Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient Population. Adult hospitalized patients with Atrial Fibrillation and Flutter. This guideline excludes pregnant women.
Objectives. The purpose of these inpatient care guidelines is to provide an evidence-based blue print for the acute care of patients with atrial fibrillation (AF) and atrial flutter (AFL) at Michigan Medicine. It is hoped that this standardization of care will result in improved patient outcomes, shorter length of hospital stay, lower readmission rates, and overall cost savings for the system and patients.
Key Points
Clinical Presentation
Patients presenting with palpitations, irregular pulse, chest pain, dyspnea, fatigue, lightheadedness, syncope, cardio-embolic disease and new or recurrent heart failure should be evaluated for AF/AFL. While AF may be asymptomatic and found incidentally, AFL is usually highly symptomatic.
Diagnosis
Electrocardiogram (ECG) is essential in the diagnosis of AF/AFL. The initial evaluation is summarized in Table 1 and should include:
Table 1
Diagnostic evaluation of atrial fibrillation and atrial flutter.
- Physical exam
- Laboratory evaluation: CBC, basic metabolic profile, magnesium, thyroid-stimulating hormone, and cardiac enzymes as indicated
- Imaging: Chest X-ray, echocardiogram
- Continuous telemetry monitoring in the hospital
Treatment
Initial treatment of AF/AFL depends on hemodynamic stability:
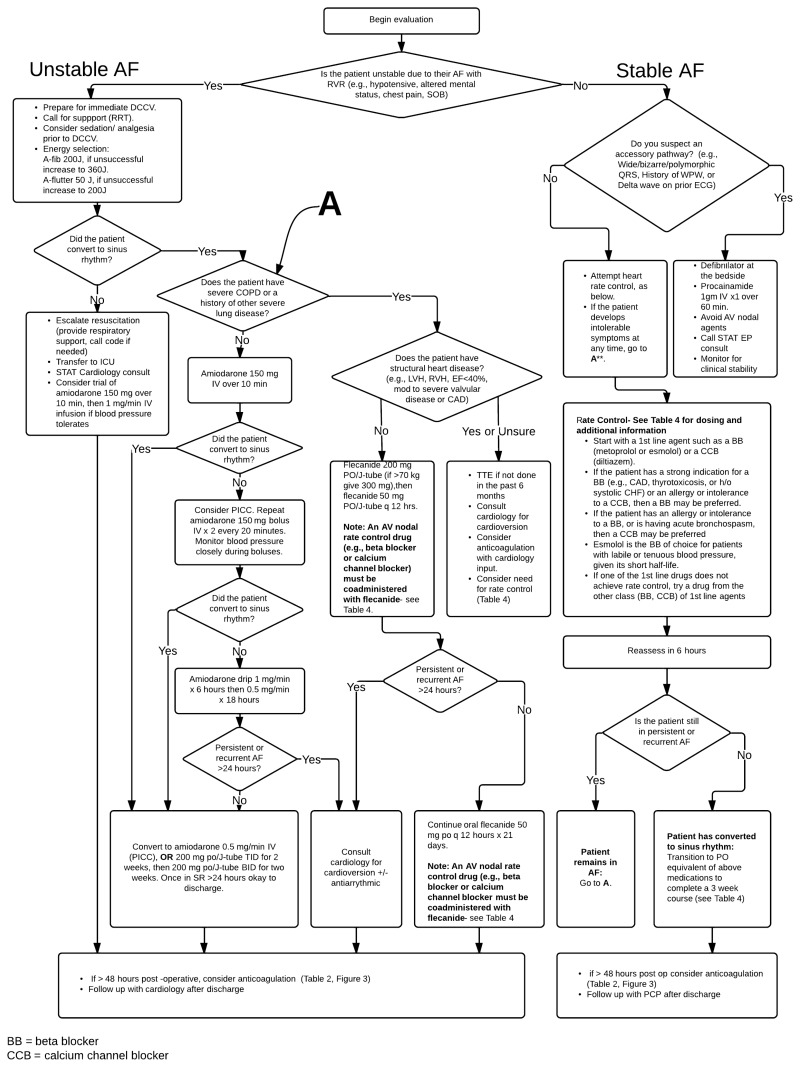
Unstable AF/AFL (Figure 1)
- Begin resuscitation and consider other conditions contributing to instability [I,D]
- If instability is due to AF/AFL, immediate direct current cardioversion (DCCV) is recommended [I,B]
Stable AF/AFL (Figure 2):
- For ED patients: Screen for early cardioversion in the Emergency Department (Figure 4) [II,A]
- If the patient’s heart rate or symptoms are not controlled with rate control medications, consider a rhythm control strategy – [I,B]
Figure 2
Management of STABLE atrial fibrillation and atrial flutter with rapid ventricular response.
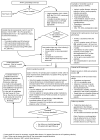
Figure 4
Michigan Medicine Emergency Department evaluation and management of AF/AFL.
Table 2
CHA2DS2VASc score and annual stroke risk.
Table 3
Pharmacologic agents useful for rate control in patients with AF/AFL,.
Box 6
Management of AF in patients with cardiac comorbidities.
Box 2
Rate control summary.
Box 3
Criteria for consulting EP.
Box 4
DC cardioversion (DCCV).
Box 1
Anticoagulation summary. Note: paroxysmal AF/AFL is associated with increased risk for cardioembolic stroke, similar to persistent and permanent AF/AFL.)
Discharge planning and follow-up is summarized in Figure 2 (Box 5).
Box 5
Discharge and follow-up recommendations for Michigan Medicine.
Footnotes
- *
Strength of recommendation:
I = generally should be performed; II = may be reasonable to perform; III = generally should not be performed.
Levels of evidence reflect the best available literature in support of an intervention or test:
A=randomized controlled trials; B=controlled trials, no randomization; C=observational trials; D=opinion of expert panel.
Clinical Problem and Management Issues
Incidence
Atrial fibrillation (AF) and atrial flutter (AFL) are the most common sustained arrhythmias in the U.S., affecting 2.5 million adults with the majority of patients over the age of 65. The burden of AF/AFL is projected to increase over the next few years with forecasts 12 million people in the US by 2030. More than 450,000 hospitalizations with AF/AFL as the primary diagnosis occur annually in the US. (https://www.cdc.gov/heartdisease/atrial_fibrillation.htm).
Rationale for Recommendations
AF/AFL is prevalent in the population of patients evaluated in the Emergency Department (ED) and admitted to the University Hospital. Given the vast spectrum of patient presentations and the breadth of treatment options, management of AF patients is inherently complex. Patients presenting with AF may receive inconsistent care. These guidelines have been developed to support consistent care delivery for patients with AF across the ED and inpatient services. These guidelines are applicable to all inpatients with notable exception of patients on the Cardiology, Cardiac Intensive Care or Cardiac Surgery Services. Many of the recommendations in this guideline are based on information from guidelines from the American College of Cardiology, American Heart Association, and the Heart Rhythm Society.4,5
Diagnosis
Electrocardiographic documentation is essential to establish the diagnosis of AF/AFL. AF is characterized by the replacement of consistent P waves by rapid oscillations or fibrillatory waves that vary in amplitude, shape, and timing, associated with an irregular, frequently rapid ventricular response. Patients in AF/AFL can rarely have regular cardiac cycles (R-R intervals), in the presence of AV block, ventricular tachycardia, or AV junctional tachycardia.
AF may be detected on a 12 lead surface ECG or an ambulatory cardiac monitor. AF may or may not be associated with rapid ventricular response depending on intrinsic atrio-ventricular conduction system, autonomic tone, and medications.
AF may occur alone or be associated with other arrhythmias, notably AFL and atrial tachycardia (AT). AFL in the typical form is characterized by a saw-tooth pattern of regular atrial activation called flutter waves on the ECG, particularly visible in leads II, III, aVF, and V1.
Definitions and Classification
AF and AFL are supraventricular tachyarrhythmias. AF is characterized by uncoordinated atrial activation with consequent deterioration of atrial mechanical function. Typical AFL is a macro-reentrant atrial arrhythmia utilizing the cavotricuspid isthmus. AFL may result from treatment to prevent recurrent AF.
Many of the recommendations in this document apply to both AF and AFL, especially as they relate to rate control and anticoagulation.
There are a number of types of AF/AFL:
- First detected - new onset
- Paroxysmal – self-terminating, episodes generally last 7 days or less (most less than 24 hours)
- Persistent – not self-terminating, episodes usually last longer than 7 days, or require cardioversion
- Permanent – cardioversion failed to acutely convert or was not attempted
- Recurrent – both paroxysmal AF/AFL and persistent AF/AFL may be recurrent
- Post-operative – Post-operative AF/AFL usually occurs within one week of the surgical procedure
Causes and Medical Conditions Associated with AF/AFL
Incidence of AF/AFL increases with increasing age. Many patients have structural heart disease such as coronary artery disease (CAD), hypertensive heart disease, valvular heart disease (especially mitral regurgitation or stenosis), or dilated or hypertrophic cardiomyopathy. Other comorbidities include obesity with or without sleep apnea, diabetes, and bronchopulmonary disease. AF/AFL may be caused by “reversible” causes such as post-operative state, pneumonia, pulmonary embolism, or hyperthyroidism.
Treatment
Clinical Approaches: Unstable and Stable AF/AFL
The approach to the evaluation and management of AF/AFL depends on the clinical circumstances of the arrhythmia. For instance, patients with unstable AF/AFL require immediate supportive care for stabilization, and often require emergent interventions to terminate the arrhythmia (rhythm control). On the other hand, patients with stable AF/AFL may not require emergent treatment, but might need measures to control their heart rate (rate control) and prevent strokes (anticoagulation). Stable patients may not always require interventions to terminate the arrhythmia (rhythm control), although that may be appropriate is some cases.
