From: The TRP Calcium Channel and Retinal Degeneration

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
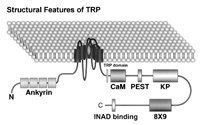
Putative domain structure and topology of Drosophila TRP. TRP contains six putative transmembrane helices S1-S6 and a putative pore region between S5 and S6, S3-S6 show sequence fragments identical to the equivalent regions of the dihydropyridine receptor (a vertebrate voltage-gated Ca2+ channel). TRP and all TRP homologue channels have 3–4 consensus ankyrin motifs towards the amino terminus. There is a highly conserved TRP domain with still unknown function and one putative calmodulin-binding site (CaM) in the TRP sequence. The TRP protein has a curious and unique hydrophilic sequence near the carboxyl terminus consisting of nine repeats of the sequence: DKDKKPG/AD (8×9), a PEST sequence, a proline-rich region with the dipeptide KP, repeating 27 times (KP), an INAD binding domain at the end of the C-terminus. (From Minke and Selinger 1996, with permission from Current Opinion in Neurobiology)
From: The TRP Calcium Channel and Retinal Degeneration

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.