NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Constitutional chromosome abnormalities are seen frequently in infertile men. The consequences of these abnormalities in sperm are outlined. Infertile men with a normal chromosome karyotype also have an increased frequency of chromosome abnormalities in their sperm and a review of these studies is presented. New research methods such as single sperm PCR and immunocytogenetic analysis of the synaptonemal complex have presented exciting new ways to study the etiology of chromosome abnormalities in humans and may provide prognostic information that assists in the management of infertile patients.
Introduction
Chromosome abnormalities are surprisingly common in humans, with an incidence of approximately 0.6% present in newborns, 6% in stillbirths and more than 60% in spontaneous abortions. Most chromosome abnormalities are lethal and are lost early in embryologic development, manifesting as infertility or spontaneous abortions. Some chromosomal abnormalities survive to term, for example trisomy 13, 18, 21 and aneuploidy for the sex chromosomes as well as certain chromosomal rearrangements such as translocations and inversions. Individuals with these chromosome anomalies can have mental and physical disabilities, infertility, behavioural problems and/or impaired sexual development.
Most chromosome abnormalities have their origin during meiosis, thus information on the causes of chromosome abnormalities and factors affecting their frequency are best studied in human gametes. During the past two decades there has been an explosion of information on the chromosome complement of human spermatozoa. This has been stimulated by the advent of new techniques: human sperm karyotyping using the hamster oocyte system, fluorescence in situ hybridization (FISH) analysis, single sperm polymerase chain reaction (PCR) analysis, and synaptonemal complex (SC) analysis.
Rudak et al1 first reported the use of hamster ova to reactivate human sperm allowing the analysis of pronuclear chromosomes. Other laboratories followed with the fist publications on the frequency and type of chromosome abnormalities in human sperm.2 - 5 Human sperm karyotyping, using the hamster system, provides detailed information about each individual chromosome, permitting analysis of both numerical and structural abnormalities. However, there are significant disadvantages of this approach: sperm must be capable of fertilizing hamster oocytes, the technique is very difficult, time-consuming, expensive and the data yield is small. In fact, only 12 laboratories world-wide have had success with this technique despite many efforts.
Fluorescent in situ hybridization analysis with chromosome-specific DNA probes provides a faster, cheaper, easier alternative for detecting numerical abnormalities (aneuploidy) in human sperm.6 - 8 Also, sperm with defects in fertilization ability and/or motility can be assessed using FISH analysis.9 - 11 Single sperm PCR is a difficult, time-consuming technique but it can be used in a very powerful manner for specific studies on recombination and the etiology of chromosome abnormalities.12 , 13 Finally, a new immunocytogenetic technique allows assessment of early meiosis including the fidelity of chromosome pairing and recombination. 14 - 16 Some of the studies on infertile men, using these various techniques, will be summarized in this review.
Studies in Infertile Men
Infertile men have an increased risk of chromosome abnormalities in their sperm; these abnormalities can be passed on to their offspring. This has become more clinically relevant with the advent of intracytoplasmic sperm injection (ICSI) since even men with extremely poor sperm parameters can successfully father a pregnancy. The chromosome abnormalities in sperm can occur because of constitutional chromosome abnormalities in the somatic cells of the infertile men (e.g., translocation or sex chromosomal aneuploidy) or because of a susceptibility to nondisjunction in chromosomally normal infertile men.
Men with Constitutional Chromosome Abnormalities
Constitutional chromosome abnormalities are relatively common in humans. These can be numerical chromosome abnormalities, such as men with an extra sex chromosome or structural abnormalities, such as translocations. Many of these abnormalities are associated with infertility and an increased risk of pregnancy loss. The frequency of constitutional chromosomal abnormalities in infertile men varies between 2 and 14%, depending on the severity of the infertility and the nature of the pathology.17 Constitutional abnormalities have been studied using the hamster oocyte system, which provides precision in the human sperm karyotypes. 18,19 In the past decade, FISH analysis has been utilized with centromeric and telomeric probes to determine chromosome segregations and take advantage of the larger sample sizes to assess interchromosomal effects.20 An interchromosomal effect refers to the possibility that abnormalities in the segregation of one chromosome might affect the segregation of other chromosomes. For example, aneuploidy of a sex chromosome might affect the segregation of an autosome.
