Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Objective: This guideline document aims to improve the effectiveness, efficiency, and safety of loop diuretic administration for heart failure (HF) patients in order to reduce symptoms, shorten length of stay, decrease repeat hospitalizations, and potentially improve survival.
Patient Population: All patients with decompensated heart failure regardless of ejection fraction.
Excluded Patients: Patients with end-stage renal disease, cirrhosis, nephrotic syndrome, or hypoalbuminemia with a serum albumin less than 3 g/dL.
Strength of Evidence Classification
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Level of Evidence Classification
- A = systematic reviews of randomized controlled trials with or without meta-analysis,
- B = randomized controlled trials,
- C = systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control),
- D = individual observation studies (case study/case series),
- E = expert opinion regarding benefits and harm
Guideline Summary
| Loop Diuretic Initiation Recommendations *[SOR, LOE] | Link to discussion |
|---|---|
| For diuretic naïve patients, an initial furosemide dose of 40 mg to 80 mg is recommended, administered as an IV bolus with dose selection based on degree of fluid overload, kidney function, and age. [I, E] | Q1 |
| For patients who have been on loop diuretic therapy as an outpatient, the oral loop diuretic dose should be converted to an oral furosemide equivalent (Table 2). The IV dose should be at least ≥ 1 to 2.5 times higher than the oral furosemide equivalent. Example: a patient taking furosemide 40 mg PO twice daily should receive at least 40 mg IV twice daily. Do not use IV doses lower than the outpatient oral dose (Figure 1). [I, B] | Q2 |
| The initial frequency of IV furosemide should be twice daily. Adhere to this frequency even if patients only take oral diuretics once daily at home. [I, B] | Q4 |
| Sodium intake should be restricted to ≤ 2,000 mg daily. [I, E] | Q5 |
| Fluid intake should be restricted to ≤ 2,000 ml daily. [I, E] | Q5 |
| Monitoring Efficacy and Safety of Loop Diuretic Therapy | Q# |
| Daily weights and accurate, frequent measures of patient intake and urine output are imperative for effective diuresis. Vital signs should be monitored at least every four hours. [I, E] | Q6 |
| Metabolic panels should be obtained at least daily in all patients receiving intravenous diuresis to monitor renal function and electrolytes. Consider obtaining metabolic panels twice daily in patients with characteristics that increase the risk of acute kidney injury or arrhythmias. Electrolyte supplementation may be required. [I, E] | Q7 |
| We recommend an initial diuresis target of at least 1 kilogram of weight loss daily in most patients. Alternative targets may be considered based on symptoms, vital signs, hemodynamics, renal function, electrolytes, and other factors. [I, E] | Q8 |
| While data is mixed, prioritize daily standing weights in the assessment of diuretic effectiveness, especially since weights are followed in the outpatient setting. [I, E] | Q10 |
| Immediately “double the dose” and utilize (Figure 1) when the initial diuretic dose is not effective. [I, B, E] | Q12 |
| Our algorithm defines diuretic resistance as failing to achieve any of the following despite escalation of IV diuretic bolus doses: a urine output of > 300 mL two hours after diuretic dosing, a urine output of 1200 mL eight hours after diuretic dosing, or a daily weight loss of < 1 kg. This term is commonly used when bolus loop diuretics are unable to achieve adequate diuresis. In cases of diuretic resistance, a continuous infusion of loop diuretic or the addition of a thiazide-like diuretic can be considered. [I, E] | Q13 |
| In cases of diuretic resistance, a continuous infusion of loop diuretic is one option to consider. Initial continuous infusion dosing should be made in accordance with (Figure 2). [I, B] | Q14 |
| Use metolazone as a first-line therapy when thiazide diuretics are used to augment bolus or continuous infusion intravenous loop diuretics. See Table 3 for dosing. Check metabolic panels twice daily and supplement electrolytes to prevent electrolyte derangements. [I, B] | Q15 |
| Diuretic resistance should prompt providers to evaluate for the presence of cardiorenal syndrome, low cardiac output, or cardiogenic shock. [I, E] | Q16 |
| The pathophysiology of kidney dysfunction in cardiorenal syndrome includes both renal under-perfusion and renal venous congestion. Therefore, decongestive therapy with diuretics is a mainstay of management. [I, E] | Q17 |
| Diuresis commonly results in an elevated serum creatinine, but most frequently does not reflect significant kidney damage. Modest increases should be monitored closely, but do not necessitate discontinuing diuretics when there is evidence of persistent congestion, as stability and improvement often occur with successful decongestion. [I, E] | Q18 |
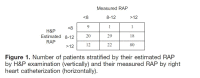
| Right heart catheterization for hemodynamic assessment should be considered when patients are not improving with standard heart failure therapies. Consider expert consultation in these cases. Other pharmacologic therapies for heart failure can affect diuretic effectiveness. [I, E] | Q20 |
| Transition to Oral Loop Diuretic Therapy | |
| Euvolemia should be achieved before transitioning to oral diuretics. Relying on symptom resolution alone may lead to inadequate decongestion. [I, E] | Q22 |
| Given that aspects of the physical examination lack sensitivity for identifying increased filling pressures in the setting of heart failure, use all available data (history, vitals, weights, exam, laboratory data, echocardiography, etc.) when assessing volume status, filling pressures, and perfusion. Filling pressures and perfusion should be categorized for all patients. Targeting a previous “dry weight” often results in inadequate diuresis and should not be used as the paramount factor in establishing euvolemia. Right heart catheterization may be needed in select instances. [I, E] | Q23 |
| Consider the following factors when determining an appropriate oral diuretic dose to maintain euvolemia: current IV dosing regimen during admission, renal function, previous home diuretic dose, and reason for decompensation. [I, E] | Q25 |
| Monitor patients for at least 24 hours on oral loop diuretic therapy before discharge to ensure that the loop diuretic regimen selected achieves adequate urine output and maintains stable weight, renal function, and electrolytes. [I, E] | Q26 |
| Prescribe a rescue diuretic dose before discharge. This allows patients and outpatient providers to respond quickly upon evidence of fluid retention and may prevent decompensation and rehospitalization. Educate patients to notify providers immediately when a rescue dose is used. [I, E] | Q27 |
| Include discharge weight and rescue diuretic dose for the early post-discharge period in the discharge documentation. [I, E] | Q28 |
| All patients should be provided with Michigan Medicine Heart Failure self-care education including the Heart Failure Action Plan (Appendix A). [I, E] | Q29 |
Guideline Overview
This document is intended to guide the administration of loop diuretic from the beginning of an admission for decompensated heart failure (HF), to the point of discharge. Therefore, it will focus not only on diuresis for the purpose of fluid removal but also on establishing an effective oral diuretic regimen for outpatient care. Loop diuretics are the focus of this guideline as they are used in the vast majority of cases of acute decompensated heart failure (ADHF).
Controlled trials of loop diuretic therapy have demonstrated that these agents increase sodium excretion and decrease fluid retention in patients with HF. Loop diuretic therapy in HF has never been shown to improve mortality directly. Nevertheless, loop diuretics are essential for improving symptoms related to fluid retention in HF. Inappropriate use of loop diuretics can lead to persistent fluid retention, volume depletion, hypotension, and acute kidney injury all of which can affect the ability to optimize other therapies for HF known to improve survival.
In an effort to make these guidelines as clinically useful as possible, we have provided recommendations to formulate the Michigan Medicine Inpatient Diuresis Guide. This guideline is designed to assist with the following objectives:
- Select an appropriate initial dose of intravenous (IV) loop diuretic
- Recognize when diuresis is not achieving adequate fluid and weight loss
- Guide providers in adjusting diuretic therapy to achieve adequate fluid and weight loss
- Ensure appropriate vital sign and laboratory monitoring occurs
- Align care team members with goal of shortening length of stay and improving outcomes
Importantly, the guide below should never replace clinical judgment. Should a patient not respond to an initial diuretic dose, providers are strongly encouraged to reassess patient volume status and consider causes of diuretic resistance such as low cardiac output, cardiogenic shock, cardiorenal syndrome, hypoalbuminemia, etc. Several questions in this document seek to educate readers on these causes of diuretic resistance. In some cases, subspecialty consultation may be required. For trainees, we encourage frequent diuretic assessments in conjunction with your attending, especially if a patient is not responding to IV diuresis.
Content used to design the Michigan Medicine Inpatient Diuresis Guide will be presented in a question-based format with the intent of addressing commonly encountered questions clinicians face from admission to discharge. Questions are organized in the following sections:
- Part One: Loop Diuretic Initiation
- Part Two: Monitoring Efficacy and Safety of Loop Diuretic Therapy
- Part Three: Transition to Oral Loop Diuretic Therapy
Michigan Medicine Inpatient Diuresis Guide

Figure 1
Loop Diuretic Initiation and Dose Titration.
Figure 2
Approach to the Diuretic Resistant Patient.
Table 1
Transitioning to Oral Diuretic Therapy.
Table 2
Equivalent Doses of Loop Diuretics.

Figure 3
Volume Removal in Heart Failure.
While providers can adjust diuretic therapy frequently during a hospitalization, variation in sodium and fluid intake can compromise diuretic effectiveness. This can be even more challenging to address in the outpatient setting when diuretic dosing cannot be adjusted as frequently.
Table 3
Thiazide-Like Diuretics.
Table 4
Sensitivity and Specificity of Signs and Symptoms for Congestion.
Table 5
Pharmacokinetics and Pharmacodynamics of Loop Diuretics Given Orally.
Table 6
Sample Outpatient Diuretic Plan.
Part One. Loop Diuretic Initiation
Q1: How should a provider choose an initial loop diuretic dose in a diuretic naïve patient?
Recommendation:
- For diuretic naïve patients, an initial furosemide dose of 40 mg to 80 mg administered as an IV bolus with dose selection based on degree of fluid overload, kidney function, and age.
- Refer to Michigan Medicine Diuretic Guide for more details.
Patients who have not been on diuretics as an outpatient, an initial furosemide dose can vary based on the degree of fluid overload, kidney function, and age, though 40 to 80 mg given IV, is recommended.1 Loop diuretics are often noted to have a “threshold effect” or dose-response curve, which means there is little response until a threshold is achieved.2 Doses that pass that threshold will rapidly approach a maximum or ceiling for which higher levels will not increase the maximal rate of diuresis.2
Effective IV diuretic action requires delivery to the kidney; secretion into the tubule lumen; and binding to the transport protein. Patients with chronic kidney disease (CKD) have compromised diuretic secretion into the tubule lumen for which a higher threshold diuretic dose will be required.3
Additionally, the maximal natriuretic effect of diuretic agents is reduced with CKD because the filtered sodium load decreases though the kidneys maintain urinary salt excretion through suppression of sodium reabsorption along the nephron.
Q2: How should a provider choose an initial diuretic dose in a patient who has previously received loop diuretic therapy?
Recommendations:
- For patients previously prescribed loop diuretic therapy as an outpatient, the oral dose should be converted to an oral furosemide equivalent.
- IV dosing should be at least ≥ 1 to 2.5 times higher than the oral furosemide equivalent. Example: a patient taking furosemide 40 mg PO twice daily should receive at least 40 mg IV twice daily.
- Do not use IV doses lower than the outpatient oral dose.
- Please see Michigan Medicine Diuretic Guide.
Route of administration
IV loop diuretic therapy is the mainstay of treatment for fluid removal in patients hospitalized with ADHF.4 Gastrointestinal absorption of any loop diuretic may be altered during ADHF.5 While total bioavailability may be maintained, slower absorption can impair natriuresis, especially given the threshold for natriuresis is increased during an episode of ADHF. IV loop diuretics should be administered without delay, as early therapy has been associated with better outcomes.6
Initial dose
For patients who have been on loop diuretic therapy as an outpatient, convert the total oral daily dose to an oral furosemide equivalent and administer IV at least 1 to 2.5 times the chronic oral daily diuretic dose.1
Observational studies demonstrate associations between high doses of loop diuretics and adverse outcomes, including renal failure. However, these studies may be confounded with their observational nature as a higher dose of diuretics may be a marker for illness severity.7 The DOSE study was designed to address 2 common questions regarding IV loop diuretic therapy in patients hospitalized with HF: 1): whether higher-dose loop diuretics (specifically furosemide) is preferable to lower dose and 2) whether continuous infusion is preferable to intermittent IV boluses.8 DOSE was a randomized study with 308 patients hospitalized with HF and signs/symptoms of congestion using a 2 × 2 factorial design. Patients were randomized to IV boluses every 12H or a continuous infusion and to low dose (equal to patient’s home daily oral dose) or high dose (equal to 2.5 times the home daily oral dose). There was no statistical difference between bolus and continuous infusion in patients’ global assessment of symptoms or in the mean change in creatinine level. In the high versus low dose strategy, there was no statistical difference in patients’ global assessment of symptoms or change in serum creatinine. The high dose strategy was associated with greater net fluid loss, weight loss, and relief from dyspnea, though this was balanced by a higher proportion of patients in the high dose group with worsening renal function (24% vs 14%; p=0.04). Importantly, a subsequent analysis of the DOSE trial did not identify worsening renal function as a risk factor for readmission or death following discharge.8
Equivalent doses
Although the equivalent doses of diuretics have been debated, it is generally accepted that 1 mg bumetanide = 20 mg torsemide= 40 mg furosemide.9 These were the equivalent doses used in the DOSE trial.
Initial dosing administration strategy
Initial diuretic dosing may be administered as intermittent boluses or a continuous infusion.9 The DOSE trial showed no difference in outcomes between intermittent boluses or continuous infusions. It may be reasonable to initiate initial diuresis with intermittent boluses administered every 8 to 12 hours, which may be transitioned to a continuous infusion if urine output goals are not met.10,11 Some potential advantages to a continuous infusion versus intermittent dosing include the loop diuretic concentration being maintained above the diuretic threshold and the avoidance of high peak levels, which are associated with toxicity.12 Given they often require closer monitoring (especially with electrolytes), it is recommended to initiate diuresis with intermittent IV boluses given Q12H and adjust frequency or convert to infusion as needed to achieve daily fluid goals.
Q3: Should one loop diuretic be preferred over another?
Recommendation:
- IV furosemide is recommended as the initial IV loop diuretic barring contraindications.
