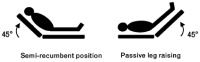Early Recognition and Initial Management of Sepsis in Adult Patients
Authors
Jessie King, MD, PhD, Lead, Carol E Chenoweth, MD, Peter C England, MD, Andrew Heiler, MBA, RN, Michael T Kenes, PharmD, FCCM, BCPS, BCCCP, Krishnan Raghavendran, MD, Winnie Wood, MSN, RN, CNS, and Shiwei Zhou, MD.1 Clinical Guidelines Oversight: Megan Mack, MD, Dave Wesorick, MD, and April Proudlock, BBA, RN.
Affiliations
 Quality Department Guidelines Program
Quality Department Guidelines ProgramFor more information: 734-936-9771
Patient Population: Sepsis is medical emergency, associated with high morbidity and mortality, and early recognition and standardized treatment of sepsis saves lives1. This guideline is intended for adult patients.
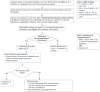
We recommend following the Surviving Sepsis Campaign’s International Guidelines for the Management of Sepsis and Septic Shock (2021)2, on which this guideline is based. We also endorse the SEP-1 treatment bundle (Figure 1) as standard of care for the initial management of a patient with sepsis.
Definitions: Sepsis is a syndrome characterized by life-threatening organ dysfunction caused by a dysregulated host response to infection.1 SEP3 clinically defined sepsis as acute organ dysfunction (e.g., ≥2 new SOFA points), plus evidence of infection2. However for billing, documentation, and treatment purposes, the older definitions of sepsis are still used.
Sepsis: two Systemic Inflammatory Response syndrome (SIRS) criteria plus evidence of infection.
Severe sepsis: Sepsis with evidence of organ dysfunction.
Septic shock: Sepsis with persistent hypotension, despite fluid resuscitation.
Key Points
Recognition of sepsis
- Severe sepsis and septic shock are medical emergencies, and we recommend that sepsis improvement programs be established for all hospital units/services at Michigan Medicine with the goal of standardizing and improving early recognition, resuscitation, and treatment. (I-C)
- Currently, screening tools and alerts have been put in place to help identify patients developing sepsis. We recommend that clinicians review the data in the sepsis navigator when the alert is received to determine whether the patient should be treated for sepsis. (I-E)
Initial Approach to the Sepsis Patient: The SEP-1 Bundle
- Figure 1 shows a recommended approach to early recognition and initial therapy for sepsis
Antibiotics
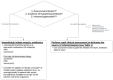
- Figure 2 summarizes the approach to empiric antibiotic recommendations for sepsis patients.
- Sepsis patients with nosocomial infections, organ dysfunction, signs of hypoperfusion or shock (including elevated lactate) presumed to be the result of infection, and/or immunosuppression should be treated with the immediate initiation of broad-spectrum antibiotics, while stable sepsis patients can undergo a rapid clinical evaluation to identify the source of sepsis before initiating empiric antibiotics. (I-C)
- Antimicrobial Stewardship disease state treatment guidelines should be utilized to select empiric antimicrobial therapy and to ensure appropriate dosing (Table 1). (I-E)
- MRSA nasal swabs, when negative, can eliminate the need for MRSA coverage in sepsis patients. (I-E)
- For adults with possible septic shock or a high likelihood for sepsis, we recommend administering antimicrobials immediately, ideally within 1 h of recognition. (I-C)
- For adults with possible sepsis without shock, we suggest a time-limited course of rapid investigation, and if concern for infection persists, the administration of antimicrobials within 3 h from the time when sepsis was first recognized. (I-C)
- For adults with a low likelihood of infection and without shock, we suggest deferring antimicrobials while continuing to closely monitor the patient. (II-E)
Resuscitation-Fluids (Figure 1)
- For adults with sepsis induced hypoperfusion, defined as a lactate ≥ 4 or hypotension (MAP < 65, SBP<90 or reduction in SBP of 40mmHg or more):
- ○ We suggest that 30 mL/kg (adjusted body weight for BMI > 30) of intravenous (IV) crystalloid fluid be given within the first 3 hours of resuscitation (I-E)
- ○ We also suggest consideration of fluid resuscitation in patients with mild lactate elevation (2-4) and/or signs/symptoms of volume-depletion (I-C).
- ○ We recommend using balanced crystalloids (lactated ringers) over normal saline for sepsis resuscitation (I-B)
- ○ We suggest considering albumin in patients who received large volume of crystalloids and have a low serum albumin over using crystalloids alone as discussed in the text (below) (II-C)
- ○ History of heart failure and liver failure are not contraindications to fluid resuscitation. For patients at risk for poor tolerance of fluids (e.g., reduced cardiac function, aortic stenosis, end-stage renal disease), however, we suggest frequent reassessment of intravascular volume status, with total volume of fluid-resuscitation based on response to therapy (II-E)
Resuscitation - Reassessment (Figure 1)
- After the initial fluid bolus, reassessment should include physical examination and repeat lactate level. Mean arterial pressure (MAP) should be used to determine the need for vasopressors, if not already started (see below), and additional fluids. (I-E)
- Useful physical examination maneuvers include the passive leg raise with subsequent assessment of perfusion (Figure 3), or assessment of capillary refill. (II-E)
- In the ICU, other hemodynamic parameters may be followed as well.
Resuscitation - Vasopressors and Steroids (Figure 1)
- For adults with septic shock, we recommend using norepinephrine as the first-line agent over other vasopressors (Starting dose: 0.05-0.15 mcg/kg/min, titrated to effect). (I-B)
- For adults with septic shock on norepinephrine with inadequate MAP levels, we suggest adding vasopressin at a rate of 0.03 units/min instead of escalating the dose of norepinephrine. In our practice, vasopressin is usually started when the dose of norepinephrine is in the range of 0.25-0.5 mcg/kg/min. (II-E)
- For adults with septic shock and inadequate MAP levels despite norepinephrine and vasopressin, we suggest adding epinephrine. (II-E)
- When using vasopressors peripherally we recommend they be delivered via a high-quality IV (18+g; in forearm or upper arm; ultrasound-confirmed; and with a nursing check per PIV policy to ensure proper functioning of the IV). The appropriateness of peripheral administration should be reassessed at least daily, transition to central administration in patients with high or escalating vasopressor dosing. (II-C)
- For adults with septic shock with a persistent norepinephrine requirement (e.g., ≥4 hours of NE at any dosage), we suggest using stress-dose steroids (i.e. IV hydrocortisone 50mg q6hr +/- oral fludrocortisone 50 mcg q24hr). (II-C)
- For adults with septic shock, we recommend using invasive monitoring of arterial blood pressure via radial arterial catheter over non-invasive monitoring, as soon as practical (II-E). If radial artery access is not feasible, then non-invasive monitoring vs alternative arterial access can be determined on a case-by-case basis.
Source Control
- When an anatomical area is identified as responsible for sepsis/septic shock, intervention to achieve source control increases the patient’s likelihood of survival. (I-C)
- Procedures for source control should be done as early as feasible, within 6-12 hours of admission, or as soon as possible after resuscitation. Some patients may not achieve hemodynamic stability without adequate source control. (I-C)
- Patients should be monitored for adequacy of source control after procedures/interventions are made, with the expectation of clinical improvement within 48 hours of definitive treatment. (II-E)
- For patients where intervention cannot be performed or such interventions do not provide complete source control, Infectious Disease should be consulted for assistance in management. (I-E)
De-resuscitation
- A positive fluid balance after resuscitation for sepsis is associated with worsened clinical outcomes. Extraneous or superfluous fluid administration (i.e., maintenance fluids or intravenous medications/carriers when enteral administration is acceptable) should be avoided. Achievement of a negative volume status (using diuresis or dialysis), once stable, should be achieved and guided by the utilization of physiologic parameters. (I-E)
De-escalation of Antibiotics
- For adults with an initial diagnosis of sepsis or septic shock and adequate source control, where optimal duration of therapy is unclear, we suggest using clinical evaluation to decide when to discontinue antimicrobials.
- Serial procalcitonin measurements can help support the discontinuation of empiric antibiotics, as outlined in this Procalcitonin Guideline (I-E)
Post-Discharge
- Survivors of life-threatening illnesses, such as sepsis and septic shock should be screened for the physical, mental, and cognitive dysfunction that characterizes post-intensive care syndrome and referred to specialists, as appropriate. (II-C)
- After a sepsis hospitalization, patients should follow up with an appropriate healthcare provider, depending on discharge circumstance (e.g., PCP, physical medicine and rehabilitation, or another appropriate specialist), and receive post-hospital care to promote recovery (e.g., physical therapy, occupational therapy, etc.), as appropriate. (I-E)
Sepsis in Vulnerable Populations - Congestive Heart Failure
- Fluid resuscitation should not be withheld due to a history of heart failure. However, patients at risk for poor tolerance of fluid (e.g., reduced LVEF or severe diastolic dysfunction) should have frequent reassessment of clinical status and intravascular volume during the course of fluid resuscitation, with ultimate volume guided by clinical response.
Sepsis in Vulnerable Populations - Neutropenia
- Neutropenic patients should be closely monitored for signs/symptoms of infection as they are particularly vulnerable to the development of sepsis and septic shock. (I-E)
- The hematological malignancy population has an increased risk of antibiotic resistance, and therefore antibiotic choice should be guided by the Neutropenic Fever Guidelines. Anti-pseudomonal antibiotics are recommended. (I-C)
- Source control in the neutropenic population is no different than in non-neutropenic patient in theory, though the least invasive method should be used, due to challenges with wound healing and concomitant thrombocytopenia. (I-E)
- Stimulation of neutrophil production with any of the various colony stimulating factor formulations is not recommended due to the risk of immune reconstitution syndrome which can threaten clinical stability (II-E)
Sepsis in Vulnerable Populations - Cirrhosis
- There should be a high level of suspicion for infection/sepsis in cirrhotic patients admitted to the hospital.