Sex Chromosomal Abnormalities
Men with a 47,XYY karyotype generally produce normal children but there has been no systematic study of children born to those men. Theoretically 50% of the sperm cells should be abnormal. In a study of 75 sperm karyotypes from a 47,XYY male we found no sperm disomic for a sex chromosome.21 Our results supported the hypothesis that the extra sex chromosome is eliminated during spermatogenesis. FISH analysis on the same male with 10,000 sperm studied demonstrated a small but significant increase for XY disomy to 0.6%.22 Similar FISH studies by other laboratories have demonstrated increased frequencies of sperm aneuploidy for the sex chromosomes ranging from 0.3%23 to 15%24 (see ref. 25 for a review). However, when only the more stringent three-colour FISH studies are assessed, the frequency of 24,YY or 24,XY sperm was ≤1%.25 There has been no compelling evidence for an interchromosomal effect in these men.
Men with Klinefelter syndrome (47,XXY) or mosaic variants (e.g., 47,XXY/46,XY) generally have severe oligozoospermia or azoospermia and sperm can sometimes be obtained by a testicular biopsy. FISH analysis has demonstrated that the frequency of aneuploidy for the sex chromosomes varies from 1.5%26 to 7%27 in sperm from Klinefelter mosaics and 2%28 to 25%29 in the sperm of men who appear to have a nonmosaic 47,XXY karyotype (for review see ref. 17). Chromosomally normal offspring as well as conceptions with a 47,XXY karyotype have been reported in Klinefelter Syndrome males who have fathered a pregnancy through intracytoplasmic sperm injection (ICSI).17
Translocations
Robertsonian translocation carriers have a fusion of the long arms of two acrocentric chromosomes resulting in a balanced state with 45 chromosomes. Pairing of the chromosomes at meiosis can lead to chromosomally balanced and unbalanced gametes. Sperm karyotyping studies have demonstrated that 3-27% of the spermatozoa are unbalanced.30 FISH studies in 13 Robertsonian translocation heterozygotes have shown similar frequencies of imbalance varying from 7%31 to 26%.20 Interchromosomal effects were reported in 4 studies (e.g., see refs. 32, 33) but none of the increased disomy frequencies were higher than 1%.
Reciprocal translocations occur when there are exchanges of chromosome material between any chromosomes. During meiosis, 4 chromosomes must pair in reciprocal translocation heterozygotes and the resulting segregations have a higher frequency of unbalanced chromosomes than Robertsonian translocations. Sperm karyotyping studies of over 30 reciprocal translocation heterozygotes have shown that 19-77% of spermatozoa are unbalanced.18 FISH analyses of chromosome segregations in over 30 carriers have reported frequencies of unbalanced chromosomes ranging from 49%-81%.34 , 35 In one interesting study, 4 male family members of a kindred segregating a chromosome 15; 17 translocation, were studied by FISH analysis.36 The segregation patterns were very similar in all 4 men with approximately 50% of sperm chromosomally unbalanced. This demonstrates the reproducibility of the method. Because the frequency of chromosome abnormality is very high, some men carrying reciprocal translocations have undergone preimplantation genetic diagnosis (PGD) in order to implant only chromosomally normal or balanced embryos. Studies comparing the frequency of chromosome abnormalities in sperm and embryos from reciprocal translocation carriers show a very close agreement in the abnormality frequencies.20 Indeed, it has been shown that the incidence of abnormal sperm from translocation carriers is predictive of the proportion of aneuploid embryos they will produce during a PGD cycle. This in turn has a direct influence on the patient's chances of becoming pregnant. Many FISH studies have assessed the possibility of an interchromosomal effect in reciprocal translocation heterozygotes. An increased frequency for at least one type of disomy was reported in 58% of carriers.17 Disomy frequencies were 1.5 to 6.6 times those observed in control donors. However, preliminary evidence from studies of preimplantation genetic diagnosis suggests an interchromosomal effect for Robertsonian translocations, but not for reciprocal translocation.37