IV furosemide is favored as the initial IV loop diuretic unless there are contraindications. IV torsemide is no longer available. IV bumetanide has not been proven to be more efficacious compared to IV furosemide when administered in equipotent dosages. Additionally, continuous infusions of bumetanide have been associated with myalgias.10 Based on the fact that myalgias in patients with HF receiving continuous IV bumetanide were 29-fold higher than reported in the package labeling, it may be preferrable to use IV furosemide continuous infusions or add a thiazide-like diuretic prior to utilizing IV bumetanide continuous infusions, especially at higher doses. IV furosemide has also been reported to have acute vascular effects causing venodilation which may also have a symptomatic benefit for patients admitted with ADHF.11
Patients with an allergy to sulfa antibiotics may safely use loop diuretics. Studies have shown that cross-reactivity between antibiotic sulfonamides and nonantibiotic sulfonamides may not occur or, at the very least, the risk for reactivity is extremely low.12–14 Mechanisms of cross-reaction due to antibody production are unlikely to occur with nonantibiotic sulfonamides.
Q4: What should be the initial frequency of diuretic dosing?
Recommendation:
- Initial frequency of IV furosemide is twice daily.
- Use this frequency even if patients take oral diuretics once daily at home.
Both furosemide and bumetanide are short-acting; therefore, they are most effective when dosed twice daily.2 When administered once daily, there are periods where the concentration in the tubular fluid is below a therapeutic level for which there is post-diuretic sodium retention. The initial natriuretic effect from one of these shorter acting loop diuretics tends to wane after 3 to 6 hours for which a daily dose would have 16 to 21 hours for the sodium and water losses to be reversed by the kidneys.2 Once daily dosing may create a phenomenon of post-diuretic NaCl retention in some patients. Where the urinary NaCl excretion is below baseline, which remains present until the another diuretic dose is administered.12 For this reason, the DOSE trial utilized bolus administration twice daily.2 If patients are having adequate urine output with a dose of diuretic (threshold dose), consider increasing to every 8 hours to meet daily fluid goals.1
Q5: What additional non-pharmacologic interventions should be implemented?
Recommendation:
- Sodium restriction of ≤ 2,000 mg daily
On average, Americans consume between 3,400 and 3,700 mg/day of sodium.13 Guidelines vary in regards to specific recommendations for sodium restriction for HF varying between 1,500 and 3,000 mg/day to no recommendation at all.9,14,15 Patients with HF may have difficulty adhering to low-sodium diets and self-reported adherence may be inaccurate.16,17 Interestingly, compared to healthy controls, HF patients have been shown to have impaired recognition of salt taste.18
The neurohormonal activation that occurs in HF promotes sodium and fluid retention.19–21 Therefore, sodium restriction has the potential to improve symptoms and decrease hospitalizations by decreasing congestion. Through blood pressure reduction, sodium restriction may also delay HF progression. On the other hand, sodium restriction has been proposed to be detrimental in HF as it can increase neurohormonal activation by further decreasing effective circulatory volume.22,23 This may lead to worsening ventricular function and congestion. Furthermore, sodium restriction has been associated with decreased calorie, macronutrient, and micronutrient intake which worsens overall nutrition status and has been associated with poorer quality of life and increased risk of readmission. 24,25 These competing effects of sodium restriction may partially explain why randomized clinical trials have yet to definitively demonstrate that sodium restriction is either beneficial or detrimental to HF patients.
Sodium restriction interventions below 1500 mg/day and between 2000-3000 mg/day have been shown to improve symptoms, NYHA class, natriuretic peptide levels, and quality of life in outpatients with HF.21(p),24,26 Salt intake has also been positively correlated with natriuretic peptide levels which are prognostic in HF.27 Clinical studies that have provided patients with low-sodium meals (sodium 1150 mg/day) have demonstrated that sodium restriction can improve blood pressure, arterial stiffness, ventricular-arterial coupling, diastolic function, and oxidative stress in hypertensive HF with preserved ejection fraction (HFpEF).28,29 While prospective observational studies have associated high-sodium intake (> 3000 mg/day) with hospitalizations and mortality, particularly in patients with more symptomatic HF with reduced ejection fraction (HFrEF),30–33 randomized trials have yet to demonstrate benefit for hospitalizations or mortality. Inpatient sodium restriction is commonly recommended for patients with ADHF, though studies are lacking. In two trials separately assessing patients with HFpEF and HFrEF, a sodium restriction of < 800 mg/day and a fluid restriction of < 800 mL/day did not improve weight, congestion score, or readmission rates compared to control.34,35
In contrast, randomized trials from a single Italian research group have associated sodium restriction (1840 vs. 2760 mg/day) achieved with dietician-prescribed meal plans with increased risk of readmissions over 180 days and higher levels of neurohormonal activation in recently discharged patients with HFrEF.22,23,36 However, these studies also randomized patients to different fluid restrictions (1,000 vs. 2,000 mL/day) and high doses of furosemide (125 vs 250 mg furosemide twice daily). Furthermore, adjustments to diuretic therapy and fluid restriction were prohibited after discharge which could have contributed to hypovolemia in sodium-restricted patients. Lastly, rates of optimal medical therapy utilization were low; this is relevant given that such therapies can block the effects of neurohormonal activation associated with sodium restriction.37,38 Based on these concerns, it is difficult from these studies to understand the effect of sodium restriction in isolation. A meta-analysis including studies from the above group found a low-sodium diet to be associated with increased readmission and mortality risk; however, it was later retracted out of concern for the validity of the data reported.39,40 Duplication of research data was also suspected.41 In contrast to the observational studies cited above, one prospective observational study using food frequency questionnaires to assess sodium intake demonstrated that a sodium restriction of < 2500 mg/day was associated with an increased risk of the primary outcome of HF hospitalization or death, particularly for patients with milder NYHA class II symptoms.37 Another study found that self-reported low-sodium intake is associated with higher mortality in HF patients.42 However, the studies did not adjust for prior hospitalizations and acknowledged the possibility of reverse causality (that is, sicker patients were told to consume less sodium) to explain the outcomes observed.37,38
Importantly, studies evaluating sodium restriction in HF have varied in regards to the following: sodium-restriction targeted, intervention to reduce sodium intake, and assessments of baseline sodium intake and adherence to the prescribed intervention (food questionnaires, diaries, urine sodium excretion, and other tools utilized).43 Food-frequency questionnaires, 24-hour food recall, and measurements of urine sodium excretion (especially one-time samples) also have inherent limitations (recall bias, incomplete recording, etc.) that can misclassify patient sodium intake.17,44 Studies have also included fluid restriction and diuretic interventions that make it challenging to isolate the effect of sodium restriction. All of these issues have very likely contributed to the mixed results observed in studies of sodium restriction in HF.
Given that evidence is lacking to support our specific recommendation above, providers must recognize that patients may benefit from individualized goals. Furthermore, especially given concerns that a low sodium diet can negatively affect overall nutrition, it may be more appropriate to recommend a particular diet (for example, the DASH diet) rather than recommend sodium restriction alone. Lastly, regardless of what level of sodium intake is recommended, patients should be educated that taking in a consistent amount of sodium each day can facilitate appropriate diuretic dosing. In other words, significant variation in day-to-day sodium intake can make it challenging for providers to prescribe a diuretic regimen to prevent fluid retention (Figure 2).
Recommendation:
- Fluid restriction of ≤ 2,000 mL daily
Data are lacking to support a recommendation for fluid restriction in the setting of decompensated HF.45 The 2013 ACC/AHA Guidelines recommend a 1.5 to 2.0 liter/day fluid restriction in stage D HF based solely on expert opinion.9 The European Society of Cardiology (ESC) recently made a similar recommendation recognizing that there is a gap in evidence on this topic.46 Both guidelines recommend that fluid restrictions should be tailored to the specific patient. That is, a fluid restriction of 2 liters/day is likely adequate for most patients though patients with diuretic resistance or hyponatremia may be more likely to benefit from this degree or even greater fluid restriction (1.5 L/day). In contrast to the guidelines above, similar to sodium restriction, fluid restriction has been proposed to be detrimental in HF through increased neurohormonal activation by further decreasing effective circulatory volume.22,23,47
We will review studies of fluid restriction in HF in the paragraphs below. While these data are conflicting, our guideline recommends a fluid restriction of ≤ 2,000 mL daily. This is based on expert opinion and is consistent with our current practice at Michigan Medicine. We have chosen this recommendation as it is in agreement with the ACC/AHA and ESC guidelines for the management of HF cited above. Lastly, we have recommended this degree of a fluid restriction as it can facilitate successful diuresis in the inpatient setting. While the Michigan Medicine Advanced Heart Failure Program recommends this same degree of fluid restriction in the outpatient setting, physicians should consider whether this same degree of restriction is appropriate for maintaining euvolemia in all outpatients.
To review, there are few data supporting a benefit of fluid restriction in HF. Studies to date are limited by heterogeneity and small sample sizes.48 A small, single-blind, randomized study of 67 patients admitted for decompensated HF showed that a fluid restriction of 1 L/day compared to no restriction did not result in a shorter duration of IV diuresis, lower diuretic doses, or lower natriuretic peptide levels after diuresis. However, the group that did not receive a fluid restriction consumed a mean of only 392 mL/day greater than the restricted group (1467 mL/days in no restriction vs. 1074 mL/day) in restricted group.49 Another study of stable outpatients with HFrEF did not show a benefit in regards to symptoms or hospitalizations using a 1.5 L/day fluid restriction compared to a 30 to 35 mL/kg/day fluid restriction.50 In contrast, the SALT-HF trial included 46 HF patients with hyponatremia (sodium < 137 mg/dL) found that a fluid restriction of less than 1 L/day resulted in improved quality of life 60 days after discharge.51 Given the small size of these studies and the discrepant findings, more research is clearly needed to determine whether fluid restriction is appropriate in both the inpatient and outpatient settings.
One challenge when reviewing the literature relates to the fact that some studies have evaluated fluid restriction with concurrent sodium restriction making it difficult to isolate the effect of fluid restriction alone.26 For example, in two trials separately assessing patients with HFpEF and HFrEF, a sodium restriction of < 800 mg/day and a fluid restriction of < 800 mL/day did not improve weight, congestion score, or readmission rates compared to control.34,35 The discussion regarding sodium restriction above references several additional studies acknowledging the limitations of these studies in isolating a benefit of either sodium restriction or fluid restriction in isolation. Please note the additional concerns regarding these studies described in the discussion above.22,23,36
Be cognizant that patients often experience thirst in the setting of fluid restriction. Common approaches to relieving thirst can include providing ice chips. Chewing gum has been found to effectively relieve thirst in this setting.52
Part Two. Monitoring Efficacy and Safety of Loop Diuretic Therapy
Q6: (Safety) What monitoring is required from Nursing for safe and effective diuresis?
Recommendations:
- Daily weights and accurate, frequent measures of patient intake and urine output are imperative for effective diuresis.
- Vital signs should be monitored at least every four hours.
In general, HF is perceived as a condition of volume overload. Although problems with sodium homeostasis underly the state of volume overload, diuretic therapy is initiated with the goal of unloading the volume overloaded patient. In this context, it is important to objectively measure if the diuretics are effectively unloading the patient. Objective evidence of diuretic effectiveness versus resistance can be corroborated using daily weights and urine output.
Daily weights in the hospital can be obtained through a morning standing weight, or a bed weight. In general, a morning standing weight is usually more accurate because there are less variables to confound the results.53 In order to minimize variables during standing daily weights, patients should be weighed at approximately the same time each day, wearing the same amount of clothing. Other variables such as the telemetry box should be controlled. Alternatively, for patients who cannot stand on a scale, a bed weight can be measured. Bed weight accuracy from day to day relies on consistency with regards to what is being weighed. For example, it is imperative that bedding and other objects on the bed are consistently the same so that only the patient’s weight is being measured. From an institutional standpoint, it should be noted that initial weights taken in the Michigan Medicine emergency department are often patient self-reported weights.
Given the inherent complexities of obtaining accurate weights, it is important to correlate weight loss with urine output. This necessitates accurate input and urine output measurements. The basic principle is that 1 L of urine should equal 1 kg (2.2 lbs.) of weight. Urine output should be measured by having the patient urinate into a container that allows for accurate measurement. Foley catheters for accurate output assessment are discouraged except in extreme cases. Every effort should be made to communicate with the nursing team about alternatives to foley catheter placement. The measurement of urine output serves at least two roles. First, a 24-hour urine output can be compared to the patient’s intake to get a net urine balance. This value is correlated with the daily weight measurement, and these values can help determine if diuretic management is effective. Interestingly, studies reveal that even with stringent efforts to obtain accurate input and urine output measurements, there is often a poor correlation between weights and net urine output.54 Therefore, urine output and daily weights must be viewed as pieces of data in the context of the entire clinical scenario. Second, urine output should be measured and recorded on an ongoing basis, ideally hourly after the diuretic is given, as this allows for timely assessment of diuretic effectiveness and prompt escalation as needed. Adequate dosing of IV diuretics should have a rapid time to onset (within 10 minutes), reach peak serum concentration by 2 hours, and should last approximately 6 hours.4 Thus, assessment of the diuretic response can be assessed as early as 2 hours after the dose is given. A urine output of 150 mL/hour is considered an adequate response.55–57 Based on diuretic pharmacokinetics, an inadequate urine output at 2-6 hours after a diuretic dose should be considered an inadequate response and the dose should be escalated promptly so that excessive time is not lost with inadequate diuresis.
Fluid intake is usually restricted to 2 L or 64 ounces per day. At Michigan Medicine, half of the daily fluid allowance (one liter) is used by the kitchen, and the other half is distributed to the patient by the nursing staff. Fluid intake totals are tallied and documented at the end of a shift which can be either 8 or 12 hours.
Although ADHF is often perceived as a “volume overload” problem with care focusing on removing fluid, a key pathophysiologic underpinning of ADHF is the accumulation of excessive sodium. Furthermore, the clinical correlation between urine output and daily weight change is often weak making the adequacy of diuretic response unclear. As a result, recent attention has been focused on measuring sodium output as an indicator of diuretic response.58–61 A spot urine sodium output of < 50-70mEq/L after 2 hours is considered an inadequate response to a diuretic and should prompt a dose escalation.4,62 While additional studies are needed, this objective measure of diuretic responsiveness may play a prominent role in future ADHF management.
Vital signs should be measured at least every 4 hours and sooner if there is a change in clinical status. Hypotension or sinus tachycardia should be regarded as important clinical events and should prompt nursing to escalate to the physician team to define the underlying cause. Hypotension and/or sinus tachycardia could herald several clinical scenarios: hypovolemia due to overdiureses, low cardiac output or cardiogenic shock due to worsening HF, excessive afterload reduction, and other causes unrelated to ADHF (blood loss, infection/sepsis). The underlying cause must be identified so that a tailored response can be utilized.
Q7: Safety) What laboratory monitoring is required for safe and effective diuresis?
Recommendations:
- Metabolic panels should be obtained at least daily in all patients receiving IV diuresis to monitor renal function and electrolytes.