- It is reasonable to consider albumin administration in addition to balanced crystalloids in patients who meet criteria for fluid resuscitation and require vasopressors (e.g. ≤3.2) (II-C)
- Severe liver disease can be considered an immunocompromised or hemodynamically at-risk state, and therefore represent a vulnerable population with regards to the development of sepsis or septic shock.
Table 1
Overview of the Initial Evaluation and Empiric Antibiotics for the Septic Patient.
Table 2
Vasoactive Agents.
Table 3
Resuscitative Fluids.
Footnotes
- *
Strength of Recommendation Classification
I = Generally should be performed; II = May be reasonable to perform; III = Generally should not be performed
Level of Evidence Classification
A = systematic reviews of randomized controlled trials with or without meta-analysis; B = randomized controlled trials; C = systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control); D = individual observation studies (case study/case series); E = expert opinion regarding benefits and harm
Clinical Background and Rationale for Recommendations
Recognition of sepsis
Severe sepsis and septic shock are medical emergencies and delays in treatment lead to increased morbidity and mortality.3–5 Sepsis improvement programs highlight awareness of the disease and lead to improved recognition through education. The Electronic Health Record (EHR) can be utilized to help with screening and alerting providers about potential sepsis cases. However, these systems have traditionally performed sub-optimally6,7 and thus provider education and recognition is crucial to improved sepsis recognition and care.
Sepsis care should be standardized across the hospital since many studies have demonstrated improved clinical outcomes with bundled sepsis treatment.8–10 A large retrospective study evaluated compliance with a 2013 statewide mandate for bundled sepsis care in New York.5 This analysis showed that bundled, protocolized sepsis care was associated with improved clinical outcomes. A similar but larger study involving 1,012,410 patients among 506 hospitals located in five states demonstrated improved sepsis outcomes, including mortality, with protocolized sepsis care.11
Quick sequential organ failure assessment, or qSOFA, is a set of three variables developed in 2016 to predict mortality in patients with known or suspected sepsis.2 The three variables include a Glasgow Coma Score < 15, a respiratory rate ≥ 22 breaths/minute, and a systolic blood pressure ≤ 100 mmHg. If a patient has two or more variables present at a given time, then the patient is qSOFA positive. Studies have shown that qSOFA is a better predictor of mortality in patients with suspected or known sepsis when compared to other screening tools such as SIRS, NEWS or MEWS. However, the goal of a screening tool is high sensitivity, so that clinicians can identify patients with the disease process of interest, not prediction of mortality. 12
Here at Michigan Medicine, we are actively screening our patients for sepsis on presentation to the ED and on the inpatient floors. When patients screen positive with a concern for sepsis or who meet criteria for severe sepsis, a BPA and page are pushed to the First Contact. We recommend that the First Contact review the data in the Sepsis Navigator to understand why the BPA fired and to evaluate whether sepsis treatment should be initiated in the patient. Consideration of patient symptoms, laboratory values, and underlying risk of infection in determination of whether the patient should be treated for sepsis.
Initial Approach to the Sepsis Patient: The SEP-1 Bundle
- The “SEP-1 Bundle” is an all-or-none treatment bundle for severe sepsis/septic shock that was instituted as a quality metric by the Center for Medicare and Medicaid Services (CMS) in the interest of standardizing sepsis care. According to the bundle, there are tasks to be completed within 3 hours and 6 hours of suspicion/recognition of sepsis in order to “pass” the metric. These specifically are:3 Hour Bundle: measure lactate, draw blood cultures, start appropriate antimicrobial therapy, give fluid resuscitation (30 mL/kg) for hypotension or lactate >4, start pressors if hypotension is profound during or persistent after the fluid resuscitation
- 6 Hour Bundle: Repeat lactate if initial lactate >2, Repeat volume status and tissue perfusion assessment after fluid resuscitation, titrate pressors to goal MAP>65mmHg
Lactate
Lactate levels are important in the identification13–15, risk stratification16–18 and resuscitation19–22 of patients with sepsis, and an elevated lactate may be a sign of occult shock.16,18
Lactate has been studied as a method to identify patients with sepsis. In comparison to other lab markers, including white blood cell count, neutrophil count, and procalcitonin, lactate has been shown to be a better predictor of severe sepsis and septic shock.13–15
All patients with suspected or confirmed sepsis should have a lactate checked upon time of recognition.16 Lactate levels are important in the risk stratification5,6 and resuscitation7 of patients with sepsis, and elevated lactate may be a sign of occult shock.23 Elevated lactate levels have been associated with increased mortality for both intermediate lactate elevations (≥ 2) and high lactates (≥ 4).16,17 Thus, elevated lactate levels are concerning in patients with sepsis and should suggest to providers the need for fluid resuscitation, hemodynamic support, and additional resources such as rapid response intervention or a higher level of care.
Lactate should be rechecked to evaluate the effectiveness of resuscitation if the initial lactate is greater than or equal to 2.24 Lactate clearance can be confounded by patient comorbidities, especially cirrhosis, or use of beta-agonists (i.e., albuterol or epinephrine) and should be interpreted within a broad clinical context and not as a single marker of resuscitation.25 Other markers of resuscitation such as central venous oxygen saturation26 and capillary perfusion27 have been shown to be non-inferior to lactate reduction and may aid in evaluation of resuscitation, along with lactate reduction (see Resuscitation & Reassessment).
Blood cultures
A blood culture “set” includes 2 bottles, 1 aerobic and 1 anaerobic. A blood culture “site” refers to the location from which the culture is drawn. When infection is suspected, at least 2 sets of blood cultures should be drawn (aerobic & anaerobic). Blood cultures should be drawn from 2 separate peripheral sites in most patients. However, if the patient has an indwelling central catheter that is suspected to be infected, cultures should be performed through each lumen of the catheter, in addition to the peripheral site.
As antibiotic administration significantly decreases the sensitivity of blood cultures, blood cultures should be obtained prior to the initiation of antibiotics, unless the patient is unstable or critically ill, in which case antimicrobials should be initiated immediately, regardless of whether blood cultures have been obtained.28 How long to wait for cultures to be drawn in the setting of severe sepsis is at this discretion of the clinician, but it seems reasonable to start antibiotics if cultures are unable to be obtained within 45 minutes.
Antibiotics
Antimicrobial therapy should be selected to include coverage for likely organisms at the presumed site of infection. Local data (https://www.med.umich.edu/asp/) should be utilized to tailor antimicrobial therapy. This includes local antibiograms (https://labportal.med.umich.edu/portal/apps/antibiogram/) and treatment guidelines according to disease state, which are held and maintained on the Antimicrobial Stewardship website (https://www.med.umich.edu/asp/adult.html). Patients with a reported Beta-lactam allergy should be considered for referral to the Beta-lactam Evaluation Team during their hospitalization (https://www.med.umich.edu/asp/pdf/adult_guidelines/Beta-lactam-Evaluation-and-Empiric.pdf).
Antimicrobial therapy should be prioritized for septic patients with presumed infection. Antibiotics should be targeted to cover presumed organisms based upon likely organisms at the presumed source of infection and tailored based upon local prevalence of, and risk factors for, resistance among those organisms. While risk factors for resistant organisms vary depending on geographic location of included study patients, specific type of infection, and number of variables collected, general risk factors include patients with a higher severity of illness, those with previous broad-spectrum antimicrobial exposure, those with exposure to high-risk healthcare settings (i.e. prolonged hospitalization, long-term care facilities, dialysis centers, etc.), immunosuppression, and previous recent resistant organisms.29–31 Double-coverage of gram-negative organisms, while exposing patients to additional antimicrobial agents and their adverse effects, is often used to maximize the chances that a suspected or proven bacteria is susceptible to one of the two antimicrobials until microbiologic susceptibilities result. The benefit of double-coverage likely is maximized in patients with highest risk of resistant organisms or in areas with a high local prevalence of resistant organisms. In patients at a high risk of immediate morbidity and mortality from their infection (i.e., septic shock with escalating vasopressor requirement), the benefit of double coverage likely outweighs the immediate risks, in contrast to patients at low risk of morbidity and mortality from infection, where judicious waiting for culture results to tailor antibiotics will not lead to additional harm.32 De-escalation strategies should be tailored based upon a patient’s clinical status, as well as laboratory or stewardship resources available to rapidly detect or rule-out bacterial organisms.
Antimicrobial delivery should be optimized to ensure rapid achievement of therapeutic targets and concentrations at the presumed site of infection. Strategies to optimize the pharmacokinetic-pharmacodynamic parameters should be utilized when able and include extended (or continuous)-infusion beta-lactams, and extended-interval aminoglycosides.33
MRSA accounts for a small percentage of culture positive infections in critically ill patients (i.e., 5%), yet a majority of critically ill septic patients receive anti-MRSA antibiotics.34 Individual risk factors for MRSA include recent IV antibiotics, history of recurrent skin infections or chronic wounds, presence of invasive devices, hemodialysis, recent hospital admission, severity of illness, and most importantly, prior history of MRSA infection or colonization. Failure to cover for MRSA in a patient with a true MRSA infection may be harmful, but unnecessary coverage for MRSA in a patient without MRSA may also be harmful.35 MRSA nasal swab testing is often used to avoid initiation of MRSA-targeted antibiotics, as it has a high negative predictive value for not only respiratory infections (96.5%), but also other systemic infections as well.36 Prior MRSA nasal swabs within the previous 60 days have also performed well when examining the durability of a previous negative result.37 Importantly, a positive MRSA nasal swab does not indicate an active MRSA infection or necessitate the use of anti-MRSA antibiotics. Rather, a positive swab indicates that the patient is colonized with MRSA, and that this organism should be covered if a S. aureus infection is presumed.
