From: Regulation of the Rod Photoreceptor Cyclic Nucleotide-Gated Channel

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
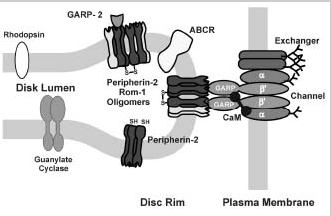
Diagram depicting the interaction of the rod channel with other ROS proteins as revealed by immunoprecipitation and cross-linking studies. In the plasma membrane, the heterotetrameric cGMP-gated channel (depicted as consisting of two α-subunits and two β-subunits) interacts with Na/Ca-K exchanger39,55,56 previously shown to exist as a dimer in the membrane.58 The β-subunit of the channel binds endogenous CaM in a Ca2+-dependent manner as shown in this study. The channel:exchanger complex also interacts with peripherin-2-containing oligomers in the rim region of disc membranes. This interaction is mediated by the GARP part of channel β-subunit. GARP2 and less abundant GARP1 (not shown) are confined to the rim region of disc membranes through their interactions with peripherin-2 homomeric and peripherin-2:rom1 heteromeric disulfide-linked oligomeric and core complexes.38,39 Disc proteins ABCR and GC1 (guanylate cyclase) do not form stable associations with the cyclic GMP-gated channel or soluble GARP proteins.
From: Regulation of the Rod Photoreceptor Cyclic Nucleotide-Gated Channel

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.