoma by sex, England 2001-2003 to 2008-2010. Three year averages
B.11.2. HMRN incidence for NHL
Table 31 shows the expected UK incidence of the individual diseases which make up NHL, and the age-standardised rates for males, females and persons. It shows that two thirds of all NHL consists of diffuse large B-cell lymphoma (48.5%) and follicular lymphoma (18.1%). HMRN estimate just over 10,000 cases of NHL per year in the UK.
Table 31
Expected incidence of Non-Hodgkin lymphoma (HMRN data).
Figure 62 shows incidence of NHL by quintile of deprivation in English national data from NCRS, there is no significant difference in incidence of NHL by quintile of deprivation for either males or females.
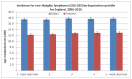
Figure 62
Incidence of NHL by quintile of deprivation.
B.11.3. Mortality
Table 32 shows trends in age-standardised mortality from NHL; age-standardised mortality fell significantly between 2001-03 and 2008-10 for both males and females.
Table 32
Mortality – Non Hodgkin lymphoma by sex, England 2001-2003 to 2008-2010. Three year averages.
Figure 63 presents age-standardised mortality rates for NHL by quintile of deprivation, whilst there is no relationship between deprivation and incidence of NHL, the mortality rate from NHL is significantly higher in the most deprived quintile compared to the least deprived for both males and females (6.7 per 100,000 in the most deprived quintile compared to 6.0 in the least for males, and 4.4 per 100,000 in the most deprived quintile compared to 3.5 in the least for females).
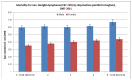
Figure 63
Age-standardised mortality rates for NHL by quintile of deprivation.
B.11.4. Survival
Table 33 shows five-year relative survival for NHL as a whole, and the various conditions that are grouped together as NHL from the Haematological Malignancies Research Network (HMRN).
Table 33
5-year relative survival (%) (HMRN data).
Figures 64 and 65 show trends in relative survival for all NHL combined in English national data from NCRS. There was an increase in relative survival at 5 years among males (all ages) from 54% (95% CI: 53-55%) for individuals diagnosed in 2000-03 to 65% (95% CI: 64-66%) for those diagnosed in 2008-10. Among female patients (all ages) with NHL there was an increase in relative survival at 5 years from 56% (95% CI: 55-56%) for individuals diagnosed in 2000-03 to 67% (95% CI: 66-68%) for those diagnosed in 2008-10.
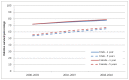
Figure 64
Relative 1 and 5 year survival - Non Hodgkin lymphoma, by sex , diagnosed in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010 in England.
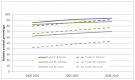
Figure 65
Trends in relative survival rates for Non-Hodgkin lymphoma diagnosed in persons in the periods 2000-2003, 2004-2007 and 2008-2010 followed up to end of 2010, by age group in England.
Figure 66 shows one- and five-year relative survival of patients with NHL by quintile of deprivation, relative survival is significantly lower in the most deprived quintile compared to the least deprived quintile, at both one (72.4% compared to 78.9%) and five years (54.3% compared to 61.3%).
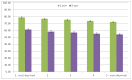
Figure 66
1 and 5-year survival of patients (persons) diagnosed with Non-Hodgkin lymphoma in England, 2000-2007 by deprivation (IMD2004).
B.11.5. Routes to diagnosis
Figure 67 shows a breakdown of referrals for NHL by presentation route. It shows that the highest proportion of referrals came to diagnosis via a GP referral (34.2%), followed by emergency admissions (26.9%). The proportion of emergency admissions for NHL is significantly higher than all malignancies combined (22.9%).

Figure 67
Referrals for NHL by presentation route.
Figure 68 shows one-, two- and three-year relative survival estimates for NHL by presentation route. In common with other haematological malignancies emergency presentations had significantly poorer relative survival at all time intervals, compared to all other presentation routes.
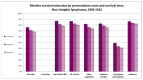
Figure 68
One-, two- and three-year relative survival estimates for NHL by presentation route.
Figure 69 shows one-year relative survival estimates for NHL by age and presentation route. Tere is significantly lower one-year relative survival amongst patients presenting as an emergency for all age groups when compared to all routes as a whole. The effect of the emergency presentation on relative survival seems particularly acute in the older age group, with one-year relative survival in the 85+ emergency presentations group (20%), being less than half that for 85+ patients in all groups combined (46%).
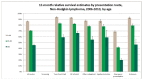
Figure 69
One-year relative survival estimates for NHL by age and presentation route.
As part of the National Audit of Cancer Diagnosis in Primary Care (Royal College of General Practitioners, 2011), participating practices were asked to count all consultations relating to the presenting problem that was associated with the patient's cancer. The majority of patients included in the audit had consulted their GP once or twice (66%), and only 15% had consulted their GP three or more times. For lymphoma patients 22% of patients had consulted their GP three times or more, and 8% more than five times (Figure 70).
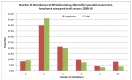
Figure 70
Number of attendances at GP before being referred for specialist assessment.
References
- Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, Richards M. Routes to diagnosis for cancer – determining the patient journey using multiple routine data sets. British Journal of Cancer. 2012;107:1220–1226. www
.bjcancer.com . [PMC free article: PMC3494426] [PubMed: 22996611] [CrossRef] - National Institute for Clinical Excellence. Guidance on Cancer Services – Improving Outcomes in Haematological Cancers – The Manual. 2003.
- NCIN. Blood Cancers Data Quality Report National Cancer Data Repository (NCDR). 2010.
- NCIN. Registration for Blood Cancers in England: comparison of routine data with a specialist population-based register. 2013. http://www
.ncin.org.uk/view?rid=2166 - Royal College of General Practitioners. National Audit of cancer diagnosis in primary care. 2011.
Footnotes
- a
Quality Health – National Cancer Patient Experience Survey: https://www
.quality-health .co.uk/surveys /national-cancer-patient-experience-survey - b
Haematological Malignancies Research Network (HMRN) website - https://www
.hmrn.org/ [accessed August 2015] - c
Quality Health – National Cancer Patient Experience Survey: https://www
.quality-health .co.uk/surveys /national-cancer-patient-experience-survey
- Needs Assessment - Addendum to Haematological CancersNeeds Assessment - Addendum to Haematological Cancers
- Inga sp. 75 TP-2009 isolate 212CC internal transcribed spacer 1, partial sequenc...Inga sp. 75 TP-2009 isolate 212CC internal transcribed spacer 1, partial sequence; 5.8S ribosomal RNA gene, complete sequence; and internal transcribed spacer 2, partial sequencegi|262071491|gb|GU013460.1|Nucleotide
- ow93d11.s1 Soares_fetal_liver_spleen_1NFLS_S1 Homo sapiens cDNA clone IMAGE:1654...ow93d11.s1 Soares_fetal_liver_spleen_1NFLS_S1 Homo sapiens cDNA clone IMAGE:1654389 3', mRNA sequencegi|3254007|gnl|dbEST|1772322|gb|AI0 .1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