Infertile Men with a Normal Somatic Karyotype
Infertile men with a constitutional chromosomal abnormality clearly have an increased risk for sperm with chromosome anomalies. Recently, it has been determined that even infertile men with a normal somatic karyotype produce sperm with a higher frequency of chromosome abnormalities. Early studies of infertile men studied patients with mixed types of infertility or oligoaesthenoteratozoospermia (OAT). The great majority of studies that employed multicolour FISH found a significantly increased frequency of chromosome aneuploidy for the autosomes and particularly for the sex chromosomes (e.g., see refs. 6, 38-40, for review see ref. 17). Reports based on prenatal diagnosis of ICSI pregnancies have indicated a risk of de novo chromosome abnormalities of approximately 2%41 and sex chromosomal abnormalities have been shown to be of paternal origin.42
These studies tended to group all types of infertility together but it is possible that some subsets of infertility have an increased risk while others do not. Some studies have started to address this question. Our laboratory has found that men with oligozoospermia (<20 million sperm/ml) demonstrated an inverse relationship between sperm concentration and the frequency of chromosome abnormalities.42 Men with teratozoospermia (>70% abnormal forms) but normal sperm concentration or with aesthenozoospermia (<50% motile sperm) and a normal sperm concentration also had an increased risk of sperm chromosome abnormalities.10,43 Other laboratories have also determined that various perturbations in spermatogenesis leading to abnormal semen profiles lead to sperm chromosome abnormalities.44 Furthermore, some researchers have suggested that abnormalities of the centrosome may exist in surgically retrieved sperm, a problem that may lead to increased mitotic nondisjunction and mosiacism in resulting embryos.45
In the last few years there have been a number of studies on testicular sperm, obtained by biopsy for ICSI from azoospermic patients (no sperm in the ejaculate). There has been concern that these infertile men might have greatly elevated frequencies of chromosome abnormalities in their sperm. In fact, studies to date have indicated that those men tend to have elevations in the frequencies of sperm chromosome abnormalities similar to that seen in infertile men with sperm in the ejaculate.45- 47 However, it must be emphasized that the majority of studies on testicular biopsies are based on very small sample sizes of men and sperm, and individual men might have quite varied frequencies of sperm chromosome abnormalities.
Recombination Analysis by Single Sperm Typing
Our studies and those of others have demonstrated that the frequency of aneuploidy in sperm is elevated for the sex chromosomes compared to the autosomes.48- 51 The XY bivalent normally has only one crossover in the pseudoautosomal region during meiosis. If the frequency of recombination is reduced or absent for these chromosomes, they may be particularly susceptible to nondisjunction. Indeed, it has been shown that 47,XXY of paternal origin is associated with a decreased recombination frequency.52 For a direct test of whether recombination is associated with nondisjunction in human sperm, we performed single sperm PCR analysis for a sex specific locus (STS/STS pseudogene) and a pseudoautosomal locus (DXY515).12 Individual unisomic sperm (23,X or Y) were isolated using a FACStarPlus flow cytometer into PCR tubes. To identify disomic 24,XY sperm, 3-colour FISH analysis was performed with probes for chromosomes X, Y and 1. The 24,XY cells were identified using fluorescence microscopy, each disomic sperm was scraped off the slide using a glass needle attached to a micromanipulator and then put into a PCR tube. Heminested PCR analysis of the two markers was performed to determine the frequency of recombination. The frequency of recombination between the two DNA markers was 38% for the normal unisomic sperm compared to 25% for the 24,XY disomic sperm that had undergone nondisjunction. This difference was highly significant and demonstrates that lack of recombination in the pseudoautosomal region is associated with XY nondisjunction and the production of aneuploid sperm.