Because of the differences between these groups, this guideline will divide the discussion into 2 sections:
- Unstable AF/AFL
- Stable AF/AFL
There are also special considerations for populations presenting to the Emergency Department, or those who experience AF postoperatively (with special consideration for thoracic surgery).
Unstable AF/AFL
AF/AFL can be defined as unstable when it results in altered mental status, ischemic chest discomfort, acute heart failure, or other signs of shock or hemodynamic instability. Patients with unstable AF/AFL require rapid stabilization. Differentiating between hemodynamic instability that is a direct result of the arrhythmia and instability in the setting of AF/AFL but secondary to another cause (such as sepsis, hypovolemia, ACS or PE) will help to determine the focus of treatment efforts.
An overview of initial management of unstable AF is illustrated in Figure 1, and includes cardiac monitoring, supplying supplemental oxygen, obtaining large-bore IV access, and addressing intravascular volume depletion with IV fluid administration, provided the patient does not have an acute exacerbation of heart failure.
At the same time, a focused history and physical should be conducted, with special attention paid to the duration and nature of symptoms, comorbidities, and identifying reversible causes of AF/AFL. Underlying causes of hemodynamic instability other than the arrhythmia should be sought and treated, including early resuscitation for sepsis, blood products for severe anemia, cardiac catheterization for ACS, anticoagulation and possible thrombolysis for pulmonary embolism. Supportive treatment with vasoactive agents may also be necessary. Although cardioversion should be considered in such patients, it is important to recognize that many of the critically ill patients seen in the ED may have chronic AF or additional comorbidities that may lead to failed or short-lived effects of cardioversion. The main focus should be placed at treating the underlying condition causing the hemodynamic instability.
In patients who are hemodynamically unstable as a result of AF/AFL without other causes for shock or hypotension, immediate synchronized direct current cardioversion (DCCV) is first-line treatment. For AF, the recommended initial energy level is 200 joules (J), which can be increased to 360 J if lower energy levels are unsuccessful. For AFL, the recommended initial energy level is 50 J, which can be increased to 200 J if unsuccessful.
If cardioversion is repeatedly unsuccessful, a STAT CCU consult should be placed to help direct care. Resuscitative efforts should also be escalated to include consideration of respiratory support, additional vasoactive agents, and calling a code if needed. A trial of amiodarone with an intravenous loading dose of 150mg over 10 minutes followed by a 1mg/min IV infusion can also be considered.
If a patient with unstable AF/AFL is successfully cardioverted with resolution of clinical instability, post-cardioversion treatment should then transition to diagnostic evaluation as directed by Table 1 as well as considering anticoagulation (Figure 3) and placing an EP consult for assistance with long-term rhythm management of AF/AFL.
Stable AF/AFL with RVR
Stable AF/AFL with rapid ventricular response (RVR) can be defined as AF/AFL with HR > 110 at rest with no signs of instability listed above. Stable AF/AFL can be new, chronic, or paroxysmal, and differentiating between these classes can have important long-term management implications. In this section, we will focus on the evaluation and management of any type of AF with RVR.
Evaluation of New-Onset AF/AFL. The evaluation of AF/AFL is intended to discover the cause of the arrhythmia, and clinical features of the patient that might impact treatment (See Table 1).
Management of New-Onset AF/AFL. The management of stable, new-onset AF/AFL with RVR can be divided into these general categories:
- Treatment of underlying conditions
- Consideration of an accessory pathway
- Control of heart rate
- Consideration of rhythm control
- Anticoagulation
- Subspecialty consultation as needed
- Discharge planning and follow-up
Often, several of these categories of treatment will be applied, in parallel. However, the main goals of AF/AFL with RVR management are symptomatic improvement (via rate/or rhythm control), and prevention of thromboembolic complications (with antiplatelet or anticoagulant medications). An overview of the management of stable AF/AFL is illustrated in Figure 2.
Treatment of underlying conditions. Patients with AF/AFL with RVR can have a number of underlying acute conditions (infectious, hypovolemia, anemia, etc.) that may be driving the tachycardic response. These should be suspected and appropriately evaluated early, as treatment of these underlying conditions is key to resolving the RVR in such patients.
Consideration of an accessory pathway. Some patients with AF/AFL will present with features suggestive of an accessory pathway associated with Wolff-Parkinson-White (WPW) Syndrome. The ECG of a patient with preexcitation during AF/AFL typically shows varying degrees of preexcitation, variable RR intervals and variable (bizarre) QRS morphologies. This represents a special circumstance in the management of AF/AFL and necessitates an urgent EP consult. 6
Drugs contraindicated in patients with accessory pathways include digoxin, and non-dihydropyridine calcium channel antagonists (eg, verapamil, diltiazem), which slow conduction across the AV node, and can result in paradoxical acceleration of the ventricular rate, hypotension, or ventricular fibrillation. Beta-blockers are ineffective and may cause hypotension. When the arrhythmia is associated with hemodynamic compromise, early DCCV is indicated (see Figure 1). In hemodynamically stable patients with preexcited AF/AFL, procainamide is recommended to restore sinus rhythm. Further management should be guided by consultation with EP. Of note, any patient with preexcitation and syncope, with or without history of AF/AFL, warrants inpatient EP consultation.
Control of heart rate. One of the primary management goals for patients with AF/AFL with RVR is the control of the patient’s heart rate, as shown in Figure 2, and summarized in Box 2. In the short-term, the goal of controlling the heart rate is to control any symptoms that the patient may be experiencing. Rate control medications slow conduction through the AV node, thus slowing ventricular rates in rapid AF/AFL. In the absence of pre-excitation syndromes, non-dihydropyridine calcium channel antagonists and beta-blockers are most commonly used, and can safely achieve rate control in most patients. Table 3 summarizes important information about the medications that are currently recommended for controlling heart rate. Asymptomatic patients, or mildly symptomatic patients, can be treated with oral medications, which can be rapidly titrated to control heart rate. The doses of both metoprolol tartrate and short-acting diltiazem can be increased every 4-6 hours to achieve heart rate control. For more highly symptomatic patients, heart rate control and symptom relief can be hastened by the addition of intermittent IV injections of these same medications, as shown in Table 3. Continuous IV medications should not be frequently required, and should be reserved for situations in which the use of oral medications and intermittent IV injections fail to control the heart rate or symptoms.
It is important to note that drugs with a negative inotropic effect (e.g., beta-blockers, and non-dihydropyridine calcium channel-blockers) are contraindicated in patients with decompensated HFrEF and hypoperfusion.4,7 Signs of hypoperfusion may include acute kidney or liver injury, elevated lactate level, cool extremities, poor response to diuretics, or hypotension.8 Although beta-blockers are recommended in the treatment of HFrEF, they should only be initiated in patients with compensated disease. Continuation of oral home beta-blockers is recommended in stable patients with decompensated HFrEF and normal perfusion. These patients may even tolerate cautious/slow up-titration when performed alongside other treatments, although rapid titration to treat RVR is not recommended. When using beta-blockers in patients with compensated HFrEF, start low doses (e.g., metoprolol tartrate 6.25 mg PO BID, carvedilol 3.125 mg BID or metoprolol succinate 12.5 mg PO daily) and titrate gradually (e.g., weekly). Alternative strategies of rate control are required when managing AF patients with decompensated HFrEF (See Box 6). Cardiology consultation is recommended for patients presenting with decompensated systolic heart failure and AF/AFL with RVR. Digoxin is a useful rate control agent in this population, as discussed more below.
In patients without HFrEF, beta-blockers should be considered early for the treatment of new-onset, rapid AF/AFL. Beta-blockers are very effective for rate control, achieving the specified heart rate endpoints in 70% of patients compared with 54% with use of calcium channel antagonists in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial.9 Beta-blockers also have additional indications for comorbidities that are commonly found in patients with AF/AFL, such as hypertension, CAD, or heart failure, although they may not be well tolerated by patients with acute bronchospasm or severe emphysema. Table 3 describes some of the beta-blockers that are commonly used for AF/AFL. Oral beta-blockers may cause hypotension and should not be used in the setting of a labile or tenuous blood pressures. In this setting, esmolol infusion is preferred due to its short onset of action and rapid clearance. Of note, propranolol is most useful in the setting of thyrotoxicosis.
Non-dihydropyridine calcium channel antagonists, such as diltiazem and verapamil, are another effective class of medications for heart rate control in AF/AFL. Calcium channel antagonists should be used in the setting of a contraindication to beta-blockers such as an allergy or bronchoconstriction. Diltiazem, given its rapid onset, tends to be more popular, and has been shown in a randomized controlled trial to be more effective in controlling the ventricular rate than amiodarone or digoxin.10
Should a patient with rapid AF/AFL not achieve adequate control of their heart rate following an initial trial of one of the AV nodal blockers, the other class of AV node blockade should subsequently be substituted or added. Co-administration of oral beta-blockers and non-dihydropyridine calcium channel antagonists for long term heart rate control may be necessary, and is generally safe with the appropriate monitoring. If both calcium channel antagonists and beta-blockers prove to be ineffective, a rhythm-control strategy should be considered.
Digoxin has been largely replaced by more effective AV nodal blockers, but remains useful as an adjunct in patients with heart failure. Digoxin has little, if any, effect on blood pressure, so it can also be useful in hypotensive patients. Therefore, digoxin can be an effective short-term tool for acutely controlling heart rate in patients with AF/AFL with RVR and concomitant decompensated HFrEF (see Table 3).
Digoxin tends to exert its rate controlling effect by enhancing vagal tone. Therefore, it is often ineffective in circumstances of increased sympathetic activity, such as exercise or critical illness.11 Therefore, digoxin is often combined with another AV nodal blocker when used chronically.
Amiodarone is known to slow ventricular rates during AF/AFL. It is generally indicated only when maintenance of sinus rhythm is desired given its significant toxicities, but can be used for rate control in some circumstances (see Table 3).