Timing of Antibiotics
Early administration of effective antimicrobials has been shown to reduce mortality in patients with sepsis or septic shock in observational or retrospective studies.4,5,23,38 However, the importance of early antimicrobial treatment, must be tempered against the adverse effects of indiscriminate overuse of antibiotics, such as allergic or hypersensitivity reactions, renal dysfunction, Clostridioides difficile infections and antimicrobial resistance.39
In multiple large-scale studies of sepsis, the association of early antimicrobial administration and reduced mortality are strongest for patients with septic shock, and therefore support use of early administration of antimicrobials for patients with septic shock, in whom the risk of delayed, inadequate, or avoidance of treatment is the highest..5,40,41
In the above studies, the association of timing of antimicrobials in patients with sepsis without shock and mortality, is less consistent. Therefore, in patients with sepsis without shock, rapid assessment of the patient for infectious or non-infectious source for sepsis within 3 hours is recommended. The decision whether antimicrobials should be deferred or administered may be based on findings in the rapid assessment.
When the decision to initiate antimicrobial agents is made, significant effort should be placed on ensuring rapid administration to the patient. This includes leveraging institutional strategies such as optimization of automated dispensing cabinets for medication availability closest to patient, ordering as “STAT” compared with “routine”, and prioritizing administration once medication is at the patient’s bedside.
Resuscitation
Fluids
Septic shock is classically designated as a distributive shock characterized by a profound immune response and release of vasodilatory substances resulting in inappropriate vascular dilation and tissue hypoperfusion. However, the over-reactive inflammatory response is commonly secondary to a separate disease process that frequently results in some period of poor volume intake, increased insensible loss (e.g. increased minute ventilation, hyperthermic losses secondary to fever) and potential GI losses, all contributing to intravascular depletion and hypovolemia. Lastly, the inflammatory response has been associated with myocardial dysfunction and decreased cardiac output. These separate but potentially additive causes for hypoperfusion makes septic shock difficult to protocolize. However, given the likelihood for intravascular depletion and hypovolemia it is suggested that initial volume resuscitation be attempted if evidence of tissue hypoperfusion.
The overarching goal of fluid therapy is to improve oxygen delivery to the tissue (DO2) by increasing stroke volume (SV) and cardiac output (CO). The goal of fluid resuscitation (or an endpoint for fluid therapy) is not to increase the mean arterial pressure (MAP).42 A patient is deemed “fluid responsive” when SV increases by 10-15% after a fluid challenge (i.e., 250-500 mL); such patients should receive judicious fluids until the appropriate increase in SV/CO subsides. Administration of fluids to patients deemed not fluid responsive only serves to overload the patient with unnecessary fluid which subsequently impairs organ function.
Fluid resuscitation has shown benefit in experimental studies by improving microvascular prefusion,43(p) improving cardiac output and oxygen delivery,44,45 and improved organ function.46 However, the data around impact of initial fluid administration on clinical outcomes is less robust. 30 mL/kg was utilized in the landmark paper, Early Goal Directed Therapy47 (EGDT), in which significant mortality benefit was demonstrated by protocolized sepsis care. The PROMISE,48 PROCESS49 and ARISE50 trials evaluated bundled sepsis care and did not focus on the timing and amount of fluid given and thus provide no specific insight into the benefit of early or large volume fluid resuscitation. The previously mentioned large clinical trials that were published post-EGDT showed no significant difference in “usual care” compared to protocolized EGDT, but the volumes administered in each of these trials did approach the similar 30 mL/kg given in EGDT. More recently the Classic trial51 and Clovers trial52 attempted to evaluate restrictive versus fluid resuscitation in septic patients. Unfortunately these papers do not provide additional guidance on initial fluid resuscitation as both studies do not account for fluid resuscitation received prior to randomization which was often 2-3 liters. It is thus unclear based on these large clinical trials whether fluid administration alone has benefit for patients with tissue hypoperfusion.
There is observational data53,54 that demonstrate clinical outcome benefits to bundled sepsis care and are frequently referenced in the discussion of early large volume fluid resuscitation, however none of these studies focuses specifically on early fluid administration alone.
There are current concerns in the medical community that over-administration of intravascular fluids to hospitalized patients is associated with worse clinical outcomes.55,56 However, these studies focus on large volume administration over longer durations of time and do not apply to the initial 30mL/kg fluid resuscitation. Ongoing volume administration, which is discussed below, is more likely an area with greater risk of harm to a patient. Thus, we continue to suggest the administration of 30 ml/kg of fluid resuscitation be administered in the first 3 hours of sepsis onset, especially given the potential for hypovolemia in our septic patients. Further research evaluating specific volume targets and time of administration is needed.
Given clinical concerns for over-resuscitation in our morbidly obese population, it is reasonable for the initial volume of resuscitation to be based on ideal or adjusted body weight, instead of actual weight. Literature57 currently is sparse on this subject.
The choice of fluids for resuscitation are typically separated into crystalloids and colloids. Crystalloids have the advantage of being more readily available and lower in cost when compared to colloids. Colloids have the theoretical benefit of increasing oncotic pressure intravascularly and thus preventing extravasation of the fluid into the extravascular space.
There have been several large trials that have attempted to address which resuscitative fluid, colloid (albumin) or crystalloid, is superior in critically ill patients. These studies show no clear benefit of one over the other in mortality at 28 days.58–60 Thus, given the cost difference and lack of clear benefit, we recommend crystalloids as first-line fluid for resuscitation in septic patients.
Several studies have shown benefit to balanced solutions (e.g. lactated ringers, Plasma-Lyte, or normosol) over normal saline, including reduction in major adverse kidney events within 30 days (MAKE-30),61 and reduced in-hospital mortality in patients with sepsis.62 Finally a meta-analysis63 of nearly 35,000 patients was recently published in the New England Journal that provides a signal for mortality reduction with the use of balanced crystalloids. The clinical impact or separation of effect is likely more pronounced in patients who require large volume resuscitation compared those with more conservative fluid needs. Regardless, choice of fluid therapy should be individualized for each patient. Balanced fluids likely produce less elevations in serum chloride levels, whereas 0.9% NaCl is likely advantageous for those at risk of cerebral edema or dysnatremia. Lacking robust, definitive evidence, and apart from a strong indication to choose one agent over another, we suggest administration of balanced crystalloids for patients with sepsis over 0.9% NaCl. Albumin may have a role in sepsis management, but not as a first line resuscitative fluid as discussed above. In patients who have received large volumes of crystalloids, the addition of albumin may have clinical benefit. The ALBIOS59 study, in which patients received approximately 3000 to 4000 mL of fluid over the seven day study period, demonstrated that patients receiving albumin, in addition to crystalloid, had a statistically higher mean arterial pressure and lower net fluid balance. A more recent retrospective analysis by Gomez et al.64 compared patients who received normal saline with 5% albumin versus normal saline alone in patients that received > 60 ml/kg of fluid resuscitation in 24 hours. This study demonstrated a lower adjusted hospital 30-day mortality in patients that received albumin. Additionally, those in the albumin arm had a lower incidence of renal injury. Thus, albumin may provide some benefit when providing large volume resuscitation. Further analysis of what constitutes large volume and timing of albumin administration is necessary for a stronger recommendation to be made.
Given the limited availability of the blood product albumin, and its associated cost, efforts have been taken to produce synthetic colloids (i.e., hydroxyethyl starches, gelatins, dextrans). Unfortunately, multiple studies have shown the harm of using synthetic colloids in a variety of clinical settings for resuscitation in comparison to crystalloids.65–69 These are resuscitation fluids which contain large molecules aimed at increasing intravascular oncotic pressure with the physiologic goal of keeping resuscitative fluid from extravasating into extravascular tissue. However, these studies all found that patients receiving synthetic colloids have higher rates of AKI and need for renal replacement therapy, and failed to find a mortality benefit in favor of colloids.
Pre-existing conditions that make patients more vulnerable to fluid overload (heart failure, cirrhosis, ESRD, etc) are generally not a contraindication to initial fluid resuscitation (30 mL/kg) and reduced volumes do not lead to lower rates of intubation, or increased ventilator-free days as demonstrated in one retrospective propensity-score matched analysis.70 There is a mortality benefit to initial volume resuscitation in these patients,71,72 however, and if lower volumes are being considered, appropriate documentation of their current volume status and contraindication to fluid is recommended.
Ongoing management after Initial Resuscitation: Reassessment and Support
Since the original Goal Directed Therapy article was published,47 there have been numerous studies trying to elucidate the best method by which to assess a patient’s volume status in the setting of sepsis. Many methods, including Swan-Ganz catheter,73 measurement of central venous pressure (CVP),74 and mixed venous oxygenation75 are invasive and are correlated with mortality outcomes, but have not been definitively shown to improve survival. Newer non-invasive technologies for measuring cardiac output have been developed, however these require additional training and are not widely available outside of the intensive care units.
After the initial 30 mL/kg bolus, we recommend reassessment of the clinical status of the patient. Lactate should be rechecked to evaluate the effectiveness of resuscitation if the initial lactate is greater than or equal to 2.24 If there is recurrent or refractory hypotension, we recommend performing a physical examination that includes whether the patient remains responsive to additional fluid resuscitation which may be accomplished through an additional small volume bolus (250-500 mL given rapidly, over 10 minutes), or by performing a passive leg raise (see Figure 3), and then followed by objective measures of SV/CO (non-invasive cardiac output systems, ECHO, esophageal doppler, etc.). When measurement of CO or SV is not possible, a >15% increase in pulse pressure could indicate that the patient is fluid responsive utilizing a passive leg-raise test for 60–90 seconds.
Other markers of resuscitation such as central venous oxygen saturation26 and capillary perfusion27 have been shown to be non-inferior to lactate reduction and may aid in evaluation of resuscitation. Physical exam findings such as skin mottling, and poor capillary refill indicate ongoing poor perfusion.