- — Consider obtaining metabolic panels twice daily in some patients (see below).
- Supplement electrolytes when necessary.
Metabolic panels should be obtained at least daily in all patients receiving IV diuresis for decompensated HF to monitor renal function and electrolytes. This is universally recommended by guidelines though based on expert opinion.9 For many patients, twice daily metabolic panels should be considered especially in patients with the following characteristics that increase the risk of acute kidney injury or arrhythmias:
- ▪ Low cardiac output
- ▪ Chronic renal insufficiency
- ▪ Right ventricular dysfunction
- ▪ Chronic kidney disease
- ▪ Electrolyte abnormalities at baseline
- ▪ Prescribed electrolytes supplementation as an outpatient
- ▪ Require high doses of loop diuretic therapy or thiazide-like diuretic augmentation
- ▪ Experiencing substantial diuresis losing several liters of fluid daily
- ▪ History of ventricular arrhythmias
Given that many HF patients have these characteristics, twice daily metabolic panels are often warranted. When obtained, results can allow for appropriate diuretic adjustments to avoid adverse events. For example, overdiuresis can result in acute kidney injury which can increase length of stay. Alternatively, electrolyte abnormalities can provoke arrhythmias.
Please note that many patients will require supplemental potassium to maintain normal potassium levels and avoid arrhythmias in the setting of IV diuresis. Patients requiring potassium supplementation as outpatients will often need supplementation continued throughout admission. If supplementation is given daily during the hospitalization, the provider should consider standing doses to avoid recurrent hypokalemia and acute dosing of potassium. This is especially the case when thiazide-like diuretics such as metolazone are used to enhance diuresis. Such diuretics can provoke significant hypokalemia and additional potassium supplementation should be considered pre-emptively at the time of dosing thiazide-like diuretics. In general, we recommend supplementation to a goal serum potassium of > 4 mmol/L. It should be noted that hypomagnesemia can prevent adequate potassium repletion. An appropriate goal level for serum magnesium is not well established, though we recommend aiming for a level above 2 mg/dL in the presence or history of ventricular arrhythmias.
Q8: Safety) What rate of daily fluid removal is recommended?
Recommendation:
- An initial diuresis target of at least 1 kilogram of weight loss daily in most patients.
- Alternative targets may be considered based on patient symptoms, vital signs, hemodynamics, renal function, electrolytes, and other factors.
Data are lacking to support a specific rate of diuresis for patients with decompensated HF. No randomized trial has evaluated one rate of diuresis against another. Retrospective studies evaluating diuresis for HF often do not report weight loss or urine output among subjects. Presumably, this is because the retrospective acquisition of such data can be challenging, and the accuracy of such data may be questionable. Randomized clinical trials that have included HF patients receiving diuresis have reported variable rates of weight loss though have exceeded rates of 1 kg/day.2,63–65 Total amount of weight loss with diuresis in these trials ranged from 3 to 8 kg total.2,63–68
We recommend at least 1 kilogram of weight loss daily in most patients. This corresponds to a net fluid loss of 1L assuming accurate intake and output measures are obtained. Considering the recommendation that patients have a 2-L fluid restriction (see question #5), we recommend targeting at least 3 liters of urine output to achieve this daily weight loss goal. We have recommended providers follow weight as a diuresis target rather than intake and output measures because reliably measuring intake and output can be difficult in some patients.
Efficient resolution of congestion improves symptoms and can shorten length of stay, therefore, a faster rate of diuresis may be preferred in some situations. For example, a faster rate may be selected in patients with respiratory failure from pulmonary edema. Alternatively, a faster rate may be selected for patients with profound fluid overload. We strongly recommend closely monitoring renal function and electrolytes with twice daily metabolic panels in cases when more aggressive diuresis goals are set. Providers should slow the rate of diuresis in such cases if hypotension, acute kidney injury, or significant electrolyte abnormalities develop.
In the following instances, a slower rate of diuresis (daily weight loss of 0.5 to 1.0 kg) may be preferred to avoid hypotension, acute kidney injury, and other adverse outcomes:
- ▪ Patients experiencing an initial worsening of renal function with IV diuresis
- ▪ Patients with chronic kidney disease especially in advanced stages
- ▪ Patients with moderate-severe right ventricular systolic dysfunction
- ▪ Patients with hypoalbuminemia
- ▪ Patients with HFpEF with a narrow window for optimal preload
One reason why evidence is lacking to support a specific rate for diuresis relates to changes in renal function observed during management of decompensated HF. Worsening renal function (serum creatinine increase of ≥ 0.3 mg/dL; WRF) is a common marker of congestion and is not always a marker of complete decongestion when it occurs with diuresis.69 Meaning, decreasing or discontinuing diuresis may not always be appropriate in the setting of WRF. Diuretic management in the setting of WRF remains dependent on the degree of congestion present, the degree of WRF observed, and evaluating for low cardiac output and other contributors to WRF. WRF is often transient and resolves during the post-discharge period. In these cases, data suggests patients may be at lower risk of adverse outcomes compared to patients with persistent worsening renal function, especially when complete decongestion is achieved.67,68,70–73 Please see question 18 for more details.
Q9: (Safety) Should loop diuretic therapy be held in the setting of hypotension?
Recommendation:
- Hypotension in the setting of IV diuresis should warrant consideration of volume depletion. Hypotension may also be a marker of low cardiac output or even cardiogenic shock.
- In these instances, specialty consultation is warranted.
- Generally, loop diuretics have a neutral effect on blood pressure based on their pharmacologic characteristics.
Assuming the patient is volume overloaded, loop diuretics are unlikely to cause hypotension. They typically have a neutral impact on blood pressure or may improve it by offloading excessive preload on the heart.74 In studies investigating diuresis in patients with ADHF, there were no differences in hypotension even when utilizing high-dose diuretics.2,75 It has been suggested that when hypotension does occur with the use of IV diuresis during admission for ADHF, it is often the consequence of volume depletion, not the diuretic use itself.76 More specifically, volume depletion lowers preload resulting in a lower stroke volume and hypotension. IV furosemide has also been reported to cause acute venodilation which could decrease preload and lower blood pressure in the setting of volume depletion.11 Importantly, loop diuretics do not affect systemic vascular resistance.
Please note that there is no specific systolic or diastolic blood pressure measurement that is used to define hypotension in a HF patient. Some patients with HFrEF can be asymptomatic with no evidence of malperfusion while maintaining systolic blood pressures in the 80-100’s mmHg. Symptoms, laboratory data, markers of perfusion, and other factors should always be considered when determining whether significant hypotension is present.
Q10: Why should weight be prioritized as a marker of effective diuresis over net intake/output?
Recommendation:
- While data is mixed, prioritize daily standing weights in the assessment of diuretic effectiveness, especially since weights are followed in the outpatient setting.
Weight and net fluid input and output are commonly used metrics to gauge the effectiveness of diuresis. However, these measures do not always correlate consistently with each other and both are imperfect measures.54 Net input/output is subject to inaccuracies due to factors such as unrecorded intake, missed voids secondary to incontinence, or errors in urine collection. Accurate daily weights may also be challenging to obtain due to timing in relation to meals or urination/defecation, not using the same scale, or the use of standing versus bed weights. Despite the limitations of these measurements, prioritize weight as a marker of diuresis effectiveness. Some studies have found that early weight loss in acute decompensated HF correlates with improvements in congestion and outcomes, though this finding is not consistent across all studies.77 An average weight loss of 4 - 8 kg during a hospitalization for ADHF has been reported in recent trials.1 It is important that a patient being treated for ADHF is weighed daily, at the same time each morning (prior to breakfast), preferably using a standing weight on a consistent scale.
Q11: What should be considered evidence of an effective diuretic dose and frequency?
Recommendations:
- In the setting of volume overload, a urine output of at least 150 mL/hr.
- IV loop diuretics should be given at least twice daily in this setting, based on their relatively short half-life.
Despite the widespread use of IV diuretic therapy in the setting of decompensated HF, guidelines supporting their use are based mostly on expert opinion.9,78 Data are also lacking to support any one definition of an effective dose response. Currently, nurse-based measurement of urine output remains the standard of care to assess diuretic responsiveness. Experts have proposed algorithms aiming to achieve a urine output of 150 mL/hour with the assessment of urine output occurring within 2-6 hours of each diuretic dose.4 This approach aims for a total urine output of 3,600 mL daily which is reasonable assuming a fluid restriction of 2,000 mL is utilized.79 Change in weight can also be utilized as a marker of effective diuresis; however, weight is usually measured once daily and relying on this alone may lead to less timely modifications of diuretic dosing. Please note that the timing of urine output measurement for diuretic efficacy assessment may be partly determined by patient level of care and frequency of nursing assessments. It also should be noted that weight loss resulting from diuresis does not always correlate with net fluid output.54
We recommend at least twice daily bolus dosing of IV loop diuretics. This is based on the relatively short half-life of loop diuretics. Diuretics response usually decreases within six hours of each dose which allows for rebound sodium in fluid retention over the remaining hours of the day.4 For this reason, twice daily dosing is recommended. Continuous infusion may be preferred in some instances as it prevents rebound sodium retention observed with bolus dosing.
Spot urine sodium measurements obtained two hours after IV loop diuretic administration are currently being studied as an alternative to assessing diuretic efficacy. However, clinical experience with this approach is lacking. In a recent study, a urine-sodium based approach to guide diuresis resulted in greater urine output, net fluid output, and weight loss.79
Q12: What should be done if the initial loop diuretic dose is not meeting fluid removal goal?
Recommendation:
- Immediately “double the dose” and utilize the Michigan Medicine Diuretic Guideline when the initial diuretic dose is not effective.
Loop diuretics must be dosed to achieve a threshold concentration to result in natriuresis and fluid removal. Increasing loop diuretic doses further will eventually reach a “ceiling” of sodium excretion at which point continuing to increase the dose does not result in further sodium excretion and volume loss.62 However, using higher doses of loop diuretic does allow for the concentration of diuretic to remain above the threshold concentration level for a longer period of time, which promotes more natriuresis. In a similar fashion, giving multiple doses of loop diuretic can increase the amount of time the diuretic concentration remains above threshold level resulting in significant natriuresis. Therefore, more frequent IV loop diuretic doses increase natriuresis once an effective dose is determined. Lastly, in the setting of decompensated HF, the relationship between diuretic dose and sodium excretion is shifted rightward (Table 1).4
In light of the above relationships between diuretic dose and natriuretic response, experts advocate for doubling the dose of IV loop diuretic if an adequate response of at least 150 mL/hour is not achieved (see question #11).4,80 Furthermore, based on pharmacokinetics described above, the higher dose of IV loop diuretic should be given immediately upon recognizing that the prior dose was ineffective. Once an effective bolus dose of loop diuretic has been defined, dosing at least every 12 hours is recommended.4,80
Data supports that using higher doses of IV loop diuretic therapy may be more effective. In the Diuretic Optimization Strategies Evaluation (DOSE) trial, 308 patients with acute decompensated HF (mean ejection fraction 35%) were randomized to low-dose versus high-dose loop diuretic therapy.2 The low-dose group received a total IV furosemide dose equivalent to the total outpatient oral loop diuretic dose. The high-dose group received a total IV furosemide dose 2.5 times the total outpatient oral loop diuretic dose. Patients in both groups were additionally randomized to receive doses either by bolus infusion every 12 hours or by continuous infusion. The high-dose strategy was associated with greater symptom relief, weight loss, and net fluid loss with no significant difference in mean change in creatinine level. Patients in the high-dose group were more likely to experience worsening renal function defined by a rise in creatinine greater than 0.3 mg/dL. This later was shown not to be associated with worse outcomes similar to other studies.8,70,72
Q13: What is diuretic resistance and what strategies can be taken to address it?
Recommendation:
- Our algorithm defines diuretic resistance as failing to achieve any of the following: a urine output of > 300 mL two hours after diuretic dosing, a urine output of 1200 mL eight hours after diuretic dosing, or a daily weight loss of < 1 kg. This term is commonly used when bolus loop diuretics are unable to achieve adequate diuresis.
- In cases of diuretic resistance, a continuous infusion of loop diuretic or the addition of a thiazide-like diuretic can be considered.
Diuretic resistance, or the failure to achieve an adequate diuretic response, is a term that can apply to any situation in which inadequate urine output is observed following a diuretic dose. Our algorithm defines diuretic resistance as failing to achieve any of the following: a urine output of > 300 mL two hours after diuretic dosing, a urine output of 1200 mL eight hours after diuretic dosing, or a daily weight loss of < 1 kg. This term is commonly used when bolus loop diuretics are unable to achieve adequate diuresis. Diuretic resistance can be due to pre-renal and intrinsic renal mechanisms. Pre-renal mechanisms include renal venous congestion and low cardiac output. Therefore, appropriate optimization of hemodynamics can improve diuretic response. Intrinsic renal mechanisms include inadequate concentration of loop diuretic as well as compensatory distal tubule sodium reabsorption.4
In the setting of diuretic resistance, a continuous infusion of IV loop diuretic should be considered to achieve an adequate concentration of loop diuretic. This approach is more likely to maintain the diuretic concentration above the threshold concentration for natriuresis for a longer duration of time. Furthermore, continuous infusions prevent rebound sodium retention which can occur several hours after a bolus infusion of a loop diuretic. A continuous infusion should be preceded by a loading dose to achieve a steady-state serum loop diuretic concentration rapidly.80 Please see question #14 for more details.
Another strategy to address diuretic resistance is to add a thiazide-like diuretic. These agents block the sodium-chloride cotransporter which is involved in the compensatory reabsorption of sodium at the distal tubule.4,80 See question #15 for more details.
Q14: How should continuous infusions of loop diuretic therapy be initiated?
Recommendation:
- Initial continuous infusion dosing should be made in accordance with the Michigan Medicine Diuretic Guideline.
It is reasonable to utilize intermittent IV loop diuretic bolus dosing every 12 hours as an initial strategy. If diuretic goals are not met with furosemide 160 mg IV dosing every 8 – 12 hours (diuretic resistance), a continuous infusion should be considered.1 While the DOSE trial did not demonstrate any significant differences between continuous infusions compared with bolus dosing, patients on continuous infusions were less likely to require diuretic dose increases or the addition of a thiazide-type diuretic.2 Some patients may have a greater natriuretic effect with a continuous infusion and there may be advantages from a pharmacodynamic standpoint including maintenance of the loop diuretic above the threshold while avoiding high peak levels that may put the patient at risk for toxicity.4
The DOSE trial utilized continuous infusions with initial dosing from 1 (low intensity cohort) to 2.5 (high intensity cohort) times the chronic oral home diuretic dose. Importantly, the total daily dose of loop diuretic was equivalent between the continuous infusion and bolus dosing treatments in the DOSE trial. In clinical practice, continuous loop diuretic infusions very often result in a higher total daily dose of loop diuretic compared to bolus dosing resulting in more significant diuresis. Continuous infusions should be preceded by a loading dose to ensure rapid achievement of loop diuretic steady-state.80 There is no well-established dosing strategy for continuous infusions.