Table 3 provides a review of drugs used for control of ventricular rate in AF/AFL, their side effects, and contraindications. Although short-term control of heart rate is aimed at controlling symptoms, long-term control of heart rate is also important to prevent the development of a tachycardia-induced cardiomyopathy, as a sustained, uncontrolled tachycardia may lead to deterioration of ventricular function. Fortunately, this tachycardia-induced cardiomyopathy tends to resolve within 6 months of rate or rhythm control.
For heart rate that cannot be controlled well with medications after 48 hours, a rhythm-control strategy should be considered.
Defining adequate rate control. Based on the results of the RACE II trial, a resting heart rate <110 bpm indicates adequate rate control.12 Treatment to achieve strict rate control of heart rate (80 bpm at rest or 110 bpm during a 6-minute walk) is not beneficial compared to achieving a resting heart rate 110 bpm in patients with persistent AF/AFL who have stable ventricular function (left ventricular ejection fraction ≥ 40) and no or acceptable symptoms related to AF/AFL. Of note, long-term (> 3 years) effects on ventricular function were not evaluated in this study, therefore periodic monitoring of LV function is recommended.
Consideration of rhythm control. A rhythm-control strategy is one in which the goal is to return the patient to a normal sinus rhythm via cardioversion and can be applied acutely, or as part of chronic management. Rhythm-control is most strongly indicated in hospital patients who are unstable because of the AF/AFL (see Figure 1), or those whose heart rate or symptoms cannot be adequately controlled with a rate-control strategy (see Figure 2).
In the hospital, cardioversion is typically accomplished by the use of direct-current cardioversion (DCCV). DCCV can be performed with or without TEE, as shown in Box 4. Of note, several studies have now documented the utility of cardiac computed tomographic angiography (CTA) in ruling out left atrial/left atrial appendage (LA/LAA) thrombus prior to cardioversion. Although most guidelines recommend TEE for this purpose, CTA is an alternative test for this, and has some advantages, including its noninvasive nature, and ability to perform it without sedation and in the setting of oral, esophageal, or gastric diseases. When performed with delayed imaging, the sensitivity of CTA for LA/LAA thrombus is nearly 100% (compared to TEE). CTA does not provide all of the information that is provided by TEE (it does not demonstrate spontaneous echo contrast, chamber function, or valvular function). But, if there is no other indication for TEE, CTA is a viable alternative for ruling out LA/LAA thrombus before cardioversion.36
At Michigan Medicine, any provider can order a cardioversion, and EP consultation is not required. However, once a rhythm-control strategy is adopted, an EP consultation can be very helpful in guiding the care of these patients. Box 3 in Figure 2 provides guidance on when EP consultation is most likely to be helpful.
There are several antiarrhythmic medications that may be used for rhythm control (see appendix A). The clinical use of these medications is a complex endeavor, typically done in consultation with EP or cardiology, although some of these agents may be given prior to consultation for unstable patients, or for patients on certain specialty services (e.g., Cardiac Surgery, Thoracic Surgery).
Previous clinical trials showed no convincing evidence that a rhythm-control strategy is associated with a morbidity or mortality benefit when compared to a rate-control strategy. The AFFIRM trial found no difference in mortality or stroke rate between elderly patients with permanent AF assigned to one strategy or the other.9 The RACE (Rate Control vs. Electrical cardioversion for recurrent persistent AF) trial found rhythm control with cardioversion and antiarrhythmic medications not inferior to rate control for prevention of death and morbidity.13 The trial data do not necessarily apply to younger patients without heart disease or to patients whose dependency upon sinus rhythm is likely to change over time. Subsequent development of ventricular hypertrophy, systolic heart failure, or pulmonary disease may affect patient’s symptomatic status. Such a patient may not feel different in sinus rhythm when initially evaluated but if left in AF/AFL may face difficulties in the future with an increase in difficulty restoring sinus rhythm because of atrial remodeling. Therefore, eventual attempts at rhythm control are sometimes considered in these patients in follow-up, but they are not usually part of the decision-making for inpatients.
The EAST-AFNET 4 trial, a more recent trial comparing rhythm vs. rate control strategies in patients with recently diagnosed first-onset atrial fibrillation (within 12 months), did demonstrate a significantly lower risk for cardiovascular death and stroke in the rhythm control group, challenging the conventional teaching on this point.14 This study underscores the importance of a comprehensive management strategy for patients with AF, including dedicated follow-up. However, it does not impact the inpatient management of AF, as the rhythm control approach was enacted within a year of the diagnosis of AF.
For inpatients, the main indications for an immediate rhythm control strategy remain a.) the inability to achieve adequate HR control with medications, or b.) the inability to medically control the patient’s symptoms.
Treating the patient that spontaneously converts to sinus rhythm. These patients represent a special challenge for the clinician, because it is often unclear whether the AF/AFL was a transient rhythm related to a specific stressor, or if it represents a paroxysm of AF/AFL that will recur in the future. Acute illness that results in transient AF/AFL may indicate a predilection in such patients for future paroxysms of AF/AFL, therefore we recommend close follow-up and monitoring of these patients. (Our recommended approach to postoperative AF/AFL can be found in the Special Considerations: Postoperative AF/AFL section, below.) In most cases, these patients may be treated as if they have paroxysmal AF/AFL, a condition that poses a stroke risk similar to chronic AF. For patients with AF/AFL that convert spontaneously, we recommend these considerations:
- Consider anticoagulation, as per the Anticoagulation section below (see Figure 3).
- Consider the use of an oral rate control agent, depending on the pre- and post-conversion heart rates.
- Provide education to these patients about AF/AFL, including instructions about how to recognize the rhythm, and what to do if it recurs.
- Patients who present with new-onset AF/AFL should have follow up with EP, even if they spontaneously convert to sinus rhythm in the hospital (see the Discharge Planning and Follow-Up section below, and Figure 2, box 5).
- Regardless of whether or not anticoagulation is started initially, the decision can be reassessed in follow up, based on the cardiac event monitor results, and the patient’s CHA2DS2-VASc score, bleeding risk, and preferences.
Ablative therapies. AF ablation with pulmonary vein isolation may be considered for symptomatic patients who have failed at least one antiarrhythmic agent and in those patients who do not wish life-long antiarrhythmic drug therapy. AF ablation is more efficacious and is associated with improved quality of life compared to antiarrhythmic medication. However, risks and benefits of an invasive procedure must be carefully weighed against the benefits of maintaining sinus rhythm.
AFL ablation of typical AFL is a safe, well-tolerated procedure with complete elimination of the arrhythmia in >95% of patients, and should be considered a first line treatment in appropriately selected patients with recurrent typical AFL.15
AV nodal ablation does not result in conversion to sinus rhythm, and it is merely designed to control ventricular response to AF. AV nodal ablation should not be attempted without a prior trial of medication to control the ventricular rate. Catheter AV nodal ablation results in complete AV block and requires implantation of a permanent pacemaker. AV nodal ablation may be a viable option for elderly or frail patients in whom pharmacologic rate control is complicated by hypotensive episodes.
Anticoagulation. Due to the increased risk of stroke and systemic embolism with AF/AFL, anticoagulation is utilized in almost all patients undergoing acute management with electrical or pharmacologic cardioversion, and for long-term stroke prevention based on additional risk factors. This section will present recommendations for anticoagulation of patients with AF/AFL in the following circumstances:
- Acute anticoagulation with cardioversion
- Acute anticoagulation of new-onset AF/AFL, without cardioversion
- Long-term anticoagulation
These recommendations are illustrated in the algorithm in Figure 3. For post-thoracic surgery patients presenting with AF/AFL, refer to Appendix C. In all cases, the risk of bleeding should be considered, and contraindications to anticoagulation should be recognized. While the most common absolute contraindication to anticoagulation therapy is active bleeding, clinicians should weight the risks and benefits of anticoagulation therapy for all patients, including those with recent bleeding, known bleeding diathesis, recent trauma or surgery, thrombocytopenia, history of intracranial hemorrhage, and known high risk bleeding lesions (e.g, intracranial or spinal malignancy).16,17
Left atrial appendage occlusion. (Watchman Device). In patients with indications for long-term anti-coagulation with concomitant bleeding risks, atrial appendage occlusion devices (Watchman Device) can be considered18, via an outpatient referral to the EP Watchman Clinic. Following Watchman device implant, patients are typically placed on an oral anticoagulant and aspirin for 6-7 weeks, and then transitioned only to daily clopidogrel (75 MG daily) for 6 months to device implant. The patient will require follow-up TEE to verify that there is no significant peri-device flow or leak. After 6 months, the patient can be transitioned to daily aspirin. There are off-label exceptions to altering the anticoagulation management post-device implant, but this would require careful multidisciplinary consultation.
Acute anticoagulation with cardioversion. Anti-coagulation is especially important when attempts are made to convert AF/AFL to sinus rhythm. The following anticoagulation recommendations should be used regardless of cardioversion strategy (i.e. pharmacologic or electrical). In addition, AF/AFL that is determined to be less than 48 hours in duration may proceed to cardioversion because the likelihood of prior thrombus formation is low. Even in this clinical scenario, post-cardioversion anticoagulation is generally indicated. Patients in AF/AFL for longer than 48 hours (or if the duration in unknown) have an elevated risk of having developed an atrial thrombus. Therefore, these patients should undergo a TEE- or CT-guided cardioversion. Alternatively, these patients may be maintained on therapeutic anticoagulation for a minimum of three weeks prior to undergoing planned cardioversion. The following recommendations are summarized in Figure 3:
- If the patient has a history of ischemic stroke in the past 2 weeks, consult neurology for anticoagulation recommendations. Figure 3 lists some possible contraindications to anticoagulation.
- If the patient has valvular AF/AFL (moderate to severe mitral stenosis or mechanical heart valve), parenteral anticoagulation as a bridge to warfarin is recommended. Selection of parenteral anticoagulant depends on renal function:
- - If the estimated creatinine clearance is ≥ 30 ml/min, then anticoagulation with subcutaneous enoxaparin 1 mg/kg twice daily can be used in place of IV unfractionated heparin, with transition to warfarin therapy.