There is recent evidence that after initial fluid resuscitation, there is no difference in outcomes when additional fluids are given or vasopressors are initiated. 76
Vasopressors and Steroids
Norepinephrine is recommended as a first-line agent in the treatment of septic shock and we suggest against dopamine use. Both observational studies77–79 and a multicenter RCT80 suggested norepinephrine to be superior to dopamine in terms of 28 day mortality, likely driven by an increase in arrhythmias due to dopamine. This finding was confirmed in a subsequent meta-analysis in 2012.81
Studies have evaluated additional agents including vasopressin, epinephrine, and phenylephrine in comparison to norepinephrine as a first-line vasopressor in septic shock. There was no significant difference between vasopressin and norepinephrine82,83 in terms of 28-day mortality, and when vasopressin is used in conjunction with norepinephrine there may be a survival benefit over high dose norepinephrine monotherapy.82 Additionally, in meta-analysis,84 vasopressin was found to cause less tachyarrhythmias than norepinephrine. A RCT85 comparing norepinephrine and dobutamine to epinephrine alone in the treatment of septic shock found no difference in 28-day mortality between groups or in the incidence of side effects. These results were subsequently confirmed in a large prospective observational study.86
We suggest starting peripheral vasopressors to achieve MAP goals instead of delaying initiation until central access may be achieved. However, central access should be obtained if ongoing vasopressor support is needed. Traditionally, vasopressor administration mandated immediate placement of a central venous catheter in either the internal jugular, femoral or subclavian veins (to avoid tissue injury, should extravasation occur). However, this frequently led to delays in vasopressor administration which placed patients at risk for ongoing hypotension and continued organ injury. A number of case reports and case series65–67,87–89 had documented the risk of complications from peripherally administered vasopressors, however the majority of these studies were very old. More recently, an observational study68, two systematic reviews69,90 and one meta-analysis91 have demonstrated low incidence of serious adverse injury secondary to vasopressor administration through a peripheral IV. The incidence of extravasation seems to be greater when IVs are placed distal to the antecubital fossa. Thus, we suggest starting peripheral vasopressors to achieve MAP goals instead of delaying initiation until central access may be achieved. However, central access should be obtained if ongoing vasopressor support is needed.
For adults with septic shock requiring norepinephrine for at least 4 hours at doses of ≥ 0.25 μg/kg/min, we suggest using stress dose steroids (i.e. IV hydrocortisone 50mg q6hr plus/minus oral fludrocortisone 50 mcg q24hr).
All of the major trials on stress dose steroids in septic shock have consistently shown faster resolution of shock with the administration of steroids, with several demonstrating a mortality benefit. Sepsis is defined by a dysregulated host response to infection involving unregulated inflammation, organ dysfunction and hemodynamic instability. In addition, there is evidence that the hypothalamic-pituitary-adrenal (HPA) axis becomes dysregulated in sepsis.92 Steroids have thus been an attractive treatment option to counteract the widespread inflammatory cascade and re-store the dysregulated HPA access.
In the early 2000’s two large RCTs90,91 demonstrated a faster resolution of shock (i.e. shorter duration of vasopressor therapy). One study90 which enrolled sicker patients (63% 28-day mortality in placebo group) demonstrated a mortality benefit from steroid therapy.
Two more recent trials92,93 have since been published which have reinforced the administering of stress dose steroids in patients with septic shock. Both of these trials demonstrated a shorter time to shock reversal with steroids, and again a reduction in mortality for the trial93 that enrolled sicker patients.
For adults with sepsis with hypotension, non-invasive blood pressure cuff measurements can be inaccurate compared to invasive monitoring11–15, particularly in patients with elevated BMI. Arterial catheters for arterial blood pressure measurement allow continuous, real-time measurements so that therapeutic decisions can be made with accurate and immediate information. While there are associated risks to invasive arterial monitoring16, these are small and using ultrasound guidance may increase placement success rates and decrease complication rates.17,18
Resuscitation Targets
Septic shock is characterized by inappropriate arterial vasodilation secondary to an exaggerated inflammatory response in the patient. This inappropriate arterial vasodilation causes a reduction in the MAP and can lead to decreased organ perfusion if MAP falls below the autoregulatory zone of each organ. Decreased organ perfusion leads to ischemia and organ failure if not corrected. Fluid administration aims to improve cardiac output and maintain MAP but for many patients with septic shock volume administration will not be sufficient and vasopressor support will be required to raise MAP and organ perfusion pressure.
The goal MAP recommended by surviving sepsis guidelines24 is equal to or greater than 65. This MAP goal was first based on small clinical studies94,95 in which biomarkers (lactate, creatinine), cardiac index and urine output were observed following changes in NE infusions in patients with septic shock. These studies showed no significant benefit in lactate reduction, creatinine or urine output when MAP was raised from 65 to 85 with vasopressors.
In 2014 the SEPSISPAM96 study was published which compared 28-day mortality between MAP goals of 65 to 70 mmHg against those with a goal of 80-85 mmHg. The study found no significant difference in 28-day mortality and patients with the higher MAP target experienced longer duration on vasopressors and an increased rate of atrial fibrillation. Interestingly, upon subgroup analysis, patients with chronic hypertension demonstrated less adverse renal outcomes when randomized to a higher MAP target. Two large retrospective studies97,98 published following SEPSISPAM demonstrated significant increases in hospital mortality and the incidence of AKI when MAP was < 65 in patients with septic shock.
Additionally an individual patient-data meta-analysis99 performed on previous studies, of which SEPSISPAM was the majority of the patient data included, showed a non-significant trend for increased mortality in patients > 65 when higher MAP targets were chosen. This led to the more recent 65 trial100 which was a RCT comparing permissive hypotension (MAP 60-65 mmHg) to usual MAP goals in patients older than 65 years of age requiring vasopressor support in the ICU for vasodilatory shock. The study found no significant difference between groups in terms of 90-day mortality, AKI or vasopressor duration. The mean difference in blood pressure between groups was approximately six, thus it is not surprising that no significant difference was found.
Thus, given the current available data, an initial MAP goal of ≥ 65 mmHg for patients in septic shock seems the best initial goal for all patients with septic shock. The SEPSISPAM and 65 trial remind us that individualized care is important and that adjusting the MAP goal based on individual patient data may be reasonable.
Ongoing Management
Source control
Definition: Source control is defined as the identification and possible removal of the anatomical area responsible for sepsis and septic shock. Excluding areas that are not responsible for the initiation of sepsis is additionally an important element of source control. Source control is identified as a key element of the surviving sepsis guidelines established and now widely implemented across the globe.24
Clinical Decision making in source control: Adequate source control is associated with increased survival.101,102 There are a multitude of factors that determine the clinical approach to performance of a procedure in patients with sepsis.103 The timing of the procedure is often dependent on the presence of hemodynamic stability. In patients with profound septic shock and vasoplegia, source control procedures should be undertaken as soon as the patient is resuscitated. While there is limited data to suggest the appropriate timing of the performance of procedures for source control, it is our recommendation based on a multitude of smaller studies that it be performed within 6-12 hours from admission.104,105 It is also clear from clinical observations that some patients may not achieve hemodynamic stability despite maximal resuscitative efforts without adequate source control.106
Treatment alternatives: Source control in a critically ill patient could include many different treatment paradigms.
- Drainage of closed space infection including abscess-either surgically or with interventional procedures performed with radiologic assistance. Some examples include drainage of infected cholecystitis or pyelonephritis.
- Surgical removal of infected organs or medical devices. For example, appendicitis, perforated sigmoid diverticulitis, infected necrotic pancreas in patients with pancreatic necrosis, and ischemic bowel.
- Repair of a perforation. For example, perforated ulcers of the stomach, duodenum and small bowel.
- Surgical exclusion of continued source of infections such as diversion of fecal stream. For example, performance of a diverting ileotomy or colostomy in patients with perforated diverticulitis and cancers.
- Surgical debridement for necrotizing soft tissue infections
The selection of the type of procedure depends on multiple factors. A risk-benefit analysis dictates the timing and the location of performance of the procedure. In general, the least invasive procedure is the first line of therapy. Additional factors include clinical expertise, availability of interventions and other logistic institutional factors, patient preference and the nature of clinical status.107 The nature of intra-operative procedures depends on hemodynamic and respiratory stability, presence of severe metabolic acidosis, and hypothermia with diffuse coagulopathy. In some patients, anatomic resection of the infected area is not possible. A diversion or drainage of the infected bed is the suggested alternative. Additionally, a surgical procedure such as a laparotomy may be warranted to exclude specific causes of septic shock where further radiologic studies may not be possible. Most procedures are either performed in the operating room or in interventional radiology suites. However, in patients with extreme hemodynamic instability and respiratory distress despite maximal ventilatory support, performance of a bedside procedure in the ICU, is recommended.
Concept of damage control: Evidence of damage control or performance of an abbreviated operation was first initiated in trauma patients with severe non-compressible hemorrhage in the chest or abdomen. Over the last several decades mortality benefits have been observed and now extended to diverse types of traumatic insults.108,109 These principles have been adapted for patients with septic shock where source control has been undertaken. The basic principles of the initial procedure involve arresting areas of hemorrhage or contamination with luminal bowel contents in the operating room. Such maneuvers in emergency general surgery include resection or stapling of small and large bowel. The next steps involve resuscitation in the ICU where facets of coagulopathy, lactic acidosis and hypothermia are addressed through resuscitative measures and goal directed therapy. The final steps include return to the operating room where definitive surgical procedures along with closure of the abdomen is achieved, once the patient is appropriately resuscitated. The definitive procedure may be performed at any time but typically between 48-72 hours after resuscitation in the ICU. There are a number of observational and retrospective case studies evaluating damage control laparotomy versus conventional single stage surgery. A recent meta-analysis of 5 non-randomized and 16 observational studies suggested no difference in crude mortality rates between a damage control versus conventional single stage surgical intervention.110 However, the standardized mortality ratio displayed clear benefit for damage control surgery. In presence of severe septic shock, metabolic acidosis and coagulopathy, we strongly suggest taking a damage control approach to surgery and resuscitation.