The Comparison of Diuretic Strategies for Combination Nephron Blockade [IV Thiazide vs Oral Thiazide vs Tolvaptan] in Acute Heart Failure Complicated by Loop Diuretic Resistance (3T trial) was a study done in 60 patients hospitalized with ADHF and IV loop diuretic resistance as defined by total urine output < 2 liters in the 12 hours prior to enrollment to an IV furosemide equivalent dose ≥ 240 mg/day over the preceding 12 hours.75 The majority of patients in this trial were HFrEF (77%) and received an average of 612 ± 439 mg/day of IV furosemide equivalents prior to randomization. Patients in the 3T trial (Comparison of Diuretic Strategies for Combination Nephron Blockade [IV Thiazide vs Oral Thiazide vs Tolvaptan] in Acute Heart Failure Complicated by Loop Diuretic Resistance) were initiated on furosemide with a 100 mg IV bolus followed by 20 mg/hr in those resistant to IV furosemide doses of 240-479 mg/day and 100 mg IV bolus followed by 30 mg/hr in those resistant to ≥ 480 mg/day in 24 hours prior to enrollment.75 No adverse events attributed to these infusions were noted in the trial. The CARESS trial utilized a stepped pharmacological approach grouped by patients receiving loop diuretic doses per day ≤ 80mg (40 mg intravenous bolus + 5 mg/hr); 81-160mg (80 mg intravenous bolus + 10mg/hr); 161-240mg (80 mg intravenous bolus + 20 mg/hr); or > 240 mg (80mg intravenous bolus + 30 mg/hr).64 No patients experienced hypotension from the loop diuretic infusion dosing in the CARESS trial and there were significantly more adverse events in the ultrafiltration cohort compared to the pharmacological diuretic cohort.
In accordance with the Michigan Medicine Diuretic Guideline, if patients are resistant to IV bolus administration or not meeting fluid goals at escalating IV bolus frequencies, consider initiating a continuous IV furosemide infusion at 20 mg/hr. A furosemide bolus of 160 mg IV should be given at the time of infusion initiation so that steady-state may be achieved rapidly. Double the dose of furosemide infusion every 4 hours as needed until a urine output of 150 mL/hr is achieved. Bolus furosemide administration of 160 mg IV with each dose change may allow for diuresis goal to be achieved sooner. Furosemide infusions in clinical trials have not exceeded 40 mg/hr. Please note that doses up to 160 mg/hr can be used at our institution; however, physician discretion and attention to safety markers (urine output, electrolyte abnormalities, hypotension, etc.) is strongly advised.
Q15: How should loop diuresis be augmented with thiazide diuretic therapy?
Recommendation:
- Metolazone should be utilized first-line when needed to augment loop diuresis.
- Check twice daily metabolic panels and supplement electrolytes in this setting to prevent electrolyte derangements. (See Table for dosing.)
Distal tubular sodium reabsorption is theorized to be one mechanism of diuretic resistance. Therefore, if a patient displays diuretic resistance or is not meeting fluid goals, it is reasonable to trial combination nephron blockade with the addition of a thiazide-like diuretic. The dose of loop diuretic at which augmentation should be added is unknown.4 Metolazone is often first-line though other types of thiazide diuretics or thiazide-like diuretics seem to have equal efficacy when administered in equipotent doses.81 The use of IV chlorothiazide did not achieve greater weight loss compared to oral metolazone in a prospective trial of strategies for treating diuretic resistance. Therefore, metolazone should be trialed prior to escalation to chlorothiazide due to the cost difference.75 Differences between agents are summarized below.82 Chlorthalidone is generally not recommended to augment diuresis given greater reduction in blood pressure and electrolyte wasting compared to other thiazide diuretics.
Based on the goal to block sodium reabsorption with thiazide-like diuretics, we advise giving these agents 30 minutes prior to bolus dosing of a loop diuretic. The addition of these agents may result in robust diuresis; therefore, close monitoring for hypokalemia, hyponatremia, and acute kidney injury is required. For this reason, we recommend twice daily basic metabolic panels when using thiazide augmentation and, if appropriate, giving pre-emptive potassium supplementation at the time of thiazide dosing to prevent hypokalemia. In addition, these agents have longer half-lives than the loop diuretics with which they are given. While daily administration is reasonable for inpatients, the long-acting nature and potential for electrolyte derangements should motivate providers to consider every other day or as needed dosing in the outpatient setting. As with loop diuretics, we recommend using the lowest dose needed for effective diuresis to avoid the adverse effects of these agents.
Q16: What are markers that poor renal perfusion is contributing to ineffective diuresis?
Recommendation:
- Diuretic resistance should prompt providers to evaluate for the presence of cardiorenal syndrome, low cardiac output, or cardiogenic shock.
Diuretic resistance can be due to decreased renal blood flow. In the majority of these cases, it is a result of increased central venous pressure in the setting of HF. This is one characteristic of cardiorenal syndrome which will be discussed in more detail in the following questions. Importantly, even in the setting of a mild- to moderately-reduced cardiac output, increased central venous pressure is the primary hemodynamic mechanism responsible for cardiorenal syndrome.69
In select cases, diuretic resistance can be observed in the setting of severely reduced cardiac output. In this case, decreased renal blood flow is the result of a severely reduced cardiac output. In these instances, hemodynamics can be optimized to improve cardiac output and renal perfusion allowing for a patient to “warm up in order to dry out.”83 See question #24 for more details. Such severe reductions in renal blood flow can occur in the presence or absence of cardiogenic shock. Providers must recognize cardiogenic shock immediately to prevent multi-organ failure.
Given that worsening renal function can be a sign of both cardiorenal syndrome and cardiogenic shock, clinicians must evaluate for other signs of cardiogenic shock in any patient that presents with decompensated HF, especially if diuretic resistance is present. By definition, cardiogenic shock is a low cardiac output state resulting from cardiac dysfunction leading to severe end-organ hypoperfusion associated with tissue hypoxia.84 Physical examination findings suggestive of cardiogenic shock include tachycardia, narrow pulse pressure, cool extremities, altered mental status, and oliguria. In addition to elevated creatinine, laboratory findings suggestive of cardiogenic shock include metabolic acidosis, elevated lactate, and severe transaminitis. Please note congestive hepatopathy secondary to congestive HF can result in a mild transaminitis (usually < 3 times the upper limit of normal) in the absence of overt cardiogenic shock. It is often also associated with an increased bilirubin and alkaline phosphatase level. It must be recognized that transaminases peak 1-3 days after a hemodynamic insult occurs; therefore, an isolated measurement of transaminases may not reflect ongoing tissue hypoperfusion.84
Importantly, cardiogenic shock can occur in the absence of hypotension. Systemic blood pressure is determined by cardiac output and systemic vascular resistance. Patients presenting with cardiogenic shock can display markedly elevated measures of systemic vascular resistance that maintain a systolic blood pressure above 90 or even 100 mmHg despite severely reduced cardiac outputs. Furthermore, stable HF patients on medical therapy can have lower blood pressures (< 100 mmHg) in the absence of shock. In short, blood pressure measurements should not be viewed as a reliable means to evaluate the presence or absence of cardiogenic shock.
We recommend that providers use a combination of the above markers when non-invasively assessing hemodynamics in a patient with HF. In some cases, providers should consider right heart catheterization to invasively measure hemodynamics, recognizing that patients can vary in what degree of decline in cardiac output is required to result in cardiogenic shock. Simply, there is no threshold cardiac output measurement below which cardiogenic shock occurs. In any patient in which cardiogenic shock is suspected, emergent cardiology consultation should be obtained. Please see questions #20, #21, and #24 for more information.
Q17: What is cardiorenal syndrome and how should it be treated?
Recommendation:
- Decongestive therapy with diuretics is a mainstay of management of cardiorenal syndrome.
Cardiorenal syndrome (CRS) is a global term referring to conditions that affect the function of both the heart and the kidneys. There is increasing recognition of the cross-talk that occurs between these organs, and the fact that acute or chronic dysfunction in one of these organ systems frequently leads to damage in the other. Five different subtypes of CRS have been described: type 1 (acute CRS), type 2 (chronic CRS), type 3 (acute renocardiac), type 4 (chronic renocardiac), type 5 (secondary CRS due to systemic disease affecting both organ systems).85 This guideline focuses on management of types 1 and 2 CRS, whereby cardiac dysfunction leads to worsening renal function (WRF).
Type 1 and 2 CRS are characterized by the development of WRF or acute kidney injury (AKI) in the setting of acute and/or chronic cardiac disease. Type 1 CRS can occur with both acute HF and acute coronary syndrome, and complicates nearly 1 in 4 patients with these conditions.86 The pathophysiology of types 1 and 2 CRS includes aspects of both reduced renal perfusion and increased renal congestion. A traditional view has been that reduced cardiac output leads to impaired renal perfusion with resultant activation of the renin-angiotensin-aldosterone system (RAAS); subsequently, increased sodium and fluid retention further contribute to worsening cardiac congestion and reduced cardiac function in a vicious cycle. However, there is now recognition that HF (including with preserved ejection fraction) also leads to reduced renal venous return, increased renal vascular resistance, and reduced renal function.87 Supporting this is the consistent observation that elevations in right heart pressures (e.g. right atrial pressure or central venous pressure) correlate with kidney dysfunction and AKI risk in patients with cardiac disease.88–90
The management of type 1 and 2 CRS involves multiple components (e.g. RAAS blockade, optimization of cardiac output) and a comprehensive review is beyond the scope of this guideline, but a central component is decongestion therapy with diuretics. Decongestion can improve both renal perfusion and reduce renal venous hypertension, both leading to improvement in kidney function. Therefore, while diuretics are often considered to be harmful for kidney function, a hallmark of types 1 and 2 CRS is improvement in kidney function with appropriate diuresis.85 At the same time, close monitoring of kidney function is warranted, as over-diuresis can also occur and lead to WRF/AKI.
As discussed in other sections, loop diuretics are first-line therapy for decongestion in CRS, both in acute and chronic situations. In addition, sodium-glucose co-transporter 2 inhibitors (SGLT2i’s) have been shown in multiple clinical trials to improve outcomes in patients with both HFpEF or HFrEF,91 and therefore should also be part of CRS management. While this class of meds can be considered a diuretic, they work on the proximal tubule and therefore typically have weak diuretic potency; so while the mechanism of benefit is unclear, it is likely not due to a significant decongestive effect. Nevertheless, the SGLT2i’s can potentiate the effect of other diuretics and lead to volume depletion in some patients, so cautious titration of diuretics is advised for patients on combination diuretic therapy.
In cases where diuretics fail to achieve appropriate decongestion, extra-corporeal therapies (e.g. ultrafiltration) have been used. Several clinical trials have compared the efficacy of ultrafiltration versus diuretics, with mixed results.64,65 At present, there is insufficient evidence to recommend ultrafiltration therapy as part of the routine management of CRS.85 While ultrafiltration therapy may be beneficial in some cases, there is currently no established way to identify which patients will have better outcomes with ultrafiltration therapy compared to standard diuretic approaches, and some clinical trials have demonstrated harm associated with extracorporeal therapy. At present, ultrafiltration therapy should be reserved for patients with true diuretic failure and/or patients who otherwise need dialysis for clearance due to advanced renal failure.
Q18: Should a rise in creatinine be expected with effective diuresis?
Recommendation:
- Diuresis commonly results in a rise in serum creatinine, but most such rises do not reflect significant kidney damage.
- Modest increases should be observed closely but do not necessitate stopping diuretics in the setting of persistent evidence of congestion, as stability and improvement often occur with successful decongestion.
A rise in serum creatinine (SCr) commonly occurs in the setting of diuresis for acute HF. However, not all of these rises reflect significantly worsening renal function and in many cases these changes are transient and may not be associated with worse outcomes. The clinical challenge lies in distinguishing when kidney injury is occurring versus relatively benign changes in SCr.
Significant AKI can occur from several mechanisms during diuresis, most notably the development of frank hypotension and severe intravascular volume depletion leading to renal ischemia. Risk factors for this include concomitant therapy with ACEI/ARB, hypotension, and more significant baseline kidney dysfunction. Signs suggestive of AKI include falling urine output with decreasing responsiveness to diuretics after initial response; evidence of other tissue ischemia, such as lactic acidosis; larger rises in SCr; and progressive or persistent worsening kidney function not responsive to holding diuretics.
Fortunately, the more common scenario is relatively modest rises in SCr (<0.5-1.0mg/dL) occurring during diuresis. Because creatinine is distributed throughout total body water and usually generated at a steady state, a significant change in fluid balance can result in changes in SCr not related to a change in glomerular filtration rate (GFR). Diuretic therapy can also lead to sympathetic and RAAS activation, which can lead to physiologic decreases in GFR (and thus SCr rise) without kidney injury. Indeed, studies using biomarkers of renal tubular injury have found that diuresis-associated changes in SCr may not reflect kidney damage.92 In other studies, an early modest rise in SCr with diuresis has been shown to be a positive prognostic factor: a post-hoc analysis of the DOSE trial found that nearly 20% of participants experienced WRF (defined as SCr rise >0.3mg/dL) within the first 72 hours of diuretic therapy, but this was actually associated with improved clinical outcomes.8 However, the literature is mixed, with most observational studies linking WRF during HF hospitalization with worse overall outcomes.93 Clinicians should have a low threshold for basic evaluation (e.g. urinalysis, urine sediment exam) to rule out intrinsic causes of kidney injury/WRF such as allergic interstitial nephritis or acute tubular necrosis, which can also occur in this setting.
As noted in previous sections, decongestion therapy is an integral component of acute HF management. In patients hospitalized for acute HF, persistent congestion is associated with worse outcomes, including recurrent hospitalization and mortality.94 Similarly, persistent WRF – particularly in the setting of persistent congestion – is also associated with worse prognosis as compared to transient WRF.95 Therefore, evidence of persistent congestion is an indication to continue diuretic therapy even in the setting of initial mild increases in SCr, as appropriate decongestion can lead to improved renal function.
Q19: Can other agents be added to enhance diuresis?
Recommendation:
- There is insufficient data to recommend diuretic augmentation with the following agents: acetazolamide, tolvaptan, dopamine, or higher doses of spironolactone.