- - If the estimated creatinine clearance is < 30 ml/min, then IV unfractionated heparin with transition to warfarin therapy is preferred.
- If the patient has nonvalvular AF/AFL, a direct oral anticoagulant (DOAC) is recommended over parenteral therapy bridging to warfarin, but is also dependent on renal function:
- - If the estimated creatinine clearance is ≥ 30 ml/min, a DOAC is preferred (Appendix B). Subcutaneous enoxaparin 1 mg/kg twice daily with transition to warfarin therapy can be selected in patients that are not candidates for DOAC (cost, drug-drug interactions, etc.)
- - If the estimated creatinine clearance is < 30 ml/min, IV unfractionated heparin with transition to warfarin therapy may be preferred due to less experience with DOAC in this clinical setting. If a DOAC is selected, apixaban is preferred.
- If the patient is on antiplatelet therapy, see discussion in the Special Populations section below.
- Once a patient has undergone cardioversion, all patients should be anticoagulated for at least four weeks after cardioversion, with subsequent long-term anticoagulation therapy based on risk factors, as outlined below (See Long-term anticoagulation.)
Acute anticoagulation of new-onset AF/AFL without cardioversion. The decision for acute anticoagulation of AF/AFL patients who are not selected for initial conversion to sinus rhythm should be based on individual stroke risk, as assessed in nonvalvular AF/AFL by the CHA2DS2-VASc score (Table 2).1 Generally, patients with a high risk for stroke (CHA2DS2-VASc score ≥ 2 [male] or 3 [female]) should be initiated on therapeutic anticoagulation acutely while those with a lower stroke risk (CHA2DS2-VASc score 0 [male] or 1 [female]) do not require immediate therapeutic anticoagulation. Of note, the DOACs have a rapid onset of action and do not require bridging with parenteral agents. An overview for the management of anticoagulation in this setting is illustrated in Figure 3, with the specifics depending on patient characteristics, as follows:
- If the patient has a history of ischemic stroke in the past 2 weeks, consult neurology for anticoagulation recommendations. Figure 3 lists some possible contraindications to anticoagulation.
- If the patient has valvular AF/AFL, they are at increased risk for stroke irrespective of their CHA2DS2-VASc score and should be acutely anticoagulated with a parenteral agent determined by renal function:
- - If the estimated creatinine clearance is ≥ 30 ml/min, then anticoagulation with subcutaneous enoxaparin 1 mg/kg twice daily can be used in place of IV unfractionated heparin, with transition to warfarin therapy.
- - If the estimated creatinine clearance < 30 ml/min, then IV unfractionated heparin with transition to warfarin therapy is preferred.
- - DOACs are not recommended in this patient population, especially patients with mechanical valve replacement.
- If the patient has nonvalvular AF/AFL, a direct oral anticoagulant (DOAC) is recommended over warfarin +/- parenteral therapy, but is also dependent on renal function:
- - If the estimated creatinine clearance is ≥ 30 ml/min, a DOAC is preferred (Appendix B). Warfarin therapy can be selected in patients that are not candidates for DOACs (cost, drug-drug interactions, etc.)
- - Parenteral therapy as a bridge to therapeutic warfarin should be considered if the reduction in stroke outweighs the risk of bleeding complications:
- ▪ Ischemic stroke within past 3 months, bridging recommended
- ▪ Remote ischemic stroke (> 3 months) or CHA2DS2-VASc score ≥ 7, bridging optional based on bleeding risk and patient preference
- ▪ CHA2DS2-VASc score 0-6 without history of TIA/stroke, bridging not recommended
- - If the estimated creatinine clearance is < 30 ml/min and the benefit of stroke reduction from anticoagulation is felt to outweigh the risks of bleeding, warfarin therapy may be preferred due to less experience with DOACs in this clinical setting. See above to determine need for parenteral bridging with warfarin. If a DOAC is selected, apixaban is preferred.
- If the patient is on antiplatelet therapy, see the discussion in the Special Populations section below.
- If the patient is on antiplatelet therapy, see the discussion in the Special Populations section below.
Long-term anticoagulation. Anticoagulation should be continued indefinitely, or until the patient develops a contraindication, even if antiarrhythmic agents appears to maintain sinus rhythm. The need for long-term anticoagulation for prevention of stroke/TIA and systemic embolism is determined based on the patient’s thrombotic risk assessment. Derived from the CHADS2 score, the CHA2DS2-VASc score is a well-studied risk stratification scheme used in the 2012 European Society of Cardiology AF guidelines.19 The CHA2DS2-VASc score accounts for additional risk factors not included in the CHADS2 score and appears to present the advantage of being able to better identify truly low risk patients (CHA2DS2-VASc score of 0 [male] or 1 [female]).1 Given this, for most clinical scenarios we recommend using CHA2DS2-VASc score to determine a patient’s thrombotic risk. In addition to clinical risk stratification, patient and family preferences should be taken into account in decisions about anticoagulant therapy. Of note, prior studies have indicated that when compared to physicians, patients generally place more value on stroke prevention rather than avoiding bleeding. Table 2 shows the CHA2DS2-VASc scoring system and the adjusted rate of stroke per 100 person-years.
The following are recommendations for long-term anticoagulation in AF/AFL patients:
- Patients with valvular AF/AFL are candidates for long-term anticoagulation, regardless of their CHA2DS2-VASc score.
- If the CHA2DS2-VASc score is 0 (male) or 1 (female), it is reasonable to omit anticoagulant therapy
- If CHA2DS2-VASc score is 1 (male) or 2 (female), patients may be treated with an oral anticoagulant or no medication. Risks and benefits should be discussed with the patient.
- If the CHA2DS2-VASc score is ≥ 2, then long term oral anticoagulation is preferred. Oral anticoagulation with a DOAC is preferred over adjusted-dose warfarin unless the patient is not well suited for a DOAC. (see below – Special Populations)20
For patients that are at higher risk for stroke, oral anticoagulation with warfarin is more effective for stroke prevention than anti-platelet therapy (aspirin-clopidogrel combination therapy or aspirin monotherapy). Warfarin should be adjusted to an INR range of 2.0 to 3.0 in most patients with AF/AFL. Warfarin should be adjusted to an INR range of 2.5-3.5 in patients with AF and mechanical mitral valves. The most recent North American and European guidelines on management of atrial fibrillation no longer recommend the use of antiplatelet therapy for stroke prevention in AF/AFL.21,22
The DOACs have demonstrated similar to increased efficacy in stroke prevention and a similar to reduced rate of major bleeding when compared to adjusted-dose warfarin therapy in phase 3 clinical trials of patients with AF/AFL.
Dabigatran. The RE-LY trial compared two doses of dabigatran (150 and 110 mg twice daily) to open-label, adjusted-dose warfarin.23 The majority of patients had a CHADS2 score greater than 2. Dabigatran 150 mg twice daily was found to be superior to warfarin in reduction of stroke or systemic embolism with a similar rate of major bleeding. Life-threatening and intracranial bleeding were significantly lower with dabigatran, whereas more patients suffered gastrointestinal bleeding with high-dose dabigatran. Although rare, the rate of myocardial infarction was higher in dabigatran-treated patients.
Rivaroxaban. Rivaroxaban was compared with adjusted-dose warfarin in the ROCKET-AF trial.24 Patients included had an average CHADS2 score of 3.5. Rivaroxaban was found to be noninferior to warfarin for prevention of stroke or systemic embolism, and the composite of major and non-major clinically relevant bleeding events (primary safety endpoint) was similar. Critical, intracranial and fatal bleeding was significantly lower in the rivaroxaban group, while gastrointestinal bleeding was significantly higher. Within 30 days of stopping the trial, an increase in stroke and systemic embolism occurred in the rivaroxaban group leading to the recommendation for avoidance of abrupt cessation of rivaroxaban in the absence of adequate anticoagulation (an effect also seen with apixaban).
Apixaban. Apixaban was compared to adjusted-dose warfarin in the ARISTOTLE trial.25 The stroke risk of patients was similar to that seen in the RE-LY trial. The trial found a significant reduction in stroke or systemic embolism in the apixaban group compared to warfarin and a reduction in all bleeding indices. The rates of gastrointestinal bleeding and myocardial infarction were not increased. Apixaban was also compared to aspirin 81-324 mg daily in patients at high risk for stroke (average CHADS2 score of 2) but who were deemed “unsuitable” vitamin K antagonist candidates (AVERROES Study26). The most frequent reasons for “unsuitability” were patient refusal and low likelihood of INR measurement at requested intervals, and few patients were included for bleeding history or bleeding risk. The AVERROES trial was terminated early due to significant reduction in stroke or systemic embolism in the apixaban group with no difference in major bleeding events, although minor bleeding was more common in the apixaban group.
Edoxaban. Edoxaban was compared to adjusted-dose warfarin in the ENGAGE AF-TIMI 48 study.27 Edoxaban was shown to be non-inferior to well-managed warfarin overall, with lower risk of stroke or systemic embolism in patients randomized to edoxaban 60 mg daily as compared to patients treated with warfarin. The risk of major bleeding was also lower for patients randomized to edoxaban as compared to warfarin.
Pharmacokinetic and other relevant parameters of the DOACs are compared in Appendix B.28 While all of the agents undergo renal and hepatic metabolism, dabigatran is primarily eliminated renally and rivaroxaban and apixaban undergo more extensive hepatic metabolism. Although these agents have fewer drug-drug interactions than warfarin therapy, there are still clinically relevant interactions to be aware of including P-glycoprotein interactions with all of the agents and CYP3A4 interactions with rivaroxaban and apixaban.