Adequacy of source control: There is little evidence in literature that suggest how to conclude whether source control has been successful or further interventions are needed. For patients where the operative source control has been definitively performed (examples sigmoid colectomy and appendectomy) it is expected that patients will respond with clinical improvement within 48 hours of the intervention. If the patient does not respond to what was thought to be a definitive procedure, further imaging or surgery may be warranted. However, for clinical scenarios where definitive source control is not surgically attainable (for example, uncontrolled enteric fistula with ongoing leak into the abdomen that has been subjected to multiple laparotomies), a combination of non-surgical and long term antibiotics may be necessary. Removal of potentially infected lines and tubes should be performed after initial resuscitation as part of an overall source control plan19. For intravascular lines, these should be removed when alternative intravascular access has been established. There may be circumstances in which it is not practical to remove an intravascular line, and the patient should be treated through the line for an extended period of time. We recommend Infectious Disease consultation in this circumstance.
De-Resuscitation
Patients who recover from septic shock often have a positive fluid balance.111,112 After initial resuscitation and stabilization, de-escalation of fluid management and optimization of volume status is required. The goals of de-escalation include restoration of organ function by attaining negative fluid balanced (i.e. progressing toward net even for the hospitalization) through goal-directed fluid removal. This is achieved through use of diuretics or renal replacement therapy and the limitation of fluid intake, including medication carriers and diluents.
While data continues to emerge, it is clear that prolonged positive fluid balance is associated with worsened organ function and clinical outcomes, and that de-resuscitation (i.e. achieving a negative fluid balance after stabilization) is associated with improved clinical outcomes.113,114 Fluid balance therefore is a practice-dependent and modifiable risk factor for clinical outcomes, including mortality. Patients should be assessed frequently and early after initial stabilization utilizing physiologic data (i.e. pre-admission volume status, dynamic and static hemodynamic parameters, assessment of organ function, etc.) when available to guide aggressive volume removal.115
De-escalation of Antibiotics
While early antimicrobial treatment is important for improving mortality in patients with septic shock, limiting the continued overuse of broad spectrum antimicrobials is also important to decrease potential for development of antimicrobial resistant microorganisms.116 Once a microbial pathogen and susceptibilities had been identified, or a non-infectious cause for acute illness is identified, stopping unnecessary antimicrobials and/or narrowing the spectrum of antimicrobials to target only the identified organisms is recommended. Michigan Medicine Antimicrobial Stewardship guidelines provide appropriate target based treatment guidelines: University of Michigan Health System (umich.edu) If infection has been ruled out, discontinuing all antimicrobials is recommended. De-escalation is generally safe, may decrease cost associated with unnecessary antibiotic use, and can decrease selection pressure for antimicrobial resistance, C.difficile infection and antibiotic adverse events. A recent meta-analysis reviewed 13 studies and found improved short-term mortality and decreased hospital length of stay in patients who had antimicrobial de-escalation.117
An anterior nares screen for methicillin-resistant Staphylococcus aureus (MRSA) can be a useful tool for discontinuation of anti-MRSA antibiotics, such as vancomycin.36 A negative MRSA nasal screen may be used to guide the safe discontinuation of anti-MRSA antimicrobials.
Resolution of sepsis physiology may lag behind control of the infection, and procalcitonin can aid in the de-escalation and discontinuation of antimicrobials in a safe and timely manner. Measurement of serum procalcitonin may assist with de-escalation of antimicrobials during treatment of sepsis. A meta-analysis of 14 RCTs assessing the use of procalcitonin compared to controls to guide antimicrobial therapy duration in patients with sepsis suggested improved mortality in those patients managed with use of procalcitonin. In these studies there was no difference in lCU or hospital length of stay. Antibiotic exposure was consistently lower in patients managed using procalcitonin, with minimal adverse effects.24 Based on benefit, with minimal undesirable effects, procalcitonin is suggested for use, along with clinical evaluation, to guide duration of antimicrobial treatment in patients with sepsis or septic shock and adequate source control.
Post-discharge
Post-intensive care syndrome (PICS), or post-hospital syndrome, is a collection of problems that patients can experience after surviving a life-threatening illness, like sepsis or septic shock.118,119 The three domains include alterations in physical function, mental health, and cognitive impairment. More than half of all survivors of critical illness and sepsis will suffer from at least one of these problems.120,121
These problems can be identified through normal patient (or caregiver)-healthcare provider interactions, asking specific questions, or formal testing in each of the three domains. Patients with pre-existing health problems in a specific domain are more likely to develop problems in that area post-hospitalization.122 Patients with more severe illness are also more likely to develop PICS.
Treatment involves identification of specific symptoms, providing therapy, as able, and referral to specialized providers (i.e. physical therapy, psychiatric services, occupational therapy, etc.).123 Even with identification and treatment, PICS symptoms are often present at six to twelve months post-hospitalization. For example, in one randomized controlled study, cognitive impairment at 12 months post discharge was seen in 34% and 24% of patients, comparable to moderate traumatic brain injury and mild Alzheimer’s disease, respectively.124
PICS not only affects patients, but also their caregivers. It is important to counsel caregivers and suggest referral to their own healthcare providers for support.125 Structure for follow up for sepsis patients after discharge is evolving, and we recommend discussing follow up options with your Care Management team.
Sepsis in Vulnerable Populations
Certain groups of patients are more vulnerable to sepsis and more likely to die from sepsis. These include patients with history of heart failure, cirrhosis, or neutropenia. Patients with heart failure and cirrhosis are less likely to get standard-of-care treatment, especially with regards to fluid resuscitation in the setting of hypotension or elevated lactate. Patients with neutropenia are vulnerable to opportunistic infections as well as community acquired ones, and are profoundly immunosuppressed, limited in their ability to fight these infections at all. Early, and broad spectrum, antibiotics are critical to their survival. Specific considerations for these patient populations are addressed below.
Heart Failure
Sepsis accounts for almost 25% of the deaths in patients with chronic heart failure20,21 and patients with CHF have higher mortality rates than patients without CHF who are admitted for sepsis.22,23
While chronic heart failure is not an exception to the SEP1 Bundle mandate by CMS, septic heart failure patients are less likely to receive adequate fluid resuscitation than non-heart failure patients72 The 3 hour bundle has been shown to reduce mortality in heart failure patients24. A large prospective observational study demonstrated that the subgroup of patients with heart failure had reduced mortality when compliant with the 3 hour bundle24, and this included the 30 mL/kg fluid bolus, when applicable.
Studies around treatment of sepsis in patients with decompensated heart failure are lacking.
Neutropenia
Immunocompromised states are associated with lower sepsis survival, with neutropenia conveying a particularly high risk of mortality in critically ill sepsis patients.25 Neutropenia is most frequently associated with malignancy and/or chemotherapy and these patients frequently have additional risk factors such as recent surgeries, central venous catheters, and pre-treatment with antibiotics. National guidelines around management of sepsis in neutropenic cancer patients were published in 2018.26 These guidelines suggest we follow standard of care (SEP1 bundle) for the initial treatment of sepsis in the neutropenic population. At Michigan Medicine, hematological malignancy patients have increased risk of antibiotic resistance and therefore antibiotic choice should be adjusted per the Neutropenic Fever Guidelines, with anti-pseudomonal antibiotics recommended. There may be a role for antifungal treatment in the setting of prolonged neutropenia for patients not on fungal prophylaxis, given their increased susceptibility to invasive fungal infections.
Source control in the setting of neutropenia or pancytopenia should be no different than in non-neutropenic patients, though it is recommended that the least invasive procedure is used. Surgical interventions, however, should not be delayed due to pancytopenia, but rather blood products should be transfused to the surgeon’s specifications to mitigate associated risk.26 There is no evidence that granulocyte transfusion is beneficial.
Routine use of G-CSF or GM-CSF is not recommended in patients with neutropenic sepsis, as rapid neutropenia recovery carries a risk of respiratory deterioration due to ARDS.27,28
Cirrhosis
Patients with cirrhosis and septic shock experience higher mortality than those without cirrhosis.29 This may be due, in part, to altered hemodynamics at baseline in cirrhosis patients, as it is known they have increased renal and neurological organ dysfunction in the setting of sepsis. Unfortunately, there have not been many studies looking at the best practices in management of sepsis in this vulnerable population. Cirrhosis does not exclude patients from standard treatment bundles (SEP1), however there has been debate amongst physicians as to the best fluid for resuscitation in this unique population. A recent single-center randomized controlled trial of albumin infusion (250 mL of 5% solution) versus 30 mL/kg of normal saline demonstrated improved blood pressure (MAP > 65) and lactate clearance at 3 hours as well as improved mortality at 6 days with albumin resuscitation as compared to normal saline.30 However, the average MELD-Na score in both groups was over 33, and 30-day and 60-day mortality rates were not reported. Although albumin can be used for volume expansion in patients with cirrhosis, the evidence supporting this approach is limited. Therefore, in cirrhosis patients with sepsis, we recommend initial fluid resuscitation with 30 mL/kg of crystalloids, as recommended for patients without cirrhosis. It may be reasonable to add albumin in some circumstances as well.
Literature, Guidelines, and Performance Measures
Strategy for Literature Search
Within the Medline (Ovid) database, the following search strategy was used.
- Sepsis/or septicemia or septic or blood poison* or bloodstream infection* or pyaemia* or bacterernia*or endotoxemia* or fungemia* or candidemia*
- Not exp neonatal sepsis
- exp animals/not humans/not exp adolescents/or exp child/or exp infant/not adult
The Main search retrieved 13,772 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follow:
- Sepsis Main -Guidelines, total results were 765
- Sepsis Main -Clinical Trials, total results were 3863
- Sepsis Main -Cohort Studies, total results were 9144
Within the Cochrane Database of Systematic Reviews, 55 reviews were found using the strategy in the search strategies document.
The search was conducted in components each keyed to a specific causal link in a formal problem structure (available upon request). The search was supplemented with very recent clinical trials known to expert members of the panel. Negative trials were specifically sought. The search was a single cycle.
Level of evidence supporting a diagnostic method or an intervention:
- A= systematic reviews of randomized controlled trials
- B= randomized controlled trials
- C=systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (e.g., cohort, cross-sectional, case control)
- D= individual observation studies (case or case series)
- E =opinion of expert panel.