Data to support the use of other agents in patients with diuretic resistance are lacking.4 Acetazolamide (500 mg IV daily) was compared to placebo in 519 patients with ADHF, clinical signs of volume overload, and an N-terminal pro-B-type natriuretic peptide level >250 pg/mL. The primary endpoint of successful decongestion (defined as the absence of signs of volume overload) occurred in 42.4% patients in the acetazolamide group and 30.5% in the placebo group (risk ratio, 1.46; p<0.001). Patients randomized to acetazolamide experienced a 500 mL greater diuretic effect on the second day after randomization. Though these findings were significant, the primary endpoint of successful decongestion based on physical exam is subjective and known to be difficult to evaluate at the bedside. Knowing the limitations of the study, it is reasonable to add on acetazolamide to a loop diuretic in those that are diuretic resistant knowing there may be an additive natriuretic effect.96 Higher doses of mineralocorticoid antagonists have natriuretic effects though ATHENA-HF investigated high-dose spironolactone in patients admitted with HF and found no difference in net urine output or weight change compared to the use of low dose spironolactone or placebo.97 While tolvaptan showed equivalent efficacy to chlorothiazide and metolazone in the 3T trial, given its cost and side effect profile, it should be avoided for routine use as adjunctive therapy for diuresis.75 There is no clear data to support low-dose dopamine. While the 2013 ACC/AHA guidelines support its use in conjunction with loop diuretic therapy to improve diuresis and preserve renal function as a IIb recommendation, these clinical benefits have not been observed in randomized trials such as ROSE-AHF and ROPA-DOP.9,97,98 As this guideline excludes patients with an albumin < 3g/dL, there is no evidence to support the use of albumin to augment diuresis. It is also not supported within the Michigan Medicine Adult Albumin Guidelines.
Q20: When should right heart catheterization be considered?
Recommendations:
- Right heart catheterization (RHC) should be considered when patients are not improving with standard HF therapies.
- Consider expert consultation.
RHC is an invasive procedure in which a pulmonary artery catheter is placed to measure intracardiac filling pressures and calculate cardiac output (CO). RHC can obtain these data at a single time point. Alternatively, a pulmonary artery catheter can be left in place to obtain serial data to guide therapy in the intensive care unit setting. RHC is often used to evaluate the severity and etiology of pulmonary hypertension. It is also used to determine a candidacy for advanced HF therapies and to optimize patients prior to such therapies. Importantly, a randomized trail did not demonstrate improved outcomes with the routine use of RHC for hemodynamic-guided therapy in patients hospitalized with severe, symptomatic, advanced HF.63 Therefore, the guidelines recommend the following mostly based on expert opinion:
Class I (Should be performed): Invasive hemodynamic monitoring with a pulmonary artery catheter should be performed to guide therapy in patients who have respiratory distress or evidence of impaired perfusion in whom the adequacy or excess of intracardiac filling pressures cannot be determined from clinical assessment.9
Class IIa (Reasonable to perform): Invasive hemodynamic monitoring can be useful for carefully selected patients with acute HF who have persistent symptoms despite empiric adjustment of standard therapies and...
- ▪ Fluid status, perfusion, or systemic or pulmonary vascular resistance is uncertain.
- ▪ Systolic blood pressure remains low or is associated with symptoms
- ▪ Renal function is worsening with therapy
- ▪ Require parenteral vasoactive agents
- ▪ May need consideration for mechanical circulatory support or transplant
Class III (No Benefit): Routine use of invasive hemodynamic monitoring is not recommended in normotensive patients with acute decompensated HF with symptomatic response to diuretics and vasodilators.
As suggested by the guidelines cited above, there are instances in which RHC should be considered. Most simply, RHC should be considered when patients are not improving with standard HF therapies. This includes instances in which patients are not responding to IV diuresis, also known as diuretic resistance. In these cases, RHC provides data that may not be appreciated on history and physical examination. Question #24 explains how “mismatch” and low cardiac output states can be missed in the absence of RHC data. Once this data is obtained, results can direct diuresis as well as afterload reduction and potentially inotrope therapy.
Q21: How should right heart catheterization data be used to guide diuresis?
Recommendation:
- Right heart catheterization provides an assessment of hemodynamics that can assist in the management of decompensated HF.
- Diuretic therapy affects hemodynamics as well as several other evidence-based pharmacologic therapies for HF.
RHC is an invasive procedure in which a pulmonary artery catheter is placed to measure intracardiac filling pressures and calculate cardiac output. As stated in Question #24, clinicians must recognize that filling pressures are the result of myocardial systolic function, diastolic function, plasma volume, venous capacitance, and other factors. “Congestion” in HF is best described as increased intracardiac filling pressures leading to symptoms. This can be due to volume overload but also due to redistribution of blood volume such as from the splanchnic capacitance vasculature to the effective circulatory volume.
Improving symptoms in HF is most directly related to lowering left-sided filling pressures [left ventricular end-diastolic pressure (LVEDP) and left atrial pressure]. Preload most directly refers to left ventricular end-diastolic pressure which is elevated in the setting of decompensated HF. Through decreasing effective circulatory volume, IV diuresis lowers preload (left-sided filling pressures). Patients differ in their left ventricular intravascular pressure-volume relationships. As a result, pressure measurements from RHC do not predict the exact amount of volume that needs to be diuresed to improve symptoms. Remember, pressures not volumes are being assessed with the assessment of jugular venous distension and with right heart catheterization.80
While we recognize that this guideline focuses on IV diuresis, it should be appreciated that changes in variables such as afterload and contractility can also affect preload independent of diuresis. Understanding these variables can assist providers in diuresing patients safely and effectively. Particularly for HFrEF, afterload reduction can be very effective at improving intracardiac filling pressures; it can also enhance diuretic responsiveness by improving renal perfusion. However, in some instances, opportunities for afterload reduction can easily be missed. It is critical for providers to recognize that patients with normal or even lower blood pressure can potentially benefit from afterload reducing medications. These include Angiotensin-converting enzyme (ACE) inhibitors, Angiotensin II receptor blockers (ARBs), and Angiotensin Receptor-Neprilysin Inhibitors (ARNi) all of which have been shown to improve outcomes such as mortality in HFrEF. Depending on the situation, titration of afterload-reducing medications may need to be done carefully, particularly in patients with lower blood pressure. RHC provides a calculation of systemic vascular resistance (SVR) which is an assessment of left ventricular afterload. In decompensated HF, patients can have significantly elevated SVR. An increased SVR can compensate for a low CO to ensure maintenance of an adequate systemic blood pressure (BP) because BP = CO × SVR. When afterload reduction is added to patients with these hemodynamics, SVR reduction can be met with an increased CO without a drop in blood pressure, assuming the left ventricle has adequate contractile reserve to augment its CO when afterload is reduced. In this way, even patients with normal or lower BP can tolerate afterload-reducing medication without hypotension. When patients do experience hypotension with the initiation of afterload-reducing medications, it can be inferred that minimal contractile reserve is present, which explains why patients that cannot tolerate medical therapy for HF have worse outcomes.
Please note that patients with HFpEF do not experience significant improvements in CO (augmentation of stroke volume) in the setting of afterload reduction. This is due to the increased arterial elastance (steeper slope of the end-systolic pressure volume relationship) associated with HFpEF. In such patients, afterload reduction can be met with significant hypotension without significant hemodynamic benefit.
In less common instances, particularly in the setting of a low CO, inodilator (inotrope + vasodilator = inodilator) therapies can be implemented to improve CO and lower left-sided filling pressures. These agents include dobutamine and milrinone both of which directly increase contractility (inotrope) and decrease SVR (vasodilator). Advanced Heart Failure consultation should be strongly considered in these cases. Further discussion on the use of these therapies is beyond the scope of this guideline.
It should be noted that the above interventions on preload, afterload, and contractility may be challenging to implement in more tenuous patients such as those with lower blood pressure, end-organ dysfunction, or cardiogenic shock. Advanced Heart Failure consultation should be considered in these cases. In addition, such patients often benefit from serial RHC measurements in the intensive care setting to guide therapy.
Part Three. Transitioning to Oral Diuretic Therapy
Q22: When should a patient be transitioned to oral diuretic therapy?
Recommendation:
- Euvolemia should be achieved before transitioning to oral diuretics.
- Relying on symptom resolution alone may lead to inadequate decongestion.
It is ideal for patients to be fully decongested prior to transition to oral diuretics and discharge. Studies have demonstrated that patients who are discharged with residual congestion have higher rates of rehospitalization and death,99 yet it is not uncommon that patients are discharged before reaching euvolemia.100 The provider should recognize that improvement in symptoms of congestion (such as dyspnea) often occur early in the treatment of ADHF and will precede resolution of the signs of congestion. Reliance on symptom relief alone to guide diuresis will usually result in premature transition to an oral diuretic regimen.80 Therefore, the provider must ensure the patient has achieved a euvolemic volume status by symptoms and signs of congestion prior to instituting oral diuretics. In some cases, providers may elect to transition a patient to oral diuretics prior to euvolemia being achieved. This may be in an effort to shorten what would otherwise be a prolonged hospital stay. In these cases, it is imperative that an effective oral diuretic be established prior to discharge as outlined in Questions 25 and 26.
Q23: What markers should be utilized to establish that euvolemia has been achieved?
Recommendation:
- Use all data available (history, vitals, weights, exam, laboratory data, echocardiography, etc.) when evaluating for euvolemia.
- Targeting a previous “dry weight” often results in underdiuresis and should not be used as the paramount factor to establishing euvolemia.
Determining at which point euvolemia has been reached is challenging and requires a multi-parameter assessment of the patient’s volume status. Improvement/resolution in symptoms of resting dyspnea, bendopnea, orthopnea, and edema should be assessed, but as aforementioned, are insufficient in isolation to determine euvolemia. The physical exam should demonstrate resolution of jugular venous distention, lower extremity and abdominal edema, hepatosplenomegaly, and rales. However, when considering the physical exam in the HF patient, there are some caveats to note including the expected persistence of edema in patients with low albumin states, and the absence of “classic” signs of volume overload despite elevated filling pressures in patients with chronic HF.1 Of the clinical signs, jugular venous pressure is the most useful parameter and ideally should be < 8 cm H2O in the euvolemic patient.80 Targeting a previous “dry weight” often results in underdiuresis and should not be used as the paramount factor to establishing euvolemia.
Diagnostic testing may help the provider to determine volume status, but should be used as an adjunct to the physical exam. Laboratory data might demonstrate reduction in natriuretic peptides (BNP and NT-proBNP), hemoconcentration, and a transient rise in creatinine once euvolemia is obtained. In regards to natriuretic peptides, while an elevation in natriuretic peptides is quite sensitive in the diagnosis of HF, there is no data to support BNP-guided diuresis in the hospitalized patient with acute decompensated HF.101 We do recommend obtaining a BNP on discharge, as BNP after the treatment of acute HF has prognostic value. Imaging may also be used to support the volume status assessment. Chest imaging in the euvolemic patient should demonstrate resolution of pulmonary edema and pleural effusions on X-ray, and a decrease in B-lines on point of care lung ultrasound. Point of care ultrasound can also be used to assess jugular venous pressure and inferior vena cava dimensions. Echocardiography may also show a non-dilated and collapsible inferior vena cava suggesting low-normal right atrial pressure.80
In select cases where there is discrepancy between these parameters and volume status remains unclear, a right heart catheterization may be warranted to determine filling pressure.
Q24: How can the physical examination in HF patients deceive clinicians?
Recommendation:
- Given that aspects of the physical examination lack sensitivity for identifying increased filling pressures in the setting of decompensated HF, we recommend using all data available (history, vitals, weights, exam, laboratory data, echocardiography, etc.) when assessing volume status, filling pressures, and perfusion.
- RHC may be needed in select cases.
The history and physical examination are essential tools in the assessment and management of decompensated HF. However, there are instances in which relying on specific findings may misguide even the most experienced physicians. This concept has been supported by manuscripts presenting data from the ESCAPE trial, a randomized trial evaluating the use of pulmonary artery catheters in the management of decompensated HF.63
Drazner et al. evaluated the utility of individual components of the history and physical examination.102 More specifically, the sensitivity, specificity, and predictive values for each component were evaluated in patients with a pulmonary capillary wedge pressure greater than 22 mmHg (this is a moderately elevated value that is well above normal). The study demonstrated that jugular venous distension and orthopnea are more sensitive findings while other findings such as rales and edema are not very sensitive (see below).
Jugular venous distension clearly guides the management of any HF patient. However, there are limitations to this exam finding. First, it can be challenging to assess on examination. Drazner, et al. compared physician estimations of right atrial pressure based on history and examination with right atrial pressure measured by right heart catheterization. Discrepancies were observed:
Jugular venous distension is also limited in that it serves only as an assessment of right atrial pressure. As a result, it is only a surrogate of left-sided intracardiac filling pressures such as the pulmonary capillary wedge pressure, which itself is a surrogate of left ventricular end diastolic pressure. Importantly, elevated left-sided filling pressures lead to symptoms of dyspnea and orthopnea in HF. In many cases, an elevated jugular venous distension (elevated right atrial pressure) corresponds to elevated left-sided pressures. In fact, studies have shown that right atrial pressure and pulmonary capillary pressure are correlated, and that pulmonary capillary wedge pressure is usually two times the value of the right atrial pressure.103,104
When this relationship is not present, a phenomenon known as mismatch is present. This can complicate treatment of decompensated HF. Mismatch occurs when right- and left-sided intracardiac filling pressures are not proportional to each other. In other words, either the right atrial pressure or pulmonary capillary is normal while the other is abnormal. Campbell et al. evaluated 537 patients with advanced HF undergoing RHC.105 A total of 389 patients (72%) had right atrial and pulmonary capillary wedge pressures that matched (low or high for both measures). However, 148 patients (28%) had mismatch. When evaluating only patients with at least one elevated pressure, 43% of patients had mismatch. High-left mismatch occurs when the pulmonary capillary wedge pressure is elevated though the right atrial pressure is normal. This can lead to providers failing to appropriately decongest a patient if the jugular venous distension is used solely to guide diuresis. High-right mismatch occurs when the right atrial pressure is elevated though the pulmonary capillary wedge pressure is normal. This can lead to acute kidney injury if a provider diureses a patient based on elevated jugular venous distension when in fact the left ventricle may be becoming underfilled. To make matters even more challenging, the relationship between right and left-sided pressures can change with diuresis.104
The above discussions illustrate that individual components of the history and physical examination can be misleading. We recommend using all data available (history, vitals, weights, exam, laboratory data, echocardiography, etc.) when assessing volume status. In some instances, RHC may be needed to define filling pressures and guide management.