DOACs are preferred over adjusted-dose warfarin therapy, given the outcome data and the reduction in monitoring requirements, dietary interactions, and amount of drug interaction. A few exceptions exist due to a lack of data; including patients with advanced liver or kidney disease, or/and valvular AF/AFL. In addition, a trial comparing dabigatran to warfarin in patients with mechanical heart valves was terminated early due to increased risk of thromboembolic and bleeding complications with dabigatran therapy.29 Warfarin is preferred in these patient populations until more data is available. (See below - Special Populations)
When prescribing DOACs, clinicians must be aware that these agents can be quite costly, and that they are not covered uniformly by all insurance companies. Therefore, when these agents are prescribed, it is critical that clinicians assure that the patient will be able to obtain the medication, and that cost (or insurance coverage issues) is not a barrier to medical compliance after discharge. Determination of an individual’s co-pay for DOACs is done in conjunction with pharmacy and should be done prior to discharge.
Special Populations for Anticoagulation
Some circumstances require special consideration of anticoagulants.
- If the patient has valvular AF (moderate or severe mitral stenosis or mechanical valve replacement), then oral anticoagulation with adjusted-dose warfarin is strongly preferred over other antithrombotic strategies. This is particularly true for patients with mechanical valves, for whom use of DOACs is contraindicated.
- If the patient has AF/AFL and an ischemic stroke within the previous 2 weeks, then consultation with the Stroke Team is recommended for anticoagulation decision.
- If the patient has an estimated creatinine clearance < 30 ml/min, then when oral anticoagulation is deemed appropriate, adjusted-dose warfarin therapy is preferred over other antithrombotic strategies due to the limited data with DOACs in this patient population. Use of apixaban or rivaroxaban are FDA-approved options for patients who strongly prefer the use of a DOAC. When parenteral anticoagulation is desired, unfractionated heparin is preferred over low molecular weight heparin.
- If a patient with AF/AFL also has an indication for antiplatelet therapy, the management will depend on the precise indication for the antiplatelet agent(s):
- If the patient has AF/AFL, and the indication for antiplatelet therapy is primary or secondary prevention of cardiovascular disease, and the patient has not had ACS within the previous year, and has not had coronary stent placement within the last year, then oral anticoagulation alone is sufficient (no antiplatelet agent is needed in addition to the oral anticoagulant)3031.
- If the patient has AF/AFL and ACS within the previous year, without stent placement, then when oral anticoagulation is selected, oral anticoagulation plus single antiplatelet therapy (usually P2Y12 inhibitor) is preferred over triple therapy (oral anticoagulation, P2Y12 inhibitor, and aspirin). When combined with an oral anticoagulant, clopidogrel is the preferred P2Y12 inhibitor agent to reduce bleeding risk31.
- If the patient has AF/AFL and ACS with stent placement, then when oral anticoagulation is selected, triple therapy should be used for as short a duration as possible (up to 1 month) after stent placement. Dual therapy with an oral anticoagulant and a P2Y12 inhibitor can be continued for up to 1 year following stent placement. When combined with an oral anticoagulant, clopidogrel is the preferred P2Y12 inhibitor agent to reduce bleeding risk. Consultation with general cardiology should be considered in such patients31.
- ACC and AHA recommend targeting an INR of 2 to 2.5 for patients receiving triple therapy, but the effectiveness and safety of this approach compared with the conventional INR range of 2 to 3 remains unproven.
- Low dose aspirin is often recommended, in addition to anticoagulation therapy, in patients with mechanical heart valves, or those with bioprosthetic heart valves and additional risk factors.
- For any patient taking combined anticoagulant + antiplatelet therapy, consideration should be made for gastroprotection with the use of proton pump inhibitor therapy.
- Recommendations for postoperative AF are discussed in a separate section below.
Transient AF/AFL that resolves and was caused by “reversible” causes such as post-operative state, pneumonia, pulmonary embolism, or hyperthyroidism in otherwise low risk patients may not require long term anticoagulation. We recommend initiating anticoagulation at the time of diagnosis for most of these patients, based on their CHA2DS2-VASc score, unless bleeding risk is high. We also recommend prescribing a cardiac event monitor at discharge for these patients to assess their burden of AF/AFL, which may help to determine their long-term need for anticoagulation (For details, see Figure 2, Box 5).
Subspecialty Consultation. Consultation of subspecialty services, including general cardiology and electrophysiology (EP), is commonly indicated for hospitalized patients with AF/AFL. Although, these services can be consulted at any time at the discretion of the primary service, the following guidance will try to describe the clinical situations for which cardiology consultation will be indicated, and when EP consultation might be helpful.
General cardiology consultation is indicated when the atrial fibrillation is accompanied by other heart disease, such as acute ischemia, decompensated heart failure, or other complex cardiac disease, as shown in Figure 2. General cardiology is also the most appropriate consultation for assistance with postoperative atrial fibrillation.
EP consultation can be particularly helpful when questions arise about rhythm control (Figure 2 and Box 3). Although EP consultation is not required to order DCCV, the complexity of a rhythm-control strategy makes EP consultation appropriate in many of these patients.
Approach to Refractory Paroxysmal AF/AFL
The goal of management of a patient with refractory paroxysmal AF/AFL, especially in the setting of left atrial enlargement or long history of AF/AFL duration, is to reduce the recurrence rate or delay recurrence as much as possible. Thus, not all AF/AFL recurrences should be considered a therapeutic failure and prompt a change in strategy. When recurrences occur, the impact of AF/AFL recurrence on patient quality of life and adverse events such as repeat hospitalizations should be evaluated. This might involve changing antiarrhythmic agents, considering catheter ablation, concurrently using multiple treatment modalities, or abandoning the goal of rhythm control altogether. Patients should be educated on the pros and cons of ED visits vs. outpatient management based on patient’s individual risk profile and disease pattern. In addition to traditional risk factors for AF/AFL, obstructive sleep apnea has emerged as a significant predictor of AF/AFL recurrence. Patients at risk for sleep apnea (snoring, large BMI, large neck size, daytime sleepiness or fatigue) should be referred for sleep study, and those already diagnosed with OSA should be encouraged to adhere to their CPAP.
Approach to Refractory Persistent/Permanent AF/AFL
Not infrequently, patients with AF/AFL who were previously well rate controlled may present with worsening symptoms. Possible explanations for worsened clinical status may include noncompliance with medication or dietary indiscretion, which may trigger heart failure symptoms with fluid retention, hypoxia, and tachycardia. Patients should be questioned about caffeine use, recent changes to medications, and use of over the counter cold remedies, herbal and nutritional supplements. Thyroid function may need to be reassessed, especially in patients on amiodarone. Patients compliant with oral beta-blockers should be considered for adding oral calcium channel antagonists and vice versa (see Table 4). In cases of recurrent or persistently increased heart rate, AV nodal ablation with a pacemaker should be considered.
Discharge Planning and Hospital Follow-Up
Patients hospitalized with AF/AFL should be given education about AF/AFL during their hospital stay, an informational packet is available from the CVC library and can also be found on 7A in the UH. Appropriate follow-up should be arranged upon discharge from the hospital or ED. General guidance for Michigan Medicine patients is given in Box 5 in Figure 2.
Patients that are discharged directly from the ED (or the Medical Short Stay Unit) should have expedited follow up, to ensure appropriate heart rate control, medication and symptom tolerance, and appropriate anticoagulation management. At Michigan Medicine, this can be accomplished via the Priority EP follow-up clinic.
Before discharge, it should be confirmed that the patient will be able to obtain all new prescriptions, and that there will not be insurance issues. At Michigan Medicine, the Transitions-of-Care Pharmacy Tech can assist with this assessment (pager 34978).
We recommend that floor patients who are on rate-control medications should have a hall-walk on their day of discharge to verify that their heart rates are reasonably controlled when ambulating prior to discharge.
If the patient is discharged on an anticoagulant drug (i.e., warfarin or a DOAC), they should be referred to an anticoagulation clinic (either at Michigan Medicine or via the PCP). To ensure proper adjustment and monitoring of warfarin, and to assist with education and compliance for the DOAC’s.
At the time of discharge, we recommend outpatient cardiac event monitoring, for selected patients. This monitoring should only be ordered at the time of discharge if the results can be directed to the appropriate follow-up cardiologist or EP physician. (At Michigan Medicine, cardiac event monitoring can be accomplished by prescribing a 2-week Ziopatch, if the patient will be following up with a Michigan Medicine physician.) Cardiac event monitoring is recommended for these patients:
- All patients with new onset AF/AFL, including those who are treated with cardioversion or cardiovert spontaneously (to monitor for recurrence), and those that are discharged in atrial fibrillation (to monitor rhythm and rate control)
- Any patient with suspected paroxysmal AF/AFL who would benefit from documenting the frequency and duration of paroxysms
- Any patient with concern for inadequate rate control, or complications of rate control (e.g., bradycardia, heart block)
- Patients treated for TIA or stoke that may be a consequence of undetected AF/AFL
Special Considerations: Emergency Department (ED) Management
The acute ED management of AF/AFL is variable, and few specific treatment recommendations exist. Traditionally, management strategies have focused on rate control of rapid AF/AFL, and admission to the hospital for continued administration of rate control agents and decisions for anticoagulation, and these remain reasonable in many patient populations. Recent studies suggest that for some category of low-risk patients, management via rhythm control is also a reasonable strategy. This section will detail recommendations specific to the ED patient with AF/AFL.
Unstable ED Patient
The evaluation and management of unstable patients with AF/AFL are covered in Figure 1 and the accompanying text above.
Stable ED Patient
Selected, stable patients with AF/AFL can sometimes receive all of their treatment in the ED, avoiding hospital admission (Figure 4).
Rate Control. Most patients who present to the ED with AF/AFL and rapid ventricular response will require medical treatment to slow their heart rate, as shown in Figure 2.
Rhythm Control. A select group of patients with AF/AFL with rapid ventricular response will meet criteria for DCCV in the ED (see Figure 4). These patients would be generally healthy, with known onset of AF/AFL in the last 48 hours or with known anticoagulation for greater than 3 weeks. They should not have failed prior cardioversion attempt for AF/AFL.