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Health System to which the content is most relevant: Emergency Medicine, Family Medicine, General Medicine, Infectious Disease, Gastroenterology, and Radiology. Medication recommendations were reviewed by the Pharmacy and Therapeutics Committee. The Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers endorsed the final version.
References
- 1.
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi:10.1001/jama.2016.0287 [PMC free article: PMC4968574] [PubMed: 26903338] [CrossRef]
- 2.
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. doi:10.1001/jama.2016.0288 [PMC free article: PMC5433435] [PubMed: 26903335] [CrossRef]
- 3.
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Critical Care Medicine. 2014;42(8):1749-1755. doi:10.1097/CCM.0000000000000330 [PubMed: 24717459] [CrossRef]
- 4.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. [PubMed: 16625125]
- 5.
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. Journal of Medicine. 2017;376(23):2235-2244. doi:10.1056/NEJMoa1703058 [PMC free article: PMC5538258] [PubMed: 28528569] [CrossRef]
- 6.
- Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 10(6):396-402. [PMC free article: PMC4477829] [PubMed: 25758641]
- 7.
- Warttig S, Alderson P, Evans DJ, Lewis SR, Kourbeti IS, Smith AF. Automated monitoring compared to standard care for the early detection of sepsis in critically ill patients. [Review]. Cochrane Database of Systematic Reviews. 2018;1:CD012404. doi:10.1002/14651858.CD012404.pub2 [PMC free article: PMC6353245] [PubMed: 29938790] [CrossRef]
- 8.
- Warstadt NM, Caldwell JR, Tang N, Mandola S, Jamin C, Dahn C. Quality initiative to improve emergency department sepsis bundle compliance through utilisation of an electronic health record tool. BMJ Open Qual. 2022;11(1):e001624. [PMC free article: PMC8739442] [PubMed: 34992053]
- 9.
- Milano PK, Desai SA, Eiting EA, Hofmann EF, Lam CN, Menchine M. Sepsis Bundle Adherence Is Associated with Improved Survival in Severe Sepsis or Septic Shock. Journal of Emergency Medicine. 2018;19(5):774-781. doi:10.5811/westjem.2018.7.37651 [PMC free article: PMC6123087] [PubMed: 30202487] [CrossRef]
- 10.
- Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010;38(2):668-678. [PMC free article: PMC3383776] [PubMed: 20029343]
- 11.
- Kahn JM, Davis BS, Yabes JG, et al. Association Between State-Mandated Protocolized Sepsis Care and In-hospital Mortality Among Adults With Sepsis. JAMA. 2019;322(3):240-250. doi:10.1001/jama.2019.9021 [PMC free article: PMC6635905] [PubMed: 31310298] [CrossRef]
- 12.
- Churpek MM, Snyder A, Han X, Sokol S, Pettit N, Howell MD, Edelson DP. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am J Respir Crit Care Med. 2017;195(7):906-911. [PMC free article: PMC5387705] [PubMed: 27649072]
- 13.
- Karon BS, Tolan NV, Wockenfus AM, Block DR, Baumann NA, Bryant SC, Clements CM. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin Biochem. 2017;50(16-17):956-958. [PubMed: 28552399]
- 14.
- Ljungstrom L, Pernestig AK, Jacobsson G, Andersson R, Usener B, Tilevik D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS ONE [Electronic Resource]. 2017;12(7):e0181704. doi:10.1371/journal.pone.0181704 [PMC free article: PMC5519182] [PubMed: 28727802] [CrossRef]
- 15.
- Contenti J, Corraze H, Lemoel F, Levraut J. Effectiveness of arterial, venous, and capillary blood lactate as a sepsis triage tool in ED patients. Journal of Emergency Medicine. 2015;33(2):167-172. [PubMed: 25432592]
- 16.
- Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2021 Zhang;37(5):1670-1677. [PubMed: 19325467]
- 17.
- Liu, G. & Haijin, lv & An, Y. & Wei, X. & Yi, X. & Yi, H.. Early lactate levels for prediction of mortality in patients with sepsis or septic shock: A meta-analysis. Int J of Clin Exp Med. 2017;10(1):37-47.
- 18.
- Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892-1899. [PubMed: 17618418]
- 19.
- Pan J, Peng M, Liao C, Hu X, Wang A, Li X. Relative efficacy and safety of early lactate clearance-guided therapy resuscitation in patients with sepsis: A meta-analysis. [Review]. Medicine. 2019;98(8):e14453. doi:10.1097/MD.0000000000014453 [PMC free article: PMC6408023] [PubMed: 30813144] [CrossRef]
- 20.
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Medicine. 2015;41(10):1862-1863. doi:10.1007/s00134-015-3955-2 [PubMed: 26154408] [CrossRef]
- 21.
- Simpson SQ, Gaines M, Hussein Y, Badgett RG. Early goal-directed therapy for severe sepsis and septic shock: A living systematic review. J Crit Care. 2016;36:43-48. [PubMed: 27546746]
- 22.
- Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med32. 2004;32(8):1637-1642. [PubMed: 15286537]
- 23.
- Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable,. Crit Care. 2004;8(2):R60-R65. [PMC free article: PMC420024] [PubMed: 15025779]
- 24.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med47. 2021;47(11):1181-1247. [PMC free article: PMC8486643] [PubMed: 34599691]
- 25.
- McCallister Reid, Nuppnau Mark, Michael W. Sjoding, Robert P. Dickson, Rishi Chanderraj. In septic patients, initial lactate clearance is highly confounded by comorbidities and poorly predicts subsequent lactate trajectory. Chest. Published online 2023. [PMC free article: PMC10548455] [PubMed: 37087051]
- 26.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739-746. [PMC free article: PMC2918907] [PubMed: 20179283]
- 27.
- Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321(7):654-664. doi:10.1001/jama.2019.0071 [PMC free article: PMC6439620] [PubMed: 30772908] [CrossRef]
- 28.
- Scheer CS, Fuchs C, Grundling M, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clinical Microbiology & Infection. 2019;25(3):326-331. doi:10.1016/j.cmi.2018.05.016 [PubMed: 29879482] [CrossRef]
- 29.
- Lat I, et al, DEFINE study group and the Discovery Research Network. A Multicenter, Prospective, Observational Study to Determine Predictive Factors for Multidrug-Resistant Pneumonia in Critically Ill Adults: The DEFINE Stu. Pharmacotherapy. 2018;39(3):253-260. [PubMed: 30101412]
- 30.
- Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of Exposure to Antipseudomonal beta-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Journal of Human Pharmacology. 2019;39(3):261-270. doi:10.1002/phar.2201 [PMC free article: PMC6507412] [PubMed: 30506852] [CrossRef]
- 31.
- Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med. 2001;134(4):298-314. [PubMed: 11182841]
- 32.
- Sjovall F, Perner A, Hylander Moller M. Empirical mono- versus combination antibiotic therapy in adult intensive care patients with severe sepsis - A systematic review with meta-analysis and trial sequential analysis. [Review]. Journal of Infection. 2017;74(4):331-344. doi:10.1016/j.jinf.2016.11.013 [PubMed: 27919645] [CrossRef]
- 33.
- Williams P, Beall G, Cotta MO, Roberts JA. Antimicrobial dosing in critical care: A pragmatic adult dosing nomogram. Int J Antimicrob Agents. 2020;55(2):105837. [PubMed: 31722224]
- 34.
- Vincent JL,, et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA. 2020;323(15):478-148. [PMC free article: PMC7093816] [PubMed: 32207816]
- 35.
- Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. [PubMed: 30621854]
- 36.
- Carr AL, Daley MJ, Givens Merkel K, Rose DT. Clinical Utility of Methicillin-Resistant Staphylococcus aureus Nasal Screening for Antimicrobial Stewardship: A Review of Current Literature. Pharmacotherapy. 2018;38(12):1216-1228. [PubMed: 30300441]
- 37.
- Mallidi MG, Slocum GW, Peksa GD, DeMott JM. Impact of Prior-to-Admission Methicillin-Resistant Staphylococcus aureus Nares Screening in Critically Ill Adults With Pneumonia. Ann Pharmacother. 2022;56(2):124-130. [PubMed: 34096323]
- 38.
- Kalil AC, Johnson DW, Lisco SJ, Sun J. Early Goal-Directed Therapy for Sepsis: A Novel Solution for Discordant Survival Outcomes in Clinical Trials. [Review]. Critical Care Medicine. 2017;45(4):607-614. doi:10.1097/CCM.0000000000002235 [PubMed: 28067711] [CrossRef]
- 39.
- Klompas M, Calandra T, Singer M. Antibiotics for Sepsis-Finding the Equilibrium. JAMA. 2018;320(14):1433-1434. [PubMed: 30242350]
- 40.
- Liu VX, Fielding-Singh V, Greene JD, et al. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med. 2017;196(7):856-863. doi:10.1164/rccm.201609-1848OC [PMC free article: PMC5649973] [PubMed: 28345952] [CrossRef]
- 41.
- Peltan ID, Brown SM, Bledsoe JR, et al. ED Door-to-Antibiotic Time and Long-term Mortality in Sepsis. Chest. 2019;155(5):938-946. doi:10.1016/j.chest.2019.02.008 [PMC free article: PMC6533450] [PubMed: 30779916] [CrossRef]
- 42.
- Ueyama H, Kiyonaka S. Predicting the Need for Fluid Therapy-Does Fluid Responsiveness Wor. J Intensive Care. 2017;5(34). [PMC free article: PMC5461727] [PubMed: 28603624]
- 43.
- Ospina-Tascon G, Neves AP, Occhipinti G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949-955. [PubMed: 20221744]
- 44.
- Pottecher J, Deruddre S, Teboul JL, et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010;36(11):1867-1874. [PubMed: 20725823]
- 45.
- Gilbert EM, Haupt MT, Mandanas RY, et al. The effect of fluid loading, blood transfusion, and catecholamine infusion on oxygen delivery and consumption in patients with sepsis. Rev Respir Dis. 1986;134(5):873-878. [PubMed: 3777684]
- 46.