Furthermore, clinicians must recognize that filling pressures are the result of myocardial systolic function, diastolic function, plasma volume, and venous capacitance. “Congestion” in HF is best described as increased intracardiac filling pressures leading to symptoms. This can be due to volume overload but also to redistribution of blood volume such as from the splanchnic capacitance vasculature to the effective circulatory volume. Therefore, the additional of neurohormonal medical therapies and afterload reduction can relieve congestion independent of diuresis. Importantly, this also means that a pressure measurement does not predict the exact amount of volume that needs to be diuresed to improve symptoms --- patients differ in their left ventricular intravascular pressure-volume relationships. Remember, pressures not volumes are being assessed by jugular venous distension and RHC.80
Clinicians similarly struggle with the bedside assessment of CO. It is encouraged that all providers classify patients with acute decompensated HF by categorized left-sided filling pressures and perfusion.106 When available, pulmonary capillary wedge pressure is the marker used to categorize left-sided filling pressure; however, on most occasions, clinicians must use clinical and examination data to estimate this. Patients are deemed “wet” if the pulmonary capillary wedge pressure is categorized as elevated. In regards to perfusion, when available, cardiac index (CO divided by body surface area) can be used for categorization. On most occasions, this must be judged in the absence of RHC data. A patient is deemed to be “cold” in the presence of decreased perfusion. Such patients need improved perfusion so that they can “warm up in order to dry out”.83
In a recent study by Narang and colleagues, physicians evaluated the above profile in 97 patients with acute decompensated advanced HF prior to RHC. Physicians from interns to HF specialists were given access to all patient clinical data. Despite this, clinical assessments demonstrated a positive predictive value of 74.7% and negative predictive valve of only 50.4% for the “cold and wet” profile. In the majority of cases, providers were unable to correctly categorize the degree of elevation of the following hemodynamic measurements: right atrial pressure (43.4% accurate), pulmonary capillary wedge pressure (32.6% accurate), cardiac index (47.4% accurate). Physician level of training did not impact the predictive power of clinical assessment. Provider agreement was poor. These findings argue that select patients benefit from the invasive measurement of hemodynamics using RHC.107 Indeed, in this study of advanced HF patients, RHC altered management in 71% of cases. See question #21 for more details.
Q25: How should an oral diuretic dose to maintain euvolemia be chosen?
Recommendation:
- Several factors should be taken into consideration when determining an appropriate oral diuretic dose to maintain euvolemia: current IV dosing regimen during admission, renal function, previous home diuretic dose, and reason for decompensation.
The ideal choice of loop diuretic for patients with chronic HF is unknown.98 Several factors should be taken into consideration when determining an appropriate oral diuretic dose to maintain euvolemia: current IV dosing regimen during admission, renal function, previous home diuretic dose, and reason for decompensation. Dosing conversion from IV to oral route needs to be taken into consideration (See Tables 1 and 2 above). Depending on degree of residual congestion, patients may have their loop diuretic dose response curve shifted to the right (see Figure 4). Therefore, higher doses of oral loop diuretic may be needed. Both furosemide and bumetanide are short-acting and are therefore often more effective when given twice daily to reduce periods when the concentration in the tubular fluid is below a therapeutic level.98 Torsemide and bumetanide have more predictable bioavailability than furosemide.108
Torsemide is favored by some given its longer duration and more predictable bioavailability, particularly for patients with CKD or diuretic resistance. Retrospective data and a small, randomized trial suggest improved outcomes with torsemide compared to furosemide. Torsemide was recently compared to furosemide in the TRANSFORM-HF (Torsemide comparison with furosemide for management of heart failure) trial.98 This study randomized patients hospitalized with ADHF regardless of ejection fraction to furosemide (n = 1428) or torsemide (n = 1431). The primary outcome of all-cause mortality occurred in 26.2% of the furosemide group vs 26.1% of the torsemide group (p = 0.77). The secondary composite outcome of all-cause mortality or hospitalization occurred in 49.3% of the furosemide group vs 47.3% of the torsemide group (p = 0.11).109 Thus, more data is needed to support the use of any one loop diuretic over another.
Transition to oral diuretics should also prompt consideration for potassium supplementation at home.1 The potassium supplementation will be lower for oral therapy intended to maintain rather than decrease net fluid balance. It is preferable to test the potassium replacement schedule upon switching to oral diuretics. One should consider changes to therapy that may alter potassium elimination (ACEI, ARB, ARNI, and aldosterone antagonists) and consider conditions that are at risk for hyperkalemia, such as CKD.
Q26: How should the efficacy and safety of an oral diuretic dose be evaluated?
Recommendation:
- Patients should be monitored for at least 24 hours on oral loop diuretic therapy before discharge to ensure that the loop diuretic regimen selected achieves adequate urine output and maintains stable weight, renal function, and electrolytes.
We strongly recommend that patients are monitored for at least 24 hours on oral diuretic therapy before discharge. Readmissions after admission for decompensated HF are very common and often due to inadequate decongestion or recurrent congestion. For this reason, it is imperative that patients receive an oral diuretic regimen capable of maintaining euvolemia.
Of note, it is important that oral diuretic therapy be assessed after a suitable period of time off of IV diuretic therapy. Patients should be off of IV diuretics for at least six hours prior to assessing the efficacy of an oral diuretic dose. Additionally, thiazide diuretics such as metolazone have a longer half-life compared to loop diuretics. Providers must recognize that response to an oral loop diuretic can be affected by recent thiazide diuretic exposure.
Several variables can be considered when determining an adequate oral diuretic dose. Prior to discharge, it is most appropriate to ensure that patients maintain a stable weight on oral diuretic therapy. Physician discretion is needed in determining whether once or twice daily dosing of an oral diuretic is needed. This may depend on the current volume status and renal function of the patient. Furosemide and bumetanide are more likely to require twice daily dosing since they both have a shorter duration of action compared to torsemide. If given twice daily, patients should be instructed to take the second dose in the early afternoon to avoid urinating overnight. Effective oral diuretics usually result in urination without 45 minutes of dosing with several voids in the following hours. This response can be promoted by having patients take oral diuretic in a recumbent position on an empty stomach. In addition to monitoring weight and urine output, renal function and electrolytes should be monitored closely. Perturbations in either may require alterations in oral diuretic dosing to avoid electrolytes abnormalities or kidney injury which could contribute to readmission. Change in supplemental potassium dosing may be required.
While establishing an oral diuretic before discharge is supported by guidelines (Yancy 2013, Hollenberg 2019),1,9 few studies have sought to evaluate a benefit from this. A retrospective study of 123 patients from a single academic center revealed lower HF readmission rates among patients observed on oral diuretic therapy for over 24 hours prior to discharge.110
Q27: What is a rescue diuretic dose and how should it be selected?
Recommendation:
- A rescue diuretic dose should be prescribed before discharge. This allows patients and outpatient providers to respond quickly to evidence of fluid retention to prevent decompensation and rehospitalization.
- Patients must notify providers immediately when a rescue dose is used.
Despite the best efforts of inpatient providers, it can be difficult to select an appropriate oral diuretic regimen for outpatient use. This is partly due to the limited time available to assess the efficacy of an oral diuretic. It is also related to changes in patient sodium and fluid consumption after discharge. As a result, diuretic therapy is frequently adjusted in the first few weeks after discharge.
Therefore, it is imperative for inpatient providers to prescribe a rescue diuretic dose for patients.1 This allows for patients and outpatient providers to respond quickly to evidence of fluid retention to prevent decompensation and rehospitalization. The selection of a rescue diuretic dose depends on a patient’s maintenance diuretic dose. Assuming an efficacious diuretic dose is being used, when a patient is prescribed a loop diuretic with a daily frequency, twice daily dosing should be considered. In patients taking an efficacious loop diuretic twice daily for maintenance, a thiazide diuretic booster could be considered for as needed dosing. Please see question #26 for more information regarding determining the efficacy of an oral diuretic dose.
Regardless of the selected rescue diuretic regimen, patient education is incredibly important. Patients must be educated on the signs and symptoms of fluid retention so that the rescue diuretic regimen can be effectively utilized, and providers can be notified. Rescue diuretic prescriptions may be particularly useful to direct providers at rehabilitation facilities that may be less familiar with the patient.
We recommend the using the green-yellow-red HF zones (see Appendix) for educating patients on when to contact providers and when to utilize the rescue diuretic dose. When patients are in the “yellow zone,” a rescue diuretic dose should be considered. For patients in the “red zone,” referral to an emergency department is most appropriate.
Please note that patients may require recommendations for potassium supplementation should they require a rescue diuretic regimen.
Please note it is best for all patients to notify their providers should they use the rescue diuretic dose.
We encourage providers to document the discharge diuretic plan in the discharge summary (Table 6).
Q28: What should be considered essential documentation at the time of discharge?
Recommendation:
- Discharge summaries should include the information listed below.
The transition of care from the inpatient to the outpatient setting is a critical time for HF patients and their caregivers. The discharge summary is an important document that conveys what happened during the hospital stay and sets the stage for maintenance and optimization of care by outpatient providers. Care facilities (subacute rehabilitation, skilled nursing centers, etc.) are dependent on the discharge summary as a guide to help them provide ongoing care. Critical information for the discharge summary includes the following:1
- ▪ Admission weight
- ▪ Discharge weight (this should be the patient’s dry weight in almost all cases)
- ▪ Oral diuretic dose recommended at discharge
- ▪ 24-hour urine output on oral diuretic therapy alone (if available)
- ▪ Rescue diuretic dose and triggers
- ▪ Renal function at discharge
- ▪ Admission and discharge natriuretic peptide levels
- ▪ Goal-directed medical therapy for HF initiated and/or contraindications to initiation
- ▪ Follow-up laboratory work, testing, and appointments (should ideally be scheduled and on the AVS and Discharge Summary at the time of discharge)
- ▪ Contact number the patient should call if they have questions
- ▪ Self-care instructions including daily weights and vital sign monitoring
- ▪ Diet recommendations (usually 2,000 mg sodium and 2-liter fluid restrictions per day)
- ▪ A copy of the Heart Failure Action Plan and other relevant educational materials (see Q29)
For discharge documentation, please consider using the following smartphrase which includes some of the information above: “.HFDISCHARGEDOC.”
Q29: What education regarding self-care and diuresis should be provided to patients?
Recommendation:
- All patients should be provided with Michigan Medicine Heart Failure self-care education prior to discharge.
Patients can use the link below to access all Michigan Medicine Heart Failure education materials.
Heart Failure | Patient Education Clearinghouse (umich.edu)
We also recommend that patients watch the following short online videos:
- What is Heart Failure?
- Commonly Prescribed Medications For People with Heart Failure
- What is a Fluid Restriction?
- What is a Sodium Restriction?
These can be accessed using the link below:
Literature, Guidelines, and Performance Measures
Strategy for Literature Search
The literature search for this guideline was conducted prospectively using the major keywords of “diuretics” or “diuresis” or “heart failure”. Results were limited to humans, adults, and published in the English language, for dates ranging from January 2011 to August 2021 on Medline. Results were limited to Guidelines, Clinical Trials, and Cohort Studies.
Additional key words included: diures*, diuretic*, frusemide*, Lasix, errolon, furanthril, furantral, bumetanide, bumenthanide, bumex, drenural, fordiuran, miccil, bumedyl, torsemide, demadex, tolvaptan, heart failure, cardiac failure, myocardial failure, heart decompensation, fluid restriction and heart failure, sodium restriction and heart failure, acute kidney injury/or cardio renal syndrome or worsening renal function, or worsening kidney function, loop diuretic* or high ceiling diuretic* or sodium potassium chloride cotransporter inhibitor* or Na-K-Cl transporter inhibitor*, diuretic* resistance or resistant or response*.
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. The search was a single cycle. Conclusions were based on prospective randomized clinical trials if available, to the exclusion of other data; if randomized controlled trials were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size.
The main search retrieved 29,833 references for the search terms “diuretic” and “heart failure”. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results were as follows:
- Diuretics/Heart Failure - Guidelines, total results were 1813
- Diuretics/Heart Failure - Clinical Trials, total results were 13642
- Diuretics/Heart Failure – Cohort Studies, total results were 14378
Related National Performance Measures
There are no national performance measures associated with Diuretic Management in Decompensated Heart Failure.
Funding
The development of this guideline was funded by the University of Michigan Health System
Guideline Development Team and Disclosures
The multidisciplinary guideline development team represented the following units of the University of Michigan Health System:
- Internal Medicine: David A.Bozaan, MD
- Cardiology: Matthew C. Konerman, MD, Adam B. Stein, MD, Jessica Guidi, MD
- Nephrology: Michael Heung, MD
- Pharmacy Services: Sarah Adie, PharmD
- Inpatient Clinical Guidelines Oversight: Megan R. Mack, MD, David H. Wesorick, MD, April Proudlock, BBA, RN, Ellen Patrick, MA
- Literature search service: Taubman Health Sciences Library
Disclosures
The University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
Relevant personal financial relationships with commercial entities: None
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Health System to which the content is most relevant: Emergency Medicine, General Medicine, Infectious Disease, Neurosurgery, Cardiology, Cardiac Surgery, Stroke, Pharmacy Services, and Thoracic Surgery. Medication recommendations were reviewed by the Pharmacy and Therapeutics Committee. The final version was endorsed by the Clinical Practice Committee of the University of Michigan Faculty Group Practice and the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
Appendix A. Michigan Medicine Heart Failure Action Plan
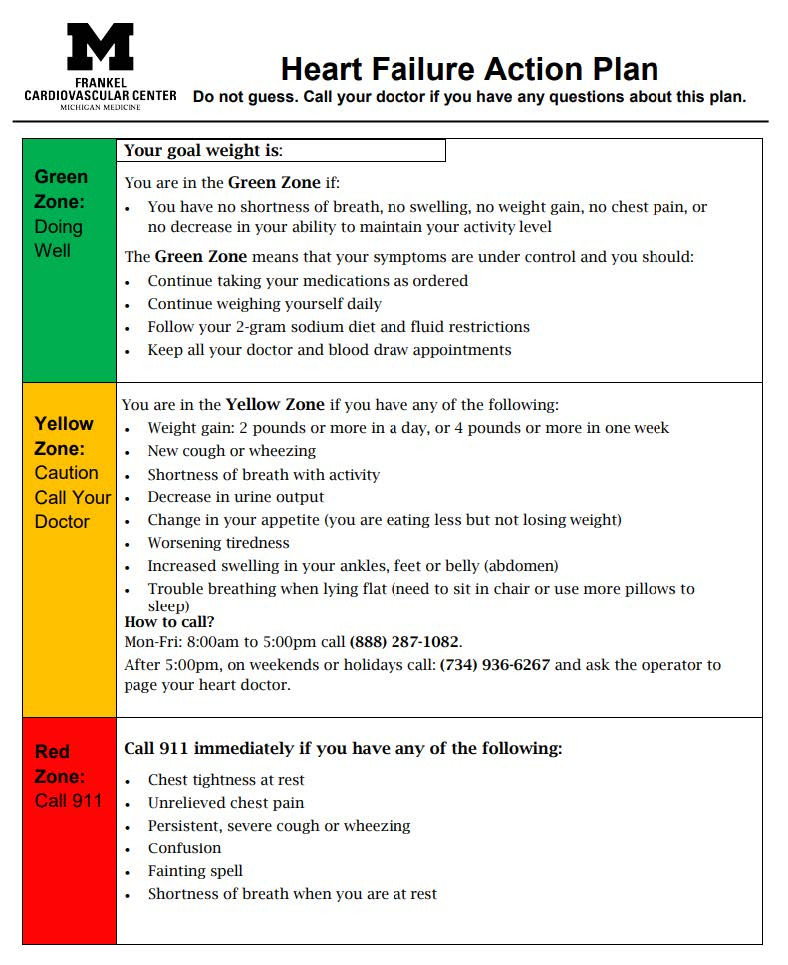
References
- 1.
- Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, Reed BN, Roy CL, Storrow AB. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74(15):1966–2011. doi:10.1016/j.jacc.2019.08.001 [PubMed: 31526538] [CrossRef]
- 2.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. Journal of Medicine. 2011;364(9):797–805. doi:10.1056/NEJMoa1005419 [PMC free article: PMC3412356] [PubMed: 21366472] [CrossRef]
- 3.
- Wu W, Bush KT, Nigam SK. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci Rep. 2017;7(1):4939. doi:10.1038/s41598-017-04949-2 [PMC free article: PMC5504054] [PubMed: 28694431] [CrossRef]
- 4.
- Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic Therapy for Patients With Heart Failure: JACC State-of-the-Art Review. [Review]. Journal of the American College of Cardiology. 2020;75(10):1178–1195. doi:10.1016/j.jacc.2019.12.059 [PubMed: 32164892] [CrossRef]
- 5.
- Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102(3):314–8. [PubMed: 3970471]
- 6.
- Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007;107(1):44–51. [PubMed: 16741357]
- 7.
- Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, Adams KF Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–1069. [PMC free article: PMC3127394] [PubMed: 17719273]
- 8.
- Brisco MA, Zile MR, Hanberg JS, et al. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. Journal of Cardiac Failure. 2016;22(10):753–760. doi:10.1016/j.cardfail.2016.06.423 [PMC free article: PMC5435117] [PubMed: 27374839] [CrossRef]
- 9.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;52(16):e147–239. [PubMed: 23747642]
- 10.
- Xiong L, Chinaeke EE, Lu K, Lenihan DJ, Merritt TE, Cox ZL. Evaluation of Severe Myalgia Induced by Continuous-Infusion Bumetanide in Patients with Acute Heart Failure. Journal of Human Pharmacology. 2019;39(8):854–860. doi:10.1002/phar.2297 [PubMed: 31225921] [CrossRef]
- 11.
- Jhund PS, McMurray JJ, Davie AP. The acute vascular effects of frusemide in heart failure. Br J Clin Pharmacol. 2000;50(1):9–13. [PMC free article: PMC2014966] [PubMed: 10886111]
- 12.
- Ellison DH, Felker GM. Diuretic Treatment in Heart Failure. N Engl J Med. 2017;377(20):1964–1975. [PMC free article: PMC5811193] [PubMed: 29141174]
- 13.
- Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr. 2010;92(5):1172–1180. [PMC free article: PMC2954449] [PubMed: 20826631]
- 14.
- Appel L, Frohlich E, Hall J, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–1143. [PubMed: 21233236]
- 15.
- Vest AR, Chan M, Deswal A, et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. Journal of Cardiac Failure. 2019;25(5):380–400. doi:10.1016/j.cardfail.2019.03.007 [PubMed: 30877038] [CrossRef]
- 16.
- Riegel B, Lee S, Hill J, et al. Patterns of adherence to diuretics, dietary sodium and fluid intake recommendations in adults with heart failure. Heart & Lung. 2019;48(3):179–185. doi:10.1016/j.hrtlng.2018.12.008 [PMC free article: PMC6486853] [PubMed: 30638609] [CrossRef]
- 17.
- Colin-Ramirez E, Arcand J, Ezekowitz JA. Estimates of Dietary Sodium Consumption in Patients With Chronic Heart Failure. [Review]. Journal of Cardiac Failure. 2015;21(12):981–988. doi:10.1016/j.cardfail.2015.08.345 [PubMed: 26386452] [CrossRef]
- 18.
- Cohen LP, Hummel SL, Maurer MS, Lopez-Pintado S, Wessler JD. Salt Taste Recognition in a Heart Failure Cohort. Journal of Cardiac Failure. 2017;23(7):538–544. doi:10.1016/j.cardfail.2017.05.001 [PubMed: 28499978] [CrossRef]
- 19.
- Billingsley HE, Hummel SL, Carbone S. The role of diet and nutrition in heart failure: A state-of-the-art narrative review. [Review]. Progress in Cardiovascular Diseases. 2020;63(5):538–551. doi:10.1016/j.pcad.2020.08.004 [PMC free article: PMC7686142] [PubMed: 32798501] [CrossRef]
- 20.
- Khan MS, Jones DW, Butler J. Salt, No Salt, or Less Salt for Patients With Heart Failure?. [Review]. Journal of Medicine. 2020;133(1):32–38. doi:10.1016/j.amjmed.2019.07.034 [PubMed: 31419422] [CrossRef]
- 21.
- Colin-Ramirez E, Ezekowitz JA. Salt in the diet in patients with heart failure: what to recommend. [Review]. Current Opinion in Cardiology. 2016;31(2):196–203. doi:10.1097/HCO.0000000000000253 [PubMed: 26595701] [CrossRef]
- 22.
- Paterna. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clinical Science. Published online 2008. [PubMed: 17688420]
- 23.
- Paterna. Medium Term Effects of Different Dosage of Diuretic, Sodium, andFluid Administration on Neurohormonal and Clinical Outcome inPatients With Recently Compensated Heart Failure. American Journal of Cardiology. Published online 2009. [PubMed: 19101237]
- 24.
- Colin-Ramirez. Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition. Published online 2004. [PubMed: 15474877]
- 25.
- Bilgen. Insufficient Calorie Intake Worsens Post-Discharge Quality of Life and Increases Readmission Burden in Heart Failure. JACC: Heart Failure. Published online 2020. [PMC free article: PMC9210452] [PubMed: 32653445]
- 26.
- Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M. Salt and fluid restriction is effective in patients with chronic heart failure. Journal of Heart Failure. 2013;15(11):1304–1310. doi:10.1093/eurjhf/hft097 [PubMed: 23787719] [CrossRef]
- 27.
- Sadanaga T, Ando K, Hirota S, et al. B-type natriuretic peptide levels are decreased by reducing dietary salt intake in patients with compensated heart failure with preserved ejection fraction. Internal Medicine Journal. 2013;43(6):663–667. doi:10.1111/imj.12063 [PubMed: 23279137] [CrossRef]
- 28.
- Hummel SL, Seymour EM, Brook RD, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60(5):1200–1206. doi:10.1161/HYPERTENSIONAHA.112.202705 [PMC free article: PMC3522520] [PubMed: 23033371] [CrossRef]
- 29.
- Hummel SL, Seymour EM, Brook RD, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circulation: Heart Failure. 2013;6(6):1165–1171. doi:10.1161/CIRCHEARTFAILURE.113.000481 [PMC free article: PMC4017662] [PubMed: 23985432] [CrossRef]
- 30.
- Arcand J, Ivanov J, Sasson A, et al. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Journal of Clinical Nutrition. 2011;93(2):332–337. doi:10.3945/ajcn.110.000174 [PubMed: 21084647] [CrossRef]
- 31.
- Lennie TA, Song EK, Wu JR, et al. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. Journal of Cardiac Failure. 2011;17(4):325–330. doi:10.1016/j.cardfail.2010.11.008 [PMC free article: PMC3073718] [PubMed: 21440871] [CrossRef]
- 32.
- Son YJ, Lee Y, Song EK. Adherence to a sodium-restricted diet is associated with lower symptom burden and longer cardiac event-free survival in patients with heart failure. Journal of Clinical Nursing. 2011;20(21–22):3029–3038. doi:10.1111/j.1365-2702.2011.03755.x [PubMed: 21707808] [CrossRef]
- 33.
- Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event-free survival in patients with mild heart failure. Journal of Cardiovascular Nursing. 2014;13(6):541–548. doi:10.1177/1474515113517574 [PMC free article: PMC4393886] [PubMed: 24366983] [CrossRef]
- 34.
- Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Internal Medicine. 2013;173(12):1058–1064. doi:10.1001/jamainternmed.2013.552 [PubMed: 23689381] [CrossRef]
- 35.
- Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, et al. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: Results from a randomized clinical trial. Nutrition. 2018;1:111–117. doi:10.1016/j.nut.2018.02.007 [PubMed: 29793053] [CrossRef]
- 36.
- Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145(3):459–466. [PubMed: 12660669]
- 37.
- Doukky R, Avery E, Mangla A, et al. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC Heart Failure. 2016;4(1):24–35. doi:10.1016/j.jchf.2015.08.007 [PMC free article: PMC4705447] [PubMed: 26738949] [CrossRef]
- 38.
- Hummel. Dietary Sodium Restriction in Heart Failure: A Recommendation Worth its Salt?∗. JACC Heart Failure. Published online 2016. [PubMed: 26738950]
- 39.
- DiNicolantonio. Retraction notice. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta-analysis. Heart. Published online 2013. [PubMed: 22914535]
- 40.
- DiNicolantonio. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta-analysis. Heart. Published online 2013. [PubMed: 22914535]
- 41.
- Expression of Concern: Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clinical Science. Published online 2020. [PubMed: 32677681]
- 42.
- Kessing D, Denollet J, Widdershoven J, Kupper N. Self-Care and All-Cause Mortality in Patients With Chronic Heart Failure. JACC Heart Failure. 2016;4(3):176–183. doi:10.1016/j.jchf.2015.12.006 [PubMed: 26874385] [CrossRef]
- 43.
- Kalogeropoulos A, Papadimitriou L, Georgiopoulou VV, Dunbar SB, Skopicki H, Butler J. Low- Versus Moderate-Sodium Diet in Patients With Recent Hospitalization for Heart Failure: The PROHIBIT (Prevent Adverse Outcomes in Heart Failure by Limiting Sodium) Pilot Study. Circulation: Heart Failure. 2020;13(1):e006389. doi:10.1161/CIRCHEARTFAILURE.119.006389 [PMC free article: PMC7233690] [PubMed: 31959014] [CrossRef]
- 44.
- Cobb. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. Published online 2014. [PubMed: 24515991]
- 45.
- Miller RK, Thornton N. Does Evidence Drive Fluid Volume Restriction in Chronic Heart Failure?. [Review]. Nursing Clinics of North America. 2017;52(2):261–267. doi:10.1016/j.cnur.2017.01.003 [PubMed: 28478874] [CrossRef]
- 46.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726.
- 47.
- Parrinello G, Di Pasquale P, Licata G, Torres D, Giammanco M, Fasullo S, Mezzero M, Paterna S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15(10):864–873. [PubMed: 19944363]
- 48.
- Li Y, Fu B, Qian X. Liberal versus restricted fluid administration in heart failure patients. A systematic review and meta-analysis of randomized trials. [Review]. International Heart Journal. 2015;56(2):192–195. doi:10.1536/ihj.14-288 [PubMed: 25740394] [CrossRef]
- 49.
- Travers B, O’Loughlin C, Murphy NF, Ryder M, Conlon C, Ledwidge M, McDonald K. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13(2):128–132. [PubMed: 17395053]
- 50.
- Holst M, Strömberg A, Lindholm M, Willenheimer R. Liberal versus restricted fluid prescription in stabilised patients with chronic heart failure: result of a randomised cross-over study of the effects on health-related quality of life, physical capacity, thirst and morbidity. Scand Cardiovasc J. 2008;42(5):316–322. [PubMed: 18609051]
- 51.
- Albert NM, Nutter B, Forney J, Slifcak E, Tang WH. A randomized controlled pilot study of outcomes of strict allowance of fluid therapy in hyponatremic heart failure (SALT-HF). J Card Fail. 2013;19(1):1–9. [PubMed: 23273588]
- 52.
- Allida SM, Shehab S, Inglis SC, Davidson PM, Hayward CS, Newton PJ. A RandomisEd ControLled TrIal of ChEwing Gum to RelieVE Thirst in Chronic Heart Failure (RELIEVE-CHF). Heart, Lung & Circulation. 2021;30(4):516–524. doi:10.1016/j.hlc.2020.09.004 [PubMed: 33032897] [CrossRef]
- 53.
- Gerl H, Miko A, Nelson M, Godaire L. Are In-Bed Electronic Weights Recorded in the Medical Record Accurate? Medsurg Nurs. 2016;25(3):177–181. [PubMed: 27522846]
- 54.
- Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, Coca SG, Tang WH. Substantial Discrepancy Between Fluid and Weight Loss During Acute Decompensated Heart Failure Treatment. Am J Med. 2015;128(7):776–783. [PMC free article: PMC4475432] [PubMed: 25595470]
- 55.
- Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G, Greenberg BH, Pang PS, Levin B, Hua TA, Severin T, Ponikowski P, Metra M; RELAX-AHF Investigators. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail. 2014;16(11):1230–1240. [PMC free article: PMC4489340] [PubMed: 25287144]
- 56.
- Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35(19):1284–1293. [PubMed: 24585267]
- 57.
- Kula AJ, Hanberg JS, Wilson FP, Brisco MA, Bellumkonda L, Jacoby D, Coca SG, Parikh CR, Tang WHW, Testani JM. Influence of Titration of Neurohormonal Antagonists and Blood Pressure Reduction on Renal Function and Decongestion in Decompensated Heart Failure. Circ Heart Fail. 2016;9(1):e002333. [PMC free article: PMC4741376] [PubMed: 26699390]
- 58.