Initial rhythm control attempt may be pharmacologic. The best-studied agent in ED populations is procainamide, 1gm IV over 1 hour. Studies show that this results in an approximately 60% cardioversion rate. Procainamide should be held for SBP < 90mmHg, and is contraindicated in patient with renal failure (CrCl < 30), severe liver disease, or lupus. If cardioversion does not occur with procainamide, electrical cardioversion with procedural sedation may be administered. For AF, 200J synchronized cardioversion is recommended, and for AFL, 50J synchronized cardioversion is recommended. Anterior-lateral electrode positioning is more effective than anterior-posterior positioning for biphasic cardioversion of AF.36 If there is no success with the first attempt, higher energy levels (360J for AF or 200J for AFL) may be attempted. For patients who fail cardioversion after these attempts, a rate control strategy should then be utilized, as illustrated in Figure 2, with subsequent admission to the hospital.
Patients with successful cardioversion should be monitored for 3 hours for reoccurrence. Anticoagulation should be initiated per CHA2DS2-VASc score (Table 2, Figure 3). Referrals should be placed for outpatient EP consult, with a preceding transthoracic echocardiogram if they have not had one in the prior 6 months, and the patient may be discharged home.
Criteria for Hospitalization
Inpatient hospitalization is indicated for patients with highly symptomatic, recurrent or new onset AF/AFL, especially if it is poorly tolerated and either rate control or rhythm control are urgently needed. AF/AFL may complicate or exacerbate an acute illness, which would also indicate the need for hospitalization. Well tolerated AF/AFL with controlled rates, whether new onset or recurrent, does not in itself automatically necessitate inpatient admission. Many patients may be treated in the ED with outpatient follow-up with the Electrophysiology Service.
Factors favoring discharge from the Emergency Department:
- Patients <60 years of age with lone AF
- Other patients with no structural heart disease and tolerable symptoms
- Well controlled ventricular rate during AF/AFL
- Established EP follow-up, living close
Factors favoring inpatient hospitalization:
- Patients with history of coronary artery disease, cardiomyopathy, or heart failure symptoms
- Patients with chest pain or dyspnea, if the cardiac cause of these complaints cannot be definitively ruled out in the ED.
- Inability or difficulty achieving rate control despite appropriate AV nodal agents
- Hypotension upon attempting to rate control
- Embolic event or high risk of thromboembolism (valvular AF/AFL)
- Intolerable symptoms requiring cardioversion and antiarrhythmic drug initiation, unless patient is a candidate for outpatient initiation of antiarrhythmic medication.
- Patients with non-cardiac causes of AF/AFL (hyperthyroidism, pulmonary embolism, pneumonia)
- Lack of efficacy using the current strategy (Figure 4)
- Pre-excitation on ECG or syncope
- Concomitant ventricular arrhythmias (multiple runs of non-sustained ventricular tachycardia)
- Prolonged pause or ventricular asystole seen on telemetry (> 5 seconds)
Special Considerations: Postoperative AF/AFL
AF/AFL that occurs in the postoperative setting requires special consideration. Possible etiologies for postoperative AF/AFL should be considered based on the type of operation performed and potential perioperative events/complications. Many patients with postoperative AF/AFL will spontaneously convert back to sinus rhythm, and many of those patients will never have a recurrence of atrial fibrillation again. Therefore, postoperative AF/AFL is often treated with a rhythm control strategy. However, a subgroup of patients who suffer from postop AF/AFL will be found to have underlying heart disease, and go on to have recurrences. The optimal clinical approach to a patient with postoperative AF/AFL is not certain, but general recommendations will be provided in this section.
Approach to the Patient with Postoperative AF/AFL
For the purposes of this discussion, we will divide postop AF/AFL into categories based on the type of surgery with which it is associated.
Post-cardiac surgery. AF/AFL is an extremely common complication of cardiac surgery. For these patients, the approach is often one of aggressive rhythm control, and this population is outside of the scope of this guideline.
Post-non-cardiac, thoracic surgery. Similarly, AF/AFL is a common complication of non-cardiac, thoracic surgery. AF occurs in approximately 3% of this population, with the highest incidence after pneumonectomy and esophagectomy (21%). Because AF/AFL is so common in this patient population, it is often managed directly by the surgical service, and the management focuses on acute restoration of sinus rhythm. Appendix C outlines our institutional approach to these patients.
Amiodarone is often used for rhythm control in the thoracic surgery protocol. Amiodarone was effective in cardioverting 76-86% of patients postoperatively, and can be given IV, followed by oral dosing. Amiodarone should be avoided in patients with severe lung disease or after a pneumonectomy. If the patient requires an IV drip, a central venous catheter (e.g. PICC line) should be considered to avoid the risk of IV infiltration and subsequent skin necrosis.
Post non-cardiac, non-thoracic surgery. Lastly, there is a large population of non-cardiac, non-thoracic surgery patients that will suffer from postoperative AF/AFL. The remainder of this section will present an approach to these patients.
As with non-postoperative AF/AFL, the underlying cause of the rhythm should be sought. In addition to the standard evaluation (Table 1), postoperative patients are at risk for rapid fluid shifts, bleeding, thromboembolic disease, and other causes.
Patients with unstable postoperative AF/AFL should be treated as shown in Figure 1. For stable patients, treatment should first focus on controlling the heart rate as in Figure 2 for the first 24 hours. The approach for patients who remain in AF/AFL for > 24 hours post-op includes cardiology consultation for consideration of rhythm control in the majority of patients (see more below).
Patients who spontaneously convert back to sinus rhythm within a 24-hour period may be treated somewhat differently. In these patients, it is unclear if the AF/AFL represents an isolated event, or if it marks the patient as being at risk for future AF/AFL. For these patients, the focus of treatment should be on assessing the risk for future AF/AFL. One approach to these patients might be to monitor them on telemetry while they are in the hospital, and to provide them with cardiac event monitoring at discharge, as per the Discharge Planning and Hospital Follow-up section (Figure 2, box 5). It is useful to provide education to these patients about AF/AFL, including instructions about how to recognize the rhythm, and what to do if it recurs. These patients should be given non-urgent follow up with their PCP (or a cardiologist) after hospital discharge with the intention of specifically following up on possible recurrence of AF/AFL. Ideally, this problem should be added to the discharge summary problem list to facilitate adequate follow-up. For patients with transient postoperative AF/AFL anticoagulation is often withheld (see more below).
The optimal approach to postoperative AF/AFL is uncertain, and that is especially true when considering rhythm control for non-cardiac, non-thoracic surgery patients. Some experts feel that the majority of patients who develop AF/AFL after surgery should be treated with a rhythm control strategy. This is because postoperative AF/AFL is a potentially reversible condition. That is, many of these patients will respond to treatment, and be cured of their AF/AFL. However, rhythm control should only be considered an urgent matter if the patient is unstable, highly symptomatic, or difficult to rate control with medications. Also, there are several reasons that a rhythm control strategy might be delayed in the postoperative setting. For example, attempts at cardioversion require systemic anticoagulation therapy to prevent embolic disease, and some postoperative patients may not be good candidates for immediate anticoagulation. In those cases, attempts at rhythm control might be delayed. We recommend a cardiology consult for most patients with postoperative AF/AFL that lasts > 24 hours after surgery. The consultant will be able to assist in this decision-making as it pertains to the value and timing of a possible rhythm control strategy.
Management of Anticoagulation for Postoperative AF/AFL
The optimal approach to anticoagulation in patients with transient atrial fibrillation after noncardiac surgery is uncertain. Low quality observational trials suggest that postoperative atrial fibrillation is associated with an increased risk for stroke, but it is unclear if this applies to only brief episodes.32,33 For patients in AF/AFL for more than 48 hours after surgery, anticoagulation should be considered, as discussed in the Anticoagulation section of this document. However, in postoperative patients, anticoagulation should always be discussed with the surgical service, considering the risk of bleeding in the postoperative patient. In some cases where the patient is at high risk for recurrent AF/AFL, or high risk for stroke with AF/AFL, it may be appropriate to provide anticoagulation therapy, even if the duration of the postoperative AF/AFL is < 48 hours. Regardless of whether or not anticoagulation is started initially, the decision can be reassessed in follow up, based on the cardiac event monitor results, and the patient’s CHA2DS2-VASc score, bleeding risk, and preferences.
Special Considerations: Management of Preoperative Anticoagulation in AF/AFL
Preoperative Considerations for Warfarin Patients
Because warfarin has a long half-life, it requires discontinuation several days prior to procedures where normal hemostasis is desired. The decision to maintain therapeutic anticoagulation up until the time of the procedure (“bridging”) should be based upon the risk for development of stroke or systemic embolism, and adapted based on individual patient circumstances. General recommendations are as follows:
- Patients at low risk for thromboembolism: no-bridging strategy is preferred over bridging with UFH or LMWH. Low risk patient populations include: CHA2DS2-VASc score of 0 to 6 (assuming no prior history of stroke or TIA)
- Patients at intermediate risk for thromboembolism: bridging or no-bridging strategy with UFH or LMWH is determined based on individual patient- and surgery-related factors. Moderate risk patient populations include: CHA2DS2-VASc score of 7 or greater or remote history of stroke or TIA.
- Patients at high risk for thromboembolism: Bridging with UFH or LMWH is preferred over no anticoagulation during interruption of warfarin therapy. High-risk patient populations include: stroke or TIA within prior 3 months, or valvular AF/AFL (i.e., moderate to severe MS or a mechanical heart valve in any position).