- Pranskunas A, Koopmans M, Koetsier PM, et al. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med39. 2013;39(4):612-619. [PMC free article: PMC3607718] [PubMed: 23263029]
- 47.
- Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. [PubMed: 11794169]
- 48.
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. Journal of Medicine. 2015;372(14):1301-1311. doi:10.1056/NEJMoa1500896 [PubMed: 25776532] [CrossRef]
- 49.
- Investigators P, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. Journal of Medicine. 2014;370(18):1683-1693. doi:10.1056/NEJMoa1401602 [PMC free article: PMC4101700] [PubMed: 24635773] [CrossRef]
- 50.
- ARISE Investigators, Group ACT, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. Journal of Medicine. 2014;371(16):1496-1506. doi:10.1056/NEJMoa1404380 [PubMed: 25272316] [CrossRef]
- 51.
- Tine S. Meyhoff, M.D., Peter B. Hjortrup, M.D., Ph.D., Jørn Wetterslev, M.D., Ph.D., Praleene Sivapalan, M.D., Jon H. Laake, M.D., Ph.D., Maria Cronhjort, M.D., Ph.D., Stephan M. Jakob, M.D., Ph.D., Maurizio Cecconi, M.D., Marek Nalos, M.D., Ph.D., Marlies Ostermann, M.D., Ph.D., Manu Malbrain, M.D., Ph.D., Ville Pettilä, M.D., Ph.D., et al, for the CLASSIC Trial Group. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N Engl J Med. 2022;386:2459-2470. doi:10.1056/NEJMoa2202707 [PubMed: 35709019] [CrossRef]
- 52.
- George Walker. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. NEJM. Published online 2023. doi:10.1056/NEJMoa2212663 [PMC free article: PMC10685906] [PubMed: 36688507] [CrossRef]
- 53.
- Armen SB, Freer CV, Showalter JW, et al. Improving Outcomes in Patients With Sepsis. Journal of Medical Quality. 2016;31(1):56-63. doi:10.1177/1062860614551042 [PMC free article: PMC5731488] [PubMed: 25216849] [CrossRef]
- 54.
- Levy MM, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222-231. [PMC free article: PMC2826633] [PubMed: 20069275]
- 55.
- Marik PE, Byrne L, van Haren F. Fluid resuscitation in sepsis: the great 30 mL per kg hoax. [Review]. Journal of Thoracic Disease. 2020;1(Suppl 1):S37-S47. doi:10.21037/jtd.2019.12.84 [PMC free article: PMC7024756] [PubMed: 32148924] [CrossRef]
- 56.
- FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495. [PubMed: 21615299]
- 57.
- Antal O, Ştefănescu E, Mleşniţe M, Bălan AM, Hagău N. Initial Fluid Resuscitation Following Adjusted Body Weight Dosing in Sepsis and Septic Shock. J Crit Care Med (Targu Mures). 2019;5(4):130-135. [PMC free article: PMC6942448] [PubMed: 31915718]
- 58.
- Finfer S, SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256. [PubMed: 15163774]
- 59.
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. Journal of Medicine. 2014;370(15):1412-1421. doi:10.1056/NEJMoa1305727 [PubMed: 24635772] [CrossRef]
- 60.
- Annane D, CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809-1817. [PubMed: 24108515]
- 61.
- Self WH, Semler MW, Wanderer JP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med. 2018;378(9):819-828. doi:10.1056/NEJMoa1711586 [PMC free article: PMC5846618] [PubMed: 29485926] [CrossRef]
- 62.
- Semler MW, Self WH, SMART Investigators and the Pragmatic Critical Care Research Group. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378(9):829-839. [PMC free article: PMC5846085] [PubMed: 29485925]
- 63.
- Hammond DA, et al. Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. nn Pharmacother. 2020;54(1):5-13. [PubMed: 31364382]
- 64.
- Gomez H, et al. Effects of 5% Albumin Plus Saline Versus Saline Alone on Outcomes From Large-Volume Resuscitation in Critically Ill Patients. Crit Care Med. 2021;49(1):79-90. [PMC free article: PMC7746571] [PubMed: 33165027]
- 65.
- Kahn JM, et al. Skin necrosis after extravasation of low-dose vasopressin administered for septic shock. Crit Care Med. 2002;30(8):1899-1901. [PubMed: 12163813]
- 66.
- Dünser MW, et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med. 2003;31(5):1394-1398. [PubMed: 12771608]
- 67.
- Bunker N, Higgins D. Peripheral administration of vasopressin for catecholamine-resistant hypotension complicated by skin necrosis. Crit Care Med. 2006;34(3):935. [PubMed: 16505698]
- 68.
- Medlej K, et al. Complications from Administration of Vasopressors Through Peripheral Venous Catheters: An Observational Study. J Emerg Med. 2018;54(1):47-53. [PubMed: 29110979]
- 69.
- Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care. 2015;30(3):653.e9-17. [PubMed: 25669592]
- 70.
- Khan RA, Khan NA, Bauer SR, et al. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients With Sepsis, Heart Failure, End-Stage Renal Disease, and Cirrhosis. Chest. 2020;157(2):286-292. doi:10.1016/j.chest.2019.09.029 [PubMed: 31622591] [CrossRef]
- 71.
- Taenzer AH, Patel SJ, Allen TL, et al Improvement in Mortality With Early Fluid Bolus in Sepsis Patients With a History of Congestive Heart Failure. 2020;4(5):537-541. doi:10.1016/j.mayocpiqo.2020.05.008 [PMC free article: PMC7557190] [PubMed: 33083702] [CrossRef]
- 72.
- Acharya R, Patel A, Schultz E, Bourgeois M, Kandinata N, Paswan R, Kafle S, Sedhai YR, Younus U. Fluid resuscitation and outcomes in heart failure patients with severe sepsis or septic shock: A retrospective case-control study. PLoS One. 2021;16(8):e0256368. doi:10.1371/journal.pone.0256368. PMID: 34411178; PMCID: PMC8376054 [PMC free article: PMC8376054] [PubMed: 34411178] [CrossRef]
- 73.
- Velissaris D, Karamouzos V, Kotroni I, Pierrakos C, Karanikolas M. The Use of Pulmonary Artery Catheter in Sepsis Patients: A Literature Review. [Review]. Journal of Clinical Medicine Research. 2016;8(11):769-776. [PMC free article: PMC5047014] [PubMed: 27738477]
- 74.
- Chen H, Zhu Z, Zhao C, et al. Central venous pressure measurement is associated with improved outcomes in septic patients: an analysis of the MIMIC-III database. Crit Care. 2020;24(1):433. doi:10.1186/s13054-020-03109-9 [PMC free article: PMC7358999] [PubMed: 32665010] [CrossRef]
- 75.
- Sandeep Gajbe, Sona Dave, Rajesh Kasimahanti. Central Venous Oxygen Saturation as a Surrogate Marker for Outcome in Critically ill Patients-A Prospective Observational Cohort Study. J Anest & Inten Care Med. 2018;6(5):555698. doi:10.19080/JAICM.2018.06.555698 [CrossRef]
- 76.
- National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network; Shapiro NI, Douglas IS, Brower RG, Brown SM, Exline MC, Ginde AA, Gong MN, Grissom CK, Hayden D, Hough CL, Huang W, Iwashyna TJ, Jones AE, Khan A, Lai P, Liu KD, Miller CD, Oldmixon K, Park PK, Rice TW, Ringwood N, Semler MW, Steingrub JS, Talmor D, Thompson BT, Yealy DM, Self WH. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 2023;9;388(6):499-510. [PMC free article: PMC10685906] [PubMed: 36688507]
- 77.
- Sakr Y, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med. 2006;34(3):589-597. [PubMed: 16505643]
- 78.
- Boulain T, et al. Dopamine therapy in septic shock: detrimental effect on survival? J Crit Care. 2009;(4):575-582. [PubMed: 19327324]
- 79.
- Martin C, et al. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28(8):2758-2765. [PubMed: 10966247]
- 80.
- De Backer D, et al, SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779-789. [PubMed: 20200382]
- 81.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Critical Care Medicine. 2012;40(3):725-730. doi:10.1097/CCM.0b013e31823778ee [PubMed: 22036860] [CrossRef]
- 82.
- Russell JA, Wellman H, Walley KR. Vasopressin versus norepinephrine in septic shock: a propensity score matched efficiency retrospective cohort study in the VASST coordinating center hospital. Journal of intensive care. 2018;1:73. doi:10.1186/s40560-018-0344-2 [PMC free article: PMC6240281] [PubMed: 30473792] [CrossRef]
- 83.
- Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316(5):509-518. doi:10.1001/jama.2016.10485 [PubMed: 27483065] [CrossRef]
- 84.
- McIntyre WF, et al. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA. 2018;319(18):1889-1900. [PMC free article: PMC6583502] [PubMed: 29801010]
- 85.
- Annane D, et al, CATS Study Group. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370(9588):676-684. [PubMed: 17720019]
- 86.
- Myburgh JA, et al, CAT Study investigators. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34(12):2226-2234. [PubMed: 18654759]
- 87.
- PERLOW S, et al. Skin necrosis following intravenous use of norepinephrine; report of six cases. Am J Surg. 1956;92(4):566-570. [PubMed: 13362725]
- 88.
- HUMPHREYS J, et al. Skin necrosis following intravenous noradrenaline. Br Med J. 1955;2(4950):1250-1252. [PMC free article: PMC1981272] [PubMed: 13269844]
- 89.
- GREENWALD HP,. Tissue necrosis following subcutaneous infiltration with nor-epinephrine; report of two cases. N Engl J Med. 1952;246(7):252-253. [PubMed: 14890849]
- 90.
- Tian DH, et al. Safety of peripheral administration of vasopressor medications: A systematic review. Emerg Med Australas. 2020;32(2):220-227. [PubMed: 31698544]
- 91.
