From: Molecular Interaction Network of the Hsp90 Chaperone System

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
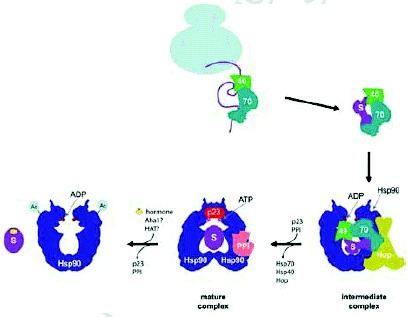
The Hsp90 functional cycle. A nascent polypeptide chain of a steroid hormone receptor, for example, is initially recognized by the Hsp70/Hsp40 system. Hop (yeast Sti1) binds both Hsp70 and Hsp90 to form the intermediate complex allowing the transfer of the substrate protein to Hsp90. Subsequently, p23 (yeast Sba1) and other cyclophilins and immunophilins (indicated by PPI) bind Hsp90, releasing Hsp70/Hsp40 and Hop. In the resulting mature complex, Hsp90 is stabilized in the ATP state by p23. If the substrate is a protein kinase, Cdc37 is also involved in stabilizing this complex (not shown). Upon entry of the hormone into the cell, the receptor binds the hormone and is released from Hsp90. The release is promoted by Aha1 which enhances the ATPase activity of the chaperone and, in mammalian cells, is also possibly promoted by the acetylation of the chaperone.
From: Molecular Interaction Network of the Hsp90 Chaperone System

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.