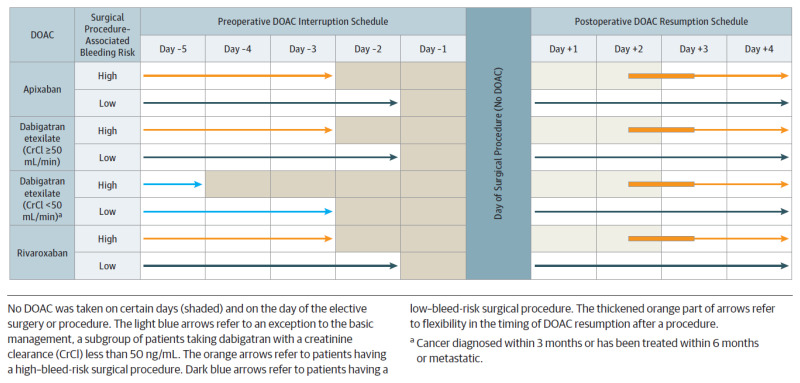
Preoperative Considerations for DOAC Patients
DOACs have a shorter half-life compared to warfarin which allows for reduced duration of interruption prior to procedure. The timing of interruption depends on the bleeding risk of the patient/procedure and thrombotic risk of the patient. Recommendations are specific to each DOAC based on pharmacokinetics, dosing frequency and dependence on renal clearance (Appendix D.). Parenteral anticoagulation is generally not indicated during the interruption period due to the short half-live of DOACs.
Special Considerations: Recent Stroke/CVA
In patients with recent ischemic stroke (< 2 weeks prior), Stroke Team consultation should be considered before initiating anticoagulation. Multiple factors such as size of stroke, time since the event, other bleeding risk, presence of intracardiac thrombus on echo, neurologic impairment, petechial hemorrhage in the infarct will impact on the recommendations.
Literature, Guidelines and Performance Measures
Strategy for Literature Search
The literature search for this guideline was conducted prospectively using the major keywords of “atrial fibrillation” or “atrial flutter.” Results were limited to humans, adults, and published in the English language, for dates ranging from March 2013 to December 2019 on Medline. Results were limited to Guidelines, Clinical Trials, and Cohort Studies.
Additional key words included: rate control, rhythm control, procedures, cardioversion, transesophageal echocardiography, ablation, antiarrhythmic medications, established drug therapies, novel drug therapies, stroke prevention, anticoagulation/antiplatelet therapy, clinical classification systems/risk calculators, and post-operative.
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. The search was a single cycle. Conclusions were based on prospective randomized clinical trials if available, to the exclusion of other data; if randomized controlled trials were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size.
The main search retrieved 10,748 references for the search terms “atrial fibrillation” and “atrial flutter”. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results were as follows:
- Atrial Fibrillation/Atrial Flutter - Guidelines, total results were 478
- Atrial Fibrillation/Atrial Flutter - Clinical Trials, total results were 4617
- Atrial Fibrillation/Atrial Flutter – Cohort Studies, total results were 5653
Related National Guidelines
The UMHS Clinical Guideline on Atrial Fibrillation and Atrial Flutter is generally consistent with other guidelines published nationally and internationally, including:
- January C, Wann LS, etal. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J The American College of Cardiology. 2014;(64):1-76.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. Circulation. 2019;140(2):e125-e151.
Related National Performance Measures
National programs that have clinical performance measures of atrial fibrillation and atrial flutter, primarily related to ambulatory care, include the following.
- Centers for Medicare & Medicaid Services, CMS179, ADE Prevention and Monitoring: Warfarin Time in Therapeutic Range, for adult patients with atrial fibrillation who are on chronic warfarin therapy. The measure includes Average percentage of time that patients in the measure population have INR results within the therapeutic range (i.e., TTR).
- National Quality Forum (NQF) Warfarin Therapy for Patients with Atrial Fibrillation. (NQF# 0084). Percent of all patients ≥ 18 years old with a diagnosis of heart failure and paroxysmal or chronic atrial fibrillation who were prescribed warfarin therapy.
- NQF. Ischemic stroke – Anticoagulation for A-fib/flutter. (NQF# 0436). Ischemic stroke patients with atrial fibrillation/flutter who are prescribed anticoagulation therapy at hospital discharge
Disclosures
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
No team member reported a conflict of interest.
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Health System to which the content is most relevant: Emergency Medicine, General Medicine, Infectious Disease, Neurosurgery, Cardiology, Cardiac Surgery, Stroke, Pharmacy Services, and Thoracic Surgery. Medication recommendations were reviewed by the Pharmacy and Therapeutics Committee. The final version was endorsed by the Clinical Practice Committee of the University of Michigan Faculty Group Practice and the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
Initial Release: May, 2014
Major Revision: November, 2021
Inpatient Clinical Guidelines Oversight
Megan R Mack, MD
David H Wesorick, MD
April Proudlock,BBA, RN
Literature search service: Taubman Health Sciences Library
© Regents of the University of Michigan
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient. These recommendations are intended for UH/CVC providers.
References
- 1.
- Odum LE, Cochran KA, Aistrope DS SK. The CHADS2 versus the new CHA2DS2-VASc scoring systems for guiding antithrombotic treatment of patients with atrial fibrillation: review of the literature and recommendations for use. Pharmacotherapy. 2012;Mar;32(3):285–296. doi:10.1002/j.1875-9114.2012.01023.x [PubMed: 22392459] [CrossRef]
- 2.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Anderson JL Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman E, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW, Harold JG, Jacobovitz S, Oetgen WJ, May C, Scholtz A, Bradfield L, Ramadhan HJL, ed. Circulation. 2014;130(23):2071–2104. doi:https://dx
.doi.org/10 .1161/CIR.0000000000000040 [PubMed: 24682348] - 3.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612 [PubMed: 32860505] [CrossRef]
- 4.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Anderson JL Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman E, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW, Harold JG, Jacobovitz S, Oetgen WJ, May C, Scholtz A, Bradfield L, Ramadhan HJL, ed. Circulation. 2014;130(23):e199–267. doi:https://dx
.doi.org/10 .1161/CIR.0000000000000041 [PMC free article: PMC4676081] [PubMed: 24682347] - 5.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. Circulation. 2019;140(2):e125–e151. doi:https://dx
.doi.org/10 .1161/CIR.0000000000000665 [PubMed: 30686041] - 6.
- Fengler BT, Brady WJ, Plautz CU. Atrial fibrillation in the Wolff-Parkinson-White syndrome: ECG recognition and treatment in the ED. Am J Emerg Med. 2007;25(5):576–583. doi:10.1016/j.ajem.2006.10.017 [PubMed: 17543664] [CrossRef]
- 7.
- Gorenek B (Chair), Halvorsen S, Kudaiberdieva G, et al. Atrial fibrillation in acute heart failure: A position statement from the Acute Cardiovascular Care Association and European Heart Rhythm Association of the European Society of Cardiology. Eur Hear J Acute Cardiovasc Care. 2020;9(4):348–357. doi:10.1177/2048872619894255 [PubMed: 31976747] [CrossRef]
- 8.
- Teerlink John R., Khalid Alburikan MM and JER. Acute Decompensated Heart Failure Update. Curr Cardiol Rev. 2015;11:53–62. doi:https://doi
.org/10.2174 /1573403X09666131117174414 [PMC free article: PMC4347210] [PubMed: 24251454] - 9.
- Olshansky B, Rosenfeld LE, Warner AL, et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: Approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43(7):1201–1208. doi:10.1016/j.jacc.2003.11.032 [PubMed: 15063430] [CrossRef]
- 10.
- Siu CW, Lau CP, Lee WL, Lam KF TH. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;Jul;37(7):2174–2179. [PubMed: 19487941]
- 11.
- Farshi R, Kistner D, Sarma JSM, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33(2):304–310. doi:10.1016/S0735-1097(98)00561-0 [PubMed: 9973007] [CrossRef]
- 12.
- Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJV den BMRII. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;15(362):1363–1373. [PubMed: 20231232]
- 13.
- Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, WAAF and CHFI. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;Jun 19;358(25):2667–2677. doi:10.1056/NEJMoa0708789 [PubMed: 18565859] [CrossRef]
- 14.
- Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi:10.1056/nejmoa2019422 [PubMed: 32865375] [CrossRef]
- 15.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. doi:10.1093/europace/euw295 [PubMed: 27567465] [CrossRef]
- 16.
- David A Garcia M, Mark Crowther, MD Ms. Risks and prevention of bleeding with oral anticoagulants. UpToDate. https://www
.uptodate .com/contents/risks-and-prevention-of-bleeding-with-oral-anticoagulants. Published 2021. - 17.
- Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematol (United States). 2016;2016(1):620–624. doi:10.1182/asheducation-2016.1.620 [PMC free article: PMC6142471] [PubMed: 27913537] [CrossRef]
- 18.
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. Holmes DR Chinitz L, Douglas P, Gurley J, Reddy VY, Sick P, Turi ZG, Hustead ST, Asinger RW, Shear W, Pulling C, Lew B, Plucinski D, Rogers E, Neuzil P, Sievert H, Mobius-Winkler S, Sick P, Tucker K, Doshi S, Swarup V, Bahu M, Quesada R, Huber K, Reddy VBM, ed. JAMA. 2014;312(19):1988–1998. doi:https://dx
.doi.org/10 .1001/jama.2014.15192 [PubMed: 25399274] - 19.
- John Camm A, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–2747. doi:10.1093/eurheartj/ehs253 [PubMed: 22922413] [CrossRef]
- 20.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. J Am Coll Cardiol. 2019;74(1):104–132. doi:10.1016/j.jacc.2019.01.011 [PubMed: 30703431] [CrossRef]
- 21.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi:10.1093/eurheartj/ehw210 [PubMed: 27567408] [CrossRef]
- 22.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. J Am Coll Cardiol. 2019;74(1):104–132. doi:https://dx
.doi.org/10 .1016/j.jacc.2019.01.011 [PubMed: 30703431] - 23.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD WLR-LSC and I. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;Sep 17;361(12):1139–1151. [PubMed: 19717844]
- 24.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KACRRAI. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;Sep 8;365(10):883–891. [PubMed: 21830957]
- 25.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J WLAC and I. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;Sep 15;365(11):981–992. [PubMed: 21870978]
- 26.