- Tran QK, et al. Complication of vasopressor infusion through peripheral venous catheter: A systematic review and meta-analysis. Am J Emerg Med. 2020;38(11):2434-2443. [PubMed: 33039229]
- 92.
- Annane D. The Role of ACTH and Corticosteroids for Sepsis and Septic Shock: An Update. [Review]. Frontiers in Endocrinology. 2016;1:70. doi:10.3389/fendo.2016.00070 [PMC free article: PMC4913096] [PubMed: 27379022] [CrossRef]
- 93.
- Annane D, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862-871. [PubMed: 12186604]
- 94.
- LeDoux D, et al. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28(8):2729-2732. [PubMed: 10966242]
- 95.
- Bourgoin A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33(4):780-786. [PubMed: 15818105]
- 96.
- Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. Journal of Medicine. 2014;370(17):1583-1593. doi:10.1056/NEJMoa1312173 [PubMed: 24635770] [CrossRef]
- 97.
- Maheshwari K, Nathanson BH, Munson SH, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Medicine. 2018;44(6):857-867. doi:10.1007/s00134-018-5218-5 [PMC free article: PMC6013508] [PubMed: 29872882] [CrossRef]
- 98.
- Vincent JL, et al. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care. 2018;8(1):107. [PMC free article: PMC6223403] [PubMed: 30411243]
- 99.
- Lamontagne F, Day AG, Meade MO, et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Medicine. 2018;44(1):12-21. doi:10.1007/s00134-017-5016-5 [PubMed: 29260272] [CrossRef]
- 100.
- Lamontagne F, et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA. 2020;323(10):938-949. [PMC free article: PMC7064880] [PubMed: 32049269]
- 101.
- Kim H, Chung SP, Choi SH, et al. Impact of timing to source control in patients with septic shock: A prospective multi-center observational study. J Crit Care. 2019;53:176-182. doi:10.1016/j.jcrc.2019.06.012. Epub 2019 Jun 17. PMID: 31247517. [PubMed: 31247517] [CrossRef]
- 102.
- Martinez ML, Ferrer R, Torrents E, et al. Impact of Source Control in Patients With Severe Sepsis and Septic Shock. Crit Care Med. 2017;45(1):11-19. doi:10.1097/CCM.0000000000002011. PMID: 27611975. [PubMed: 27611975] [CrossRef]
- 103.
- Jimenez MF, Marshall JC; International Sepsis Forum. Source control in the management of sepsis. Intensive Care Med. 2001;27 Suppl 1:S49-62. [PubMed: 11307370]
- 104.
- Azuhata T, Kinoshita K, Kawano D, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18(3):R87. doi:10.1186/cc13854 [PMC free article: PMC4057117] [PubMed: 24886954] [CrossRef]
- 105.
- Buck DL, Vester-Andersen M, Møller MH; Danish Clinical Register of Emergency Surgery. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013;100(8):1045-1049. [PubMed: 23754645]
- 106.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. doi:10.1086/649554. Erratum in: Clin Infect Dis. 2010 Jun 15;50(12):1695. Dosage error in article text. PMID: 20034345. [PubMed: 20034345] [CrossRef]
- 107.
- Bloos F, Ruddel H, Thomas-Ruddel D, et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Medicine. 2017;43(11):1602-1612. doi:10.1007/s00134-017-4782-4 [PubMed: 28466151] [CrossRef]
- 108.
- Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51(2):261-269. [PubMed: 11493783]
- 109.
- Rotondo MF, Schwab CW, McGonigal MD, et al. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375-382. [PubMed: 8371295]
- 110.
- Haltmeier T, Falke M, Quaile O, Candinas D, Schnüriger B. Damage-control surgery in patients with nontraumatic abdominal emergencies: A systematic review and meta-analysis. J Trauma. 2022;92(6):1075-1085. [PubMed: 34882591]
- 111.
- Bouchard JE, Mehta RL. Fluid balance issues in the critically ill patient. Contrib Nephrol. 2010;164:69-78. [PubMed: 20427995]
- 112.
- Mitchell KH, Carlbom D, Caldwell E, Leary PJ, Himmelfarb J, Hough CL. Volume Overload: Prevalence, Risk Factors, and Functional Outcome in Survivors of Septic Shock. Annals of the American Thoracic Society. 2015;12(12):1837-1844. doi:10.1513/AnnalsATS.201504-187OC [PMC free article: PMC4722831] [PubMed: 26394090] [CrossRef]
- 113.
- Tigabu BM, Davari M, Kebriaeezadeh A, Mojtahedzadeh M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review. Journal of Critical Care. 2018;1:153-159. doi:10.1016/j.jcrc.2018.08.018 [PubMed: 30199843] [CrossRef]
- 114.
- Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit Care Med. 2020;48(12):1862-1870. [PubMed: 33009098]
- 115.
- Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. [Review]. Intensive Care Medicine. 2017;43(2):155-170. doi:10.1007/s00134-016-4573-3 [PubMed: 27734109] [CrossRef]
- 116.
- Arulkumaran N, Routledge M, Schlebusch S, Lipman J, Conway Morris A. Antimicrobial-associated harm in critical care: a narrative review. Intensive Care Med. 2020;46(2):225-235. [PMC free article: PMC7046486] [PubMed: 31996961]
- 117.
- Tabah A, Bassetti M, Kollef MH, Zahar JR, Paiva JA, Timsit JF, Roberts JA, Schouten J, Giamarellou H, Rello J, De Waele J, Shorr AF, Leone M, Poulakou G, Depuydt P, Garnacho-Montero J. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020;36(2):245-265. [PubMed: 31781835]
- 118.
- Kosinski S, Mohammad RA, Pitcher M, Haezebrouck E, Coe AB, Costa DK, Prescott HC, Iwashyna TJ, McSparron JI. What Is Post-Intensive Care Syndrome (PICS)? Am J Respir Crit Care Med. 2020;201(8):P15-P16. [PubMed: 32293204]
- 119.
- Needham DM, Davidson J, Cohen H,, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502-509. [PubMed: 21946660]
- 120.
- Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, Thompson JL, Chandrasekhar R, Ely EW, Brummel NE. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit Care Med. 2018;46(9):1393-1401. [PMC free article: PMC6095801] [PubMed: 29787415]
- 121.
- Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, Hopkins RO; NIH NHLBI ARDS Network. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567-576. [PMC free article: PMC3827703] [PubMed: 23805899]
- 122.
- Mikkelsen ME, Still M, Anderson BJ, Bienvenu OJ, Brodsky MB, Brummel N, Butcher B, Clay AS, Felt H, Ferrante LE, Haines KJ, Harhay MO, Hope AA, Hopkins RO, Hosey M, Hough CTL, Jackson JC, Johnson A, Khan B, Lone NI, MacTavish P, McPeake J, Montgomery-Yates A, Needham DM, Netzer G, Schorr C, Skidmore B, Stollings JL, Umberger R, Andrews A, Iwashyna TJ, Sevin CM. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit Care Med. 2020;48(11):1670-1679. [PubMed: 32947467]
- 123.
- Geense WW, van den Boogaard M, van der Hoeven JG, Vermeulen H, Hannink G, Zegers M. Nonpharmacologic Interventions to Prevent or Mitigate Adverse Long-Term Outcomes Among ICU Survivors: A Systematic Review and Meta-Analysis. Crit Care Med. 2019;47(11):1607-1618. [PubMed: 31464769]
- 124.
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316. [PMC free article: PMC3922401] [PubMed: 24088092]
- 125.
- Zante B, Camenisch SA, Schefold JC. Interventions in Post-Intensive Care Syndrome-Family: A Systematic Literature Review. Crit Care Med. 2020;48(9):e835-e840. [PubMed: 32590386]
- 128.
- Velissaris D, Karamouzos V, Kotroni I, Pierrakos C, and Karanikolas M. The Use of Pulmonary Artery Catheter in Sepsis Patients: A Literature Review J Clin Med Res. 2016 Nov; 8(11): 769–776. [PMC free article: PMC5047014] [PubMed: 27738477]
- 129.
- Chen H, Zhu Z, Zhao C, Guo Y, Chen D, Wei Y, Jin J. Central venous pressure measurement is associated with improved outcomes in septic patients: an analysis of the MIMIC-III database. Crit Care. 2020 Jul 14;24(1):433 [PMC free article: PMC7358999] [PubMed: 32665010]
- 130.
- Gajbe S, Sona Dave S, and Kasimahanti R. Central Venous Oxygen Saturation as a Surrogate Marker for Outcome in Critically ill Patients-A Prospective Observational Cohort Study. J Anest & Inten Care Med 6(5): JAICM.MS.ID.555698 (2018)
- 131.
- Khan RA, Khan NA, Bauer SR, Li M, Duggal A, Wang X, Reddy AJ. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients With Sepsis, Heart Failure, End-Stage Renal Disease, and Cirrhosis. Chest. 2020 Feb;157(2):286-292. [PubMed: 31622591]
- 132.
- Taenzer AH, Patel SJ, Allen TL, Doerfler ME, Park TR, Savitz LA, Park JG; Members of the High Value Healthcare Collaborative. Improvement in Mortality With Early Fluid Bolus in Sepsis Patients With a History of Congestive Heart Failure. Mayo Clin Proc Innov Qual Outcomes. 2020 Aug 19;4(5):537-541. [PMC free article: PMC7557190] [PubMed: 33083702]
- 133.
- Khan RA, Khan NA, Bauer SR, Li M, Duggal A, Wang X, Reddy AJ. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients With Sepsis, Heart Failure, End-Stage Renal Disease, and Cirrhosis. Chest. 2020 Feb;157(2):286-292. [PubMed: 31622591]
- 134.
- Acharya R, Patel A, Schultz E, Bourgeois M, Kandinata N, Paswan R, Kafle S, Sedhai YR, Younus U. Fluid resuscitation and outcomes in heart failure patients with severe sepsis or septic shock: A retrospective case-control study. PLoS One. 2021 Aug 19;16(8):e0256368. [PMC free article: PMC8376054] [PubMed: 34411178]
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Created: November 2023.
Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/