Synaptonemal Complex Analysis
The discovery that lack of recombination is associated with nondisjunction is significant because it provides a definite molecular correlate with aneuploidy. The next logical step is to assess recombination in other chromosomes. However, this is extremely difficult and time-consuming by single sperm PCR analysis. Luckily new immunocytogenetic techniques allow assessment of recombination and chromosome pairing by visualization of the synaptonemal complex in early meiosis.15,16,53 Various important meiotic structures can be identified by the use of immunofluorescence. Antibodies against SCPI (transverse elements) or SCP3 (lateral elements) can be used to visualize the synaptonemal complexes (SCs, the proteinaceous structure linking homologous chromosomes in prophase of meiosis 1). The centromere can be localized with CREST antisera. Most importantly, recent studies have demonstrated that antibodies against the DNA mismatch repair protein MLH1 identify the sites of meiotic exchange in SCs in both mouse54,55 and human spermatocytes15,16 (fig. 1).
To determine if some of the defects in infertile men are caused by irregularities in chromosome pairing and recombination during prophase of meiosis 1, we have initiated studies in men with azoospermia using the newly developed immunocytogenetic technique. We have analyzed meiotic cells (obtained by testicular biopsy) in azoospermic men. Some men had no meiotic cells at all, one man had all cells in the zygotene stage with sister chromatids paired but a block in the ability to progress to pachytene (synapsis of homologous chromosomes) and several men had pachytene cells with a decreased frequency of recombination foci and a variety of abnormalities in chromosome pairing. Our preliminary results suggest that meiotic abnormalities of chromosome pairing and recombination are a significant cause of male infertility.
Conclusions
Cytogenetic analysis of somatic cells, sperm cells and testicular tissue are all important in the diagnosis of male infertility and in determining the etiology of chromosome abnormalities.
Acknowledgements
Renée H. Martin holds the Canada Research Chair in Genetics and her research is supported by grant MA-7961 from the Canadian Institutes of Health Research.
References
- 1.
- Rudak E, Jacobs P, Yanagimachi R. Direct analysis of the chromosome constitution of human spermatozoa. Nature. 1978;274:911–913. [PubMed: 683334]
- 2.
- Martin R, Lin C, Balkan W. et al. Direct chromosomal analysis of human spermatozoa: preliminary results from 18 normal men. Am J Hum Genet. 1982;34(3):459–468. [PMC free article: PMC1685332] [PubMed: 7081223]
- 3.
- Martin R, Balkan W, Burns K. et al. The chromosome constitution of 1000 human spermatozoa. Hum Genet. 1983;63(4):305–309. [PubMed: 6683243]
- 4.
- Brandriff B, Gordon L, Ashworth L. et al. Chromosomes of human sperm: variability among normal individuals. Hum Genet. 1985;70:18–24. [PubMed: 3838965]
- 5.
- Mikamo K, Kamiguchi Y, Tateno H. Spontaneous and in vitro radiation-induced chromosome aberrations in human spermatozoa: Application of a new methodIn: Mendelsohn ML, Albertini RJ, eds.Mutation and the Environment Part B: Metabolism, Testing Methods, and ChromosomesToronto: John Wiley & Sons, Inc,1990447–456. [PubMed: 2392450]
- 6.
- Moosani N, Pattinson H, Carter M. et al. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil Steril. 1995;64(4):811–817. [PubMed: 7672155]
- 7.
- Shi Q, Martin R. Spontaneous frequencies of aneuploid and diploid sperm in 10 normal Chinese men: assessed by multicolor fluorescence in situ hybridization. Cytogenet Cell Genet. 2000;90(1-2):79–83. [PubMed: 11060453]
- 8.