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F YSASC and I. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;Mar 3;364(9):806–817. [PubMed: 21309657]
- 27.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. Braunwald E Giugliano RP, Ruff CT, Morin SE, Hoffman EB, Murphy SA, Deenadayalu N, Grip L, Mercuri M, Lanz H, Patel I, Curt V, Duggal A, Hanyok J, Dave J, Morgan D, Choi Y, Shi M, Jin J, Xie J, Crerand W, Kappelhof J, Maxwell W, Skinner M, Patel S, Betch AEM, ed. N Engl J Med. 2013;369(22):2093–2104. doi:https://dx
.doi.org/10.1056/NEJMoa1310907 [PubMed: 24251359] - 28.
- (MAQI2) MAQII. Anticoagulation Toolkit. https:
//anticoagulationtoolkit.org/. Published 2020. - 29.
- John W. Eikelboom, M.D., Stuart J. Connolly, M.D., Brueckmann Martina, M.D., Christopher B. Granger, M.D., Arie P. Kappetein, M.D., Ph.D., Mack Michael J., M.D., Jon Blatchford, C. Stat., Devenny Kevin, B.Sc., Friedman Jeffrey, M.D., Guiver Kelly, M.Sc., R MD. Dabigatran versus Warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi:10.1056/nejmoa1300615 [PubMed: 23991661] [CrossRef]
- 30.
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med. 2019. doi:https://dx
.doi.org/10 .1001/jamainternmed.2019.2431 [PMC free article: PMC6686768] [PubMed: 31380891] - 31.
- Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, Marine JE, Mehran R, Messe SR, Patel NS, Peterson BE, Rosenfield K, Spinler SA TV. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of. J Am Coll Cardiol. 2021;77(5):629–658. [PubMed: 33250267]
- 32.
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol. 2018;72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088 [PubMed: 30336826] [CrossRef]
- 33.
- Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616–622. doi:https://dx
.doi.org/10 .1001/jama.2014.9143 [PMC free article: PMC4277813] [PubMed: 25117130] - 34.
- Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125(2):381–389. doi:10.1161/CIRCULATIONAHA.111.019927 [PubMed: 22249528] [CrossRef]
- 35.
- Dan GA, Martinez-Rubio A, Agewall S, et al. Antiarrhythmic drugs-clinical use and clinical decision making: A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Soci. Europace. 2018;20(5):731–732. doi:10.1093/europace/eux373 [PubMed: 29438514] [CrossRef]
- 36.
- Pathan F, Hecht H, Narula J, Marwick TH. Roles of transesophageal echocardiography and cardiac computed tomography for evaluation of left atrial thrombus and associated pathology: A review and critical analysis. JACC: Cardiovascular Imaging. 2018;11(4)616–27. https://www
.sciencedirect .com/science/article /pii/S1936878X18301049?via%3Dihub [PubMed: 29622180]
Appendix A. Commonly used antiarrhythmic drugs34,35
| Drug | Daily Dose | Comments |
|---|---|---|
| Flecainide (Class IC) | 200 to 300 mg in two divided doses |
|
| Propafenone (Class IC) | 450 to 900 mg in two or three divided doses depending on formulation |
|
| Sotalol (Class III) | 80 to 320 mg in two divided doses |
|
| Dofetilide (Class III) | 250 to 1000 mg in two divided doses |
|
| Amiodarone (Class III) | Loading dose: Loading dose: (~10 gm total) 200 mg TID x 14 days, then 200 mg BID x 14 days. A higher daily dose over a shorter time period may be used if tolerated. Maintenance dose: 200mg daily IV may be substituted for first 24 hours of therapy: 150mg IV bolus x 1, followed by IV infusion of 1mg/min x 6 hours, then 0.5mg/min for 18 hours. |
|
| Dronedarone (Class III) | 400 mg Q12H |
|
| Ibutilide (Class III) | ≥ 60 kg: 1 mg IVPB over 10 minutes < 60 kg: 0.01 mg/kg IVPB over 10 minutes If arrhythmia doesn’t terminate within 10 minutes after end of initial infusion, may repeat dose over 10 minutes |
|
| Disopyramide (Class IA) | 400 to 750 mg Q6h for immediate release formulation and Q12h for controlled release |
|
| Procainamide (Class IA) | 1gm IV over 1 hour for cardioversion Maintenance infusion available but usually reserved for life-threatening arrhythmias |
|
Appendix B28. Direct oral anticoagulants used for stroke prophylaxis in atrial fibrillation and atrial flutter**
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Mechanism of action | Direct thrombin inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor |
| Tmax (hours) | 1-3 | 2-4 | 3-4 | 1-2 |
| Metabolism | Phase II – glucoronidation | CYP3A4, CYP3A5, CYP2J2 | CYP3A4, CYP3A5 | Minimal via hydrolysis, conjugation, oxidation by CYP3A4 |
| Excretion | 80% renal | 2/3 liver, 1/3 renal | 25% renal, 75% fecal | 50% renal, 50% fecal |
| Elimination half-life (hours) | 12-17 | 5-9 | 9-14 | 10-14 |
| Dose | 150 mg PO BID | 20 mg PO daily | 5 mg PO BID | 60 mg daily; Avoid use if CrCl >95 ml/min |
| Dose – Renal Impairment | CrCl 15-30: 75mg BID CrCl <15: not recommended | CrCl ≤50: 15 mg daily | Any two: SCr ≥1.5; age ≥80 or weight <60kg: 2.5 mg BID | CrCl 15-50: 30 mg daily CrCl <15: not recommended |
| Dose – Hepatic Impairment | Moderate-severe: not recommended | Child-Pugh B or C, or coagulopathy: not recommended | Severe impairment: not recommended | Moderate-severe: not recommended |
| Drug Interactions | Ketoconazole or dronedarone & CrCl 30-50 ml/min: 75mg BID; P-gp inhibitor & CrCl 15-30 ml/min: not recommended; P-gp inducer: avoid | Strong dual- inhibitors or inducers of P-gp and CYP3A4: avoid; Combined P-gp and weak-moderate inhibitors of CYP3A4, & CrCl 15-50ml/min: use caution | Strong dual-inhibitors of P-gp and CYP3A4: reduce dose to 2.5mg BID or avoid if 2.5mg BID is already indicated based on above factors; Strong dual-inducers: avoid | Strong P-gp inducers: avoid |
Footnotes
- **
Referenced package inserts for specific dosing information.
Appendix D. Perioperative Management of DOAC30**
Footnotes
- **
Patients with estimated CrCl <30 mL/min may require longer interruption and/or pre-operative anti-Xa/IIa levels to ensure adequate clearance prior to procedure
APPROVALS
| P&T | Date: 8/17/2021 |
| ECCA | Date: 11/9/2021 |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Created: May 2014; Last Update: November 2021.
- Clinical Problem and Management Issues
- Diagnosis
- Treatment
- Special Considerations: Emergency Department (ED) Management
- Special Considerations: Postoperative AF/AFL
- Special Considerations: Management of Preoperative Anticoagulation in AF/AFL
- Special Considerations: Recent Stroke/CVA
- Literature, Guidelines and Performance Measures
- Disclosures
- References
- Commonly used antiarrhythmic drugs
- Direct oral anticoagulants used for stroke prophylaxis in atrial fibrillation and atrial flutter
- Management of acute atrial fibrillation/flutter after thoracic surgery
- Perioperative Management of DOAC
- APPROVALS
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Rates and implications for hospitalization of patients ≥65 years of age with atrial fibrillation/flutter.[Am J Cardiol. 2012]Rates and implications for hospitalization of patients ≥65 years of age with atrial fibrillation/flutter.Naccarelli GV, Johnston SS, Dalal M, Lin J, Patel PP. Am J Cardiol. 2012 Feb 15; 109(4):543-9. Epub 2011 Nov 24.
- CHA(2)DS(2)-VASc and readmission with new-onset atrial fibrillation, atrial flutter, or acute cerebrovascular accident.[Int J Cardiol. 2021]CHA(2)DS(2)-VASc and readmission with new-onset atrial fibrillation, atrial flutter, or acute cerebrovascular accident.Rutland J, Ayoub K, Etaee F, Ogunbayo G, Darrat Y, Marji M, Masri A, Elayi CS. Int J Cardiol. 2021 Jan 15; 323:72-76. Epub 2020 Aug 13.
- Characteristics of patients presenting to emergency department for primary atrial fibrillation or flutter at an academic medical center.[Indian Heart J. 2021]Characteristics of patients presenting to emergency department for primary atrial fibrillation or flutter at an academic medical center.Sadaf MI, O'Bryan J, Biese K, Chen S, Deyo Z, Mendys P, Sears SF, Tuttle H, Walker TJ, Gehi AK. Indian Heart J. 2021 Sep-Oct; 73(5):588-593. Epub 2021 Aug 24.
- Review Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation. A meta-analysis.[J Interv Card Electrophysiol. ...]Review Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation. A meta-analysis.Romero J, Diaz JC, Di Biase L, Kumar S, Briceno D, Tedrow UB, Valencia CR, Baldinger SH, Koplan B, Epstein LM, et al. J Interv Card Electrophysiol. 2017 Apr; 48(3):307-315. Epub 2017 Jan 9.
- Canadian Cardiovascular Society atrial fibrillation guidelines 2010: management of recent-onset atrial fibrillation and flutter in the emergency department.[Can J Cardiol. 2011]Canadian Cardiovascular Society atrial fibrillation guidelines 2010: management of recent-onset atrial fibrillation and flutter in the emergency department.Stiell IG, Macle L, CCS Atrial Fibrillation Guidelines Committee. Can J Cardiol. 2011 Jan-Feb; 27(1):38-46.
- Inpatient Management of Acute Atrial Fibrillation and Atrial Flutter in Non-Preg...Inpatient Management of Acute Atrial Fibrillation and Atrial Flutter in Non-Pregnant Hospitalized Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...