- Ferreira JP, Girerd N, Bettencourt Medeiros P, Bento Ricardo M, Almeida T, Rola A, Zannad F, Rossignol P, Aragão I. Lack of Diuretic Efficiency (but Not Low Diuresis) Early in An Acutely Decompensated Heart Failure Episode Is Associated with Increased 180-Day Mortality. Cardiorenal Med. 2017;7(2):137–149. [PMC free article: PMC5465746] [PubMed: 28611787]
- 59.
- Singh D, Shrestha K, Testani JM, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. Journal of Cardiac Failure. 2014;20(6):392–399. doi:10.1016/j.cardfail.2014.03.006 [PMC free article: PMC4067259] [PubMed: 24704538] [CrossRef]
- 60.
- Testani JM, Hanberg JS, Cheng S, et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients With Heart Failure. Circulation: Heart Failure. 2016;9(1):e002370. doi:10.1161/CIRCHEARTFAILURE.115.002370 [PMC free article: PMC4741370] [PubMed: 26721915] [CrossRef]
- 61.
- Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7(5):766–772. [PubMed: 25037309]
- 62.
- Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–155. [PubMed: 30600580]
- 63.
- Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW; ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. [PubMed: 16204662]
- 64.
- Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. Journal of Medicine. 2012;367(24):2296–2304. doi:10.1056/NEJMoa1210357 [PMC free article: PMC3690472] [PubMed: 23131078] [CrossRef]
- 65.
- Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–83. [PubMed: 17291932]
- 66.
- Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M; RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. [PubMed: 23141816]
- 67.
- Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–272. [PMC free article: PMC3025294] [PubMed: 20606118]
- 68.
- van der Meer P, Postmus D, Ponikowski P, et al. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. Journal of the American College of Cardiology. 2013;61(19):1973–1981. doi:10.1016/j.jacc.2012.12.050 [PubMed: 23500313] [CrossRef]
- 69.
- Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. [PMC free article: PMC2856960] [PubMed: 19215833]
- 70.
- Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16(7):541–547. [PubMed: 20610229]
- 71.
- Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF. Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J. 2014;168(6):891–900. [PubMed: 25458653]
- 72.
- Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circulation: Heart Failure. 2012;5(1):54–62. doi:10.1161/CIRCHEARTFAILURE.111.963413 [PubMed: 22167320] [CrossRef]
- 73.
- Greene SJ, Gheorghiade M, Vaduganathan M, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Journal of Heart Failure. 2013;15(12):1401–1411. doi:10.1093/eurjhf/hft110 [PMC free article: PMC4199468] [PubMed: 23845795] [CrossRef]
- 74.
- Abraham J, Blumer V, Burkhoff D, Pahuja M, Sinha SS, Rosner C, Vorovich E, Grafton G, Bagnola A, Hernandez-Montfort JA, Kapur NK. Heart Failure-Related Cardiogenic Shock: Pathophysiology, Evaluation and Management Considerations: Review of Heart Failure-Related Cardiogenic Shock. J Card Fail. 2021;27(10):1126–1140. [PubMed: 34625131]
- 75.
- Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Failure. 2020;8(3):157–168. doi:10.1016/j.jchf.2019.09.012 [PMC free article: PMC7058489] [PubMed: 31838029] [CrossRef]
- 76.
- Palazzuoli A, Ruocco G, Ronco C, McCullough PA. Loop diuretics in acute heart failure: beyond the decongestive relief for the kidney. Critical Care (London, England). 2015;1:296. doi:10.1186/s13054-015-1017-3 [PMC free article: PMC4559070] [PubMed: 26335137] [CrossRef]
- 77.
- Groarke JD, Stevens SR, Mentz RJ, et al. Clinical Significance of Early Fluid and Weight Change During Acute Heart Failure Hospitalization. Journal of Cardiac Failure. 2018;24(9):542–549. doi:10.1016/j.cardfail.2017.12.013 [PubMed: 29337281] [CrossRef]
- 78.
- Ponikowski. ESC GUIDELINES2016 ESC Guidelines for the diagnosis andtreatment of acute and chronic heart failure. European Journal of Heart Failure. Published online 2016.
- 79.
- Rao VS, Ivey-Miranda JB, Cox ZL, et al. Natriuretic Equation to Predict Loop Diuretic Response in Patients With Heart Failure. Journal of the American College of Cardiology. 2021;77(6):695–708. doi:10.1016/j.jacc.2020.12.022 [PMC free article: PMC8114781] [PubMed: 33573739] [CrossRef]
- 80.
- Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–155. [PubMed: 30600580]
- 81.
- Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–34. [PubMed: 21029871]
- 82.
- Givertz MM, Teerlink JR, Albert NM, et al. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. [Review]. Journal of Cardiac Failure. 2013;19(6):371–389. doi:10.1016/j.cardfail.2013.04.002 [PubMed: 23743486] [CrossRef]
- 83.
- Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287(5):628–640. [PubMed: 11829703]
- 84.
- Chioncel O, Ambrosy AP, Bubenek S, et al. Epidemiology, pathophysiology, and in-hospital management of pulmonary edema: data from the Romanian Acute Heart Failure Syndromes registry. Journal of Cardiovascular Medicine. 2016;17(2):92–104. doi:10.2459/JCM.0000000000000192 [PubMed: 25252041] [CrossRef]
- 85.
- Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139(16):e840–e878. [PubMed: 30852913]
- 86.
- Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, Herck I, Decruyenaere J, Hoste EA. Acute Kidney Injury in Cardiorenal Syndrome Type 1 Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2016;6(2):116–28. [PMC free article: PMC4789882] [PubMed: 26989397]
- 87.
- Husain-Syed F, Gröne HJ, Assmus B, Bauer P, Gall H, Seeger W, Ghofrani A, Ronco C, Birk HW. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC heart failure. 2021;8(1):183–203. [PMC free article: PMC7835563] [PubMed: 33258308]
- 88.
- Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582–588. [PubMed: 19215832]
- 89.
- Gambardella I, Gaudino M, Ronco C, Lau C, Ivascu N, Girardi LN. Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg. 2016;23(5):800–805. [PubMed: 27422971]
- 90.
- Khoury S, Steinvil A, Gal-Oz A, et al. Association between central venous pressure as assessed by echocardiography, left ventricular function and acute cardio-renal syndrome in patients with ST segment elevation myocardial infarction. Clinical Research in Cardiology. 2018;107(10):937–944. doi:10.1007/s00392-018-1266-7 [PubMed: 29744618] [CrossRef]
- 91.
- Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail. Published online 2022. [PMC free article: PMC8934917] [PubMed: 35112512]
- 92.
- Ahmad T, Jackson K, Rao VS, et al. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation. 2018;137(19):2016–2028. doi:10.1161/CIRCULATIONAHA.117.030112 [PMC free article: PMC6066176] [PubMed: 29352071] [CrossRef]
- 93.
- Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. [Review]. European Heart Journal. 2014;35(7):455–469. doi:10.1093/eurheartj/eht386 [PubMed: 24164864] [CrossRef]
- 94.
- Metra M, Cotter G, Senger S, Edwards C, Cleland JG, Ponikowski P, Cursack GC, Milo O, Teerlink JR, Givertz MM, O’Connor CM, Dittrich HC, Bloomfield DM, Voors AA, Davison BA. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circ Heart Fail. 2018;11(5)::e004644. doi:doi:10.1161/CIRCHEARTFAILURE.117.004644 [PubMed: 29748350] [CrossRef]
- 95.
- Wattad M, Darawsha W, Solomonica A, et al. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Journal of Cardiology. 2015;115(7):932–937. doi:10.1016/j.amjcard.2015.01.019 [PubMed: 25700802] [CrossRef]
- 96.
- Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, Tartaglia K, Chenot F, Moubayed S, Dierckx R, Blouard P, Troisfontaines P, Derthoo D, Smolders W, Bruckers L, Droogne W, Ter Maaten JM, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M; ADVOR Study Group. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N Engl J Med. 2022;387(13):1185–1195. [PubMed: 36027559]
- 97.
- Sharma K, Vaishnav J, Kalathiya R, et al. Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine: The ROPA-DOP Trial. JACC Heart Failure. 2018;6(10):859–870. doi:10.1016/j.jchf.2018.04.008 [PubMed: 30098962] [CrossRef]
- 98.
- Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic Therapy for Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(10):1178–1195. [PubMed: 32164892]
- 99.
- Ambrosy AP, Vaduganathan M, Mentz RJ, et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. American Heart Journal. 2013;165(2):216–225. doi:10.1016/j.ahj.2012.11.004 [PubMed: 23351825] [CrossRef]
- 100.
- Rubio-Gracia J, Demissei BG, Ter Maaten JM, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Journal of Cardiology. 2018;1:185–191. doi:10.1016/j.ijcard.2018.01.067 [PubMed: 29544928] [CrossRef]
- 101.
- Stienen S, Salah K, Moons AH, Bakx AL, van Pol P, Kortz RAM, Ferreira JP, Marques I, Schroeder-Tanka JM, Keijer JT, Bayés-Genis A, Tijssen JGP, Pinto YM, Kok WE. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure: PRIMA II Randomized Controlled Trial (Can NT-ProBNP-Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?). Circulation. 2018;137(16):1671–1683. [PubMed: 29242350]
- 102.
- Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, Young JB, Califf RM, Nohria A. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1(3):170–177. [PMC free article: PMC2724723] [PubMed: 19675681]
- 103.
- Drazner MH, Hamilton MA, Fonarow G, Creaser J, Flavell C, Stevenson LW. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132. [PubMed: 10598737]
- 104.
- Drazner MH, Velez-Martinez M, Ayers CR, Reimold SC, Thibodeau JT, Mishkin JD, Mammen PP, Markham DW, Patel CB. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail. 2013;6(2):264–270. [PubMed: 23392790]
- 105.
- Campbell P, Drazner MH, Kato M, Lakdawala N, Palardy M, Nohria A, Stevenson LW. Mismatch of right- and left-sided filling pressures in chronic heart failure. J Card Fail. 2011;17(7):561–568. [PubMed: 21703528]
- 106.
- Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41(10):1797–1804. [PubMed: 12767667]
- 107.
- Narang N, Chung B, Nguyen A, et al. Discordance Between Clinical Assessment and Invasive Hemodynamics in Patients With Advanced Heart Failure. Journal of Cardiac Failure. 2020;26(2):128–135. doi:10.1016/j.cardfail.2019.08.004 [PMC free article: PMC7108664] [PubMed: 31442494] [CrossRef]
- 108.
- Teerlink JR, Alburikan K, Metra M, Rodgers JE. Acute decompensated heart failure update. Curr Cardiol Rev. 2015;11(1):53–62. [PMC free article: PMC4347210] [PubMed: 24251454]
- 109.
- Bavry Anthony A., MD, MPH, FACC. Torsemide Comparison With Furosemide for Management of Heart Failure - TRANSFORM-HF. National Heart, Lung, and Blood Institute. Published online 2022.
- 110.
- Laliberte B, Reed BN, Devabhakthuni S, Watson K, Ivaturi V, Liu T, Gottlieb SS. Observation of Patients Transitioned to an Oral Loop Diuretic Before Discharge and Risk of Readmission for Acute Decompensated Heart Failure. J Card Fail. 2017;23(10):746–752. [PubMed: 28688888]
Approvals
P&T: Oct 2022
ECCA: Dec 2022
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Created: December 2022.
- Guideline Summary
- Guideline Overview
- Michigan Medicine Inpatient Diuresis Guide
- Loop Diuretic Initiation
- Monitoring Efficacy and Safety of Loop Diuretic Therapy
- Transitioning to Oral Diuretic Therapy
- Literature, Guidelines, and Performance Measures
- Michigan Medicine Heart Failure Action Plan
- References
- Approvals
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Effects of nesiritide and predictors of urine output in acute decompensated heart failure: results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure).[J Am Coll Cardiol. 2013]Effects of nesiritide and predictors of urine output in acute decompensated heart failure: results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure).Gottlieb SS, Stebbins A, Voors AA, Hasselblad V, Ezekowitz JA, Califf RM, O'Connor CM, Starling RC, Hernandez AF. J Am Coll Cardiol. 2013 Sep 24; 62(13):1177-83. Epub 2013 Jun 7.
- Rationale and Design of the "Safety and Efficacy of the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial:" A Double-Blind, Randomized, Placebo-Controlled Study to Determine the Effect of Combined Diuretic Therapy (Loop Diuretics With Thiazide-Type Diuretics) Among Patients With Decompensated Heart Failure.[J Card Fail. 2016]Rationale and Design of the "Safety and Efficacy of the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial:" A Double-Blind, Randomized, Placebo-Controlled Study to Determine the Effect of Combined Diuretic Therapy (Loop Diuretics With Thiazide-Type Diuretics) Among Patients With Decompensated Heart Failure.Trullàs JC, Morales-Rull JL, Casado J, Freitas Ramírez A, Manzano L, Formiga F, CLOROTIC investigators. J Card Fail. 2016 Jul; 22(7):529-36. Epub 2015 Nov 11.
- Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure.[Circ Heart Fail. 2014]Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH. Circ Heart Fail. 2014 Mar 1; 7(2):261-70. Epub 2013 Dec 30.
- Review Effectiveness of continuous infusions of loop diuretics for severe heart failure.[J Cardiovasc Med (Hagerstown)....]Review Effectiveness of continuous infusions of loop diuretics for severe heart failure.Howard PA, Dunn MI. J Cardiovasc Med (Hagerstown). 2006 Jan; 7(1):5-10.
- Review Impact of Loop Diuretic on Outcomes in Patients with Heart Failure and Reduced Ejection Fraction.[Curr Heart Fail Rep. 2022]Review Impact of Loop Diuretic on Outcomes in Patients with Heart Failure and Reduced Ejection Fraction.Antonietta CM, Calvi E, Faggiano A, Maffeis C, Bosisio M, De Stefano M, Carugo S, Faggiano P. Curr Heart Fail Rep. 2022 Feb; 19(1):15-25. Epub 2022 Jan 17.
- Michigan Medicine Inpatient Diuretic Guideline for Patients with Acute Decompens...Michigan Medicine Inpatient Diuretic Guideline for Patients with Acute Decompensated Heart Failure
- LOC130066171 [Homo sapiens]LOC130066171 [Homo sapiens]Gene ID:130066171Gene
- LOC127893736 [Homo sapiens]LOC127893736 [Homo sapiens]Gene ID:127893736Gene
- nas-27 Zinc metalloproteinase nas-27 [Caenorhabditis elegans]nas-27 Zinc metalloproteinase nas-27 [Caenorhabditis elegans]Gene ID:188809Gene
- TRBJ1-3 T cell receptor beta joining 1-3 [Homo sapiens]TRBJ1-3 T cell receptor beta joining 1-3 [Homo sapiens]Gene ID:28633Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...