- Wyrobek A, Robbins W, Mehraein Y. et al. Detection of sex chromosomal aneuploidies X-X, Y-Y, and X-Y in human sperm using two-chromosome fluorescence in situ hybridization. Am J Med Genet. 1994;53(1):1–7. [PubMed: 7802028]
- 9.
- Martin R, Ernst S, Rademaker A. et al. Analysis of sperm chromosome complements before, during, and after chemotherapy. Cancer Genet Cytogenet. 1999;108(2):133–136. [PubMed: 9973940]
- 10.
- Hristova R, Ko E, Greene C. et al. Chromosome abnormalities in sperm from infertile men with aesthenoteratozoospermia. Biol Reprod. 2002;66:1781–1783. [PubMed: 12021062]
- 11.
- Robbins WA, Meistrich ML, Moore D. et al. Chemotherapy induces transient sex chromosomal and autosomal aneuploidy in human sperm. Nat Genet. 1997;16(1):74–8. [PubMed: 9140398]
- 12.
- Shi Q, Spriggs E, Field L. et al. Single sperm typing demonstrates that reduced recombination is associated with the production of aneuploid 24,XY human sperm. Am J Med Genet. 2001;99(1):34–38. [PubMed: 11170091]
- 13.
- Shi Q, Spriggs E, Field L. et al. Absence of age effect on meiotic recombination between human X and Y chromosomes. Am J Hum Genet. 2002;71:254–261. [PMC free article: PMC379158] [PubMed: 12046006]
- 14.
- Sun F, Oliver-Bonet M, Liehr T. et al. Human male recombination maps for individual chromosomes. Am J Hum Genet. 2004;74(3):521–531. [PMC free article: PMC1182265] [PubMed: 14973780]
- 15.
- Barlow AL, Hultén MA. Crossing over analysis at pachytene in man. Euro J Hum Genet. 1998;6(4):350–358. [PubMed: 9781043]
- 16.
- Lynn A, Koehler KE, Judis L. et al. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science. 2002;296:2222–2225. [PubMed: 12052900]
- 17.
- Shi Q, Martin R. Aneuploidy in human spermatozoa: FISH analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction. 2001;121(5):655–666. [PubMed: 11427153]
- 18.
- Martin R, Spriggs E. Sperm chromosome complements in a man heterozygous for a reciprocal translocation 46,XY,t(9;13)(q21.1;q21.2) and a review of the literature. Clin Genet. 1995;47(1):42–46. [PubMed: 7774043]
- 19.
- Martin R. Sperm chromosome analysis in a man heterozygous for a paracentric inversion of chromosome 14 (q24.1q32.1). Am J Hum Genet. 1999;64(5):1480–1484. [PMC free article: PMC1377889] [PubMed: 10205284]
- 20.
- Escudero T, Abdelhadi I, Sandalinas M. et al. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril. 2003;79(Suppl 3):1528–1534. [PubMed: 12801555]
- 21.
- Benet J, Martin R. Sperm chromosome complements in a 47,XYY man. Hum Genet. 1988;78(4):313–315. [PubMed: 3360445]
- 22.
- Martin R, McInnes B, Rademaker A. Analysis of aneuploidy for chromosomes 13, 21, X and Y by multicolour fluorescence in situ hybridisation (FISH) in a 47,XYY male. Zygote. 1999;7(2):131–134. [PubMed: 10418106]
- 23.
- Chevret E, Rousseaux S, Monteil M. et al. Meiotic behaviour of sex chromosomes investigated by three-colour FISH on 35,142 sperm nuclei from two 47,XYY males. Hum Genet. 1997;99(3):407–412. [PubMed: 9050931]
- 24.
- Mercier S, Morel F, Roux C. et al. Analysis of the sex chromosomal equipment in spermatozoa of a 47,XYY male using two-colour fluorescence in-situ hybridization. Mol Hum Reprod. 1996;2(7):485–488. [PubMed: 9239657]
- 25.
- Shi Q, Martin R. Multicolor fluorescence in situ hybridization analysis of meiotic chromosome segregation in a 47,XYY male and a review of the literature. Am J Med Genet. 2000;93(1):40–46. [PubMed: 10861680]
- 26.
- Lim A, Fong Y, Yu S. Estimates of sperm sex chromosome disomy and diploidy rates in a 47,XXY/ 46,XY mosaic Klinefelter patient. Hum Genet. 1999;104:405–409. [PubMed: 10394932]
- 27.
- Kruse R, Guttenbach M, Shartmann B. et al. Genetic counselling in a patient with XXY/XXXY/XY mosaic Klinefelter's syndrome: estimate of sex chromosome aberrations in sperm before intracytoplasmic sperm injection. Fertil Steril. 1998;69:432–485. [PubMed: 9531882]
- 28.
- Rives N, Joly G, Machy A. et al. Assessment of sex chromosome aneuploidy in sperm nuclei from 47,XXY and 46,XY/47,XXY males: comparison with fertile and infertile males with normal karyotype. Mol Hum Reprod. 2000;6(2):107–112. [PubMed: 10655452]
- 29.
- Estop A, Munne S, Cieply K. et al. Meiotic products of a Klinefelter 47,XXY male as determined by sperm fluorescence in-situ hybridization analysis. Hum Reprod. 1998;13(1):124–127. [PubMed: 9512242]
- 30.
- Martin R. Sperm cell—genetic aspectsIn: Grudzinskas JG, Yovich JL, Simpson JL et al, eds.Cambridge reviews in human reproductionCambridge, England: Cambridge University Press,1995104–121.
- 31.
- Fryndman N, Romana S, Le Lorc'h M. et al. Assisting reproduction of infertile men carrying a Robertsonian translocation. Hum Reprod. 2001;16:2274–2277. [PubMed: 11679503]
- 32.
- Rousseaux S, Chevret E, Monteil M. et al. Sperm nuclei analysis of a Robertsonian t(14q21q) carrier, by FISH, using three plasmids and two YAC probes. Hum Genet. 1995;96(6):655–660. [PubMed: 8522322]
- 33.
- Morel F, Roux C, Bresson JL. FISH analysis of the chromosomal status of spermatozoa from three men with 45,XY,der(13;14)(q10;q10) karyotype. Mol Hum Reprod. 2001;7:483–488. [PubMed: 11331672]
- 34.
- Giltay J, Kastrop P, Tiemessen C. et al. Sperm analysis in a subfertile male with a Y;16 translocation, using four-color FISH. Cytogenet Cell Genet. 1999;84:67–72. [PubMed: 10343106]
- 35.
- Geneix A, Schubert B, Force A. et al. Sperm analysis by FISH in a case of t(17;22)(q11;q12) balanced transloction. Hum Reprod. 2002;17:325–331. [PubMed: 11821272]
- 36.
- Cora T, Acar H, Kaynak M. Molecular cytogenetic detection of meiotic segregation patterns in sperm nuclei of carriers of 46,XY,t(15;17)(q21;q25). J Androl. 2002;23:793–798. [PubMed: 12399524]
- 37.
- Gianaroli L, Magli MC, Ferraretti AP. et al. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17(12):3301–3207. [PubMed: 12456624]
- 38.
- McInnes B, Rademaker A, Greene C. et al. Abnormalities for chromosomes 13 and 21 detected in spermatozoa from infertile men. Hum Reprod. 1998;13(1O):2787–2790. [PubMed: 9804231]
- 39.
- Pang M, Hoegerman S, Cuticchia A. et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14(5):1266–1273. [PubMed: 10325276]
- 40.
- Vegetti W, Van AsscheE, Frias A. et al. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in situ hybridization in infertile men. Hum Reprod. 2000;15:351–365. [PubMed: 10655307]
- 41.
- Van SteirteghemA, Bonduelle M, Devroey P. et al. Follow-up of children born after ICSI. Hum Reprod Update. 2002;8(2):111–116. [PubMed: 12099626]
- 42.
- Van OpstalD, Los F, Ramlakhan S. et al. Determination of the parent of origin in nine cases of prenatally detected chromosome aberrations found after intracytoplasmic sperm injection. Hum Reprod. 1997;12:682–686. [PubMed: 9159424]
- 43.
- Templado C, Hoang T, Greene C. et al. Aneuploid spermatozoa in infertile men: teratozoospermia. Mol Reprod Dev. 2002;61:200–204. [PubMed: 11803555]
- 44.
- Pellestor F, Girardet A, Coignet L. et al. Cytogenetic analysis of meiotic segregation in sperm from two males heterozygous for reciprocal translocations using PRINS and humster techniques. Cytogenet Cell Genet. 1997;78:202–208. [PubMed: 9465888]
- 45.
- Silber S, Escudero T, Lenahan K. et al. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79:30–8. [PubMed: 12524060]
- 46.
- Burello N, Calogero AE, De Palma A. et al. Chromosome analysis of epididymal and testicular spermatozoa in patients with azoospermia. Euro J Hum Genet. 2002;10:362–366. [PubMed: 12080387]
- 47.
- Levron J, Aviain-Goldring A, Madgar I. et al. Sperm chromosome abnormalities in men with severe male factor infertility who are undergoing in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2001;76:479–484. [PubMed: 11532468]
- 48.
- Spriggs E, Rademaker A, Martin R. Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet. 1996;58(2):356–362. [PMC free article: PMC1914531] [PubMed: 8571962]
- 49.
- Martin R, Ko E, Rademaker A. Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am J Med Genet. 1991;39(3):321–331. [PubMed: 1867285]
- 50.
- Williams BJ, Ballenger CA, Malter HE. et al. Non-disjunction in human sperm: results of fluorescence in situ hybridization studies using two and three probes. Hum Mol Genet. 1993;2:1929–1936. [PubMed: 8281157]
- 51.
- Scarpato R, Naccarati A, Mariani M. et al. Aneuploidy and diploidy rates in sperm of five men after three-colour hybridization: indication of X chromosome-associated autosome 2 aneuploidy. Mutat Res. 1998;412(3):227–233. [PubMed: 9600690]
- 52.
- Hassold T, Sherman S, Pettay D. et al. XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet. 1991;49(2):253–260. [PMC free article: PMC1683286] [PubMed: 1867189]
- 53.
- Sun F, Kozak G, Scott S. et al. Meiotic defects in a man with non-obstructive azoospermia: Case report. Hum Reprod. 2004;19(8):1770–3. [PubMed: 15205399]
- 54.
- Baker SM, Plug AW, Prolla TA. et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13(3):336–42. [PubMed: 8673133]
- 55.
- Anderson LK, Reeves A, Webb LM. et al. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics. 1999;151(4):1569–1579. [PMC free article: PMC1460565] [PubMed: 10101178]
Figures
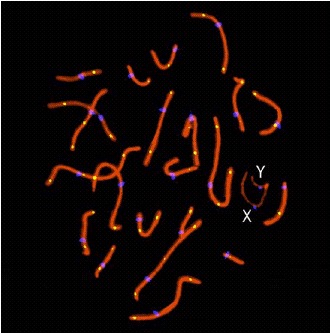
Figure 1
Example of human spermatocyte in pachytene stage. Chromosome bivalents are paired with 22 mature autosomal synaptonemal complexes (red) and sex chromosomes (marked X and Y). Centromeres are shown in blue (CREST). The MLH1 foci (yellow) indicate recombination sites. A color version of this figure may be viewed at http://www.Eurekah.com.